Abstract
Alcohol Use Disorder (AUD) is a debilitating disorder that manifests as problematic patterns of alcohol use. At the core of AUD’s behavioral manifestations are the profound structural, physiological, cellular, and molecular effects of alcohol on the brain. While the field has made considerable progress in understanding the neuromolecular targets of alcohol we still lack a comprehensive understanding of alcohol’s actions and effective treatment strategies. Drosophila melanogaster is a powerful model for investigating the neuromolecular targets of alcohol because flies model many of the core behavioral elements of AUD and offer a rich genetic toolkit to precisely reveal the in vivo molecular actions of alcohol. In this review, we focus on receptors and channels that are often targeted by alcohol within the brain. We discuss the general roles of these proteins, their role in alcohol-associated behaviors across species, and propose ways in which Drosophila models can help advance the field.
Keywords: Alcohol, AUD, receptors, channels, Drosophila
Introduction
Uncontrolled or abusive alcohol consumption is an undisputed global health concern with significant social costs and economic burdens.1 Individuals suffering from Alcohol Use Disorder (AUD) often display persistent patterns of alcohol use that escalates from abuse to dependence. Underlying these maladaptive behaviors are short and long-term changes to neurotransmitters, receptors, synapses, and circuits. Understanding the neuromolecular targets of alcohol and how they are altered is critical to the development of novel AUD treatment strategies.
Ethanol, the chemical component that underlies alcohol’s psychoactive effects, has a notoriously promiscuous pharmacology. Within the nervous system, ethanol can directly bind to neuromolecular targets, allosterically modifying receptors and ion channels, and inevitably causing a multitude of cascading effects on nearly every neurotransmitter-signaling system.2 For the purpose of this review we will use the term “alcohol” when generally referring to the intoxicating substance that animals consume, or are exposed to, and the term “ethanol” for the specific chemical component that has measurable effects within the brain.
Drosophila have been used to study various endophenotypes of human AUD for over 20 years.3,4 Strikingly, flies exhibit maladaptive behavioral patterns similar to humans that suffer from AUD. For example, following prior experience flies will voluntarily consume alcohol reaching pharmacologically relevant internal levels and escalate alcohol intake.5,6 This consumption is not dependent on the caloric properties of ethanol.5,7 Flies find the pharmacological properties of alcohol rewarding,8 and are willing to work or overcome aversive stimuli in order to gain access to it.5,8 Once intoxicated flies become socially disinhibited9 and they develop both rapid tolerance to a single exposure as well as chronic tolerance following repeated exposures.10 Chronic alcohol administration can also lead to withdrawal-like behavior, such as seizures.11
Drosophila have significantly contributed to our overall neuromolecular and genetic understanding of AUD. For lists of evolutionarily conserved genes in both Drosophila and mammals that are implicated in alcohol-associated behaviors, we refer readers to Berger et al, Kaun et al, Devineni et al, and Rodan and Rothenfluh.12-15 Decades of postmortem tissue analysis, cell culture experiments, and animal models of AUD have implicated receptors and channels in the nervous system, including GABA, glutamate, dopamine, serotonin, Ca2+ channels, and K+ channels. This review will emphasize how established and recently developed genetic and experimental tools may be leveraged in Drosophila to further reveal the precise in vivo molecular actions of ethanol. We focus on the most prominent receptors and channels associated with AUD and conclude by discussing generalizable approaches that will surely advance our understanding of AUD.
Gamma Aminobutyric Acid (GABA) Receptors
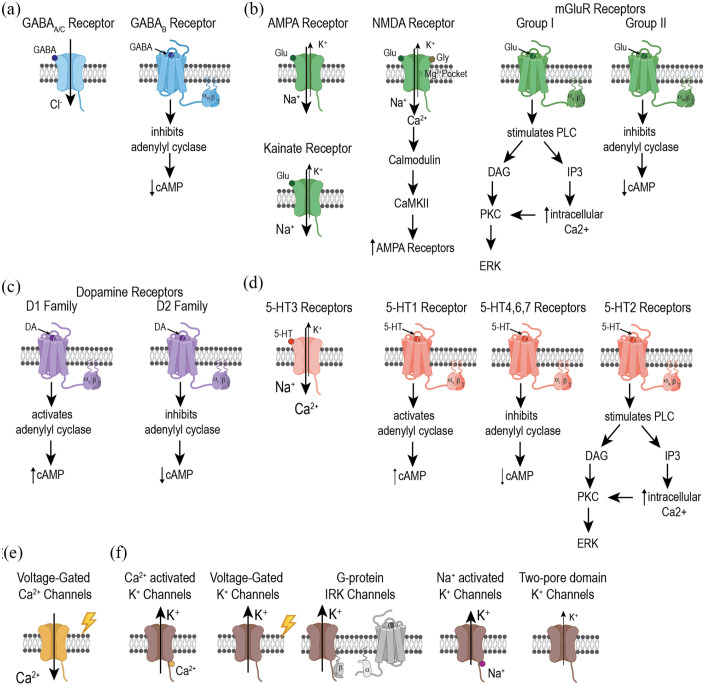
GABA is the major inhibitory neurotransmitter in the mammalian and fly nervous systems. Both human and fly GABA receptors include GABAA and GABAB (Figure 1a; Table 1). A subclass of GABAA receptors made entirely of rho (ρ) subunits is often called either GABAA-rho or GABAC; however, there is little evidence that GABAA-rho/GABAC receptors play a significant role in AUD. The super family of GABAA receptors are ligand-gated ion channels comprised of 5 protein subunits that form a central Cl− pore. GABAB receptors are metabotropic G-protein-coupled receptors (GPCRs). Early work estimates approximately 1 in 5 cortical neurons to be GABAergic in primates.16 Single cell transcriptomics estimates the fly central brain to be approximately 10% (10 000) GABAergic neurons.17
Figure 1.
Alcohol-related receptors, channels, and their downstream pathways. (a) GABA receptors are classified as either ionotropic (GABAA/C) or metabotropic (GABAB). GABAA/C receptors are gated chloride-conducting ion channels whereas GABAB receptors activate Gi/o proteins which inhibit adenylyl cyclase and decrease cAMP. (b) Glutamate receptors are classified as either ionotropic (AMPA, Kainate, and NMDA) or metabotropic (mGluRs) receptors. AMPA, Kainate, and NMDA receptors are all gated sodium-conducting cation channels, however, NMDA receptors also conduct calcium. mGluR are classified as groups I and II. Group I mGluRs activate Gq proteins which activate the PLC signaling pathway, whereas group II mGluRs activate Gi/o proteins which inhibit adenylyl cyclase and decrease cAMP. (c) Dopamine receptors are classified as D1- or D2-family members, which are both metabotropic receptors. However, D1 receptors activate Gs proteins thereby increasing cAMP, whereas D2 receptors activate Gi proteins thereby decreasing cAMP. (d) 5-HT receptors are classified as either ionotropic (5-HT3) or metabotropic (5HT1, 5-HT4,6,7, and 5-HT2) cation-permeable channel. 5-HT3 receptors are gated sodium-conducting cation channels. 5-HT3 receptors are not present in Drosophila. 5-HT metabotropic receptors activate either Gs, Gi, or Gq proteins to influence adenylyl cyclase and PLC signaling. (e) Calcium channels are gated by voltage. (f) Potassium channels are a diverse family that can be activated by Ca2+, voltage, the G βγ protein complex, and Na+. SLO2 is the fly homolog of the Na+ activated potassium channel, however it is not Na+ activated. Potassium channels also include two-pore domain K+ channels.
Table 1.
Receptors and channels associated with alcohol use in humans and Drosophila.
