ABSTRACT
Articular cartilage consists of an extracellular matrix including many proteins as well as embedded chondrocytes. Articular cartilage formation and function are influenced by mechanical forces. Hind limb unloading or simulated microgravity causes articular cartilage loss, suggesting the importance of the healthy mechanical environment in articular cartilage homeostasis and implying a significant role of appropriate mechanical stimulation in articular cartilage degeneration. Mechanosensitive ion channels participate in regulating the metabolism of articular chondrocytes, including matrix protein production and extracellular matrix synthesis. Mechanical stimuli, including fluid shear stress, stretch, compression and cell swelling and decreased mechanical conditions (such as simulated microgravity) can alter the membrane potential and regulate the metabolism of articular chondrocytes via transmembrane ion channel-induced ionic fluxes. This process includes Ca2+ influx and the resulting mobilization of Ca2+ that is due to massive released Ca2+ from stores, intracellular cation efflux and extracellular cation influx. This review brings together published information on mechanosensitive ion channels, such as stretch-activated channels (SACs), voltage-gated Ca2+ channels (VGCCs), large conductance Ca2+-activated K+ channels (BKCa channels), Ca2+-activated K+ channels (SKCa channels), voltage-activated H+ channels (VAHCs), acid sensing ion channels (ASICs), transient receptor potential (TRP) family channels, and piezo1/2 channels. Data based on epithelial sodium channels (ENaCs), purinergic receptors and N-methyl-d-aspartate (NMDA) receptors are also included. These channels mediate mechanoelectrical physiological processes essential for converting physical force signals into biological signals. The primary channel-mediated effects and signaling pathways regulated by these mechanosensitive ion channels can influence the progression of osteoarthritis during the mechanosensory and mechanoadaptive process of articular chondrocytes.
KEYWORDS: Ion channel, mechanical force, articular chondrocyte, membrane potential, osteoarthritis
Introduction
Articular chondrocytes are subjected to phasic stimulation by mechanical forces that is due to normal walking/mobility in all manners. These biomechanical forces cause articular cartilage deformation. Articular cartilage is a layer of low-friction, load-bearing tissue that functions as a cushion for sensing body weight and exercise. Beneficial mechanical stimuli promote cartilage regeneration and prevent or attenuate osteoarthritis progression. In contrast, harmful mechanical stimuli can disrupt cartilage homeostasis and can accelerate cartilage degeneration. Moreover, chondrocyte senescence is associated with age-related osteoarthritis characterized by impaired cartilage repair [1].
The synovium is a thin sheet of cellular, well-vascularized connective tissue that lines the synovial joint cavity. Fibroblast-like synoviocytes in the synovium synthesize and secrete hyaluronan (HA) into the joint fluid to ensure/promote joint lubrication, which is essential for articular cartilage health. Increased intra-articular osmotic pressure can drastically increase the hydraulic conductance of the stretched synovial lining (increased area, reduced thickness) [2]. The major part of the hydraulic resistance to fluid transport between blood and joint cavity is generated by the synovial lining and interstitial HA, which can conserve intra-articular lubricant and lead to outflow buffering via the osmotic pressure of an HA concentration polarization layer on the synovial surface [3–10]. Static stretch stimulated HA secretion in primary rabbit synoviocyte cultures from microdissected synovial intima [11]. The activation of Ca2+ influx-dependent activation of the PKCɑ-MEK-ERK1/2 cascade is importantly involved in mechanically induced HA secretion by static stretch in synoviocytes [12], which suggests that mechanically induced Ca2+ influx and Ca2+-dependent kinases can regulate synovial HA secretion [13]. Mechanically induced HA secretion by cyclic joint movement may protect joints against the damaging effects of repetitive joint use and replace the HA lost during periods of immobility, which may contribute to the clinical benefit of exercise therapy in moderate osteoarthritis [14]. The epithelial sodium channel (ENaC) channel blocker amiloride inhibited mechanically induced HA secretion in synoviocytes [15]. Ca2+-dependent kinases are major regulators of synovial HA secretion and fibroblast-like synoviocytes cultured from the inner synovium of rabbits exhibit voltage-dependent inward and outward currents involving voltage-gated (Kv1.1) K+ channels and L-type VGCCs [13].
Cyclic tensile strain upregulates the mRNA levels of cyclooxygenase-2 (COX-2), collagen 2 and aggrecan (ACAN) and induces the release of nitric oxide and prostaglandin E 2 in monolayer cultures of porcine articular chondrocytes. In addition, anabolic modulator transforming growth factor beta3 (TGFβ3), catabolic modulator TGFβ1 and matrix metalloproteinase-1 (MMP-1) all increase over the duration of static mechanical stretch [16]. Physiologically, dynamic compression-induced membrane strain has been shown to have anabolic effects on chondrocytes and to maintain articular cartilage in a healthy state [17]. Ion channel-mediated flux of Ca2+ can regulate the proliferation of human chondrocytes [18]. Mechanical forces stimulate the synthesis and release of matrix proteins and glycosaminoglycan in articular cartilage via complex molecular mechanisms, including mechanical regulation of ion channels such as K+ channels, Ca2+ channels and Na+/K+ pumps and stretch-activated channels (SACs). The common denominator of this channel activity is often mechanical activation of the Ca2+ signaling pathways [19–27]. A lack of mechanosensitive ion channels, such as ENaC and transient receptor potential vanilloid 4 (TRPV4) has been shown to impair the mechanical sensitivity of chondrocytes in response to mechanical membrane strain induced by hypotonic solution [23,28–30]. Mechanical forces such as compression, strain, extracellular matrix (ECM) deformation, substrate deflection and fluid shear stress in the mechanical cartilage environment can significantly alter the expression and activity of membrane mechanosensitive ion channels resulting in intracellular cation mobilization of chondrocytes and regulation of the proliferation and differentiation of immature chondrocytes and survival of hypertrophic chondrocytes [19,31–36]. Some mechanosensitive ion channels sense and transduce mechanical transduction in response to physiological levels of mechanical force. However, abnormal or excessive mechanosensitive ion channel activity may accelerate the deterioration of chondrocytes and ECM in response to injurious levels of mechanical stimulus.
For example, repeated overloading leads to oxidant-dependent mitochondrial dysfunction in chondrocytes, and may result in destabilization of cartilage and osteoarthritis (OA) by disrupting chondrocyte anabolic responses to mechanical stimuli [37]. Mechanical stimulation (such as physiological compression) significantly modulates the expression of collagen 2, ACAN, the SOX9 transcription factor, cartilage oligomeric matrix protein, collagen degradation marker C2C and vascular endothelial growth factor in OA human cartilage [38]. Excessive mechanical loading of subchondral osteoblasts altered the phenotypic characteristics of chondrocytes accompanied by upregulation of MMP genes and downregulation of proteoglycan and collagen in porcine chondrocytes [39]. Appropriate expression of ion channels is vital for the formation of extracellular matrix, for instance, K+ channel gene transcription is altered in OA [27,40].
There are differences in electrophysiological properties and gene expression between human chondrocytes and chondroprogenitors derived from normal and osteoarthritic cartilage [41]. Elucidating the mechanosensitive molecular mechanisms by which these mechanical stimuli regulate chondrocyte membrane ion channels and joint homeostasis will provide insights into the pathophysiological process of OA [42–44]. However, the mechanobiology of articular chondrocytes is still not well understood partly because of the complexity of the mechanotransduction process in articular cartilage [43,45]. Adopting shorter step lengths during daily activity and when walking for exercise can reduce mechanical stimuli associated with articular cartilage degenerative processes in adults with and without obesity [46]. Mechanically induced release of growth factor responds quickly to mechanical damage and repairs ligament tissue by activating transcription factor 2 accompanied by accelerated repair of ligament injury repair, promoted ligament fibroblast migration of OA, decreased MMP-2 activity and remitted cell deformation [47]. Mechanically induced release of growth factor may protect articular chondrocytes against harmful cell stress responses and prevent OA progression. Walking with reduced step length may benefit adults at risk for disability because of knee osteoarthritis suggesting that physiologic joint loading helps maintain cartilage integrity. However, both disuse and overuse can result in cartilage degradation. The primary characteristics of OA are destruction of articular cartilage, ECM degradation, dysfunction of chondrocytes, osteophyte formation and subchondral bone alterations [48]. An osteophyte is a fibrocartilage-capped bony outgrowth that is one of the features of OA [49]. Moreover, osteophytes are thought to originate from progenitor cells (residing in the periosteum at the boundary of bone and cartilage) that undergo a process of chondrogenesis and finally endochondral ossification [50]. Osteophytes can be induced with a single mechanical impact applied to the periosteum in rat knees, which indicates that moderate trauma to the periosteal layer of the joint may play a role in osteophyte development [51]. Periarticular osteophyte formation may erode articular cartilage by disrupting chondrocyte functions accelerating ECM degradation in the context of OA progression (Figure 1).
Figure 1.
Conceptual illustration of the cellular mechanotransduction mechanism mediated by ion channels in articular chondrocytes
The mechanosensory processes in chondrocytes
Chondrocytes are the unique cells found in all types of cartilage. Key to their function is the ability to respond to mechanical loads with changes in metabolic activity. This mechanosensory and mechanotransductive process is, in part, mediated through the activity of a range of expressed mechanosensitive transmembrane ion channels.
The following transmembrane ion channels have been reported to be expressed in human, equine, bovine, chicken, and murine chondrocytes: transient receptor potential (TRP) family channels, including TRPV1, TRPV2, TRPV3, TRPV4, TRPV6 and TRPP1/2; large conductance Ca2+-activated K+ channels (BKCa channels); small-conductance Ca2+-activated K+ channels (SKCa); SACs; voltage-activated H+ channels (VAHCs); T/L-type voltage-gated Ca2+ channels (VGCCs); Piezo1/2 channels; epithelial sodium channels (ENaCs); purinergic receptors; Ca2+ release-activated Ca2+ channels (CRACs) and chloride channels.
