Abstract
Background
COVID-19 can be accompanied by acute neurological complications of both central and peripheral nervous systems (CNS and PNS). In this study, we estimate the frequency of such complications among hospital inpatients with COVID-19 in Assiut and Aswan university hospitals.
Materials and Methods
We screened all patients with suspected COVID-19 admitted from 1 June to 10 August 2020 to the university hospitals of Assiut and Aswan in Upper Egypt. Clinical and laboratory tests, CT/MRI of the chest and brain, and neurophysiology study were performed for each patient if indicated.
Results
439 patients had confirmed/probable COVID-19; neurological manifestations occurred in 222. Of these, 117 had acute neurological disease and the remainder had nonspecific neuropsychiatric symptoms such as headache, vertigo, and depression. The CNS was affected in 75 patients: 55 had stroke and the others had convulsions (5), encephalitis (6), hypoxic encephalopathy (4), cord myelopathy (2), relapse of multiple sclerosis (2), and meningoencephalitis (1). The PNS was affected in 42 patients: the majority had anosmia and ageusia (31) and the others had Guillain-Barré syndrome (4), peripheral neuropathy (3), myasthenia gravis (MG, 2), or myositis (2). Fever, respiratory symptoms, and headache were the most common general symptoms. Hypertension, diabetes mellitus, and ischemic heart disease were the most common comorbidities in patients with CNS affection.
Conclusion
In COVID-19, both the CNS and PNS are affected. Stroke was the most common complication for CNS, and anosmia and/or ageusia were common for PNS diseases. However, there were 6 cases of encephalitis, 2 cases of spinal cord myelopathy, 2 cases of MG, and 2 cases of myositis.
Keywords: COVID-19, Cerebrovascular stroke, Myelopathy, Spinal cord infarct, Transverse myelitis, Guillain-Barré syndrome, Myositis, Central nervous system, Peripheral nervous system, Anosmia, Ageusia, Seizure, Encephalitis
Introduction
In most patients, COVID-19 presents with a mild flu-like illness. Elderly patients with comorbidities, such as hypertension, diabetes, or lung and cardiac disease, are more likely to have severe disease and deaths. Neurological complications are frequently reported in severely or critically ill patients with comorbidities. Both central and peripheral nervous systems (CNS and PNS) can be affected [1, 2]. The SARS-CoV-2 virus is thought to enter the brain either via a hematogenous route [3] or via the olfactory system [4].
Most previous publications included case reports or a series of patients with stroke, encephalitis, seizures, transverse myelitis, and cranial neuropathies. However, in many of these, details were relatively sparse or vague (e.g., dizziness, vertigo, headache, and others), reflecting the challenge of studying such patients. A few studies showed the benefits of identifying patients with neurological complications across centers [1, 2]. A large series of 214 patients from Wuhan reported neurological symptoms in 78 patients.
There is little knowledge available concerning the acute neurological complications of COVID-19 in Egypt. This retrospective cohort study represents suspected COVID-19 patients admitted during the period from 1 June to 10 August 2020 to the university hospitals at Assiut and Aswan in order to estimate the following:
The proportion of acute neurological and psychiatric complications in 439 patients with COVID-19
The relative frequency of CNS and PNS involvement
The types of comorbidity associated with neurological complications
Materials and Methods
Patients with suspected COVID-19 were admitted during the period from 1 June to 10 August 2020 at the 2 largest university hospitals in Upper Egypt (Assiut University Hospital − El-Raghy Hospital, Neurology, Psychiatry, and Neurosurgery Department, Intensive Care Unit, and Chest Department − and Aswan University Hospital − Chest Department, Intensive Care Unit, and Emergency Unit) having quarantine areas of isolation for those patients. Many patients with confirmed or suspected COVID-19 had mild symptoms and were told to isolate at home. All cases admitted as inpatients had moderate to severe symptoms of COVID-19. Thus, our cohort will underrepresent patients with milder symptoms, such as mild cough, sore throat, headache, fever, fatigue, reduced taste and smell, or others. Neurologists were encouraged to report cases with neurological symptoms and signs of COVID-19 and to admit them for investigation to the Neurology, Psychiatry, and Neurosurgery hospital. Computed tomography (CT) of the chest to demonstrate the ground-glass opacity appearance of the lungs with consolidation and laboratory investigations, including blood gases, complete blood picture, serum D-dimer, ferritin, C-reactive protein, renal and liver functions, and coagulation profile, were performed. For patients with neurological manifestations, CT or MRI of brain or spine, nerve conduction, and electromyography (EMG)of the upper and lower limbs were performed if needed. Written informed consent was obtained from each patient or relative, and Local Ethical Committee of Assiut University Hospital approved the study before the IRB approved the study.
Diagnosis and Evidence of COVID-19
Reverse transcription polymerase chain reaction (RT-PCR) is the gold standard diagnostic procedure for confirming SARS-CoV-2 virus infection. RT-PCR testing was performed with nasopharyngeal swabs using swabs with a synthetic tip and an aluminum or plastic shaft. Swabs were placed immediately into sterile tubes containing 2–3 mL of viral transport media. The samples were stored at 2–8°C for up to 72 h.
