Graphical abstract
Keywords: Cardiac I/R injury, Melatonin, Melatonin receptors, Left ventricular function, Mitochondria, Cardiomyocyte death
Abstract
Introduction
Previous studies reported the beneficial effects of pretreatment with melatonin on the heart during cardiac ischemia/reperfusion (I/R) injury. However, the effects of melatonin given after cardiac ischemia, as well as its comparative temporal effects are unknown. These include pretreatment, during ischemia, and at the onset of reperfusion. Also, the association between melatonin receptors and cardiac arrhythmias, mitochondrial function and dynamics, autophagy, and mitophagy during cardiac I/R have not been investigated.
Objectives
We tested two major hypotheses in this study. Firstly, the temporal effect of melatonin administration exerts different cardioprotective efficacy during cardiac I/R. Secondly, melatonin provides cardioprotective effects via MT2 activation, leading to improvement in cardiac mitochondrial function and dynamics, reduced excessive mitophagy and autophagy, and decreased cardiac arrhythmias, resulting in improved LV function.
Methods
Male rats were subjected to cardiac I/R, and divided into 4 intervention groups: vehicle, pretreatment with melatonin, melatonin given during ischemia, and melatonin given at the onset of reperfusion. In addition, either a non-specific melatonin receptor (MT) blocker or specific MT2 blocker was given to rats.
Results
Treatment with melatonin at all time points alleviated cardiac I/R injury to a similar extent, quantified by reduction in infarct size, arrhythmia score, LV dysfunction, cardiac mitochondrial dysfunction, imbalance of mitochondrial dynamics, excessive mitophagy, and a decreased Bax/Bcl2 ratio. In H9C2 cells, melatonin increased %cell viability by reducing mitochondrial dynamic imbalance and a decrease in Bax protein expression. The cardioprotective effects of melatonin were dependent on MT2 activation.
Conclusion
Melatonin given before or after ischemia exerted equal levels of cardioprotection on the heart with I/R injury, and its beneficial effects on cardiac arrhythmias, cardiac mitochondrial function and dynamics were dependent upon the activation of MT2.
Introduction
Acute myocardial infarction (AMI) imposes an enormous burden on both global economics and health [1], [2]. Reperfusion therapy is the treatment of choice for AMI, as it is an effective method of restoring blood to the injured myocardium [3], [4]. Although reperfusion therapy is the most effective treatment, the reperfusion procedure itself can trigger additional injury to the heart, a process known as cardiac ischemia/reperfusion (I/R) injury [3], [4]. Cardiac I/R injury can result in an excess of free radical generation, which in turn leads to the death of cardiomyocytes, an increase in myocardial infarct size, and left ventricular (LV) dysfunction [3], [4]. For this reason, pharmacological intervention which can effectively alleviate reperfusion injury when given after cardiac ischemia is essential and is applicable to a clinical setting.
Melatonin is an endogenous hormone that is primarily synthesized and secreted by the pineal gland [5]. Although the major role of melatonin is to regulate biological rhythms in the body, also being involved in the sleep-awake pattern, it is also effective as an anti-aging and anti-cancer chemical. In addition, it has been shown to confer cardioprotection in several cardiac diseases [5], [6], [7], [8]. Although findings are still controversial, many reports from in vitro, in vivo and clinical reports have shown that melatonin could reduce cardiac I/R injury beyond its usual role as a regulator of circadian rhythms [9], [10], [11], [12], [13], [14], [15], [16], [17]. Despite these reports, comparative investigations into the temporal effect of melatonin administration during cardiac I/R are currently not available.
The temporal effects of melatonin on the heart with I/R injury are unknown and the mechanisms responsible for its benefits are still unclear. Previous studies have shown that the effects of melatonin rely on the activation of melatonin membrane receptors, melatonin receptor 1 and 2 (MT1 and MT2), and also it has intracellular actions independent of melatonin membrane receptors [5], [18]. In the cardiac I/R setting, cardioprotective effects of melatonin have been mainly attributed to the activation of melatonin membrane receptors [11], [19], [20], [21]. In a previous study by Han et al, it was clearly demonstrated that MT2, but not MT1, was associated with cardioprotective effects in cardiac I/R injury [11]. However, there are several knowledge gaps acknowledged in that study. First, the temporal effects of melatonin administration and MT2 activation during cardiac I/R which have potential significant clinical applications are not known. In addition, any association between different times of MT2 activation and the effects on cardiac mitochondrial function, mitochondrial dynamics, mitophagy and autophagy during cardiac I/R was not investigated. It is also known that reperfusion arrhythmias are important complications in cardiac I/R [22]. Whether temporal MT2 activation during cardiac I/R affects the incidence of cardiac arrhythmias is not known. Finally, the contribution of melatonin receptor-independent effects on cardioprotective mechanisms against cardiac I/R injury has never been investigated.
In this study, we investigated the effects of melatonin given at different time points during cardiac I/R, including pretreatment, during ischemia or at the onset of reperfusion, and also the roles of melatonin receptors on the heart with cardiac I/R injury. Two major hypotheses were tested in this study. First, temporal effect of melatonin administration exerts different levels of cardioprotective efficacy during cardiac I/R. Second, melatonin provides cardioprotective effects via MT2 activation, leading to improved cardiac mitochondrial function and dynamics, reduced excessive mitophagy and autophagy, and decreased cardiac arrhythmias, resulting in improved LV function.
Methods
Ethical approval
All animal procedures and experimental protocols described in this study were performed under the NIH guidelines (Guide for the care and use of laboratory animals) and authorized by the Institutional Animal Care and Use Committees of the Faculty of Medicine, Chiang Mai University, Thailand (permit no. 17/2561). Eight-week old male Wistar rats (n = 72), weighing 250–300 g, were purchased from Nomura Siam International, Bangkok, Thailand. All rats were kept in a regulated temperature and humidity with a light/dark period of 12-hours. The rats were fed with a standard laboratory rat diet and water ad libitum. After 1 week of acclimatization, rats were subjected to cardiac I/R protocol and divided into 4 groups, a vehicle group (0.5% ethanol in normal saline solution) (n = 12), and 3 melatonin treatment groups (n = 12/group). In the melatonin treatment groups, dosage of melatonin given to the rats was 10 mg/kg, via i.v. injection, at: 1) 15 min prior to cardiac ischemia, or 2) 15 min after left anterior descending artery (LAD) ligation (i.e. during ischemia), or 3) at the onset of reperfusion. In addition, 2 additional groups were used to investigate the mechanisms of melatonin (n = 12/group). In these groups, either a non-specific melatonin receptor (MT) blocker (Luzindole, 1 mg/kg, i.v. injection) or a specific MT2 blocker (4-PPDOT, 1 mg/kg, i.v. injection) was given to the rats 5 min prior to melatonin treatment and before cardiac I/R induction. Since there is no specific MT1 blocker available, the non-specific melatonin receptor blocker was chosen to be used together with a specific MT2 blocker. Melatonin was dissolved in normal saline solution containing 0.5% ethanol. The dose of melatonin, Luzindole and 4-PPDOT were chosen to be in accordance with the results from a previous study [12], [13], [20]. At the end of cardiac I/R protocol, rats were euthanized, and the hearts were rapidly removed for further study. The detailed methodology is described in the supplementary section and a summary of the experimental protocol of this in vivo study is shown in Supplementary Fig. 1A.
