Abstract
Hair follicles cyclically regenerate throughout adult mammalian life, owing to a resident population of epithelial hair follicle stem cells. Stem cell (SC) activity drives bouts of follicle growth, which are periodically interrupted by follicle regression and rest. These phases and the transitions between them are tightly spatiotemporally coordinated by signaling crosstalk between stem/progenitor cells and the various cell types of the microenvironment, or niche. The dermal papilla (DP) is a cluster of specialized mesenchymal cells that have long been recognized for important niche roles in regulating hair follicle SC activation as well as progenitor proliferation and differentiation during follicle growth. In addition to the DP, the mesenchyme of the murine pelage follicle is also comprised of a follicle-lining smooth muscle known as the dermal sheath (DS), which has been far less studied than the DP yet may be equally specialized and important for hair cycling. In this review, we define the murine pelage DS in comparison to human DS and discuss recent work that highlights the emergent importance of the DS in the hair follicle SC niche. Last, we examine potential therapeutic applications for the DS in hair regeneration and wound healing.
Keywords: Dermal sheath, hair follicle mesenchyme, stem cell niche, epithelial-mesenchymal interactions
1 |. INTRODUCTION
The stages of the hair follicle cycle are categorized into periods of regression (catagen), rest (telogen), and growth (anagen) (1). Growth is fueled by resident epithelial stem cells of the hair follicle (2). Stem cell (SC) activity is coordinated by the collective input of molecular signals that derive from diverse cell types of the microenvironment, or niche (3–8). The hair follicle mesenchyme is represented by two distinct compartments called the dermal papilla (DP) and dermal sheath (DS), and the DP has garnered substantial attention over the past nearly 60 years since the discovery of its hair regenerative and inductive properties in grafting and transplantation assays (9–16). Whereas investigations over the last few decades have yielded remarkable insights into DP niche functions, less has been revealed about DS function. In fact, in comparison to the DP, the DS remains scarcely studied and little is known about its role during homeostatic hair follicle cycling.
In this review, we discuss the DS as an emerging component of the hair follicle SC niche and potential DS-based therapeutic applications. To standardize the terminology for optimizing clarity in future studies, we begin by defining the DS compartment and its associated structures and highlight key differences between human and rodent pelage follicle anatomy. Next, we summarize initial efforts that have illuminated the DS as an indispensable niche component and proceed by discussing other possible niche roles. Finally, we posit the manifold ways by which achieving an in-depth understanding of the DS niche may inform improved cell-based and pharmacological therapies to treat hair loss and promote wound healing.
2 |. DEFINING THE DERMAL SHEATH
2.1 –. The murine dermal sheath is a follicle-lining smooth muscle
The murine pelage DS is thought to also originate from the embryonic dermal condensate (DC) precursor of the DP (17), and in the mature follicle the DS tightly encapsulates the growing anagen hair follicle. The DS is separated from the follicular epithelium by a thin basement membrane comprised of basal lamina secreted by the epithelium and two layers of collagen fibers likely produced by the DS, which are situated between the basal lamina and DS. The inner collagen layer is oriented parallel to the hair follicle axis while collagen fibers of the outer layer extend orthogonally to those of the inner layer (18).
Since the discovery of alpha smooth muscle actin (αSMA) expression in the murine DS nearly 30 years ago (19), αSMA expression has been the most widely recognized DS molecular marker (13). Furthermore, robust αSMA expression in the DS led to the hypothesis that the DS is comprised of a contractile cell type. The molecular signature was recently defined for DS isolated from the dorsal pelage of murine back skin, which revealed expression of smooth muscle molecular machinery including genes expressed exclusively in smooth muscle and not in contractile myofibroblasts (20). Thus, murine pelage DS is broadly defined as a follicle-lining smooth muscle of fibroblastic origin.
2.2 –. The human dermal sheath resides within the connective tissue sheath
Dorsal murine pelage follicles and human hair follicles contain comparably organized epithelial and mesenchymal lineages and the cycle-dependent functions of these lineages seem to be largely conserved, rendering the murine pelage a crucial model for uncovering the dynamic regulation of human hair cycling (21, 22). However, key differences do exist, such as divergent stage durations and interfollicular synchronization.
Importantly, the outermost layer of the human hair follicle – the connective tissue sheath (CTS) – is more layered and complex than the outermost layer of the murine pelage follicle (Figure 1). The innermost CTS layer is composed of longitudinal collagen fibers that extend in an orientation parallel to the hair axis and directly borders the basal lamina secreted by the hair follicle epithelium (23). The mesenchymal cells in the middle layer are – as in murine follicles – marked by αSMA+ immunoreactivity and likely also represent the contractile DS (23). These cells tightly line the inner longitudinal collagen layer and reside embedded within transversely oriented collagen fibers (23, 24). The outer layer of the CTS, comprised of non-uniformly oriented collagen fibers, contains a variety of cell types including blood vessels, immune cells, fat cells, and sparsely intermingled αSMA- fibroblasts distinct from the DS. Human DS, akin to murine DS, wraps around the entire anagen bulb and extends distally up to the bulge, the anatomically distinct compartment containing the hair follicle SCs (2, 25–29).
Figure 1.