| Family/subtype | Receptor | Genes | Comments and References | |
|---|---|---|---|---|
| Human | Drosophila | |||
| GABA type A, ionotropic | GABAA/C | GABRA1, GABRA2, GABRA3, GABRA4, GABRA5, GABRA6, GABRB1, GABRB2, GABRB3, GABRD, GABRE, GABRG1, GABRG2, GABRG3, GABRP, GABRQ, GABRR1, GABRR2, GABRR3 | Rdl, Lcch3, Grd, CG12344, CG8916 | Stilwell et al.26
Kim et al.33 No specific work on GABAA and alcohol in flies |
| GABA type B, class C metabotropic GPCRS | GABAB | GABBR1, GABBR2 | GABA-B-R1, GABA-B-R2, GABA-B-R3 | Dzitoyeva et al.30
Manev and Dzitoyeva32 Ranson et al.31 Fisher et al.140 |
| Glutamate ionotropic | NMDA | GRIN1, GRIN2A, GRIN2B, GRIN2C, GRIN2D, GRIN3A, GRIN3B | Nmdar1, Nmdar2 | Maiya et al.40
Troutwine et al.41 |
| AMPA | GRIA1, GRIA2, GRIA3, GRIA4 | *Non-NMDA neuromuscular junction receptors: GluRIIA, GluRIIB, GluRIIC, GluRIID, GluRIIE | *Ionotropic receptors that conduct Ca2+ upon glutamate binding, but do not respond to AMPA or kainite | |
| Kainate | GRIK1, GRIK2, GRIK3, GRIK4 | |||
| delta | GRID1, GRID2 | |||
| Glutamate class C metabotropic GPCRS | mGluR1-8 | GRM1, GRM2, GRM3, GRM4, GRM5, GRM6, GRM7, GRM8 | mGluR, mtt* | *Lacks Glu binding residues |
| Dopamine class A rhodopsin-like GPCRS | D1 family (Gs-coupled) | DRD1, DRD5 | Dop1R1, Dop1R2, DopEcR* | *Also responsive to ecdysteroids |
| D2 family (Gi-coupled) | DRD2, DRD3, DRD4 | Dop2R | ||
| Serotonin (5-HT) ionotropic | HTR3 | HTR3A, HTR3B, HTR3C, HTR3D, HTR3E | – | |
| Serotonin (5-HT) class A rhodopsin-like GPCRS | HTR1-2,4-7 | HTR1A, HTR1B, HTR1D, HTR1E, HTR1F, HTR2A, HTR2B, HTR2C, HTR4, HTR5A, HTR5BP, HTR6, HTR7 | 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2B, 5-HT7, Octα2R* | *A receptor for octopamine (analogous to adrenaline) that also responds to 5-HT106 |
| Voltage-gated calcium channels | Ca2+ channel alpha subunits | CACNA1A, CACNA1B, CACNA1C, CACNA1D, CACNA1E, CACNA1F, CACNA1G, CACNA1H, CACNA1I, CACNA1S | cac | High-voltage activated: L-type CaV1.2 (α1C), CACNA1C L-type CaV1.3 (α1D), CACNA1D N-type CaV2.2 (α1B), CACNA1B P/Q-type Cav2.1 (α1A), CACNA1A R-type CaV2.3 (α1E), CACNA1E Low-voltage activated: CaV3.1 (α1G), CACNA1G CaV3.2 (α1H), CACNA1H |
| Ca2+ channel alpha2delta and beta subunits | CACNA2D1, CACNA2D2, CACNA2D3, CACNA2D4 and CACNB1, CACNB2, CACNB3, CACNB4 | Ca-Ma2d, stj, stol, Ca-β | ||
| Ca2+ channel gamma subunits | CACNG1, CACNG2, CACNG3, CACNG4, CACNG5, CACNG6, CACNG7, CACNG8 | Ca-α1D, Ca-α1T | ||
| Potassium channels | Ca2+-activated K channels | KCNMA1, KCNN1, KCNN2, KCNN2, KCNN3, KCNN4 | SK, slo, SLO2 | |
| K+-activated K channels | KCNA1, KCNA2, KCNA3, KCNA4, KCNA5, KCNA6, KCNA7, KCNA10, KCNB1, KCNB2, KCNC1, KCNC2, KCNC3, KCNC4, KCND1, KCND2, KCND3, KCNF1, KCNG1, KCNG2, KCNG3, KCNG4, KCNH1, KCNH2, KCNH3, KCNH4, KCNH5, KCNH6, KCNH8, KCNQ1, KCNQ2, KCNQ2, KCNQ3, KCNQ4, KCNQ5, KCNS1, KCNS2, KCNS3, KCNV1, KCNV2 | Ih*, eag, Elk, KCNQ, sei, Sh, Shab, Shal, Shaw, Shawl | *Hyperpolarization-activated cyclic nucleotide-gated | |
| K+-inward rectifying channels | KCNJ1, KCNJ2, KCNJ3, KCNJ4, KCNJ5, KCNJ6, KCNJ8, KCNJ9, KCNJ10, KCNJ11, KCNJ12, KCNJ13, KCNJ14, KCNJ15, KCNJ16, KCNJ18 | Irk1, Irk2, Irk3 | includes G-protein coupled inward rectifying potassium channels (GIRKs); Bodhinathan and Slesinger128 | |
| K+-two pore domain channels | KCNK1, KCNK2, KCNK3, KCNK4, KCNK5, KCNK6, KCNK7, KCNK9, KCNK10, KCNK12, KCNK13, KCNK15, KCNK16, KCNK17, KCNK18 | galene, Ork1, sand, Task6, Task7, CG1688, CG10864, CG34396, CG42594, CG43155 | ||
| Na+-activated K channels | KCNT1, KCNT2 | SLO2 homolog, but not sodium-activated | ||
GABA signaling in AUD
Alcohol consumption significantly alters GABAergic signaling particularly in mammalian brain regions like the ventral tegmental area (VTA), central amygdala (CeA), and the globus pallidus (GP). Ethanol acts on GABA receptors to increase presynaptic GABA release and acts as a GABA-mimetic, which potentiates inhibitory GABA currents post-synaptically. These effects ultimately contribute to short-term CNS depression and long-term homeostatic excitation that occurs during withdrawal.
GABA Type A channels in AUD
It is well established that GABAA is involved in mediating the effects of ethanol in mammals.18 Ethanol at low to moderate levels (up to 30 mM) acts as an indirect GABA agonist by binding to extracellular domains of δ subunit-containing GABAA receptors.19 This positive allosteric modulation causes sustained hyperpolarization and tonic silencing of GABAA-expressing neurons. Significant work has also identified specific roles for α1- and α2-containing GABAA receptors in regulating addictive behaviors.20 In contrast, increased tolerance is associated with a downregulation of GABAA receptors.21
The human genome has 19 GABAA subunit genes, whereas Drosophila has 5. The fly Resistance to dieldrin (Rdl) gene encoding the ~600 amino acid Rdl receptor is the most intensely studied and shows 46% amino acid sequence similarity to the human GABAA subunit pi, GABRP.22 Rdl is primarily expressed in the fly nervous system throughout development23 and Rdl-containing receptors show similar electrophysiological and pharmacological properties to that of fast-acting inhibitory transmission.24 In adult flies, Rdl is highly expressed within the antennal lobes, the mushroom body, optic lobes, ventrolateral protocerebrum, and the central complex.25 A direct role for Rdl or the other GABAA subunits in modulating flies’ response to ethanol has yet to be described. However, similar to mammalian GABAA receptors, Rdl is sensitive to picrotoxin, a noncompetitive GABAA antagonist that acts as a convulsant.26 This suggests that Rdl mutants may be useful in revealing the understudied mechanisms of withdrawal-induced seizures, neurotoxicity, and neurodegeneration following chronic alcohol exposure. Flies, therefore, offer a tractable model in which to perform pharmacologic screens and targeted GABAA subunit knockdowns to further reveal how ethanol impacts GABAergic signaling and animal behavior.
GABA Type B GPCRs in AUD
GABAB GPCRs have also become an important focus of human AUD research. Pharmacologic targeting of GABAB receptors with baclofen, a GABAB agonist, can suppress alcohol drinking and withdrawal in rodents and human alcoholics.27 There are many proposed mechanisms for baclofen’s therapeutic actions in the context of AUD, but most of these hypotheses require further investigation.
In Drosophila, GABAB receptors are encoded by Gαi-coupled subunits D-GABA-B-R1, D-GABA-B-R2, and D-GABA-B-R3 (dGB1-3), which inhibits downstream cAMP second messenger signaling upon ligand binding. Both dGB1 and dGB2 are homologous to mammalian GABAB receptors and the conserved nature of their intracellular trafficking has very recently been investigated.28 dGB3 is ~47% amino acid sequence similar to human GABBR222 and is expressed in a similar, albeit slightly different, spatiotemporal pattern to Rdl.29 Like mammals, the fly GABAB receptors play a role in behavioral response to alcohol.30,31 Interestingly, baclofen has reportedly no effect in flies,32 but recent work has demonstrated that pharmacologic agonism or antagonism of GABAB can bidirectionally influence flies’ alcohol tolerance.31
In addition to the GABA receptor subunits, other regulators of GABAergic transmission require investigation. Rogdi, an atypical leucine zipper named after one of Pavlov’s dogs, was recently shown to control GABA transmission in mammals.33 However, Rogdi’s role in AUD has not yet been investigated. Interestingly, fly rogdi mutants show reduced ethanol tolerance in a genetic screen investigating the overlap between long-term memory mutants and abnormal alcohol responses.12 To our knowledge, no further investigation or directed forward genetic approaches have been performed to study Rogdi’s role in AUD.
Given the immense diversity of GABA receptors and their distribution throughout the nervous system, one distinct advantage for employing fly genetic tools is to further delineate the cell- and receptor-type specific functions of GABA receptors in the context of alcohol response.
Glutamate Receptors
Glutamate is the major excitatory neurotransmitter in the mammalian nervous systems and a mediator of neural plasticity. Glutamate neurotransmission is tightly regulated because overstimulation can lead to seizures. Similar to GABA receptors, human glutamate receptors include both ionotropic channels (AMPA, Kainate, NMDA) and 3 groups of metabotropic GPCRs (mGluRs 1-8) (Figure 1b; Table 1). In contrast to mammals, Drosophila ionotropic channels include N-methyl-D-aspartate (NMDA) receptors and insect-specific glutamate-gated chloride channels (GluCls), which are similar to mammalian glycine receptors. For simplicity, our discussion will focus on NMDA receptors and group I and II mGluRs. Single cell transcriptomics estimates the fly central brain is approximately 24% (24 000) glutamatergic neurons.17
Glutamate signaling in AUD
In contrast to GABA, alcohol has an inhibitory effect on glutamate activity in mammals. Acute exposure to alcohol reduces glutamatergic activity while also stimulating GABAergic activity. Chronic alcohol exposure has the reverse effects.34 Early micro-dialysis studies in the striatum reported a decrease in extracellular concentrations in response to alcohol,35 whereas extracellular glutamate significantly increases following alcohol withdrawal.36 Ethanol-mediated disruption of the balance between excitation and inhibition is also controlled by glial cells, which play a crucial role in maintaining glutamatergic tone. Astrocytic glutamate transporters EAAT1 and EAAT2 function to clear extracellular glutamate.37 For a review on the role of mammalian glutamatergic signaling in AUD see Goodwani et al.38
NMDA channels in AUD
NMDA receptors are one of the 3 types of mammalian ionotropic glutamate receptors essential in neuronal plasticity and alcohol response. They are highly conserved ligand-gated cation channels permeable to positively charged ions like Ca2+ and Na+ and thus mediate rapid excitatory neurotransmission.
The human genome contains 7 genes encoding NMDA receptor subunits, whereas flies have 2, Nmdar1 and Nmdar2, that are 65% and 47% amino acid sequence similar to human GRIN1 and GRIN2D, respectively.22 There are many conserved molecular and physiological characteristics between vertebrate and fly NMDA receptors.39
In Drosophila, a mutant named intolerant was identified in a genetic screen for abnormal ethanol sensitivity and tolerance. This mutation is a novel allele of fly Discs large 1 (dlg1), a conserved homolog of mammalian PSD-95 and SAP97, which are thought to play a role in the post-synaptic localization of NMDA receptors.40 Subsequent analysis of loss-of-function Nmdar1 fly mutants showed reduced rapid and chronic tolerance to ethanol further bolstering conservational evidence of NMDARs’ sensitivity to ethanol. Recent CRISPR-based techniques have also been used to replace the fly Nmdar1 sequence with nonsynonmous point mutations that reduce ethanol sensitivity in mice.41 Point mutations affecting either a transmembrane domain or the calcium-binding site influenced behavioral response to ethanol and increased consumption.