In vitro study
H9C2 cells were incubated in Dulbecco’s modified Eagle’s medium-F12 (DMEM) containing 10% fetal bovine serum (FBS) and antibiotics (100 U/mL of penicillin and 100 µg/mL of streptomycin) at 37 °C in a 5% CO2 incubator overnight. H9C2 cells were divided into those exhibiting a normal condition and those in a hypoxia/reoxygenation (H/R) injury condition. Cells in each condition were divided into 12 groups: 1. normal control, 2. vehicle (0.05% ethanol in PBS), 3. pretreatment with melatonin, 4. siRNA control, 5. MT1 siRNA, 6. MT2 siRNA, 7. H/R alone, 8. H/R being pretreated with melatonin, 9. H/R in H9C2 with MT1 siRNA, 10. H/R in H9C2 with MT2 siRNA, 11. H/R being pretreated with melatonin in H9C2 with MT1 siRNA, and 12. H/R being pretreated with melatonin in H9C2 with MT2 siRNA. For melatonin treatment groups, H9C2 cells were pretreated with 1000 µM of melatonin for 15 min and subsequently subjected to the H/R injury protocol. The detailed methodology is described in the supplementary section. The diagram depicting the experimental protocol of in vitro study is shown in Supplementary Fig. 1B.
Statistical analysis
All data are presented as the mean ± SEM. Statistical analysis was performed using GraphPad Prism 7.0 software. A one-way-ANOVA followed by an LSD post hoc test was used to test the difference between groups. The level of statistical significance was set at p < 0.05.
Results
Melatonin treatment at all time points effectively reduced myocardial infarct size and decreased Bax/Bcl2 ratio through the activation of MT2
MT2 protein expression following melatonin treatment was investigated. The results from the western blot revealed that MT2 protein expression increased slightly in melatonin treated groups, however, the level of increase was not statistically significant (Supplementary figure 2). Next, to test whether melatonin given at different time points could reduce myocardial damage against cardiac I/R injury, the percentage myocardial infarct size/area at risk (AAR) was determined. Our results showed that the AAR did not differ between groups (approximately 35% in all groups). Treatment with melatonin at all time points significantly reduced %infarct size/AAR, in comparison to the vehicle group, accounting for approximately 37% reduction (Fig. 1A). To evaluate the role of melatonin receptor activation in the regulation of myocardial infarct size during cardiac I/R, either Luzindole or 4-PPDOT was given to the rats prior to melatonin treatment. The results showed that the %infarct size/AAR was reversed to the same level as that in the groups treated with vehicle (Fig. 1A), indicating that MT2 activation is required for melatonin-mediated infarct size reduction in rats with cardiac I/R.
Fig. 1.
The effects of melatonin administration at different time points and the roles of melatonin receptors on myocardial infarct size and cell death pathways during cardiac I/R injury. (A) Representative and quantitative analysis of myocardial infarct size by Evan blue/TTC staining (n = 5–6/group); (B) cleaved-caspase3/total-caspase3 (n = 5–6/group); (C) Bax/Bcl2 ratio (n = 5–6/group); (D) Beclin1/Actin (n = 5–6/group); (E) LC3II/Actin (n = 5–6/group); (F) p62/Actin (n = 5–6/group). *p < 0.05 vs. vehicle. Abbreviations: I/R: ischemia reperfusion injury; AAR: area at risk; I: ischemic area; R: remote area; P-Mel: pretreatment with melatonin; I-Mel: melatonin administration during myocardial ischemia; R-Mel: melatonin administration at onset of reperfusion; MelLuz: melatonin and Luzindole administration; MelDot: melatonin and 4-PPDOT administration.
The expression of proteins associated with apoptosis and autophagy was determined. In the case of apoptosis, the protein expression of cleaved caspase3/caspase3 was not different between groups (Fig. 1B), whereas Bax/Bcl-2 ratio was significantly decreased in all melatonin treatment groups, when compared with the vehicle group (Fig. 1C). Administration of Luzindole or 4-PPDOT increased the Bax/Bcl-2 ratio in rats with cardiac I/R, when compared with melatonin treatment groups (Fig. 1C).
In the case of autophagy, melatonin treatment at all time points significantly reduced protein expression of Beclin1, and LC3II and increased p62 in rats with cardiac I/R, when compared with the vehicle group (Fig. 1D-F). However, Luzindole and 4-PPDOT did not alter the expression of autophagic proteins, when compared with the melatonin treatment group (Fig. 1D-F).
Melatonin treatment at all time points attenuated LV dysfunction through the activation of MT2
Data from invasive LV function assessment showed that cardiac I/R caused LV dysfunction in the vehicle group as indicated by increased HR, LVEDP and -dP/dt, and decreased SV, LVESP, and + dP/dt during the ischemic period, when compared with their baselines (Fig. 2A-F). During reperfusion, vehicle treated rats had increased LVEDP and -dP/dt, and decreased SV, LVESP, and + dP/dt, when compared with their baselines, and these parameters were no different from the ischemic period (Fig. 2A-F). During ischemia, pretreatment with melatonin and melatonin treatment during ischemia significantly preserved SV, LVESP, LVEDP, and ± dP/dt during the ischemic period (Fig. 2B-F). After reperfusion, treatment with melatonin at all time points led to increased SV, LVESP, and + dP/dt, together with reduced LVEDP and -dP/dt, when compared to their respective baselines (Fig. 2B-F). Administration of Luzindole and 4-PPDOT inhibited the improvement of LV function by melatonin (Fig. 2B-F).
Fig. 2.
