Abstract
Purpose:
Based on synergistic effects between green tea polyphenon E (PPE) and epidermal growth factor receptor-tyrosine kinase inhibitor in preclinical studies, we conducted a phase 1b study of the PPE and erlotinib combination in patients with advanced premalignant lesions (APL) of the oral cavity and larynx.
Patients and Methods:
Patients were treated with a fixed dose of PPE (200mg 3x day) and dose escalation of erlotinib (50, 75, 100 mg daily) for 6-months (6-M) with tissue biopsy at baseline and 6-M. Primary endpoints were safety and toxicity, secondary endpoints were evaluation of pathologic response, cancer free survival (CFS), overall survival (OS) and biomarker modulation.
Results:
Among 21 enrolled patients, 19 began treatment and 17 completed 6 months of treatment with PPE and erlotinib. Main characteristics of treated patients: 15 severe dysplasia or carcinoma in situ; 17 oral cavity. Only skin rash was associated with DLT and MTD. Recommended doses for phase 2 studies are PPE 600mg daily plus erlotinib 100mg daily for 6-M. Pathologic responses in 17 evaluable patients: pCR (47%) and pPR (18%). The 5-year CFS and OS were 66.3% and 93%, respectively. Among tested biomarkers, only pERK was correlated with response to treatment.
Conclusion:
Treatment with PPE and erlotinib combination was well tolerated in patients with APLs of the head and neck, and showed a high rate of pathologic response with excellent CFS. This combination deserves further investigation for the chemoprevention and/or prevention of second primary tumors in early stage head and neck cancer.
Keywords: Chemoprevention, green tea PPE, erlotinib, premalignant lesions, phase 1b
Introduction
Squamous cell carcinoma of the head and neck (SCCHN) is the sixth most common cancer worldwide. In the United States, head neck cancer accounts for 3% of all cancers, with approximately 60,000 new cases and more than 12,000 deaths estimated in 2020 (1). Despite advances in conventional therapies, including surgery, radiation, and chemotherapy, molecularly targeted agents, and more recently developed immunotherapy, the overall survival rate for SCCHN has only marginally improved in the past three decades (2, 3). Thus, the development of effectively preventive approaches is highly desirable to reduce the incidence and mortality of SCCHN.
The most studied agents for chemoprevention of head and neck cancer are vitamin A and its analogs. In a double-blind randomized study using either high-dose 13-cis-retinoic acid (13-cRA) or placebo for the treatment of oral premalignant lesions, clinical and pathological responses were observed in more than 50% of those treated with high-dose 13-cRA (4). However, toxicity was significant and the duration of response was limited. Follow up clinical trials attempted to use a long-term low dose of 13-cRA to achieve similar efficacy with reduced toxicity (5, 6), however, the results were disappointingly negative (5–7). Because retinoids and interferons are known to be highly synergistic, a three-drug combination regimen of 13-cRA, interferon-alpha, and alpha-tocopherol (biochemoprevention) was tested in patients with advanced premalignant lesions or locally advanced SCCHN in an adjuvant setting (8–10). The results of these pilot studies were promising, but validation in large-scale clinical trials has not been conducted.
Molecularly targeted agents have also been investigated for their chemopreventive potential. Cyclooxygenase-2 (COX-2) is frequently expressed in neoplastic cells of head and neck, lung, colon, and breast cancer tissues. Almost 100-fold greater expression levels of COX-2 mRNA were found in SCCHN cells compared with normal oral mucosa (11). Thus, selective COX-2 inhibitors have been considered as promising agents for chemoprevention (12), but the cardiovascular side-effects of these agents raise concerns about their use in the preventative setting (13). The epidermal growth factor receptor (EGFR) is also a potential target for chemoprevention given its overexpression in a wide variety of malignant tumors, including SCCHN (39). However, single agent EGFR-TKI erlotinib did not show activity in the prevention of oral cancers in a multi-institutional, placebo-controlled randomized phase 3 study in patients with premalignant lesions of the oral cavity (EPOC) (14).
In general, combination strategies have proven more effective than single agents in the prevention and treatment of cancer, since combination therapies not only enhance clinical responses but also diminish the probability of developing drug resistance (24). Combining lower doses of effective drugs may also reduce the risk of side effects whilst eluding drug resistance and improving efficacy, which is a particularly important strategy in cancer chemopreventive approaches (29). Although there have been some promising results with combination chemoprevention strategies for cancer (10, 26), the use of multiple agents has mainly been studied in the context of cancer treatment. The development of SCCHN is a multi-step process involving multiple signal transduction pathways and complicated cross-talks among the pathways (27). Combined treatments using appropriate multi-targeted agents may be more effective than targeting single molecules in overcoming the variability and complexity of genetic alterations in SCCHN (28).
In preclinical studies, our group found that combined treatment with COX-2 inhibitor (celecoxib, Celebrex) and EGFR-TKI (erlotinib) significantly inhibited tumor cell growth as compared with each single agent both in vitro and in vivo (15, 16). Based on these results, we conducted a phase I pilot clinical trial to treat premalignant lesions of SCCHN with the combination, which showed promising efficacy but significant toxicity (17, 18). Natural compounds may offer improved safety profiles, a particularly important consideration for chemoprevention approaches. Green tea (Camellia sinensis) is one of the most widely consumed beverages worldwide. The cancer preventive activities of green tea have received a great deal of attention from researchers and the general public. We observed in preclinical studies that the combination of green tea polyphenon E (PPE) and EGFR-TKI erlotinib synergistically inhibits tumor growth (54). Epidemiological studies from different countries have reported promising results in reducing human cancer risk (19–22), while other studies, however, are not consistent with these observations (23, 24). Therefore, definitive conclusions on the cancer preventive effects of green tea cannot be reached due to various confounding factors, and further studies in the clinical trial setting are clearly warranted (25).
In the current study, we tested the combination of green tea PPE and EGFR-TKI erlotinib in a phase 1b study. Although toxicity and pharmacokinetics have been studied for either erlotinib or green tea PPE as single agents in humans, no clinical studies have been conducted combining these two agents in the chemoprevention setting in head and neck cancer. Therefore, we conducted a phase 1b clinical trial with dose escalation in patients with advanced premalignant lesions of the oral cavity and larynx along with biomarker studies as a major project of the NCI-funded Head and Neck Cancer SPORE (Specialized Program of Research Excellence) Program.