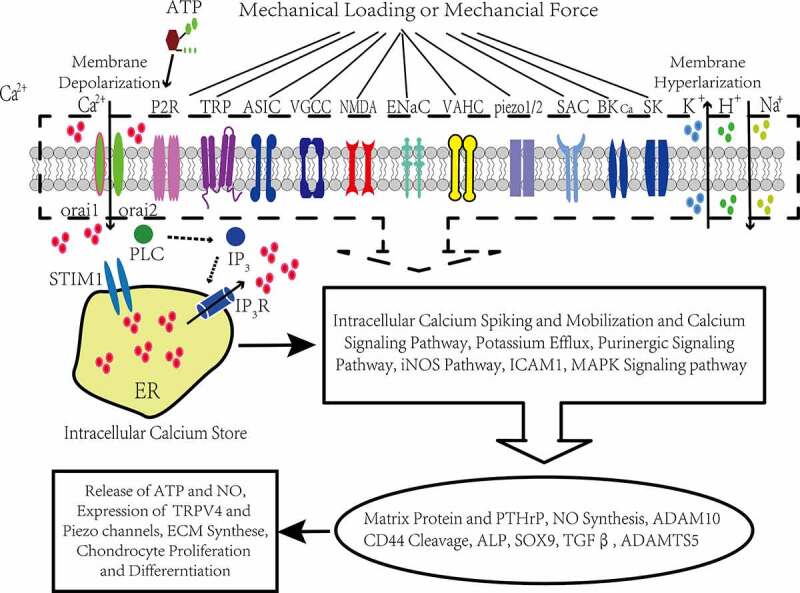
Physiological ion homeostasis is fundamental to routine chondrocyte functions, ion transport through membrane channels is vital for electrophysiological properties manifested by altered membrane potential and mechanically induced Ca2+ influx and activation of the Ca2+-related signaling pathways in response to mechanical stimulation [52], and compression increases ACAN-mRNA via Ca2+/calmodulin-dependent signaling processes in bovine articular chondrocytes (BACs) [53]. K+ channel subfamilies, such as BKCa channels, SKCa channels and Kv subtype channels, have all been identified in articular chondrocytes and participate in mechanotransduction, cell volume regulation, apoptosis and chondrogenesis [40]. TRPV4 and Piezo1/2 channels mediate mechanical modulation of articular chondrocytes and are involved in hypertrophic chondrocyte and ECM degeneration. Many studies have demonstrated that chondrocytes, as nonexcitable cells, sense and respond to a variety of mechanical forces by different morphological and metabolic changes, and that these forces are essential for the mechanical properties of articular cartilage [35,54–60]. In addition to ion channels, cytoskeletal elements, connexin and pannexin-based hemichannels form mechanosensitive units along with adjacent purinergic receptors, and mediate the mechanotransduction process of chondrocytes involving ATP release and intracellular Ca2+ oscillation [21,45,61,62]. ENaCs and SACs participate in the mechanical electrophysiological process of the chondrocyte membrane along with cytoskeletal proteins, ɑ5β1 integrins via mitogen-activated protein kinase (MAPK) signaling, tyrosine kinases and phospholipase C (PLC), which phosphorylate inositol 1,4,5-trisphosphate (IP3), focal adhesion kinase (FAK), paxillin (a focal adhesion complex adapter protein) and β-catenin [63–68]. The cyclical pressure-induced strain activated SKCa channels and SACs involving the phosphorylation of p125, p90, p70, FAK, β-catenin and ɑ5 integrin in normal human articular chondrocytes [56]. ENaCs and VGCCs colocalized with β1 integrins mediate mechanotransduction processes along with the cytoskeleton in murine chondrocytes [69]. Mechanical stimuli activate MAPK signals and alter ACAN gene expression most likely via ion channels [23,70]. The association of mechanosensitive ion channels with integrin, cytoskeletal and signaling complexes is vital for physiological mechanically induced responses of chondrocytes including inducing intracellular anion oscillations and activating mechanical signaling pathways. Maintenance of cell volume is essential for survival and the membrane potential may be vital for regulating chondrocyte volume by changing the osmotic pressure for ionic flux [71]. Mechanically induced cell volume changes induce ATP release in chondrocytes, and purinergic receptors are activated [72], and a Gq-mediated PLC enzymatic reaction is triggered, which produces IP3 that binds to IP3 receptors and induces Ca2+ release from the endoplasmic reticulum [21]. The depletion of intracellular Ca2+ stores causes subsequent store-operated Ca2+ entry (SOCE) through CRACs consisting of Orai1, Orai2 and stromal interaction molecule 1 (STIM1) and causes membrane hyperpolarization [21,73–76]. CRAC-modified mesenchymal stem cells (MSCs) have distinct differentiation fates to adipocytes, osteoblasts, and chondrocytes from multipotent mesenchymal stem cells [77].
In summary, these channels can sense and respond to environmental factors especially mechanical cues, such as ECM deformation and membrane strain [23,27,35,56,57,78–86]. As shown in Table 1, ion channels that are involved in the mechanosensory and mechanotransductive processes of articular chondrocytes are summarized and the mechanosensory and mechanotransductive processes are briefly described.
Table 1.
The mechanosensory and mechanotransductive process of articular chondrocytes mediated by ion channels
| Study | Cell/Tissue | Ion channel and mechanical stimuli | Mechanosensory and mechanotransductive process and mechanoadaptation |
|---|---|---|---|
| Wu et al (2000) | Growth plate chondrocytes | SACs, L-type VGCCs, stretch-induced cartilage matrix deformation | Synthesis of matrix protein, chondrocyte proliferation and differentiation |
| Srinivasan et al. (2015) | T-type VGCC KO mice and WT mice, MC3T3-E1 | T-type VGCCs, dynamic compression | Matrix protein, mechanically induced OA phenotype |
| Parisi et al. (2018) | Embryonic chick hindlimb explants of chick articular joint | SACs, L-type VGCCs, dynamic compression | Ca2+ influx, mechanically induced cellular responses |
| Tanaka et al. (2005) | rat growth plate chondrocytes | Ca2+ channels sensitive to nifedipine, mechanical strain | Parathyroid hormone-related protein, chondrocyte maturation and matrix formation |
| Yellowley et al. (2002) | BACs | SACs, membrane stretch | Ca2+ influx, altered membrane potential |
| Chowdhury et al (2006) | BACs | Purinergic 2 receptor ion channels, SACs, dynamic compression | ATP release, Ca2+ influx, inhibition of NO-induced catabolic effect on articular cartilage |
| Millward-Sadler et al (2004) | Normal human chondrocytes | P2Y2 purinergic receptor, SK channels, mechanical stimulation at 0.33 Hz for 20 minutes | ATP release, membrane hyperpolarization |
| Lee et al. (2000) | Normal human articular chondrocytes | SK channels, SACs, 0.33 Hz cyclical pressure-induced strain | Phosphorylation of p125, p90, and p70, FAK, β-catenin and integrin |
| Mouw et al. (2007) | BACs | K+ channels, Ca2+ channels, SACs, static or dynamic compression | Ca2+ signaling pathways, synthesis of glycosaminoglycan and matrix protein |
| Sánchez et al(2003) | BACs | SACs, VAHCs, membrane stretch | Na+ influx, H+ efflux, membrane depolarization |
| Sanchez, et al(2011) | Human articular chondrocytes | BKCa channels, membrane stretch | K+ efflux, membrane, hyperpolarization, matrix turnover |
| Sanchez et al (2010) | Articular chondrocytes from healthy human and OA patients | BKCa channels, SACs, membrane stretch | K+ efflux, Na+ influx, altered membrane potential |
| Hdud et al. (2014) | Equine articular chondrocytes | TRPV4, BKCa channels, membrane stretch | ERK1/2 and p38 MAPK protein phosphorylation, altered expression of TRPV4 and BKCa channels |
| Sanchez et al. 2011 | Human articular chondrocytes | BKCa channels, membrane stretch | K+ efflux, membrane hyperpolarization |
| Lee et al. (2014) | Chondrocytes of femoral condyles from skeletally mature female pigs | Piezo1/2, cell strain in smooth lateral expanded cells under vertical compression(injurious level of strain) | Ca2+ influx, altered membrane potential, maladaptive chondrocyte responses, cartilage degeneration |
| O’Conor et al. (2014) | Articular cartilage chondrocytes from the femur and ulnas of skeletally immature pigs | TRPV4, dynamic compressive loading | Ca2+ influx, enhanced articular cartilage matrix synthesis and mechanical property |
| Zelenski et al. (2015) | Murine articular chondrocytes | TRPV4, hypotonic stress | Ca2+ influx and Ca2+ signaling pathways |
| Somogyi et al. (2015) | Chicken and murine articular chondrocytes | TRPV3 receptor, uniaxial cyclic compressive force | Increased expression of TRPV3 receptor, matrix synthesis |
| Kobayakawa et al. (2016) |
HCS-2/8 cells, a human chondrocyte line | TRPV4, cyclic tensile strain | Activation of TRPV4, up-regulation of ADAM10-regulation of CD44 cleavage |
| Servin-Vences et al. (2017) | Primary chondrocytes from mice, HEK-293 cells | TRPV4, Piezo1, substrate-deflection, membrane stretch | Ca2+ influx, mechanical transduction of substrate-deflection and membrane stretch |
| Lv, M, et al. (2018) | Murine articular chondrocytes | TRPV4, T-type VGCCs, SACs, ECM deformation and membrane strain induced by compression | Intracellular Ca2+ oscillation |
| Yang et al. (2018) | Human articular chondrocytes from OA patients | Piezo1, cyclic stretch | Up-regulation of piezo1 protein |
| Karamesinis et al. (2017) | ATDC5 cell line derived from mouse teratocarcinoma cells | TRPP1,TRPP2, continuous hydrostatic pressure | Up-regulation of TRPP1 and TRPP2, regulation of chondrocyte differentiation |
| Du et al. (2020) | Primary chondrocytes from mice | TRPV4, piezo2, cyclic tensile strain | Ca2+ influx, altered membrane potential |
| Liu et al. (2013) | MSCs | SACs, mechanical stimulation applied on cultured MSCs by silicon nanowire | Ras/Raf/MEK/ERK signaling cascades, adhesion, chondrocyte proliferation, and differentiation of MSCs |
| Shimazaki, A., et al. (2006) | Human normal and OA articular chondrocytes | NMDA receptor(ligand-gated ion channels),mechanical stimuli at 0.33 Hz | Membrane hyperpolarization |
| Mouw, J. K et al. (2007) | BACs | Ca2+ channels, ATP-dependent Ca2+ pumps, dynamic compression | Synthesis of protein and sulfated glycosaminoglycan |
| Xu, B et al. (2019) | Rat articular chondrocyte | TRPV4, membrane stretch | Ca2+ influx |
| Valhmu, W. B et al. (2002) | BACs | CRACs, compressive stress of 0.1 MPa for 1 h | Compression-induced ACAN mRNA |
Mechanical responses of SACs, VGCCs and VAHCs in chondrocytes
Articular chondrocytes dwell in an environment that continuously changes their osmolarity as a consequence of mechanical stimulation. ECM deformation and hypotonic solution can generate membrane strain and result in physiological adaptation of cell volume and shape in chondrocytes. Matrix deformation-induced membrane stretching induces Ca2+ influx in BACs through SACs [82,83,87]. Gadolinium chloride, an SAC blocker, causes rat chondrosarcoma cells to undergo dedifferentiation accompanied by the downregulation of ACAN, SOX9, collagen 2 and collagen 9; and the upregulation of collagen 1 and fibronectin [88]. Pharmacological blockade of SACs and VGCCs can eliminate the effects of mechanical stimulation on the growth and shape development of chick joints [82]. Mechanical stimulation by silicon nanowires regulates adhesion, chondrocyte proliferation, and differentiation of MSCs partially via activation of SACs and Ras/Raf/MEK/ERK signaling cascades [68]. Cell swelling-induced membrane strain caused activation of a volume-sensitive outwardly rectifying Cl – current followed by a regulatory volume decrease in freshly dissociated rat articular chondrocytes and influenced the mechanical properties of BACs [89,90]. Membrane strains induced by hypotonic solution increase intracellular Ca2+ and trigger membrane depolarization in BACs via SACs [91,92]. T-type VGCCs mediate mechanically induced osteoblast-derived factor release from MC3T3-E1 cells sheared by fluid shear stress, and the conditioned-media obtained from sheared MC3T3-E1 cells may promote the early OA phenotype in chondrocytes [34]. Cyclic mechanical strain enhanced the expression of parathyroid hormone-related proteins, which promoted chondrocyte maturation and ECM formation via Ca2+ channels sensitive to nifedipine in chondrocytes [32]. Fluid shear stress elevated the mRNA levels of collagen Xɑ1, alkaline phosphatase (ALP), MMP13, ACAN, and collagen IIɑ1 in chondrocytes via activation of T-type VGCCs in osteoblasts and enhanced the interaction between subchondral osteoblasts and articular chondrocytes. These changes result in enhanced cartilage degeneration and subchondral bone resorption [34]. SACs and T/L-type VGCCs mediate the mechanical transduction process of immature chondrocytes in response to mechanical stretch induced by articular cartilage matrix deformation [82,93]. Mechanical stretching causes articular cartilage matrix deformation and promotes chondrocyte proliferation and chondrocyte differentiation of immature chondrocytes through SACs and L-type VGCCs and increases the mRNA and protein expression of CMP/Matrilin-1 and collagen ɑ1(X) [93].
In summary, mechanical cues are transduced, at least in part, through mechanosensitive Ca2+ channels, and both SACs and VGCCs participate in cartilage development and may exert synergistic effects of mechanotransduction [82]. Hypotonic solution induces membrane depolarization and intracellular alkalinization of BACs via Na+ influx and H+ efflux by SACs and VAHCs [94]. VAHCs in BACs mediate electrophysiological transduction and have specific mechanosensitive properties in response to osmotic challenges [95].
