Abstract
Objectives
Accumulating evidence has implicated that brain derived neurotrophic factor (BDNF) is thought to be involved in the pathophysiology of depression, but its correlation with ketamine’s antidepressant efficacy focusing on Chinese individuals with depression is not known. This study was aim to determine the correlation of plasma BDNF (pBDNF) concentrations and ketamine’s antidepressant efficacy.
Methods
Ninety-four individuals with depression received six intravenous infusions ketamine (0.5 mg/kg). Remission and response were defined as Montgomery-Asberg Depression Rating Scale (MADRS) scores less than 10 and a reduction of 50% or more in MADRS scores, respectively. Plasma was collected at baseline and at 24 h and 2 weeks after completing six ketamine infusions (baseline, 13 d and 26 d).
Results
A significant improvement in MADRS scores and pBDNF concentrations was found after completing six ketamine infusions compared to baseline (all ps < 0.05). Higher baseline pBDNF concentrations were found in ketamine responders/remitters (11.0 ± 6.2/10.1 ± 5.8 ng/ml) than nonresponders/nonremitters (8.0 ± 5.5/9.2 ± 6.4 ng/ml) (all ps < 0.05). Baseline pBDNF concentrations were correlated with MADRS scores at 13 d (t = − 2.011, p = 0.047) or 26 d (t = − 2.398, p = 0.019) in depressed patients (all ps < 0.05). Subgroup analyses found similar results in individuals suffering from treatment refractory depression.
Conclusion
This preliminary study suggests that baseline pBDNF concentrations appeared to be correlated with ketamine’s antidepressant efficacy in Chinese patients with depression.
Keywords: Ketamine, Brain-derived neurotrophic factor, Depression, Predictors, Response
Introduction
Accumulating evidence suggests that glutamatergic abnormalities are associated with the pathophysiology of mood disorders (Yüksel & Öngür, 2010). Numerous early studies had consistently reported that an antagonist of glutamatergic N-methyl-D-aspartate (NMDA) receptors ketamine at subanesthetic doses could result in fast-acting and sustained antidepressant effects in individuals suffering from unipolar and bipolar depression (Na & Kim, 2021; Phillips et al., 2020). For example, ketamine’s repeated administration had quick and enduring antidepressant and antisuicidal effects in depressed patients (Kryst et al., 2020).
The precise mechanisms underlying subanesthetic intravenous ketamine’s antidepressant actions are still incompletely understood (Rong et al., 2018). A recent animal study found that blockade of NMDA receptors increased the induction of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor expression in models of depression, and subsequent activation of the mammalian target of rapamycin (mTOR) pathway was needed for the rapid and robust antidepressant action of ketamine (Li et al., 2010). Growing evidence implicated neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), played an important in the pathophysiology of mood disorders (Duman, 2004; Duman & Monteggia, 2006). BDNF is a key protein in facilitating and supporting memory growth and neuronal survival (Leal, Bramham & Duarte, 2017). Rapid and transient upregulation of BDNF reversed or blocked atrophy and cell loss in patients with depression, and it may be a critical component in subanesthetic intravenous ketamine’s antidepressant actions (Haile et al., 2014).
In general, BDNF plays a role in the pathophysiology of schizophrenia (Singh et al., 2020) and mood disorders (Molendijk et al., 2014; Sagud et al., 2016). For example, several studies found that individuals suffering from depression had lower serum BDNF concentrations and pBDNF concentrations than that of healthy subjects (Molendijk et al., 2014; Sagud et al., 2016) and recovered after successful antidepressant therapy (Brunoni, Lopes & Fregni, 2008; Polyakova et al., 2015). Central and peripheral BDNF is positively correlated with the response and remission of antidepressant treatment (Lee & Kim, 2010). Notably, Kurita et al. (2012) reported that remitted than nonremitted depressed patients appeared to have higher pBDNF concentrations, and these concentrations were associated with the Montgomery-Asberg Depression Rating Scale (MADRS) scores.
BDNF as a predictor of ketamine’s antidepressant efficacy in individuals suffering from treatment-refractory depression (TRD) has been investigated, but with inconsistent findings. For example, several open-label studies on ketamine and BDNF found a negative association of the increase in BDNF following a single ketamine infusion with the severity of depression (Cornwell et al., 2012; Duncan et al., 2013). Another study found that BDNF did not mediate single subanesthetic intravenous ketamine’s antidepressant efficacy (Machado-Vieira et al., 2009). However, no studies had been published to examine the relationship of pBDNF concentrations and serial subanesthetic intravenous ketamine infusions’ antidepressant efficacy in Chinese individuals suffering from depression.