RNA extraction of SARS-CoV-2 was performed using the QIAamp Viral RNA Mini Kit (Cat. No. 52904) supplied by QIAGEN, Germany. Sample preparation using QIAcube instruments follows the same steps as the manual procedure (i.e., lyse, bind, wash, and elute).
Pathogen detection of SARS-CoV-2 RNA used the TaqManTM 2019-nCoV Control Kit v1 (Cat. No. A47532) supplied by QIAGEN, Germany, on the Applied Biosystems 7500 Fast RT-PCR System, Foster City, CA, USA. It amplified and detected 3 viral genomic regions, reducing the risk of false negatives including the N protein (nucleocapsid gene), S protein (spike gene), and open reading frame-1ab (ORF1ab) genes. Applied BiosystemsTM TaqManTM 2019-nCoV Control Kit v1 (Cat. No. A47533) is a synthetic positive control that contains target sequences for each of the assays included in the TaqManTM 2019-nCoV Assay Kit v1 (Cat. No. A47532).
Evidence of SARS-CoV-2 infection was defined as follows:
1. Definite COVID-19 if patients came with clinical symptoms of infection and had positive respiratory sample PCR (e.g., nasal or throat swab)
2. Probable COVID-19 if clinical symptoms of infection and chest CT were consistent with COVID-19 and 1 or 2 laboratory investigations were positive (lymphopenia, high serum ferritin, and D-dimer Level) but PCR was negative or unavailable
Diagnosis of Neurological Disorders
When patients met >1 specific clinical case definition (e.g., seizures and encephalitis or stroke), the underlying causal diagnosis was considered primary and complications of that diagnosis were considered as secondary features (e.g., encephalitis or cerebrovascular stroke would be considered primary and seizures as secondary).
Definition of Major Neurological Diseases
Cerebrovascular Disease
Stroke was defined according to the WHO criteria as a syndrome of rapidly developing clinical signs of focal (or global) disturbance of cerebral function, with symptoms lasting 24 h or longer or leading to death, with no apparent cause other than of vascular origin [5], and documented by CT brain as ischemic or hemorrhagic stroke.
Subarachnoid Hemorrhage
It refers to bleeding into the subarachnoid space as documented by CT brain scan. The classic presentation can include sudden onset of severe headache (the classic feature), accompanying nausea or vomiting, symptoms of meningeal irritation, photophobia and visual changes, focal neurologic deficits, and sudden loss of consciousness at the ictus.
Cerebral Venous Sinus Thrombosis
The classic presentation can include the following: headache, blurred vision, fainting or loss of consciousness, loss of control over movement in part of the body, seizures, and coma.
Encephalitis
Symptoms include high fever, headache, sensitivity to light, stiff neck and back, vomiting, altered mental state, confusion and, in severe cases, seizures (clinical or electroencephalographic evidence), paralysis, and coma with exclusion of iatrogenic factors, such as sedatives and antipsychotics. Encephalitis documented by the presence of brain edema and signs of inflammations in MRI.
Hypoxic Encephalopathy
Symptoms include altered sensorium ranging from confusion, delirium, and stupor to coma due to prolonged hypoxia with exclusion of iatrogenic factors, such as sedatives and antipsychotics.
Meningitis
It is characterized by symptoms such as headache, fever, stiff neck, and manifestation of increased intracranial tension.
Transverse Myelitis
Transverse myelitis is a disorder caused by inflammation of the spinal cord. It is characterized by symptoms and signs of neurologic dysfunction in motor and sensory tracts on both sides of the spinal cord. The involvement of motor and sensory control leads to altered sensation, weakness, and sometimes urinary or bowel dysfunction.
Guillain-Barré Syndrome
Guillain-Barré syndrome (GBS) is defined as an acute illness characterized by clinical symptoms beginning with progressive ascending weakness (usually beginning peripherally in the limbs), impairment of position and vibration sense, reduced or absent tendon reflexes with neurophysiological evidence of prolonged distal and F wave latencies, and reduced motor conduction velocities.
Statistical Analysis
The collected data were revised, coded, tabulated, and introduced to a PC using Statistical Package for the Social Sciences (SPSS 25). Number and percent or means ± SD were used to represent data. The level of significance was set at p < 0.05. Patients with neurological manifestations were classified into 3 groups: patients with acute CNS disease (n = 75), patients with acute PNS disease (n = 42), and patients with nonspecific neuropsychiatric manifestations (n = 105).