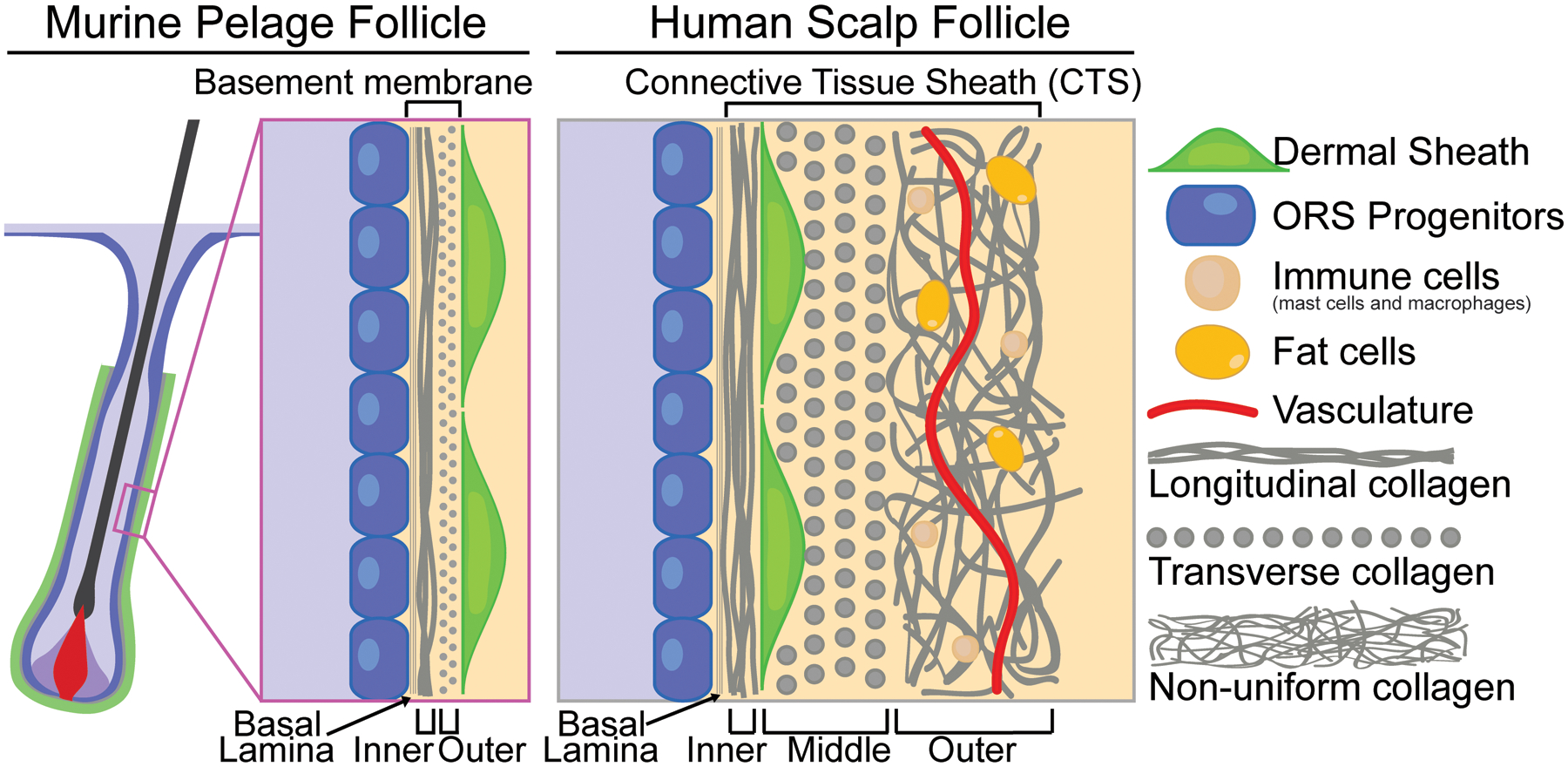
Dermal sheath in dorsal murine pelage follicles and human scalp follicles. In dorsal murine pelage follicles (left panel), the DS is the outermost layer and is separated from the ORS progenitors by a thin basement membrane composed of basal lamina and two collagen layers. In human scalp follicles, the outermost layer is the CTS (right panel), which is composed of three collagenous layers of differing orientations. The transverse middle layer houses the DS that closely lines the longitudinal inner layer, while the outer layer of multidirectional collagen contains heterologous cell types including immune cells, fat cells, and vasculature.
It is likely the DS secretes components of the basement membrane in growing murine pelage follicles due to high DS expression of collagens and other basement membrane proteins (20). Similarly, it seems probable that the DS lays down at least the innermost CTS layer in growing human follicles as robust immunoreactivity for COL4A1 is observed in this compartment (30, 31). The mechanisms by which these connective tissues are remodeled and removed during catagen regression are unknown, although two theories that have long been hypothesized are the involvement of macrophages surrounding the hair follicle and residing within the outer CTS layer (18, 32), and of digestive enzymes secreted by either the DS or follicular epithelium (24) (Figure 3). Functionally testing these possibilities will be fascinating areas of future investigation.
Figure 3.
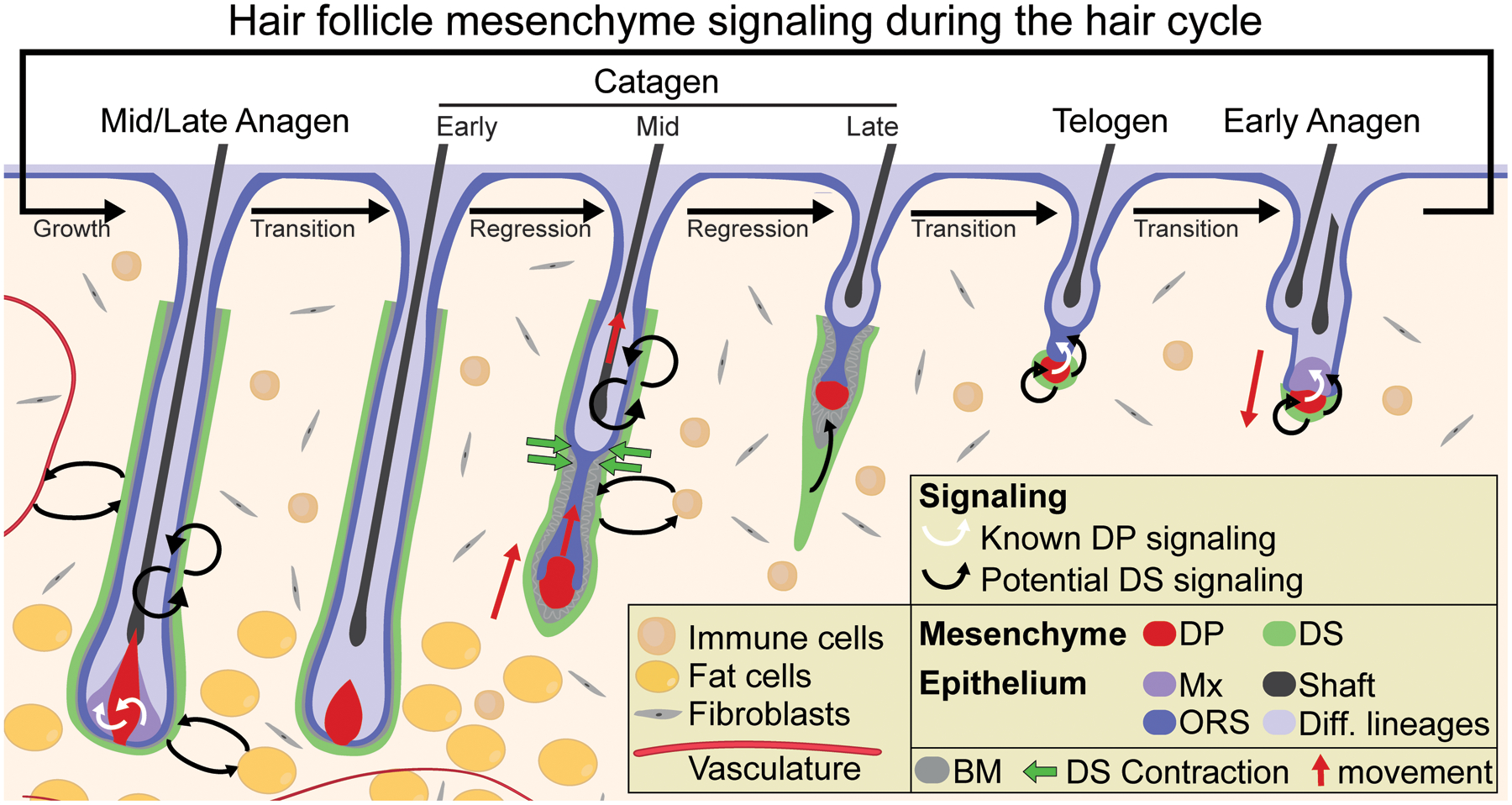
Known DP signaling and potential DS signaling during the hair cycle. During the growth phase (anagen), the DP signals to epithelial progenitors to instruct their proliferation and upward differentiation. During the regression phase (catagen), dermal sheath contraction at the interface of the club hair and epithelial strand (the “bottleneck”) powers follicle regression and upward movement of the DP. DS contraction facilitates the relocation of the DP to its stem cell-adjacent position by the resting phase (telogen). During follicle regeneration at the onset of the next growth phase, the DP provides activating signals to the SCs to trigger their proliferation. Currently, there are no known roles for the DS as a signaling niche during the hair cycle although many intriguing possibilities exist, including to regulate: progenitor proliferation during follicle growth, progenitor apoptosis and basement membrane remodeling during follicle regression, and SC quiescence/activation during follicle rest either through direct paracrine signaling or indirectly by relaying molecular signals through the DP. It is also possible that the DS – located at the interface of the extrafollicular environment – might engage in molecular crosstalk with various cell types in the dermis including fibroblasts, immune cells, adipocytes, and vasculature. DS = dermal sheath; DP = dermal papilla; Mx = matrix; ORS = outer root sheath progenitors; BM = basement membrane.
