Abstract
Metabolic engineering has allowed the production of a diverse number of valuable chemicals using microbial organisms. Many biological challenges for improving bio-production exist which limit performance and slow the commercialization of metabolically engineered systems. Dynamic metabolic engineering is a rapidly developing field that seeks to address these challenges through the design of genetically encoded metabolic control systems which allow cells to autonomously adjust their flux in response to their external and internal metabolic state. This review first discusses theoretical works which provide mechanistic insights and design choices for dynamic control systems including two-stage, continuous, and population behavior control strategies. Next, we summarize molecular mechanisms for various sensors and actuators which enable dynamic metabolic control in microbial systems. Finally, important applications of dynamic control to the production of several metabolite products are highlighted, including fatty acids, aromatics, and terpene compounds. Altogether, this review provides a comprehensive overview of the progress, advances, and prospects in the design of dynamic control systems for improved titer, rate, and yield metrics in metabolic engineering.
Keywords: Dynamic metabolic control, Dynamic metabolic engineering, Synthetic biology, Biosensors, Genetic circuits
1. Introduction
In research laboratory settings, metabolic engineering has enabled the production of a vast array of metabolite products including fuels (Jiang et al., 2018, 2017; Yan and Pfleger, 2020), chemicals (Bai et al., 2019; Bowen et al., 2016; Reed and Alper, 2018), medicines (Cao et al., 2020), and polymer precursors (Zheng et al., 2020), using dozens of microbial species from bacteria (Becker et al., 2018; Pontrelli et al., 2018) to eukarya (Abdel-Mawgoud et al., 2018; Lian et al., 2018) and archaea domains (Crosby et al., 2019). However, commercial production of these compounds in industrial scales has been lagging, largely due to the inability of the engineered strains to maintain stable performance at large scales while meeting stringent titer, rate, and yield (TRY) requirements.
Metabolic engineers have faced a myriad of challenges in forcing engineered microbes to over-produce metabolite products. Engineered metabolic pathways utilize shared host machinery, including RNA polymerases, ribosomes, ATP, cofactors, and other native metabolites. Competition on cellular resources is sensitive to fermentation conditions and can cause metabolic burden (Kurland and Dong, 1996), improper cofactor balance (Bentley et al., 2016; Charusanti et al., 2010), or accumulation of metabolites to toxic levels (Kizer et al., 2008), all of which can interfere with the growth and desired metabolic objective of the engineered microbes. Additionally, cells growing in large-scale bioreactors often experience different and changing microenvironments, leading to heterogeneity in their performance (Delvigne et al., 2014; Pigou and Morchain, 2015). These issues constrain metabolite production and give advantage to fast-growing, yet non-productive isogenic cells or mutant strains (Rugbjerg et al., 2018a), which ultimately lowers overall TRY performance (Zhuang et al., 2013). Optimizing strain performance through design-build-test cycles is lengthy and costly. The cost of commercializing a metabolite product was estimated to range from $100 million to $1 billion (Crater and Lievense, 2018; Wehrs et al., 2019).
Dynamic metabolic engineering seeks to address these challenges through the development of genetically encoded control systems which allow microbes to autonomously adjust their metabolic flux in response to the external environment and/or internal metabolic state. The concept is inspired by natural metabolic control systems which microbes use to maintain homeostasis, coordinate metabolic flux (Chubukov et al., 2014; Kochanowski et al., 2017), and adapt metabolism to changing environments (Chin et al., 2008) and stresses (Jozefczuk et al., 2010). These dynamic control systems are in contrast to static control systems traditionally used in metabolic engineering, where metabolic pathways are expressed constitutively and are tuned by the choice of promoters, ribosome binding sites, and gene copy numbers (Holtz and Keasling, 2010). Since the first demonstration of enhanced lycopene production two decades ago (Farmer and Liao, 2000), dynamic metabolic engineering has become a popular strategy. Recent advances in synthetic biology and systems biology have provided the tools for dynamic metabolic engineering while control theory has provided new design principles for expanding beyond natural control systems. There are multiple examples where dynamic metabolic control has provided microbes remarkable robustness in different fermentation conditions and improved TRY performance (Anesiadis et al., 2008; Cress et al., 2015; Liu et al., 2018).
In this work, we seek to provide a comprehensive review of the theory and practice of dynamic metabolic engineering, focusing on its improvement to TRY metrics. In the first section, we will present theoretical works that discuss the benefits of incorporating dynamic metabolic control as well as works that provide guidance on design choices for the construction of control systems. In the second section, we will review the construction and engineering of sensors and control mechanisms, referred to as actuators, the two fundamental components of dynamic metabolic control systems. Finally, we will review metabolic pathways to which dynamic metabolic engineering has been applied with the focus on control topologies.
2. Theoretical insights and design choices for metabolic dynamic control
Several design choices need to be made before implementing a dynamic control system in a metabolic pathway. In this section, we summarize the theoretical concepts and benefits of three popular dynamic control strategies: two-stage metabolic switches, continuous metabolic control, and population behavior control. Additionally, for each section we highlight theoretical works which elucidate the design choices for improving TRY metrics.
2.1. Two-stange metabolic control systems
A two-stage metabolic switch is a straightforward yet effective dynamic control strategy for overcoming numerous trade-offs inherent in engineered metabolic bioprocesses (Burg et al., 2016; Venayak et al., 2015). A two-stage metabolic switch decouples the competing tasks of biomass accumulation and metabolite overproduction. In the first stage, cells are engineered to focus on rapid cell growth, usually with minimal production of the product. In the second stage, cell growth is minimized while substrate fluxes are funneled into product formation. This contrasts with a one-stage process where biomass accumulation and product formation occur concurrently. One early example of two-stage process is the batch production of glycerol and ethanol in Escherichia coli (Gadkar et al., 2005). The work found that glycerol concentration could be improved by 30% with a flux switch during production as compared to maintaining constant glycerol flux throughout the batch process (Fig. 1A). In the modeled one-stage production, biomass accumulated more slowly than the optimal two-stage production, leading to slower volumetric productivity and a lower glycerol titer. This demonstrates the value of a two-stage production for optimizing productivity, which can be a critical consideration for commercializing a bioprocess (Zhuang et al., 2013).
Fig. 1. Overview of theoretical benefits of dynamic metabolic control.
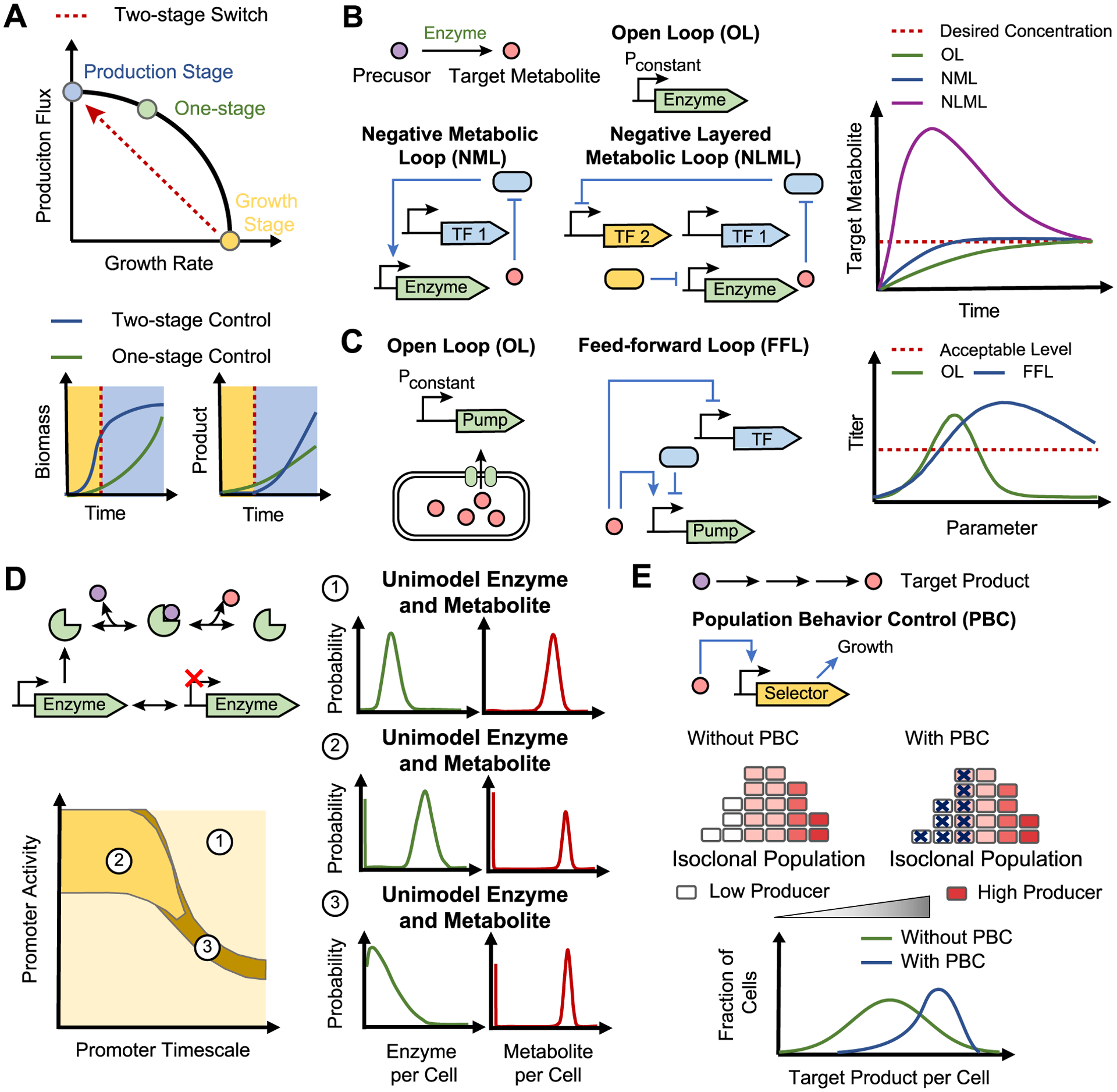
(A) Comparison of one-stage control with two-stage dynamic control strategy. Lower graphs show theoretical biomass and product formation over time for each strategy (one-stage control, green line; two-stage control, blue line), with yellow and blue shaded regions marking the growth and production phases respectively (Gadkar et al., 2005). (B) Comparison of open loop strategy (green) with continuous dynamic control strategies (NML, blue; NLML, purple) for accelerating metabolic response to input (Liu and Zhang, 2018). Blue oval shapes represent metabolite-responsive transcription factors. Graph shows metabolite concentration over time for each architecture with the desired metabolite concentration in red. (C) Comparison of open loop control (green) with a continuous dynamic control (blue) for reducing parametric sensitivity (Dunlop et al., 2010). Graph shows final titers for each architecture for a range of parameter values. A hypothetical acceptable production titer (red line) is shown. (D) Stochastic gene expression and stochastic enzyme kinetics cause cell-to-cell variation in enzyme and metabolite levels. Lower graph shows parameter values for three distinct metabolite distribution regimes: one unimodal and two bimodal regimes (Tonn et al., 2019). Side graphs show single-cell distribution for enzyme (green) and metabolite (red) concentrations corresponding to each regime. (E) Comparison of static control (green) with population behavior control (blue) for selecting high-performing individual cells. Graph shows distribution of single-cell metabolite concentrations for each strategy (Xiao et al., 2016).
2.1.1. Choosing between a two-stage or one-stage fermentation process
Not all two-stage bioprocesses can outperform one-stage fermentation. Important factors that affect the decision to implement either a one-stage or two-stage bioprocess include strain performance during slow-growing or non-growing conditions, economic constraints (Klamt et al., 2018), as well as metabolic network dynamics (Jabarivelisdeh and Waldherr, 2018). For example, slow-growing and stationary phase cells often have reduced glucose uptake rates which can decrease substrate utilization for product formation (Burg et al., 2016; Harder et al., 2018). This trade-off in substrate utilization and growth was explored by Klamt et al. (2018). The model showed that reducing the glucose uptake rate in the production phase below approximately 4 mmol/gDW/h, which fell in the experimentally observed range of 0.5–4.5 mmol/gDW/h, caused the two-stage process to have lower volumetric productivity than a comparable one-stage process with the same yield and initial conditions.
The mode of bioprocess operation (e.g. fed-batch versus batch) also affects the choice between one-stage and two-stage processes. Yegorov et al. developed a kinetic model to study cellular reactions governing both shared cell resources (e.g. transcription, translation) and bio-production (e.g. enzymatic reactions in an engineered pathway) (Yegorov et al., 2019). The model was used to find the optimal RNA polymerase expression rate for maximizing either biomass or product formation under constant or limited substrate environments. This work uncovered that in the case of a constant nutritional environment, which can be found in fed-batch and continuous bioprocess, a high RNA polymerase activity to maximize both cell growth and production is preferred, motivating a one-stage fermentation. However, when the nutrient is limited, such as in a batch process, RNA polymerase activity needs to be reduced to shut down cellular replication and focus cellular resources on the expression of product formation enzymes. Thus, batch processes can benefit most from using a two-stage process.
