Abstract
Background: Patients with hematologic malignancies (HM) often receive aggressive care at the end of life (EOL). Early palliative care (PC) has been shown to improve EOL care outcomes, but its benefits are less established in HM than in solid tumors.
Objectives: We sought to describe the use of billed PC services among Medicare beneficiaries with HM. We hypothesized that receipt of early PC services (rendered >30 days before death) may be associated with less aggressive EOL care.
Design: Retrospective cohort analysis
Setting/Subjects: Using the Surveillance, Epidemiology, and End Results-Medicare registry, we studied patients with leukemia, lymphoma, myeloma, myelodysplastic syndrome, or myeloproliferative neoplasm who died between 2001 and 2015.
Measurements: We described trends in the use of PC services and evaluated the association between early PC services and metrics of EOL care aggressiveness.
Results: Among 139,191 decedents, the proportion receiving PC services increased from 0.4% in 2001 to 13.3% in 2015. Median time from first encounter to death was 10 days and 84.3% of encounters occurred during hospitalizations. In patients who survived >30 days from diagnosis (N = 120,741), the use of early PC services was more frequent in acute leukemia, women, and black patients, among other characteristics. Early PC services were associated with increased hospice use and decreased health care utilization at the EOL.
Conclusion: Among patients with HM, there was an upward trend in PC services, and early PC services were associated with less aggressive EOL care. Our results support the need for prospective trials of early PC in HM.
Keywords: end-of-life care; hematologic malignancy; Medicare; palliative care; population; Surveillance, Epidemiology, and End Results
Introduction
Despite advances in systemic therapy, many patients with hematologic malignancies (HM) eventually die of their disease.1 Advanced age is a major risk factor for disease-related mortality in HM.2 Patients with HM can experience high physical and psychological symptom burden, decline in functional status and quality of life, and more aggressive end-of-life (EOL) care than patients with solid organ cancers.3 These findings highlight the need for better EOL care in patients with HM.
Palliative care (PC) is a medical specialty well-suited for providing high-quality EOL care. PC specialists provide complex symptom management, effective communication, spiritual, emotional, and social assessments, family-centered care, and focus on quality of life.3 PC services provided earlier in the disease course reduce the aggressiveness of EOL care for patients with advanced solid tumor malignancies.4 Such benefits of early PC, however, have not been thoroughly studied in HM.5
Several population-level studies in patients with solid tumors have shown that PC was associated with less aggressive EOL care, including increased hospice use and decreased health care utilization.6–8 In contrast, studies measuring similar associations in HM were either qualitative or were limited by selective single-institution settings and patient populations.9 To our knowledge, no population-level studies have examined the association between PC services and the aggressiveness of EOL care among patients with HM.
Our study had two objectives. First, we sought to describe the use of billed PC services among Medicare beneficiaries with HM in terms of frequency, trends over time, and characteristics of the associated encounters. Second, we hypothesized that receipt of early PC services (>30 days before death) may be associated with less aggressive EOL care, increased hospice use, and decreased health care utilization, as suggested by research in solid tumors.4,8
We defined “early” PC services as >30 days before death since many of the outcome measures involve increased health care utilization within the last 30 days of life. Although some patients with HM may experience a rapid decline, when PC services, such as advance care planning, are provided well before such decline, it is more likely that these services will have an observable impact on the aggressiveness of EOL care.
Methods
Data
We identified the study cohort using the National Cancer Institute's (NCI) Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. SEER is a population-based cancer registry that collects data from individual cancer registries representing ∼34% of the United States population.10 The sociodemographic profile in the registry is representative of the entire United States population for participating states. The SEER-Medicare database successfully links SEER records to corresponding Medicare enrollment and claims data for >95% of individuals aged 65 or older at diagnosis.
