Abstract
Background
Cutaneous manifestations in hospitalized children with SARS-CoV-2 have not been studied systematically.
Objective
To describe the mucocutaneous involvement in pediatric patients with COVID-19 admitted to a pediatric institution in Madrid (Spain), located in a zone reporting among the highest prevalence of COVID-19 in Europe.
Methods
A descriptive, analytical study was conducted on a series of 50 children hospitalized with COVID-19 between March 1, 2020, and November 30, 2020.
Results
Twenty-one patients presented with mucocutaneous symptoms: 18 patients with macular and/or papular exanthem, 17 with conjunctival hyperemia, and 9 with red cracked lips or strawberry tongue. Eighteen patients fulfilled criteria for multisystem inflammatory syndrome in children. Patients with mucocutaneous involvement tended to be older and presented to the emergency department with poor general status and extreme tachycardia, higher C-reactive protein and D-dimer levels, and lower lymphocyte counts than patients without skin signs. Mucocutaneous manifestations pose a higher risk of admission to the pediatric intensive care unit (odds ratio, 10.24; 95% confidence interval, 2.23-46.88; P = .003).
Conclusions
Children hospitalized with COVID-19 frequently had mucocutaneous involvement, with most symptoms fulfilling criteria for multisystem inflammatory syndrome in children. Patients with an exanthem or conjunctival hyperemia at admission have a higher probability of pediatric intensive care admission than patients without mucocutaneous symptoms.
Key words: COVID-19, pediatric dermatology, multisystem inflammatory syndrome, SARS-CoV-2
Capsule Summary.
-
•
Macular and/or papular exanthems, conjunctival hyperemia, red cracked lips, or strawberry tongue appear in 42% of children admitted for COVID-19, mostly in the context of multisystem inflammatory syndrome.
-
•
Mucocutaneous manifestations are associated with a higher risk of admission to a pediatric intensive care unit and should warrant prompt attention.
Introduction
COVID-19 in previously healthy children is usually a mild or asymptomatic disease, practically without associated mortality.1 Fever and respiratory symptoms were the most frequent manifestations in early pediatric series from China and Italy2 , 3; however, the involvement of other organs and systems has been described.4 Gastrointestinal and mucocutaneous manifestations are frequent in multisystem inflammatory syndrome in children (MIS-C), the most serious condition associated with SARS-CoV-2 infection in the pediatric population.5 , 6
Based on findings in adult patients, skin manifestations of COVID-19 have been classified under 5 categories: acral pseudochilblain, vesicular eruptions, urticarial lesions, macular and/or papular eruptions, and livedo or necrosis.7 Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks.8 , 9 The other cutaneous manifestations of COVID-19 in children have been the subject of case reports or small case series.10
The mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and the implications on the clinical course have not yet been extensively described. The objective of this study was to describe mucocutaneous manifestations in children hospitalized for COVID-19.
Methods
Study design
We conducted a descriptive, analytical study of pediatric patients with COVID-19 admitted from March 1 to November 30, 2020, to a single pediatric institution in Madrid (Spain). Approval was obtained from the institution's Ethics Committee and Board, and standard informed consent to record images was obtained.
Inclusion criteria
Patients from zero to 18 years of age admitted to the hospital with a COVID-19 diagnosis were eligible if they met at least 1 of the following criteria:
-
•
Positive reverse transcriptase polymerase chain reaction (RT-PCR) for SARS-CoV-2 collected from a nasopharyngeal and oropharyngeal swab; or
-
•
Clinical suspicion (symptoms compatible with MIS-C or symptoms compatible with COVID-19 with a close contact having tested positive for COVID-19) and a positive immunoglobulin-M enzyme-linked immunosorbent assay for SARS-CoV-2.
Exclusion criteria
Patients were not eligible if they had a positive RT-PCR result in the emergency department but infection with SARS-CoV-2 was not directly related to the cause of admission. Patients with cancer and undergoing chemotherapy were also excluded.
