Abstract
Background
Advance care planning (ACP) is a process that supports adults in understanding and sharing their personal values, life goals, and preferences regarding future medical care. We examined the current status of ACP and end‐of‐life (EOL) communication between oncologists and patients with metastatic breast cancer.
Materials and Methods
We conducted a survey among 41 institutions that specialize in oncology by using an online tool in October 2019. Participants (118 physicians) from 38 institutions completed a 39‐item questionnaire that measured facility type and function; physicians’ background and clinical approach, education about EOL communication, and understanding about ACP; and the current situation of ACP and EOL discussions.
Results
Ninety‐eight responses concerning physicians’ engagement in ACP with patients were obtained. Seventy‐one (72%) answered that they had engaged in ACP. Among these, 23 (33%) physicians used a structured format to facilitate the conversation in their institutions, and only 6 (8%) settled triggers or sentinel events for the initiation of ACP. In the multivariable analysis, only the opportunity to learn communication skills was associated with physicians’ engagement with ACP (odds ratio: 2.8, 95% confidence interval: 1.1–7.0). The frequency and timing of communication about ACP and EOL care with patients substantially varied among the oncologists. Communication about patients’ life expectancy was less frequent compared with other topics.
Conclusion
The opportunity to improve EOL communication skills promoted physicians’ engagement with ACP among patients with metastatic/advanced breast cancer. However, there were still substantial variabilities in the method, frequency, and timing of ACP and EOL communication among the oncologists.
Implications for Practice
This study found that the opportunity to improve end‐of‐life (EOL) communication skills promoted physicians’ engagement in advance care planning (ACP) among patients with metastatic/advanced breast cancer. All oncologists who treat said patients are encouraged to participate in effective education programs concerning EOL communication skills. In clinical practice, there are substantial variabilities in the method, frequency, and timing of ACP and EOL communication among oncologists. As recommended in several clinical guidelines, the authors suggest a system that identifies patients who require conversations about their care goals, a structured format to facilitate the conversations, and continuous measurement for improving EOL care and treatment.
Keywords: Advance care planning, End‐of‐life discussion, Metastatic breast cancer, Communication, Patient‐centered care
Short abstract
The purpose of advance care planning is to enable individuals to make plans about their future health care and improve discussions about end‐of‐life care and treatment. This article examines the current status of advance care planning for patients with advanced metastatic breast cancer to determine factors associated with physician engagement in institutions that specialize in oncology.
Introduction
Although the development of new targeted therapies and immune therapies has improved survival outcomes among patients, metastatic breast cancer (MBC) is still difficult to cure [1, 2, 3]. Therefore, the main goal of MBC treatment remains to prolong survival and improve patients’ quality of life (QOL). However, treatment expectations differ greatly between patients and physicians. More than half of patients with metastatic colon or lung cancer considered that the purpose of systemic therapy was to cure the disease [4], and patients with MBC are also not provided with adequate information about the disease [5, 6].
Although discussing end‐of‐life (EOL) care is one of the important elements in the communication between clinicians and patients with noncurable cancer [7], it often occurs late in the course of their illness, and some patients have no EOL discussions before death [8, 9, 10]. Failing to have these discussions is associated with intensive treatment (e.g., chemotherapy, admission in intensive care unit) near EOL and underuse of medical resources such as palliative care service [11, 12]. There are many reasons for the less frequent EOL communication between patients and physicians [13, 14, 15, 16]. Some physicians may have little confidence in their EOL communication ability, particularly related to uncertainty about prognosis and fears over providing an inaccurate prediction. Physicians also may fear that sharing bad news could lead to patients’ experiencing depression and a loss of hope. Other physicians may fear not knowing how to answer patients’ questions during EOL discussions [13].
The purpose of advance care planning (ACP) is to enable individuals to make plans about their future health care and increase the quantity and quality of discussions about EOL care and treatment. ACP has been shown to improve the consistency of care with patients’ goals among various patient populations [17, 18]. However, there are several definitions and concepts of ACP, which make it difficult for clinical practitioners and researchers to understand what ACP means. Therefore, multidisciplinary panels of international experts recently met to develop a consensus definition of ACP in Europe, the U.S., and Asia [19, 20, 21].
