Abstract
Evaluation and effective management of asthma, and in particular severe asthma, remains at the core of pulmonary practice. Over the last 20–30 years, there has been increasing appreciation that “severe asthma” encompasses multiple different subgroups or phenotypes, each with differing presentations. Using clinical phenotyping, in combination with rapidly advancing molecular tools and targeted monoclonal antibodies (human knockouts), the understanding of these phenotypes, and our ability to treat them, have greatly advanced. Type-2 (T2)-high and -low severe asthmas are now easily identified. Fractional exhaled nitric oxide and blood eosinophil counts can be routinely employed in clinical settings to identify these phenotypes and predict responses to specific therapies, meeting the initial goals of precision medicine. Integration of molecular signals, biomarkers, and clinical responses to targeted therapies has enabled identification of critical molecular pathways and, in certain phenotypes, advanced them to near-endotype status. Despite these advances, little guidance is available to determine which class of biologic is appropriate for a given patient, and current “breakthrough” therapies remain expensive and even inaccessible to many patients. Many of the most severe asthmas, with and without T2-biomarker elevations, remain poorly understood and treated. Nevertheless, conceptual understanding of “the severe asthmas” has evolved dramatically in a mere 25 years, leading to dramatic improvements in the lives of many.
Keywords: asthma, cytokines, biologic therapy, type 2 asthma, eosinophils
Compared with the understanding of many core pulmonary diseases, the understanding of asthma and severe asthma has improved exponentially over the last 20–30 years, including understanding concerning the application of precision-medicine approaches. Despite these advances, much remains to be biologically and immunologically clarified. Current “breakthrough” therapies are expensive and often inaccessible, and many patients remain poorly understood and treated. Yet conceptual understanding of “the severe asthmas” is now vastly different from what it was a mere 25 years ago, with accompanying dramatic improvement in the lives of many.
Definition
Advances in the understanding of severe asthma accelerated ∼20 years ago when severe asthma was recognized as a distinct grouping of entities. Severe asthma was specifically defined, first in 2000 by an American Thoracic Society (ATS) workshop, then by the World Health Organization (for a more global definition with relevance to poorly developed and developing countries), and most recently by the European Respiratory Society (ERS)–ATS guidelines (1–3). Underlying obstructions with reversibility or reactivity, current or previous, remain at the heart of identifying severe asthma, such that confirmation of an asthma diagnosis, by original ATS definitions, is mandatory (4). This need to link to current or historical reversible obstruction is sometimes lost when evaluating symptomatic patients, despite guideline-appropriate therapy, leading to inaccurate diagnoses. In addition, lack of reversibility to normal should not invoke a diagnosis of chronic obstructive pulmonary disease; rather, it is a hallmark of severe asthmatic disease (1).
Once an asthma diagnosis is established, ERS–ATS–defined severe asthma (Box 1) is “asthma which requires treatment with high-dose corticosteroids (CSs), plus a second controller, to remain controlled or which remains uncontrolled despite this therapy” (1). Control is defined by symptoms, exacerbations, and degree of obstruction. Yet the current severe asthma definition remains an umbrella definition (5). It can encompass smokers, ex-smokers, and nonsmokers and a broad range of underlying pathobiologies or molecular phenotypes. Dissecting them is critical to improving treatment.
Box 1. European Respiratory Society–American Thoracic Society Definition of Severe Asthma
Severe asthma is defined as asthma that requires treatment with high-dose inhaled corticosteroids (≥1,000 μg of fluticasone propionate or equivalent) plus a second controller (and/or systemic corticosteroids) to prevent it from becoming “uncontrolled” or as asthma that remains “uncontrolled” despite this therapy.
Inadequate control is defined by any of the following:
• Poor symptom control: ACQ (Asthma Control Questionnaire) score >1.5 or ACT (Asthma Control Test) score <20 (or “not well controlled” according to National Asthma Education and Prevention Program or Global Initiative for Asthma guidelines) over 3 months of evaluation
• Frequent severe exacerbations: two or more systemic corticosteroid bursts (>3 d each)
• Serious exacerbations: at least one hospitalization or ICU stay or mechanical ventilation in the previous year
• Airflow limitation: FEV1 < 80% predicted (in presence of reduced FEV1/FVC) after bronchodilator medication is withheld
Prevalence
Despite improved definitions, the current prevalence of severe asthma remains poorly understood. The best estimates range from 4% to 10% of the asthma population (6, 7). Much of this uncertainly is due to a disconnect between clinical diagnosis and physiologic confirmation and the necessity of determining whether patients are treated and adhering to treatment appropriately. Severe asthma, although more common in the very young, as compared with older children, appears to become less common in adolescence and early adulthood, only to appear or reappear in midadult life (8, 9). Although it is predominantly a male disease in childhood, severe asthma, like adult asthma overall, largely affects females (8, 10), perhaps because of resolution in boys and increased adult incidence in women. In three different cohorts, 45–50% of patients with severe asthma reported adult-onset (>12 yr old) disease, the majority of whom were females (8, 11, 12). In contrast, resolution of severe asthma (not asthma itself) was observed in 60% of children in SARP (Severe Asthma Research Program), 3–4 years after enrollment (median age 11 yr old) (13). Little is understood regarding this temporary or perhaps permanent resolution. However, anecdotally, many patients report childhood disease, resolution, and subsequent second worsening in adulthood for unclear reasons.
Clinical Evaluation
With five biologic therapies available for severe asthma, an accurate phenotypic diagnosis and subsequent assessment are paramount. A detailed history should include questions on the timeline for onset of respiratory symptoms and their severity. If asthma was not diagnosed in a patient in childhood or if a patient does not remember the diagnosis, frequent school absence or bronchitic episodes are suggestive of early/childhood-onset asthma (EOA). Comorbidities from sinus disease, with or without nasal polyps, to gastroesophageal reflux disease and atopic dermatitis (or other rashes) should be assessed (see Box 2 for a more complete list, associated symptoms, and treatments). Triggers should be addressed, although “allergic” symptoms often poorly relate to specific IgE testing. In adolescent and adult patients, smoking/vaping history and occupational history are essential. Finally, in severe asthma, personal or family history of asthma and autoimmune disease, including autoimmune thyroid disease, should be recorded.
Box 2. Common Comorbidities Associated with Severe Asthma
Comorbidities, symptoms of which can also mimic asthma
• Gastroesophageal reflux: symptoms include cough, nocturnal awakening, and chest tightness
• Chronic rhinosinusitis: symptoms include cough, upper-airway wheeze, and sputum production
• Obesity: symptoms include shortness of breath
• Vocal-cord dysfunction: symptoms include wheeze, shortness of breath, and chest tightness
• Anxiety: symptoms include shortness of breath and chest tightness
Comorbidities, treatment of which may improve underlying asthma
• Allergic reactions: potential treatments include decreasing exposure, rhinitis therapies, and anti-IgE or anti-IL4R therapies
• Obesity: potential treatments include weight loss and bariatric surgery
• Aspiration: potential treatments include swallowing precautions and gastroesophageal reflux disease treatments
• Smoking/vaping: potential treatments include smoking/vaping cessation
• Autoimmune disease: potential treatments include nonsteroidal immunosuppressive approaches
Physiologic and Laboratory Testing
The presence of current or previous reversible airway obstruction as assessed by spirometry or methacholine challenge is crucial to confirming the severe asthma diagnosis. If obstructive criteria are not met initially (FEV1 < 80% predicted before bronchodilator use in the setting of age-adjusted reduced FEV1/FVC), repeat testing, ideally with symptom worsening or medication tapering, is often helpful before methacholine challenge testing is performed. When airway obstruction is absent, but symptoms persist, it is important to consider that asthma may not be the correct diagnosis. Methacholine-challenge testing is likely to be most helpful to rule out asthma in patients with relatively normal FEV1% predicted. Figure 1A summarizes these suggestions in a flow diagram for approaching patients with a severe or difficult asthma diagnosis.
Figure 1.