Patient population
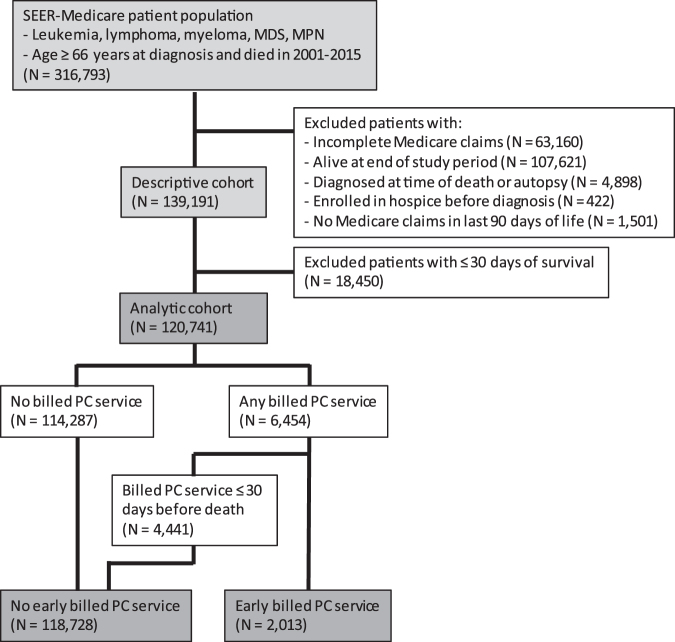
This study included Medicare beneficiaries aged 66 years or older diagnosed with leukemia, lymphoma, myeloma, myelodysplastic syndrome (MDS), or myeloproliferative neoplasm (MPN) who died between 2001 and 2015 (Fig. 1). The HM diagnoses were determined using World Health Organization's histology codes. Consistent with prior studies, our age criterion allowed for evaluation of preexisting comorbidities during one year before death.11,12 We included patients regardless of the recorded cause of death, because of known inaccuracies in the cause of death attributions in registries.12
FIG. 1.
Cohort selection. SEER, Surveillance, Epidemiology, and End Results; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; PC, palliative care. Color image is available online.
Patients who were alive at the end of the study period, patients diagnosed on autopsy or at time of death (<2 days of survival), patients who were enrolled in hospice before diagnosis, and patients without Medicare claims in the last 90 days of life were excluded. In addition, we excluded patients who did not have continuous Medicare fee-for-service (parts A and B) enrollment from 12 months before diagnosis to the time of death, or if they were enrolled in Medicare Advantage (part C) from 396 to 30 days before death, since these patients had incomplete Medicare claims data. We used the timeframe of 396 to 30 days before death to calculate the NCI-modified Charlson comorbidity index.13
Identification of billed PC services
We identified billed PC services using the International Classification of Diseases (ICD), ninth revision (ICD-9) code V66.7 and the ICD tenth revision (ICD-10) code Z51.5 (“Encounter for palliative care”) included in claims from inpatient or outpatient physician encounters (within the Medicare Carrier Claims and Hospital Outpatient files).14 In a single-center validation study, the v66.7 code demonstrated high specificity (although low sensitivity) to true specialty PC services.15 In case of multiple PC encounters, we used the first encounter as an indicator of initiation of PC services.
We categorized the location of PC services as office, acute care hospital, emergency department (ED), skilled nursing facility, or home according to the indicator included in associated claims. The medical specialties and types of health care practitioners performing PC services were also determined from claims. Rare claims with PC encounter codes from clinicians with unrelated specialties (e.g., radiology, surgery, dermatology) were excluded from the analysis.
“Early” and “late” PC services were those rendered >30 days and ≤30 days before death, respectively. “Any PC services” included all PC encounter claims regardless of the timing. Therefore, beneficiaries with “no early PC services” included both those without any PC services and those with “late PC services” (Fig. 1).
Outcomes
We examined the association between receipt of early billed PC services and the following outcomes based on National Quality Forum EOL care quality metrics: receipt of chemotherapy in the last 14 days of life, >1 ED visit in the last 30 days of life, use of intensive care unit (ICU) services in last 30 days of life, absence of hospice enrollment, and enrollment in hospice for <3 days before death.16 Additional endpoints included the following: >1 hospital admission in the last 30 days of life, death during hospitalization, and number of days on hospice.
Covariates
Covariates for analysis were selected a priori based on clinical expertise of the researchers, regardless of statistical significance. Histologic subtypes of HM were identified by morphology codes according to the World Health Organization designation, and classified as acute leukemia, chronic or unspecified leukemia, aggressive lymphoma, indolent or unspecified lymphoma, plasma cell myeloma, MDS, or MPN.17 Sociodemographic data included age, sex, race/ethnicity (as recorded by SEER), marital status, and Medicaid enrollment status (indicator of disability or poverty). To account for baseline health status, we estimated the NCI-modified Charlson comorbidity index using inpatient and outpatient claims from 396 to 30 days before death.13 This analytic timeframe was selected to allow uniform ascertainment of covariates for all decedents, regardless of their exposures or outcomes, and to account for a highly variable survival in HM under study, which ranges from weeks to many years, without introducing immortal time bias. In addition, presence of moderate-severe renal disease and performance status was estimated using a validated measure of self-reported functional status in the same time window.18 To differentiate beneficiaries who received supportive care only for the management of their HM from those who received active therapy, we also identified administration of chemotherapy within the same ascertainment timeframe, using Medicare claims as previously described.12,19
Statistical analysis
We described characteristics of patients and billed PC services using proportions for categorical variables, and medians with interquartile ranges for continuous variables. The “descriptive cohort” in this study comprised patients who met both inclusion and exclusion criteria, regardless of survival time. Within this cohort, we graphically represented trends in the use of “any billed PC services” and “early billed PC services” between 2001 and 2015.
