Abstract
Antiretroviral therapy has largely transformed HIV infection into a chronic disease condition. As such, physicians and other providers caring for individuals living with HIV infection need to be aware of the potential cardiovascular complications of HIV infection and the nuances of how HIV infection increases the risk of cardiovascular diseases, including acute myocardial infarction, stroke, peripheral artery disease, heart failure and sudden cardiac death, as well as how to select available therapies to reduce this risk. In this Review, we discuss the epidemiology and clinical features of cardiovascular disease, with a focus on coronary heart disease, in the setting of HIV infection, which includes a substantially increased risk of myocardial infarction even when the HIV infection is well controlled. We also discuss the mechanisms underlying HIV-associated atherosclerotic cardiovascular disease, such as the high rates of traditional cardiovascular risk factors in patients with HIV infection and HIV-related factors, including the use of antiretroviral therapy and chronic inflammation in the setting of effectively treated HIV infection. Finally, we highlight available therapeutic strategies, as well as approaches under investigation, to reduce the risk of cardiovascular disease and lower inflammation in patients with HIV infection.
At the end of 2017, approximately 36.9 million people were living with HIV infection, with 1.8 million becoming newly infected during that year1. The WHO recommends that all people with HIV infection receive antiretroviral therapy (ART) (Table 1). ART has transformed HIV infection into a chronic disease. As a consequence, by the year 2030, a modelling study suggests that 73% of people with HIV infection will be aged ≥50 years and 78% of individuals living with HIV infection will have cardiovascular disease (CVD)2. As shown in Fig. 1, individuals infected with HIV have been shown to be at an increased risk of CVD, including sudden cardiac death3, acute myocardial infarction (MI)4, stroke5, peripheral artery disease and heart failure with either reduced or preserved ejection fraction6. This Review focuses on coronary heart disease in the setting of HIV infection.
Table 1 |.
Commonly used antiretroviral drugs
| Class | Mechanism of action179 | Generic name | Abbreviation | Brand name | Year of FDA approval |
|---|---|---|---|---|---|
| Protease inhibitors | Selectively bind to HIV proteases and inhibit cleavage of Gag-Pro-Pol polyproteins in HIV-infected cells, resulting in the production of immature, non-infectious virions | Atazanavir | ATV | Reyataz | 2003 |
| Darunavir | DRV | Prezista | 2006 | ||
| Fosamprenavir | FOS-APV, FPV | Lexiva | 2003 | ||
| Ritonavir | RTV | Norvir | 1996 | ||
| Saquinavir | SQV | Invirase | 1995 | ||
| Tipranavir | TPV | Aptivus | 2005 | ||
| Nucleoside reverse-transcriptase inhibitors | Inhibit viral replication through competitive binding to the HIV enzyme reverse transcriptase, leading to termination of DNA chain elongation | Abacavir | ABC | Ziagen | 1998 |
| Emtricitabine | FTC | Emtriva | 2003 | ||
| Lamivudine | 3TC | Epivir | 1995 | ||
| Tenofovir fumarate disoproxil | TDF | Viread | 2001 | ||
| Zidovudine | AZT | Retrovir | 1987 | ||
| Non-nucleoside reverse-transcriptase inhibitors | Prevent HIV reverse transcriptase from adding new nucleotides to the DNA chain, leading to a decline in viral replication | Doravirine | DOR | Pifeltro | 2018 |
| Efavirenz | EFV | Sustiva | 1998 | ||
| Etravirine | ETR | Intelence | 2008 | ||
| Nevirapine | NVP | Viramune | 1996 | ||
| Rilpivirine | RPV | Edurant | 2011 | ||
| CCR5 antagonist | Entry inhibitor; binds to the cell membrane receptor CCR5, thereby blocking the entry of CCR5-tropic viruses into CD4+ T cells | Maraviroc | MVC | Selzentry | 2007 |
| Integrase inhibitors | Prevent binding of the pre-integration complex to the host cell DNA, terminating the integration step of HIV replication | Dolutegravir | DTG | Tivicay | 2013 |
| Raltegravir | RAL | Isentress | 2007 | ||
| Fusion inhibitor | Prevents viral fusion to CD4+ T cells by binding to the HIV envelope glycoprotein 41 | Enfuvirtide | T-20 | Fuzeon | 2003 |
| Pharmacokinetic enhancer | Protease inhibitors combined with integrase inhibitors to improve potency of the antiviral agent and to decrease pill burden by increasing trough drug concentration, drug half-life and peak concentration (Cmax) in plasma | Cobicistat | COBI | Tybost | 2014 |
| Post-attachment inhibitor | Monoclonal antibody that binds to the CD4 molecule and blocks viral entry into the cell | Ibalizumab | IBA | Trogarzo | 2018 |
CCR5, CC-chemokine receptor 5.
Fig. 1 |. Overview of changes in HIV treatment and HIV-associated cardiovascular diseases.
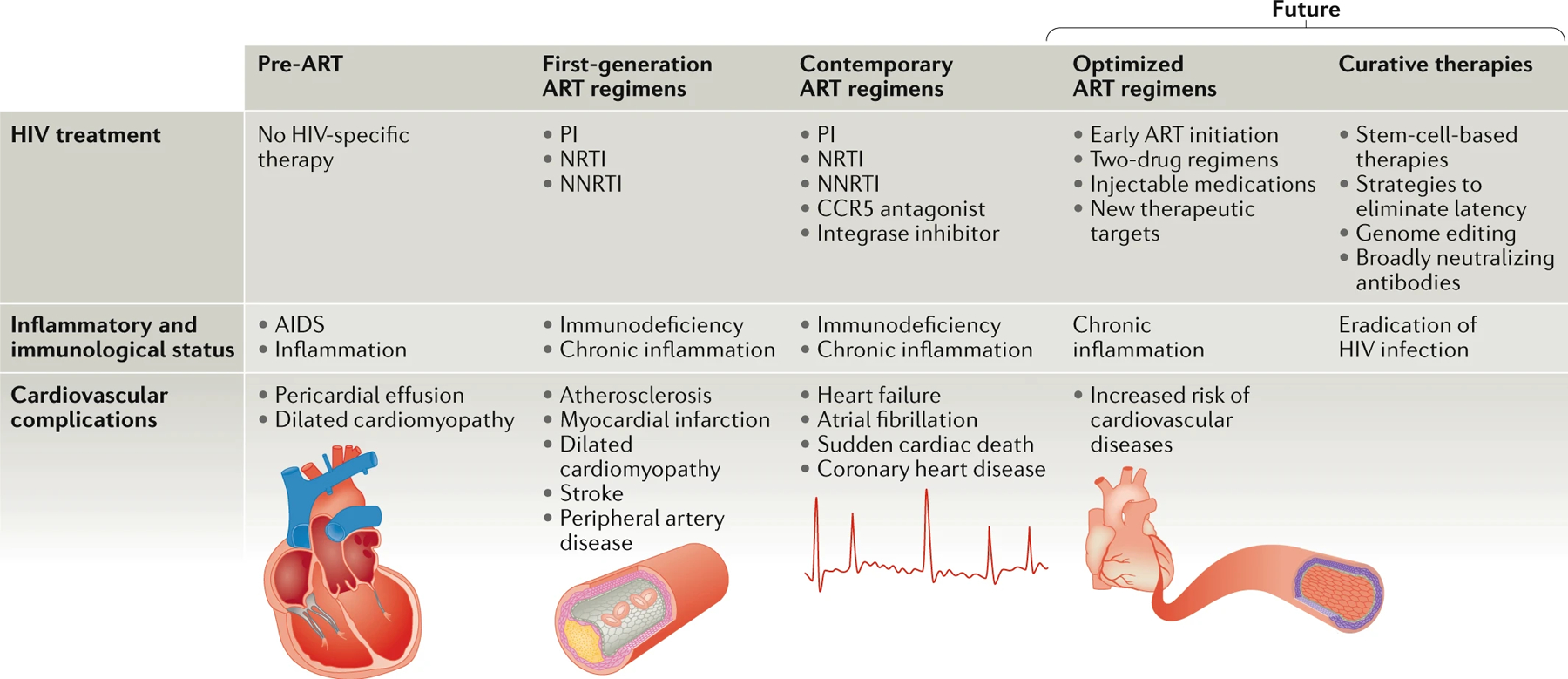
The types of cardiovascular complications associated with HIV infection have changed in the pre-antiretroviral therapy (ART) and ART eras and are likely to continue evolving in the future as new medications and treatment approaches emerge. In the pre-ART era, dilated cardiomyopathy and pericardial effusions were the most commonly reported cardiovascular issues in patients infected with HIV14,176. After the introduction of protease inhibitors (PIs) in the late 1990s, atherosclerotic complications including myocardial infarction were described177,178. More recently, reports of heart failure and rhythm abnormalities are now emerging in the setting of HIV infection3,6. In the future, among individuals with access to ART, HIV infection will be a chronic disease state with increased risk of coronary artery disease19. CCR5, CC-chemokine receptor 5; NNRTI, non-nucleoside reverse-transcriptase inhibitor ; NRTI, nucleoside reverse-transcriptase inhibitor.
Although ART controls the HIV infection, the disease is not cured; individuals must continue taking ART indefinitely, and, in this setting, chronic inflammation and immune activation persist, leading to downstream consequences including the development of atherosclerotic CVD (ASCVD)7. The population living with HIV is ageing, and, although traditional CVD risk factors are crucial contributors to CVD, HIV-specific factors, including ART, chronic inflammation and immune activation, have a role in HIV-associated CVD, including atherosclerosis. The atherosclerosis associated with HIV infection differs in important ways from atherosclerosis in the non-HIV setting in terms of both aetiological factors and clinical presentation. The low-grade inflammation associated with HIV infection accelerates atherogenesis through a variety of mechanisms. For example, atherosclerotic plaques in patients with HIV infection are more likely to be non-calcified and more prone to rupture than atherosclerotic plaques in patients without HIV infection8,9. Around one-half of MI events among patients with HIV infection are related to an imbalance in cardiac oxygen demand and supply rather than to atherothrombotic disease10. Finally, individuals infected with HIV who develop acute coronary syndrome (ACS) are, on average, a decade younger than uninfected patients with ACS and are more likely to be men who smoke and have low levels of HDL cholesterol (HDL-C)11.
In this Review, we address the epidemiology and clinical features of CVD in people living with HIV infection and discuss the mechanisms of HIV-associated ASCVD and the management of CVD risk factors in people with HIV infection. Although much of this information is similar to that in people with CVD without HIV infection, important differences exist, particularly on the potential future therapies for CVD in people living with HIV.
Epidemiology of CVD in HIV infection
Before the advent of ART in the late 1990s, HIV infection almost always progressed to AIDS, with frequent opportunistic infections leading to death. Cardiomyopathy with systolic dysfunction was common in patients with AIDS and was associated with high short-term mortality (Table 2). For example, in one small study from the pre-ART era, median survival was 101 days in patients with AIDS with dilated cardiomyopathy compared with 472 days in patients with AIDS with normal ventricular function12. Cardiomyopathy was attributed to direct HIV infection of the myocardium with or with-out myocarditis, co-infection with other viruses such as coxsackievirus B3 and Cytomegalovirus (CMV), opportunistic infections and nutritional disorders13. Pericardial effusions were also common and presaged a high short-term mortality14.
Table 2 |.