2.3 –. Dermal sheath remodeling during hair cycling
During the hair follicle cycle, the DS of the dorsal murine pelage undergoes dynamic remodeling. During anagen, the proliferating DS encases the growing follicle and closely juxtaposes its epithelial counterpart, the outer root sheath (ORS) progenitors. The ORS, which derives mainly from the more proliferative hair follicle SCs of the telogen germ but also to some extent from the more quiescent hair follicle SCs of the bulge (33–36), gives rise to the pool of matrix transit amplifying cells (TAC) that fully engulf the DP by mid-anagen (1). Separated from the ORS by just a thin basement membrane, the top of anagen DS begins below the bulge and extends downward, wrapping around the entirety of the hair bulb (Figure 2). At the base of the follicle is the DS “cup,” which is contiguous with the DP through a connecting stalk of cells. The DS cup expresses the DS marker αSMA and lacks the DP marker LEF1 (37), and recent transcriptomic analyses of the murine pelage DS, DS cup, and DP further reveals the distinct molecular signatures of these mesenchymal compartments (38–40). Thus, the DS and its cup are contiguous with, but morphologically and molecularly distinct from, the DP.
Figure 2.
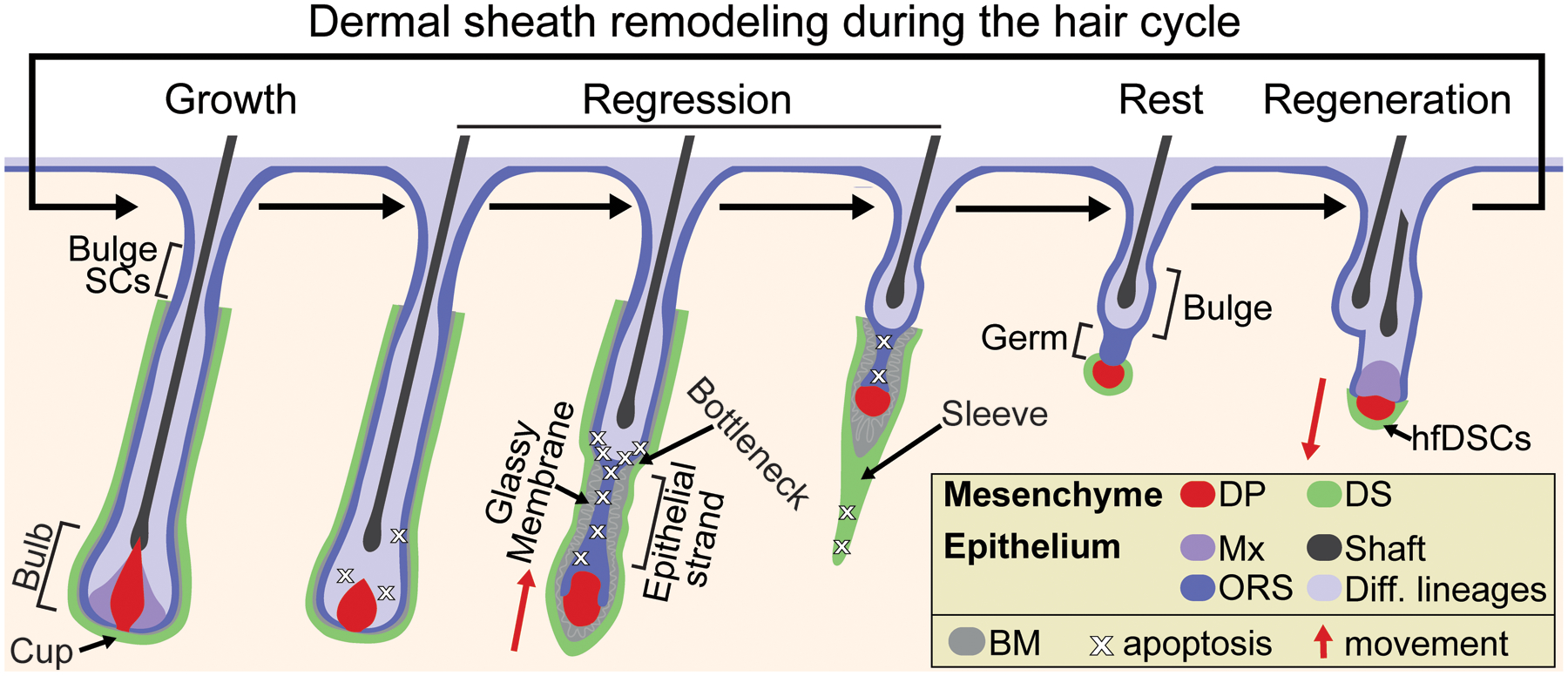
Dynamic remodeling and morphology of the dermal sheath during the hair cycle. During the growth phase (anagen), expanding DS tightly wraps the follicle and is closely juxtaposed with the epithelial ORS progenitors, with the proximal bulbar DS cells referred to as the DS cup. During the regression phase (catagen), the DS more loosely encases the epithelial strand around which restructuring of the basement membrane occurs (referred to as “glassy”). Apoptotic DS cells trail the regressing follicle in the DS “sleeve,” and by the resting phase (telogen) only a few DS cells persist and intimately surround the DP, which are bipotent stem cells termed hair follicle dermal stem cells (hfDSCs). DS = dermal sheath; DP = dermal papilla; Mx = matrix; ORS = outer root sheath progenitors; BM = basement membrane.