Because there have been few reports about the current status of ACP and whether oncologists’ specialty and experience affect their attitude toward EOL discussions, we examined the current status of ACP and EOL discussions with patients with MBC to determine what factors are associated with physicians’ engagement with ACP in institutions that specialize in oncology. Existing literature indicates, to date, that the beliefs of physicians and their attitudes toward EOL discussions are possible factors associated with the timing [12, 22, 23]. However, these findings come from less recent works conducted among physicians of patients with other types of metastatic/advanced cancers. Our study is, to the best of our knowledge, the first survey on the current status of ACP for patients with advanced MBC per the views of physicians who specialize in cancer treatment and care.
Materials And Methods
Participants
We conducted a survey about the current status of ACP and EOL communication between patients with breast cancer and physicians. The target population of our survey was all physicians who treat breast cancer, including surgical oncologists, medical oncologists, and palliative care physicians in 45 institutions that belong to the Japan Clinical Oncology Group (JCOG). JCOG is the largest Japanese cooperative group for cancer treatment that is fully funded by national research grants [24, 25] The breast cancer study group of JCOG (JCOG‐BCSG) has conducted more than 20 clinical trials, mainly for patients with MBC, since 1985.
Instrument Development
The questionnaire was designed based on an extensive literature review and evaluated through several meetings and emails by focus‐group members of JCOG‐BCSG. The focus‐group comprised 6 medical oncologists, 1 palliative care specialist, 2 nurses, and 15 surgical oncologists who specialized in MBC treatment. We also obtained feedback about the design and contents of the questionnaire from members of several patient advocacy groups. Based on both forms of feedback, we revised the questionnaire. We developed the questionnaire using an online survey tool, Survey Monkey (SurveyMonkey Inc., San Mateo, CA). We did not collect any identifiable information from physicians; thus, we deemed that our survey did not require approval from an institutional review board. After confirmation that the survey questions worked properly by focus‐group members, we started to collect responses in October 2019. Using our mailing list, we sent invitations twice to 385 physicians at 45 institutions with the Web link and QR code.
Study Outcome Measures
The questionnaire consisted of 39 questions, including facility type and function (seven questions), physicians’ background (three questions), physicians’ situation and clinical approach to patients with metastatic and recurrent cancer (e.g., number of outpatients per day, timing of referral to palliative care clinic; five questions), opportunity for education about EOL communication (two questions), physicians’ understanding about ACP (three questions), current situation of ACP (13 questions), and the current situation of EOL discussion (six questions; see supplemental data). These questions about facility type and function, physicians’ background, physicians’ situation and clinical approach to patients with metastatic and recurrent cancer, and opportunity for education about EOL communication could potentially contribute to physicians’ engagement with ACP. We investigated associations between these questions and a question about the physicians’ engagement with ACP (question 20 in supplemental data). As shown in Table 1, each organization had a slightly different definition of ACP. We asked physicians what definition was closest to what they believed by masking the organization in the questionnaire.
Table 1.
Definitions of advance care planning from several organizations
| Organization (country) | Definition |
|---|---|
| Institute of Medicine (U.S.) [39] | A process of discussion of end‐of‐life care, clarification of related values and goals, and embodiment of preferences through written documents (e.g., advance directive, physicians’ orders for life‐sustaining treatment) and medical orders |
| Ministry of Health, Labour, and Welfare (Japan) | A process in which the individual repeatedly discusses end‐of‐life treatment and care with the family and care teams, in advance |
| Sudore and colleagues (U.S. and other countries) [19] | Advance care planning is a process that supports adults at any age or stage of health in understanding and sharing their personal values, life goals, and preferences regarding future medical care |
| European Association for Palliative Care (Europe) [21] | Advance care planning enables individuals to define goals and preferences for future medical treatment and care, to discuss these goals and preferences with family and health care providers, and to record and review these preferences if appropriate |
Statistical Analysis
The variable types of responses to the questionnaires on facility and physician factors were categorical or binary. Therefore, for the univariable analyses, we performed chi‐square tests to investigate which factors were associated with physicians’ engagement with ACP. Following this, the adjusted odds ratios of physicians’ engagement with ACP were reported from a multivariable logistic regression model by using the variables that were significant in the univariable analysis (i.e., facility type, existence of palliative care ward in the institution, and opportunity to learn communication skills in EOL discussion). Two‐tailed values of p < .05 denoted significance. Statistical analyses were performed using STATA version 16.0 (STATA Corp., College Station, TX).