Mechanical responses of BKCa channels in chondrocytes
Voltage-gated potassium channels play an important role in regulating the membrane potential of primary equine and elephant articular chondrocytes and may mediate the electromechanotransduction process [96]. The BKCa channel is present in the chondrocyte membrane and is highly selective for K+, and its gating is dependent on intracellular Ca2+ [27,80]. Blocking Ca2+-activated K+ channels significantly reduces the SOCE induced by Ca2+ addition after store-depletion of intracellular Ca2+ by thapsigargin in OUMS-27 cells derived from human chondrosarcoma [97]. The membrane strain induced by hypotonic solution promotes K+ efflux and membrane hyperpolarization by activating BKCa channels in human chondrocytes [56,80]. Hypertonic solution induced membrane hyperpolarization via K+ efflux by BKCa channels in human articular chondrocytes [80]. Activation of BKCa channels in human chondrocytes by hypertonic challenge may affect the synthesis and degeneration of the articular cartilage matrix via several metabolic signaling pathways [80]. The total stretch-activated membrane current is induced by the BKCa channels present in equine articular chondrocytes, as recorded by using patch-clamp electrophysiology technology [98]. TREK-2 is a mammalian two-pore domain K+ channel and is important for mechanosensation and can sense and transduce a broad profile of forces within the membrane [99]. Although hypertonic solution induces membrane hyperpolarization, in articular chondrocytes from healthy humans via K+ efflux by BKCa channels, chondrocytes from OA patients fail to respond to this kind of mechanical stimulation [100]. Stretch triggers membrane hyperpolarization via BKCa channels in isolated primary equine chondrocytes [98]. Hypertonic solutions phosphorylate p38 MAPK accompanied by altered expression of BKCa channels in equine articular chondrocytes [101].
Mechanical responses of TRP family channels in chondrocytes
TRPV4 channels mediate significant signal transduction to osmotic and mechanical stimuli in live cells [102]. The membrane strain induced by hypotonic solution was found to activate the TRPV4 channel, and mechanically induced Ca2+ influx and SOX9 transcription are associated with the synthesis of articular cartilage [29,30,103]. Membrane stretching activates TRPV4 ion channels and induces Ca2+ influx in rat articular chondrocytes [104]. TRPV4 ion channels play a role in chondrocyte pathological processes induced by injurious levels of loading [105]. Adamalysin-like metalloproteinases with a TS motif (ADAMTS), MMPs and a disintegrin and metalloproteinase (ADAM) were reported to be expressed in cartilage and participate in the destruction of cartilage in OA by regulating ACAN expression [106,107]. Dynamic compression enhances articular cartilage matrix synthesis by upregulating the TGFβ3, collagen 2ɑ1 and ACAN genes and downregulating the ADAMTS5 gene. These effects require Ca2+ influx induced by mechanical activation of the TRPV4 ion channel [33,108]. The ECM of articular cartilage is a key factor in the development and progression of OA and HA plays a key role in articular cartilage lubrication and preventing ECM loss in articular cartilage in addition to its other excellent physicochemical properties, such as regulation of cell adhesion and cell motility, and manipulation of cell differentiation and proliferation [109,110]. Excessive cyclic tensile strain enhances the expression of ADAM10, which induces cleavage of CD44 a transmembrane protein that serves as an HA receptor [81]. Mechanically induced CD44 cleavage can lead to the loss of extracellular matrices in chondrocytes, which is mediated by TRPV4 in HCS-2/8 cells, a human chondrocytic cell line [81]. Treatment with the TRPV4 agonist 4ɑPDD improves the mechanical properties of articular cartilage constructs [111]. Similar to the anti-inflammatory effects in cells and explants responding to osmotic loading, cyclic tensile strain inhibited IL-1β-mediated NO and PGE2 release by activating TRPV4 channels in articular chondrocytes [112]. Isolated chondrocytes present a regulatory volume decrease, and TRPV4 plays a significant role in this process [113].
GSK1,016,790A (a selective TRPV4 agonist) promoted chondrogenesis, as evidenced by the upregulation of SOX9 and ACAN via intracellular adhesion molecule-1 [114], which demonstrated that the TRPV4 ion channel participates in cartilage growth. TRPV4 gene KO mice showed osteoarthritic knee joint degeneration and the isolated chondrocytes lost the capacity to respond to mechanical strain [29,30,103,115,116]. Chondrocytes from TRPV4 KO mice failed to respond to hypotonic stress and showed inhibition of mechanically induced Ca2+ influx [29,30,103]. Adipose-derived stem cells from TRPV4 knockdown mice tend to undergo adipogenic and osteogenic differentiation and resist chondrogenic differentiation, and TRPV4 knockdown mice develop severe osteoarthritis [117]. The TRPV4 agonist GSK1016790A in the absence of mechanical loading similarly enhanced anabolic and suppressed catabolic gene expression, and potently increased matrix biosynthesis and construct mechanical properties. Inhibition of TRPV4 during dynamic loading prevented acute, mechanically mediated regulation of proanabolic and anticatabolic genes, and furthermore, blocked the loading-induced enhancement of matrix accumulation and mechanical properties [33]. Pharmacological blockade and gene knockdown of TRPV4 attenuate chondrocyte apoptosis and upregulation of FAS-associated protein and cleaved caspase-3, caspase-6, caspase-7, and caspase-8 in rat OA anterior cruciate-ligament transection models [104]. Pharmaceutical activation of TRPV4 inhibited IL-1β-mediated NO release and prevented cartilage degradation and loss of mechanical properties [112]. Furthermore, activation of TRPV4 increases TRPV4 cilia translocation and modulates the expression of soluble tubulin and cilia length, suggesting the potential of TRPV4 manipulation as a novel therapeutic mechanism to suppress proinflammatory signaling and OA cartilage degradation [112]. TRPV4 contributes to the sensation of pain, which is due to hypoosmotic stimuli and inflammatory mechanical hyperalgesia, where TRPV4 sensitization by intracellular signaling leads to pain behaviors in mice [118,119]. A lack of TRPV4-mediated cartilage mechanotransduction in adulthood mice attenuates the severity of aging-associated OA. However, depletion of chondrocyte TRPV4 failed to block OA development following destabilization of the medial meniscus [116]. Impaired osmotic regulation and pressure sensation and increased knee OA scores are observed in TRPV4 KO mice [120]. Intra-articular injection of the TRPV4 antagonist RN-1734 into the knee joint appears to attenuate the responses of the sensitized C-fibers of the acutely inflamed joint to innocuous and noxious mechanical stimulation. These findings suggest that TRPV4 ion channel localization to afferent nerves may be involved in OA mechanical allodynia [121].
Other TRP channels
Continuous hydrostatic pressure promotes runt-related transcription factor 2 production and phosphorylation of SOX9 in ATDC5 cells accompanied by the upregulation of TRPP1 and TRPP2 in ATDC5 cells [84]. Rat TRPA1 channels transiently expressed in human embryonic kidney 293 cells are activated by hypertonic solution supporting a role for TRPA1 in mechanosensation [122]. TRPA1 blockade by TCS 5,861,528 or gene depletion of TRPA1 inhibits acute inflammation, cartilage changes and joint pain by suppressing iodoacetate-induced upregulation COX-2 [123]. Upregulated expression of TRPA1 participates in the occurrence mechanism of mechanical hyperalgesia induced by OA [119]. TRPA1 is associated with the OA driving inflammatory cytokine IL-6, which is supported by high expression of IL-6, IL-6 cytokine family leukemia inhibitory factor and IL-11 in WT chondrocytes and significant downregulation of IL-6 in TRPA1 KO mice. Furthermore, treatment with a TRPA1 antagonist significantly downregulated the expression of IL-6 in chondrocytes from WT mice and OA patients, which indicates that TRPA1 regulates the synthesis of the OA driven inflammatory cytokine IL-6 in chondrocytes and may serve as a possible therapeutic target for OA [124]. TRPV3 receptors mediate the mechanical transduction process and the uniaxial cyclic compressive force increases the expression of the TRPV3 receptor in chondrocytes [78]. The mRNA expression of TRPV3 is the highest among TRPV family channels in chicken and mouse tissue samples [78]. Although the mRNA expression of TRPV3 is significantly induced by mechanical load as revealed by semiquantitative RT-PCR analyses, the mRNA expression of other TRPV channels fails to show any significant alterations upon mechanical load [78]. The resting membrane potential of chondrocytes may be partially controlled by TRPV5 and the positive resting membrane potential may play a protective role in chondrocytes responding to hypotonic solution with minimum changes in cell volume [71,125].
Mechanical responses of Piezo1/2 channels in chondrocytes
Piezo is a type of mechanically sensitive ion channel that is necessary for cells to respond to mechanical stimuli and can convert mechanical signals sensed by the membrane into intracellular electrical or chemical signals. Crosslinking between the cap and blade residues inhibits mechanical gating of the Piezo1 channel [126]. Cyclic stretch increased the expression of Piezo1 channels in human articular chondrocytes from OA patients [127]. Cholesterol enrichment or depletion by methyl-β-cyclodextrin or disruption of membrane cholesterol organization by dynasore can impair the function of Piezo1 channels. These findings highlight the importance of plasma membrane localization and organization of Piezo1 channels and indicate that mechanical activation of the Piezo1 channel is directly dependent on the membrane composition and lateral organization of membrane cholesterol domains [128]. Similar to mechanically induced Piezo1 activation, Piezo1 activation is countered by the K+ channel TRAAK (K2P4.1). This action is also responsive to mechanical forces [129]. This finding suggests that although membrane tension is the direct mediator of force, other mechanosensitive ion channels can actually play important indirect roles by influencing which regions of the cell membrane experience changes in tension when forces are applied to a cell. Interestingly, mechanical stimulation of the lipid bilayer alone is sufficient to activate Piezo1 channels, which indicates that Piezo1 channels functioning as molecular force transducers in the cellular membrane may not rely on alternate cellular components to sense mechanical stimuli [130,131]. Electrophysiology experiments of Piezo1/2 channels confirm that Piezo1/2 channels function as significant frequency filters. The effectiveness of mechanical transduction varies with the stimulus frequency, waveform, and duration presented by patch-clamp recordings, which suggests the potential contributions of Piezo1/2 channels in transducing repetitive mechanical stimuli [132]. In addition to transduction of extracellular mechanical forces, Piezo1 channels may be activated by cell-generated forces induced by Myosin II phosphorylation by Myosin Light Chain Kinase [133]. Application of a negative pressure failed to activate Piezo2 channels in human Merkel cell carcinoma cells and human embryonic kidney 293 T cells, but both positive and negative pressure activated Piezo1 channels in a similar manner [134]. We also note that moderate cold can potentiate the conversion of mechanical force into excitatory current because of the cold sensitivity of Piezo2 channels and Piezo2 channels are evolutionarily conserved as cutaneous mechanoreceptors [135], which may provide evidence for the involvement of Piezo2 channels in chondrocyte mechanotransduction process. TRPV4 and Piezo1 channels mediate compression-induced chondrocyte membrane strain and result in intracellular Ca2+ concentration oscillation in the cytoplasm [35,86]. The physiological level of intermittent tensile strain caused TRPV4-dependent membrane potential, but injurious levels of intermittent tensile strain triggered Piezo2-dependent membrane potential. In conclusion, these findings thus reveal the key role of TRPV4 and Piezo2 ion channels in mechanoelectrical transduction in primary murine chondrocytes [85].