As the hair cycle transitions from growth to regression, TACs exit proliferation and finish differentiation. The thinning hair bulb opens and a condensed DP gradually emerges from its engulfment as early catagen progresses (1). By mid-catagen, the club hair of the regressing follicle has formed and interfaces with an epithelial strand at a region of the follicle that has been termed the “bottleneck” (20), containing progenitors with robust apoptotic activity (41, 42). While the DS maintains tight juxtaposition with the ORS from the bottleneck proximally up to the bulge, the DS more loosely lines the epithelial strand where a thicker “glassy” basement membrane develops characterized by the corrugation, pleating and fragmentation of collagen fibers (18, 32, 43) (Figure 2). Intact DS surrounds the unenclosed and compacted DP and a short distal “tail” or “sleeve” of DS trails the DP. In human hair follicles, the CTS tail has been referred to as “streamer” or “stela” (22, 44–46), although it is unclear how similar the murine DS sleeve and human CTS streamer are in terms of cellular dynamics and physiological function. In murine pelage follicles, the DS sleeve lengthens as more cells are added to it as catagen progresses. It can be detected by NCAM+ immunoreactivity (47) or via immunoreactivity with other markers such as αSMA (20), which distinguishes the final stage of catagen and telogen, when the DS sleeve can no longer be detected after undergoing apoptotic pruning during the late stages of catagen (1, 37). In turn, human CTS streamers are believed to remain into telogen and have been speculated to serve as guiding tract for the next follicle downgrowth (45, 46), but evidence for such a physiological mechanism is lacking. By telogen of murine follicles, only a few original DS cup cells (typically ~3–6 depending on age) persist in close proximity to the DP that have the remarkable ability to replenish the DS and contribute cells to the DP of growing hair follicles (37, 39).
3 |. HIERARCHICAL ORGANIZATION OF THE DERMAL SHEATH
3.1 –. The dermal sheath harbors long-lived, self-renewing stem cells
Recent studies have revealed that, like the hair follicle epithelium, expansion of the hair follicle mesenchyme during follicle growth involves a hierarchy of cell production. The few DS cells that remain after catagen and tightly wrap the telogen DP are a SC population that self-renews and replenishes the entire DS of each follicle during the following anagen hair growth, and have been termed hair follicle dermal stem cells (hfDSCs) (37) (Figure 2). Initial EdU pulse-chase experiments have revealed label-retaining cells in the DS cup at the base of the anagen follicle over consecutive adult hair cycles (37), suggesting that the SCs reside in the DS cup during anagen. This early finding opens the door to many exciting questions for future investigation, which include addressing the spatiotemporal dynamics of hfDSC activation during follicle growth, the symmetry of divisions, and whether slow-cycling hfDSCs give rise to a pool of faster-cycling progenitors that in turn differentiate into mature DS.
3.2 –. Dermal sheath progenitors can contribute to the dermal papilla
It has been shown that hfDSCs also can contribute to the anagen DP and therefore are bipotent (37). The first clues that the DS might harbor a bipotent SC came from foundational experiments demonstrating the capacity of the DS cup to restore pre-existing amputated hair follicles and induce de novo hair follicle formation upon transplantation (48–51), which collectively strongly suggested that DS cup may be able to adopt DP fate. Recent lineage tracing studies uncovered bipotent hfDSCs that are able to self-renew and reconstitute the entire DS and contribute to the DP over multiple adult hair cycles (37, 39). Single-cell transcriptomics and lineage trajectory analyses of the mesenchyme support the earlier described bipotent state of hfDSCs (37), which can give rise to both DS and DP (39).
Since switching to smaller hair types occurs after experimentally reducing DP cell numbers (52) and recruitment of hfDSC progeny into the DP to some extent regulates hair growth and type (37), it is an intriguing possibility that hfDSC exhaustion or loss of plasticity may be a mechanism of DP depletion and follicle miniaturization and hair loss. Indeed, it was recently discovered that hfDSC numbers decrease with age, which correlates with smaller DPs and thinner pelage coat (39). Therefore, illuminating precise dynamics of hfDSC-DS-DP lineage relationships during hair cycling as well as defining the mechanisms that maintain hfDSC plasticity and self-renewal will be important.
4 |. THE DERMAL SHEATH AS A CRITICAL STEM CELL NICHE
The regulation of stem and progenitor cells within the hair follicle is tightly coordinated through the collective interactions of numerous intrinsic and extrinsic niche signals. Amongst these, the requirement for the DP in hair cycling has long been appreciated due to pioneering HF amputation experiments (10), observations in hairless and Vdr knockout mice (53–55), and recent in vivo laser ablation studies (56). Many studies have subsequently uncovered mechanisms by which DP niche signals regulate hair cycling (6, 15, 57–62). All the while, despite enduring hypotheses that the DS represents an equally specialized mesenchymal niche (48–51), potential DS niche functions have been scarcely studied.
4.1 –. DS contraction reunites dermal papilla with epithelial stem cells
The DS and its functional smooth muscle contractility were recently shown to be definitively required for homeostatic hair cycling in the dorsal murine pelage (20). Cytotoxic ablation of DS resulted in stalled catagen regression, thereby preventing relocation of the DP to its telogen hair follicle SC-adjacent position where its inductive signals are ultimately required for hair regeneration (20, 56). While it had long been hypothesized that a “contraction force” during catagen might relocate the DP (63), the lack of DS-specific genetic tools precluded testing of this hypothesis and possible mechanisms underlying putative contraction-mediated remodeling remained unclear. To address this gap, DS-specific genetic targeting was established in the skin and revealed that DS centripetal contraction at the club-epithelial strand bottleneck pushes the hair shaft upwards and the epithelial strand serves as a stable tether to translate this force into upward pulling on the DP (20) (Figure 3), demonstrating the DS is a functional component of the hair follicle stem cell niche.
4.2 –. Dermal sheath engagement in crosstalk with epithelial progenitors
The epithelial progenitors – ensheathed and intimately apposed with the mesenchyme – are in prime position to engage in crosstalk with the DS. Epithelial-mesenchymal interaction is a widespread mechanism by which cell fate choices are regulated throughout embryonic development (64). They are also a cornerstone of SC regulation in the classical SC niche model (8, 65), in which signaling crosstalk underlies hair follicle formation, growth and regeneration (4, 6, 21, 66, 67). Analogous to the intestinal epithelial crypt in which pericryptic fibroblasts and smooth muscle cells have an established niche role of secreting regulatory signals to epithelial stem cells (68–70), there exists an intriguing possibility of progenitor-DS crosstalk in the murine pelage hair follicle (71) (Figure 3). This possibility is supported by observations in epithelial mutants that exhibit alterations in the timing of anagen or catagen entry, such as in Runx1 (72, 73) and Gata6 (74) conditional knockouts respectively. In these mutants and others, perturbations in the epithelium that influence stem/progenitor cell expansion or regression also appear to modulate DS activity, suggestive of synchronous crosstalk between the two compartments. As DS-specific genetic tools continue to be generated and made widely available, many fascinating biological insights into the DS as a signaling niche will likely be revealed. Such potential epithelial-mesenchymal signaling might be key to the regulation of progenitor expansion and fate choices during follicle growth and progenitor apoptosis during follicle regression.