Results
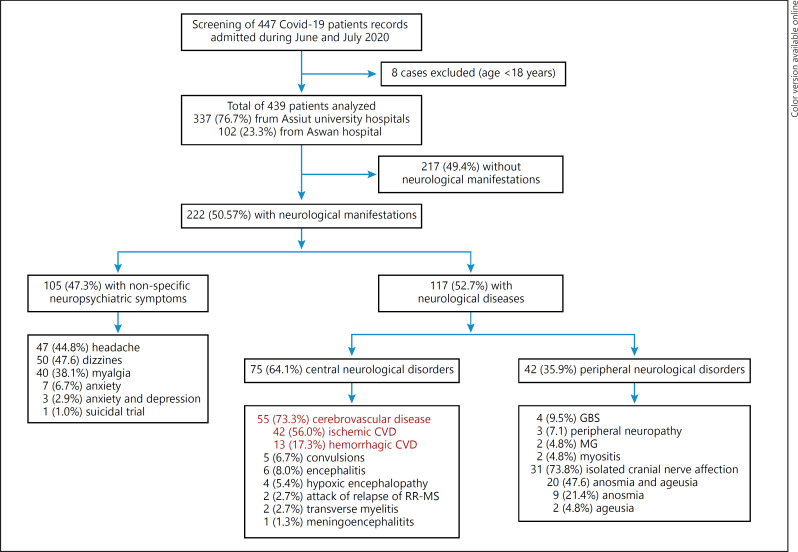
Out of 439 patients with confirmed/probable COVID-19, 222 (50.6%) had neurological manifestations. 117 (52.7%) of these had acute neurological disease: 75 (64.1%) with central neurological disorders and 42 (35.9%) with acute peripheral neurological disorders; the remaining 105 patients had nonspecific symptoms such as dizziness, headache, depression, and anxiety (Fig. 1, flowchart). The most common CNS disease was cerebrovascular disease (CVD; 55 patients [73.3%]), mostly ischemic stroke (42 cases: 9.6% of 439), while 13 patients (3% of 439) had hemorrhagic CVD. There was 1 case (0.22%) of subarachnoid hemorrhage and 1 case of subdural hematoma with intracerebral hemorrhage.
Fig. 1.
Flowchart of studied patients. GBS, Guillain-Barré syndrome; MG, myasthenia gravis; MS, multiple sclerosis; CVD, cerebrovascular disease.
Encephalitis was recorded in 6 patients (8%), followed by convulsions (5 patients [6.7%]), hypoxic encephalopathy (4 patients [5.4%]), transverse myelitis (2 patients [2.7%]), relapse of multiple sclerosis (RR-MS; 2 patients [2.7%]), and meningoencephalitis (1 patient [1.3%]). Of the 41 cases with PNS disease, 31 (73.8%) had isolated cranial nerve affection (anosmia and/or ageusia), 4 (9.5%) had GBS, 3 (7.1%) had peripheral neuropathy, 2 (4.8%) had myasthenia gravis (MG), and 2 (4.8%) had myositis (Fig. 1, flowchart).
Table 1 details the demographics and symptomatology of the patients. Patients with acute CNS disorders were significantly older than the other groups. There was a significant difference between the percent of definite/probable COVID-19 between patients with CNS disorders versus those with PNS disorders. The most common presenting symptoms were fever, respiratory symptoms and signs, and headache followed by fatigue and malaise, GIT symptoms, dizziness, and/or vertigo; the least frequent symptom was sore throat. The percentage of symptoms was significantly higher in patients with PNS because most patients with acute CNS disease had disturbed consciousness, making it impossible to assess them in detail (most of them had cerebrovascular stroke).
Table 1.
Patient details and results of chest CT
| Variable | Patients with definite neurological disease, N = 117 | Patients with acute CNS disease, n = 75 | Patients with acute PNS disease, n = 42 | p value |
|---|---|---|---|---|
| Age, mean±SD, years | 55.1±17.2 | 59.5±17.5 | 48.3±14.3 | 0.001 |
| Sex, M/F | 61/56 | 41/34 | 20/22 | |
| Ratio, % | 52.1/47.9 | 54.7/45.3 | 47.6/52.4 | 0.600 |
| Definite COVID-19, n (%) | 55 (47.0) | 23 (30.7) | 32 (76.2) | |
| Probable COVID-19, n (%) | 62 (53.0) | 52 (69.3) | 10 (33.8) | 0.0001 |
| Presenting of general symptoms, n (%) | ||||
| Fever | 101 (86.3) | 70 (93.3) | 31 (73.8 | 0.003 |
| Sore throat | 7 (6.0) | 0 (0) | 7 (16.7) | 0.0001 |
| Fatigue and malaise | 30 (25.6) | 14 (18.7) | 16 (38.1) | 0.003 |
| Headache | 36 (30.8) | 21 (28) | 15 (35.7) | 0.380 |
| Dizziness and/or vertigo | 15 (12.8) | 4 (5.3) | 11 (26.2) | 0.001 |
| GIT symptoms and signs | 29 (24.8) | 17 (22.7) | 12 (28.6) | 0.321 |
| Respiratory symptoms and signs | 100 (85.5) | 60 (80.0) | 40 (95.2) | 0.781 |
| CT chest: positive bilateral/unilateral GGO | 98 (83.8) | 69 (87.3) | 29 (69) | 0.043 |
CNS, central nervous system; PNS, peripheral nervous system; GIT, gastrointestinal tract; GGO, ground-glass opacities with consolidation; CT, computed tomography.