The present study was performed to examine the correlation of pBDNF concentrations and six subanesthetic intravenous ketamine’s antidepressant efficacy (0.5 mg/kg) administered thrice weekly over two weeks in Chinese individuals suffering from unipolar and bipolar depression. In this study, we hypothesized that serial intravenous subanesthetic ketamine would increase pBDNF concentrations, and baseline pBDNF concentrations would be associated with ketamine’s antidepressant efficacy in individuals suffering from depression.
Methods
Study sample
Data of the current study were collected from an open-label clinical study, which examined serial intravenous subanesthetic ketamine’s antidepressant and antisuicidal efficacy in individuals suffering from depression and was performed between November 2016 to December 2017 (registration number: ChicCTR-OOC-17012239) (Zheng et al., 2018). The Ethics Committee of the Affiliated Brain Hospital of Guangzhou Medical University approved the current trial’s protocol (Ethical Application Ref: 2016-030) and written informed consent was obtained from all participants.
All subjects were recruited based on the following inclusion criteria: (1) aged between 18 and 65 years, without psychotic symptoms; (2) diagnosis of unipolar or bipolar depression by a certified psychiatrist according to the Structured Clinical Interview for DSM-V (SCID-5) criteria, with a score of 17 or more for the Hamilton Depression Rating Scale (HAMD-17) (Hamilton, 1960); (3) suffering from TRD, which was defined as nonresponse to 2 or more antidepressant treatments, or experiencing suicidal ideation as measured with the Scale for Suicidal Ideations (Beck, Kovacs & Weissman, 1979); (4) had no a history of neurological diseases (e.g., dementia), drug or alcohol abuse; (5) negative urine toxicology; (6) were not pregnant or breast feeding; and (7) had no any unstable medical illness (e.g., cerebrovascular diseases).
Treatment
All patients received a thrice-weekly ketamine treatment regimen for 2 weeks, with a follow-up period of two weeks. The method for repeated ketamine infusions was described in detail in our early trial (Zheng et al., 2018). Briefly, vital signs and clinical status of participants were routinely monitored, and each subject received six intravenous infusions of 0.5 mg/kg ketamine over 40 min. During the study period, all subjects continued taking psychotropic agents.
Response and remission
The MADRS (Montgomery & Asberg, 1979; Zhong et al., 2011) was used to assess depressive symptoms at baseline, 1 d after the sixth infusion (13 d), and 2 weeks after the last ketamine treatments (26 d). Remission and response were defined as MADRS scores less than 10 (Zimmerman, Posternak & Chelminski, 2004) and a reduction of 50% or more in MADRS scores, respectively.
Measurement of pBDNF concentrations
Plasma was collected at baseline, 13 d and 26 d, which were stored at −80 °C until further use. In accordance with the manufacturer’s instructions, in this study a commercially available enzyme-linked immunosorbent assay (ELISA) kit (EMD Millipore Corporation, MA, USA) was used to measure pBDNF concentrations.
Statistical analysis
The Mann–Whitney U test was conducted to analyze nonnormally distributed continuous data, and independent t tests were applied for normally distributed continuous data. For categorical variables, the Fisher’s exact test or Chi-squared test were applied for comparisons between groups (responders versus nonresponders and remitters versus nonremitters). Changes in pBDNF concentrations and MADRS scores over time and subgroup differences (responders/nonresponders and remitters/nonremitters) were examined using linear mixed models. Bivariate correlation analysis was applied in order to determine the correlation of baseline pBDNF concentrations and MADRS scores at 13 d and 26 d in individuals suffering from unipolar or bipolar depression. Multiple linear regression were also used to examine the independent association of baseline pBDNF concentrations and MADRS scores at 13 d and 26 d. MADRS scores were entered as the dependent variable, while Baseline pBDNF concentrations were entered as independent variables and other variables including age, gender, body weight, body mass index, psychiatric family history, previous hospitalization, psychiatric comorbidity, and age of onset were entered as covariate variables. Furthermore, an additional analysis was also performed on a subsample of patients with TRD in this study. IBM SPSS version 23 software (IBM Corporation, Armonk, NY, USA) was used in this study, and significance was set as p-value less than 0.05.
Results
Ninety-four individuals (aged 18 to 62 years) with unipolar or bipolar depression who provided a baseline blood sample were enrolled. Of these patients, 81.9% (77/94) fulfilled the diagnostic criteria of TRD. Baseline pBDNF concentrations with a mean value of 10.1 ng/ml, ranged from 0.9 to 27.2 ng/ml.
Treatment outcome and BDNF
After the last ketamine treatments, the rates of response and remission were 68.1% (64/94) and 51.1% (48/94), respectively. The rates of response and remission for patients with TRD were 68.8% (53/77) and 51.9% (40/77), respectively, after completion of six ketamine infusions. Higher baseline pBDNF concentrations were found in ketamine responders/remitters (11.0 ± 6.2/10.1 ± 5.8 ng/ml) than nonresponders/nonremitters (8.0 ± 5.5/9.2 ± 6.4 ng/ml) (all ps<0.05, Table 1).