(A) Suggested flow diagram for the initial assessment of a patient with difficult asthma, before confirmation of diagnosis of severe asthma, based on current or previous evidence for airway obstruction, with or without reversibility. (B) Suggested flow diagram for the initial evaluation of a patient after confirming a diagnosis of severe asthma. Each algorithm includes initial and continued assessment and treatment of risk factors and comorbid conditions, as well as prescription of and adherence to standard therapies at each step. FeNO-Hi is characterized by >24 ppb, and Eos-Hi is characterized by 300 cells/μl, although in some cases, ≥150 cells/μl may be sufficient (see text for further details). ACO = asthma–COPD overlap; AG = asthmatic granulomatosis; COPD = chronic obstructive pulmonary disease; CRP = C-reactive protein; CT = computed tomography; EGPA = eosinophilic granulomatosis with polyangiitis; Eos = eosinophils; eval = evaluation; FeNO = fraction of exhaled NO; Hi = high; HP = hypersensitivity pneumonitis; HS = Hi-sensitivity; LAMA = long-acting muscarinic antagonist; Lo = low; Neg = negative; OCS = oral corticosteroid; Pos = positive; pred = predicted; T2 = type 2; VATS = video-assisted thoracoscopic surgery.
Together with spirometry, biomarkers for type 2 (T2) inflammation, specifically blood eosinophils and fraction of exhaled NO (FeNO) should be evaluated. Historical blood eosinophilia should be ascertained in electronic health records and in relation to CS treatment. Limited data demonstrate changes in blood eosinophils over time, such that a single low blood eosinophil count should not categorize a patient as having low eosinophils (14). This is particularly important if the initial presentation is during or up to 4 weeks after a CS burst. Similarly, FeNO concentrations above 24 ppb (or even 20 ppb), on high-dose inhaled CSs (ICSs), or particularly systemic CSs, are not normal, and may indicate systemic CS-dependent disease (15, 16). In biologic treatment trials of severe asthma with reversible obstruction (typically 20–25% improvement in FEV1), persistently elevated T2 biomarkers identify the most exacerbation-prone patients with severe asthma (17–20). Depending on the severity and frequency of symptoms, exacerbations, and response to therapies, even patients who meet physiologic criteria for asthma may benefit from further testing, including plethysmographic lung-function testing (including FRC, residual volume, and conductance) and diffusing capacity, CT imaging (tree-in-bud, bronchiectasis, ground-glass opacities), and blood testing (antinuclear antibodies and antineutrophilic cytoplasmic antibodies, erythrocyte sedimentation rate, CRP [C-reactive protein]), quantitative immunoglobulins, and IgE/specific IgE testing). Figure 1B is a suggested schematic approach to evaluation of physiologically confirmed severe asthma.
Additional Diagnostic Approaches
Every patient evaluation requires assessment of adherence, typically assessed through pharmacy records or relatively inexpensive electronic monitoring (21, 22). However, poor adherence does not fully explain severe disease. Understanding responses to systemic CSs can be helpful, with T2 inflammation and late-onset disease being associated with the best responses (23). CS responsiveness also identifies patients in whom T2 biologic therapies may work best, or who may benefit from low-dose oral CSs when biologics are unavailable (24). Those who require continuous systemic CSs or who never have elevated T2 biomarker concentrations are likely to have a more complicated form of asthma or something other than asthma (23). Consideration should be given to aspiration, hypersensitivity pneumonitis, immunodeficiency, or autoimmune airway disease (Figures 1A and 1B), which may benefit from video-assisted thoracoscopic biopsies (25). Persistence of T2 biomarkers despite the use of systemic CSs should precipitate evaluation for eosinophilic granulomatosis with polyangiitis (EGPA) (in the presence of systemic symptoms), asthmatic granulomatosis (without systemic symptoms), or autoimmune-associated airway disease (26–28). Autoimmune-associated asthma remains to be fully defined and characterized but could include asthma associated with autoimmune thyroid disease, rheumatoid arthritis, inflammatory bowel disease, or Sjögren’s syndrome, often with accompanying elevated CRP and/or sedimentation rates.
Clinical and Molecular Biomarkers and Phenotyping
T2 Phenotyping
Currently, after an asthma diagnosis is confirmed, the most informative clinical phenotyping is determination of T2 status (Figure 1B). Minimum blood eosinophil concentrations to identify T2-high (T2-Hi) CS-treated disease range from 150 to 300 eosinophils/μl, whereas minimum FeNO concentrations range from 20 to 25 ppb (16, 17, 29). Although the relation of these cut points to lung eosinophils or T2 inflammation in severe asthma is less clear (30, 31), treatment with IL-4 receptor–targeted antibodies is efficacious in patients meeting these cut points, supportive of active T2 immunity, even with lower T2 biomarkers (17). The prevalence of T2-Hi severe asthma is unclear, but nearly 65% of patients enrolled in a European study, U-BIOPRED (Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes), had elevated sputum eosinophils despite high-dose CSs (32). Accurate T2 phenotyping in patients on systemic CSs may require CS tapering and reevaluation with symptom onset/worsening (Figure 1B). Patients who repeatedly have no elevations in T2 biomarkers would be considered T2-low (T2-Lo).
Age at Onset
Although T2 phenotyping is well established and predictive of treatment responses, additional clinical features also may determine responses to therapy, including age at onset of symptoms (Figure 1B). Age at onset is consistently associated with differing clinical asthma characteristics, despite similar elevations in T2 biomarkers (11, 33–35). EOA (typically before ages of 13–18 yr) is consistently associated with allergic symptoms/specific IgE to environmental allergens. Despite elevated allergic biomarkers, T2 biomarker concentrations are variable. Like EOA, later-onset asthma (LOA) can be associated or not with T2 inflammation. However, unlike EOA, when T2 biomarkers are elevated, LOA T2-Hi asthma associates with even higher blood and lung eosinophil concentrations, more nasal polyps, and less specific IgE (12, 34). Obesity may be seen across all groups, but a subgroup with LOA is associated with very low T2 and allergic/IgE biomarker concentrations (11, 36). Molecular or even physiologic mechanisms remain poorly understood (37, 38). These profound differences in EOA versus LOA with similar T2-biomarker variability suggest different underlying molecular mechanisms.
Plasma IL-6
Plasma IL-6 concentrations have also been associated with different clinical characteristics. They are not associated with T2 inflammation and, indeed, appear to be independent of it (39). IL-6 concentrations are associated with more exacerbation-prone disease, obesity, and peripheral blood neutrophilia in cross-sectional analysis and have more recently been described to be predictive of asthma exacerbations, which are, again, independent of blood eosinophils (40). Although IL-6 is correlated with body mass index and age, IL-6 concentrations predicted exacerbations independently of these. Intriguingly, plasma IL-6 concentrations are not associated with increases in lung IL-6, suggesting a systemic immune source, and in fact, lung IL-6 transsignaling is associated with T2 inflammation (41). IL-6 concentrations are highly correlated with blood CRP concentrations, suggesting that these concentrations could be used to identify this at-risk group (42). Higher CRP concentrations have previously been associated with an increased risk of asthma, but severity was not addressed (43). However, until IL-6 concentrations are standardized, high-sensitivity CRP concentrations could potentially be substituted as a marker of more complex asthma, whether it is T2-Hi or T2-Lo asthma (Figure 1B).