The “analytic cohort” comprised patients who survived >30 days from diagnosis, and was used for further analyses to avoid mortality bias (excluded patients with shorter survival times would not be able to receive early PC services according to our definition). The association between covariates and receipt of early PC services was examined in a mixed-effects multivariable logistic regression that included the indicator of calendar year of death, using a random intercept for the Health Services Area to account for local variation in billing intensity, and reporting adjusted odds ratios. The association between receipt of early PC services and EOL care outcomes was examined in analogous mixed-effects generalized linear models for binary outcomes (robust Poisson) or count outcomes (negative binomial), reporting adjusted relative risk or duration, respectively. All model estimates are provided with 95% confidence intervals. However, considering a very large dataset, we considered both statistical and clinical significance (i.e., relative and absolute effect sizes) when interpreting the results. Statistical analysis was conducted using Stata/MP 15.1 (StataCorp LLC, College Station, TX).
The Institutional Review Board of Rhode Island Hospital deemed this study exempt from review. This study did not require patient consent because patient information was deidentified in administrative data.
Results
Of 139,191 decedents with HM (descriptive cohort), 7270 (5.2%) received any billed PC services and 2013 (1.7%) received early billed PC services. The proportion of beneficiaries receiving any billed PC services increased from 0.4% in 2001 to 13.3% in 2015 (Fig. 2A). Median time from first PC encounter to death was 10 days. Of all PC encounters, 84.3% occurred during hospital admissions and 5.9% during office visits. The proportion of patients with early billed PC services increased from 0.2% in 2001 to 4.3% in 2015. Median time from the first early PC encounter to death was 82 days. Of these encounters, 74.9% occurred during hospital admissions, and 14.8% during office visits. The proportion of billed PC services (any or early) were similar among patients with HM of different histologies (Fig. 2B).
FIG. 2.
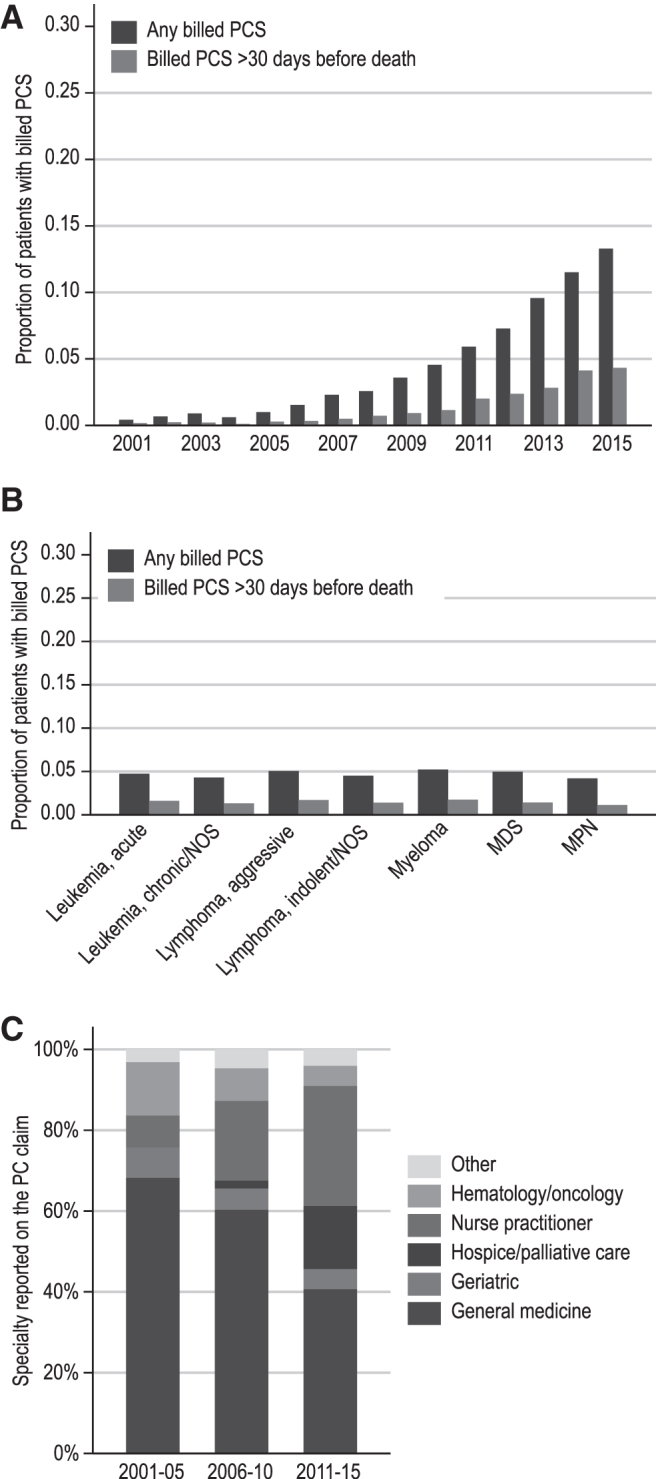
(A) Trends in the proportion of patients receiving billed PC services by year of death. (B) Proportion of patients receiving billed PC services based on histology type. (C) Trends in specialties reported on PC encounter claims. The Y-axes of (A, B) are truncated at 30% for clarity. PCS, palliative care services; NOS, not otherwise specified. Color image is available online.