Results
During the 2‐week data collection period, we obtained responses from 118 physicians (90 surgical oncologists, 25 medical oncologists, and 4 palliative care physicians) from 41 institutions across Japan. There were 20 physicians (17%) who did not complete half of the questions. Among them, 18 physicians (15%) were not engaged in treating patients with MBC or associated with outpatient clinics. Thus, they were not given the opportunity to answer questions on ACP. A total of 98 (83%) physicians from 38 institutions completed half of the questions, and 89 (75%) physicians completed the last question. In the survey, 94 (80%) oncologists had more than 10 years of experience, and 66 (56%) and 35 (30%) oncologists saw more than 10 or 20 patients with MBC per week, respectively.
Physicians’ Understanding and Engagement with ACP
There were 98 responses concerning physicians’ engagement in ACP with patients. Seventy‐eight (80%) physicians answered that they understood well or almost understood what ACP means, and 20 physicians (20%) answered that they had just heard about ACP. The ACP definition (Table 1) that physicians considered most appropriate was as follows, from most to least appropriate: the definition of European Association for Palliative Care (n = 35, 36%), Sudore and colleagues (n = 28, 29%), Ministry of Health, Labour, and Welfare of Japan (n = 21, 21%), and Institute of Medicine (n = 14, 14%). All respondents (n = 98) considered that the process of ACP is necessary in their clinical practice.
Seventy‐one (72%) physicians answered that they had engaged in ACP. Table 2 shows the associations between physicians’ engagement with ACP and the examined variables. In univariate analyses, physicians’ engagement with ACP was less frequent in cancer centers (p = .003), facilities with palliative care wards (p = .049), and physicians without the opportunity to learn communication skills (p = .02). There was no association between ACP engagement and the following background characteristics of physicians: physicians’ department and experience in oncology, average number of outpatients per day, average number of patients with MBC in charge per week, and average consultation time with outpatients with MBC. In the multivariable analysis, only the opportunity to learn communication skills was associated with physicians’ engagement with ACP (odds ratio: 2.8; 95% confidence interval: 1.1–7.0). There were three main types of reasons for not engaging in ACP. First, physicians answered that there was no systematic approach concerning ACP in their institution (n = 9). Second, physicians did not understand how to initiate ACP with patients and families (n = 7). Finally, physicians did not have enough time and human resources in their institution (n = 5).
Table 2.
Physicians’ engagement with advance care planning and institution‐ and physician‐related factors
| Factor | Physicians’ engagement with advance care planning | p value | |
|---|---|---|---|
| Engaged, n (%) | Not engaged, n (%) | ||
| Facility factor | |||
| Facility type | |||
| Academic university | 22 (31) | 5 (18) | .003 |
| Cancer center | 18 (25) | 13 (48) | |
| National hospital | 6 (8) | 8 (30) | |
| Municipal/prefectural hospital | 8 (11) | 0 (0) | |
| Medical cooperation | 8 (11) | 1 (3.7) | |
| Other | 9 (12) | 0 (0) | |
| Supportive/palliative care clinic | |||
| Yes | 60 (85) | 24 (89) | .58 |
| No | 11 (15) | 3 (11) | |
| Palliative care ward | |||
| Yes | 24 (34) | 15 (56) | .049 |
| No | 47 (66) | 12 (44) | |
| Physician factor | |||
| Department | |||
| Surgical oncology | 52 (73) | 21 (84) | .47 |
| Medical oncology | 17 (24) | 4 (16) | |
| Palliative care | 2 (3) | 0 (0) | |
| Experience in oncology, years | |||
| <10 | 12 (17) | 4 (15) | .20 |
| 10–19 | 31 (44) | 17 (63) | |
| ≥20 | 28 (39) | 6 (22) | |
| Average number of outpatients in charge per day | |||
| <20 | 13 (18) | 7 (26) | .70 |
| 20–39 | 35 (50) | 13 (48) | |
| ≥40 | 22 (31) | 7 (26) | |
| Average number of patients with metastatic/advanced breast cancer in charge per week | |||
| <10 | 23 (32) | 10 (37) | .91 |
| 10–19 | 22 (31) | 8 (30) | |
| ≥20 | 26 (37) | 9 (33) | |
| Average consultation time with outpatients with metastatic/advanced breast cancer, min | |||
| <10 | 21 (30) | 11 (40) | .29 |
| 10–30 | 50 (70) | 16 (60) | |
| Opportunity to learn about communication skills in end‐of‐life discussions | |||
| Yes | 45 (63) | 10 (37) | .02 |
| No | 26 (37) | 17 (63) | |
| Total | 71 | 27 | |
Among the 71 physicians who had engaged in ACP across 34 institutions, the process of ACP was engaged in by individuals (n = 33; 21 institutions), medical or surgical oncology departments (n = 14; 11 institutions), palliative care services (n = 1), or institutionwide (n = 23; 11 institutions). Twenty‐three (33%) physicians used a pamphlet or a structured format to facilitate the conversation, and only six (8%) physicians settled triggers or sentinel events (e.g., initial diagnosis of metastatic disease, relapse, or progression) for the initiation of ACP for patients with MBC.