Complex Immune Phenotypes
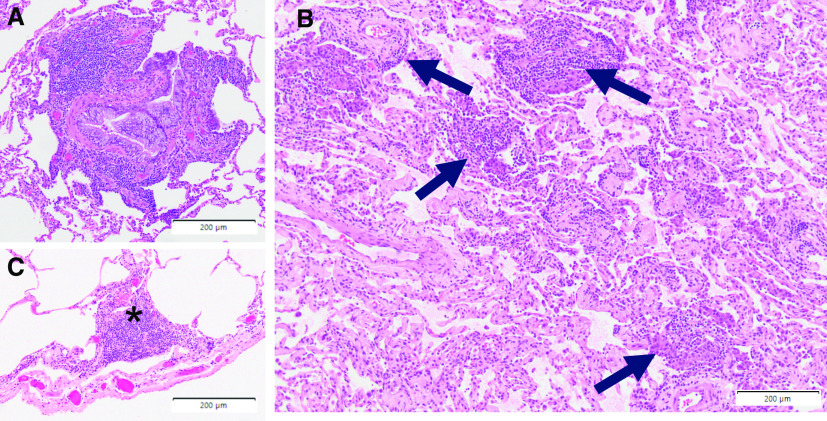
Whether systemic inflammation in addition to T2 immunity contributes to more severe or complex disease is unknown. However, patients with mixed immune pathways and biomarker elevations may also contribute to a very poorly defined phenotype of T2-plus asthma, many of whom may have a family or personal history of autoimmunity (27). CRP elevations are often seen in EGPA, as well as asthmatic granulomatosis, with concomitant evidence for elevations in eosinophils and FeNO (27, 28). Not surprisingly, these patients are variably responsive to T2-targeted biologics alone. Figure 2 depicts pathology from a patient with T2-Hi asthma, autoimmune thyroid disease, and inflammatory bowel disease, noting changes of both asthma and autoimmunity.
Figure 2.
Distal lung (VATS) tissue from a patient with physiologically defined T2-Hi severe asthma with background autoimmune thyroid and inflammatory bowel disease, potentially representing T2 complex asthma. (A) Evidence of severe eosinophilic and profoundly lymphocytic airway disease with goblet-cell hyperplasia. (B) Concurrent scattered interstitial perivascular mononuclear inflammatory-cell aggregates (identified by arrows). (C) Lymphocytic pleuritic inflammation characteristic of autoimmune lung disease (the asterisk identifies the inflammation). Scale bars, 200 μm. Hi = high; T2 = type 2; VATS = video-assisted thoracoscopic surgery.
Long-Term Trajectories
Little is understood regarding long-term trajectories of any of these clinically identifiable biologic-associated phenotypes. Longitudinal SARP data suggest that children with severe asthma, which is EOA by definition, may resolve their severe asthma, with high blood eosinophils being the best predictor of remission (13). Exacerbation-associated asthma, at least before T2 biologic intervention, appears to be a consistent characteristic of a poorly defined phenotype or poorly defined phenotypes, with baseline blood eosinophil concentrations, elevations of both eosinophils and neutrophils in sputum, and plasma IL-6 concentrations all being predictive of exacerbation-prone disease (40, 44). Elevations in both sputum eosinophils and neutrophils have also been associated with greater lung-function decline (44).
Biologic Implications of Current Therapeutic Approaches
Standard Controller Therapies
Step-up therapy and current ERS–ATS guidelines all recommend high-dose ICSs, with additional controller agents (long-acting β-agonists [LABAs]), long-acting muscarinic antagonists (LAMAs), or leukotriene modifiers as central therapies for patients with severe asthma. Treatment with some combination of these, with high-dose ICSs, is often required before biologic therapies can be started. Yet, the evidence to suggest these combinations are more than modestly better than lower doses in patients with severe asthma is limited. The only step-up study of medium- to high-dose ICS, in combination with a LABA, showed only small improvements in control with step-up to higher-dose ICSs plus a LABA (45). With recent control standards and increasing severity, less than 50% achieved well-controlled status (46). Similarly, severe exacerbations were not significantly reduced with high-dose salmeterol–fluticasone versus fluticasone alone in patients who entered the U.S. Food and Drug Administration (FDA)-mandated LABA safety study with poorly controlled asthma (47). Thus, even in controlled clinical-trial settings, efficacy is generally incremental. Another variation of ICS–LABA treatment is treatment with “maintenance and reliever therapy,” in which the combination of single-inhaler formoterol and an ICS is used both as controller and as reliever. This reliever approach reduces asthma exacerbations, as compared with either formoterol or terbutaline alone, in patients with mild-to-moderate or, possibly, more severe asthma (48–50). Pooled analyses and meta-analyses also support the efficacy of this approach in patients with Global Initiative for Asthma (GINA) step 4 asthma, with suggestions that severe exacerbations may be reduced by ∼30% (51–53). GINA recommends low-dose combination ICS–LABA treatment (specifically formoterol) as a reliever for asthma described by GINA steps 3–5 (54). Because of the complexities of the U.S. medical system, this approach is often financially challenging. Finally, the specific efficacy of this approach in symptomatic patients already on high-dose ICSs with a LABA (ERS–ATS severe asthma definition) remains to be determined.
Interestingly, BAL-fluid cells of patients receiving LABAs/ICSs combined with daily short-acting β-agonists show marked suppression of cyclic-AMP pathway genes, critical for host defense and repair, suggesting a potential long-term negative impact, which requires further investigation (55). β2-receptor polymorphisms could also play a role in poor responses, as rare SNPs have been associated with severe, exacerbation-prone disease, although the mechanisms for the effect remain to be determined (56). Finally, high-dose ICSs may not reach diseased smaller airways, given observed differences in T2 gene signatures in proximal and distal airways (57).
Tiotropium added to LABA–ICS treatment led to lung-function improvements and a 21% reduction in exacerbations compared with LABA–ICS treatment alone, whereas symptom control was not consistently improved (58). A triple LAMA–LABA–ICS combination (with umeclidinium) is now FDA approved for asthma on the basis of improvement in FEV1% predicted but was not studied in currently defined severe asthma (trial 4, NCT 02924688). Leukotriene modifiers have never been studied in severe asthma, and none of these medications have been phenotypically evaluated.
Macrolide Therapy
Two azithromycin trials have been performed in patients with more severe asthma on combination therapy, with differing target populations and results. The first study (N = 109) failed to reduce exacerbations or bronchitic infections in the total population. However, a predefined subgroup analysis of patients with blood eosinophil concentrations ≤200/μl demonstrated a significant reduction in these events (59). In contrast, a larger study reported a 41% reduction in asthma exacerbations with the addition of a higher azithromycin dose in a group with moderate-to-severe asthma, as well as in a subgroup with severe asthma (60, 61). In contrast to the smaller study, greater efficacy was seen in patients with eosinophilic inflammation. Emergence of macrolide-resistant bacterial strains occurred in both, suggesting patients should be monitored appropriately. Whether efficacy is through antibiotic or antiinflammatory effects (or both) is unknown. However, consideration of a trial of macrolide therapy should be entertained in both patients with T2-Hi asthma and patients with T2-Lo asthma before advancing to biologic therapy.
Targeted Biologic Therapies
Unlike the above therapies, the five approved monoclonal antibodies target three known biologic pathways, creating, in the simplest of terms, human “knockouts.” All show evidence of greater efficacy with increasing elevations of T2 biomarkers. These therapies differ in terms of targets, biomarkers, treatment of comorbid conditions, and, importantly, patient responses.
Anti-IgE
Given the association of allergy with IgE, it is not surprising that anti-IgE (omalizumab) was targeted to an allergic asthma phenotype. Consistent with this approach, it limits asthmatic responses to allergen challenge in mild asthma (62, 63). Clinical trials then targeted patients with moderate-to-severe asthma on medium- to higher-dose ICSs alone with elevated total IgE concentrations as well as at least one specific IgE. Patients were excluded because of combinations of high body mass index and total IgE, but within these parameters, omalizumab consistently reduced asthma exacerbations (64, 65), with small improvements in quality of life and lung function. When studied in patients with severe asthma as defined today, treatment with omalizumab reduced exacerbations by 25% compared with placebo treatment, with small improvements in quality of life (66). IgE concentrations did not predict responses, but when patients were stratified in a post hoc analysis by T2 biomarkers, exacerbations were reduced to a greater degree in those with the presence of both modestly elevated IgE and T2 biomarkers, despite similar IgE concentrations (16). Exacerbations overall were more common in the T2-Hi placebo groups, such that omalizumab lowered the rate to that of the T2-Lo group. This poor relation of IgE concentrations with traditional T2 biomarkers calls into question the overall biology driving elevated IgE. In the absence of elevated T2 biomarkers, involvement of other cell types, including GATA-3–independent IL-4 production by follicular T-helper cells, could also trigger B-cell proliferation and isotype switching (67, 68). Interestingly, anti-IgE has been suggested to decrease virus-related exacerbations in children through promotion of type 1 IFN expression by plasmacytoid dendritic cells because of reduced surface expression of FceRI, supporting an impact of anti-IgE beyond mast cells/basophils (69, 70). Omalizumab also recently showed efficacy in patients with nasal polyps with elevated IgE, suggesting that patients with both asthma and nasal polyps could dually benefit (71). Median blood eosinophil concentrations were well over 300/μl, consistent with ongoing T2 inflammation with elevated IgE, corresponding to the best-responding phenotype.