Patients and Methods
Study design
This phase 1b chemoprevention study recruited all patients in a single institution at Emory University Winship Cancer Institute and was conducted in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS) and performed after approval by the Institutional Review Board (IRB) of Emory University Winship Cancer Institute. The primary objectives of the study were to explore dose-limiting toxicity (DLT) and maximum tolerated dose (MTD) and assess safety of the combined treatment with green tea PPE and erlotinib in patients with advanced premalignant lesions of the head and neck. Based on simulation studies of the feasibility parameter alpha, in this study we adopted the Escalation with Overdose Control (EWOC) design, which is an effective means of controlling the frequency of overdosing in a phase 1 trial (30). The secondary objectives were to assess pathologic response, overall survival (OS) and cancer free survival (CFS), and to assess whether relevant biomarkers were modulated by the treatment in tissues or cytobrushed specimens. Pathological response was defined as follows: pathological complete response (pCR) is defined by complete disappearance of dysplasia from the epithelium, pathological partial response (pPR) by improvement of dysplasia at least one degree (i.e., severe dysplasia becomes moderate or mild dysplasia), pathological stable disease (pSD) by minor focal improvement or no changes of dysplasia, and pathological progressive disease (pPD) by worsening of at least one degree of dysplasia or development of invasive squamous cell carcinoma. The final pathological responses were evaluated by pathologists by comparing the biopsied tissues at 6 months to the pre-treatment initial biopsied tissues.
The key eligibility criteria included: documented histology of premalignant lesions of the oral cavity or larynx including advanced premalignant lesions (mild dysplasia, moderate dysplasia, severe dysplasia or carcinoma in situ); definitively treated T1–2N0 with oral premalignant lesions; ECOG performance status of 0 or 1; at least 18 years of age; normal bone marrow, renal and hepatic functions; ability to swallow the pills of green tea PPE and erlotinib; adequate contraception and negative pregnancy test for women of child-bearing potential; consent to have re-biopsy for pathologic responses and biomarker studies using tissue; cytobrushed or blood samples; and signed written informed consent form. For biomarker studies, we recruited non-smoking normal individuals as controls.
Treatment
Treatment schedule included a fixed dose of green tea PPE (200 mg) orally administered three times a day, and erlotinib administered orally daily with dose escalation from 50mg [level 1], to 75mg [level 2] to 100mg [level 3] for 6 months. Because of the potential toxicities of erlotinib, such as skin rash and diarrhea, the dose of erlotinib in this study was limited to 100mg per day after consultation with the FDA when the IND was filed. For all enrolled patients, a tissue biopsy was obtained before treatment for diagnosis and a second biopsy was obtained after 6 months of treatment to evaluate pathologic response and biomarker levels, while biopsy at 3 months or 12 months was optional. We also performed cytobrush of the APL lesions and normal buccal mucosa at baseline, 3, 6, and 12 months for biomarker studies.
Biomarker studies:
Biopsy specimens were collected at baseline, 6 months and 12 months (optional) intervals. Formalin-fixed paraffin embedded (FFPE) biopsy tissue sections (4-micron thickness) were subjected to immunohistochemical staining, and all sections were pre-heated for 30 minutes and washed through a series of xylene and alcohol treatments, followed by antigen retrieval using 1X citrate buffer in a microwave. The slides were cooled at room temperature and quenched using 3% hydrogen peroxide in distilled water. The sections were washed and then blocked using 2.5% normal horse serum following the instructions from the manufacturer (Vectastain Kit, Vector Laboratories, Burlingame, CA). Tissue sections were stained using primary antibodies of pERK (4370, Cell Signaling Technology, Danvers, MA), pS6 (4857, Cell Signaling Technology, Danvers, MA) and Ki-67 (ab92742, Cambridge, MA.), and incubated overnight at 40C. Sections were washed and incubated with corresponding secondary antibodies, visualized by DAB and counterstained with hematoxylin. Positive signals were counted in three random fields under ×200 magnification and were quantified using weighted index (= Intensity × % of positive staining). The intensity of staining was scored as negative (0), weak (1+), intermediate (2+), and strong (3+) positivity. The quantification was determined blindly and independently by two investigators (including the pathologist).
Statistical methods:
Statistical analysis was conducted using SAS Version 9.4, and SAS macros developed by the Biostatistics and Bioinformatics Shared Resource at Winship Cancer Institute (31). The significance level was set at P < 0.05. Descriptive statistics for patients’ baseline characteristics and toxicity profile were reported. The univariate association of pathological response was assessed by chi-square test for categorical biomarkers and ANOVA for numerical biomarkers. Kaplan-Meier method and log-rank test were used to estimate 5-year event-free rate with 95% confidence interval.
Results
Patient characteristics and toxicity evaluation
We enrolled 21 patients from February 2011 to November 2017 at Emory Winship Cancer Institute after all eligibility criteria were met and patients signed the IRB-approved informed consent form. We also recruited 6 never smokers with no history of cancer to serve as controls for future studies. After obtaining informed consent, we collected cytobrushed saliva samples from these control subjects and banked them at −80C for future biomarker studies using next generation sequencing or other molecular studies. Among 21 enrolled patients, 2 were ineligible, 19 patients began treatment, and 17 completed 6 months of treatment (2 patients discontinued treatment due to personal and social reasons within 2 months of treatment). The characteristics of these 19 patients are listed in Table 1. Briefly, there were 9 males and 10 females; median age of 64 years; 10 former or current smokers, 9 never smokers and none had any other risk factors (i.e., betel nut chewing, mal-fitting of denture or any occupational hazards); 15 patients with severe dysplasia or carcinoma in situ (CIS), 2 with moderate dysplasia, 2 with mild dysplasia; 13 prior surgical resections; and 17 patients had APL of the oral cavity and 2 of the larynx. The initial 3 patients were treated with fixed dose of green tea PPE 200mg, three times a day in combination with a dose escalation of erlotinib 50mg a day (level 1), 4 patients received erlotinib 75mg a day (level 2), and 12 patients received erlotinib, 100mg a day (level 3).