Effect of antiretroviral therapy on the risk of cardiovascular disease
| Study (year) | Study population | ART | Number of patients | Follow-up | Cardiovascular end points | Findings | Refs |
|---|---|---|---|---|---|---|---|
| Bozzette et al. (2003) | Patients with HIV infection who received care at a VA centre | Combination therapy with PIs, nucleoside analogues and NNRTIs | 36,766 | 8.5 years | CVD and cerebrovascular disease | Use of ART was associated with a reduction in the risk of CVD | 180 |
| D:A:D study group (2003) | Patients with HIV infection | Combination regimen including a PI or an NNRTI | 23,468 | 2.2 years | MI | Use of ART was associated with a 26% relative increase in rate of MI per year of exposure | 181 |
| SMART (2006) | Patients with well-controlled HIV infection; cohort from 33 countries | Continuous ART versus episodic use of ART | 5,472 | 16 months | Opportunistic disease or death from any cause; major cardiovascular, renal or hepatic disease | Continuous ART reduced the risk of CVD compared with episodic use of ART | 182 |
| D:A:D study group (2007) | Patients with HIV infection | PIs or NNRTIs | 23,437 | 5.2 years | MI | Exposure to PIs was associated with a higher rate of MI per year of exposure | 25 |
| D:A:D study group (2008) | Patients with HIV infection | NRTIs | 33,347 | 7.2 years | MI | Use of abacavir or didanosine in the previous 6 months was associated with increased risk of MI | 28 |
| Stein et al. (2015) | Patients with HIV infection without known CVD or diabetes mellitus who were initiating their first ART | NRTI, PI or integrase inhibitor | 328 | 6.4 years | Changes in carotid artery IMT | Atazanavir had a protective effect, with slower carotid IMT progression in the setting of high plasma bilirubin levels compared with other ART regimens | 183 |
| START (2015) | Patients with HIV infection; cohort from 35 countries | Immediate initiation of ART versus deferred initiation of ART | 4,299 | 6.4 years | MI, stroke, coronary revascularization or CVD-related death | Early initiation of ART did not significantly reduce the incidence of the cardiovascular end point | 184 |
| Marconi et al. (2018) | Individuals with or without HIV infection and without known CVD | NRTI, PI or NNRTI | 96,381 | 8.8 years | CVD including acute MI, heart failure and stroke | Decreased risk of CVD in the setting of high plasma bilirubin levels irrespective of HIV infection status | 120 |
| Elion et al. (2018) | Patients with HIV infection | NRTI | 8,265 | 12 years | Type 1 and type 2 MI | Use of abacavir in the past 6 months was associated with increased risk of MI | 31 |
| D:A:D study group (2018) | Patients with HIV | PIs | 49,709 | >15 years | CVD | Use of ritonavir-boosted darunavir but not ritonavir-boosted atazanavir was associated with increased risk of CVD | 27 |
ART, antiretroviral therapy ; CVD, cardiovascular disease; IMT, intima–media thickness; MI, myocardial infarction; NNRTI, non-nucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor ; VA , Veterans Affairs.
The incidence of HIV-associated cardiomyopathy has decreased from the pre-ART era, from 25.6 to 3.9 cases per 1,000 person-years15. The phenotype of cardiomyopathy has also changed markedly, from symptomatic systolic dysfunction with left ventricular dilatation in the pre-ART era to asymptomatic systolic or diastolic dysfunction detected by echocardiography in ART-treated patients with HIV infection. Whereas older ARTs, such as nucleoside reverse-transcriptase inhibitors (NRTIs), were implicated in mitochondrial toxicity leading to dilated cardiomyopathy16, more recent studies have suggested that protease inhibitor use is related to increased cardiovascular mortality and 30-day re-admission for heart failure17.
A 2018 systematic review of 80 longitudinal studies of CVD in HIV cohorts, which included 793,635 individuals living with HIV, concluded that the risk of MI and stroke in patients with HIV infection was increased by 2.16 (95% CI 1.68–2.77) compared with uninfected individuals18. The magnitude of this increased risk is similar to that associated with major CVD risk factors, such as diabetes mellitus and hypertension19.Given that the prevalence of HIV infection is much lower than the prevalence of these traditional CVD risk factors, the overall influence of HIV infection on the risk of CVD would, at first glance, seem to be low; however, the global burden of HIV-associated MI and stroke has tripled over the past two decades and now accounts for 2.6 disability-adjusted life-years on an annual basis18.
The burden of traditional CVD risk factors is high in people with HIV infection. A study published in 2015 on the large VACS VC cohort showed that <2% of patients with HIV had optimal levels of traditional CVD risk factors20. However, the increased risk of MI in people with HIV infection persists after adjustment for these risk factors; in the same cohort, the increased risk of MI was approximately 50% after adjusting for Framingham risk factors, comorbidities and substance abuse4. The risk of MI was higher in those with higher HIV-1 RNA levels in blood (≥500 copies per ml) and lower CD4+ T cell counts (<200 cells per μl), but the risk was still elevated in those with low HIV-1 RNA levels and high CD4+ T cell counts4. Although most of the study participants were men, the risk of MI was also significantly increased in women with HIV infection21.
As with MI, the risk of stroke is also increased in people with HIV infection18. Globally, the risk of stroke in patients with HIV infection is approximately double that in individuals without HIV infection18, but, in one study from the USA, the HR was 1.40 (95% CI 1.17–1.69), decreasing to 1.21 (95% CI 1.01–1.46) after adjustment for demographic and stroke risk factors5. A much higher risk of stroke has been reported in African patients with HIV, in whom initiation of ART is usually delayed after infection, with the highest risk of stroke being during the first 6 months after starting ART22. Risk factors for stroke in African patients with HIV infection compared with uninfected patients with stroke included younger age, large-artery disease and a low CD4+ T cell count after initiation of ART23.
The relationship between use of ART and the risk of MI is not straightforward. Case reports of MI in individuals with HIV infection began to appear soon after the introduction of ART24 (Table 2). Early protease inhibitors were associated with the incidence of MI in a 2007 report, in which each cumulative year of protease inhibitor use was associated with a 10% increase in the risk of MI, even after adjusting for the changes in blood lipid levels caused by the protease inhibitor therapy25. Some of the newer protease inhibitors are likely to be safer. More recent reports indicate that among two widely used, recent-generation protease inhibitors, atazanavir was not associated with an increased risk of CVD compared with darunavir26,27.
Abacavir, a widely prescribed NRTI, was reported in several observational cohorts to be associated with an increased risk of MI28–31, possibly because this drug increases vascular inflammation and platelet reactivity32. However, other studies, including an FDA meta-analysis33, do not show an increased risk of MI with abacavir. Nevertheless, US guidelines recommend that abacavir be avoided or used with caution in individuals at high risk of CVD34.
The START trial35 demonstrated unequivocally that starting ART in adults with HIV infection and a CD4+ T cell count of >500 cells per μl reduced mortality and serious AIDS-related and non-AIDS-related events compared with starting ART therapy after the CD4+ T cell count had declined to 350 cells per μl. However, the incidence of cardiovascular events was low in this study, and, although a nonsignificant trend towards a reduction in the risk of cardiovascular events with early initiation of the treatment was observed, no definitive benefit was demonstrated. In substudies of the START trial, early initiation of ART did not improve arterial elasticity36 and led to increased total and LDL cholesterol (LDL-C) levels in plasma but also to increased HDL-C levels and lower use of antihypertensive medications compared with delayed ART initiation37. Therefore, the effect of early initiation of ART on the risk of CVD remains unknown.
Earlier initiation of ART and the use of safer forms of ART from a CVD risk perspective might have resulted in a decrease in the risk of MI over the past decade, but additional studies with longer-term follow-up will be needed to evaluate definitively the effect of early initiation of ART on cardiovascular events. Some studies have reported a declining relative risk of MI in the setting of HIV infection38, which might be related to the use of newer protease inhibitors27, better control of HIV infection39 or modification of traditional CVD risk factors over time40, including smoking41. Thus far, no studies have reported on the effect of integrase inhibitors on cardiovascular events in HIV infection.
Clinical features of HIV-associated CVD
Early reports of coronary heart disease among individuals with HIV infection were remarkably consistent in the described clinical characteristics11,33,42–47. Patients with HIV infection and coronary heart disease in these studies were >90% men and had a mean age of 42–50 years, about a decade younger than patients with ACS without HIV infection in the reports with control groups11. More than half were current smokers11,33,42–44,46,47, and, in studies including blood lipid measurements, plasma HDL-C levels were lower in patients with coronary heart disease and HIV infection than in uninfected patients with coronary heart disease11,43. As would be expected in the population with HIV infection, single-vessel disease was common11,43,46,47, and, in those with ACS, risk scores were lower than in individuals with ACS without HIV infection11. Therefore, as expected, in-hospital mortality in patients with ACS and HIV infection was not high for that era: 4.8% (9 of 189 patients) in the studies in which this outcome was reported11,42,43,46,47.
Coronary angioplasty in these early studies was as effective in patients with HIV infection as in patients without HIV infection; however, restenosis was more common in the setting of HIV infection11,43. The wide-spread use of coronary stents has reduced the incidence of restenosis, and drug-eluting stents have been shown to reduce cardiovascular events in patients with or without HIV infection48. Nevertheless, in a small study in which follow-up coronary angiography was performed routinely in individuals with HIV infection undergoing coronary stenting, the incidence of restenosis with drug-eluting stents was 19%49. Total CD8+ T cell count, but not CD4+ T cell count, was associated with restenosis, as was plasma C-reactive protein (CRP) level at follow-up (but not baseline)49. These results suggest that persistent inflammation in patients with HIV infection might be causally related to restenosis.
In a contemporary cohort of 226 patients with HIV infection who were followed up for 3 years after hospitalization for ACS, the risk of recurrent ACS was not increased compared with individuals without HIV infection (HR 1.08, 95% CI 0.76–1.54)50. HIV infection was significantly associated with all-cause mortality, but this association was not present among individuals with CD4+ T cell counts ≥500 cells per μl. These intermediate-term results are encouraging; however, longer-term follow-up studies of patients with HIV infection and coronary heart disease are lacking.
Imaging studies have provided insight into the features and pathophysiology of HIV-associated coronary heart disease. Overall stenosis was less severe among men with ACS and HIV infection than in matched, uninfected individuals with ACS, although the numbers of diseased coronary vessels and lesions were similar in both groups51. In another study, plaque burden assessed as lesion severity in intracoronary ultrasonography findings was also lower in patients with ACS and HIV infection than in patients with ACS without HIV infection52. Furthermore, hyperechoic, non-calcified coronary plaques were much more common in patients with HIV (100% versus 35%; P < 0.05).
A higher prevalence of non-calcified coronary lesions among patients with HIV than in individuals without HIV infection was also reported in a meta-analysis of individuals without coronary symptoms who were evaluated with coronary CT9. In nine studies with 1,229 patients infected with HIV and 1,029 controls without HIV infection, the rates of non-calcified coronary plaques were higher in the HIV group (58% versus 17%; OR 3.26, 95% CI 1.30–8.18). Some evidence suggests that these non-calcified plaques are particularly amenable to LDL-C lowering with statin therapy. In a small clinical trial in which patients with HIV infection were randomly assigned to receive atorvastatin or placebo and followed up for 1 year, atorvastatin significantly reduced non-calcified coronary plaque volume compared with placebo53. Why individuals infected with HIV have a higher prevalence of non-calcified coronary plaques has yet to be established but does not seem to be related to ART54 and might be due to chronic inflammation and immune activation in treated HIV infection55,56.
Mechanisms of HIV-related atherogenesis
The pathogenesis of atherosclerosis in the setting of HIV infection is complex and poorly understood. Underlying mechanisms for HIV-associated ASCVD include the effects of the HIV proteins on immune and vascular cells, the immunodeficiency caused by the HIV infection, co-infection with CMV, microbial translocation from the gut, chronic inflammation and immune cell activation. These factors and their interrelationships are depicted in Fig. 2 and are summarized below.
Fig. 2 |. Pathophysiology and management of HIV-associated atherosclerotic cardiovascular disease.
