Abstract
Although our current knowledge of the pathophysiology of COVID-19 is still fragmentary, the information so far accrued on the tropism and life cycle of its etiological agent SARS-CoV-2, together with the emerging clinical data, suffice to indicate that the severe acute pulmonary syndrome is the main, but not the only manifestation of COVID-19. Necropsy studies are increasingly revealing underlying endothelial vasculopathies in the form of micro-haemorrhages and micro-thrombi. Intertwined with defective antiviral responses, dysregulated coagulation mechanisms, abnormal hyper-inflammatory reactions and responses, COVID-19 is disclosing a wide pathophysiological palette. An additional property in categorising the disease is the combination of tissue (e.g. neuro- and vasculo-tropism) with organ tropism, whereby the virus preferentially attacks certain organs with highly developed capillary beds, such as the lungs, gastrointestinal tract, kidney and brain. These multiple clinical presentations confirm that the acute respiratory syndrome as described initially is increasingly unfolding as a more complex nosological entity, a multiorgan syndrome of systemic breadth. The neurological manifestations of COVID-19, the focus of this review, reflect this manifold nature of the disease.
Keywords: COVID-19, SARS-CoV-2, Neurotropism, Brain, Neurological complications, Viral infection, Central nervous system
Highlights
-
•
The original severe acute pulmonary syndrome is the main, but not the only manifestation of COVID-19.
-
•
One year of basic and clinical research indicate that COVID-19 is a much more complex multisystemic nosological entity.
-
•
SARS-CoV-2 tropism for vascular endothelium, epithelial mucosae and nervous tissue determines pathophysiological mechanisms.
1. Introduction
Due to a multiplicity of factors including the presenting symptomatology and the kindredness of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with other related coronaviruses (CoVs) like severe acute respiratory syndrome CoV (SARS-CoV) and the Middle East respiratory syndrome CoV (MERS-CoV), the CoV disease 2019 (COVID-19) was initially categorized as severe pneumonia (Sun et al., 2020). Most cases of the disease, especially those admitted to intensive care units (ICUs), were diagnosed, and treated as such (Wang et al., 2020a; Zhou et al., 2020a; Arentz et al., 2020; Chen et al., 2020a; Jain and Yuan, 2020). The categorization of severity -mild, not requiring respiratory aid; moderate, requiring some form of respiratory assistance; and severe, requiring obligatory mechanical respiratory support-was also dominated by the pulmonary pathology and respiratory dysfunction (Mao et al., 2020). However, as we learn more about the new viral disease, limiting it to a severe acute respiratory syndrome has proved to be neither an accurate not a comprehensive description of this nosological entity. Coronaviruses (CoVs) as a family cause respiratory, gastrointestinal, nephrological, and neurological disease, but SARS-CoV-2 can adopt any of these manifestations and several more.
As the pandemic enters its second year, the evolution of the disease has given rise to additional clinical forms associated with long-term chronic variants of the disease (post-COVID-19 syndrome, long COVID-19, long-haulers) (Antonini, 2020; Baig, 2020; Heneka et al., 2020; Fotuhi et al., 2020), thus blurring the initial circumscription of COVID-19 to an acute syndrome, as the acronym of its causative agent conveys. The persistence of the disease can be manifest as lingering symptoms (e.g. dysosmias persisting for months) or as new symptoms not present during the acute phase (e.g. confusion, memory loss). In a study conducted on 478 COVID-19 patients 4 months after hospital discharge, 51% had at least one new symptom: 31% fatigue, 21% cognitive symptoms and 16 new-onset dyspnoea (Group TWCftCS, 2021).
Undoubtedly the most important sequelae of the long-term and chronic forms of the disease are of a psychological and neuropsychiatric nature and are already being manifested in the increased incidence among COVID-19 patients in the mid- and long-term post-infective phases of the disease (Rogers et al., 2020). Psychological burnout, “pandemic fatigue” and in more severe cases post-critical illness or post-traumatic chronic stress syndrome (PTSD) are affecting an alarming number of patients. PTSD has been associated with the depression of the immune system that may follow acute exacerbated immune responses (Liang et al., 2020a). An early study of 714 clinically stable post-COVID-19 patients indicated that 96.2% of them suffered from PTSD (Bo et al., 2020).
An additional group of post-acute COVID-19 patients consists of persistently infected but asymptomatic and pre-symptomatic carriers (Rasmussen and Popescu, 2021) and patients with only mild symptoms. The former set of patients constitutes an important source of contagion (see recent review in (Gao et al., 2021)) which is difficult to assess from an epidemiological viewpoint. Asymptomatic carriage has been coined the Achilles’ heel of COVID-19 control strategies (Gandhi et al., 2020). Immunocompromised patients are another source of contagion, shedding virions for several months after remission of the acute phase of the disease (Avanzato et al., 2020). Millions of immunosuppressed patients suffering acquired immunodeficiency syndrome (~26 million in Africa alone) and tuberculosis are at risk of contracting severe forms of COVID-19.
Reviews and literature meta-analyses on the neurological aspects of COVID-19 (Berger, 2020; Zubair et al., 2020; De Felice et al., 2020; Montalvan et al., 2020; Gklinos, 2020; Leonardi et al., 2020; Vonck et al., 2020) point to two types of neurological affectation, namely para-infectious and post-infectious sequelae, and three main pathogenic mechanisms of central nervous system (CNS) affectation in COVID-19, with some possible overlap: a) direct primary damage of the CNS parenchyma, a rare occurrence; b) hyper-inflammatory response syndrome (cytokine release syndrome, “cytokine storm”), mostly observed in severe forms of COVID-19, overemphasized during the initial months of the pandemic but statistically not a frequent occurrence either according to ref (Mudd et al., 2020) and c) systemic sepsis, which may evolve to complications like viral encephalitis or viral encephalopathy, and death.
I have recently discussed the current knowledge of the SARS-CoV-2 structure and biology with a view to developing prophylactic or therapeutic drugs (Barrantes, 2020a) and some entry points and routes followed by the virus to produce pathogenic effects on the nervous system (Barrantes, 2020b). In addition to the bronchopulmonary epithelium, two important primary sources are the nasal and the intestinal mucosae, because of the anatomical vicinity to the forebrain of the former and the extensive surface and hence greater capacity to produce massive replication and release of virions into the bloodstream of the latter. In this review I critically examine possible pathophysiological scenarios underlying the neurological manifestation of the disease. To do so I exploit the still fragmentary knowledge so far gained on SARS-CoV-2, focusing on the direct and indirect pathogenic mechanisms exerted by the virus on the endothelial cell and on the intestinal tract-CNS connection.
The reader is referred to recent reviews on COVID-19 general clinical aspects (Richardson et al., 2020; Guan et al., 2020), endocrinological features (Stefan et al., 2021), neuropsychiatric aspects (Rogers et al., 2020; Alonso-Lana et al., 2020; Wang et al., 2021a, 2021b), brain-immune axis of the disease (Wang et al., 2021b; Peters et al., 2021), drug repurposing ((Barrantes, 2020a; Cavasotto and Di Filippo, 2021) microbiology (Fung and Liu, 2019) and epidemiology (Su et al., 2016) of Coronaviruses, and of SARS-CoV-2 biology and evolutionary aspects (Li et al., 2020a) or physicochemical aspect impinging on airborne transmission of the virus (Scheller et al., 2020).
2. Evolution of the COVID-19 clinical and neurological picture
Typical reports of COVID-19 presentation among individuals admitted to hospital during the initial months of the pandemic included lower respiratory tract infection and fever, dry cough, and dyspnoea (Huang et al., 2020). Fever is still the most frequent sign, observed in up to 90% of patients (Guan et al., 2020), followed by bilateral lung infiltration with ground-glass opacity (50%) in thoracic CT scans, and lymphopenia, a possible consequence of the combination of T lymphocyte destruction by the virus and hampered lymphopoiesis (Wang et al., 2020a; Guan et al., 2020). But in addition to the dominant pulmonary affectation, other clinical manifestations reflecting involvement of other organs have become progressively apparent. Gastrointestinal (Jin et al., 2020; Parasa et al., 2020; Zhou et al., 2020b; Ding and Liang, 2020), multi-organ thrombotic and thromboembolic disease (Bikdeli et al., 2020) (Jain and Yuan, 2020), and sepsis with microcirculatory compromise (Colantuoni et al., 2020; Magro et al., 2020) were rapidly added to the predominant respiratory syndrome. Detailed accounts of the respiratory affectation and other clinical aspect of the disease can be found in recent reviews (Richardson et al., 2020; Guan et al., 2020; del Rio and Malani, 2020; Wiersinga et al., 2020).
The neurological compromise in COVID-19 patients was already described in early studies: of 214 COVID-19 patients 36.4% presented neurological symptoms (Mao et al., 2020). A similar study found that 36% of patients reported CNS symptoms (impaired consciousness) and peripheral symptoms (e.g. paraesthesias) (Wu et al., 2020a). A larger study (1099 patients) described other neurological manifestations like myalgias (14.9%) and headache (13.6%) (Guan et al., 2020). One of the first European studies found a higher percentage (57.4%) of neurological manifestations among 841 Spanish COVID-19 patients, predominantly male, with myalgias and headaches as main symptoms (Romero-Sánchez et al., 2020). Myopathies with elevated creatine kinase levels and axonal polyneuropathies had already been described for SARS patients 2–4 weeks into the course of the virosis (Tsai et al., 2004, 2005). In COVID-19, a reported case of myopathy with high creatine kinase levels was found to be a type I interferonopathy in response to SARS-CoV-2 infection (Manzano et al., 2020). Rhabdomyolysis, Guillain-Barré syndrome (De Felice et al., 2020; Wu et al., 2020a; Carod-Artal, 2020; Pleasure et al., 2020) and myoclonus (Rábano-Suárez et al., 2020) have also been reported. Unlike the syndrome caused by other viruses that induce demyelinating forms of Guillain-Barré, COVID-19 is associated with axonal or sensory pathologies. Importantly, it became apparent that the type of neurological presentations in COVID-19 patients was associated with the severity of the disease; stroke, seizures, ataxia, depressed consciousness and myopathies occur more frequently in the severe forms (45.5%) than in the less severe forms (30.2%) (Mao et al., 2020) and constitute in several cases life-threatening neurological complications of the disease (Carod-Artal, 2020).
Neurodegenerative diseases and particularly Alzheimer disease and Parkinson disease share some common clinical manifestations with COVID-19: 1) dysosmias at early stages of Alzheimer disease (see literature meta-analysis in (Silva et al., 2018)) or both anosmia and dysgeusia in Parkinson disease (Tarakad and Jankovic, 2017; Poewe et al., 2017; Otero-Losada et al., 2020); 2) histopathological alterations of the olfactory mucosa early on (Talamo et al., 1989) (Perry et al., 2003) and subsequent degeneration of the olfactory bulb (Murphy et al., 1990) in the course of both diseases, the latter progressing in parallel with the evolution from mild cognitive impairment to full dementia (Bathini et al., 2019). Pathophysiological findings of aggregated α-synuclein have been tentatively associated with hyposmia in Parkinson disease (Poewe et al., 2017). The possibility has recently been formulated that sustained inflammation and increased levels of α-synuclein in the course of COVID-19, as observed with West Nile virus and Western encephalitis virus infections, leads to the formation of aggregates akin to those of Parkinson disease (Brundin et al., 2020). Intensive research in the Alzheimer disease field has been devoted to developing strategies to deliver putative therapeutic agents into the brain; e.g. intranasal application has been used in these attempts to circumvent the blood-brain barrier (BBB) in an effort to reduce systemic effects on other organs, avoid first-pass hepatic metabolism, and exploit the higher bioavailability and faster pharmacokinetics of the intransal route (Erdő et al., 2018). Cell-penetrating peptides have been employed to catalyse the nasal-to-brain transport of macromolecules. Upon conjugation with a low molecular weight protamine, administration of nanoparticles and proteins into brain was shown to be facilitated by the conjugates (Lin et al., 2016).
Parkinson disease as a neurological comorbidity was initially considered a rare occurrence (Sulzer et al., 2020), but more recently single-case reports indicate the development of (probably) progressive parkinsonism 2–5 weeks after infection with SARS-CoV-2. In one such case the patient presented myoclonus and an asymmetrical hypokinetic-rigid syndrome after severe COVID-19 (Méndez-Guerrero et al., 2020). Two other cases also followed severe COVID-19 (Cohen et al., 2020; Faber et al., 2020); the three patients showed brain imaging manifestations of nigrostriatal dopamine system hypoactivity. Although it cannot be established whether the SARS-CoV-2 infection acted as a trigger or catalyser of the parkinsonism or is a mere coincidental occurrence, the motor symptomatology is highly suggestive of a causative relationship (Brundin et al., 2020). Merello and coworkers discuss three different scenarios of transient or permanent parkinsonism following viral infections (Merello et al., 2020). The pathophysiology of Parkinson disease associated with COVID-19 is still not clear, but SARS-CoV-2 infection may trigger α-synuclein upregulation, followed by immune reactions resulting from α-synuclein deposition; glial compromise may ensue, further amplifying the immune response as a product of cytokine and chemokine production by the microglia. Microglia cell response is also known to be elicited by α-synuclein (Awogbindin et al., 2020).
Cases of myasthenia gravis have been reported in association with COVID-19 (Restivo et al., 2020; Finsterer et al., 2020), raising the possibility that autoimmune antibodies against SARS-CoV-2 epitopes might also react against the nicotinic acetylcholine receptor or other molecular constituents of the neuromuscular junction (Paz and Barrantes, 2019).
Patients with severe forms of COVID-19 and requiring ICU care are more prone to develop multiple organ system dysfunction, presenting in some cases complications of a neurological nature, such as ischemic and cerebrovascular disease (Mao et al., 2020; Wu et al., 2020a; Li et al., 2020b; Sharifi-Razavi et al., 2020; Pezzini and Padovani, 2020). Multicentre retrospective-prospective studies of neurological manifestations in COVID-19 are beginning to appear (Ferrarese et al., 2020). This type of study is needed to better ascertain the actual incidence of neurological compromises in the viral disease on a more solid statistical basis.
Chronic immune dysregulation is often invoked as a factor that predisposes patients with Down syndrome to be more vulnerable to pulmonary infections and higher mortality rates from pneumonia and sepsis. Thus trisomy 21 patients may exhibit a higher risk of developing severe forms of COVID-19 in case of SARS-CoV-2 infection (Espinosa, 2020).
3. Mild neurological symptoms: dysosmias and dysgeusias
The hundreds to thousands of genes of odour receptors make the olfactory system unique among sensory systems (Brann and Datta, 2020). Loss of smell (anosmia) and taste (dysgeusia) are by now well-established early symptoms of COVID-19, although during the early months of the pandemic these neurological symptoms were not necessarily associated with the disease and were initially reported by otorhinolaryngologists (Lüers et al., 2020). One of the earliest reports of dysosmias was the finding of hyposmia among COVID-19 patients in Wuhan, China (Mao et al., 2020). Of 10,069 patients studied in Tehran, Iran, 48.2% exhibited anosmia or hyposmia, with abrupt installation of the anosmia in 76% of the cases. Of these latter patients, 83.4% also exhibited dysgeusia (Bagheri et al., 2020). Olfactory and gustatory disorders were also reported in 34% of COVID-19 patients in Milan, Italy (Giacomelli et al., 2020). A telephone survey in Treviso and Belluno provinces in Italy analysed 202 individuals who tested positive for SARS-CoV-2; 64% reported dysosmias or dysgeusias, more frequently among female patients, and appearing before other symptoms in 12% of the cases (Spinato et al., 2020). In Munich, Germany, in a study of 9 patients, 4 presented hyposmia, anosmia, dysgeusia or ageusia (Wölfel et al., 2020). A psychophysical study jointly undertaken on COVID-19 patients in 12 hospitals across Europe showed a very high (~76%) incidence of these symptoms and established correlations between olfactory and gustatory dysfunctions, with dysosmias observed earlier than dysgeusias (Lechien et al., 2020). As the pandemic passed the 6-month mark, anosmia and hyposmia appeared more frequently in otherwise asymptomatic (Boscolo-Rizzo et al., 2020), mildly symptomatic (Spinato et al., 2020; Lechien et al., 2020), or as first-presenting symptoms (Boscolo-Rizzo et al., 2020; Lee et al., 2020a; Gautier and Ravussin, 2020), or even as the only symptoms (Hjelmesæth and Skaare, 2020). A statistical study performed in Teheran subjected 60 confirmed COVID-19 patients and 60 matched control individuals to the Univ. of Pennsylvania Smell Identification Test (UPSIT), a validated 40-odorant assay. Virtually all (98%) COVID-19 patients tested positive for dysosmias: anosmia (25%), severe microsmia (33%), moderate microsmia (27%), mild microsmia (13%) and one patient was normosmic (2%) (Moein et al., 2020). One distinctive feature of the dysosmias in COVID-19 is that they appear relatively early during the disease, in contrast with what was observed in SARS. A second characteristic is that they are seldom accompanied by rhinorrhoea.