Frequency and Timing of Communication about EOL and Resource Use
The frequency of communication about ACP and EOL with MBC patients is shown in Figure 1. Communication about life expectancy was less frequent compared with the other topics. We did not find any association between the frequency of communication and physicians’ background.
Figure 1.
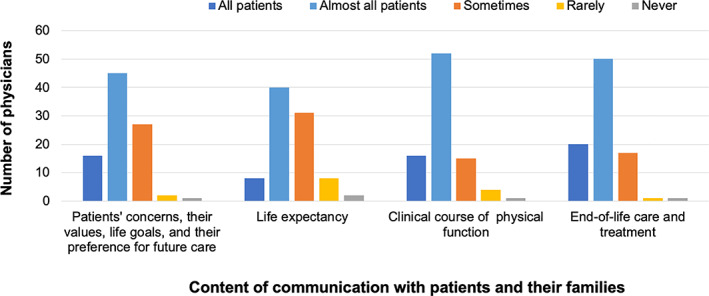
Frequency of communication with patients with metastatic breast cancer.
As shown in Figure 2, there were few discussions with patients about their values, goals, and preference for future care, which were performed mostly when little anticancer therapy remained (n = 33; 37%) or as soon as possible after the diagnosis of metastasis (n = 29; 33%). Most physicians documented the content of discussions with patients as a brief summary (n = 54; 61%), whereas many others (n = 31; 35%) documented them in detail in their medical chart.
Figure 2.
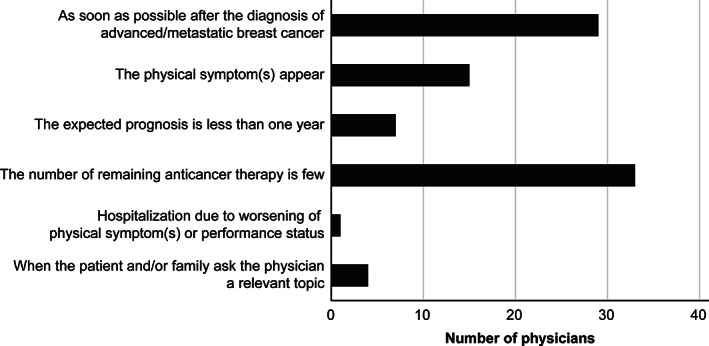
Appropriate timing of conversations with patients concerning patients’ values, goals, and preferences for future care (n = 89; choice of one appropriate timing).
Figure 3 shows the appropriate timing of referral to palliative care departments. Reasons included psychological (n = 54; 54%) or physical (e.g., poor pain control; n = 74; 74%) support, the late line (e.g., fourth or fifth line) of anticancer therapy (n = 26; 26%), and before withdrawal of anticancer therapy or before transfer to the palliative care ward (n = 14; 14%).
Figure 3.
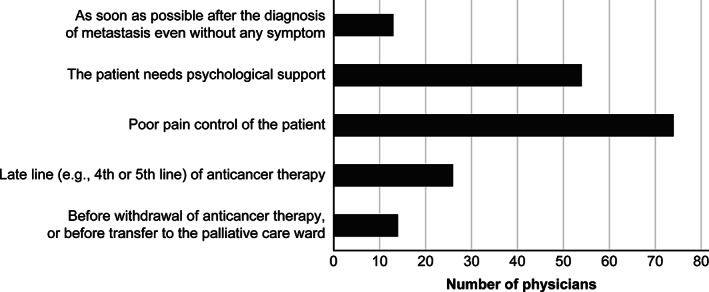
Appropriate timing for referring patients to a palliative care department (n = 100; choice of two appropriate timings).