2.1.2. Choosing valves for two-stage switch
To implement a two-stage process, appropriate metabolic reactions must be controlled. Although several well-known strain design algorithms are available for static genetic interventions (Burgard et al., 2003; Ranganathan et al., 2010), these may not be appropriate for two-stage switchable systems. An algorithm for identifying reactions that can be either knocked-out or controlled to achieve near theoretical maximum yield in two metabolic states was developed (Venayak et al., 2018b). This algorithm was applied to identify metabolic valves that can switch from 90% maximum biomass yield to 90% maximum product yield. For 87 organic products that can be derived from E. coli metabolism, 56 of them can be switched using a single switchable valve. A small number of valves in glycolysis, the TCA cycle, and oxidative phosphorylation were found particularly useful for the decoupled production. Thus, this strategy could be highly beneficial for identifying appropriate control valves computationally.
2.1.3. Engineering bistability for two-stage switchable systems
While many biological control systems can be used to switch between two-state, systems which exhibit bistability have additional benefits (Ferrell, 2002; Mitrophanov and Groisman, 2008; Tiwari et al., 2011; Veening et al., 2008). One general property of bistable systems is hysteresis, a memory-like property where the threshold of the signal output response curve is different depending on the recent history of the input signal (Ferrell, 2002). Hysteresis can allow the input signal to be reduced without switching back to the growth state. Additionally, bistability enables a slow response to input signals that are near the switching threshold (Tiwari et al., 2011). This property allows bistable switches to filter out mild, transient changes in the input signal which may be typical of a heterogenous bioreactor. A general design principle for a bistable metabolic system is to have a positive feedback loop along with non-linear kinetics, such as using a cooperative promoter, or ultrasensitive enzyme kinetics (Angeli et al., 2004). One example of bistability in metabolic engineering is the application of a metabolic toggle-switch, which uses mutually repressive transcription factors to lock cells in one of two metabolic states until a metabolic switching signal is applied (Bothfeld et al., 2017; Venayak et al., 2018a). Bistability in metabolic uptake rate could be useful for implementing a two-stage production since the extracellular nutrient concentration could be different in growth and production stage. Several positive feedback architectures for generating bistable metabolite uptake rate were explored (Oyarzún and Chaves, 2015). It was found that using an activator-repressor topology, where the metabolite activates its own uptake and represses its consumption, had the largest parameter space for generating bistability.
2.2. Continuous metabolic control systems
Continuous metabolic control is a dynamic metabolic control strategy where microbes continuously sense and respond to changing signals from its external environment or internal metabolic state. Continuous metabolic control has received increasing attention in recent years (He et al., 2016; Liu et al., 2018) due to its numerous potential benefits to metabolic engineering including automatic balancing of metabolic fluxes (Zhang et al., 2012), alleviating toxicity from intermediate accumulation, accelerating dynamic response (Liu and Zhang, 2018), reducing sensitivity to metabolic perturbation (Oyarzún and Stan, 2013), and making systems robust to parameter uncertainty (Dunlop et al., 2010). An early example of continuous metabolic control is the automatic balancing of free fatty acid (FFA) and ethanol flux in the production of fatty acid ethyl esters (FAEE) in E. coli (Zhang et al., 2012). The FAEE production system had numerous challenges, including ethanol toxicity, competition for metabolite intermediates with native pathways, and futile cycling of FFA activation. The work found that sensing acyl-CoA, a key pathway intermediate, and feedback to the ethanol and FFA producing modules allowed cells to maintain tight control over FAEE production, with a three-fold increase in yield compared to a strain without continuous metabolic control. Additionally, a kinetic model of the FAEE control system revealed that continuous metabolic control of the pathway modules could improve productivity compared to constitutive control over a wide range of parameters. Global sensitivity analysis indicated that the most important parameters are those associated with the accumulation of toxic intermediates and the synthesis of burdensome proteins. Due to the numerous potential benefits, continuous metabolic control could improve the performance of a large range of metabolic pathways.
2.2.1. Choosing valves for continuous dynamic control
Implementing a continuous metabolic control first requires choosing the metabolic reactions to be controlled. Currently, unlike in two-state switching, generally applicable algorithms for identifying the best metabolic reactions for dynamic control have not been developed. The challenge comes from the fact that the problems solved by continuous dynamic control are often pathway-specific, such as to avoid the accumulation of a toxic pathway intermediate or to optimize the expression of a burdensome enzyme. Thus, implementation of continuous dynamic control requires preexisting knowledge on rate-limiting steps, pathway bottlenecks, and intermediate accumulation. Metabolic control analysis (MCA) can potentially be used to identify reactions that have a high degree of control over pathway flux, which would suggest that tight control over these nodes may have the most impact on the pathway (for review of MCA see (He et al., 2016)).
2.2.2. Choosing control topology
Common control topologies in dynamic metabolic engineering are positive feedback, negative feedback, and feedforward. Deciding which topology to use is generally specific to the system and requires a detailed kinetic model to evaluate which topologies will provide the largest gains in TRY metrics. Simulating all possible topologies with many parameters was demonstrated as a method for choosing the best continuous control system (Stevens and Carothers, 2015). In this work, production of p-aminostyrene (p-AS) was simulated for 729 unique possibilities for the control topologies along with 2000 parameter values for each topology. The p-AS yield from each simulation was compared to the median yield from the static control topology to identify topologies which generally improve yields. This work found that only 19% of topologies had higher median yields than static control, however, the top 5% of architecture had median yields of nearly 3 times higher than static control. Although some topologies could generally improve yield, the spread of performance within a topology could be more than 100-fold. In general, there is no topology that will always outperform other topologies in dynamic metabolic control. Thus, choosing the best topology highly depends on the controlled system and would require extensive modeling to uncover the maximum performance.
2.2.3. Accelerating metabolite dynamics through engineered feedback control
In dynamic metabolic systems, rapid response to the switching signal is desired because it allows microbes to reach a productive metabolic state more rapidly, thus potentially improving overall TRY metrics. Incorporating continuous metabolic control into these systems has been recognized as a potential strategy for altering the metabolite dynamics (Schmitz et al., 2017). Several feedback architectures and their parameters have been explored at the theoretical level for accelerating response to metabolic signals.
Metabolic switching using a conventional open loop (OL) pathway topology in response to an input signal, such as induction of enzyme expression, is generally slow. It was found that for an OL topology, it takes 2.48 cell cycles for the metabolite concentration to rise to 50% of its steady-state concentration (defined as the metabolite rise-time) after the step-up input signal (Fig. 1B). Liu et al. built and studied three closed loop feedback systems of different topologies: a negative gene loop (NGL), a negative metabolic loop (NML), and a negative layered metabolic loop (NLML) (Liu and Zhang, 2018) (Fig. 1B). While all three loops had some ability to decrease the rise-time for metabolite concentrations compared to the OL, the NLML, where metabolite concentration is feedback through a genetic inverter then an enzyme controller, was capable of dramatically accelerating rise-times by 11.8-fold (Fig. 1B). However, this rapid increase in rise-time was accompanied by a large metabolite concentration overshoot, where metabolite concentration rises above the steady-state before settling. Through tuning the many parameters, it was found that for NLML, faster rise times were generally correlated with larger overshoots. Using a high maximum promoter strength of the genetic inverter and a low threshold of the enzyme controller were found to be the most efficient for decreasing the rise-time.
In addition to step-up inputs, rapid response to a step-down input signal, or removal of a step-up input signal, could be beneficial in allowing switching systems to recover to their initial states. The recovery dynamics of a metabolite uptake architecture were studied (Hartline et al., 2020). In this architecture, the enzyme for extracellular uptake is controlled by a positive feedback loop where the intracellular metabolite activates the expression of the uptake enzyme by sequestering the metabolite-responsive transcription factor (MRTF) during the step-up response. It was found that once the extracellular metabolite concentration drops, the expression of the uptake enzyme will continue due to the delay in dropping the intracellular metabolite concentration, thus leading to a long time to drop uptake pathway enzyme concentrations to 50% of its initial level, called the recovery time. The recovery dynamics can be accelerated by rapidly releasing the MRTF from sequestration. The rate of release of MRTF depends on the amount of MRTF stored during the high extracellular metabolite state and by the rate of consumption of the intracellular metabolite. MRTF promoter strength was identified as a key parameter for tuning pathway recovery time. Further, incorporating a negative autoregulatory loop could help reduce the overall resource utilization for actuating the recovery dynamics. These modeling results were additionally confirmed through re-engineering and testing of the E. coli fatty acid uptake system.
2.2.4. Improving metabolic robustness through engineered feedback control
In designing a robust metabolically engineered bioprocess, two types of uncertainties should be accounted for: microbial environmental perturbation and parameter uncertainty. Engineering negative feedback in the metabolic control system has been explored as an avenue to make engineered microbes robust to both kinds of uncertainties.
Microbial environmental perturbation arises due to the dynamic and heterogeneous microenvironments that microbes encounter in a bioreactor (Lara et al., 2006). Such perturbations can cause major concentration changes for metabolites involved in an engineered pathway (Kresnowati et al., 2006; Taymaz-Nikerel et al., 2013). Metabolite feedback was shown to help make metabolite levels less sensitive to these metabolic fluctuations (Oyarzún and Stan, 2013, 2012). Using strong, tight promoters allowed the system to further minimize the decrease in product concentration, however, strong promoters could also lead to oscillatory dynamics. Although these theoretical studies are promising, experimental implementation of these control systems have not been performed. Compared to well-controlled lab-scale fermentation, effects from these control systems are probably more dramatic in large-scale fermenters, where microbial environmental uncertainties are more pronounced. Although, metabolic engineers currently focus on lab-scale optimizations, we expect these robust metabolic control systems to demonstrate experimental improvements in the near future.
Parameter uncertainty arises due to the use of biological parts, such as promoters and ribosome binding sites, which have uncertain performance characteristics, and therefore requires extensive fine-tuning to achieve desired system performance. Metabolite feedback has also been explored as a mechanism to make systems less sensitive to uncertainty in system parameters (Dunlop et al., 2010; Harrison and Dunlop, 2012). Toxic biofuel production was modeled as an example system, and the effects of feedback architecture on parameter sensitivity were explored (Dunlop et al., 2010). In this model system, the biofuel product is toxic to cell growth, which hinders further biofuel production. Cellular efflux pumps were modeled to remove biofuel from the cell, reducing the biofuel toxicity. However, over-expression of membrane-bound efflux pumps can be toxic too, thus requiring careful balance of efflux pump expression and biofuel production. For the case of no feedback control in the expression of efflux pumps, the model revealed a narrow range of promoter strengths had a nearly maximal amount of biofuel production (Fig. 1C). Parameter sensitivity analysis of three other controller architectures demonstrated that including feedback in general reduced the sensitivity of biofuel production to system parameters variation. Further, it was found that the feedforward loop topology was robust to parameter uncertainty for all five model parameters (Fig. 1C). Based on this modeling work, an experimental library of feedback systems was constructed and tested for enhanced tolerance to the biofuel pinene (Siu et al., 2018). Several members of this library were shown to have enhanced tolerance to pinene, despite having varying promoter strengths and number of transcription factor binding sites in the promoter. These results highlight how incorporating feedback control into pathway design can make the system more robust to parametric uncertainties.
2.3. Control of metabolite heterogeneity
Metabolite heterogeneity, the cell-to-cell variation in metabolite levels in isogenic populations (Schmitz et al., 2017), has only recently been recognized to strongly affect overall TRY performance (Lv et al., 2019). Microbes have numerous stochastic mechanisms that give rise to cell-to-cell differences in mRNA, proteins, metabolic levels, and flux activities (Ackermann, 2015; Raj and van Oudenaarden, 2008; Takhaveev and Heinemann, 2018). Here, we discuss theoretical insights on the impact of metabolic heterogeneity on metabolic bioprocesses and how dynamic metabolic control can reduce or exploit metabolite heterogeneity for improved bioprocess performance.
2.3.1. Stochasticity in metabolic pathways
Stochasticity in biology can lead to large heterogeneity in metabolic activity. Using experimental measurements of the single-cell proteomic data (Taniguchi et al., 2010), the impact of cell-to-cell variation on protein concentration to E. coli population growth rate has been assessed by a genome-scale FBA model (Labhsetwar et al., 2013). The model produced a population with a broad distribution of growth rates. Cells at the extremes of the growth rate distribution had different metabolic fluxes, where the fast-growing cells tended to prefer using the Entner-Doudoroff pathway, while slow-growing cells utilized the glycolysis pathway more predominantly. These stochastic effects could be important for production, particularly if production is growth-coupled or if the product is derived from a pathway intermediate which varies strongly between cells.
Additionally, large metabolite heterogeneity can arise from enzyme variation even within one metabolic reaction (Tonn et al., 2019). Stochastic analysis of a model metabolic pathway with reversible Michaelis-Menten kinetics revealed several regimes of bimodal metabolite distributions (Fig. 1D). In the first regime, promoters with slow switching but high expression rates generate a bimodal distribution in enzyme levels, leading to bimodality in metabolites. In the second regime, enzymes are unimodally expressed at a low level. Occasionally, enzyme concentration could reach near-zero, which caused the product to become depleted, so a bimodal distribution in metabolite levels was generated (Fig. 1D). In engineered systems, enzymes are typically highly expressed, making it unlikely for the second bimodality regime to occur. However, many products are derived from native metabolites, which could exhibit this mode of bimodality. In these cases, a cell may stochastically have low levels of production pathway substrate, which may impact bioprocess productivity. These theoretical studies have raised the concern that metabolite heterogeneity impacts bioprocess robustness (Delvigne and Goffin, 2014) and thus warrant strategies to cope with heterogeneity (Binder et al., 2017).