Table 1:
Patient Characteristics
| Characteristics | No. Patients N=19 (%) |
|---|---|
| Age: | |
| Median (Range) | 64 (33–78) |
| Sex: | |
| Male | 9 (47.4) |
| Female | 10 (52.6) |
| Smoking or other risk factors: | |
| Active/Former Smokers | 10 (52.6) |
| Never Smokers | 9 (47.4) |
| Betel nut chewers or other risks | 0 (0) |
| Site: | |
| Buccal Mucosa | 2 (10.5) |
| Floor of Mouth | 1 (5.3) |
| Gingiva | 1 (5.3) |
| Larynx | 2 (10.5) |
| Pharynx | 1 (5.3) |
| Oral Tongue | 12 (63.2) |
| Tissue Histology: | |
| Mild | 2 (10.5) |
| Moderate | 2 (10.5) |
| Severe/CIS | 15 (78.9) |
The cumulative toxicity of all three doses are tabulated in Table 2. At dose level 1, we observed skin rash and dryness, indigestion, nausea, fatigue, anemia and thrombocytopenia, which were all grade 1 and resolved after treatment, but we did not observe any DLT. Only 1 patient developed grade 3 skin rashes on erlotinib 100mg dose (level 3); the drug was withheld for 2 weeks and restarted at 75mg dose without any further dose reduction. Two patients developed grade 3 hypertension (1 at level 2 and 1 at level 3), which resolved after withholding both green tea PPE and erlotinib for one week and resuming the drug with one dose lower level without further problems. One patient developed grade 2 epistaxis likely related to erlotinib with dryness of the nostril, which resolved without any significant issue. Significant DLTs were skin rash and hypertension (level 3) which were resolved after withholding erlotinib for one week and the MTD may have not been reached (the dose of erlotinib in this study was, however, limited to 100mg per day after consultation with the FDA when the IND was filed). Therefore, the recommended doses for future phase II studies would be erlotinib 100mg per day and green tea PPE 200mg three times a day.
Table 2:
Toxicity Evaluation
| Side Effects (N = 19 Patients) |
G1 No (%) | G2 No (%) | G3 No (%) |
|---|---|---|---|
| Rash (skin) | 16 (84) | 1 (5) | 1 (5) |
| Pruritus/Dry skin | 13 (68) | 1 (5) | 0 |
| Fatigue | 10 (53) | 1 (5) | 0 |
| Diarrhea | 10 (53) | 1 (5) | 0 |
| Hair thinning | 4 (21) | 0 | 0 |
| Anxiety/sleep disorder | 3 (16) | 0 | 0 |
| Tongue pain | 3 (16) | 0 | 0 |
| Indigestion | 3 (16) | 0 | 0 |
| Nausea | 4 (21) | 0 | 0 |
| Epistaxis | 1 (5) | 1 (5) | 0 |
| Anemia | 3 (16) | 0 | 0 |
| Thrombocytopenia | 1 (5) | 0 | 0 |
| SGOT/SGPT elevation | 8 (42) | 0 | 0 |
| Hypertension | 2 (11) | 1 (5) | 2 (11) |
| Hyperglycemia | 5 (26) | 0 | 0 |
| Hypokalemia | 2 (11) | 0 | 0 |
G1 - Grade 1, G2 - Grade 2, G3 - Grade 3.
Pathological response and survival
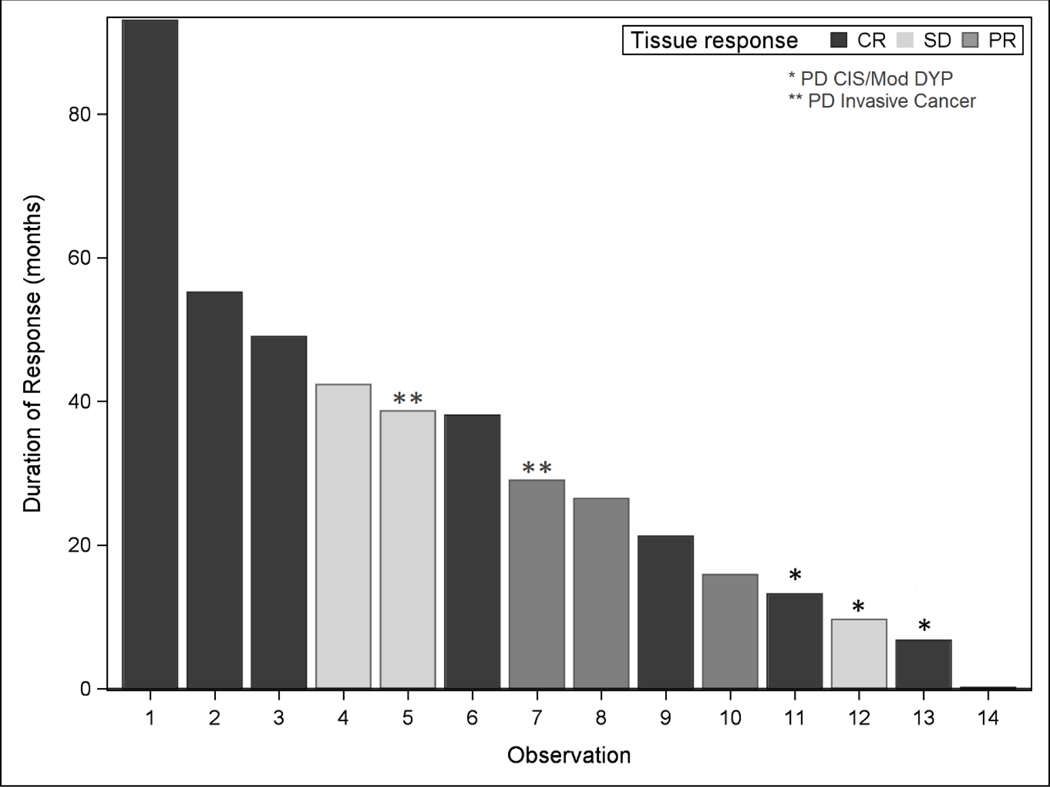
Among the 19 patients enrolled, 2 patients dropped out within 2 months of treatment (without undergoing biopsy) and 17 patients completed 6 months of treatment with green tea PPE and erlotinib. All 17 patients underwent repeat biopsy at 6 months for pathologic evaluation and biomarker studies. Pathology specimens from each patient before and after treatment were assessed by the 3 assigned head and neck pathologists and reviewed again by the study designated pathologist (QS) to ensure no discrepancies. In fact there were no disagreements in pathologic grading for all participating subjects. Among 17 pathologically evaluable patients, 8 patients achieved pCR (47%), 3 pPR (18%), 3 pSD (18%) and 3 pPD (18%), with an overall major pathologic response of 65% (11/17). Figure 1 shows examples of complete and partial pathologic responses. In the subset analysis, among 13 patients with severe dysplasia or carcinoma in situ histology, 6 patients achieved pCR, 2 had pPR, 2 had pSD and 3 had pPD. For this subset of very advanced APL, we obtained major pathologic responses of 62% (6 pCR and 2 pPR, 8/13 patients) which is remarkable, and a similar rate as in the overall population. The median duration of response was 28 months among all responders, 30 months among subjects with CR (N=8), and 27 months among subjects with PR (N=3) (P=NS). During the follow up period of time, the number of recurrences or PD in the CR group was 2/8 (25%), while that in the PR group was 1/3 (33%) and 2/3 (66%) in the SD group (because of small sample size, the p value was not significant). Two patients developed invasive cancer among all responders. The recurrences (both pre-malignant and invasive cancer) developed in the same sites as the initial premalignant lesions (please see detail for each responder in Figure 2).