IL-4–Receptor Blockade
IL-4Rα blockade promotes broader reduction of T2 immunity, limiting the activity of both IL-4 and IL-13 and downstream pathways. Like anti-IgE, IL-4Rα blockade, through the IL-4 mutant molecule pitrakinra, inhibited the late allergic asthmatic response in patients with mild asthma, supporting the efficacy in allergic inflammation (72). In moderate-to-severe asthma, as currently defined (medium- to high-dose combination therapy), dupilumab, an IL-4Rα antibody, reduced exacerbations and improved lung function, with responses again dependent on T2 biomarker elevations. Dupilumab reduced exacerbations (∼50%) compared with placebo in patients with relatively low eosinophil and FeNO thresholds (≥150/μl or ≥25 ppb), with dupilumab being the only biologic for which FeNO is a predictive biomarker. No efficacy is seen in the absence of elevations in either of these, but the greatest impact is in those with elevations in both (17). Elevations in FeNO and blood eosinophils consistently define patients with more exacerbation-prone disease. FeNO also appears to be an important response biomarker, declining in relation to improving FEV1 (18, 73). Although the biologic changes that drive improvement with anti–IL-4Rα are unknown, the parallel improvements in FeNO and lung function suggest that epithelial cells are directly affected, potentially reducing mucus/mucus plugs associated with T2 inflammation (74). Dupilumab also reduces oral CS dependency, with reduction in oral CS doses of 70% in the dupilumab group, compared with 42% in the placebo group (75). Although patients were not recruited with respect to baseline eosinophil concentrations, the median eosinophil number was in fact over 300/μl, supporting the concept that systemic CS reliance identifies a T2-Hi phenotype.
Phenotypically, dupilumab efficacy may be greater in later-onset T2-Hi asthma, particularly in those with eosinophil concentrations ≥300/μl (76). These patients are likely enhanced for nasal polyps, and dupilumab is approved by the FDA for their treatment (34, 77). Although atopic dermatitis/eczema has long been considered an IgE-mediated disease, anti-IgE has not shown efficacy. In contrast, dupilumab was initially FDA approved for treatment of atopic dermatitis, suggesting that signaling downstream of IL-4Rα is pathogenic, whereas downstream IgE is not (78). Thus, treatment with dupilumab could be initially considered in patients with both EOA and atopic dermatitis. Unlike nasal polyps, atopic dermatitis is typically associated with EOA as part of an “atopic march,” yet its association with severe asthma is unclear. This improvement of these widely different comorbidities by dupilumab suggests that alternate IL-4/13 pathways may drive these two diseases.
Peripheral blood eosinophils increase in approximately one-third of dupilumab-treated patients, typically returning to baseline within 3 months, but rare cases of EGPA have been reported (18). In fact, in the later-stage trials, patients with eosinophil counts of >1,500/ml at baseline were excluded, suggesting counts at this level and above as exclusionary for treatment. It has been suggested that blood eosinophils increase because of inhibition of IL-4/13–mediated chemokine-driven trafficking into the airways (79). However, treatment with an anti–IL-13 antibody (tralokinumab) failed to impact endobronchial-tissue eosinophils (80). Thus, whether efficacy of dupilumab is determined by reduction in tissue eosinophils remains uncertain.
Anti–IL-5/5R
Eosinophils have been associated with asthma for well over 100 years. Like many asthma therapies, anti–IL-5 was initially trialed in an allergen-challenge model (81). Unlike either anti-IgE or IL-4Rα blockade, anti–IL-5 did not inhibit allergic asthmatic responses, despite depleting blood eosinophils to a level near zero, suggesting that IL-5 plays a less important role in allergic responses, as compared with IgE and IL-4/13.
Although entry criteria for the clinical trials with the three FDA-approved biologics, mepolizumab, reslizumab (anti–IL-5 molecules), and benralizumab (anti-IL5R), varied somewhat, the ability of all three to reduce asthma exacerbations is remarkably similar and on the order of 40–50% (19, 20, 82–84). Improvements in lung function and patient-reported outcomes are variable. Although all three antibodies profoundly reduce peripheral eosinophils, the impact on lung tissue and sputum eosinophils is also less clear (30, 82, 85). In all cases, however, efficacy increases with increasing severity and blood eosinophilia (84, 86). Similar to the efficacy of anti-IL4R approaches, efficacy in this approach is greater in patients with LOA, and this is again supportive of the differing importance and drivers of T2 inflammation in EOA (and allergic asthma) compared with LOA (19, 24, 87). Both mepolizumab and benralizumab reduce the need for systemic CSs (∼70% reduction in dose), and, in fact, chronic use of oral CSs also predicts a better response to therapy (24, 88, 89). Consistent with their association with eosinophilic/T2-Hi LOA, 30–35% of patients in the CS-sparing trials reported nasal polyps. Thus, an overall image of the best responders to anti–IL-5/5R (and IL-4Rα) (LOA, eosinophilic asthma, exacerbation-prone asthma, and CS-dependent asthma with nasal polyps) is emerging and is consistent with findings of clinical phenotyping studies.
In addition, sufficient data exist to determine whether anti-IgE or dupilumab should be used initially in patients with childhood-onset allergic asthma. However, either is potentially preferable to IL-5–targeted therapies, given the lack of efficacy of anti–IL-5 in allergen-challenge models. Finally, despite matching biomarker and phenotype to biologic therapy, many patients will not respond to any of the currently available antibodies for unknown pathologic reasons.
Biologic Implications of T2-Lo Therapies
As noted earlier, T2-Lo asthma is defined by the persistence of low concentrations of T2 biomarkers. In fact, repeated measurements, with reductions in background systemic CSs, suggest that the percentages may be relatively small, if an asthma diagnosis is confirmed. However, this definition of T2-Lo asthma allows for a wide range of potential underlying pathobiologies. To this point, no definitive evidence exists to support specific biologic or other therapies for these patients. Therefore, of necessity, the approaches are either nonspecific or targeted to physical or physiologic characteristics associated with T2-Lo asthma. Airway obstruction in the absence of T2 biomarkers may improve with LAMAs, when added to combination therapy, with no evidence to suggest added benefit in patients with increased eosinophils (58, 90). Based on small studies, patients with late-onset T2-Lo asthma may improve with weight loss (bariatric surgery) (91), whereas patients with severe asthma and low eosinophils may respond to azithromycin (59, 61).
Perhaps the most obvious treatment for severe T2-Lo asthma is bronchial thermoplasty, which was developed to specifically ablate airway smooth muscle, rather than to target inflammatory processes, and therefore should work at least as well in T2-Lo asthma as in T2-Hi asthma (92). However, no studies of bronchial thermoplasty to date have stratified patients by baseline T2 status. Whether bronchial thermoplasty is more efficacious in T2-Lo or T2-Hi severe asthma remains controversial, with one recent study suggesting that responses to bronchial thermoplasty may be more effective in patients with greater baseline eosinophil concentrations, in contrast to another suggesting the opposite (93, 94). Importantly, the actual mechanism for efficacy of bronchial thermoplasty remains unclear, with inconsistent relationships between reduction in airway smooth muscle and symptom control. Additional studies, including studies evaluating inflammatory, epithelial, and neuronal function, are needed in well-characterized patients to fully understand the biologic implications for the efficacy of bronchial thermoplasty in T2-Lo or T2-Hi severe asthma.
Integrating Clinical, Molecular, and Therapeutic Phenotypes to Understand Mechanisms
The previous sections suggest different underlying immune mechanisms for recognizable phenotypes. Despite this, broader genetic or molecular mechanisms for these phenotypes remain poorly understood.
