Abstract
Viral nanotechnology exploits the prefabricated nanostructures of viruses, which are already abundant in nature. With well-defined molecular architectures, viral nanocarriers offer unprecedented opportunities for precise structural and functional manipulation using genetic engineering and/or bio-orthogonal chemistries. In this manner, they can be loaded with diverse molecular payloads for targeted delivery. Mammalian viruses are already established in the clinic for gene therapy and immunotherapy, and inactivated viruses or virus-like particles have long been used as vaccines. More recently, plant viruses and bacteriophages have been developed as nanocarriers for diagnostic imaging, vaccine and drug delivery, and combined diagnosis/therapy (theranostics). The first wave of these novel virus-based tools has completed clinical development and is poised to make an impact on clinical practice.
Keywords: viral nanotechnology, plant viruses, bacteriophages, virus-like particles, drug delivery, imaging, vaccines, immunotherapy, nanomedicine
1. INTRODUCTION TO VIRAL NANOTECHNOLOGY
Nanomedicine is the use of nanoscale materials for medical applications. Some nanomedical formulations have already progressed through clinical development and are now approved in humans or companion animals (1); however, development and testing of novel nanomaterials are critical for further advancement of the field. These nanocarrier-based formulations are designed to optimize the delivery of diagnostic and/or therapeutic agents. This is achieved by improving the solubility, stability, and/or bioavailability of the cargo or by targeting disease sites and thus reducing off-target systemic toxicity. However, the nanocarrier may also play an active role by interacting with the immune system and enhancing its response to a vaccine. In the case of therapeutic vaccines and immunotherapies, the nanocarrier may even play a direct role in the eradication of an established disease. The development pipeline features several broad categories of nanocarriers, including liposomes, protein scaffolds, polymer micelles, dendrimers, and inorganic/metallic nanoparticles, with an even more diverse payload repertoire encompassing small-molecule drugs, nucleic acids, biologics, and contrast agents (2).
One promising category of nanocarriers is based on viruses. These ubiquitous nucleoprotein particles are naturally occurring nanomaterials that have perfected the art of cargo delivery to specific host cells during evolution, making them ideal for the development of nanocarriers. The size and shape of viruses vary according to the species, although most fall within the range of 20–500 nm with icosahedral or helical structural configurations. Helical viruses can be flexible filaments or rigid rods, whereas other viruses have more complex head-and-tail structures or outer envelopes (Figure 1).
Figure 1.
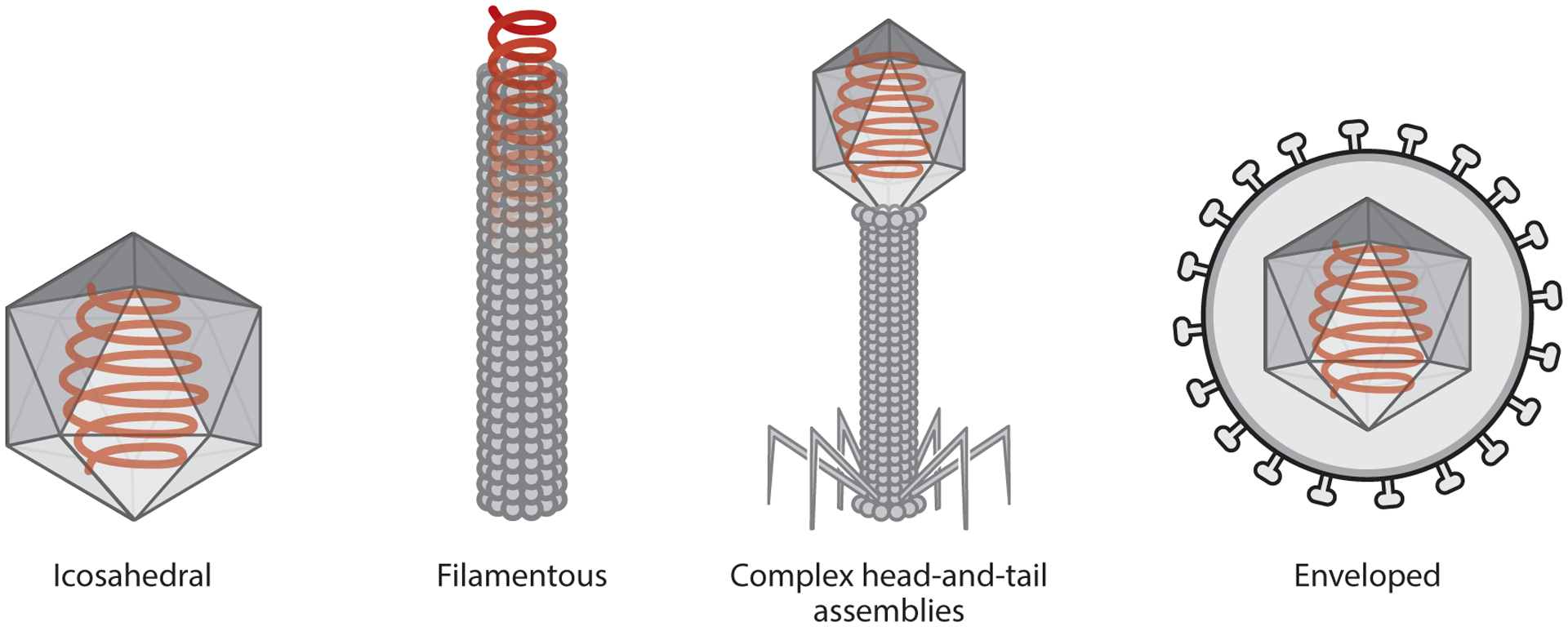
The structure of a virus depends on the species, and there is great diversity in nature, but the four basic configurations are icosahedral, filamentous (rigid or flexible), head-and-tail assemblies, and enveloped viruses.
Viruses have been conventionally used as vaccines, occasionally in their native form but more often as inactivated (e.g., heat-killed or chemically inactivated) or otherwise attenuated preparations and more recently as subunit vaccines. The development of viruses as delivery vehicles has broadened their applications in medicine and beyond, including in materials science (3, 4). In addition to their natural ability to deliver cargo to target cells, the replication of viruses in their natural host(s) or heterologous expression systems offers a low-cost production platform. Furthermore, the structure of the viral nucleocapsid is genetically encoded, which means that viral preparations are clonally identical. This provides an inbuilt quality control step that cannot yet be matched by synthetic materials (5). Finally, the nucleoprotein architecture of viruses is amenable to structural alterations introduced by genetic engineering (6). The endogenous structure can be modified through addition, deletion, or replacement of amino acids to alter the surface charge, or to introduce reactive side chains for chemical conjugation or for introduction of specific ligands or epitopes. Once optimized, such genetically engineered viruses can be replicated to make identical copies without batch-to-batch variations, just like the native virus. The modification of amino acid side chains can be achieved using various bio-orthogonal chemistries, allowing the conjugation of diverse ligands and small-molecule reagents to the external or internal surfaces, in some cases simultaneously (7, 8). The structural homogeneity and robustness of viruses ensures that chemical modifications can be introduced with atomic precision and the modified particles have a long shelf life, in some cases remaining stable at room temperature for years (this is particularly evident for plant viruses and bacteriophages) (9–12).
The medical applications of mammalian viruses are based on host-specific infection, replication, and propagation. Therefore, they cater to a highly specific niche of gene therapy and virotherapy (13). However, mammalian viruses pose the risk of undesirable events such as reversion of attenuated versions to virulent forms (e.g., by mutation and/or recombination) or the integration of partial or complete virus sequences into the host genome. These drawbacks can be overcome by the use of plant viruses and bacteriophages, which cannot infect or replicate within mammalian cells and therefore provide nanocarriers with inbuilt safety. However, plant viruses and bacteriophages are still perceived as foreign antigens by the mammalian immune system and are therefore immunogenic. This can be advantageous in certain immunotherapy scenarios, but undesirable immunogenicity can be avoided by shielding the particles with molecules such as polyethylene glycol (PEG), a strategy also used with synthetic nanocarriers (14).
In this review, we highlight recent developments in the use of viral nanocarriers for the delivery of vaccines, drugs, and imaging reagents and in next-generation immunotherapy. We focus on plant viruses and bacteriophages but also draw on informative examples involving mammalian viruses and nonviral protein nanoparticles.
2. VIRAL NANOCARRIERS FOR THE DELIVERY OF SMALL MOLECULES, PROTEINS, AND NUCLEIC ACID THERAPEUTICS
Viruses that have been repurposed to carry a medically relevant payload are defined as viral nanoparticles (VNPs) if they retain the virus genome or virus-like particles (VLPs) if the genome is absent (15). As depicted in Figure 2, the molecular cargo can be tethered to the external or internal surface of the capsid by covalent or noncovalent interactions with the coat proteins (8, 16, 17) or can be packaged inside the capsid by programmed self-assembly (18, 19) or infusion (20, 21).
Figure 2.
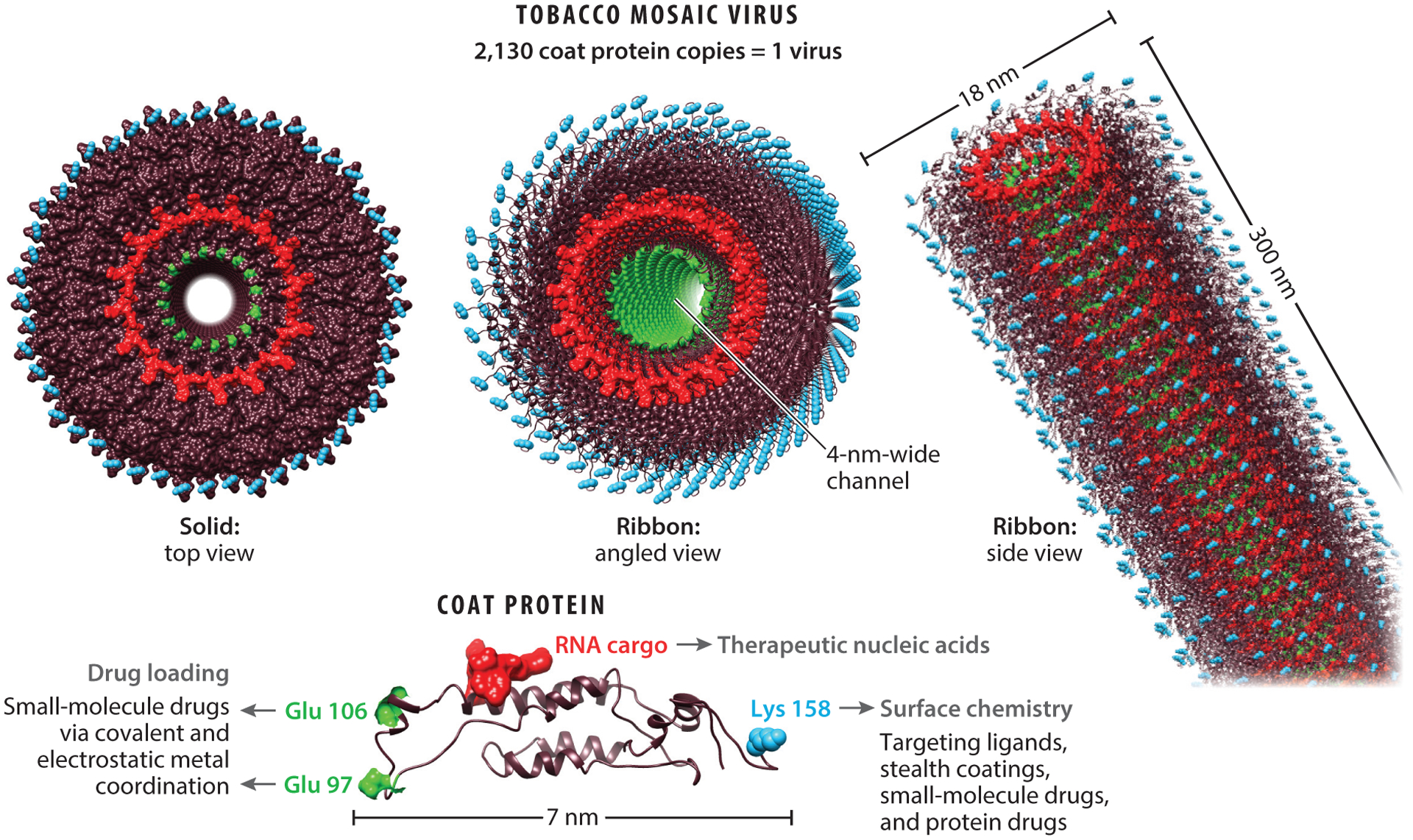
Gene and drug delivery by tobacco mosaic virus (TMV). Similar design and engineering concepts have been established for various filamentous and icosahedral plant viruses and bacteriophages.
2.1. Viral Nanocarriers for Drug Delivery
Viral nanocarriers have been developed for the delivery of diverse therapeutic payloads including small-molecule drugs, protein drugs, enzymes (to convert prodrugs into their active ingredient), photosensitizers, and combinations thereof. In concert with targeting ligands, such as peptides, antibodies, and aptamers, these formulations improve the specificity and efficacy of treatment while minimizing systemic toxicity. Some prominent examples are discussed below.
The use of bacteriophages carrying small-molecule cancer drugs dates back more than 10 years. In one of the earliest studies, filamentous bacteriophage fUSE5 was genetically engineered to express the immunoglobulin G (IgG)-binding domain of staphylococcal protein A (the ZZ domain), and the resulting fUSE5-ZZ bacteriophage particles were loaded with hygromycin or doxorubicin by conjugation to peptide DFK (which is sensitive to the protease cathepsin B), allowing controlled drug release in target cells (22). The resulting particles could be targeted by mixing them with specific antibodies due to the high-affinity binding between the ZZ domain and IgG fragment crystallizable (Fc) domain. The particles were initially targeted using antibodies specific for two members of the epidermal growth factor receptor (EGFR) family, EGFR/ErbB-1 and HER2/neu/ErbB-2, resulting in specific interactions with cells displaying those receptors followed by receptor-mediated endocytosis, intracellular degradation of the virus carrier, and drug release. This increased the cytotoxicity of the payload by ~1,000-fold compared with the free drug (22). Similarly, bacteriophage M13 was loaded with doxorubicin via the DFK peptide tethered to the P8 coat protein and also achieved targeted drug delivery and enhanced efficacy (22). In an alternative approach, a core-shell nano-assembly comprising bacteriophage M13 conjugated to folic acid (thus targeting the folic acid receptor) and block copolymer PCL-P2VP for the entrapment of doxorubicin molecules was taken up by tumor cells and degraded in the acidic environment of the endosome to release the drug (23). In this case, the bacteriophage was not a drug carrier per se but a targeting reagent that associated with the hydrogel-like nano-assembly via multivalent interactions. Other bacteriophages, including MS2, lambda, and HK97, have also been used for targeted drug delivery, increasing the cytotoxicity of their payload at lower drug concentrations and reducing off-target effects (24, 25).