2.3.2. Controlling metabolite heterogeneity using feedback loops
Several papers have explored the impact of feedback on the amount of metabolite heterogeneity in the system (Borri et al., 2016, 2015; Oyarzún et al., 2015). In these papers, a metabolic enzyme is continuously controlled by a promoter and the enzyme converts a substrate into a final product. Two main feedback loop architectures have been considered: enzyme autoregulation or end-product feedback. Compared to a constitutive system with an identical mean product level, both enzyme autoregulation and end-product feedback could always reduce metabolite noise, with stronger feedback leading to stronger noise reduction (Borri et al., 2015). Additionally, feedback sensitivity was identified as a critical parameter to achieve large noise reduction for a wide range of promoters and feedback strengths (Borri et al., 2016, 2015; Oyarzún et al., 2015). This body of work provides an excellent theoretical foundation for the engineering of feedback systems for reduction of metabolite heterogeneity in the one-step pathways.
2.3.3. Controlling metabolite heterogeneity using population behavior control
Population behavior control is an emerging dynamic metabolic control strategy for controlling metabolite heterogeneity in engineered microbes (Lv et al., 2019). In this control paradigm, metabolite concentration is measured by a sensor which transmits this signal into a growth-modulating actuator (Fig. 1E). The actuator can provide a growth advantage to high-producing cells or steer low-producers towards self-destruction. In one example (Xiao et al., 2016), a FFA-based sensor-actuator was used to drive the expression of tetracycline efflux pumps in FFA-producing E. coli. Cells that stochastically produced low intracellular concentrations of FFA were unable to express sufficient efflux pumps, which caused cell death in the presence of tetracycline. Under the control of FAA sensor-actuator, the cell population displayed an increase mean FFA concentration and a 4.5-fold enhancement in FFA titer in a fermenter (Fig. 1E). This control strategy also proved useful to reduce phenotypic heterogeneity caused by genetic instability (Rugbjerg et al., 2018b). Here, a mevalonic acid (MVA)-based sensor-actuator was used to drive the expression of folP and glm, which are growth-critical genes for E. coli in a broad context. This control system eliminated cells that had low mevalonic acid production due to genetic heterogeneity, thus extending the productive lifetime of the bioprocess to 95 cell generations. Given the demonstrated promise of this control paradigm, we expect future developments in control theory for biological systems to address the benefits and limitations of population behavior control for dynamic metabolic engineering.
3. Sensors and actuation
Once a dynamic metabolic control topography has been selected, the individual parts and mechanisms need to be implemented. The essential parts of dynamic metabolic controls include sensors that detect metabolic or environmental signals, and actuators that change the output of the metabolic system in response to the sensor.
3.1. Sensing in metabolic control
Sensors are extremely useful in several metabolic engineering applications, which have been reviewed elsewhere (Liu et al., 2015a). This section will focus on sensors that have been demonstrated in dynamic metabolic engineering applications.
3.1.1. Sensing exogenous chemical signals
Common chemical inducers:
Chemical inducers are traditionally used in open-loop pathways to induce the expression of metabolic enzymes but are also useful in two-stage switchable systems (Fig. 2A). Generally, cells are grown in a bioreactor to accumulate biomass, and a chemical inducer is added to halt growth as well as upregulate flux to a desired pathway (Burg et al., 2016; Soma et al., 2014). While being easy to use, chemical inducers can be expensive, must be added at a pre-determined time, and can be difficult to remove if switching the metabolic state back is desired.
Fig. 2. Overview of sensing mechanisms used in dynamic metabolic control.

(A) A chemical inducer binds to a repressor that undergoes a conformational change and releases itself from binding to DNA, thus allowing RNA polymerase to promote gene expression (Soma et al., 2014). (B) As cell density increases, more AHL molecules are released into the extracellular environment until the concentration is high enough to trigger a quorum response (Gupta et al., 2017). (C) Thermolabile repressor cI857 changes conformation and DNA binding activity in response to temperature (Zhou et al., 2012). (D) Light responsive transcription factors are created by fusing light-sensitive protein domains with DNA binding domains (Milias-Argeitis et al., 2016). (E) The Pgas promoter is able to detect and respond to low pH levels (Yin et al., 2017). (F) FadR is a repressor transcription factor that binds to acyl-CoA thus acting as a sensor for acyl-CoA (Zhang et al., 2012). (G) The L-Trp biosensor is based on a metabolite-responsive signal peptide TnaC (Fang et al., 2016). Without L-Trp, the operon undergoes the Rho-dependent transcriptional termination. L-Trp can prevent the release of the ribosome at the tnaC stop codon, blocking Rho from binding to the mRNA, so that RNA polymerase can continue to transcribe the downstream GOI.
Nutrient sensing:
Many large scale bioprocesses choose to use various nutrients as the switching signal to reduce cost (Scalcinati et al., 2012). Glucose depletion, for example, is an attractive signal for two-stage fermentation. As glucose becomes completely consumed, cell growth ceases. Glucose-dependent promoters, such as those identified through genome-wide transcription datasets (Maury et al., 2018), have been used to induce metabolite production (Bothfeld et al., 2017). Other nutrient-based switching systems include systems induced by nitrogen-starvation (Sonderegger et al., 2005), phosphorus-starvation (Chubukov and Sauer, 2014), maltose (Liu et al., 2017), and ammonia (Xiao et al., 2017).
Quorum sensing (QS):
QS is an attractive signal target for dynamic control since it naturally acts as a measure of cell population density which is linked to cell growth (Tan and Prather, 2017). When cell density passes a threshold, specific small molecules accumulate and can be used to induce a response to dynamically balance cell growth and target production while synchronizing cell activity in a population. Extensive work has been done to engineer QS for dynamic metabolic control. For example, in the gram-positive bacteria Bacillus subtilis, bifunctional and modular QS was engineered using the Phr60-Rap60-Spo0A QS system to improve production of menaquinone-7, a type of vitamin K (Cui et al., 2019). The quorum signal response can be tuned to different cell densities (He et al., 2017), thus enabling growth-to-production switching at different quorum for improved production of isopropanol (Soma and Hanai, 2015) (Fig. 2B), myo-inisitol (MI), and glucaric acid (Gupta et al., 2017).
3.1.2. Environmental signals
Temperature switches:
Temperature-inducible systems have some benefits because the temperature of a vessel can be controlled externally, reducing the risk of contamination. Further, heat induction can be easily timed. Two heat-inducible systems extensively used in metabolic engineering are the λ phage promoters (i.e., PL and PR), both of which are regulated by the thermolabile cI857 repressor (Harder et al., 2018; Valdez-Cruz et al., 2010; Zhou et al., 2016, 2012) (Fig. 2C). Cold-inducible switches have also been developed via directed evolution (Zheng et al., 2019). Temperature induction also has limitations: (1) heat can alter protein folded structures and folding dynamics; (2) large or rapid change in temperature can trigger heat- or cold-shock responses and alter the global regulatory and metabolic networks; and (3) implementing temperature change in large fermenters uniformly can be slow due to heat transfer limitations.
Light switches:
Optogenetic switches allow control strategies using visible light signals which is low cost and generally non-toxic. Light sensors are often derived from the photoreceptors domain of specialized proteins that undergo a conformational change in response to light (Milias-Argeitis et al., 2016). In an engineered Saccharomyces cerevisiae system, the light-responsive Phy/PIF module was adapted to allow red and far-red light pulses to switch on and off transcription of Gal4-responsive genes, enabling in silico feedback control (Milias-Argeitis et al., 2011) (Fig. 2D). Another engineered S. cerevisiae system used light to control fermentation with the Erythrobacter litoralis EL222 optogenetic transcription system, switching from a light-induced growth phase to a dark-induced production phase (Zhao et al., 2018). In an engineered E. coli system, the light sensor histidine kinase CcaS was used to respond to red and green light to control growth in an automated optogenetic feedback control system (Milias-Argeitis et al., 2016). A limitation of optogenetic switches is the problem of light-delivery since dense cell cultures scatter light significantly.
Other environmental switches:
Several other environmental signals have also been used in dynamic metabolic controls. The Pgas promoter functions efficiently at pH 2.0 but becomes inactive above pH 5.0, thus was used to produce organic acid in Aspergillus niger, which further reduced pH of cell cultures (Yin et al., 2017) (Fig. 2E). The promoter of the cell wall glycoprotein CCW14 from S. cerevisiae was also engineered for a low-pH response to improve production of lactic acid (Rajkumar et al., 2016). Additionally, the oxygen-inducible nar promoter from the narGHJI operon in E. coli responds to anaerobic conditions and was used to produce 2,3-butanediol and 1,3-propanediol in E. coli when cell culture was switched to anaerobic conditions (Hwang et al., 2017).
3.1.3. Metabolite sensing
There is a high demand for endogenous metabolite sensors when building dynamic metabolic control systems. When engineered microbes can adjust their metabolism according to their intracellular metabolic status, each cell is spontaneously controlled even without synchronization at the population level.
Metabolite-responsive transcription factors (MRTFs):
MRTFs are the most commonly used proteins for developing endogenous metabolite sensors. An MRTF usually binds to a specific metabolite and undergoes a conformational change that alters its DNA binding activity. Using the MRTF’s cognate promoters or incorporating its operator sites into engineered promoters, transcription of metabolic genes can be controlled by the detected endogenous metabolite. Examples of MRTF-based sensors used in dynamic metabolic control include a FadR-based acyl-CoA sensor for FAEE production (Zhang et al., 2012) (Fig. 2F), FapR-based malonyl-CoA sensors for FFA and 3-hydroxypropionic acid (3-HP) production (Liu et al., 2015b; Xu et al., 2014; David et al., 2016), and a MexR-based pinene sensor for pinene production (Siu et al., 2018). The effector specificity of the MRTF can be altered through site-directed mutagenesis of the metabolite-binding pocket and screened by FACS as demonstrated in a HucR-based vanillin sensor for vanillin and ferulic acid production (Liang et al., 2020). Additionally, synthetic MRTFs can be created by fusing the DNA binding domain of a known TF with the ligand-binding domain from another enzyme (Chou and Keasling, 2013).
Stress-responsive promoters:
For metabolites that cause cellular stresses, native promoters up- or down-regulated by the accumulation of the metabolite can be used as sensors. In E. coli, the accumulation of farnesyl pyrophosphate (FPP) from the engineered isoprenoid pathway is toxic (Martin et al., 2003). Stress-responsive promoters identified by genome-wide transcriptional analysis were used to control FPP-consuming enzymes, increasing amorphadiene production and improving growth (Dahl et al., 2013). Similarly, ergosterol-responsive promoters were used to dynamically regulate the expression of ERG9 in S. cerevisiae, leading to increased amorphadiene production (Yuan and Ching, 2015).
Other types of sensing:
Besides MRTFs and stress-responsive promoters, aptamers present another mechanism by which a signal can be sensed (Liu et al., 2015a). While many aptamers exist and bind to a wide range of chemicals, the major hurdle is pairing metabolite-binding of an aptamer to an actuating mechanism while enabling tight control over gene expression and without disrupting protein function (Stevens and Carothers, 2015). Additionally, some metabolite-responsive signal peptides (also called leader peptide), such as TnaC that regulate the expression of downstream tnaAB expression in response to tryptophan, have been used as sensors for dynamic metabolic engineering (Fang et al., 2016) (Fig. 2G).
3.2. Actuation in metabolic control
In metabolic engineering, actuation usually involves controlling the abundance or activity of pathway enzymes in response to signals from the corresponding sensor. This section will discuss actuation strategies at different levels and highlight advantages and disadvantages with respect to the speed of flux response, and cellular economy utilization.
3.2.1. Transcriptional actuation
In addition to chemical inducers that are mostly used in two-stage fermentation (Burg et al., 2016), MRTFs can be directly used to repress or activate gene expression from their cognate promoters, enabling transcriptional actuation according to sensor signals. To obtain desirable actuation objectives, which are usually low leaky expression and high dynamic range, the cognate promoters controlled by MRTF often need to be engineered. This engineering effort can be guided by a phenomenological model that revealed the tunable parameters and constraints, such as the number and strength of repressor binding sites, in achieving the maximal dynamic range as well as low leakiness of sensor-actuators under various architectures (Mannan et al., 2017) (Fig. 3A). Another type of transcriptional control involves CRISPR interference (CRISPRi) or dCas9-mediated transcription repression. CRISPRi can be used to repress transcription of target genes upon binding to a sgRNAs, whose expression can be controlled by a sensor. For example, CRISPRi was integrated with glucosamine-6-phosphate (GlcN6P) sensors to enable sensing-actuation to control the metabolic flux of the nutraceutical N-acetylglucosamine (GlcNAc) pathway in B. subtilis (Wu et al., 2020) (Fig. 3B). Multiple orthogonal sgRNAs can be implemented into one strain to allow for multiple gene repression (Tan and Prather, 2017). When regulating multiple native genes is required, CRISPRi has advantages over MRTFs as there is no need for genome modification to change promoters of target genes. A drawback of CRISPRi is that the dCas9 expression must be carefully tuned to avoid toxicity caused by dCas9 overexpression.