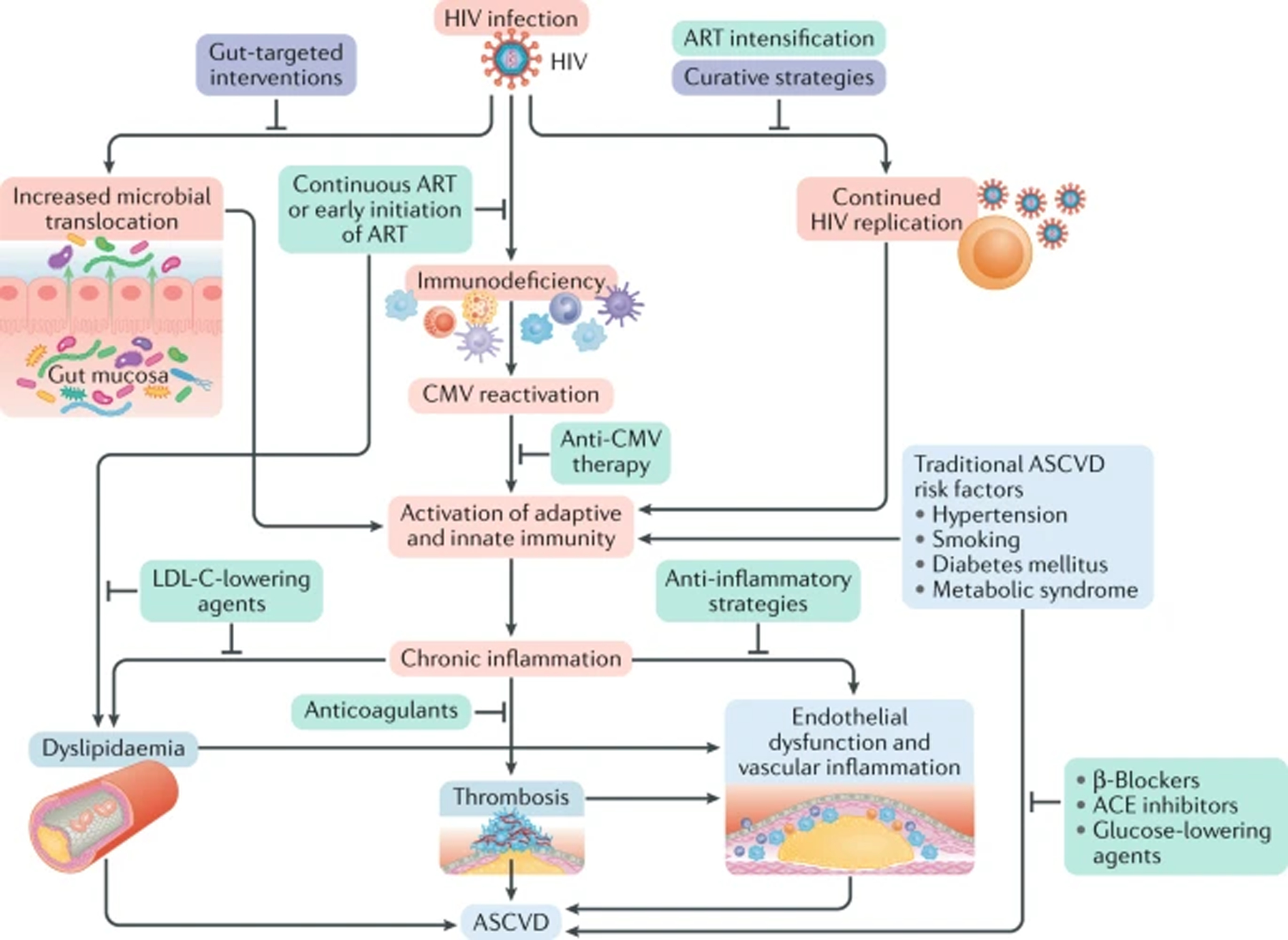
Schematic representation of the effects of HIV infection (in red) and the available strategies (in green), as well as approaches under investigation (in purple), for reducing the risk of atherosclerotic cardiovascular disease (ASCVD) and chronic inflammation in this patient population. In the setting of HIV infection, the increased microbial translocation from the gut, the continued HIV viral replication and the HIV-induced immunodeficiency, along with traditional ASCVD risk factors, contribute to immune cell activation and chronic inflammation. HIV-specific interventions to reduce the risk of ASCVD include strategies targeted at co-infections (such as Cytomegalovirus (CMV) infection), use of newer antiretroviral therapies (ARTs) and intensification of ART. Strategies aimed at eradicating the HIV infection are under investigation. Treatments targeting traditional ASCVD risk factors, such as hypertension, diabetes mellitus, smoking and metabolic syndrome, are also critical for reducing the risk of ASCVD in patients with HIV infection. Use of anticoagulants, β-blockers, angiotensin-converting enzyme (ACE) inhibitors and LDL cholesterol (LDL-C)-lowering agents (such as statins and PCSK9 inhibitors) reduce the risk of ASCVD in patients with cardiovascular disease without HIV infection and might, therefore, be useful in reducing the risk of HIV-associated ASCVD. Finally , strategies to lower inflammation, such as canakinumab, which has been reported to reduce cardiovascular events significantly in a non-HIV patient population, might also reduce the risk of HIV-associated ASCVD.
HIV proteins.
In individuals receiving ART, the HIV infection, although controlled, is not cured and therefore persists even when the virus is undetectable57, with low-level transcription of HIV genes58. HIV-encoded proteins, specifically transactivator of transcription (Tat) and negative factor (Nef), induce inflammation and endothelial dysfunction59. In addition, the HIV envelope protein gp120 has been shown to stimulate endothelin 1 production60. Therefore, by releasing low levels of certain damaging proteins, the HIV virus itself stimulates atherogenesis.
Immunodeficiency.
CD4+ T cell depletion is the hallmark of HIV infection, and nadir CD4+ T cell count is a rough marker of the severity of immunodeficiency. Nadir CD4+ T cell count was first linked to features of atherosclerosis, such as increased carotid intima-media thickness61 and increased arterial stiffness62. Soon thereafter, lower nadir CD4+ T cell counts were shown to be associated with incident MI in two cohort studies63,64. In another report in which cardiovascular events were adjudicated more stringently, type 1 MI (the atherothrombotic type) occurred more commonly in people either with lower CD4+ T cell counts or with detectable HIV RNA65. The CD4:CD8 ratio, a marker of immunosenescence, has been predictive of cardiovascular events in some66,67 but not other68 studies. Among individuals with HIV infection in New York City, USA, CVD mortality was highest among individuals with detectable viraemia (adjusted rate ratio (RR) 3.53, 95% CI 3.21–3.87), although even individuals with viral suppression had elevated CVD mortality (adjusted RR 1.53, 95% CI 1.41–1.66) compared with the general population69. These findings indicate that markers of immune system damage and viral detectability are related to cardiovascular events in patients with HIV. Immune abnormalities persist in individuals with HIV infection even after successful treatment with ART. The mechanisms linking immune system damage in HIV infection to atherosclerosis have not been elucidated. Although non-AIDS-related events, such as MI and stroke, are less common with complete viral suppression, these events still occur at rates considerably higher than in an uninfected population.
CMV co-infection.
Co-infection with CMV might contribute to HIV-associated atherosclerosis. Compared with individuals without HIV infection, patients infected with HIV consistently have a higher proportion of CMV-specific CD8+ T cells, with the highest levels seen in patients with HIV suppression who are receiving long-term ART70. CMV-specific responses seem to underlie immunosenescence or immunological ageing in HIV infection71. Co-infection with CMV is strongly linked to HIV viral persistence and might also have a role in chronic immune activation and inflammation by expansion of the HIV reservoir72. These CMV-specific T cell responses also have been shown to correlate with markers of atherosclerosis, such as carotid intima–media thickness73–75 and coronary artery calcification75. High CMV antibody titres in patients with HIV infection, and titres to other viruses such as herpes simplex virus and varicella-zoster virus, are associated with higher levels of biomarkers that accelerate inflammation and atherosclerosis74. Analogously, CMV also has an important role in the coronary atherosclerosis that develops in heart transplantation recipients76.
Gut microbial translocation.
Impairment of the gut barrier is an early feature of HIV infection, leading to microbial translocation, a process whereby microbial products leak through the intestinal barrier and cause immune activation77. Plasma levels of soluble CD14 (sCD14) and lipopolysaccharide are markers of microbial translocation that independently predict HIV disease progression78 and mortality79 in individuals who are not receiving ART. Conflicting data exist as to whether these markers are predictive of adverse outcomes in ART-treated individuals; however, gut damage and microbial translocation persist even when HIV infection is suppressed by ART77. Plasma levels of inflammatory markers, specifically IL-6 and tumour necrosis factor, have been reported to be higher in individuals with higher levels of markers of microbial translocation80. Therefore, microbial translocation is another mechanism that might contribute to the atherogenesis associated with HIV infection. However, interventions targeting this mechanism, including sevelamer81, rifaximin80, probiotic administration82 and mesalamine83, have not consistently lowered inflammatory markers or T cell activation. As these studies targeted a variety of patient populations (treated versus untreated HIV infection), evaluated different inflammatory markers and were generally short in duration, the definitive role of microbial translocation from the gut in HIV-associated ASCVD remains unclear and requires additional investigation.
Chronic inflammation and immune cell activation.
Latent HIV infection, co-infection with other viruses and microbial translocation all influence atherogenesis by increasing inflammation. HIV infection is associated with high levels in plasma of inflammation and coagulation markers, such as CRP, IL-6 and d-dimer, and these biomarkers strongly predict cardiovascular events and all-cause mortality in individuals with HIV infection84–86. Arterial and lymph node inflammation as assessed by FDG-PET and CT imaging are higher in patients with HIV infection than in individuals without HIV infection, and this increased inflammation correlates with higher circulating levels of CRP, IL-6 and activated monocytes87,88.
Inflammation offers a potential therapeutic target for the reduction of cardiovascular events in individuals with or without HIV infection; some postulated therapies to lower inflammation in HIV infection are depicted in Fig. 2. In patients with atherosclerosis without HIV infection, the beneficial effect of statin therapy is thought to be due not only to a reduction in plasma LDL-C levels but also to a reduction in inflammation89. In the presence of HIV infection, the anti-inflammatory effects of statins on markers of inflammation seem to be attenuated90,91.Changing protease-inhibitor-based regimens to integrase inhibitors does not consistently reduce inflammatory parameters92, and intensification of ART does not have a major salutary effect on inflammatory markers93,94. Short studies of aspirin therapy also had no effect on inflammatory markers in HIV infection95. Taken together, these findings suggest that strategies in addition to ART and targeting of traditional CVD risk factors are needed to reduce inflammation with the aim of lowering the risk of ASCVD in the setting of HIV infection.
Although T cell activation is a strong predictor of HIV disease progression96, inflammatory and coagulation biomarkers, including IL-6, soluble tumour necrosis factor receptor type I (sTNFRI), tumour necrosis factor receptor type II (TNFRII), kynurenine:tryptophan ratio and d-dimer, but not T cell activation, were predictive of non-AIDS events including CVD97. Some studies have implicated T cell activation in HIV infection with other indices of atherosclerosis, including carotid artery intima-media thickness98 and arterial stiffness99. The effect of T cell activation in HIV-associated vascular disease seems to be at the microvascular level, which serves as the stimulus for flow-mediated vasodilatation (a marker of macrovascular disease)100. Therefore, T cell activation in HIV infection might worsen microvascular disease, leading to endothelial dysfunction and subsequent cardiovascular events.
