Abstract
Background:
Gastroparesis (GP) is a motility disorder of the stomach presenting with upper gastrointestinal symptoms in the setting of delayed gastric emptying. Endocannabinoids are involved in the regulation of GI function including motility. However, their role in the pathophysiology of GP has not been sufficiently investigated. Our goal was to compare the circulating levels of endocannabinoids and cannabimimetic fatty acid derivatives in GP versus control subjects.
Methods:
The study compared plasma concentrations of endocannabinoids and their lipoamine and 2-acyl glycerol congeners, measured by high-pressure liquid chromatography/tandem mass spectrometry (HPLC-MS-MS), in adult patients with diabetic gastroparesis (DM-GP; n = 24; n = 16 female), idiopathic gastroparesis (ID-GP; n = 19; n = 11 female), diabetic patients without GP (DM; n = 19; n = 10 female), and healthy controls (HC; n = 18; n = 10 female). Data, presented as mean ± SEM, were analyzed with ANOVA (Sidak post hoc).
Key Results:
Endocannabinoids anandamide (AEA: 0.5 ± 0.1 nMol/L) and 2-arachidonoyl glycerol (2-AG: 2.6 ± 0.7 nMol/L) were significantly lower in female DM-GP patients vs. DM females (AEA: 2.5 ± 0.7 nMol/L and 2-AG: 9.4 ± 3.3 nMol/L). Other monoacylglycerols including 2-palmitoyl glycerol and 2-oleoyl glycerol were also lower in female DM-GP patients compared to DM females. No changes were observed in men.
Conclusions & Inferences:
Endocannabinoids and other fatty acid derivatives with cannabimimetic properties are reduced in female DM-GP patients. Since GP, particularly with diabetic etiology, is more prevalent among women and since cannabinoids are antiemetic, this decrease in levels may contribute to symptom development in these subjects. Targeting the endocannabinoid system may be a future therapeutic option in DM-GP patients.
Keywords: 2-arachidonoyl glycerol, anandamide, endocannabinoid, gastroparesis
1 ∣. INTRODUCTION
Gastroparesis (GP) is a chronic gastrointestinal (GI) motility disorder which presents with chronic refractory nausea and vomiting, epigastric discomfort, and early satiety in the setting of delayed gastric emptying without gastric outlet obstruction. The vast majority of GP cases are in women, and are idiopathic (ID-GP) or diabetic (DM-GP) in origin, while some cases have been reported in the setting of traumatic or idiopathic vagal nerve damage.1,2 Patients with GP experience significant reduction in their quality of life, and treatment options are limited.3,4
The endocannabinoid system primarily consists of the endocannabinoid ligands anandamide (AEA) and 2-arachidonoyl glycerol (2-AG), the enzymes responsible for their biosynthesis from cell membrane lipids, such as N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) and diacylglycerol lipase (DAGL), their degradative enzymes, such as fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), and their receptors, cannabinoid-1 (CB1) and cannabinoid-2 (CB2). The enzymes involved in the biosynthesis or degradation of endocannabinoids also are involved in the metabolism of other fatty acid derivatives with cannabimimetic properties. While endocannabinoids have a role in slowing GI motility and regulating GI secretion, mucosal permeability, inflammation, visceral sensation, and nociception, studies on the physiological roles of the other endogenous lipids (eg, additional 2-acyl glycerols and AEA-congeners, lipoamines) are very limited.5-7
Previous studies have described the endocannabinoid system as a potential target in treating conditions such as anorexia,8 obesity,9 cyclic vomiting syndrome, 10 and other GI disorders.5,7 Many GP patients use cannabis recreationally or medically, but whether targeting endocannabinoid system is helpful in GP is still a matter of debate.6,11,12 In addition to changes in gastric emptying, GP is also associated with enteric neuronal loss, depletion of interstitial cells of cajal, and inflammation at molecular and cellular levels such as changes in M2 macrophage population and smooth muscle fibrosis.13-16
Immunomodulatory and antifibrotic actions of endogenous cannabinoids have been demonstrated.17 Moreover, GP patients with severe nausea and abdominal pain are more likely to use cannabis and perceive it as beneficial.12 It is possible that these patients are using cannabis to ameliorate a depletion in their endogenous cannabinoid levels. Here, we tested the hypothesis that GP drives changes in circulating endocannabinoid and related lipid signaling molecules and that these changes are sex- and GP subtype–dependent.
2 ∣. METHODS
2.1 ∣. Participants and plasma collection
This study was performed at Texas Tech University Health Sciences Center El Paso after obtaining IRB approval. Patients referred to the Neurogastroenterology and Endocrinology Clinics of Texas Tech University Health Sciences Center El Paso participated in this study. Healthy controls (HCs) were included based on an advertisement. Fasting plasma samples were collected from 24 DM-GP, 19 ID-GP patients, 19 diabetic patients without GP (DM), and 18 HC. Studied subjects were 18 to 85 years old. Their demographic characteristics are presented in Table 1. Plasma samples were immediately stored at −80°C and were shipped on dry ice to the Department of Psychological & Brain Sciences of Indiana University for analysis where the lipid extraction and HPLC/MS/MS analysis was performed.