Intriguingly, it was recently uncovered that heterogeneous subpopulations within the DP mesenchyme spatially correspond to heterogenous subpopulations of epithelial progenitors in the matrix, thus forming “micro-niches” that regulate lineage fate during follicle growth (75). Analogous to this exciting mechanism of DP-progenitor crosstalk during follicle growth, it is an interesting prospect that there may exist unappreciated heterogeneity within the DS mesenchyme and epithelial progenitors during follicle regression that regulates catagen remodeling. For instance, DS-progenitor micro-niches might dictate which DS cells and progenitors survive to constitute the hair follicle SCs and hfDSCs that will facilitate ensuing regenerative cycles. While such possibilities remain untested to date, bulk and single cell RNA-sequencing of fresh-sorted DS and epithelial progenitors during late growth and regression – coupled with functional studies – will shed light on these essential questions in the future. Indeed, recent single cell transcriptomic analyses on enriched hair follicle mesenchyme during early anagen (39) and on full-thickness skin during late anagen (40) have revealed heterogeneity within the growing DS. Future analyses on populations highly enriched for DS will further parse existing heterogeneity within the compartment. Adding another layer of complexity, it will be fascinating to unravel how spatiotemporal heterogeneity in fate decisions of the bipotent stem cells that reside within the DS cup (hfDSCs) might influence DP micro-niches as well.
4.3 –. Dermal sheath modulation of extrafollicular niche constituents
In addition to the hair follicle mesenchyme, the hair follicle stem and progenitor cell niche in dorsal murine skin includes diverse cell types including neighboring nerves (76), blood vessels (77), lymphatics (78, 79), adipocytes (80, 81), immune cells (82–84), and long-range inputs from fibroblasts deep in the dermis (80). Thus, the DS – localized at the interface of epithelial progenitors and interfollicular dermis – may not only regulate epithelial progenitors through direct signaling input, but also through modulation of extrafollicular niche constituents (Figure 3). For instance, the DS may confer the hair follicle state of relative “immune privilege” (85), both as a physical barrier and through signaling crosstalk with perifollicular macrophages and mast cells.
4.4 –. Niche functions of hair follicle dermal stem cells
It was recently shown that depletion of telogen hfDSCs – the bipotent stem cells that replenish the entire DS and contribute to the DP of growing hair follicles in the murine pelage (see Chapter 3) – via cytotoxic ablation driven by αSMA-CreER resulted in delayed anagen onset (37), suggesting a requirement for hfDSCs in initiating regeneration. However, this was observed in a grafting model to avoid non-specific effects of ablating vascular smooth muscle and arrector pili muscle. In the future, once tools are developed that specifically target telogen hfDSCs, it will be important for this result to be corroborated in a non-grafting situation. Importantly, such tools would also allow hfDSC-specific gene manipulations to test how these cells might regulate anagen initiation via 1) inter-mesenchymal compartmental crosstalk to regulate DP-derived quiescence/activating signals and 2) direct quiescence/activating niche inputs to the hair follicle SCs (Figure 3). Further, hfDSC-specific tools would facilitate discovery of molecular mechanisms that control hfDSC identity and potency, which are essentially unknown outside of a possible role for PDGF signaling in hfDSC expansion (86).
4.5 –. Dermal sheath niche functions in human hair follicles
In the murine pelage, the only known niche role for the DS in the direct regulation of the hair cycle is to functionally contract during follicle regression to extrude the hair shaft and relocate the DP (20). Meanwhile, there are no known niche roles for the human DS. In the coming years, it will be essential to translate novel discoveries of DS niche functions in the murine pelage and other rodent models to human hair follicle biology. In terms of functional DS contraction, there are a few clues to indicate a similar paradigm may exist in human hair. Similar to rodent DS (87), αSMA expression is higher in the proximal region of the human anagen DS than in the distal region (23). Further, like αSMA, myosin light chain (MYL9) and myosin heavy chain (MYH11) are specifically localized to the innermost CTS fibroblasts that comprise the human DS (20). However, it remains unknown whether human DS expresses the full complement of smooth muscle molecularly machinery and whether human DS functionally contracts to power follicle regression, akin to the murine pelage system.
Similar to the murine pelage follicles, it remains a fascinating possibility that human DS might function as a signaling niche to regulate stem/progenitor cell activity through direct molecular crosstalk and indirectly through modulation of other niche constituents. For instance, expression profiling of human DS and observations of dynamic perifollicular blood capillary remodeling during human hair cycling led Kishimoto and colleagues to conclude that CD36-expressing DS cells may be associated with angiogenesis during early anagen (88), which may in turn influence progenitor activity in the growing follicle. Another study performed transcriptomic profiling of human upper DS, DS cup, and DP and revealed compartment-specific signature genes, including signaling molecules such as BMP antagonist GREM2 that the upper DS and DS cup may secrete to regulate progenitor activity (89), although such possibilities remain functionally untested.
One assay that will facilitate the study of both physical and molecular signaling DS niche functions in human hair follicle biology is human follicle organ culture (HFOC) (90). With the caveat of being an ex vivo model and therefore unable to recapitulate the broader in vivo niche, this experimental approach has yielded fruitful insights into the regulation of human hair growth, anagen-to-catagen transition, and catagen progression (91, 92). By long-term follicle culture and subsequent staging, discoveries of DS niche roles in the human hair cycle may be achieved. Utilizing agonists/antagonists of DS-specific receptors and performing siRNA knockdowns of DS-specific genes in the HFOC system will both lead to novel discoveries and validate findings from the murine pelage.
5 |. THE DERMAL SHEATH AS A FRONTIER OF TRANSLATION POTENTIAL
The DS holds largely untapped potential on multiple translational fronts. Can the DS be utilized in cell-based therapies to prevent hair loss by promoting hair follicle induction and/or regeneration? In the same vein, can the DS be exploited as a cell-based therapeutic in wound healing to enhance regeneration of fully functional skin? At the same time, pharmacological DS manipulation has never been pursued and therefore represents a novel therapeutic possibility for disorders of hair loss and overgrowth.
5.1 –. Dermal sheath cell-based therapies in hair loss
A series of early studies established the DS as a prime candidate for use in cell-based therapies to reverse hair loss. First, it was demonstrated that regeneration of amputated vibrissae follicles could be restored upon upper epidermal implantation of DS (49). Extending this work to demonstrate hair inductive capacity of the DS in a wounding scenario, Jahoda and colleagues observed de novo hair follicle formation upon co-implanting cultured DS and ORS into small ear skin wounds (93). In a non-wounding scenario, hair induction by cultured DS was later demonstrated upon implantation of murine vibrissae DS cup cells into ears and footpads (51). Collectively, these experiments revealed that implanted DS can provide hair follicle-inductive signals to remodel the epidermis and give rise to DP.
In humans, DS-induced de novo hair follicle formation was observed in a striking pilot experiment in which DS was isolated from a male scalp follicle and transplanted into female forearm skin (50). Two decades later, recently reported data highlights the promise of DS cell-based therapy in treating hair loss. First, injected human DS cup cells at the site of reconstituted human hair follicles on nude mouse skin demonstrated proper localization to the mesenchymal niche and even contributed to the DP (94). Subsequently, in a clinical human study, DS cup cells were isolated from the patients’ occipital hair follicles and then injected into randomized regions of the scalp at varying doses (95). Six- and nine months post-injection, increased hair density and diameter at DS cup injection sites were observed, although effects decreased by 12 months.