Piezo1 and Piezo2 gene KO mice presented articular cartilage defects, which indicated that Piezo1 and Piezo2 ion channels are vital to articular cartilage growth and regulate the mechanical properties of the ECM and embedded chondrocytes [136]. GsMTx4 (Piezo1/2 channel inhibitor) attenuated the deteriorated response of chondrocytes to injurious mechanical strain [105]. GsMTx4 and Piezo1/2 siRNA attenuated injurious levels of strain-induced maladaptive murine chondrocyte responses, which demonstrated that piezo1/2 participates in cartilage injury and posttraumatic OA [105,137]. GsMTx4 may play a chondroprotective role in mechanically induced articular cartilage deterioration by joint disability [33,105]. The endogenous peptide urocortin1 can maintain Piezo1 channels in a closed conformation and firmly protect articular cartilage and chondrocytes against corticotropin-releasing factor receptor 1 selective antagonist CP-154,526–induced accumulation of intracellular Ca2+, which is due to opening nonselective cation channels and cell death [138].
Mechanical responses of P2 receptors and CRACs in chondrocytes
Membrane depolarization in OA chondrocytes and membrane hyperpolarization in normal chondrocytes suggested that the purinergic signaling pathways are important mediators in OA progression [139]. Mechanically induced ATP release and an increase in extracellular ATP levels activate ligand-gated ion channel P2X receptors and some G-protein-coupled P2Y receptors, and these effects can regulate chondrocyte function and modulate intercellular communication [140]. Dynamic compression-induced ATP release in chondrocytes activates P2 receptor ion channels. For instance, the P2X7 receptors and subsequent purinergic signaling pathways, including the Ca2+ influx and Ca2+ signaling pathways, are activated, which then stimulate the synthesis and release of proteoglycans in chondrocytes and prevent compression-induced NO release of chondrocytes and articular cartilage degeneration [24,141]. Unlike other ion channels that are stimulated or activated directly by mechanical forces, P2X and P2Y receptor ion channels are both activated indirectly by a mechanically induced release of nucleotides such as ATP. Mechanically induced ATP release activates P2Y2 receptors and alters membrane potential in human chondrocytes [139]. Purinergic receptors can also confer the mechanosensitivity of the voltage-gated K+ channel KCNQ1 (also known as Kv7.1 or KvLQT1). Static compression induced the expression of ACAN mRNA in BACs, which can be dose-dependently or completely blocked by antagonists of the Ca2+/CaM signaling pathway and thapsigargin that deplete IP3-sensitive intracellular Ca2+ stores [53]. Both the amplitude and the maximal rate of rise of SOCE are significantly reduced by SOCE blockers, which decrease the mRNA expression of ACAN and collagen 2 decreases severely in chicken chondrogenic mesenchymal cells [73], which may provide substantial evidence that intracellular Ca2+ stores are vital for Ca2+ oscillations and chondrogenesis. Orai1, Orai2 and STIM1 form functional CRACs in OUMS-2 cells, and these complexes are responsible for sustained Ca2+ entry in response to histamine stimulation [74]. Histamine-induced SOCE through CRACs may also contribute to mechanically induced intracellular Ca2+ increases in chondrocytes.
Mechanical responses of NMDA receptors, ASICs, chloride channels and DEG/ENaCs in chondrocytes
One of the ionotropic glutamate receptors, the NMDA receptor, is expressed in human articular chondrocytes. This receptor may mediate mechanically induced membrane hyperpolarization [142]. Ca2+/calmodulin-dependent protein kinase II (CaMKII), which is associated with NMDA signaling, has four subunit isoforms (alpha, beta, gamma, delta). CaMKII may mediate a mechanically induced increase in intracellular Ca2+ in a wide variety of cells and tissues. The alpha – and beta-isoforms have narrow distributions restricted mainly to neuronal tissues, but the gamma – and delta-isoforms are ubiquitously expressed within neuronal tissue and articular cartilage. The CaMKII isoforms gamma and delta are expressed in both normal and OA chondrocytes and are only involved in the response of normal chondrocytes to mechanical stimulation [142]. ASIC3 is present in murine articular chondrocytes and participates in OA progression, and murine ASIC1b is permeable to K+ and functions as a mechanogating ion channel responding to membrane stretching [143]. Hypotonic stimuli significantly enhance acid-evoked membrane currents via ASIC1b in oocytes [143]. Cl – channels are known to be expressed in mammalian chondrocytes, such as voltage-dependent Cl – channels and swelling-activated Cl – channels, and these Cl – channels can participate in the regulation of resting membrane potential, cell volume, cell survival, and endochondral bone formation [144]. Chloride channels regulate chondrogenesis in chicken mandibular mesenchymal cells [145]. Hypotonic solution and extracellular acidification elicited a volume-sensitive outwardly rectifying Cl – current followed by a regulatory volume decrease and an acid-sensitive outwardly rectifying Cl – current, respectively [146]. Hypotonic solution-induced Cl – current and regulatory volume decrease responses occurred in isolated rabbit and articular chondrocytes [89,147,148]. The CIC-3 chloride channel is responsible for hypotonic solution-induced Cl – current and regulatory volume decrease in response to hypoosmotic environments [149]. Anterior cruciate ligament transection treatment results in a large increase in hypotonic-activated chloride conductance in rabbit chondrocytes and enhanced caspase-3/7 activity followed by the onset of apparent cartilage loss [150]. Hypotonic solution decreases the expression level of the ClC-7 chloride channel, which may participate in OA progression in human chondrocytes [151]. Tyrosine phosphorylation induced by the protein tyrosine kinase regulates the functional role of hypotonic solution-induced Cl – current in the regulatory volume decrease of isolated rabbit articular chondrocytes [152]. The DEG/ENaC (degenerin/epithelial sodium channel) protein family comprises related ion channel subunits from all metazoans, including humans. Members of this protein family play roles in mechanotransduction. DEG/ENaC-like ion channels participate in the modulation of canine chondrocyte volume, which can be inhibited by benzamil by reducing the influx of Na+ ions [153]. The DEG/ENaC ion channel complex may contribute to mechanically induced chondrocyte dysfunction and OA pathological changes [154].
Articular joint response to hind limb unloading or simulated microgravity
In addition to different mechanical stimuli, decreased mechanical forces or mechanical unloading situations simulated by the hind limb, joint immobilization and the random positioning system (RPM) also regulated the functions of articular chondrocytes and were accompanied by altered expression of mechanosensitive ion channels. Mechanically induced lubricant HA secretion by synoviocytes can maintain articular cartilage [155,156], which may contribute to the effects of mechanical loading of articular cartilage on the metabolism of resident chondrocytes and the synthesis of molecules to maintain the integrity of the cartilage. Moderate exercise promotes proteoglycan synthesis and maintains articular ECM homeostasis. However, articular joint immobilization may reduce HA secretion and result in decreased cartilage thickness and inhibited proteoglycan synthesis leading to ECM degeneration, which will make the articular cartilage more vulnerable to excessive mechanical stimulation. The chondrocyte phenotype was preserved when suspended clustered chondrocytes maintained a round morphology under simulated microgravity induced by RPM [157]. Progressive dedifferentiation which includes the production proportion of collagen 2/collagen 1, proteoglycan proportion of ACNA to versican (ACNA/VCAN) and a transition to fibroblast-like morphology was observed in monolayer in vitro cultured BACs except for decreased expression of the TRPV4 ion channel, which was attenuated by the RPM system that induced the suspension status of in vitro BACs [157]. Rats subjected to mechanical unloading induced by the hind limb presented increased expression of inducible NO synthase, enhanced chondrocyte apoptosis, decreased thickness of articular cartilage and compromised joint biomechanics [33]. Male C57BL/6 J mice under mechanical unloading conditions such as hind limb and joint immobilization presented with subchondral bone atrophy accompanied by osteoclast differentiation of bone marrow cells and degeneration of the cartilaginous layer without chondrocyte hypertrophy [158]. In addition, increased ALP and aggercanase activity, decreased ACAN content in calcified cartilage and decreased ALP activity in calcified cartilage were observed in male C57BL/6 J mice in the hind limb and joint immobilization groups [158]. As shown in Table 2, the expression of mechanosensitive TRPV4 channels present in articular chondrocytes is changed during progressive osteoarthritis cartilage degeneration, which is consistent with these important roles of mechanosensitive ion channels in OA progression.
Table 2.
Articular chondrocytes in response to hind limb unloading or simulated microgravity
| Study | Cell/Tissue | Decreased mechanical stimulation | Results |
|---|---|---|---|
| Wuest et al. (2018) | BACs | TRPV4, simulated microgravity induced by RPM | Altered expression of TRPV4, preserved chondrocyte phenotype |
| Basso et al(2006) | Articular and growth plate cartilage | Hind limb | Increased expression of iNOS, impaired articular cartilage, deteriorated joint biomechanics |
| Nomura et al. (2017) | Male C57BL/6 J mice | Hind limb, joint immobilization | Altered ALP and aggercanase activity, decreased ACAN content subchondral bone atrophy, cartilage degeneration |
Chondrocytes in OA
Chondrocytes are the cells within cartilage that produce and maintain the extracellular matrix. Volume regulation of chondrocytes is vital to their function and occurs in OA [113]. Articular chondrocytes are exposed to changing osmolarity and compressive loads. Ion channels implicated in volume control are changed in chondrocytes from osteoarthritic cartilage [113]. In addition to mechanically induced chondrocyte phenotype changes, some specific mechanosensitive ion channel agonists or antagonists and gene knockdown technology can also alter the chondrocyte phenotype and may be involved in OA pathological progression and OA pain. As shown in Table 3, some mutations in TRPV4 cause severe developmental abnormalities, such as skeletal dysplasia and arthropathy [118]. Differential regulation of ion channels such as BKCa and TRPV4 at the functional level and expression level is observed in early OA synovial fluid mesenchymal progenitor cells [159,160], which suggests that mechanosensitive ion channels participate in OA progression.
Table 3.