Table 1. Comparison of baseline sample characteristics between responders and nonresponders and between remitters and nonremitters.
| Variables | Total (n = 94) | Response after six ketamine infusions | Remission after six ketamine infusions | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Responders (n = 64) | Nonresponders (n = 30) | Statistics | Remitters (n = 48) | Nonremitters (n = 46) | Statistics | ||||
| N (%) | N (%) | N (%) | X2 | p | N (%) | N (%) | X2 | p | |
| Female | 50 (53.2) | 35 (54.7) | 15 (50.0) | 0.2 | 0.67 | 22 (45.8) | 28 (60.9) | 2.1 | 0.14 |
| Employment | 38 (40.4) | 29 (45.3) | 9 (30.0) | 2.0 | 0.16 | 22 (45.8) | 16 (34.8) | 1.2 | 0.28 |
| Married | 50 (53.2) | 35 (54.7) | 15 (50.0) | 0.2 | 0.67 | 27 (56.3) | 23 (50.0) | 0.4 | 0.54 |
| Mean (SD) | Mean (SD) | Mean (SD) | T/Z | p | Mean (SD) | Mean (SD) | T/Z | p | |
| Age (years) | 34.6 (11.6) | 35.1 (11.2) | 33.4 (12.5) | −0.7 | 0.50 | 34.8 (10.9) | 34.3 (12.4) | −0.2 | 0.83 |
| Education (years) | 12.4 (3.3) | 12.8 (3.2) | 11.4 (3.4) | −2.0 | 0.049 | 12.6 (3.2) | 12.1 (3.4) | −0.6 | 0.53 |
| BMI (kg/m2) | 22.4 (3.6) | 22.5 (3.5) | 22.2 (3.8) | −0.4 | 0.66 | 22.7 (3.9) | 22.1 (3.2) | 0.8 | 0.43 |
| Duration of illness (months) | 102.5 (98.3) | 106.3 (101.7) | 94.5 (91.9) | —a | 0.51 | 107.3 (101.4) | 97.6 (95.9) | —a | 0.58 |
| Baseline MADRS scores | 31.9 (7.6) | 31.8 (7.6) | 32.2 (7.6) | 0.3 | 0.80 | 30.6 (7.3) | 33.3 (7.7) | −1.8 | 0.08 |
| pBDNF concentrations (ng/ml) | 10.1 (6.2) | 11.0 (6.2) | 8.0 (5.5) | —a | 0.01 | 10.1 (5.8) | 9.2 (6.4) | —a | 0.045 |
Notes.
Mann–Whitney U test.
Bolded values are p<0.05.
- pBDNF
- plasma brain derived neurotrophic factor
- BMI
- Body Mass Index
- MADRS
- Montgomery-Asberg Depression Rating Scale
- SD
- standard deviation
Linear mixed models showed that MADRS scores and pBDNF concentrations exhibited significant time main effects between responders and nonresponders and between remitters and nonremitters (Table 2). Ketamine produced a significant change in MADRS scores and pBDNF concentrations at 13 d and 26 d when compared to baseline (Figs. 1 and 2). Similar results were found in patients with TRD (Table S1, Figs. S1 and S2).
Table 2. Comparison of MADRS scores and pBDNF concentrations between responders and nonresponders and between remitters and nonremitters in patients with unipolar and bipolar depression using linear mixed model analysis.
| Outcomes | Variables | Group-by-time interaction | Time main effect | Group main effect | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Responders vs. nonresponders | MADRS scores | 59.79 | <0.001 | 223.39 | <0.001 | 59.32 | <0.001 |
| pBDNF concentrations (ng/ml) | 0.04 | 0.837 | 8.55 | <0.001 | 3.90 | 0.024 | |
| Remitters vs. nonremitters | MADRS scores | 74.95 | <0.001 | 263.13 | <0.001 | 29.52 | <0.001 |
| pBDNF concentrations (ng/ml) | 0.02 | 0.888 | 6.40 | 0.003 | 2.61 | 0.079 | |
Notes.
Bolded values are p < 0.05.
- pBDNF
- plasma brain derived neurotrophic factor
- MADRS
- Montgomery-Asberg Depression Rating Scale
Figure 1. Change in depressive symptoms in patients with unipolar and bipolar depression.
