Abstract
Identifying the susceptibility factors of the emotional response to COVID-19 is highly significant for the psychological epidemic-crisis intervention, and autistic-related traits (ATs) is likely to be one of the candidate factors. The current study explored the relationships between ATs, emotional response to COVID-19, and the behavioural immune system (BIS) measured by trait pathogen avoidance and COVID-19 risk perception in the general population. The results showed that ATs predicted increased negative emotions directly and indirectly by enhancing the activation tendency of BIS and COVID-19 risk perception. The findings provide a candidate hypothesis for the reaction characteristics to pathogen threats in individuals with ASD and expand the understanding of individual differences in response to COVID-19.
Keywords: Autism spectrum disorder, Autistic traits, Negative emotions, Behavioural immune system, Perceived risk, COVID-19
1. Introduction
The novel coronavirus disease (COVID-19) has caused a global pandemic. Due to its highly contagious nature as well as the uncertainty and low predictability of the pandemic, the public has been shrouded in epidemic stress (Zhao et al., 2020). A series of negative consequences of anti-epidemic measures (e.g. increased social isolation and routines change) are likely to further intensify this situation (Brooks et al., 2020). Enhanced negative emotions, such as fear, anxiety and anger, are one of the main manifestations of epidemic stress (Li et al., 2020).
Some idiosyncratic or cognitive psychological factors may be particularly relevant for understanding the emotional responses to COVID-19. Humans beings are evolutionarily equipped with a set of adaptive psychological mechanisms, namely the behavioural immune system (BIS), to promote disease avoidance (Ackerman et al., 2018; Murray and Schaller, 2016). The BIS does not exist as a binary but rather as a spectrum with significant variability in sensitivity or reactivity across individuals; therefore, it can be assessed using a chronic personality trait (Terrizzi et al., 2013), such as trait pathogen avoidance or the activation tendency of BIS, as measured by the Perceived Vulnerability to Disease (PVD) questionnaire (Duncan et al., 2009). When faced with potential pathogen threats (e.g. the COVID-19 pandemic), the BIS initiates negative emotions and enhances risk perception to promote the discovery of potential infection sources in time and avoid infection (Li et al., 2020; Makhanova and Shepherd, 2020). On the other hand, an individual's cognitive assessment of threats (e.g. risk perception) in the environment might also result in negative emotions (Zheng et al., 2019).
Indeed, recent studies have shown that the levels of trait pathogen avoidance and COVID-19 risk perception are associated with increased emotional response to COVID-19 (Li et al., 2020; Makhanova and Shepherd, 2020; Shook et al., 2020; Yildirim and Guler, 2021). According to the hypothesis that the dispositional psychological structure often influences an individual's response to stressors (Haruvi-Lamdan et al., 2019), we infer that in addition to the direct path mentioned above, the activation tendency of BIS may further indirectly increase the emotional response to COVID-19 by enhancing its risk perception.
Identifying the susceptibility factors for experiencing negative emotions during the COVID-19 pandemic is highly significant for the psychological epidemic-crisis intervention. Recent studies have shown that some dimensions in personality traits (e.g. neuroticism), pre-existing medical conditions and mental disorders are the susceptibility factors of increased COVID-19-related stress (Aschwanden et al., 2020; Asmundson et al., 2020; Nikcevic et al., 2021). Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental condition, showing features of difficulties in social functions, unusually stereotypic behaviours and restricted interests (Baron-Cohen et al., 2001). Theoretically, some co-occurring and endogenous characteristics associated with ASD, such as lower tolerance of uncertainty and unexpected change as well as poor emotional regulation, are likely to make individuals with ASD more susceptible to the emotional impacts of the COVID-19 pandemic (Cassidy et al., 2020; Oakley et al., 2020). However, such argument lacks empirical evidence.
It has been long recognised that the severity of ASD symptoms fall along a spectrum, and these non-clinical, significant manifestations of autistic phenotypes in the general population are known as subclinical autistic traits (ATs) (Baron-Cohen et al., 2001; Zhao et al., 2019). ATs are also considered to be the sixth independent personality trait, and individuals with high ATs in the general population can be regarded as the analogue model of ASD (Wakabayashi et al., 2006). Identifying and quantifying the cognitive-emotional differences associated with ATs not only provide an opportunity to evaluate ASD models in samples with more variability and effective control of comorbidities (Amos et al., 2019) but also provide advanced evidence for the ASD-related feature profiles that have not yet been mined (Zhao et al., 2020) as well as an understanding of the psychological structure of the typical developing individuals (TD) (Landry and Chouinard, 2016). Therefore, exploring the relationship between ATs and epidemic-related outcomes is a potential window to understand the characteristics of emotional response to COVID-19 among individuals with ASD. The purpose of our present study was to explore the relationship between ATs and negative emotions during COVID-19 in the general population and further understand this association through BIS and COVID-19 risk perception.
Following the basic logic of the ATs research field (Amos et al., 2019; Baron-Cohen et al., 2001; Zhao et al., 2020), namely that the research hypothesis about the cognitive and behavioural characteristics related to ATs usually mirrors clinical ASD characteristics, the present study takes the evidence from the clinical ASD population, which may be related to the response to pathogen threat, as an argument for the association between ATs and BIS and COVID-19 risk perception.
Accumulating evidence implicates immunological disturbances in ASD at both systemic and cellular levels. For example, an unbalanced cytokine profile (e.g. enhanced pro-inflammatory and reduced anti-inflammatory cytokine concentration) (Goines and Ashwood, 2013; Masi et al., 2015) and decreased numbers of CD4+ T-cells (Bjørklund et al., 2016) have been identified in individuals with ASD. Furthermore, ATs in the general population were also associated with the abnormal biological immune system profiles similar to those among individuals diagnosed with ASD (Saresella et al., 2009). In sum, these findings suggest that the biological immune system dysfunction may be related to ASD onset or regulate ATs level (Bjørklund et al., 2016). A compensatory relationship between the BIS and biological immune system may exist: when the biological immune system is limited, the behavioural management of pathogens or the enhancement of trait pathogen avoidance may be the preferred (Ackerman et al., 2018). It is suggested that the vulnerability of the biological immune system in ASD may prompt the mobilisation of BIS to protect the host from pathogens or that ATs may be associated with enhanced BIS tendencies.
The prevalence of medical illnesses, such as autoimmune disease, allergy, infections and cardiovascular diseases, is substantially higher in individuals with ASD than that in the general population (Bjørklund et al., 2016; Croen et al., 2015; Oakley et al., 2020). A recent study showed that past and recent disease experiences are associated with increased BIS activation tendency (Makhanova et al., 2020). Therefore, the increased disease experience associated with ASD may contribute to the link between ATs and BIS. Furthermore, as indicated by life history theory (Mittal et al., 2015), the self-protection model and the risk-as-feelings model (Loewenstein et al., 2001), suggesting past disease experience, shape an individual's perception of disease susceptibility (Makhanova et al., 2020), and increased severe disease experiences may increase the perceived risk of COVID-19 among individuals with ASD or high ATs.