The effects of melatonin administration at different time points and the roles of melatonin receptors on LV function during cardiac I/R injury. (A) Heart rate (n = 5–6/group); (B) Stroke volume (n = 5–6/group); (C) Left ventricular end systolic pressure (n = 5–6/group); (D) Left ventricular end diastolic pressure (n = 5–6/group); (E) + dP/dt max (n = 5–6/group); (F) -dP/dt min (n = 5–6/group). *p < 0.05 vs. its respective baseline. Abbreviations: P-Mel: pretreatment with melatonin; I-Mel: melatonin administration during myocardial ischemia; R-Mel: melatonin administration at onset of reperfusion; MelLuz: melatonin and Luzindole administration; MelDot: melatonin and 4-PPDOT administration.
Melatonin treatment at all time points reduced cardiac arrhythmias through the activation of MT2.
Melatonin treatment at all time points reduced arrhythmia score during cardiac I/R, and the antiarrhythmic effect of melatonin was negated by Luzindole and 4-PPDOT (Fig. 3A). In addition, the ratio of p-Cx43ser368/Cx43 protein expression was determined to assess gap junction function. Our results showed that p-Cx43ser368/Cx43 was decreased in the ischemic area of the heart in vehicle treated rats, compared with the remote areas in the same rats (Fig. 3B). Treatment with melatonin at all time points increased the expression of proteins p-Cx43ser368/Cx43, when compared with the vehicle group (Fig. 3B). Administration of Luzindole and 4-PPDOT reduced the beneficial effects of melatonin on p-Cx43ser368/Cx43 in rats with cardiac I/R (Fig. 3B).
Fig. 3.
The effects of melatonin administration at different time points and the roles of melatonin receptors on cardiac arrhythmias and gap junction function during cardiac I/R injury. (A) Quantitative analysis of arrhythmia score based on the criteria of Curtis and Walker (n = 5–6/group); (B) p-Cx43ser368/Cx43 (n = 5–6/group). *p < 0.05 vs. vehicle. Abbreviations: I: ischemic area; R: remote area; P-Mel: pretreatment with melatonin; I-Mel: melatonin administration during myocardial ischemia; R-Mel: melatonin administration at onset of reperfusion; MelLuz: melatonin and Luzindole administration; MelDot: melatonin and 4-PPDOT administration.
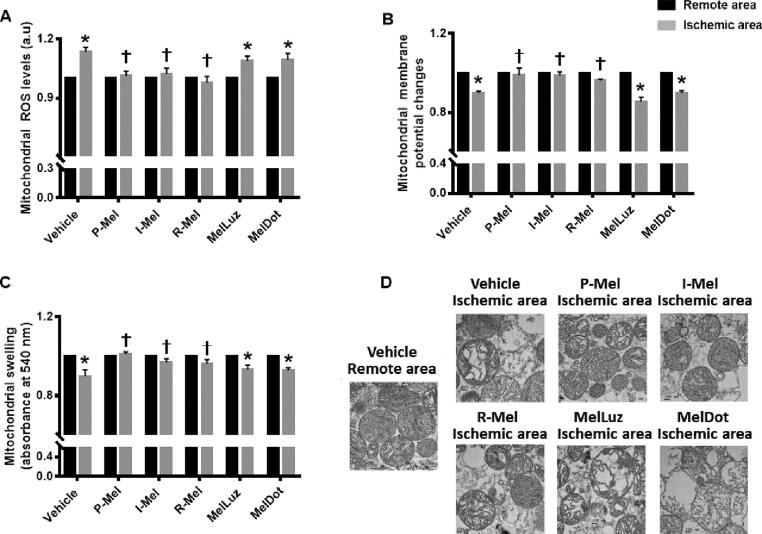
Melatonin treatment at all time points attenuated cardiac mitochondrial dysfunction and mitochondrial dynamic imbalance through activation of MT2.
We subsequently investigated the effects of melatonin on cardiac mitochondrial function, mitochondrial dynamics, and mitophagy. Our results demonstrated that cardiac I/R induced dysfunction in cardiac mitochondria as evidenced by an increase in mitochondrial ROS levels, and depolarization and swelling in mitochondria in myocardial tissue from the ischemic area, when compared with tissue from a remote area (Fig. 4A-D). Treatment with melatonin at all time points similarly reduced mitochondrial ROS levels, and depolarization and swelling in mitochondria in the ischemic zone, compared with an ischemic area from the vehicle group (Fig. 4A-C). Administration of Luzindole and 4-PPDOT blocked the positive effects of melatonin on cardiac mitochondrial function in rats with cardiac I/R (Fig. 4A-C). Consistent with the mitochondrial function data, transmission electron micrographs of cardiac mitochondria exhibited swollen mitochondria in cardiomyocytes from the ischemic area in the vehicle group, and indicated that treatment with melatonin effectively suppressed mitochondrial swelling, the micrographs showing well-organized cristae structure. In contrast, administration of Luzindole and 4-PPDOT inhibited the protective effect of melatonin on mitochondrial morphology (Fig. 4D).
Fig. 4.
The effects of melatonin administration at different time points and the roles of melatonin receptors on cardiac mitochondrial function during cardiac I/R injury. (A) Mitochondrial ROS level (n = 5–6/group); (B) Mitochondrial membrane potential changes (n = 5–6/group); (C) Mitochondrial swelling (n = 5–6/group); (D) Representative images of mitochondrial morphology taken by transmission electron microscopy (n = 5–6/group). *p < 0.05 vs. the remote area, †p < 0.05 vs ischemic area of the vehicle group. Abbreviations: P-Mel: pretreatment with melatonin; I-Mel: melatonin administration during myocardial ischemia; R-Mel: melatonin administration at onset of reperfusion; MelLuz: melatonin and Luzindole administration; MelDot: melatonin and 4-PPDOT administration; ROS: reactive oxygen species.
In addition to mitochondrial function, we also investigated the temporal effects of melatonin administration on cardiac mitochondrial dynamic protein expression including mitochondrial fission markers (p-Drp1ser616/Drp1) and mitochondrial fusion markers (Mfn1, Mfn2, and OPA1). Our results revealed that the expression of p-Drp1ser616/Drp1 was significantly suppressed by melatonin treatment at all time points (Fig. 5A). In addition, melatonin treatment at all time points significantly increased the expression of Mfn1, Mfn2 and OPA1 proteins, when compared with the vehicle group (Fig. 5B-D). Administration of Luzindole and 4-PPDOT abolished the protective effects of melatonin on cardiac mitochondrial dynamics (Fig. 5A-D).