In addition to the dysosmias, chemesthetic function (i.e. sensitivity to chemical compounds like menthol or capsaicin in mucosae via peripheral nociceptive receptors of somatosensory neuronal cells) was reduced (−37%) in the course of the disease (Parma et al., 2020). Qualitatively distorted sensory perceptions like parosmias, or phantom chemosensory perceptions like phatogeusias and phanthosmias were rarely observed.
The American Academy of Otolaryngology issued a statement warning that patients presenting this symptomatology in the absence of other manifestations of respiratory disease such as allergic rhinitis, acute or chronic rhinosinusitis, should be alert to the possibility of COVID-19 infection (Anosmia, Hyposmia, and Dysgeusia Symptoms of Coronavirus Disease, https://www.entnet.org/content/coronavirus-disease-2019-resources (2020). Isolated sudden onset anosmia (ISOA) has been postulated as a presentation syndrome of SARS-CoV-2 infection not accompanied by other symptoms in two case reports in which the individuals tested positive for COVID-19 (Gane et al., 2020) (Eliezer et al., 2020); in the latter case the anosmia was sudden and complete. A detailed quantitative study of dysosmias and dysgeusia can be found in ref. (Mercante et al., 2020).
Some COVID-19 patients who recovered from severe pneumonia have also referred long-term anosmia as a sequel of the disease and hyposmia, remarkably, only seldom (Lüers et al., 2020; Carfì et al., 2020). Long-lasting dysosmias are an infrequent presentation of SARS: there is a case report of residual and severe form of anosmia in a female patient infected with SARS-CoV about 3 weeks after onset of symptomatology (Hwang, 2006). Post-viral disease olfactory loss appears to be associated with a higher incidence of cranial neuropathies (Jitaroon et al., 2020).
Using an app launched in the UK to record symptoms of putative COVID-19 infected persons through digital media, of ca. 1.5 million surveyed individuals only 1702 had undergone an RT-PCR test: 579 tested positive. Of these, ca. 60% reported loss of smell and taste, leading the authors to suggest that these symptoms are a strong predictor of COVID-19 in non-tested individuals (Menni et al., 2020). Odour tests are available for olfaction memory dysfunctions (see e.g. (Frank and Murphy, 2020)) and are likely to be used more frequently to assess the degree of olfactory sense loss and recovery in affected COVID-19 patients. A statistically significant correlation was found between the decrease in interleukin-6 and recovery of smell after the olfactory dysfunction episode (Cazzolla et al., 2020).
4. Invasion of the CNS
4.1. Transcriptomics of the mucosal epithelia
One of the rapidly growing areas of research in COVID-19 is that of the virus-host cell interactome, aimed at identifying the proteins that interact with the SARS-CoV-2 proteins and/or RNA. The genome of the virus codes for about 28 proteins (4 structural proteins and 16 non-structural proteins, nsps). The genes coding for the nsps are scattered among the genes coding for the structural proteins in most CoVs (Fung and Liu, 2019). The connectivity maps are complex, with sub-networks and nodes involving between 330 and 400 possible interactions of these 28 proteins with the many more host cell proteins and cellular processes (Gordon et al., 2020a, 2020b; Díaz, 2020), including signalling mechanisms (Suryawanshi et al., 2021). Identification of the viral RNA-host protein interactome also provides interesting therapeutic approaches (see review in (Schmidt et al., 2020)).
In a separate work I discuss transcriptomic studies disclosing the topographical distribution and expression at the individual cell-type level of angiotensin-converting enzyme 2 (ACE2) and its co-receptor transmembrane serine protease 2 (TMPRSS2) in the oral and nasal mucosae and along the upper aerial respiratory track (Barrantes, 2020b). In brief, and contrary to expectation, most studies report the absence of receptors from the olfactory neuronal cells but abundant expression in goblet and ciliated stem cells in the olfactory mucosa (Sungnak et al., 2020; Ziegler et al., 2020), olfactory support cells and stem cells, and in vascular pericytes of the olfactory bulb (Chen et al., 2020b). A meta-analysis of RNA-Seq libraries with ~29,000 single cells found ACE2/TMPRSS2 expression in sustentacular cells, and ACE2 alone in a subset of the sustentacular cells in human olfactory neuroepithelium (Fodoulian et al., 2020). Using a golden Syrian hamster model, the sustentacular cells were shown to be the main target after intranasal SARS-CoV-2 instillation, leading these authors to conclude that sustentacular cell injury suffices to account for the dysfunctional odour perception (Bryche et al., 2020). The death of sustentacular cells would deprive olfactory neurons of the cytochrome P450 detoxifying activity and cilia that the former cells provide (Bilinska and Butowt, 2020). All in all, the evidence appears to indicate that SARS-CoV-2 exhibits tropism for various cell types in the olfactory epithelium, leading to the death of sustentacular cells in a hamster model, and possibly affecting several other cell types in humans. SARS-CoV-2 may remain in the mucosa only and sustain the pathophysiological effects in the periphery, accounting for the dysosmias, and may utilize this entry point as they make their way to the CNS. Anatomopathological studies showing frontal cortex involvement and correlative neuropsychiatric symptoms are already pointing in this direction (Rogers et al., 2020; Kotfis et al., 2020).
4.2. The neural olfactory route
In animal model systems, the neurotropic herpes simplex virus type I (HSVI) and mouse hepatitis virus (MHV) were early reported to enter the CNS via olfactory neurons. After reaching the olfactory bulb, they spread trans-neuronally along different neural pathways (Barnett et al., 1993). Interestingly, the spread of the two viruses, infecting a unique and only partially overlapping set of connections of the olfactory bulb, suggests a marked tropism. Both viruses infected dopaminergic neurons in the ventral tegmental area, but MHV produced a more widespread infection in the A10 group, as well as infecting A8 and A9. In contrast, only HSV infected noradrenergic neurons in the locus coeruleus. The results led the authors to hypothesize that the differential pattern of viral spread could be due to virus uptake by specific neurotransmitter systems, although other factors could not be discarded, such as differences in the distribution of the synapses or the cellular receptor for the two viruses. See also review by ref (van Riel et al., 2015) for a discussion of trans-synaptic viral transport. Swine hemagglutinating encephalomyelitis virus also propagates centripetally using a trans-synaptic pathway and causes deadly acute encephalitis (Li et al., 2013). Bornavirus BoDV-1 is another virus that reaches the CNS via the olfactory pathway (Sauder and Staeheli, 2003), as shown in vivo in adult rats and in vitro using olfactory mucosal cell cultures (Kupke et al., 2019).
Other viruses that can use the nasal olfactory epithelium as entry point and the olfactory nerve as a shortcut into the CNS include influenza A virus, herpesviruses, poliovirus, paramyxoviruses, vesicular stomatitis virus, rabies virus, parainfluenza virus, adenoviruses, Japanese encephalitis virus, West Nile virus, chikungunya virus, La Crosse virus, mouse hepatitis virus, and bunyaviruses (van Riel et al., 2015).
Among CoVs, hOC43-CoV has been reported to infect the CNS via the nasal mucosa causing encephalitis with vacuolation in neuronal cells and strong microglial reactivity in infected areas (Jacomy and Talbot, 2003). In an animal model system, the hOC43-CoV apparently propagates from the nasal cavity to the olfactory bulb and piriform cortex to ultimately reach the brain stem; in cell culture, an axonal neuron-to-neuron propagation is followed (Dubé et al., 2018).
4.3. The olfactory ensheathing cell route
The nasal mucosae have quite a variety of cells that viruses can exploit, including the specialized olfactory-ensheathing glial cells of the olfactory epithelium region. An additional route which to my knowledge has not been contemplated for SARS-CoV-2 entry into the CNS is that followed by some picornaviruses (among which are the common cold rhinoviruses) and Bornaviruses. Picornavirus infection induces temporal release of multiple extracellular vesicles, by which means they escape control by their host cells and spread infection (van der Grein et al., 2019). In the case of the Bornaviruses, which can cause fatal encephalitis in humans, the study of Kupke et al. (2019) showed that viral antigens were still present in the olfactory ensheathing cells four days after infection with BoDV-1. This route could be operative upon infection of any of the potential cell targets transiting along extracellular paths as discussed in this review.
Instead of uptake by olfactory neurons and subsequent axonal transport along the olfactory neural pathway, bypassing the extracellular epithelial barrier appears to be the path chosen by small protein molecules like the nerve growth factor (NGF). Upon intranasal administration of 125I-labelled-NGF, the fast appearance of radioactive material is observed in olfactory bulbs, brain, and brain stem (Frey et al., 1997).
4.4. The transmucosal aneural route
Alternative routes for CNS invasion by SARS-CoV-2 have been proposed. The frequent alteration of smell associated with SARS-CoV-2 infection does not appear to be correlated with correspondingly frequent neurological signs of infection of the olfactory bulb, olfactory cortical areas, or rostral regions of the brain. As analysed above, dysosmias are an early symptom, and may appear in individuals otherwise asymptomatic. It may well be that the infection is contained in the nasal cavity. Locally, SARS-CoV infection leads to the disruption of the ciliated epithelium and ciliary dyskinesia, which affect mucociliary clearance and contribute distally to the pulmonary disease (Bustamante-Marin and Ostrowski, 2017). Meinhardt and coworkers (Meinhardt et al., 2021) demonstrated the presence of SARS-CoV-2 RNA and protein in anatomically distinct regions of the nasopharynx and brain, (Meinhardt et al., 2021). SARS-CoV-2 virions were found inside the cytoplasm of ciliated cell and endothelial cells, where they disrupt kinocilia. SARS-CoV-2 can subsequently enter the nervous system by crossing the neural–mucosal interface, i.e. a transmucosal tract. Distinct thromboembolic ischemic infarction lesions were found in brain.
5. SARS-CoV-2 receptors in brain
Even before the enzymes of the renin-angiotensin-aldosterone system (RAAS) were identified in the CNS, it was known that infusion of angiotensin II could increase blood pressure, and the presence of renin in brain suggested that angiotensin could be synthesized locally (reviewed in ref (Xu et al., 2011)). Early immunohistochemical work by the group of Lazartigues identified the presence of ACE2 in neuronal cell bodies of rat brain but not in glia (Doobay et al., 2007). These authors identified ACE2 positivity in the subfornical organ, and mRNA/protein expression was found to be inversely correlated in the nucleus of the tractus solitarius/dorsal motor nucleus of the vagus and the rostral ventrolateral medulla oblongata. They further argued that the finding of ACE2 expression in brain structures involved in the control of cardiovascular function suggests that the enzyme may play a role in the central regulation of blood pressure and autonomic nervous system diseases, such as hypertension (Doobay et al., 2007). Expression of ACE2 was not only found in the nucleus of the tractus solitarius but also in other areas associated with the central regulation of blood pressure, like the paraventricular nucleus (Xia and Lazartigues, 2010). Interestingly, this occurrence of ACE in a specialized group of CNS structures lacking a proper BBB, the so-called circumventricular organs, may point to a direct route for SARS-CoV-2 to gain access to the brain from the general circulation. In addition, inputs to the circumventricular organs sense and integrate signals for fluid balance (e.g. angiotensin II), metabolic control (e.g. leptin) and immune regulation (e.g. IL-1β and IL-6) which, as we will see, play important roles in COVID-19, and their outputs can directly influence individual CNS neurons via efferent projections to autonomic control centres in the hypothalamus and medulla (Ferguson et al., 2014).
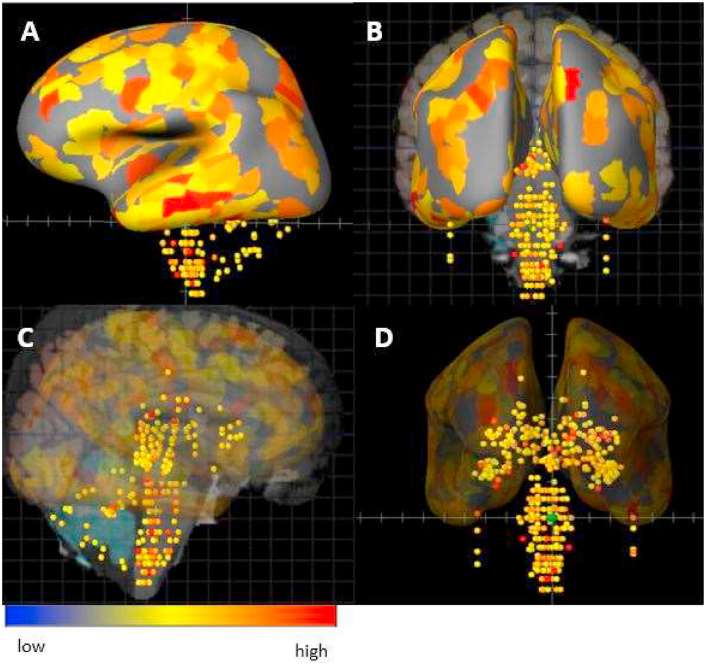
Recent RNA-Seq studies have dissected the multiple CNS localizations of ACE2 mRNA, pointing to potential sites for SARS-CoV-2 binding. ACE2 has been found to be highly expressed in the substantia nigra, choroid plexus and ventricles, olfactory bulb (Chen et al., 2020b) and various cortical regions, including middle temporal gyrus, posterior cingulate cortex, and frontal and motor areas (Fig. 1). Another recent study showed that ACE2 is widely expressed in vessels of different calibres in post-mortem frontal cortex, and is significantly increased in the brain vasculature of patients with a history of dementia or hypertension (Buzhdygan et al., 2020). Interestingly, when the authors tested the effect of the SARS-CoV-2 S1 protein subunit in an in vitro microfluidics model system of the BBB, the spike protein induced a proinflammatory condition in the endothelial cells.
Fig. 1.
Distribution of ACE2 gene mRNA in adult human brain regions. The illustration shows the distribution of the SARS-CoV-2 host-cell receptor using the probe ACE2-A_23_P252981 in a human sample (patient HO351.2001). The dots display the intensity of ACE2 mRNA expression in more than 100 localizations including the telencephalon, cerebral cortex, limbic lobe and hippocampal formation in a sagittal view of the cortex (A, solid representation; C, transparent rendering of the cortical mass to enable visualization of the internal structures (e.g. hippocampus, cerebellum and brain stem) and rostro-caudal coronal view enabling visualization of surface and internal (midbrain) structures (B,D). The same visualization protocol is followed: (B, solid representation; D, transparent rendering of the cortical mass to enable visualization of the internal structures). The colour code reflects the intensity as high (red), neutral (yellow) or low (blue). Obtained from the Allen Human Protein Atlas (http://www.proteinatlas.org) and the Human Brain Atlas (http://human.brain-map.org) through Brain Explorer (https://human.brain-map.org/static/brainexplorer) (Hawrylycz et al., 2012). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In vivo studies using human ACE2 transgenic mice and brain organoids (“minibrains”) have disclosed the ability of SARS-CoV-2 to infect neurons and cause their death (Song et al., 2020; Yang et al., 2020). Dopaminergic neurons derived from human-induced pluripotent cells appear to be particularly rich in ACE2, making them more vulnerable to SARS-CoV-2 infection, whereas cortical neurons showed relatively low expression levels of the enzyme (Yang et al., 2020). Electron microscope examination of a brain sample from a COVID-19 necropsy revealed 80–110 nm viral particles inside vesicles -presumably of endosomal nature- in endothelial and neuronal cell bodies of the frontal cortex, a finding that may correlate with the clinical picture of delirium observed in some patients (Rogers et al., 2020; Kotfis et al., 2020; Kennedy et al., 2020). The presence of the virus was evidenced also by RT-PCR of brain tissue (Paniz-Mondolfi et al., 2020). In this single-case report, neuropsychiatric symptoms correlated with the post-mortem histology; during hospitalization, the 74-year-old patient had episodes of confusion and agitation and became combative, suggesting frontal cortex involvement. SARS-CoV-2 RNA has also been found in a case of encephalopathy (Moriguchi et al., 2020). A series of necropsies of 32 COVID-19 patients showed (micro)thrombotic/thromboembolic signatures in the CNS and olfactory mucosa. The latter regions exhibited 124% higher levels of virion load than the lower respiratory tract (Meinhardt et al., 2021), suggesting that SARS-CoV-2 may have used the nasal epithelium as entry point and followed a centripetal route to reach the brain. Nuclear magnetic resonance bilateral signals compatible with viral infection were observed in the two olfactory bulbs and the posterior gyrus rectus, a cortical brain area associated with olfaction, in a COVID-19 patient having symptoms of anosmia and dysgeusia (Politi et al., 2020).