To sum up, although individuals with ASD are theoretically considered more susceptible to the negative emotions caused by the COVID-19 pandemic due to some co-occurrence or endogenous characteristics (Cassidy et al., 2020; Oakley et al., 2020), this inference needs empirical support. Based on the hypothesis of continuous distribution of autism symptoms (Baron-Cohen et al., 2001), the present study aims to explore the relationship between ATs and the emotional response to COVID-19 pandemic as well as the role of idiosyncratic (e.g. trait pathogen avoidance) and cognitive (e.g. COVID-19 risk perception) factors in this association; its aim is to provide candidate evidence for the profile of the characteristics and mechanisms of autistic individuals’ response to pathogen threat and to promote the understanding of susceptibility factors for COVID-19-related stress. Based on the existing literature, we hypothesise the following: (1) ATs are associated with enhanced negative emotions, COVID-19 risk perception and high trait levels of pathogen avoidance; (2) trait pathogen avoidance is associated with increased negative emotions and COVID-19 risk perception; (3) finally, COVID-19 risk perception is associated with increased negative emotions. Based on the mutual predictive relationship among these variables, we further assume that BIS and COVID-19 risk perception mediate the relationship between ATs and negative emotions during COVID-19 – namely the independent mediating mechanisms of BIS and COVID-19 risk perception and a chain-mediating mechanism from BIS to COVID-19 risk perception.
2. Methods
2.1. Participants
The Ethics Committee of the Department of Psychology at Shanghai Normal University approved this study. A total of 723 university students were recruited online (see detailed recruitment procedure below). After eliminating questionnaires that consistently selected the highest or lowest score or gave a wrong response to a question used to detect whether the participants answered the questionnaire carefully, 684 (381 females, mean age = 22.43 years, SD = 2.45, range = 18–31 years old) questionnaires were analysed. As only 34 participants were in Wuhan, China (the city with the most serious epidemic in China) at the time of the questionnaire survey, no separate analysis was conducted. The sample characteristics are shown in Table 1 .
Table 1.
Sample background characteristics.
| % (n) | % (n) | ||
|---|---|---|---|
| Gender | Nationality | ||
| Male | 44.30 (303) | Minority nationality | 5.70 (39) |
| Female | 55.70 (381) | Han nationality | 94.30 (645) |
| Major | RL during outbreak | ||
| Medical Science | 17.84 (122) | Central China | 21.35 (146) |
| Pedagogy | 23.39 (160) | North China | 13.30 (91) |
| Psychology | 25.15 (172) | East China | 32.02 (219) |
| Business Administration | 10.09 (69) | South China | 19.88 (136) |
| Biology | 14.18 (97) | Northwest China | 13.45 (92) |
| Law | 9.36 (64) | ||
| RA during outbreak | Only child or not | ||
| Rural areas | 33.63 (230) | Yes | 41.20 (282) |
| Urban areas | 66.37 (454) | Not | 58.80 (402) |
Note: RL = Region of location; RA= Residential area.
The nature of the study was explained to all participants, and each gave informed consent. All participants were self-reportedly free from any psychiatric, neurological or other serious medical illness, and no direct relatives had an ASD diagnosis.
2.2. Materials and measures
2.2.1. Autism Spectrum Quotient (AQ)
The Chinese version of AQ is a reliable instrument for quantifying ATs in both clinical and non-clinical samples in mainland China (Zhang et al., 2016). Following the original scale (Baron-Cohen et al., 2001), the Chinese version of AQ includes 50 items covering five areas: imagination, attention switching, attention to detail, social skills and communication. Considering that the continuous scale is more reliable than the 0/1 scoring scale and increases the likelihood of obtaining a better approximation of continuous distribution (Zhang et al., 2016; Zhao et al., 2020), we used the continuous (4-point Likert) scale. A high AQ score indicates a high autistic load. In this study, the alpha internal reliability (henceforth ‘α’) of the overall AQ was 0.71.
2.2.2. The PVD scale
The PVD questionnaire (Duncan et al., 2009) is a 15-item scale (originally in English) that assesses the activation tendency of BIS (Ackerman et al., 2018) and includes two subscales: germ aversion (GA) and perceived infectability (PI). GA is composed of eight items and reflects an individual's emotional and behavioural responses to situations where disease-causing microorganisms might be transmitted (e.g. ‘It really bothers me when people sneeze without covering their mouths’), while PI has seven items and reflects an individual's general perception of their susceptibility to disease (e.g. ‘I am more likely than the people around me to catch an infectious disease’). The scale ranged from 1 (Strongly disagree) to 5 (Strongly agree). The PVD questionnaire has been widely applied to different countries and cultural contexts, such as the United States (Makhanova and Shepherd, 2020), China (Wu and Chang, 2012) and Spain (Díaz et al., 2016). Considering that the internal consistency of the GA subscale in some studies was relatively poor, α < 0.6 (Díaz et al., 2016; Wu and Chang, 2012), and that GA and PI subscales theoretically reflect different aspects of trait pathogen avoidance (Duncan et al., 2009) and predict different behaviours in empirical research (Makhanova and Shepherd, 2020), the present study used the two subscales separately. The reliability of the GA and PI in this study were α = 0.64 and α = 0.74, respectively.
2.2.3. COVID-19 risk perception
According to the protection motivation theory and the health belief model (Bults et al., 2011; De Zwart et al., 2007), the COVID-19 risk perception can be evaluated by two sub-dimensions: perceived severity (a person's perception of the severity of COVID-19 transmission and the disease threatening their health) and perceived vulnerability (a person's perception of their probability of being infected with COVID-19). This construct and the corresponding measurement items have been widely used to assess the risk perception of severe acute respiratory syndrome, avian influenza and COVID-19 (Bults et al., 2011; De Zwart et al., 2007; Walrave et al., 2020; Yildirim and Guler, 2020). In the original scale (Bults et al., 2011), three items each were used to measure perceived severity and perceived vulnerability respectively. The scale ranged from 1 (Not severe at all) to 5 (Very severe). A high score indicates a high perceived risk load. The Chinese version was translated from the original English version (Bults et al., 2011) through strict back-translation; thus, it can be considered linguistically equivalent to the original version.
In order to further validate the applicability of the risk perception model to the COVID-19 context and our sample, half of our data were used for exploratory factor analysis (EFA), and the other half were used for confirmatory factor analysis (CFA). Principal component analysis extracted two factors and explained 59.86% of the total variation.