Fig. 5.
The effects of melatonin administration at different time points and the roles of melatonin receptors on cardiac mitochondrial dynamics and mitophagy during cardiac I/R injury. (A) p-Drp1/Drp1 (n = 5–6/group); (B) Mfn1/Actin (n = 5–6/group); (C) Mfn2/Actin (n = 5–6/group); (D) OPA1/Actin (n = 5–6/group); (E) PINK1/Actin (n = 5–6/group); (F) Parkin/Actin (n = 5–6/group). *p < 0.05 vs. vehicle. Abbreviations: I: ischemic area; R: remote area; P-Mel: pretreatment with melatonin; I-Mel: melatonin administration during myocardial ischemia; R-Mel: melatonin administration at onset of reperfusion; MelLuz: melatonin and Luzindole administration; MelDot: melatonin and 4-PPDOT administration; Drp1: dynamin related protein 1; Mfn: mitofusin, OPA: optic atrophy protein.
The effects of melatonin on PINK1/Parkin dependent mitophagy were also determined. The cardiac I/R-induced excessive mitophagy was significantly suppressed by melatonin treatment at all time points, as shown by downregulation of PINK1 and Parkin protein expression (Fig. 5E-F). Interestingly, the regulatory role of melatonin on mitophagy is through melatonin receptors in an independent manner, since Luzindole and 4-PPDOT did not affect the expression of PINK1 and Parkin proteins, when compared with melatonin treatment groups. (Fig. 5E-F).
Melatonin receptor 2 activation was required to protect cardiomyocytes against hypoxia/reoxygenation (H/R) injury
To confirm the mechanistic role of melatonin and melatonin receptors in rats with cardiac I/R, H9C2 cells were used and subjected to the H/R protocol. MT1 and MT2 siRNA were used to test the role of melatonin receptor regulation during H/R. Successful siRNA was confirmed by a decrease in MT1 and MT2 mRNA expression in H9C2 cells (Supplementary Fig. 3). In the normal cells, silencing of MT1 and MT2 with or without melatonin treatment did not affect % cell viability (Fig. 6A). H/R successfully reduced the cell viability to approximately 50% (Fig. 6A). MT1 and MT2 silencing did not aggravate H/R induced cell viability reduction (Fig. 6A). Melatonin treatment in H9C2 cells with or without MT1 silencing reversed %cell viability to approximately 70%. However, the %cell viability was comparable between the H/R alone group versus melatonin treatment in H9C2 cells with MT2 siRNA (Fig. 6A). Survival kinase, apoptotic protein and mitochondrial dynamics proteins were determined in H9C2 cells with H/R. The representative western blots are shown in Fig. 6B. H/R significantly suppressed expression of p-ERK1/2/ERK1/2 when compared with the normal control group and silencing of MT1 and MT2 did not aggravate a reduction of p-ERK1/2/ERK1/2 (Fig. 6C). It was found that melatonin treatment with or without MT1 silencing potentially enhanced p-ERK1/2/ERK1/2 protein expression. In contrast, melatonin treatment with MT2 silencing led to a reduction in p-ERK1/2/ERK1/2 expression, findings which were similar to the H/R group (Fig. 6C). Furthermore, H/R led to a remarkable increase in Bax, p-Drp1ser616/Drp1 expression, and a decrease in Mfn2 expression, when compared with normal controls (Fig. 6D-F). Melatonin treatment with or without MT1 silencing significantly reversed all of these parameters. These beneficial effects of melatonin were not observed in H9C2 cells with MT2 siRNA (Fig. 6D-F).
Fig. 6.
The effects of melatonin administration and the roles of its receptors on cell viability, survival, apoptosis, and mitochondrial dynamics in H9C2 cells subjected to H/R injury. (A) %cell viability (n = 5/group); (B) p-ERK/ERK (n = 5/group); (C) Bax/Actin (n = 5/group); (D) p-Drp1ser616/Drp1 (n = 5/group); (E) Mfn2/Actin (n = 5/group). *p < 0.05 vs. vehicle; †p < 0.05 vs melatonin. Abbreviations: H/R: hypoxia/reoxygenation; Veh: vehicle; Mel: melatonin; MT: melatonin receptor; Drp1: dynamin related protein; Mfn: mitofusin.
Discussion
The major findings from this study are as follows: i) cardiac I/R led to infarct size expansion, cardiac arrhythmias, and LV dysfunction through cardiac mitochondrial dysfunction, an imbalance in mitochondrial dynamics, and excessive mitophagy which led to excessive autophagy and apoptotic cell death; ii) the results in the in vivo study demonstrated for the first time that melatonin administration at all time points effectively reduced infarct size, cardiac arrhythmias, and LV dysfunction by improving cardiac mitochondrial function, mitochondrial dynamic balance, and mitophagy, leading to a reduction in cardiac cell death; iii) results from the in vitro study showed that melatonin treatment increased %cell viability via the activation of the survival kinase pathway (ERK1/2), decreasing mitochondrial dynamic imbalance, and cardiac cell death, and iv) the positive effects of melatonin, as mentioned above, were abrogated in the presence of both the MT2 receptor blocker and MT2 siRNA.
Cardiac I/R injury was first reported by Jennings and colleagues in 1960 [23]. It is characterized by cell swelling, myocardial contracture, and the disruption of myocardial sarcolemma, when the tissue has only been subjected to 30 min of ischemia followed by reperfusion for 1 h [23], [24]. Subsequent to this report, numerous pieces of scientific research into the detrimental effects of cardiac I/R injury have been published from that date to the present day [24]. Molecular and cellular mechanisms underlying cardiac I/R are complex, for example we know that cardiac ischemia induces the accumulation of sodium, hydrogen, and calcium ions intracellularly, resulting in intracellular acidosis [25]. Reperfusion induces rapid alteration of the flux of these ions, together with a rapid renormalization of intracellular pH, which leads to an enhancement of cytotoxicity and the initiation of apoptotic cell death, infarct size expansion, arrhythmias, and worsening LV dysfunction [25]. However, reperfusion remains the only effective strategy to salvage an ischemic myocardium. Therefore, several therapeutic strategies have been established to decrease myocardial susceptibility to cardiac I/R including melatonin treatment.