6. Neurological complications and mortality among COVID-19 patients
SARS-CoV-2 is a highly pathogenic virus, and a non-negligible proportion of COVID-19 patients experience deterioration of their physical condition that often involves vital organs and can precipitate fatal outcomes. About 15% of the COVID-19 patients evolve to a severe form of the disease and 5–6% become critically ill (Wang et al., 2020a; Guan et al., 2020; Huang et al., 2020). Non-neurological severe complications of COVID-19 may lead to atypical acute respiratory syndrome, pulmonary embolism, myocardial infarction, arterial or venous thrombosis of any-calibre blood vessels, acute kidney failure or stroke (Wang et al., 2020a; Zhou et al., 2020a; Arentz et al., 2020; Chen et al., 2020a; Jain and Yuan, 2020; Piazza and Morrow, 2020). Older age and comorbidities are among the main predictive factors of fatal outcomes (Zhou et al., 2020a; Jain and Yuan, 2020; Wu et al., 2020b; Tang et al., 2020). Statistics of the risk scores predicting critical illness are beginning to emerge. The retrospective analysis with the largest casuistic (72,314 individuals) indicates a case-fatality rate of 2.3% (Wu and McGoogan, 2020).
A study on 1590 patients hospitalized in China identified 10 variables as independent predictive risk factors of neurological complications, among which unconsciousness was the main neurological sign (Liang et al., 2020b). Necropsies of SARS patients have also detected viral particles in neuronal cell bodies in the cerebral cortex and hypothalamus (Ding et al., 2003; Gu et al., 2005).
As the pandemic and the length of the recovery period of convalescent patients extend in time, new aspects of the disease become apparent, such as debilitating fatigue and gastrointestinal disarrays, or a constellation of neuropsychological signs involving cognitive alterations and mental exertion that resemble some manifestations of myalgic encephalomyelitis and/or chronic fatigue syndrome/PTSD, as observed in some survivors of the 2013 SARS epidemic (Liang et al., 2020a; Tansey et al., 2007).
Statistically, the leading cause of death among hospitalized COVID-19 patients is an acute and atypical pneumonia, usually associated with an overreactive inflammatory response. Respiratory failure was initially reported as the main cause of death (69.5%) among 81 patients in Wuhan (Zhang et al., 2020), with ARDS reported to be strongly associated with mortality in severely ill COVID-19 patients (Yang and Jin, 2020). Pulmonary arterial thrombosis of small and mid-sizes vessels has also been reported as a cause of death (Lax et al., 2020). A recent meta-analysis using an analytics platform (OpenSAFELY) covering 40% of all patients in England (more than 17 million adults and ~11,000 COVID-19 patients) found that COVID-19-related deaths were statistically associated with the following risk factors: older age, being male, obesity (Lockhart and O’Rahilly, 2020) and its association with diabetes, severe forms of asthma (Jackson et al., 2020), chronic respiratory disease, chronic heart disease, autoimmune diseases and reduced kidney function among the most salient comorbidities (Williamson et al., 2020). Black (Bunyavanich et al., 2020; Rodgers and Gibbons, 2020) and South Asian people appear to be at higher risk.
Although the pulmonary picture has dominated the characterization of the disease, together with the atypical form ARDS appearing as a likely terminal complication, obitus of COVID-19 patients may be due to several other single-organ compromises (with heart, kidneys and brain being highly susceptible to fatal failure) or, most plausibly, multi-organ failure in severely ill patients. Remarkably, some of the most severe neurological complications of COVID-19 like acute disseminated encephalomyelitis with haemorrhagic compromise do not appear to correlate with the severity of the respiratory clinical picture (Paterson et al., 2020). Increased neurological disability among multiple sclerosis patients was consistently associated with increased risk for worse clinical severity and outcome of COVID-19 (Salter et al., 2021). The more we learn about COVID-19, the more likely it is that the disease will ultimately be described as a complex multi-factorial, multi-organ syndrome of systemic breadth with underlying common pathologies of varied intensities, possibly of an endothelial nature. The following sub-sections analyse severe neurological complications of COVID-19: cerebrovascular disease and stroke, encephalopathy, encephalitis, abnormal inflammatory response, and direct attack of the CNS -including specific nuclei that may be responsible for peripheral lethal outcomes of the disease.
6.1. Cerebrovascular disease
A retrospective study of 3 hospitals in Wuhan reported that 36.4% of the COVID-19 patients had neurological manifestations, of whom 5.7% had acute cerebrovascular diseases, impaired consciousness (14.8%) and encephalopathy (2.8%) (Mao et al., 2020). Of the cardiovascular injuries, 5% were acute ischaemic strokes, 0.5% venous thrombosis, and 0.5% cerebral haemorrhages. This clinical picture appears to be reproduced in another single-centre study (Li et al., 2020b). In another study, 2.6% of 229 non–critically ill hospitalized patients and 35.3% of 170 hospitalized critically ill patients had thrombotic complications (Piazza and Morrow, 2020). The proportion of COVID-19 patient with stroke was found to be 1.8%, with an in-hospital mortality of 34.4%, in a multicentre study (Fridman et al., 2020).
Strokes are a rather uncommon complication in CNS infections of viral origin, but silent brain infarcts are likely to occur in COVID-19 patients, especially in those in ICUs. Silent infarcts are observed in 20% of otherwise healthy elderly individuals and ~50% in selected series with diabetes and/or cardiovascular comorbidities (see review in ref (Vermeer et al., 2007)).
Massive intracerebral haemorrhage with intraventricular and subarachnoid haemorrhage have been reported in elderly individuals with COVID-19 in Iran (Sharifi-Razavi et al., 2020). Presentations of COVID-19 in the form of acute stroke have also been reported; a retrospective analysis of 1916 COVID-19 patients admitted to emergency departments in New York hospitals found that 18% had an acute ischemic stroke (Merkler et al., 2020). The vascular damage leading to stroke in all these cases was associated with large vessel disease (Avula et al., 2020; Lee et al., 2020b). The marked endothelial dysregulation appears to be at the root of the high thrombogenic activity in COVID-19 patients (Marini and Gattinoni, 2020). Remarkably, stroke can be the first presenting clinical picture in asymptomatic young COVID-19 patients, as compiled in a meta-analysis of 125 cases from Canada, USA and Iran (Fridman et al., 2020). These authors also found that older age, other chronic comorbidities, and the severity of COVID-19 respiratory symptoms are associated with an extremely elevated (~59%) risk of death. A systemic prothrombotic phenotype has been reported in COVID-19 patients, involving immunothrombosis in the genesis of inflammatory microvascular thrombi containing neutrophil extracellular traps associated with platelets and fibrin, neutrophil-platelet aggregates and a distinct neutrophil and platelet activation pattern in blood, which changes with disease severity (Nicolai et al., 2020). In this phenotypic classification, severely affected COVID-19 patients are characterized by excessive platelet and neutrophil activation compared to healthy controls. In most of the COVID-19 cases with complications of a cerebrovascular nature, the pre-existing comorbidities analysed in the preceding section -especially hypertension and diabetes- constitute a frequent finding, these risk factors often leading to fatal outcomes. Guidelines for endovascular stroke treatment during the COVID-19 pandemic have recently appeared (Ospel and Goyal, 2020).
6.2. Encephalopathy
Neurological and neuropsychiatric symptoms reported in several cases of COVID-19 include confusion, attentional and/or memory impairment, lethargy and delirium (Rogers et al., 2020; Kotfis et al., 2020; Kennedy et al., 2020; Nguyen et al., 2020) and seizures (Carod-Artal, 2020; Lahiri and Ardila, 2020), some of which are suggestive of encephalopathy (Espinosa et al., 2020; Filatov et al., 2020). Although acute encephalopathy is usually a most severe complication in paediatric viral infections (Mizuguchi et al., 2007), it can also be present in adult patients. Acute disseminated encephalopathy is a frequent complication of viral infections. Anatomopathological examination of 18 post-mortem brains showed only hypoxic lesions and no signs of encephalitis or other changes referable to the virus (Solomon et al., 2020). In contrast, similar analyses have found parenchymal abnormalities like subcortical micro- and macro-haemorrhages, and oedematous changes suggestive of encephalopathy (Coolen et al., 2020). Glial fibrillary acidic protein and neurofilament light chain protein, two neurochemical pathological markers of CNS glial and neuronal injuries, respectively, were found in blood plasma of COVID-19 patients (Kanberg et al., 2020). A study of a cohort of 799 COVID-19 patients indicated that 113 of them (~20%) developed hypoxic/ischemic encephalopathy (Chen et al., 2020c). A recent study of 43 patients with COVID-19-related neurological disorders lists encephalopathies with delirium and psychosis and no MRI or CSF abnormalities as the leading finding (29/43 cases) (Paterson et al., 2020).
Acute disseminated encephalomyelitis is usually a post-infectious syndrome characterized by perivenous oedema, demyelination and macrophage-lymphocyte infiltration, and has been observed in other viroses like influenza associated with intracerebral cytokine release syndrome and disruption of the BBB (Rossi, 2008). A recent case report illustrates the lesions revealed in the necropsy of a COVID-19 patient who presented a vascular and acute disseminated encephalomyelitis. The lesions resembled both vascular and demyelinating aetiologies. Haemorrhagic white matter lesions were observed throughout the cerebral hemispheres with surrounding axonal injury and macrophages. Rare neocortical micro-infarcts were also present (Reichard et al., 2020). A possible link between the pulmonary localization of SARS-CoV-2 and encephalomyelitic complications of COVID-19 has recently been postulated (Alharthy et al., 2020).
Neurological complications are more common in older patients with comorbidities, particularly hypertension, who presented less typical symptoms and exhibited the more severe forms of the disease (45.5%) compared to patients with milder presentations (30.2%) and more typical symptoms like fever and cough (Mao et al., 2020). Intracerebral haemorrhages constitute a severe complication of hypertensive patients, particularly elderly patients. In COVID-19 intracerebral haemorrhages are rare; only three cases out of 1200 hospital admissions in Russia were recently reported (Pavlov et al., 2020). Encephalopathies as complications of COVID-19 patients without apparent penetration of the virus in the brain parenchyma have been reported (Espinosa et al., 2020; Filatov et al., 2020), and a more rare viral penetration of the brain stem has also been documented (Li et al., 2020c).
The auto-amplifying, hyper-production of cytokines characteristic of the cytokine release syndrome is a complicating factor in acute encephalopathies accompanied by vasogenic brain oedema, such as is found in Reye-like syndrome, haemorrhagic shock, encephalopathy syndrome, and acute necrotizing encephalopathy (Mizuguchi et al., 2007). An acute haemorrhagic necrotizing encephalopathy with bilateral involvement of the thalami and temporal lobes has recently been reported in a female COVID-19 patient (Poyiadji et al., 2020). This type of encephalopathy is a rare complication of influenza or other viral infections with disruption of the BBB but without direct viral infection.
6.3. Encephalitis
In general, acute encephalitis of infectious or immune causes (or both) is most common in children but is also observed in adults and may conduce to acute encephalopathy, which in severe cases has a high mortality. Encephalitis produced by SARS-CoV infection was reported during the SARS epidemic, associated with tonic-clonic convulsions (Lau et al., 2004) or intractable seizures (Barcelo-Coblijn et al., 2003). A case of meningitis and encephalitis in a 24-year old male was reported in Japan. The acute case, accompanied by convulsions and unconsciousness, was diagnosed as aseptic encephalitis. SARS-CoV-2 RNA was found in the cerebrospinal fluid (Moriguchi et al., 2020). Other cases of encephalitis have now been reported (Ye et al., 2020).
In investigating the likely sources of encephalitis, one should focus attention on those organs that are susceptible to and/or have high capacity to produce virions; one such niche is the intestinal tract. A meta-analysis indicates 12% of COVID-19 patients manifest gastrointestinal symptoms, including diarrhoea, nausea or vomiting, and 45% test positive for SARS-CoV-2 in faeces (Parasa et al., 2020). Interestingly, among 58 COVID-19 positive children in the U.K., 52% presented diarrhoea and 53% abdominal pain and serious cardiovascular signs that led to the diagnosis of paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (Whittaker et al., 2020). The enterocyte appears to be prone to infection because of its high expression of ACE2 and TMPRSS2 (Ziegler et al., 2020), and has been shown to be extremely efficacious at replicating and shedding SARS-CoV-2 virions. Additionally, enterocytes have a strong interferon type-II response and exacerbated cytokine responses (Lamers et al., 2020; Stanifer et al., 2020). The gene coding for the viral receptor, ACE2, is a human interferon-stimulated gene expressed, among other tissues, in ileal absorptive enterocytes (Ziegler et al., 2020). The affectation of the gastrointestinal tract includes the oesophagus; bleeding caused by SARS-CoV-2 infection has been reported (Li et al., 2020d). The mRNA transcriptomic profiling studies finding highest expression levels of ACE2 in intestinal enterocytes, and the observation that ~60% of ileal enterocytes express the enzyme, lends support to the possible intestinal source of SARS-CoV-2 virions (see Fig. 2 below).
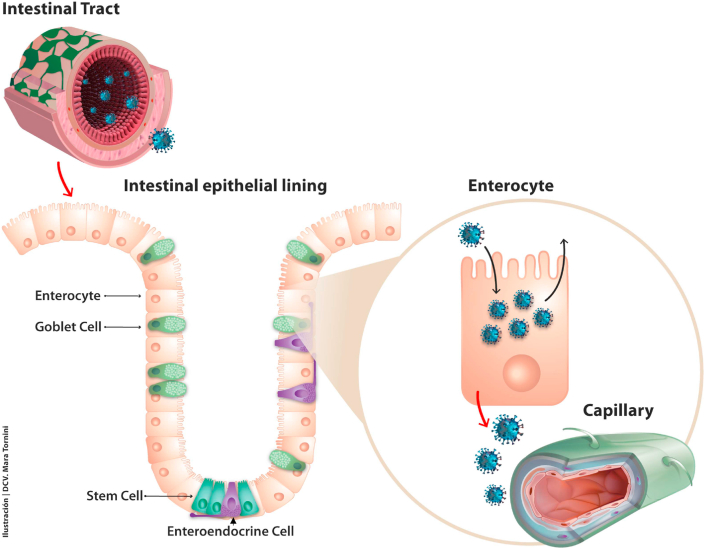
Fig. 2.
The intestinal tract offers SARS-CoV-2 multiple entry points and routes. Various types of epithelial cells are found in the intestinal epithelial lining. Enterocytes are by far the most abundant, and they express higher amounts of ACE2 than the pulmonary alveolar cells (Xu et al., 2020b) and also express higher amounts of TMPRSS4, whereas secretory cells like the goblet cells express more TMPRSS2, its isoform (Zang et al., 2020). These two proteases also enhance membrane fusion, facilitating virion entry into the cells by endocytosis (arrow on apical enterocyte plasmalemma) through a yet uncharacterized mechanism. SARS-CoV-2 replicates most efficaciously in the enterocyte, and exocytic shedding can occur from the apical and/or the basal membrane (red arrow). Fluorescent-labelled SARS-CoV-2 particles are mostly found in mature villous absorptive enterocytes and to a lesser extent in undifferentiated stems cells (Zang et al., 2020). Enteroendocrine cells (shown in purple) exhibit lower expression levels of ACE2 and TMPRSS2/4. Once in the submucosa SARS-CoV-2 virions can find their way to the capillaries, also rich in ACE2 in their endothelial cells and pericytes (depicted in green in the lower-right corner), to reach the general circulation. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
A recent study using a murine model of COVID-19 found SARS-CoV-2 in the brain parenchyma of human ACE2-carrying transgenic mice. Some of these mouse brains exhibited a mutation, C23525T (H644Y), in the S1 region of the spike glycoprotein gene (Jiang et al., 2020).
6.4. Direct attack of CNS and cardiorespiratory nuclei by SARS-CoV-2
The shortest possible route for secondary infection of the CNS following primary attack of the nasal epithelium is the anterograde pathway from the olfactory mucosa via olfactory nerve to reach the olfactory bulb, a path that has been extensively discussed at the hypothetical level in the context of COVID-19, although there is still no firm evidence that the virus follows this route (see review in Barrantes, 2020b). An alternative route following primary attack of the nasal mucosa was early postulated for the vesicular stomatitis virus infection in infant rats, whereby the virus could be transported from the olfactory epithelium to the reticular core neurons in the median raphe, the ventral and horizontal diagonal band and the locus coeruleus (Mohammed et al., 1993). The hypothesis was recently reformulated in the context of COVID-19 (Li et al., 2020c).