This result is consistent with the original structural hypothesis, except for the item ‘Do you think that, in general, you are susceptible to getting the COVID-19 if you take no preventive measures?’, which originally belonged to perceived vulnerability and was assigned to perceived severity. It seems reasonable that, when the spread of the epidemic is perceived to be more serious, it is easier to be infected without taking preventive measures. The result of CFA supported the two-factor model of the COVID-19 risk perception: χ2 = 13.06; df = 6; χ2/df = 2.18; RMSEA = 0.059; GFI = 0.99; CFI = 0.97; TLI = 0.93. The reliability of perceived severity and perceived vulnerability obtained in this sample were α =0.70 and α = 0.65, respectively.
2.2.4. Negative emotions
Three kinds of negative emotions, namely fear, anger and anxiety, were included to explore the emotional response to COVID-19. The emotion types and measurement forms are consistent with the existing COVID-19 studies (Huang et al., 2020; Li et al., 2020; Makhanova and Shepherd, 2020). Three emotional words (‘scared’ or ‘恐惧的’ in Chinese, ‘afraid’ or ‘害怕的’ and ‘worried’ or ‘担心的’) were used to assess fear-related emotions. Two emotional words each were used to assess emotions related to anger (‘irritable’ or ‘易怒的’ in Chinese and ‘angry’ or ‘生气的’) and anxiety (‘upset’ or ‘心神不宁的’ in Chinese and ‘dysphoria’ or ‘烦躁不安的’). The participants were asked to rate the extent to which they experienced the above emotions in response to the outbreak of COVID-19 in China on a 5-point scale, ranging from 1 (Not at all) to 5 (Very much).
In order to further explore the structure of individuals’ emotional response to COVID-19, half of our data were used for EFA, and the other half were used for CFA. Principal component analysis extracted single factor and explained 65.67% of the total variation. The result of CFA supported the single-factor model: χ2 = 18.40; df = 12; χ2/df = 1.53; RMSEA = 0.040; GFI = 0.99; CFI = 0.99; TLI = 0.99. The reliability of this sample was α = 0.92.
Additionally, this study employed a demographic survey that included questions, as shown in Table 1. Participants’ health was examined by asking the following questions:
-
1)
Have you been diagnosed with any mental health condition (e.g. schizophrenia, depression or anxiety)?
-
2)
Have you been diagnosed with any neurological disease (e.g. epilepsy or meningitis)?
-
3)
Have you been diagnosed with any serious medical illness (e.g. head injury or heart disease)?
-
4)
Have you or your relatives been diagnosed with ASD?
-
5)
Have you or your relatives been diagnosed with COVID-19?
2.3. Procedure
On 20 January 2020, the National Health Commission in China officially listed COVID-19 as a type B infectious disease, marking the beginning of the comprehensive upgrade of epidemic prevention and increased general public concern. The data collection period was from 17 to 27 February 2020. Participants completed an online questionnaire hosted by Wenjuanxing (https://www.wjx.cn/). We contacted those in charge of student online contact groups (WeChat or QQ), such as student monitors, and asked for their consent to share the questionnaire link to their online class group. On the front page of the online questionnaire, we explained the purpose of this study in detail and clarified the number of items in the questionnaire as well as the approximate time taken to complete the questionnaire. Participants were also informed about the voluntary nature of the study, and their data were guaranteed anonymity and confidentiality. Once participants agreed to participate in the survey, they could click on the start button to automatically jump to the formal survey questions. After the participants completed and submitted the survey, the data were automatically uploaded to the system. The participants were given RMB 2 for their participation.
2.4. Statistical analysis
Data analysis was conducted using SPSS Statistics 19.0, Mplus7.0, and Amos 20.0. Gender differences were consistently found in ATs (Baron-Cohen et al., 2001), trait pathogen avoidance (Díaz et al., 2016; Duncan et al., 2009) and COVID-19 risk perception (Huang et al., 2020); thus, an independent samples t-test was used to analyse the possible gender differences in the current sample. Based on our hypothesis, Pearson correlations were used to analyses the bivariate correlations between variables of interest. In the test of the mediating effect, the bootstrap method (based on 5000 bootstrapped resamples) was used to test the statistical significance of the indirect effects (Zhao et al., 2020). If the 95% bootstrap confidence interval (CI) for the indirect effect was entirely above or below zero, then the indirect effect was considered significant; otherwise, the opposite was true.
3. Results
3.1. Test of validity and common method bias
To assess the discriminant validity of variables, we performed confirmatory factor analysis using the maximum likelihood estimation method, with Mplus version 7.0. Table 2 shows that the six-factor model fit the data best, which suggested that the questionnaires had good construct validity. Considering the subjective data collected in the present study, the results may have been influenced by common methodological biases (CMB). We controlled for CMB through procedural remedies and statistical remedy analysis. First, the principles of ‘no right or wrong’ and anonymity were emphasised; the question order was counterbalanced across the questionnaire survey. Second, the unmeasured latent method construct was further used to test the CMB (Richardson et al., 2009). The results showed that the fitting indices of the model were acceptable after adding a method factor (χ2 = 1011.22; df = 448; χ2/df = 2.26; SRMR = 0.056; CFI = 0.90; TLI = 0.88; RMSEA = 0.0431; 90% CI [0.039 0.046]). However, the corrected chi-square difference was less than the critical value (0.05), possibly indicating statistical non-significance.
Table 2.
Confirmatory factor analysis and model comparison.
| Model | χ2 | df | SRMR | CFI | TLI | RMSEA 90% CI |
|---|---|---|---|---|---|---|
| Six-factor modela | 949.74 | 472 | 0.053 | 0.92 | 0.91 | 0.038 [0.035 0.042] |
| Five-factor modelb | 1271.23 | 479 | 0.064 | 0.86 | 0.85 | 0.049 [0.046, 0.052] |
| Four-factor modelc | 1582.51 | 486 | 0.071 | 0.80 | 0.78 | 0.057 [0.054 0.061] |
| Three-factor modeld | 2810.01 | 492 | 0.092 | 0.58 | 0.56 | 0.083 [0.080 0.086] |
| Single-factor modele | 3035.64 | 528 | 0.095 | 0.55 | 0.52 | 0.087 [0.084, 0.090] |
Note: a = Each variable is independent;b= Combining AQ and negative emotions as one factor
=Combining AQ, negative emotions and perceived infectability as one factor
= Combining AQ, negative emotions, perceived vulnerability and germ aversion as one factor
= Combining all items into one factor.
3.2. Gender differences among variables
Table 3 describes the gender differences among variables. The level of ATs in males was marginally significantly higher than in females. Fear, anxiety, anger, total negative emotions, perceived severity, perceived vulnerability and total COVID-19 perceived risk in females were significantly higher than in males (Table 3).
Table 3.
