Abstract
Chimeric antigen receptor (CAR)‐T cell therapy has shown salient efficacy in cancer immunotherapy, particularly in the treatment of B cell malignancies. However, the efficacy of CAR‐T for solid tumors remains inadequate. In this study, we displayed that c‐met is an appropriate therapeutic target for papillary renal cell carcinoma (PRCC) using clinical samples, developed an anti‐human c‐met CAR‐T cells, and investigated the anti‐tumor efficacy of the CAR‐T cells using an orthotopic mouse model as pre‐clinical research. Administration of the anti‐c‐met CAR‐T cells induced marked infiltration of the CAR‐T cells into the tumor tissue and unambiguous suppression of tumor growth. Furthermore, in combination with axitinib, the anti‐tumor efficacy of the CAR‐T cells was synergistically augmented. Taken together, our current study demonstrated the potential for clinical application of anti‐c‐met CAR‐T cells in the treatment of patients with PRCC.
Keywords: axitinib, CAR‐T cells, c‐met, orthotopic model, papillary renal cell carcinoma
Administration of the anti‐human c‐met CAR‐T cells induced unambiguous suppression of the tumor growth in an orthotopic model of papillary renal cell carcinoma.
1. INTRODUCTION
Papillary renal cell carcinoma (PRCC) is the second most common type of renal cancer after clear cell renal cell carcinoma (CCRCC), accounting for approximately 10%‐20% of renal cancers. 1 , 2 , 3 It is classified into 2 main subtypes, type‐1 and type‐2. Type 1 is characterized by papillae and tubular structures covered with small cells containing basophilic cytoplasm and a small, uniform, oval nuclei. It is often multifocal but rarely recurs or metastasizes, and has a good prognosis, however in advanced cases the prognosis is poor. 4 , 5 , 6 Type 2 is characterized by papillae covered with large cells containing eosinophilic cytoplasm and large, spherical nuclei with prominent nucleoli. The prognosis of type 2 is generally more unfavorable compared with that of type 1 as it is often found as metastases. 4 , 5 , 6 In the treatment of advanced cases of PRCC, like that of CCRCC, tyrosine kinase inhibitors (TKIs) and/or mechanistic target of rapamycin inhibitors have sometimes been used empirically in clinical practice. However, the scientific evidence on their therapeutic efficacy has not been thoroughly demonstrated, and there are currently no effective forms of therapy for patients with advanced disease. 4 , 5 , 7 , 8
Hepatocyte growth factor receptor, or c‐mesenchymal‐epithelial transition factor (c‐met) is a cell surface protein tyrosine kinase. 9 , 10 , 11 , 12 It plays important roles not only in embryogenesis, organ development and differentiation but also in tumor cell migration, proliferation, and invasion in a variety of cancers including breast cancer, lung cancer, and renal cancer. 9 , 10 , 11 , 13 , 14 , 15 , 16 , 17 Based on these findings, drugs that target c‐met, such as TKIs and antibody‐drug conjugates, are currently under development, and crizotinib has been approved for non–small‐cell lung cancer in some countries. 18 , 19 Several previous reports have demonstrated that c‐met is also expressed in PRCC, 4 , 20 and clinical trials with TKIs targeting c‐met have been conducted. However, cases in which complete remission of the tumor was induced by such treatments were found to be rare. 21 , 22 Therefore, the establishment of novel therapies aimed at a radical cure of advanced PRCC is an important issue.
CAR is an engineered antigen receptor consisted of 3 components: an immunoglobulin single‐chain variable fragment (scFv) whose light and heavy chains are derived from a monoclonal antibody specific for a cancer cell surface antigen, a transmembrane domain, and intracellular signaling domains derived from costimulatory molecules such as CD3ζ, CD28, and 4‐1BB (CD137). 23 , 24 In many clinical cases, CAR‐T cells are generated by transfecting the CAR gene into autologous peripheral blood T cells prepared from patients using a lentiviral or retroviral vector. Unlike common T cells, CAR‐T cells are hidden from HLA restriction when detecting and attacking tumor cells. CAR‐T cell therapy targeting CD19 has exhibited impressive therapeutic efficacy in several B cell malignancies, and has been approved in many countries. 25 , 26 , 27 , 28 , 29 , 30 Anti‐c‐met CAR‐T cells have been developed, and clinical trials are being conducted for several types of tumors including breast cancer. 31 In addition, in general, the efficacy of CAR‐T cell therapy against solid tumors is reported to be limited, and many hurdles remain for its clinical application. 32 , 33 , 34 , 35
In this study, we generated an anti‐human c‐met CAR‐T cells with human T cells, and examined whether the CAR‐T cells exhibited therapeutic efficacy against PRCC. For this purpose, we established an orthotopic cancer model in which a human PRCC cell line positive for c‐met was injected into the kidneys of immunodeficient mice. Our current study revealed that human anti‐c‐met CAR‐T cells apparently induced therapeutic effects in the model. Moreover, we also demonstrated that the anti‐tumor activity of the CAR‐T cells was synergistically augmented in combination with axitinib.
2. MATERIALS AND METHODS
2.1. Patient samples
All the patients whose specimens were used in this study provided informed consent, and the use of tumor samples was approved by the Institutional Review Board of Yamaguchi University. The patient baseline characteristics are displayed in Table 1. The number of the patients was 33 (19 men and 14 women), and the median age was 66 y old. Based on the nature of the tumor cells, PRCC is classified as either type 1, type 2, or oncocytic variants, which show characteristics of both type 1 and type 2. 36 In this study, the proportion of type 1, type 2, and oncocytic variants was 24.2%, 57.6% and 18.2%, respectively. Patients had undergone radical or partial nephrectomy at 7 clinical centers in Yamaguchi prefecture between 2006 to 2016. Each diagnostic sample was reviewed by one of the authors (YN) 37 , 38 , 39 , 40 and at least one other independent pathologist. All the pathologists, including YN, were blinded to any clinical data.
TABLE 1.