Group I and II metabotrophic glutamate receptors in AUD
The metabotrophic glutamate receptors (mGluRs) mediate slow excitatory and inhibitory effects through intracellular G-protein signaling thereby causing a wide range of physiological effects. In mammals, Group I (mGluR1/5) and Group II (mGluR2/3) mGluRs are widely studied for their roles in alcohol dependence processes. Group I mGluRs are predominantly localized post-synaptically and cause slow excitatory neurotransmission by stimulating Gq/PLC/IP3 signaling that increases release of intracellular Ca2+ stores. Group II mGluRs are largely presynaptic and cause slow inhibitory neurotransmission by Gi/o protein signaling that decreases intracellular cAMP. Both Group I and II mGluRs influence alcohol self-administration, conditioned place preference for alcohol, and withdrawal. They are promising therapeutic targets for allosteric modulation in the pharmacologic treatment of AUD.38
The human genome encodes 8 mGluRs, whereas flies only have 1 functional mGluR that is 65% amino acid sequence similar to human GRM3.22 The fly mGluR signals with both Gi and Gq proteins; it regulates synaptic plasticity and is required for higher order behaviors like social interaction and memory.42 In contrast to work in mammals, mGluR in the fly has not yet been directly investigated in the context of alcohol. Thus, it is unclear whether it plays an analogous role in alcohol-related behaviors. However, the fly Homer protein, a homolog of mammalian Homer1 that is known to interact with Group I mGluRs, was found to be an important mediator of ethanol sensitivity and tolerance.43 The role of Homer family proteins in mediating the localization and function of mGluRs is of great interest in the AUD field because of its role as a postsynaptic density scaffold protein and involvement in alcohol-induced behavioral plasticity.44 The precise spatial and temporal genetic tools in flies may help reveal Homer-mGluR dynamics that are associated with alcohol-induced synaptic plasticity and circuitry changes.
Dopamine Receptors
Dopamine is a biogenic amine associated with many behaviors, including motor function, learning and memory, arousal, and reward. Dysfunction of the dopaminergic system is an underlying cause of numerous neurological conditions, such as Parkinson’s disease and drug addiction. The dopamine receptors are all G-protein coupled and classified into two families. The D1 family activates adenylyl cyclase and cAMP signaling upon dopamine binding, whereas the D2 family receptors inhibit these intracellular signals (Figure 1c; Table 1).
Dopaminergic signaling in AUD
The mesencephalon is a midbrain region composed of roughly 90% of the total dopaminergic neurons in the human brain; there are nearly 600 000 midbrain dopaminergic cells.45 These neurons are subdivided into well-characterized circuitry systems including the substantia nigra projections to the basal ganglia (nigrostriatal pathway) and ventral tegmental area (VTA) projections to the nucleus accumbens (mesolimbic pathway) or the cortex (mesocortical pathway). Flies have roughly 300 dopaminergic neurons organized into 15 major clusters that broadly innervate the adult central brain with significant projections to the mushrooom body (MB) and central complex.46
Countless human and rodent studies have explored the relationship between the dopaminergic signaling and alcohol abuse with evidence amassed from anatomical, physiological, pharmacologic, genetic, and behavioral research. Acute alcohol administration is thought to significantly increase firing of dopamine neurons within the VTA by targeting hyperpolarization-activated cyclic nucleotide-gated cation channels (HCN), modulating potassium channels, and altering L-type Ca2+ channels.47 This results in a prominent net increase in dopamine release within the ventral striatum and is thought to play a crucial role in the initiation of alcohol reinforcement.
Dopaminergic receptors in AUD
Within the mammalian striatum, populations of medium spiny neurons (MSNs) are defined by the expression of different types of dopamine receptors as well as opioid peptides. Approximately half of MSNs express excitatory D1 receptors whereas the remaining half express inhibitory D2 receptors.48 In addition to being expressed in MSNs, D2 receptors are located on the presynaptic terminal of dopaminergic neurons and function as auto-receptors to regulate the release of dopamine.49
A large body of preclinical and clinical AUD studies report reduced D2 receptor ligand binding, suggesting that levels of the D2 receptor are significantly reduced in the striatum of humans with AUD and may serve as a biomarker to predict relapse during recovery.50 Furthermore, high levels of D2 receptor in individuals might serve a protective role against developing AUD.51 In rodents, D2 receptors are critical for alcohol reinforcement52 and habitual alcohol seeking.53 Overexpression of D2 receptors in rodents reduces alcohol self-administration;54 the role of D1 receptors in alcohol dependence is less consistent. Recent work, however, suggests that chronic alcohol exposure disrupts the balance between D1 and D2 signaling pathways in MSNs of the striatum leading to a more robust behavioral response to ethanol and resiliency to sedation.55
D1 dopamine GPCRs in AUD
There are 2 human D1 receptors (DRD1 and DRD5). The fly genome encodes 4 total dopamine receptors and 2 have been functionally classified as D1-like: Dop1R1 (aka dumb, DA1, DopR) has 48% amino acid sequence similarity to DRD5 and Dop1R2 (aka DAMB) has 45% amino acid sequence similarity to ADRB1.22 A third unique dopamine and non-canonical ecdysone GPCR called DopEcR can also activate cAMP signaling.56 DopEcR has 46% amino acid sequence similarity to human orphan GPCR GPR52.22 Similar to mammalian studies, D1-like receptors have also been implicated in alcohol-related behaviors in flies.
Like mammals, when flies are exposed to low doses of alcohol they increase locomotor stimulation and become more disinhibited.57 Both mammalian DRD1 and fly Dop1R1 are implicated in modulating the locomotor response to alcohol intoxication.55,58 Knockdown of Dop1R1 in the fly brain significantly reduces ethanol-induced increases in locomotor activity. The central complex, homologous to the mammalian basal ganglia,59 is a system of brain regions that play a critical role in behavioral responses including locomotor response, sleep, and learning and memory. Interestingly, restoring expression of Dop1R1 in a subset of central complex neurons (ellipsoid body) rescues ethanol-induced locomotor defects in Dop1R1 mutants.58
Despite Dop1R1’s involvement in modulating the locomotor response to alcohol intoxication, knockdown of Dop1R1 within requisite MB circuits does not appear to affect alcohol-associated preference.60 However, expression of Dop1R1 within a separate subset of central complex neurons (dorsal fan-shaped body) is required for alcohol avoidance in naïve flies and the expression of Dop1R1 within the MB is required for experience-dependent alcohol preference in a voluntary consumption assay.61 Given the DRD1 hypersensitivity described in mammalian models,62 an overexpression model would be informative for exploring the role of Dop1R1 in chronic alcohol seeking behaviors.
When flies are exposed to substantial doses of alcohol they lose postural control and eventually sedate.15,57,63,64 Recently, DopEcR, the unique GPCR that binds dopamine and the major insect steroid hormones called ecdysteroids,56 was found to be required in alcohol-induced sedation.65 DopEcR mutants took longer to succumb to the sedative effects of alcohol, whereas neuronal overexpression of DopEcR significantly reduced the time to sedation. Further investigation suggested that DopEcR mediates an ecdysone-induced promotion of sedation via EGFR/ErK signaling inhibition65 and may also mediate a dopaminergic signal that suppresses ethanol-induced hyperactivity.66 DopEcR has been compared to vertebrate GPER1, the non-canonical estrogen receptor, which also responds to dopamine in heterologous expression systems.67 GPER1 influences various nervous system functions including synaptic plasticity and neuroprotection,68 but to our knowledge its role in AUD has not been determined. Thus, the role of steroid hormones and their interaction with dopamine receptors in mammals requires further investigation.
D2 dopamine GPCRs in AUD
The human genome encodes 3 D2 receptors (DRD2, DRD3, and DRD4). The fly genome only has 1 D2 receptor, Dop2R (aka D2R). Dop2R shows 46% amino acid sequence similarity to human DRD2.22 For an extensive discussion of dopamine receptor homology, pharmacology, and signaling mechanisms see Karam et al.69
The role of D2-like receptors within the central complex has not been explored, however, D2-like receptors have an identified role within MB circuitry in establishing alcohol-associated preference.60,70 Knockdown of Dop2R in a population of dopamine neurons innervating the MB suggest that the regulation of dopamine release via dopamine autoreceptors is critical to the development of alcohol-associated preference in flies.60 Knocking down Dop2R in all of the cholinergic MB neurons also significantly reduces preference for alcohol-associated odor cues.70 Dop2R is also required in a single glutamatergic MB output neuron during consolidation of alcohol-associated preference.60 Although mammalian work on D2 receptor function has not yet reached this level of circuit precision analysis, these fly studies suggest a potentially diverse functional repertoire of D2-like receptors in alcohol-related behaviors. Thus, seemingly incongruent results between mammals and flies likely provide insight to the complexity and importance of how D2-like receptors and others uniquely modify responses to alcohol with circuit specificity.
Further analysis via RNA sequencing of isolated MB nuclei revealed that repeated alcohol-cue training caused lasting changes in the MB nuclear transcriptome. Most notable was a switch in expression of two Dop2R receptor isoforms that differ in a single amino acid—the alternative inclusion of a serine in the third cytoplasmic loop.70 This site in the receptor is a conserved place of interaction for G-proteins and thus has implications for downstream signaling.71 Recent work demonstrates that particular neurotransmitter-producing populations have distinct RNA editing signatures,72 suggesting that flies may be useful for testing site- and context-specific isoform expression in the context of alcohol exposure.
Serotonin Receptors
The serotonergic (5-hydroxytryptamine or 5-HT) system is involved in nearly every aspect of mammalian physiology including neurogenesis, motor control, sleep, mood, and cognition; it also plays a key role in regulating alcohol consumption, dependence, and withdrawal. Similar to GABA and glutamate receptors, 5-HT receptors come as either ligand-gated ion channels (5-HT3) or metabotropic GPCRs (Figure 1d; Table 1). However, flies do not have a homologous 5-HT3 ligand-gated ion channel and so for the purposes of this review we will focus our attention on 5-HT GPCRs.