Gender differences among variables.
| Variables | Gender | M | SD | t | d |
|---|---|---|---|---|---|
| 1. Autistic traits | Male | 118.87 | 9.72 | 1.87#1 | 0.14 |
| Female | 117.53 | 9.08 | |||
| 2. Fear | Male | 7.79 | 2.91 | -4.37⁎⁎⁎ | 0.39 |
| Female | 8.75 | 2.82 | |||
| 3. Anxiety | Male | 4.67 | 2.07 | -3.06⁎⁎ | 0.24 |
| Female | 5.14 | 1.92 | |||
| 4. Anger | Male | 4.31 | 2.05 | -2.82⁎⁎ | 0.21 |
| Female | 4.74 | 1.95 | |||
| 5. Total negative emotions | Male | 16.77 | 6.26 | -3.94⁎⁎⁎ | 0.30 |
| Female | 18.64 | 6.05 | |||
| 6. Perceived severity | Male | 14.77 | 3.39 | -4.04⁎⁎ | 0.31 |
| Female | 15.73 | 2.75 | |||
| 7. Perceived vulnerability | Male | 4.69 | 1.64 | -1.73#2 | 0.13 |
| Female | 4.90 | 1.46 | |||
| 8. Total perceived risk | Male | 19.70 | 4.00 | -3.05⁎⁎ | 0.23 |
| Female | 20.56 | 3.42 | |||
| 9. Germ aversion | Male | 19.51 | 4.31 | -1.21 | 0.10 |
| Female | 19.92 | 4.50 | |||
| 10. Perceived infectability | Male | 25.92 | 4.02 | -0.60 | 0.05 |
| Female | 26.11 | 4.11 | |||
| 11.Total BIS | Male | 45.43 | 6.30 | -1.20 | 0.10 |
| Female | 46.03 | 6.66 |
Note: * p <0.05
p = 0.06
p = 0.08
p < 0.01
p < 0.001. These symbols also apply to the tables below.
3.3. Correlations
Table 4 summarises the correlations between the measures. ATs were positively correlated with fear, anxiety, anger, total negative emotions, perceived vulnerability, total COVID-19 perceived risk and BIS-PI. Different types of negative emotions were positively correlated with the sub-dimensions and total scores of perceived risk and trait pathogen avoidance. Finally, perceived severity was positively correlated with BIS-germ aversion, and perceived vulnerability was positively correlated with BIS-PI.
Table 4.
Bivariate correlations between variables of interest.
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. ATs | 1.00 | |||||||||
| 2. Fear | .16⁎⁎ | 1.00 | ||||||||
| 3. Anxiety | .24⁎⁎ | .82⁎⁎ | 1.00 | |||||||
| 4. Anger | .21⁎⁎ | .62⁎⁎ | .68⁎⁎ | 1.00 | ||||||
| 5. Total NEs | .22⁎⁎ | .93⁎⁎ | .92⁎⁎ | .83⁎⁎ | 1.00 | |||||
| 6. COVID-19 PR_PS | -.04 | .35⁎⁎ | .22⁎⁎ | .10* | .27⁎⁎ | 1.00 | ||||
| 7. COVID-19 PR_PV | .23⁎⁎ | .33⁎⁎ | .37⁎⁎ | .24⁎⁎ | .35⁎⁎ | .21⁎⁎ | 1.00 | |||
| 8. Total COVID-19 PR | .08* | .44⁎⁎ | .36⁎⁎ | .20⁎⁎ | .39⁎⁎ | .90⁎⁎ | .58⁎⁎ | 1.00 | ||
| 9. BIS_GA | .05 | .18⁎⁎ | .11⁎⁎ | .12⁎⁎ | .16⁎⁎ | .13⁎⁎ | .01 | .09* | 1.00 | |
| 10. BIS_PI | .16⁎⁎ | .20⁎⁎ | .22⁎⁎ | .14⁎⁎ | .21⁎⁎ | .01 | .22⁎⁎ | .12⁎⁎ | .17⁎⁎ | 1.00 |
| 11.Total BIS | .14⁎⁎ | .25⁎⁎ | .22⁎⁎ | .17⁎⁎ | .24⁎⁎ | .09* | .16⁎⁎ | .14⁎⁎ | .74⁎⁎ | .79⁎⁎ |
Note: ATs = Autistic traits, NEs = Negative emotions, PS = Perceived severity, PV = Perceived vulnerability, COVID-19 PR = COVID-19 perceived risk, GA = Germ aversion, PI = Perceived infectability.
3.4. Relations between ATs and negative emotions: the mediating effects of BIS and perceived risk
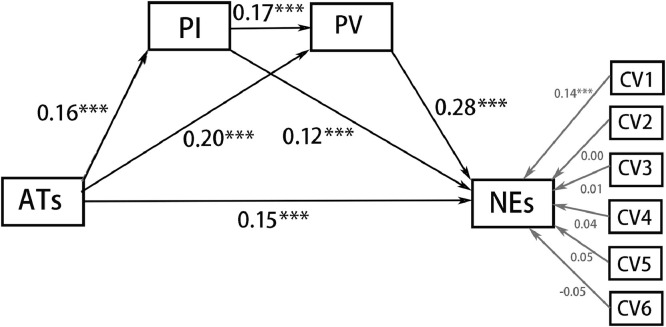
Based on the results of the correlation analysis and our hypothesis that BIS and perceived risk of COVID-19 mediate the relationship between ATs and negative emotions, we used Amos 20.0 to test the mediating model. The scores on all variables in the path analysis were converted to z-scores. After controlling for the effect of the control variable (see Fig. 1 ), the regression coefficients of each path were found to be significant. All the standardised loadings and their significance in the model are shown in Fig. 1. ATs positively predicted PV, PI and negative emotions. PV positively predicted PI and negative emotions, and PI positively predicted negative emotions. Furthermore, the indirect effects of ATs on negative emotions through PV and PI were significant. Table 6 shows the point estimate of each indirect effect, the 95% CI of each point estimation and the proportions of the indirect effect to the total effect for each mediation model.
Fig. 1.
The mediating model of PI and PV between ATs and negative emotions (NEs).
Note: CV1 = Gender; CV2 = Nationality; CV3 = Major; CV4 = Region of location during outbreak; CV5 = only child or not; CV6 = Residential area during outbreak.
Table 6.
Mediating effects of each sub-model.
| Model | Effect | SE | BootLLCI | BootULCI | Ratio |
|---|---|---|---|---|---|
| Total indirect effect | .055 | .010 | .037 | .077 | 26.83% |
| Mod 1. ATs→PI→NEs | .012 | .006 | .004 | .028 | 5.54% |
| Mod 2. ATs→PV→NEs | .037 | .009 | .023 | .056 | 18.05% |
| Mod 3. ATs→PI→PV→NEs | .005 | .002 | .002 | .010 | 2.44% |
Our model showed gender differences in some variables, which indicates that gender may regulate the mediating model. Therefore, the invariance of multi-group structural equational model in Amos 20.0 was conducted to evaluate whether the mediation model was cross-gender equivalent. The test results are shown in Table 5 . The fitting indices of the unconstrained model (Model A) were good and met the preconditions for the subsequent equivalence test (Table 5). The results of model comparison suggested no significant difference between the fitting indices of model A and the model with defining path loading equal (Model A vs. Model B) as well as between the fitting indices of model B and the model with setting covariance and path loading equal (Model B vs. Model C). This indicated that the null hypothesis of invariance should not be rejected under moderate loose criterion (Wasti et al., 2007). The models of males and females were not equal in residual; however, the residual equivalence is excessively strict (Schmitt and Kuljanin, 2008), and the present study focused more on path loading. Thus, the mediation model can be considered to have cross-gender equivalence.
