Significance
Although PCa depends on AR signaling, the effect of androgen deprivation therapy is temporary and eventually leads to lethal castration-resistant prostate cancer (CRPC). The present study revealed that finasteride, abiraterone, and enzalutamide all increased EGFR nuclear translocation and increased proliferation. In men with BPH, treatment with finasteride led to nuclear expression of EGFR, and treatment with finasteride together with isoflavones (ERβ agonists) inhibited finasteride-induced nuclear EGFR. Of clinical significance is the increase in ERβ expression in advanced PCa upon short-term use of abiraterone, because it suggests a window in time when ERβ agonists may be effective in advanced PCa. Thus, ERβ agonists, by preventing EGFR nuclear translocation in PCa, might be useful in preventing development of tyrosine kinase driven cancer.
Keywords: nuclear receptor, prostate cancer, EGFR, PTEN, ADT
Abstract
Knockout of ERβ in the mouse leads to nuclear expression of epidermal growth factor receptor (EGFR) in the prostate. To examine whether ERβ plays a similar role in the human prostate, we used four cohorts of men: 1) a Swedish cohort of normal prostates and PCa (prostate cancer) of different Gleason grades; 2) men with benign prostatic hyperplasia (BPH) treated with the 5α-reductase inhibitor, finasteride, and finasteride together with the ERβ agonists, soy isoflavones; 3) men with PCa above Gleason grade 4 (GG4), treated with ADT (androgen deprivation therapy) and abiraterone (AA), the blocker of androgen synthesis for different durations; and 4) men with GG4 PCa on ADT or ADT with the AR (androgen receptor) blocker, enzalutamide, for 4 mo to 6 mo. In men with BPH, finasteride treatment induced EGFR nuclear expression, but, when finasteride was combined with isoflavones, EGFR remained on the cell membrane. In GG4 patients, blocking of AR for 4 mo to 6 mo resulted in loss of ERβ and PTEN expression and increase in patients with nuclear EGFR from 10 to 40%. In the men with GG4 PCa, blocking of adrenal synthesis of testosterone for 2 mo to 7 mo had the beneficial effect of increasing ERβ expression, but, on treatment longer than 8 mo, ERβ was lost and EGFR moved to the nucleus. Since nuclear EGFR is a predictor of poor outcome in PCa, addition of ERβ agonists together with abiraterone should be considered as a treatment that might sustain expression of ERβ and offer some benefit to patients.
Prostate cancer is the second most frequent cancer and the fifth leading cause of cancer death in men, with about 1.3 million new cases and 359,000 associated deaths worldwide in 2018 (1). Androgen deprivation therapy (ADT) is the standard treatment for advanced or metastatic prostate cancer (PCa). ADT is the use of luteinizing hormone-releasing hormone agonists or antagonists to cause a chemical castration. Although ADT is very effective in the short term, a third of PCa will develop resistance to ADT, termed castration-resistant prostate cancer (CRPC) through numerous mechanisms which either reinstate androgen receptor (AR) signaling activity or promote androgen independence (2). The androgen synthesis inhibitor abiraterone acetate targets the adrenal to inhibit the synthesis of dehydroepiandrosterone (DHEA) in the adrenal and in the tumor itself, while enzalutamide is an AR blocker (3–6). However, although these treatments increase survival in patients with lethal prostate cancer, their effectiveness is also temporary (7). There is a need for alternative druggable targets for PCa.