TABLE 1.
Demographic characteristics of the study subjects
| Control (n = 18) |
Diabetes (n = 19) |
Idiopathic gastroparesis (n = 19) |
Diabetic gastroparesis (n = 24) |
|
|---|---|---|---|---|
| Femalea | 10 | 10 | 11 | 16 |
| Age (year)b | 53.1 (2.5) | 58.7 (3.2) | 54.9 (3.8) | 54.9 (2.3) |
| Body mass indexb | 29.9 (1.5) | 30.9 (2.2) | 29.4 (1.9) | 28.4 (1.2) |
| Hemoglobin A1cb | - | 7.5 (0.2) | - | 8.1 (0.3) |
| Total symptom scoreb | - | - | 14.6 (1.6) | 14.7 (1.5) |
| Type I diabetesa | - | 6 | - | 7 |
| Antidepressants and Narcoticsa | - | - | n = 1 SSRIc n = 1 TCAd |
n = 3 Tramadol |
Number (n).
Mean (standard error of mean).
Selective serotonin reuptake inhibitor.
Tricyclic antidepressant.
In the GP groups, the diagnosis was confirmed based on the standardized 4-hour gastric emptying test where a radiolabeled meal retention of >60% at 2 hours and >10% at 4 hours was indicative of GP.18 In GP patients, total symptom score which was the sum of severity (score: 0-4) for each major symptom (vomiting, nausea, early satiety, bloating, postprandial fullness, and epigastric pain) during the last 2 weeks before sample collection was calculated.19 None of the subjects had any organic GI disorder such as peptic ulcer or inflammatory bowel disease, cancer, allergy, autoimmune, infectious diseases or were receiving antibiotics, immunosuppressants or non-steroidal anti-inflammatory drugs. Any subject with a history of cannabis use was excluded from the study. Patients were also asked to stop taking antidepressants and narcotics at least 72 hours before collection of the blood samples.
2.2 ∣. Lipid extraction and HPLC/MS/MS analysis
Plasma was aliquoted into 150 μL volumes for each participant and then added to 2 mL of (HPLC)-grade methanol (Thermo Fisher Scientific). Solutions were spiked with 500 picomoles deuterium-labeled N-arachidonoyl glycine (d8NAGly; Cayman Chemical) to determine extraction efficiency and centrifuged at 19,000 × g for 20 minutes at 24°C. Supernatants were diluted with HPLC water (purified in house) to make a 15% supernatant solution. Lipid extractions were performed as previously described using C18 solid-phase extraction columns (Agilent).20-28 Briefly, columns were conditioned with 5 mL HPLC methanol followed by 2.5 mL HPLC water. Then, the supernatant/water solution was loaded onto the column. Impurities were washed off with 2.5 mL HPLC water. A series of 5 elutions with 1.5 mL 40%, 60%, 75%, 85%, and 100% methanol were collected.
As previously described,20-29 extracts were analyzed using an Applied Biosystems API 3000 triple quadrupole mass spectrometer (Foster City, CA, USA). 20 μL from each elution were chromatographed using a 2.1 × 50 mm XDB-C18 reversed phase HPLC analytical column with a 3.5 micron particle size (Agilent) using optimized mobile phase ingredients (mobile phase A: 80% water, 20% methanol with 1 mmol/L ammonium acetate; mobile phase B: 100% methanol with 1 mmol/L ammonium acetate). Two Shimadzu 10ADvp pumps provided pressure for gradient elution. Analysis of the HPLC/MS/MS data was performed using Analyst software (Applied Biosystems).20-29 Chromatograms displaying the retention time of analytes matching programmed parent and fragment ion masses were generated by running each sample using a multiple reaction monitoring (MRM) method. Retention times were then compared to those from standards for the suspected compound. If retention times matched, then concentrations were determined by calculating the area under the curve for the unknown and comparing it to the calibration curve obtained from the standards. Extraction efficiency was calculated using the recovery vial spiked with 500 pmol d8NAGly as a standard, and analyte levels were adjusted for extraction efficiency.
Data were analyzed using GraphPad Prism 6.1 software (GraphPad Software, Inc) and presented as mean ± standard error of the mean (SEM). Normality was tested based on D'Agostino-Pearson omnibus test, and statistical comparisons were based on one-way analysis of variance (ANOVA) and Sidak post hoc.
3 ∣. RESULTS
No statistical difference in the baseline characteristics of the study subjects was observed (Table 1). Fasting plasma samples from 24 DM-GP (n = 16 women), 19 ID-GP patients (n = 11 women), 19 diabetic patients without GP (DM; n = 10 women), and 18 HC (n = 10 women) were analyzed. Plasma concentrations of the endocannabinoid AEA were significantly decreased in women with DM-GP (Figure 1). There were no significant differences in AEA across the four groups in men, suggesting a sex-dependent effect.