Table 5.
The fitting indices of model equivalence and the results of nested model comparison.
| Model | χ2 | df | CFI | TLI | RMSEA | Model C | ∆χ2 | ∆df | p | ∆CFI | ∆TLI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 68.904 | 46 | .932 | .893 | .027 | ||||||||
| B | 82.872 | 57 | .923 | .902 | .026 | (1) | 13.969 | 11 | >.05 | -0.009 | 0.009 | ||
| C | 84.453 | 58 | .921 | .902 | .026 | (2) | 1.581 | 1 | >.05 | -0.002 | 0 | ||
| D | 107.925 | 66 | .875 | .863 | .031 | (3) | 23.47 | 8 | <.05 | -0.046 | -0.039 | ||
Note: A = Unconstrained, B = Structural weights, C = Structural covariances, D = Structural residuals; the grey shaded part showed the comparison results, Model C = Model comparison, (1) = Model A vs. Model B, (2) = Model B vs. Model C, (3) = Model C vs. Model D.
4. Discussion
To our knowledge, this is the first study that explored the association between ATs and the negative emotional response to the COVID-19 pandemic and the mediating mechanism between them from the perspective of the activation tendency of BIS and COVID-19 risk perception. Our path analysis revealed that ATs were directly associated with increased negative emotional response to COVID-19 and indirectly through complex mediation by trait pathogen avoidance and COVID-19 risk perception.
Gender differences were found in several variables of interest; in line with previous studies (Baron-Cohen et al., 2001; Zhao et al., 2019), males were found to display more ATs than females. Early studies propose that some endogenous epigenetic or neuroendocrine factors ‘protect’ females from developing ASD or high ATs (Werling and Geschwind, 2013; Zhao et al., 2020). However, recent researchers believe that current gender inequality measurements have difficulty detecting the female autistic phenotype (Lai et al., 2015), thus exaggerating the gender differences of ATs to a certain extent. Consistent with recent findings on the emotional impact of COVID-19 (Huang et al., 2020), our results also suggested that females felt more negative emotions than males during COVID-19. This gender difference in emotional response might be partly explained by the greater perceived risk among females as revealed in the findings of the present and recent studies (Galasso et al., 2020). For instance, the increased perceived risk of COVID-19 leads them to experience more fear of infection.
ASD spectrum is likely to be endogenously associated with an impaired stress mechanism, especially in the social stressful situation (Bishop-Fitzpatrick et al., 2017; Hirvikoski and Blomqvist, 2015). Although the atypical stress mechanism in individuals with ASD is likely to be regulated by the context category (Taylor and Corbett, 2014), there is a lack of description of whether these individuals exhibit distinct emotional responses to pathogen threat related stressors (e.g. the current COVID-19 pandemic). Through an analogue ASD approach, our findings suggest that ATs predicted increased anger, fear and anxiety response to COVID-19, which is in line with individuals with ASD reporting greater fear of infection than TD controls (Reinvall et al., 2016). Our results are further supported by a recent findings showing that, compared with the low ATs group, the negative emotions among the high ATs group during the COVID-19 outbreak are significantly higher than those before (Zhao et al., 2020). Our results provide a candidate hypothesis for the characteristics of the emotional response to COVID-19 among the clinical ASD population and expand the understanding of personality susceptibility factors of epidemic stress in the general population.
It is well-documented that autism spectrum, including clinical ASD and ATs in the general population, is associated with a dysfunction of the biological immune system (Goines and Ashwood, 2013; Masi et al., 2015; Saresella et al., 2009). However, the relationship between ASD/ATs and BIS has not been identified. Our findings, which show that ATs are associated with a greater likelihood of activating the BIS, especially with increased PI, fill this gap. This result is consistent with the hypothesis of functional compensation of the two immune systems (Ackerman et al., 2018), wherein the dysfunction of the biological immune system associated with ATs/ASD leads to an enhanced tendency to trigger emotion and cognition to navigate pathogen avoidance and prophylactic behaviour against pathogen threats. Furthermore, our results may also be explained from the perspective of empiricism. Recent studies have shown that past disease experience were associated with the activation tendency of the BIS and enhanced risk perception and emotional response to COVID-19 (Makhanova et al., 2020; Yan et al., 2020). Individuals with ASD are more vulnerable to medical conditions than TD controls (Bjørklund et al., 2016; Li and Zhou, 2016). Therefore, we speculated that the increased illness experience associated with ASD/ATs would sensitise individuals with ASD or high ATs to the threat of pathogens. Studies directly measuring disease experience and BIS activation tendency in both the clinical and the general population are needed to validate this speculation.
ASD or ATs seem to be associated with an attenuated threat or risk perception, especially in the domain of social threats (Ewing et al., 2015; Santos et al., 2012; Sasson et al., 2016). However, in the context of pathogenic threats, our findings revealed an opposite picture wherein ATs predict increased COVID-19 risk perception, which further suggests that the threat perception profiles associated with ASD/ATs are likely to be context-dependent. Our results are consistent with a recent study revealing that individuals with ASD reported greater perceived risk of COVID-19 than the general population (Oakley et al., 2020). They are supported by life history theory (Mittal et al., 2015) as well as by the self-protection model and the risk-as-feelings model (Makhanova et al., 2020), wherein past cognitive and emotional experiences in a threat situation constitute the navigation factors of risk perception in the current similar situation (Loewenstein et al., 2001; Tanu and Kakkar, 2019). Individuals diagnosed with ASD are frequently affected by pathogen-related illnesses (Bjørklund et al., 2016; Sakamoto et al., 2015); as such, to avoid these unpleasant illness experiences, it may be optimal for individuals with ASD or high ATs to improve their risk perception of infection by pathogens. More direct measurement is needed to validate this speculation.