A comprehensive understanding of the DS niche will likely be the first step of developing and improving DS cell-based therapeutics, since recapitulating the in vivo niche when culturing DP is crucial to maintaining its hair-inductive activity, for example by culturing DP as spheroids (96) or in the presence of Wnt and BMP ligands (14, 15). For DS, culturing in presence of PDGF (86) or Wnt-enhancing RSPO ligands (38) might augment expansion of DS in culture while retaining HF inductive capacity. In the future, combining bulk and single cell transcriptomics will reveal candidate genes and signaling pathways that confer DS hair inductivity – prime for exploitation in cell-based therapeutics – as well as the full scope of DS heterogeneity.
5.2 –. Dermal sheath cell-based therapies in wound healing
A provocative, longstanding hypothesis is that DS cells participate in wound healing by giving rise to wound healing myofibroblasts and/or contributing to the dermis (97), yet few data in support of this theory have been reported. Still, it was shown that DS cells implanted into wounds in rat body skin incorporate into the dermis (98) and that, in the murine pelage, grafted and genetically tagged follicle-associated dermal cells migrate out into the dermis in response to punch wounds (99). More recently, it was demonstrated that cultured human DS accelerates wound healing in diabetic mice (100). Together, these intriguing data hint at the possibility that DS may contribute to wound healing in vivo.
Therefore, it is an appealing possibility that the DS is a plastic cell type with potential to be utilized and engineered in cell-based therapies for enhancement of wound healing.
While it was recently shown that the DS becomes differentially recruited into DP fate during wound-induced hair growth, suggestive of increased DS plasticity during wounding (101), it is unknown whether the DS or its resident hfDSCs differentiate into non-hair follicle mesenchymal lineages in vivo. Future investigations should address whether the DS emigrates from its hair follicle niche to assist in healing and, if so, identify the DS-derived cell types that contribute to healing.
For instance, it is plausible that the DS serves as a progenitor pool for wound healing fibroblasts (97). Another intriguing possibility is that the DS gives rise to adipocyte precursors and regulates fat regeneration during wound healing, which itself can contribute to functional wound healing (102, 103). This hypothesis stems from exciting recent data that demonstrates large adult mouse wounds can regenerate cutaneous fat from myofibroblasts – a phenomenon restricted to regions where new hair follicles form (104). Crucially, dermal cells only differentiated into adipocytes in vitro if isolated from hair follicle-containing regions and lineage tracing used αSMA-CreER, which also drives robust recombination in the DS (37). While the authors excluded the DP, vascular smooth muscle, and panniculus carnosus muscle as possible contributors of adipocyte precursor generation, they did not address potential contributions from the DS. In light of such possibilities, it is tantalizing to consider how DS plasticity might be leveraged to facilitate regeneration of skin that harbors all the appendages and cell types – including adipocytes – of healthy skin.
5.3 –. Pharmacological DS manipulation: novel therapeutic possibilities
While recent studies have begun to probe the possibilities of utilizing DS in cell-based therapies, the prospect of pharmacologically manipulating the DS remains wholly unexplored. Based on recent data that revealed an indispensable role of DS contraction in hair follicle regression (20) (see Chapter 3.1), pharmacological modulation of DS contraction may be a novel therapeutic avenue for disorders of hair loss and overgrowth (Figure 4). Considering DS contraction is the driving force underlying upward extrusion of the hair shaft, topical or intradermal administration of small molecule contraction inhibitors may effectively allow the hair shaft to be retained and slow the progression of hair loss. Conversely, topical or intradermal administration of contraction agonists may facilitate the extrusion of hair shafts in regions of unwanted hair overgrowth. Further uncovering of the molecular mechanisms controlling homeostatic DS contraction during the hair cycle will likely facilitate pharmacological targeting of specific DS receptors. As future studies continue unraveling specialized DS niche functions, many more therapeutic possibilities will likely become evident. For instance, once molecular signals are deciphered that regulate DS proliferation and contribution to the DP, it is conceivable that pharmacological approaches could be taken to modulate DP size and influence hair growth.
Figure 4.
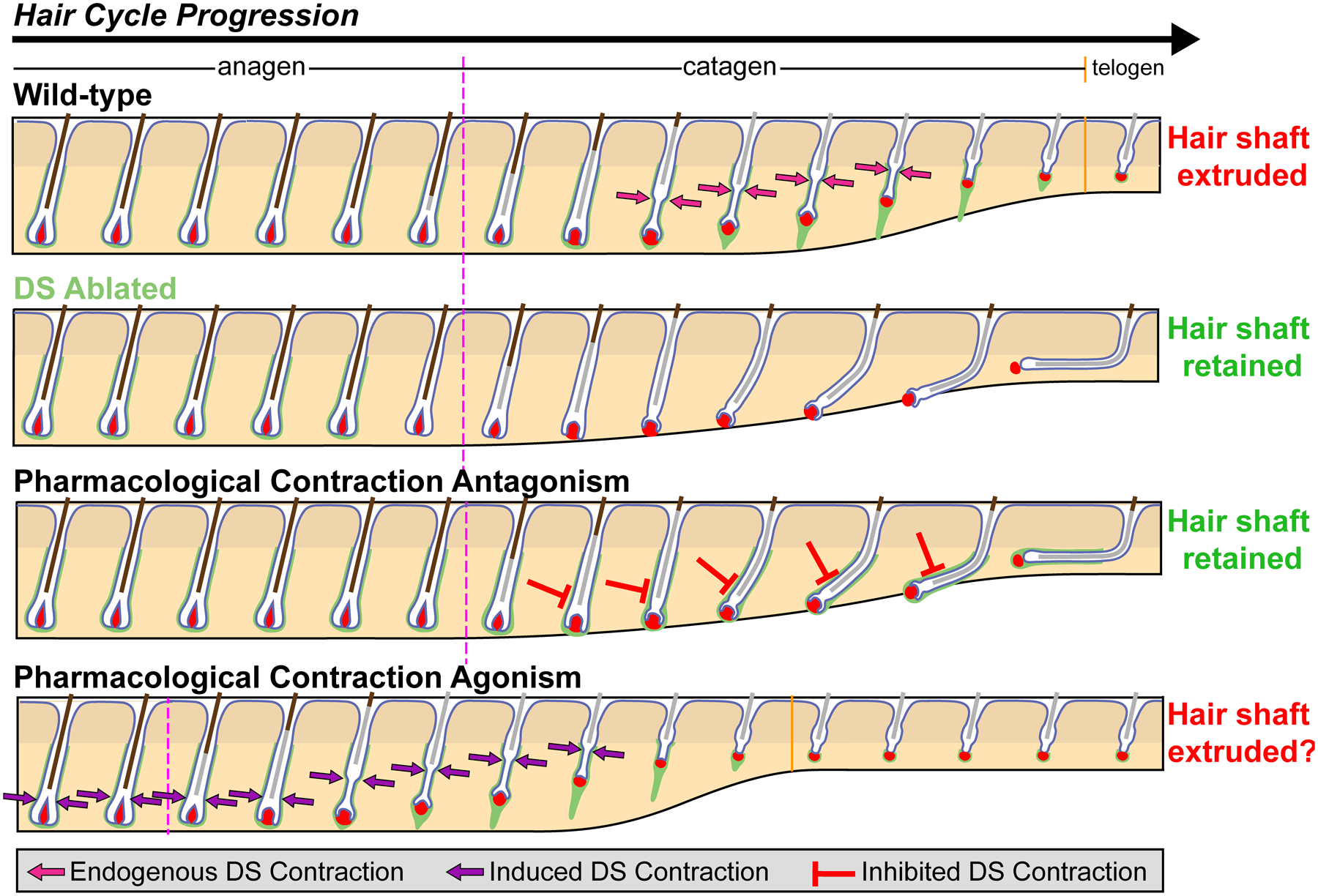
Pharmacological manipulation of DS contraction as a novel therapeutic possibility. During homeostatic hair cycle (Wild-type), the DS functions as a smooth muscle that contracts at the interface of the club hair and epithelial strand (termed the “bottleneck”) to push the hair shaft upward, thereby extruding it during follicle regression (20). When the DS is cytotoxically ablated (DS Ablated), the hair shaft is retained as follicles fail to regress. Similarly, when smooth muscle contraction is pharmacologically inhibited, the hair shaft is also retained as follicles again fail to regress. On the other hand, it may be possible to also induce hair shaft extrusion via pharmacological contraction, although the potential for this approach remains untested.