Drugs such as doxorubicin and cisplatin have also been delivered by plant viruses. One of the simplest formulations was based on potato virus X (PVX), which was able to deliver doxorubicin by exploiting hydrophobic interactions involving π−π stacking of the planar drug molecules and polar amino acids in the PVX coat protein (17). Targeted drug delivery systems have also been developed using VLPs derived from Johnson grass chlorotic stripe mosaic virus (JgCSMV) covalently modified on the external surface with folic acid as a targeting ligand and with doxorubicin infused into the central cavity (26). These concepts can be applied broadly to other drug targets and viral platforms. For example, the external surface of tobacco mosaic virus (TMV) has been modified with mannose or lactose as targeting ligands, and the central channel was loaded with cisplatin, exploiting the interaction between platinum and glutamic acid residues, 4,200 of which line the TMV central channel (27). Similarly, ~2,000 molecules of the platinum-based drug candidate phenanthriplatin were loaded into TMV using noncovalent interactions to achieve efficient delivery and antitumor activity against breast cancer xenografts in mice (20). The negative charge of the TMV channel has also been exploited to achieve the electrostatic coupling of nonplatinum drugs such as mitoxantrone, a type II topoisomerase inhibitor (28).
An additional beneficial aspect of viruses is their ability to adopt new conformations depending on the environment, meaning that the structure of VNPs and VLPs can be influenced by the salt concentration, pH, and temperature of the medium during assembly. The design space of TMV particles is particularly intriguing because self-assembly under different conditions can yield rods of a defined length, icosahedral capsids, and complex assemblies such as stars and boomerangs (29). Nanoparticle shape and size govern differential cellular interactions and pharmacokinetics in vivo (30, 31); therefore, such shape transitions can be used to meet customized delivery needs (32). For example, doxorubicin-loaded TMV nano-disks outperformed spherical and filamentous counterparts for the delivery of drugs to intracranial glioma in mice (33).
In addition to small-molecule drugs, high-aspect-ratio (aspect ratio of nanoparticles is defined as the ratio of length to width) VNPs and VLPs are particularly efficient for delivery of biologics (proteins and nucleic acids), which are challenging to deliver as free reagents because they are often targeted by the immune system. The rigid rods formed by TMV (300 × 18 nm) have the tendency to marginate when traveling through blood vessels, making them ideal for the treatment of vascular disease, such as targeting thrombi even without integrated molecular recognition chemistry (34). TMV has therefore been engineered to carry protein-based drugs such as streptokinase and tissue plasminogen activator, encouraging thrombus resolution and reducing the risk of bleeding (35, 36). Similarly, the flexible nanofilaments formed by PVX tend to concentrate in tumors and have been used to deliver tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), which binds to death receptors DR4 and DR5 overexpressed on cancer cells and induces caspase-dependent apoptosis (37). PVX-TRAIL was assembled by interactions between nickel-coordinated nitrilotriacetic acid modules displayed on the PVX surface and TRAIL with an N-terminal His6 tag, resulting in the accommodation of ~500 TRAIL molecules per PVX particle. The multivalent PVX-TRAIL formulation was significantly more efficacious than soluble TRAIL against human triple-negative breast cancer in an athymic mouse model (37). In this case, multivalent display (rather than delivery) enhanced drug efficacy by promoting the crosslinking of DR4/DR5 and strongly triggering the proapoptotic signaling pathway in cancer cells.
Proteins can also be encapsulated in viral cages, and this can be achieved with high precision. For example, bacteriophage P22 VLPs enable protein loading with controlled cargo stoichiometry and packaging density. In a recent example, streptavidin was linked to the P22 scaffold protein to program the assembly of a hybrid capsid consisting of the capsid protein and chimeric scaffolding protein, thus allowing the internal presentation of streptavidin. The assembly of a mixture of native and streptavidin-linked scaffold proteins allows the number of streptavidin molecules loaded per particle to be controlled precisely. This in turn provides control over the density of biotinylated molecular cargo that can be loaded in or tethered to the viral capsid (38).
Cancer drugs can be administered as inactive prodrugs to prevent off-target toxicity, relying on endogenous enzymes to convert them into active drugs. However, the efficacy of this approach is dependent on endogenous enzyme expression and activity, which vary between individuals and between cells in the same individual, especially in the heterogeneous tumor environment. To achieve consistent drug doses, viral nanocarriers containing enzymes have been developed to perform specific biochemical reactions in situ, and these can be targeted to tumor cells to avoid off-target effects caused by systemic prodrug conversion. For example, the programmed disassembly and reassembly of cowpea chlorotic mottle virus (CCMV) in the presence of cytochrome P450 (CYP) enzyme (39) and the differential expression of bacteriophage P22 coat proteins and CYP enzyme in Escherichia coli each resulted in the encapsidation of ~100 CYP molecules in the virus (40). When the P22-CYP particles were conjugated to folic acid, they achieved the targeted conversion of prodrug tamoxifen in tumor cells (40). More recently, complementary single-stranded nucleic acid tags were used to achieve the co-encapsidation of multiple enzymes in a single CCMV particle, resulting in the confinement of a functional enzymatic cascade (41).
Virus particles have also been proven useful for the delivery of photosensitizers used in photodynamic therapy (PDT) and photothermal therapy (PTT). In the former approach, a photosensitizer can be activated by light to produce reactive oxygen species that kill cells by inducing oxidative stress, whereas the latter achieves the same aim by triggering an increase in temperature. Photosensitizers must be incorporated into nanocarriers to improve their solubility and biocompatibility and to allow cell-specific targeting. For example, bacteriophage MS2 was modified internally with ~180 porphyrin molecules and externally with aptamers targeting Jurkat cells, resulting in the photo-dependent elimination of this cell population (42). CCMV-based PDT nanocarriers have been developed by assembling the viral coat proteins around phthalocyanine macrocycle dendrimers (43). Furthermore, the internal channel of TMV has been loaded with the photosensitizer 5-(4-ethynylphenyl)-10,15,20-tris(4-methylpyridin-4-ium-1-yl)porphyrin-zinc(II)triiodide (Zn-EpPor), allowing the uptake of the photosensitizer by endocytosis followed by endolysosomal drug release, thus enhancing the efficacy of PDT in melanoma cells compared with the free drug (44). PTT has been implemented via the programmed assembly of gold nanoparticles (AuNP) on the capsids of bacteriophage T7 carrying a ligand that targets prostate cancer cells. The T7-AuNP clusters were taken up by the target cells and localized within the endosomal compartments, resulting in targeted temperature increase that killed the cells efficiently in response to light (45). New developments in wirelessly activated implantable photonic devices (46, 47) can overcome the current limitations of delivering lights to deeper tissues for in vivo PDT/PTT using surgical or endoscopic insertion of optics fibers (48).
2.2. Phage Nanocarriers and Phage Therapy
Bacteriophages play a special role when targeting infectious diseases because they can act both as nanocarriers and as antibacterial agents in their own right. Filamentous phages were developed for the targeted delivery of antibiotics ~15 years ago, with early examples including particles loaded with chloramphenicol and neomycin to prevent the growth of E. coli, Streptococcus pyogenes, and Staphylococcus aureus (49, 50). Along these lines, a recent study demonstrated that conjugating azithromycine to bacteriophage Qβ enabled the trafficking and rapid accumulation of the antibiotic-laden VLPs in mouse lungs, and this could be developed as a strategy for pulmonary drug delivery (51). However, the prevalence of multidrug-resistant bacteria has increased the demand for new antimicrobial strategies because the development of new antibiotics can take more than 10 years from discovery to approval (52, 53). Phage therapy, which has long been applied in the ex-Soviet Bloc (54, 55), is now re-emerging in the West. The resurgence of phage therapy is attributed to improved knowledge of phage biology, phage interactions with the host immune system (56, 57), development of standard protocols (58), and technological advancements in production, purification, characterization, and handling (59). Recent developments include use of bacteriophage or phage cocktails in animal models against several clinically important pathogens including Pseudomonas aeruginosa (60), Clostridium difficile (61), multidrug-resistant E. coli O25:H4ST 131 strain (62), and Vibrio parahaemolyticus (63). Phage therapy has been also evaluated in human patients; for example, at the Eliava Institute of Bacteriophages in Georgia and the Institute of Immunology and Experimental Therapy in Poland, patients were treated with phages for antibiotic unresponsive diabetic foot ulcers (64), and at the University College London Ear Institute and the Royal National Throat, Nose and Ear Hospital in London (UK), patients received phage therapy to treat chronic otitis (65). More recently, at the University of California, San Diego, phage therapy was successfully used for the treatment of a faculty member infected with multidrug-resistant Acinetobacter baumannii who had failed to respond to 4 months of conventional antibiotic therapy (66). This was followed by the treatment of further patients (67, 68).
In addition to the use of wild-type bacteriophages, next-generation phage therapies use genetically modified bacteriophage. For example, removal of the immunity repressor gene 45 of bacteriophage ZoeJ, which is required for lysogeny, leads to improved lytic activity of the phage in Mycobacterium abscessus (69). Another new direction is the incorporation of bacteriophages into hydrogels to achieve sustained delivery, as recently demonstrated using bacteriophage PA5 to kill P. aeruginosa PA01 over prolonged treatment periods (70). Besides tackling antibody-resistant pathogens and overcoming adverse reactions, phage therapy offers several advantages. In contrast to antibiotics, phages can penetrate bacterial biofilms via enzymatic degradation (e.g., by extracellular polymeric substance depolymerase) of the biofilm (71, 72). Phages also show both species and strain specificity and are therefore less likely to affect the gut microbiome (73, 74). However, this strain specificity can also limit the general applicability of phage for infective wounds colonized by several strains of bacteria. Phage cocktails can overcome these shortcomings but present challenging issues that are beyond the scope of this review.
2.3. Viruses and Virus-Like Particles for Gene Therapy
Gene therapy involves the delivery of nucleic acids to disrupt, replace, or repair nonfunctional or dysfunctional genes, thus restoring the functionality of native proteins. Nearly 3,000 gene therapy clinical trials have been conducted thus far (75), several of which have led to approved products (Table 1). Because viruses evolved naturally to protect their nucleic acid cargo and shuttle it between cells, they remain the most efficient gene delivery vectors, accounting for nearly 70% of all gene therapy trials. The operational complexity and therapeutic efficacy of viral vectors remain unmatched (76–78). The major gene therapy vectors are based on adenoviruses, adeno-associated viruses, lentiviruses/retroviruses, and herpesviruses. Plant viruses and bacteriophage have also been developed as safer alternatives to mammalian viral vectors that carry the inherent risk of insertional mutagenesis and genotoxicity (79). Plant viruses and bacteriophages can also be produced inexpensively on a large scale in their native hosts or in heterologous production systems, avoiding the time-consuming and expensive cultivation of mammalian cells.
Table 1.
Approved gene therapies
| Trade name (generic name) | Viral vector | Delivered gene/modification | Condition | Reference |
|---|---|---|---|---|
| ZOLGENSMA (onasemnogene abeparvovec-xioi) | AAV9 | Survival motor neuron 1 | spinal muscular atrophy | 80 |
| LUXTURNA (voretigene neparvovec-rzyl) | AAV2 | RPE 65 | retinal dystrophy | 81 |
| Gendicine | adenovirus | TP53 | head and neck cancer | 82 |
| Strimveli | gamma-retrovirus | Adenosine deaminase | severe combined immunodeficiency | 83 |
| KYMRIAH (tisagenlecleucel) | lentivirus | genetically modified autologous T cells encoding an anti-CD19 chimeric antigen receptor | acute lymphoblastic leukemia | 84 |
| YESCARTA (axicabtagene ciloleucel) | retrovirus | large B cell lymphoma | 85 | |
| Zalmoxis | retrovirus | genetically modified allogeneic T cells encoding a truncated form of the human low affinity nerve growth factor receptor and the herpes simplex virus type 1 thymidine kinase | hematopoietic stem cell transplant | 85 |
| Zynteglo | lentivirus | genetically modified autologous CD34+ cells encoding β-globin gene (T87Q mutant) | transfusion-dependent β-thalassemia | 86 |
| Imlygic (talimogene laherparepvec/T-VEC) | herpes simplex virus type 1 | Granulocyte-macrophage colony-stimulating factor | advanced melanoma | 87 |
Abbreviation: AAV, adeno-associated virus.
A recent example of gene therapy involving a plant virus was the use of CCMV to deliver gfp messenger RNA (mRNA) [encoding green fluorescent protein (GFP)] to mammalian cells. The RNA cargo was stabilized by encapsulation and was released into the cytoplasm when co-delivered with the reagent Lipofectamine 2000 and then expressed, resulting in intracellular green fluorescence (88). CCMV has also been used to deliver mammalian replicons (89, 90), enabling the amplification and expression of constructs encoding a range of model antigens (91). Similarly, TMV was loaded with gfp mRNA as proof of concept for a vaccine delivery platform, resulting in an immune response to GFP in the immunized mice (92).
Nucleic acid therapeutics are not restricted to gene therapy and also extend to the delivery of small regulatory RNAs such as short interfering RNAs (siRNAs), microRNAs (miRNAs), and anti-miRNA oligonucleotides. Early proof-of-concept work showed that the cell-penetrating peptide M-lycotoxin L17E facilitated the uptake of CCMV VLPs loaded with siRNAs to knock down the expression of GFP and the forkhead box transcription factor FOXA1 (93). Similarly, TMV vectors displaying the human immunodeficiency virus (HIV) transacting activator of transduction (TAT) peptide (YGRKKRRQRRR) were able to deliver GFP-specific siRNA to mouse epidermal stem cells in vitro and metastatic hepatocellular carcinoma in vivo. The TAT peptide facilitated the lysosomal/endosomal escape of the vector, releasing the siRNA in the cytoplasm (94). In a recent study, a VLP/RNA interference nanocomplex based on bacteriophage Qβ was designed to downregulate the hepatocyte growth factor receptor gene (c-MET). In malignant brain tumor cells, hepatocyte growth factor binds to c-MET to protect cells from the damage caused by alkylating agents such as temozolomide (TMZ). The nanocarrier was modified to display a cell-penetrating peptide and an apolipoprotein E (ApoE) peptide, allowing passage across the blood-brain barrier. The suppression of c-MET expression worked synergistically with TMZ in mouse models of intracranial glioblastoma (95). The encapsulation of MS2 phage RNA is mediated by a 19-nucleotide stem-loop structure known as the pac site, which interacts with the coat protein. Therefore, nucleotides, drugs, and proteins conjugated to RNA molecules containing a pac site are efficiently packaged into the self-assembling capsid. Using this strategy, antisense RNA complementary to the p120 mRNA of myelogenous leukemia cells was packed into MS2 capsids displaying transferrin and delivered to leukemia cells expressing the transferrin receptor, increasing the cytotoxicity of the formulation compared with free antisense RNA (96).