Detailed matching of molecular and immune profiling with clinical phenotypes is still lacking. Almost all animal models focus on a T-helper cell/allergen–driven disease, more representative of milder asthma, for which overlap with severe asthma phenotypes is poor. Although animal models of severe, poorly CS-responsive asthma, with an increasingly identified type 1 immune response, have been developed, the relation to specific human phenotypes continues to be an area of active investigation (95–98). Compartment differences further complicate analyses, as proximal (sputum), distal (BAL), and endobronchial (biopsy or brush) findings may all differ. Numerous studies have, however, suggested additional immune processes, alone or in combination with T2 pathways, including type 1 and Th17 processes, as well as innate immunity. The patterns vary by approach and compartments studied, often with limited association with specific clinical characteristics (32, 96, 99–102). The presence of neutrophils has been linked to Th17, inflammasome, and IL-6 pathways, with little overlap with T2 pathways (32, 99, 101). Despite this, therapeutics specifically targeting neutrophils, even in patients with neutrophilic inflammation, have not shown efficacy (103–105). Given the common observation of combined increases in lung eosinophils and neutrophils with more severe disease, it is conceivable that targeting both cells may be more important (44, 106).
There is also controversy on cellular sources of T2 cytokines, including classic T-helper cell type 2 effector cells, tissue-resident memory T cells, T2 innate lymphoid cells (ILC2s), and even nonlymphoid sources, including mast cells/basophils, eosinophils, and monocyte macrophages. Given the differing responses to T2 biologics in the face of similar T2-biomarker elevations, differing mixes of immune cells may be contributing. Thus, the understanding of the underlying molecular mechanisms for various phenotypes will benefit from incorporation of responses to T2 biologics.
Only two consistently recognizable phenotypes to date have specific identifiable mechanisms associated with them. The first includes a severe variation of EOA, with or without T2 inflammation (Figure 3). EOA is typically associated with a stronger family history of asthma and associated genetics, in which 17q12-21 is consistently identified as a susceptibility locus (107). Targeted evaluation of SNPs in this locus identified genetic relationships to epithelial-cell gene expression of GSDMB (gasdermin-B) and more severe EOA (108). Epithelial expression of GSDMB, part of a family of proteins associated with cell-death pathways, correlates strongly with type 1 and 2 IFN pathway genes, suggesting a role in type 1 immunity and, perhaps, viral infections, in the risks for severe EOA. Patients with EOA can respond to all T2-specific biologics, although not as robustly as in LOA (24, 76, 87). Thus, although EOA can be hallmarked by high T2-associated gene expression, T2 cytokines, in particular IL-5, the blocking of which is also ineffective in allergen challenge, may not play as big a role (81). Lung lymphocytes (broadly identified) may also be less numerous in these patients, suggesting nonlymphoid sources for IL-4/13 with less IL-5 (100). The association with allergy, IgE, and specific IgE suggests that mast cells/basophils (or other FcεR1+ cells) may be a source, which awaits further investigation.
Figure 3.
Schematic representation of the potential mechanisms defining the clinical–molecular phenotype of early-onset T2-Hi (or T2-Lo) asthma. Question marks and dashed arrows represent speculative relationships. The more bold dashed line represents the boundary of a lymph node. APC = antigen-presenting cell; Hi = high; iNOS = inducible nitric oxide synthase; Lo = low; T2 = type 2; Tfh = T-follicular helper cell; Th0 = T-helper cell type 0; Th2 = T-helper cell type 2.
The best-characterized clinical–molecular phenotype is T2-Hi LOA, which is associated with eosinophilia, nasal polyps, CS-dependent disease, and, to a lesser extent, aspirin sensitivity (Figure 4). As emphasized, these patients respond remarkably better to IL-4Rα– and IL-5/5R–targeted therapy than do patients with EOA. Several studies have suggested activation of ILC2s, generating IL-5 and IL-13 in asthmatic airways and nasal polyps (109, 110),which is supported by the efficacy of anti-IL4Rα and, to a limited degree, anti–IL-5 in nasal polyps (111). Recent success with anti-TSLP (anti–thymic stromal lymphopoietin), an epithelial alarmin, in moderate-to-severe asthma supports a role for TSLP-activated ILC2s in at least some asthma phenotypes, with considerable additional interest in the alarmin IL-33 (112). Mast cells, seen in association with the subgroup with aspirin-exacerbated respiratory disease, also express TSLP and IL-33 receptors and likely contribute increased cysteinyl leukotrienes (113–115). Mast-cell involvement may explain the efficacy of anti-IgE in these patients as well (71). Recently, a loss-of-function genetic mutation in 15LO1 (15 lipoxygenase-1), an enzyme prominent in T2-Hi epithelial cells, virtually eliminated the likelihood of developing nasal polyps (116). 15LO1 is tightly linked to a newly identified form of programmed cell death termed ferroptosis as well as to compensatory autophagy (117, 118). It is strongly induced by IL-4/13 and is critical for expression of eosinophilic chemokines, including eotaxin-3 (118, 119). Thus, this eosinophilic LOA phenotype is the first to closely meet the definition of an endotype, or “a subgroup of a condition which is defined by a distinct functional or pathobiological mechanism” (120). Although additional endotypes will clearly be identified, including those related to T2-Lo disease, inflammasomes, T cells (including type 1 cells), and autoimmunity, further investigation with targeted biologic interventions is needed.
Figure 4.
Schematic representation of the potential mechanisms defining the clinical–molecular phenotype (or perhaps endotype) of late-onset T2-Hi asthma, showing the central roles of epithelial cells, ILC2s, and mast cells. Question marks represent speculation. 15LO1 = 15 lipoxygenase-1; Hi = high; ILC2 = T2 innate lymphoid cell; iNOS = inducible nitric oxide synthase; ST2 = IL-33 receptor; T2 = type 2; TSLP = thymic stromal lymphopoietin.
Conclusions
Integrated approaches including clinical and molecular phenotyping in relation to responses to biologic therapy have remarkably improved our understanding of the phenotypes, and even endotypes, of the severe asthmas. Patients whose asthma remains uncontrolled on medium- to high-dose ICSs, despite standard approaches, should be biologically phenotyped into T2-Hi or T2-Lo categories through measurement and monitoring of blood eosinophils and FeNO in relation to their age at onset. However, details of the immune cells and their drivers in relation to these phenotypes remain to be better understood, and many patients, especially those with T2-Hi asthma in association with other immune factors, or T2-Lo asthma, are still poorly treated. Concerted study of biology, immune pathways, and clinical outcomes, with deference to specific therapies, is needed to continue to improve the understanding and treatment of the severe asthmas.
Supplementary Material
Acknowledgments
Acknowledgment
The author thanks BioRender for assistance with figure development and thanks Drs. Anuradha Ray and Merritt Fajt for their helpful suggestions, as well as Dr. Humberto Trejo-Bittar for the pathologic images in Figure 2.
Footnotes
Supported by NIH grants P01 AI106684-06, R01-HL146002, AI-145406-01A1, and HL153058-01 and by the Dellenback Fund.