Canakinumab, a monoclonal antibody targeting IL-1β, was evaluated in a randomized, placebo-controlled trial involving 10,061 patients with previous MI and a high-sensitivity CRP (hsCRP) level of ≥2 mg/l (reF.101).The three doses of canakinumab that were tested all reduced hsCRP levels, ranging from 26% reduction with the 50 mg dose to 41% with the 300 mg dose, but did not affect LDL-C levels. At the intermediate and higher doses, canakinumab reduced the primary composite end point of CVD-related death, MI and stroke. Patients who had a hsCRP reduction to <2 mg/l had significant reductions in cardiovascular events, CVD-related mortality and all-cause mortality, whereas no significant reduction in these outcomes was observed in patients who did not achieve the hsCRP reduction to this level102. Similar findings were reported for individuals who achieved on-treatment IL-6 levels below the study median value of 1.65ng/l — namely, a 32% reduction in major adverse cardiovascular events, a 52% reduction in CVD-related mortality and a 48% reduction in all cause mortality103. Canakinumab therapy was associated with a higher incidence of fatal infection than placebo101.
Would canakinumab reduce cardiovascular events in individuals infected with HIV? In a small study in patients with HIV infection, our group has shown that canakinumab therapy significantly reduced plasma IL-6 and hsCRP levels, with no effect on CD4, CD8 or RNA viral levels104. Inflammation in the bone marrow and arterial inflammation were reduced after a single dose of canakinumab. By contrast, in another study published in 2018, low-dose methotrexate significantly lowered the CD8+ T cell count but had no effect on inflammatory markers or endothelial function105. However, low-dose methotrexate did favourably affect specialized ultrasonography indices in the brachial artery106. These preliminary studies with inflammatory biomarkers as surrogate end points will hopefully lead to clinical trials on anti-inflammatory treatments with cardiovascular events as end points in the population living with HIV.
As previously noted, markers of inflammation and coagulation predict cardiovascular events in individuals with HIV infection84–86. A summary of markers studied in different cohorts of patients with HIV infection and the association with clinical outcomes is shown in Table 3. The strong relationship between inflammatory and coagulation markers is striking and apparent even in the setting of diverse HIV-infected populations including men, women and older and younger individuals. In combined data from three large cohorts of patients with HIV infection, IL-6 and d-dimer levels in plasma were independently associated with the risk of serious non-AIDS events or death, with an estimated 37% reduction in non-AIDS events or death resulting from a 25% decrease in IL-6 or d-dimer levels107. Aside from prediction of clinical events, plasma levels of soluble CD163 (sCD163), a marker of macrophage activity, was associated with higher levels of arterial inflammation in HIV infection87.
Table 3 |.
Associations of inflammatory and coagulation biomarkers with mortality in HIV infection
| Study (year) | Study population | Number of patients | Follow-up | Findings | Refs |
|---|---|---|---|---|---|
| SMART (2008) | Patients with well-controlled HIV infection; cohort from 33 countries | 5,472 | 3,700 person-years |
|
84,185 |
| FRAM (2010) | Patients with HIV infection | 922 | 5 years | Fibrinogen and CRP levels were strong and independent predictors of mortality | 186 |
| ALLRT (2014) | Patients with HIV infection who had achieved virological suppression within 1 year after ART initiation | 143 | 48–64 weeks after ART initiation | High IL-6, sTNFRI, sTNFRII and d-dimer levels in plasma and KT ratio at 1 year were associated with increased risk of non-AIDS events | 97 |
| VACS (2016) | Patients with HIV infection and individuals without infection | 2,350 | 6.9 years | HIV infection was associated with elevated IL-6, sCD14 and d-dimer levels in plasma, which are associated with mortality | 187 |
| MACS (2016) | Patients with well-controlled HIV infection | 670 | Up to 18 years | IL-6 and sCD14 levels in plasma were predictive of mortality | 188 |
| START (2017) | Patients with HIV infection; cohort from 35 countries | 4,299 | 3.2 years | Baseline IL-6 and d-dimer levels in plasma were associated with the risk of AIDS, serious non-AIDS events or death | 189 |
ART, antiretroviral therapy ; CRP, C-reactive protein; CVD, cardiovascular disease; hsCRP, high-sensitivity C-reactive protein; KT, kynurenine:tryptophan; sCD14; soluble CD14; sTNFRI, soluble tumour necrosis factor receptor type I; sTNFRII, soluble tumour necrosis factor receptor type II.
In the general population, the immune system has been implicated in atherosclerosis as well as in conduction system disorders, myocarditis and heart failure with preserved ejection fraction108. In particular, the role of haematopoietic stem cells, inflammation and macrophages has been associated with these conditions; therefore, it is plausible that immune system abnormalities that are present in HIV infection underlie HIV-associated CVDs. HIV infects CD4+ T cells, most of which reside in secondary lymphoid tissues, such as the lymph nodes109. Myeloid cells, which include monocytes, dendritic cells and macrophages, have a crucial role in HIV disease pathogenesis110,111. Macrophage infiltration has been observed in the hearts of monkeys infected with simian immunodeficiency virus (SIV), and macrophage infiltration correlated with fibrosis and cardiac pathology112. Anti-inflammatory therapy with natalizumab (a monoclonal antibody against the cell adhesion molecule α4 integrin) reduced the number of macrophages in cardiac tissue as well as cardiac pathology in this animal model113. Monocyte and macrophage markers, including sCD163 and sCD14 levels in plasma and CD14+CD16+ cell counts, have been linked to subclinical atherosclerosis in HIV infection in a variety of studies113.
A 2018 study showed that biomarkers in HIV infection can be grouped into clusters, with each cluster related to a cardiac phenotype114. For example, the inflammatory phenotype is characterized by higher levels of CRP, IL-6 and d-dimer, whereas the cardiac phenotype comprised a clustering of higher levels of protein ST2 (also known as IL-1 receptor-like 1), N-terminal pro-B-type natriuretic peptide and growth/differentiation factor 15. Diastolic dysfunction was common in the inflammatory group of biomarkers, and pulmonary hypertension was more common in the cardiac group of markers. Both phenotypes were associated with a threefold increase in mortality over a 6.9-year follow-up after adjustment for other prognostic variables. Biomarker clusters in patients with HIV might be helpful for selecting patients for appropriate therapy to reduce cardiovascular events.
ART-related mechanisms.
The most obvious mechanism by which ART increases the risk of CVD is through worsening of blood lipid levels. The increase in LDL-C levels with protease inhibitors seems to result from increased cholesterol absorption rather than increased synthesis115. Whereas some ARTs increase LDL-C levels, other ARTs, particularly older protease inhibitors, induce hypertriglyceridaemia, with ritonavir being the worst culprit116. Interestingly, in the large D:A:D study117, even after adjustment for blood lipid levels, cumulative exposure to the NRTIs abacavir or didanosine or to the protease inhibitors lopinavir–ritonavir or indinavir was associated with an increased risk of MI. Newer protease inhibitors, as well as the integrase inhibitor raltegravir and the virus-entry inhibitor maraviroc, have favourable effects on lipid levels, particularly compared with older types of ARTs118.
ART might also increase the risk of ASCVD through other mechanisms. Insulin resistance, lipodystrophy and other patterns of fat distribution can contribute to atherogenesis. The NRTI abacavir has been linked to an increased risk of MI in some28–31 but not all studies33. This increase in risk has been attributed to increased platelet reactivity32 and to endothelial dysfunction119. In the D:A:D study26, a difference in the risk of MI was noted between the widely used protease inhibitors, atazanavir and darunavir, with atazanavir being associated with a lower risk of MI than darunavir. This reduced risk of MI has been suggested to reflect a protective effect of atazanavir given the bilirubin-raising activity of the drug120. Overall, we emphasize that current ART regimens are associated with a much lower risk of CVD than older ART regimens.
Understanding the role of inflammation and inflammatory biomarkers in the general population with coronary heart disease has taken several decades to establish121,122, including ascertaining which markers have prognostic importance as opposed to being on the causal pathway. Although research on inflammatory biomarkers of the risk of CVD in HIV infection has been performed, much additional work is needed to identify the best biomarker in the setting of HIV infection along with the effect of HIV-related factors (including immune dysfunction) and traditional CVD risk factors. Identifying how ART, treatments for CVD and potential anti-inflammatory strategies change these inflammatory pathways and biomarkers will also be critical to advance the field. Of note, risk calculators used in the general population are inaccurate in patients infected with HIV and systematically underestimate risk123. Using non-biased methods, such as proteomics, which have been used in the general population, might be helpful to develop HIV-specific risk scores124.
Management of CVD risk in HIV infection
Dyslipidaemia.
The onset of HIV infection is associated with a decline in total cholesterol, LDL-C and HDL-C levels125. In a study comparing patients with HIV infection with matched uninfected controls, the group with HIV infection had lower HDL-C and LDL-C levels and higher triglyceride, CRP and IL-6 levels126. Because starting ART is now recommended at the time of initial HIV diagnosis, the lipid pattern of untreated HIV infection is seen only among individuals who live in resource-limited settings and, therefore, do not have access to ART. Of note, in a meta-analysis of 80 studies, the greatest risk of CVD in HIV infection was found to be in sub-Saharan Africa and the Asia Pacific regions18.
The effect of ART on blood lipid levels varies across the classes of ART drugs and between drugs within the same class, as broadly summarized in Table 4 (of note, this summary is not meant to be comprehensive). The effects of individual drugs are difficult to ascertain because HIV treatment typically requires three or four drugs. Generally, protease inhibitors, NRTIs and non-NRTIs (NNRTIs) all increase triglyceride levels and can increase LDL-C levels. LDL-C and triglyceride levels increase more with dual than with single protease inhibitor therapy.
Table 4 |.
Effect of antiretroviral therapies on blood lipid levels190
| Class | Drug | Effect on blood lipids | Refs |
|---|---|---|---|
| Protease inhibitors | Atazanavir | Increases HDL-C and decreases LDL-C levels | 191 |
| Darunavir | Increases HDL-C levels | 191 | |
| Fosamprenavir | Hypertriglyceridaemia | 192 | |
| Ritonavira | Increases HDL-C levels | 191 | |
| Saquinavir | Neutral | 192 | |
| Tipranavir | Dyslipidaemia | 192 | |
| NRTIs | Abacavir | Increases total cholesterol, LDL-C and HDL-C levels | 193 |
| Lamivudine | Increases total cholesterol, LDL-C and HDL-C levels | 193 | |
| Tenofovir fumarate disoproxil | Lowers LDL levels | 194 | |
| Zidovudine | Hypertriglyceridaemia | 194 | |
| NNRTIs | Efavirenz | Increases total cholesterol, LDL-C, HDL-C and triglyceride levels | 193 |
| Nevirapine | Neutral or decreases lipid levels | 195 | |
| Rilpivirine | Neutral | 193 | |
| Integrase inhibitors | Dolutegravir | Neutral | 133 |
| Raltegravir | Increases HDL levels | 191 |
HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; NNRTI, non-nucleoside reverse-transcriptase inhibitor ; NRTI, nucleoside reverse-transcriptase inhibitor.
Although ritonavir is a protease inhibitor, this drug is generally used as a pharmacokinetic enhancer.
Important differences have been described between protease inhibitors; in a report from the D:A:D study116, ritonavir and ritonavir-containing regimens increased triglyceride and LDL-C levels more, saquinavir caused less abnormally low HDL-C levels and nelfinavir was associated with fewer patients presenting with a high total cholesterol:HDL-C ratio compared with other protease inhibitors. In another study, LDL-C level increased by a mean of 2.0 mmol/l with initiation of ritonavir, 0.8 mmol/l with indinavir and 1.2 mmol/l with nelfinavir127. Ritonavir, but not the other two protease inhibitors, was associated with very elevated plasma triglyceride levels. Ritonavir can sometimes cause extreme hypertriglyceridaemia, with levels >10 mmol/l, and result in pancreatitis. Lower doses of ritonavir than those used in the study are now usually used, but hypertriglyceridaemia is also seen with combinations of ritonavir-saquinavir and ritonavir-lopinavir. Compared with these older protease inhibitors, use of atazanavir and darunavir is associated with more favourable lipid profiles128.