CoVs have been reported to follow neural routes other than those originating in the nasal mucosa, e.g. synaptic routes from mechano- and chemo-receptors in the lower respiratory airways and lungs to ultimately reach the medulla oblongata (Li et al., 2020c; Harberts et al., 2011). Among other CNS localizations, ACE2 is found in the brainstem cardiorespiratory nuclei (Xu et al., 2011; Doobay et al., 2007). These routes and receptor localizations provide an explanation for two associated phenomena of different severity: 1) the paradoxical “happy hypoxia” syndrome, i.e. the remarkably low blood O2 levels in some COVID-19 patients and 2) the possibility that death by respiratory failure may in fact be due to a central cause – failure of the cardiorespiratory centres in the brain stem- rather than the atypical “CARDS” pulmonary complications. A study of 32 COVID-19 necropsies showed SARS-CoV-2 RNA in the respiratory and cardiovascular regulatory centres in the medulla oblongata (Meinhardt et al., 2021).
Another hypothetical affectation of a CNS nucleus postulates infection of the oropharynx, centripetal virus migration along the facial and the vagus nerves, up to the nucleus tractus solitarius. Inflammation of this nucleus is further suggested to be responsible, together with a defective hypothalamic-pituitary-adrenal axis, for the abnormal cytokine production (Ur and Verma, 2020).
7. Dysregulated inflammatory responses in COVID-19
7.1. The pro-inflammatory cytokine release syndrome and the key role of interleukins
In the CNS, low levels of interleukin (IL) 6 (IL-6) produced by glial cells have been associated with physiological regulation of synaptic transmission and plasticity, in turn associated with the maintenance of cognitive functions (Yirmiya and Goshen, 2011). In contrast, elevated levels of IL-6 are observed in several neurodegenerative and psychiatric diseases (Gruol, 2015). IL-6 is also considered to be a “master player” in the pro-inflammatory cytokine family, inducing the expression of a variety of proteins involved in acute inflammation (Uciechowski and Dempke, 2020). Neuroinflammation can be envisaged as the reactive response of the CNS to altered homeostasis observed under a wide spectrum of pathological conditions, either systemic and/or affecting the CNS, including infectious diseases (Ransohoff et al., 2015). Viral infections can also lead to a massive, disproportionate, or uncontrolled systemic syndrome consisting of pro-inflammatory cytokine production (hypercytokinemia) and release -the cytokine release syndrome (Behrens and Koretzky, 2017). Some CoVs have been reported to induce this syndrome. In SARS, this is associated with the hyperproduction of IL-1β, IL-6, IL-12 A, interferon-γ, interferon-γ inducible protein 10 (IP10) and MCP1. In MERS, augmentation of TNF, IL-15, IFN and IL-17. A has been reported (Channappanavar and Perlman, 2017). These CoV-associated immunological profiles led to the suggestion that SARS-CoV-2 could also trigger a hyper-inflammatory cytokine release syndrome (Mehta et al., 2020). Indeed, early reports indicated that some COVID-19 patients exhibited an abnormal cytokine profile (Huang et al., 2020) associated with disease severity and respiratory failure in particular (Moore and June 2020). The profile involved macrophage activation and increased levels of IL-2, IL-2R, IL-7, IL-8, IL-9, IL-10, granulocyte colony stimulating factor, IP-10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, tumour necrosis factor-α (Huang et al., 2020) and elevated ferritin (Ruan et al., 2020). The latter is a clinical laboratory predictor of possible fatal outcome. IL-1β and tumour necrosis factor activate glucuronidases that enzymatically digest the complex and extensive glycocalyx barrier covering the endoluminal endothelial cell surface, and upregulate hyaluronic acid synthase 2, an enzyme that increases hyaluronic acid deposition in the extracellular space, thus contributing to water and electrolyte retention and oedema (Teuwen et al., 2020). This syndrome is particularly dangerous in older patients who have a basal “inflammaging” (increased basal inflammation) status and multi-organ accumulation of senescent cells (Akbar and Gilroy, 2020) that may predispose them to a dysfunctional immune response. This coincides with the decline in macrophage activity, naïve T cell production and effector memory T cell competence in the elderly (Li et al., 2019). Along these lines, a recent study indicates that when accounting for cofounding factors, the cytokine storm is a relatively rare (3–4%) event among COVID-19 patients, affecting predominantly elderly patients who require ventilatory assistance (Mudd et al., 2020).
The hyper-inflammatory syndrome in COVID-19 has been described to follow the trans-route (Moore and June 2020), mainly involving the pro-inflammatory IL-6 family. IL-6 levels in plasma were found to be a strong predictive marker of upcoming respiratory failure in hospitalized COVID-19 patients (Herold et al., 2020).
Recent transcriptome analyses addressing characterization of host cell responses to SARS-CoV-2 infection revealed unexpected differences between the responses to this virus and responses to other respiratory viruses having similar tropism. Using a human adenocarcinoma epithelial cell assay, the study disclosed two interesting findings: despite having undetectable levels of ACE2 and the TMPRSS2 protease, a) the alveolar epithelial cells supported virion replication and b) SARS-CoV-2 replication produced a muted antiviral response, with unusually low production of interferons and higher than normal levels of IL-6 (Blanco-Melo et al., 2020). The in vitro assay was validated by infection of ferrets with SARS-CoV-2; the animals showed similar muted responses, with transcriptional profiles showing diminished cytokine responses.
Auto-antibodies against type I interferons have been observed in patients with life-threatening cases of COVID-19 (Bastard et al., 2020). Activated immune cell infiltration of the brain parenchyma may include, however, the Ly6Glo subset of neutrophil-like cells with the capacity to induce CNS neural regeneration (Sas et al., 2020).
As the pandemic extends in time, the lifetime of cytokine responses in the disease is beginning to be characterized. Inflammatory cytokines persist for several weeks in the cerebrospinal fluid of oncological patients with neurological manifestations of COVID-19 after SARS-CoV-2 infection (Remsik et al., 2021).
7.2. Disrupted metabolic pathways associated with excessive cytokine production
A recent report has identified a carbohydrate metabolic pathway required for activating influenza virus-induced cytokine release syndrome (Wang et al., 2020b). The transcription factor interferon regulatory factor 5 (IRF5) is required to induce the pro-inflammatory cytokine production observed in influenza virus infection. IRF5-induced inflammatory response enhances glucose metabolism because more energy is required by immune cells for the cytokine response and because the virus requires carbohydrates for replication. The hexosamine biosynthetic pathway is the common metabolic requirement for both processes (Wang et al., 2020b). These findings raise the possibility that a similar process occurs in the cytokine pro-inflammatory syndrome associated with other viral infections, including COVID-19. A recent study conducted on 1122 COVID-19 patients in the United States found that those with diabetes or hyperglycaemia have a four-fold greater mortality than those without these comorbidities. The IRF5 cascade also induces pro-inflammatory cytokine production that eventually leads to the cytokine release syndrome.
The effect of aging on the immune response has been analysed in a recent viewpoint article (Akbar and Gilroy, 2020). Briefly, the combination of a basal inflammatory status in older individuals and multi-organ accumulation of senescent cells would be key predisposing/exacerbating factors for dysregulated immune responses in COVID-19 elderly patients. Thus, although hyper-inflammatory responses have dominated recent views about the immune response in COVID-19, this and other findings suggest that insufficient immune responses may also be operative. For example, the non-structural protein nsp1 from SARS-CoV-2 effectively induces a nearly complete shutdown of the host protein translation upon binding to the 40S small ribosomal subunit, thereby blocking innate immune responses that would otherwise facilitate clearance of the infection (Thoms et al., 2020).
Recent immune-metabolic profiling has identified populations of T cells and myeloid cells with unique gene programmes and metabolic profiles linked to mitochondrial dysfunction and apoptosis and which correlate with lymphopenia and COVID-19 severity (Thompson et al., 2021). CD4+ T cells, CD8+ T cells, and neutralizing antibodies synergically contribute to control SARS-CoV-2 infection (Sette and Crotty, 2021).
7.3. Immune profile as a marker of COVID-19 severity
One important question is what marks the transition from the mild-to-moderate to the severe (10–20%) forms of the disease (Jain and Yuan, 2020). Single-cell RNA sequencing and single-cell proteomics of blood mononuclear cells showed changes in immune cell composition and activation over time. In one study, HLA-DRhighCD11chigh (human leukocyte antigen -DR isotype) inflammatory monocytes with an interferon-stimulated gene signature were found to be elevated in mild COVID-19. The severe form of the disease showed neutrophil precursor cells, pointing to emergency myelopoiesis, dysfunctional mature neutrophils expressing PD-L1 and abnormal oxidative response, and HLA-DRlow monocytes, indicating marked affectation of the myeloid lineage and resulting in continuous tissue inflammation and ineffective host defence (Schulte-Schrepping et al., 2020). In a second study, the disappearance of non-classical CD14lowCD16high monocytes and accumulation of HLA-DRlow were accompanied by massive release of calprotectin (Silvin et al., 2020). These authors suggest the use of flow cytometry assays to detect decreases in non-classical monocytes and predict the evolution of COVID-19 cases. Both survivors and non-survivors of severe COVID-19 develop robust IgM and IgA responses accompanied by defective Fcγ receptor binding and Fc effector activity, indicating deficient humoral immunity; moderate COVID-19 patients develop sluggish delayed responses that eventually mature (Zohar et al., 2020).
A multi-omics analysis of blood plasma collected from COVID-19 patients during the first week of infection following diagnosis revealed changes in the immune cell repertoire, increases in inflammatory markers and loss of metabolites and metabolic processes as the disease evolved from mild to moderate severity (Su et al., 2020). A transcriptomics study characterized the single-cell RNA profile of peripheral mononuclear cells of COVID-19 patients, revealing defective antigen presentation in monocytes and high interferon responsiveness in lymphocytes, and suppression of genes involved in cytotoxic activity in both NK and CD8 lymphocytes, thus explaining the reduced viral clearance of severe COVID-19 patients (Yao et al., 2020). Single-cell RNA-sequencing of host factors has also disclosed changes in cholesterol biosynthesis in the disease: increased cholesterol synthesis correlates with SARS-CoV-2 resistance (Daniloski et al., 2020). Another study from the same authors surveyed >20,000 potential anti-SARS-CoV-2 compounds, identifying some that induced cholesterol biosynthesis as potential viral inhibitors.
As we see, the immune response in COVID-19 patients is highly heterogeneous, particularly in hospitalized patients with severe forms, with some patients displaying robust CD8 T cell and/or CD4 T cell activation and proliferation, while ~20% of patients exhibit minimal responses (Mathew et al., 2020); the former group probably corresponds to those patients presenting high tissular viral load in autopsies (Xu et al., 2020a). The immune response is doubly anomalous: SARS-CoV-2 replication produces in some cases a muted antiviral response, with negligible production of interferon type-I and II in response to the virus infection, but also pathologically elevated cytokine levels - IL-6 in particular (Blanco-Melo et al., 2020). This configures a severely abnormal innate antiviral response coupled to the dysregulated inflammatory cytokine production discussed in previous sections. The diagnostic signature recently associated with this condition is configured by increased IP-10, IL-10 and IL-6, a triad that anticipates subsequent clinical progression (Laing et al., 2020). According to these authors, this triad of pro-inflammatory markers constitutes a signature that predicts length of hospitalization; its use is recommended for diagnostic purposes such as early risk-based stratification of patients. They also suggest targeting the triad as a therapeutic strategy that may contribute to the success of dexamethasone treatment. Peripheral blood neutrophil profiling is also emerging as a predictive diagnosis of disease course and its use is suggested for patient risk stratification (Aschenbrenner et al., 2021).
8. A unifying hypothesis of SARS-CoV-2 affectation of the CNS
As SARS-CoV-2 paths in the human organism are dissected and alterations compromising different organs are increasingly disclosed, the casuistic of neuropathological findings in necropsies of COVID-19 patients reveal that the most common findings are neuroinflammatory alterations of the brain stem (Matschke et al., 2020). The notion of common underlying pathophysiological mechanisms and multiple target organs is gaining strength. COVID-19 symptomatology obeys the singularities of the different organs under viral attack, but the conjunction of abnormal immune responses, coagulopathies involving alteration of the coagulation factors, platelet activation and stasis constitute a constellation of factors pointing to virus-induced endothelial bed damage leading to micro-thromboembolism as a common noxa. Genome-wide association studies (GWAS) are beginning to disclose genetic factors associated with disease severity and life-threatening COVID-19, mostly involving two key immune signalling pathways: interferon-mediated antiviral signalling and chemokine-mediated inflammatory signalling (McCoy et al., 2020). Other genes include ethnicity and specific genes like SLC6A20, LZTFL1, CCR9, FYCO1, CXCR6, XCR1 ABO, FOXP4 or CCR2 (Ellinghaus et al., 2020), and gene clusters near the gene coding for tyrosine kinase 2 (TYK2), within the gene encoding dipeptidyl peptidase 9 (DPP9), or the interferon receptor gene IFNAR2 (Pairo-Castineira et al., 2020).
This leads us to formulate a unifying hypothesis of SARS-CoV-2 severe affectation of the CNS. Analysis of the possible virus entry points makes apparent that despite the physical vicinity of the nasal and oral mucosae to the brain, the local infection may not suffice to result in a robust systemic virion shedding and ensuing viremia (Barrantes, 2020a, 2020b). In fact, only two primary entry points fulfil the requisite massive supply of virions to affect the CNS in a severe form: the pulmonary and the intestinal mucosae, the latter with twice the surface and expressing higher amounts of ACE2 than the pulmonary lining (Xu et al., 2020b). In this final section I bring together the two requirements, namely the enteric entry point and the damaged endothelial cell to elaborate on a unifying hypothesis linking the gastrointestinal tract primary infection to the CNS affectation.
8.1. The gastrointestinal tract-brain axis
The digestive tract is a key SARS-CoV-2 entry point and various routes emanating from the enteric tract can be followed by the virions to reach the CNS (Barrantes, 2020b). Clinical studies have underscored the importance of this route (Jin et al., 2020; Parasa et al., 2020; Zhou et al., 2020b; Ding and Liang, 2020; Trottein and Sokol, 2020) and proteomic and immunohistochemical studies have corroborated several aspects of these hypotheses, providing evidence for robust expression of receptors and co-receptors in enterocytes as well as in neurons and glial cells of the enteric nervous system (Deffner et al., 2020; Briguglio et al., 2020; Liang et al., 2020c) and distinct stem cell clusters in the proximal and distal small intestine (Liang et al., 2020c).
A cryo-EM study has solved the structure of the full-length human ACE2 with or without the receptor-binding domain (RBD) of the SARS-CoV-2 spike (S) protein in the presence of a neutral amino acid transporter, B°AT1 (Yan et al., 2020). B°AT1 is the major luminal sodium-dependent neutral amino acid transporter of the small intestine and kidney proximal tubule. Interestingly, to be expressed in the small intestine, B°AT1 critically requires to be associated with collectrin (Tmem27), a protein homologous to the transmembrane region of ACE2 (Camargo et al., 2009). ACE2 is essential for the intestinal uptake of the amino acid tryptophan. As is well known, this amino acid is the precursor of 5-hydroxytryptamine, the neurotransmitter serotonin. ACE2 is also necessary for exercise-dependent modulation of pro-mitotic adult neurogenesis in rodent adult hippocampus (Klempin et al., 2018). These are two examples of the multiple functions displayed by the SARS-CoV-2 receptor in its pleotropic roles in these two organs and the possible interrelationship via intestine-brain neural or circulatory system connections (see Fig. 2 below).
The high receptor capacity of the enteric mucosae, especially that lining the duodenum and ileum, with expression of ACE2 and the two isoforms of the serine protease TMRSS2 and TMPRSS4 (Grasselli et al., 2020) (higher than that in the bronchoalveolar epithelium (Xu et al., 2020b)), together with the extensive surface area of the intestinal mucosa (ca. 250 m2) make the intestinal tract a massive source of virion uptake, replication, and shedding that can be fed into the intestinal lumen or the bloodstream, and reach elevated viral titres, inducing the production of excess levels of pro-inflammatory cytokines. Clinical presentations of intestinal disease in COVID-19 are increasingly being reported (Jin et al., 2020; Parasa et al., 2020) and pathological findings of intestinal damage are observed in 45% of COVID-19 necropsies (Bryce et al., 2020). It is currently not known to what extent the microbiota of the gastrointestinal tract plays a role in the infectious mechanism or whether chronic inflammatory bowel conditions constitute a risk factor (Zhou et al., 2020b). The inflammatory status may also apply to the endothelial cells of the intestinal microcirculation capillaries. Once these barriers are surpassed, the virions in the circulatory stream can reach any organ. A recent in vitro study using human intestinal epithelial cells showed the very efficient infection of these cells by SARS-CoV-2 (Stanifer et al., 2020). The virions are rapidly inactivated by a medium resembling the content of the colon, leading to the suggestion that the viral mRNA that transits through the large intestine is not infectious (Zang et al., 2020), thus making the faecal-oral transmission (Ding and Liang, 2020) less tenable for SARS-CoV-2.