Finally, mammalian viruses (97, 98) and bacteriophage P22 (99) have been used to facilitate gene editing with the powerful CRISPR/Cas9 system by shuttling the components into target cells. For example, bacteriophage P22 VLPs have been produced by expressing the coat protein and a truncated scaffold protein genetically fused to Cas9 in E. coli, followed by spontaneous self-assembly with the synthetic guide RNA (sgRNA). This co-expression system allowed the temporal decoupling of the cargo and capsid and facilitated the assembly of the scaffold-Cas9 fusion protein with the sgRNA cargo prior to coat protein expression, resulting in the encapsulation of ~20 Cas9 enzymes per capsid. The encapsulated Cas9 achieved target-specific double-stranded DNA cleavage and was protected from proteolysis (99).
2.4. Viruses and Virus-Like Particles for Molecular Imaging
Molecular imaging involves the use of markers or labels to highlight cellular or tissue-level structures, providing information about biological processes and also enabling early diagnoses, accurate prognoses, and personalized therapy (100). Ideal molecular imaging techniques achieve the optimal signal-to-noise ratio at the target site with minimal toxicity. Viruses carrying imaging reagents are advantageous because their short circulation and retention time make them easier to eliminate than synthetic nanoparticles. Viruses can be tailored to carry a wide range of contrast agents and/or fluorescent labels, and the addition of aptamers, peptides, or antibodies to the external surface of the virus allows the targeting of particular cells and tissues. Viral nanocarriers have therefore been developed for multiple imaging modalities, including fluorescence imaging, magnetic resonance imaging (MRI), positron emission tomography (PET), and computed tomography (CT) (101) (Figure 3).
Figure 3.
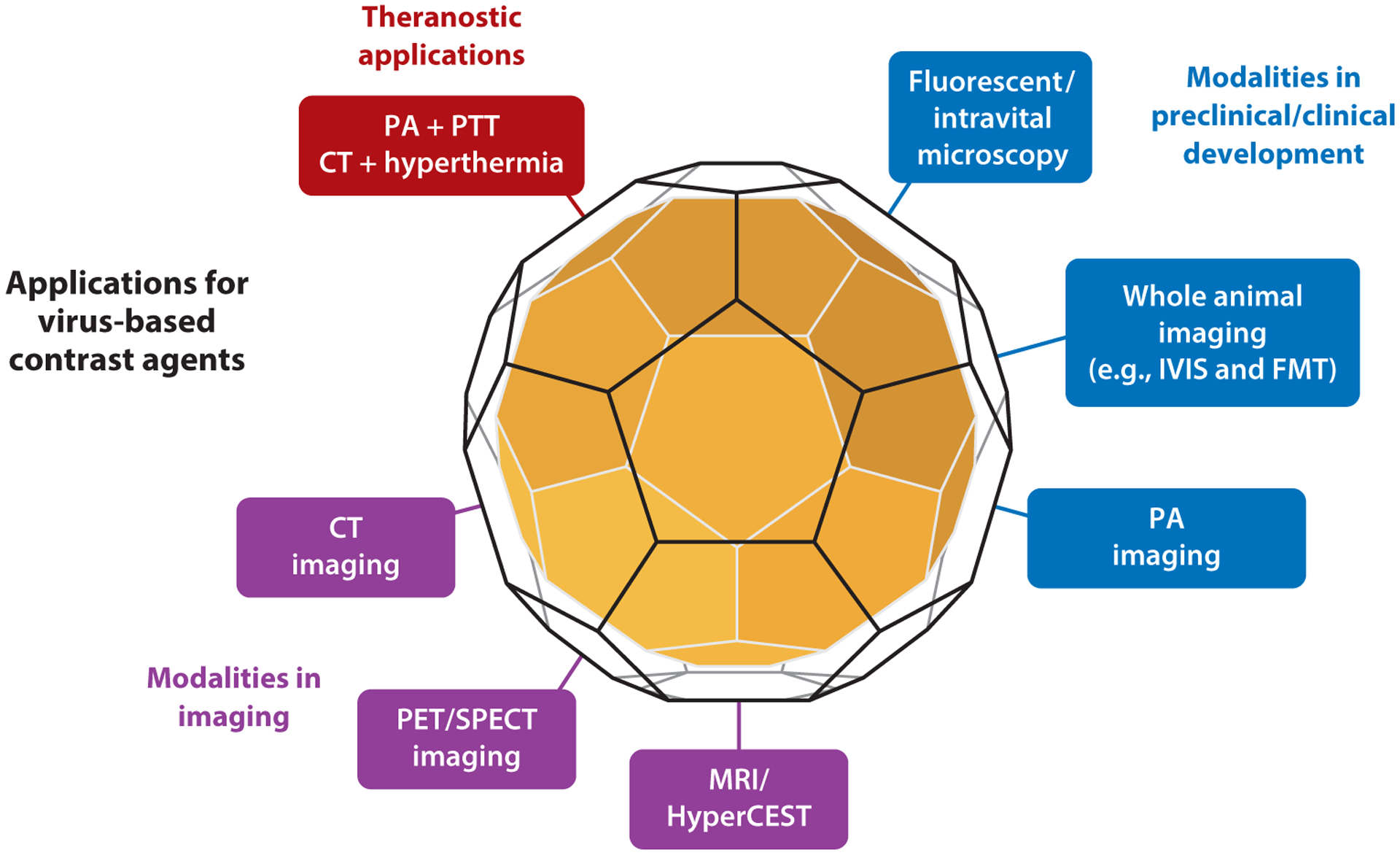
Viral nanocarriers have been developed as a versatile platform for multiple diagnostic imaging modalities and theranostic applications. Abbreviations: CT, computed tomography; FMT, fluorescence molecular tomography; HyperCEST, hyperpolarized xenon chemical exchange saturation transfer; IVIS, in vivo imaging system; MRI, magnetic resonance imaging; PA, photoacoustic imaging; PET, position emission tomography; PTT, photothermal therapy; SPECT, single photon emission computed tomography.
The use of VNPs/VLPs for fluorescence imaging initially required the conjugation or encapsidation of many fluorescent dye molecules per particle. However, genetic engineering has also been used to produce viral particles carrying intrinsically fluorescent proteins, such as GFP and mCherry (102). Furthermore, a combination of genetic engineering and programmed self-assembly has been used to prepare polyomavirus particles simultaneously loaded with GFP and m-Ruby3, which are suitable for fluorescence resonance energy transfer experiments (103). Preclinical imaging in live animals has been achieved using VLPs by incorporating near-infrared (NIR) fluorescent dyes and/or NIR2 contrast agents such as single-walled carbon nanotubes and quantum dots (104–106).
In one example of virus-based fluorescence imaging, fluorescent cowpea mosaic virus (CPMV) particles have been used to image the tumor neovasculature by intravital two-photon laser scanning microscopy, exploiting the natural interaction between CPMV and the endothelial protein vimentin (107). However, other targets can be selected by adding appropriate ligands to the CPMV surface. For example, the use of peptide ligands specific for epidermal growth factor-like domain 7 was sufficient to retarget CPMV, allowing its use as a diagnostic reagent for the stratification of prostate cancer (108).
Several groups have recently developed fluorescent VNPs/VLPs for cardiovascular imaging in order to stratify atherosclerotic plaques, thus improving the accuracy of prognoses and reducing the frequency of strokes and heart attacks. TMV particles loaded with the NIR dye Cy5 and conjugated to peptides targeting S100A9 (myeloid-related protein 14) have been used to detect the presence of macrophage-rich atherosclerotic lesions in ApoE−/− mice (109). Similarly, MS2 VLPs carrying the NIR fluorescent dye AlexaFluor 680 and displaying antibodies specific for vascular cell adhesion molecule were used to detect atherosclerotic plaques in the descending aorta and aortic arch (110). Although many VLPs are designed to bind membrane proteins on target cells, others target components of the extracellular matrix. For example, collagen and secreted protein acidic and rich in cysteine (SPARC) were targeted with fluorescent M13 VLPs for deep tissue imaging in lung cancer, and SPARC was also used for the fluorescence-guided resection of ovarian cancer using M13 particles carrying single-walled carbon nanotubes (111).
Viruses modified with MRI, PET, and CT contrast agents are promising formulations for clinical development. MRI is the most frequently used noninvasive diagnostic technique. Given the rapid recent progress in cryogen-free superconducting magnet technology, the current trend in clinical MRI involves the use of ultrahigh magnetic field strengths (≥7.0 T) to achieve a higher signal-to-noise ratio and greater spatial and temporal resolution (112). The signal-to-noise ratio in MRI is improved by contrast agents that selectively shorten the T1 or T2 relaxation times in the region of interest. VNPs/VLPs carrying such contrast agents can therefore improve the quality of MRI by concentrating these molecules at the target site. Simultaneously, the toxicity of the contrast agents is reduced because the VNPs/VLPs are cleared more rapidly than the free molecules. Accordingly, viruses and their VLPs have been loaded with contrast agents for both T1 and T2 imaging. For example, bacteriophage P22 has been used to encapsulate gadolinium-tetraazacyclododecane (Gd-DOTA) and Mn3+ chelators, leading to higher T1 relaxivities (113, 114). Similarly, TMV was loaded with Gd-DOTA and conjugated to vascular cell adhesion molecule 1 in order to target the corresponding receptors, allowing the detection and imaging of atherosclerotic plaques at submicromolar doses. The high aspect ratio of the TMV nanoparticles promoted ligand-receptor interactions by enhanced margination to vessel walls, while the slower tumbling and enhanced relaxivity of the Gd-DOTA increased the signal-to-noise ratio and imaging sensitivity (115). For T2 imaging, brome mosaic virus (BMV) and bacteriophage M13 have been loaded with iron oxide nanoparticles. The BMV VLPs formed a shell around an iron oxide core, increasing the T2 relaxivity by 4–6.5-fold compared with commercially available T2 contrast agents (116). The iron oxide–loaded M13 VLPs were also conjugated to SPARC peptides for the targeted imaging of prostate cancer (117).
Bimodal contrast agents allow the use of multiple imaging techniques. In a key example, the internal cavity of TMV particles was loaded with a dysprosium (Dy3+) complex to increase T2 relaxivity during MRI but also with the NIR fluorescent dye Cy7.5 (118). The external surface was conjugated to the peptide DGEA via a PEG linker to target integrin α2β1 on prostate cancer cells and achieved high relaxivity during ultrahigh-field MRI (119). Interestingly, physalis mottle virus particles loaded with MRI contrast agents to target prostate cancer cells were cleared more slowly than TMV and therefore allowed tumor imaging for several days (106). Such longitudinal imaging modalities may be useful to follow disease progression and/or therapeutic responses.
Chemical exchange saturation transfer (CEST) and HyperCEST imaging are newer MRI modalities using reagents based on xenon in which exogenous nuclei are selectively saturated and enhanced water signals are observed due to saturation transfer to the surrounding bulk water (120, 121). Bacteriophage MS2, M13, and fd have been used to develop VNP/VLP formulations to exploit these new techniques. For example, cyptophane cages bound to filamentous M13 achieved HyperCEST contrast enhancement with sensitivities as low as 230 fM, 50-fold more sensitive than free cyptophane (122). Similarly, icosahedral MS2 particles containing 125 cyptophane molecules improved contrast, with sensitivity as low as 0.7 pM (123). In a more recent development, cyptophane-loaded MS2 particles were targeted to Burkitt’s lymphoma cells using an aptamer specific for the membrane protein mIgM (124). Furthermore, cyptophane-loaded filamentous fd particles targeting EGFR/ErbB-1 have been used for the selective imaging of liver cancer cells (125). The unprecedented sensitivity of these new MRI modalities could significantly increase the accuracy of preclinical and clinical imaging.
In another new development, TMV was loaded with a metal-free paramagnetic nitroxide organic radical contrast agent (ORCA) to develop MRI and electron paramagnetic resonance probes for superoxide detection in vitro with enhanced r1 and r2 relaxivities. The probes therefore functioned as both T1 and T2 contrast agents, making them suitable for preclinical and clinical MRI scanners. Furthermore, bimodal TMV probes with fluorescent dyes conjugated to the internal surface and ORCA to the outer surface allowed the fluorophore to be used as a concentration marker regardless of the ORCA oxidation state (126).
PET imaging is based on the detection of radiotracers and has been used to map the biodistribution of bacteriophage MS2 in vivo by covalently attaching [18F] fluorobenzaldehyde or by loading the particle with DOTA-[64Cu] (127). Similarly, the biodistribution and tumor homing ability of MS2 particles targeting EGFR/ErbB-1 in a breast cancer model (128) and T7 particles displaying RGD peptides (129) were also investigated by PET imaging after labeling the particles with 64Cu. PET imaging has been also used to confirm successful gene delivery using viral vectors. For example, vectors expressing the HSV-1 thymidine kinase reporter system can be traced by PET imaging by monitoring the phosphorylation of radiolabeled thymidine analogs because the accumulation of radioactive tracer is proportional to the quantity and activity of the enzyme (130). Finally, gold-coated CPMV particles were recently developed for CT imaging, which uses scattered X-rays for three-dimensional visual reconstruction and tissue segmentation. The gold-coated CPMV particles improved the sensitivity of the technique, reducing the scan time to less than 2 min and achieving a resolution of nearly 150 HU (131).