ERβ is well expressed in both epithelial and stromal cells of the normal human prostate (8, 9), but expression is lost as cancer progresses above Gleason grade 3 (GG3). In PCa cell lines, ERβ expression is undetectable at very low cycle threshold values above 28. In the PCa cell line, PC3, forced expression of ERβ inhibits epithelial−mesenchymal transition (EMT) by destabilizing hypoxia-inducible factor 1-α (HIF-1α) and decreasing vascular endothelial growth factor-mediated nuclear localization of snail, a transcription factor that regulates EMT (10). ERβ also promotes apoptosis by up-regulating the expression of p53-up-regulated modulator of apoptosis (11). In mice, treatment with the ERβ agonist (LY3201) down-regulated AR signaling in the ventral prostate (VP), and this was accompanied by down-regulation of the AR driver, RAR-related orphan receptor c. At the same time, the AR corepressor dachshund family (DACH1) was up-regulated (12). LY3201 treatment of wild-type (WT) mice also resulted in phosphatase and tensin homolog (PTEN) nuclear translocation (12). When ERβ is completely removed from the mouse genome (ERβcrispr−/− mice), there is epithelial hyperplasia, fibroplasia, and stromal overgrowth and intra ductal cancer-like lesions in the VP. These phenotypes are accompanied by an increase in P63 and Ki67, and loss of Purα and DACH1, two AR repressors (13). Thus, activating ERβ in the VP should be considered to be a target for the treatment of early-stage PCa to prevent cancer progression. Gehrig et al. (14) investigated the effect of an ERβ-specific ligand on VCaP cells in the presence or absence of ADT. Their study demonstrated that ERβ activation decreased expression of both AR and the AR splice variant AR-V7. Since VCaP cells are derived from bone metastasis of PCa, ERβ may also be effective for CRPC treatment.
The epidermal growth factor receptor (EGFR), one of four members of the human epidermal growth factor receptor (HER) family of receptor tyrosine kinases, is overexpressed in many malignancies, including PCa (15, 16). Around 30% of PCa patients overexpress EGFR, which is associated with bone metastasis (17, 18). Although EGFR is one of the most targeted receptors in cancer treatment, small tyrosine kinase inhibitors against EGFR such as Lapatinib, Erlotinib, and Gefitinib only have limited effectiveness in PCa (19). There are many mechanisms of resistance to EGFR inhibitors, including changes in intracellular trafficking, and EGFR nuclear translocation. Nuclear EGFR functions as a cotranscription factor for some genes involved in cell proliferation and angiogenesis, and as a tyrosine kinase to activate and stabilize proliferating cell nuclear antigen (PCNA) and DNA-dependent protein kinase (20).
Benign prostate hyperplasia (BPH) is defined by gland enlargement with stromal overgrowth and proliferation of epithelial cells in the transitional zone of prostate (21). Androgen/AR signaling plays an important role in BPH, and the 5α-reductase inhibitor (finasteride) which decreases the synthesis of dihydrotestosterone (DHT) from testosterone is widely used in the treatment of BPH (22).
In the present study, we used immunohistochemistry to examine AR, ERβ, and EGFR expression in PCa. We report that, in BPH patients, treatment with isoflavone, a phytoestrogen, inhibited finasteride-induced EGFR nuclear translocation. In PCa, 1) expression of ERβ was negatively correlated with EGFR nuclear translocation, 2) ADT+enzalutamide treatment promoted EGFR nuclear translocation, and 3) EGFR nuclear translocation was positively correlated with a higher rate of recurrence in PCa.
Results
Nuclear Expression of EGFR in ERβcrispr−/− Mice.
A previous study has demonstrated nuclear localization of EGFR in epithelial and stromal cells in ERβ−/− mouse uterus (23). We now show that, in mice in which there is complete deletion of the ERβ gene (ERβcrispr−/− mice), EGFR is expressed in the nuclei of the epithelium of the VP.
ERβ Agonist Is Associated with Inhibition of Finasteride-Induced EGFR Nuclear Translocation in BPH.