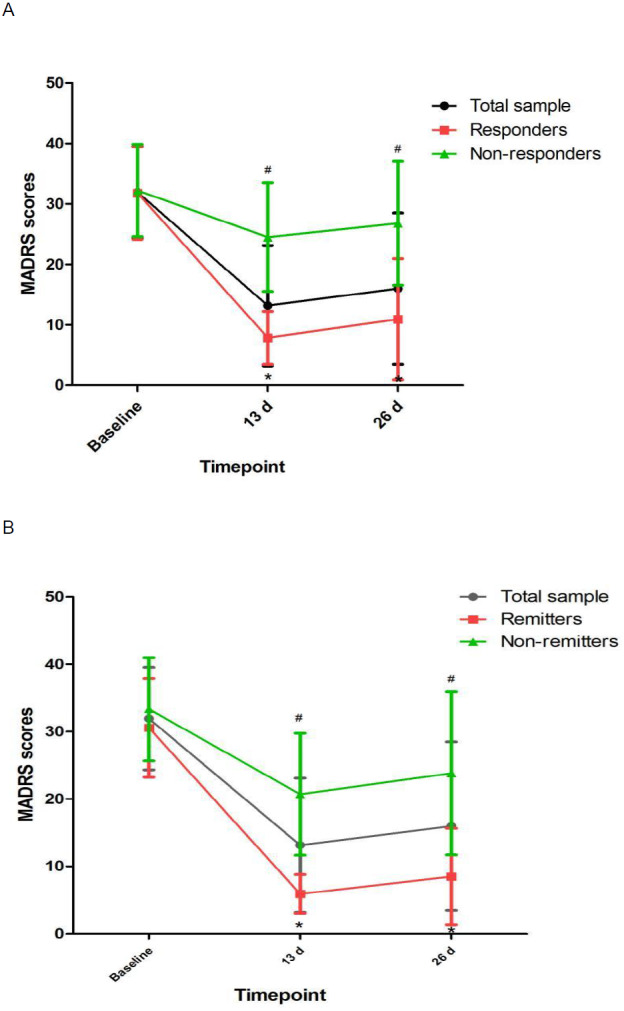
# Significant difference was found when comparing baseline to the indicated times (p < 0.05). ∗ Significant difference was found between responders and nonresponders and between remitters and nonremitters at the indicated times (p < 0.05). Abbreviations: MADRS, the Montgomery-Asberg Depression Rating Scale.
Figure 2. Change in pBDNF concentrations in patients with unipolar and bipolar depression.
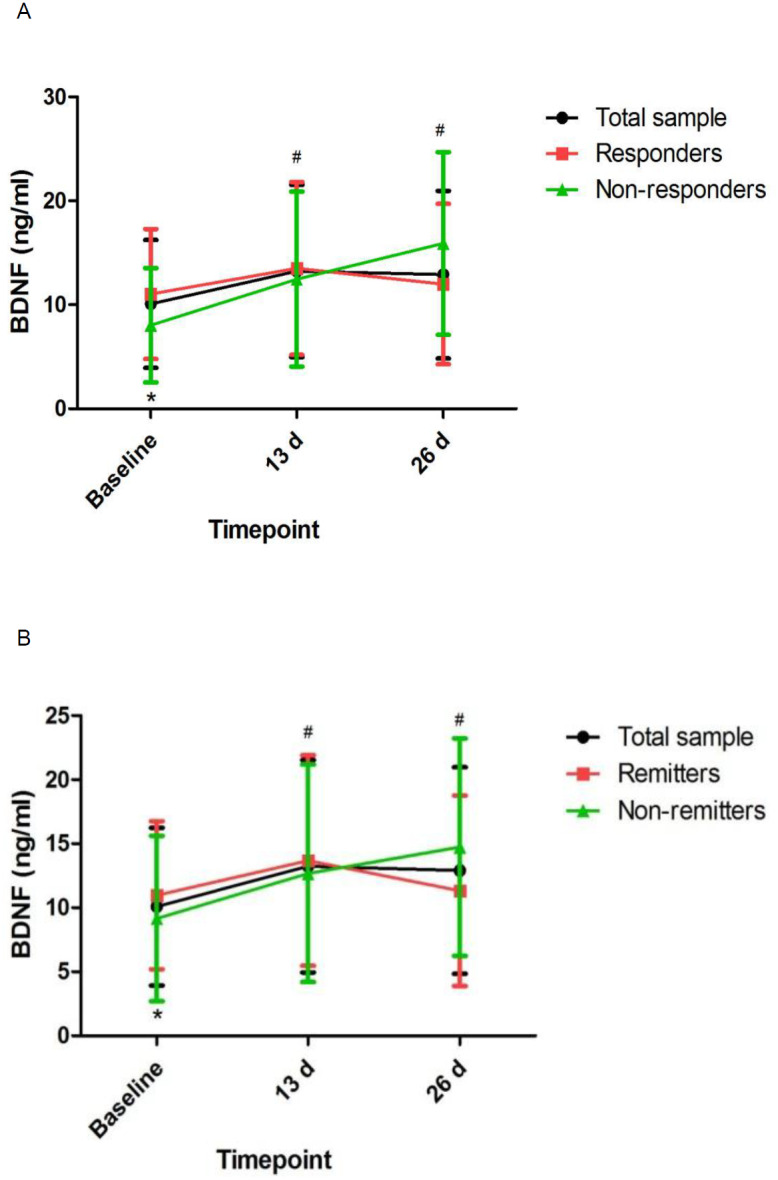
# Significant difference was found when comparing baseline to the indicated times (p < 0.05). ∗ Significant difference was found between responders and nonresponders and between remitters and nonremitters at the indicated times (p < 0.05). Abbreviations: pBDNF, plasma brain derived neurotrophic factor.