Patient baseline characteristics
| Factor | Number | % |
|---|---|---|
| Age | ||
| 66≧ | 19 | 57.60% |
| 66< | 14 | 42.40% |
| Gender | ||
| Male | 24 | 72.70% |
| Female | 9 | 27.30% |
| Subtype | ||
| Type‐1 | 8 | 24.20% |
| Type‐2 | 19 | 57.60% |
| Oncocytic variant | 6 | 18.20% |
| cT | ||
| T1a | 19 | 57.60% |
| T1b | 8 | 24.20% |
| T2 | 6 | 18.20% |
| ≧T3 | 0 | 0% |
| cN | ||
| 0 | 33 | 100% |
| ≧1 | 0 | 0% |
| cM | ||
| 0 | 33 | 100% |
| 1 | 0 | 0% |
2.2. Mice and cell line
Male and female NOD. Cg‐Prkdc scid Il2rgtm1Wjl/SzJ (NSG) mice (6 wk old) were purchased from SLC (Shizuoka, Japan) and used for this study. The mice were maintained under specific pathogen‐free conditions in the animal facility at Yamaguchi University. A498, a human renal carcinoma cell line, was purchased from the American Type Culture Collection (Manassas, VA, USA). A498‐Luc, which stably expresses luciferase, and KMS11, a human myeloma cell line, were purchased from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). A498 was transduced with retroviral vector expressing GFP to establish A498‐GFP clones. Culture medium used for the A498 lines was Eagle's Minimum Essential Medium supplemented with 10% FCS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Culture medium used for KMS11 was RPMI‐1640 medium supplemented with 10% FCS, 100 U/ml penicillin, 100 μg/mL streptomycin, 50 μmol/L of 2‐mercaptoethanol, 25 mmol/L HEPES, and 2 mmol/L l‐glutamine.
2.3. Retroviral vectors for gene transfection into human T cells
Human PBMCs were collected from healthy volunteers, under the institutional approval of Yamaguchi University. The constructs of anti‐c‐met single‐chain variable fragment (scFv) were generated based on previous reports. 41 The scFv was fused with the transmembrane domain of human CD8α, and cytoplasmic regions of human CD28, 4‐1BB (CD137), and CD3ζ, to construct a 3rd generation CAR, which was then cloned into pMSGV1 to generate retroviral vectors. 41 , 42 Transduction of the CAR‐expressing retroviral vectors into human T cells was conducted as previously described, 41 , 43 with some modifications. Briefly, GP2‐293 packaging cells (Clontech, Mountain View, CA) were transfected with the CAR‐expressing plasmid together with p‐Ampho retrovirus packaging plasmid (Clontech) using Lipofectamine® Reagent (Thermo Fisher Scientific). Culture supernatants containing retroviral vectors were harvested and used for gene transduction. Activated healthy donor‐derived PBMCs were infected with viral supernatants in the presence of RetroNectin® (TaKaRa Bio, Kusatsu, Japan). Cells were incubated with OpTmizer (Gibco) supplemented with OpTmizer CTS, CTS Immune Cell serum replacement, l‐glutamine (Gibco), penicillin‐streptomycin sulfate, and amphotericin B for 5 d in the presence of IL‐2. Transduction efficiency of CAR was assessed using flow cytometry.
2.4. Flow cytometry
APC‐conjugated anti‐c‐met mAb (clone 95 106, R&D) was used to detect surface c‐met. CAR‐transduced T cells were stained with 6‐His tagged human c‐met fusion protein (R&D Systems) and secondary PE‐conjugated anti‐6‐His mAb (clone RM146, Abcam, Cambridge, UK), together with APC‐conjugated anti‐CD8α mAb (clone RPA‐T8, BioLegend). Zombie Yellow viability dye (BioLegend) and PE‐conjugated anti‐CD45 mAb (clone HI30, BioLegend) were used for the in vitro co‐culture assay. Human TruStain FcX (BioLegend) was used to block nonspecific binding of antigen‐specific antibodies with Fcγ receptors. Flow cytometry data were acquired using EC800 (SONY) or CytoFLEX (Beckman Coulter), and analyzed using FlowJo software (FlowJo, LLC.).
2.5. In vitro cytotoxicity assay
For in vitro cytotoxicity assay, CAR‐T or untransduced (hereafter referred to as UTD) T cells (1 × 105 /well) were co‐cultured with tumor cells at an effector to target (E:T) ratio of 1:3 or 1:5 for 48 h. The cultured cells were harvested and stained with Zombie Yellow viability dye and anti‐CD45 mAb, followed by flow cytometric analysis to detect the residual tumor cells and T cells. Concentrations of interferon (IFN)‐γ in the culture supernatants were measured by ELISA kits (BioLegend). To analyze the kinetics of the in vitro cytotoxicity, CAR‐T or UTD‐T cells (3 × 104 cells/well) were co‐cultured with A498‐GFP at an E:T ratio of 1:1 for 48 h, and images were taken on an IncuCyte S3 system (Sartorius) every 30 min.
2.6. In vivo orthotopic mouse model of PRCC
On day 0, 8 × 105 A498‐Luc with Matrigel (Corning) were injected into the subcapsular space of the left kidneys of NSG mice under anesthesia. On day 17, 1 × 106 or 3 × 106 CAR‐T or UTD‐T cells were injected intravenously (iv) through the tail vein. In some experiments, axitinib was administered orally once daily for 1 wk starting on day 17 after tumor inoculation. The dose (600 μg/mouse) was determined based on previous studies, in which the administration of axitinib alone was reported to induce weak or moderate anti‐tumor effects across several cancer models. 44 , 45 , 46 , 47 Tumor burden was periodically measured using the IVIS Spectrum In Vivo Imaging System (Perkin Elmer), and analyzed using Living Image Software (Perkin Elmer). In some experiments, tumor masses of A498‐Luc grown on the kidneys of NSG mice were resected and used for histological analyses.
2.7. Histopathological analysis
Formalin‐fixed and paraffin‐embedded tumor tissues derived from the patients or tumor‐bearing mice were applied to hematoxylin and eosin (H&E) staining conducted by Sojinkai (Ube, Japan). To assess the expression of c‐met on the patient specimens, immunohistochemistry (IHC) was performed using rabbit anti‐c‐met mAb (clone EPR19067, Abcam). The intensity of c‐met expression was classified into 4 levels (0 to + 3) based on the decision of pathologists and without prior information concerning clinical data and experimental settings. To evaluate the infiltration of CD8+ T cells into tumor tissues in our murine model, IHC with rabbit anti‐CD8 polyclonal Ab (Abcam) was conducted. Microscopy analyses for H&E stained samples and IHC sections were conducted using a BZ‐X710 fluorescence microscope (KEYENCE).
2.8. RNA in situ hybridization
To investigate the infiltration of CAR‐T cells into tumor tissues in our murine model, RNA in situ hybridization was carried out using RNA scope 2.5 HD Duplex detection Kit (Advanced Cell Diagnostics). The probe complementary to nucleotides of anti‐c‐met CAR scFv was designed and synthesized at Advanced Cell Diagnostics. Hybridization was performed in accordance with the manufacturer’s instruction, and then counterstaining was conducted with hematoxylin. Microscopic analysis was conducted with BZ‐X710 (KEYENCE).