FIGURE 1.
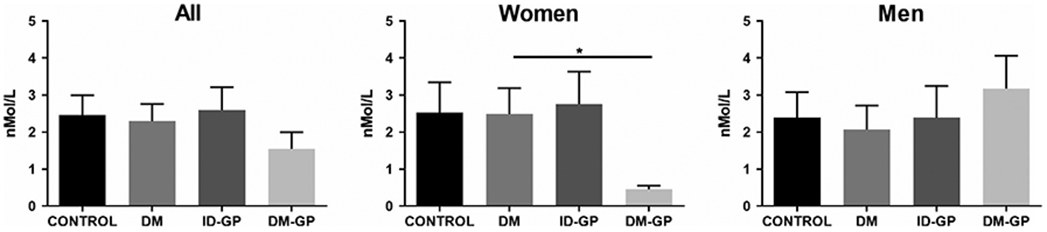
Anandamide levels in plasma. Women with DM-GP had lower plasma AEA levels (one-way ANOVA P < .05; * Sidak post hoc P < .05 in DM-GP vs. DM). AEA, anandamide; DM, diabetes mellitus; ID-GP, idiopathic gastroparesis; and DM-GP, diabetic gastroparesis
There were no significant changes between groups in the additional N-acyl ethanolamines screened: palmitoyl ethanolamine (PEA), oleoyl ethanolamine (OEA), stearoyl ethanolamine (SEA) and docosahexaenoyl ethanolamine (DEA) (Figure 2), as well as linoleoyl ethanolamine (LEA).
FIGURE 2.
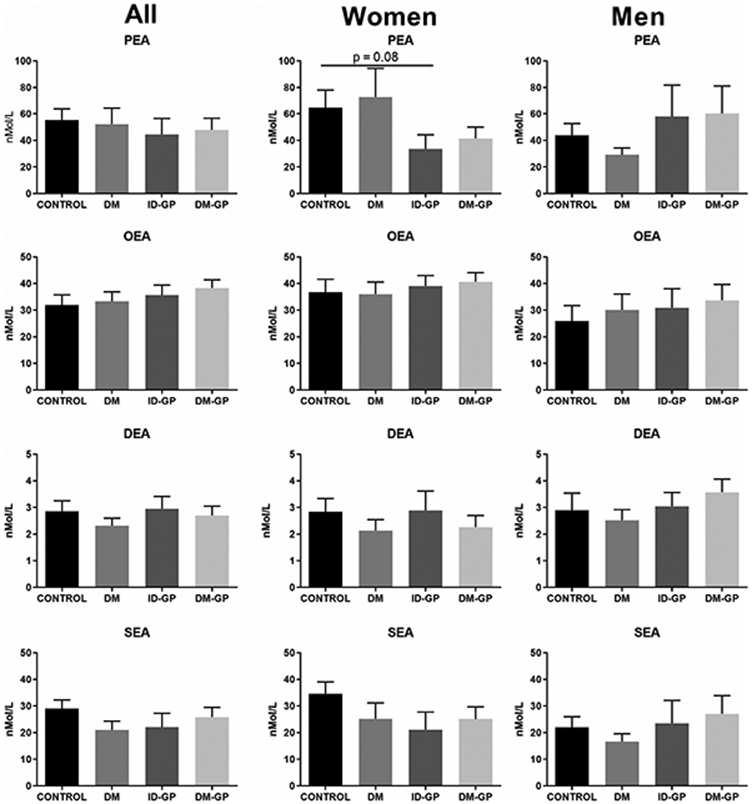
N-acyl ethanolamines in plasma. Palmitoyl ethanolamine (PEA), oleoyl ethanolamine (OEA), docosahexaenoyl ethanolamine (DEA), and stearoyl ethanolamine (SEA) in gastroparesis vs. control. DM, diabetes mellitus; DM-GP, diabetic gastroparesis; ID-GP, idiopathic gastroparesis
Like what was observed with AEA, the plasma endocannabinoid 2-AG was significantly decreased in women with DM-GP (Figure 3). Importantly, this pattern was also observed with additional 2-acyl glycerols such as 2-palmitoyl glycerol (2-PG), 2-oleoyl glycerol (2-OG), and 2-linoleoyl glycerol (2-LG) (Figure 4).
FIGURE 3.