Fig. 3. Overview of actuator mechanisms in dynamic metabolic control.

(A) MRTF-based transcriptional actuation (Mannan et al., 2017). (B) CRISPRi-based transcriptional actuation (Wu et al., 2020). (C) Antisense RNA-based post-transcriptional actuation (Yang et al., 2018). asRNA, antisense RNA. (D) RNA-interference-based post-transcriptional actuation. In S. cerevisiae, RNA hairpins are cleaved by a Dicer protein to create fragments, which interact with the Argonaute protein to destroy target mRNAs, thus preventing translation (Williams et al., 2015). dsRNA, double-stranded RNA; siRNA, small interfering RNA. (E) A post-translation actuation mechanism based on the TevP proteinase (Gao et al., 2019).
3.2.2. Post-transcriptional actuation
Gene expression can also be controlled post-transcriptionally using either antisense RNAs or RNA interference (RNAi). RNA repression has been used to regulate proB, glnA, and argB for their role in regulating intracellular ATP concentration (Chen et al., 2015). Hybridization between the antisense RNA and the RBS or open reading frame of the mRNA prevents translation or enhances mRNA degradation, ultimately decreasing gene expression. Antisense RNAs have been employed in E. coli for simultaneous regulation of multiple genes, autonomously redistributing carbon flux between native metabolism and an engineered muconic acid (MA) biosynthesis pathway (Yang et al., 2018) (Fig. 3C). In S. cerevisiae, the Argonaute (AGO1) and Dicer (DCR1) genes from yeast Saccharomyces castellii were used in conjunction with QS to degrade targeting mRNAs, enabling dynamic regulation of the shikimate pathway increasing para-hydroxybenzoic acid (PHBA) production (Williams et al., 2015) (Fig. 3D).
3.2.3. Post-translation actuation
Post-translational control of enzyme activity by a metabolite of interest can provide rapid control over the flux of a pathway and can be ideal for dynamic metabolic systems (Venayak et al., 2015). However, engineering allosteric enzymes for specific reactions is extremely challenging because novel allosteric interactions require high selectivity and robust output upon metabolite binding. Existing post-translation actuation mechanisms mostly focus on regulating enzyme degradation rate by adding degradation tags to target enzymes. For example, a TevP protease-based dynamic regulation circuit was constructed to create an ON and OFF switch that accumulates and degrades target proteins, respectively, depending on the degradation tags used. The protease was placed under growth- or stationary-phase promoters, allowing the system to respond to the growth stage and improving shikimate production (Gao et al., 2019) (Fig. 3E).
4. Applications of dynamic metabolic control
As theories and molecular biology tools are developed, an increasing number of dynamic metabolic control systems were developed for numerous pathways to benefit bioproduction (Table 1). In this section, we will summarize the pathways and metabolic nodes being dynamically regulated.
Table 1.
Dynamic metabolic control in metabolic engineering.a
| Hostb | Control Strategyc | Sensing signal | Actuation leveld | Target pathwaye | Performance improvement | Reference | |
|---|---|---|---|---|---|---|---|
| 1 | EC | TS, CM | ACP | TX | Terpenoid (lycopene) | 18-fold (yield) | Farmer and Liao (2000) |
| 2 | EC | CM | Fatty acyl-CoA | TX | Fatty acid derivative (FAEE) | 2-fold (titer) | Zhang et al. (2012) |
| 3 | SC | TS | Glucose | TX | Terpenoid (α-santaleane) | 1.5-fold (titer) | Scalcinati et al. (2012) |
| 4 | EC | TS | Temperature | TX | Lactate | 1.3-fold (titer) | Zhou et al. (2012) |
| 5 | EC | CM | Stress (FPP) | TX | Terpenoid (amorphadiene) | 2-fold (titer) | Dahl et al. (2013) |
| 6 | EC | CM | Malonyl-CoA | TX | Free fatty acid | 2.1-fold (titer) | Xu et al. (2014) |
| 7 | EC | TS | QS (AHL) | TX | 1,4-butanediol | No static control | Liu and Lu (2015) |
| 8 | EC | CM | Malonyl-CoA | TX | Free fatty acid | 1.3-fold (titer) | Liu et al. (2015b) |
| 9 | EC | TS | QS (AHL) | TX | Isopropanol | 3.7-fold (titer); 3.1-fold (yield) | Soma and Hanai (2015) |
| 10 | SC | TS | QS (pheromone) | RNAi | Aromatic (PHBA) | 2.4-fold (titer) | Williams et al. (2015) |
| 11 | SC | TS | Glucose | TX | Terpenoid (carotenoid) | 1.3-fold (content) | Xie et al. (2015) |
| 12 | SC | CM | Ergosterol | TX | Terpenoid (amorphadiene) | 5-fold (titer) | Yuan and Ching (2015) |
| 13 | CG | TS | Lysine | TL | Lysine | 1.9-fold (yield) | Zhou and Zeng (2015a, b) |
| 14 | SC | TS, CM | Glucose, Malonyl-CoA | TX | 3-HP | 10-fold (titer) | David et al. (2016) |
| 15 | EC | TS | Glucose, hydroxycinnamoyl-CoA | TX | Vanillic acid | 5-fold (titer) | Lo et al. (2016) |
| 16 | EC | TS | Glucose, fatty acyl-CoA | TX | Fatty acid derivative (FAEE) | 2.4-fold (titer) | |
| 17 | EC | TS, CM | Stress (FPP) | TX | Terpenoid (zeaxanthin) | 2-fold (content); 2.1-fold (titer) | Shen et al. (2016) |
| 18 | EC | PB | Fatty acyl-CoA | TX | Free fatty acid | 4.5-fold (titer); 2.5-fold (yield) |
Xiao et al. (2016) |
| 19 | EC | PB | Tyrosine | TX | Tyrosine | 2.6-fold (titer) | |
| 20 | EC | TS | QS (AHL) | TX | MI MI derivative (Glucaric acid) Shikimate |
5.6-fold (titer); 4-fold (titer) ND to 105 mg/L |
Gupta et al. (2017) |
| 21 | EC | TS | QS (AHL), Phase | TX | PHB | 2.9-fold (content) | He et al. (2017) |
| 22 | EC | TS | Dissolved oxygen | TX | Lactate 2,3-butanediol 1,3-propanediol |
No static control | Hwang et al. (2017) |
| 23 | EC | TS | QS (AHL) | TX | Terpenoid (bisabolene) | 1.4-fold (titer) | Kim et al. (2017) |
| 24 | EC | CM | Ammonium | TX | Cyanophycin | 1.4-fold (titer) | Xiao et al. (2017) |
| 25 | AR | TS | Low pH | TX | Itaconate | 6-fold (titer) | Yin et al. (2017) |
| 26 | EC | TS, CM | QS (AHL), MI | TX | MI derivative (glucaric acid) | 4-fold (titer) | Doong et al. (2018) |
| 27 | EC | TS | Temperature | TX | Itaconate | 1.5-fold (titer); 1.9-fold (productivity) |
Harder et al. (2018) |
| 28 | STR | TS | Cell phase | TX | Polyketide (actinorhodin) Polyketide (oxytetracycline) |
1.3-fold (titer) 9.1-fold (titer) |
Li et al. (2018) |
| 29 | SC | TS | Glucose | TX | 3-HP | 1.7-fold (titer) | Maury et al. (2018) |
| 30 | BS | CM | GlcN6P | TX | GlcNAc | 2-fold (titer) | Niu et al. (2018) |
| 31 | SC | TS, CM | Ergosterol, glucose | PD, TX | Terpenoid (linalool) Terpenoid (limonene) |
44-fold (titer) ND to 76 mg/L |
Peng et al. (2018) |
| 32 | EC | TS | MA | TX, asRNA | Aromatic (MA) | 4.7-fold (titer) | Yang et al. (2018) |
| 33 | SC | TS | Light | TX | Isobutanol 2-MBOH |
4-fold (titer) 3-fold (titer) |
Zhao et al. (2018) |
| 34 | CG | CM | Isoleucine | Attenu-ation | 4-HIL | 2.3-fold (titer); 2.5-fold (yield) |
Zhang et al. (2018) |
| 35 | BS | TS, CM | QS (Phr60) | TX | Terpenoid (menaquinone-7) | 40-fold (titer) | Cui et al. (2019) |
| 36 | EC | TS | QS (AHL) | TX | Aromatic (naringenin) salicylic acid |
15-fold (titer) 1.8-fold (titer) |
Dinh and Prather (2019) |
| 37 | EC | TS | Phase | PD | Shikimate | 8.5-fold (titer) | Gao et al. (2019) |
| 38 | EC | TS | Oscillator | PD | Xylonate | 2-fold (titer) | |
| 39 | EC | TS | QS (AHL), stress | TX | Terpenoid (oxygenated taxanes) | 2.4-fold (titer) | Glasscock et al. (2019) |
| 40 | EC | PB | 4-HB | TX | Phenol | 1.8-fold (titer) | Guo et al. (2019) |
| 41 | EC | PB | Tyrosine | TX | Phenol | 2.7-fold (titer) | |
| 42 | EC | TS | QS | TX | Aromatic (4-HPAA) | 1.5-fold (titer) | Shen et al. (2019) |
| 43 | EC | PB | Tryptophan | TX | Tryptophan | 1.5-fold (titer) | Wang et al. (2019) |
| 44 | EC | PB | Phenylalanine | TX | Phenylalanine | 1.5-fold (titer) | |
| 45 | EC | TS | Phenylalanine | TX | Phenylalanine | 1.4-fold (titer) | Wu et al. (2019) |
| 46 | EC | TS | QS (AHL) | GS | Xylonate Shikimate |
1.4-fold (titer) 12.3-fold (titer) |
Gao et al. (2020) |
| 47 | EC | PB | 4-HB | TX | PCA | 1.2-fold (titer) | Guo et al. (2020) |
| 48 | EC | TS | QS (AHL) | TX | PHB ALA |
6-fold (titer) 12-fold (titer) |
Gu et al. (2020) |
| 49 | EC | TS | Vanillin | TX | Aromatic (vanillin) | 1.2-fold (titer) | Liang et al. (2020) |
| 50 | YL | CM, PB | Malonyl-CoA, naringenin | TX, CRISPRi | Aromatic (naringenin) | 1.8-fold (titer) | Lv et al. (2020) |
| 51 | STR | TS | QS (GBL) | TX | Polyketide (rapamycin) | 6.6-fold (titer) | Tian et al. (2020) |
| 52 | BS | CM | GlcN6P | TX, CRISPRi | GlcNAc | 1.6-fold (titer) | Wu et al. (2020) |
| 53 | EC | PB | Tryptophan | TX | Tryptamine | 2.1-fold (titer) | Wang et al. (2020) |
Examples using only traditional inducers are excluded. Examples are sorted by the year when the literature was published. In one literature, if a sensor-actuator system is applied to multiple pathways, these applications are considered as one example of dynamic metabolic control.
Host abbreviations: EC, Escherichia coli; SC, Saccharomyces cerevisiae; CG, Corynebacterium glutamicum; AR, Aspergillus niger; BS, Bacillus subtilis; STR, Streptomyces species; YL, Yarrowia lipolytica.
Strategy abbreviations: TS, two-stage metabolic control; CM, continuous metabolic control; PB, population behavior control.
Actuation level abbreviations: TX, transcription; TL, translation; PD, protein degradation; asRNA, antisense RNA; GS, genetic sequence; CRISPRi, CRISPR interference; RNAi, RNA interference.
Other abbreviations: QS, quorum sensing; AHL, N-acyl homoserine lactones; GBL, γ-butyrolactones; ACP, acetyl phosphate; MI, myo-inositol; MA, muconic acid; FAEE, fatty acid ethyl ester; PHBA, para-hydroxybenzoic acid; 3-HP, 3-hydroxypropionic acid; 4-HPAA, 4-hydroxyphenylacetic acid; PHB, poly-β-hydroxybutyrate; GlcN6P, glucosamine-6-phosphate; GlcNAc, N-acetylglucosamine; 4-HIL, 4-hydroxyisoleucine; 2-MBOH, 2-methyl-1-butanol; 4-HB, 4-hydroxybenzoate; PCA, protocatechuic acid; ALA, 5-aminolevulinic acid; ND, not detectable.
4.1. Applications in pathways branching from glycolysis
Glucaric acid is a precursor to detergents and nylons but has been proven difficult to produce in high quantities due to the carbon flux competition with glycolysis at the glucose-6-phosphate (G6P) branching point. To solve this competition, phosphofructokinase (PFK), the flux controlling enzyme in the upper glycolysis, needs to be repressed in the glucaric acid production phase. A QS system was used to achieve this dynamic repression once the cells reach a desired density, thereby switching from a growth phase to a production phase (Gupta et al., 2017). Another problem in this pathway is the low stability and activity of MI oxygenase (MIOX) (Fig. 4A). To delay the activity decline of MIOX, a MI biosensor was then used to upregulate the expression of MIOX upon the buildup of sufficient MI (Doong et al., 2018). The two dynamic control strategies collectively led to improved glucaric acid production at a titer of nearly 2 g/L in E. coli.