As schematically shown in Fig. 2, virions can be either endocytosed by enterocytes or find their way between epithelial intercellular junctions and subsequently the endothelial gaps (Pober and Sessa, 2007), to reach the submucosal capillary network and the general circulation via the hepatic portal system. SARS-CoV has been reported to enter cells in complex with ACE2 via a pH-, clathrin- and caveolin1-dependent endocytic process which apparently occurs in cholesterol/sphingolipid-rich membrane lipid domains (the so-called “lipid rafts”) (Wang et al., 2008). More recent experimental evidence indicates that SARS-CoV-2 follows a specific endocytic pathway, namely the clathrin and dynamin-independent, pH-dependent CLIC/GEEC endocytic pathway. Endosomal acidification inhibitors like BafilomycinA1 and NH4Cl, which inhibit this pathway, strongly block viral S protein uptake (Prabhakara et al., 2020).
I have suggested the enteric nervous system as an alternative centripetal viral route exploiting the submucosal or the myenteric plexuses (Barrantes, 2020b), a hypothesis that has subsequently found experimental support (Deffner et al., 2020). SARS-CoV-2 infection of the these plexuses and neurons may constitute intermediate stations in the virus track to the CNS, as is the case with the neurotropic varicella zoster virus (Gershon and Gershon, 2013). SARS-CoV-2 might also undergo latency in neurons, as is observed with the varicella virus. The human dorsal root ganglion (DRG) sympathetic neuron expresses the ACE2 gene (Ray et al., 2018) (Shiers et al., 2020) as well as the MRGPRD and Nppb genes, the former of which is selectively expressed in a subset of nociceptive receptors with peripheral nerve branching in colon (Hockley et al., 2019) or meninges (von Buchholtz et al., 2020) together with the ACE2 gene (Shiers et al., 2020). The centripetal retrograde pathway to the CNS followed by the virus would be even more direct if infection involved the DRG neuron (Barrantes, 2020b; De Virgiliis and Di Giovanni, 2020).
8.1.1. The blood-brain barrier (BBB) and endothelial integrity
The BBB is a multicellular stratified natural fence composed of brain capillary endothelial cells surrounded by pericytes, astrocyte end-feet and the extracellular basal lamina. Permeation of the barrier is severely restricted to all but a few small hydrophobic molecules, although permeability is not linearly related to the size of the solutes present in the blood stream (see reviews in (Abbott et al., 2010; Zlokovic, 2008; Rustenhoven and Kipnis, 2019)). It is increasingly being appreciated that the barrier is not purely anatomical, but also involves immune, electrolyte, water transport, and metabolic barriers (Begley, 2004). Because of the BBB, the brain as an organ is effectively isolated from the rest of the body’s economy. The process of acquiring such a sophisticated barrier in the course of phylogenetic evolution is conjectured to derive from the need to isolate neuronal excitability from ionic fluxes stemming from blood plasma (Abbott, 2005; Obermeier et al., 2016).
The endothelium fulfils multiple physiological roles, acting as an immunological barrier and regulating vascular permeability through its semi-permeable interface. The vascular endothelium growth factor (VEGF) is a major protagonist in endothelial physiology (Li et al., 2020e). Upon activation of VEGF-A receptor, a downstream metabolic cascade involving cadherin phosphorylation participates in the regulation of endothelial permeability. Endothelial cells perform autocrine and paracrine functions, producing angiogenic factors as well as vasoconstrictor factors like endothelin-1 to regulate vascular tone together with vasodilatory mechanisms mediated by nitric oxide and ACE2. The brain capillary bed is in relatively close topographic contact with glial and nerve cells, particularly astroglial endings surrounding the pericytes and endothelial cells (Fig. 3).
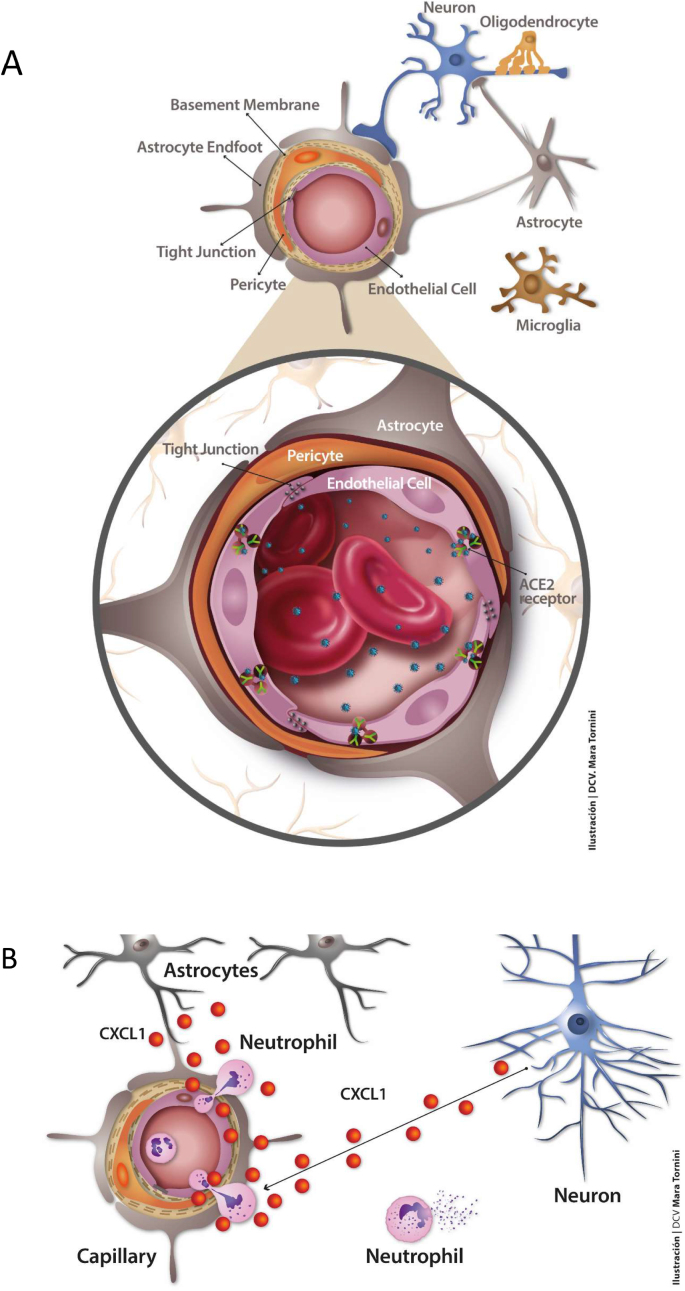
Fig. 3.
The key dysfunctional unit in brain: the capillary endothelial cell-pericyte. Upper figure: SARS-CoV-2 virions (blue particles) have been found in infected endothelial cells in necropsy samples of frontal cerebral cortex from a COVID-19 patient (Paniz-Mondolfi et al., 2020). Mechanisms for viral crossing of the BBB include disruption of the tight junctions sealing contiguous endothelial cells (Pober and Sessa, 2007), transcytosis (Rhea et al., 2021) and/or endocytic internalization of the virus upon binding to ACE2. Other receptors present in brain vasculature have been invoked (Cantuti-Castelvetri et al., 2020; Daly et al., 2020). The viral load into the blood stream is highly variable (Zheng et al., 2020). Pericytes (Brann et al., 2020) and astrocytes (Chen et al., 2020b; Xia and Lazartigues, 2008) possess ACE2 receptor capacity that could further spread the virus in the brain parenchyma once the BBB has been surpassed. SARS-CoV-2 S1 protein has recently been shown to trespass the BBB in a murine model, reaching all regions of the brain (Rhea et al., 2021). Lower figure: Another salient pathological aspect of endothelial dysfunction is related to the overexpression of astrocyte-derived cytokine CXCL1 and neutrophil, activated immune cell, and monocyte infiltration into the brain. These manifestations are observed in herpes simplex (HSV-1) infection associated with viral encephalitis. The chemokine (C-X-C motif) ligand 1 (CXCL1) is produced by astrocytes in response to HSV-1 and by astrocytes and neurons in response to IL-1α (Michael et al., 2020) and forms part of the SARS-CoV-2 hyper-neuroinflammatory response. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Direct passage of virions into the brain parenchyma could in principle occur in areas rich in fenestrated capillaries, i.e. lacking a proper BBB and thus allowing exchange between the blood and the cerebrospinal fluid, as is the case with the so-called circumventricular organs in the vicinity of the 3rd and 4th ventricles (Xu et al., 2011). Another possible mechanism for crossing the BBB is via macrophages, as is the case with the HIV-1 virus (Barker and Vaidya, 2020).
8.2. Dysfunctional endothelial cells at the root of pro-thrombotic/thromboembolic and pro-inflammatory reactions
8.2.1. The vascular endothelial growth factor cascade
When IL-6 binds to the soluble variant of its receptor, the latter forms a complex with the signal-transducing molecule gp130, present at the surface of essentially all cells in the organism. This complex then activates the release of various factors, including platelet-secreted vascular endothelial growth factor (VEGF) and monocyte chemoattractant protein-1, inhibiting E-cadherin in endothelial cells (Moore and June 2020; Teuwen et al., 2020). This, in turn, loosens the tight junctions between endothelial cells (“endothelial gaps”) (Pober and Sessa, 2007), leading to increased vascular permeability and leakage, and possibly precipitating the cytokine release syndrome (Behrens and Koretzky, 2017) in brain. Another implication of VEGF in COVID-19 is its reported ability to activate the neuropilin-1 receptor, a process that increases SARS-CoV-2 spike protein engagement. Indeed, VEGF-A is not only an angiogenic growth factor but is also a trigger that activates neuronal firing related to pain sensing. Neuropilins are expressed on normal blood and lymphatic endothelial cells, playing roles in the formation of blood and lymphatic vascular networks during angiogenesis. They act as co-receptors of VEGF receptors and support angiogenic signalling (Alghamdi et al., 2020). Given the ability of the SARS-CoV-2 spike protein, but not that of SARS-CoV or MERS-CoV, to bind to the b1b2 domain of the neuropilin-1 receptor (Cantuti-Castelvetri et al., 2020; Daly et al., 2020), SARS-CoV-2 infection might interfere with VEGF-A/neuropilin-1 receptor pain sensing (Moutal et al., 2020).
8.2.2. The bradykinin cascade
Pro-inflammatory mechanisms also affect the endothelial cells, which intervene directly and indirectly in coagulation. Another abnormal mechanism intervening in endothelial cells is the bradykinin B1 cascade, initiated by activation of bradykinin B1 receptors present in the endothelial cells. Upregulated by pro-inflammatory cytokines (van de Veerdonk et al., 2020), this cascade could also be operative in brain. ACE2 is required to inactivate des-Arg9 bradykinin, the potent endogenous ligand of B1 receptors. If ACE2 is “knocked-out” by SARS-CoV-2 binding, local vascular leakage could ensue, leading to angioedema. Importantly, direct experimental evidence of the presence of SARS-CoV-2 in the endothelium of COVID-19 patients demonstrating endothelial viral infection and diffuse lymphocytic endotheliitis is now available (Varga et al., 2020).
Under normal conditions, endothelial nitric oxide synthase releases nitric oxide, with its vasodilator and anti-thrombotic effects; one of the hallmarks of endothelial dysfunction in COVID-19 is the diminished activity of this enzyme, with concomitant nitric oxide deficiency (Green, 2020). Endothelial dysfunction shifts the delicate equilibrium of endothelial homeostasis towards reduced vasodilation, a pro-inflammatory status, and pro-thrombotic conditions, i.e. conditions akin to those found in endotheliitis. Inflammation, an early protective mechanism against diverse noxa, is tightly regulated to provide a balanced response (see recent review by (Weavers and Martin, 2020). The multimolecular protein complexes called inflammasomes play an important role in this mechanism; upon activation, the enzyme caspase-1 cleaves the inactive cytokine precursors pro-IL-1β and pro-IL-18 to produce their active forms (Seoane et al., 2020).
There is increasing evidence that in COVID-19, adhesion molecules are upregulated, cytokines such as macrophage chemoattractant peptide-1 are generated, inflammatory cells infiltrate the brain parenchyma (Fig. 3), and plasminogen activator inhibitor-1 contributes to the inflammatory response and pro-thrombotic status. SARS-CoV-2 in complex with ACE2 leads to depletion of the receptor in infected cells, lowering the level of angiotensin 1-7 and increasing the level of angiotensin II, the latter further inducing vasoconstriction and pro-inflammatory and pro-coagulant effects (Abassi et al., 2020). Native anticoagulant mechanisms such as those involving protein C, tissue factor pathway inhibitor (TFPI) or antithrombin are often altered in COVID-19. Spliced isoforms of TFPI are expressed differentially by endothelial cells (TFPIβ) and platelets (TFPIα) (Mast, 2016). The activated form of the pro-enzyme serine protease protein C, i.e. activated protein C, displays potent anti-coagulant and anti-thrombotic activities (Griffin et al., 2018). The dysfunctional status of pulmonary endothelial cells and biomarkers in COVID-19 is reviewed in ref. (Kaur et al., 2020).
Although many of the micro-vasculopathy alterations in COVID-19 are centred on the endothelial cell, pericytes are also affected, particularly in the pulmonary parenchyma, where post-mortem studies have revealed apoptosis and lower numbers of pericytes (Cardot-Leccia et al., 2020).
8.3. Coagulopathies
Coagulation abnormalities affect endothelial functions, looping feed-back mechanisms that lead to mutual activation and amplification of both pathologies. Plasma proteins intervening in the acute phase of coagulation and fibrinolysis are elevated in inflammation, whereas endogenous natural anticoagulatory mechanisms are inhibited (Connors and Levy, 2020; Arachchillage and Laffan, 2020). Pro-inflammatory cytokines further induce changes in the plasmalemma of endothelial cells and circulating blood cells towards a coagulant-prone status, including thrombin formation within small blood vessels, configuring a thrombotic microangiopathy that is beginning to be corroborated in COVID-19 necropsies (Meinhardt et al., 2021). Post-mortem studies of COVID-19 patients found that neutrophil activator marker CD177 was highly upregulated in neutrophils in microvascular thrombi of patients having severe forms of COVID-19, whereas less severe cases showed lesser levels of the activator. The authors also found a highly activated subpopulation of platelets (Nicolai et al., 2020). Post-mortem anatomopathological examination of COVID-19 patients in Germany showed that the cause of death of older patients (>65 years-old) was cardiorespiratory failure, whereas patients younger than 65 years died either of massive intracranial haemorrhage or pulmonary embolism, consistent with the coagulopathy increasingly found in COVID-19 (von Weyhern et al., 2020). Clinically, the abnormal coagulant-pro status is manifested in prolonged prothrombin time and is accompanied by mild thrombocytopenia. D-dimer is higher in deceased patients, suggesting the association of coagulopathies with poor prognosis (Wu et al., 2020b; Tang et al., 2020; Paterson et al., 2020; Marini and Gattinoni, 2020; Arachchillage and Laffan, 2020; Shi et al., 2020; Chen et al., 2020d). The thrombotic pathology involves micro- and macro-thromboses in the lungs and other organs, including brain (Zhou et al., 2020a), with lesions of both venous and arterial systems (Bikdeli et al., 2020; Klok et al., 2020). The latter study conducted on 181 ICU patients showed 31% incidence of thrombotic complications. Some of these pathologies had already been observed with SARS patients who presented hypercoagulable status and large artery ischemic strokes (Tsai et al., 2005). Histopathological findings of intravascular fibrin thrombi in medium size arteries, arterioles and capillaries were observed in essentially all necropsies of 67 COVID-19 individuals in New York (Bryce et al., 2020). This picture includes the CNS, where multifocal microthrombi, petechial microhaemorrhages, and acute infarctions were observed in 6/20 cases, affecting the frontal, parietal or occipital lobes. A recent study using radiolabelled spike protein unambiguously shows that the viral protein crosses the BBB presumably by an adsorptive transcytosis mechanism involving ACE2, reaches all regions of the brain and is partly retained by the brain endothelial cells (Rhea et al., 2021). Recent transcriptomic studies of necropsy samples from 144 COVID-19 patients have shown elevated expression of cathepsin L1, rather than ACE2, in pulmonary tissue, and stressed the multi-organ nature of the disease, with dysregulation of angiogenesis, coagulation and fibrosis (Nie et al., 2021).
A further complication of thrombotic nature in COVID-19 patients, akin to the antiphospholipid syndrome, has recently been disclosed. Importantly, pro-thrombotic IgG and IgM autoantibodies against phospholipids or phospholipid-binding proteins, like anti-cardiolipin and anti-phosphatidyl serine, were found in 52% of hospitalized COVID-19 patients, correlating with disease severity (Zuo et al., 2020). These autoantibodies are known to activate endothelial cells, platelets and neutrophils and are usually correlated with neutrophil hyperactivity, including the release of neutrophil remnants, the so-called extracellular traps, adding to the pro-thrombotic status.