Figure 1:
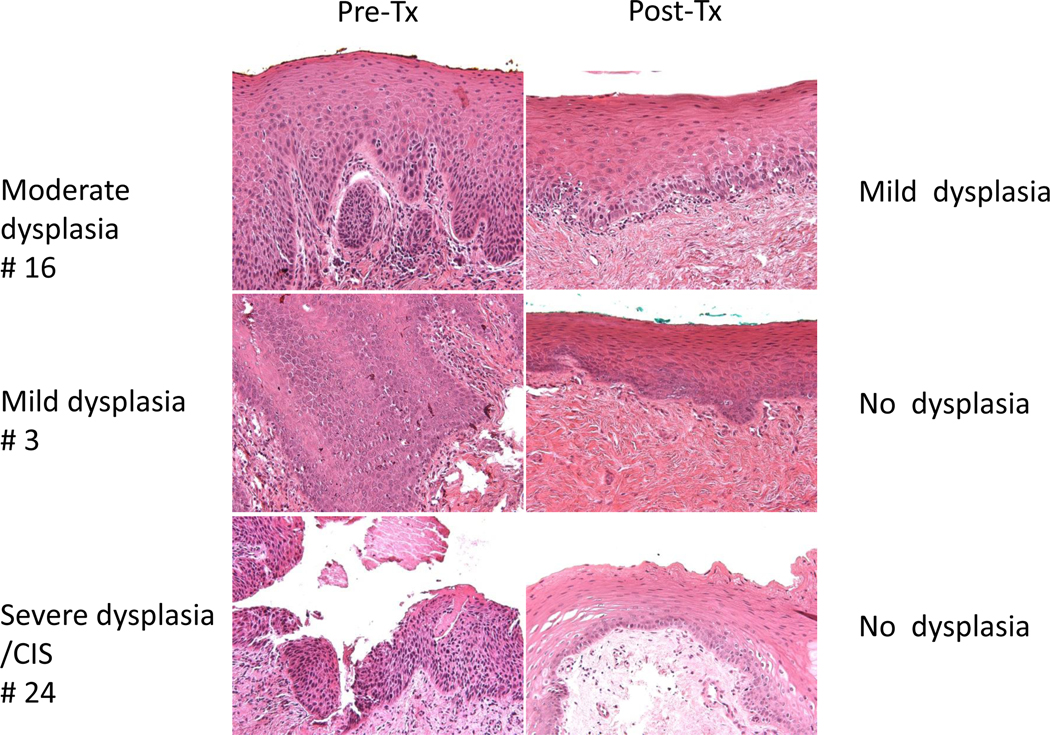
Three example cases of pathologic responses of premalignant lesions of the oral cavity before treatment (Pre-Tx) and after 6 months of treatment (Post-Tx) with green tea PPE and erlotinib. Case #16 was moderate dysplasia before treatment (Pre-Tx) that became mild dysplasia (pathologic partial response) after treatment (Post-Tx). Case #3 was mild dysplasia Pre-Tx and improved to no dysplasia Post-Tx (pathologic complete response). Case #24 was severe dysplasia/carcinoma in situ (CIS) Pre-Tx that also improved to no dysplasia Post-Tx (pathologic complete response) (200X Magnification).
Figure 2:
The figure represents duration (months) of response for each responder among 17 pathologically evaluable patients: 8 CRs, 3 PRs and 3 SDs. Five patients developed progressive disease during the follow-up: * Indicates progressive disease without developing invasive cancer (3 cases) and ** indicates progressive disease with developing invasive cancer (2 cases).
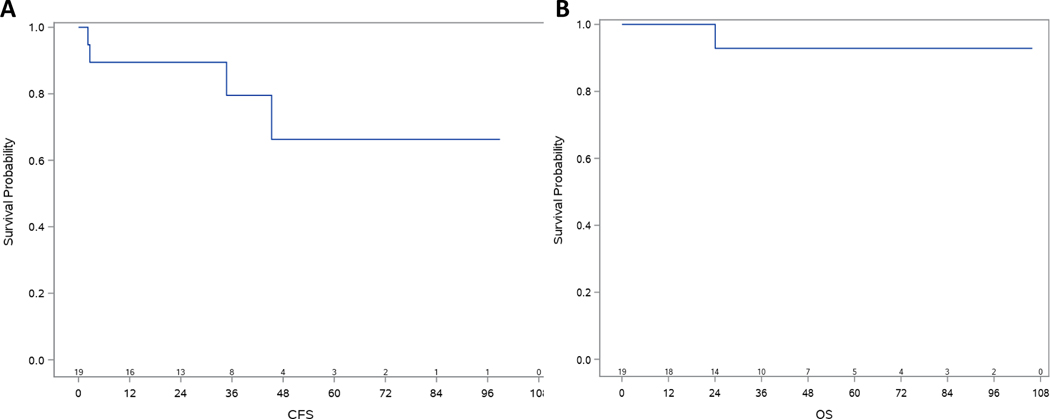
The median follow up time was 44.2 months with a 95% CI of (21.3, 59.3) months. The median overall survival has not been reached and only one patient died of non-cancer related diseases (Figure 3A). The median cancer free survival (Figure 3B) has also not been reached. The 5-year cancer free survival and 5-year overall survival were 66.3% (95% C.I., 29.0%, 87.2%) and 92.9% (95% C.I., 59.1%, 99.0%), respectively.
Figure 3:
Kaplan-Meier plots of overall survival (OS) (A) and cancer free survival (CFS) (B) of the 19 patients. The 5-year OS was 93% (95% CI, 59.1%−99%) and median OS was not reached. The 5-year CFS was 66.3% (95% CI, 29.0%−87.2%) and median CFS was not reached. The median follow up was 44.2 months.
Biomarker studies
We studied biomarkers in biopsied tissues collected before treatment (baseline samples) and 6 months post treatment (mandatory). Immunohistochemical (IHC) staining was performed based on tissue availability for Ki-67, pERK, and pS6 and quantified by two independent investigators. Positive signals were counted in three random fields as described in the Methods section.