Table 2 indicates that 79 (67.5%) cases out of 117 had one or more comorbidity risk factors; these were significantly more common in patients with CNS disorders than PNS disorders, particularly renal impairment and previous neurological disease (particularly CVS). In general, the commonest comorbidities were hypertension followed by diabetes and ischemic heart disease and neurological disease.
Table 2.
Comorbidities among studied groups
| Patients with definite neurological disease, N = 117, n (%) | Patients with acute CNS disease, n = 75, n (%) | Patients with acute PNS disease, n = 42, n (%) | p value | |
|---|---|---|---|---|
| Comorbidities | 79 (67.5) | 57 (76) | 22 (52.4) | 0.008 |
| Hypertension | 53 (45.3) | 38 (50.7) | 15 (36.7) | 0.119 |
| Ischemic heart | 20 (17.1) | 14 (18.7) | 6 (14.3) | 0.546 |
| Diabetes mellitus | 41 (35) | 25 (33.3) | 16 (38.1) | 0.605 |
| Impaired liver function | 6 (5.1) | 6 (8) | 0 | 0.218 |
| Impaired renal function | 10 (8.4) | 9 (12) | 1 (2.4) | 0.074 |
| Chest disease | 4 (3.4) | 2 (2.7) | 2 (4.8) | 0.550 |
| Neurological disease (types)* | 14 (12.0) | 11 (14.7) | 3 (7.1) | 0.229 |
CNS, central nervous system; PNS, peripheral nervous system; PD, Parkinson's disease; AD, Alzheimer's disease; MG, myasthenia gravis. * Ischemic stroke, hemorrhagic stroke, epilepsy, PD, PD and AD, AD, brain tumor, MG, CKD, and PD.
Overall, 55 cases had definite COVID-19 and 62 cases had probable COVID-19 (32 cases with unavailable PCR [51.6%] and 30 cases with negative PCR [48.4%] out of a total of 117 cases with neurological disorders). Comparison between definite and probable COVID-19 cases is shown in Table 3. The age of probable cases was significantly higher than that of definite cases (60.3 ± 15.2 vs. 51.5 ± 17.1, p = 0.004). There was no significant difference between the symptoms of the 2 groups except for sore throat and fatigue and malaise which occurred more frequently in definite cases. For acute CNS cases, CVS was more frequent in probable than definite COVID-19 cases (12 definite and 43 probable cases). In PNS cases, the only significant difference was between cases of isolated CN affection as most of them had positive PCR (29 definite and 2 probable).
Table 3.
Definite COVID-19 versus probable COVID-19
| Variable | Patients with definite COVID-19, n = 55 | Patients with probable COVID-19, n = 62 | p value |
|---|---|---|---|
| Age, mean±SD, years | 51.5±17.1 | 60.3±15.2 | 0.004 |
| Sex, M/F | 28/27 | 35/26 | 0.485 |
| Ratio, % | 50.9/49.1 | 57.4/42.6 | |
| Presenting of general symptoms, n (%) | |||
| Fever | 47 (85.5) | 54 (87.1) | 0.796 |
| Sore throat | 6 (10.9) | 1 (1.6) | 0.034 |
| Fatigue and malaise | 20 (36.4) | 10 (16.1) | 0.012 |
| Headache | 21 (38.1) | 15 (24.2) | 0.102 |
| Dizziness and/or vertigo | 9 (16.4) | 4 (6.5) | 0.089 |
| GIT symptoms and signs | 11 (20) | 18 (29) | 0.259 |
| Respiratory symptoms and signs | 48 (87.3) | 52 (83.9) | 0.602 |
| CNS, n (%) | |||
| Cerebrovascular stroke | 12 (21.8) | 43 (69.4) | <0.001 |
| Convulsion | 4 (7.3) | 1 (1.6) | 0.131 |
| Encephalitis | 2 (3.6) | 4 (6.5) | 0.491 |
| Hypoxic encephalopathy | 2 (3.6) | 2 (3.2) | 0.903 |
| Cord myelopathy | 2 (3.6) | 0 | 0.130 |
| Attack of relapse of MS | 0 | 2 (3.2) | 0.179 |
| Meningoencephalitis | 1 (1.8) | 0 | 0.286 |
| PNS, n (%) | |||
| GBS | 2 (3.6) | 2 (3.2) | 0.903 |
| Peripheral neuropathy | 0 | 3 (4.8) | 0.098 |
| Myositis | 1 (1.8) | 1 (1.6) | 0.932 |
| Myasthenia | 0 | 2 (3.2) | 0.179 |
| Isolated cranial nerves (anosmia and ageusia) | 29 (52.7) | 2 (3.2) | <0.001 |
| Comorbidities, n (%) | |||
| Hypertension | 20 (36.4) | 33 (53.2) | 0.067 |
| Ischemic heart | 12 (21.8) | 16 (25.8) | 0.614 |
| Diabetes mellitus | 23 (41.8) | 18 (29.0) | 0.148 |
| Impaired liver function | 3 (5.5) | 3 (4.8) | 0.880 |
| Impaired renal function | 2 (3.6) | 8 (12.9) | 0.074 |
| Chest disease | 3 (5.5) | 1 (1.6) | 0.254 |
| Neurological disease (types) | 5 (9.1) | 9 (14.5) | 0.367 |
GIT, gastrointestinal tract; GBS, Guillain-Barré syndrome; CNS, central nervous system; PNS, peripheral nervous system; PD, Parkinson's disease; AD, Alzheimer's disease; MG, myasthenia gravis; MS, multiple sclerosis.