Viruses loaded simultaneously with imaging reagents and drugs can be used for both diagnosis and therapy, a concept known as theranostics. Some theranostic modalities are constitutive, meaning that the imaging reagents and drugs are active simultaneously. Examples include a multifunctional bacteriophage M13 conjugated with chemotherapy, fluorophores, and targeting ligands designed for simultaneous imaging and drug delivery to prostate cancer cells (132) as well as tri-functional simian virus 40 VNPs displaying the peptide CGNKRTRGC to target atherosclerotic plaques and encapsulating NIR quantum dots (QD800) for imaging and the anticoagulant drug Hirulog for atherosclerotic imaging and therapy (133). A more refined approach is conditional or induced therapy, in which the drug is activated after imaging by applying an external trigger. This is beneficial because it allows for therapeutic decisions based on the imaging results (e.g., therapy can be withheld if the imaging results reveal that treatment is unnecessary). For example, TMV particles were loaded with MRI contrast agents and coated with polydopamine for photoacoustic imaging and PTT, the latter based on strong NIR absorption with high photothermal conversion efficiency (134). Similarly, hepatitis B virus core (HBc) particles were loaded with indocyanine green for image-guided cancer phototherapy and also conjugated to the RGD peptide for tumor targeting. The VLPs accumulated in U87MG tumors and facilitated sensitive NIR fluorescence/photoacoustic imaging followed by PDT/PTT for tumor ablation upon irradiation with an NIR laser (135). HBc VLPs have also been used to develop multifunctional theranostic agents carrying iron oxide and the cancer drug methotrexate (MTX) (136). The MTX was chemically conjugated to the iron oxide shell-core nanoparticles and embedded in the capsid. The iron oxide component enabled T2-weighted MRI as well as PTT for tumor ablation in response to an NIR laser, whereas the MTX killed the tumor cells with or without laser exposure. The particles were therefore suitable for image-guided therapy. When injected into 4T1 tumors in mice and irradiated with the NIR laser, the particles achieved synergistic tumor ablation by PTT and chemotherapy.
3. VIRUSES AS IMMUNOMODULATORS
Viral nucleocapsids act as pathogen-associated molecular patterns (PAMPs), which are danger signals that activate the innate immune system by triggering multiple signaling pathways (137). Cell surface receptors Toll-like receptor (TLR) 2 and TLR4 have been implicated in recognition of viral proteins (138–140), whereas intracellular receptors TLR3, TLR7, TLR8, and TLR9 can recognize viral nucleic acids (141). Although plant viruses and bacteriophages are noninfectious in mammals, their nucleocapsid imparts similar immunological potency via TLRs (142–144). Furthermore, the size range of most viruses (20–500 nm) allows VLPs to drain into the lymph nodes and to be captured by innate immune cells to prime the immune system, making them ideal for the development of vaccines and immunotherapy platforms.
3.1. Virus-Like Particles as Platforms for Vaccine Development
The prevention of infectious diseases by vaccination is arguably the greatest medical achievement in history. While viral diseases smallpox and rinderpest have been eradicated by vaccination, others such as poliomyelitis, rubella, yellow fever, and measles have been brought under control to a great extent (145). However, efficacious vaccines remain unavailable for many prevalent diseases, such as HIV/acquired immunodeficiency syndrome and malaria, and have not yet been developed for many emerging and resurgent diseases, such as coronaviruses including SARS-CoV, MERS-CoV, and the more recent SARS-CoV-2 (146–148). Therapeutic and prophylactic vaccines are also being developed for cancer, chronic diseases, and substance abuse.
Most vaccines are based on live, inactivated, or attenuated whole pathogens. The disadvantage of this approach is that the antigens required to prime immune memory are inextricably linked to the original pathogen, which incurs a risk of reversion to virulence and also means that the properties of the delivery platform cannot be changed. The introduction of subunit vaccines was the first step toward separating the antigen from the delivery platform, but this also removed the advantages of the virus as a self-contained adjuvant. The more recent development of recombinant VLPs has expanded the versatility of vaccines by combining antigens with the most potent immunostimulatory virus-based nanocarriers (149–151). Several VLP-based vaccines have been approved or are undergoing clinical evaluation (Table 2). While VLP vaccines allow for surface display of antigens and induce neutralizing antibody response, in the absence of de novo synthesis of viral antigens, they often induce a weak cytotoxic T cell response as compared with live vaccines.
Table 2.
Clinical trials of VLP-based vaccines
| Company | Trial number(s) | Phase(s) | Route | Formulation | Disease | Antigen | VLP vector | Status |
|---|---|---|---|---|---|---|---|---|
| Merck Sharp & Dohme | NCT00943722/NCT01984697 | III | Intramuscular | Nonavalent HPV VLP (V503), no adjuvant specified | HPV | L1 capsid protein | HPV6, 11, 16, 18, 31, 33, 45, 52 and 58 | Completed (2017) |
| Fraunhofer Center for Molecular Biotechnology | NCT02013687 | I | Intramuscular | Pfs25-CP VLP with alhydrigel | Malaria | Pfs25 | Alfalfa mosaic virus | Completed (2017) |
| Fred Hutchinson Cancer Research Center | NCT03051516 | IV | Intramuscular | Nonavalent HPV VLP (Gardasil 9), NaCl, aluminum hydroxyphosphate sulfate, L-histidine, sodium borate, pol, polysorbate 80 | High-grade Squamous intraep-ithelial lesions | L1 capsid protein | HPV6, 11, 16, 18, 31, 33, 45, 52 and 58 | Active |
| Merck Sharp & Dohme | NCT03158220 | III | Intramuscular | Aluminum hydroxyphosphate sulfate, yeast protein, NaCl, sodium borate, pol, polysorbate 80 | HPV | L1 capsid protein | HPV6, 11, 16, 18, 31, 33, 45, 52 and 58 | Completed (2019) |
| Shanghai Zerun Biotechnology Co., Ltd. | NCT02740777, NCT02733068 | II/III | Intramuscular | Bivalent VLP combination with aluminum phosphate, in a histidine buffer with sodium chloride and Tween-80 | HPV | L1 capsid protein | HPV 16 and 18 | Active |
| Takeda | NCT02669121, NCT03039790 | II | Intramuscular | Bivalent VLP combination with aluminum hydroxide | Norovirus | VP1 capsid protein | Gl.1 norovirus and GII.4 norovirus | Completed (2019) |
| Medicago | NCT03301051 | III | Intramuscular | Quadrivalent VLP combination in phosphate buffer with sodium chloride and Tween-80 | Influenza A virus | H1, H3, B hemagglutinin | Influenza A virus | Completed (2019) |
| Medicago | NCT02768805, NCT02233816, NCT02236052 | II | Intramuscular | Quadrivalent VLP combination in phosphate buffer with sodium chloride and Tween-80 | Influenza A virus | Unspecified | Influenza A virus | Completed (2019) |
| National Institute of Allergy and Infectious Disease | NCT02562482 | II | Intramuscular | VRC-CHKVLP059– 00-VP VLP in phosphate buffered saline | CHIKV | E1, E2, and CHIKV, capsid protein | Chikungunya virus | Completed (2019) |
| Sentinext Therapeutics Sdn Bhd | ACTRN12617001027303 | I | Intramuscular | Alhydrogel and aluminum hydroxide in Bis-Tris buffer with NaCl | Hand, foot, and mouth disease | EV-A71 | Enterovirus 71 | Active |
Abbreviations: CHIKV, chikungunya virus; HPV, human papillomavirus; VLP, virus-like particle.
3.1.1. Virus-like particle–based vaccines against infectious diseases.
VLP-based vaccines have been approved for the prevention of hepatitis B virus, hepatitis E virus, and human papillomavirus (HPV) infections, the latter also preventing HPV-derived cervical cancer. Other VLPs are undergoing clinical trials to prevent infections with influenza A virus (https://www.clinicaltrials.gov/ identifiers NCT02768805, NCT02233816), chikungunya virus (NCT02562482), human cytomegalovirus (NCT02826798), and Norwalk virus (NCT02669121, NCT03039790, NCT02661490). VLPs based on plant viruses and bacteriophage have also been developed to present the HPV L2 epitope, including grapevine fanleaf virus and bacteriophage MS2, leading to the production of HPV-neutralizing antibodies in preclinical studies (152, 153). Similarly, a VLP displaying the Ebola virus glycoprotein and matrix protein (VP40) was used in conjugation with the TLR4 agonist glucopyranosyl lipid adjuvant to enhance immunogenicity and promote durable protection against a mouse-adapted Ebola virus strain (154). Plant and bacteriophage VLPs have also been used to develop vaccines against Zika virus, influenza A virus, Norwalk virus, and the bacterial diseases brucellosis, anthrax, and bubonic plague (155). In a recent study, bacteriophage Qβ displaying the cell-transversal protein from the human malaria parasite Plasmodium vivax (PvCeITOS) was evaluated as a preclinical malaria vaccine and produced PvCeITOS-specific antibodies and CD8+ T cells in mice (156).
3.1.2. Virus-like particle–derived vaccines against cancer.
Cancer vaccines are designed to prime the immune system to recognize tumor-associated antigens and launch an endogenous adaptive immune response that can eliminate primary tumors as well as any residual or recurring disease by inducing immune memory. Tumor antigens are often overexpressed self-antigens, and cancer vaccines must therefore overcome self-tolerance by providing potent adjuvants, which can be achieved by presenting the vaccines as VLPs (157, 158).
Several VLP-based vaccines have been developed targeting HER2/neu/ErbB-2, some of which accomplished a true virus-like display of the correctly folded multi-epitope antigen to induce a strong polyclonal antibody response. For example, using the Spy-tag/Spy-catcher ligation method, Acinetobacter bacteriophage AP205 VLPs were engineered to display multivalent human HER2 on the particle surface, overcoming B cell tolerance and inducing a strong polyclonal anti-HER2 autoantibody response (159). Prophylactic vaccination reduced the spontaneous development of mammary carcinomas by 50–100% in human HER2 transgenic mice and inhibited the growth of HER2+ tumors implanted in wild-type mice. The comparison of vaccine platforms for HER2 epitope display based on VLPs differing in shape and structure revealed that icosahedral CPMV nanoparticles outperformed PVX filaments in terms of lymphatic draining and uptake by antigen presenting cells (APCs) (160). Efficacy studies showed that the CPMV-HER2 vaccine candidate delayed the progression of primary tumors and metastasis in mice, thereby prolonging survival (161).
Antibodies and T cell responses generated by VLPs target not only the epitope but also the VLP carrier. To overcome this drawback and focus the immune system solely on the epitope, the Steinmetz lab recently deployed a heterologous prime-boost strategy in which the different antigen delivery systems are used sequentially to boost immunity against a shared antigen (162, 163). Thus, we used distinct VLP carriers presenting the same epitope for primary immunization and subsequent boosts. We used three icosahedral plant viruses—CPMV, CCMV, and sesbania mosaic virus—displaying an HER2 epitope. The heterologous prime-boost significantly enhanced the HER2-specific immune response and induced an efficacious Th1-predominant response, as indicated by delayed tumor progression and improved survival (164).
Bacteriophage Qβ has been engineered to present tumor-associated carbohydrate antigens including the poorly immunogenic monomeric Tn antigen (165) and the ganglioside antigen GM2 (166). Recently, a Qβ-MUC1 glycopeptide vaccine targeting the epithelial cell marker mucin 1 was shown to induce high titers of anti-MUC1 IgG antibodies in MUC1 transgenic mice, conferring protection against primary and metastatic breast cancers (167, 168).
Current strategies for the development of cancer vaccines rely on the identification and targeting of neoantigens, thus offering the potential for patient-specific treatments (169). A multi-target vaccine approach based on bacteriophage Qβ VLPs was recently evaluated against B16F10 murine melanoma, comparing vaccines containing germline epitopes identified by immunopeptidomics with mutated epitopes predicted by whole exome sequencing and a third set consisting of a mix of these antigens. This study indicated that the mixture of germline and mutated epitopes achieved the greatest therapeutic efficacy (170).
3.1.3. The diverse applications of virus-like particle–based vaccine platforms.
VLP-based vaccines have been developed to treat a range of disorders, including cardiovascular, neurological, and autoimmune diseases. In the context of cardiovascular diseases, proprotein convertase subtilisin/kexin type 9 (PCSK9) is a secreted protein that controls cholesterol homeostasis by enhancing the degradation of the low-density lipoprotein receptor. Mutations that increase PCSK9 activity can lead to hypercholesterolemia, atherosclerosis, and early-onset cardiovascular diseases. Monoclonal antibodies targeting PCSK9 reduce its activity. Accordingly, the vaccination of mice and macaques with bacteriophage Qβ VLPs displaying PCSK9-derived peptides resulted in high titers of PCSK9-specific antibodies, inhibiting the enzyme and lowering the levels of cholesterol, phospholipids, and triglycerides (171). In the context of neurological disorders, a VLP based on HPV displaying the β-amyloid 11–28 epitope (Aβ 11–28) was recently used as a multivalent scaffold to develop a candidate vaccine against a mouse model of Alzheimer’s disease. The chimeric VLP elicited Aβ-specific antibodies that bound to β-amyloid plaques in a mouse model (172). In other examples, a cucumber mosaic virus (CuMV)-based vaccine displaying recombinant nerve growth factor (NGF) generated antibodies against NGF, a key factor underlying the chronic pain associated with osteoarthritis. Antibodies against NGF are potent analgesics, and a recent study showed that the vaccine induced high titers of anti-NGF antibodies in mice that had undergone partial meniscectomy to induce osteoarthritis, leading to the reversal of pain behavior (173). In the context of autoimmune diseases, interleukin 17 (IL-17) mediates the release of proinflammatory cytokines in a wide range of cells, and aberrant T helper cells producing this molecule (Th17) are implicated in several autoimmune disorders, including arthritis and multiple sclerosis. To mimic the blockade of IL-17 by monoclonal antibodies, mice were immunized with bacteriophage Qβ VLPs displaying IL-17. The vaccine overcame self-tolerance against IL-17 and generated high titers of anti-IL-17 antibodies, which slowed the progression of the disease (174). Vaccination strategies have also been explored for contraception. Previously, several mammalian viral vectors including recombinant myxoma virus, ectromelia virus, and cytomegalovirus-based contraception vaccines have been evaluated (175–177). Using similar approaches, contraception vaccines based on plant virus–derived VNPs were used to induce antibodies that blocked the ability of sperm to interact with the zona pellucida of the egg. JgCSMV particles were engineered to display the sperm peptide YLP12 and zona pellucida epitope ZP3, leading to a significant loss of fertility in immunized mice (178).
VLPs have also been developed into veterinary vaccines. For example, VLPs based on CuMV have been used as a vaccine scaffold for the major cat allergen Fel d1. Cats vaccinated with the VLPs produced high-affinity antibodies against Fel d1 with strong neutralizing effects in vitro and in vivo, which could potentially protect pet owners with allergies (179). CuMV VLPs have also been developed into a vaccine against insect-bite hypersensitivity in horses using equine IL-5 as the antigen (180).