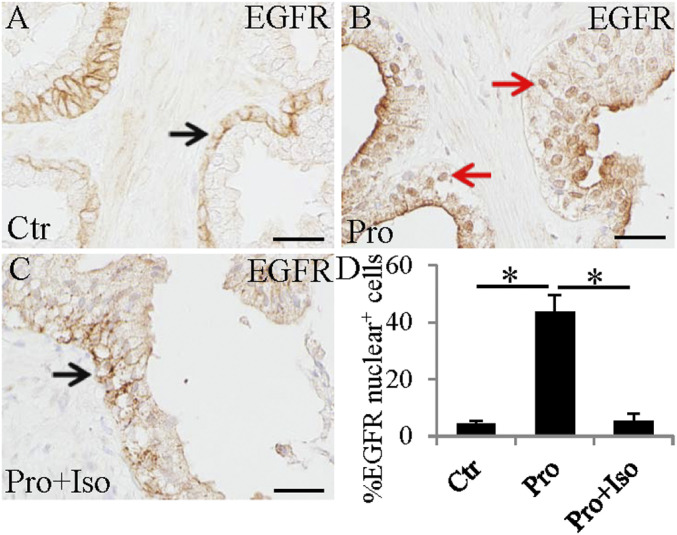
To investigate whether ERβ agonists play a role in EGFR nuclear translocation, we used formalin-fixed, paraffin-embedded (FFPE) slides from the study by Alonso-Magdalena et al. (24). In this study, BPH patients were treated with placebo (Ctr group), or with finasteride, a 5α-reductase inhibitor (Fin group), or Fin+isoflavone (a phytoestrogen, an ERβ agonist) (Fin+Iso group). There were eight patients in each group. We found that, in the Ctr group, EGFR was mostly expressed on the cell membrane of epithelial cells (Fig. 1A). In the Fin group, there were many EGFR nuclear-positive epithelial cells (Fig. 1B). In the Fin+Iso group, nuclear EGFR-expressing cells were rare (Fig. 1 C and D). Thus, by inhibiting the formation of DHT, the endogenous ERβ agonist, 3β-Adiol, is lost, and EGFR moves into the nucleus.
Fig. 1.
Activity of ERβ inhibits finasteride (Pro = Proscar) induced EGFR nuclear translocation in BPH. In BPH, EGFR was mainly expressed in the cell membrane (black arrow) (A). Finasteride treatment increased nuclear expression of EGFR (red arrows) (B). Combined treatment with finasteride and isoflavone decreased expression of EGFR in nuclei (black arrow) (C). (Scale bars, 50 μm). Statistical analyses of EGFR nuclear-positive cells in control, finasteride, or finasteride+isoflavone treated BPH (*P < 0.01) (D).
Expression of ERβ and AR in Normal Prostate and PCa.
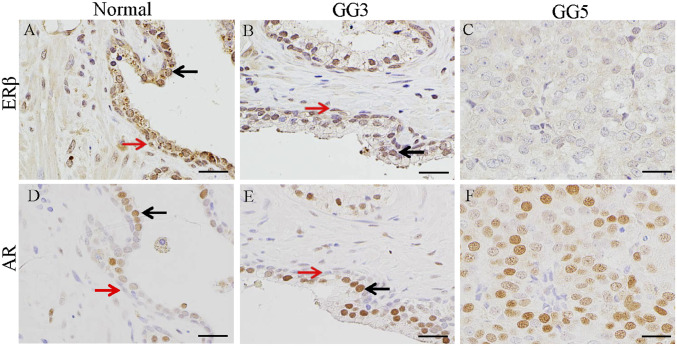
It has been previously shown that expression of ERβ is maintained in PCa of GG3 but is diminished in higher Gleason grades (10, 25). In the normal prostate (Fig. 2A), ERβ is abundantly expressed in the prostate epithelium, both luminal (black arrow) and basal cells (red arrow) as well as some stromal cells. The expression of ERβ was evident in GG3 PCa (Fig. 2B). In GG5, ERβ was not detectable (Fig. 2C). In the normal prostate, AR was well expressed in luminal epithelial cells (black arrow) and some stromal cells (Fig. 2D). Basal cells (red arrows) did not express AR (Fig. 2D). AR expression was maintained in cancer of GG3 and GG5 (Fig. 2 E and F).
Fig. 2.
Expression of ERβ and AR in prostate and prostate cancers. ERβ was expressed in both luminal epithelial cells (black arrow) and myoepithelial cells (red arrow) in normal prostate (A). In GG3 prostate cancer, there were many relatively normal ducts where ERβ was still expressed in cells in luminal layer (black arrow) and myoepithelial cells (red arrow) (B); however, ERβ was lost in GG5 prostate cancer (C). AR was mainly expressed in luminal epithelial cells (black arrow), not in myoepithelial cells (red arrow) in normal prostate (D). In apparently normal ducts in GG3 prostate cancer, cells in luminal layer (black arrow) expressed AR; basal epithelial cells (red arrow) did not express AR (E). AR was highly expressed in cancer cells in GG5 prostate cancer (F). (Scale bars, 50 μm.)