The mean serum level of D-dimer was 2.737 ± 6.63 mg/L (range 0.19–35.2 mg/L). A significantly higher level was seen (8.77 ± 11.2: range 0.46–35.2 mg/L) in patients with acute CNS disease than with acute PNS disease (0.79 ± 0.92: range 0.19–3.6 mg/L) (p = 0.0001). The mean serum ferritin was 544.7 ± 723.4 ng/mL (range 10–3,859 ng/mL). It was nonsignificantly higher in patients with acute CNS disease (787.2 ± 1,204.3: range 143–3,959 ng/mL) than in patients with PNS disease (463.9 ± 480.6: range 10–1,819.7 ng/mL) (p = 0.251). The mean percent lymphocytes was 18.6 ± 12.5 (range 2.5–59.3). Patients with acute CNS disease had a significantly lower level (16 ± 12.3: range 2.5–59.3) than those with PNS disease (23.1 ± 11.8: range 7.98–49.8) (p = 0.003).
Discussion
Available pieces of evidence suggest that the SARS-CoV-2 virus can traverse the blood-brain barrier and enter the brain, possibly via a hematogenous route. It may also enter transneuronally via the olfactory system, across the cribriform plate [4]. Angiotensin-converting enzyme 2 receptors that are present on endothelial cells of cerebral vasculature act as cell entry points for virus [3].
Cerebrovascular Disease
Many cerebrovascular events were identified in our study (55 cases [73.3%] out of 75 patients with CNS symptoms; or 12.5% out of the whole sample of 439 COVID-19 patients). This is consistent with previous cohorts and case reports of acute COVID-19 complications [2, 6, 7]. Most of them were ischemic stroke (42 cases: 9.6% of 439), while 13 patients (3% of 439) had hemorrhagic CVD; out of the latter, 1 case had subarachnoid hemorrhage and another had subdural hematoma with intracerebral hemorrhage. Li and coworkers [8], in a retrospective study, noted that out of 221 patients, 13 (5.9%) developed CVD after infection. Of these, 11 (84.6%) were diagnosed with ischemic stroke, 1 (7.7%) with cerebral venous sinus thrombosis, and 1 (7.7%) with cerebral hemorrhage. In a case series of 12 patients [9], Reddy et al. [9] found that 10 patients had ischemic stroke, of which 1 suffered hemorrhagic transformation and 2 had intracerebral hemorrhage [6]. Beyrouti and coworkers [6], in a report of 6 severely affected patients with large cerebral infarcts, noted elevated D-dimer levels (≥1,000 µg/L), indicating a coagulopathy. Few other studies have reported cerebrovascular complications in COVID-19 [2, 10]. Other small case series have described patients with COVID-19 and concurrent stroke [7, 11].
The pathophysiological mechanisms that underlie cerebrovascular ischemic events in COVID-19 could potentially be related to vasculopathy, with a report of SARS-CoV-2 endotheliitis-related cerebrovascular events [12]. In addition, there is an increase of conventional stroke risk during sepsis [13, 14]. Comorbidities, such as diabetes and hypertension, enhance expression of the angiotensin-converting enzyme 2 receptor in the brain and neurotropism of the SARS-CoV-2 virus [15]. In COVID-19, alterations in blood pressure control are another proposed mechanism that has been suggested to explain the increased risk of cerebral vascular complications. Ordinarily, angiotensin-converting enzyme 2 signaling lowers blood pressure [16]. Competitive blockage of angiotensin-converting enzyme 2 by the SARS-CoV-2 virus downregulates angiotensin-converting enzyme 2 expression leading to uncontrolled blood pressure, and the enhanced possibility of cerebrovascular accidents is proposed as another mechanism [16].
Cord Myelopathy
Spinal cord involvement is rare in COVID-19. In the present study, we reported 2 cases with spinal cord involvement out of 439 cases (0.5%) with PCR positive for COVID-19. The first patient came with acute onset of flaccid paraplegia and a sensory level at T4 associated with retention of urine, 10 days after flu-like symptoms. The MRI revealed a picture of transverse myelitis. The second patient presented with acute quadriplegia with preservation of deep sensation and a history of fever, headache, and insomnia 3 days previously. The MRI showed cervicodorsal myelopathy of the anterior 2/3 of the cord extending from C4 to T4 most probably caused by secondary occlusion of the anterior spinal artery following acute COVID-19 pneumonia. Only 4 cases of transverse myelitis have been reported previously [17, 18, 19, 20]. It is probably due to heightened inflammation following a cytokine storm brought on by COVID-19 [20].