NNRTIs also increase LDL-C levels but do not decrease HDL-C levels13. Among NNRTIs, efavirenz was associated with slightly more patients developing hypercholesterolaemia and hypertriglyceridaemia than nevirapine116. Efavirenz has been associated with greater increases in total cholesterol and LDL-C but not total cholesterol:HDL-C ratio compared with the atazanavir-ritonavir combination129. Compared with the NNRTI rilpivirine, efavirenz is associated with higher total cholesterol, HDL-C, LDL-C and triglyceride levels130. The newer formulation of the NRTI tenofovir (tenofovir alafenamide) is associated with higher levels of both LDL-C and HDL-C, but similar total cholesterol:HDL-C ratio, compared with the older formulation (tenofovir disoproxil fumarate), which has a lipid-lowering effect131.
Newer ARTs, such as the integrase inhibitors, the CC-chemokine receptor 5 (CCR5)-co-receptor antagonist maraviroc and second-generation protease inhibitors, such as atazanavir, favourably affect lipid levels, especially compared with older ARTs, and are associated with improvements in surrogate markers of atherosclerosis, such as flow-mediated vasodilatation and carotid intima-media thickness132. The integrase inhibitors dolutegravir and raltegravir seem to affect blood lipids in a similar way133.
Across various studies, the prevalence of hyperlipidaemia among people living with HIV ranges from 28% to 80%, with hypertriglyceridaemia being the most common abnormality134. Intrinsic differences in study populations and the evolution of ART over time are probable explanations for this broad range. Most of the studies described here were of fairly short duration and were usually done in North American or European populations. However, ART is now initiated most often in people living in sub-Saharan Africa, where data defining the metabolic effects of treatment are scarce. In a 2018 meta-analysis of 14 trials and including a total of 21,023 individuals from this region, ART was associated with an increased risk of hypertriglyceridaemia (RR 2.05, 95% CI 1.51–2.77); however, no consistent associations were observed between ART and raised blood pressure, blood glucose levels, glycated haemoglobin (HbA1c) levels or other blood lipids135
In one study, hypertriglyceridaemia in patients with HIV infection was related to their higher intake of total fat, saturated fat and cholesterol compared with individuals without HIV infection136. Saturated fat intake was strongly correlated with triglyceride levels, suggesting that dietary modification to decrease saturated fat intake might be a logical approach to control high triglyceride levels in patients with HIV infection.
Until recently, guidelines for cholesterol management have not specifically addressed individuals living with HIV. The 2016 ESC/European Atherosclerosis Society (EAS) guidelines devote a short section to individuals with HIV infection and recommend dietary changes and exercise, as well as switching, when feasible, to a more ‘lipid-friendly’ ART137. The guidelines also state that statin therapy should be considered to achieve the target LDL-C level of 2.8 mmol/l, the same target that is recommended for other patients at high risk of CVD. The US National Lipid Association recommended considering HIV infection as an independent risk factor for selecting drug therapy to lower LDL-C levels138. The 2018 ACC/AHA guidelines state that HIV infection can be considered a CVD risk enhancer, which would favour starting moderate-intensity or high-intensity statin therapy139.
Many clinical trials have documented that LDL-C lowering, usually with statin therapy, reduces the risk of cardiovascular events across a broad spectrum of patients without HIV infection. Similar data are not yet available for people with HIV infection; however, the REPRIEVE trial140, launched in 2015, will address this issue. As previously noted, in a small, randomized trial in patients with HIV infection, atorvastatin significantly reduced non-calcified coronary plaque volume compared with placebo during a follow-up of 1 year53.
Drug–drug interactions are important considerations when initiating lipid-lowering drugs in patients infected with HIV. A systematic review of 18 statin trials in patients with HIV infection receiving ART demonstrated that statin therapy can be administered safely in this patient population141. Use of lovastatin and simvastatin with protease inhibitors is contraindicated owing to the risk of rhabdomyolysis from high statin levels in blood134. Use of ritonavir with protease inhibitors seems to increase the area under the curve for atorvastatin as well; therefore, guidelines from the Infectious Diseases Society of America suggest starting at lower doses of atorvastatin in individuals receiving protease inhibitor-based regimens142. Rosuvastatin levels in blood increase when used with atazanavir-ritonavir and lopinavir-ritonavir; therefore, the dose of rosuvastatin should be limited to 10 mg when used in combination with these drugs143. Pravastatin and fluvastatin are safer to use in combination with ART but do not lower LDL-C levels as much as atorvastatin or rosuvastatin. Pravastatin and fluvastatin were widely used after the introduction of ART but are less popular now owing to the growing realization, reflected in contemporary guidelines, that greater degrees of LDL-C lowering yield greater reductions in the risk of cardiovascular events144. Pitavastatin might be a good compromise for some people with HIV infection; this drug is metabolized via glucuronidation, thereby avoiding drug–drug interactions145, and, at higher doses, pitavastatin shows moderate LDL-C lowering. In one randomized study in patients with HIV infection, pitavastatin (4 mg per day) reduced LDL-C level by 31% and pravastatin (40 mg per day) reduced LDL-C level by 21% from baseline levels, with similarly low rates of adverse effects for both statin treatments146. According to a 2018 study, as many as 50% of individuals with HIV infection were eligible for statin therapy on the basis of at least one US guideline, but not all the eligible patients were prescribed statins147. Even when individuals are receiving statin therapy, those with HIV infection might not achieve as much lipid lowering as individuals without HIV infection; namely, a meta-analysis of studies in people living with HIV infection demonstrated that only a low percentage achieved the expected reduction in LDL-C level after initiation of statin therapy148. From 2007 to 2014, the percentage of individuals with HIV infection taking a contraindicated (owing to their ART) statin decreased; however, this decreasing trend was attenuated in 2015 owing to an increase in the use of the ART cobicistat149.
In individuals with HIV infection who do not tolerate statins, ezetimibe is a safe option, albeit with limited LDL-C lowering efficacy150. Ezetimibe can be used in addition to a maximally tolerated statin dose for individuals with HIV infection who are at a very high risk of CVD and do not achieve satisfactory LDL-C lowering with statin therapy alone. Use of bile acid sequestrants is not appropriate in the setting of HIV infection because these agents increase plasma triglyceride levels and their effect on the absorption of ARTs is unknown151. Individuals with HIV infection often have a high pill burden, and an add-on therapy might present additional challenges.
Switching ART to drugs that do not adversely affect blood lipids is worthwhile as long as viral suppression is maintained. Switching from older protease inhibitors to integrase inhibitors improves blood lipid levels, but at the cost of an increased rate of virological failure152 and, therefore, is not recommended for individuals with a history of virological failure. In another study of patients with HIV infection and high risk of CVD, continuing on a ritonavir-boosted protease inhibitor regimen or switching to dolutegravir (an integrase inhibitor) was associated with similar rates of virological failure after 48 weeks; however, total cholesterol, LDL-C and triglyceride levels all improved (P < 0.0001) in the dolutegravir group153. For those not already taking a statin, adding statin therapy is probably preferable to switching the type of ART; in one study, adding rosuvastatin (10 mg per day) was better tolerated and yielded better blood lipid results than switching154.
The common problem of hypertriglyceridaemia in patients with HIV infection should first be addressed by reducing intake of carbohydrates, including alcohol. When plasma triglyceride levels exceed 10 mmol/l, pancreatitis is a serious risk and immediate treatment is required, but lower levels of hypertriglyceridaemia are not benign because they probably increase the risk of CVD. Fibrates are widely used to lower triglyceride levels in people without HIV infection but have a drug–drug interaction with statins and some types of ART. A change in ART to drugs that induce less hypertriglyceridaemia is often the best approach.
Diabetes and the metabolic syndrome.
Whether HIV infection itself is associated with an increased risk of diabetes or whether the increased risk is related only to specific ART drugs has been controversial. Protease inhibitors and the thymidine analogue stavudine can cause insulin resistance118; however, these drugs are now not often used owing to their toxicity. In a large cohort study from Denmark, the risk of diabetes among patients with HIV infection was nearly triple that of the general population in 1996–1999, but this excess was absent in 1999–2010 (reF.155). Conversely, a cross-sectional study from sub-Saharan Africa published in 2018 reported a higher prevalence of diabetes and a HbA1c level of ≥6% in people living with HIV infection compared with uninfected controls156.
Lipodystrophy is a syndrome that results in central adiposity from fat accumulation in the dorsocervical region, increased or preserved visceral fat and peripheral fat loss157 and can be divided into lipoatrophy and lipohypertrophy158. Excess visceral adiposity is associated with insulin resistance among individuals with or without HIV infection159. Lipodystrophy develops in 20–35% of patients taking older protease inhibitors or the NRTIs didanosine or stavudine160,161; however, newer protease inhibitors, such as atazanavir, do not seem to cause lipodystrophy37,160,161. Lipodystrophy is often associated with insulin resistance, impaired glucose tolerance, hypertriglyceridaemia, low HDL-C levels and hypertension.
Reported rates of metabolic syndrome in people with HIV infection range from 8.5% to 52%, with rates at the lower end of this range seen in multicentre studies in which patients had less exposure to ART and rates at the higher end found in Latin American countries118. Metabolic syndrome usually develops in the first 3 years after starting an ART regimen that includes lopinavir-ritonavir or stavudine. According to most studies, metabolic syndrome is a predictor of cardiovascular events and death in people living with HIV infection, as in individuals without the infection118. Among the general population, development of chronic kidney disease is increased in the setting of metabolic syndrome162, and blood pressure is a component of metabolic syndrome using Adult Treatment Panel III (ATP III) criteria163.
The prevalence of hypertension and chronic kidney disease does not seem to be higher than normal in people with HIV infection, with the exception of a higher incidence of chronic kidney disease among patients exposed to some ART regimens164. Nevertheless, hypertension and prehypertension have been shown to be risk factors for cardiovascular events in people with HIV infection, just as in individuals without the infection165. Similarly, chronic kidney disease, defined as either albuminuria or a decreased glomerular filtration rate, is associated with an increased risk of cardiovascular events in patients with HIV infection166. Among 35,357 patients with HIV in the D:A:D cohort, lower glomerular filtration rate was strongly associated with a higher risk of CVD167.
Smoking.
The prevalence of cigarette smoking among individuals with HIV infection is extremely high. In a large cohort study from Denmark, nearly half of people with HIV infection smoked compared with one-fifth of people without HIV infection168. All-cause mortality was much higher among smokers than nonsmokers with HIV infection. For example, an individual aged 35 years and living with HIV infection had a median life expectancy of 62.6 years (95% CI 59.9–64.6 years) if a smoker and 78.4 years (95% CI 70.8–84.0 years) if a nonsmoker. More life-years were lost owing to smoking than to HIV (12.3 versus 5.1). The population-attributable risk of death due to smoking was 61.5% in individuals with HIV and 34.2% among people without the infection. These statistics emphasize the importance of stopping smoking for patients with HIV infection.
Smoking cessation programmes are reported to have the same modest success rates in people with HIV infection as has been reported in individuals without HIV infection. In a meta-analysis of eight trials including 1,822 patients with HIV who were smokers, behavioural interventions increased abstinence rates by half (relative risk 1.51, 95% CI 1.17–1.95)169, and a meta-analysis of smoking cessation interventions demonstrated that long-term outcomes were similar among individuals with or without HIV infection170. In one study, physicians trained to provide smoking cessation counselling and treatment for individuals living with HIV infection succeeded in significantly increasing smoking cessation rates and decreasing relapse rates171. Potential drug-drug interactions between ART and drugs for smoking cessation have not been well studied. Studies of varenicline, bupropion and nicotine-replacement therapy in people with HIV infection have generally been small, short and uncontrolled but have shown similar safety and success rates to reports in individuals without HIV infection172,173.