Fig. 4. Representative pathways with engineered metabolic regulation.

(A) Glucaric acid production in E. coli (Doong et al., 2018). AHL, N-acyl homoserine lactones; PFK-1, phospho-fructokinase-1; G6P, glucose-6-ph osphate; F6P, fructose-6-phosphate; MI, myo-inositol; MIOX, myo-inositol oxygenase. (B) 4-HIL production in C. glutamicum (Zhang et al., 2018). α-KG, α-ketoglutarate; SUCC, succinyl-CoA; SUC, succinate; OAA, oxaloacetate; Ile, L-isoleucine; 4-HIL, 4-hydroxyiso leucine; OdhA, α-ketoglutarate dehydrogenase complex E1 subunit. (C) MA production in E. coli (Yang et al., 2018). E4P, erythrose 4-phosphate; PEP, phosphoenolpyruvate; DAHP, 3-deoxy-d-ara-bino-heptulosonate-7-phosphate; MA, muconic acid; AAA, aromatic amino acid; EntC, isochorismate synthase; PchB, isochorismate pyruvate lyase; PykF, pyruvate kinase I; PykA, pyruvate kinase II; Ppc, phosphoenolpyruvate carboxylase. (D) Naringenin production in Y. lipolytica (Lv et al., 2020). LEU2, 3-isopropylmalate dehydrogenase; FAS1, fatty acid synthetases Fas1p; FAS2, fatty acid synthetases Fas2p; FabD, malonyl-CoA-ACP transacylase. β-IPM, 3-isopropylmalate; KIC, α-ketoi-socarpoate (E) Monoterpene production in S. cerevisiae (Peng et al., 2018). DMAPP, dimethylallyl pyrophosphate; IPP, isopentenyl pyrophosphate; GPP, geranyl pyrophosphate; FPP, farnesol pyrophosphate; Erg20p, FPP synthase. (F) Xylonate production in E. coli (Gao et al., 2020). PPP, pentose phosphate pathway; XylA, xylose isomerase; CcxylB, xylose dehydrogenase from Caulobacter crescentus; Dashed line, multiple enzymatic steps; blue line, engineered regulatory interaction; Circle, the signal molecule in the dynamic control system. Pathways and metabolites in bold are essential to the host.
Another important metabolic precursor from the central glycolysis is fructose-6-phosphate (F6P), which can be used to synthesize GlcNAc, a food supplement and nutraceutical. Dynamically downregulating PFK-1 in the glycolysis pathway and phosphoglucosamine mutase in the competitive peptidoglycan pathway was effective in GlcNAc overproduction (Niu et al., 2018; Wu et al., 2020). Controlling G6P dehydrogenase and GlcN6P N-acetyltransferase in response to the GlcN6P level has also been demonstrated to enhance GlcNAc production (Wu et al., 2020).
4.2. Applications in pathways branching from the TCA cycle
Many platform chemicals are synthesized from pyruvate. Meanwhile pyruvate is also converted to acetyl-CoA, an essential metabolite for cell growth. To minimize the competition on pyruvate between bio-production and cell growth, two-stage metabolic switches were used to activate the transcription of pathway genes only in the production phase for several pyruvate-derived chemicals, such as lactate (Hwang et al., 2017; Zhou et al., 2012), 2,3-butanediol (Hwang et al., 2017), and iso-butanol (Zhao et al., 2018).
Slowing down the TCA cycle is another widely used dynamic control strategy. For example, repressing the citrate synthase dynamically has been used to enhance the production of chemicals derived from acetyl-CoA and oxaloacetate, such as isopropanol (Soma et al., 2014; Soma and Hanai, 2015), poly-β-hydroxybutyrate (Gu et al., 2020; He et al., 2017), and lysine (Zhou and Zeng, 2015a, 2015b). Similarly, dynamically downregulating the isocitrate dehydrogenase was exploited by using a temperature-dependent system with PR and the repressor cI857 to increase itaconate production, a cis-aconitate derived product (Harder et al., 2018). Reactions in the TCA cycle can also be modulated by the concentration of the substrate in the target pathway. For example, 4-hydroxyisoleucine (4-HIL) is synthesized from isoleucine and α-keto-glutarate. To enhance 4-HIL synthesis, the expression of α-ketoglutarate dehydrogenase complex was repressed by an attenuator when the level of isoleucine is high (Zhang et al., 2018) (Fig. 4B).
4.3. Applications in pathways involving shikimate and aromatics
The shikimate pathway provides the building blocks for the biosynthesis of various aromatics in microbes. To improve the production of shikimate or shikimate-derived aromatics, several metabolic nodes have been regulated by dynamic control strategies. The shikimate pathway starts with the condensation of phosphoenolpyruvate (PEP) and erythrose-4-phosphate. Dynamic downregulation of the pathways from PEP to the TCA cycle through antisense RNAs can increase the flux of the shikimate pathway (Yang et al., 2018) (Fig. 4C). Shikimate and chorismate are important intermediates in the biosynthesis of aromatic amino acids (AAAs, i.e., phenylalanine, tyrosine, and tryptophan), which are essential to cell growth and many other valuable aromatic acids. Additionally, shikimate is a key ingredient in the formulation of drug Tamiflu for the treatment of influenza. To accumulate shikimate, dynamic control has been used to block the expression of shikimate kinase in the production phase (Gao et al., 2020, 2019; Gupta et al., 2017). To enhance the production of other aromatic acids, dynamical control via repressing the conversion of chorismate to AAA biosynthesis and activating the target aromatic acid pathway has shown success in the production of MA (Yang et al., 2018) (Fig. 4C), PHBA (Williams et al., 2015), salicylic acid (Dinh and Prather, 2019), and 4-hydroxyphenylacetic acid (Shen et al., 2019). Many aromatics are secondary metabolites that can be toxic to the host when accumulated. Dynamic upregulation of the secondary biosynthetic pathway has also been applied to improve the production of these metabolites, such as naringenin (Dinh and Prather, 2019), vanillin (Liang et al., 2020), and vanillic acid (Lo et al., 2016).
4.4. Applications in pathways consuming Malonyl-CoA
Malonyl-CoA serves as a rate-limiting precursor in fatty acid chain elongation, and has received much attention as a node for dynamic metabolic engineering for the oleochemical family of compounds (David et al., 2016; Xu, 2020). In E. coli, malonyl-CoA is synthesized from acetyl-CoA by acetyl-CoA carboxylase (ACC) regulon. However, over-expression of ACC leads to significant toxicity, caused by either depletion of the acetyl-CoA metabolite or due to imbalanced expression of the ACC subunits, thus motivating the engineering of several dynamic control schemes. Sensing of malonyl-CoA is typically achieved through the MRTF FapR, whose binding site fapO can be incorporated into engineered promoters. In one control scheme, an engineered phage PA1 promoter with fapO operator was used to form a layered negative metabolic feedback loop, where the expression of ACC was negatively regulated by malonyl-CoA via a LacI intermediate (Liu et al., 2015b). The negative metabolic feedback improved both cell growth and FFA production compared to the unregulated strains. In another scheme, two engineered promoters with fapO operator were developed, which can be induced and repressed by malonyl-CoA respectively (Xu et al., 2014). One promoter was used to activate ACC expression, leading to malonyl-CoA production whenever malonyl-CoA concentration was low, representing a negative metabolic loop. Another promoter was used to drive expression of the downstream fatty acid synthase (FAS) module, leading to FAS expression and malonyl-CoA consumption only when the malonyl-CoA level was high. This control scheme led to oscillatory malonyl-CoA dynamics and an improved fatty acid titer over the uncontrolled strain.
Downregulating fatty acid synthesis has emerged as a new strategy for producing many other malonyl-CoA-derived chemicals. An endogenous QS system coupled with CRISPRi that simultaneously downregulates key nodes in the TCA cycle, fatty acid and AAA synthesis pathways was applied to enhance rapamycin production in an industrial Streptomyces species (Tian et al., 2020). In another example, fatty acid synthesis is negatively autoregulated through CRISPRi driven by fatty acid-inducible promoters to increase naringenin production in Yarrowia lipolytica (Lv et al., 2020) (Fig. 4D). A second layer of dynamic control was also developed to further improve genetic stability in long-term naringenin production. In this Y. lipolytica strain, 3-isopropylmalate dehydrogenase (LEU2), which is involved in leucine synthesis, was controlled by a naringenin-inducible promoter, which couples the naringenin production with cell growth (Fig. 4D).
4.5. Applications in pathways producing terpenes and terpenoids
Dynamic control has been widely used in terpene and terpenoid production. The building blocks of terpenes and terpenoids are isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which need to be synthesized from either acetyl-CoA through the MVA pathway, or from glyceraldehyde 3-phosphate and pyruvate through the methylerythritol phosphate (MEP) pathway. In E. coli, overexpression of the terpenoid pathway can lead to growth retardation (Farmer and Liao, 2000; Kim et al., 2017; Martin et al., 2003). Both the MVA (Kim et al., 2017) and MEP pathways (Farmer and Liao, 2000) have been dynamically activated to improve terpenoid production in E. coli. Intermediates of the terpenoid pathway, including 1-hydroxy-2-methyl-2-butenyl 4-diphosphate in the MEP pathway, 3-hydroxy-3-methyl-glutaryl-CoA in the MVA pathway, IPP, and FPP, are also toxic to host cells when accumulated to high concentrations (Li et al., 2017; Martin et al., 2003). Dynamic control has been used to avoid accumulation of these intermediates (Cui et al., 2019; Dahl et al., 2013; Shen et al., 2016). Additionally, some terpenoids, such as sterol in fungi and undecaprenyl pyrophosphate in Bacillus species, are essential to the microbial hosts. Dynamic control has been used to balance the synthesis of both these essential terpenoids and the final products (Cui et al., 2019; Peng et al., 2018; Scalcinati et al., 2012; Xie et al., 2015; Yuan and Ching, 2015). For example, to increase monoterpene production, FPP synthase (Erg20p) was downregulated by ergosterol through protein degradation tag (Peng et al., 2018) (Fig. 4E). Synthesis of complex terpenoids, such as Taxol, requires oxygenation, which is often catalyzed by cytochrome P450. However, P450 expression is often burdensome to host cells. A stress-responsive promoter with a transcriptional switch has also been applied to optimize the P450 expression level, resulting in increased titers in oxygenated Taxol precursor (Glasscock et al., 2019). For a complex terpenoid pathway, multiple metabolic nodes can be simultaneously regulated, as shown in the production of menaquinone-7 using a bifunctional QS system (Cui et al., 2019).
4.6. Applications in pathways utilizing xylose
Bioproduction from renewable feedstock has led to developments in the utilization of xylan or xylose as a substrate. Xylan utilization was improved in E. coli by engineering both extracellular xylan degradation and intracellular xylose conversion (Gao et al., 2020). In this case, extracellular xylan degradation was first improved by tuning the expression of xylanases and xylose transporters. Xylose can be converted to xylulose, which can be further utilized in pentose phosphate pathway to support the cell growth. Xylose can also be converted to xylonate, a high-value platform chemical in industry. To increase xylonate production, a QS system was used to downregulate the xylose isomerase (encoded by xylA) and upregulate xylose dehydrogenase (encoded by ccxylB) once the cells reach a desired density (Fig. 4F). Efficient xylose utilization is also hampered by carbon catabolite repression. To solve this problem, a protease-based inverter was used to block glucose utilization in the production phase for xylonate production (Gao et al., 2019). Another issue in xylonate production is pH homeostasis. Hydrolysis of xylonolactone to xylonate by lactonase can acidify the cell, impairing cell viability (Nygård et al., 2014). To achieve intracellular pH homeostasis, a protease-based oscillator was built to periodically slow down the hydrolysis, which further led to an improved xylonate titer (Gao et al., 2019). Xylose has also been used for 1,4-butanediol production in E. coli. The pathway from xylose to 1,4-butanediol requires additional reducing equivalents from native metabolism. To delay the competition of reducing equivalents, a QS system was exploited for dynamic control of the 1,4-butanediol biosynthesis pathway (Liu and Lu, 2015).
5. Conclusions
Dynamic control has been used for overcoming numerous challenges in metabolic engineering, including toxic metabolite accumulation, unbalanced pathway flux, and production heterogeneity, with new applications still being developed. Theoretical work has elucidated the fundamentals on the dynamics and robustness of controlled metabolic systems and has illustrated the choice of control valves and topology. Synthetic biology has provided delicate tools to construct sensors for detecting changing environments and actuators for precise control of cellular metabolism. Altogether, these models and tools have established a strong foundation for future research in dynamic metabolic engineering.