Discussion
To our knowledge, this is the first survey on the status of ACP for patients with advanced MBC, as per the views of physicians who specialize in cancer treatment and care. In our survey, most physicians agreed with the concepts and the definition of ACP by Sudore and colleagues and the European Association for Palliative Care (EAPC), which do not focus on EOL care—rather, they emphasize the importance of personal values, life goals, and preferences regarding future medical care (Table 1). In contrast, the definitions of ACP by the Institute of Medicine and the Ministry of Health and Welfare of Japan focused on discussions about EOL treatment or care. More than one‐third of physicians considered these definitions better than the two definitions by Sudore et al. and EAPC.
The National Comprehensive Cancer Network guidelines describe practical interventions and evaluations during the ACP process [26]. In those guidelines, several types of interventions are recommended according to patients’ estimated life expectancy. If the estimated life expectancy is months to years, the oncology team first should assess patients’ decision‐making capacity and identify a surrogate decision‐maker, if necessary; then, they should initiate discussions about patients’ personal values and care preferences. Only when the estimated life expectancy is weeks should the oncology team determine patients’ and their caregivers’ preferences for the location of patients’ death and reconfirm patients’ values and EOL decisions. Although the appropriate timing of initiating ACP processes is controversial [27], care providers should offer interventions that are tailored to patients’ needs.
In our survey, more than 70% were surgical oncologists because only a small number of institutions have a medical oncology department in Japan [28]; therefore, surgical oncologists—who are qualified to treat breast cancer—typically play the chief role in the systemic treatment of MBC. Seventy‐two percent of the oncologists responded that they were engaged in ACP processes. The only factor that was associated with physicians’ ACP engagement was the opportunity to learn communication skills in EOL discussion. The two most frequent reasons for not engaging in ACP were not having a systematic approach in their institution and not understanding the method to provide ACP to patients. All oncologists who offer treatment for patients with metastatic/advanced cancer are encouraged to participate in effective education programs concerning EOL communication skills [29].
Among the physicians who responded that they were engaged in ACP processes with patients, half were engaged individually (not organizationally). Few physicians used a pamphlet or a structured format to facilitate these conversations, and there was not a standard process of ACP in most institutions. Moreover, there was substantial variability in the frequency and timing for initiating EOL treatment and care discussions. Previous studies investigating EOL discussion between physicians and patients with other types of metastatic/advanced cancers, also showed similar variability; the beliefs of physicians regarding EOL discussion were reported to be associated with the timing [8, 22].
Communication about life expectancy was less frequent compared with the other topics. Although we did not find any association between physicians’ ACP attitudes and their background characteristics, Mori and colleagues found that oncologists’ own perceptions about what is important for a “good death,” perceived difficulty in estimating the prognosis, and discomfort in talking about death influence their attitudes toward EOL discussions [23]. As recommended in several clinical guidelines [7, 30], there is a need to develop a system that identifies patients who require conversations about their care goals, a structured format to facilitate said conversations, and continuous measurement for improving EOL care and treatment in each institution.
In several randomized controlled trials, researchers investigated the influence of ACP among patients with metastatic cancers on improved EOL conversation quality and patients’ anxiety and depression [31, 32, 33, 34, 35]. However, these studies were unable to demonstrate whether the conversations resulted in care that aligned with patients’ goals or whether they lead to improved QOL during treatment. In addition, most ACP interventions in the clinical trials focused on communication about the EOL care and treatment for the patients whose expected prognosis was less than 1 year. It is not yet clear what kind of ACP program is appropriate and when we should start interventions for patients with advanced cancer [27, 36, 37, 38]; therefore, further clinical research is required to offer optimal ACP tailored to individual patients with metastatic/advanced cancer.
There are several limitations to our study. First, the target population of our questionnaire includes oncologists from the major cancer centers and universities that belong to JCOG‐BCSG. Therefore, our results are not generalizable to those who provide community‐level ACP. In addition, the perspectives of nurses and patients should also be investigated, although our previous study revealed that physicians and nurses noted similar ACP experiences (45 of 71 nurses completed 90% of the survey; data not shown). Second, the response rate of the physicians to our questionnaire is not so high (31%), and the sample size of medical oncologists and palliative physicians was also small in this survey; thus, we did not have enough power to detect ACP differences per specialty. However, 41 out of 45 institutions (91%) responded to the questionnaire, indicating the current status of ACP across Japan. A further study should expand the target population.