6 |. CONCLUSION AND PERSPECTIVES
The DS, now recognized for its critical importance in hair cycling, is a fascinating cell type that has long lived in the shadow of its mesenchymal neighbor – the DP – and been largely overlooked recently by the hair research community. It is an exciting prospect that this decade will see revived focus on the DS and the birth of a new era unraveling DS niche functions. By generating DS-specific genetic tools and combining bulk and single cell transcriptomics, live imaging, and lineage tracing, the field should see a revolution in our understanding of the DS niche in the coming years (105). This, in turn, will be the key to unlocking effective DS cell-based and pharmacological therapies to combat hair loss and promote regeneration of healthy skin during wound healing.
ACKNOWLEDGMENTS
We thank Nivedita Saxena and all members of our laboratory for helpful comments on the manuscript and valuable discussions. P.M. was supported by NIH/NIAMS grant R01AR071047. N.H. was supported by training grants T32GM007280 from NIH/NIGMS, T32HHD075735 from NIH/NIDCR, and F30AR070639 from NIH/NIAMS. M.R. was supported by grants from NIH/NIAMS (R01AR071047; R01AR063151) and the New York State Department of Health (NYSTEM-C029574; NYSTEM-C32561GG). We apologize to all colleagues whose relevant work we could not discuss due to space limitations.
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflicting interests.
REFERENCES
- 1.Müller-Röver S et al. , J. Invest. Dermatol 117, 3–15 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Cotsarelis G, Sun TT, Lavker RM, Cell 61, 1329–1337 (1990). [DOI] [PubMed] [Google Scholar]
- 3.Lee J, Tumbar T, Semin. Cell Dev. Biol 23, 906–916 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu Y, Li L, Fuchs E, Nat. Med 20, 847–856 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzales KAU, Fuchs E, Dev. Cell 43, 387–401 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sennett R, Rendl M, Semin. Cell Dev. Biol 23, 917–927 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein J, Horsley V, Cell. Mol. Life Sci 69, 2573–2582 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rezza A, Sennett R, Rendl M, Curr. Top. Dev. Bio 107, 333–372 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Cohen J, J. Embryol. Exp. Morphol 9, 117–127 (1961). [PubMed] [Google Scholar]
- 10.Oliver RF, J. Embryol. Exp. Morphol 18, 43–51 (1967). [PubMed] [Google Scholar]
- 11.Kollar EJ, J. Invest. Dermatol 55, 374–378 (1970). [DOI] [PubMed] [Google Scholar]
- 12.Jahoda CAB, Horne KA, Oliver RF, Nature 311, 560–562 (1984). [DOI] [PubMed] [Google Scholar]
- 13.Yang C-C, Cotsarelis G, J. Dermatol. Sci 57, 2–11 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishimoto J, Burgeson RE, Morgan BA, Genes Dev 14, 1181–1185 (2000). [PMC free article] [PubMed] [Google Scholar]
- 15.Rendl M, Polak L, Fuchs E, Genes Dev 22, 543–557 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds AJ, Jahoda CAB, Development. 115, 587–593 (1992). [DOI] [PubMed] [Google Scholar]
- 17.Grisanti L et al. , J. Invest. Dermatol 133, 344–353 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parakkal PF, J. Cell Biol 40, 561–564 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jahoda CAB, Reynolds AJ, Chaponnier C, Forester JC, Gabbiani G, J. Cell Sci 99, 627–636 (1991). [DOI] [PubMed] [Google Scholar]
- 20.Heitman N et al. , Science 367, 161–166 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider MR, Schmidt-Ullrich R, Paus R, Curr. Biol 19, R132–R142 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Oh JW et al. , J. Invest. Dermatol 136, 33–44 (2016). [Google Scholar]
- 23.Urabe A, Furumura M, Imayama S, Nakayama J, Hori Y, Arch. Dermatol. Res 284, 246–249 (1992). [DOI] [PubMed] [Google Scholar]
- 24.Ito M, Sato Y, Arch. Dermatol. Res 282, 434–441 (1990). [DOI] [PubMed] [Google Scholar]
- 25.Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y, Cell 104, 233–245 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM, Cell 102, 451–461 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Morris RJ et al. , Nat. Biotechnol 22, 411–417 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Tumbar T et al. , Science 303, 359–363 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E, Cell 118, 635–648 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Weber L, Kriegf T, Muller PK, Kirsch E, Br. J. Dermatol 106, 267–273 (1982). [DOI] [PubMed] [Google Scholar]
- 31.Messenger AG, Elliott K, Temple A, Randall VA, J. Invest. Dermatol 96, 93– 97 (1991). [DOI] [PubMed] [Google Scholar]
- 32.De Weert J, Kint A, Geerts ML, Arch. Dermatol. Res 272, 79–92 (1981). [DOI] [PubMed] [Google Scholar]
- 33.Hsu YC, Pasolli HA, Fuchs E, Cell 144, 92–105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rompolas P, Mesa KR, Greco V, Nature 502, 513–518 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xin T, Gonzalez D, Rompolas P, Greco V, Nat. Cell Biol 20, 1361–1369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T, Cell Stem Cell 5, 267–278 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahmani W et al. , Dev. Cell 31, 543–558 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Hagner A et al. , iScience. 23, 101019 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin W et al. , Dev. Cell 53, 185–198 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Joost S et al. , Cell Stem Cell 26, 441–457 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Lindner G et al. , Am. J. Pathol 151, 1601–1617 (1997). [PMC free article] [PubMed] [Google Scholar]
- 42.Mesa KR et al. , Nature 522, 94–97 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Couchman JR, Gibson WT, Dev. Biol 108, 290–298 (1985). [DOI] [PubMed] [Google Scholar]
- 44.Commo S, Bernard BA, Br. J. Dermatol 137, 31–38 (1997). [PubMed] [Google Scholar]
- 45.Horenstein MG, Jacob JS, J. Cutan. Pathol 35, 1115–1120 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Sinclair R et al. , J. Invest. Dermatol. Symp. Proc 8, 56–64 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Müller-Röver S, Peters EJM, Botchkarev VA, Panteleyev A, Paus R, J. Histochem. Cytochem 46, 1401–1409 (1998). [DOI] [PubMed] [Google Scholar]