Hypoxic Encephalopathy
There were 4 confirmed (nasopharyngeal swab) COVID-19 cases (0.25% of the total or 5.4% of those with CNS symptoms) who presented with altered sensorium ranging from confusion, delirium, and stupor to coma due to prolonged hypoxia and low oxygen saturation. They came to the hospital complaining of headache, altered mental status, fever, and cough. Upon examination, the patients were found to be encephalopathic, not responding to any commands. Noncontrast brain CT scan showed diffuse brain edema with effacement of cerebrospinal fluid (CSF) spaces, whilst electroencephalography showed bilateral mild diffuse slowing of background activity.
Encephalitis
The SARS-CoV-2 virus has the potential to enter the brain by traveling from the nasal mucosa to the olfactory bulb and spreading to the piriform cortex. Xiang and coworkers [21] in Beijing, China, claimed to isolate the first SARS-CoV-2 virus in CSF. So far, there have been 7 additional reported cases of SARS-CoV-2-associated encephalitis, encephalopathy, or meningitis. In the present study, 6 cases had features consistent with encephalitis. The SARS-CoV-2 virus was identified in a throat swab in 4 cases, while the other 2 cases were probable COVID-19 with positive chest CT that demonstrated the ground-glass appearance of the lungs and leukocytosis and lymphopenia. Neuroimaging was normal. Unfortunately we were unable to examine the CSF.
Seizures
Seizures are not a common manifestation in COVID-19. In the present study, there were 19 cases out of 439 (4.3%). In 3 of them (0.68%), the seizures were novel; another 2 patients had a known history of controlled epilepsy and had experienced exacerbation of the seizures with COVID-19 (0.46%). In the other 14 patients (3.19%), the seizures were associated with other primary pathology (5 patients had recent ischemic stroke, 2 patients had recent hemorrhagic stroke, 6 patients had encephalitis, and 1 patient had an old brain tumor). All our cases were diagnosed on clinical and electroencephalographic evidence. In contrast to our results, a multicentric Chinese retrospective study noted that among 304 COVID-19 patients (108 with severe disease), none had acute symptomatic seizures or status epilepticus despite the presence of severe metabolic alterations. Mao and coworkers [2] noted that among 214 patients admitted to intensive care units, there was only a single case of seizure. Vollono and colleagues [22] published a report of nonconvulsive status epilepticus triggered by COVID-19, in an elderly patient. The higher frequency of seizures in COVID-19 patients in our study may be related to the omission of mild cases and specialty admission of cases with neurological manifestations to the Neurology, Psychiatry, and Neurosurgery Department, Assiut University.
Guillain-Barré Syndrome
Postinfectious autoimmune reactions can affect neuronal cells. The SARS-CoV-2 virus epitopes bear a structural resemblance to several human proteins. Molecular mimicry between virus epitope and myelin basic protein results in autoimmune postinfectious demyelinating syndromes [23].
In the present study, GBS is a frequently encountered neurological complication of COVID-19. Four cases presented with classical clinical manifestations of acute symmetric flaccid weakness with absent reflexes and numbness of 4 limbs, 2-3 weeks after COVID-19. Nerve conduction, F waves, H reflex, and electromyography confirmed the presence of severe demyelinating polyradiculoneuropathy. Zhao and coworkers [24] described the first patient of COVID-19 presenting with GBS.
Myositis and COVID-19
In the present study, 2 (4.8%) cases had myositis. The first patient came to the hospital with a 3- to 4-day history of fever and lower respiratory tract symptoms (cough, sputum, and dyspnea). He then developed respiratory distress, bulbar symptoms, neck and proximal muscle weakness, and pain of all 4 limbs with no sensory impairment. PCR was positive for COVID-19 with hypoxemia, anemia, leukocytosis, lymphopenia, with high levels of creatine kinase (CPK), ferritin, and D-dimer. A chest CT showed bilateral ground-glass opacity. The patient needed noninvasive mechanical ventilation. EMG confirmed the clinical diagnosis of myositis. The 2nd patient had a 7-day history of fever and cough. She developed severe muscle pain and weakness of 4 limbs, and EMG confirmed the clinical diagnosis of myositis. Her laboratory data showed decreased PCO2, anemia, leukocytosis, neutropenia, and lymphopenia with high CPK and ferritin. CT chest showed bilateral ground-glass opacity. PCR was not performed.
Zhang et al. [25] reported a patient with COVID-19-associated inflammatory myopathy, presenting with facial, bulbar, and proximal limb weakness. Beydon and coworkers [26] recently described myositis in a critically ill patient of COVID-19. The patient presented with acute myalgia, difficulty in waking, and proximal weakness. CPK (25,384 IU/L) was markedly elevated. Four days later, the patient became febrile and tested positive for the SARS-CoV-2 virus.