PC services were billed most commonly by health care practitioners identified as general medicine (internal and family medicine) physicians (45.6%), nurse practitioners (NPs) (27.0%), and hospice and palliative medicine (HPM) physicians (12.3%; Fig. 2C). The overall volume of billed PC services increased over time across all clinician-types, whereas the proportion of claims from HPM physicians and NPs increased and the proportion of claims from other physicians decreased.
Further analysis was conducted on the 120,741 (86.7%) beneficiaries who survived >30 days from the HM diagnosis (analytic cohort). Within this group, we compared characteristics of patients who received early billed PC services (N = 2013) to those with no early billed PC services (N = 118,728; Table 1). In a multivariable model, early billed PC services were rendered significantly more frequently to patients with acute leukemia (compared with other HM subtypes), women, black patients, patients with higher comorbidity indices or poor performance statuses, patients with end-stage renal disease, and patients who received chemotherapy at any time after HM diagnosis. Early billed PC services were less frequent among patients aged 80 and older compared with younger individuals.
Table 1.
Characteristics of Patients Who Did or Did Not Receive Early Billed Palliative Care Services, and Association of Early Billed Palliative Care Services with These Characteristics in Multivariable Models (Cohort Limited to Survivors of >30 Days)
| Characteristic | Total patients | Palliative care |
Multivariable modela |
||
|---|---|---|---|---|---|
| No early billed PC services | Early billed PC services | OR (95% CI) | p-Value | ||
| N | 120,741 | 118,728 | 2013 | ||
| Histology group, N (%) | |||||
| Leukemia, acute | 11,241 (9.3) | 11,025 (9.3) | 216 (10.7) | Reference | |
| Leukemia, chronic/NOS | 17,424 (14.4) | 17,164 (14.5) | 260 (12.9) | 0.59 (0.49–0.71) | <0.001 |
| Lymphoma, aggressive | 22,110 (18.3) | 21,721 (18.3) | 389 (19.3) | 0.75 (0.63–0.90) | 0.001 |
| Lymphoma, indolent/NOS | 24,066 (19.9) | 23,693 (20.0) | 373 (18.5) | 0.62 (0.52–0.74) | <0.001 |
| Myeloma | 18,674 (15.5) | 18,324 (15.4) | 350 (17.4) | 0.71 (0.59–0.85) | <0.001 |
| Myelodysplastic syndrome | 21,162 (17.5) | 20,814 (17.5) | 348 (17.3) | 0.62 (0.52–0.74) | <0.001 |
| Myeloproliferative neoplasm | 6064 (5.0) | 5987 (5.0) | 77 (3.8) | 0.46 (0.35–0.61) | <0.001 |
| Age group, at death, N (%) | |||||
| 65 to <70 years | 6429 (5.3) | 6309 (5.3) | 120 (6.0) | Reference | |
| 70 to <75 years | 17,782 (14.7) | 17,489 (14.7) | 293 (14.6) | 0.87 (0.70–1.09) | 0.23 |
| 75 to <80 years | 25,137 (20.8) | 24,709 (20.8) | 428 (21.3) | 0.87 (0.70–1.07) | 0.19 |
| 80 to <85 years | 29,376 (24.3) | 28,961 (24.4) | 415 (20.6) | 0.68 (0.55–0.84) | <0.001 |
| 85–90 years | 25,242 (20.9) | 24,801 (20.9) | 441 (21.9) | 0.78 (0.63–0.97) | 0.03 |
| ≥90 years | 16,775 (13.9) | 16,459 (13.9) | 316 (15.7) | 0.73 (0.58–0.92) | 0.01 |
| Sex, N (%) | |||||
| Men | 65,077 (53.9) | 64,044 (53.9) | 1033 (51.3) | Reference | |
| Women | 55,664 (46.1) | 54,684 (46.1) | 980 (48.7) | 1.11 (1.01–1.23) | 0.03 |
| Race/ethnicity, N (%) | |||||
| White | 102,017 (84.5) | 100,400 (84.6) | 1617 (80.3) | Reference | |
| Hispanic | 5938 (4.9) | 5846 (4.9) | 92 (4.6) | 0.92 (0.74–1.16) | 0.50 |