As we mentioned above, sodium, hydrogen, and calcium ions regulate cardiac I/R injury. Calcium overload substantially contributes to the pathogenesis of cardiac I/R injury [26]. Treatment with melatonin has been shown to attenuate cardiac I/R-induced calcium overload and reverse the increased calcium transient amplitude [27]. Melatonin also alleviated cardiac I/R injury via the suppressing of IP3R-[Ca2+]cytosol/ VDAC[Ca2+]mitochondria axis to maintain calcium homeostasis in a MAPK/ERK-dependent manner [28], [29]. Additionally, the transmembrane sodium gradient is essential for both excitability of the cardiac cell and the regulation of the cytoplasmic concentration of hydrogen and calcium ions [26]. Future studies are needed to investigate the temporal effects of melatonin administration on the flux of sodium and hydrogen ions in cardiomyocytes with I/R injury.
Several reports have been published which demonstrate the potential cardioprotective effects of melatonin given prior to myocardial ischemia against cardiac I/R injury [16], [21], [30], [31]. The major advantages of treatment with melatonin are infarct size reduction [16], [21], [30], [31], attenuated LV dysfunction [16], [21], [30], [31], decreased apoptotic cell death and oxidative stress suppression [5], [6], [7], [8]. Consistent with previous studies, our data in this study demonstrated that pretreatment with melatonin significantly reduced infarct size (an approximate 30% reduction) and improved LV function. Melatonin treatment prior to acute myocardial ischemia is not clinically relevant, however the initiation of melatonin treatment after myocardial ischemia has already occurred is deemed to be clinically significant. The results of this study showed that melatonin given during the ischemic period and immediately at the onset of reperfusion both resulted in similar cardioprotective effects to those shown in pretreatment in reducing infarct size and LV dysfunction. Although several pharmacological interventions have been proposed to provide cardioprotection in cases of acute cardiac I/R injury [32], [33], [34], [35], [36], there are only few drugs currently available, which can provide similar cardioprotective effects when they are given before or after cardiac I/R [35], [36]. In our study, melatonin is one of those few. The results of our study emphasized that melatonin could be used as both preventive and therapeutic strategies in the case of cardiac I/R injury.
Mechanistically, the formation of infarcts and the degree of infarct size indicates both myocardial cell death and many other types of cell death occur during cardiac I/R injury through both apoptosis and autophagy [25]. According to previous studies, pretreatment with melatonin markedly reduced infarct size by suppressing the expression of apoptosis and autophagy related proteins [5], [6], [7], [8]. Apoptosis plays a crucial role in the mediation of cardiomyocyte death, resulting in infarct size expansion and LV dysfunction in the model of cardiac I/R injury [26]. Several studies reported that melatonin treatment reduced apoptosis significantly in cardiac I/R injury models [15], [16], [17], [37], [38], [39]. A difference in the timing of administration might account for some controversial results observed in our experiments, as melatonin was given at 15 min prior to cardiac I/R, in contrast to previous reports in which melatonin was given at least 30 min before ischemia. However, there was a study which reported that melatonin given at a dose of 20 mg/kg during ischemia significantly reduced apoptosis as indicated by a decrease in caspase3 activity and apoptotic index (%) [11]. However, our results showed for the first time that melatonin treatment at various time points potentially enhanced the expression of the anti-apoptotic protein Bcl-2, while suppressing the expression of Bax, a pro-apoptotic protein. Furthermore, the initiation of autophagy requires Beclin1 to form phagosomes, which then bind with LC3II, leading to the expansion of the phagosomes [40]. The activation of p62 is a final step of autophagy, as it delivers ubiquitinated cargos for autophagic degradation and acts as a substrate during autophagic degradation [40]. Excessive autophagy was reported in cardiac I/R, as indicated by increasing Beclin1, LC3II, and reducing p62 levels [26]. According to our results, treatment with melatonin decreased Beclin1, LC3II, along with an increase in p62 level, suggesting that melatonin mitigated the excessive autophagy in cardiac I/R. Collectively, our results demonstrated that melatonin treatment before or after cardiac ischemia shared similar efficacy in infarct size reduction mainly via the reduction in apoptotic cell death and excessive autophagy.
Disturbance of cardiac rhythm, including lethal ventricular arrhythmias, are common consequences of cardiac I/R injury [22]. Cx43 is ventricular gap junction-associated protein crucial for cell to cell communication, ensuring coordination of electrical excitation and synchronic contraction for each heartbeat [41]. Cx43 has multiple sites of phosphorylation, and dephosphorylation of Cx43 at ser368 has previously been reported as having an association with arrythmias [41]. Melatonin exerted an antiarrhythmic potential, which could be due to its antioxidant effects [42], [43]. A recent study has reported that melatonin prevented Cx43 dephosphorylation and its abnormal topology in a model of low potassium perfused rat hearts [42]. Our current study indicated that melatonin attenuated cardiac I/R injury-induced arrhythmias and the potential mechanism involved in this situation was that melatonin increased Cx43 phosphorylation at ser368. Furthermore, an increase in the incidence of cardiac arrhythmias has been associated with depolarization of cardiac mitochondria caused by an increase in cardiac mitochondrial ROS level [44]. Therefore, due to the antioxidant properties of melatonin, melatonin could reduce arrhythmias by directly scavenging these ROS, thus decreasing cardiac mitochondrial depolarization as observed in our study. It is worthy of note that the outcomes of the various melatonin administration time points showed identical antiarrhythmic properties.
Cardiac I/R injury is associated with dysfunctional mitochondria, as accompanied by increased production of mitochondrial ROS, depolarization of mitochondria, and mitochondrial swelling [25]. Dysfunctional cardiac mitochondria were observed in the ischemic myocardium in this study. As cardiac mitochondrial ROS levels exceed a critical limit, the mitochondrial permeability transition pores (mPTP) are activated and open, resulting in the release of free radicals across the mitochondrial membrane [45]. The prolonged mPTP opening causes cardiac mitochondrial swelling and subsequently mitochondrial rupture [45]. In this study, treatment with melatonin was shown to attenuate cardiac mitochondrial dysfunction through a reduction of ROS level in the cardiac mitochondria. This finding was consistent with a previous report which showed that pretreatment with melatonin had cardioprotective effects via the reduction of mitochondrial ROS level [43]. A decrease of ROS level in cardiac mitochondria following treatment with melatonin led to the amelioration of cardiac mitochondrial depolarization and cardiac mitochondrial swelling, resulting in an improvement in LV function. Importantly, all administrative time points of melatonin showed the same level of efficacy in the attenuation of cardiac mitochondrial dysfunction and LV dysfunction.