Conclusion
We found the opportunity to learn about communication skills concerning EOL discussion, which promoted physicians’ engagement with ACP among patients with MBC. However, there were still substantial variabilities in the method, frequency, and timing of ACP and EOL communication that were dependent on beliefs and attitudes of oncologists. It is crucial to promote educational opportunities and a cross‐organizational system for facilitating ACP and EOL communication in each institution. Further randomized clinical trials to investigate and disseminate optimal ACP, tailored to the individual needs of patients with metastatic/advanced cancer, are required.
Author Contributions
Conception/design: Yasuaki Sagara, Masanori Mori, Sena Yamamoto, Keiko Eguchi, Tsuguo Iwatani, Yoichi Naito, Takahiro Kogawa, Kiyo Tanaka, Haruru Kotani, Hiroyuki Yasojima, Yukinori Ozaki, Emi Noguchi, Minoru Miyasita, Naoto Kondo, Tadahiko Shien, Hiroji Iwata
Provision of study material or patients: Yasuaki Sagara, Tsuguo Iwatani, Yoichi Naito, Takahiro Kogawa, Kiyo Tanaka, Haruru Kotani, Hiroyuki Yasojima, Yukinori Ozaki, Emi Noguchi, Minoru Miyasita, Naoto Kondo, Tadahiko Shien, Hiroji Iwata
Data analysis and interpretation: Yasuaki Sagara, Masanori Mori, Sena Yamamoto, Tsuguo Iwatani, Yoichi Naito, Takahiro Kogawa, Kiyo Tanaka, Haruru Kotani, Hiroyuki Yasojima, Yukinori Ozaki, Emi Noguchi, Minoru Miyasita, Naoto Kondo, Naoki Nikura, Masakazu Toi, Tadahiko Shien, Hiroji Iwata
Manuscript writing: Yasuaki Sagara, Masanori Mori, Sena Yamamoto, Tsuguo Iwatani, Yoichi Naito, Takahiro Kogawa, Kiyo Tanaka, Haruru Kotani, Hiroyuki Yasojima, Yukinori Ozaki, Emi Noguchi, Minoru Miyasita, Naoto Kondo, Naoki Nikura, Masakazu Toi, Tadahiko Shien, Hiroji Iwata
Final approval of manuscript: Yasuaki Sagara, Masanori Mori, Sena Yamamoto, Keiko Eguchi, Tsuguo Iwatani, Yoichi Naito, Takahiro Kogawa, Kiyo Tanaka, Haruru Kotani, Hiroyuki Yasojima, Yukinori Ozaki, Emi Noguchi, Minoru Miyasita, Naoto Kondo, Naoki Niikura, Masakazu Toi, Tadahiko Shien, Hiroji Iwata
Disclosures
Yoichi Naito: Eli Lilly & Co., Chugai, Pfizer, AstraZeneca (C/A), Roche H: Chugai, Pfizer, Eli Lilly & Co., Eisai, Novartis, Taiho, Fuji FIlm Toyama Chemostry, Gardant, Shionogi (RF); Masakazu Toi: Chugai, Takeda, Pfizer, Kyowa‐Kirin, Taiho, Eisai, Daiichi‐Sankyo, AstraZeneca, JBCRG Association, Astellas, Shimadzu, Nippon‐Kayaku, AFI Technology (RF), Chugai, Takeda, Pfizer, Kyowa‐Kirin, Taiho, Eisai, Daiichi‐Sankyo, AstraZeneca, Eli Lilly & Co., Merck Sharp & Dohme, Genomic Health, Novartis, Konica Minolta, Bristol‐Myers Squibb, Shimadzu, Yakult, Nippon‐Kayaku (H), Kyowa‐Kirin, Daiichi‐Sankyo, Bristol‐Myers Squibb, Athenex Oncology, Bertis (SAB), JBCRG Association, KBCRN Association, OOTR (NPO) (other—member of board of directors); Hiroji Iwata: Chugai, Novartis, AstraZeneca, Pfizer, Eli Lilly & Co., Daiichi‐Sankyo, Merck Sharp & Dohme, Eisai, Kyowa Hakko Kirin, Bayer, Boehringer Ingelheim, Nihon Kayaku, Sanofi (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting Information
Acknowledgments
We acknowledge the Japan Clinical Oncology Group, members of patient advocacy groups, and all participating physicians who made this study possible. This report was supported in part by the National Cancer Center Research and Development Fund (29‐A‐3) from the Ministry of Health, Labour, and Welfare and the Practical Research for Innovative Cancer Control (20ck0106307h0003) from the Japan Agency for Medical Research and Development, AMED. Grammar and language assistance was provided by professional editors at Editage, a division of Cactus Communications (www.editage.com).