9. Concluding remarks
The unfolding picture of COVID-19 is increasingly revealing its multifaceted, systemic and complex nature. Both the virus and the host organism contribute to the complexity of the connections and epiphenomenological clinical forms, with numerous partnerships between SARS-CoV-2 RNA and/or proteins and host-cell processes. Mutants of the virus with varying infectivity, different glycan moieties masking antibody targets on the viral surface and risk factors like age, comorbidities such as hypertension, cardiac or renal diseases and obesity, intermix to produce a wide palette of clinical outcomes both at the level of the individual and the population. The emerging genetic factors, including ethnicity and specific genes related to immune and inflammatory pathways, contribute to the pathogenesis and may influence the outcome of the disease. There are still many aspects of this complex nosological entity that remain to be elucidated and its severity in many cases and pandemic scale warrant further investigation in years to come.
Author contributions
I conceived and designed the study, searched the literature, interpreted the data and wrote the manuscript. I conceived the illustrations and had technical help to produce them.
Ethical approval
The research has been given ethical approval.
Declaration of competing interest
The author declares no conflict of interest.
Acknowledgements
This work was written within the framework of the grant PICT 2015–2654 from the Ministry of Science, Technology and Innovative Production of Argentina.
References
- Abassi Z., Higazi A.A.R., Kinaneh S., Armaly Z., Skorecki K., Heyman S.N. ACE2, COVID-19 infection, inflammation, and coagulopathy: missing pieces in the puzzle. Front. Physiol. 2020;11:574753. doi: 10.3389/fphys.2020.574753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott N.J. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell. Mol. Neurobiol. 2005;25:5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott N.J., Patabendige A.A.K., Dolman D.E.M., Yusof S.R., Begley D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Akbar A.N., Gilroy D.W. Aging immunity may exacerbate COVID-19. Science. 2020;369:256–257. doi: 10.1126/science.abb0762. (New York, NY) [DOI] [PubMed] [Google Scholar]
- Alghamdi A.A.A., Benwell C.J., Atkinson S.J., Lambert J., Johnson R.T., Robinson S.D. NRP2 as an emerging angiogenic player; promoting endothelial cell adhesion and migration by regulating recycling of α5 integrin. Front Cell Dev Biol. 2020;8:395. doi: 10.3389/fcell.2020.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharthy A., Faqihi F., Memish Z.A., Karakitsos D. Lung injury in COVID-19—an emerging hypothesis. ACS Chem. Neurosci. 2020;11:2156–2158. doi: 10.1021/acschemneuro.0c00422. [DOI] [PubMed] [Google Scholar]
- Alonso-Lana S., Marquié M., Ruiz A., Boada M. Cognitive and neuropsychiatric manifestations of COVID-19 and effects on elderly individuals with dementia. Front. Aging Neurosci. 2020;12:588872. doi: 10.3389/fnagi.2020.588872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A. Health care for chronic neurological patients after COVID-19. Lancet Neurol. 2020;19:562–563. doi: 10.1016/S1474-4422(20)30157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arachchillage D.R., Laffan M. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis : JTH. 2020;18:1233–1234. doi: 10.1111/jth.14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. J. Am. Med. Assoc. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbrenner A.C., Mouktaroudi M., Krämer B., Oestreich M., Antonakos N., Nuesch-Germano M. Disease severity-specific neutrophil signatures in blood transcriptomes stratify COVID-19 patients. Genome Med. 2021;13:7. doi: 10.1186/s13073-020-00823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzato V.A., Matson M.J., Seifert S.N., Pryce R., Williamson B.N., Anzick S.L. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183:1901–1912. doi: 10.1016/j.cell.2020.10.049. e1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avula A., Nalleballe K., Narula N., Sapozhnikov S., Dandu V., Toom S. COVID-19 presenting as stroke. Brain Behav. Immun. 2020;87:115–119. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awogbindin I.O., Ishola I.O., St-Pierre M.K., Carrier M., Savage J.C., Di Paolo T. Remodeling microglia to a protective phenotype in Parkinson’s disease? Neurosci. Lett. 2020;735:135164. doi: 10.1016/j.neulet.2020.135164. [DOI] [PubMed] [Google Scholar]
- Bagheri S.H.R., Asghari A.M., Farhadi M., Shamshiri A.R., Kabir A., Kamrava S.K. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak. Med. J. Islam. Repub. Iran. 2020;2020(15 Jun):34–62. doi: 10.34171/mjiri.34.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A.M. Chronic COVID syndrome: need for an appropriate medical terminology for long-COVID and COVID long-haulers. J. Med. Virol. 2020;93:2555–2556. doi: 10.1002/jmv.26624. [DOI] [PubMed] [Google Scholar]
- Barcelo-Coblijn G., Hogyes E., Kitajka K., Puskas L.G., Zvara A., Hackler L., Jr. Modification by docosahexaenoic acid of age-induced alterations in gene expression and molecular composition of rat brain phospholipids. ProcNatlAcadSciUSA. 2003;100:11321–11326. doi: 10.1073/pnas.1734008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker C.T., Vaidya N.K. Modeling HIV-1 infection in the brain. PLoS Comput. Biol. 2020;16 doi: 10.1371/journal.pcbi.1008305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett E.M., Cassell M.D., Perlman S. Two neurotropic viruses, herpes simplex virus type 1 and mouse hepatitis virus, spread along different neural pathways from the main olfactory bulb. Neuroscience. 1993;57:1007–1025. doi: 10.1016/0306-4522(93)90045-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes F.J. While we wait for a vaccine against SARS-CoV-2, why not think about available drugs? Front. Physiol. 2020;11:820. doi: 10.3389/fphys.2020.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes F.J. Central nervous system targets and routes for SARS-CoV-2: current views and new hypotheses. ACS Chem. Neurosci. 2020;11:2793–2803. doi: 10.1021/acschemneuro.0c00434. [DOI] [PubMed] [Google Scholar]
- Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. J. Exp. Med. 2020;18 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathini P., Mottas A., Jaquet M., Brai E., Alberi L. Progressive signaling changes in the olfactory nerve of patients with Alzheimer’s disease. Neurobiol. Aging. 2019;76:80–95. doi: 10.1016/j.neurobiolaging.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Begley D.J. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol. Ther. 2004;104:29–45. doi: 10.1016/j.pharmthera.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Behrens E.M., Koretzky G.A. Review: cytokine storm syndrome: looking toward the precision medicine era. Arthritis & rheumatology. 2017;69:1135–1143. doi: 10.1002/art.40071. Hoboken, NJ. [DOI] [PubMed] [Google Scholar]
- Berger J.R. COVID-19 and the nervous system. J. Neurovirol. 2020;26:143–148. doi: 10.1007/s13365-020-00840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinska K., Butowt R. Anosmia in COVID-19: a bumpy road to establishing a cellular mechanism. ACS Chem. Neurosci. 2020;11:2152–2155. doi: 10.1021/acschemneuro.0c00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Melo D., Nilsson-Payant B.E., Liu W.-C., Uhl S., Hoagland D., Møller R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo H.-X., Li W., Yang Y., Wang Y., Zhang Q., Cheung T. Posttraumatic stress symptoms and attitude toward crisis mental health services among clinically stable patients with COVID-19 in China. Psychol. Med. 2020;27:1–2. doi: 10.1017/S0033291720000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo-Rizzo P., Borsetto D., Spinato G., Fabbris C., Menegaldo A., Gaudioso P. New onset of loss of smell or taste in household contacts of home-isolated SARS-CoV-2-positive subjects. Eur. Arch. Oto-Rhino-Laryngol. : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2020;277:2637–2640. doi: 10.1007/s00405-020-06066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann D.H., Datta S.R. Finding the brain in the nose. Annu. Rev. Neurosci. 2020;43:277–295. doi: 10.1146/annurev-neuro-102119-103452. [DOI] [PubMed] [Google Scholar]
- Brann D.H., Tsukahara T., Weinreb C., Lipovsek M., Van den Berge K., Gong B. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Science advances. 2020;6 doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briguglio M., Bona A., Porta M., Dell’Osso B., Pregliasco F.E., Banfi G. Disentangling the hypothesis of host dysosmia and SARS-CoV-2: the bait symptom that hides neglected neurophysiological routes. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin P., Nath A., Beckham J.D. Is COVID-19 a perfect storm for Parkinson’s disease? Trends Neurosci. 2020;43:931–933. doi: 10.1016/j.tins.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce C., Grimes Z., Pujadas E., Ahuja S., Beasley M.B., Albrecht R. The Mount Sinai COVID-19 Autopsy Experience. medRxiv : the Preprint Server for Health Sciences. 2020. Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. 2020.2005.2018.20099960. [Google Scholar]
- Bryche B., St Albin A., Murri S., Lacôte S., Pulido C., Ar Gouilh M. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav. Immun. 2020;89:579–586. doi: 10.1016/j.bbi.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunyavanich S., Grant C., Vicencio A. Racial/ethnic variation in nasal gene expression of transmembrane serine protease 2 (TMPRSS2) J. Am. Med. Assoc. 2020;324:17386. doi: 10.1001/jama.2020.17386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante-Marin X.M., Ostrowski L.E. Cilia and mucociliary clearance. Cold Spring Harbor perspectives in biology. 2017;9:a028241. doi: 10.1101/cshperspect.a028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzhdygan T.P., DeOre B.J., Baldwin-Leclair A., Bullock T.A., McGary H.M., Khan J.A. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier. Neurobiol. Dis. 2020;146:105131. doi: 10.1016/j.nbd.2020.105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo S.M., Singer D., Makrides V., Huggel K., Pos K.M., Wagner C.A. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology. 2009;136:872–882. doi: 10.1053/j.gastro.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. 2020;370:856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardot-Leccia N., Hubiche T., Dellamonica J., Burel-Vandenbos F., Passeron T. Pericyte alteration sheds light on micro-vasculopathy in COVID-19 infection. Intensive Care Med. 2020;46:1777–1778. doi: 10.1007/s00134-020-06147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. J. Am. Med. Assoc. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carod-Artal F.J. Neurological complications of coronavirus and COVID-19. Rev. Neurol. 2020;70:311–322. doi: 10.33588/rn.7009.2020179. [DOI] [PubMed] [Google Scholar]
- Cavasotto C.N., Di Filippo J.I. In silico drug repurposing for COVID-19: targeting SARS-CoV-2 proteins through docking and consensus ranking. Molecular informatics. 2021;40 doi: 10.1002/minf.202000115. [DOI] [PubMed] [Google Scholar]
- Cazzolla A.P., Lovero R., Lo Muzio L., Testa N.F., Schirinzi A., Palmieri G. Taste and smell disorders in COVID-19 patients: role of interleukin-6. ACS Chem. Neurosci. 2020;11:2774–2781. doi: 10.1021/acschemneuro.0c00447. [DOI] [PubMed] [Google Scholar]
- Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Deng C., Chen X., Zhang X., Chen B., Yu H. Ocular manifestations and clinical characteristics of 534 cases of COVID-19 in China: a cross-sectional study. Acta Ophtalmol. 2020;98:e951–e959. doi: 10.1111/aos.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Wang K., Yu J., Howard D., French L., Chen Z. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in human and mouse brain. Front. Neurol. 2020;11:573095. doi: 10.3389/fneur.2020.573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.H., Young M.T., Gounley J., Stanley C., Bhowmik D. 2020. Distinct Structural Flexibility within SARS-CoV-2 Spike Protein Reveals Potential Therapeutic Targets. 2020.2004.2017.047548. [Google Scholar]
- Cohen M.E., Eichel R., Steiner-Birmanns B., Janah A., Ioshpa M., Bar-Shalom R. A case of probable Parkinson’s disease after SARS-CoV-2 infection. Lancet Neurol. 2020;19:804–805. doi: 10.1016/S1474-4422(20)30305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni A., Martini R., Caprari P., Ballestri M., Capecchi P.L., Gnasso A. COVID-19 sepsis and microcirculation dysfunction. Front. Physiol. 2020;11:747. doi: 10.3389/fphys.2020.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;137:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen T., Lolli V., Sadeghi N., Rovai A., Trotta N., Taccone F.S. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020;95 doi: 10.1212/WNL.0000000000010116. e2016-e2027. [DOI] [PubMed] [Google Scholar]
- Daly J.L., Simonetti B., Klein K., Chen K.-E., Williamson M.K., Antón-Plágaro C. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370:861–865. doi: 10.1126/science.abd3072. (New York, NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniloski Z., Jordan T.X., Wessels H.-H., Hoagland D.A., Kasela S., Legut M. Identification of required host factors for SARS-CoV-2 infection in human cells. Cell. 2020;184:92–105.e116. doi: 10.1016/j.cell.2020.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice F.G., Tovar-Moll F., Moll J., Munoz D.P., Ferreira S.T. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the central nervous system. Trends Neurosci. 2020;43:355–357. doi: 10.1016/j.tins.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Virgiliis F., Di Giovanni S. Lung innervation in the eye of a cytokine storm: neuroimmune interactions and COVID-19. Nat. Rev. Neurol. 2020;16:645–652. doi: 10.1038/s41582-020-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffner F., Scharr M., Klingenstein S., Klingenstein M., Milazzo A., Scherer S. Histological evidence for the enteric nervous system and the choroid plexus as alternative routes of neuroinvasion by SARS-CoV2. Front. Neuroanat. 2020;14:596439. doi: 10.3389/fnana.2020.596439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio C., Malani P.N. COVID-19—new insights on a rapidly changing epidemic. J. Am. Med. Assoc. 2020;323:1339–1340. doi: 10.1001/jama.2020.3072. [DOI] [PubMed] [Google Scholar]
- Díaz J. SARS-CoV-2 molecular network structure. Front. Physiol. 2020;11:870. doi: 10.3389/fphys.2020.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Liang T.J. Is SARS-CoV-2 also an enteric pathogen with potential fecal-oral transmission? A COVID-19 virological and clinical review. Gastroenterology. 2020;159:53–61. doi: 10.1053/j.gastro.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Wang H., Shen H., Li Z., Geng J., Han H. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J. Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doobay M.F., Talman L.S., Obr T.D., Tian X., Davisson R.L., Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé M., Le Coupanec A., Wong A.H.M., Rini J.M., Desforges M., Talbot P.J. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J. Virol. 2018;92 doi: 10.1128/JVI.00404-18. e00404-00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliezer M., Hautefort C., Hamel A.-L., Verillaud B., Herman P., Houdart E. Sudden and complete olfactory loss function as a possible symptom of COVID-19. JAMA Otolaryngology–Head & Neck Surgery. 2020;146:674–675. doi: 10.1001/jamaoto.2020.0832. [DOI] [PubMed] [Google Scholar]
- Ellinghaus D., Degenhardt F., Bujanda L., Buti M., Albillos A., Invernizzi P. Genomewide association study of severe covid-19 with respiratory failure. N. Engl. J. Med. 2020;383:1522–1534. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdő F., Bors L.A., Farkas D., Á Bajza, Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018;143:155–170. doi: 10.1016/j.brainresbull.2018.10.009. [DOI] [PubMed] [Google Scholar]
- Espinosa J.M. Down syndrome and COVID-19: a perfect storm? Cell Rep Med. 2020;1:100019. doi: 10.1016/j.xcrm.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa P.S., Rizvi Z., S P., Hindi F., Filatov A. Neurological complications of coronavirus disease (COVID-19): encephalopathy, MRI brain and cerebrospinal fluid findings: case 2. Cureus. 2020;12 doi: 10.7759/cureus.7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber I., Brandão P.R.P., Menegatti F., de Carvalho Bispo D.D., Maluf F.B., Cardoso F. Coronavirus disease 2019 and parkinsonism: a non-post-encephalitic case. Movement disorders. official journal of the Movement Disorder Society. 2020;35:1721–1722. doi: 10.1002/mds.28277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A.V., De Luca LA, Jr . In: Neurobiology of Body Fluid Homeostasis: Transduction and Integration. Menani J.V., Johnson A.K., editors. CRC Press/Taylor & Francis Group, LLC; Boca Raton (FL): 2014. Circumventricular organs: integrators of circulating signals controlling hydration, energy balance, and immune function. [PubMed] [Google Scholar]
- Ferrarese C., Silani V., Priori A., Galimberti S., Agostoni E., Monaco S. An Italian multicenter retrospective-prospective observational study on neurological manifestations of COVID-19 (NEUROCOVID) Neurol. Sci. : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2020;41:1355–1359. doi: 10.1007/s10072-020-04450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov A., Sharma P., Hindi F., Espinosa P.S. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12 doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J., Scorza F.A., Scorza C.A. Diagnosing myasthenic crisis in SARS-CoV-2 infected patients requires adherence to appropriate criteria. J. Neurol. Sci. 2020;417:117062. doi: 10.1016/j.jns.2020.117062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodoulian L., Tuberosa J., Rossier D., Boillat M., Kan C.-D., Pauli V. SARS-CoV-2 receptor and entry genes are expressed by sustentacular cells in the human olfactory neuroepithelium. iScience. 2020;23:101839. doi: 10.1016/j.isci.2020.101839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotuhi M., Mian A., Meysami S., Raji C.A. Neurobiology of COVID-19. J. Alzheim. Dis. 2020;76:3–19. doi: 10.3233/JAD-200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank C., Murphy C. The brief form of the California odor learning test 3. Front. Neurosci. 2020;14:173. doi: 10.3389/fnins.2020.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey W.H., Liu J., Chen X., Thorne R.G., Fawcett J.R., Ala T.A. Delivery of 125I-NGF to the brain via the olfactory route. Drug Deliv. 1997;4:87–92. [Google Scholar]