The effect of smoking cessation on the rates of subsequent cardiovascular events was reported for a large cohort in the D:A:D study174. At baseline, 11,951 participants (44%) were currently smoking and during the follow-up period, 69% of them stopped smoking at least once. The incidence RR for MI compared with never smokers decreased from 3.73 (95% CI 2.46–5.64) within the first year of smoking cessation to 3.00 (95% CI 1.84–4.88) within 1–2 years, to 2.62 (95% CI 1.42–4.83) at 2–3 years and to 2.07 (95% CI 1.19–3.63) after >3 years. Similar trends were seen for total coronary events and total cardiovascular events. Although cardiovascular event rates were still approximately double the rate in never smokers after 3 years, the rates were much lower than the rates in individuals who continued smoking, making smoking cessation a very desirable therapeutic goal.
Conclusions
In summary, atherosclerosis in the setting of HIV infection continues to be an important health concern that has implications on mortality, particularly in the future as this patient population continues to age. In the 20 years since the initial descriptions of MI were reported in patients with HIV infection175, ART has transformed HIV infection into a chronic disease condition, and progress has been made in determining the contribution of ART, chronic inflammation and immune activation as well as traditional CVD risk factors to this disease process. However, much work remains to elucidate further the underlying mechanisms for HIV-associated ASCVD as well as therapeutic strategies to lower the risk of CVD and treat CVD in patients with HIV infection, which will include implementation studies to improve outcomes and CVD management for people living with HIV infection.
CD4+ T cell counts.
T cell subset that has a role in the immune system response against pathogens, infections and illnesses. a normal CD4+ T cell count is 500–1,500 cells per μl of plasma. CD4+ T cells are the main target cell of HIV, and the CD4+ T cell count is used to monitor the status of the HIV infection and the efficacy of the antiretroviral therapy.
Nadir CD4+ T cell count.
The lowest CD4+ T cell count an individual has had, which serves as a marker for immunodeficiency.
Immunosenescence.
Changes to the immune system that can be associated with age.
Viraemia.
Presence of viral particles in the blood.
Latent HIV infection.
A dormant or non-replicative HIV infection within a cell; in this state, the virus is not actively infecting other cells and individuals do not usually have noticeable symptoms.
Virological failure.
Refers to the failure of the HIV treatment to supress the virus completely; the virus is detectable in the blood (>200 copies per ml). This failure can occur as a result of drug resistance, drug toxicity or noncompliance with antiretroviral therapy.
Key points.
As improvements to antiretroviral therapies have led to better control of HIV infection (although not cured it), individuals with HIV infection are now ageing, and cardiovascular disease is an important health concern in this patient population.
Traditional risk factors including dyslipidaemia, hypertension, cigarette smoking, diabetes mellitus and metabolic syndrome are common among people with HIV infection and increase the risk of cardiovascular disease.
In addition to traditional risk factors, characteristics related to HIV infection, including low CD4+ T cell count, nadir CD4+ T cell count and viral detectability, and some antiretroviral therapies are independently associated with increased risk of cardiovascular disease.
In the setting of treated suppressed HIV replication, chronic inflammation and immune activation persist and are strongly predictive of mortality and cardiovascular events.
Potential strategies to reduce the risk of cardiovascular disease in patients with HIV infection include targeting traditional risk factors, initiation of antiretroviral therapy to reduce inflammation and other approaches to lower inflammation, including gut-related interventions, statin therapy and immune modulators.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Redfield MM Heart failure with preserved ejection fraction. N. Engl. J. Med 375, 1868–1877 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Smit M et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect. Dis 15, 810–818 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng ZH et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J. Am. Coll. Cardiol 59, 1891–1896 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freiberg MS et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern. Med 173, 614–622 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow FC et al. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J. Acquir. Immune Defic. Syndr 60, 351–358 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freiberg MS et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol 2, 536–546 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeks SG, Lewin SR & Havlir DV The end of AIDS: HIV infection as a chronic disease. Lancet 382, 1525–1533 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanni MV et al. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS 27, 1263–1272 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Ascenzo F et al. High prevalence at computed coronary tomography of non-calcified plaques in asymptomatic HIV patients treated with HAART: a meta-analysis. Atherosclerosis 240, 197–204 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Crane HM et al. Types of myocardial infarction among human immunodeficiency virus-infected individuals in the United States. JAMA Cardiol 2, 260–267 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsue PY et al. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation 109, 316–319 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Currie PF et al. Heart muscle disease related to HIV infection: prognostic implications. BMJ 309, 1605–1607 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsue PY & Waters DD Heart failure in persons living with HIV infection. Curr. Opin. HIV AIDS 12, 534–539 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Eisenberg MJ, Gordon AS & Schiller NB HIV-associated pericardial effusions. Chest 102, 956–958 (1992). [DOI] [PubMed] [Google Scholar]
- 15.Remick J et al. Heart failure in patients with human immunodeficiency virus infection: epidemiology, pathophysiology, treatment, and future research. Circulation 129, 1781–1789 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanuma J et al. Dilated cardiomyopathy in an adult human immunodeficiency virus type 1-positive patient treated with a zidovudine-containing antiretroviral regimen. Clin. Infect. Dis 37, e109–e111 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Alvi RM et al. Protease inhibitors and cardiovascular outcomes in patients with HIV and heart failure. J. Am. Coll. Cardiol 72, 518–530 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah ASV et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV. Circulation 138, 1100–1112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsue PY & Waters DD Time to recognize HIV infection as a major cardiovascular risk factor. Circulation 138, 1113–1115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paisible AL et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J. Acquir. Immune Defic. Syndr 68, 209–216 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Womack JA et al. HIV infection and cardiovascular disease in women. J. Am. Heart Assoc 3, e001035 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benjamin LA et al. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: a case-control study. Neurology 86, 324–333 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamin LA et al. The role of human immunodeficiency virus-associated vasculopathy in the etiology of stroke. J. Infect. Dis 216, 545–553 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry K, Melroe H, Huebesch J, Hermundson J & Simpson J Atorvastatin and gemfibrozil for protease-inhibitor-related lipid abnormalities. Lancet 352, 1031–1032 (1998). [DOI] [PubMed] [Google Scholar]
- 25.Friis-Moller N et al. Class of antiretroviral drugs and the risk of myocardial infarction. N. Engl. J. Med 356, 1723–1735 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Monforte A et al. Atazanavir is not associated with an increased risk of cardio-or cerebrovascular disease events. AIDS 27, 407–415 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Ryom L et al. Cardiovascular disease and use of contemporary protease inhibitors: the D:A:D international prospective multicohort study.Lancet HIV 5, e291–e300 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Sabin CA et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet 371, 1417–1426 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabin CA et al. Is there continued evidence for an association between abacavir usage and myocardial infarction risk in individuals with HIV? A cohort collaboration. BMC Med 14, 61 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcus JL et al. Use of abacavir and risk of cardiovascular disease among HIV-infected individuals. J. Acquir. Immune Defic. Syndr 71, 413–419 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Elion RA et al. Recent abacavir use increases risk of type 1 and type 2 myocardial infarctions among adults with HIV. J. Acquir. Immune Defic. Syndr 78, 62–72 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez A et al. Cardiovascular toxicity of abacavir: a clinical controversy in need of a pharmacological explanation. AIDS 31, 1781–1795 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Ding X et al. No association of abacavir use with myocardial infarction: findings of an FDA meta-analysis. J. Acquir. Immune Defic. Syndr 61, 441–447 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Saag MS et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA 320, 379–396 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundgren JD et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N. Engl. J. Med 373, 795–807 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker JV et al. Early antiretroviral therapy at high CD4 counts does not improve arterial elasticity: a substudy of the strategic timing of antiretroviral treatment (START) trial. Open Forum Infect. Dis 3, ofw213 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker JV et al. Changes in cardiovascular disease risk factors with immediate versus deferred antiretroviral therapy initiation among HIV-positive participants in the START (Strategic Timing of Antiretroviral Treatment) trial. J. Am. Heart Assoc 6, e004987 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein DB et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin. Infect. Dis 60, 1278–1280 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Delaney JA et al. Cumulative human immunodeficiency viremia, antiretroviral therapy, and incident myocardial infarction. Epidemiology 30, 69–74 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shahmanesh M et al. The cardiovascular risk management for people living with HIV in Europe: how well are we doing? AIDS 30, 2505–2518 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen LD et al. Myocardial infarction among Danish HIV-infected individuals: population-attributable fractions associated with smoking. Clin. Infect. Dis 60, 1415–1423 (2015). [DOI] [PubMed] [Google Scholar]
- 42.David MH, Hornung R & Fichtenbaum CJ Ischemic cardiovascular disease in persons with human immunodeficiency virus infection. Clin. Infect. Dis 34, 98–102 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Matetzky S et al. Acute myocardial infarction in human immunodeficiency virus–infected patients. Arch. Intern. Med 163, 457–460 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Escaut L et al. Coronary artery disease in HIV infected patients. Intensive Care Med. 29, 969–973 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Mehta NJ & Khan IA HIV-associated coronary artery disease. Angiology 54, 269–275 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Ambrose JA et al. Frequency of and outcome of acute coronary syndromes in patients with human immunodeficiency virus infection. Am. J. Cardiol 92, 301–303 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Varriale P, Saravi G, Hernandez E & Carbon F Acute myocardial infarction in patients infected with human immunodeficiency virus. Am. Heart J 147, 55–59 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Ren X et al. Comparison of outcomes using bare metal versus drug-eluting stents in coronary artery disease patients with and without human immunodeficiency virus infection. Am. J. Cardiol 104, 216–222 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Schneider S et al. Association of increased CD8+ and persisting C-reactive protein levels with restenosis in HIV patients after coronary stenting. AIDS 30, 1413–1421 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Marcus JL et al. Recurrence after hospitalization for acute coronary syndrome among HIV-infected and HIV-uninfected individuals. HIV Med 20, 19–26 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Dwyer EJ et al. Lower coronary plaque burden in patients with HIV presenting with acute coronary syndrome. Open Heart 3, e000511 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peyracchia M et al. Evaluation of coronary features of HIV patients presenting with ACS: the CUORE, a multicenter study. Atherosclerosis 274, 218–226 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Lo J et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2, e52–e63 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas GP et al. Associations between antiretroviral use and subclinical coronary atherosclerosis. AIDS 30, 2477–2486 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Post WS et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann. Intern. Med 160, 458–467 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Metkus TS et al. HIV infection is associated with an increased prevalence of coronary noncalcified plaque among participants with a coronary artery calcium score of zero: multicenter AIDS Cohort Study (MACS). HIV Med 16, 635–639 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deeks SG et al. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat. Med 22, 839–850 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krikke M et al. The role of T cells in the development of cardiovascular disease in HIV-infected patients. Atherosclerosis 237, 92–98 (2014). [DOI] [PubMed] [Google Scholar]