Data collection
Each patient received an identification code to blind investigators to individual patient data and to facilitate anonymous data collection. Epidemiologic, clinical, and testing data were retrieved from the electronic medical records of the admitted patients. Epidemiologic data included sex, age, length of hospital stay, pediatric intensive care unit (PICU) admission, and a record of close contact with suspected or confirmed cases of COVID-19. Clinical data included a history of chronic disease; previous treatment; signs (appearance, heart rate, oxygen saturation); and symptoms (fever, rhinorrhea, cough, respiratory distress, thoracic pain, sore throat, abdominal pain, vomiting, diarrhea, rash, conjunctival hyperemia without secretion, red cracked lips, strawberry tongue, headache, irritability, drowsiness, myalgia, anosmia, or ageusia). Reason for admission was classified into 6 categories: respiratory, gastrointestinal, neurologic, MIS-C, neonates with fever, and protracted fever. Analytical determination on admission included leukocyte, neutrophil, and lymphocyte counts; lactate dehydrogenase; D-dimer; C-reactive protein; and procalcitonin.
Definitions
We followed the case definition for MIS-C as described in the Health Advisory on MSS-C, published by the Centers for Disease Control.11 The case definition for MIS-C was as follows:
-
•
An individual younger than 21 years age, presenting with fever, laboratory evidence of inflammation, and clinical evidence of severe illness requiring hospitalization, with multisystem (>2) organ involvement (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, or neurologic), and
-
•
No alternative plausible diagnoses, and
-
•
Positive for current or recent SARS-CoV-2 infection as determined by an RT-PCR, serology, or antigen test, or exposure to a suspected or confirmed COVID-19 case no more than 4 weeks prior to the onset of symptoms.
Statistical analysis
Data were analyzed using Stata version 15.0 (StataCorp). Variables with normal distribution were reported as mean and standard deviation. Variables that did not meet the normality requirements were reported as median and interquartile range. Variables within categories were expressed as percentages. Two-tailed t tests were used to compare means between groups. Chi-squared tests were used to compare proportions. A stepwise logistic regression analysis was used to calculate the risk of admission to a PICU. A P value < .05 was considered statistically significant.
Results
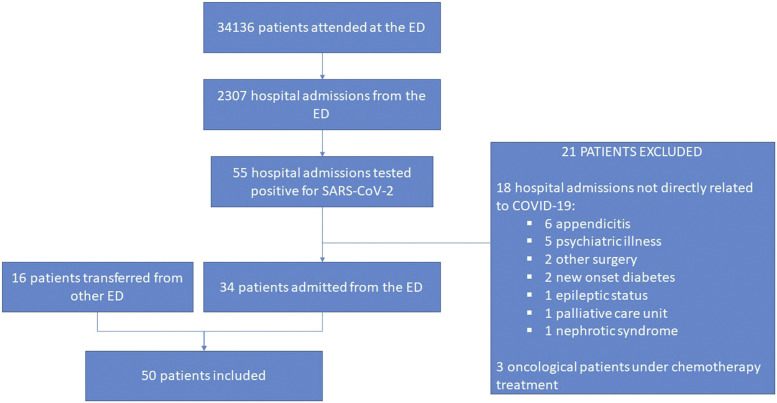
Fifty patients were included in the study (Fig 1 ). Forty-four (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met the clinical criteria for suspicion and had a negative RT-PCR with a positive immunoglobulin serology. Thirty-four patients (68%) had a close contact with a suspected or confirmed case of COVID-19. In the remaining 16 (32%), the origin of the infection remained unknown.
Fig 1.
Flowchart of patients included in the study. ED, Emergency department.
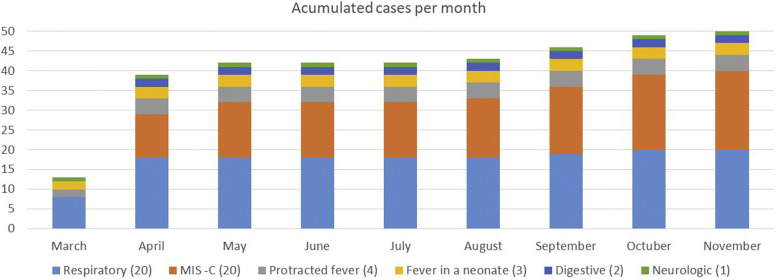
The main reasons for admission were respiratory illness (40%) and MIS-C (40%). The evolution of admitted COVID-19 patients during the pandemic is shown in Fig 2 .
Fig 2.