In the near future, we expect this field to continue to grow, especially along the lines of solving several unaddressed challenges. Firstly, current dynamic metabolic control often relies on extensive trial-and-error for choosing topology and tuning of the control parameters, which is labor-intensive. Although several control topologies may help to improve production, the most optimal topology can be difficult to predict. In addition, improved TRY performance is only seen within a narrow range of parameter settings. Addressing these challenges requires precise control design algorithms, which connect the dynamics of control systems with overall metabolite production metrics to quickly identify the desirable control topology and parameters for experimental implementation. Second, for control systems that are not performing adequately, there is a lack of diagnostic tools for assessing dynamic problems, for example long delays, overshoot, slow dynamics, and oscillations. This lack of diagnostic tools exacerbates the trial-and-error search for good control systems. Third, although a key advantage of implementing dynamic controls is to respond to heterogeneous microenvironments and metabolite heterogeneity, models of control system often do not account for these heterogeneities. These phenomena may be important in the choice of topologies and parameters. Additionally, methods for rapidly assessing the performance of control systems when subjected to these heterogeneities are lacking. Development of models and methods to account for heterogeneity could be critical for the scale-up and commercialization of many bioprocesses, where large heterogeneities exist. Finally, as the most important component of dynamic control, sensors are still lacking for many metabolites of interest, which limits the application of dynamic control. General methods to engineer sensors specific to a metabolite of interest are strongly needed. New design strategies beyond known MRTFs, responsive promoters, and riboswitches, are particularly interesting. Solving these grand challenges will provide exciting avenues for research at the nexus of control theory, systems and synthetic biology, and metabolic engineering.
Acknowledgements
This work is supported by the National Institute of General Medical Sciences of the National Institutes of Health [grant number R35GM133797]. AS and YH are supported by the National Human Genome Research Institute training grant [grant number T32 HG000045].
Abbreviations
- AAA
aromatic amino acid
- ACC
acetyl-CoA carboxylase
- DMAPP
dimethylallyl pyrophosphate
- FPP
farnesyl pyrophosphate
- FAEE
fatty acid ethyl esters
- FFA
free fatty acid
- GlcN6P
glucosamine-6-phosphate
- G6P
glucose-6-phosphate
- GOI
gene of interest
- IPP
isopentenyl pyrophosphate
- MCA
metabolic control analysis
- MRTF
metabolite-responsive transcription factor
- MA
muconic acid
- MEP
methylerythritol phosphate
- MVA
mevalonic acid
- MI
myo-inisitol
- MIOX
myo-inisitol oxygenase
- GlcNAc
N-acetylglucosamine
- NGL
negative gene loop
- NLML
negative layered metabolic loop
- NML
negative metabolic loop
- OL
open loop
- p-AS
p-aminostyrene
- PHBA
para-hydroxybenzoic acid
- PFK
phosphofructokinase
- QS
quorum sensing
- RNAi
RNA interference
- TRY
Titer, Rate, and Yield
- 3-HP
3-hydroxypropionic acid
- 4-HIL
4-hydroxyisoleucine
Footnotes
Declaration of competing interest
None.
References
- Abdel-Mawgoud AM, Markham KA, Palmer CM, Liu N, Stephanopoulos G, Alper HS, 2018. Metabolic engineering in the host Yarrowia lipolytica. Metab. Eng 50, 192–208. 10.1016/j.ymben.2018.07.016. [DOI] [PubMed] [Google Scholar]
- Ackermann M, 2015. A functional perspective on phenotypic heterogeneity in microorganisms. Nat. Rev. Microbiol 13, 497–508. 10.1038/nrmicro3491. [DOI] [PubMed] [Google Scholar]
- Anesiadis N, Cluett WR, Mahadevan R, 2008. Dynamic metabolic engineering for increasing bioprocess productivity. Metab. Eng 10, 255–266. 10.1016/j.ymben.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Angeli D, Ferrell JE, Sontag ED, 2004. Detection of multistability, bifurcations, and hysteresis in a large class of biological positive-feedback systems. Proc. Natl. Acad. Sci. U. S. A 101, 1822–1827. 10.1073/pnas.0308265100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai W, Geng W, Wang S, Zhang F, 2019. Biosynthesis, regulation, and engineering of microbially produced branched biofuels. Biotechnol. Biofuels 12, 1–12. 10.1186/s13068-019-1424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Rohles CM, Wittmann C, 2018. Metabolically engineered Corynebacterium glutamicum for bio-based production of chemicals, fuels, materials, and healthcare products. Metab. Eng 50, 122–141. 10.1016/j.ymben.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Bentley GJ, Jiang W, Guamán LP, Xiao Y, Zhang F, 2016. Engineering Escherichia coli to produce branched-chain fatty acids in high percentages. Metab. Eng 38, 148–158. 10.1016/j.ymben.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Binder D, Drepper T, Jaeger KE, Delvigne F, Wiechert W, Kohlheyer D, Grünberger A, 2017. Homogenizing bacterial cell factories: analysis and engineering of phenotypic heterogeneity. Metab. Eng 42, 145–156. 10.1016/j.ymben.2017.06.009. [DOI] [PubMed] [Google Scholar]
- Borri A, Palumbo P, Singh A, 2016. Impact of negative feedback in metabolic noise propagation. IET Syst. Biol 10, 179–186. 10.1049/iet-syb.2016.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borri A, Palumbo P, Singh A, 2015. Metabolic noise reduction for enzymatic reactions: the role of a negative feedback. In: Proc. IEEE Conf. Decis. Control 54rd IEEE, pp. 2537–2542. 10.1109/CDC.2015.7402598. [DOI] [Google Scholar]
- Bothfeld W, Kapov G, Tyo KEJ, 2017. A glucose-sensing toggle switch for autonomous, high productivity genetic control. ACS Synth. Biol 6, 1296–1304. 10.1021/acssynbio.6b00257. [DOI] [PubMed] [Google Scholar]
- Bowen CH, Bonin J, Kogler A, Barba-Ostria C, Zhang F, 2016. Engineering Escherichia coli for conversion of glucose to medium-chain ω-hydroxy fatty acids and α,ω-dicarboxylic acids. ACS Synth. Biol 5, 200–206. 10.1021/acssynbio.5b00201. [DOI] [PubMed] [Google Scholar]
- Burg JM, Cooper CB, Ye Z, Reed BR, Moreb EA, Lynch MD, 2016. Large-scale bioprocess competitiveness: the potential of dynamic metabolic control in two-stage fermentations. Curr. Opin. Chem. Eng 14, 121–136. 10.1016/j.coche.2016.09.008. [DOI] [Google Scholar]
- Burgard AP, Pharkya P, Maranas CD, 2003. Optknock: a bilevel programming framework for identifying gene knockout strategies for microbial strain optimization. Biotechnol. Bioeng 84, 647–657. 10.1002/bit.10803. [DOI] [PubMed] [Google Scholar]
- Cao M, Gao M, Suástegui M, Mei Y, Shao Z, 2020. Building microbial factories for the production of aromatic amino acid pathway derivatives: from commodity chemicals to plant-sourced natural products. Metab. Eng 58, 94–132. 10.1016/j.ymben.2019.08.008. [DOI] [PubMed] [Google Scholar]
- Charusanti P, Conrad TM, Knight EM, Venkataraman K, Fong NL, Xie B, Gao Y, Palsson B, 2010. Genetic basis of growth adaptation of Escherichia coli after deletion of pgi, a major metabolic gene. PLoS Genet. 6 10.1371/journal.pgen.1001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lou S, Fan L, Zhang X, Tan T, 2015. Control of ATP concentration in Escherichia coli using synthetic small regulatory RNAs for enhanced S-adenosylmethionine production. FEMS Microbiol. Lett 362, 1–8. 10.1093/femsle/fnv115. [DOI] [PubMed] [Google Scholar]
- Chin C-S, Chubukov V, Jolly ER, DeRisi J, Li H, 2008. Dynamics and design principles of a basic regulatory architecture controlling metabolic pathways. PLoS Biol. 6, e146. 10.1371/journal.pbio.0060146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou HH, Keasling JD, 2013. Programming adaptive control to evolve increased metabolite production. Nat. Commun 4, 1–8. 10.1038/ncomms3595. [DOI] [PubMed] [Google Scholar]
- Chubukov V, Gerosa L, Kochanowski K, Sauer U, 2014. Coordination of microbial metabolism. Nat. Rev. Microbiol 12, 327–340. 10.1038/nrmicro3238. [DOI] [PubMed] [Google Scholar]
- Chubukov V, Sauer U, 2014. Environmental dependence of stationary-phase metabolism in Bacillus subtilis and Escherichia coli. Appl. Environ. Microbiol 80, 2901–2909. 10.1128/AEM.00061-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crater JS, Lievense JC, 2018. Scale-up of industrial microbial processes. FEMS Microbiol. Lett 365, 1–5. 10.1093/femsle/fny138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress BF, Trantas EA, Ververidis F, Linhardt RJ, Koffas MAG, 2015. Sensitive cells: enabling tools for static and dynamic control of microbial metabolic pathways. Curr. Opin. Biotechnol 10.1016/j.copbio.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Crosby JR, Laemthong T, Lewis AM, Straub CT, Adams MW, Kelly RM, 2019. Extreme thermophiles as emerging metabolic engineering platforms. Curr. Opin. Biotechnol 59, 55–64. 10.1016/j.copbio.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Cui S, Lv X, Wu Y, Li J, Du G, Ledesma-Amaro R, Liu L, 2019. Engineering a bifunctional Phr60-Rap60-Spo0A quorum-sensing molecular switch for dynamic fine-tuning of menaquinone-7 synthesis in Bacillus subtilis. ACS Synth. Biol 8, 1826–1837. 10.1021/acssynbio.9b00140. [DOI] [PubMed] [Google Scholar]
- Dahl RH, Zhang F, Alonso-Gutierrez J, Baidoo E, Batth TS, Redding-Johanson AM, Petzold CJ, Mukhopadhyay A, Lee TS, Adams PD, Keasling JD, 2013. Engineering dynamic pathway regulation using stress-response promoters. Nat. Biotechnol 31, 1039–1046. 10.1038/nbt.2689. [DOI] [PubMed] [Google Scholar]
- David F, Nielsen J, Siewers V, 2016. Flux control at the malonyl-CoA node through hierarchical dynamic pathway regulation in Saccharomyces cerevisiae. ACS Synth. Biol 5, 224–233. 10.1021/acssynbio.5b00161. [DOI] [PubMed] [Google Scholar]
- Delvigne F, Goffin P, 2014. Microbial heterogeneity affects bioprocess robustness: dynamic single-cell analysis contributes to understanding of microbial populations. Biotechnol. J 9, 61–72. 10.1002/biot.201300119. [DOI] [PubMed] [Google Scholar]
- Delvigne F, Zune Q, Lara AR, Al-Soud W, Sørensen SJ, 2014. Metabolic variability in bioprocessing: implications of microbial phenotypic heterogeneity. Trends Biotechnol. 32, 608–616. 10.1016/j.tibtech.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Dinh CV, Prather KLJ, 2019. Development of an autonomous and bifunctional quorum-sensing circuit for metabolic flux control in engineered Escherichia coli. Proc. Natl. Acad. Sci. U. S. A 116, 25562–25568. 10.1073/pnas.1911144116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doong SJ, Gupta A, Prather KLJ, 2018. Layered dynamic regulation for improving metabolic pathway productivity in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A 115, 2964–2969. 10.1073/pnas.1716920115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop MJ, Keasling JD, Mukhopadhyay A, 2010. A model for improving microbial biofuel production using a synthetic feedback loop. Syst. Synth. Biol 4, 95–104. 10.1007/s11693-010-9052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Wang T, Zhang C, Bai J, Zheng X, Zhao X, Lou C, Xing XH, 2016. Intermediate-sensor assisted push-pull strategy and its application in heterologous deoxyviolacein production in Escherichia coli. Metab. Eng 33, 41–51. 10.1016/j.ymben.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Farmer WR, Liao JC, 2000. Improving lycopene production in Escherichia coli by engineering metabolic control. Nat. Biotechnol 18, 533–537. 10.1038/75398. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, 2002. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol 14, 140–148. 10.1016/S0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- Gadkar KG, Doyle FJ, Edwards JS, Mahadevan R, 2005. Estimating optimal profiles of genetic alterations using constraint-based models. Biotechnol. Bioeng 89, 243–251. 10.1002/bit.20349. [DOI] [PubMed] [Google Scholar]
- Gao C, Guo L, Ding Q, Hu G, Ye C, Liu J, Chen X, Liu L, 2020. Dynamic consolidated bioprocessing for direct production of xylonate and shikimate from xylan by Escherichia coli. Metab. Eng 60, 128–137. 10.1016/j.ymben.2020.04.001. [DOI] [PubMed] [Google Scholar]
- Gao C, Hou J, Xu P, Guo L, Chen X, Hu G, Ye C, Edwards H, Chen J, Chen W, Liu L, 2019. Programmable biomolecular switches for rewiring flux in Escherichia coli. Nat. Commun 10, 1–12. 10.1038/s41467-019-11793-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasscock CJ, Lazar JT, Biggs BW, Arnold JH, Kang M-K, Tullman-Ercek D, Tyo KEJ, Lucks JB, 2019. Dynamic control of pathway expression with riboregulated switchable feedback promoters. bioRxiv 529180. 10.1101/529180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Jiang W, Mu Y, Huang H, Su T, Luo Y, Liang Q, Qi Q, 2020. Quorum sensing-based dual-function switch and its application in solving two key metabolic engineering problems. ACS Synth. Biol 9, 209–217. 10.1021/acssynbio.9b00290. [DOI] [PubMed] [Google Scholar]