5-HT signaling in AUD
There are roughly 60 000 serotoninergic neurons in the human central brain, most of which reside in the raphe nuclei of the brainstem.73 5-HT neurons from the dorsal raphe nucleus are thought to modulate dopaminergic neurons in the VTA and enhance ethanol-induced increases in firing.74 Flies have a relatively simple serotoninergic system with around 80 neurons spread into various clusters within the adult central brain,75 yet the serotoninergic system functions similarly in regulating mood, motivation, and response to alcohol.
Early work suggested that deficits in 5-HT were associated with AUD and motivation to seek alcohol.76,77 Loss of 5-HT neurotransmission increased alcohol consumption and enhanced vulnerability to dependence.78 Acute alcohol exposure increases extracellular 5-HT levels,79 whereas chronic exposure decreases 5-HT levels in the CNS. The reduction following chronic alcohol exposure may be the result of accelerated 5-HT reuptake by the serotonin transporter (SERT) or due to dysfunctional 5-HT release from the raphe nuclei.80 Similarly, in flies reduced motivation to seek rewards is associated with decreases in 5-HT levels within the brain.81,82 Interestingly, a depression-like state in flies could be ameliorated by lithium-chloride treatment, a commonly prescribed antidepressant.81 These works demonstrate the conserved role of 5-HT in modulating internal states and motivation across species. For a comprehensive review on the role of 5-HT signaling in alcohol addiction see Belmer et al.80
5-HT GPCRs in AUD
The human genome encodes 13 different 5-HT GPCRs (HTR1A, HTR1B, HTR1D, HTR1E, HTR1F, HTR2A, HTR2B, HTR2C, HTR4, HTR5A, HTR5BP, HTR6, HTR7). In general, activation of 5-HT1 receptors subtypes result in neuronal inhibition and inhibition of second messenger adenylate cyclase, whereas activation of 5-HT2, 4, 6, and 7 results in neuronal excitation and activates second messenger adenylate cyclase or phospholipase C signaling.83 Further, 5-HT1A and 1B are identified as autoreceptors, which are localized to the presynaptic membrane of serotonergic neurons.84 A number of 5-HT GPCRs have been implicated in AUD.
The 5-HT1 autoreceptors have an identified role in modulating alcohol consumption. Early studies found that 5-HT1A antagonists attenuated alcohol consumption,85,86 whereas mice lacking 5-HT1B displayed increased alcohol consumption.87 More recent work suggests that chronic ethanol consumption in rodents hypersensitizes the autoreceptor 5-HT1A88 and differentially alters expression levels of 5-HT1A in a regionally specific manner.89,90
The 5-HT2 receptors are also implicated in alcohol reinforcement and consumption. Early work reported that 5-HT2 antagonists decrease acute ethanol reinforcement91 and alcohol consumption.92 More recent work suggests that different subtypes of 5-HT2 receptors play distinct roles. For instance, drugs that include antagonists for 5-HT2C are reported to decrease voluntary ethanol consumption.93,94 Of course receptors often do not work in isolation. Cyproheptadine, a potent 5-HT2 receptor antagonist, when used in combination with Prazosin, a α1β-adrenergic antagonist, reversed alcohol preference in mice, suggesting that adrenergic and serotonergic transmission work cooperatively to support alcohol-associated behaviors.95
The role of 5-HT2C receptors appears to be similarly regionally specific with levels of 5-HT2C receptors being increased in the NAc following chronic exposure, and treatment with antagonists inhibit intake and behavioral sensitization in mice.96,97 These data underscore the importance of obtaining an understanding of the actions of ethanol on serotonin receptors with circuitry specificity. New evidence has also emerged showing that alterations in post-transcriptional editing of 5-HT2C mRNA may participate in the development of AUD as well as other psychiatric conditions.98 Specifically, the 5-HT2C mRNA can be RNA edited at several different positions99 causing amino acid substitutions that influence receptor activity.100 To what extent RNA editing impacts mammalian AUD is still being determined.
In Drosophila there are 5 genes encoding 5-HT GPCRs—5-HT1A, 5-HT1B, 5-HT2A, 5-HT2B, and 5-HT7—which range from 37%-52% amino acid sequence similarity compared to their human homologs.22 Similar to mammals, 5-HT in flies mediates diverse processes including short- and long-term memory, circadian rhythm, aggression, and courtship behavior. The fly 5-HT receptors are expressed in various regions of the adult protocerebrum, including the mushroom body, central complex, and optic lobes.101,102
In regard to modeling AUD, knockdown of fly protein kinase C (PKC) in 5-HT and dopaminergic neurons resulted in ethanol resistance, a phenotype that was mitigated by pharmacological inhibition of serotonin reuptake.103 5-HT has also been shown to bidirectionally influence olfactory attraction to alcohol.104 A recent study also found that 5-HT signaling in flies was required for dietary yeast-induced increases in alcohol consumption and resistance to sedation.105 This study highlights the conserved link between 5-HT, diet, and alcohol-related behavior across species. It is interesting that both adrenergic and serotonergic transmission influence mammalian alcohol-associated behaviors as it was recently discovered that flies have a novel adrenergic-like receptor (DmOcta2R).106 This receptor is activated by 5-HT as well as its cognate ligand octopamine, which is analogous to norepinephrine and implicated in alcohol attraction and sedation.10,107 Perhaps further characterization of this unique receptor’s cellular mechanisms will reveal conserved interactions between these neurotransmitter systems in AUD.
Voltage-Gated Calcium Channels
Voltage-gated calcium channels (VGCCs) are voltage sensitive ion channels embedded in the membrane of excitable cells that regulate the rapid entry of Ca2+ during depolarization. At the core of these channels is a principal pore-forming α1 subunit and up to 3 supporting α2δ, β, and γ subunits. There are 5 classified types of high-voltage-activated channels (L-type, P/Q-type, N-type, R-type, and T-type) and 3 low-voltage-activated channels each composed of a sole α1 subunit (Figure 1e; Table 1).
Ca2+ channels in AUD
VGCCs play a wide range of roles in physiological and pathophysiological conditions, particularly in controlling neuronal excitability. They are common therapeutic drug targets108 and implicated in acute and chronic effects of alcohol as well as withdrawal. Acute alcohol-induced inhibition of VGGCs may induce a compensatory upregulation during chronic alcohol intoxication and this upregulation may be revealed during withdrawal. For reviews on VGCCs in AUD see N’Gouemo.109
Each type of VGCC has been examined in models of AUD, although L-type current is the most investigated. Inhibition of L-type VGCCs decreases alcohol consumption and mediates alcohol-seeking behavior.110 However, human alcohol-related clinical trials using L-type blockers and modulators showed conflicting effects on alcohol intake, withdrawal, and abstinence.111-113 Additionally, gabapentin, which binds the α2δ subunit, suppresses central amygdala (CeA) activity and promotes abstinence in human alcoholics.113 Chronic alcohol exposure leads to increased P-type current in the cerebellum,114 and P/Q-type VGGCs also mediate the ethanol- and CRF-sensitivity of GABAergic synapses in the CeA.115 N-type current is affected by both acute and chronic ethanol in vitro and acute exposure in mice lacking functional N-type VGCCs show increased ethanol-induced ataxia, resistance to righting reflex, reduced ethanol consumption, and conditioned place preference compared to wildtype.116 Low or high concentrations of ethanol can also enhance or decrease T-type current, respectively in thalamic brain slices.117 These findings suggest that there are various roles for VGCCs in modulating responses to ethanol and in the development of alcohol reward and preference.
Humans have 9 genes encoding VGCC α1 subunits, all of which are expressed in the CNS.118 In flies there is only 1 α1 gene, cacophony (cac), which shows 55% amino acid sequence similarity to CACNA1B.22 Furthermore, humans also have 16 genes encoding the α2δ, β, and γ auxiliary subunits, whereas flies have 6. The fly cac mutants are highly studied in neuromuscular synapse regulation and in various behaviors including seizures,119-121 but there have been no direct studies published on the role of cacophony in fly alcohol response. A recent study, however, identified a new mechanism downstream of Ca2+ influx by which intoxicating levels of ethanol inhibit presynaptic release.122 This presynaptic modulation required Unc13 proteins, which are known to interact with vesicle fusion machinery and VGCCs.123 The work is consistent with ethanol causing homeostatic synaptic changes that lead to functional alcohol tolerance. The simplicity of having fewer VGCC genes in flies has been helpful in unraveling the functional contributions of different VGCC auxiliary subunits. For example, there are distinct roles between D-type Ca-α1D subunits and the α2δ subunits straightjacket and CG4587.124 Thus, flies offer a possible model in which to characterize any redundancy or exclusive functions of VGCC subunits in neuromodulation following alcohol exposure.
RNA editing also factors into the final protein products and function of VGCCs.125 For instance, editing of human CACNA1D in the CNS influences calmodulin (CaM) interactions and behavior in mice.126 RNA editing is quite common in invertebrates with ~4% of transcripts being edited and two-thirds of those causing nonsynonmous (nonsyn.) substitutions.23 In the adult fly central brain neurons there were 9 detected editing sites in cac (7 nonsyn.), 7 in Ca-α1D (5 nonsyn.), 6 in Ca-α1D (2 nonsyn.), 5 in Ca-β (4 nonsyn.), and 2 in stj (2 nonsyn.).72 Thus, flies can likely be used to determine the extent to which alcohol affects RNA editing of VGCC subunits, or conversely how edited channels influence response to alcohol.
Potassium Channels
Potassium channels (KCNs) are found in most cell types and control a wide variety of cell functions. KCNs have a K+-selective pore and are sub-classified into 4 classes, either Ca2+-activated (KCNN), K+-activated (KCNA), inwardly rectifying (KCNJ), 2 pore domain channels (KCNK), or Na+-activated (KCNT) (Figure 1f; Table 1). Regulation of K+ flux is critical for setting or resetting the resting membrane potential, thus controlling the sharp action potential of excitable cells. KCNs are tetrameric complexes and properties of their gating and inactivation ultimately control the channel’s conductance.