2.9. Animal study approval
All mouse studies were conducted in accordance with national guidelines for the humane treatment of animals and were approved by the Institutional Animal Care and Use Committee (IACUC) at Yamaguchi University.
2.10. Statistics
Significances were determined by two‐sided Student unpaired t tests. P values less than .05 were considered as significant.
3. RESULTS
3.1. c‐met expression in PRCC clinical samples
To validate whether c‐met could be an appropriate candidate as a CAR target in PRCC, we first analyzed the expression levels of c‐met in clinical samples using immunohistochemistry. As shown in Figure 1 and Table 2, expression of c‐met was found in almost all samples (97%) regardless of the PRCC types. Particularly in type 1 and type 2, which represent the majority of PRCC, 100% and 95% of samples exhibited + 2 or higher intensity of c‐met expression, respectively (Table 2). In contrast, in normal renal tissues, the expression of c‐met was not detected, except for in some renal tubules where subtle expression was observed (Figure S1). These results suggested that c‐met would be a promising CAR target in PRCC.
FIGURE 1.
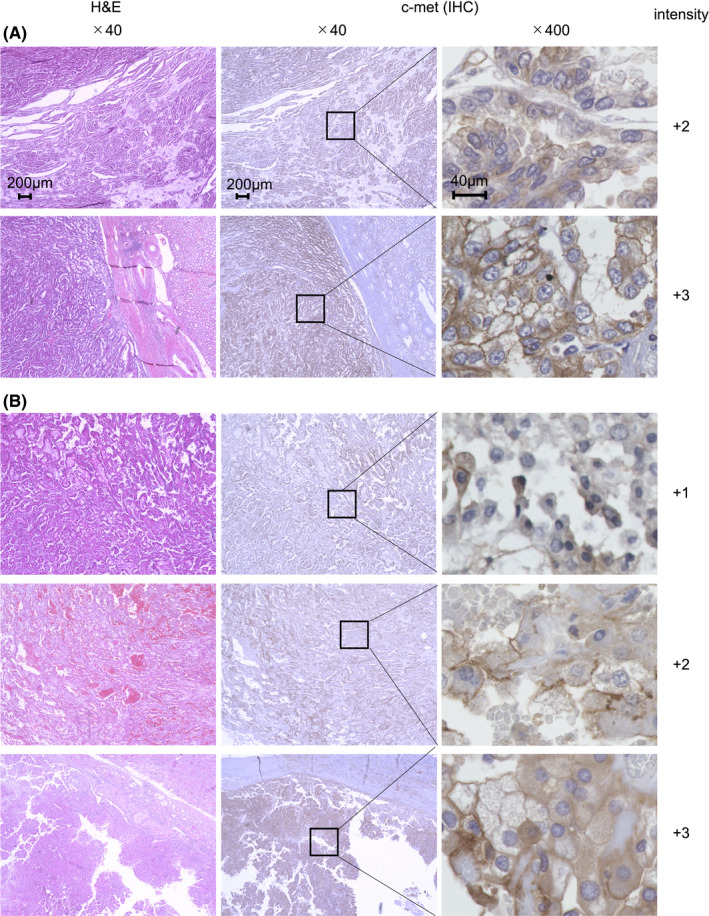
Expression of c‐met in clinical samples of PRCC. H&E staining and immunohistochemistry (IHC) for c‐met were conducted on clinical specimens of type 1 (A) and type 2 (B) PRCC. In IHC analysis, rabbit anti‐human c‐met mAb were employed for primary staining. Microscopic examination of H&E and IHC staining samples were conducted at ×40 or ×400 magnification. The values of c‐met intensity were indicated. Representative images are displayed
TABLE 2.
Expression of c‐met on clinical specimens
| PRCC subtype | c‐met protein expression (using IHC) | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Type‐1 | 0/8 (0%) | 0/8 (0%) | 4/8 (50.0%) | 4/8 (50.0%) |
| Type‐2 | 0/19 (0%) | 1/19 (5.3%) | 3/19 (15.8%) | 15/19 (78.9%) |
| Oncocytic variant | 1/6 (16.7%) | 1/6 (16.7%) | 0/6 (0%) | 4/6 (66.6%) |
| Total | 1/33 (3.0%) | 2/33 (6.1%) | 7/33 (21.2%) | 23/33 (69.7%) |
3.2. In vitro anti‐tumor response of the anti‐human c‐met CAR‐T cells
To investigate the tumoricidal efficacy of CAR‐T cells, we constructed an anti‐human c‐met CAR containing signaling motifs consisting of CD28, 4‐1BB and CD3ζ sequences (Figure S2A). When human peripheral blood T cells were transduced with retroviral vectors encoding anti‐c‐met CAR, the transduction efficiencies were approximately 60‐75% (Figure S2B).
Next, we assessed anti‐tumor effects of the CAR‐T cells in response to a human tumor cell line expressing endogenous c‐met on the cell surface. As a target tumor, a human renal carcinoma cell line A498, on which c‐met was highly expressed (Figure S2C), was employed. KMS11, a human myeloma cell line, was used as a c‐met‐negative control. When co‐cultured with those tumor cells, the CAR‐T cells, but not UTD‐T cells, induced not only significant reduction of the number of residual A498 but also an increase in the number of T cells, confirming the anti‐tumor capacity of the CAR‐T cells (Figure 2A, B). In contrast, the CAR‐T cells displayed no tumoricidal activity against KMS11, strongly suggesting the specificity of the anti‐c‐met CAR (Figure 2A, B). Moreover, significant production of IFN‐γ by the CAR‐T cells, but not by the UTD‐T cells, was induced during co‐culture with A498, while low levels of IFN‐γ were secreted during co‐culture with KMS11, verifying again the reactivity of the CAR‐T cells specific for the c‐met‐positive tumor (Figure 2C). In addition, we analyzed the kinetics of the tumoricidal effects exerted by the CAR‐T cells. The induction of anti‐tumor activity was found in the early phase of the co‐culture, and the activity persisted for more than 48 h (Figure 2D). Collectively, all these data from in vitro assays suggested that the anti‐c‐met CAR‐T cells exerted effective anti‐tumor activity specific for c‐met.
FIGURE 2.