3.1.4. Virus-like particle–based in situ vaccines for cancer therapy.
The immunostimulatory properties of VLPs are ideal for immunotherapy, either relying on the intrinsic properties of the virus or by exploiting them as nanocarriers for an immunostimulatory cargo. VLPs can also be used for in situ vaccination or oncolytic virotherapy. The latter exploits the tendency of some viruses to replicate selectively within cancer cells and trigger their lysis, thereby exerting anticancer effects by directly debulking the tumor and by instigating antitumor immune responses (181). For example, talimogene laherparepvec (T-VEC) is a live oncolytic herpesvirus that displays granulocyte stimulating factor, which is approved for the treatment of inoperable metastatic melanoma (182). T-VEC is injected directly into the tumor, where it preferentially replicates in dividing cells and triggers apoptosis, as well as promotes local and systemic immune responses. The lysis of cancer cells releases tumor-associated antigens that are sampled by infiltrating APCs, thus customizing the vaccination to the individual tumor (183, 184). These innate responses also prime the adaptive immune system, leading to systemic antitumor immunity at local and remote disease sites. Several other oncolytic viruses are undergoing clinical trials, including poliovirus, Newcastle disease virus, vaccinia virus, and measles virus (181).
To be effective, cancer immunotherapies must overcome the immunosuppressive tumor microenvironment (TME) (185). In situ vaccination refers to the administration of immunotherapy directly to a tumor or metastatic site to relieve immunosuppression in the TME and induce a systemic antitumor immune response (Figure 4). Recently, CPMV and papaya mosaic virus (PapMV) were shown to modulate the TME when applied as in situ vaccines. The efficacy of in situ CPMV was established in syngeneic mouse tumor models of melanoma; ovarian, colon, and breast cancer (186–188); and glioma (189) as well as in companion dogs with oral melanoma (190). The administration of CPMV to tumors leads to a cascade of events including the infiltration and activation of innate immune cells, the increased secretion of proinflammatory cytokines, and the recruitment and repolarization of immune cells to antitumor phenotypes leading to tumor cell death. Tumor antigens are then released and taken up by tumor-infiltrating APCs as discussed above for oncolytic viruses, leading to the augmentation of local and systemic antitumor responses mediated by antitumor CD8+ T cells. However, unlike oncolytic viruses, the potency of in situ vaccines based on plant viruses is driven by their immunostimulatory multivalent capsids, which are recognized as PAMPs. Although PapMV immunotherapy is attributed primarily to the encapsidated nucleic acid, which activates TLR7 signaling, both the capsid and RNA of CPMV are required for a potent effect. In subsequent studies, a combination of the CPMV in situ vaccine and cyclophosphamide (191) or radiation treatment (192) was shown to elicit a synergistic effect, leading to long-term antitumor responses and immune memory in mouse models of melanoma and ovarian and breast cancer. In another study, filamentous PVX particles were used as an in situ vaccine combined with doxorubicin in a mouse melanoma model. The direct injection of nonconjugated PVX and doxorubicin into the tumor achieved a synergistic effect, slowing tumor progression and improving survival compared with the PVX or doxorubicin treatments alone (193).
Figure 4.
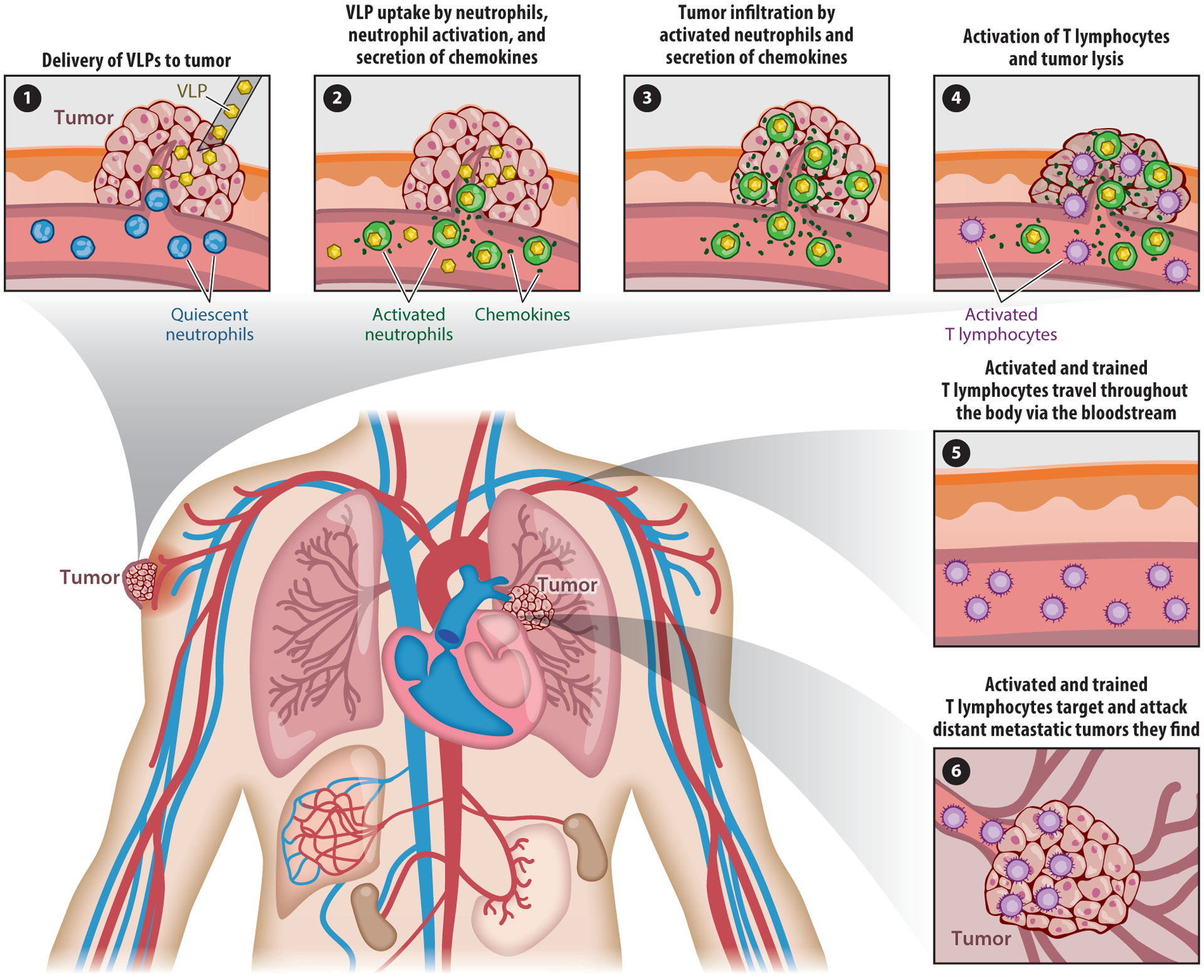
Virus-like particle (VLP)-based in situ vaccination strategy. (❶) Direct administration of VLPs into a tumor. (❷) Infiltration and activation of neutrophils, followed cytokine/chemokine secretion, leading to (❸) the activation of T cells and tumor lysis (both neutrophils and T cells can lyse tumor cells). (❹) Local priming of tumor antigen-specific T cells results in the eradication of tumor cells at distant metastatic sites (❺, ❻).
Bacteriophage M13 targeting carcinoembryonic antigen has been used as an in situ vaccine in a mouse model of colorectal cancer (194). The cytotoxic response and immune memory were attributed to TLR9 signaling based on the single-stranded DNA genome of the virus. Bacteriophage Qβ VLPs and VLPs loaded with immunostimulatory CpG oligonucleotides have also shown promise as in situ vaccines (158). The encapsulation and delivery of immunostimulatory molecules such as CpG oligonucleotides has several advantages, including the protection of the cargo from premature degradation and the effective targeting of innate immune cells. Not only is this more efficient, but also it avoids the off-target effects of systemic immunomodulators, including systemic inflammatory responses, autoimmune disease–like symptoms, and organ dysfunction (195, 196). Indeed, several nanocarrier-based drug delivery strategies have been repurposed for immunotherapy (197, 198). Mammalian viruses or their components have been used to deliver immunotherapeutic agents, including HBc particles displaying tumor antigens and carrying CpG oligonucleotides that showed efficacy against fibrosarcoma in mouse models (199). Lentiviral vectors displaying breast tumor antigens have been designed to target dendritic cells using a sindbis virus glycoprotein that selectively binds the surface protein DC-SIGN (200, 201). Similarly, bacteriophages have been used in preclinical and clinical studies to present tumor antigens while encapsulating CpG oligonucleotides, resulting in the preferential uptake of VLPs by APCs and strong effector T cell responses in mice (202).
4. CHALLENGES AND NEW DIRECTIONS
VNPs and VLPs offer a remarkable combination of beneficial properties. The size and shape of the particles facilitate their vascular transport, cellular uptake, and interactions. Their robust structure can withstand harsh chemical and physical conditions, yet they remain biocompatible and biodegradable, and to a great extent they conform to the structure-function rules that apply to synthetic nanomaterials. Accordingly, large doses of viruses are well tolerated, but proteolytic degradation ensures their rapid and complete clearance. The genetically predetermined architecture minimizes structural variations and allows precision engineering to generate new structures that interact predictably with biological systems.
As discussed in this review, plant viruses and bacteriophages provide an added layer of safety over mammalian viruses, but they remain immunogenic. Therefore, they are susceptible to accelerated clearance and reduced therapeutic efficacy upon repeat administrations. For example, the immunostimulatory effects of PapMV were completely abolished after multiple treatments, attributed in part to the appearance of PapMV-specific antibodies (203). Such effects are not unique to viral platforms and have been observed for other protein-based therapeutics (204, 205). Several surface passivation strategies have been developed to shield the viral surface from non-specific adsorption of serum proteins or to prevent recognition by antibodies including coating viral surfaces with polymers such as PEG (14) or camouflaging with albumin (206). Other methods can be adapted from synthetic virology, where the selective alteration of immunodominant protein domains has been shown to modulate viral tropism and immunogenicity (207, 208).
Multiple administrations of viral nanocarriers can also be abolished via slow release implants, scaffolds, and patches. Recently, a solvent free melt-encapsulation strategy was developed to load VNPs based on bacteriophage Qβ into poly(lactic-coglycolic) acid implants. As subcutaneous implants, the biodegradable polymers released VLPs over time without affecting the structural integrity of the particles and generated an anti-VLP immune response comparable to that achieved by multiple injections of the soluble particles (209). Similarly, the slow release of a CPMV-based in situ vaccine in a mouse model of ovarian cancer was as effective as multiple injections of soluble CPMV (210). In another example, bacteriophage M13 was embedded in responsive polymers by grafting NIPAM-cophenylboronic acid onto the M13 surface. The temperature-responsive gelation behavior of the polymer was exploited to incorporate insulin into the M13-polymer conjugate at 4°C, followed by conversion to a hydrogel at 37°C. This insulin-hydrogel formulation showed glucose-dependent release behavior, and hydrophilic conversion of the hydrogel matrix in the presence of glucose was used to tailor the insulin release rate (211). The development of VLP-based oral vaccines is another significant area for future exploration. For example, VLPs based on influenza A virus displaying an intestinal protozoan antigen showed remarkable stability to temperature and pH changes, enabling vaccination by oral delivery (212).
Scalable production platforms based on plants and microbes offer an inexpensive and reliable approach to produce large quantities of VNPs and VLPs for research as well as preclinical and clinical development. This will allow the development of ever more innovative and versatile virus-based diagnostic and therapeutic reagents in the future.