In PCa, Loss of ERβ Is Accompanied by an Increase of EGFR Nuclear Translocation and Loss of PTEN.
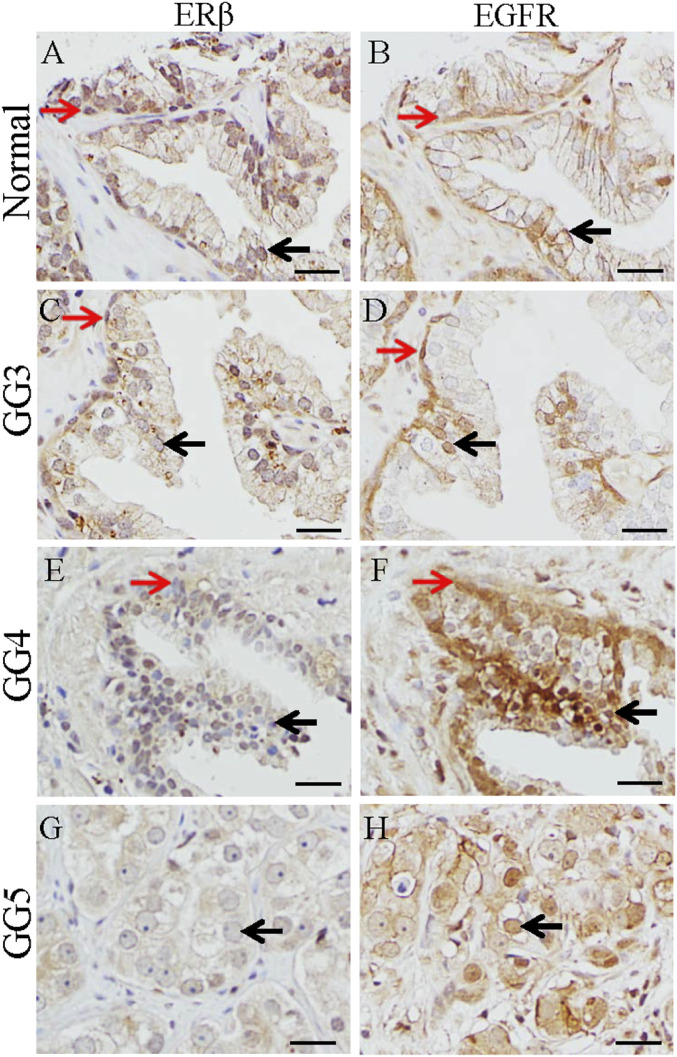
Nuclear translocation of EGFR is found in many cancers (26, 27). Here we show that ERβ is expressed in nuclei of the basal cells (red arrow) and luminal epithelium (black arrow) in the normal prostate (Fig. 3A), and, in the same areas, EGFR is expressed on the cell membrane (red arrow) (Fig. 3B). In GG3 PCa, ERβ expression in epithelial cells was lower than in the normal prostate (Fig. 3C), and EGFR was expressed in nuclei of some epithelial cells (Fig. 3D). There was a marked decrease of ERβ expression in GG4 PCa (Fig. 3E), and EGFR was expressed in cell nuclei (red arrow) (Fig. 3F). ERβ was lost in GG5 PCa (Fig. 3G), and EGFR was located in the nuclei of cancer cells (black arrow) (Fig. 3H).
Fig. 3.
Expression of ERβ and EGFR in prostate and prostate cancers. In the normal prostate, ERβ was expressed in nuclei of the basal (red arrow) and luminal epithelium (black arrow) (A), and EGFR was located in the cell membrane of the basal (red arrow) and luminal epithelial cells (black arrow) (B). In GG3, ERβ expression in cells in luminal layer (black arrow) and basal layer (red arrow) was decreased (C), and EGFR was present in nuclei of some cells in luminal layer (black arrow) and basal layer (red arrow) (D). In GG4, expression of ERβ was very low in cells of luminal layer (black arrow) and basal layer (red arrow) (E), and EGFR was present in nuclei of cells in luminal layer (black arrow) and basal layer (red arrow) (F). In GG5, ERβ was lost (black arrow) (G), and EGFR was present in the nuclei of cancer cells (black arrow) (H). (Scale bars, 50 μm).