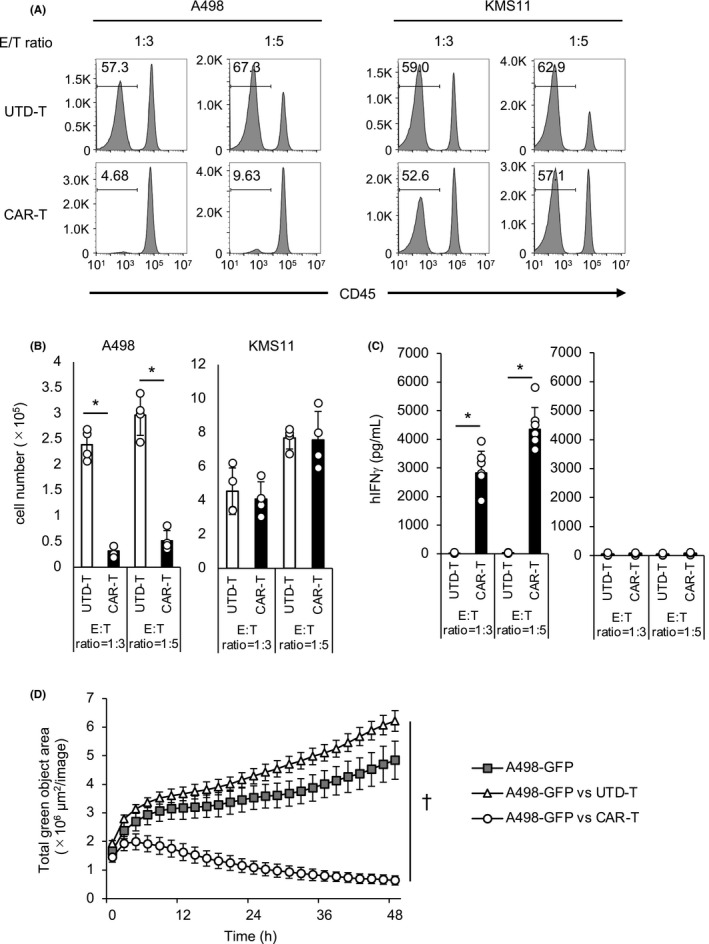
c‐met‐specific responses of the CAR‐T cells in vitro. A‐C, Anti‐c‐met CAR‐T or UTD‐T cells were co‐cultured with c‐met‐positive PRCC line A497 or c‐met‐negative myeloma line KMS11 for 2 d. The effector to target (E:T) ratios were 1:3 or 1:5. The percentages (A) and the numbers (B) of the residual tumor cells were analyzed using flow cytometry. The population negative for CD45 was considered as tumor cells. N = 4. C, The supernatants of the co‐cultured cells were harvested and the concentration of IFN‐γ was assessed using ELISA. N = 6. B,C, Data are shown as mean ± SEM of triplicate samples. D, A498‐GFP were co‐cultured with/without CAR‐T or UTD‐T cells at an E:T ratio of 1:1 for 48 h, and images were taken every 30 min. Data are shown as mean ± SEM. B‐D, * and † represent P <.05 and .001, respectively, calculated by two‐sided t test. Representative data from independent experiments are shown
3.3. Anti‐tumor efficacy of the anti‐c‐met CAR‐T cells in an orthotopic model of human PRCC
To investigate the therapeutic capacity of the anti‐c‐met CAR‐T cells, we established an orthotopic model of human PRCC. On day 0, A498‐Luc was injected into the subcapsular space of the left kidneys of immunodeficient NSG mice, and then the CAR‐T or UTD‐T cells were intravenously administered on day 17. Growth of the tumor was evaluated using bioluminescence imaging assessed with IVIS. Treatment with 3 × 106 CAR‐T cells induced an apparent suppression of tumor growth, and complete tumor regression was achieved in approximately 60% of the mice (Figure 3). Histological analysis with the resected tumors revealed that administration of the CAR‐T cells induced dense infiltrations of lymphocytes including CD8‐positive T cells and CAR‐T cells (Figure 4). By contrast, such infiltrations were elicited infrequently after the injection of UTD‐T cells, consistent with the ineffectiveness of the treatment (Figure 4). These results suggested the therapeutic potential of the anti‐c‐met CAR‐T cells in the orthotopic model of human PRCC. In addition, treatment with 1 × 106 CAR‐T cells exhibited only a temporary suppression of the tumor, and eventually resulted in the uncontrolled outgrowth, as seen for UTD‐T cells (Figure 3), indicating that there remains room for improvement for the anti‐c‐met CAR‐T cell therapy.
FIGURE 3.
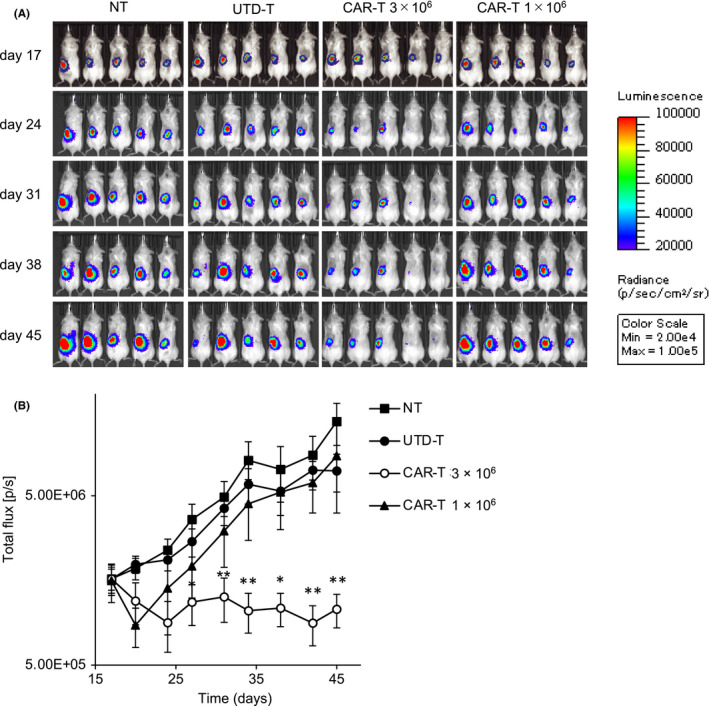
Anti‐tumor effects of the anti‐c‐met CAR‐T cells in orthotopic model of human PRCC. A498‐Luc (8 × 105 cells) with Matrigel were injected into the subcapsular space of left kidney of immunodeficient NSG mice on day 0, followed by iv injection of 1 × 106 or 3 × 106 CAR‐T or UTD‐T cells on day 17, or left nontreated (NT). Tumor growth was assessed using IVIS twice a week. A, Representative bioluminescence images of the mice are shown. B, Total flux of whole‐body bioluminescence measured using IVIS is shown as mean ± SEM. Data from 2 independent experiments are combined (NT, UTD‐T, CAR‐T 3 × 106: N = 10 per each group, CAR‐T 1 × 106: N = 5). * and ** represent P values <.05 and <.01, respectively, calculated by two‐sided t test
FIGURE 4.
