Abstract
This commentary focuses on the results of the study by Pietrantonio et al., which evaluated the clinical conundrum of triplet versus doublet chemotherapy in combination with targeted therapy for metastatic left‐sided RAS/BRAF wild‐type colorectal cancer and appears in this issue. Both FOLFOXIRI [fluorouracil, leucovorin, oxaliplatin, and irinotecan] plus bevacizumab and FOLFOX [fluorouracil, leucovorin, and oxaliplatin] plus panitumumab have shown impressive activity in this population; however, the two have not been directly compared. The article by Pietrantonio et al. presents a propensity score‐adjusted analysis using information from five previous randomized trials and provides best available evidence comparing these regimens. This commentary will discuss their results and how their findings fit in current treatment paradigms.
Short abstract
This commentary focuses on recently reported results of a study evaluating the clinical conundrum of triplet versus doublet chemotherapy in combination with targeted therapy for metastatic left‐sided RAS/BRAF wild‐type colorectal cancer.
Introduction
Fluoropyrimidine‐based doublet chemotherapy has remained the treatment backbone in metastatic colorectal cancer for the past 2 decades. Efforts to improve on the combination include the addition of bevacizumab or anti‐epidermal growth factor receptor (EGFR) agents [1, 2] and intensification with the triplet regimen fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI), with or without targeted therapy [3, 4, 5]. The importance of tumor sidedness has emerged in recent years, and post hoc analyses demonstrate improved outcomes with anti‐EGFR agents compared with bevacizumab for left‐sided primaries when used with doublet chemotherapy [6]. The converse is true for right‐sided tumors, with a survival advantage favoring bevacizumab [6]. For left‐sided colorectal cancers that are RAS/BRAF wild type, current guidelines support first‐line doublet plus anti‐EGFR or triplet chemotherapy with or without bevacizumab; however, there are limited data comparing the two strategies [7].
Figure 1.
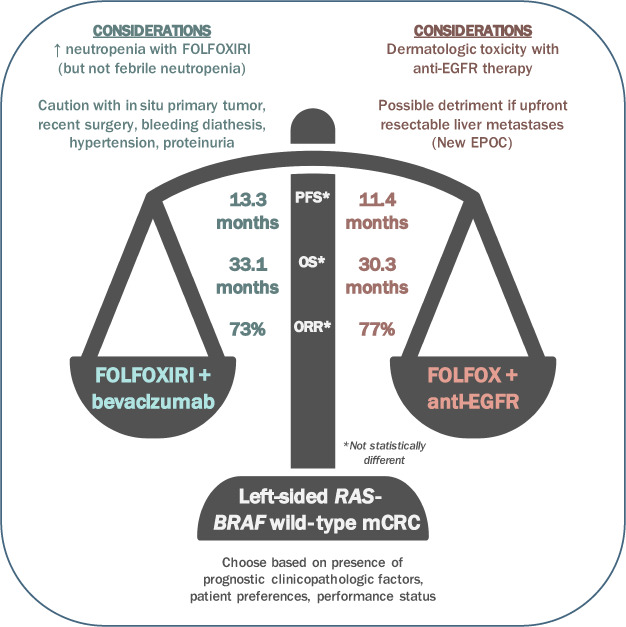
Considerations for choosing FOLFOXIRI + bevacizumab versus FOLFOX + anti‐EGFR therapy for left‐sided RAS/BRAF wild‐type mCRC. Abbreviations: EGFR, epidermal growth factor receptor; FOLFOX, fluorouracil, leucovorin, and oxaliplatin; FOLFOXIRI, fluorouracil, leucovorin, oxaliplatin, and irinotecan; mCRC, metastatic colorectal cancer; ORR, overall response rate; OS, overall survival; PFS, progression‐free survival.
Left‐Sided RAS/BRAF Wild‐Type Tumors: FOLFOXIRI + Bevacizumab Versus FOLFOX + Anti‐EGFR
Pietrantonio et al. conducted a propensity‐matched retrospective analysis composed of five phase II/III trials, Valentino, TRIBE, TRIBE2, STEAM, and CHARTA, comparing FOLFOXIRI + bevacizumab versus fluorouracil, leucovorin, and oxaliplatin (FOLFOX) + panitumumab in left‐sided RAS/BRAF wild‐type tumors [8]. This study addressed an important knowledge gap, as there are no randomized head‐to‐head trials specifically evaluating this question. In the analysis, no difference was observed in progression‐free survival (PFS) between FOLFOX + panitumumab and FOLFOXIRI + bevacizumab (11.4 vs. 13.3 months, adjusted hazard ratio [HR] 0.82, p = .11); overall survival (OS) was also similar (30.3 vs. 33.1 months, adjusted HR 0.80, p = .14). Response rates (77% vs. 73%, adjusted OR 0.79, p = .40) and disease control rates (95% vs. 97%, adjusted OR 1.09, p = .89) were similar for FOLFOX + panitumumab and FOLFOXIRI + bevacizumab, respectively. Rates of secondary resection of metastases did not differ between the two groups. Regarding toxicity, neutropenia was more prevalent in the triplet group (48% vs. 26%, p = .03), but febrile neutropenia rates were similar (6% vs. 3%, p = .24). Considerations for choosing between the two regimens are summarized in Figure 1.