- 59.Wang T et al. Increased cardiovascular disease risk in the HIV-positive population on ART: potential role of HIV-Nef and Tat. Cardiovasc. Pathol 24, 279–282 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ehrenreich H et al. Potent stimulation of monocytic endothelin-1 production by HIV-1 glycoprotein 120. J. Immunol 150, 4601–4609 (1993). [PubMed] [Google Scholar]
- 61.Hsue PY et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation 109, 1603–1608 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Ho JE et al. The association of CD4+ T-cell counts and cardiovascular risk in treated HIV disease. AIDS 26, 1115–1120 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lichtenstein KA et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin. Infect. Dis 51, 435–447 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Triant VA et al. Association of immunologic and virologic factors with myocardial infarction rates in a U. S. Health Care System. J. Acquir. Immune Defic. Syndr 55, 615–619 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Drozd DR et al. Increased risk of myocardial infarction in HIV-infected individuals in North America compared with the general population. J. Acquir. Immune Defic. Syndr 75, 568–576 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Serrano-Villar S et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+T cell activation, and increased risk of non-AIDS morbidity and mortality. PLOS Pathog. 10, e1004078 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serrano-Villar S et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLOS ONE 9, e85798 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trickey A et al. CD4: CD8 ratio and CD8 count as prognostic markers for mortality in human immunodeficiency virus–infected patients on antiretroviral therapy: the Antiretroviral Therapy Cohort Collaboration (ART-CC). Clin. Infect. Dis 65, 959–966 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hanna DB et al. Trends in cardiovascular disease mortality among persons with HIV in New York City, 2001–2012. Clin. Infect. Dis 63, 1122–1129 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hunt PW et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J. Infect. Dis 197, 126–133 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Naeger DM et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLOS ONE 5, e8886 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Christensen-Quick A, Vanpouille C, Lisco A & Gianella S Cytomegalovirus and HIV persistence: pouring gas on the fire. AIDS Res. Hum. Retroviruses 33, S23–S30 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hsue PY et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T cell responses. AIDS 20, 2275–2283 (2006). [DOI] [PubMed] [Google Scholar]
- 74.Masia M et al. Increased carotid intima-media thickness associated with antibody responses to varicella-zoster virus and cytomegalovirus in HIV-infected patients. PLOS ONE 8, e64327 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knudsen A et al. Coronary artery calcium and intima-media thickness are associated with level of cytomegalovirus immunoglobulin G in HIV-infected patients. HIV Med 20, 60–62 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Johansson I et al. Cytomegalovirus infection and disease reduce 10-year cardiac allograft vasculopathy-free survival in heart transplant recipients. BMC Infect. Dis 15, 582 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tincati C, Douek DC & Marchetti G Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res. Ther 13, 19 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marchetti G et al. Microbial translocation predicts disease progression of HIV-isnfected antiretroviral-naive patients with high CD4+cell count. AIDS 25, 1385–1394 (2011). [DOI] [PubMed] [Google Scholar]
- 79.Sandler NG et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J. Infect. Dis 203, 780–790 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reus Bañuls S et al. Association between inflammatory markers and microbial translocation in patients with human immunodeficiency virus infection taking antiretroviral treatment [Spanish]. Med. Clin. (Barc.) 142, 47–52 (2014). [DOI] [PubMed] [Google Scholar]
- 81.Sandler NG et al. Sevelamer does not decrease lipopolysaccharide or soluble CD14 levels but decreases soluble tissue factor, low-density lipoprotein (LDL) cholesterol, and oxidized LDL cholesterol levels in individuals with untreated HIV infection. J. Infect. Dis 210, 1549–1554 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ishizaki A et al. Effects of short-term probiotic ingestion on immune profiles and microbial translocation among HIV-1-infected Vietnamese children. Int. J. Mol. Sci 18, 2185 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Somsouk M et al. The immunologic effects of mesalamine in treated HIV-infected individuals with incomplete CD4+ T cell recovery: a randomized crossover trial. PLOS ONE 9, e116306 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuller LH et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLOS Med 5, e203 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borges AH et al. Interleukin 6 is a stronger predictor of clinical events than high-sensitivity C-reactive protein or D-dimer during HIV infection. J. Infect. Dis 214, 408–416 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nordell AD et al. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J. Am. Heart Assoc 3, e000844 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Subramanian S et al. Arterial inflammation in patients with HIV. JAMA 308, 379–386 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tawakol A et al. Association of arterial and lymph node inflammation with distinct inflammatory pathways in human immunodeficiency virus infection. JAMA Cardiol 2, 163–171 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ridker PM Residual inflammatory risk: addressing the obverse side of the atherosclerosis prevention coin. Eur. Heart J 37, 1720–1722 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Fichtenbaum CJ, Yeh T-M, Evans SR & Aberg JA Treatment with pravastatin and fenofibrate improves atherogenic lipid profiles but not inflammatory markers in ACTG 5087. J. Clin. Lipidol 4, 279–287 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Toribio M et al. Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in HIV. AIDS 31, 797–806 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kelesidis T et al. Changes in inflammation and immune activation with atazanavir-, raltegravir-, darunavir-based initial antiviral therapy: ACTG 5260s. Clin. Infect. Dis 61, 651–660 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim CJ et al. Impact of intensified antiretroviral therapy during early HIV infection on gut immunology and inflammatory blood biomarkers. AIDS 31, 1529–1534 (2017). [DOI] [PubMed] [Google Scholar]
- 94.Hatano H et al. A randomized controlled trial assessing the effects of raltegravir intensification on endothelial function in treated HIV infection. J. Acquir. Immune Defic. Syndr 61, 317–325 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O’Brien MP et al. A randomized placebo controlled trial of aspirin effects on immune activation in chronically human immunodeficiency virus infected adults on virologically suppressive antiretroviral therapy. Open Forum Infect. Dis 4, ofw278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fahey JL et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N. Engl. J. Med 322, 166–172 (1990). [DOI] [PubMed] [Google Scholar]
- 97.Tenorio AR et al. Soluble markers of inflammation and coagulation but not T cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J. Infect. Dis 210, 1248–1259 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Longenecker CT et al. Markers of inflammation and CD8 T cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 14, 385–390 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaplan RC et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J. Infect. Dis 203, 452–463 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sinha A et al. Role of T-cell dysfunction, inflammation, and coagulation in microvascular disease in HIV. J. Am. Heart Assoc 5, e004243 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ridker PM et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med 377, 1119–1131 (2017). [DOI] [PubMed] [Google Scholar]
- 102.Ridker PM et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet 391, 319–328 (2018). [DOI] [PubMed] [Google Scholar]
- 103.Ridker PM et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur. Heart J 39, 3499–3507 (2018). [DOI] [PubMed] [Google Scholar]
- 104.Hsue PY et al. IL-1beta inhibition reduces atherosclerotic inflammation in HIV infection. J. Am. Coll. Cardiol 72, 2809–2811 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hsue PY et al. Safety and impact of low-dose methotrexate on endothelial function and inflammation in individuals with treated human immunodeficiency virus: AIDS Clinical Trials Group Study A5314. Clin. Infect. Dis 10.1093/cid/ciy781 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stein JH et al. Brachial artery echogenicity and grayscale texture changes in HIV-infected individuals receiving low-dose methotrexate. Arterioscler. Thromb. Vasc. Biol 38, 2870–2878 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grund B et al. Relevance of interleukin-6 and D-dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLOS ONE 11, e0155100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Swirski FK & Nahrendorf M Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat. Rev. Immunol 18, 733–744 (2018). [DOI] [PubMed] [Google Scholar]
- 109.Deeks SG, Overbaugh J, Phillips A & Buchbinder S HIV infection. Nat. Rev. Dis. Primers 1, 15035 (2015). [DOI] [PubMed] [Google Scholar]
- 110.Campbell JH, Hearps AC, Martin GE, Williams KC & Crowe SM The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. AIDS 28, 2175–2187 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wacleche VS, Tremblay CL, Routy JP & Ancuta P The biology of monocytes and dendritic cells: contribution to HIV pathogenesis. Viruses 10, E65 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Walker JA et al. Elevated numbers of CD163+ macrophages in hearts of simian immunodeficiency virus-infected monkeys correlate with cardiac pathology and fibrosis. AIDS Res. Hum. Retroviruses 30, 685–694 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Walker JA et al. Anti-alpha4 integrin antibody blocks monocyte/macrophage traffic to the heart and decreases cardiac pathology in a SIV infection model of AIDS. J. Am. Heart Assoc 4, e001932 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Scherzer R et al. Association of biomarker clusters with cardiac phenotypes and mortality in patients with HIV infection. Circ. Heart Fail 11, e004312 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leyes P et al. Increased cholesterol absorption rather than synthesis is involved in boosted protease inhibitor-associated hypercholesterolaemia. AIDS 32, 1309–1316 (2018). [DOI] [PubMed] [Google Scholar]
- 116.Fontas E et al. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different antiretroviral drugs associated with different lipid profiles? J. Infect. Dis 189, 1056–1074 (2004). [DOI] [PubMed] [Google Scholar]
- 117.Worm SW et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J. Infect. Dis 201, 318–330 (2010). [DOI] [PubMed] [Google Scholar]
- 118.Nix LM & Tien PC Metabolic syndrome, diabetes, and cardiovascular risk in HIV. Curr. HIV/AIDS Rep 11, 271–278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hsue PY et al. Association of abacavir and impaired endothelial function in treated and suppressed HIV-infected patients. AIDS 23, 2021–2027 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marconi VC et al. Bilirubin is inversely associated with cardiovascular disease among HIV-positive and HIV-negative individuals in VACS (Veterans Aging Cohort Study). J. Am. Heart Assoc 7, e007792 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maseri A Inflammation, atherosclerosis, and ischemic events — exploring the hidden side of the moon. N. Engl. J. Med 336, 1014–1016 (1997). [DOI] [PubMed] [Google Scholar]
- 122.Hansson GK Inflammation and atherosclerosis: the end of a controversy. Circulation 136, 1875–1877 (2017). [DOI] [PubMed] [Google Scholar]
- 123.Triant VA et al. Cardiovascular risk prediction functions underestimate risk in HIV infection. Circulation 137, 2203–2214 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ganz P et al. Development and validation of a protein-based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA 315, 2532–2541 (2016). [DOI] [PubMed] [Google Scholar]