In addition to mitochondrial function, mitochondrial dynamics also play an essential role in the pathogenesis of cardiac I/R injury [3], [4], [25]. Previous studies have shown that an inhibition of mitochondrial fission promoted cardioprotection in the case of cardiac I/R injury [35]. Enhancing mitochondrial fusion by using a mitochondrial fusion promoter also led to improvement in LV function in a cardiac I/R injury setting [36]. With regard to the outcome of mitochondrial dynamics, our results demonstrated that cardiac I/R injury provoked mitochondrial fission and suppressed the mitochondrial fusion process. This was followed by mitochondrial dysfunction and apoptosis initiation. Melatonin suppressed mitochondrial fission and enhanced mitochondrial fusion, as evidenced by the inactivation of the corresponding fission markers (Drp1 and p-Drp1ser616) and the activation of fusion related markers (Mfn1, Mfn2, and OPA1). In addition to mitochondrial dynamics, mitophagy homeostasis is reported as being a further contributor to the pathogenesis of cardiac I/R injury [3], [4], [25]. Consistent with previous studies, our results revealed that cardiac I/R injury causes excessive mitophagy as indicated by PINK1 and Parkin upregulation, and this deteriorative effect could be reversed by treatment with melatonin.
Extracellular signal-regulated kinase 1/2 (ERK1/2) is a member of MAPK family, which controls many physiological and pathological processes [46]. ERK1/2 is involved in a reperfusion injury salvage kinase (RISK) pathway [47]. In the setting of cardiac I/R, phosphorylation of ERK1/2 has been shown to exert beneficial effects by reducing apoptosis, oxidative stress, and inflammation [46]. The activation of the RISK pathway was demonstrated to occur at the onset of reperfusion, thereby preventing the opening of mitochondrial permeability transition pores (mPTP), a phenomenon reported to occur within the first 15 min of reperfusion [48]. However, the activation of ERK1/2 showed both beneficial and harmful effects in cardiac I/R injury [46]. The activation of ERK1/2 instigated by single administration of some drugs/agents exerted cardioprotection against I/R injury [47], [49], [50], while prolonged ERK1/2 activation was shown to induce cardiac hypertrophy following cardiac I/R injury [50], [51].
Additionally, this study focused on the roles of melatonin receptors including MT1 and MT2 in the setting of cardiac I/R. The differing chemical characteristics in MT1 and MT2, including molecular structure, pharmacological characteristics, and chromosomal sites have been well described in previous studies [11], [52]. These differences might underlie their specific roles in cardioprotection during cardiac I/R. Therefore, Luzindole, a nonselective ligand with an affinity 15- to 25-times higher for the MT2 [53], and 4-PPDOT , a selective MT2 ligand with a greater affinity 300- to 1,500-fold higher for this receptor [53] were used in this study. Our results from both in vivo and in vitro studies clearly demonstrated that the beneficial effects of melatonin in reducing LV dysfunction and infarct size were dependent upon the activation of MT2, but not MT1. These findings are consistent with a previous report by Han and colleagues [11]. In addition, our present study clarified that the activation of MT2 is essential for melatonin-mediated antiarrhythmic effects, and improved both mitochondrial function and mitochondrial dynamics. Furthermore, our results demonstrated that excessive autophagy and mitophagy were not affected by melatonin membrane receptors. These findings suggest a novel role of melatonin membrane receptor-independent effects on the reduction of excessive autophagy and mitophagy in the heart during cardiac I/R injury. This is supported by a previous study that demonstrated that the expression of the retinoid-related orphan receptors (RORs) which is located on the nuclear membrane was involved in the pathogenesis of cardiac I/R [54]. Taking note of the findings of previous studies, we postulated that melatonin modulated autophagy and mitophagy acted through the activation of RORα. However, our current study has a limitation with regard to this fact and we could not further our investigation at this point as specific RORα blockers or siRNA are not commercially available for use in rats. A summary of the proposed mechanism exerted by melatonin is illustrated in Supplementary Fig. 4.
In summary, our study provides clear novel evidence for the first time that the administration of melatonin at different time points including pretreatment, during ischemia, and at the onset of reperfusion exerts cardioprotective effects against cardiac I/R injury in rats. In addition, we have shown that MT2 activation improved cardiac mitochondrial function and dynamics, and decreased ventricular arrhythmias and infarct size, resulting in attenuated LV dysfunction. However, melatonin reduced excessive autophagy and mitophagy in a melatonin membrane receptor-independent manner. These findings provide important data for the transference of melatonin treatment into future clinical use.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Senior Research Scholar grant from the National Research Council of Thailand (SCC); Thailand Research Fund grant: TRG6280005 (NA); the Royal Golden Jubilee Program PHD/0107/2560 (KS and NC); the NSTDA Research Chair grant from the National Science and Technology Development Agency Thailand (NC), and the Chiang Mai University Center of Excellence Award (NC).