Who Is a Candidate for the Triplet Regimen?
In practice, triplet therapy is considered for fit patients with cancers exhibiting aggressive behavior and/or poor prognostic features. This may include tumors with RAS or BRAF V600E mutations, poorly differentiated and signet ring histology, right sidedness, and/or a high disease burden. The addition of bevacizumab is another consideration depending on RAS/BRAF mutational status, tumor sidedness, and the presence of an in situ primary tumor. A recent meta‐analysis by Cremolini et al. comparing triplet‐bevacizumab versus doublet‐bevacizumab showed a 4.5‐month OS benefit favoring the triplet group [9]. Notably, the benefit was not observed in the BRAF V600E subgroup (HR 1.11, 95% confidence interval 0.75–1.73) [9]. A separate meta‐analysis with overlapping trials also did not find a PFS or OS benefit in BRAF‐mutated tumors, but the OS analysis only included one trial and PFS analysis included the VOLFI study containing FOLFOXIRI + panitumumab, which may have confounded results [10].
The optimal regimen for BRAF‐mutated tumors remains undetermined. Given the benefit of combination BRAF with or without MEK inhibitors and anti‐EGFR therapy in the refractory setting [11], the phase II ANCHOR study is currently evaluating the combination of encorafenib, binimetinib, and cetuximab in the first‐line setting. Preliminary data show an objective response rate of 50% and disease control rate of 85% [12]. However, there remains a role for chemotherapy in cases for which rapid treatment initiation is required and mutational status results are pending. Although there are mixed results around the use of FOLFOXIRI + bevacizumab for the first‐line treatment of BRAF V600E‐mutated colorectal cancer in the recent meta‐analyses, it intuitively makes sense that one may want to use one's most aggressive option first, as these patients are prone to rapid deterioration and treatment attrition is high. A recent population‐based study showed that only 26% of patients with BRAF V600E‐mutated colorectal cancer receive second‐line therapy [13].
Another setting to consider the use of FOLFOXIRI + bevacizumab combination is conversion therapy for initially unresectable liver disease, particularly in RAS‐mutant disease. In the Pietrantonio et al. study of left‐sided RAS/BRAF tumors, a benefit was not observed in secondary resection of metastases with FOLFOXIRI + bevacizumab compared with FOLFOX + panitumumab at 22% versus 18% (p = .514) [8], but the role of anti‐EGFR with FOLFOX is controversial based on data from the New EPOC trial [14]. In this study of patients with upfront resectable or borderline resectable KRAS wild‐type liver metastases, the addition of cetuximab to chemotherapy was associated with worse PFS and OS outcomes [14].
Challenges with Triplet Chemotherapy
The inclusion of additional antineoplastic agents, particularly chemotherapy, comes at a cost of additional adverse events. Clinically important adverse events are rates of neutropenia and febrile neutropenia, the latter reported to be 6% in the Pietrantonio et al. study [8]. Of note, prophylactic granulocyte colony‐stimulating factor (GCSF) was not implemented in the included five studies. Clinicians will need to provide patient counseling and education around this potential complication and anticipate the need for prophylactic GCSF use. In addition, treatment intensity with triplet chemotherapy may inevitably require de‐escalation of care to maintenance therapy. The phase II Valentino study in the analysis compared maintenance strategies of 5‐fluorouracil‐panitumumab and panitumumab alone [15], and the other studies also varied in terms of duration of induction therapy. Interpretation of the results may be impacted by these factors. In practice, the decision to switch to maintenance therapy will need to take into account an individual's tumor response, cumulative toxicity, and patient preferences.
Phase II data from the VOLFI trial supports the addition of anti‐EGFR treatment to triplet chemotherapy [5], but current evidence is more robust supporting the addition of bevacizumab. However, caution must be taken with bevacizumab for patients with the following characteristics: in situ primary tumor, recent surgery, bleeding diathesis, and hypertension, which can be common scenarios for this patient population. On the other hand, anti‐EGFR therapy with cetuximab or panitumumab is generally less restrictive in terms of patient selection; however, for some patients the dermatologic complications of long‐term anti‐EGFR therapy can be significant. This can be partially managed with prophylactic tetracycline use, topical ointments, and patient education but should not be underestimated. In fact, prophylactic use of antibiotics has been linked with improved outcomes in some studies in both colorectal and lung cancer when patients are undergoing anti‐EGFR therapy [16, 17].