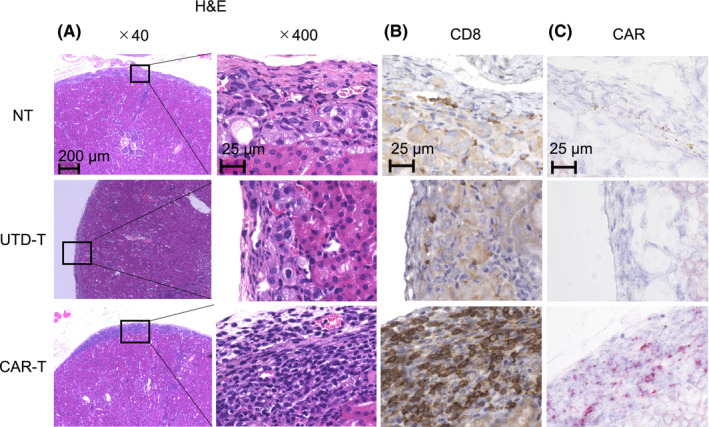
Infiltration of CD8+ lymphocytes including the CAR‐T cells into the tumor tissue. Kidneys were resected on day 21 from the mice treated with the same procedure as in Figure 3, and H&E staining (A), IHC (B) or RNA in situ hybridization (C) were performed. B, In IHC analysis, rabbit anti‐human CD8 polyclonal Ab was employed for primary staining. Positive cells were visualized in brown. C, In RNA in situ hybridization analysis, the probe complementary to nucleotides of anti‐c‐met CAR scFv was used. Positive cells were visualized in red. Microscopic examinations were conducted at ×40 or ×400 magnification. Representative images are displayed
3.4. Augmentation of anti‐c‐met CAR‐T cell therapy in combination with axitinib
Axitinib is a multi‐target inhibitor of vascular endothelial growth factor receptor 1 (VEGFR1), VEGFR2, VEGFR3, platelet‐derived growth factor receptor β, and c‐Kit, and is used widely in the treatment of CCRCC. In addition, to our knowledge, there are no results of large clinical studies on the treatment for PRCC with axitinib, which therefore has yet to be generalized for clinical use in PRCC patients. It has been reported recently that combination therapy with axitinib and pembrolizumab significantly prolonged periods of both overall survival and progression‐free survival (PFS) in patients with advanced CCRCC compared with monotherapy with sunitinib. 48 , 49 These reports prompted us to investigate the potential of combination therapy with anti‐c‐met CAR‐T cells and axitinib in the PRCC orthotopic model. Axitinib induced only a small anti‐tumor effect when used as a single agent or in combination with UTD‐T cells (Figure 5). In sharp contrast, the combination of axitinib and anti‐c‐met CAR‐T cells exerted obvious suppression of tumor growth (Figure 5). It is noteworthy that the dose of the CAR‐T cells was 1 × 106, and at this dose tumor growth could not be controlled without axitinib (Figure 3). These results strongly suggested that the combination of anti‐c‐met CAR‐T cells and axitinib synergistically augmented therapeutic capacity in the PRCC orthotopic model.
FIGURE 5.
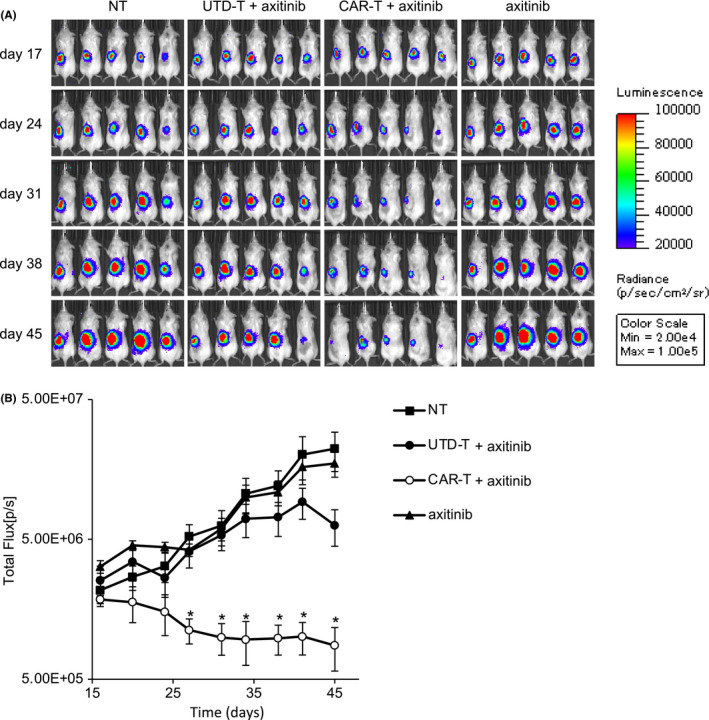
Augmentation of the anti‐tumor effects of the CAR‐T cells in combination with axitinib. A498‐Luc (8 × 105 cells) with Matrigel were injected into the subcapsular space of left kidney of immunodeficient NSG mice on day 0, followed by iv injection of 1 × 106 CAR‐T or UTD‐T cells on day 17, or left nontreated (NT). Axitinib (600 μg/mouse) was orally administered each day for 1 wk starting on day 17 after tumor inoculation. Tumor growth was assessed using IVIS twice week. A, Representative bioluminescence images of the mice are shown. B, Total flux of whole‐body bioluminescence measured using IVIS is shown as mean ± SEM. Data from 2 independent experiments are combined (N = 5 per each group). * represents P <.05 calculated using two‐sided t test
4. DISCUSSION
In this study, we first evaluated the adequacy of c‐met as a therapeutic target for PRCC with clinical specimens, and found that c‐met was expressed in almost all our clinical samples. This finding prompted us to generate anti‐human c‐met CAR‐T cells, and to evaluated their anti‐tumor capacity in a murine orthotopic PRCC model, in which human tumor cells were injected into the subcapsular space of kidneys of immunodeficient mice. The administration of CAR‐T cells induced significant anti‐tumor effect against the established solid tumor. To our knowledge, this is the first report demonstrating the therapeutic potential of anti‐c‐met CAR‐T cells against PRCC. Moreover, we displayed that the anti‐tumor efficacy of the CAR‐T cells was synergistically enhanced in combination with axitinib. This is also the first report in terms of clinical benefit for the combination of CAR‐T cells and axitinib.