- Fridman S., Bullrich M.B., Jimenez-Ruiz A., Costantini P., Shah P., Just C. Stroke Risk, phenotypes, and death in COVID-19: systematic review and newly reported cases. Neurology. 2020;95:e3373–e3385. doi: 10.1212/WNL.0000000000010851. [DOI] [PubMed] [Google Scholar]
- Fung T.S., Liu D.X. Human coronavirus: host-pathogen interaction. Annu. Rev. Microbiol. 2019;73:529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles’ heel of current strategies to control covid-19. N. Engl. J. Med. 2020;382:2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gane S.B., Kelly C., Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020;58:299–301. doi: 10.4193/Rhin20.114. [DOI] [PubMed] [Google Scholar]
- Gao Z., Xu Y., Sun C., Wang X., Guo Y., Qiu S. A systematic review of asymptomatic infections with COVID-19. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2021;54:12–16. doi: 10.1016/j.jmii.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J.F., Ravussin Y. A new symptom of COVID-19: loss of taste and smell. Obesity. 2020;28:848. doi: 10.1002/oby.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon A.A., Gershon M.D. Pathogenesis and current approaches to control of varicella-zoster virus infections. Clin. Microbiol. Rev. 2013;26:728–743. doi: 10.1128/CMR.00052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin. Infect. Dis. 2020;71:889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gklinos P. Neurological manifestations of COVID-19: a review of what we know so far. J. Neurol. 2020;267:2485–2489. doi: 10.1007/s00415-020-09939-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D.E., Hiatt J., Bouhaddou M., Rezelj V.V., Ulferts S., Braberg H. vol. 370. Science; New York, NY: 2020. (Comparative Host-Coronavirus Protein Interaction Networks Reveal Pan-Viral Disease Mechanisms). eabe9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region. Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S.J. Covid-19 accelerates endothelial dysfunction and nitric oxide deficiency. Microb. Infect. 2020;22:149–150. doi: 10.1016/j.micinf.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J.H., Zlokovic B.V., Mosnier L.O. Activated protein C, protease activated receptor 1, and neuroprotection. Blood. 2018;132:159–169. doi: 10.1182/blood-2018-02-769026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group TWCftCS Four-month clinical status of a cohort of patients after hospitalization for COVID-19. J. Am. Med. Assoc. 2021 doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol D.L. IL-6 regulation of synaptic function in the CNS. Neuropharmacology. 2015;96:42–54. doi: 10.1016/j.neuropharm.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harberts E., Yao K., Wohler J.E., Maric D., Ohayon J., Henkin R. Human herpesvirus-6 entry into the central nervous system through the olfactory pathway. Proc. Natl. Acad. Sci. U.S.A. 2011;108:13734–13739. doi: 10.1073/pnas.1105143108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylycz M.J., Lein E.S., Guillozet-Bongaarts A.L., Shen E.H., Ng L., Miller J.A. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M.T., Golenbock D., Latz E., Morgan D., Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimer’s Res. Ther. 2020;12:69. doi: 10.1186/s13195-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold T., Jurinovic V., Arnreich C., Hellmuth J.C., Bergwelt-Baildon M., Klein M. Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients. J. Allergy Clin. Immunol. 2020;146:128–136. doi: 10.1016/j.jaci.2020.05.008. e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmesæth J., Skaare D. Loss of smell or taste as the only symptom of COVID-19. Tidsskrift for den Norske laegeforening : tidsskrift for praktisk medicin. ny raekke. 2020;140:7. doi: 10.4045/tidsskr.20.0287. [DOI] [PubMed] [Google Scholar]
- Hockley J.R.F., Taylor T.S., Callejo G., Wilbrey A.L., Gutteridge A., Bach K. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut. 2019;68:633–644. doi: 10.1136/gutjnl-2017-315631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C.S. Olfactory neuropathy in severe acute respiratory syndrome: report of A case. Acta Neurol. Taiwanica. 2006;15:26–28. [PubMed] [Google Scholar]
- Jackson D.J., Busse W.W., Bacharier L.B., Kattan M., O’Connor G.T., Wood R.A. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J. Allergy Clin. Immunol. 2020;146:203–206. doi: 10.1016/j.jaci.2020.04.009. e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacomy H., Talbot P.J. Vacuolating encephalitis in mice infected by human coronavirus OC43. Virology. 2003;315:20–33. doi: 10.1016/S0042-6822(03)00323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain V., Yuan J.-M. Systematic review and meta-analysis of predictive symptoms and comorbidities for severe COVID-19 infection. Int. J. Publ. Health. 2020;25:1–14. doi: 10.1007/s00038-020-01390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R.-D., Liu M.-Q., Chen Y., Shan C., Zhou Y.-W., Shen X.-R. Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell. 2020;182 doi: 10.1016/j.cell.2020.05.027. 50-58.e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Lian J.S., Hu J.H., Gao J., Zheng L., Zhang Y.M. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitaroon K., Wangworawut Y., Ma Y., Patel Z.M. Evaluation of the incidence of other cranial neuropathies in patients with postviral olfactory loss. JAMA Otolaryngology–Head & Neck Surgery. 2020;146:1–6. doi: 10.1001/jamaoto.2020.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanberg N., Ashton N.J., Andersson L.-M., Yilmaz A., Lindh M., Nilsson S. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020;95:e1754–e1759. doi: 10.1212/WNL.0000000000010111. [DOI] [PubMed] [Google Scholar]
- Kaur S., Tripathi D.M., Yadav A. The enigma of endothelium in COVID-19. Front. Physiol. 2020;11:989. doi: 10.3389/fphys.2020.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M., Helfand B.K.I., Gou R.Y., Gartaganis S.L., Webb M., Moccia J.M. Delirium in older patients with COVID-19 presenting to the emergency department. JAMA Network Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.29540. e2029540-e2029540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempin F., Mosienko V., Matthes S., Villela D.C., Todiras M., Penninger J.M. Depletion of angiotensin-converting enzyme 2 reduces brain serotonin and impairs the running-induced neurogenic response. Cell. Mol. Life Sci. : CM. 2018;75:3625–3634. doi: 10.1007/s00018-018-2815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotfis K., Williams Roberson S., Wilson J.E., Dabrowski W., Pun B.T., Ely E.W. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit. Care. 2020;24:176. doi: 10.1186/s13054-020-02882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupke A., Becker S., Wewetzer K., Ahlemeyer B., Eickmann M., Herden C. Intranasal borna disease virus (BoDV-1) infection: insights into initial steps and potential contagiosity. Int. J. Mol. Sci. 2019;20:1318. doi: 10.3390/ijms20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri D., Ardila A. COVID-19 pandemic: a neurological perspective. Cureus. 2020;12 doi: 10.7759/cureus.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing A.G., Lorenc A., del Molino del Barrio I., Das A., Fish M., Monin L. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 2020;26:1623–1635. doi: 10.1038/s41591-020-1038-6. [DOI] [PubMed] [Google Scholar]
- Lamers M.M., Beumer J., van der Vaart J., Knoops K., Puschhof J., Breugem T.I. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. doi: 10.1126/science.abc1669. New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K.K., Yu W.C., Chu C.M., Lau S.T., Sheng B., Yuen K.Y. Possible central nervous system infection by SARS coronavirus. Emerg. Infect. Dis. 2004;10:342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax S.F., Skok K., Sechner P., Kessler H.H., Kaufmann N., Koelblinger C. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Internal med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Oto-Rhino-Laryngol. : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Min P., Lee S., Kim S.W. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J. Kor. Med. Sci. 2020;35:e174. doi: 10.3346/jkms.2020.35.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.W., Yusof Khan A.H.K., Ching S.M., Chia P.K., Loh W.C., Abdul Rashid AMi Stroke and novel coronavirus infection in humans: a systematic review and meta-analysis. Front. Neurol. 2020;11:579070. doi: 10.3389/fneur.2020.579070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi M., Padovani A., McArthur J.C. Neurological manifestations associated with COVID-19: a review and a call for action. J. Neurol. 2020;267:1573–1576. doi: 10.1007/s00415-020-09896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hirano N., Hayashida T., Taniguchi T., Sugita Y. Neurotropic virus tracing suggests a membranous-coating-mediated mechanism for transsynaptic communication. J. Comp. Neurol. 2013;521:203–212. doi: 10.1002/cne.23171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Yao D., Zeng X., Kasakovski D., Zhang Y., Chen S. Age related human T cell subset evolution and senescence. Immun. Ageing : Ital. Am. 2019;16:24. doi: 10.1186/s12979-019-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Giorgi E.E., Marichannegowda M.H., Foley B., Xiao C., Kong X.-P. Emergence of SARS-CoV-2 through recombination and strong purifying selection. Science advances. 2020;6 doi: 10.1126/sciadv.abb9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang M., Zahou Y. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5:279–284. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Huang S., Lu J., Lai R., Zhang Z., Lin X. Upper gastrointestinal bleeding caused by SARS-CoV-2 infection. Am. J. Gastroenterol. 2020;115:1541–1542. doi: 10.14309/ajg.0000000000000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Redfors B., Sáinz-Jaspeado M., Shi S., Martinsson P., Padhan N. Suppressed vascular leakage and myocardial edema improve outcome from myocardial infarction. Front. Physiol. 2020;11:763. doi: 10.3389/fphys.2020.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Zhu Y., Fang Y. COVID-19 and post-traumatic stress disorder: a vicious circle involving immunosuppression. CNS Neurosci. Ther. 2020;26:876–878. doi: 10.1111/cns.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W., Liang H., Ou L., Chen B., Chen A., Li C. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180:1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W., Feng Z., Rao S., Xiao C., Xue X., Lin Z. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69:1141–1143. doi: 10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- Lin T., Liu E., He H., Shin M.C., Moon C., Yang V.C. Nose-to-brain delivery of macromolecules mediated by cell-penetrating peptides. Acta Pharm. Sin. B. 2016;6:352–358. doi: 10.1016/j.apsb.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart S.M., O’Rahilly S. When two pandemics meet: why is obesity associated with increased COVID-19 mortality? Med. Plus. 2020;1:33–42. doi: 10.1016/j.medj.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüers J.-C., Klußmann J.P., Guntinas-Lichius O. Die Covid-19-Pandemie und das HNO-Fachgebiet: worauf kommt es aktuell an? Laryngo-Rhino-Otol. 2020;99:287–291. doi: 10.1055/a-1095-2344. [DOI] [PubMed] [Google Scholar]
- Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. : J. Lab. Clin. Med. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano G.S., Woods J.K., Amato A.A. Covid-19–Associated myopathy caused by type I interferonopathy. N. Engl. J. Med. 2020;383:2389–2390. doi: 10.1056/NEJMc2031085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Wang M., Chen S., He Q., Chang J., Hong C. 2020. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: a Retrospective Case Series Study. 2020.2002.2022.20026500. [Google Scholar]
- Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. J. Am. Med. Assoc. 2020:2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- Mast A.E. Tissue factor pathway inhibitor: multiple anticoagulant activities for a single protein. Arterioscler. Thromb. Vasc. Biol. 2016;36:9–14. doi: 10.1161/ATVBAHA.115.305996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D., Giles J.R., Baxter A.E., Oldridge D.A., Greenplate A.R., Wu J.E. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369 doi: 10.1126/science.abc8511. New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy K., Peterson A., Tian Y., Sang Y. Immunogenetic association underlying severe COVID-19. Vaccines. 2020;8:700. doi: 10.3390/vaccines8040700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- Méndez-Guerrero A., Laespada-García M.I., Gómez-Grande A., Ruiz-Ortiz M., Blanco-Palmero V.A., Azcarate-Diaz F.J. Acute hypokinetic-rigid syndrome following SARS-CoV-2 infection. Neurology. 2020;95:e2109–e2118. doi: 10.1212/WNL.0000000000010282. [DOI] [PubMed] [Google Scholar]
- Menni C., Valdes A., Freydin M.B., Ganesh S., El-Sayed Moustafa J., Visconti A. Loss of smell and taste in combination with other symptoms is a strong predictor of COVID-19 infection. Nat. Med. 2020;26:1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercante G., Ferreli F., De Virgilio A., Gaino F., Di Bari M., Colombo G. Prevalence of taste and smell dysfunction in coronavirus disease 2019. JAMA Otolaryngology–Head & Neck Surgery. 2020;146:723–728. doi: 10.1001/jamaoto.2020.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merello M., Bhatia K.P., Obeso J.A. SARS-CoV-2 and the risk of Parkinson’s disease: facts and fantasy. Lancet Neurol. 2020;20:94–95. doi: 10.1016/S1474-4422(20)30442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkler A.E., Parikh N.S., Mir S., Gupta A., Kamel H., Lin E. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA neurology. 2020;77:1–7. doi: 10.1001/jamaneurol.2020.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael B.D., Bricio-Moreno L., Sorensen E.W., Miyabe Y., Lian J., Solomon T. Astrocyte- and neuron-derived CXCL1 drives neutrophil transmigration and blood-brain barrier permeability in viral encephalitis. Cell Rep. 2020;32:108150. doi: 10.1016/j.celrep.2020.108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi M., Yamanouchi H., Ichiyama T., Shiomi M. Acute encephalopathy associated with influenza and other viral infections. Acta Neurol. Scand. Suppl. 2007;186:45–56. [PubMed] [Google Scholar]
- Moein S.T., Hashemian S.M.R., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020;10:944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A.H., Norrby E., Kristensson K. Viruses and behavioural changes: a review of clinical and experimental findings. Rev. Neurosci. 1993;4:267–286. doi: 10.1515/revneuro.1993.4.3.267. [DOI] [PubMed] [Google Scholar]
- Montalvan V., Lee J., Bueso T., De Toledo J., Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin. Neurol. Neurosurg. 2020;194:105921. doi: 10.1016/j.clineuro.2020.105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B.J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. (New York, NY) [DOI] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutal A., Martin L.F., Boinon L., Gomez K., Ran D., Zhou Y. SARS-CoV-2 Spike protein hijacks VEGF-A/Neuropilin-1 receptor signaling to induce analgesia. Pain. 2020;162:243–252. doi: 10.1097/j.pain.0000000000002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd P.A., Crawford J.C., Turner J.S., Souquette A., Reynolds D., Bender D. Distinct inflammatory profiles distinguish COVID-19 from influenza with limited contributions from cytokine storm. Science advances. 2020;6 doi: 10.1126/sciadv.abe3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C., Gilmore M.M., Seery C.S., Salmon D.P., Lasker B.R. Olfactory thresholds are associated with degree of dementia in Alzheimer’s disease. Neurobiol. Aging. 1990;11:465–469. doi: 10.1016/0197-4580(90)90014-q. [DOI] [PubMed] [Google Scholar]
- Nguyen S., Major K., Cochet C., Bizzozzero T., Barbarossa L., Bosshard W. [COVID-19 infection in the elderly in French-speaking Switzerland: an inventory of beliefs, convictions and certainties] Rev. Med. Suisse. 2020;16:835–838. [PubMed] [Google Scholar]
- Nicolai L., Leunig A., Brambs S., Kaiser R., Weinberger T., Weigand M. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X., Qian L., Sun R., Huang B., Dong X., Xiao Q. Multi-organ proteomic landscape of COVID-19 autopsies. Cell. 2021;184:775–791. doi: 10.1016/j.cell.2021.01.004. e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier B., Verma A., Ransohoff R.M. The blood-brain barrier. Handb. Clin. Neurol. 2016;133:39–59. doi: 10.1016/B978-0-444-63432-0.00003-7. [DOI] [PubMed] [Google Scholar]