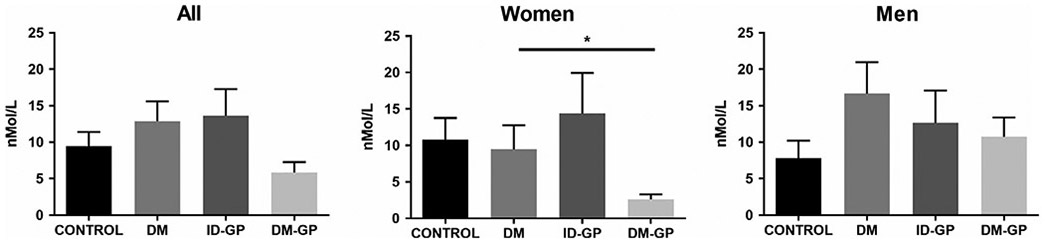
Plasma levels of 2-AG in gastroparesis vs. control (one-way ANOVA P < .05; * Sidak post hoc P < .05 in DM-GP vs. DM). DM, diabetes mellitus; DM-GP: diabetic gastroparesis; ID-GP, idiopathic gastroparesis
FIGURE 4.
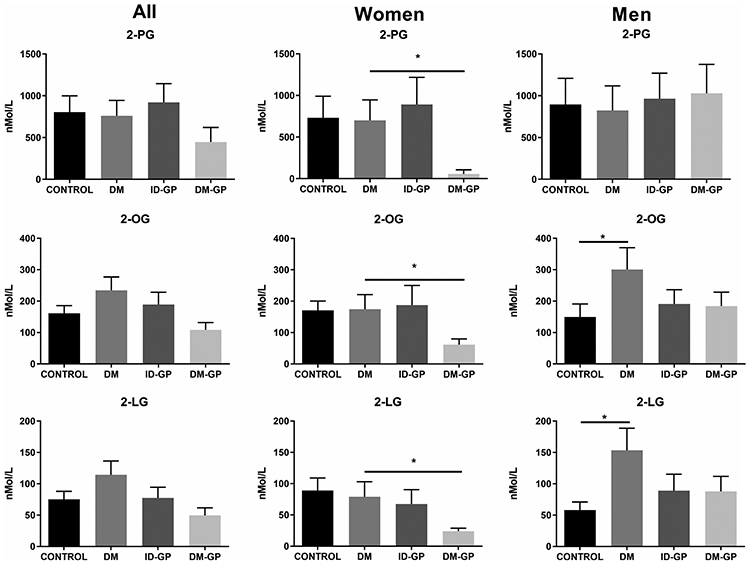
Plasma 2-acyl glycerols in gastroparesis vs. control (one-way ANOVA P < .05; * Sidak post hoc P < .05 in DM-GP vs. DM). DM, diabetes mellitus; DM-GP: diabetic gastroparesis; ID-GP, idiopathic gastroparesis; 2-PG, 2-palmitoyl glycerol; 2-OG, 2-oleoyl glycerol; 2-LG, 2-linoleoyl glycerol
Circulating levels of the free fatty acids arachidonic acid, linoleic acid, and oleic acid were significantly higher in women with DM-GP vs. DM only (Figure 5). There was also a significant reduction in arachidonic acid levels in women with ID-GP versus healthy controls, representing one of the few differences in circulating lipid levels between healthy controls and GP patients in the study.
FIGURE 5.
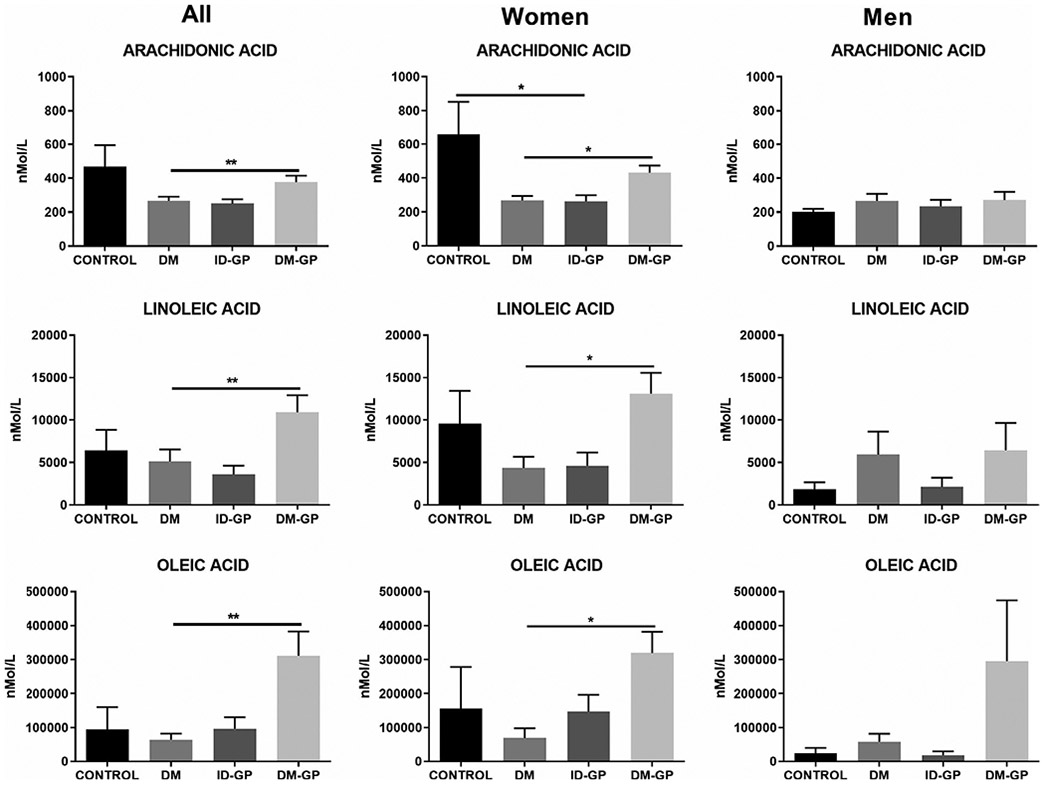
Free fatty acids in plasma across different patient populations (one-way ANOVA P < .05; Sidak post hoc * P < .05 and **P < .01 in DM-GP vs. DM). DM, diabetes mellitus; DM-GP, diabetic gastroparesis; ID-GP, idiopathic gastroparesis
4 ∣. DISCUSSION
The plant Cannabis has two major active components, tetrahydrocannabinol (THC) and cannabidiol (CBD), which have been used for the treatment of GI disorders presenting with nausea, vomiting, abdominal pain, and inflammation.5,7,30-36 Based on recent studies, 11.7%-46.7% of GP patients reported current or past use of cannabinoids including THC, dronabinol, and/or CBD. Patients with severe symptoms were more likely to use Cannabis and those who were using Cannabis (THC) reported more improvement in their GP symptoms compared to CBD alone.11,12
The endocannabinoid system, which includes endocannabinoid ligands (anandamide and 2-arachidonoylglycerol), enzymes responsible for their biosynthesis and degradation, and the cannabinoid receptors 1 and 2 (CB1 and CB2), is involved in the regulation of GI function including motility, mucosal permeability, inflammation, visceral sensation, and nociception.5 Stimulation of the CB1 and CB2 receptors with their agonists inhibits GI motility/peristalsis, decreases inflammation, and reduces visceral pain. These effects are moderated in the central nervous system (CNS), enteric nervous system, and through the gut-brain interaction.5-7,30,31,34