The role of ion channels in osteoarthritis
| Study | Cell/Tissue | Ion channel and intenvention | Results |
|---|---|---|---|
| Funabashi et al. (2010) | OUMS-27 cell line | SK channels, CRAC channels, histamine | K+ efflux through SK channels, increased intracellular Ca2+ concentration via nonselective cation channels including CRAC channels, membrane hyperpolarization |
| Li et al. (2017) | Human chondrocytes from OA patients | Piezo1, GsMTx4, a PIEZO-blocking peptide | Suppressed expression of apoptosis-related genes |
| Sooampon et al. (2013) | Human periodontal ligament (HPDL) cells, human osteoblasts | Piezo1, GsMTx4 | Ca2+ influx, attenuated deteriorated response of chondrocytes to injurious mechanical strain |
| O’Conor et al. (2016) | Conditional knockout (cKO) mice | Gene knock down of TRPV4 channel | Decreased total periarticular bone volume, reduced severity of aging-associated OA |
| Ogawa et al. (2019) | The ATDC5 cell line | TRPV4, GSK1016790A, a selective TRPV4 agonist | Activation of TRPV4-ICAM-1-up-regulation of chondrogenic marker genes including SOX9 and ACAN, HA facilitated TRPV4-induced chondrogenesis |
| Srinivasan et al. (2015) | KO and WT mice | Gene knock down of T-type VGCCs | Enhanced cartilage degeneration and subchondral bone resorption |
| Kuduk, S. D., et al. (2010), Izumi, M., et al. (2012) | KO and WT mice, rat osteoarthritis models | Gene knock down of ASIC-3, the ASIC3 inhibitor A-317,567, ASIC3 selective peptide blocker (APETx2) | Altered expression of ASIC3 in knee joint afferents, reversed osteoarthritis pain and mechanical hyperalgesia |
| Schuelert, N., et al. (2010) | Rat osteoarthritis models | The cannabinoid CB2 receptor agonist GW405833 | Reduced mechanosensitivity of afferent nerve fibers in control joints, nociceptive responses in OA joints |
| Shimazaki, A., et al. (2006) | Human normal and OA articular chondrocytes | NMDA receptor(ligand-gated ion channels), CaMKII inhibitor | Inhibited membrane potential, upregulation of aggrecan mRNA |
| Clark et al. (2010) | KO mice | Gene knock down of TRPV4 | Severe OA degeneration |
| Lee et al. (2014) | Murine chondrocytes | Piezo1, GsMTx4, siRNA | Attenuated maladaptive chondrocyte responses |
| Zelenski et al. (2015) | Murine articular chondrocytes | Gene knock down of TRPV4 | Suppressed mechanically induced Ca2+ influx and formation of pericellular matrix |
| Moilanen, L. J., et al. (2015) | Murine articular chondrocytes | Pharmacological blockade and gene knock down of TRPA1 | Suppressed iodoacetate-induced OA |
| O’Conor, C. J., et al. (2016) | cKO mice | Gene knock down of TRPV4 | Reduced severity of aging-associated OA |
| He, B. H., et al. (2017) | OA mice | Mechanosensitive ion channels, the selective inhibitor GsMTx4 | Reduced activation of dorsal horn nociceptive circuits and primary mechanical allodynia |
| Xing, R., et al. (2017) | OA mice | TRPA1, TRPV4 | Upregulation of TRPA1 and TRPV4 mechanical hyperalgesia induced by OA |
| Parisi, C., et al. (2018) | Chick joint | Pharmacological blockade of SACs, VGCCs, | Removed effects of mechanical stimulation on joint cartilage growth and shape development |
| Raouf, R., et al. (2018) | Rat DRG nerons | Piezo2, overexpression and gene knock down of annexin A6 | Attenuated mechanical hyperalgesis induced by OA |
| Richter, F., et al. (2019) | Rat DRG nerons | TRPV4, agonist 4αPDD, GSK 1016790 A and antagonist RN-1734 | Altered mechanonociception of the normal and inflamed joint |
| Xu, B., et al. (2019) | Rat articular chondrocytes | Pharmacological blockade and gene knock down of TRPV4 | Attenuated cartilage degeneration in rat OA anterior cruciate-ligament transection (ALCT) models |
| Nummenmaa, E., et al. (2020) | Rat articular chondrocytes, human OA chondrocytes | Pharmacological blockade and gene knock down of TRPA1 | Downregulation of the pro-inflammatory cytokine interleukin-6 (IL-6), IL-6 family cytokines leukemia inhibitory factor (LIF) and IL-11 |
| Fu, S., et al. (2021) | Rat articular chondrocytes | Pharmacological blockade or activation of TRPV4 | Pro-inflammatory signaling and cartilage degradation |
GsMTx4 inhibits the activation of nociceptors followed by mechanical allodynia by weakening the sensitization of mechanosensitive ion channels in OA mice [161], which indicates that Piezo1/2 channels also participate in OA mechanical hyperalgesia in addition to OA cartilage degeneration. Annexin A6 is a membrane-associated Ca2+-binding protein. Annexin A6-deficient mice showed increased sensitivity to mechanical stimuli and increased activity of Piezo2 channels that mediate a rapidly adapting mechanogated current linked to proprioception and touch in sensory neurons. Overexpression of Annexin A6 may attenuate OA mechanical pain by inhibiting rapidly adapting currents induced by Piezo2 channels [162]. Intra-articular injection of VGCC inhibition may lessen OA changes, such as enhanced cartilage degeneration and subchondral bone resorption, in both posttraumatic and age-related OA [34]. Dysregulation of NMDA-CaMKII signaling may contribute to the onset and progression of osteoarthritis [142]. Upregulation of pain-related neurochemical markers such as ASIC3 is observed in joint afferents of a rat OA model induced by intra-articular injection of monoiodoacetate [163]. The ASIC3 inhibitor attenuated mechanical hyperalgesia by inhibiting the expression of ASIC3 in knee joint afferents and ASIC3 KO mice reversed mechanical hyperalgesia in a rat osteoarthritis model, which indicates the involvement of ASICs in osteoarthritis pain via the central nervous system [164,165]. The intracellular injection of the CB2 receptor agonist GW405833 into synoviocytes attenuates osteoarthritis knee joint pain in a rat osteoarthritis model [166].
In summary, mechanical stimulus mechanically induced intracellular cation mobilization via ion channels, such as TRPV4, ASIC3 and Piezo1/2, and these mechanosensitive channels participate in mechanically induced alteration of articular chondrocytes and the maintenance of ECM homeostasis in joints. Ion channels may serve as potential biomarkers for OA [167]. The intensification of ion channels may prevent or attenuate osteoarthritis progression. Dysregulation of the mechanical responses of chondrocytes may participate in the progression of OA [100].
Conclusion and perspectives
During joint movement, the mechanical load of body weight is the most physiological stimulus affecting cartilage. Body weight can influence of the ECM in applied animal experiments. Excessive obesity may induce injurious levels of mechanical stimulation and thus accelerate joint aging. However, significantly decreased mechanical stimuli, such as hind limb unloading, can also cause pathological degeneration of articular cartilage because of a lack of sufficient physiological mechanical stimulation. Moderate exercise under healthy body weight may exert chondroprotective effects and stimulate the anabolic metabolism of articular cartilage in physiological mechanical contexts. Obesity or fracture of the articular surface can result in a quantitative imbalance between anabolic and catabolic activity, along with ECM catabolic metabolism, particularly in swelling articular cartilage tissue, which leads to the development of OA and compromised mechanical resilience of articular cartilage. As a result, it is important to investigate the effects of deformation pressure or shear force on articular cartilage homeostasis. These data can unveil the pathophysiological properties of OA progression by exploring the molecular mechanism by which mechanoreceptors participate in the mechano-adaptation process.
In many different cell biomechanical studies, there is a direct link between mechanoreceptors at the cell surface and intercellular biochemical signaling, which in turn controls downstream effector molecules [168]. Among the mechanoreceptors in the cell membrane, mechanosensitive ion channels are essential for the transduction of mechanical stimuli into biologically relevant signals. The innate force-sensing ability of mechanosensitive channels transduces mechanical signals. Furthermore, mechanical stimuli can induce alteration in chondrocyte metabolism and influence the homeostasis of articular cartilage. Although physiological expression of ion channels is essential for articular ECM formation, differentially expressed transmembrane channels on chondrocyte surface membranes (plasmalemma) are relevant to OA [27,83,141]. The effects of mechanosensitive ion channels may delay OA cartilage degeneration and attenuate OA-induced mechanical allodynia. The molecular mechanisms by which ion channels participate in the mechanotransduction process are not clear, and the interaction relationships among some of these ion channels are unknown. More research is needed to define the electrophysiological properties of individual ion channels that can participate in mechanically altered metabolism of chondrocytes. The molecular mechanisms by which mechanical stimuli prevent articular cartilage degeneration and promote articular cartilage repair may yield insights into the rationale of possible mechanical therapy, such as the potential for therapeutic low-intensity pulsed ultrasound for OA [169,170].
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81874017 and 81960403 and 82060405 and 82060413); National Science Foundation of Gansu Province of China (20JR5RA320); Lanzhou Science and Technology Plan Program (2018-3-52); Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (CY2017-QN12, CY2017-ZD02); The Fundamental Research Funds for the Central Universities (lzujbky-2020-kb17).
Funding Statement
This work was supported by the The National Natural Science Foundation of China [81874017]; The National Natural Science Foundation of China [81960403]; The National Natural Science Foundation of China [82060405]; National Science Foundation of Gansu Province of China [20JR5RA320]; The National Natural Science Foundation of China [82060413]; Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital [CY2017-QN12]; Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital [CY2017-ZD02]; The Fundamental Research Funds for the Central Universities [lzujbky-2020-kb17]; Lanzhou Science and Technology Plan Program [2018-3-52].
Authors’s contributions
Kun Zhang, Yayi Xia and Lifu Wang conceived and designed the idea to this paper; Yuanjun Teng, Xuening Liu, Qiong Yi and Zhongcheng Liu participated in its design and coordination and supervised the paper. Yuanjun Teng, Dacheng Zhao, Dechen Yu and Xiangyi Chen collected and analyzed the references and drafted the paper. Bin Geng supervised the framework of the article. All authors read and approved the final version of the manuscript. Kun Zhang was the first author of this article. Kun Zhang and Lifu Wang contributed equally to this work.
Disclosure statement
There are no relevant financial or non-financial competing interests
References
- [1].Martin JA, Buckwalter JA.. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):106–110. [DOI] [PubMed] [Google Scholar]
- [2].Price FM, Levick JR, Mason RM. Changes in glycosaminoglycan concentration and synovial permeability at raised intra-articular pressure in rabbit knees. J Physiol. 1996;495(3):821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Levick JR. Microvascular architecture and exchange in synovial joints. Microcirculation. 1995. September;2(3):217–233. [DOI] [PubMed] [Google Scholar]
- [4].Coleman PJ, Scott D, Abiona A, et al. Effect of depletion of interstitial hyaluronan on hydraulic conductance in rabbit knee synovium. J Physiol. 1998;509(3):695–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Scott D, Coleman PJ, Mason RM, et al. Direct evidence for the partial reflection of hyaluronan molecules by the lining of rabbit knee joints during trans-synovial flow. J Physiol. 1998;508(2):619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Coleman PJ, Scott D, Mason RM, et al. Role of hyaluronan chain length in buffering interstitial flow across synoviumin rabbits. J Physiol. 2000;526(2):425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Scott D, Coleman PJ, Mason RM, et al. Action of polysaccharides of similar average mass but differing molecular volume and charge on fluid drainage through synovial interstitium in rabbit knees. J Physiol. 2000. November 1;528(Pt 3):609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Scott D, Coleman PJ, Mason RM, et al. Concentration dependence of interstitial flow buffering by hyaluronan in synovial joints. Microvasc Res. 2000. May;59(3):345–353. [DOI] [PubMed] [Google Scholar]