| Black | 8146 (6.7) | 7949 (6.7) | 197 (9.8) | 1.45 (1.22–1.71) | <0.001 |
| American Indian/Alaska Native | 355 (0.3) | 349 (0.3) | cc | 0.66 (0.28–1.54) | 0.34 |
| Asian/other | 4285 (3.5) | 4184 (3.5) | 101 (5.0) | 0.99 (0.78–1.25) | 0.93 |
| Marital status, N (%) | |||||
| Married | 61,058 (50.6) | 60,073 (50.6) | 985 (48.9) | Reference | |
| Widowed | 35,340 (29.3) | 34,781 (29.3) | 559 (27.8) | 0.98 (0.87–1.10) | 0.70 |
| Unmarried/domestic partner | 15,588 (12.9) | 15,277 (12.9) | 311 (15.4) | 1.01 (0.88–1.16) | 0.83 |
| Unknown | 8755 (7.3) | 8597 (7.2) | 158 (7.8) | 1.01 (0.85–1.21) | 0.89 |
| Medicaid enrollment status, N (%) | 20,456 (16.9) | 20,060 (16.9) | 397 (19.7) | 0.90 (0.79–1.02) | 0.11 |
| NCI comorbidity index,b median (IQR) | 2.9 (1.2–4.9) | 2.9 (1.3–4.8) | 3.7 (1.7–6.2) | 1.09 (1.07–1.11) | <0.001 |
| Poor performance status,bN (%) | 42,993 (35.6) | 41,880 (35.3) | 1113 (55.3) | 2.10 (1.90–2.32) | <0.001 |
| Moderate-severe renal disease,bN (%) | 34,591 (28.6) | 33,706 (28.4) | 885 (44.0) | 1.15 (1.03–1.28) | 0.01 |
| Receipt of chemotherapy,bN (%) | 43,639 (36.1) | 42,792 (36) | 847 (42.1) | 1.39 (1.26–1.54) | <0.001 |
Multivariable model adjusting for patient and disease characteristics.
Ascertained within the timeframe of 396 to 30 days before death.
Category combined with “Asian/other” due to cell size being <10.
PC, palliative care; OR, odds ratio; NCI, National Cancer Institute; CI, confidence interval; IQR, interquartile range; NOS, not otherwise specified.
We observed significant differences in the EOL care outcomes among patients who did or did not receive early billed PC services (Table 2). Recipients of early billed PC services had a relative 20% higher rate of using hospice services before death, 50% longer average hospice length of stay (if enrolled), and 15% higher chance of using hospice services for >3 days before death. Furthermore, receipt of early billed PC services was associated with a statistically significant and clinically meaningful decrease in aggressive care at the EOL, as measured by each of the following outcomes: >1 ED visit or hospitalization, ICU admission, death in the acute hospital setting, or chemotherapy use in the last 14 days of life.
Table 2.
Claims-Based End-Of-Life Care Metrics among Medicare Beneficiaries Who Did or Did Not Receive Early Billed Palliative Care Services, and Association of Early Billed Palliative Care Services with These Metrics in Multivariable Models
| EOL care metric | No early billed PC services | Early billed PC services | Adjusted RR or durationa | 95% CIa |
|---|---|---|---|---|
| N | 118,728 | 2013 | ||
| Death on hospice, % | 47 | 64 | 1.20 | 1.15–1.24 |
| Days on hospice, median | 9 | 22 | 1.50 | 1.41–1.59 |
| >3 days on hospice, % | 73 | 84 | 1.15 | 1.12–1.18 |
| >1 ED visit, last 30 days, % | 13 | 11 | 0.77 | 0.69–0.86 |
| >1 hospitalization, last 30 days, % | 14 | 9 | 0.71 | 0.61–0.83 |
| ICU admission, last 30 days, % | 37 | 28 | 0.74 | 0.69–0.80 |
| Inpatient death, % | 38 | 27 | 0.78 | 0.73–0.83 |
| Chemotherapy use, last 14 days, % | 7 | 4 | 0.59 | 0.47–0.74 |
Coefficient for early billed PC services from a multivariable model adjusting for hematologic malignancy histology, patients' age, sex, race, marital status, Medicaid coinsurance, comorbidities, performance status, and year of death.