ACKNOWLEDGMENTS
This work was supported in part by the following grants to N.F.S.: CAREER DMR 1841848 from the National Science Foundation; R01 CA202814, R01 HL137674, R01CA224605, and U01CA218292 from the National Institutes of Health (NIH); and 128319-RSG-15-144-01-CDD from the American Cancer Society. C.E.B. is supported by the University of California, San Diego, Clinician Scientist Radiology Residency Program grant T32EB005970 from the NIH.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Ventola CL. 2017. Progress in nanomedicine: approved and investigational nanodrugs. Pharm. Ther 42:742–55 [PMC free article] [PubMed] [Google Scholar]
- 2.Shi J, Kantoff PW, Wooster R, Farokhzad OC. 2017. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17:20–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yildiz I, Shukla S, Steinmetz NF. 2011. Applications of viral nanoparticles in medicine. Curr. Opin. Biotechnol 22:901–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen AM, Steinmetz NF. 2016. Design of virus-based nanomaterials for medicine, biotechnology, and energy. Chem. Soc. Rev 45:4074–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koudelka KJ, Pitek AS, Manchester M, Steinmetz NF. 2015. Virus-based nanoparticles as versatile nanomachines. Annu. Rev. Virol 2:379–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glasgow J, Tullman-Ercek D. 2014. Production and applications of engineered viral capsids. Appl. Microbiol. Biotechnol 98:5847–58 [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Qiao J, Niu Z, Wang Q. 2012. Natural supramolecular building blocks: from virus coat proteins to viral nanoparticles. Chem. Soc. Rev 41:6178–94 [DOI] [PubMed] [Google Scholar]
- 8.Pokorski JK, Steinmetz NF. 2011. The art of engineering viral nanoparticles. Mol. Pharm 8:29–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merabishvili M, Vervaet C, Pirnay JP, De Vos D, Verbeken G, et al. 2013. Stability of Staphylococcus aureus phage ISP after freeze-drying (lyophilization). PLOS ONE 8(7):e68797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malik DJ, Sokolov IJ, Vinner GK, Mancuso F, Cinquerrui S, et al. 2017. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci 249:100–33 [DOI] [PubMed] [Google Scholar]
- 11.Manohar P, Ramesh N. 2019. Improved lyophilization conditions for long-term storage of bacteriophages. Sci. Rep 9(1):15242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollings M, Stone OM. 1970. The long-term survival of some plant viruses preserved by lyophilization. Ann. Appl. Biol 65(3):411–18 [Google Scholar]
- 13.Fukuhara H, Ino Y, Todo T. 2016. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 107:1373–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulati NM, Stewart PL, Steinmetz NF. 2018. Bioinspired shielding strategies for nanoparticle drug delivery applications. Mol. Pharm 15:2900–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Kan-Davelaar HE, van Hest JC, Cornelissen JJ, Koay MS. 2014. Using viruses as nanomedicines. Br. J. Pharmacol 171:4001–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Choi H, Bae Y, Kang S. 2019. Development of target-tunable P22 VLP-based delivery nanoplatforms using bacterial superglue. Biotechnol. Bioeng 116:2843–51 [DOI] [PubMed] [Google Scholar]
- 17.Le DH, Lee KL, Shukla S, Commandeur U, Steinmetz NF. 2017. Potato virus X, a filamentous plant viral nanoparticle for doxorubicin delivery in cancer therapy. Nanoscale 9:2348–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manuel-Cabrera CA, Vallejo-Cardona AA, Padilla-Camberos E, Hernandez-Gutierrez R, Herrera-Rodriguez SE, Gutierrez-Ortega A. 2016. Self-assembly of hexahistidine-tagged tobacco etch virus capsid protein into microfilaments that induce IgG2-specific response against a soluble porcine reproductive and respiratory syndrome virus chimeric protein. Virol. J 13:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thrane S, Janitzek CM, Agerbaek MO, Ditlev SB, Resende M, et al. 2015. A novel virus-like particle based vaccine platform displaying the placental malaria antigen VAR2CSA. PLOS ONE 10:e0143071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czapar AE, Zheng YR, Riddell IA, Shukla S, Awuah SG, et al. 2016. Tobacco mosaic virus delivery of phenanthriplatin for cancer therapy. ACS Nano 10:4119–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vernekar AA, Berger G, Czapar AE, Veliz FA, Wang DI, et al. 2018. Speciation of phenanthriplatin and its analogs in the core of tobacco mosaic virus. J. Am. Chem. Soc 140:4279–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bar H, Yacoby I, Benhar I. 2008. Killing cancer cells by targeted drug-carrying phage nanomedicines. BMC Biotechnol. 8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suthiwangcharoen N, Li T, Li K, Thompson P, You S, Wang Q. 2011. M13 bacteriophage-polymer nanoassemblies as drug delivery vehicles. Nano Res. 4:483–93 [Google Scholar]
- 24.Karimi M, Mirshekari H, Moosavi Basri SM, Bahrami S, Moghoofei M, Hamblin MR. 2016. Bacteriophages and phage-inspired nanocarriers for targeted delivery of therapeutic cargos. Adv. Drug Deliv. Rev 106:45–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashley CE, Carnes EC, Phillips GK, Durfee PN, Buley MD, et al. 2011. Cell-specific delivery of diverse cargos by bacteriophage MS2 virus-like particles. ACS Nano 5:5729–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thong QX, Biabanikhankahdani R, Ho KL, Alitheen NB, Tan WS. 2019. Thermally-responsive virus-like particle for targeted delivery of cancer drug. Sci. Rep 9:3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Liu B, Gao S, Wang Z, Tian Y, et al. 2017. Glyco-decorated tobacco mosaic virus as a vector for cisplatin delivery. J. Mater. Chem. B 5:2078–85 [DOI] [PubMed] [Google Scholar]
- 28.Lin RD, Steinmetz NF. 2018. Tobacco mosaic virus delivery of mitoxantrone for cancer therapy. Nanoscale 10:16307–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lomonossoff GP, Wege C. 2018. TMV particles: the journey from fundamental studies to bionanotechnology applications. Adv. Virus Res 102:149–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowden SD, Salmond GPC. 2006. Exploitation of a β-lactamase reporter gene fusion in the carbapenem antibiotic production operon to study adaptive evolution in Erwinia carotovora. Microbiology 152(Part 4):1089–97 [DOI] [PubMed] [Google Scholar]
- 31.Tan J, Shah S, Thomas A, Ou-Yang HD, Liu Y. 2013. The influence of size, shape and vessel geometry on nanoparticle distribution. Microfluid Nanofluidics 14(1–2):77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shukla S, Eber FJ, Nagarajan AS, DiFranco NA, Schmidt N, et al. 2015. The impact of aspect ratio on the biodistribution and tumor homing of rigid soft-matter nanorods. Adv. Healthc. Mater 4:874–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finbloom JA, Aanei IL, Bernard JM, Klass SH, Elledge SK, et al. 2018. Evaluation of three morphologically distinct virus-like particles as nanocarriers for convection-enhanced drug delivery to glioblastoma. Nanomaterials 8:E1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen AM, Wang Y, Jiang K, Hsu GC, Gao H, et al. 2015. Shaping bio-inspired nanotechnologies to target thrombosis for dual optical-magnetic resonance imaging. J. Mater. Chem. B 3:6037–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitek AS, Wang Y, Gulati S, Gao H, Stewart PL, et al. 2017. Elongated plant virus-based nanoparticles for enhanced delivery of thrombolytic therapies. Mol. Pharm 14:3815–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitek AS, Park J, Wang Y, Gao H, Hu H, et al. 2018. Delivery of thrombolytic therapy using rod-shaped plant viral nanoparticles decreases the risk of hemorrhage. Nanoscale 10:16547–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le DHT, Commandeur U, Steinmetz NF. 2019. Presentation and delivery of tumor necrosis factor-related apoptosis-inducing ligand via elongated plant viral nanoparticle enhances antitumor efficacy. ACS Nano 13:2501–10 [DOI] [PubMed] [Google Scholar]
- 38.Sharma J, Uchida M, Miettinen HM, Douglas T. 2017. Modular interior loading and exterior decoration of a virus-like particle. Nanoscale 9:10420–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez-Sanchez L, Cadena-Nava RD, Palomares LA, Ruiz-Garcia J, Koay MS, et al. 2014. Chemotherapy pro-drug activation by biocatalytic virus-like nanoparticles containing cytochrome P450. Enzyme Microb. Technol 60:24–31 [DOI] [PubMed] [Google Scholar]
- 40.Tapia-Moreno A, Juarez-Moreno K, Gonzalez-Davis O, Cadena-Nava RD, Vazquez-Duhalt R. 2017. Biocatalytic virus capsid as nanovehicle for enzymatic activation of Tamoxifen in tumor cells. Biotechnol. J 12:1600706. [DOI] [PubMed] [Google Scholar]
- 41.Brasch M, Putri RM, de Ruiter MV, Luque D, Koay MS, et al. 2017. Assembling enzymatic cascade pathways inside virus-based nanocages using dual-tasking nucleic acid tags. J. Am. Chem. Soc 139:1512–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephanopoulos N, Tong GJ, Hsiao SC, Francis MB. 2010. Dual-surface modified virus capsids for targeted delivery of photodynamic agents to cancer cells. ACS Nano 4:6014–20 [DOI] [PubMed] [Google Scholar]
- 43.Setaro F, Brasch M, Hahn U, Koay MS, Cornelissen JJ, et al. 2015. Generation-dependent templated self-assembly of biohybrid protein nanoparticles around photosensitizer dendrimers. Nano Lett. 15:1245–51 [DOI] [PubMed] [Google Scholar]
- 44.Lee KL, Carpenter BL, Wen AM, Ghiladi RA, Steinmetz NF. 2016. High aspect ratio nanotubes formed by tobacco mosaic virus for delivery of photodynamic agents targeting melanoma. ACS Biomater. Sci. Eng 2:838–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh MH, Yu JH, Kim I, Nam YS. 2015. Genetically programmed clusters of gold nanoparticles for cancer cell-targeted photothermal therapy. ACS Appl. Mater. Interfaces 7:22578–86 [DOI] [PubMed] [Google Scholar]
- 46.Bansal A, Yang F, Xi T, Zhang Y, Ho JS. 2018. In vivo wireless photonic photodynamic therapy. PNAS 115(7):1469–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim A, Zhou J, Samaddar S, Song SH, Elzey BD, et al. 2019. An implantable ultrasonically-powered micro-light-source (μlight) for photodynamic therapy. Sci. Rep 9(1):1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoon I, Li JZ, Shim YK. 2013. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc 46(1):7–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yacoby I, Bar H, Benhar I. 2007. Targeted drug-carrying bacteriophages as antibacterial nanomedicines. Antimicrob. Agents Chemother 51:2156–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yacoby I, Shamis M, Bar H, Shabat D, Benhar I. 2006. Targeting antibacterial agents by using drug-carrying filamentous bacteriophages. Antimicrob. Agents Chemother 50:2087–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crooke SN, Schimer J, Raji I, Wu B, Oyelere AK, Finn MG. 2019. Lung tissue delivery of virus-like particles mediated by macrolide antibiotics. Mol. Pharm 16:2947–55 [DOI] [PubMed] [Google Scholar]
- 52.Marston HD, Dixon DM, Knisely JM, Palmore TN, Fauci AS. 2016. Antimicrobial resistance. JAMA 316:1193–204 [DOI] [PubMed] [Google Scholar]
- 53.Thorpe KE, Joski P, Johnston KJ. 2018. Antibiotic-resistant infection treatment costs have doubled since 2002, now exceeding $2 billion annually. Health Aff. 37:662–69 [DOI] [PubMed] [Google Scholar]
- 54.Gordillo Altamirano FL, Barr JJ. 2019. Phage therapy in the postantibiotic era. Clin. Microbiol. Rev 32:e00066–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin DM, Koskella B, Lin HC. Phage therapy: an alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther 8(3):162–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Belleghem JD, Dabrowska K, Vaneechoutte M, Barr JJ, Bollyky PL. 2018. Interactions between bacteriophage, bacteria, and the mammalian immune system. Viruses 11(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hodyra-Stefaniak K, Miernikiewicz P, Drapała J, Drab M, Jończyk-Matysiak E, et al. 2015. Mammalian host-versus-phage immune response determines phage fate in vivo. Sci. Rep 5:14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cui Z, Guo X, Feng T, Li L. 2019. Exploring the whole standard operating procedure for phage therapy in clinical practice. J. Transl. Med 17(1):373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Merabishvili M, Pirnay JP, Verbeken G, Chanishvili N, Tediashvili M, et al. 2009. Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLOS ONE 4(3):e4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watanabe R, Matsumoto T, Sano G, Ishii Y, Tateda K, et al. 2007. Efficacy of bacteriophage therapy against gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob. Agents Chemother 51(2):446–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramesh V, Fralick JA, Rolfe RD. 1999. Prevention of Clostridium difficile-induced ileocecitis with Bacteriophage. Anaerobe 5(2):69–78 [Google Scholar]
- 62.Pouillot F, Chomton M, Blois H, Courroux C, Noelig J, et al. 2012. Efficacy of bacteriophage therapy in experimental sepsis and meningitis caused by a clone O25b:H4-ST131 Escherichia coli strain producing CTX-M-15. Antimicrob. Agents Chemother 56(7):3568–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jun JW, Shin TH, Kim JH, Shin SP, Han JE, et al. 2014. Bacteriophage therapy of a Vibrio parahaemolyticus infection caused by a multiple-antibiotic-resistant O3:K6 pandemic clinical strain. J. Infect. Dis 210(1):72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fish R, Kutter E, Wheat G, Blasdel B, Kutateladze M, Kuhl S. 2016. Bacteriophage treatment of intransigent diabetic toe ulcers: a case series. J. Wound Care 25(Suppl. 7):S27–3326949862 [Google Scholar]
- 65.Wright A, Hawkins CH, Anggård EE, Harper DR. 2009. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol 34(4):349–57 [DOI] [PubMed] [Google Scholar]
- 66.Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, et al. 2017. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob. Agents Chemother 61:e00954–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.LaVergne S, Hamilton T, Biswas B, Kumaraswamy M, Schooley RT, Wooten D. 2018. Phage therapy for a multidrug-resistant Acinetobacter baumannii craniectomy site infection. Open Forum Infect. Dis 5:ofy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aslam S, Pretorius V, Lehman SM, Morales S, Schooley RT. 2019. Novel bacteriophage therapy for treatment of left ventricular assist device infection. J. Heart Lung Transplant 38:475–76 [DOI] [PubMed] [Google Scholar]
- 69.Dedrick RM, Guerrero-Bustamante CA, Garlena RA, Russell DA, Ford K, et al. 2019. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med 25:730–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rubalskii E, Ruemke S, Salmoukas C, Aleshkin A, Bochkareva S, et al. 2019. Fibrin glue as a local drug-delivery system for bacteriophage PA5. Sci. Rep 9:2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abedon ST. 2015. Ecology of anti-biofilm agents I: antibiotics versus bacteriophages. Pharmaceuticals (Basel.) 8(3):525–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hughes KA, Sutherland IW, Jones MV. 1998. Biofilm susceptibility to bacteriophage attack: the role of phage-borne polysaccharide depolymerase. Microbiology 144(Part 11):3039–47 [DOI] [PubMed] [Google Scholar]