- 125.Riddler SA et al. Impact of HIV infection and HAART on serum lipids in men. JAMA 289, 2978–2982 (2003). [DOI] [PubMed] [Google Scholar]
- 126.Fourie CM, Van Rooyen JM, Kruger A & Schutte AE Lipid abnormalities in a never-treated HIV-1 subtype C-infected African population. Lipids 45, 73–80 (2010). [DOI] [PubMed] [Google Scholar]
- 127.Periard D et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation 100, 700–705 (1999). [DOI] [PubMed] [Google Scholar]
- 128.Ucciferri C et al. Improved metabolic profile after switch to darunavir/ritonavir in HIV positive patients previously on protease inhibitor therapy. J. Med. Virol 85, 755–759 (2013). [DOI] [PubMed] [Google Scholar]
- 129.Daar ES et al. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann. Intern. Med 154, 445–456 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Molina JM et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet 378, 238–246 (2011). [DOI] [PubMed] [Google Scholar]
- 131.Sax PE et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J. Acquir. Immune Defic. Syndr 67, 52–58 (2014). [DOI] [PubMed] [Google Scholar]
- 132.Srinivasa S & Grinspoon SK Metabolic and body composition effects of newer antiretrovirals in HIV-infected patients. Eur. J. Endocrinol 170, R185–R102 (2014). [DOI] [PubMed] [Google Scholar]
- 133.Raffi F et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 381, 735–743 (2013). [DOI] [PubMed] [Google Scholar]
- 134.Libby P, Bonow RO, Mann DL & Zipes DP Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, 2-Volume Set (Elsevier Health Sciences, 2007). [Google Scholar]
- 135.Ekoru K et al. HIV treatment is associated with a two-fold higher probability of raised triglycerides: pooled analyses in 21 023 individuals in sub-Saharan Africa. Glob. Health Epidemiol. Genom 3, e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Joy T et al. Dietary fat intake and relationship to serum lipid levels in HIV-infected patients with metabolic abnormalities in the HAART era. AIDS 21, 1591–1600 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Landmesser U et al. 2017 update of ESC/EAS Task Force on practical clinical guidance for proprotein convertase subtilisin/kexin type 9 inhibition in patients with atherosclerotic cardiovascular disease or in familial hypercholesterolaemia. Eur. Heart J 39, 1131–1143 (2017). [DOI] [PubMed] [Google Scholar]
- 138.Jacobson TA NLA task force on statin safety-2014 update. J. Clin. Lipidol 8, S1–S4 (2014). [DOI] [PubMed] [Google Scholar]
- 139.Grundy SM et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J. Am. Coll. Cardiol 10.1016/j.jacc.2018.11.002 (2018). [DOI] [Google Scholar]
- 140.Gilbert JM, Fitch KV & Grinspoon SK HIV-related cardiovascular disease, statins, and the REPRIEVE trial. Top. Antivir. Med 23, 146–149 (2015). [PMC free article] [PubMed] [Google Scholar]
- 141.Feinstein MJ, Achenbach CJ, Stone NJ & Lloyd-Jones DMA Systematic review of the usefulness of statin therapy in HIV-infected patients. Am. J. Cardiol 115, 1760–1766 (2015). [DOI] [PubMed] [Google Scholar]
- 142.Dube MP et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin. Infect. Dis 37, 613–627 (2003). [DOI] [PubMed] [Google Scholar]
- 143.Busti AJ et al. Effects of atazanavir/ritonavir or fosamprenavir/ritonavir on the pharmacokinetics of rosuvastatin. J. Cardiovasc. Pharmacol 51, 605–610 (2008). [DOI] [PubMed] [Google Scholar]
- 144.Waters DD & Boekholdt SM An evidence-based guide to cholesterol-lowering guidelines. Can. J. Cardiol 33, 343–349 (2017). [DOI] [PubMed] [Google Scholar]
- 145.Mosepele M, Molefe-Baikai OJ, Grinspoon SK & Triant VA Benefits and risks of statin therapy in the HIV-infected population. Curr. Infect. Dis. Rep 20, 20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aberg JA et al. Pitavastatin versus pravastatin in adults with HIV-1 infection and dyslipidaemia (INTREPID): 12 week and 52 week results of a phase 4, multicentre, randomised, double-blind, superiority trial. Lancet HIV 4, e284–e294 (2017). [DOI] [PubMed] [Google Scholar]
- 147.Levy ME, Greenberg AE, Magnus M, Younes N & Castel A Evaluation of statin eligibility, prescribing practices, and therapeutic responses using ATP III, ACC/AHA, and NLA dyslipidemia treatment guidelines in a large urban cohort of HIV-infected outpatients. AIDS Patient Care STDS 32, 58–69 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Burkholder GA et al. Low-density lipoprotein cholesterol response after statin initiation among persons living with human immunodeficiency virus. J. Clin. Lipidol 12, 988–998 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rosenson RS, Colantonio LD, Burkholder GA, Chen L & Muntner P Trends in utilization of statin therapy and contraindicated statin use in HIV-infected adults treated with antiretroviral therapy from 2007 through 2015. J. Am. Heart Assoc 7, e010345 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wohl DA et al. Ezetimibe alone reduces low-density lipoprotein cholesterol in HIV-infected patients receiving combination antiretroviral therapy. Clin. Infect. Dis 47, 1105–1108 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.[No authors listed]. Corrigendum to: ‘2016 ESC/EAS Guidelines for the Management of Dyslipidaemias’. Eur. Heart J 39, 1254 (2018). [DOI] [PubMed] [Google Scholar]
- 152.Eron JJ et al. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trials. Lancet 375, 396–407 (2010). [DOI] [PubMed] [Google Scholar]
- 153.Gatell JM et al. Switching from a ritonavir-boosted protease inhibitor to a dolutegravir-based regimen for maintenance of HIV viral suppression in patients with high cardiovascular risk. AIDS 31, 2503–2514 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee FJ et al. Rosuvastatin versus protease inhibitor switching for hypercholesterolaemia: a randomized trial. HIV Med 17, 605–614 (2016). [DOI] [PubMed] [Google Scholar]
- 155.Rasmussen LD et al. Risk of diabetes mellitus in persons with and without HIV: a Danish nationwide population-based cohort study. PLOS ONE 7, e44575 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mathabire Rücker SC et al. High rates of hypertension, diabetes, elevated low-density lipoprotein cholesterol, and cardiovascular disease risk factors in HIV-infected patients in Malawi. AIDS 32, 253–260 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lake JE & Currier JS Metabolic disease in HIV infection. Lancet Infect. Dis 13, 964–975 (2013). [DOI] [PubMed] [Google Scholar]
- 158.Mirza FS, Luthra P & Chirch L Endocrinological aspects of HIV infection. J. Endocrinol. Invest 41, 881–899 (2018). [DOI] [PubMed] [Google Scholar]
- 159.Grunfeld C et al. Association of upper trunk and visceral adipose tissue volume with insulin resistance in control and HIV-infected subjects in the FRAM study. J. Acquir. Immune Defic. Syndr 46, 283–290 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Martinez E et al. Risk of lipodystrophy in HIV-1-infected patients treated with protease inhibitors: a prospective cohort study. Lancet 357, 592–598 (2001). [DOI] [PubMed] [Google Scholar]
- 161.Rakotoambinina B et al. Lipodystrophic syndromes and hyperlipidemia in a cohort of HIV-1-infected patients receiving triple combination antiretroviral therapy with a protease inhibitor. J. Acquir. Immune Defic. Syndr 27, 443–449 (2001). [DOI] [PubMed] [Google Scholar]
- 162.Kurella M, Lo JC & Chertow GM Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J. Am. Soc. Nephrol 16, 2134–2140 (2005). [DOI] [PubMed] [Google Scholar]
- 163.Grundy SM et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112, 2735–2752 (2005). [DOI] [PubMed] [Google Scholar]
- 164.Mocroft A et al. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV-positive individuals with a normal baseline estimated glomerular filtration rate: a prospective international cohort study. Lancet HIV 3, e23–e32 (2016). [DOI] [PubMed] [Google Scholar]
- 165.Armah KA et al. Prehypertension, hypertension, and the risk of acute myocardial infarction in HIV-infected and -uninfected veterans. Clin. Infect. Dis 58, 121–129 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Choi AI et al. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation 121, 651–658 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ryom L et al. Renal impairment and cardiovascular disease in HIV-positive individuals: the D:A:D study. J. Infect. Dis 214, 1212–1220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Helleberg M et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin. Infect. Dis 56, 727–734 (2013). [DOI] [PubMed] [Google Scholar]
- 169.Keith A, Dong Y, Shuter J & Himelhoch S Behavioral interventions for tobacco use in HIV-infected smokers: a meta-analysis. J. Acquir. Immune Defic. Syndr 72, 527–533 (2016). [DOI] [PubMed] [Google Scholar]
- 170.Pool ER, Dogar O, Lindsay RP, Weatherburn P & Siddiqi K Interventions for tobacco use cessation in people living with HIV and AIDS. Cochrane Database Syst. Rev 6, CD011120 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Huber M et al. Outcome of smoking cessation counselling of HIV-positive persons by HIV care physicians. HIV Med 13, 387–397 (2012). [DOI] [PubMed] [Google Scholar]
- 172.Cui Q et al. Safety and tolerability of varenicline tartrate (Champix(®)/Chantix(®)) for smoking cessation in HIV-infected subjects: a pilot open-label study. AIDS Patient Care STDS 26, 12–19 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Balfour L et al. An HIV-tailored quit-smoking counselling pilot intervention targeting depressive symptoms plus nicotine replacement therapy. AIDS Care 29, 24–31 (2017). [DOI] [PubMed] [Google Scholar]
- 174.Petoumenos K et al. Rates of cardiovascular disease following smoking cessation in patients with HIV infection: results from the D:A:D study(*). HIV Med 12, 412–421 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Behrens G, Schmidt H, Meyer D, Stoll M & Schmidt RE Vascular complications associated with use of HIV protease inhibitors. Lancet 351, 1958 (1998). [DOI] [PubMed] [Google Scholar]
- 176.Himelman RB, Chung WS, Chernoff DN, Schiller NB & Hollander H Cardiac manifestations of human immunodeficiency virus infection: a two-dimensional echocardiographic study. J. Am. Coll. Cardiol 13, 1030–1036 (1989). [DOI] [PubMed] [Google Scholar]
- 177.Vittecoq D, Escaut L & Monsuez JJ Vascular complications associated with use of HIV protease inhibitors. Lancet 351, 1959 (1998). [DOI] [PubMed] [Google Scholar]
- 178.Holmberg SD et al. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet 360, 1747–1748 (2002). [DOI] [PubMed] [Google Scholar]
- 179.Fletcher CV Overview of antiretroviral agents used to treat HIV. UpToDate https://www.uptodate.com/contents/overview-of-antiretroviral-agents-used-to-treat-hiv (2018). [Google Scholar]
- 180.Bozzette SA, Ake CF, Tam HK, Chang SW & Louis TA Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N. Engl. J. Med 348, 702–710 (2003). [DOI] [PubMed] [Google Scholar]
- 181.Friis-Moller N et al. Combination antiretroviral therapy and the risk of myocardial infarction. N. Engl. J. Med 349, 1993–2003 (2003). [DOI] [PubMed] [Google Scholar]
- 182.El-Sadr WM et al. CD4+ count-guided interruption of antiretroviral treatment. N. Engl. J. Med 355, 2283–2296 (2006). [DOI] [PubMed] [Google Scholar]
- 183.Stein JH et al. A prospective, randomized clinical trial of antiretroviral therapies on carotid wall thickness: AIDS Clinical Trial Group Study A5260s. AIDS 29, 1775–1783 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N. Engl. J. Med 373, 795–807 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Duprez DA et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLOS ONE 7, e44454 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tien PC et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J. Acquir. Immune Defic. Syndr 55, 316–322 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.So-Armah KA et al. Do biomarkers of inflammation, monocyte activation, and altered coagulation explain excess mortality between HIV infected and uninfected people? J. Acquir. Immune Defic. Syndr 72, 206–213 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wada NI et al. Inflammatory biomarkers and mortality risk among HIV-suppressed men: a multisite prospective cohort study. Clin. Infect. Dis 63, 984–990 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Baker JV et al. Systemic inflammation, coagulation, and clinical risk in the START trial. Open Forum Infect. Dis 4, ofx262 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.U.S. Department of Health and Human Services. FDA-approved HIV medicines. AIDSinfo https://aidsinfo.nih.gov/understanding-hiv-aids/fact-sheets/21/58/fda-approved-hiv-medicines (updated 12 Apr 2019). [Google Scholar]
- 191.Ofotokun I et al. Comparison of the metabolic effects of ritonavir-boosted darunavir or atazanavir versus raltegravir, and the impact of ritonavir plasma exposure: ACTG 5257. Clin. Infect. Dis 60, 1842–1851 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bergersen BM Cardiovascular risk in patients with HIV Infection: impact of antiretroviral therapy. Drugs 66, 1971–1987 (2006). [DOI] [PubMed] [Google Scholar]
- 193.Tebas P et al. Lipid levels and changes in body fat distribution in treatment-naive, HIV-1-infected adults treated with rilpivirine or efavirenz for 96 weeks in the ECHO and THRIVE trials. Clin. Infect. Dis 59, 425–434 (2014). [DOI] [PubMed] [Google Scholar]
- 194.Crane HM et al. Impact of NRTIs on lipid levels among a large HIV-infected cohort initiating antiretroviral therapy in clinical care. AIDS 25, 185–195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Maggi P et al. Cardiovascular risk factors in patients on long-term treatment with nevirapine-or efavirenz-based regimens. J. Antimicrob. Chemother 66, 896–900 (2011). [DOI] [PubMed] [Google Scholar]