- Guo X, Li Z, Wang X, Wang J, Chala J, Lu Y, Zhang H, 2019. De novo phenol bioproduction from glucose using biosensor-assisted microbial coculture engineering. Biotechnol. Bioeng 116, 3349–3359. 10.1002/bit.27168. [DOI] [PubMed] [Google Scholar]
- Guo X, Wang X, Chen T, Lu Y, Zhang H, 2020. Comparing E. coli mono-cultures and co-cultures for biosynthesis of protocatechuic acid and hydroquinone. Biochem. Eng. J 156, 107518. 10.1016/j.bej.2020.107518. [DOI] [Google Scholar]
- Gupta A, Reizman IMB, Reisch CR, Prather KLJ, 2017. Dynamic regulation of metabolic flux in engineered bacteria using a pathway-independent quorum-sensing circuit. Nat. Biotechnol 35 10.1038/nbt.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder BJ, Bettenbrock K, Klamt S, 2018. Temperature-dependent dynamic control of the TCA cycle increases volumetric productivity of itaconic acid production by Escherichia coli. Biotechnol. Bioeng 115, 156–164. 10.1002/bit.26446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison ME, Dunlop MJ, 2012. Synthetic feedback loop model for increasing microbial biofuel production using a biosensor. Front. Microbiol 3, 1–9. 10.3389/fmicb.2012.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline CJ, Mannan AA, Liu D, Zhang F, Oyarzún DA, 2020. Metabolite sequestration enables rapid recovery from fatty acid depletion in Escherichia coli. mBio 11, 1–11. 10.1128/mBio.03112-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Murabito E, Westerhoff HV, 2016. Synthetic biology and regulatory networks: where metabolic systems biology meets control engineering. J. R. Soc. Interface 13. 10.1098/rsif.2015.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Chen Y, Liang Q, Qi Q, 2017. Autoinduced AND gate controls metabolic pathway dynamically in response to microbial communities and cell physiological state. ACS Synth. Biol 6, 463–470. 10.1021/acssynbio.6b00177. [DOI] [PubMed] [Google Scholar]
- Holtz WJ, Keasling JD, 2010. Engineering static and dynamic control of synthetic pathways. Cell 140, 19–23. 10.1016/j.cell.2009.12.029. [DOI] [PubMed] [Google Scholar]
- Hwang HJ, Kim JW, Ju SY, Park JH, Lee PC, 2017. Application of an oxygen-inducible nar promoter system in metabolic engineering for production of biochemicals in Escherichia coli. Biotechnol. Bioeng 114, 468–473. 10.1002/bit.26082. [DOI] [PubMed] [Google Scholar]
- Jabarivelisdeh B, Waldherr S, 2018. Optimization of bioprocess productivity based on metabolic-genetic network models with bilevel dynamic programming. Biotechnol. Bioeng 115, 1829–1841. 10.1002/bit.26599. [DOI] [PubMed] [Google Scholar]
- Jiang W, Gu P, Zhang F, 2018. Steps towards ‘drop-in’ biofuels: focusing on metabolic pathways. Curr. Opin. Biotechnol 53, 26–32. 10.1016/j.copbio.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Jiang W, Qiao JB, Bentley GJ, Liu D, Zhang F, 2017. Modular pathway engineering for the microbial production of branched-chain fatty alcohols. Biotechnol. Biofuels 10, 1–15. 10.1186/s13068-017-0936-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozefczuk S, Klie S, Catchpole G, Szymanski J, Cuadros-Inostroza A, Steinhauser D, Selbig J, Willmitzer L, 2010. Metabolomic and transcriptomic stress response of Escherichia coli. Mol. Syst. Biol 6, 1–16. 10.1038/msb.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EM, Woo HM, Tian T, Yilmaz S, Javidpour P, Keasling JD, Lee TS, 2017. Autonomous control of metabolic state by a quorum sensing (QS)-mediated regulator for bisabolene production in engineered E. coli. Metab. Eng 44, 325–336. 10.1016/j.ymben.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Kizer L, Pitera DJ, Pfleger BF, Keasling JD, 2008. Application of functional genomics to pathway optimization for increased isoprenoid production. Appl. Environ. Microbiol 74, 3229–3241. 10.1128/AEM.02750-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamt S, Mahadevan R, Hädicke O, 2018. When do two-stage processes outperform one-stage processes? Biotechnol. J 13 10.1002/biot.201700539. [DOI] [PubMed] [Google Scholar]
- Kochanowski K, Gerosa L, Brunner SF, Christodoulou D, Nikolaev YV, Sauer U, 2017. Few regulatory metabolites coordinate expression of central metabolic genes in Escherichia coli. Mol. Syst. Biol 13, 903. 10.15252/msb.20167402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresnowati MTAP, Van Winden WA, Almering MJH, Ten Pierick A, Ras C, Knijnenburg TA, Daran-Lapujade P, Pronk JT, Heijnen JJ, Daran JM, 2006. When transcriptome meets metabolome: fast cellular responses of yeast to sudden relief of glucose limitation. Mol. Syst. Biol 2 10.1038/msb4100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland CG, Dong H, 1996. Bacterial growth inhibition by overproduction of protein. Mol. Microbiol 21, 1–4. 10.1046/j.1365-2958.1996.5901313.x. [DOI] [PubMed] [Google Scholar]
- Labhsetwar P, Cole JA, Roberts E, Price ND, Luthey-Schulten ZA, 2013. Heterogeneity in protein expression induces metabolic variability in a modeled Escherichia coli population. Proc. Natl. Acad. Sci. U. S. A 110, 14006–14011. 10.1073/pnas.1222569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara AR, Galindo E, Ramírez OT, Palomares LA, 2006. Living with heterogeneities in bioreactors: understanding the effects of environmental gradients on cells. Mol. Biotechnol 34, 355–381. 10.1385/MB:34:3:355. [DOI] [PubMed] [Google Scholar]
- Li Q, Fan F, Gao X, Yang C, Bi C, Tang J, Liu T, Zhang X, 2017. Balanced activation of IspG and IspH to eliminate MEP intermediate accumulation and improve isoprenoids production in Escherichia coli. Metab. Eng 44, 13–21. 10.1016/j.ymben.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Li S, Wang J, Xiang W, Yang K, Li Z, Wang W, 2018. An autoregulated fine-tuning strategy for titer improvement of secondary metabolites using native promoters in Streptomyces. ACS Synth. Biol 7, 522–530. 10.1021/acssynbio.7b00318. [DOI] [PubMed] [Google Scholar]
- Lian J, Mishra S, Zhao H, 2018. Recent advances in metabolic engineering of Saccharomyces cerevisiae: new tools and their applications. Metab. Eng 50, 85–108. 10.1016/j.ymben.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Liang C, Zhang X, Wu J, Mu S, Wu Z, Jin J-M, Tang S-Y, 2020. Dynamic control of toxic natural product biosynthesis by an artificial regulatory circuit. Metab. Eng 57, 239–246. 10.1016/j.ymben.2019.12.002. [DOI] [PubMed] [Google Scholar]
- Liu D, Evans T, Zhang F, 2015a. Applications and advances of metabolite biosensors for metabolic engineering. Metab. Eng 31, 35–43. 10.1016/j.ymben.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Liu D, Mannan AA, Han Y, Oyarzún DA, Zhang F, 2018. Dynamic metabolic control: towards precision engineering of metabolism. J. Ind. Microbiol. Biotechnol 45, 535–543. 10.1007/s10295-018-2013-9. [DOI] [PubMed] [Google Scholar]
- Liu D, Xiao Y, Evans BS, Zhang F, 2015b. Negative feedback regulation of fatty acid production based on a malonyl-CoA sensor–actuator. ACS Synth. Biol 4, 132–140. 10.1021/sb400158w. [DOI] [PubMed] [Google Scholar]
- Liu D, Zhang F, 2018. Metabolic feedback circuits provide rapid control of metabolite dynamics. ACS Synth. Biol 7, 347–356. 10.1021/acssynbio.7b00342. [DOI] [PubMed] [Google Scholar]
- Liu S. De, Wu YN, Wang TM, Zhang C, Xing XH, 2017. Maltose utilization as a novel selection strategy for continuous evolution of microbes with enhanced metabolite production. ACS Synth. Biol 6, 2326–2338. 10.1021/acssynbio.7b00247. [DOI] [PubMed] [Google Scholar]
- Liu H, Lu T, 2015. Autonomous production of 1,4-butanediol via a de novo biosynthesis pathway in engineered Escherichia coli. Metab. Eng 29, 135–141. 10.1016/j.ymben.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Lo TM, Chng SH, Teo WS, Cho HS, Chang MW, 2016. A two-layer gene circuit for decoupling cell growth from metabolite production. Cell Syst 3, 133–143. 10.1016/j.cels.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Lv Y, Gu Y, Xu J, Zhou J, Xu P, 2020. Coupling metabolic addiction with negative autoregulation to improve strain stability and pathway yield. Metab. Eng 61, 79–88. 10.1016/j.ymben.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y, Qian S, Du G, Chen J, Zhou J, Xu P, 2019. Coupling feedback genetic circuits with growth phenotype for dynamic population control and intelligent bioproduction. Metab. Eng 54, 109–116. 10.1016/j.ymben.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Mannan AA, Liu D, Zhang F, Oyarzún DA, 2017. Fundamental design principles for transcription-factor-based metabolite biosensors. ACS Synth. Biol 6, 1851–1859. 10.1021/acssynbio.7b00172. [DOI] [PubMed] [Google Scholar]
- Martin VJJ, Pitera DJ, Withers ST, Newman JD, Keasling JD, 2003. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat. Biotechnol 21, 796–802. 10.1038/nbt833. [DOI] [PubMed] [Google Scholar]
- Maury J, Kannan S, Jensen NB, Öberg FK, Kildegaard KR, Forster J, Nielsen J, Workman CT, Borodina I, 2018. Glucose-dependent promoters for dynamic regulation of metabolic pathways. Front. Bioengy Biotechnol 6, 1–12. 10.3389/fbioe.2018.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milias-Argeitis A, Rullan M, Aoki SK, Buchmann P, Khammash M, 2016. Automated optogenetic feedback control for precise and robust regulation of gene expression and cell growth. Nat. Commun 7 10.1038/ncomms12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milias-Argeitis A, Summers S, Stewart-Ornstein J, Zuleta I, Pincus D, El-Samad H, Khammash M, Lygeros J, 2011. In silico feedback for in vivo regulation of a gene expression circuit. Nat. Biotechnol 29, 1114–1116. 10.1038/nbt.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrophanov AY, Groisman EA, 2008. Positive feedback in cellular control systems. Bioessays 30, 542–555. 10.1002/bies.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu T, Liu Y, Li J, Koffas M, Du G, Alper HS, Liu L, 2018. Engineering a glucosamine-6-phosphate responsive glmS ribozyme switch enables dynamic control of metabolic flux in Bacillus subtilis for overproduction of N-acetylglucosamine. ACS Synth. Biol 7, 2423–2435. 10.1021/acssynbio.8b00196. [DOI] [PubMed] [Google Scholar]
- Nygård Y, Maaheimo H, Mojzita D, Toivari M, Wiebe M, Resnekov O, Gustavo Pesce C, Ruohonen L, Penttilä M, 2014. Single cell and in vivo analyses elucidate the effect of xylC lactonase during production of D-xylonate in Saccharomyces cerevisiae. Metab. Eng 25, 238–247. 10.1016/j.ymben.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Oyarzún DA, Chaves M, 2015. Design of a bistable switch to control cellular uptake. J. R. Soc. Interface 12. 10.1098/rsif.2015.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarzún DA, Lugagne JB, Stan G-BV, 2015. Noise propagation in synthetic gene circuits for metabolic control. ACS Synth. Biol 4, 116–125. 10.1021/sb400126a. [DOI] [PubMed] [Google Scholar]
- Oyarzún DA, Stan G-BV, 2013. Synthetic gene circuits for metabolic control: design trade-offs and constraints. J. R. Soc. Interface 10, 20120671. 10.1098/rsif.2012.0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarzún DA, Stan G-BV, 2012. Design tradeoffs in a synthetic gene control circuit for metabolic networks. In: 2012 American Control Conference. (ACC) IEEE, pp. 2743–2748. 10.1109/ACC.2012.6314705. [DOI] [Google Scholar]
- Peng B, Nielsen LK, Kampranis SC, Vickers CE, 2018. Engineered protein degradation of farnesyl pyrophosphate synthase is an effective regulatory mechanism to increase monoterpene production in Saccharomyces cerevisiae. Metab. Eng 47, 83–93. 10.1016/j.ymben.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Pigou M, Morchain J, 2015. Investigating the interactions between physical and biological heterogeneities in bioreactors using compartment, population balance and metabolic models. Chem. Eng. Sci 126, 267–282. 10.1016/j.ces.2014.11.035. [DOI] [Google Scholar]