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Dawood S, Broglio K, Gonzalez‐Angulo AM et al. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. J Clin Oncol 2008;26:4891–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dafni U, Grimani I, Xyrafas A et al. Fifteen‐year trends in metastatic breast cancer survival in Greece. Breast Cancer Res Treat 2010;119:621–631. [DOI] [PubMed] [Google Scholar]
- 3. Schmid P, Adams S, Rugo HS et al. Atezolizumab and nab‐paclitaxel in advanced triple‐negative breast cancer. N Engl J Med 2018;379:2108–2121. [DOI] [PubMed] [Google Scholar]
- 4. Weeks JC, Catalano PJ, Cronin A et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med 2012;367:1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lux MP, Bayer CM, Loehberg CR et al. Shared decision‐making in metastatic breast cancer: Discrepancy between the expected prolongation of life and treatment efficacy between patients and physicians, and influencing factors. Breast Cancer Res Treat 2013;139:429–440. [DOI] [PubMed] [Google Scholar]
- 6. Cardoso F, Spence D, Mertz S et al. Global analysis of advanced/metastatic breast cancer: Decade report (2005‐2015). Breast 2018;39:131–138. [DOI] [PubMed] [Google Scholar]
- 7. Gilligan T, Coyle N, Frankel RM et al. Patient‐clinician communication: American Society of Clinical Oncology consensus guideline. J Clin Oncol 2017;35:3618–3632. [DOI] [PubMed] [Google Scholar]
- 8. Mack JW, Cronin A, Taback N et al. End‐of‐life care discussions among patients with advanced cancer: A cohort study. Ann Intern Med 2012;156:204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Narang AK, Wright A, Nicholas LH. Trends in advance care planning in patients with cancer. JAMA Oncol 2015;2410:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mori M, Yoshida S, Shiozaki M et al. Talking about death with terminally‐ill cancer patients: What contributes to the regret of bereaved family members? J Pain Symptom Manage 2017;54:853–860.e1. [DOI] [PubMed] [Google Scholar]
- 11. Morita T, Akechi T, Ikenaga M et al. Late referrals to specialized palliative care service in Japan. J Clin Oncol 2005;23:2637–2644. [DOI] [PubMed] [Google Scholar]
- 12. Mack JW, Cronin A, Keating NL et al. Associations between end‐of‐life discussion characteristics and care received near death: A prospective cohort study. J Clin Oncol 2012;30:4387–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buckman R. Breaking bad news: Why is it still so difficult? Br Med J 1984;288:1597–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaasa S, Loge JH, Aapro M et al. Integration of oncology and palliative care: A Lancet Oncology Commission. Lancet Oncol 2018:588–653. [DOI] [PubMed] [Google Scholar]
- 15. Pfeil TA, Laryionava K, Reiter‐Theil S et al. What keeps oncologists from addressing palliative care early on with incurable cancer patients? An active stance seems key. The Oncologist 2015;20:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Travers A, Taylor V. What are the barriers to initiating end‐of‐life conversations with patients in the last year of life? Int J Palliat Nurs 2016;22:454–462. [DOI] [PubMed] [Google Scholar]
- 17. Houben CHM, Spruit MA, Groenen MTJ et al. Efficacy of advance care planning: A systematic review and meta‐analysis. J Am Med Dir Assoc 2014;15:477–489. [DOI] [PubMed] [Google Scholar]
- 18. Detering KM, Hancock AD, Reade MC et al. The impact of advance care planning on end of life care in elderly patients: Randomised controlled trial. Br Med J 2010;340:c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sudore RL, Lum HD, You JJ et al. Definition from a multidisciplinary Delphi panel. J Pain Symptom Manag 2017;53:821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin CP, Cheng SY, Mori M et al. 2019 Taipei declaration on advance care planning: A cultural adaptation of end‐of‐life care discussion. J Palliat Med 2019;22:1175–1177. [DOI] [PubMed] [Google Scholar]
- 21. Rietjens JAC, Sudore RL, Connolly M et al. Definition and recommendations for advance care planning: An international consensus supported by the European Association for Palliative Care. Lancet Oncol 2017;18:e543–e551. [DOI] [PubMed] [Google Scholar]