All three biomarkers were analyzed in the baseline tissues, however, no differential expression was observed based on the degree of dysplasia, perhaps related to the small sample size (data not shown). We analyzed the expression of the markers and correlations between the expression of any biomarkers and pathologic response. Interestingly, pERK expression at baseline was significantly correlated with pathologic response (p=0.014) (Figure 4 & Table S1), while expression of Ki-67 and pS6 were not associated with pathologic responses (p=0.92 and p=0.68, respectively) (Table S1). We also analyzed whether there was any change in the 3 biomarkers after the treatment for 6-months and any correlation in the change to pathologic responses. It was observed that pERK was clearly reduced particularly in the CR patients and Ki67 was also reduced in most cases after the treatment (Figures 4, S1A, S1B, and Table S1), although we did not obtain a statistically significant correlation between the reductions of ERK and Ki67 and pathologic responses due to the small sample size (Table S1).
Figure 4:
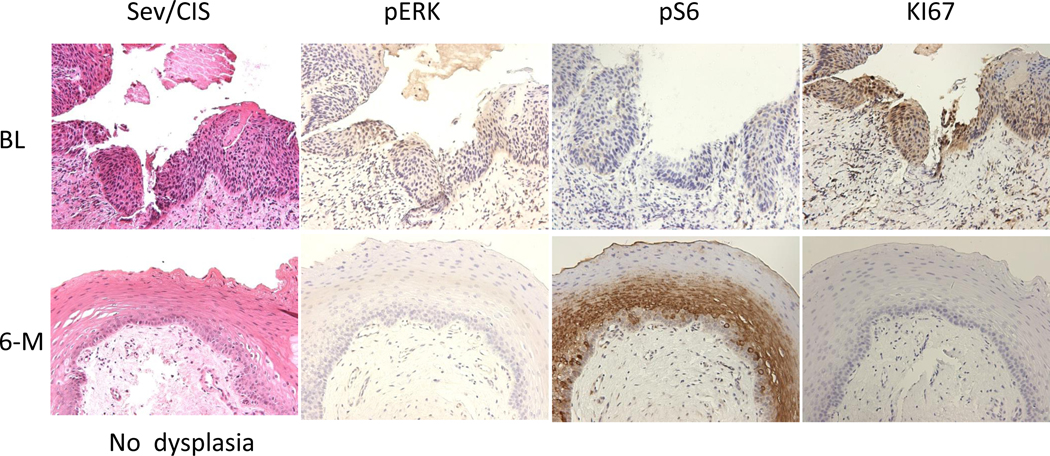
Biomarker modulation of Case #24 (severe dysplasia/CIS) at baseline (Pre-Tx) became no dysplasia (pathologic complete response). pERK was highly expressed in the baseline (BL) tissue which was downregulated after 6 months of treatment (6-M). PS6 was not expressed at baseline but increased after 6 months, while Ki-67 was highly expressed at baseline (BL) and was downregulated after 6 months of treatment (6-M) (200X Magnification).
Discussion
The concept of chemoprevention in head and neck cancer was born many decades ago (32) and multiple agents including retinoids have been extensively studied. In particular, high dose retinoids have been well documented to effectively prevent invasive cancer in premalignant lesions of the head and neck, but demonstrated significant toxicity (4, 5, 33), while low doses of retinoids did not prevent second primary tumor development when given in an adjuvant setting in early stage head and neck cancer in a large placebo controlled phase III randomized study (34–36). Many other studies with different chemopreventive agents have been conducted in the past without much success (14, 37, 38). Therefore, as of today, no standard approaches have been established for the chemoprevention of head and neck cancer.
As molecularly targeted agents have been extensively developed for cancer therapeutics, orally active agents with limited but tolerable toxicities (i.e., EGFR-TKI [erlotinib]) may be good candidates for chemoprevention in SCCHN. EGFR overexpression has been documented extensively in a wide variety of malignant tumors, including SCCHN (39). Overexpression of EGFR and its ligand TGF-alpha was observed in 80 to 90% of SCCHN specimens. Several studies, including our own, have demonstrated that EGFR overexpression correlates with reduced disease-free and overall survival and increased risk of disease recurrence and metastasis (40). Importantly, we found that EGFR expression was upregulated in a stepwise manner from normal human oral epithelium adjacent to tumor and remained elevated throughout the histologic progression from hyperplasia to dysplasia to carcinoma in situ, and further progressed to squamous cell carcinoma (39), suggesting that EGFR may serve as an excellent target for chemoprevention. There are several potential approaches to block EGFR signaling pathways in carcinogenesis. Among these studies, many synthetic and semi-synthetic compounds have been identified as EGFR-selective TKIs, which induce significant growth inhibition of human tumor xenografts. Oral administration of erlotinib to mice significantly reduced the level of EGFR autophosphorylation in human tumor xenografts, and daily administration of erlotinib markedly inhibited the growth of the HN5 human head and neck carcinoma, as well as A431 squamous epidermoid carcinoma xenografts (41, 42). However, the chemopreventive activity of erlotinib has only recently been evaluated in human cancers (14, 17, 18). Phase I pharmacokinetic (PK) studies of erlotinib have been conducted in healthy volunteers and in individuals with a variety of solid tumors (43–48) with excellent oral bioavailability. Previously, erlotinib was tested in patients with high-risk oral premalignant lesions (OPLs) defined by specific loss of heterozygosity (LOH) profiles in the Erlotinib Prevention of Oral Cancer (EPOC) study as a multi-institutional randomized, placebo-controlled double-bind trial with erlotinib (150 mg/day) or placebo for 12 months. One hundred and fifty subjects were randomized with 75 each to the placebo and erlotinib groups. The primary study endpoint of 3-year cancer free survival rates in placebo- and erlotinib- treated patients were disappointingly similar, 74% and 70%, respectively (P= 0.45). The study, therefore, concluded that single agent erlotinib did not improve cancer free survival in high-risk patients with LOH-positive or high-EGFR-gene-copy-number OPLs, thus this study does not support single agent erlotinib use in this setting (14).
Green tea is a non-fermented product containing four major polyphenols: epigallo-cathechin-gallate (EGCG), epicatechin gallate (ECG), epigallo-catechin (EGC), and epicatechin (EC) based on HPLC analysis (49, 50). EGCG is the most abundant and active catechin and has received by far the most attention among the green tea extracts. Several studies have reported the broad inhibitory activity of green tea and its constituents against carcinogenesis (51–55). Multiple signal transduction pathways have been heavily studied in the inhibition of tumor growth and suppression of carcinogenesis by EGCG and other tea polyphenols (51–56). This inhibitory effect of EGCG has also been attributed to the inhibition of AP-1 activity, possibly due to the inhibition of mitogen-activated protein kinase (MAPK) activities (57–59). In our biomarker studies of our phase 1b trial, phosphorylated ERK was shown to be significantly correlated with pathologic response. In fact, this finding is compatible with the findings of preclinical studies: EGCG was shown to inhibit the activation of PI3K/AKT, which represents cellular signaling cascades of MAPK/ERK in cell growth, proliferation and survival (57).