- Ospel J.M., Goyal M. Endovascular stroke treatment during the COVID-19 pandemic. Nat. Rev. Neurol. 2020;16:351–352. doi: 10.1038/s41582-020-0371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Losada M., Kobiec T., Udovin L., Chevalier G., Quarracino C., Menéndez Maissonave C. Parkinson’s disease in the era of a novel respiratory virus pandemic. Front. Neurol. 2020;11:995. doi: 10.3389/fneur.2020.00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pairo-Castineira E., Clohisey S., Klaric L., Bretherick A.D., Rawlik K., Pasko D. Genetic mechanisms of critical illness in Covid-19. Nature. 2020;591:92–98. doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J. Central nervous system involvement by severe acute respiratory syndrome coronavirus -2 (SARS-CoV-2) J. Med. Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasa S., Desai M., Thoguluva Chandrasekar V., Patel H.K., Kennedy K.F., Roesch T. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta-analysis. JAMA Network Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma V., Ohla K., Veldhuizen M.G., Niv M.Y., Kelly C.E., Bakke A.J. More than smell. COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem. Senses. 2020;45:609–622. doi: 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov V., Beylerli O., Gareev I., Torres Solis L.F., Solís Herrera A., Aliev G. COVID-19-Related intracerebral hemorrhage. Front. Aging Neurosci. 2020;12:600172. doi: 10.3389/fnagi.2020.600172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz M.L., Barrantes F.J. Autoimmune attack of the neuromuscular junction in myasthenia gravis: nicotinic acetylcholine receptors and other targets. ACS Chem. Neurosci. 2019;10:2186–2194. doi: 10.1021/acschemneuro.9b00041. [DOI] [PubMed] [Google Scholar]
- Perry G., Castellani R.J., Smith M.A., Harris P.L., Kubat Z., Ghanbari K. Oxidative damage in the olfactory system in Alzheimer’s disease. Acta Neuropathol. 2003;106:552–556. doi: 10.1007/s00401-003-0761-7. [DOI] [PubMed] [Google Scholar]
- Peters E.M.J., Schedlowski M., Watzl C., Gimsa U. To stress or not to stress: brain-behavior-immune interaction may weaken or promote the immune response to SARS-CoV-2. Neurobiology of stress. 2021;14:100296. doi: 10.1016/j.ynstr.2021.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzini A., Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020;16:636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza G., Morrow D.A. Diagnosis, management, and pathophysiology of arterial and venous thrombosis in COVID-19. J. Am. Med. Assoc. 2020;324:2548–2549. doi: 10.1001/jama.2020.23422. [DOI] [PubMed] [Google Scholar]
- Pleasure S.J., Green A.J., Josephson S.A. The spectrum of neurologic disease in the severe acute respiratory syndrome coronavirus 2 pandemic infection: neurologists move to the frontlines. JAMA neurology. 2020;77:679–680. doi: 10.1001/jamaneurol.2020.1065. [DOI] [PubMed] [Google Scholar]
- Pober J.S., Sessa W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- Poewe W., Seppi K., Tanner C.M., Halliday G.M., Brundin P., Volkmann J. Parkinson disease. Nature reviews Disease primers. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- Politi L.S., Salsano E., Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA neurology. 2020;77:1028–1029. doi: 10.1001/jamaneurol.2020.2125. [DOI] [PubMed] [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated acute hemorrhagidc necrotizing encephalopathy: CT and MRI features. Radiology. 2020;296:E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakara C., Godbole R., Sil P., Jahnavi S., van Zanten T.S., Sheth D. 2020. Niclosamide Inhibits SARS-CoV2 Entry by Blocking Internalization through pH-dependent CLIC/GEEC Endocytic Pathway. 2020.2012.2016.422529. [Google Scholar]
- Rábano-Suárez P., Bermejo-Guerrero L., Méndez-Guerrero A., Parra-Serrano J., Toledo-Alfocea D., Sánchez-Tejerina D. Generalized myoclonus in COVID-19. Neurology. 2020;95:e767–e772. doi: 10.1212/WNL.0000000000009829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff R.M., Schafer D., Vincent A., Blachère N.E., Bar-Or A. Neuroinflammation: ways in which the immune system Affects the brain. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2015;12:896–909. doi: 10.1007/s13311-015-0385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen A.L., Popescu S.V. SARS-CoV-2 transmission without symptoms. 2021;371:1206–1207. doi: 10.1126/science.abf9569. [DOI] [PubMed] [Google Scholar]
- Ray P., Torck A., Quigley L., Wangzhou A., Neiman M., Rao C. Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research. Pain. 2018;159:1325–1345. doi: 10.1097/j.pain.0000000000001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remsik J., Wilcox J.A., Babady N.E., McMillen T.A., Vachha B.A., Halpern N.A. Inflammatory leptomeningeal cytokines mediate COVID-19 neurologic symptoms in cancer patients. Canc. Cell. 2021;39:276–283. doi: 10.1016/j.ccell.2021.01.007. e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restivo D.A., Centozone D., Alesina A., Marchese-Ragona R. Myasthenia gravis associated with SARS-CoV-2 infection. Ann. Intern. Med. 2020;173:1027–1028. doi: 10.7326/L20-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea E.M., Logsdon A.F., Hansen K.M., Williams L.M., Reed M.J., Baumann K.K. The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nat. Neurosci. 2021;24:368–378. doi: 10.1038/s41593-020-00771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. J. Am. Med. Assoc. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers G.P., Gibbons G.H. Obesity and hypertension in the time of COVID-19. J. Am. Med. Assoc. 2020;324:1163–1165. doi: 10.1001/jama.2020.16753. [DOI] [PubMed] [Google Scholar]
- Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. The lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á, Layos-Romero A., García-García J. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95 doi: 10.1212/WNL.0000000000009937. e1060-e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A. Imaging of acute disseminated encephalomyelitis. Neuroimaging Clin. 2008;18:149–161. doi: 10.1016/j.nic.2007.12.007. ix) [DOI] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustenhoven J., Kipnis J. Bypassing the blood-brain barrier. Science. 2019;366:1448–1449. doi: 10.1126/science.aay0479. New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter A., Fox R.J., Newsome S.D., Halper J., Li D.K.B., Kanellis P. Outcomes and risk factors associated with SARS-CoV-2 infection in a north American registry of patients with multiple sclerosis. JAMA neurology. 2021 doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sas A.R., Carbajal K.S., Jerome A.D., Menon R., Yoon C., Kalinski A.L. A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat. Immunol. 2020;21:1496–1505. doi: 10.1038/s41590-020-00813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauder C., Staeheli P. Rat model of borna disease virus transmission: epidemiological implications. J. Virol. 2003;77:12886–12890. doi: 10.1128/JVI.77.23.12886-12890.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller C., Krebs F., Minkner R., Astner I., Gil-Moles M., Wätzig H. Physicochemical properties of SARS-CoV-2 for drug targeting, virus inactivation and attenuation, vaccine formulation and quality control. Electrophoresis. 2020;41:1137–1151. doi: 10.1002/elps.202000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N., Lareau C.A., Keshishian H., Ganskih S., Schneider C., Hennig T. The SARS-CoV-2 RNA–protein interactome in infected human cells. Nature microbiology. 2020;6:339–353. doi: 10.1038/s41564-020-00846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Schrepping J., Reusch N., Paclik D., Baßler K., Schlickeiser S., Zhang B. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182:1419–1440. doi: 10.1016/j.cell.2020.08.001. e1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane P.I., Lee B., Hoyle C., Yu S., Lopez-Castejon G., Lowe M. The NLRP3–inflammasome as a sensor of organelle dysfunction. J. Cell Biol. 2020;219 doi: 10.1083/jcb.202006194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Razavi A., Karimi N., Rouhani N. COVID-19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect. 2020;35:100669. doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in wuhan, China. JAMA Cardiology. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiers S., Ray P., Wangzhou A., Esteves Tatsui C., Rhines L., Li Y. ACE2 expression in human dorsal root ganglion sensory neurons: implications for SARS-CoV-2 virus-induced neurological effects. Pain. 2020;161:2494–2501. doi: 10.1097/j.pain.0000000000002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M.M.E., Mercer P.B.S., Witt M.C.Z., Pessoa R.R. Olfactory dysfunction in Alzheimer’s disease Systematic review and meta-analysis. Dementia & neuropsychologia. 2018;12:123–132. doi: 10.1590/1980-57642018dn12-020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvin A., Chapuis N., Dunsmore G., Goubet A.G., Dubuisson A., Derosa L. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell. 2020;182:1401–1418. doi: 10.1016/j.cell.2020.08.002. e1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon I.H., Normandin E., Bhattacharyya S., Mukerji S.S., Keller K., Ali A.S. Neuropathological features of covid-19. N. Engl. J. Med. 2020;383:989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Zhang C., Israelow B., Lu P., Weizman O.-E., Liu F. Neuroinvasive potential of SARS-CoV-2 revealed in a human brain organoid model. J. Exp. Med. 2020;218 [Google Scholar]
- Spinato G., Fabbris C., Polesel J., Cazzador D., Borsetto D., Hopkins C. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. J. Am. Med. Assoc. 2020;323:2089–2090. doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanifer M.L., Kee C., Cortese M., Zumaran C.M., Triana S., Mukenhirn M. Critical role of type III interferon in controlling SARS-CoV-2 infection in human intestinal epithelial cells. Cell Rep. 2020;32:107863. doi: 10.1016/j.celrep.2020.107863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan N., Birkenfeld A.L., Schulze M.B. Global pandemics interconnected — obesity, impaired metabolic health and COVID-19. Nat. Rev. Endocrinol. 2021;17:135–149. doi: 10.1038/s41574-020-00462-1. [DOI] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Chen D., Yuan D., Lausted C., Choi J., Dai C.L. Multi-omics resolves a sharp disease-state shift between mild and moderate COVID-19. Cell. 2020;183:1479–1495. doi: 10.1016/j.cell.2020.10.037. e1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D., Antonini A., Leta V., Nordvig A., Smeyne R.J., Goldman J.E. COVID-19 and possible links with Parkinson’s disease and parkinsonism: from bench to bedside. NPJ Parkinson’s disease. 2020;6:18. doi: 10.1038/s41531-020-00123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P., Lu X., Xu C., Sun W., Pan B. Understanding of COVID-19 based on current evidence. J. Med. Virol. 2020;92:548–551. doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawanshi R.K., Koganti R., Agelidis A., Patil C.D., Shukla D. Dysregulation of cell signaling by SARS-CoV-2. Trends Microbiol. 2021;29:224–237. doi: 10.1016/j.tim.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamo B.R., Rudel R., Kosik K.S., Lee V.M., Neff S., Adelman L. Pathological changes in olfactory neurons in patients with Alzheimer’s disease. Nature. 1989;337:736–739. doi: 10.1038/337736a0. [DOI] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis : JTH. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey C.M., Louie M., Loeb M., Gold W.L., Muller M.P., de Jager J. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch. Intern. Med. 2007;167:1312–1320. doi: 10.1001/archinte.167.12.1312. [DOI] [PubMed] [Google Scholar]
- Tarakad A., Jankovic J. Anosmia and ageusia in Parkinson’s disease. Int. Rev. Neurobiol. 2017;133:541–556. doi: 10.1016/bs.irn.2017.05.028. [DOI] [PubMed] [Google Scholar]
- Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E.A., Cascino K., Ordonez A.A., Zhou W., Vaghasia A., Hamacher-Brady A. Metabolic programs define dysfunctional immune responses in severe COVID-19 patients. Cell Rep. 2021;34:108863. doi: 10.1016/j.celrep.2021.108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoms M., Buschauer R., Ameismeier M., Koepke L., Denk T., Hirschenberger M. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science. 2020;369:1249–1255. doi: 10.1126/science.abc8665. (New York, NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottein F., Sokol H. Potential causes and consequences of gastrointestinal disorders during a SARS-CoV-2 infection. Cell Rep. 2020;32:107915. doi: 10.1016/j.celrep.2020.107915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai L.K., Hsieh S.T., Chao C.C., Chen Y.C., Lin Y.H., Chang S.C. Neuromuscular disorders in severe acute respiratory syndrome. Arch. Neurol. 2004;61:1669–1673. doi: 10.1001/archneur.61.11.1669. [DOI] [PubMed] [Google Scholar]
- Tsai L.K., Hsieh S.T., Chang Y.C. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol. Taiwanica. 2005;14:113–119. [PubMed] [Google Scholar]
- Uciechowski P., Dempke W.C.M. Interleukin-6: a masterplayer in the cytokine network. Oncology. 2020;98:131–137. doi: 10.1159/000505099. [DOI] [PubMed] [Google Scholar]
- Ur A., Verma K. Cytokine storm in COVID19: a neural hypothesis. ACS Chem. Neurosci. 2020;11:1868–1870. doi: 10.1021/acschemneuro.0c00346. [DOI] [PubMed] [Google Scholar]
- van de Veerdonk F., Netea M.G., van Deuren M., van der Meer J.W., de Mast Q., Bruggemann R.J. Kinins and cytokines in COVID-19: a comprehensive pathophysiological approach. Preprints. 2020;2020:2020040023. [Google Scholar]
- van der Grein S.G., KAY Defourny, Rabouw H.H., Galiveti C.R., Langereis M.A., Wauben M.H.M. Picornavirus infection induces temporal release of multiple extracellular vesicle subsets that differ in molecular composition and infectious potential. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riel D., Verdijk R., Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J. Pathol. 2015;235:277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer S.E., Longstreth W.T., Koudstaal P.J. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- von Buchholtz L.J., Lam R.M., Emrick J.J., Chesler A.T., Ryba N.J.P. Assigning transcriptomic class in the trigeminal ganglion using multiplex in situ hybridization and machine learning. Pain. 2020;161:2212–2224. doi: 10.1097/j.pain.0000000000001911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Weyhern C.H., Kaufmann I., Neff F., Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395:e109. doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonck K., Garrez I., De Herdt V., Hemelsoet D., Laureys G., Raedt R. Neurological manifestations and neuro-invasive mechanisms of the severe acute respiratory syndrome coronavirus type 2. Eur. J. Neurol. 2020;27:1578–1587. doi: 10.1111/ene.14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in wuhan, China. J. Am. Med. Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Fang P., He R., Li M., Yu H., Zhou L. O-GlcNAc transferase promotes influenza A virus–induced cytokine storm by targeting interferon regulatory factor–5. Sci Adv. 2020;6 doi: 10.1126/sciadv.aaz7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Davis P.B., Gurney M.E., Xu R. COVID-19 and dementia: analyses of risk, disparity, and outcomes from electronic health records in the US. Alzheimers Dement. 2021:1–10. doi: 10.1002/alz.12296. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.C., Su K.P., Pariante C.M. The three frontlines against COVID-19: brain, behavior, and immunity. Brain Behav. Immun. 2021;93:409–414. doi: 10.1016/j.bbi.2021.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weavers H., Martin P. The cell biology of inflammation: from common traits to remarkable immunological adaptations. J. Cell Biol. 2020;219 doi: 10.1083/jcb.202004003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. J. Am. Med. Assoc. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J. Am. Med. Assoc. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. J. Am. Med. Assoc. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia Ja, Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H., Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J. Neurochem. 2008;107:1482–1484. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H., Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr. Hypertens. Rep. 2010;12:170–175. doi: 10.1007/s11906-010-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Sriramula S., Lazartigues E. ACE2/ANG-(1-7)/Mas pathway in the brain: the axis of good. Am. J. Physiol. 2011;300:R804–R817. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory medicine. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. (New York, NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Jin Z. An acute respiratory infection runs into the most common noncommunicable epidemic—COVID-19 and cardiovascular diseases. JAMA Cardiology. 2020;5:743–744. doi: 10.1001/jamacardio.2020.0934. [DOI] [PubMed] [Google Scholar]
- Yang L., Han Y., Nilsson-Payant B.E., Gupta V., Wang P., Duan X. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell stem cell. 2020;27:125–136. doi: 10.1016/j.stem.2020.06.015. e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C., Bora S.A., Parimon T., Zaman T., Friedman O.A., Palatinus J.A. Cell type-specific immune dysregulation in severely ill COVID-19 patients. Cell Rep. 2020;34:108590. doi: 10.1016/j.celrep.2020.108590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Ren Y., Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav. Immun. 2020;88:945–946. doi: 10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R., Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Zang R., Castro M.F.G., McCune B.T., Zeng Q., Rothlauf P.W., Sonnek N.M. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Zhou X., Qiu Y., Feng F., Feng J., Jia Y. Clinical characteristics of 82 death cases with COVID-19. PloS One. 2020;15 doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Zhao N., Shu Y., Han S., Chen B., Shu X. Effect of gastrointestinal symptoms in patients with COVID-19. Gastroenterology. 2020;158:2294–2297. doi: 10.1053/j.gastro.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035. doi: 10.1016/j.cell.2020.04.035. e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zohar T., Loos C., Fischinger S., Atyeo C., Wang C., Slein M.D. Compromised humoral functional evolution tracks with SARS-CoV-2 mortality. Cell. 2020;183:1508–1519. doi: 10.1016/j.cell.2020.10.052. e1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA neurology. 2020;77:1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y., Estes S.K., Ali R.A., Gandhi A.A., Yalavarthi S., Shi H. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]