- 73.Mai V, Ukhanova M, Reinhard MK, Li M, Sulakvelidze A. 2015. Bacteriophage administration significantly reduces Shigella colonization and shedding by Shigella-challenged mice without deleterious side effects and distortions in the gut microbiota. Bacteriophage 5(4):e1088124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galtier M, De Sordi L, Maura D, Arachchi H, Volant S, et al. 2016. Bacteriophages to reduce gut carriage of antibiotic resistant uropathogens with low impact on microbiota composition. Environ. Microbiol 18(7):2237–45 [DOI] [PubMed] [Google Scholar]
- 75.Beitelshees M, Hill A, Rostami P, Jones CH, Pfeifer BA. 2017. Pressing diseases that represent promising targets for gene therapy. Discov. Med 24:313–22 [PMC free article] [PubMed] [Google Scholar]
- 76.Xi S, Grandis J. 2003. Gene therapy for the treatment of oral squamous cell carcinoma. J. Dent. Res 82:11–16 [DOI] [PubMed] [Google Scholar]
- 77.Sidransky D 1995. Molecular genetics of head and neck cancer. Curr. Opin. Oncol 7:229–33 [DOI] [PubMed] [Google Scholar]
- 78.Mali S 2013. Delivery systems for gene therapy. Indian J. Hum. Genet 19:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.David RM, Doherty AT. 2015. Viral vectors: the road to reducing genotoxicity. Toxicol. Sci 155(2):315–25 [DOI] [PubMed] [Google Scholar]
- 80.Rao VK, Kapp D, Schroth M. 2018. Gene therapy for spinal muscular atrophy: an emerging treatment option for a devastating disease. J. Manag. Care Spec. Pharm 24:S3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Russell S, Bennett J, Wellman JA, Chung DC, Yu Z-F, et al. 2017. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet 390:849–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang W-W, Li L, Li D, Liu J, Li X, et al. 2018. The first approved gene therapy product for cancer Ad-p53 (Gendicine): 12 years in the clinic. Hum. Gene Ther 29:160–79 [DOI] [PubMed] [Google Scholar]
- 83.Monaco L, Faccio L. 2017. Patient-driven search for rare disease therapies: the Fondazione Telethon success story and the strategy leading to Strimvelis. EMBO Mol. Med 9:289–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng P-P, Kros JM, Li J. 2018. Approved CAR T cell therapies: ice bucket challenges on glaring safety risks and long-term impacts. Drug Discov. Today 23:1175–82 [DOI] [PubMed] [Google Scholar]
- 85.Ghani K, Boivin-Welch M, Roy S, Dakiw-Piaceski A, Barbier M, et al. 2019. Generation of high-titer self-inactivated γ-retroviral vector producer cells. Mol. Ther.-Methods Clin. Dev 14:90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karponi G, Zogas N. 2019. Gene therapy for beta-thalassemia: updated perspectives. Appl. Clin. Genet 12:167–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rehman H, Silk AW, Kane MP, Kaufman HL. 2016. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J. Immunother. Cancer 4:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Azizgolshani O, Garmann RF, Cadena-Nava R, Knobler CM, Gelbart WM. 2013. Reconstituted plant viral capsids can release genes to mammalian cells. Virology 441:12–17 [DOI] [PubMed] [Google Scholar]
- 89.Gitlin L, Hagai T, LaBarbera A, Solovey M, Andino R. 2014. Rapid evolution of virus sequences in intrinsically disordered protein regions. PLOS Pathog. 10:e1004529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ball LA, Amann JM, Garrett BK. 1992. Replication of nodamura virus after transfection of viral RNA into mammalian cells in culture. J. Virol 66:2326–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Biddlecome A, Habte HH, McGrath KM, Sambanthamoorthy S, Wurm M, et al. 2019. Delivery of self-amplifying RNA vaccines in in vitro reconstituted virus-like particles. PLOS ONE 14:e0215031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou Y, Maharaj PD, Mallajosyula JK, McCormick AA, Kearney CM. 2015. In planta production of Flock House virus transencapsidated RNA and its potential use as a vaccine. Mol. Biotechnol 57:325–36 [DOI] [PubMed] [Google Scholar]
- 93.Lam P, Steinmetz NF. 2019. Delivery of siRNA therapeutics using cowpea chlorotic mottle virus-like particles. Biomater. Sci 7:3138–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tian Y, Zhou M, Shi H, Gao S, Xie G, et al. 2018. Integration of cell-penetrating peptides with rod-like bionanoparticles: virus-inspired gene-silencing technology. Nano Lett. 18:5453–60 [DOI] [PubMed] [Google Scholar]
- 95.Pang H-H, Huang C-Y, Chou Y-W, Lin C-J, Zhou Z-L, et al. 2019. Bioengineering fluorescent virus-like particle/RNAi nanocomplexes act synergistically with temozolomide to eradicate brain tumors. Nanoscale 11:8102–9 [DOI] [PubMed] [Google Scholar]
- 96.Wu M, Sherwin T, Brown WL, Stockley PG. 2005. Delivery of antisense oligonucleotides to leukemia cells by RNA bacteriophage capsids. Nanomedicine 1:67–76 [DOI] [PubMed] [Google Scholar]
- 97.Ehrke-Schulz E, Schiwon M, Leitner T, Dávid S, Bergmann T, et al. 2017. CRISPR/Cas9 delivery with one single adenoviral vector devoid of all viral genes. Sci. Rep 7:17113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao J, Bergmann T, Zhang W, Schiwon M, Ehrke-Schulz E, Ehrhardt A. 2019. Viral vector-based delivery of CRISPR/Cas9 and donor DNA for homology-directed repair in an in vitro model for canine hemophilia B. Mol. Ther.-Nucleic Acids 14:364–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qazi S, Miettinen HM, Wilkinson RA, McCoy K, Douglas T, Wiedenheft B. 2016. Programmed self-assembly of an active P22-Cas9 nanocarrier system. Mol. Pharm 13:1191–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu M, Shu J. 2018. Multimodal molecular imaging: current status and future directions. Contrast Media Mol. Imaging 2018:1382183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schwarz B, Douglas T. 2015. Development of virus-like particles for diagnostic and prophylactic biomedical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 7:722–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shukla S, Dickmeis C, Fischer R, Commandeur U, Steinmetz NF. 2018. In planta production of fluorescent filamentous plant virus-based nanoparticles. Methods Mol. Biol 1776:61–84 [DOI] [PubMed] [Google Scholar]
- 103.Dashti NH, Abidin RS, Sainsbury F. 2018. Programmable in vitro coencapsidation of guest proteins for intracellular delivery by virus-like particles. ACS Nano 12:4615–23 [DOI] [PubMed] [Google Scholar]
- 104.Jung B, Anvari B. 2013. Virus-mimicking optical nanomaterials: near infrared absorption and fluorescence characteristics and physical stability in biological environments. ACS Appl. Mater. Interfaces 5:7492–500 [DOI] [PubMed] [Google Scholar]
- 105.Leeuw TK, Reith RM, Simonette RA, Harden ME, Cherukuri P, et al. 2007. Single-walled carbon nanotubes in the intact organism: near-IR imaging and biocompatibility studies in Drosophila. Nano Lett. 7:2650–54 [DOI] [PubMed] [Google Scholar]
- 106.Hu H, Masarapu H, Gu Y, Zhang Y, Yu X, Steinmetz NF. 2019. Physalis mottle virus-like nanoparticles for targeted cancer imaging. ACS Appl. Mater. Interfaces 11:18213–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leong HS, Steinmetz NF, Ablack A, Destito G, Zijlstra A, et al. 2010. Intravital imaging of embryonic and tumor neovasculature using viral nanoparticles. Nat. Protoc 5:1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cho C-F, Yu L, Nsiama TK, Kadam AN, Raturi A, et al. 2017. Viral nanoparticles decorated with novel EGFL7 ligands enable intravital imaging of tumor neovasculature. Nanoscale 9:12096–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Park J, Gao H, Wang Y, Hu H, Simon DI, Steinmetz NF. 2019. S100A9-targeted tobacco mosaic virus nanoparticles exhibit high specificity toward atherosclerotic lesions in ApoE−/− mice. J. Mater. Chem. B 7:1842–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aanei IL, Huynh T, Seo Y, Francis MB. 2018. Vascular cell adhesion molecule-targeted MS2 viral capsids for the detection of early-stage atherosclerotic plaques. Bioconjug. Chem 29:2526–30 [DOI] [PubMed] [Google Scholar]
- 111.Ceppi L, Bardhan NM, Na Y, Siegel A, Rajan N, et al. 2019. Real-time single-walled carbon nanotube-based fluorescence imaging improves survival after debulking surgery in an ovarian cancer model. ACS Nano 13:5356–65 [DOI] [PubMed] [Google Scholar]
- 112.Nakada T 2007. Clinical application of high and ultra high-field MRI. Brain Dev. 29:325–35 [DOI] [PubMed] [Google Scholar]
- 113.Quazi S, Leipold LO, Abedin MJ, Johnson B, Prevelige P, et al. 2013. P22 viral capsids as nanocomposite high-relaxivity MRI contrast agents. Mol. Pharm 10:11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Quazi S, Uchida M, Usselman R, Shearer R, Edwards E, Douglas T. 2014. Manganese(III) porphyrins complexed with P22 virus-like particles as T1-enhanced contrast agents for magnetic resonance imaging. J. Biol. Inorg. Chem 19:237–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bruckman MA, Jiang K, Simpson EJ, Randolph LN, Luyt LG, et al. 2014. Dual-modal magnetic resonance and fluorescence imaging of atherosclerotic plaques in vivo using VCAM-1 targeted tobacco mosaic virus. Nano Lett. 14:1551–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Malyutin AG, Cheng H, Sanchez-Felix OR, Carlson K, Stein BD, et al. 2015. Coat protein-dependent behavior of poly(ethylene glycol) tails in iron oxide core virus-like nanoparticles. ACS Appl. Mater. Interfaces 7:12089–98 [DOI] [PubMed] [Google Scholar]
- 117.Ghosh D, Lee Y, Thomas S, Kohli AG, Yun DS, et al. 2012. M13-templated magnetic nanoparticles for targeted in vivo imaging of prostate cancer. Nat. Nanotechnol 7:677–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hu H, Zhang Y, Shukla S, Gu Y, Yu X, Steinmetz NF. 2017. Dysprosium-modified tobacco mosaic virus nanoparticles for ultra-high-field magnetic resonance and near-infrared fluorescence imaging of prostate cancer. ACS Nano 11:9249–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Norek M, Peters JA. 2011. MRI contrast agents based on dysprosium or holmium. Prog. Nucl. Magn. Reson. Spectrosc 59:64–82 [DOI] [PubMed] [Google Scholar]
- 120.Jayapaul J, Schroder L. 2019. Nanoparticle-based contrast agents for 129Xe HyperCEST NMR and MRI applications. Contrast Media Mol. Imaging 2019:9498173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Y, Dmochowski IJ. 2016. An expanded palette of xenon-129 NMR biosensors. Acc. Chem. Res 49:2179–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stevens TK, Palaniappan KK, Ramirez RM, Francis MB, Wemmer DE, Pines A. 2013. HyperCEST detection of a 129Xe-based contrast agent composed of cryptophane-A molecular cages on a bacteriophage scaffold. Magn. Reson. Med 69:1245–52 [DOI] [PubMed] [Google Scholar]
- 123.Meldrum T, Seim KL, Bajaj VS, Palaniappan KK, Wu W, et al. 2010. A xenon-based molecular sensor assembled on an MS2 viral capsid scaffold. J. Am. Chem. Soc 132:5936–37 [DOI] [PubMed] [Google Scholar]
- 124.Jeong K, Netirojjanakul C, Munch HK, Sun J, Finbloom JA, et al. 2016. Targeted molecular imaging of cancer cells using MS2-based 129Xe NMR. Bioconjug. Chem 27:1796–801 [DOI] [PubMed] [Google Scholar]
- 125.Palaniappan KK, Ramirez RM, Bajaj VS, Wemmer DE, Pines A, Francis MB. 2013. Molecular imaging of cancer cells using a bacteriophage-based 129Xe NMR biosensor. Angew. Chem. Int. Ed. Engl 52:4849–53 [DOI] [PubMed] [Google Scholar]
- 126.Dharmarwardana M, Martins AF, Chen Z, Palacios PM, Nowak CM, et al. 2018. Nitroxyl modified tobacco mosaic virus as a metal-free high-relaxivity MRI and EPR active superoxide sensor. Mol. Pharm 15:2973–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Farkas ME, Aanei IL, Behrens CR, Tong GJ, Murphy ST, et al. 2013. PET imaging and biodistribution of chemically modified bacteriophage MS2. Mol. Pharm 10:69–76 [DOI] [PubMed] [Google Scholar]
- 128.Aanei IL, ElSohly AM, Farkas ME, Netirojjanakul C, Regan M, et al. 2016. Biodistribution of antibody-MS2 viral capsid conjugates in breast cancer models. Mol. Pharm 13:3764–72 [DOI] [PubMed] [Google Scholar]
- 129.Li Z, Jin Q, Huang C, Dasa S, Chen L, et al. 2011. Trackable and targeted phage as positron emission tomography (PET) agent for cancer imaging. Theranostics 1:371–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brader P, Kelly K, Gang S, Shah JP, Wong RJ, et al. 2009. Imaging of lymph node micrometastases using an oncolytic herpes virus and [18F]FEAU PET. PLOS ONE 4:e4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aljabali AAA, Zoubi MSA, Al-Batanyeh KM, Al-Radaideh A, Obeid MA, et al. 2019. Gold-coated plant virus as computed tomography imaging contrast agent. Beilstein J. Nanotechnol 10:1983–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ghosh D, Kohli AG, Moser F, Endy D, Belcher AM. 2012. Refactored M13 bacteriophage as a platform for tumor cell imaging and drug delivery. ACS Synth. Biol 1:576–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sun X, Li W, Zhang X, Qi M, Zhang Z, et al. 2016. In vivo targeting and imaging of atherosclerosis using multifunctional virus-like particles of Simian virus 40. Nano Lett. 16:6164–71 [DOI] [PubMed] [Google Scholar]
- 134.Hu H, Yang Q, Baroni S, Yang H, Aime S, Steinmetz NF. 2019. Polydopamine-decorated tobacco mosaic virus for photoacoustic/magnetic resonance bimodal imaging and photothermal cancer therapy. Nanoscale 11:9760–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shan W, Chen R, Zhang Q, Zhao J, Chen B, et al. 2018. Improved stable indocyanine green (ICG)-mediated cancer optotheranostics with naturalized hepatitis B core particles. Adv. Mater 30:e1707567. [DOI] [PubMed] [Google Scholar]
- 136.Zhang Q, Shan W, Ai C, Chen Z, Zhou T, et al. 2018. Construction of multifunctional Fe3O4-MTX@HBc nanoparticles for MR imaging and photothermal therapy/chemotherapy. Nanotheranostics 2:87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mohsen MO, Gomes AC, Vogel M, Bachmann MF. 2018. Interaction of viral capsid-derived virus-like particles (VLPs) with the innate immune system. Vaccines 6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bieback K, Lien E, Klagge IM, Avota E, Schneider-Schaulies J, et al. 2002. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J. Virol 76(17):8729–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ge Y, Mansell A, Ussher JE, Brooks AE, Manning K, et al. 2013. Rotavirus NSP4 triggers secretion of proinflammatory cytokines from macrophages via toll-like receptor 2. J. Virol 87(20):11160–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, et al. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol 1(5):398–401 [DOI] [PubMed] [Google Scholar]