Currently, several agents for PRCC are under development or in clinical trials. Savolitinib, a TKI targeting c‐met, has been shown to exert drastic anti‐tumor effects against PRCC using patient‐derived xenograft (PDX) models. 50 In the PDX models, savolitinib displayed anti‐tumor activity superior to that of sunitinib or crizotinib, which are approved for renal cell carcinoma and non–small‐cell lung cancer, respectively. Furthermore, promising results in the open‐label, randomized phase 3 clinical trial on savolitinib for PRCC (SAVOIR, ClinicalTrials.gov identifier: NCT03091192) have been reported. 51 In this clinical trial, PFS of savolitinib‐treated group was longer than in the sunitinib‐treated group, although median PFS was not statistically different between the 2 groups (7.0 mo for savolitinib vs 5.6 mo for sunitinib), however it is known that long‐term monotherapy with TKI often results in treatment resistance in tumors. Conversely, immunotherapies including CAR‐T cell therapy are expected to achieve potent clinical efficacy, which can often last for long periods without inducing resistance to treatment. 25 , 26 , 27 , 28 , 29 , 30 Therefore, CAR‐T cell therapy, including combination therapy with axitinib, would be a potential therapeutic option for advanced PRCC relapsed and/or refractory to conventional therapies including TKIs.
Although mechanisms underlying augmentation of therapeutic efficacy induced by the combination of CAR‐T cells and axitinib remain to be elucidated, infiltration of CAR‐T cells into tumors might be increased by the action of axitinib. Generally, insufficient accumulation of intravenously injected CAR‐T cells into tumor tissues is one of the major causes for the inefficiency of CAR‐T cell therapy against solid tumors. In addition, blockade of the interaction between vascular endothelial growth factor (VEGF) and VEGF receptor (VEGFR) with antibodies or TKIs including axitinib has been reported to normalize tumor vasculature consisting of immunosuppressive endothelial cells and to enhance T cell‐endothelial cell interaction, resulting in the enhancement of T cell infiltration in tumor tissues. 52 , 53 , 54 , 55 Furthermore, axitinib has the potential to reduce the immunosuppressive capacity of monocytic myeloid‐derived suppressor cells (MDSCs). 53 Interestingly, axitinib has also been reported to induce the differentiation of MDSCs into an antigen‐presenting phenotype. 47 , 53 Precise investigation of these mechanisms would be difficult in the current study, because the transferred human T cell including anti‐c‐met CAR‐T cells would not develop physiological interactions with mouse endothelial cells or antigen‐presenting cells due to the differences between species in xenogeneic mouse models. Further experiments using syngeneic models or clinical specimens are required and are planned for future studies.
The expression of c‐met is not strictly confined to cancer, and c‐met is found on several normal cell types, including epithelial cells, hepatocytes, and neurons. 10 , 56 Therefore, injection of anti‐c‐met CAR‐T cells might induce adverse events due to on‐target or off‐tumor toxicity. As we employed xenograft murine models in this study, the induction of adverse events could not be assessed. A previous report on an early‐phase clinical trial for metastatic breast cancer using anti‐c‐met CAR‐T cells demonstrated that no drug‐related adverse events greater than grade 1 were induced. 31 Nevertheless, it may be expected that some genetic modifications to improve safety, such as integration of suicide gene system, 57 , 58 , 59 should be applied on CAR‐T cells. Alternatively, decreasing the number of CAR‐T cells administered may also be beneficial in reducing the risk of adverse events. Combination with other agents including immune checkpoint inhibitors, TKIs such as axitinib, or chemotherapeutic drugs would help to reduce the required numbers of CAR‐T cells, as shown here.
Graft‐versus‐host disease due to xenogeneic T cell responses is inevitable in tumor models in which human CAR‐T cells are implanted into immunodeficient mice. 60 In addition, CAR‐T cells were generated from healthy donor‐derived T cells in this study, and, therefore, allogeneic reactions of CAR‐T cells were presumed to be induced due to HLA mismatch. Therefore, the anti‐tumor effects in our in vivo model might be the sum of both tumor‐specific and nonspecific responses, indicating a possible overestimation. However, as the UTD‐T cells exerted only a small anti‐tumor effect in our models, the nonspecific responses of the CAR‐T cells due to xenogeneic and/or allogeneic reactions would be negligible.
In this study, we used conventional CAR‐T cells and found that a relatively large number of CAR‐T cells (3 × 106/mouse) were required to induce sufficient anti‐tumor responses in the orthotopic model. To improve the therapeutic efficacy, we combined CAR‐T cells with axitinib. Conversely, novel CAR‐T cells that conferred unique functions have been generated to exert therapeutic effects more efficiently against solid tumors. To overcome the immunosuppressive environment of tumor tissues, CAR‐T cells with the capacity to produce anti‐PD‐1 scFv have been developed. 61 , 62 In another concept, to enhance infiltration, accumulation, and survival of CAR‐T cells in solid tumors, we have recently reported CAR‐T cells expressing IL‐7 and CCL19 simultaneously. 63 It would also be reasonable to convert the conventional anti‐c‐met CAR‐T cells employed in this study to such next‐generation CAR systems to enhance anti‐tumor capacity.
In conclusion, in this study, we demonstrated for the first time that anti‐c‐met CAR‐T cell therapy can be applied to PRCC and that anti‐tumor efficacy of CAR‐T cells can be enhanced by combination with axitinib. Although careful evaluation with other models, such as syngeneic models or PDX models, is still required, our current results clearly support and promote translational research to develop novel therapies using anti‐c‐met CAR‐T cells against intractable PRCC.
DISCLOSURE
Koji Tamada and Yukimi Sakoda hold stocks in Noile‐Immune Biotech Inc and receive remuneration from Noile‐Immune Biotech Inc. Koji Tamada received lecture fees from Ono Pharmaceutical, MSD, and Chugai Pharmaceutical. Hideyasu Matsuyama received a lecture fee from MSD. Koji Tamada received research funds from Noile‐Immune Biotech Inc and Chugai Pharmaceutical. Yukimi Sakoda received a research fund from Noile‐Immune Biotech. Other authors declare no conflict of interest.