In the current study, we observed that circulating endocannabinoid and related lipid levels were altered in GP, mainly in women and in DM-GP patients. The changes observed could partially underlie the symptom profile of these patients, as decreased endocannabinoid levels may reduce their potential protective mechanisms in controlling emesis. Chouker et al reported lower blood endocannabinoid levels among human subjects with motion sickness while undergoing parabolic flight maneuvers.37 In pregnant women who had hyperemesis gravidarum, no changes in plasma AEA, OEA, or PEA were detected compared with matched pregnant controls.38 In another study, which compared serum endocannabinoids and related lipids OEA and PEA in cyclic vomiting syndrome (CVS) patients, no change was observed in AEA and 2-AG levels in CVS versus control; however, serum levels of OEA and PEA were higher during the sick compared with well-phase CVS.39
Van Sickle et al showed that methanandamide, a stable analog of anandamide, inhibits morphine 6 glucuronide induced emesis in ferrets through a CB1-mediated mechanism. 40 URB597, which is an inhibitor of FAAH and potentially enhances anandamide levels through blocking its degradation, has some CB1-dependent antiemetic effects in animal models,41 while, in a model of cisplatin-induced emesis, anandamide was ineffective in controlling emesis when given alone.41 Although the body of literature mainly supports the protective role of anandamide in controlling emesis, discrepancies in results might be due to the differences in models used and suggest that levels of AEA do not always directly correlate with nausea and vomiting. Furthermore, effects of FAAH inhibitors extend beyond their effects on AEA,24,27,42 such that AEA might not have been the only modulator in studies where FAAH inhibitors showed a decrease in emesis.
Additional N-acyl ethanolamines are synthetized and degraded within similar pathways as anandamide. For example, NAPE-PLD and FAAH, respectively, function as biosynthetic and metabolic enzymes for all N-acyl ethanolamines screened in our study,22,24,43-46 and are not specific to anandamide. Given that there were no concurrent decreases in other N-acyl ethanolamines in the DM-GP-women in the current study, NAPE-PLD or FAAH inhibition appears an unlikely mechanism underlying the decrease in AEA.
We did not observe any change in PEA level in GP patients versus their controls. In a recent study in CVS patients, PEA was increased suggesting a potential protective role against emesis. Rock and colleagues showed that FAAH inhibition reduces acute nausea in rats through a PPARα-mediated effect. Remarkably, the available clinical data support PEA as an effective analgesic compound with minimal side effects.47 In a study by Cremon et al, PEA combined with polydatin, which reduces mast cell activation, markedly improved abdominal pain severity in irritable bowel syndrome without any serious adverse effect. This supports the therapeutic roles of PEA in GI disorders.48 Whether PEA can help with nausea and vomiting needs further investigation.