Evolution of children admitted to the authors' institution with COVID-19 throughout the pandemic. Respiratory disease consisted of pneumonia (9 cases), bronchiolitis (7 cases), and upper respiratory tract infection (4 cases). Gastrointestinal involvement consisted of prolonged diarrhea (1 case) and recurrent abdominal pain (1 case). Neurologic disease was due to multiple thrombosis in 1 patient. MIS-C, Multisystem inflammatory syndrome in children.
A general description of the sample is shown in Table I . Twenty-one patients (42%) exhibited mucocutaneous symptoms, including 18 patients with exanthem, 17 with conjunctival hyperemia without secretion, and 9 with red cracked lips or strawberry tongue (Figs 3 and 4 ).
Table I.
Characteristics of hospitalized children with COVID-19
| Characteristics | All cases (n = 50) | Mucocutaneous involvement YES (n = 21) | Mucocutaneous involvement NO (n = 29) | P value |
|---|---|---|---|---|
| Age median (interquartile range) | 9.5 (4.7-12) | 9.4 (5.1-11.7) | 4 (0.1-12.2) | <.001 |
| Age group, n (%) | .024 | |||
| <2 years | 15 (30) | 2 (9.5) | 13 (44.8) | |
| 2-10 years | 16 (32) | 11 (52.3) | 5 (17.2) | |
| >10 years | 19 (38) | 8 (38.1) | 11 (37.9) | |
| Sex, n (%) | .916 | |||
| Male | 29 (58) | 12 (57.1) | 17 (58.6) | |
| Female | 21 (42) | 9 (42.8) | 12 (41.4) | |
| Underlying medical condition, n (%) | 5 (10) | 1 (4.7) | 4 (13.7) | .293 |
| Reason for admission, n (%) | <.001 | |||
| Respiratory | 20 (40) | 1 (4.7) | 19 (65.5) | |
| Digestive | 2 (4) | 0 | 2 (6.9) | |
| Neurologic | 1 (2) | 0 | 1 (3.4) | |
| Multisystem inflammatory syndrome | 20 (40) | 18 (85.7) | 2 (6.9) | |
| Fever in a neonate | 3 (6) | 1 (4.7) | 2 (6.9) | |
| Protracted fever | 4 (8) | 1 (4.7) | 3 (10.3) | |
| PICU admission, n (%) | 28 (56) | 17 (80.9) | 9 (31.3) | <.001 |
| Median days of stay (interquartile range) | 8 (3-10) | 10 (8-12) | 4 (3-8) | <.001 |
| Symptoms at the ED, n (%) | ||||
| Fever | 45 (90) | 21 (100) | 24 (82.7) | .044 |
| Respiratory (rhinorrhea, cough, respiratory distress, thoracic pain, sore throat) | 31 (62) | 8 (38.1) | 23 (79.3) | .003 |
| Gastrointestinal (abdominal pain, vomiting, diarrhea) | 32 (64) | 19 (90.4) | 13 (44.8) | <.001 |
| Neurological (headache, irritability, drowsiness) | 18 (36) | 8 (38.1) | 10 (34.5) | .792 |
| Myalgia | 7 (14) | 3 (14.2) | 4 (13.8) | .960 |
| Anosmia, ageusia | 3 (6) | 1 (4.7) | 2 (6.9) | .753 |
| Signs at the ED, n (%) | ||||
| Altered appearance | 16 (32) | 12 (57) | 4 (13.8) | .001 |
| Extreme tachycardia∗ | 18 (36) | 14 (66) | 4 (13.8) | .001 |
| Oxygen saturation < 94% | 7 (14) | 0 | 7 (24.1) | .003 |
| Laboratory values median (interquartile range) | ||||
| Leukocytes (cells/μL) | 7940 (5510-12240) | 7880 (7015-1197) | 8510 (4910-13027) | .659 |
| Neutrophils (cells/μL) | 6200 (3340-9400) | 6875 (6252-10105) | 4080 (1937-7747) | .159 |
| Lymphocytes (cells/μL) | 870 (520-2130) | 510 (362-772) | 2415 (1005-3885) | <.001 |
| D-dimer (μg/mL) | 2.22 (1.18-3.71) | 4.04 (2.29-11.19) | 1.39 (0.44-2.39) | <.001 |
| Lactate dehydrogenase (UI/L) | 301 (268-351) | 297 (253-1197) | 303 (264-486) | .419 |
| C-reactive protein (mg/dL) | 11.47 (0.77-20.68) | 25.46 (12.36-34.12) | 0.85 (0.20-8.69) | <.001 |
| Procalcitonin (ng/mL) | 2.06 (0.63-6.46) | 2.61 (1.39-4.64) | 0.14 (0.06-1.84) | <.001 |
ED, Emergency department; PICU, pediatric intensive care unit.