Correlation of BDNF and MADRS scores
Correlation analyses showed significant associations between pBDNF concentrations at baseline and MADRS scores at 13 d and 26 d in depressed patients (all p s<0.05; Table 3). The significant association of pBDNF concentrations at baseline and MADRS scores at 13 d (t = −2.011, p = 0.047) and 26 d (t = −2.398, p = 0.019) remained in multiple regression analysis. Similar results were found in patients with TRD (Table S2).
Table 3. Correlation of baseline pBDNF concentrations and MADRS scores at 13 d or 26 d in patients with unipolar and bipolar depression.
| Variables | MADRS scores at 13 d | MADRS scores at 26 d |
|---|---|---|
| pBDNF concentrations (ng/ml) | r = − 0.220 | r = − 0.278 |
| p=0.033 | p=0.007 |
Notes.
Bolded values are p<0.05.
- pBDNF
- plasma brain derived neurotrophic factor
- MADRS
- the Montgomery-Asberg Depression Rating Scale
- r
- Pearson coefficient of correlation
Discussion
This is the first study to determine pBDNF concentrations after six subanesthetic intravenous ketamine in Chinese individuals suffering from unipolar and bipolar depression and to investigate the correlation of pBDNF concentrations at baseline and six subanesthetic intravenous ketamine’s antidepressant efficacy. The following main findings included: (1) ketamine increased pBDNF at 13 d and 26 d compared to baseline; (2) responders/remitters had significantly higher baseline pBDNF concentrations than nonresponders/nonremitters; (3) MADRS scores showed significant improvement at both time points across the total sample compared to baseline; (4) baseline pBDNF concentrations were related with MADRS scores; and (5) additional analysis of patients with TRD also found that pBDNF concentrations were related with the antidepressant outcome of ketamine in patients with TRD.
Consistent with an animal study after single ketamine infusion (Pytka et al., 2018), our study demonstrated that ketamine increased pBDNF concentrations after six ketamine infusions. Although nonresponders/nonremitters had significantly lower pBDNF concentrations at baseline than responders/remitters, repeated ketamine infusions failed to significantly increase pBDNF concentrations in responders/remitters when compared to nonresponders/nonremitters. Similarly, a previous study found no changes in pBDNF concentrations in individuals suffering from TRD after completion of an intravenous infusion of ketamine compared to baseline (Machado-Vieira et al., 2009). However, Haile et al. found that pBDNF concentrations were significantly increased following a single ketamine infusion in responders compared to nonresponders (Haile et al., 2014). Therefore, these findings should be confirmed by randomized controlled trials.
The observed rapid reduction in MADRS scores lasted up to 2 weeks, replicating the previous findings (Rasmussen et al., 2013; Shiroma et al., 2014). However, the primary objective of this study is to examine the association of baseline pBDNF concentrations and six subanesthetic intravenous ketamine’s antidepressant efficacy. Several studies examined the association of pBDNF concentrations with the antidepressant response of a single infusion of ketamine, but these findings are inconsistent (Haile et al., 2014; Lee & Kim, 2010). For instance, one study reported that pBDNF concentrations were related with the severity of depression (Haile et al., 2014). However, Machado-Vieira et al.’s study reported a negative finding on the association of pBDNF concentrations and ketamine’s antidepressant efficacy (Machado-Vieira et al., 2009).
Notably, several animal studies reported that increased hippocampal and cortical BDNF expression can partly accounting for ketamine’s antidepressant-like efficacy (Autry et al., 2011; Réus et al., 2011). pBDNF concentrations were lower in individuals suffering from depression compared to healthy controls (Kishi et al., 2017; Munno et al., 2013) and increased after receiving antidepressants (Munno et al., 2013; Polyakova et al., 2015), electroconvulsive therapy (Luan et al., 2020; Piccinni et al., 2009), and repeated transcranial magnetic stimulation (Yukimasa et al., 2006). Therefore, neurotrophic factors, such as BDNF, might be involved in ketamine’s antidepressant mechanism. Notably, BDNF is implicated in the regulation of synaptic plasticity, including the synaptic recruitment of AMPA receptors. Growing studies indicate that synaptic plasticity is altered in individuals with depression (Machado-Vieira, Zarate Jr & Manji, 2006; Schloesser et al., 2008; Zarate Jr, Singh & Manji, 2006), and ketamine’s antidepressant efficacy may be attributed to the synaptic potentiation of neural circuits mediated by increased AMPA-to-NMDA glutamate receptors (Maeng & Zarate Jr, 2007).