All p values were <0.0001.
EOL, end-of-life; RR, relative risk; ED, emergency department; ICU, intensive care unit.
Discussion
To our knowledge, this is the first population-level study describing the use of billed PC services in patients with HM. We found that the use of PC services was overall low, regardless of specific histology, although it exponentially increased over time. Most PC services were initiated late in the disease course. The low proportion of patients with recorded PC services and increase in billing for such services are consistent with observations from Medicare beneficiaries with solid tumors.7,11,20–22 The upward trend in billed PC services may be related to rapid change in practice patterns after landmark clinical trials showed benefits of early integrated PC, as well as the recognition of HPM as a separate board-certified subspecialty in 2006.4,23–25 Better recognition of PC as a component of supportive care for cancer patients, rather than terminal care in the last days of life may be contributing to these changes.26 Alternatively, the observed trends may simply reflect changes in billing practices. The increased proportion of HPM physicians and NPs billing for PC services over time may also reflect the expansion of PC programs using dedicated NPs and board-certified HPM physicians.27,28 However, hospitals often use the ICD-9/ICD-10 billing codes to reflect any inpatient clinician's documentation of EOL, terminal, comfort, and/or hospice care.7 Therefore, the increase in NP claims may also be related to general workforce changes in acute care settings.28
In our study, receipt of early billed PC services among older patients with HM was associated with less aggressive EOL care, increased hospice use, and decreased health care utilization. These findings support the American Society of Clinical Oncology's provisional opinion statement and subsequent clinical guideline, which endorse early integration of PC services into standard oncologic care.4,29,30 Our observations mirror findings from a prior study of Medicare beneficiaries with solid organ cancers, which suggested that earlier PC encounters were associated with reductions in terminal chemotherapy use and an increase in duration of hospice length of stay.8 Within the HM space, Selvaggi et al. found that implementation of a PC service on the bone marrow transplant unit was associated with an increase in hospice referrals.9 Although our observations suggest benefits of early integration of PC in patients with HM and associated EOL care outcomes, more research is needed in this area, particularly prospective clinical trials.
Our study differs in design compared with prior studies identifying PC services in Medicare claims. Rather than study EOL care outcomes for recipients of any billed PC services, we focused on receipt of early PC services. When PC services are provided late and close to the EOL, they are often less influential on the aggressiveness of EOL care. Consistent with these observations, the EOL care outcomes were seemingly worse for patients receiving “late” PC services than for those who died without any PC services (Supplementary Table S1 and Supplementary Fig. S1). These findings may be because patients already receiving aggressive care are referred for specialty PC with the goal of comfort care upon rapid terminal deterioration. In other words, late PC services occur in conjunction with more aggressive EOL care, but not as the cause for these outcomes. Prior literature suggests prognostic uncertainty in HM as one of the reasons for late PC referrals.5,31 One way to overcome this barrier is to implement prognosis-independent early integration of PC with standard hematologic care, as currently piloted in our and other institutions.5 We note that the minimum timeframe to consider “early” PC services in HM is uncertain and may differ than in solid tumors.32,33 Further research is also needed to explore the minimum timeframe necessary to achieve meaningful benefits for PC intervention.
Another important difference is that prior studies identified inpatient PC services using codes from the inpatient Medicare Provider Analysis and Review files. Because these files provide only summary ICD codes associated with the entire hospital admission, the exact dates of PC services are not available. Some researchers choose the last day of hospitalization to represent the date of PC services.8 This approach is not practical in HM, as patients are often hospitalized for >30 days due to infection or complications of chemotherapy. In contrast, we used claims collected from physician, physician assistant, and NP billing, which include the exact day of service. The trade-off of our approach was potential lower sensitivity to detect all PC services, as the relevant ICD-9/ICD-10 codes may not be consistently recorded by practitioners on their claims.