- [9].Scott D, Bertin K, Poli Aet al. Interstitial pressure gradients around joints; location of chief resistance to fluid drainage from the rabbit knee. Exp Physiol. 2001;86:739–747. [DOI] [PubMed] [Google Scholar]
- [10].Lu Y, Levick JR, Wang W. The mechanism of synovial fluid retention in pressurized joint cavities. Microcirculation. 2005. Oct-Nov;12(7):581–595. [DOI] [PubMed] [Google Scholar]
- [11].Momberger TS, Levick JR, Mason RM. Hyaluronan secretion by synoviocytes is mechanosensitive. Matrix Biol. 2005. December;24(8):510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Momberger TS, Levick JR, Mason RM. Mechanosensitive synoviocytes: a Ca2+ -PKCalpha-MAP kinase pathway contributes to stretch-induced hyaluronan synthesis in vitro. Matrix Biol. 2006. July;25(5):306–316. [DOI] [PubMed] [Google Scholar]
- [13].Large RJ, Hollywood MA, Sergeant GP, et al. Ionic currents in intimal cultured synoviocytes from the rabbit. Am J Physiol Cell Physiol. 2010. November;299(5):C1180–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ingram KR, Wann AK, Angel CK, et al. Cyclic movement stimulates hyaluronan secretion into the synovial cavity of rabbit joints. J Physiol. 2008. March 15;586(6):1715–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ingram KR, Wann AK, Wingate RM, et al. Signal pathways regulating hyaluronan secretion into static and cycled synovial joints of rabbits. J Physiol. 2009. September 1;587(Pt 17):4361–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huang J, Ballou LR, Hasty KA. Cyclic equibiaxial tensile strain induces both anabolic and catabolic responses in articular chondrocytes. Gene. 2007. December 1;404(1–2):101–109. [DOI] [PubMed] [Google Scholar]
- [17].Kaupp JA, Weber JF, Waldman SD. Mechanical stimulation of chondrocyte-agarose hydrogels. J Vis Exp. 2012. October;27(68):e4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wohlrab D, Wohlrab J, Reichel H, et al. Is the proliferation of human chondrocytes regulated by ionic channels? J Orthop Sci. 2001;6:155–159. [DOI] [PubMed] [Google Scholar]
- [19].Mouw JK, Imler SM, Levenston ME. Ion-channel regulation of chondrocyte matrix synthesis in 3D culture under static and dynamic compression. Biomech Model Mechanobiol. 2007. January;6(1–2):33–41. [DOI] [PubMed] [Google Scholar]
- [20].Zhu M, Zhou S, Huang Z, et al. Ca2+-dependent endoplasmic reticulum stress regulates mechanical stress-mediated cartilage thinning. J Dent Res. 2016. July;95(8):889–896. [DOI] [PubMed] [Google Scholar]
- [21].Suzuki Y, Yamamura H, Imaizumi Y, et al. K(+) and Ca(2+) channels regulate Ca(2+) signaling in chondrocytes: an illustrated review. Cells. 2020. June 29;9(7). DOI: 10.3390/cells9071577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xu BY, Jin Y, Ma XH, et al. The potential role of mechanically sensitive ion channels in the physiology, injury, and repair of articular cartilage. J Orthop Surg. 2020;28(3):2309499020950262. . [DOI] [PubMed] [Google Scholar]
- [23].Mobasheri A, Carter SD, Martin-Vasallo P, et al. Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol Int. 2002;26(1):1–18. [DOI] [PubMed] [Google Scholar]
- [24].Chowdhury TT, Knight MM. Purinergic pathway suppresses the release of .NO and stimulates proteoglycan synthesis in chondrocyte/agarose constructs subjected to dynamic compression. J Cell Physiol. 2006. December;209(3):845–853. [DOI] [PubMed] [Google Scholar]
- [25].Li J, Zhao Q, Wang E, et al. Dynamic compression of rabbit adipose-derived stem cells transfected with insulin-like growth factor 1 in chitosan/gelatin scaffolds induces chondrogenesis and matrix biosynthesis. J Cell Physiol. 2012. May;227(5):2003–2012. [DOI] [PubMed] [Google Scholar]
- [26].Das P, Schurman DJ, Lane Smith R. Nitric oxide and G proteins mediate the response of bovine articular chondrocytes to fluid-induced shear. J Orthop Res. 1997;15(1):87–93. [DOI] [PubMed] [Google Scholar]
- [27].Asmar A, Barrett-Jolley R, Werner A, et al. Membrane channel gene expression in human costal and articular chondrocytes. Organogenesis. 2016. April 2;12(2):94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].O’Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflugers Arch. 2005. October;451(1):193–203. [DOI] [PubMed] [Google Scholar]
- [29].Clark AL, Votta BJ, Kumar S, et al. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis Rheum. 2010. October;62(10):2973–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Guilak F, Leddy HA, Liedtke W. Transient receptor potential vanilloid 4: the sixth sense of the musculoskeletal system? Ann N Y Acad Sci. 2010. March;1192:404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hall AC, Horwitz ER, Wilkins RJ. The cellular physiology of articular cartilage. Exp Physiol. 1996;81(3):535–545. [DOI] [PubMed] [Google Scholar]
- [32].Tanaka N, Ohno S, Honda K, et al. Cyclic mechanical strain regulates the PTHrP expression in cultured chondrocytes via activation of the Ca2+ channel. J Dent Res. 2005;84:64–68. [DOI] [PubMed] [Google Scholar]
- [33].O’Conor CJ, Leddy HA, Benefield HC, et al. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci U S A. 2014;111:1316–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Srinivasan PP, Parajuli A, Price C, et al. Inhibition of T-type voltage sensitive calcium channel reduces load-induced OA in mice and suppresses the catabolic effect of bone mechanical stress on chondrocytes. PloS One. 2015;10(5):e0127290. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Servin-Vences MR, Moroni M, Lewin GR, et al. Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. Elife. 2017. January 30;6. DOI: 10.7554/eLife.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ji Q, He C. Extracorporeal shockwave therapy promotes chondrogenesis in cartilage tissue engineering: a hypothesis based on previous evidence. Med Hypotheses. 2016. June;91:9–15. [DOI] [PubMed] [Google Scholar]
- [37].Coleman MC, Ramakrishnan PS, Brouillette MJ, et al. Injurious loading of articular cartilage compromises chondrocyte respiratory function. Arthritis Rheumatol (Hoboken). 2016. March;68(3):662–671. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dolzani P, Assirelli E, Pulsatelli L, et al. Ex vivo physiological compression of human osteoarthritis cartilage modulates cellular and matrix components. PloS One. 2019;14(9):e0222947. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lin YY, Tanaka N, Ohkuma S, et al. Applying an excessive mechanical stress alters the effect of subchondral osteoblasts on chondrocytes in a co-culture system. Eur J Oral Sci. 2010. April;118(2):151–158. . [DOI] [PubMed] [Google Scholar]
- [40].Mobasheri A, Lewis R, Ferreira-Mendes A, et al. Potassium channels in articular chondrocytes. Channels. 2012. Nov-Dec;6(6):416–425. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kachroo U, Livingston A, Vinod E, et al. Comparison of electrophysiological properties and gene expression between human chondrocytes and chondroprogenitors derived from normal and osteoarthritic cartilage. Cartilage. 2020. July;11(3):374–384. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sun HB. Mechanical loading, cartilage degradation, and arthritis. Ann N Y Acad Sci. 2010. November;1211:37–50. [DOI] [PubMed] [Google Scholar]
- [43].Chen C, Tambe DT, Deng L, et al. Biomechanical properties and mechanobiology of the articular chondrocyte. Am J Physiol Cell Physiol. 2013. December 15;305(12):C1202–8. [DOI] [PubMed] [Google Scholar]
- [44].Matta C, Mobasheri A, Gergely P, et al. Ser/Thr-phosphoprotein phosphatases in chondrogenesis: neglected components of a two-player game. Cell Signal. 2014. October;26(10):2175–2185. . [DOI] [PubMed] [Google Scholar]
- [45].Blain EJ. Involvement of the cytoskeletal elements in articular cartilage homeostasis and pathology. Int J Exp Pathol. 2009. February;90(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Milner CE, Meardon SA, Hawkins JL, et al. Walking velocity and step length adjustments affect knee joint contact forces in healthy weight and obese adults. J Orthop Res. 2018. October;36(10):2679–2686. [DOI] [PubMed] [Google Scholar]
- [47].Ma Y, Song Y, Li L, et al. Mechano growth factor pretreatment yield mechanical stimuli induced cell stress responses in ligament fibroblasts of osteoarthritis via activating ATF-2. Biotechnol Lett. 2020. August;42(8):1337–1349. [DOI] [PubMed] [Google Scholar]
- [48].Silawal S, Triebel J, Bertsch T, et al. Osteoarthritis and the complement cascade. Clin Med Insights. 2018;11:1179544117751430. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wong S, Chiu K, Yan C. Review article: osteophytes. J Orthop Surg. 2016;24(3):403–410. [DOI] [PubMed] [Google Scholar]
- [50].Van Der Kraan PM, Van Den Berg WB. Osteophytes: relevance and biology. Osteoarthritis Cartilage. 2007;15(3):237–244. [DOI] [PubMed] [Google Scholar]
- [51].Venne G, Tse MY, Pang SC, et al. Mechanically-induced osteophyte in the rat knee. Osteoarthritis Cartilage. 2020. June;28(6):853–864. [DOI] [PubMed] [Google Scholar]
- [52].Mobasheri A, Mobasheri R, Francis MJ, et al. Ion transport in chondrocytes: membrane transporters involved in intracellular ion homeostasis and the regulation of cell volume, free [Ca2+] and pH. Histol Histopathol. 1998;13:893–910. [DOI] [PubMed] [Google Scholar]
- [53].Valhmu WB, Raia FJ. myo-Inositol 1,4,5-trisphosphate and Ca2+/calmodulin-dependent factors mediate transduction of compression-induced signals in bovine articular chondrocytes. Biochem J. 2002;361:689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ramage L, Nuki G, Salter DM. Signalling cascades in mechanotransduction: cell-matrix interactions and mechanical loading. Scand J Med Sci Sports. 2009. August;19(4):457–469. [DOI] [PubMed] [Google Scholar]
- [55].Servin-Vences MR, Richardson J, Lewin GR, et al. Mechanoelectrical transduction in chondrocytes. Clin Exp Pharmacol Physiol. 2018. May;45(5):481–488. [DOI] [PubMed] [Google Scholar]
- [56].Lee HS, Millward-Sadler SJ, Wright MO, et al. Integrin and mechanosensitive ion channel-dependent tyrosine phosphorylation of focal adhesion proteins and beta-catenin in human articular chondrocytes after mechanical stimulation. J Bone Miner Res. 2000. August;15(8):1501–1509. [DOI] [PubMed] [Google Scholar]
- [57].Wang JH, Thampatty BP. An introductory review of cell mechanobiology. Biomech Model Mechanobiol. 2006. March;5(1):1–16. [DOI] [PubMed] [Google Scholar]
- [58].Zhao Z, Li Y, Wang M, et al. Mechanotransduction pathways in the regulation of cartilage chondrocyte homoeostasis. J Cell Mol Med. 2020. May;24(10):5408–5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. [DOI] [PubMed] [Google Scholar]
- [60].Barrett-Jolley R, Lewis R, Fallman R, et al. The emerging chondrocyte channelome. Front Physiol. 2010;1:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lee W, Guilak F, Liedtke W. Role of piezo channels in joint health and injury. Curr Top Membr. 2017;79:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Nourse JL, Pathak MM. How cells channel their stress: interplay between Piezo1 and the cytoskeleton. Semin Cell Dev Biol. 2017. November;71:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Trujillo E, Alvarez De La Rosa D, Mobasheri A, et al. Sodium transport systems in human chondrocytes. II. Expression of ENaC, Na+/K+/2Cl- cotransporter and Na+/H+ exchangers in healthy and arthritic chondrocytes. Histol Histopathol. 1999. October;14(4):1023–1031. [DOI] [PubMed] [Google Scholar]