Toxicity is always an important issue when studying cancer chemopreventive agents. When green tea polyphenols are administered orally to animals (mice and rats etc.), no toxicity is observed even at high doses (500mg/kg body weight). However, liver toxicity and thiol conjugates of EGCG can be observed when EGCG is administered in mice (58). In human studies, it was reported that good bioavailability of free EGCG can be achieved by taking green tea PPE capsules, which were well tolerated up to a dose of 800mg EGCG (59). Nevertheless, there have been some concerns regarding the safe use of green tea extracts as a weight reduction aid (60). However, an encouraging report by Bettuzzi, et al., (61) demonstrated that daily oral administration of 600 mg of tea catechins for one year prevented prostate cancer development in subjects with high grade PIN without any toxicity profile. Furthermore, our previous study conducted at M.D. Anderson Cancer Center indicated that a phase I trial of oral green tea extract in adult patients with solid tumors led only to tolerable toxicity which was caffeine related (62). These observations strongly support that green tea polyphenols may serve as a good candidate for chemoprevention.
Importantly, green tea polyphenols, particularly, the combination of EGCG and erlotinib may synergistically inhibit carcinogenesis and tumor progression through targeting multiple signal transduction pathway molecules, such as EGFR, AKT, MAPKs, or NF-κB (54, 63). In fact, our current phase 1b trial clearly showed that the combination of green tea PPE and EGFR-TKI (erlotinib) was well tolerated in patients with advanced oral premalignant lesions in the mouth cavity and larynx. After 6 months of intervention, we obtained remarkably high pathologic responses including complete response (47%, 8/17), partial response (18%, 3/17), and stable disease (18%, 3/17), while only 18% (3/17) of patients had progressive disease. In particular, the most advanced premalignant lesions (severe dysplasia or carcinoma in situ) showed also very high pathologic major responses (62%) with excellent median duration of response (33.6 months) in a subset analysis. Therefore, our combined treatment is clearly more active in terms of pathologic response than green tea extract alone, which was reported to yield a pathologic response in 6 of 28 patients (21% with all partial response) (64). As shown in the EPOC study and stated previously, treatment with single agent erlotinib did not show any impact on oral cancer free survival compared to the placebo group (14). Therefore, we believe that our combination of green tea PPE and erlotinib appears to be much more effective and synergistic in preventing the progression of APLs to invasive cancer in the head and neck, although the finding is based on a small pilot phase 1 study. Thus, based on the encouraging results we are convinced that this combination well deserves to move forward in larger chemoprevention studies targeting premalignant lesions of the head and neck, and/or chemoprevention of second primary tumors in an adjuvant setting in early stage (stages I and II) head and neck cancer that is definitively treated with surgery, radiation therapy or both. Currently there are no standard approaches in an adjuvant setting to treat these patients who have developed early stage head and neck cancer, yet there is a good chance of recurrence or development of SPT over the years to come.
Finally, we performed a limited biomarker study using the biopsied tissues, which appears to be a critical component in such chemoprevention studies. Of the markers tested, pERK was the only marker predictive of outcome of response in our pilot study. Based on the small sample size of the current study, however, it is not surprising that the associations of other biomarkers were not statistically significant. Clearly, such biomarker studies should be incorporated into future larger sample sized clinical studies to better understand the biology of chemoprevention studies. Using the collected cytobrushed samples (banked at −80C) from the enrolled treated and control subjects we plan to conduct additional biomarker studies.
Supplementary Material
Translational Relevance.
We observed in preclinical studies that the combination of green tea polyphenon E (PPE) and epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) synergistically inhibits tumor growth. This report describes the phase I clinical evaluation of the combination, green tea PPE and EFGR-TKI (erlotinib), in patients with advanced premalignant lesions (APLs) of the oral cavity and larynx. The doublet combination with dose escalation of erlotinib and fixed dose of PPE was well tolerated in patients with premalignant lesions and demonstrated a safety profile consistent with that of the individual agents. No unexpected safety concerns were noted. The combination demonstrated promising pathologic responses, including more than 50% of patients with pathologic complete and partial responses after 6 months of treatment. This combination, therefore, deserves further investigation for chemoprevention and/or prevention of second primary tumors in early stage head and neck squamous cell carcinoma.
Acknowledgements:
Our study was supported by NCI CA P50 128613 (HNC SPORE), P30CA138292, Mitsui Norin Co, Ltd for supply of green tea PPE and Astellas Pharma, Inc. for erlotinib. Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University. We appreciate the editorial comments and review by Dr. Anthea Hammond.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors declare no potential conflicts of Interest to disclose.