Limitations of our study include its retrospective nature, which allows us to examine association but not causation. Another limitation is the reliance on billing codes, which were originally used to document “comfort care,” “terminal care,” “end-of-life care,” or “hospice care,” and thus reflect disease severity for reimbursement purposes.7 This care is not differentiated in billing claims from true specialty PC, which is now recognized as expert symptom management, in-depth conversations about goals of care, advance care planning, and psychosocial support. Therefore, we could not discern from claims which components of PC were being provided. To address this limitation, we defined our exposure of interest as “billed PC services” for a more accurate representation of the content of these codes. Other studies have also used the ICD codes as a surrogate of specialty PC services.6–8,11,20,22,34,35 Ruck et al. state that “the V66.7 code [is] a blunt instrument (…), but one that still provides basic information about PC utilization”.7 Our decision to examine early PC services (rendered >30 days before death) decreases the chance that these services represent documentation of “comfort care” or “terminal care,” rather than true specialty PC services, although we acknowledge that receipt of early PC is likely underreported in claims to begin with.
EOL care outcomes data, such as hospice use and health care utilization, are important quantitative measurements of the aggressiveness of EOL care. However, these metrics originate from clinicians' and administrators' points of view. Further research should focus on whether or not these metrics matter to patients and their families. An analysis of patient- and caregiver-reported outcomes would serve to better elucidate what EOL care quality means to patients with HM and to evaluate how specialty PC influences this care.
Conclusion
In conclusion, the use of billed PC services among Medicare beneficiaries with HM has increased in recent years, but most encounters still occur within days of death and in the inpatient setting. Early receipt of PC services was associated with less aggressive EOL care, increased hospice use, and decreased health care utilization suggesting benefits similar to those observed in solid tumors. Since causation is uncertain in retrospective data, our results strongly support prospective trials of early PC for patients with HM.
Supplementary Material
Acknowledgments
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U58DP003862–01 awarded to the California Department of Public Health. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc., and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Presented in part at the 61st American Society of Hematology Annual Meeting and Exposition, Orlando, FL, December 7–10, 2019, and at the 2019 American Society of Clinical Oncology Supportive Care in Oncology Symposium, San Francisco, CA, October 2019.
Disclaimer
The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Funding Information
This work was supported by the American Cancer Society (grant number 128608-RSGI-15-211-01-CPHPS).
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Morikawa M, Shirai Y, Ochiai R, Miyagawa K: Barriers to the collaboration between hematologists and palliative care teams on relapse or refractory leukemia and malignant lymphoma patients' care: A qualitative study. Am J Hosp Palliat Care 2016;33:977–984 [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Institute: SEER cancer statistics review 1975–2015. https://seer.cancer.gov/csr/1975_2015/ (Last accessed April15, 2020)
- 3. LeBlanc TW, El-Jawahri A: When and why should patients with hematologic malignancies see a palliative care specialist? Hematol Am Soc Hematol Educ Program 2015;2015:471–478 [DOI] [PubMed] [Google Scholar]
- 4. Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733–742 [DOI] [PubMed] [Google Scholar]
- 5. LeBlanc TW, Roeland EJ, El-Jawahri A: Early palliative care for patients with hematologic malignancies: Is it really so difficult to achieve? Curr Hematol Malig Rep 2017;12:300–308 [DOI] [PubMed] [Google Scholar]
- 6. Jang RW, Krzyzanowska MK, Zimmermann C, et al. : Palliative care and the aggressiveness of end-of-life care in patients with advanced pancreatic cancer. J Natl Cancer Inst 2015;107:dju424. [DOI] [PubMed] [Google Scholar]
- 7. Ruck JM, Canner JK, Smith TJ, Johnston FM: Use of inpatient palliative care by type of malignancy. J Palliat Med 2018;21:1300–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Triplett DP, LeBrett WG, Bryant AK, et al. : Effect of palliative care on aggressiveness of end-of-life care among patients with advanced cancer. J Oncol Pract 2017;13:e760–e769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Selvaggi KJ, Vick JB, Jessell SA, et al. : Bridging the gap: A palliative care consultation service in a hematological malignancy-bone marrow transplant unit. J Community Support Oncol 2014;12:50–55 [DOI] [PubMed] [Google Scholar]
- 10. NCI SEER Program Overview 2018. https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf (Last accessed April15, 2020)