- Pontrelli S, Chiu TY, Lan EI, Chen FYH, Chang P, Liao JC, 2018. Escherichia coli as a host for metabolic engineering. Metab. Eng 50, 16–46. 10.1016/j.ymben.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Raj A, van Oudenaarden A, 2008. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell 135, 216–226. 10.1016/j.cell.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar AS, Liu G, Bergenholm D, Arsovska D, Kristensen M, Nielsen J, Jensen MK, Keasling JD, 2016. Engineering of synthetic, stress-responsive yeast promoters. Nucleic Acids Res. 44 10.1093/nar/gkw553e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan S, Suthers PF, Maranas CD, 2010. OptForce: an optimization procedure for identifying all genetic manipulations leading to targeted overproductions. PLoS Comput. Biol 6, e1000744 10.1371/journal.pcbi.1000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed KB, Alper HS, 2018. Expanding beyond canonical metabolism: interfacing alternative elements, synthetic biology, and metabolic engineering. Synth. Syst. Biotechnol 3, 20–33. 10.1016/j.synbio.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugbjerg P, Myling-Petersen N, Porse A, Sarup-Lytzen K, Sommer MOA, 2018a. Diverse genetic error modes constrain large-scale bio-based production. Nat. Commun 9 10.1038/s41467-018-03232-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugbjerg P, Sarup-Lytzen K, Nagy M, Sommer MOA, 2018b. Synthetic addiction extends the productive life time of engineered Escherichia coli populations. Proc. Natl. Acad. Sci. U. S. A 115, 2347–2352. 10.1073/pnas.1718622115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalcinati G, Knuf C, Partow S, Chen Y, Maury J, Schalk M, Daviet L, Nielsen J, Siewers V, 2012. Dynamic control of gene expression in Saccharomyces cerevisiae engineered for the production of plant sesquitepene α-santaleane in a fed-batch mode. Metab. Eng 14, 91–103. 10.1016/j.ymben.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Schmitz AC, Hartline CJ, Zhang F, 2017. Engineering microbial metabolite dynamics and heterogeneity. Biotechnol. J 12, 1700422. 10.1002/biot.201700422. [DOI] [PubMed] [Google Scholar]
- Shen HJ, Cheng BY, Zhang YM, Tang L, Li Z, Bu YF, Li XR, Tian GQ, Liu JZ, 2016. Dynamic control of the mevalonate pathway expression for improved zeaxanthin production in Escherichia coli and comparative proteome analysis. Metab. Eng 38, 180–190. 10.1016/j.ymben.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Shen YP, Fong LS, Yan ZB, Liu JZ, 2019. Combining directed evolution of pathway enzymes and dynamic pathway regulation using a quorum-sensing circuit to improve the production of 4-hydroxyphenylacetic acid in Escherichia coli. Biotechnol. Biofuels 12, 1–11. 10.1186/s13068-019-1438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu Y, Fenno J, Lindle JM, Dunlop MJ, 2018. Design and selection of a synthetic feedback loop for optimizing biofuel tolerance. ACS Synth. Biol 7, 16–23. 10.1021/acssynbio.7b00260. [DOI] [PubMed] [Google Scholar]
- Soma Y, Hanai T, 2015. Self-induced metabolic state switching by a tunable cell density sensor for microbial isopropanol production. Metab. Eng 30, 7–15. 10.1016/j.ymben.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Soma Y, Tsuruno K, Wada M, Yokota A, Hanai T, 2014. Metabolic flux redirection from a central metabolic pathway toward a synthetic pathway using a metabolic toggle switch. Metab. Eng 23, 175–184. 10.1016/j.ymben.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Sonderegger M, Schümperli M, Sauer U, 2005. Selection of quiescent Escherichia coli with high metabolic activity. Metab. Eng 7, 4–9. 10.1016/j.ymben.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Stevens JT, Carothers JM, 2015. Designing RNA-based genetic control systems for efficient production from engineered metabolic pathways. ACS Synth. Biol 4, 107–115. 10.1021/sb400201u. [DOI] [PubMed] [Google Scholar]
- Takhaveev V, Heinemann M, 2018. Metabolic heterogeneity in clonal microbial populations. Curr. Opin. Microbiol 45, 30–38. 10.1016/j.mib.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Tan SZ, Prather KLJ, 2017. Dynamic pathway regulation: recent advances and methods of construction. Curr. Opin. Chem. Biol 10.1016/j.cbpa.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Choi PJ, Li GW, Chen H, Babu M, Hearn J, Emili A, Xie XS, 2010. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science 329, 533–538. 10.1126/science.1188308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taymaz-Nikerel H, De Mey M, Baart G, Maertens J, Heijnen JJ, van Gulik W, 2013. Changes in substrate availability in Escherichia coli lead to rapid metabolite, flux and growth rate responses. Metab. Eng 16, 115–129. 10.1016/j.ymben.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Tian J, Yang G, Gu Y, Sun X, Lu Y, Jiang W, 2020. Developing an endogenous quorum-sensing based CRISPRi circuit for autonomous and tunable dynamic regulation of multiple targets in Streptomyces. Nucleic Acids Res. 1–39 10.1093/nar/gkaa602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A, Ray JCJ, Narula J, Igoshin OA, 2011. Bistable responses in bacterial genetic networks: designs and dynamical consequences. Math. Biosci 231, 76–89. 10.1016/j.mbs.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonn MK, Thomas P, Barahona M, Oyarzún DA, 2019. Stochastic modelling reveals mechanisms of metabolic heterogeneity. Commun. Biol 2, 1–9. 10.1038/s42003-019-0347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez-Cruz NA, Caspeta L, Pérez NO, Ramírez OT, Trujillo-Roldán MA, 2010. Production of recombinant proteins in E. coli by the heat inducible expression system based on the phage lambda pL and/or pR promoters. Microb. Cell Factories 9, 1–16. 10.1186/1475-2859-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening J-W, Smits WK, Kuipers OP, 2008. Bistability, epigenetics, and bet-hedging in bacteria. Annu. Rev. Microbiol 62, 193–210. 10.1146/annurev.micro.62.081307.163002. [DOI] [PubMed] [Google Scholar]
- Venayak N, Anesiadis N, Cluett WR, Mahadevan R, 2015. Engineering metabolism through dynamic control. Curr. Opin. Biotechnol 10.1016/j.copbio.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Venayak N, Raj K, Jaydeep R, Mahadevan R, 2018a. An optimized bistable metabolic switch to decouple phenotypic states during anaerobic fermentation. ACS Synth. Biol 7, 2854–2866. 10.1021/acssynbio.8b00284. [DOI] [PubMed] [Google Scholar]
- Venayak N, von Kamp A, Klamt S, Mahadevan R, 2018b. MoVE identifies metabolic valves to switch between phenotypic states. Nat. Commun 9, 1–9. 10.1038/s41467-018-07719-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cabales A, Li Z, Zhang H, 2019. Biosensor-assisted high performing cell selection using an E. coli toxin/antitoxin system. Biochem. Eng. J 144, 110–118. 10.1016/j.bej.2019.01.016. [DOI] [Google Scholar]
- Wang X, Policarpio L, Prajapati D, Li Z, Zhang H, 2020. Developing E. coli-E. coli co-cultures to overcome barriers of heterologous tryptamine biosynthesis. Metab. Eng. Commun 10, e00110 10.1016/j.mec.2019.e00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrs M, Tanjore D, Eng T, Lievense J, Pray TR, Mukhopadhyay A, 2019. Engineering robust production microbes for large-scale cultivation. Trends Microbiol 27, 524–537. 10.1016/j.tim.2019.01.006. [DOI] [PubMed] [Google Scholar]
- Williams TC, Averesch NJH, Winter G, Plan MR, Vickers CE, Nielsen LK, Krömer JO, 2015. Quorum-sensing linked RNA interference for dynamic metabolic pathway control in Saccharomyces cerevisiae. Metab. Eng 29, 124–134. 10.1016/j.ymben.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Wu J, Liu Y, Zhao S, Sun J, Jin Z, Zhang D, 2019. Application of dynamic regulation to increase L-phenylalanine production in Escherichia coli. J. Microbiol. Biotechnol 29, 923–932. 10.4014/jmb.1901.01058. [DOI] [PubMed] [Google Scholar]
- Wu Y, Chen T, Liu Y, Tian R, Lv X, Li J, Du G, Chen J, Ledesma-Amaro R, Liu L, 2020. Design of a programmable biosensor-CRISPRi genetic circuits for dynamic and autonomous dual-control of metabolic flux in Bacillus subtilis. Nucleic Acids Res. 48, 996–1009. 10.1093/nar/gkz1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Bowen CH, Liu D, Zhang F, 2016. Exploiting nongenetic cell-to-cell variation for enhanced biosynthesis. Nat. Chem. Biol 12, 339–344. 10.1038/nchembio.2046. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Jiang W, Zhang F, 2017. Developing a genetically encoded, cross-species biosensor for detecting ammonium and regulating biosynthesis of cyanophycin. ACS Synth. Biol 6, 1807–1815. 10.1021/acssynbio.7b00069. [DOI] [PubMed] [Google Scholar]
- Xie W, Ye L, Lv X, Xu H, Yu H, 2015. Sequential control of biosynthetic pathways for balanced utilization of metabolic intermediates in Saccharomyces cerevisiae. Metab. Eng 28, 8–18. 10.1016/j.ymben.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Xu P, 2020. Branch point control at malonyl-CoA node: a computational framework to uncover the design principles of an ideal genetic-metabolic switch. Metab. Eng. Commun 10.1016/j.mec.2020.e00127e00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Li L, Zhang F, Stephanopoulos G, Koffas M, 2014. Improving fatty acids production by engineering dynamic pathway regulation and metabolic control. Proc. Natl. Acad. Sci. U. S. A 111, 11299–11304. 10.1073/PNAS.1406401111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Pfleger BF, 2020. Revisiting metabolic engineering strategies for microbial synthesis of oleochemicals. Metab. Eng 58, 35–46. 10.1016/j.ymben.2019.04.009. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lin Y, Wang J, Wu Y, Zhang R, Cheng M, Shen X, Wang J, Chen Z, Li C, Yuan Q, Yan Y, 2018. Sensor-regulator and RNAi based bifunctional dynamic control network for engineered microbial synthesis. Nat. Commun 9, 1–10. 10.1038/s41467-018-05466-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegorov I, Mairet F, de Jong H, 2019. Optimal control of bacterial growth for the maximization of metabolite production. J. Math. Biol 78, 985–1032. 10.1007/s00285-018-1299-6. [DOI] [PubMed] [Google Scholar]
- Yin X, Shin HD, Li J, Du G, Liu L, Chen J, 2017. Pgas, a low-pH-induced promoter, as a tool for dynamic control of gene expression for metabolic engineering of Aspergillus niger. Appl. Environ. Microbiol 83, 1–14. 10.1128/AEM.03222-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Ching CB, 2015. Dynamic control of ERG9 expression for improved amorpha-4,11-diene production in Saccharomyces cerevisiae. Microb. Cell Factories 14, 1–10. 10.1186/s12934-015-0220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li Y, Ma J, Liu Y, He J, Li Y, Zhu F, Meng J, Zhan J, Li Z, Zhao L, Ma Q, Fan X, Xu Q, Xie X, Chen N, 2018. High production of 4-hydroxyisoleucine in Corynebacterium glutamicum by multistep metabolic engineering. Metab. Eng 49, 287–298. 10.1016/j.ymben.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Zhang F, Carothers JM, Keasling JD, 2012. Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat. Biotechnol 30, 354–359. 10.1038/nbt.2149. [DOI] [PubMed] [Google Scholar]
- Zhao EM, Zhang Y, Mehl J, Park H, Lalwani MA, Toettcher JE, Avalos JL, 2018. Optogenetic regulation of engineered cellular metabolism for microbial chemical production. Nature 555, 683–687. 10.1038/nature26141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Chen JC, Ma YM, Chen GQ, 2020. Engineering biosynthesis of polyhydroxyalkanoates (PHA) for diversity and cost reduction. Metab. Eng 58, 82–93. 10.1016/j.ymben.2019.07.004. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Meng F, Zhu Z, Wei W, Sun Z, Chen J, Yu B, Lou C, Chen GQ, 2019. A tight cold-inducible switch built by coupling thermosensitive transcriptional and proteolytic regulatory parts. Nucleic Acids Res 47, e137. 10.1093/nar/gkz785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L-B, Zeng A-P, 2015a. Engineering a lysine-ON riboswitch for metabolic control of lysine production in Corynebacterium glutamicum. ACS Synth. Biol 4, 1335–1340. 10.1021/acssynbio.5b00075. [DOI] [PubMed] [Google Scholar]
- Zhou L-B, Zeng A-P, 2015b. Exploring lysine riboswitch for metabolic flux control and improvement of L-lysine synthesis in Corynebacterium glutamicum. ACS Synth. Biol 4, 729–734. 10.1021/sb500332c. [DOI] [PubMed] [Google Scholar]
- Zhou L, Deng C, Cui WJ, Liu ZM, Zhou ZM, 2016. Efficient L-alanine production by a thermo-regulated switch in Escherichia coli. Appl. Biochem. Biotechnol 178, 324–337. 10.1007/s12010-015-1874-x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Niu DD, Tian KM, Chen XZ, Prior BA, Shen W, Shi GY, Singh S, Wang ZX, 2012. Genetically switched D-lactate production in Escherichia coli. Metab. Eng 14, 560–568. 10.1016/j.ymben.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Zhuang K, Yang L, Cluett WR, Mahadevan R, 2013. Dynamic strain scanning optimization: an efficient strain design strategy for balanced yield, titer, and productivity. DySScO strategy for strain design. BMC Biotechnol. 13 10.1186/1472-6750-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