Supporting information
Fig S1‐S2
ACKNOWLEDGMENTS
The authors would thank Daisuke Umezu and Yasunori Iida for excellent advice on the study. The authors would also thank Hiromi Kurosawa, Mihoko Ida, Nana Okada, Makiko Miyamoto, Satoshi Tatekabe, Nanami Nakamura for their excellent technical supports.
Mori J‐I, Adachi K, Sakoda Y, et al. Anti‐tumor efficacy of human anti‐c‐met CAR‐T cells against papillary renal cell carcinoma in an orthotopic model. Cancer Sci. 2021;112:1417–1428. 10.1111/cas.14835
Contributor Information
Keishi Adachi, Email: fairkid@yamaguchi-u.ac.jp.
Koji Tamada, Email: fairkid@yamaguchi-u.ac.jp, Email: ktamada@yamaguchi-u.ac.jp.
REFERENCES
- 1. Wang L, Williamson SR, Wang M, et al. Molecular subtyping of metastatic renal cell carcinoma: implications for targeted therapy. Mol Cancer. 2014;13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang XJ, Tan MH, Kim HL, et al. A molecular classification of papillary renal cell carcinoma. Cancer Res. 2005;65:5628‐5637. [DOI] [PubMed] [Google Scholar]
- 3. Sanders ME, Mick R, Tomaszewski JE, Barr FG. Unique patterns of allelic imbalance distinguish type 1 from type 2 sporadic papillary renal cell carcinoma. Am J Pathol. 2002;161:997‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Linehan WM, Spellman PT, Ricketts CJ, et al. Comprehensive Molecular Characterization of Papillary Renal‐Cell Carcinoma. N Engl J Med. 2016;374:135‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vikram R, Ng CS, Tamboli P, et al. Papillary renal cell carcinoma: radiologic‐pathologic correlation and spectrum of disease. Radiographics. 2009;29(3):741–754. 10.1148/rg.293085190 [DOI] [PubMed] [Google Scholar]
- 6. Delahunt B, Eble JN, McCredie MR, Bethwaite PB, Stewart JH, Bilous AM. Morphologic typing of papillary renal cell carcinoma: comparison of growth kinetics and patient survival in 66 cases. Hum Pathol. 2001;32:590‐595. [DOI] [PubMed] [Google Scholar]
- 7. Courthod G, Tucci M, Di Maio M, Scagliotti GV. Papillary renal cell carcinoma: a review of the current therapeutic landscape. Crit Rev Oncol Hematol. 2015;96:100‐112. [DOI] [PubMed] [Google Scholar]
- 8. Aparicio LMA, Fernandez IP, Cassinello J. Tyrosine kinase inhibitors reprogramming immunity in renal cell carcinoma: rethinking cancer immunotherapy. Clin Transl Oncol. 2017;19:1175‐1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bylicki O, Paleiron N, Assié JB, Chouaïd C. Targeting the MET‐signaling pathway in non‐small‐cell lung cancer: evidence to date. Onco Targets Ther. 2020;13:5691‐5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sakai K, Aoki S, Matsumoto K. Hepatocyte growth factor and Met in drug discovery. J Biochem. 2015;157:271‐284. [DOI] [PubMed] [Google Scholar]
- 11. Organ SL, Tsao MS. An overview of the c‐MET signaling pathway. Ther Adv Med Oncol. 2011;3:S7‐S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bottaro DP, Rubin JS, Faletto DL, et al. Identification of the hepatocyte growth factor receptor as the c‐met proto‐oncogene product. Science. 1991;251:802‐804. [DOI] [PubMed] [Google Scholar]
- 13. Ghoussoub RA, Dillon DA, D'Aquila T, Rimm EB, Fearon ER, Rimm DL. Expression of c‐met is a strong independent prognostic factor in breast carcinoma. Cancer. 1998;82:1513‐1520. [DOI] [PubMed] [Google Scholar]
- 14. Awad MM, Oxnard GR, Jackman DM, et al. MET Exon 14 mutations in non‐small‐cell lung cancer are associated with advanced age and stage‐dependent MET genomic amplification and c‐Met overexpression. J Clin Oncol. 2016;34:721‐730. [DOI] [PubMed] [Google Scholar]
- 15. Linehan WM, Vasselli J, Srinivasan R, et al. Genetic basis of cancer of the kidney: disease‐specific approaches to therapy. Clin Cancer Res. 2004;10:6282s‐6289s. [DOI] [PubMed] [Google Scholar]
- 16. Linehan WM, Srinivasan R, Schmidt LS. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol. 2010;7:277‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gibney GT, Aziz SA, Camp RL, et al. c‐Met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann Oncol. 2013;24:343‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK‐positive lung cancer. N Engl J Med. 2013;368:2385‐2394. [DOI] [PubMed] [Google Scholar]
- 19. Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK‐positive non‐small‐cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sweeney P, El‐Naggar AK, Lin SH, Pisters LL. Biological significance of c‐met over expression in papillary renal cell carcinoma. J Urol. 2002;168:51‐55. [PubMed] [Google Scholar]
- 21. Schöffski P, Wozniak A, Escudier B, et al. Crizotinib achieves long‐lasting disease control in advanced papillary renal‐cell carcinoma type 1 patients with MET mutations or amplification. EORTC 90101 CREATE trial. Eur J Cancer. 2017;87:147‐163. [DOI] [PubMed] [Google Scholar]
- 22. Choueiri TK, Plimack E, Arkenau HT, et al. Biomarker‐based phase II trial of Savolitinib in patients with advanced papillary renal cell cancer. J Clin Oncol. 2017;35:2993‐3001. [DOI] [PubMed] [Google Scholar]
- 23. Maude SL, Teachey DT, Porter DL, Grupp SA. CD19‐targeted chimeric antigen receptor T‐cell therapy for acute lymphoblastic leukemia. Blood. 2015;125:4017‐4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T‐cells forward. Nat Rev Clin Oncol. 2016;13:370‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor‐modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor‐modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509‐1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B‐Cell Lymphoma. N Engl J Med. 2019;380:45‐56. [DOI] [PubMed] [Google Scholar]
- 29. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T‐cell therapy in refractory large B‐Cell Lymphoma. N Engl J Med. 2017;377:2531‐2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 results of ZUMA‐1: a multicenter study of KTE‐C19 anti‐CD19 CAR T cell therapy in refractory aggressive Lymphoma. Mol Ther. 2017;25:285‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tchou J, Zhao Y, Levine BL, et al. Safety and efficacy of intratumoral injections of Chimeric Antigen Receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol Res. 2017;5:1152‐1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Newick K, O'Brien S, Moon E, Albelda SM. CAR T Cell Therapy for Solid Tumors. Annu Rev Med. 2017;68:139‐152. [DOI] [PubMed] [Google Scholar]