Heart rate ≥ 99th percentile for age.
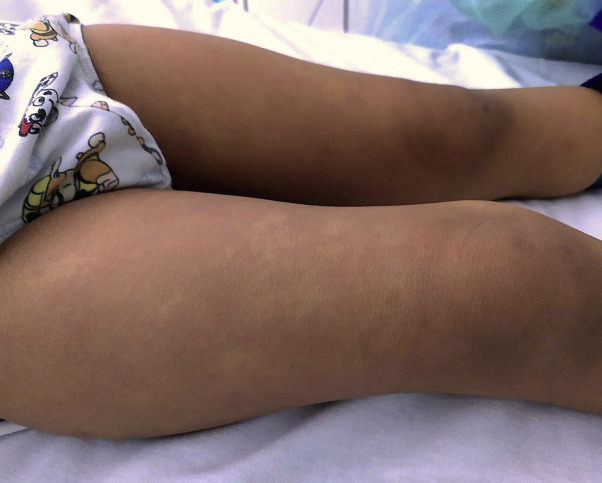
Fig 3.
Multisystem inflammatory syndrome. Confluent exanthem on thighs.
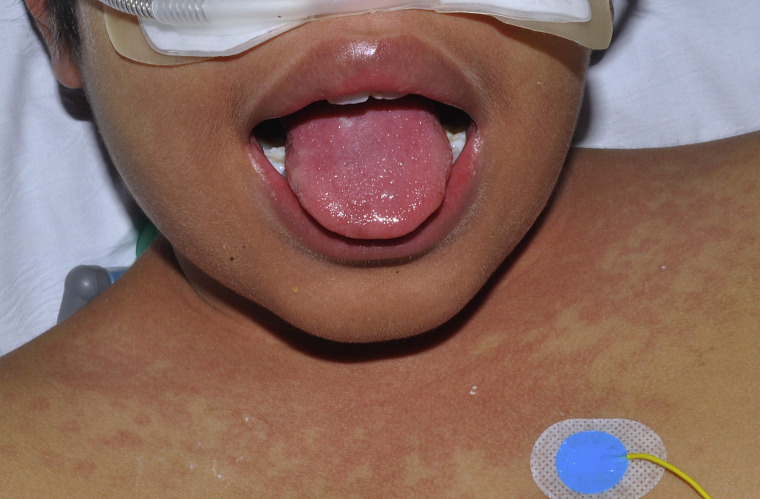
Fig 4.
Multisystem inflammatory syndrome. Macular and papular eruption on the neck and strawberry tongue.
The exanthem was described as macular and/or papular in all 18 cases, with confluent erythematous macules and papules that might become diffuse or show a lacy or reticulated pattern. All patients had mucocutaneous symptoms at the time of diagnosis in the emergency department, with variable progression throughout hospital admission. The limbs (78%) and trunk (72%) were the most commonly involved areas, and palms and soles (55%), genitals/groins (50%), and face (33%) were affected less frequently. One patient had an isolated acral ischemic lesion with a diameter of 5 mm, which appeared on 1 finger after recovery.
Patients with mucocutaneous symptoms tended to be older than those without skin signs and presented to the emergency department with a poor general status and extreme tachycardia. Fever and gastrointestinal symptoms were more frequent and respiratory symptoms were infrequent. Eighty-six percent of patients fulfilled the criteria for MIS-C (18 patients). An exanthem was also found in 1 patient admitted because of respiratory disease, 1 with protracted fever, and 1 febrile neonate. Of the 20 patients admitted because of MIS-C (40%), only 2 did not present mucocutaneous symptoms. Patients with mucocutaneous involvement also had higher C-reactive protein, procalcitonin, and D-dimer levels and lower lymphocyte counts in their first laboratory test on presentation to the emergency department. They were admitted to the PICU more frequently, and length of stay was longer than for patients without mucocutaneous involvement. All patients admitted to the PICU with mucocutaneous symptoms were classified as MIS-C.