Consistent with assumptions suggesting that the BIS triggers adaptive negative emotions and cognition to promote pathogen avoidance (Ackerman et al., 2018; Murray and Schaller, 2016) and the empirical findings in the context of the COVID-19 pandemic (Makhanova and Shepherd, 2020; Shook et al., 2020), our results showed that the trait of BIS activation tendency predicted increased perceived risk and negative emotions (e.g. fear, anxiety and anger). As studies have revealed (Zheng et al., 2019), our results also suggested that perceived risk was associated with increased negative emotions. Given that the relationship between anger and BIS and risk perception is not fully understood, the following discussion focuses on this topic. Activating the BIS requires individuals to weigh its advantages and disadvantages because the activation of BIS can avoid infection; however, one must also bear the cost of reducing social interaction and changing daily living habits. The BIS is sensitive to this cost-effectiveness (Mark et al., 2011); thus, the positive relationship between anger and BIS activation tendency may reflect the incidental emotional response to the cost of activating the BIS. Anger is intrinsically related to the perceived controllability of risk (Bernardo et al., 2019). The relevant responsible units in Hubei Province officially confirmed on 20 January that COVID-19 could be transmitted from person to person, which was not consistent with previous reports (Li et al., 2020). This may have made the public feel that the current epidemic risk was controllable if the information released at that time were more accurate. Therefore, this risk perception of negative events that were supposed to be controllable led to increased anger.
As per the findings mentioned above, the activation tendency of BIS and risk perception are not only the distal and proximal precursors of negative emotions experienced during COVID-19 but also the enhanced aspects associated with ATs. Therefore, trait pathogen avoidance and perceived risk are assumed to be mediators between ATs and enhanced negative emotions. Through a path modelling approach, the results of the present study revealed three mediation mechanisms. More concretely, ATs predict increased negative emotions by independently increasing the activation tendency of BIS and risk perception and through their complex chain mediation. It should be noted that the total mediating effect explained 26.83% of the total variance, which implies that negative emotions are enhanced mainly by the direct influence of ATs or that other mediators between ATs and negative emotions exist. For instance, studies have shown how the non-adaptive use of emotion regulation strategies mediates the relationship between ATs and negative emotions (Zhao et al., 2020). Therefore, the exploration of the enhancement of the association between ATs and negative emotions by atypical emotion regulation during COVID-19 may be of interest. Additionally, anti-epidemic measures (e.g. self-isolation and social distancing) force the public to make rapid changes in daily living habits. ASD or ATs are associated with lower tolerance of uncertainty and unexpected change (Cassidy et al., 2020; Wigham et al.,2015), making it difficult for individuals with high ATs to effectively manage change, thus experiencing more negative emotions.
The present study has limitations. First, although our results were either directly or indirectly supported by previous studies, the reliability of the perceived vulnerability subscale in our model was not ideal, which may have affected the reliability of our findings. Second, the current study was cross-sectional; thus, causal conclusions cannot be drawn. Experimental designs, which may explore the cognitive and emotional differences between high and low AT groups in specific tasks under pathogen threat priming situations, may be of immense help to consolidate our findings. Third, although our findings may provide a candidate hypothesis for the response characteristics and mechanisms related to pathogenic threats in ASD, the model revealed in the present study could differ in ASD populations. For instance, the disease experience in the clinical ASD population may be more frequent than that in the general population. Based on the existing research findings (Makhanova et al., 2020), we infer that the association between clinical ASD and BIS and COVID-19 risk perception may be stronger than that between ATs and these variables in the general population. Fourth, although the correlation strength between the core variables in our study is very similar to those from similar research topic (Abdelrahman, 2020; Makhanova and Shepherd, 2020), these correlations are relatively weak. Future research might aim to better explain these relationship. Furthermore, when our results are applied to clinical practice, they must be explained cautiously. Lastly, negative emotions and risk perception during the epidemic are likely to change dynamically with its development. Therefore, our model may only reflect phenomena during the acute outbreak of COVID-19. Longitudinal designs may provide a dynamic description of the mediation model.
In conclusion, theoretical deduction and previous findings suggested that ASD is likely to be associated with an increased emotional response to pathogen threats. Through an ‘analogy-ASD’ approach in the general population, the present study revealed a positive association between ATs and negative emotional response to the COVID-19 pandemic and its mediating mechanism from the perspective of the BIS and risk perception. The present findings extend our understanding of the BIS profile and threat perception characteristics associated with autism spectrum, and the individual differences in response to COVID-19. Moreover, as we mentioned in the limitations, some important topics must be further explored.
CRediT authorship contribution statement
Xudong Zhao: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Wendian Shi: Conceptualization, Methodology, Writing – review & editing. Xiujun Li: Investigation, Visualization, Data curtion. Wenrui Li: Investigation, Formal analysis. Chunbo Li: Conceptualization, Methodology, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies.
Ethical approval
All participants were informed about the study and provided electronic informed consent before the study. The study was approved by the Ethics Committee of the Department of Psychology at Shanghai Normal University.
References
- Abdelrahman M. Personality traits, risk perception, and protective behaviors of Arab residents of Qatar during the COVID-19 pandemic. Int. J. Ment. Health Addict. 2020:1–12. doi: 10.1007/s11469-020-00352-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman J.M., Hill S.E., Murray D.R. The behavioral immune system: Current concerns and future directions. Soc. Personal. Psychol. Compass. 2018;12(2):e12371. doi: 10.1111/spc3.12371. [DOI] [Google Scholar]
- Amos G.A., Byrne G., Chouinard P.A., Godber T. Autism traits, sensory over-responsivity, anxiety, and stress: a test of explanatory models. J. Autism Dev. Disord. 2019;49(1):98–112. doi: 10.1007/s10803-018-3695-6. [DOI] [PubMed] [Google Scholar]