- 33. Zhang E, Gu J, Xu H. Prospects for chimeric antigen receptor‐modified T cell therapy for solid tumors. Mol Cancer. 2018;17:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guedan S, Ruella M, June CH. Emerging cellular therapies for cancer. Annu Rev Immunol. 2019;37:145‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17:147‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lefèvre M, Couturier J, Sibony M, et al. Adult papillary renal tumor with oncocytic cells: clinicopathologic, immunohistochemical, and cytogenetic features of 10 cases. Am J Surg Pathol. 2005;29:1576‐1581. [DOI] [PubMed] [Google Scholar]
- 37. Ishihara H, Yamashita S, Liu YY, et al. Genetic and epigenetic profiling indicates the proximal tubule origin of renal cancers in end‐stage renal disease. Cancer Sci. 2020;111:4276‐4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mikami S, Kuroda N, Nagashima Y, et al. Classification of solid renal tumor with oncocytic/eosinophilic cytoplasm: is hybrid oncocytic/chromophobe renal tumor a subtype of oncocytoma, chromophobe renal cell carcinoma, or a distinct tumor entity? Ann Transl Med. 2019;7:S350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ohashi R, Schraml P, Angori S, et al. Classic chromophobe renal cell carcinoma incur a larger number of chromosomal losses than seen in the eosinophilic subtype. Cancers (Basel). 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Furuya M, Hasumi H, Yao M, Nagashima Y. Birt‐Hogg‐Dubé syndrome‐associated renal cell carcinoma: histopathological features and diagnostic conundrum. Cancer Sci. 2020;111:15‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tamada K, Geng D, Sakoda Y, et al. Redirecting gene‐modified T cells toward various cancer types using tagged antibodies. Clin Cancer Res. 2012;18:6436‐6445. [DOI] [PubMed] [Google Scholar]
- 42. Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843‐851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T‐cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wilmes LJ, Pallavicini MG, Fleming LM, et al. AG‐013736, a novel inhibitor of VEGF receptor tyrosine kinases, inhibits breast cancer growth and decreases vascular permeability as detected by dynamic contrast‐enhanced magnetic resonance imaging. Magn Reson Imaging. 2007;25:319‐327. [DOI] [PubMed] [Google Scholar]
- 45. Fenton BM, Paoni SF. The addition of AG‐013736 to fractionated radiation improves tumor response without functionally normalizing the tumor vasculature. Cancer Res. 2007;67:9921‐9928. [DOI] [PubMed] [Google Scholar]
- 46. Hu‐Lowe DD, Zou HY, Grazzini ML, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG‐013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008;14:7272‐7283. [DOI] [PubMed] [Google Scholar]
- 47. Yuan H, Cai P, Li Q, et al. Axitinib augments antitumor activity in renal cell carcinoma via STAT3‐dependent reversal of myeloid‐derived suppressor cell accumulation. Biomed Pharmacother. 2014;68:751‐756. [DOI] [PubMed] [Google Scholar]
- 48. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for advanced renal‐cell carcinoma. N Engl J Med. 2019;380:1116‐1127. [DOI] [PubMed] [Google Scholar]
- 49. Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non‐randomised, open‐label, dose‐finding, and dose‐expansion phase 1b trial. Lancet Oncol. 2018;19:405‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schuller AG, Barry ER, Jones RD, et al. The MET inhibitor AZD6094 (Savolitinib, HMPL‐504) induces regression in papillary renal cell carcinoma patient‐derived xenograft models. Clin Cancer Res. 2015;21:2811‐2819. [DOI] [PubMed] [Google Scholar]
- 51. Choueiri TK, Heng DYC, Lee JL, et al. Efficacy of Savolitinib vs Sunitinib in patients with MET‐driven papillary renal cell carcinoma: the SAVOIR phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1247‐1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171‐6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Du Four S, Maenhout SK, De Pierre K, et al. Axitinib increases the infiltration of immune cells and reduces the suppressive capacity of monocytic MDSCs in an intracranial mouse melanoma model. Oncoimmunology. 2015;4:e998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang Y, Yuan J, Righi E, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109:17561‐17566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Georganaki M, van Hooren L, Dimberg A. Vascular targeting to increase the efficiency of immune checkpoint blockade in cancer. Front Immunol. 2018;9:3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cui JJ. Targeting receptor tyrosine kinase MET in cancer: small molecule inhibitors and clinical progress. J Med Chem. 2014;57:4427‐4453. [DOI] [PubMed] [Google Scholar]
- 57. Duong CP, Yong CS, Kershaw MH, Slaney CY, Darcy PK. Cancer immunotherapy utilizing gene‐modified T cells: from the bench to the clinic. Mol Immunol. 2015;67:46‐57. [DOI] [PubMed] [Google Scholar]
- 58. Lal S, Lauer UM, Niethammer D, Beck JF, Schlegel PG. Suicide genes: past, present and future perspectives. Immunol Today. 2000;21:48‐54. [DOI] [PubMed] [Google Scholar]
- 59. Greco R, Oliveira G, Stanghellini MT, et al. Improving the safety of cell therapy with the TK‐suicide gene. Front Pharmacol. 2015;6:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Walsh NC, Kenney LL, Jangalwe S, et al. Humanized mouse models of clinical disease. Annu Rev Pathol. 2017;12:187‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nakajima M, Sakoda Y, Adachi K, Nagano H, Tamada K. Improved survival of chimeric antigen receptor‐engineered T (CAR‐T) and tumor‐specific T cells caused by anti‐programmed cell death protein 1 single‐chain variable fragment‐producing CAR‐T cells. Cancer Sci. 2019;110:3079‐3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rafiq S, Yeku OO, Jackson HJ, et al. Targeted delivery of a PD‐1‐blocking scFv by CAR‐T cells enhances anti‐tumor efficacy in vivo. Nat Biotechnol. 2018;36:847‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, Tamada K. IL‐7 and CCL19 expression in CAR‐T cells improves immune cell infiltration and CAR‐T cell survival in the tumor. Nat Biotechnol. 2018;36:346‐351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S2