K+ channels in AUD
Several KCNs are implicated in various alcohol-associated responses. Ethanol alters voltage/calcium-gated large conductance potassium (BK, slo) channels leading to perturbations in physiology and behavior. Slo channels have ethanol-binding sites and are generally inhibited by ethanol, but their responses vary depending on subunit composition, modification by phosphorylation, and the lipid microenvironment.127 G-protein-gated inwardly rectifying potassium channels (GIRKs) regulate neuronal responses in the brain reward circuit and are possible targets for AUD therapeutics.128 Ethanol enhances GIRK currents in VTA neurons129 and GIRK knockout mice show various behaviors associated with alcohol withdrawal, intake, self-administration, and changes in motor response.130 The K+-activated (Shaw) channels have crucial residues necessary for inhibition by ethanol. Acute alcohol can increase the expression of two-pore potassium channel KCNK12 in the VTA and knockdown causes increased alcohol consumption.131
Humans have 78 genes encoding the KCN subunits, whereas flies have 26. The fly large-conductance BK channel slowpoke (slo) has 64% amino acid sequence similarity to KCNMA1.22 Following its discovery in flies, slo was first identified for its role in alcohol response in a screen for ethanol-resistance in C. elegans.132 Expression of slo is increased after alcohol-induced sedation leading to a counter-intuitive increase in excitability following neuroadaptive homeostasis.133,134 Furthermore, slo is required for the development of functional tolerance and withdrawal-associated increases in seizure susceptibility.135
To our knowledge, many of the fly’s K+-activated and 3 Irk channels have not been implicated in alcohol-induced responses, but all show ~50%-70% similarity to human homologs.22 There also seem to be no apparent fly Na+-activated (KCNT) homologs although SLO2 shows 53% amino acid sequence similarity. The single KCNQ channel in flies shows 51% amino acid sequence similarity to human KCNQ4; it’s more sensitive to acute ethanol block than it is in mammals and KCNQ null flies display increased sensitivity and tolerance to the sedative effects of alcohol.136 Lastly, there is one particular KCN tool routinely used in fly neurogenetic studies. Overexpression of the human KCNJ2 inwardly rectifying potassium channel (often called Kir2.1) can hyperpolarize neurons of interest thereby inhibiting their activity. In addition to using KCN expression to control neuronal silencing, flies also afford a model in which to study the role of KCN modulation by ethanol.
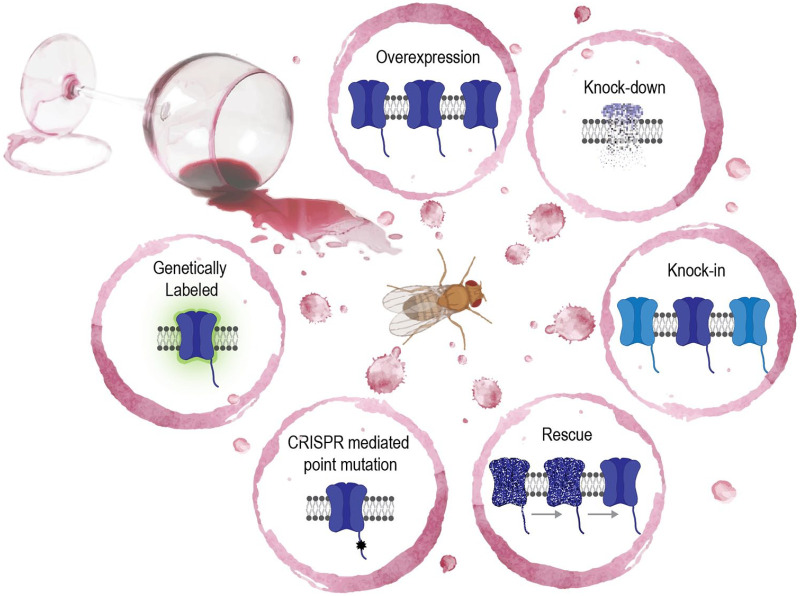
Genetic Tools for All Receptors/Ion Channels
A clear benefit to using Drosophila as a model system is its genetic tractability and simplicity. There are rich mutant and transgenic tools available that provide the opportunity to control, visualize, and measure molecules in vivo (Figure 2). Here we provide examples of established as well as recently developed tools and discuss how they might be employed in studying the aforementioned receptors and channels in the context of alcohol.
Figure 2.
General tools available for investigating alcohol-associated receptors, channels, and other proteins.
Tools for precise spatiotemporal control and visualization
A distinct advantage of using flies is the ability to use refined intersectional genetic strategies. There are multiple binary transcriptional factor/enhancer systems such as the GAL4/UAS, LexA/LexAop, and QF/QUAS systems that can be used to target multiple cell types simultaneously for circuit level analyses. The split-GAL4 and split-LexA systems, which separately express the activation and DNA-binding domains can further refine targeting even to single cell resolution.137 Various genetic mosaicism techniques can also be readily performed to test cell autonomy and clonal patches.138 Furthermore, there is an impressive versatile collection of nearly 7400 MiMIC gene trap lines, which provide essential mutagenetic, reversible, and replaceable endogenous insertions throughout the Drosophila genome.139
The recently designed “FlpStop” approach provides a means to control cell-type-specific genetic disruption or rescue of endogenous gene function.140 Specifically, a FlpStop cassette lies dormant within a gene of interest’s intron, which can then be inverted via cell-type specific expression of Flp recombinase revealing premature transcription and translation stop signals and fluorescently labeling genetically modified cells. As proof-of-principle candidates, both Rdl and Gad1, the glutamic acid decarboxylase 1 enzyme required for GABA synthesis, were used to showcase conditional null targeting. Although GABAB receptors were not modified in this study, another recent site-specific knock-in (“KI”) approach was performed on GABA-B-R1, GABA-B-R3, and VGAT, the vesicular GABA transporter.141 The “KI” cassette introduced a self-cleaving T2A peptide and GAL4 or LexA transcription factor sequence prior to a gene of interest’s stop codon. This method beautifully revealed the specific adult neural expression patterns of these, and almost 200 other neurotransmitters, neuropeptides, and receptors.
Further aiding in visualization methods, a recent T2A-GAL4 insertion library has been created, which endogenously modified 75 of the 113 fly genes encoding neurotransmitter receptors.142 Specifically, T2A-GAL4 integrated upstream of stop codons results in a pre-mRNA that self-cleaves thereby producing an unmodified receptor plus a yeast GAL4 transcription factor. The GAL4 transcription factor can then activate any UAS-based reporter or effector transgene in a spatiotemporal specific expression fashion. For instance, Kondo et al142 demonstrate the expression patterns of ionotropic glutamate receptors in the adult brain and larval muscle tissues. They further calculate that an average adult neuron expresses 30% of known neurotransmitter receptors. The T2A-GAL4 cassettes can also be replaced with other reporter cassettes for endogenous protein tagging and activity reporters. Thus, co-receptor expression patterns and endogenous labeling of receptors can be studied in the context of alcohol exposure.
As an update to traditional protein tagging, the tissue-specific tagging of endogenous proteins (T-STEP) was created.143 The T-STEP method simultaneously RFP-tags an endogenous protein and then allows tissue-specific rippase recombination to switch the tag to a GFP signal. Given that the actions of ethanol on dopamine and serotonin receptors appear to be circuit specific, these tools would be especially helpful in resolving the changes in receptor expression in discrete circuits before and after different alcohol exposure paradigms. This approach can also be tremendously useful for determining pre- versus post-synaptic localization of different receptors. This may be especially useful considering the heterogeneity of dopamine receptors and neurons throughout the nervous system as well as the proposed relationship between D1-like and D2-like receptors expression in AUD models.
Lastly, new tools, like the fly TransTimer,144 are providing a means in which to study the real-time spatiotemporal dynamics of gene expression. TransTimer is a method that uses 2 fast-folding fluorescent proteins, where one has a shorter half-life (ie, a destabilized GFP) and the other has a longer half-life (ie, a stable RFP). Both reporters are positioned under the same promoter such that the relative relationship of the 2 signals conveys information about dynamic changes in gene expression. Tools like TransTimer can reveal in vivo transcriptional activity in real-time or in fixed immunohistochemical experiments, which are useful for lineage tracing, cell differentiation, labeling for FACS, or high-throughput sequencing methods. If applied in the AUD field, researchers could assess transcriptional and translational dynamics of particular systems concomitantly. For instance, the dynamic expression of slo across different exposure paradigms could be determined. The transcriptional regulation of other receptors can also be observed in the context of fetal alcohol models, immediate-early gene expression patterns, and in determining circadian-regulated changes that are currently undetectable with long-live reporter systems.
Visualization of neural activity
Visualizing neuronal changes in intracellular calcium are an important measure of pre- and post-synaptic activity as calcium influx often corresponds with neuronal firing and neurotransmitter exocytosis.145 The recently developed genetically-encoded calcium indicators (GECIs) provide a means to measure free intracellular Ca2+ with an extended sensor color palette and reversible photoactivation capacities.146 Another exciting, newly developed imaging tool is the genetically-encoded voltage indicator, Voltron.147 Voltron uses photostable synthetic dyes rather than fluorescent proteins to directly measure action potentials and subthreshold events that are not captured with calcium indicators. The use of synthetic dyes significantly improves the brightness and photostability of the signal. These imaging tools can be applied to any of the previously mentioned signaling pathways to better understand alcohol-induced disruptions in different circuitry in vivo.
Measuring neurotransmission
There are various ways to experimentally assess neurotransmission, including real-time voltammetry measurements, optogenetic control, and electrochemistry methods.148 Although the adult fly brain is quite small—roughly 50 µm3—measuring both real-time and tissue content levels of neurotransmitters could help reveal underlying changes that occur during alcohol exposure. Furthermore, newly developed genetically encoded fluorescent dopamine sensors, like GRABDA1m, allow for the detection of extracellular dopamine dynamics with subcellular spatial and subsecond temporal resolution in defined neurons.149 This would be especially informative in evaluating how dopamine circuits change as flies develop preference for alcohol instead of focusing on models where preference is already established. Similar tools have recently been developed for directly measuring acetylcholine release, such as GRABACh, which would be especially useful in defining the role of acetylcholine in alcohol-associated behaviors across species.150
Conclusions
Drosophila is an important model system that has significantly contributed to our understanding of the neuromolecular and genetic underpinnings of AUD. By leveraging established and recently developed genetic and experimental tools the field is better able to reveal the precise in vivo molecular actions of ethanol, and will certainly advance our understanding of AUD. We have focused on comparing the most prominent alcohol-associated receptors and channels (GABA, glutamate, dopamine, serotonin, calcium channels, and potassium channels). We conclude by describing general tools that provide nearly limitless genetic modification and control for examining the roles of many molecules, cellular processes, circuit dynamics, and complex ethological mechanisms involved with AUD. Other AUD-relevant receptors and channels not highlighted in this review include mammalian receptors for corticotropin-releasing hormone, opioids, oxytocin, glycine, neuropeptide Y, norepinephrine, and finally the Drosophila octopamine receptors. Another important consideration is the comparative analysis between transcript isoforms and the proteomic diversity of these proteins across species, which is an exciting avenue for studying molecular mechanisms of AUD.