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2020.09.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Lacey L., Tabberer M. Economic burden of post-acute myocardial infarction heart failure in the United Kingdom. Eur J Heart Fail. 2005;7(4):677–683. doi: 10.1016/j.ejheart.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen T.P., Nguyen T., Postma M. Economic Burden Of Acute Myocardial Infarction In Vietnam. Value Health. 2015;18(7):A389. [Google Scholar]
- 3.Carden D.L., Granger D.N. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190(3):255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Sanada S., Komuro I., Kitakaze M. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol. 2011;301(5):H1723–H1741. doi: 10.1152/ajpheart.00553.2011. [DOI] [PubMed] [Google Scholar]
- 5.Pandi-Perumal S.R., Trakht I., Srinivasan V., Spence D.W., Maestroni G.J., Zisapel N. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85(3):335–353. doi: 10.1016/j.pneurobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Acuna-Castroviejo D., Escames G., Venegas C., Diaz-Casado M.E., Lima-Cabello E., Lopez L.C. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71(16):2997–3025. doi: 10.1007/s00018-014-1579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominguez-Rodriguez A., Abreu-Gonzalez P., Reiter R.J. Melatonin and cardiovascular disease: myth or reality? Rev Esp Cardiol (Engl Ed). 2012;65(3):215–218. doi: 10.1016/j.recesp.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Jiki Z., Lecour S., Nduhirabandi F. Cardiovascular Benefits of Dietary Melatonin: A Myth or a Reality? Front Physiol. 2018;9:528. doi: 10.3389/fphys.2018.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dwaich K.H., Al-Amran F.G., Al-Sheibani B.I., Al-Aubaidy H.A. Melatonin effects on myocardial ischemia-reperfusion injury: Impact on the outcome in patients undergoing coronary artery bypass grafting surgery. Int J Cardiol. 2016;221:977–986. doi: 10.1016/j.ijcard.2016.07.108. [DOI] [PubMed] [Google Scholar]
- 10.Ekeloef S., Halladin N., Fonnes S., Jensen S.E., Zaremba T., Rosenberg J. Effect of Intracoronary and Intravenous Melatonin on Myocardial Salvage Index in Patients with ST-Elevation Myocardial Infarction: a Randomized Placebo Controlled Trial. J Cardiovasc Transl Res. 2017;10(5–6):470–479. doi: 10.1007/s12265-017-9768-7. [DOI] [PubMed] [Google Scholar]
- 11.Han D., Wang Y., Chen J., Zhang J., Yu P., Zhang R. Activation of melatonin receptor 2 but not melatonin receptor 1 mediates melatonin-conferred cardioprotection against myocardial ischemia/reperfusion injury. J Pineal Res. 2019;67(1) doi: 10.1111/jpi.12571. [DOI] [PubMed] [Google Scholar]
- 12.Sahna E., Parlakpinar H., Turkoz Y., Acet A. Protective effects of melatonin on myocardial ischemia/reperfusion induced infarct size and oxidative changes. Physiol Res. 2005;54(5):491–495. [PubMed] [Google Scholar]
- 13.Liu L.F., Qian Z.H., Qin Q., Shi M., Zhang H., Tao X.M. Effect of melatonin on oncosis of myocardial cells in the myocardial ischemia/reperfusion injury rat and the role of the mitochondrial permeability transition pore. Genet Mol Res. 2015;14(3):7481–7489. doi: 10.4238/2015.July.3.24. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z., Chua C.C., Gao J., Chua K.W., Ho Y.S., Hamdy R.C. Prevention of ischemia/reperfusion-induced cardiac apoptosis and injury by melatonin is independent of glutathione peroxdiase 1. J Pineal Res. 2009;46(2):235–241. doi: 10.1111/j.1600-079X.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou H., Zhang Y., Hu S., Shi C., Zhu P., Ma Q. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J Pineal Res. 2017;63(1) doi: 10.1111/jpi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu L., Li B., Zhang M., Jin Z., Duan W., Zhao G. Melatonin reduces PERK-eIF2alpha-ATF4-mediated endoplasmic reticulum stress during myocardial ischemia-reperfusion injury: role of RISK and SAFE pathways interaction. Apoptosis. 2016;21(7):809–824. doi: 10.1007/s10495-016-1246-1. [DOI] [PubMed] [Google Scholar]
- 17.Yu L.M., Di W.C., Dong X., Li Z., Zhang Y., Xue X.D. Melatonin protects diabetic heart against ischemia-reperfusion injury, role of membrane receptor-dependent cGMP-PKG activation. Biochim Biophys Acta. 2017;1864(2):563–578. doi: 10.1016/j.bbadis.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Sun H., Gusdon A.M., Qu S. Effects of melatonin on cardiovascular diseases: progress in the past year. Curr Opin Lipidol. 2016;27(4):408–413. doi: 10.1097/MOL.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genade S., Genis A., Ytrehus K., Huisamen B., Lochner A. Melatonin receptor-mediated protection against myocardial ischaemia/reperfusion injury: role of its anti-adrenergic actions. J Pineal Res. 2008;45(4):449–458. doi: 10.1111/j.1600-079X.2008.00615.x. [DOI] [PubMed] [Google Scholar]
- 20.Yu L., Sun Y., Cheng L., Jin Z., Yang Y., Zhai M. Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1. J Pineal Res. 2014;57(2):228–238. doi: 10.1111/jpi.12161. [DOI] [PubMed] [Google Scholar]
- 21.Yu L., Liang H., Lu Z., Zhao G., Zhai M., Yang Y. Membrane receptor-dependent Notch1/Hes1 activation by melatonin protects against myocardial ischemia-reperfusion injury: in vivo and in vitro studies. J Pineal Res. 2015;59(4):420–433. doi: 10.1111/jpi.12272. [DOI] [PubMed] [Google Scholar]
- 22.Di Diego J.M., Antzelevitch C. Ischemic ventricular arrhythmias: experimental models and their clinical relevance. Heart Rhythm. 2011;8(12):1963–1968. doi: 10.1016/j.hrthm.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jennings R.B., Sommers H.M., Smyth G.A., Flack H.A., Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. 1960;70:68–78. [PubMed] [Google Scholar]
- 24.Yang C.F. Clinical manifestations and basic mechanisms of myocardial ischemia/reperfusion injury. Ci Ji Yi Xue Za Zhi. 2018;30(4):209–215. doi: 10.4103/tcmj.tcmj_33_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turer A.T., Hill J.A. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106(3):360–368. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yellon D.M., Hausenloy D.J. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 27.Hu S., Zhu P., Zhou H., Zhang Y., Chen Y. Melatonin-Induced Protective Effects on Cardiomyocytes Against Reperfusion Injury Partly Through Modulation of IP3R and SERCA2a Via Activation of ERK1. Arq Bras Cardiol. 2018;110(1):44–51. doi: 10.5935/abc.20180008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y., Wang Y., Xu J., Tian F., Hu S., Chen Y. Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J Pineal Res. 2019;66(2) doi: 10.1111/jpi.12542. [DOI] [PubMed] [Google Scholar]