CME will be available for this article at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202009-3631CI on December 16, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [Published errata appear in Eur Respir J 43:1216 and Eur Respir J 52:1352020.] [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 3.Bousquet J, Mantzouranis E, Cruz AA, Aït-Khaled N, Baena-Cagnani CE, Bleecker ER, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization consultation on severe asthma. J Allergy Clin Immunol. 2010;126:926–938. doi: 10.1016/j.jaci.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 1987;136:225–244. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 5.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 6.Hekking PP, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135:896–902. doi: 10.1016/j.jaci.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 7.Backman H, Jansson SA, Stridsman C, Eriksson B, Hedman L, Eklund BM, et al. Severe asthma-a population study perspective. Clin Exp Allergy. 2019;49:819–828. doi: 10.1111/cea.13378. [DOI] [PubMed] [Google Scholar]
- 8.Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline features of the Severe Asthma Research Program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract. 2018;6:545–554, e4. doi: 10.1016/j.jaip.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zein JG, Udeh BL, Teague WG, Koroukian SM, Schlitz NK, Bleecker ER, et al. Severe Asthma Research Program. Impact of age and sex on outcomes and hospital cost of acute asthma in the United States, 2011-2012. PLoS One. 2016;11:e0157301. doi: 10.1371/journal.pone.0157301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw DE, Sousa AR, Fowler SJ, Fleming LJ, Roberts G, Corfield J, et al. U-BIOPRED Study Group. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015;46:1308–1321. doi: 10.1183/13993003.00779-2015. [DOI] [PubMed] [Google Scholar]
- 11.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. 2011;127:1486–1493, e2. doi: 10.1016/j.jaci.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113:101–108. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 13.Ross KR, Gupta R, DeBoer MD, Zein J, Phillips BR, Mauger DT, et al. Severe asthma during childhood and adolescence: a longitudinal study. J Allergy Clin Immunol. 2020;145:140–146, e9. doi: 10.1016/j.jaci.2019.09.030. [DOI] [PubMed] [Google Scholar]
- 14.Lugogo NL, Kreindler JL, Martin UJ, Cook B, Hirsch I, Trudo FJ. Blood eosinophil count group shifts and kinetics in severe eosinophilic asthma. Ann Allergy Asthma Immunol. 2020;125:171–176. doi: 10.1016/j.anai.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Wysocki K, Park SY, Bleecker E, Busse W, Castro M, Chung KF, et al. Characterization of factors associated with systemic corticosteroid use in severe asthma: data from the Severe Asthma Research Program. J Allergy Clin Immunol. 2014;133:915–918. doi: 10.1016/j.jaci.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanania NA, Wenzel S, Rosén K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804–811. doi: 10.1164/rccm.201208-1414OC. [DOI] [PubMed] [Google Scholar]
- 17.Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486–2496. doi: 10.1056/NEJMoa1804092. [DOI] [PubMed] [Google Scholar]
- 18.Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388:31–44. doi: 10.1016/S0140-6736(16)30307-5. [DOI] [PubMed] [Google Scholar]
- 19.Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. SIROCCO study Investigators. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115–2127. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 20.FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. CALIMA study Investigators. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 21.Sulaiman I, Greene G, MacHale E, Seheult J, Mokoka M, D’Arcy S, et al. A randomised clinical trial of feedback on inhaler adherence and technique in patients with severe uncontrolled asthma. Eur Respir J. 2018;51:1701126. doi: 10.1183/13993003.01126-2017. [DOI] [PubMed] [Google Scholar]
- 22.Mokoka MC, McDonnell MJ, MacHale E, Cushen B, Boland F, Cormican S, et al. Inadequate assessment of adherence to maintenance medication leads to loss of power and increased costs in trials of severe asthma therapy: results from a systematic literature review and modelling study. Eur Respir J. 2019;53:1802161. doi: 10.1183/13993003.02161-2018. [DOI] [PubMed] [Google Scholar]
- 23.Wu W, Bang S, Bleecker ER, Castro M, Denlinger L, Erzurum SC, et al. Multiview cluster analysis identifies variable corticosteroid response phenotypes in severe asthma. Am J Respir Crit Care Med. 2019;199:1358–1367. doi: 10.1164/rccm.201808-1543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bleecker ER, Wechsler ME, FitzGerald JM, Menzies-Gow A, Wu Y, Hirsch I, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J. 2018;52:1800936. doi: 10.1183/13993003.00936-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doberer D, Trejo Bittar HE, Wenzel SE. Should lung biopsies be performed in patients with severe asthma? Eur Respir Rev. 2015;24:525–539. doi: 10.1183/16000617.0045-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukherjee M, Bulir DC, Radford K, Kjarsgaard M, Huang CM, Jacobsen EA, et al. Sputum autoantibodies in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2018;141:1269–1279. doi: 10.1016/j.jaci.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 27.Wenzel SE, Vitari CA, Shende M, Strollo DC, Larkin A, Yousem SA. Asthmatic granulomatosis: a novel disease with asthmatic and granulomatous features. Am J Respir Crit Care Med. 2012;186:501–507. doi: 10.1164/rccm.201203-0476OC. [DOI] [PubMed] [Google Scholar]
- 28.Groh M, Pagnoux C, Guillevin L. Eosinophilic granulomatosis with polyangiitis (formerly Churg-Strauss syndrome): where are we now? Eur Respir J. 2015;46:1255–1258. doi: 10.1183/13993003.00963-2015. [DOI] [PubMed] [Google Scholar]
- 29.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. MENSA Investigators. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee M, Aleman Paramo F, Kjarsgaard M, Salter B, Nair G, LaVigne N, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38–46. doi: 10.1164/rccm.201707-1323OC. [DOI] [PubMed] [Google Scholar]
- 31.Hastie AT, Moore WC, Li H, Rector BM, Ortega VE, Pascual RM, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol. 2013;132:72–80. doi: 10.1016/j.jaci.2013.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossios C, Pavlidis S, Hoda U, Kuo CH, Wiegman C, Russell K, et al. Unbiased Biomarkers for the Prediction of Respiratory Diseases Outcomes (U-BIOPRED) Consortia Project Team. Sputum transcriptomics reveal upregulation of IL-1 receptor family members in patients with severe asthma. J Allergy Clin Immunol. 2018;141:560–570. doi: 10.1016/j.jaci.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 33.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. NHLBI Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu W, Bleecker E, Moore W, Busse WW, Castro M, Chung KF, et al. Unsupervised phenotyping of severe asthma research program participants using expanded lung data. J Allergy Clin Immunol. 2014;133:1280–1288. doi: 10.1016/j.jaci.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapman DG, Irvin CG, Kaminsky DA, Forgione PM, Bates JH, Dixon AE. Influence of distinct asthma phenotypes on lung function following weight loss in the obese. Respirology. 2014;19:1170–1177. doi: 10.1111/resp.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holguin F, Comhair SA, Hazen SL, Powers RW, Khatri SS, Bleecker ER, et al. An association between L-arginine/asymmetric dimethyl arginine balance, obesity, and the age of asthma onset phenotype. Am J Respir Crit Care Med. 2013;187:153–159. doi: 10.1164/rccm.201207-1270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Alwan A, Bates JH, Chapman DG, Kaminsky DA, DeSarno MJ, Irvin CG, et al. The nonallergic asthma of obesity: a matter of distal lung compliance. Am J Respir Crit Care Med. 2014;189:1494–1502. doi: 10.1164/rccm.201401-0178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, et al. NHLBI Severe Asthma Research Program. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016;4:574–584. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters MC, Mauger D, Ross KR, Phillips B, Gaston B, Cardet JC, et al. Evidence for exacerbation-prone asthma and predictive biomarkers of exacerbation frequency. Am J Respir Crit Care Med. 2020;202:973–982. doi: 10.1164/rccm.201909-1813OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jevnikar Z, Östling J, Ax E, Calvén J, Thörn K, Israelsson E, et al. Unbiased Biomarkers in Prediction of Respiratory Disease Outcomes Study Group. Epithelial IL-6 trans-signaling defines a new asthma phenotype with increased airway inflammation. J Allergy Clin Immunol. 2019;143:577–590. doi: 10.1016/j.jaci.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 42.Boss B, Neeck G. Correlation of IL-6 with the classical humoral disease activity parameters ESR and CRP and with serum cortisol, reflecting the activity of the HPA axis in active rheumatoid arthritis. Z Rheumatol. 2000;59:II62–II64. doi: 10.1007/s003930070020. [DOI] [PubMed] [Google Scholar]
- 43.Arif AA, Delclos GL, Colmer-Hamood J. Association between asthma, asthma symptoms and C-reactive protein in US adults: data from the National Health and Nutrition Examination Survey, 1999-2002. Respirology. 2007;12:675–682. doi: 10.1111/j.1440-1843.2007.01122.x. [DOI] [PubMed] [Google Scholar]
- 44.Hastie AT, Mauger DT, Denlinger LC, Coverstone A, Castro M, Erzurum S, et al. NHLBI SARP 3 Investigators. Baseline sputum eosinophil + neutrophil subgroups’ clinical characteristics and longitudinal trajectories for NHLBI Severe Asthma Research Program (SARP 3) cohort. J Allergy Clin Immunol. 2020;146:222–226. doi: 10.1016/j.jaci.2020.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. GOAL Investigators Group. Can guideline-defined asthma control be achieved? The gaining optimal asthma control study. Am J Respir Crit Care Med. 2004;170:836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 46.Bateman ED, Busse W, Pedersen SE, Bousquet J, Huang S, Zhou X, et al. Global Initiative for Asthma 2016-derived asthma control with fluticasone propionate and salmeterol: a Gaining Optimal Asthma Control (GOAL) study reanalysis. Ann Allergy Asthma Immunol. 2019;123:57–63, e2. doi: 10.1016/j.anai.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 47.Stempel DA, Yeakey AM, Pascoe SJ. Safety study of salmeterol in asthma in adults. N Engl J Med. 2016;375:1098. doi: 10.1056/NEJMc1608323. [DOI] [PubMed] [Google Scholar]
- 48.O’Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005;171:129–136. doi: 10.1164/rccm.200407-884OC. [DOI] [PubMed] [Google Scholar]
- 49.Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet. 2006;368:744–753. doi: 10.1016/S0140-6736(06)69284-2. [DOI] [PubMed] [Google Scholar]
- 50.Kuna P, Peters MJ, Manjra AI, Jorup C, Naya IP, Martínez-Jimenez NE, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract. 2007;61:725–736. doi: 10.1111/j.1742-1241.2007.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jenkins CR, Bateman ED, Sears MR, O’Byrne PM. What have we learnt about asthma control from trials of budesonide/formoterol as maintenance and reliever? Respirology. 2020;25:804–815. doi: 10.1111/resp.13804. [DOI] [PubMed] [Google Scholar]
- 52.Kew KM, Karner C, Mindus SM, Ferrara G. Combination formoterol and budesonide as maintenance and reliever therapy versus combination inhaler maintenance for chronic asthma in adults and children. Cochrane Database Syst Rev. 2013:CD009019. doi: 10.1002/14651858.CD009019.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sobieraj DM, Weeda ER, Nguyen E, Coleman CI, White CM, Lazarus SC, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA. 2018;319:1485–1496. doi: 10.1001/jama.2018.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Global Initiative for Asthma. GINA: interim guidance about COVID-19 and asthma [updated 2020 Dec 20; accessed 2021 Feb 25]. Available from: https://ginasthma.org/wp-content/uploads/2020/06/GINA-2020-report_20_06_04-1-wms.pdf.