Maintaining the bridge between translational insights across species and taking advantage of each animal models’ unique tools and systems will bring the field closer to achieving a comprehensive understanding of AUD and facilitate effective treatment strategies.
Acknowledgments
We thank Karla Kaun (Brown University) for helpful comments on earlier versions of this manuscript.
Footnotes
Funding:Financial support for the publication of this article was provided by the Department of Psychology at Bryant University.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Both authors researched and wrote the manuscript. EP generated Table 1 and KMS generated figure 1 and 2.
ORCID iD: Kristin M Scaplen  https://orcid.org/0000-0001-7493-1420
https://orcid.org/0000-0001-7493-1420
References
- 1. World Health Organization. Global status report on alcohol and health 2018. World Health Organization; 2019. [Google Scholar]
- 2. Most D, Ferguson L, Harris RA. Molecular basis of alcoholism. In: Handbook of clinical neurology. Vol 125. Elsevier; 2014:89-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93(6):997-1007. [DOI] [PubMed] [Google Scholar]
- 4. Devineni AV, Heberlein U. The evolution of Drosophila melanogaster as a model for alcohol research. Annu Rev Neurosci. 2013;36:121-138. [DOI] [PubMed] [Google Scholar]
- 5. Devineni AV, Heberlein U. Preferential ethanol consumption in Drosophila models features of addiction. Curr Biol. 2009;19(24):2126-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peru YP, Ojelade SA, Penninti PS, et al. Long-lasting, experience-dependent alcohol preference in Drosophila. Addict Biol. 2014;19(3):392-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park A, Tran T, Scheuermann EA, Smith DP, Atkinson NS. Alcohol potentiates a pheromone signal in flies. Elife. 2020;9:e59853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U. A drosophila model for alcohol reward. Nat Neurosci. 2011;14(5):612-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee HG, Kim YC, Dunning JS, Han KA. Recurring ethanol exposure induces disinhibited courtship in Drosophila. PLoS One. 2008;3(1):e1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scholz H, Ramond J, Singh CM, Heberlein U. Functional ethanol tolerance in Drosophila. Neuron. 2000;28(1):261-271. [DOI] [PubMed] [Google Scholar]
- 11. Ghezzi A, Krishnan HR, Atkinson NS. Susceptibility to ethanol withdrawal seizures is produced by BK channel gene expression. Addict Biol. 2014;19(3):332-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berger KH, Kong EC, Dubnau J, Tully T, Moore MS, Heberlein U. Ethanol sensitivity and tolerance in long-term memory mutants of Drosophila melanogaster. Alcohol Clin Exp Res. 2008;32(5):895-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaun KR, Devineni AV, Heberlein U. Drosophila melanogaster as a model to study drug addiction. Hum Genet. 2012;131(6):959-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park A, Ghezzi A, Wijesekera TP, Atkinson NS. Genetics and genomics of alcohol responses in Drosophila. Neuropharmacology. 2017;122:22-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodan AR, Rothenfluh A. The genetics of behavioral alcohol responses in Drosophila. Int Rev Neurobiol. 2010;91:25-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sahara S, Yanagawa Y, O’Leary DD, Stevens CF. The fraction of cortical GABAergic neurons is constant from near the start of cortical neurogenesis to adulthood. Journal of Neuroscience. 2012;32(14):4755-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davie K, Janssens J, Koldere D, et al. A single-cell transcriptome atlas of the aging Drosophila brain. Cell. 2018;174(4):982-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Enoch M-A. The role of GABAA receptors in the development of alcoholism. Pharmacol Biochem Behav. 2008;90(1):95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olsen RW. Analysis of γ-aminobutyric acid (GABA) type A receptor subtypes using isosteric and allosteric ligands. Neurochem Res. 2014;39(10):1924-1941. [DOI] [PubMed] [Google Scholar]
- 20. Stephens DN, King SL, Lambert JJ, Belelli D, Duka T. GABAA receptor subtype involvement in addictive behaviour. Genes Brain Behav. 2017;16(1):149-184. [DOI] [PubMed] [Google Scholar]
- 21. Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology. 1998;139(1-2):2-19. [DOI] [PubMed] [Google Scholar]
- 22. Hu Y, Flockhart I, Vinayagam A, et al. An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinf. 2011;12(1):357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Graveley BR, Brooks AN, Carlson JW, et al. The developmental transcriptome of Drosophila melanogaster. Nature. 2011;471(7339):473-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee D, Su H, O’Dowd DK. GABA receptors containing Rdl subunits mediate fast inhibitory synaptic transmission in Drosophila neurons. J Neurosci. 2003;23(11):4625-4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aronstein K, Ffrench-Constant R. Immunocytochemistry of a novel GABA receptor subunit Rdl in Drosophila melanogaster. Invert Neurosci. 1995;1(1):25-31. [DOI] [PubMed] [Google Scholar]
- 26. Stilwell GE, Saraswati S, Littleton JT, Chouinard SW. Development of a Drosophila seizure model for in vivo high-throughput drug screening. Eur J Neurosci. 2006;24(8):2211-2222. [DOI] [PubMed] [Google Scholar]
- 27. Agabio R, Colombo G. GABAB receptor ligands for the treatment of alcohol use disorder: preclinical and clinical evidence. Front Neurosci. 2014;8:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang S, Xue L, Liu X, et al. Structural basis for distinct quality control mechanisms of GABAB receptor during evolution. FASEB J. 2020;34(12):16348-16363. [DOI] [PubMed] [Google Scholar]
- 29. Okada R, Awasaki T, Ito K. Gamma-aminobutyric acid (GABA)-mediated neural connections in the Drosophila antennal lobe. J Comp Neurol. 2009;514(1):74-91. [DOI] [PubMed] [Google Scholar]
- 30. Dzitoyeva S, Dimitrijevic N, Manev H. γ-aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: adult RNA interference and pharmacological evidence. Proc Natl Acad Sci. 2003;100(9):5485-5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ranson DC, Ayoub SS, Corcoran O, Casalotti SO. Pharmacological targeting of the GABAB receptor alters Drosophila’s behavioural responses to alcohol. Addict Biol. 2020;25(2):e12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manev H, Dzitoyeva S. GABA-B receptors in Drosophila. In: Advances in Pharmacology. Vol 58. Elsevier; 2010:453-464. [DOI] [PubMed] [Google Scholar]
- 33. Kim M, Jang D, Yoo E, et al. Rogdi defines GABAergic control of a wake-promoting dopaminergic pathway to sustain sleep in Drosophila. Sci Rep. 2017;7(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rao PS, Bell RL, Engleman EA, Sari Y. Targeting glutamate uptake to treat alcohol use disorders. Front Neurosci. 2015;9:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carboni S, Isola R, Gessa G, Rossetti Z. Ethanol prevents the glutamate release induced by N-methyl-D-aspartate in the rat striatum. Neurosci Lett. 1993;152(1-2):133-136. [DOI] [PubMed] [Google Scholar]
- 36. Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol. 1995;283(1-3):177-183. [DOI] [PubMed] [Google Scholar]
- 37. Zhou Y, Danbolt NC. GABA and glutamate transporters in brain. Front Endocrinol. 2013;4:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goodwani S, Saternos H, Alasmari F, Sari Y. Metabotropic and ionotropic glutamate receptors as potential targets for the treatment of alcohol use disorder. Neurosci Biobehav Rev. 2017;77:14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xia S, Chiang A-S. NMDA receptors in Drosophila. In: Biology of the NMDA Receptor. CRC Press/Taylor & Francis; 2009. [PubMed] [Google Scholar]
- 40. Maiya R, Lee S, Berger KH, et al. DlgS97/SAP97, a neuronal isoform of discs large, regulates ethanol tolerance. PLoS One. 2012;7(11):e48967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Troutwine B, Park A, Velez-Hernandez ME, Lew L, Mihic SJ, Atkinson NS. F654A and K558Q mutations in NMDA receptor 1 affect ethanol-induced behaviors in Drosophila. Alcohol Clin Exp Res. 2019;43(12):2480-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schoenfeld BP, Choi RJ, Choi CH, et al. The Drosophila DmGluRA is required for social interaction and memory. Front Pharmacol. 2013;4:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Urizar NL, Yang Z, Edenberg HJ, Davis RL. Drosophila homer is required in a small set of neurons including the ellipsoid body for normal ethanol sensitivity and tolerance. J Neurosci. 2007;27(17):4541-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Castelli V, Brancato A, Cavallaro A, Lavanco G, Cannizzaro C. Homer2 and alcohol: a mutual interaction. Frontiers in psychiatry. 2017;8:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chinta SJ, Andersen JK. Dopaminergic neurons. Int J Biochem Cell Biol. 2005;37(5):942-946. [DOI] [PubMed] [Google Scholar]
- 46. Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits. 2009;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chu H-Y, Zhen X. Hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels in the regulation of midbrain dopamine systems. Acta Pharmacol Sin. 2010;31(9):1036-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gagnon D, Petryszyn S, Sanchez M, et al. Striatal neurons expressing D1 and D2 receptors are morphologically distinct and differently affected by dopamine denervation in mice. Sci Rep. 2017;7(1):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mercuri N, Saiardi A, Bonci A, et al. Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience. 1997;79(2):323-327. [DOI] [PubMed] [Google Scholar]
- 50. Volkow ND, Fowler JS, Wang G-J, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64(11):1575-1579. [DOI] [PubMed] [Google Scholar]