- 29.Zhu H., Jin Q., Li Y., Ma Q., Wang J., Li D. Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca(2+)]c/VDAC-[Ca(2+)]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones. 2018;23(1):101–113. doi: 10.1007/s12192-017-0827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y., Duan W., Jin Z., Yi W., Yan J., Zhang S. JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J Pineal Res. 2013;55(3):275–286. doi: 10.1111/jpi.12070. [DOI] [PubMed] [Google Scholar]
- 31.Zhai M., Li B., Duan W., Jing L., Zhang B., Zhang M. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J Pineal Res. 2017;63(2) doi: 10.1111/jpi.12419. [DOI] [PubMed] [Google Scholar]
- 32.Thummasorn S., Apaijai N., Kerdphoo S., Shinlapawittayatorn K., Chattipakorn S.C., Chattipakorn N. Humanin exerts cardioprotection against cardiac ischemia/reperfusion injury through attenuation of mitochondrial dysfunction. Cardiovasc Ther. 2016;34(6):404–414. doi: 10.1111/1755-5922.12210. [DOI] [PubMed] [Google Scholar]
- 33.Apaijai N., Chinda K., Palee S., Chattipakorn S., Chattipakorn N. Combined vildagliptin and metformin exert better cardioprotection than monotherapy against ischemia-reperfusion injury in obese-insulin resistant rats. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0102374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thummasorn S., Shinlapawittayatorn K., Chattipakorn S.C., Chattipakorn N. High-dose Humanin analogue applied during ischemia exerts cardioprotection against ischemia/reperfusion injury by reducing mitochondrial dysfunction. Cardiovasc Ther. 2017;35(5) doi: 10.1111/1755-5922.12289. [DOI] [PubMed] [Google Scholar]
- 35.Maneechote C., Palee S., Kerdphoo S., Jaiwongkam T., Chattipakorn S.C., Chattipakorn N. Differential temporal inhibition of mitochondrial fission by Mdivi-1 exerts effective cardioprotection in cardiac ischemia/reperfusion injury. Clin Sci (Lond). 2018;132(15):1669–1683. doi: 10.1042/CS20180510. [DOI] [PubMed] [Google Scholar]
- 36.Maneechote C., Palee S., Kerdphoo S., Jaiwongkam T., Chattipakorn S.C., Chattipakorn N. Balancing mitochondrial dynamics via increasing mitochondrial fusion attenuates infarct size and left ventricular dysfunction in rats with cardiac ischemia/reperfusion injury. Clin Sci (Lond). 2019;133(3):497–513. doi: 10.1042/CS20190014. [DOI] [PubMed] [Google Scholar]
- 37.Lochner A., Genade S., Davids A., Ytrehus K., Moolman J.A. Short- and long-term effects of melatonin on myocardial post-ischemic recovery. J Pineal Res. 2006;40(1):56–63. doi: 10.1111/j.1600-079X.2005.00280.x. [DOI] [PubMed] [Google Scholar]
- 38.Ceyran H., Narin F., Narin N., Akgun H., Ceyran A.B., Ozturk F. The effect of high dose melatonin on cardiac ischemia- reperfusion Injury. Yonsei Med J. 2008;49(5):735–741. doi: 10.3349/ymj.2008.49.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu L., Gong B., Duan W., Fan C., Zhang J., Li Z. Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: role of AMPK-PGC-1alpha-SIRT3 signaling. Sci Rep. 2017;7:41337. doi: 10.1038/srep41337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu W.J., Ye L., Huang W.F., Guo L.J., Xu Z.G., Wu H.L. p62 links the autophagy pathway and the ubiqutin-proteasome system upon ubiquitinated protein degradation. Cell Mol Biol Lett. 2016;21:29. doi: 10.1186/s11658-016-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue J., Yan X., Yang Y., Chen M., Wu L., Gou Z. Connexin 43 dephosphorylation contributes to arrhythmias and cardiomyocyte apoptosis in ischemia/reperfusion hearts. Basic Res Cardiol. 2019;114(5):40. doi: 10.1007/s00395-019-0748-8. [DOI] [PubMed] [Google Scholar]
- 42.Prado N.J., Egan Benova T., Diez E.R., Knezl V., Liptak B., Ponce Zumino A.Z. Melatonin receptor activation protects against low potassium-induced ventricular fibrillation by preserving action potentials and connexin-43 topology in isolated rat hearts. J Pineal Res. 2019;67(4) doi: 10.1111/jpi.12605. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y., Sun Y., Yi W., Li Y., Fan C., Xin Z. A review of melatonin as a suitable antioxidant against myocardial ischemia-reperfusion injury and clinical heart diseases. J Pineal Res. 2014;57(4):357–366. doi: 10.1111/jpi.12175. [DOI] [PubMed] [Google Scholar]
- 44.Gambardella J., Sorriento D., Ciccarelli M., Del Giudice C., Fiordelisi A., Napolitano L. Functional Role of Mitochondria in Arrhythmogenesis. Adv Exp Med Biol. 2017;982:191–202. doi: 10.1007/978-3-319-55330-6_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webster K.A. Mitochondrial membrane permeabilization and cell death during myocardial infarction: roles of calcium and reactive oxygen species. Future Cardiol. 2012;8(6):863–884. doi: 10.2217/fca.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kong T., Liu M., Ji B., Bai B., Cheng B., Wang C. Role of the Extracellular Signal-Regulated Kinase 1/2 Signaling Pathway in Ischemia-Reperfusion Injury. Front Physiol. 2019;10(1038) doi: 10.3389/fphys.2019.01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossello X., Yellon D.M. The RISK pathway and beyond. Basic Res Cardiol. 2018;113(1) doi: 10.1007/s00395-017-0662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hausenloy D.J., Yellon D.M. Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev. 2007;12(3–4):217–234. doi: 10.1007/s10741-007-9026-1. [DOI] [PubMed] [Google Scholar]
- 49.Davidson S.M., Hausenloy D., Duchen M.R., Yellon D.M. Signalling via the reperfusion injury signalling kinase (RISK) pathway links closure of the mitochondrial permeability transition pore to cardioprotection. Int J Biochem Cell Biol. 2006;38(3):414–419. doi: 10.1016/j.biocel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 50.Mensah K., Mocanu M.M., Yellon D.M. Failure to protect the myocardium against ischemia/reperfusion injury after chronic atorvastatin treatment is recaptured by acute atorvastatin treatment: A potential role for phosphatase and tensin homolog deleted on chromosome ten? J Am Coll Cardiol. 2005;45(8):1287–1291. doi: 10.1016/j.jacc.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 51.Haq S., Choukroun G., Lim H., Tymitz K.M., del Monte F., Gwathmey J. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103(5):670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- 52.Emet M., Ozcan H., Ozel L., Yayla M., Halici Z., Hacimuftuoglu A. A Review of Melatonin, Its Receptors and Drugs. The Eurasian journal of medicine. 2016;48(2):135–141. doi: 10.5152/eurasianjmed.2015.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu J., Clough S.J., Hutchinson A.J., Adamah-Biassi E.B., Popovska-Gorevski M., Dubocovich M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu Rev Pharmacol Toxicol. 2016;56:361–383. doi: 10.1146/annurev-pharmtox-010814-124742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He B., Zhao Y., Xu L., Gao L., Su Y., Lin N. The nuclear melatonin receptor RORalpha is a novel endogenous defender against myocardial ischemia/reperfusion injury. J Pineal Res. 2016;60(3):313–326. doi: 10.1111/jpi.12312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.