The overall trend in oncology is transitioning effective therapies, often in combination, from a treatment‐resistant setting to an earlier line of therapy. This leaves one wondering if we are “using up” too many lines of therapies with triplet therapy, and if it is better to prescribe drugs sequentially so we do not run out of options. However, the number of eligible patients for chemotherapy with each subsequent line of treatment declines for a variety of reasons, including declining performance status, disease progression, toxicity, and patient preference. We previously discussed how impactful this is in patients with BRAF V600E‐mutated colorectal cancer; however, even in a molecularly unselected population attrition between first and second line can be up to 40% [18]. This may mean early use of more agents can capitalize on a window of opportunity to deliver treatment, particularly in those with poor prognostic features. Furthermore, metastatic colorectal cancer can be viewed as a treatment continuum instead of distinct lines of therapies, where there is a role for maintenance therapy, drug holidays, and incorporation of local therapies [19].
Future Directions: Unanswered Questions
The microsatellite instability high (MSI‐H) population in metastatic colorectal cancer makes up ∼5% of all patients, and recently pembrolizumab was shown to double PFS (16.5 vs. 8.2 months, HR 0.60, p = .0002) compared with doublet chemotherapy with or without bevacizumab or cetuximab [20]. The triplet regimen was not a comparator arm in KEYNOTE‐177. A notable finding is that nearly 30% of patients had progressive disease as best overall response with pembrolizumab compared with 12% in the control arm. Further correlative studies are required to identify what differentiates progressive cases and to understand resistance mechanisms, but if we can identify those who are at risk of rapid progression, this patient population may represent a group that would benefit from a chemotherapy approach with FOLFOXIRI + bevacizumab if the patient is fit and poor prognostic features are present.
With regard to triplet chemotherapy plus anti‐EGFR, phase II data from VOLFI showed the addition of panitumumab to FOLFOXIRI significantly increased the overall response rate (87% vs. 60%, OR 4.47, p = .004) in patients with RAS wild type [5]. We await results from PANIRINOX and TRIPLETE, which are ongoing phase II and III trials comparing FOLFIRINOX + panitumumab versus mFOLFOX6 + panitumumab in RAS/BRAF wild‐type tumors [21, 22]. For now, if a triplet chemotherapy plus biologic is warranted, evidence remains more robust for FOLFIRINOX + bevacizumab.
Conclusion: No “Side” Prevails
We commend Pietrantonio et al. for undertaking this clinical question in the population of left‐sided RAS/BRAF wild‐type tumors, where there was a paucity of high‐quality evidence. Both FOLFOXIRI + bevacizumab and FOLFOX + anti‐EGFR are reasonable options, and clinicians must take patient preferences and the presence of poor prognostic clinicopathologic factors into account when choosing between the two. Further studies on the combination of FOLFOXIRI + anti‐EGFR are pending and may present as another option in the near future, and correlative analyses from KEYNOTE‐177 will hopefully identify how we can optimize outcomes for patients with MSI‐H colorectal cancer that progress rapidly on first‐line immunotherapy, and this is a population where further efforts to improve outcomes are needed.
Disclosures
The authors indicated no financial relationships.
Disclosures of potential conflicts of interest may be found at the end of this article.
Editor's Note: See the related article, “FOLFOXIRI‐Bevacizumab or FOLFOX‐Panitumumab in Patients with Left‐Sided RAS/BRAF Wild‐Type Metastatic Colorectal Cancer: A Propensity Score‐Based Analysis,” by Filippo Pietrantonio, Giovanni Fucà, Daniele Rossini, et al., on page 302 of this issue.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Hurwitz HI, Tebbutt NC, Kabbinavar F et al. Efficacy and safety of bevacizumab in metastatic colorectal cancer: Pooled analysis from seven randomized controlled trials. The Oncologist 2013;18:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Douillard JY, Oliner KS, Siena S et al. Panitumumab‐FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023–1034. [DOI] [PubMed] [Google Scholar]
- 3. Falcone A, Ricci S, Brunetti I et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first‐line treatment for metastatic colorectal cancer: The Gruppo Oncologico Nord Ovest. J Clin Oncol 2007;25:1670–1676. [DOI] [PubMed] [Google Scholar]
- 4. Cremolini C, Loupakis F, Antoniotti C et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first‐line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open‐label, phase 3 TRIBE study. Lancet Oncol 2015;16:1306–1315. [DOI] [PubMed] [Google Scholar]
- 5. Modest DP, Martens UM, Riera‐Knorrenschild J et al. FOLFOXIRI plus panitumumab as first‐line treatment of RAS wild‐type metastatic colorectal cancer: The randomized, open‐label, phase II VOLFI study (AIO KRK0109). J Clin Oncol 2019;37:3401–3411. [DOI] [PubMed] [Google Scholar]
- 6. Venook AP, Niedzwiecki D, Innocenti F et al. Impact of primary (1°) tumor location on overall survival (OS) and progression‐free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol 2016;34:3504. [Google Scholar]
- 7.National Comprehensive Cancer Network. Colon Cancer. Available at https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed December 10, 2020.