- Aschwanden D., Strickhouser J.E., Sesker A., Ji H.L., Terracciano A. Psychological and behavioral responses to COVID-19: the role of personality. Eur. J. Personal. 2020:2281. doi: 10.1002/per.2281. per- [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson G.J.G., Paluszek M.M., Landry C.A., Rachor G.S., McKay D., Taylor S. Do pre-existing anxiety-related and mood disorders differentially impact COVID-19 stress responses and coping? J. Anxiety Disord. 2020;74 doi: 10.1016/j.janxdis.2020.102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. The Autism-Spectrum Quotient (AQ): evidence from asperger syndrome/high-functioning autism, malesand females, scientists and mathematicians. J. Autism Dev. Disord. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bernardo F., Santos L., Dias D., Rodrigues M. Risk experience, emotions, place identity, and coping strategies in people affected by an unexpected fire (Experiencia de riesgo, emociones, identidad de lugar y estrategias de afrontamiento en personas afectadas por un incendio inesperado) PsyEcology. 2019;11(1):130–147. doi: 10.1080/21711976.2019.1643986. [DOI] [Google Scholar]
- Bishop-Fitzpatrick L., Minshew N.J., Mazefsky C.A., Eack S.M. Perception of life as stressful, not biological response to stress, is associated with greater social disability in adults with autism spectrum disorder. J. Autism Dev. Disord. 2017;47(1):1–16. doi: 10.1007/s10803-016-2910-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørklund G., Saad K., Chirumbolo S., Kern J.K., Geier D.A., Geier M.R., Urbina M.A. Immune dysfunction and neuroinflammation in autism spectrum disorder. Acta Neurobiol. Exp. (Warsz) 2016;76(4):257–268. doi: 10.21307/ane-2017-025. [DOI] [PubMed] [Google Scholar]
- Brooks S.K., Webster R.K., Smith L.E., Woodland L., Rubin G.J. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet North Am. Ed. 2020;395(10227):912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bults M., Beaujean D.J., de Zwart O., Kok G., van Empelen P., van Steenbergen J.E., Voeten H. Perceived risk, anxiety, and behavioural responses of the general public during the early phase of the Influenza A (H1N1) pandemic in the Netherlands: results of three consecutive online surveys. BMC Public Health. 2011;11(2):2. doi: 10.1186/1471-2458-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy S.A., Nicolaidis C., Davies B., Rosa S.D.R., Eisenman D., Onaiwu M.G., Waisman T.C. An expert discussion on autism in the COVID-19 pandemic. Autism Adulthood. 2020;2(2):106–117. doi: 10.1089/aut.2020.29013.sjc. . . . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen L.A., Zerbo O., Qian Y., Massolo M.L., Kripke C. The health status of adults on the autism spectrum. Autism. 2015;19(7):814–823. doi: 10.1177/1362361315577517. [DOI] [PubMed] [Google Scholar]
- Díaz A., Soriano J.F., Beleña Á. Perceived vulnerability to disease questionnaire: factor structure, psychometric properties and gender differences. Personal. Individ. Differ. 2016;101:42–49. doi: 10.1016/j.paid.2016.05.036. [DOI] [Google Scholar]
- De Zwart O., Veldhuijzen I.K., Elam G., Aro A.R., Abraham T., Bishop G.D., Brug J. Avian influenza risk perception, Europe and Asia. Emerg. Infect. Dis. 2007;13(2):290–293. doi: 10.3201/eid1302.060303. . . . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan L.A., Schaller M., Park J.H. Perceived vulnerability to disease: development and validation of a 15-item self-report instrument. Personal. Individ. Differ. 2009;47(6):541–546. doi: 10.1016/j.paid.2009.05.001. [DOI] [Google Scholar]
- Ewing L., Caulfield F., Read A., Rhodes G. Appearance-based trust behaviour is reduced in children with autism spectrum disorder. Autism. 2015;19(8):1002–1009. doi: 10.1177/1362361314559431. [DOI] [PubMed] [Google Scholar]
- Galasso V., Pons V., Profeta P., Becher M., Brouard S., Foucault M. Gender differences in COVID-19 attitudes and behavior: panel evidence from eight countries. Proc. Natl. Acad. Sci. 2020;117(44):27285–27291. doi: 10.1073/pnas.2012520117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines P.E., Ashwood P. Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicol. Teratol. 2013;36:67–81. doi: 10.1016/j.ntt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruvi-Lamdan N., Lebendiger S., Golan O., Horesh D. Are PTSD and autistic traits related? An examination among typically developing Israeli adults. Compr. Psychiatry. 2019;89:22–27. doi: 10.1016/j.comppsych.2018.11.004. [DOI] [PubMed] [Google Scholar]
- Hirvikoski T., Blomqvist M. High self-perceived stress and poor coping in intellectually able adults with autism spectrum disorder. Autism. 2015;19(6):752–757. doi: 10.1177/1362361314543530. [DOI] [PubMed] [Google Scholar]
- Huang L., Lei W., Xu F., Liu H., Yu L. Emotional responses and coping strategies in nurses and nursing students during Covid-19 outbreak: a comparative study. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.C., Lombardo M.V., Auyeung B., Chakrabarti B., Baron-Cohen S. Sex/Gender differences and autism: setting the scene for future research. J. Am. Acad. Child. Adolesc. Psychiatry. 2015;54(1):11–24. doi: 10.1016/j.jaac.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry O., Chouinard P.A. Why we should study the broader autism phenotype in typically developing populations. J. Cognit. Dev. 2016;17(4):584–595. doi: 10.1080/15248372.2016.1200046. [DOI] [Google Scholar]
- Li Q., Zhou J. The microbiota–gut–brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience. 2016;324:131–139. doi: 10.1016/j.neuroscience.2016.03.013. [DOI] [PubMed] [Google Scholar]
- Li S., Wang Y., Xue J., Zhao N., Zhu T. The impact of COVID-19 epidemic declaration on psychological consequences: a study on active weibo users. Int. J. Environ. Res. Public Health. 2020;(6):17. doi: 10.3390/ijerph17062032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein G.F., Weber E.U., Hsee C.K., Welch N. Risk as feelings. Psychol. Bull. 2001;127(2):267–286. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- Makhanova A., Shepherd M.A. Behavioral immune system linked to responses to the threat of COVID-19. Personal. Individ. Differ. 2020;167 doi: 10.1016/j.paid.2020.110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhanova A., Shepherd M.A., Plant E.A., Gerend M.A., Maner J.K. Childhood illness as an antecedent of perceived vulnerability to disease. Evol. Behav. Sci. 2020 doi: 10.1037/ebs0000238. [DOI] [Google Scholar]
- Mark S., Justin H., Park The behavioral immune system (and why it matters) Curr. Dir. Psychol. Sci. 2011;(20):99–103. [Google Scholar]
- Masi A., Quintana D.S., Glozier N., Lloyd A.R., Hickie I.B., Guastella A.J. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol. Psychiatry. 2015;20(4):440–446. doi: 10.1038/mp.2014.59. [DOI] [PubMed] [Google Scholar]
- Mittal C., Griskevicius V., Simpson J.A., Sung S., Young E.S. Cognitive adaptations to stressful environments: when childhood adversity enhances adult executive function. J. Personal. Soc. Psychol. 2015;109(4):604–621. doi: 10.1037/pspi0000028. [DOI] [PubMed] [Google Scholar]
- Murray D.R., Schaller M. The behavioral immune system: Implications for Social Cognition, Social Interaction, and Social Influence. Adv. Exp. Soc. Psychol. 2016;53:75–129. doi: 10.1016/bs.aesp.2015.09.002. [DOI] [Google Scholar]