- 48.Oliver RF, J. Embryol. Exp. Morphol 15, 331–347 (1966). [PubMed] [Google Scholar]
- 49.Horne KA, Jahoda CAB, Development 116, 563–571 (1992). [DOI] [PubMed] [Google Scholar]
- 50.Reynolds AJ, Lawrence C, Cserhalmi-Friedman PB, Christiano AM, Jahoda CAB, Nature 402, 33–34 (1999). [DOI] [PubMed] [Google Scholar]
- 51.McElwee KJ, Kissling S, Wenzel E, Huth A, Hoffmann R, J. Invest. Dermatol 121, 1267–1275 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Chi W, Wu E, Morgan BA, Development 1683, 1676–1683 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mann SJ, Anat. Rec 170, 485–499 (1971). [DOI] [PubMed] [Google Scholar]
- 54.Panteleyev AA, Paus R, Ahmad W, Sundberg JP, Christiano AM, Exp. Dermatol 7, 249–267 (1998). [DOI] [PubMed] [Google Scholar]
- 55.Bikle DD, Elalieh H, Chang S, Xie Z, Sundberg JP, J. Cell. Physiol 207, 340– 353 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Rompolas P et al. , Nature 487, 496–499 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greco V et al. , Cell Stem Cell 4, 155–169 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oshimori N, Fuchs E, Cell Stem Cell 10, 63–75 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clavel C et al. , Dev. Cell 23, 981–994 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramos R, Guerrero-Juarez CF, Plikus MV, J. Invest. Dermatol 133, 2306– 2308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morgan BA, Cold Spring Harb. Perspect. Med 4, 1–14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rendl M, Lewis L, Fuchs E, PLoS Biol 3, 1910–1924 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stenn KS, Paus R, Physiol. Rev 81, 449–494 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Ribatti D, Santoiemma M, Int. J. Dev. Biol 58, 303–306 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Scadden DT, Nature 441, 1075–1079 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Millar SE, J. Invest. Dermatol 118, 216–225 (2002). [DOI] [PubMed] [Google Scholar]
- 67.Saxena N, Mok KW, Rendl M, Exp. Dermatol 28, 332–344 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kosinski C et al. , Proc. Natl. Acad. Sci. U. S. A 104, 15418–15423 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gehart H, Clevers H, Nat. Rev. Gastroenterol. Hepatol 16, 19–34 (2019). [DOI] [PubMed] [Google Scholar]
- 70.McCarthy N et al. , Cell Stem Cell 26, 391–402 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Agabalyan NA, Rosin NL, Rahmani W, Biernaskie J, Exp. Dermatol 26, 505– 509 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Hoi CSL et al. , Mol. Cell. Biol 30, 2518–2536 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Osorio KM et al. , Development 135, 1059–1068 (2008). [DOI] [PubMed] [Google Scholar]
- 74.Wang AB, V Zhang Y, Tumbar T, EMBO J 36, 61–78 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang H, Adam RC, Ge Y, Hua ZL, Fuchs E, Cell 169, 483–496 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL, Cell Stem Cell 8, 552–565 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li KN et al. , Elife 8, 1–27 (2019). [Google Scholar]
- 78.Gur-Cohen S et al. , Science 366, 1218–1225 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peña‐Jimenez D et al. , EMBO J. 38, e101688 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Plikus MV et al. , Nature 451, 340–344 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Festa E et al. , Cell 146, 761–771 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ali N et al. , Cell 169, 1119–1129 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Castellana D, Paus R, Perez-Moreno M, PLoS Biol. 12, e1002002 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen C et al. , Cell 161, 277–290 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Christoph T et al. , Br. J. Dermatol 142, 862–873 (2000). [DOI] [PubMed] [Google Scholar]
- 86.González R et al. , npj Regen. Med 2, 1–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reynolds AJ, Chaponnier C, Jahoda CAB, Gabbiani G, J. Invest. Dermatol 101, 577–583 (1993). [DOI] [PubMed] [Google Scholar]
- 88.Yoshida Y, Soma T, Kishimoto J, Biochem. Biophys. Res. Commun 516, 945– 950 (2019). [DOI] [PubMed] [Google Scholar]
- 89.Niiyama S et al. , Acta Derm. Venereol 98, 694–698 (2018). [DOI] [PubMed] [Google Scholar]
- 90.Langan EA, Philpott MP, Kloepper JE, Paus R, Exp. Dermatol 24, 200–215 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Hawkshaw NJ et al. , PLoS Biol 16, 1–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chéret J et al. , Nat. Commun 9, 1–12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reynolds AJ, Jahoda CAB, Development 3094, 3085–3094 (1996). [DOI] [PubMed] [Google Scholar]
- 94.Yoshida Y, Soma T, Matsuzaki T, Kishimoto J, Biochem. Biophys. Res. Commun 516, 599–605 (2019). [DOI] [PubMed] [Google Scholar]
- 95.Tsuboi R et al. , J. Am. Acad. Dermatol 83, 109–116 (2020). [DOI] [PubMed] [Google Scholar]
- 96.Higgins CA, Chen JC, Cerise JE, Jahoda CAB, Christiano AM, Proc. Natl. Acad. Sci. U. S. A 110, 19679–19688 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jahoda CAB, Reynolds AJ, Lancet 358, 1445–1448 (2001). [DOI] [PubMed] [Google Scholar]
- 98.Gharzi A, Reynolds A, Jahoda CAB, Exp. Dermatol 12, 126–136 (2003). [DOI] [PubMed] [Google Scholar]
- 99.Biernaskie J et al. , Cell Stem Cell 5, 610–623 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ma D, Kua J, Lim W, Lee S, Chua A, Cytotherapy 17, 1036–1051 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Abbasi S, Biernaskie J, Exp. Dermatol 28, 419–424 (2019). [DOI] [PubMed] [Google Scholar]
- 102.Schmidt BA, Horsley V, Development 140, 1517–1527 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shook BA et al. , Cell Stem Cell 26, 880–895 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Plikus MV et al. , Science 355, 748–752 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heitman N, Saxena N, Rendl M, Curr. Opin. Cell Biol 55, 87–95 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