- 55.Weathington N, O’Brien ME, Radder J, Whisenant TC, Bleecker ER, Busse WW, et al. BAL cell gene expression in severe asthma reveals mechanisms of severe disease and influences of medications. Am J Respir Crit Care Med. 2019;200:837–856. doi: 10.1164/rccm.201811-2221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ortega VE, Hawkins GA, Moore WC, Hastie AT, Ampleford EJ, Busse WW, et al. Effect of rare variants in ADRB2 on risk of severe exacerbations and symptom control during longacting β agonist treatment in a multiethnic asthma population: a genetic study. Lancet Respir Med. 2014;2:204–213. doi: 10.1016/S2213-2600(13)70289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singhania A, Rupani H, Jayasekera N, Lumb S, Hales P, Gozzard N, et al. Altered epithelial gene expression in peripheral airways of severe asthma. PLoS One. 2017;12:e0168680. doi: 10.1371/journal.pone.0168680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367:1198–1207. doi: 10.1056/NEJMoa1208606. [DOI] [PubMed] [Google Scholar]
- 59.Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322–329. doi: 10.1136/thoraxjnl-2012-202698. [DOI] [PubMed] [Google Scholar]
- 60.Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659–668. doi: 10.1016/S0140-6736(17)31281-3. [DOI] [PubMed] [Google Scholar]
- 61.Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Efficacy of azithromycin in severe asthma from the AMAZES randomised trial. ERJ Open Res. 2019;5:00056-2019. doi: 10.1183/23120541.00056-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fahy JV, Fleming HE, Wong HH, Liu JT, Su JQ, Reimann J, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med. 1997;155:1828–1834. doi: 10.1164/ajrccm.155.6.9196082. [DOI] [PubMed] [Google Scholar]
- 63.Boulet LP, Chapman KR, Côté J, Kalra S, Bhagat R, Swystun VA, et al. Inhibitory effects of an anti-IgE antibody E25 on allergen-induced early asthmatic response. Am J Respir Crit Care Med. 1997;155:1835–1840. doi: 10.1164/ajrccm.155.6.9196083. [DOI] [PubMed] [Google Scholar]
- 64.Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 65.Solèr M, Matz J, Townley R, Buhl R, O’Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–261. doi: 10.1183/09031936.01.00092101. [DOI] [PubMed] [Google Scholar]
- 66.Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154:573–582. doi: 10.7326/0003-4819-154-9-201105030-00002. [DOI] [PubMed] [Google Scholar]
- 67.He K, Hettinga A, Kale SL, Hu S, Xie MM, Dent AL, et al. Blimp-1 is essential for allergen-induced asthma and Th2 cell development in the lung. J Exp Med. 2020;217:e20190742. doi: 10.1084/jem.20190742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50:1132–1148. doi: 10.1016/j.immuni.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gill MA, Liu AH, Calatroni A, Krouse RZ, Shao B, Schiltz A, et al. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J Allergy Clin Immunol. 2018;141:1735–1743, e9. doi: 10.1016/j.jaci.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gevaert P, Omachi TA, Corren J, Mullol J, Han J, Lee SE, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. 2020;146:595–605. doi: 10.1016/j.jaci.2020.05.032. [DOI] [PubMed] [Google Scholar]
- 72.Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007;370:1422–1431. doi: 10.1016/S0140-6736(07)61600-6. [DOI] [PubMed] [Google Scholar]
- 73.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 74.Dunican EM, Elicker BM, Gierada DS, Nagle SK, Schiebler ML, Newell JD, et al. NHLBI Severe Asthma Research Program (SARP) Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest. 2018;128:997–1009. doi: 10.1172/JCI95693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378:2475–2485. doi: 10.1056/NEJMoa1804093. [DOI] [PubMed] [Google Scholar]
- 76.Castro M, Hanania NA, Quirce S, Sher L, Maspero JF, Rice MS, et al. Dupilumab reduces severe asthma exacerbation rate and improves lung function regardless of age at onset of asthma: the LIBERTY ASTHMA QUEST study [abstract] Chest. 2019;156:A936–A939. [Google Scholar]
- 77.Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394:1638–1650. doi: 10.1016/S0140-6736(19)31881-1. [DOI] [PubMed] [Google Scholar]
- 78.Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 79.Jonstam K, Swanson BN, Mannent L, Cardell LO, Tian N, Wang Y, et al. Dupilumab reduces local type 2 pro-inflammatory biomarkers in chronic rhinosinusitis with nasal polyposis. Allergy. 2019;74:743–752. doi: 10.1111/all.13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Russell RJ, Chachi L, FitzGerald JM, Backer V, Olivenstein R, Titlestad IL, et al. MESOS study investigators. Effect of tralokinumab, an interleukin-13 neutralising monoclonal antibody, on eosinophilic airway inflammation in uncontrolled moderate-to-severe asthma (MESOS): a multicentre, double-blind, randomised, placebo-controlled phase 2 trial. Lancet Respir Med. 2018;6:499–510. doi: 10.1016/S2213-2600(18)30201-7. [DOI] [PubMed] [Google Scholar]
- 81.Leckie MJ, ten Brinke A, Khan J, Diamant Z, O’Connor BJ, Walls CM, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 82.Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, et al. Res-5-0010 Study Group. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184:1125–1132. doi: 10.1164/rccm.201103-0396OC. [DOI] [PubMed] [Google Scholar]
- 83.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 85.Laviolette M, Gossage DL, Gauvreau G, Leigh R, Olivenstein R, Katial R, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132:1086–1096, e5. doi: 10.1016/j.jaci.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Castro M, Wenzel SE, Bleecker ER, Pizzichini E, Kuna P, Busse WW, et al. Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med. 2014;2:879–890. doi: 10.1016/S2213-2600(14)70201-2. [DOI] [PubMed] [Google Scholar]
- 87.Brusselle G, Germinaro M, Weiss S, Zangrilli J. Reslizumab in patients with inadequately controlled late-onset asthma and elevated blood eosinophils. Pulm Pharmacol Ther. 2017;43:39–45. doi: 10.1016/j.pupt.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 88.Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. SIRIUS Investigators. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 89.Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. ZONDA Trial Investigators. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376:2448–2458. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- 90.Casale TB, Bateman ED, Vandewalker M, Virchow JC, Schmidt H, Engel M, et al. Tiotropium respimat add-on is efficacious in symptomatic asthma, independent of T2 phenotype. J Allergy Clin Immunol Pract. 2018;6:923–935, e9. doi: 10.1016/j.jaip.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 91.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128:508–515, e1–e2. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Castro M, Rubin AS, Laviolette M, Fiterman J, De Andrade Lima M, Shah PL, et al. AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116–124. doi: 10.1164/rccm.200903-0354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goorsenberg AWM, d’Hooghe JNS, Srikanthan K, Ten Hacken NHT, Weersink EJM, Roelofs JJTH, et al. TASMA research group. Bronchial thermoplasty induced airway smooth muscle reduction and clinical response in severe asthma: the TASMA randomized trial. Am J Respir Crit Care Med. doi: 10.1164/rccm.201911-2298OC. [online ahead of print] 28 Jul 2020. [DOI] [PubMed] [Google Scholar]