- [64].Millward-Sadler SJ, Wright MO, Lee H, et al. Altered electrophysiological responses to mechanical stimulation and abnormal signalling through alpha5beta1 integrin in chondrocytes from osteoarthritic cartilage. Osteoarthritis Cartilage. 2000. July;8(4):272–278. [DOI] [PubMed] [Google Scholar]
- [65].Salter DM, Millward-Sadler SJ, Nuki G, et al. Integrin–interleukin-4 mechanotransduction pathways in human chondrocytes. Clin Orthop Relat Res. 2001;391:49–60. [DOI] [PubMed] [Google Scholar]
- [66].Knight MM, Toyoda T, Lee DA, et al. Mechanical compression and hydrostatic pressure induce reversible changes in actin cytoskeletal organisation in chondrocytes in agarose. J Biomech. 2006;39(8):1547–1551. . [DOI] [PubMed] [Google Scholar]
- [67].Zhang M, Wang JJ, Chen YJ. Effects of mechanical pressure on intracellular calcium release channel and cytoskeletal structure in rabbit mandibular condylar chondrocytes. Life Sci. 2006. April 18;78(21):2480–2487. [DOI] [PubMed] [Google Scholar]
- [68].Liu D, Yi C, Wang K, et al. Reorganization of cytoskeleton and transient activation of Ca2+ channels in mesenchymal stem cells cultured on silicon nanowire arrays. ACS Appl Mater Interfaces. 2013. December 26;5(24):13295–13304. [DOI] [PubMed] [Google Scholar]
- [69].Shakibaei M, Mobasheri A. Beta1-integrins co-localize with Na, K-ATPase, epithelial sodium channels (ENaC) and voltage activated calcium channels (VACC) in mechanoreceptor complexes of mouse limb-bud chondrocytes. Histol Histopathol. 2003. April;18(2):343–351. [DOI] [PubMed] [Google Scholar]
- [70].Mobasheri A, Barrett-Jolley R, Shakibaei M, et al. Enigmatic roles of the epithelial sodium channel (ENaC) in articular chondrocytes and osteoblasts: mechanotransduction, sodium transport or extracellular sodium sensing? In: Kamkin A, Kiseleva I, editors. Mechanosensitivity in Cells and Tissues. Moscow: Academia Copyright © 2005, Academia Publishing House Ltd; 2005. [PubMed] [Google Scholar]
- [71].Abdul Kadir L, Stacey M, Barrett-Jolley R. Emerging roles of the membrane potential: action beyond the action potential. Front Physiol. 2018;9:1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hammami S, Willumsen NJ, Meinild AK, et al. Purinergic signalling - a possible mechanism for KCNQ1 channel response to cell volume challenges. Acta Physiol. 2013. March;207(3):503–515. [DOI] [PubMed] [Google Scholar]
- [73].Fodor J, Matta C, Olah T, et al. Store-operated calcium entry and calcium influx via voltage-operated calcium channels regulate intracellular calcium oscillations in chondrogenic cells. Cell Calcium. 2013. July;54(1):1–16. [DOI] [PubMed] [Google Scholar]
- [74].Inayama M, Suzuki Y, Yamada S, et al. Orai1-Orai2 complex is involved in store-operated calcium entry in chondrocyte cell lines. Cell Calcium. 2015. May;57(5–6):337–347. [DOI] [PubMed] [Google Scholar]
- [75].Gong X, Li G, Huang Y, et al. Synergistically regulated spontaneous calcium signaling is attributed to cartilaginous extracellular matrix metabolism. J Cell Physiol. 2019. June;234(6):9711–9722. [DOI] [PubMed] [Google Scholar]
- [76].Lunz V, Romanin C, Frischauf I. STIM1 activation of Orai1. Cell Calcium. 2019. January;77:29–38. [DOI] [PubMed] [Google Scholar]
- [77].Liu S, Takahashi M, Kiyoi T, et al. Genetic manipulation of calcium release-activated calcium channel 1 modulates the multipotency of human cartilage-derived mesenchymal stem cells. J Immunol Res. 2019;2019:7510214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Somogyi CS, Matta C, Foldvari Z, et al. Polymodal transient receptor potential vanilloid (TRPV) ion channels in chondrogenic cells. Int J Mol Sci. 2015. August 7;16(8):18412–18438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].McNulty AL, Leddy HA, Liedtke W, et al. TRPV4 as a therapeutic target for joint diseases. Naunyn-Schmiedeberg’s Arch Pharmacol. 2015. April;388(4):437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sanchez JC, Lopez-Zapata DF. The role of BKCa channels on hyperpolarization mediated by hyperosmolarity in human articular chondrocytes. Gen Physiol Biophys. 2011. March;30(1):20–27. [DOI] [PubMed] [Google Scholar]
- [81].Kobayakawa T, Takahashi N, Sobue Y, et al. Mechanical stress loading induces CD44 cleavage in human chondrocytic HCS-2/8 cells. Biochem Biophys Res Commun. 2016. September 23;478(3):1230–1235. [DOI] [PubMed] [Google Scholar]
- [82].Parisi C, Chandaria VV, Nowlan NC. Blocking mechanosensitive ion channels eliminates the effects of applied mechanical loading on chick joint morphogenesis. Philos Trans R Soc Lond B Biol Sci. 2018. September 24;373(1759):20170317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Mobasheri A, Matta C, Uzieliene I, et al. The chondrocyte channelome: a narrative review. Joint Bone Spine. 2019. January;86(1):29–35. [DOI] [PubMed] [Google Scholar]
- [84].Karamesinis K, Spyropoulou A, Dalagiorgou G, et al. Continuous hydrostatic pressure induces differentiation phenomena in chondrocytes mediated by changes in polycystins, SOX9, and RUNX2. J Orofac Orthop. 2017. January;78(1):21–31. [DOI] [PubMed] [Google Scholar]
- [85].Du G, Li L, Zhang X, et al. Roles of TRPV4 and piezo channels in stretch-evoked Ca(2+) response in chondrocytes. Exp Biol Med. 2020. February;245(3):180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lv M, Zhou Y, Chen X, et al. Calcium signaling of in situ chondrocytes in articular cartilage under compressive loading: roles of calcium sources and cell membrane ion channels. J Orthop Res. 2018. February;36(2):730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Yellowley CE, Jacobs CR, Zy LI, et al. Effects of fluid flow on intracellular calcium in bovine articular chondrocytes. Physiology. 1997. [DOI] [PubMed] [Google Scholar]
- [88].Perkins GL, Derfoul A, Ast A, et al. An inhibitor of the stretch-activated cation receptor exerts a potent effect on chondrocyte phenotype. Differentiation. 2005;73:199–211. [DOI] [PubMed] [Google Scholar]
- [89].Ponce A, Jimenez-Peña L, Tejeda-Guzman C. The role of swelling-activated chloride currents (I(CL,swell)) in the regulatory volume decrease response of freshly dissociated rat articular chondrocytes. Cell Physiol Biochem. 2012;30(5):1254–1270. [DOI] [PubMed] [Google Scholar]
- [90].Wang Z, Irianto J, Kazun S, et al. The rate of hypo-osmotic challenge influences regulatory volume decrease (RVD) and mechanical properties of articular chondrocytes. Osteoarthritis Cartilage. 2015. February;23(2):289–299. [DOI] [PubMed] [Google Scholar]
- [91].Yellowley CE, Hancox JC, Donahue HJ. Effects of cell swelling on intracellular calcium and membrane currents in bovine articular chondrocytes. J Cell Biochem. 2002;86(2):290–301. [DOI] [PubMed] [Google Scholar]
- [92].Sanchez JC, Wilkins RJ. Changes in intracellular calcium concentration in response to hypertonicity in bovine articular chondrocytes. Comp Biochem Physiol Part A Mol Integr Physiol. 2004. January;137(1):173–182. [DOI] [PubMed] [Google Scholar]
- [93].Wu QQ, Chen Q. Mechanoregulation of chondrocyte proliferation, maturation, and hypertrophy: ion-channel dependent transduction of matrix deformation signals. Exp Cell Res. 2000. May 1;256(2):383–391. [DOI] [PubMed] [Google Scholar]
- [94].Sánchez JC, Wilkins RJ. Effects of hypotonic shock on intracellular pH in bovine articular chondrocytes. Comp Biochem Physiol Part A. 2003;135(4):575–583. [DOI] [PubMed] [Google Scholar]
- [95].Sánchez JC, Powell T, Staines HM, et al. Electrophysiological demonstration of voltage-activated H+ channels in bovine articular chondrocytes. Cell Physiol Biochem. 2006;18:85–90. [DOI] [PubMed] [Google Scholar]
- [96].Mobasheri A, Gent TC, Womack MD, et al. Quantitative analysis of voltage-gated potassium currents from primary equine (Equus caballus) and elephant (Loxodonta africana) articular chondrocytes. Am J Physiol Regul Integr Comp Physiol. 2005. July;289(1):R172–80. [DOI] [PubMed] [Google Scholar]
- [97].Funabashi K, Ohya S, Yamamura H, et al. Accelerated Ca2+ entry by membrane hyperpolarization due to Ca2+-activated K+ channel activation in response to histamine in chondrocytes. Am J Physiol Cell Physiol. 2010. April;298(4):C786–97. [DOI] [PubMed] [Google Scholar]
- [98].Mobasheri A, Lewis R, Maxwell JE, et al. Characterization of a stretch-activated potassium channel in chondrocytes. J Cell Physiol. 2010. May;223(2):511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Clausen MV, Jarerattanachat V, Carpenter EP, et al. Asymmetric mechanosensitivity in a eukaryotic ion channel. Proc Natl Acad Sci U S A. 2017. October 3;114(40):E8343–e8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Sanchez JC, Lopez-Zapata DF. Effects of osmotic challenges on membrane potential in human articular chondrocytes from healthy and osteoarthritic cartilage. Biorheology. 2010;47(5–6):321–331. [DOI] [PubMed] [Google Scholar]
- [101].Hdud IM, Mobasheri A, Loughna PT. Effect of osmotic stress on the expression of TRPV4 and BKCa channels and possible interaction with ERK1/2 and p38 in cultured equine chondrocytes. Am J Physiol Cell Physiol. 2014. June 1;306(11):C1050–7. [DOI] [PubMed] [Google Scholar]
- [102].Liedtke W. TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. J Physiol. 2005. August 15;567(Pt 1):53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. 2011. December;25(6):815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Xu B, Xing R, Huang Z, et al. Excessive mechanical stress induces chondrocyte apoptosis through TRPV4 in an anterior cruciate ligament-transected rat osteoarthritis model. Life Sci. 2019. July 1;228:158–166. [DOI] [PubMed] [Google Scholar]
- [105].Lee W, Leddy HA, Chen Y, et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci U S A. 2014;111:5114–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Apte SS. Anti-ADAMTS5 monoclonal antibodies: implications for aggrecanase inhibition in osteoarthritis. Biochem J. 2016. January 1;473(1):e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Yang CY, Chanalaris A, Troeberg L. ADAMTS and ADAM metalloproteinases in osteoarthritis - looking beyond the ‘usual suspects’. Osteoarthritis Cartilage. 2017. July;25(7):1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Zelenski NA, Leddy HA, Sanchez-Adams J, et al. Type VI collagen regulates pericellular matrix properties, chondrocyte swelling, and mechanotransduction in mouse articular cartilage. Arthritis Rheumatol. 2015. May;67(5):1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Li C, Cao Z, Li W, et al. A review on the wide range applications of hyaluronic acid as a promising rejuvenating biomacromolecule in the treatments of bone related diseases. Int J Biol Macromol. 2020. December 15;165(Pt A):1264–1275. [DOI] [PubMed] [Google Scholar]
- [110].Kodama K, Takahashi H, Oiji N, et al. CANT1 deficiency in a mouse model of Desbuquois dysplasia impairs glycosaminoglycan synthesis and chondrocyte differentiation in growth plate cartilage. FEBS Open Bio. 2020. June;10(6):1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Eleswarapu SV, Athanasiou KA. TRPV4 channel activation improves the tensile properties of self-assembled articular cartilage constructs. Acta Biomater. 2013. March;9(3):5554–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Fu S, Meng H, Inamdar S, et al. Activation of TRPV4 by mechanical, osmotic or pharmaceutical stimulation is anti-inflammatory blocking IL-1β mediated articular cartilage matrix destruction. Osteoarthritis Cartilage. 2021. January;29(1):89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Lewis R, Feetham CH, Barrett-Jolley R. Cell volume regulation in chondrocytes. Cell Physiol Biochem. 2011;28(6):1111–1122. [DOI] [PubMed] [Google Scholar]
- [114].Ogawa Y, Takahashi N, Takemoto T, et al. Hyaluronan promotes TRPV4-induced chondrogenesis in ATDC5 cells. PloS One. 2019;14(8):e0219492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Garcia-Elias A, Mrkonjic S, Jung L, et al. The TRPV4 channel. Cation Channels. 2014:293–319. [DOI] [PubMed] [Google Scholar]