The other endocannabinoid compound studied here was the 2-acyl glycerol, 2-AG. 2-AG was decreased in female DM-GP patients. MAGL is the primary degradative enzyme of 2-acyl glycerols. 49,50 Increasing endogenous 2-AG levels with a selective MAGL inhibitor (JZL184) inhibited LiCl-induced vomiting in the house musk shrew through a CB1 but not a CB2-mediated mechanism.51 The effects of 2-AG and MAGL inhibition in controlling nausea were reproduced in further studies.52,53 Therefore, by decreasing 2-AG levels, nausea and vomiting may aggravate. Close analysis of the data indicated that three other 2-acyl glycerols measured in this study were decreased in DM-GP women. This is an interesting finding indicating the changes observed are in line with 2-AG levels. Of note, these 2-acyl glycerols are partially synthetized and metabolized by DAGL and MAGL, respectively. Therefore, parallel changes in their levels suggest potential alteration of these enzymes’ activity. Additionally, levels of arachidonic acid are closely related to DAGL and MAGL activity,24,50 so the concurrent increase in circulating arachidonic acid in women with DM-GP is an additional indication that these enzymes contribute to the decrease in 2-AG and other 2-acyl glycerols.
Here, it can be contended that decreasing endocannabinoid levels and their endogenous tone may hasten gastric motility and emptying in women with DM-GP. Studies have shown that there is no clear association between the severity of GP symptoms and gastric emptying, and interventions such as gastric electrical stimulation do not accelerate gastric emptying while improving the GP symptoms.54 On the other hand, decreased endocannabinoids can be considered protective or defensive in DM-GP patients to accelerate gastric emptying. In the current study, we also observed an increase in free arachidonic acid. Arachidonic acid is a mediator of inflammation either directly or through its eicosanoid metabolites; it might be cytotoxic or neurotoxic.55 Therefore, decreased endocannabinoids in DM-GP women may also be associated with increased exposure to toxic metabolites of arachidonic acid. These compounds may induce toxicity and symptoms including nausea and vomiting.
This study had some limitations such as being single-center and its small sample size. Moreover, medications or comorbidities which were not recorded may affect the fatty acid derivative levels.
In conclusion, our study shows that endocannabinoids and related lipid molecules are imbalanced in DM-GP women. This imbalance implies a loss of the protective effects of endocannabinoids on the nausea process, and a potential shift in the biochemical environment toward cytotoxicity and inflammation. Why these changes are sex- and GP subtype–dependent needs further investigation. Future studies should address the value of endocannabinoid degradation blockers such as MAGL inhibitors to boost endocannabinoid levels in GP patients and more specifically in DM-GP women. The fact that men with diabetes mellitus develop symptomatic GP without a loss of endocannabinoids argues against a major pathophysiological role of this system in men. Whether medical cannabis will be beneficial in GP patients with depleted endocannabinoids needs further investigation.
Key points.
Endocannabinoids are involved in the regulation of gastrointestinal function.
Plasma endocannabinoids anandamide and 2-AG are significantly lower in female diabetic gastroparetics compared with female diabetics without gastroparesis.
Changes in plasma endocannabinioid levels in gastroparesis are sex- and subtype-dependent.
ACKNOWLEDGEMENTS
This study was supported by Mini-Seed Grant from TTUHSC Office of Vice President for Research to Irene Sarosiek and Mohammad Bashashati and NIDA T32 fellowship (DA024628) to Emma Leishman.
Funding information
Mini-Seed Grant from TTUHSC Office of Vice President for Research; NIDA T32 fellowship, Grant/Award Number: DA024628
Footnotes
CONFLICT OF INTEREST
The authors do not have any conflict of interest regarding this article.