References:
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Munro AJ. An overview of randomised controlled trials of adjuvant chemotherapy in head and neck cancer. Br J Cancer. 1995;71(1):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359(11):1143–54. [DOI] [PubMed] [Google Scholar]
- 4.Hong WK, Endicott J, Itri LM, Doos W, Batsakis JG, Bell R, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315(24):1501–5. [DOI] [PubMed] [Google Scholar]
- 5.Lippman SM, Batsakis JG, Toth BB, Weber RS, Lee JJ, Martin JW, et al. Comparison of low-dose isotretinoin with beta carotene to prevent oral carcinogenesis. N Engl J Med. 1993;328(1):15–20. [DOI] [PubMed] [Google Scholar]
- 6.Papadimitrakopoulou VA, Hong WK, Lee JS, Martin JW, Lee JJ, Batsakis JG, et al. Low-dose isotretinoin versus beta-carotene to prevent oral carcinogenesis: long-term follow-up. J Natl Cancer Inst. 1997;89(3):257–8. [DOI] [PubMed] [Google Scholar]
- 7.Khuri FR, Lee JJ, Lippman SM, Kim ES, Cooper JS, Benner SE, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98(7):441–50. [DOI] [PubMed] [Google Scholar]
- 8.Shin DM, Khuri FR, Murphy B, Garden AS, Clayman G, Francisco M, et al. Combined interferon-alfa, 13-cis-retinoic acid, and alpha-tocopherol in locally advanced head and neck squamous cell carcinoma: novel bioadjuvant phase II trial. J Clin Oncol. 2001;19(12):3010–7. [DOI] [PubMed] [Google Scholar]
- 9.Seixas-Silva JA Jr., Richards T, Khuri FR, Wieand HS, Kim E, Murphy B, et al. Phase 2 bioadjuvant study of interferon alfa-2a, isotretinoin, and vitamin E in locally advanced squamous cell carcinoma of the head and neck: long-term follow-up. Arch Otolaryngol Head Neck Surg. 2005;131(4):304–7. [DOI] [PubMed] [Google Scholar]
- 10.Papadimitrakopoulou VA, Clayman GL, Shin DM, Myers JN, Gillenwater AM, Goepfert H, et al. Biochemoprevention for dysplastic lesions of the upper aerodigestive tract. Arch Otolaryngol Head Neck Surg. 1999;125(10):1083–9. [DOI] [PubMed] [Google Scholar]
- 11.Mestre JR, Chan G, Zhang F, Yang EK, Sacks PG, Boyle JO, et al. Inhibition of cyclooxygenase-2 expression. An approach to preventing head and neck cancer. Ann N Y Acad Sci. 1999;889:62–71. [DOI] [PubMed] [Google Scholar]
- 12.Lin DT, Subbaramaiah K, Shah JP, Dannenberg AJ, Boyle JO. Cyclooxygenase-2: a novel molecular target for the prevention and treatment of head and neck cancer. Head Neck. 2002;24(8):792–9. [DOI] [PubMed] [Google Scholar]
- 13.Dogne JM, Hanson J, Supuran C, Pratico D. Coxibs and cardiovascular side-effects: from light to shadow. Curr Pharm Des. 2006;12(8):971–5. [DOI] [PubMed] [Google Scholar]
- 14.William WN Jr., Papadimitrakopoulou V, Lee JJ, Mao L, Cohen EE, Lin HY, et al. Erlotinib and the Risk of Oral Cancer: The Erlotinib Prevention of Oral Cancer (EPOC) Randomized Clinical Trial. JAMA Oncol. 2016;2(2):209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, Zhang X, Li M, Wang Z, Wieand HS, Grandis JR, et al. Simultaneously targeting epidermal growth factor receptor tyrosine kinase and cyclooxygenase-2, an efficient approach to inhibition of squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10(17):5930–9. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Chen ZG, Choe MS, Lin Y, Sun SY, Wieand HS, et al. Tumor growth inhibition by simultaneously blocking epidermal growth factor receptor and cyclooxygenase-2 in a xenograft model. Clin Cancer Res. 2005;11(17):6261–9. [DOI] [PubMed] [Google Scholar]
- 17.Saba NF, Hurwitz SJ, Kono SA, Yang CS, Zhao Y, Chen Z, et al. Chemoprevention of head and neck cancer with celecoxib and erlotinib: results of a phase ib and pharmacokinetic study. Cancer Prev Res (Phila). 2014;7(3):283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin DM, Zhang H, Saba NF, Chen AY, Nannapaneni S, Amin AR, et al. Chemoprevention of head and neck cancer by simultaneous blocking of epidermal growth factor receptor and cyclooxygenase-2 signaling pathways: preclinical and clinical studies. Clin Cancer Res. 2013;19(5):1244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26(6):769–75. [DOI] [PubMed] [Google Scholar]
- 20.Nakachi K, Suemasu K, Suga K, Takeo T, Imai K, Higashi Y. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Jpn J Cancer Res. 1998;89(3):254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. Int J Cancer. 2004;108(1):130–5. [DOI] [PubMed] [Google Scholar]
- 22.Wu AH, Yu MC, Tseng CC, Hankin J, Pike MC. Green tea and risk of breast cancer in Asian Americans. Int J Cancer. 2003;106(4):574–9. [DOI] [PubMed] [Google Scholar]
- 23.Blot WJ, McLaughlin JK, Chow WH. Cancer rates among drinkers of black tea. Crit Rev Food Sci Nutr. 1997;37(8):739–60. [DOI] [PubMed] [Google Scholar]
- 24.Bushman JL. Green tea and cancer in humans: a review of the literature. Nutr Cancer. 1998;31(3):151–9. [DOI] [PubMed] [Google Scholar]
- 25.Yuan JM, Sun C, Butler LM. Tea and cancer prevention: epidemiological studies. Pharmacol Res. 2011;64(2):123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenner DE. Multiagent chemopreventive agent combinations. J Cell Biochem Suppl. 2000;34:121–4. [DOI] [PubMed] [Google Scholar]
- 27.Hunter KD, Parkinson EK, Harrison PR. Profiling early head and neck cancer. Nat Rev Cancer. 2005;5(2):127–35. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez CP, Adelstein DJ, Rybicki LA, Saxton JP, Lorenz RR, Wood BG, et al. Single-arm phase II study of multiagent concurrent chemoradiotherapy and gefitinib in locoregionally advanced squamous cell carcinoma of the head and neck. Head Neck. 2012;34(11):1517–23. [DOI] [PubMed] [Google Scholar]
- 29.Cai H, Scott E, Kholghi A, Andreadi C, Rufini A, Karmokar A, et al. Cancer chemoprevention: Evidence of a nonlinear dose response for the protective effects of resveratrol in humans and mice. Sci Transl Med. 2015;7(298):298ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babb J, Rogatko A, Zacks S. Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat Med. 1998;17(10):1103–20. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Nickleach DC, Zhang C, Switchenko JM, Kowalski J. Carrying out streamlined routine data analyses with reports for observational studies: introduction to a series of generic SAS ((R)) macros. F1000Res. 2018;7:1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sporn MB, Dunlop NM, Newton DL, Smith JM. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Fed Proc. 1976;35(6):1332–8. [PubMed] [Google Scholar]
- 33.Khuri FR, Kim ES, Lee JJ, Winn RJ, Benner SE, Lippman SM, et al. The impact of smoking status, disease stage, and index tumor site on second primary tumor incidence and tumor recurrence in the head and neck retinoid chemoprevention trial. Cancer Epidemiol Biomarkers Prev. 2001;10(8):823–9. [PubMed] [Google Scholar]