MG and COVID-19
In the present study, 2 patients with COVID-19 developed MG (4.8%) and required intubation for respiratory failure. One patient with previously stable MG had exacerbation of myasthenia with bilateral facial, bulbar, and masticatory symptoms and proximal weakness of 4 limbs. The second previously well patient developed MG with ptosis, bulbar manifestations, and proximal muscle weakness. The diagnosis was confirmed by a positive decrement test in neurophysiology study and elevated serum acetyl choline receptor antibody in both cases. Both patients were treated with intravenous immunoglobulin and improved. To our knowledge, only one study of Anand et al. [27] reported 5 hospitalized patients with autoimmune MG (4 with acetylcholine receptor antibodies and 1 with muscle-specific tyrosine kinase antibodies).
Smell and Taste
In the present study, 42 cases had PNS disease. The most frequent (31 cases, 73.8%) symptoms were a complete or partial loss of smell (anosmia) and taste (ageusia) consistent with recent reports of Moein et al. [28]. In a French study, Lechien and coworkers [29] reported that out of 417 mild-to-moderate COVID-19 patients, 86 and 88% of patients, respectively, reported anosmia and ageusia. The SARS-CoV-2 virus utilizes angiotensin-converting enzyme 2 receptors, present in the olfactory epithelium, to enter into the neuronal cells from where it spreads to the olfactory bulb via the olfactory nerve [30].
General Symptoms
In the present study, fever occurred in 101/117 (86.3%) patients with neurological complications with a higher frequency among patients with acute CNS versus PNS disorders (88.6 vs. 73.8%). This is consistent with other meta-analyses that reported fever as a common general manifestation in COVID-19 patients with neurological manifestations, ranging from 80.4 to 88.7% [31, 32, 33, 34].
Respiratory symptoms including cough with or without expectoration and dyspnea were also recorded in the majority of cases (100/117 patients) but were more frequent among patients with acute PNS than acute CNS diseases. Thirty-nine patients reported GIT symptoms. Respiratory symptoms were more common in the present study than in other studies [31, 32, 33, 34] ranging from 57 to 63% for cough and 45–58% for dyspnea.
Fatigue and malaise were observed in 30/117 (25.6%) patients and headache in 36/117 (30.8%) patients; both were more common in PNS diseases than in CNS diseases. Our recorded rates were lower than those in some previous meta-analyses (38–46% for fatigue) [32, 34]. In one of them with 420 mild-to-moderate COVID-19 patients, headache (70%) was the most prevalent symptom [35]. On the other hand, our recorded rate of headache was higher than the recorded rate (8–15%) in other studies [32, 33, 34]. Increased mental stress, excessive anxiety, and changes in lifestyle are possible reasons for early headaches. Belvis [36] in a recent communication opined that COVID-19-associated acute headache can be due to systemic viral infection, primary cough, headache, and tension-type headache. Early headaches respond well to acetaminophen. Headaches appearing between the 7th and the 10th day of illness may be related to cytokine storm [36]. Vertigo and dizziness were observed in 15/117 patients, while sore throat was the least frequent symptom (7/117 patients).
Conclusions
COVID-19-associated acute neurological diseases were common in our study. Acute CNS diseases were more common than PNS diseases. Cerebrovascular stroke was the most common CNS disease, and loss of smell and taste were the most common PNS symptoms. Comorbidities were common among patients with CNS diseases. These data begin to characterize the spectrum of neurological complications of COVID-19 in Egypt.
Statement of Ethics
This research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Written informed consent was obtained from each patient or relative, and Local Ethical Committee of Assiut University Hospital approved the study before IRB approval (IRB No. 17300470). The confidentiality of the patients' information was maintained during all the steps of the study.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There was no funding for this study.
Author Contributions
E.M.K.: study conception, design of the work, statistical analysis, and critical revision of the manuscript. N.A.E. and A.A.: study conception, design of the work, and drafting of the manuscript. R.K.S.: study conception and interpretation of neuroimaging. M.A.: recruited the cases and performed analysis. E.D. and H.M.H.: laboratory data acquisition. A.A.A., A.A.Z., and M.Z.: data acquisition from ICU. A.M.H. and M.K.H.: data acquisition from chest department. S.M.H.: data acquisition from COVID-19 isolation hospital, all from Assiut University Hospital. M.S. and A.S.: data acquisition from Aswan University Hospital. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgement
The authors would like to acknowledge Professor John C. Rothwell for his comments and revision of the manuscript with respect to English language, Sobell Department of Motor Neuroscience and Movement Disorders, National Hospital for Neurology and Neurosurgery, Queen Square, London, UK.