- 22. Mori M, Shimizu C, Ogawa A et al. What determines the timing of discussions on forgoing anticancer treatment? A national survey of medical oncologists. Support Care Cancer 2019;27:1375–1382. [DOI] [PubMed] [Google Scholar]
- 23. Mori M, Shimizu C, Ogawa A et al. A national survey to systematically identify factors associated with oncologists’ attitudes toward end‐of‐life discussions: What determines timing of end‐of‐life discussions? The Oncologist 2015;20:1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iwata H. The transition of breast cancer treatment and Japan Clinical Oncology Group research over two decades. Jpn J Clin Oncol 2012;42:14–20. [DOI] [PubMed] [Google Scholar]
- 25. Shimoyama M, Fukuda H, Saijo N et al. Japan Clinical Oncology Group (JCOG). Jpn J Clin Oncol 1998;28:158–162. [DOI] [PubMed] [Google Scholar]
- 26. National Comprehensive Cancer Network . Palliative Care. Version 1. 2020. Available at https://www.nccn.org/professionals/physician_gls/pdf/palliative.pdf. Accessed February 29, 2020.
- 27. Johnson S, Butow P, Kerridge I, Tattersall M. Advance care planning for cancer patients: A systematic review of perceptions and experiences of patients, families, and healthcare providers. Psychooncology 2016;25:362–386. [DOI] [PubMed] [Google Scholar]
- 28. Takiguchi Y, Sekine I, Iwasawa S et al. Current status of medical oncology in Japan‐reality gleaned from a questionnaire sent to designated cancer care hospitals. Jpn J Clin Oncol 2014;44:632–640. [DOI] [PubMed] [Google Scholar]
- 29. Fujimori M, Shirai Y, Asai M et al. Effect of communication skills training program for oncologists based on patient preferences for communication when receiving bad news: A randomized controlled trial. J Clin Oncol 2014;32:2166–2172. [DOI] [PubMed] [Google Scholar]
- 30. Bernacki RE, Block SD. Communication about serious illness care goals. JAMA Intern Med 2014;174:1994. [DOI] [PubMed] [Google Scholar]
- 31. Epstein RM, Duberstein PR, Fenton JJ et al. Effect of a patient‐centered communication intervention on oncologist‐patient communication, quality of life, and health care utilization in advanced cancer: The VOICE randomized clinical trial. JAMA Oncol 2016;10:728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paladino J, Bernacki R, Neville BA et al. Evaluating an intervention to improve communication between oncology clinicians and patients with life‐limiting cancer: A cluster randomized clinical trial of the serious illness care program. JAMA Oncol 2019;5:801–809. [DOI] [PubMed] [Google Scholar]
- 33. Bernacki R, Paladino J, Neville BA et al. Effect of the serious illness care program in outpatient oncology. JAMA Intern Med 2019;02215:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson SB, Butow PN, Bell ML et al. A randomised controlled trial of an advance care planning intervention for patients with incurable cancer. Br J Cancer 2018;119:1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang ST, Chen JS, Wen FH et al. Advance care planning improves psychological symptoms but not quality of life and preferred end‐of‐life care of patients with cancer. J Natl Compr Cancer Netw 2019;17:311–320. [DOI] [PubMed] [Google Scholar]
- 36. Lovell A, Yates P. Advance care planning in palliative care: A systematic literature review of the contextual factors influencing its uptake 2008‐2012. Palliat Med 2014;28:1026–1035. [DOI] [PubMed] [Google Scholar]
- 37.Jimenez G, Tan WS, Virk AK et al. Overview of systematic reviews of advance care planning: Summary of evidence and global lessons. J Pain Symptom Manage 2018;56:436–459.e25. [DOI] [PubMed] [Google Scholar]
- 38. Chan RJ, Webster J, Bowers A. End‐of‐life care pathways for improving outcomes in caring for the dying. Cochrane Database Syst Rev 2016;2:CD008006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Institute of Medicine . Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: National Academies Press, 2015. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting Information