- 141.Lester SN, Li K. 2014. Toll-like receptors in antiviral innate immunity. J. Mol. Biol 426(6):1246–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jobsri J, Allen A, Rajagopal D, Shipton M, Kanyuka K, et al. 2015. Plant virus particles carrying tumour antigen activate TLR7 and induce high levels of protective antibody. PLOS ONE 10(2):e0118096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Albakri MM, Veliz FA, Fiering SN, Steinmetz NF, Sieg SF. 2020. Endosomal toll-like receptors play a key role in activation of primary human monocytes by cowpea mosaic virus. Immunology 159(2):183–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Carroll-Portillo A, Lin HC. 2019. Bacteriophage and the innate immune system: access and signaling. Microorganisms 7(12):E625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Andre FE, Booy R, Bock HL, Clemens J, Datta SK, et al. 2008. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ 86:140–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fauci AS, Morens DM. 2012. The perpetual challenge of infectious diseases. N. Engl. J. Med 366:454–61 [DOI] [PubMed] [Google Scholar]
- 147.Mehand MS, Al-Shorbaji F, Millett P, Murgue B. 2018. The WHO R&D Blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antivir. Res 159:63–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Reperant LA, Osterhaus A. 2017. AIDS, Avian flu, SARS, MERS, Ebola, Zika…what next? Vaccine 35:4470–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Draper SJ, Heeney JL. 2010. Viruses as vaccine vectors for infectious diseases and cancer. Nat. Rev. Microbiol 8:62–73 [DOI] [PubMed] [Google Scholar]
- 150.Humphreys IR, Sebastian S. 2018. Novel viral vectors in infectious diseases. Immunology 153:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lauer KB, Borrow R, Blanchard TJ. 2017. Multivalent and multipathogen viral vector vaccines. Clin. Vaccine Immunol 24:e00298–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yazdani R, Shams-Bakhsh M, Hassani-Mehraban A, Arab SS, Thelen N, et al. 2019. Production and characterization of virus-like particles of grapevine fanleaf virus presenting L2 epitope of human papillomavirus minor capsid protein. BMC Biotechnol. 19:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhai L, Peabody J, Pang YS, Schiller J, Chackerian B, Tumban E. 2017. A novel candidate HPV vaccine: MS2 phage VLP displaying a tandem HPV L2 peptide offers similar protection in mice to Gardasil-9. Antivir. Res 147:116–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sunay MME, Martins KAO, Steffens JT, Gregory M, Vantongeren SA, et al. 2019. Glucopyranosyl lipid adjuvant enhances immune response to Ebola virus-like particle vaccine in mice. Vaccine 37:3902–10 [DOI] [PubMed] [Google Scholar]
- 155.Tao P, Mahalingam M, Zhu J, Moayeri M, Sha J, et al. 2018. A bacteriophage T4 nanoparticle-based dual vaccine against anthrax and plague. mBio 9:e01926–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Alves E, Salman AM, Leoratti F, Lopez-Camacho C, Viveros-Sandoval ME, et al. 2017. Evaluation of Plasmodium vivax cell-traversal protein for ookinetes and sporozoites as a preerythrocytic P. vivax vaccine. Clin. Vaccine Immunol 24:e00501–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bowen WS, Svrivastava AK, Batra L, Barsoumian H, Shirwan H. 2018. Current challenges for cancer vaccine adjuvant development. Expert Rev. Vaccines 17:207–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mohsen MO, Speiser DE, Knuth A, Bachmann MF. 2020. Virus-like particles for vaccination against cancer. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 12:e1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Palladini A, Thrane S, Janitzek CM, Pihl J, Clemmensen SB, et al. 2018. Virus-like particle display of HER2 induces potent anti-cancer responses. Oncoimmunology 7:e1408749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shukla S, Dorand RD, Myers JT, Woods SE, Gulati NM, et al. 2016. Multiple administrations of viral nanoparticles alter in vivo behavior-insights from intravital microscopy. ACS Biomater. Sci. Eng 2:829–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shukla S, Jandzinski M, Wang C, Gong X, Bonk KW, et al. 2019. A viral nanoparticle cancer vaccine delays tumor progression and prolongs survival in a HER2+ tumor mouse model. Adv. Ther 2:1800139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Woodland DL. 2004. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 25(2):98–104 [DOI] [PubMed] [Google Scholar]
- 163.Ramshaw IA, Ramsay AJ. 2000. The prime-boost strategy: exciting prospects for improved vaccination. Immunol. Today 21(4):163–65 [DOI] [PubMed] [Google Scholar]
- 164.Cai H, Shukla S, Wang C, Masarapu H, Steinmetz NF. 2019. Heterologous prime-boost enhances the antitumor immune response elicited by plant-virus-based cancer vaccine. J. Am. Chem. Soc 141:6509–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yin Z, Comellas-Aragones M, Chowdhury S, Bentley P, Kaczanowska K, et al. 2013. Boosting immunity to small tumor-associated carbohydrates with bacteriophage Qβ capsids. ACS Chem. Biol 8:1253–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yin Z, Dulaney S, McKay CS, Baniel C, Kaczanowska K, et al. 2016. Chemical synthesis of GM2 glycans, bioconjugation with bacteriophage Qβ, and the induction of anticancer antibodies. Chembiochem 17:174–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu X, Yin Z, McKay C, Pett C, Yu J, et al. 2018. Protective epitope discovery and design of MUC1-based vaccine for effective tumor protections in immunotolerant mice. J. Am. Chem. Soc 140:16596–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wu X, McKay C, Pett C, Yu J, Schorlemer M, et al. 2019. Synthesis and immunological evaluation of disaccharide bearing MUC-1 glycopeptide conjugates with virus-like particles. ACS Chem. Biol 14:2176–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sahin U, Tureci O. 2018. Personalized vaccines for cancer immunotherapy. Science 359:1355–60 [DOI] [PubMed] [Google Scholar]
- 170.Mohsen MO, Vogel M, Riether C, Muller J, Salatino S, et al. 2019. Targeting mutated plus germline epitopes confers pre-clinical efficacy of an instantly formulated cancer nano-vaccine. Front. Immunol 10:1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Crossey E, Amar MJA, Sampson M, Peabody J, Schiller JT, et al. 2015. A cholesterol-lowering VLP vaccine that targets PCSK9. Vaccine 33:5747–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gonzalez-Castro R, Acero Galindo G, Garcia Salcedo Y, Uribe Campero L, Vazquez Perez V, et al. 2018. Plant-based chimeric HPV-virus-like particles bearing amyloid-β epitopes elicit antibodies able to recognize amyloid plaques in APP-tg mouse and Alzheimer’s disease brains. Inflammopharmacology 26:817–27 [DOI] [PubMed] [Google Scholar]
- 173.von Loga IS, El-Turabi A, Jostins L, Miotla-Zarebska J, Mackay-Alderson J, et al. 2019. Active immunisation targeting nerve growth factor attenuates chronic pain behaviour in murine osteoarthritis. Ann. Rheum. Dis 78:672–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rohn TA, Jennings GT, Hernandez M, Grest P, Beck M, et al. 2006. Vaccination against IL-17 suppresses autoimmune arthritis and encephalomyelitis. Eur. J. Immunol 36:2857–67 [DOI] [PubMed] [Google Scholar]
- 175.Mackenzie SM, McLaughlin EA, Perkins HD, French N, Sutherland T, et al. 2006. Immunocontraceptive effects on female rabbits infected with recombinant myxoma virus expressing rabbit ZP2 or ZP3. Biol. Reprod 74(3):511–21 [DOI] [PubMed] [Google Scholar]
- 176.Jackson RJ, Maguire DJ, Hinds LA, Ramshaw LA. 1998. Infertility in mice induced by a recombinant ectromelia virus expressing mouse zona pellucida glycoprotein 3. Biol. Reprod 58(1):152–59 [DOI] [PubMed] [Google Scholar]
- 177.Lloyd ML, Shellam GR, Papadimitriou JM, Lawson MA. 2003. Immunocontraception is induced in BALB/c mice inoculated with murine cytomegalovirus expressing mouse zona pellucida 3. Biol. Reprod 68(6):2024–32 [DOI] [PubMed] [Google Scholar]
- 178.Choudhury S, Kakkar V, Suman P, Chakrabarti K, Vrati S, Gupta SK. 2009. Immunogenicity of zona pellucida glycoprotein-3 and spermatozoa YLP12 peptides presented on Johnson grass mosaic virus-like particles. Vaccine 27:2948–53 [DOI] [PubMed] [Google Scholar]
- 179.Thoms F, Jennings GT, Maudrich M, Vogel M, Haas S, et al. 2019. Immunization of cats to induce neutralizing antibodies against Fel d 1, the major feline allergen in human subjects. J. Allergy Clin. Immunol 144:193–203 [DOI] [PubMed] [Google Scholar]
- 180.Fettelschoss-Gabriel A, Fettelschoss V, Olomski F, Birkmann K, Thoms F, et al. 2019. Active vaccination against interleukin-5 as long-term treatment for insect-bite hypersensitivity in horses. Allergy 74:572–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Raja J, Ludwig JM, Gettinger SN, Schalper KA, Kim HS. 2018. Oncolytic virus immunotherapy: future prospects for oncology. J. Immunother. Cancer 6:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ott PA, Hodi FS. 2016. Talimogene laherparepvec for the treatment of advanced melanoma. Clin. Cancer Res 22:3127–31 [DOI] [PubMed] [Google Scholar]
- 183.Chen DS, Mellman I. 2013. Oncology meets immunology: the cancer-immunity cycle. Immunity 39:1–10 [DOI] [PubMed] [Google Scholar]
- 184.Russell L, Peng KW, Russell SJ, Diaz RM. 2019. Oncolytic viruses: priming time for cancer immunotherapy. Biodrugs 33:485–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zou W 2005. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer 5(4):263–74 [DOI] [PubMed] [Google Scholar]
- 186.Lizotte PH, Wen AM, Sheen MR, Fields J, Rojanasopondist P, et al. 2016. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol 11:295–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Murray AA, Wang C, Fiering S, Steinmetz NF. 2018. In situ vaccination with cowpea versus tobacco mosaic virus against melanoma. Mol. Pharm 15:3700–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang C, Beiss V, Steinmetz NF. 2019. Cowpea mosaic virus nanoparticles and empty virus-like particles show distinct but overlapping immunostimulatory properties. J. Virol 93:e00129–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kerstetter-Fogle A, Shukla S, Wang C, Beiss V, Harris PLR, et al. 2019. Plant virus-like particle in situ vaccine for intracranial glioma immunotherapy. Cancers 11:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hoopes PJ, Wagner RJ, Duval K, Kang K, Gladstone DJ, et al. 2018. Treatment of canine oral melanoma with nanotechnology-based immunotherapy and radiation. Mol. Pharm 15:3717–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cai H, Wang C, Shukla S, Steinmetz NF. 2019. Cowpea mosaic virus immunotherapy combined with cyclophosphamide reduces breast cancer tumor burden and inhibits lung metastasis. Adv. Sci 6:1802281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Patel R, Czapar AE, Fiering S, Oleinick NL, Steinmetz NF. 2018. Radiation therapy combined with cowpea mosaic virus nanoparticle in situ vaccination initiates immune-mediated tumor regression. ACS Omega 3:3702–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lee KL, Murray AA, Le DHT, Sheen MR, Shukla S, et al. 2017. Combination of plant virus nanoparticle-based in situ vaccination with chemotherapy potentiates antitumor response. Nano Lett. 17:4019–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Murgas P, Bustamante N, Araya N, Cruz-Gomez S, Duran E, et al. 2018. A filamentous bacteriophage targeted to carcinoembryonic antigen induces tumor regression in mouse models of colorectal cancer. Cancer Immunol. Immunother 67:183–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Weber JS, Yang JC, Atkins MB, Disis ML. 2015. Toxicities of immunotherapy for the practitioner. J. Clin. Oncol 33:2092–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shimabukuro-Vornhagen A, Godel P, Subklewe M, Stemmler HJ, Schlosser HA, et al. 2018. Cytokine release syndrome. J. Immunother. Cancer 6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Goldberg MS. 2019. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 19:587–602 [DOI] [PubMed] [Google Scholar]
- 198.Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. 2015. Synthetic nanoparticles for vaccines and immunotherapy. Chem. Rev 115:11109–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li J, Ge J, Ren S, Zhou T, Sun Y, et al. 2015. Hepatitis B surface antigen (HBsAg) and core antigen (HBcAg) combine CpG oligodeoxynucletides as a novel therapeutic vaccine for chronic hepatitis B infection. Vaccine 33:4247–54 [DOI] [PubMed] [Google Scholar]
- 200.Bryson PD, Han X, Truong N, Wang P. 2017. Breast cancer vaccines delivered by dendritic cell-targeted lentivectors induce potent antitumor immune responses and protect mice from mammary tumor growth. Vaccine 35:5842–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yang L, Yang H, Rideout K, Cho T, Joo KI, et al. 2008. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat. Biotechnol 26:326–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Storni T, Ruedl C, Schwarz K, Schwendener RA, Renner WA, Bachmann MF. 2004. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J. Immunol 172:1777–85 [DOI] [PubMed] [Google Scholar]
- 203.Chartrand K, Lebel ME, Tarrab E, Savard P, Leclerc D, Lamarre A. 2017. Efficacy of a virus-like nanoparticle as treatment for a chronic viral infection is hindered by IRAK1 regulation and antibody interference. Front. Immunol 8:1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pratt KP. 2018. Anti-drug antibodies: emerging approaches to predict, reduce or reverse biotherapeutic immunogenicity. Antibodies 7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ridker PM, Tardif JC, Amarenco P, Duggan W, Glynn RJ, et al. 2017. Lipid-reduction variability and antidrug-antibody formation with bococizumab. N. Engl. J. Med 376:1517–26 [DOI] [PubMed] [Google Scholar]
- 206.Pitek AS, Jameson SA, Veliz FA, Shukla S, Steinmetz NF. 2016. Serum albumin ‘camouflage’ of plant virus based nanoparticles prevents their antibody recognition and enhances pharmacokinetics. Biomaterials 89:89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Srivastava IK, VanDorsten K, Vojtech L, Barnett SW, Stamatatos L. 2003. Changes in the immunogenic properties of soluble gp140 human immunodeficiency virus envelope constructs upon partial deletion of the second hypervariable region. J. Virol 77:2310–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yang ZY, Chakrabarti BK, Xu L, Welcher B, Kong WP, et al. 2004. Selective modification of variable loops alters tropism and enhances immunogenicity of human immunodeficiency virus type 1 envelope. J. Virol 78:4029–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lee PW, Shukla S, Wallat JD, Danda C, Steinmetz NF, et al. 2017. Biodegradable viral nanoparticle/polymer implants prepared via melt-processing. ACS Nano 11:8777–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Czapar AE, Tiu BDB, Veliz FA, Pokorski JK, Steinmetz NF. 2018. Slow-release formulation of cowpea mosaic virus for in situ vaccine delivery to treat ovarian cancer. Adv. Sci 5:1700991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cao J, Liu S, Chen Y, Shi L, Zhang Z. 2014. Synthesis of end-functionalized boronic acid containing copolymers and their bioconjugates with rod-like viruses for multiple responsive hydrogels. Polym. Chem 5:5029–36 [Google Scholar]
- 212.Serradell MC, Rupil LL, Martino RA, Prucca CG, Carranza PG, et al. 2019. Efficient oral vaccination by bioengineering virus-like particles with protozoan surface proteins. Nat. Commun 10:361. [DOI] [PMC free article] [PubMed] [Google Scholar]