- Nikcevic A.V., Marino C., Kolubinski D.C., Leach D., Spada M.M. Modelling the contribution of the Big Five personality traits, health anxiety, and COVID-19 psychological distress to generalised anxiety and depressive symptoms during the COVID-19 pandemic. J. Affect. Disord. 2021;279:578–584. doi: 10.1016/j.jad.2020.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley, B., Tillmann, J., Ruigrok, A., Baranger, A., Takow, C., Charman, T., . . . Murphy, D. (2020). COVID-19 health and social care access for autistic people and individuals with intellectual disability: a European policy review. doi: 10.31234/osf.io/n6d3f. [DOI] [PMC free article] [PubMed]
- Reinvall O., Moisio A.-L., Lahti-Nuuttila P., Voutilainen A., Laasonen M., Kujala T. Psychiatric symptoms in children and adolescents with higher functioning autism spectrum disorders on the development and well-being assessment. Res. Autism Spectrum Disord. 2016;25:47–57. doi: 10.1016/j.rasd.2016.01.009. [DOI] [Google Scholar]
- Richardson H.A., Simmering M.J., Sturman M.C. A tale of three perspectives: Examining post hoc statistical techniques for detection and correction of common method variance. Organ. Res. Methods. 2009;12(4):762–800. [Google Scholar]
- Sakamoto A., Moriuchi H., Matsuzaki J., Motoyama K., Moriuchi M. Retrospective diagnosis of congenital cytomegalovirus infection in children with autism spectrum disorder but no other major neurologic deficit. Brain Dev. 2015;37(2):200–205. doi: 10.1016/j.braindev.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Santos A., Chaminade T., Da Fonseca D., Silva C., Rosset D., Deruelle C. Just another social scene: evidence for decreased attention to negative social scenes in high-functioning autism. J. Autism Dev. Disord. 2012;42(9):1790–1798. doi: 10.1007/s10803-011-1415-6. [DOI] [PubMed] [Google Scholar]
- Saresella M., Marventano I., Guerini F.R., Mancuso R., Ceresa L., Zanzottera M., Clerici M. An autistic endophenotype results in complex immune dysfunction in healthy siblings of autistic children. Biol. Psychiatry. 2009;66(10):978–984. doi: 10.1016/j.biopsych.2009.06.020. . . . [DOI] [PubMed] [Google Scholar]
- Sasson N.J., Shasteen J.R., Pinkham A.E. Brief report: reduced prioritization of facial threat in adults with autism. J. Autism Dev. Disord. 2016;46(4):1471–1476. doi: 10.1007/s10803-015-2664-6. [DOI] [PubMed] [Google Scholar]
- Schmitt N., Kuljanin G. Measurement invariance: review of practice and implications. Hum. Res. Manage. Rev. 2008;18(4):210–222. [Google Scholar]
- Shook N.J., Sevi B., Lee J., Oosterhoff B., Fitzgerald H.N. Disease avoidance in the time of COVID-19: the behavioral immune system is associated with concern and preventative health behaviors. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0238015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanu, Kakkar D. Influence of emotional imagery on risk perception and decision making in autism spectrum disorder. Neurophysiology. 2019;51(4):281–292. doi: 10.1007/s11062-019-09822-8. [DOI] [Google Scholar]
- Taylor J.L., Corbett B.A. A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology. 2014;49:207–228. doi: 10.1016/j.psyneuen.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrizzi J.A., Shook N.J., McDaniel M.A. The behavioral immune system and social conservatism: a meta-analysis. Evol. Hum. Behav. 2013;34(2):99–108. doi: 10.1016/j.evolhumbehav.2012.10.003. [DOI] [Google Scholar]
- Wakabayashi A., Baron-Cohen S., Wheelwright S. Are autistic traits an independent personality dimension? A study of the Autism-Spectrum Quotient (AQ) and the NEO-PI-R. Personal. Individ. Differ. 2006;41(5):873–883. doi: 10.1016/j.paid.2006.04.003. [DOI] [Google Scholar]
- Walrave M., Waeterloos C., Ponnet K. Adoption of a contact tracing app for containing COVID-19: a health belief model approach. JMIR Public Health Surveill. 2020;6(3):e20572. doi: 10.2196/20572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasti S.A., Tan H.H., Brower H.H., Önder Ç. Cross-cultural measurement of supervisor trustworthiness: an assessment of measurement invariance across three cultures. Leadersh. Q. 2007;18(5):477–489. [Google Scholar]
- Werling D.M., Geschwind D.H. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 2013;26(2):146–153. doi: 10.1097/WCO.0b013e32835ee548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigham S., Rodgers J., South M., McConachie H., Freeston M. The interplay between sensory processing abnormalities, intolerance of uncertainty, anxiety and restricted and repetitive behaviours in autism spectrum disorder. J. Autism Dev. Disord. 2015;45(4):943–952. doi: 10.1007/s10803-014-2248-x. [DOI] [PubMed] [Google Scholar]
- Wu B.-P., Chang L. The social impact of pathogen threat: How disease salience influences conformity. Personal. Individ. Differ. 2012;53(1):50–54. doi: 10.1016/j.paid.2012.02.023. [DOI] [Google Scholar]
- Yan A.F., Sun X., Zheng J., Mi B., Zuo H., Ruan G., Shi Z. Perceived risk, behavior changes and health-related outcomes during COVID-19 pandemic: findingsamong adults with and without diabetesin China. Diabetes Res. Clin. Pract. 2020;167 doi: 10.1016/j.diabres.2020.108350. . . . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim M., Guler A. COVID-19 severity, self-efficacy, knowledge, preventive behaviors, and mental health in Turkey. Death Stud. 2020:1–8. doi: 10.1080/07481187.2020.1793434. [DOI] [PubMed] [Google Scholar]
- Yildirim M., Guler A. Positivity explains how COVID-19 perceived risk increases death distress and reduces happiness. Pers. Individ. Dif. 2021;168 doi: 10.1016/j.paid.2020.110347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Sun Y., Chen F., Wu D., Tang J., Han X., Wang K. Psychometric properties of the Autism-Spectrum Quotient in both clinical and non-clinical samples: Chinese version for mainland China. BMC Psychiatry. 2016;16:213. doi: 10.1186/s12888-016-0915-5. . . . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Lan M., Li H., Yang J. Perceived stress and sleep quality among the non-diseased general public in China during the 2019 coronavirus disease: a moderated mediation model. Sleep Med. 2020 doi: 10.1016/j.sleep.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Li X., Song Y., Li C., Shi W. Autistic traits and emotional experiences in Chinese college students: mediating role of emotional regulation and sex differences. Res. Autism Spectr. Disord. 2020;77 doi: 10.1016/j.rasd.2020.101607. [DOI] [Google Scholar]
- Zhao X., Li X., Song Y., Shi W. Autistic traits and prosocial behaviour in the general population: test of the mediating effects of trait empathy and state empathic concern. J. Autism Dev. Disord. 2019;49(10):3925–3938. doi: 10.1007/s10803-018-3745-0. [DOI] [PubMed] [Google Scholar]
- Zhao X., Zhao B., Li W., Cai Y., Shi W., Li C. 2020. Autistic Traits and Gender Modulate Emotion Changes Before and During the COVID-19 Outbreak: a Preliminary Evidence Based on Quasi-Experimental Design. [DOI] [Google Scholar]
- Zheng C., Zhang J., Guo Y., Zhang Y., Qian L. Disruption and reestablishment of place attachment after large-scale disasters: the role of perceived risk, negative emotions, and coping. Int. J. Disaster Risk Reduct. 2019;40 doi: 10.1016/j.ijdrr.2019.101273. [DOI] [Google Scholar]