References
- 1.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 Jun 4;382((23)):2268–70. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Jun 1;77((6)):683–90. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020 Apr 28;9((1)):45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Natoli S, Oliveira V, Calabresi P, Maia LF, Pisani A. Does SARS-CoV-2 invade the brain? Translational lessons from animal models. Eur J Neurol. 2020 Sep;27((9)):1764–73. doi: 10.1111/ene.14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf PA, Kannel WB, Dawber TR. Prospective investigations: the Framingham study and the epidemiology of stroke. Adv Neurol. 1978;19:107–20. [PubMed] [Google Scholar]
- 6.Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020 Aug;91((8)):889–91. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020 May 14;382((20)):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5((3)):279–84. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy ST, Garg T, Shah C, Nascimento FA, Imran R, Kan P, et al. Cerebrovascular disease in patients with COVID-19: a review of the literature and case series. Case Rep Neurol. 2020 May-Aug;12((2)):199–209. doi: 10.1159/000508958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395((10223)):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S, et al. COVID-19 presenting as stroke. Brain Behav Immun. 2020 Jul;87:115–9. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020 Apr 23;382((17)):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boehme AK, Ranawat P, Luna J, Kamel H, Elkind MS. Risk of acute stroke after hospitalization for sepsis: a case-crossover study. Stroke. 2017 Mar;48((3)):574–80. doi: 10.1161/STROKEAHA.116.016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González-Pinto T, Luna-Rodríguez A, Moreno-Estébanez A, Agirre-Beitia G, Rodríguez-Antigüedad A, Ruiz-Lopez M. Emergency room neurology in times of COVID-19: malignant ischaemic stroke and SARS-CoV-2 infection. Eur J Neurol. 2020 Sep;27((9)):e35–6. doi: 10.1111/ene.14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020 Aug 6;383((6)):590–2. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu H, Rhee JW, Cheng P, Waliany S, Chang A, Witteles RM, et al. Cardiovascular complications in patients with COVID-19: consequences of viral toxicities and host immune response. Curr Cardiol Rep. 2020 Apr 21;22((5)):32. doi: 10.1007/s11886-020-01292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AlKetbi R, AlNuaimi D, AlMulla M, AlTalai N, Samir M, Kumar N, et al. Acute myelitis as a neurological complication of Covid-19: a case report and MRI findings. Radiol Case Rep. 2020 Sep;15((9)):1591–5. doi: 10.1016/j.radcr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munz M, Wessendorf S, Koretsis G, Tewald F, Baegi R, Krämer S, et al. Acute transverse myelitis after COVID-19 pneumonia. J Neurol. 2020 Aug;267((8)):2196–7. doi: 10.1007/s00415-020-09934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarma D, Bilello LA. A case report of acute transverse myelitis following novel coronavirus infection. Clin Pract Cases Emerg Med. 2020;4((3)):321–3. doi: 10.5811/cpcem.2020.5.47937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Acute myelitis after SARS-CoV-2 infection: a case report. MedRxiv. 2020 [Google Scholar]
- 21.Xiang P, Xu X, Gao L, Wang H, Xiong H, Li R. First case of 2019 novel coronavirus disease with encephalitis. ChinaXiv. 2020;202003:00015. [Google Scholar]
- 22.Vollono C, Rollo E, Romozzi M, Frisullo G, Servidei S, Borghetti A, et al. Focal status epilepticus as unique clinical feature of COVID-19: a case report. Seizure. 2020 May;78:109–12. doi: 10.1016/j.seizure.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyons-Weiler J. Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. J Transl Autoimmun. 2020;3:100051. doi: 10.1016/j.jtauto.2020.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020 May;19((5)):383–4. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Charmchi Z, Seidman RJ, Anziska Y, Velayudhan V, Perk J. COVID-19-associated myositis with severe proximal and bulbar weakness. Muscle Nerve. 2020 Sep;62((3)):E57–e60. doi: 10.1002/mus.27003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beydon M, Chevalier K, Tabaa OA, Hamroun S, Delettre A-S, Thomas M, et al. Myositis as a manifestation of SARS-CoV-2. Ann Rheum Dis. 2020 Apr 23; doi: 10.1136/annrheumdis-2020-217573. [DOI] [PubMed] [Google Scholar]
- 27.Anand P, Slama MCC, Kaku M, Ong C, Cervantes-Arslanian AM, Zhou L, et al. COVID-19 in patients with myasthenia gravis. Muscle Nerve. 2020 Aug;62((2)):254–8. doi: 10.1002/mus.26918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020 Aug;10((8)):944–50. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020 Aug;277((8)):2251–61. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beltrán-Corbellini Á, Chico-García JL, Martínez-Poles J, Rodríguez-Jorge F, Natera-Villalba E, Gómez-Corral E, et al. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur J Neurol. 2020;27((9)):1738–41. doi: 10.1111/ene.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao Y, Liu X, Xiong L, Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: a systematic review and meta-analysis. J Med Virol. 2020 Sep;92((9)):1449–59. doi: 10.1002/jmv.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020 Jun;80((6)):656–65. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020 Mar-Apr;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu J, Ji P, Pang J, Zhong Z, Li H, He C, et al. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J Med Virol. 2020 Apr 15; doi: 10.1002/jmv.25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lechien JR, Chiesa-Estomba CM, Place S, Van Laethem Y, Cabaraux P, Mat Q, et al. Clinical and epidemiological characteristics of 1,420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020 Sep;288((3)):335–44. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belvis R. Headaches during COVID-19: my clinical case and review of the literature. Headache. 2020 Jul;60((7)):1422–6. doi: 10.1111/head.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]