Adjusted for age and sex, patients with mucocutaneous involvement had a higher risk of PICU admission (odds ratio, 10.24; 95% confidence interval, 2.23-46.88; P = .003). The odds ratio for conjunctival hyperemia was 30.28 (3.25-281.69; P = .003), and the odds ratio for rash was 6.22 (1.42-27.26; P = .015). None of the patients died or experienced long-term sequelae apart from 1 patient with a coronary artery ectasia, who is currently being followed up by the Cardiology Department, and 1 patient who had myopathy that lasted for 6 months.
Discussion
The prevalence of COVID-19 within the 6.68 million people living in the community of Madrid is one of the highest in Europe.12 Our institution was designated as a pediatric referral center during the peak of the pandemic and attended the largest volume of COVID-19 and regular emergencies in the area.13 Between March and November 2020, only 0.14% of hospital admissions were because of COVID-19, but by November 30, 2020, hospital admissions grew to 52,449 patients, with 11,369 deaths in the Madrid region.14 Several mechanisms have been proposed to explain the difference in severity of SARS-CoV-2 infections between adult and pediatric patients.15
In our series of children admitted for COVID-19, mucocutaneous symptoms were fourth in frequency, after fever, respiratory symptoms, and gastrointestinal symptoms. The presence of mucocutaneous symptoms (42%) is much higher than in other series of admitted pediatric patients, which provide figures of the presence of rash (<15% patients) or conjunctivitis (<5% patients).4 As our investigation was entirely focused on the cutaneous manifestations of COVID-19, we made efforts to include data on these findings that other registries may have missed. In fact, the only previous study focused on hospitalized pediatric patients with mucocutaneous disease was by Rekhtman et al,16 who described the same proportion of mucocutaneous involvement (42%).
In our institution during the first month of the outbreak, most admissions were due to respiratory diseases and skin manifestations were infrequent. Probably for that reason, in the early pediatric literature about COVID-19 manifestations in children there is either no mention of skin involvement in Chinese series or a reported rate as low as 3% in the Italian series.2 , 3 Mucocutaneous lesions have been seen more frequently since mid-April 2020, after the first cases of MIS-C were recognized, and studies have started to reflect the presence of skin lesions.5 , 6 , 17
Mucocutaneous symptoms appeared in 6 out of 10 patients who required admission to PICU. It is known that mucocutaneous symptoms are prominent in MIS-C. Up to 90% of patients diagnosed with MIS-C had some kind of mucocutaneous involvement. This is a higher rate than reported in other studies of patients with this condition, which described percentages from 47% to 83%.5 , 6 , 16 , 17 Because this syndrome usually requires PICU admission,16 the presence of exanthem or conjunctival hyperemia in our series is an independent predictor of PICU admission in hospitalized pediatric patients with COVID-19. A similar conclusion was reached regarding with gastrointestinal manifestations, probably due to the same underlying explanation.18 In the subgroup of patients with MIS-C, Trevor et al17 found no association between the presence of mucocutaneous changes and the risk of PICU admission. Due to the small percentage of MIS-C patients without mucocutaneous disease in our series, this finding cannot be evaluated.
The presence of any mucocutaneous lesion in febrile children in the current epidemiologic context should prompt interrogation about close contacts with suspected or confirmed COVID-19 cases. The association of other symptoms, especially gastrointestinal complaints, should alert the pediatrician to the possibility of a severe COVID-19 case. The natural 4- to 5-week gap between SARS-CoV-2 infection and the development of MIS-C symptoms warrants monitoring of affected individuals.19 Attention to vital signs is key, because extreme tachycardia is a common early sign of shock. Initial blood tests should include full blood count, C-reactive protein, urea, creatinine, electrolytes, and liver function. If results from these tests support a diagnosis of MIS-C, additional investigation is recommended to determine the diagnosis and severity of the disease.20
The exanthem in admitted patients in our institution was described as macular and/or papular in all cases. Different from adult COVID-19 patients, in that no vesicular, livedoid, or necrotic lesions were found,7 except in 1 patient who had an isolated acral late ischemic lesion. At our institution, skin lesions in children with COVID-19 who did not require admission were usually located acrally,8 whereas lesions in hospitalized patients tended to appear on the trunk and limbs, with palms and soles less frequently involved. As in other series, pernio-like lesions are infrequent in hospitalized pediatric patients.16 , 17
Conjunctival hyperemia appeared in practically the same proportion as the exanthem in our series and was a better independent predictor of PICU admission than the exanthem itself. Although in adults, it has received little attention, it should be considered as an alarm sign in children with suspected or confirmed COVID-19, as it has been frequently described in patients with MIS-C.5 , 6 , 16 , 17
Our center is located in one of the regions most impacted during the pandemic in Europe. Although the fact of being a single-institution study is a limitation, we think the population is representative and offers a large cohort of hospitalized pediatric patients with COVID-19 and mucocutaneous involvement. Nevertheless, more data from other centers should be obtained in an effort to conduct a meta-analysis on patients admitted with COVID-19 and on the subgroup of MIS-C cases. As another limitation, although the study project started early in the course of the pandemic in Spain, most of the data were retrieved retrospectively.