The following limitations should be acknowledged. First, the participants continued receiving previous medications and lacked a washout period during the study, which may have affected pBDNF concentrations. However, the combination of ketamine and other antidepressants for individuals with depression is increasingly being used in the real-world clinical setting (Shiroma et al., 2014). Second, the sample size was small in the current study. Third, the possible impact of subjective evaluation was inevitable due to lack of a control group. Fourth, some comprehensive analyses, such as the mediating and moderating effect analysis, were not conducted in this study. Finally, brain BDNF concentrations and other key neurobiological mediators, such as mTOR, were not directly measured. However, BDNF crosses the blood–brain barrier, and pBDNF concentrations are closely correlated with cortical BDNF concentrations, and likely reflect brain BDNF concentrations (Pillai et al., 2010; Poduslo & Curran, 1996).
In conclusion, this preliminary study suggests that baseline pBDNF concentrations appeared to be correlated with ketamine’s antidepressant efficacy in Chinese patients with depression.
Supplemental Information
Funding Statement
This study was funded by the Science and Technology Planning Project of Liwan District of Guangzhou (202004034), Guangzhou Health Science and Technology Project (20211A011045), Guangzhou science and Technology Project of traditional Chinese Medicine and integrated traditional Chinese and Western medicine (20211A011045), China International Medical Exchange Foundation (Z-2018-35-2002), Guangzhou Clinical Characteristic Technology Project (2019TS67), and Guangdong Hospital Association (2019ZD06). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Wei Zheng, Email: zhengwei0702@163.com.
Yu-Ping Ning, Email: ningjeny@126.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Wei Zheng performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Yan-Ling Zhou conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Cheng-Yu Wang performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Xiao-Feng Lan and Bin Zhang performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Su-Miao Zhou and Su Yan analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Yu-Ping Ning conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The Affiliated Brain Hospital of Guangzhou Medical University granted ethical approval to carry out the study within its facilities (Ethical Application Ref: 2016-030).
Clinical Trial Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The Affiliated Brain Hospital of Guangzhou Medical University granted ethical approval to carry out the study within its facilities (Ethical Application Ref: 2016-030).
Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The Affiliated Brain Hospital of Guangzhou Medical University granted ethical approval to carry out the study within its facilities (Ethical Application Ref: 2016-030).
Data Availability
The following information was supplied regarding data availability:
Raw data are available as a Supplemental File.
Clinical Trial Registration
The following information was supplied regarding Clinical Trial registration:
ChicCTR-OOC-17012239
References
- Autry et al. (2011).Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, Kovacs & Weissman (1979).Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the scale for suicide ideation. Journal of Consulting and Clinical Psychology. 1979;47:343–352. doi: 10.1037/0022-006X.47.2.343. [DOI] [PubMed] [Google Scholar]
- Brunoni, Lopes & Fregni (2008).Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. The International Journal of Neuropsychopharmacology. 2008;11:1169–1180. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- Cornwell et al. (2012).Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, Zarate Jr CA. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biological Psychiatry. 2012;72:555–561. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman (2004).Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Medicine. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Duman & Monteggia (2006).Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Duncan et al. (2013).Duncan WC, Sarasso S, Ferrarelli F, Selter J, Riedner BA, Hejazi NS, Yuan P, Brutsche N, Manji HK, Tononi G, Zarate CA. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. The International Journal of Neuropsychopharmacology. 2013;16:301–311. doi: 10.1017/S1461145712000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile et al. (2014).Haile CN, Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Foulkes A, Iqbal S, Mahoney 3rd JJ, De La Garza 2nd R, Charney DS, Newton TF, Mathew SJ. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. The International Journal of Neuropsychopharmacology. 