REFERENCES
- 1.Loganathan P, Gajendran M, McCallum RW. Clinical Manifestation and Natural History of Gastroparesis. Gastrointest Endosc Clin N Am. 2019;29(1):27–38. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M, Chedid V, Ford AC, et al. Gastroparesis. Nature reviews Disease primers. 2018;4(1):41. [DOI] [PubMed] [Google Scholar]
- 3.Moshiree B, Potter M, Talley NJ. Epidemiology and Pathophysiology of Gastroparesis. Gastrointest Endosc Clin N Am. 2019;29(1):1–14. [DOI] [PubMed] [Google Scholar]
- 4.Sarosiek I, Bashashati M, McCallum RW. Safety of treatment for gastroparesis. Expert opinion on drug safety. 2016;15(7):937–945. [DOI] [PubMed] [Google Scholar]
- 5.Izzo AA, Sharkey KA. Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther. 2010;126(1):21–38. [DOI] [PubMed] [Google Scholar]
- 6.McCallum RW, Bashashati M. Cannabis for Gastroparesis: Hype or Hope? The American journal of gastroenterology. 2019;114(6):865–866. [DOI] [PubMed] [Google Scholar]
- 7.McCallum RW, Bashahati M. Cannabis in gastrointestinal disorders. Practical Gastroenterology. 2014;38(12):36–46. [Google Scholar]
- 8.Siegfried Z, Kanyas K, Latzer Y, et al. Association study of cannabinoid receptor gene (CNR1) alleles and anorexia nervosa: differences between restricting and binging/purging subtypes. Am J Med Genet B Neuropsychiatr Genet. 2004;125b(1):126–130. [DOI] [PubMed] [Google Scholar]
- 9.Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet (London, England). 2007;370(9600):1706–1713. [DOI] [PubMed] [Google Scholar]
- 10.Wasilewski A, Lewandowska U, Mosinska P, et al. Cannabinoid receptor type 1 and mu-opioid receptor polymorphisms are associated with cyclic vomiting syndrome. Am J Gastroenterol. 2017;112(6):933–939. [DOI] [PubMed] [Google Scholar]
- 11.Jehangir A, Parkman HP. Cannabinoid use in patients with gastroparesis and related disorders: Prevalence and benefit. Am J Gastroenterol. 2019;114(6):945–953. [DOI] [PubMed] [Google Scholar]
- 12.Parkman HP, Sharkey EP, Nguyen LA, et al. Marijuana Use in Patients with Symptoms of Gastroparesis: Prevalence, Patient Characteristics, and Perceived Benefit. Dig Dis Sci. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bashashati M, Moraveji S, Torabi A, et al. Pathological findings of the antral and pyloric smooth muscle in patients with gastroparesis-like syndrome compared to gastroparesis: Similarities and differences. Dig Dis Sci. 2017;62(10):2828–2833. [DOI] [PubMed] [Google Scholar]
- 14.Grover M, Bernard CE, Pasricha PJ, et al. Diabetic and idiopathic gastroparesis is associated with loss of CD206-positive macrophages in the gastric antrum. Neurogastroenterol Motil. 2017;29(6):e13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140(5):1575–1585.e1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neshatian L, Gibbons SJ, Farrugia G. Macrophages in diabetic gastroparesis–the missing link? Neurogastroenterol Motil. 2015;27(1):7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zurier RB, Burstein SH. Cannabinoids, inflammation, and fibrosis. FASEB J. 2016;30(11):3682–3689. [DOI] [PubMed] [Google Scholar]
- 18.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103(3):753–763. [DOI] [PubMed] [Google Scholar]
- 19.Lin Z, McElhinney C, Sarosiek I, Forster J, McCallum R. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci. 2005;50(7):1328–1334. [DOI] [PubMed] [Google Scholar]
- 20.Tan B, Bradshaw HB, Rimmerman N, et al. Targeted lipidomics: discovery of new fatty acyl amides. AAPS J. 2006;8(3):E461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tortoriello G, Rhodes BP, Takacs SM, et al. Targeted lipidomics in Drosophila melanogaster identifies novel 2-monoacylglycerols and N-acyl amides. PLoS One. 2013;8(7):e67865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leishman E, Mackie K, Luquet S, Bradshaw HB. Lipidomics profile of a NAPE-PLD KO mouse provides evidence of a broader role of this enzyme in lipid metabolism in the brain. Biochim Biophys Acta. 2016;1861(6):491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stuart JM, Paris JJ, Frye C, Bradshaw HB. Brain levels of prostaglandins, endocannabinoids, and related lipids are affected by mating strategies. Int. J Endocrinol 2013;2013:436252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leishman E, Cornett B, Spork K, Straiker A, Mackie K, Bradshaw HB. Broad impact of deleting endogenous cannabinoid hydrolyzing enzymes and the CB1 cannabinoid receptor on the endogenous cannabinoid-related lipidome in eight regions of the mouse brain. Pharmacol Res. 2016;110:159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leishman E, Murphy M, Mackie K, Bradshaw HB. Delta(9)-Tetrahydrocannabinol changes the brain lipidome and transcriptome differentially in the adolescent and the adult. Biochim Biophys Acta. 2018;1863(5):479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leishman E, Kunkler PE, Manchanda M, et al. Environmental toxin acrolein alters levels of endogenous lipids, including TRP agonists: a potential mechanism for headache driven by TRPA1 activation. Neurobiol Pain. 2017;1:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey LM, Slivicki RA, Leishman E, et al. A pro-nociceptive phenotype unmasked in mice lacking fatty-acid amide hydrolase. Mol Pain. 2016;12:1744806916649192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leishman E, Mackie K, Bradshaw HB. Elevated levels of arachidonic acid-derived lipids including prostaglandins and endocannabinoids are present throughout ABHD12 knockout brains: novel insights into the neurodegenerative phenotype. Front Mol Neurosci. 