- 51. Volkow ND, Wang G-J, Begleiter H, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63(9):999-1008. [DOI] [PubMed] [Google Scholar]
- 52. Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1997;21(6):1083-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Corbit LH, Nie H, Janak PH. Habitual responding for alcohol depends upon both AMPA and D2 receptor signaling in the dorsolateral striatum. Front Behav Neurosci. 2014;8:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thanos PK, Volkow ND, Freimuth P, et al. Overexpression of dopamine D2 receptors reduces alcohol self-administration. J Neurochem. 2001;78(5):1094-1103. [DOI] [PubMed] [Google Scholar]
- 55. Bocarsly ME, da Silva ESD, Kolb V, et al. A mechanism linking two known vulnerability factors for alcohol abuse: heightened alcohol stimulation and low striatal dopamine D2 receptors. Cell Rep. 2019;29(5):1147-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Srivastava DP, Yu EJ, Kennedy K, et al. Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J Neurosci. 2005;25(26):6145-6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wolf FW, Rodan AR, Tsai LT-Y, Heberlein U. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J Neurosci. 2002;22(24):11035-11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kong EC, Woo K, Li H, et al. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS One. 2010;5(4):e9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Strausfeld NJ, Hirth F. Deep homology of arthropod central complex and vertebrate basal ganglia. Science. 2013;340(6129):157-161. [DOI] [PubMed] [Google Scholar]
- 60. Scaplen KM, Talay M, Nunez KM, et al. Circuits that encode and guide alcohol-associated preference. Elife. 2020;9:e48730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ojelade SA, Butts AR, Merrill CB, et al. Dopaminergic learning and arousal circuits mediate opposing effects on alcohol consumption in Drosophila. bioRxiv. 2019:624833. [Google Scholar]
- 62. Dobbs LK, Kaplan AR, Bock R, et al. D1 receptor hypersensitivity in mice with low striatal D2 receptors facilitates select cocaine behaviors. Neuropsychopharmacology. 2019;44(4):805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rothenfluh A, Threlkeld RJ, Bainton RJ, Tsai LT, Lasek AW, Heberlein U. Distinct behavioral responses to ethanol are regulated by alternate RhoGAP18B isoforms. Cell. 2006;127(1):199-211. [DOI] [PubMed] [Google Scholar]
- 64. Parr J, Large A, Wang X, Fowler S, Ratzlaff K, Ruden D. The inebri-actometer: a device for measuring the locomotor activity of Drosophila exposed to ethanol vapor. J Neurosci Methods. 2001;107(1-2):93-99. [DOI] [PubMed] [Google Scholar]
- 65. Petruccelli E, Li Q, Rao Y, Kitamoto T. The unique dopamine/ecdysteroid receptor modulates ethanol-induced sedation in Drosophila. J Neurosci. 2016;36(16):4647-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Petruccelli E, Lark A, Mrkvicka JA, Kitamoto T. Significance of DopEcR, a G-protein coupled dopamine/ecdysteroid receptor, in physiological and behavioral response to stressors. J Neurogenet. 2020;34(1):55-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Evans PD, Bayliss A, Reale V. GPCR-mediated rapid, non-genomic actions of steroids: comparisons between DmDopEcR and GPER1 (GPR30). Gen Comp Endocrinol. 2014;195:157-163. [DOI] [PubMed] [Google Scholar]
- 68. Alexander A, Irving AJ, Harvey J. Emerging roles for the novel estrogen-sensing receptor GPER1 in the CNS. Neuropharmacology. 2017;113(Pt B):652-660. [DOI] [PubMed] [Google Scholar]
- 69. Karam CS, Jones SK, Javitch JA. Come fly with me: an overview of dopamine receptors in Drosophila melanogaster. Basic Clin Pharmacol Toxicol. 2019;126(Suppl 6):56-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Petruccelli E, Feyder M, Ledru N, Jaques Y, Anderson E, Kaun KR. Alcohol activates scabrous-notch to influence associated memories. Neuron. 2018;100(5):1209-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Picetti R, Saiardi A, Samad TA, Bozzi Y, Baik J-H, Borrelli E. Dopamine D2 receptors in signal transduction and behavior. Crit Rev Neurobiol. 1997;11(2-3):121-142. [DOI] [PubMed] [Google Scholar]
- 72. Sapiro AL, Shmueli A, Henry GL, et al. Illuminating spatial A-to-I RNA editing signatures within the Drosophila brain. Proc Natl Acad Sci U S A. 2019;116(6):2318-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6(4):557-618. [DOI] [PubMed] [Google Scholar]
- 74. Brodie MS, Trifunovic RD, Shefner SA. Serotonin potentiates ethanol-induced excitation of ventral tegmental area neurons in brain slices from three different rat strains. J Pharmacol Exp Ther. 1995;273(3):1139-1146. [PubMed] [Google Scholar]
- 75. Vallés AM, White K. Serotonin-containing neurons in Drosophila melanogaster: development and distribution. J Comp Neurol. 1988;268(3):414-428. [DOI] [PubMed] [Google Scholar]
- 76. LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: clinical evidence. Biol Psychiatry. 1994;36(5):326-337. [DOI] [PubMed] [Google Scholar]
- 77. McBride WJ, Li T-K. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12(4):339-369. [DOI] [PubMed] [Google Scholar]
- 78. Sari Y, Johnson VR, Weedman JM. Role of the serotonergic system in alcohol dependence: from animal models to clinics. In: Progress in molecular biology and translational science. Vol 98. Elsevier; 2011:401-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Helander A, Beck O, Jacobsson G, Lowenmo C, Wikstrom T. Time course of ethanol-induced changes in serotonin metabolism. Life Sci. 1993;53(10):847-855. [DOI] [PubMed] [Google Scholar]
- 80. Belmer A, Patkar OL, Pitman KM, Bartlett SE. Serotonergic neuroplasticity in alcohol addiction. Brain Plast. 2016;1(2):177-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ries A-S, Hermanns T, Poeck B, Strauss R. Serotonin modulates a depression-like state in Drosophila responsive to lithium treatment. Nat Commun. 2017;8(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Albin SD, Kaun KR, Knapp J-M, Chung P, Heberlein U, Simpson JH. A subset of serotonergic neurons evokes hunger in adult Drosophila. Curr Biol. 2015;25(18):2435-2440. [DOI] [PubMed] [Google Scholar]
- 83. Pithadia AB, Jain SM. 5-Hydroxytryptamine receptor subtypes and their modulators with therapeutic potentials. J Clin Med Res. 2009;1(2):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Starke K, Gothert M, Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev. 1989;69(3):864-989. [DOI] [PubMed] [Google Scholar]
- 85. Collins DM, Myers RD. Buspirone attenuates volitional alcohol intake in the chronically drinking monkey. Alcohol. 1987;4(1):49-56. [DOI] [PubMed] [Google Scholar]
- 86. Svensson L, Fahlke C, Hard E, Engel JA. Involvement of the serotonergic system in ethanol intake in the rat. Alcohol. 1993;10(3):219-224. [DOI] [PubMed] [Google Scholar]
- 87. Crabbe JC, Phillips TJ, Feller DJ, et al. Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nat Genet. 1996;14(1):98-101. [DOI] [PubMed] [Google Scholar]
- 88. Kelaï S, Renoir T, Chouchana L, et al. Chronic voluntary ethanol intake hypersensitizes 5-HT1A autoreceptors in C57BL/6J mice. J Neurochem. 2008;107(6):1660-1670. [DOI] [PubMed] [Google Scholar]
- 89. Chen F, Lawrence AJ. 5-HT transporter sites and 5-HT1A and 5-HT3 receptors in fawn-hooded rats: a quantitative autoradiography study. Alcohol Clin Exp Res. 2000;24(7):1093-1102. [PubMed] [Google Scholar]
- 90. Nevo I, Langlois X, Laporte AM, et al. Chronic alcoholization alters the expression of 5-HT1A and 5-HT1B receptor subtypes in rat brain. Eur J Pharmacol. 1995;281(3):229-239. [DOI] [PubMed] [Google Scholar]
- 91. Roberts AJ, McArthur RA, Hull EE, Post C, Koob GF. Effects of amperozide, 8-OH-DPAT, and FG 5974 on operant responding for ethanol. Psychopharmacology. 1998;137(1):25-32. [DOI] [PubMed] [Google Scholar]
- 92. Long TA, Kalmus GW, Bjork A, Myers RD. Alcohol intake in high alcohol drinking (HAD) rats is suppressed by FG5865, a novel 5-HT1A agonist/5-HT2 antagonist. Pharmacol Biochem Behav. 1996;53(1):33-40. [DOI] [PubMed] [Google Scholar]
- 93. Tomkins DM, Joharchi N, Tampakeras M, Martin J, Wichmann J, Higgins G. An investigation of the role of 5-HT2C receptors in modifying ethanol self-administration behaviour. Pharmacol Biochem Behav. 2002;71(4):735-744. [DOI] [PubMed] [Google Scholar]
- 94. Kasper J, Tikamdas R, Kim MS, et al. The serotonin-2 receptor modulator,(-)-trans-PAT, decreases voluntary ethanol consumption in rats. Eur J Pharmacol. 2013;718(1-3):98-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Trovero F, David S, Bernard P, Puech A, Bizot JC, Tassin JP. The combination of marketed antagonists of alpha1b-adrenergic and 5-HT2A receptors inhibits behavioral sensitization and preference to alcohol in mice: a promising approach for the treatment of alcohol dependence. PLoS One. 2016;11(3):e0151242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Andrade AL, Abrahao KP, Goeldner FO, Souza-Formigoni ML. Administration of the 5-HT2C receptor antagonist SB-242084 into the nucleus accumbens blocks the expression of ethanol-induced behavioral sensitization in Albino Swiss mice. Neuroscience. 2011;189:178-186. [DOI] [PubMed] [Google Scholar]
- 97. Yoshimoto K, Watanabe Y, Tanaka M, Kimura M. Serotonin2C receptors in the nucleus accumbens are involved in enhanced alcohol-drinking behavior. Eur J Neurosci. 2012;35(8):1368-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shirahase T, Watanabe Y, Tsujimura A, et al. Ethanol preference and drinking behavior are controlled by RNA editing in the nucleus accumbens. Front Behav Neurosci. 2018;12:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Burns CM, Chu H, Rueter SM, et al. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387(6630):303-308. [DOI] [PubMed] [Google Scholar]