- 11. Roeland EJ, Triplett DP, Matsuno RK, et al. : Patterns of palliative care consultation among elderly patients with cancer. J Natl Compr Canc Netw 2016;14:439–445 [DOI] [PubMed] [Google Scholar]
- 12. LeBlanc TW, Egan PC, Olszewski AJ: Transfusion dependence, use of hospice services, and quality of end-of-life care in leukemia. Blood 2018;132:717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klabunde CN, Potosky AL, Legler JM, Warren JL: Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258–1267 [DOI] [PubMed] [Google Scholar]
- 14. Capello CF, Meier DE, Cassel CK: Payment code for hospital-based palliative care: Help or hindrance? J Palliat Med 1998;1:155–163 [DOI] [PubMed] [Google Scholar]
- 15. Hua M, Li G, Clancy C, et al. : Validation of the V66.7 code for palliative care consultation in a single academic medical center. J Palliat Med 2017;20:372–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Quality Forum (NQF)-Endorsed Palliative Care and End-of-Life Care 2015–2016: Technical Report. https://qualityforum.org/Palliative_and_End-of-Life_Care_Project_2015–2016.aspx (Last accessed April15, 2020)
- 17. Swerdlow SH CE, Harris NL, et al. : WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed. Lyon, France: IARC Press, 2008 [Google Scholar]
- 18. Davidoff AJ, Gardner LD, Zuckerman IH, et al. : Validation of disability status, a claims-based measure of functional status for cancer treatment and outcomes studies. Med Care 2014;52:500–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olszewski AJ, Butera JN, Reagan JL, Castillo JJ: Outcomes of bendamustine- or cyclophosphamide-based first-line chemotherapy in older patients with indolent B-cell lymphoma. Am J Hematol 2019. [Epub ahead of print]; DOI: 10.1002/ajh.25707 [DOI] [PubMed] [Google Scholar]
- 20. Bhulani N, Gupta A, Gao A, et al. : Palliative care and end-of-life health care utilization in elderly patients with pancreatic cancer. J Gastrointest Oncol 2018;9:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gani F, Enumah ZO, Conca-Cheng AM, et al. : Palliative care utilization among patients admitted for gastrointestinal and thoracic cancers. J Palliat Med 2018;21:428–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mulvey CL, Smith TJ, Gourin CG: Use of inpatient palliative care services in patients with metastatic incurable head and neck cancer. Head Neck 2016;38:355–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. American Academy of Hospice and Palliative Medicine: ABMS Subsepcialty Certificaion in Hospice and Palliative Medicine. http://aahpm.org/certification/subspecialty-certification (Last accessed April15, 2020)
- 24. El-Jawahri A, LeBlanc T, VanDusen H, et al. : Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: A randomized clinical trial. JAMA 2016;316:2094–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Temel JS, Greer JA, El-Jawahri A, et al. : Effects of early integrated palliative care in patients with lung and GI cancer: A randomized clinical trial. J Clin Oncol 2017;35:834–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fadul N, Elsayem A, Palmer JL, et al. : Supportive versus palliative care: What's in a name? A survey of medical oncologists and midlevel providers at a comprehensive cancer center. Cancer 2009;115:2013–2021 [DOI] [PubMed] [Google Scholar]
- 27. Heinle R, McNulty J, Hebert RS: Nurse practitioners and the growth of palliative medicine. Am J Hosp Palliat Care 2014;31:287–291 [DOI] [PubMed] [Google Scholar]
- 28. Bryant SE: Filling the gaps: Preparing nurse practitioners for hospitalist practice. J Am Assoc Nurse Pract 2018;30:4–9 [DOI] [PubMed] [Google Scholar]
- 29. Ferrell BR, Temel JS, Temin S, et al. : Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2017;35:96–112 [DOI] [PubMed] [Google Scholar]
- 30. Smith TJ, Temin S, Alesi ER, et al. : American Society of Clinical Oncology provisional clinical opinion: The integration of palliative care into standard oncology care. J Clin Oncol 2012;30:880–887 [DOI] [PubMed] [Google Scholar]
- 31. McCaughan D, Roman E, Smith AG, et al. : Palliative care specialists' perceptions concerning referral of haematology patients to their services: Findings from a qualitative study. BMC Palliat Care 2018;17:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. El-Jawahri A, Nelson AM, Gray TF, et al. : Palliative and end-of-life care for patients with hematologic malignancies. J Clin Oncol 2020;38:944–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Odejide OO, Cronin AM, Condron N, et al. : Timeliness of end-of-life discussions for blood cancers: A national survey of hematologic oncologists. JAMA Intern Med 2016;176:263–265 [DOI] [PubMed] [Google Scholar]
- 34. Lilley EJ, Lee KC, Scott JW, et al. The impact of inpatient palliative care on end-of-life care among older trauma patients who die after hospital discharge. J Trauma Acute Care Surg 2018;85:992–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Singh T, Peters SR, Tirschwell DL, Creutzfeldt CJ: Palliative care for hospitalized patients with stroke: Results from the 2010 to 2012 national inpatient sample. Stroke 2017;48:2534–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.