2014;17:331–336. doi: 10.1017/S1461145713001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton (1960).Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi et al. (2017).Kishi T, Yoshimura R, Ikuta T, Iwata N. Brain-derived neurotrophic factor and major depressive disorder: evidence from meta-analyses. Frontiers in Psychiatry. 2017;8:308. doi: 10.3389/fphys.2017.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryst et al. (2020).Kryst J, Kawalec P, Mitoraj AM, Pilc A, Lasoń W, Brzostek T. Efficacy of single and repeated administration of ketamine in unipolar and bipolar depression: a meta-analysis of randomized clinical trials. Pharmacological Reports. 2020;72:543–562. doi: 10.1007/s43440-020-00097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita et al. (2012).Kurita M, Nishino S, Kato M, Numata Y, Sato T. Plasma brain-derived neurotrophic factor levels predict the clinical outcome of depression treatment in a naturalistic study. PLOS ONE. 2012;7:e39212. doi: 10.1371/journal.pone.0039212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal, Bramham & Duarte (2017).Leal G, Bramham CR, Duarte CB. BDNF and hippocampal synaptic plasticity. Vitamins and Hormones. 2017;104:153–195. doi: 10.1016/bs.vh.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Lee & Kim (2010).Lee BH, Kim YK. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investigation. 2010;7:231–235. doi: 10.4306/pi.2010.7.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2010).Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan et al. (2020).Luan S, Zhou B, Wu Q, Wan H, Li H. Brain-derived neurotrophic factor blood levels after electroconvulsive therapy in patients with major depressive disorder: a systematic review and meta-analysis. The Asian Journal of Psychiatry. 2020;51:101983. doi: 10.1016/j.ajp.2020.101983. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira et al. (2009).Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, Zarate Jr CA. Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. Journal of Clinical Psychiatry. 2009;70:1662–1666. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira, Zarate Jr & Manji (2006).Machado-Vieira R, Zarate Jr CA, Manji HK. Emerging novel treatments for severe mood disorders involving cellular plasticity cascades. Curr Psychos Ther Rep. 2006;4:181–190. doi: 10.1007/BF02629394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng & Zarate Jr (2007).Maeng S, Zarate Jr CA. The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Current Psychiatry Reports. 2007;9:467–474. doi: 10.1007/s11920-007-0063-1. [DOI] [PubMed] [Google Scholar]
- Molendijk et al. (2014).Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM. Molecular Psychiatry. 2014;19:791–800. doi: 10.1038/mp.2013.105. [DOI] [PubMed] [Google Scholar]
- Montgomery & Asberg (1979).Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Munno et al. (2013).Munno D, Sterpone S, Fania S, Cappellin F, Mengozzi G, Saroldi M, Bechon E, Zullo G. Plasma brain derived neurotrophic factor levels and neuropsychological aspects of depressed patients treated with paroxetine. Panminerva Medica. 2013;55:377–384. [PubMed] [Google Scholar]
- Na & Kim (2021).Na KS, Kim YK. Increased use of ketamine for the treatment of depression: benefits and concerns. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2021;104:110060. doi: 10.1016/j.pnpbp.2020.110060. [DOI] [PubMed] [Google Scholar]
- Phillips et al. (2020).Phillips JL, Norris S, Talbot J, Hatchard T, Ortiz A, Birmingham M, Owoeye O, Batten LA, Blier P. Single and repeated ketamine infusions for reduction of suicidal ideation in treatment-resistant depression. Neuropsychopharmacology. 2020;45:606–612. doi: 10.1038/s41386-019-0570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinni et al. (2009).Piccinni A, Del Debbio A, Medda P, Bianchi C, Roncaglia I, Veltri A, Zanello S, Massimetti E, Origlia N, Domenici L, Marazziti D, Dell’Osso L. Plasma Brain-Derived Neurotrophic Factor in treatment-resistant depressed patients receiving electroconvulsive therapy. European Neuropsychopharmacology. 2009;19:349–355. doi: 10.1016/j.euroneuro.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Pillai et al. (2010).Pillai A, Kale A, Joshi S, Naphade N, Raju MS, Nasrallah H, Mahadik SP. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. The International Journal of Neuropsychopharmacology. 2010;13:535–539. doi: 10.1017/S1461145709991015. [DOI] [PubMed] [Google Scholar]
- Poduslo & Curran (1996).Poduslo JF, Curran GL. Permeability at the blood–brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Research. Molecular Brain Research. 1996;36:280–286. doi: 10.1016/0169-328X(95)00250-V. [DOI] [PubMed] [Google Scholar]