- 8. Pietrantonio F, Fucà G, Rossini D et al. FOLFOXIRI‐bevacizumab or FOLFOX‐panitumumab in patients with left‐sided RAS/BRAF wild‐type metastatic colorectal cancer: A propensity score‐based analysis. The Oncologist 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cremolini C, Antoniotti C, Stein A et al. Individual patient data meta‐analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol 2020;38:3314–3324. [DOI] [PubMed] [Google Scholar]
- 10. Fedyanin M, Polyanskaya E, Elsnukaeva H et al. 466P FOLFOXIRI versus FOLFOX or FOLFIRI with targeted therapy in patients with mutant BRAF metastatic colorectal cancer: A systematic review and meta‐analysis. Ann Oncol 2020;31:S439–S440. [Google Scholar]
- 11. Kopetz S, Grothey A, Yaeger R et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E‐mutated colorectal cancer. N Engl J Med 2019;381:1632–1643. [DOI] [PubMed] [Google Scholar]
- 12. Grothey A, Tabernero J, Taieb J et al. LBA‐5 ANCHOR CRC: A single‐arm, phase 2 study of encorafenib, binimetinib plus cetuximab in previously untreated BRAF V600E‐mutant metastatic colorectal cancer. Ann Oncol 2020;31:S242–S243. [Google Scholar]
- 13. Chu JE, Johnson B, Kugathasan L et al. Population‐based screening for BRAF (V600E) in metastatic colorectal cancer reveals increased prevalence and poor prognosis. Clin Cancer Res 2020;26:4599–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bridgewater JA, Pugh SA, Maishman T et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): Long‐term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 2020;21:398–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pietrantonio F, Morano F, Corallo S et al. Maintenance therapy with panitumumab alone vs panitumumab plus fluorouracil‐leucovorin in patients with RAS wild‐type metastatic colorectal cancer: A phase 2 randomized clinical trial. JAMA Oncol 2019;5:1268–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Melosky B, Anderson H, Burkes RL et al. Pan Canadian Rash trial: A randomized phase III trial evaluating the impact of a prophylactic skin treatment regimen on epidermal growth factor receptor‐tyrosine kinase inhibitor–induced skin toxicities in patients with metastatic lung cancer. J Clin Oncol 2016;34:810–815. [DOI] [PubMed] [Google Scholar]
- 17. Sahm SW, Zahn MO, Maintz C et al. Impact of prophylactic systemic antibiotics (SA) on outcome of patients (pts) with RAS‐wildtype (RAS‐wt) metastatic colorectal carcinoma (mCRC) treated with cetuximab‐based first‐line therapy. Subgroup analysis of the German non‐interventional study ERBITAG. Ann Oncol 2019;30:v218. [Google Scholar]
- 18. Bahrabadi A, Ruan J, Gresham G et al. Predictors of treatment attrition in patients with metastatic colorectal cancer (mCRC). J Clin Oncol 2017;35:e18041. [Google Scholar]
- 19. Goldberg RM, Rothenberg ML, Van Cutsem E et al. The continuum of care: A paradigm for the management of metastatic colorectal cancer. The Oncologist 2007;12:38–50. [DOI] [PubMed] [Google Scholar]
- 20. Andre T, Shiu KK, Kim TW et al. Pembrolizumab versus chemotherapy for microsatellite instability‐high/mismatch repair deficient metastatic colorectal cancer: The phase 3 KEYNOTE‐177 study. J Clin Oncol 2020;38:LBA4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mazard T, Ghiringhelli F, Mollevi C et al. UCGI 28 panirinox: A randomized phase II study assessing panitumumab + FOLFIRINOX or mFOLFOX6 in RAS and BRAF wild type metastatic colorectal cancer patients (mCRC) selected from circulating DNA analysis. Ann Oncol 2018;29:viii204. [Google Scholar]
- 22. Borelli B, Moretto R, Lonardi S et al. TRIPLETE: A randomised phase III study of modified FOLFOXIRI plus panitumumab versus mFOLFOX6 plus panitumumab as initial therapy for patients with unresectable RAS and BRAF wild‐type metastatic colorectal cancer. ESMO Open 2018;3:e000403. [DOI] [PMC free article] [PubMed] [Google Scholar]