- [116].O’Conor CJ, Ramalingam S, Zelenski NA, et al. Cartilage-specific knockout of the mechanosensory ion channel trpv4 decreases age-related osteoarthritis. Sci Rep. 2016. July 8;6:29053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].O’Conor CJ, Griffin TM, Liedtke W, et al. Increased susceptibility of Trpv4-deficient mice to obesity and obesity-induced osteoarthritis with very high-fat diet. Ann Rheum Dis. 2013. February;72(2):300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Darby WG, Grace MS, Baratchi S, et al. Modulation of TRPV4 by diverse mechanisms. Int J Biochem Cell Biol. 2016. September;78:217–228. [DOI] [PubMed] [Google Scholar]
- [119].Xing R, Wang P, Zhao L, et al. Mechanism of TRPA1 and TRPV4 participating in mechanical hyperalgesia of rat experimental knee osteoarthritis. Arch Rheumatol. 2017. June;32(2):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Krupkova O, Zvick J, Wuertz-Kozak K. The role of transient receptor potential channels in joint diseases. Eur Cell Mater. 2017. 10;Oct(34):180–201. [DOI] [PubMed] [Google Scholar]
- [121].Richter F, Segond Von Banchet G, Schaible HG. Transient receptor potential vanilloid 4 ion channel in C-fibres is involved in mechanonociception of the normal and inflamed joint. Sci Rep. 2019. July 29;9(1):10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zhang XF, Chen J, Faltynek CR, et al. Transient receptor potential A1 mediates an osmotically activated ion channel. Eur J Neurosci. 2008. February;27(3):605–611. [DOI] [PubMed] [Google Scholar]
- [123].Moilanen LJ, Hamalainen M, Nummenmaa E, et al. Monosodium iodoacetate-induced inflammation and joint pain are reduced in TRPA1 deficient mice–potential role of TRPA1 in osteoarthritis. Osteoarthritis Cartilage. 2015. November;23(11):2017–2026. [DOI] [PubMed] [Google Scholar]
- [124].Nummenmaa E, Hämäläinen M, Pemmari A, et al. Transient receptor potential ankyrin 1 (TRPA1) is involved in upregulating interleukin-6 expression in osteoarthritic chondrocyte models. Int J Mol Sci. 2020. December 23;22:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Lewis R, Asplin KE, Bruce G, et al. The role of the membrane potential in chondrocyte volume regulation. J Cell Physiol. 2011. November;226(11):2979–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Lewis AH, Grandl J. Inactivation kinetics and mechanical gating of Piezo1 ion channels depend on subdomains within the cap. Cell Rep. 2020. January 21;30(3):870–880.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Yang QN, Cao Y, Zhou YW, et al. Expression characteristics of Piezo1 protein in stress models of human degenerative chondrocytes. China J Orthop Traumato. 2018:550–555. [DOI] [PubMed] [Google Scholar]
- [128].Ridone P, Pandzic E, Vassalli M, et al. Disruption of membrane cholesterol organization impairs the activity of PIEZO1 channel clusters. J Gen Physiol. 2020. August 3;152(8). DOI: 10.1085/jgp.201912515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Brohawn SG, Su Z, MacKinnon R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc Natl Acad Sci U S A. 2014. March 4;111(9):3614–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Syeda R, Florendo MN, Cox CD, et al. Piezo1 channels are inherently mechanosensitive. Cell Rep. 2016. November 8;17(7):1739–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Kefauver JM, Ward AB, Patapoutian A. Discoveries in structure and physiology of mechanically activated ion channels. Nature. 2020. November;587(7835):567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Lewis AH, Cui AF, McDonald MF, et al. Transduction of repetitive mechanical stimuli by Piezo1 and Piezo2 ion channels. Cell Rep. 2017. June 20;19(12):2572–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Ellefsen KL, Holt JR, Chang AC, et al. Myosin-II mediated traction forces evoke localized Piezo1-dependent Ca(2+) flickers. Commun Biol. 2019;2:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Shin KC, Park HJ, Kim JG, et al. The Piezo2 ion channel is mechanically activated by low-threshold positive pressure. Sci Rep. 2019. April 23;9(1):6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Zheng W, Nikolaev YA, Gracheva EO, et al. Piezo2 integrates mechanical and thermal cues in vertebrate mechanoreceptors. Proc Natl Acad Sci U S A. 2019. August 27;116(35):17547–17555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Zhou T, Gao B, Fan Y, et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-β-catenin. Elife. 2020. March 18;9. DOI: 10.7554/eLife.52779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Li XF, Zhang Z, Chen ZK, et al. Piezo1 protein induces the apoptosis of human osteoarthritis-derived chondrocytes by activating caspase-12, the signaling marker of ER stress. Int J Mol Med. 2017. September;40(3):845–853. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [138].Lawrence KM, Jones RC, Jackson TR, et al. Chondroprotection by urocortin involves blockade of the mechanosensitive ion channel Piezo1. Sci Rep. 2017. July 11;7(1):5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Millward-Sadler SJ, Wright MO, Flatman PW, et al. ATP in the mechanotransduction pathway of normal human chondrocytes. Biorheology. 2004;41(3–4):567–575. [PubMed] [Google Scholar]
- [140].Wei L, Mousawi F, Li D, et al. Adenosine triphosphate release and P2 receptor signaling in Piezo1 channel-dependent mechanoregulation. Front Pharmacol. 2019;10:1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Zeng D, Yao P, Zhao H. P2X7, a critical regulator and potential target for bone and joint diseases. J Cell Physiol. 2019. March;234(3):2095–2103. [DOI] [PubMed] [Google Scholar]
- [142].Shimazaki A, Wright MO, Elliot K, et al. Calcium/calmodulin-dependent protein kinase II in human articular chondrocytes. Biorheology. 2006;43(3,4):223–233. [PubMed] [Google Scholar]
- [143].Ugawa S, Ishida Y, Ueda T, et al. Hypotonic stimuli enhance proton-gated currents of acid-sensing ion channel-1b. Biochem Biophys Res Commun. 2008. March 14;367(3):530–534. [DOI] [PubMed] [Google Scholar]
- [144].Yamamura H, Suzuki Y, Imaizumi Y. Physiological and pathological functions of Cl(-) channels in chondrocytes. Biol Pharm Bull. 2018;41(8):1145–1151. [DOI] [PubMed] [Google Scholar]
- [145].Tian M, Duan Y, Duan X. Chloride channels regulate chondrogenesis in chicken mandibular mesenchymal cells. Arch Oral Biol. 2010. December;55(12):938–945. [DOI] [PubMed] [Google Scholar]
- [146].Kittl M, Winklmayr M, Helm K, et al. Acid- and volume-sensitive chloride currents in human chondrocytes. Front Cell Dev Biol. 2020;8:583131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Isoya E, Toyoda F, Imai S, et al. Swelling-activated Cl(-) current in isolated rabbit articular chondrocytes: inhibition by arachidonic acid. J Pharmacol Sci. 2009. February;109(2):293–304. [DOI] [PubMed] [Google Scholar]
- [148].Tsuga K, Tohse N, Yoshino M, et al. Chloride conductance determining membrane potential of rabbit articular chondrocytes. J Membr Biol. 2002. January 1;185(1):75–81. [DOI] [PubMed] [Google Scholar]
- [149].Yamada S, Suzuki Y, Bernotiene E, et al. Swelling-activated ClC-3 activity regulates prostaglandin E(2) release in human OUMS-27 chondrocytes. Biochem Biophys Res Commun. 2021. January 22;537:29–35. [DOI] [PubMed] [Google Scholar]
- [150].Kumagai K, Toyoda F, Staunton CA, et al. Activation of a chondrocyte volume-sensitive Cl(-) conductance prior to macroscopic cartilage lesion formation in the rabbit knee anterior cruciate ligament transection osteoarthritis model. Osteoarthritis Cartilage. 2016. October;24(10):1786–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Kurita T, Yamamura H, Suzuki Y, et al. The ClC-7 chloride channel is downregulated by hypoosmotic stress in human chondrocytes. Mol Pharmacol. 2015. July;88(1):113–120. [DOI] [PubMed] [Google Scholar]
- [152].Okumura N, Imai S, Toyoda F, et al. Regulatory role of tyrosine phosphorylation in the swelling-activated chloride current in isolated rabbit articular chondrocytes. J Physiol. 2009. August 1;587(Pt 15):3761–3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Lewis R, Feetham CH, Gentles L, et al. Benzamil sensitive ion channels contribute to volume regulation in canine chondrocytes. Br J Pharmacol. 2013. April;168(7):1584–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Gessmann R, Kourtis N, Petratos K, et al. Molecular modeling of mechanosensory ion channel structural and functional features. PloS One. 2010. September 16;5(9):e12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Sabaratnam S, Arunan V, Coleman PJ, et al. Size selectivity of hyaluronan molecular sieving by extracellular matrix in rabbit synovial joints. J Physiol. 2005. September 1;567(Pt 2):569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Sabaratnam S, Coleman PJ, Mason RM, et al. Interstitial matrix proteins determine hyaluronan reflection and fluid retention in rabbit joints: effect of protease. J Physiol. 2007. January 1;578(Pt 1):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Wuest SL, Calio M, Wernas T, et al. Influence of mechanical unloading on articular chondrocyte dedifferentiation. Int J Mol Sci. 2018;19(5):1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Nomura M, Sakitani N, Iwasawa H, et al. Thinning of articular cartilage after joint unloading or immobilization. An experimental investigation of the pathogenesis in mice. Osteoarthritis Cartilage. 2017. May;25(5):727–736. [DOI] [PubMed] [Google Scholar]
- [159].Bertram KL, Banderali U, Tailor P, et al. Ion channel expression and function in normal and osteoarthritic human synovial fluid progenitor cells. Channels. 2016;10(2):148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Suzuki Y, Ohya S, Yamamura H, et al. A new splice variant of large conductance Ca2+-activated K+ (BK) channel alpha subunit alters human chondrocyte function. J Biol Chem. 2016. November 11;291(46):24247–24260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].He BH, Christin M, Mouchbahani-Constance S, et al. Mechanosensitive ion channels in articular nociceptors drive mechanical allodynia in osteoarthritis. Osteoarthritis Cartilage. 2017. December;25(12):2091–2099. [DOI] [PubMed] [Google Scholar]
- [162].Raouf R, Lolignier S, Sexton JE, et al. Inhibition of somatosensory mechanotransduction by annexin A6. Sci Signal. 2018;11(535):eaao2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Ikeuchi M, Izumi M, Aso K, et al. Effects of intra-articular hyaluronic acid injection on immunohistochemical characterization of joint afferents in a rat model of knee osteoarthritis. Eur J Pain. 2015. March;19(3):334–340. [DOI] [PubMed] [Google Scholar]
- [164].Kuduk SD, Di Marco CN, Bodmer-Narkevitch V, et al. Synthesis, structure-activity relationship, and pharmacological profile of analogs of the ASIC-3 inhibitor A-317567. ACS Chem Neurosci. 2010. January 20;1(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Izumi M, Ikeuchi M, Ji Q, et al. Local ASIC3 modulates pain and disease progression in a rat model of osteoarthritis. J Biomed Sci. 2012. August 21;19(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Schuelert N, Zhang C, Mogg AJ, et al. Paradoxical effects of the cannabinoid CB2 receptor agonist GW405833 on rat osteoarthritic knee joint pain. Osteoarthritis Cartilage. 2010. November;18(11):1536–1543. [DOI] [PubMed] [Google Scholar]
- [167].Lewis R, Barrett-Jolley R. Changes in membrane receptors and ion channels as potential biomarkers for osteoarthritis. Front Physiol. 2015;6:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Cox CD, Bavi N, Martinac B. Biophysical principles of ion-channel-mediated mechanosensory transduction. Cell Rep. 2019. October 1;29(1):1–12. [DOI] [PubMed] [Google Scholar]
- [169].Uddin SMZ, Komatsu DE. Therapeutic potential low-intensity pulsed ultrasound for osteoarthritis: pre-clinical and clinical perspectives. Ultrasound Med Biol. 2020. April;46(4):909–920. [DOI] [PubMed] [Google Scholar]
- [170].Nasb M, Liangjiang H, Gong C, et al. Human adipose-derived Mesenchymal stem cells, low-intensity pulsed ultrasound, or their combination for the treatment of knee osteoarthritis: study protocol for a first-in-man randomized controlled trial. BMC Musculoskelet Disord. 2020. January 15;21(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]