- Polyakova et al. (2015).Polyakova M, Stuke K, Schuemberg K, Mueller K, Schoenknecht P, Schroeter ML. BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. Journal of Affective Disorders. 2015;174:432–440. doi: 10.1016/j.jad.2014.11.044. [DOI] [PubMed] [Google Scholar]
- Pytka et al. (2018).Pytka K, Głuch-Lutwin M, Kotańska M, Waszkielewicz A, Kij A, Walczak M. Single administration of HBK-15-a Triple 5-HT(1A), 5-HT(7), and 5-HT(3) receptor antagonist-reverses depressive-like behaviors in mouse model of depression induced by corticosterone. Molecular Neurobiology. 2018;55:3931–3945. doi: 10.1007/s12035-017-0605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen et al. (2013).Rasmussen KG, Lineberry TW, Galardy CW, Kung S, Lapid MI, Palmer BA, Ritter MJ, Schak KM, Sola CL, Hanson AJ, Frye MA. Serial infusions of low-dose ketamine for major depression. Journal of Psychopharmacology. 2013;27:444–450. doi: 10.1177/0269881113478283. [DOI] [PubMed] [Google Scholar]
- Réus et al. (2011).Réus GZ, Stringari RB, Ribeiro KF, Ferraro AK, Vitto MF, Cesconetto P, Souza CT, Quevedo J. Ketamine plus imipramine treatment induces antidepressant-like behavior and increases CREB and BDNF protein levels and PKA and PKC phosphorylation in rat brain. Behavioural Brain Research. 2011;221:166–171. doi: 10.1016/j.bbr.2011.02.024. [DOI] [PubMed] [Google Scholar]
- Rong et al. (2018).Rong C, Park C, Rosenblat JD, Subramaniapillai M, Zuckerman H, Fus D, Lee YL, Pan Z, Brietzke E, Mansur RB, Cha DS, Lui LMW, McIntyre RS. Predictors of response to ketamine in treatment resistant major depressive disorder and bipolar disorder. International Journal of Environmental Research and Public Health. 2018;15(4):771. doi: 10.3390/ijerph15040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagud et al. (2016).Sagud M, Nikolac Perkovic M, Vuksan-Cusa B, Maravic A, Vob Strac DS, Mihaljevic Peles A, Zivkovic M, Kusevic Z, Pivac N. A prospective, longitudinal study of platelet serotonin and plasma brain-derived neurotrophic factor concentrations in major depression: effects of vortioxetine treatment. Psychopharmacology. 2016;233:3259–3267. doi: 10.1007/s00213-016-4364-0. [DOI] [PubMed] [Google Scholar]
- Schloesser et al. (2008).Schloesser RJ, Huang J, Klein PS, Manji HK. Cellular plasticity cascades in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33:110–133. doi: 10.1038/sj.npp.1301575. [DOI] [PubMed] [Google Scholar]
- Shiroma et al. (2014).Shiroma PR, Johns B, Kuskowski M, Wels J, Thuras P, Albott CS, Lim KO. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. Journal of Affective Disorders. 2014;155:123–129. doi: 10.1016/j.jad.2013.10.036. [DOI] [PubMed] [Google Scholar]
- Singh et al. (2020).Singh J, Verma R, Raghav R, Sarkar S, Sood M, Jain R. Brain-derived neurotrophic factor (BDNF) levels in first-episode schizophrenia and healthy controls: a comparative study. The Asian Journal of Psychiatry. 2020;54:102370. doi: 10.1016/j.ajp.2020.102370. [DOI] [PubMed] [Google Scholar]
- Yukimasa et al. (2006).Yukimasa T, Yoshimura R, Tamagawa A, Uozumi T, Shinkai K, Ueda N, Tsuji S, Nakamura J. High-frequency repetitive transcranial magnetic stimulation improves refractory depression by influencing catecholamine and brain-derived neurotrophic factors. Pharmacopsychiatry. 2006;39:52–59. doi: 10.1055/s-2006-931542. [DOI] [PubMed] [Google Scholar]
- Yüksel & Öngür (2010).Yüksel C, Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biological Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate Jr, Singh & Manji (2006).Zarate Jr CA, Singh J, Manji HK. Cellular plasticity cascades: targets for the development of novel therapeutics for bipolar disorder. Biological Psychiatry. 2006;59:1006–1020. doi: 10.1016/j.biopsych.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Zheng et al. (2018).Zheng W, Zhou YL, Liu WJ, Wang CY, Zhan YN, Li HQ, Chen LJ, Li MD, Ning YP. Rapid and longer-term antidepressant effects of repeated-dose intravenous ketamine for patients with unipolar and bipolar depression. Journal of Psychiatric Research. 2018;106:61–68. doi: 10.1016/j.jpsychires.2018.09.013. [DOI] [PubMed] [Google Scholar]
- Zhong et al. (2011).Zhong BL, Wang Y, Chen HH, Wang XH. Reliability, validity and sensitivity of Montgomery-Åsberg Depression Rating Scale for patients with current major depressive disorder [in Chinese] Chinese Journal of Behavioural Medicine and Brain Sciences. 2011;20:85–87. [Google Scholar]
- Zimmerman, Posternak & Chelminski (2004).Zimmerman M, Posternak MA, Chelminski I. Derivation of a definition of remission on the Montgomery-Asberg depression rating scale corresponding to the definition of remission on the Hamilton rating scale for depression. Journal of Psychiatric Research. 2004;38:577–582. doi: 10.1016/j.jpsychires.2004.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
Raw data are available as a Supplemental File.