- 100. Niswender CM, Copeland SC, Herrick-Davis K, Emeson RB, Sanders-Bush E. RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J Biol Chem. 1999;274(14):9472-9478. [DOI] [PubMed] [Google Scholar]
- 101. Nichols CD. 5-HT2 receptors in Drosophila are expressed in the brain and modulate aspects of circadian behaviors. Dev Neurobiol. 2007;67(6):752-763. [DOI] [PubMed] [Google Scholar]
- 102. Becnel J, Johnson O, Luo J, Nassel DR, Nichols CD. The serotonin 5-HT7Dro receptor is expressed in the brain of Drosophila, and is essential for normal courtship and mating. PLoS One. 2011;6(6):e20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chen J, Zhang Y, Shen P. Protein kinase C deficiency-induced alcohol insensitivity and underlying cellular targets in Drosophila. Neuroscience. 2010;166(1):34-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Xu L, He J, Kaiser A, et al. A single pair of serotonergic neurons counteracts serotonergic inhibition of ethanol attraction in Drosophila. PLoS One. 2016;11(12):e0167518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Schmitt RE, Messick MR, Shell BC, et al. Dietary yeast influences ethanol sedation in Drosophila via serotonergic neuron function. Addict Biol. 2020;25(4):e12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Qi Y-X, Xu G, Gu G-X, et al. A new Drosophila octopamine receptor responds to serotonin. Insect Biochem Mol Biol. 2017;90:61-70. [DOI] [PubMed] [Google Scholar]
- 107. Schneider A, Ruppert M, Hendrich O, et al. Neuronal basis of innate olfactory attraction to ethanol in Drosophila. PLoS One. 2012;7(12):e52007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Belardetti F, Zamponi GW. Calcium channels as therapeutic targets. WIREs Membr Transp Signal. 2012;1(4):433-451. [Google Scholar]
- 109. N’Gouemo P. Voltage-sensitive calcium channels in the brain: relevance to alcohol intoxication and withdrawal. In: The Neuropharmacology of Alcohol. Springer; 2018:263-280. [DOI] [PubMed] [Google Scholar]
- 110. Uhrig S, Vandael D, Marcantoni A, et al. Differential roles for L-type calcium channel subtypes in alcohol dependence. Neuropsychopharmacology. 2017;42(5):1058-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Banger M, Benkert O, Roschke J, et al. Nimodipine in acute alcohol withdrawal state. J Psychiatr Res. 1992;26(2):117-123. [DOI] [PubMed] [Google Scholar]
- 112. Rush CR, Pazzaglia PJ. Pretreatment with isradipine, a calcium-channel blocker, does not attenuate the acute behavioral effects of ethanol in humans. Alcohol Clin Exp Res. 1998;22(2):539-547. [PubMed] [Google Scholar]
- 113. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gruol DL, Parsons KL. Chronic exposure to alcohol during development alters the calcium currents of cultured cerebellar Purkinje neurons. Brain Res. 1994;634(2):283-290. [DOI] [PubMed] [Google Scholar]
- 115. Varodayan FP, de Guglielmo G, Logrip ML, George O, Roberto M. Alcohol dependence disrupts amygdalar L-type voltage-gated calcium channel mechanisms. J Neurosci. 2017;37(17):4593-4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Newton PM, Messing RO. The N-type calcium channel is a novel target for treating alcohol use disorders. Channels. 2009;3(2):77-81. [DOI] [PubMed] [Google Scholar]
- 117. Carden WB, Alexander GM, Friedman DP, et al. Chronic ethanol drinking reduces native T-type calcium current in the thalamus of nonhuman primates. Brain Res. 2006;1089(1):92-100. [DOI] [PubMed] [Google Scholar]
- 118. Simms BA, Zamponi GW. Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron. 2014;82(1):24-45. [DOI] [PubMed] [Google Scholar]
- 119. Lembke KM, Law AD, Ahrar J, Morton DB. Deletion of a specific exon in the voltage-gated calcium channel gene cacophony disrupts locomotion in Drosophila larvae. J Exp Biol. 2019;222(1):191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Brusich DJ, Spring AM, James TD, Yeates CJ, Helms TH, Frank CA. Drosophila CaV2 channels harboring human migraine mutations cause synapse hyperexcitability that can be suppressed by inhibition of a Ca2+ store release pathway. PLoS Genet. 2018;14(8):e1007577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Saras A, Tanouye MA. Mutations of the calcium channel gene cacophony suppress seizures in Drosophila. PLoS Genet. 2016;12(1):e1005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Xu S, Pany S, Benny K, et al. Ethanol regulates presynaptic activity and sedation through presynaptic Unc13 proteins in Drosophila. Eneuro. 2018;5(3):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rizo J, Xu J. The synaptic vesicle release machinery. Annu Rev Biophys. 2015;44:339-367. [DOI] [PubMed] [Google Scholar]
- 124. Heinrich L, Ryglewski S. Different functions of two putative Drosophila alpha2delta subunits in the same identified motoneurons. Sci Rep. 2020;10(1):13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tariq A, Jantsch MF. Transcript diversification in the nervous system: a to I RNA editing in CNS function and disease development. Front Neurosci. 2012;6:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Huang H, Tan BZ, Shen Y, et al. RNA editing of the IQ domain in Ca(v)1.3 channels modulates their Ca(2)(+)-dependent inactivation. Neuron. 2012;73(2):304-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bukiya AN, Kuntamallappanavar G, Edwards J, Singh AK, Shivakumar B, Dopico AM. An alcohol-sensing site in the calcium- and voltage-gated, large conductance potassium (BK) channel. Proc Natl Acad Sci U S A. 2014;111(25):9313-9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bodhinathan K, Slesinger PA. Alcohol modulation of G-protein-gated inwardly rectifying potassium channels: from binding to therapeutics. Front Physiol. 2014;5:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Federici M, Nistico R, Giustizieri M, Bernardi G, Mercuri NB. Ethanol enhances GABAB-mediated inhibitory postsynaptic transmission on rat midbrain dopaminergic neurons by facilitating GIRK currents. Eur J Neurosci. 2009;29(7):1369-1377. [DOI] [PubMed] [Google Scholar]
- 130. Mayfield J, Blednov YA, Harris RA. Behavioral and genetic evidence for GIRK channels in the CNS: role in physiology, pathophysiology, and drug addiction. Int Rev Neurobiol. 2015;123:279-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. You C, Savarese A, Vandegrift BJ, et al. Ethanol acts on KCNK13 potassium channels in the ventral tegmental area to increase firing rate and modulate binge-like drinking. Neuropharmacology. 2019;144:29-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Davies AG, Pierce-Shimomura JT, Kim H, et al. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003;115(6):655-666. [DOI] [PubMed] [Google Scholar]
- 133. Ghezzi A, Pohl JB, Wang Y, Atkinson NS. BK channels play a counter-adaptive role in drug tolerance and dependence. Proc Natl Acad Sci U S A. 2010;107(37):16360-16365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Cowmeadow RB, Krishnan HR, Ghezzi A, Al’Hasan YM, Wang YZ, Atkinson NS. Ethanol tolerance caused by slowpoke induction in Drosophila. Alcohol Clin Exp Res. 2006;30(5):745-753. [DOI] [PubMed] [Google Scholar]
- 135. Li X, Ghezzi A, Pohl JB, Bohm AY, Atkinson NS. A DNA element regulates drug tolerance and withdrawal in Drosophila. PLoS One. 2013;8(9):e75549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Cavaliere S, Gillespie JM, Hodge JJ. KCNQ channels show conserved ethanol block and function in ethanol behaviour. PLoS One. 2012;7(11):e50279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Pfeiffer BD, Ngo T-TB, Hibbard KL, et al. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186(2):735-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Germani F, Bergantinos C, Johnston LA. Mosaic analysis in Drosophila. Genetics. 2018;208(2):473-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Venken KJ, Schulze KL, Haelterman NA, et al. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat Methods. 2011;8(9):737-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Fisher YE, Yang HH, Isaacman-Beck J, Xie M, Gohl DM, Clandinin TR. FlpStop, a tool for conditional gene control in Drosophila. Elife. 2017;6:e22279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Deng B, Li Q, Liu X, et al. Chemoconnectomics: mapping chemical transmission in Drosophila. Neuron. 2019;101(5):876-893.e874. [DOI] [PubMed] [Google Scholar]
- 142. Kondo S, Takahashi T, Yamagata N, et al. Neurochemical organization of the Drosophila brain visualized by endogenously tagged neurotransmitter receptors. Cell Rep. 2020;30(1):284-297.e285. [DOI] [PubMed] [Google Scholar]
- 143. Koles K, Yeh AR, Rodal AA. Tissue-specific tagging of endogenous loci in Drosophila melanogaster. Biol Open. 2016;5(1):83-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. He L, Binari R, Huang J, Falo-Sanjuan J, Perrimon N. In vivo study of gene expression with an enhanced dual-color fluorescent transcriptional timer. Elife. 2019;8:e46181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron. 2012;73(5):862-885. [DOI] [PubMed] [Google Scholar]
- 146. Akerboom J, Carreras Calderón N, Tian L, et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front Mol Neurosci. 2013;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Abdelfattah AS, Kawashima T, Singh A, et al. Bright and photostable chemigenetic indicators for extended in vivo voltage imaging. Science. 2019;365(6454):699-704. [DOI] [PubMed] [Google Scholar]
- 148. Shin M, Copeland JM, Venton BJ. Drosophila as a model system for neurotransmitter measurements. ACS Chem Neurosci. 2018;9(8):1872-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sun F, Zeng J, Jing M, et al. A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell. 2018;174(2):481-496.e419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Jing M, Li Y, Zeng J, et al. An optimized acetylcholine sensor for monitoring in vivo cholinergic activity. Nat Methods. 2020;17(11):1139-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]