- 94.Svenningsen S, Cox G, Nair P. Eosinophilia and response to bronchial thermoplasty. Am J Respir Crit Care Med. doi: 10.1164/rccm.202008-3221LE. [online ahead of print] 29 Sep 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhakta NR, Christenson SA, Nerella S, Solberg OD, Nguyen CP, Choy DF, et al. IFN-stimulated gene expression, type 2 inflammation, and endoplasmic reticulum stress in asthma. Am J Respir Crit Care Med. 2018;197:313–324. doi: 10.1164/rccm.201706-1070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, et al. High IFN-γ and low SLPI mark severe asthma in mice and humans. J Clin Invest. 2015;125:3037–3050. doi: 10.1172/JCI80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Modena BD, Bleecker ER, Busse WW, Erzurum SC, Gaston BM, Jarjour NN, et al. Gene expression correlated with severe asthma characteristics reveals heterogeneous mechanisms of severe disease. Am J Respir Crit Care Med. 2017;195:1449–1463. doi: 10.1164/rccm.201607-1407OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Voraphani N, Gladwin MT, Contreras AU, Kaminski N, Tedrow JR, Milosevic J, et al. An airway epithelial iNOS-DUOX2-thyroid peroxidase metabolome drives Th1/Th2 nitrative stress in human severe asthma. Mucosal Immunol. 2014;7:1175–1185. doi: 10.1038/mi.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015;7:301ra129. doi: 10.1126/scitranslmed.aab3142. [DOI] [PubMed] [Google Scholar]
- 100.Modena BD, Tedrow JR, Milosevic J, Bleecker ER, Meyers DA, Wu W, et al. Gene expression in relation to exhaled nitric oxide identifies novel asthma phenotypes with unique biomolecular pathways. Am J Respir Crit Care Med. 2014;190:1363–1372. doi: 10.1164/rccm.201406-1099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kuo CS, Pavlidis S, Loza M, Baribaud F, Rowe A, Pandis I, et al. U-BIOPRED Study Group. T-helper cell type 2 (Th2) and non-Th2 molecular phenotypes of asthma using sputum transcriptomics in U-BIOPRED. Eur Respir J. 2017;49:1602135. doi: 10.1183/13993003.02135-2016. [DOI] [PubMed] [Google Scholar]
- 102.Lachowicz-Scroggins ME, Dunican EM, Charbit AR, Raymond W, Looney MR, Peters MC, et al. Extracellular DNA, neutrophil extracellular traps, and inflammasome activation in severe asthma. Am J Respir Crit Care Med. 2019;199:1076–1085. doi: 10.1164/rccm.201810-1869OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294–1302. doi: 10.1164/rccm.201212-2318OC. [DOI] [PubMed] [Google Scholar]
- 104.O’Byrne PM, Metev H, Puu M, Richter K, Keen C, Uddin M, et al. Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:797–806. doi: 10.1016/S2213-2600(16)30227-2. [DOI] [PubMed] [Google Scholar]
- 105.Ray A, Kolls JK. Neutrophilic inflammation in asthma and association with disease severity. Trends Immunol. 2017;38:942–954. doi: 10.1016/j.it.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. NHLBI Severe Asthma Research Program. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133:1557–1563, e5. doi: 10.1016/j.jaci.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. GABRIEL Consortium. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li X, Christenson SA, Modean B, Li H, Busse WW, Castro M, et al. NHLBI Severe Asthma Research Program (SARP) Genetic analyses identify GSDMB associated with asthma severity, exacerbations, and antiviral pathways. J Allergy Clin Immunol. doi: 10.1016/j.jaci.2020.07.030. [online ahead of print] 11 Aug 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Walford HH, Lund SJ, Baum RE, White AA, Bergeron CM, Husseman J, et al. Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin Immunol. 2014;155:126–135. doi: 10.1016/j.clim.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ho J, Bailey M, Zaunders J, Mrad N, Sacks R, Sewell W, et al. Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin Exp Allergy. 2015;45:394–403. doi: 10.1111/cea.12462. [DOI] [PubMed] [Google Scholar]
- 111.Gevaert P, Van Bruaene N, Cattaert T, Van Steen K, Van Zele T, Acke F, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128:989–995, e1–e8. doi: 10.1016/j.jaci.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 112.Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377:936–946. doi: 10.1056/NEJMoa1704064. [DOI] [PubMed] [Google Scholar]
- 113.Israel E, Fischer AR, Rosenberg MA, Lilly CM, Callery JC, Shapiro J, et al. The pivotal role of 5-lipoxygenase products in the reaction of aspirin-sensitive asthmatics to aspirin. Am Rev Respir Dis. 1993;148:1447–1451. doi: 10.1164/ajrccm/148.6_Pt_1.1447. [DOI] [PubMed] [Google Scholar]
- 114.Nagarkar DR, Poposki JA, Comeau MR, Biyasheva A, Avila PC, Schleimer RP, et al. Airway epithelial cells activate TH2 cytokine production in mast cells through IL-1 and thymic stromal lymphopoietin. J Allergy Clin Immunol. 2012;130:225–232, e4. doi: 10.1016/j.jaci.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ro M, Lee AJ, Kim JH. 5-/12-Lipoxygenase-linked cascade contributes to the IL-33-induced synthesis of IL-13 in mast cells, thus promoting asthma development. Allergy. 2018;73:350–360. doi: 10.1111/all.13294. [DOI] [PubMed] [Google Scholar]
- 116.Kristjansson RP, Benonisdottir S, Davidsson OB, Oddsson A, Tragante V, Sigurdsson JK, et al. A loss-of-function variant in ALOX15 protects against nasal polyps and chronic rhinosinusitis. Nat Genet. 2019;51:267–276. doi: 10.1038/s41588-018-0314-6. [DOI] [PubMed] [Google Scholar]
- 117.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao J, Dar HH, Deng Y, St Croix CM, Li Z, Minami Y, et al. PEBP1 acts as a rheostat between prosurvival autophagy and ferroptotic death in asthmatic epithelial cells. Proc Natl Acad Sci USA. 2020;117:14376–14385. doi: 10.1073/pnas.1921618117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Z, Zeng M, Deng Y, Zhao J, Zhou X, Trudeau JB, et al. 15-Lipoxygenase 1 in nasal polyps promotes CCL26/eotaxin 3 expression through extracellular signal-regulated kinase activation. J Allergy Clin Immunol. 2019;144:1228–1241, e9. doi: 10.1016/j.jaci.2019.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wikipedia. Endotypes [updated 2020 Aug 16; accessed 2021 Feb 25]. Available from: https://en.wikipedia.org/wiki/Endotype#:∼:text=An%20endotype%20is%20a%20subtype,distinct%20functional%20or%20pathobiological%20mechanism.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.