Conclusions
Mucocutaneous involvement is frequent in COVID-19 pediatric patients admitted to the hospital. Most of the patients hospitalized with mucocutaneous symptoms fulfilled MIS-C criteria. As a result, patients with an exanthem or conjunctival hyperemia at admission have a higher probability of PICU admission and a longer length of stay than those without mucocutaneous symptoms.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Approval was provided by the Ethics Committee and Board of the Hospital Infantil Universitario Niño Jesús. Standard informed consent was obtained to record images.
References
- 1.Munro A.P., Faust S.N. COVID-19 in children: current evidence and key questions. Curr Opin Infect Dis. 2020;33(6):540–547. doi: 10.1097/QCO.0000000000000690. [DOI] [PubMed] [Google Scholar]
- 2.Lu X., Zhang L., Du H., et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parri N., Lenge M., Buonsenso D. Children with COVID-19 in pediatric emergency departments in Italy. N Engl J Med. 2020;383(2):187–190. doi: 10.1056/NEJMc2007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swann O.V., Holden K.A., Turtle L., et al. Clinical characteristics of children and young people admitted to hospital with COVID-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:m3249. doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldstein L.R., Rose E.B., Horwitz S.M., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whittaker E., Bamford A., Kenny J., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galván Casas C., Català A.C., Carretero Hernández G., et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colmenero I., Santonja C., Alonso-Riaño M., et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183(4):729–737. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman E.E., McMahon D.E., Lipoff J.B., et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83(2):486–492. doi: 10.1016/j.jaad.2020.05.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andina D., Belloni-Fortina A., Bodemer C., et al. Skin manifestations of COVID-19 in children: part 2. Clin Exp Dermatol. 2021;46(3):451–461. doi: 10.1111/ced.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) https://emergency.cdc.gov/han/2020/han00432.asp Available at:
- 12.Pollán M., Pérez-Gómez B., Pastor-Barriuso, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonso Cadenas J.A., Andina Martínez D., Martín Díaz M.J., et al. In response to the article «Impact of the COVID-19 pandemic in the emergency room: first findings in a hospital in Madrid». An Pediatr (Barc) 2020;94(4):270–272. doi: 10.1016/j.anpedi.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Actualización n° 262 Enfermedad por el coronavirus (COVID-19) https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_262_COVID-19.pdf Available at:
- 15.Zimmermann P., Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. 2020 doi: 10.1136/archdischild-2020-320338. [DOI] [PubMed] [Google Scholar]
- 16.Rekhtman S., Tannenbaum R., Strunk A., et al. Mucocutaneous disease and related clinical characteristics in hospitalized children and adolescents with COVID-19 and multisystem inflammatory syndrome in children. J Am Acad Dermatol. 2021;84(2):408–414. doi: 10.1016/j.jaad.2020.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young T.K., Shaw K.S., Shah J.K., et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatol. 2021;157(2):207–212. doi: 10.1001/jamadermatol.2020.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jimenez D.G., Rodríguez-Belvís M.V., Gonzalez P.F., et al. COVID-19 gastrointestinal manifestations are independent predictors of PICU admission in hospitalized pediatric patients. Pediatr Infect Dis J. 2020;39(12):e459–e462. doi: 10.1097/INF.0000000000002935. [DOI] [PubMed] [Google Scholar]
- 19.Belot A., Antona D., Renolleau S., et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25(22):2001010. doi: 10.2807/1560-7917.ES.2020.25.22.2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harwood R., Allin B., Jones C.E., et al. PIMS-TS National Consensus Management Study Group. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health. 2021;5(2):133–141. doi: 10.1016/S2352-4642(20)30304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]