- 34.Hong WK, Lippman SM, Itri LM, Karp DD, Lee JS, Byers RM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323(12):795–801. [DOI] [PubMed] [Google Scholar]
- 35.Benner SE, Pajak TF, Lippman SM, Earley C, Hong WK. Prevention of second primary tumors with isotretinoin in patients with squamous cell carcinoma of the head and neck: long-term follow-up. J Natl Cancer Inst. 1994;86(2):140–1. [DOI] [PubMed] [Google Scholar]
- 36.Bolla M, Lefur R, Ton Van J, Domenge C, Badet JM, Koskas Y, et al. Prevention of second primary tumours with etretinate in squamous cell carcinoma of the oral cavity and oropharynx. Results of a multicentric double-blind randomised study. Eur J Cancer. 1994;30A(6):767–72. [DOI] [PubMed] [Google Scholar]
- 37.Alpha-Tocopherol BCCPSG. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330(15):1029–35. [DOI] [PubMed] [Google Scholar]
- 38.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334(18):1150–5. [DOI] [PubMed] [Google Scholar]
- 39.Shin DM, Ro JY, Hong WK, Hittelman WN. Dysregulation of epidermal growth factor receptor expression in premalignant lesions during head and neck tumorigenesis. Cancer Res. 1994;54(12):3153–9. [PubMed] [Google Scholar]
- 40.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62(24):7350–6. [PubMed] [Google Scholar]
- 41.Boeckx C, Van den Bossche J, De Pauw I, Peeters M, Lardon F, Baay M, et al. The hypoxic tumor microenvironment and drug resistance against EGFR inhibitors: preclinical study in cetuximab-sensitive head and neck squamous cell carcinoma cell lines. BMC Res Notes. 2015;8:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atkinson DM, Clarke MJ, Mladek AC, Carlson BL, Trump DP, Jacobson MS, et al. Using fluorodeoxythymidine to monitor anti-EGFR inhibitor therapy in squamous cell carcinoma xenografts. Head Neck. 2008;30(6):790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ling J, Johnson KA, Miao Z, Rakhit A, Pantze MP, Hamilton M, et al. Metabolism and excretion of erlotinib, a small molecule inhibitor of epidermal growth factor receptor tyrosine kinase, in healthy male volunteers. Drug Metab Dispos. 2006;34(3):420–6. [DOI] [PubMed] [Google Scholar]
- 44.Hamilton M, Wolf JL, Rusk J, Beard SE, Clark GM, Witt K, et al. Effects of smoking on the pharmacokinetics of erlotinib. Clin Cancer Res. 2006;12(7 Pt 1):2166–71. [DOI] [PubMed] [Google Scholar]
- 45.Herbst RS, Johnson DH, Mininberg E, Carbone DP, Henderson T, Kim ES, et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23(11):2544–55. [DOI] [PubMed] [Google Scholar]
- 46.Tan AR, Yang X, Hewitt SM, Berman A, Lepper ER, Sparreboom A, et al. Evaluation of biologic end points and pharmacokinetics in patients with metastatic breast cancer after treatment with erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor. J Clin Oncol. 2004;22(15):3080–90. [DOI] [PubMed] [Google Scholar]
- 47.Smith J. Erlotinib: small-molecule targeted therapy in the treatment of non-small-cell lung cancer. Clin Ther. 2005;27(10):1513–34. [DOI] [PubMed] [Google Scholar]
- 48.Siegel-Lakhai WS, Beijnen JH, Schellens JH. Current knowledge and future directions of the selective epidermal growth factor receptor inhibitors erlotinib (Tarceva) and gefitinib (Iressa). Oncologist. 2005;10(8):579–89. [DOI] [PubMed] [Google Scholar]
- 49.Fujiki H. Green tea: Health benefits as cancer preventive for humans. Chem Rec. 2005;5(3):119–32. [DOI] [PubMed] [Google Scholar]
- 50.Yang CS, Chung JY, Yang G, Chhabra SK, Lee MJ. Tea and tea polyphenols in cancer prevention. J Nutr. 2000;130(2S Suppl):472S–8S. [DOI] [PubMed] [Google Scholar]
- 51.Masuda M, Suzui M, Lim JT, Weinstein IB. Epigallocatechin-3-gallate inhibits activation of HER-2/neu and downstream signaling pathways in human head and neck and breast carcinoma cells. Clin Cancer Res. 2003;9(9):3486–91. [PubMed] [Google Scholar]
- 52.Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005;11(7):2735–46. [DOI] [PubMed] [Google Scholar]
- 53.Shimizu M, Shirakami Y, Sakai H, Tatebe H, Nakagawa T, Hara Y, et al. EGCG inhibits activation of the insulin-like growth factor (IGF)/IGF-1 receptor axis in human hepatocellular carcinoma cells. Cancer Lett. 2008;262(1):10–8. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X, Zhang H, Tighiouart M, Lee JE, Shin HJ, Khuri FR, et al. Synergistic inhibition of head and neck tumor growth by green tea (−)-epigallocatechin-3-gallate and EGFR tyrosine kinase inhibitor. Int J Cancer. 2008;123(5):1005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pianetti S, Guo S, Kavanagh KT, Sonenshein GE. Green tea polyphenol epigallocatechin-3 gallate inhibits Her-2/neu signaling, proliferation, and transformed phenotype of breast cancer cells. Cancer Res. 2002;62(3):652–5. [PubMed] [Google Scholar]
- 56.Shimizu M, Deguchi A, Hara Y, Moriwaki H, Weinstein IB. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem Biophys Res Commun. 2005;334(3):947–53. [DOI] [PubMed] [Google Scholar]
- 57.Ju J, Hong J, Zhou JN, Pan Z, Bose M, Liao J, et al. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005;65(22):10623–31. [DOI] [PubMed] [Google Scholar]
- 58.Lambert JD, Kennett MJ, Sang S, Reuhl KR, Ju J, Yang CS. Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food Chem Toxicol. 2010;48(1):409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chow HH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, et al. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin Cancer Res. 2005;11(12):4627–33. [DOI] [PubMed] [Google Scholar]
- 60.Hursel R, Viechtbauer W, Westerterp-Plantenga MS. The effects of green tea on weight loss and weight maintenance: a meta-analysis. Int J Obes (Lond). 2009;33(9):956–61. [DOI] [PubMed] [Google Scholar]
- 61.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66(2):1234–40. [DOI] [PubMed] [Google Scholar]
- 62.Pisters KM, Newman RA, Coldman B, Shin DM, Khuri FR, Hong WK, et al. Phase I trial of oral green tea extract in adult patients with solid tumors. J Clin Oncol. 2001;19(6):1830–8. [DOI] [PubMed] [Google Scholar]
- 63.Amin AR, Khuri FR, Chen ZG, Shin DM. Synergistic growth inhibition of squamous cell carcinoma of the head and neck by erlotinib and epigallocatechin-3-gallate: the role of p53-dependent inhibition of nuclear factor-kappaB. Cancer Prev Res (Phila). 2009;2(6):538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsao AS, Liu D, Martin J, Tang XM, Lee JJ, El-Naggar AK, et al. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer Prev Res (Phila). 2009;2(11):931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.