2019;12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raboune S, Stuart JM, Leishman E, et al. Novel endogenous N-acyl amides activate TRPV1-4 receptors, BV-2 microglia, and are regulated in brain in an acute model of inflammation. Frontiers in cellular neuroscience. 2014;8:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut. 2001;48(6):859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camilleri M Cannabinoids and gastrointestinal motility: Pharmacology, clinical effects, and potential therapeutics in humans. Neurogastroenterol Motil. 2018;30(9):e13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gotfried J, Kataria R, Schey R. Review: The role of cannabinoids on esophageal function-what we know thus far. Cannabis Cannabinoid Res. 2017;2(1):252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malik Z, Baik D, Schey R. The role of cannabinoids in regulation of nausea and vomiting, and visceral pain. Curr Gastroenterol Rep. 2015;17(2):429. [DOI] [PubMed] [Google Scholar]
- 34.Schicho R, Storr M. Targeting the endocannabinoid system for gastrointestinal diseases: future therapeutic strategies. Expert Rev Clin Pharmacol. 2010;3(2):193–207. [DOI] [PubMed] [Google Scholar]
- 35.Kienzl M, Storr M, Schicho R. Cannabinoids and opioids in the treatment of inflammatory bowel diseases. Clin Transl Gastroen. 2020;11(1):e00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharkey KA, Darmani NA, Parker LA. Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur J Pharmacol. 2014;722:134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chouker A, Kaufmann I, Kreth S, et al. Motion sickness, stress and the endocannabinoid system. PLoS One. 2010;5(5):e10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gebeh AK, Willets JM, Marczylo TH, Konje JC. Plasma anandamide and related n-acylethanolamide levels are not elevated in pregnancies complicated by hyperemesis gravidarum. J Matern Fetal Neonatal Med. 2014;27(9):954–959. [DOI] [PubMed] [Google Scholar]
- 39.Venkatesan T, Zadvornova Y, Raff H, Hillard CJ. Endocannabinoid-related lipids are increased during an episode of cyclic vomiting syndrome. Neurogastroenterol Motil. 2016;28(9):1409–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Sickle MD, Oland LD, Ho W, et al. Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology. 2001;121(4):767–774. [DOI] [PubMed] [Google Scholar]
- 41.Parker LA, Limebeer CL, Rock EM, Litt DL, Kwiatkowska M, Piomelli D The FAAH inhibitor URB-597 interferes with cisplatin- and nicotine-induced vomiting in the Suncus murinus (house musk shrew). Physiol Behav. 2009;97(1):121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han B, Wright R, Kirchhoff AM, et al. Quantitative LC-MS/MS analysis of arachidonoyl amino acids in mouse brain with treatment of FAAH inhibitor. Anal Biochem. 2013;432(2):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cravatt BF, Demarest K, Patricelli MP, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98(16):9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384(6604):83–87. [DOI] [PubMed] [Google Scholar]
- 45.Leung D, Saghatelian A, Simon GM, Cravatt BF. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry. 2006;45(15):4720–4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsuboi K, Okamoto Y, Ikematsu N, et al. Enzymatic formation of N-acylethanolamines from N-acylethanolamine plasmalogen through N-acylphosphatidylethanolamine-hydrolyzing phospholipase D-dependent and -independent pathways. Bba-Mol Cell Biol L. 2011;1811(10):565–577. [DOI] [PubMed] [Google Scholar]
- 47.Gabrielsson L, Mattsson S, Fowler CJ. Palmitoylethanolamide for the treatment of pain: pharmacokinetics, safety and efficacy. Br J Clin Pharmacol. 2016;82(4):932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cremon C, Stanghellini V, Barbara MR, et al. Randomised clinical trial: the analgesic properties of dietary supplementation with palmitoylethanolamide and polydatin in irritable bowel syndrome. Aliment Pharmacol Ther. 2017;45(7):909–922. [DOI] [PubMed] [Google Scholar]
- 49.Dinh TP, Carpenter D, Leslie FM, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99(16):10819–10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nomura DK, Morrison BE, Blankman JL, et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334(6057):809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sticht MA, Long JZ, Rock EM, et al. Inhibition of monoacylglycerol lipase attenuates vomiting in Suncus murinus and 2-arachidonoyl glycerol attenuates nausea in rats. Br J Pharmacol. 2012;165(8):2425–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Limebeer CL, Rock EM, Puvanenthirarajah N, Niphakis MJ, Cravatt BF, Parker LA. Elevation of 2-AG by monoacylglycerol lipase inhibition in the visceral insular cortex interferes with anticipatory nausea in a rat model. Behav Neurosci. 2016;130(2):261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sticht MA, Limebeer CL, Rafla BR, et al. Endocannabinoid regulation of nausea is mediated by 2-arachidonoylglycerol (2-AG) in the rat visceral insular cortex. Neuropharmacology. 2016;102:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCallum RW, Lin Z, Forster J, Roeser K, Hou Q, Sarosiek I. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9(4):314–319.e311. [DOI] [PubMed] [Google Scholar]
- 55.Pompeia C, Lima T, Curi R. Arachidonic acid cytotoxicity: can arachidonic acid be a physiological mediator of cell death? Cell Biochem Funct. 2003;21(2):97–104. [DOI] [PubMed] [Google Scholar]