Expression of the tumor suppressor gene PTEN is ERβ regulated (28). We have previously reported that PTEN is down-regulated in the VP of ERβ−/− mice and that an ERβ agonist drives PTEN into the nucleus in WT mice (12). With the PCa arrays from MD Anderson Cancer Center (MDACC), we stained sequential sections for ERβ and PTEN. We found that, in cells where ERβ was expressed in the nucleus (SI Appendix, Fig. S1A), PTEN was also expressed in nuclei (SI Appendix, Fig. S1B). When ERβ was cytosolic (SI Appendix, Fig. S1C), PTEN was expressed in cytoplasm (SI Appendix, Fig. S1D). When ERβ was not expressed (SI Appendix, Fig. S1E), PTEN expression was also lost (SI Appendix, Fig. S1F). To determine whether ERβ and PTEN are expressed by the same cells, we used double immunofluorescence staining. The colocalization of ERβ, PTEN, and DAPI confirmed that ERβ and PTEN were colocalized in the same cells (SI Appendix, Fig. S1 G–J).
An Increase in EGFR Nuclear Translocation by Enzalutamide.
To investigate whether blocking AR affects EGFR nuclear translocation, we compared two tissue arrays of GG4 cancers. One (number of cores: 459) was from ADT-treated men, and the other was from ADT+enzalutamide-treated men (number of cores: 464). During staining, 24 cores from the ADT group and 22 from the ADT+enzalutamide group were lost. The percentage of EGFR-positive cores was not different between the two groups: 83 (19%) from the ADT-treated group and 103 (23%) from the ADT+enzalutamide group (Table 1). However, there was a significant increase in nuclear EGFR in the enzalutamide group. There were 9 (11%) in the ADT group and 42 (40%) in the enzalutamide-treated group (Table 2). In the EGFR nuclear-positive samples, there were significantly more PCNA-positive cells than in EGFR nuclear-negative samples (SI Appendix, Fig. S2). Thus, blocking AR had the negative effect of increasing nuclear EGFR.
Table 1.
Enzalutamide does not affect total (cytoplasmic+nuclear) EGFR expression
| Treatment | EGFR+ | EGFR− | Total | Percent EGFR positive |
| ADT | 83 | 354 | 437 | 18 |
| ADT+Enz | 103 | 337 | 440 | 23 |
| Total | 186 | 691 | 877 | 21 |
P value of the χ2 statistic is 0.095.
Table 2.
Enzalutamide increases EGFR nuclear expression
| Treatment | EGFR nuclear positive | EGFR nuclear negative | Total | Percent of samples with nuclear EGFR |
| ADT | 9 | 74 | 83 | 10 |
| ADT+Enz | 42 | 61 | 103 | 42 |
P value of the χ2 statistic is 0.000006601.
Effect of Combination of Abiraterone and Enzalutamide on Nuclear EGFR and Recurrence of PCa.
To determine whether nuclear expression of EGFR is related to PCa recurrence, we analyzed 47 patients (recruited between December 2013 and March 2015 at MDACC in Houston) who were treated with either abiraterone acetate (Abi group) or abiraterone acetate+enzalutamide (Abi+Enz group). Two patients were lost during follow-up. There was no significant difference in recurrence rate between the two treatment groups: In the Abi group, it was 56% (9 out of 16), and, in the Abi+Enz group, it was 65% (19 out of 29). Thus, addition of the AR blocker to the inhibitor of androgen synthesis did not affect recurrence. However, regardless of the treatment, recurrence rate in EGFR nuclear-positive patients was (79%). This was significantly higher than that (43%) in the nuclear EGFR-negative group of patients (Table 3).
Table 3.
Relapse rate is higher in EGFR nuclear-positive than in EGFR nuclear-negative patients
| EGFR | Relapsed | Nonrelapsed | Total | Percent of relapse |
| Nuclear positive | 11 | 3 | 14 | 78 |
| Nuclear negative | 10 | 13 | 23 | 43 |
| Total | 21 | 16 | 37 | 56 |
P value of the χ2 statistic is 0.037.
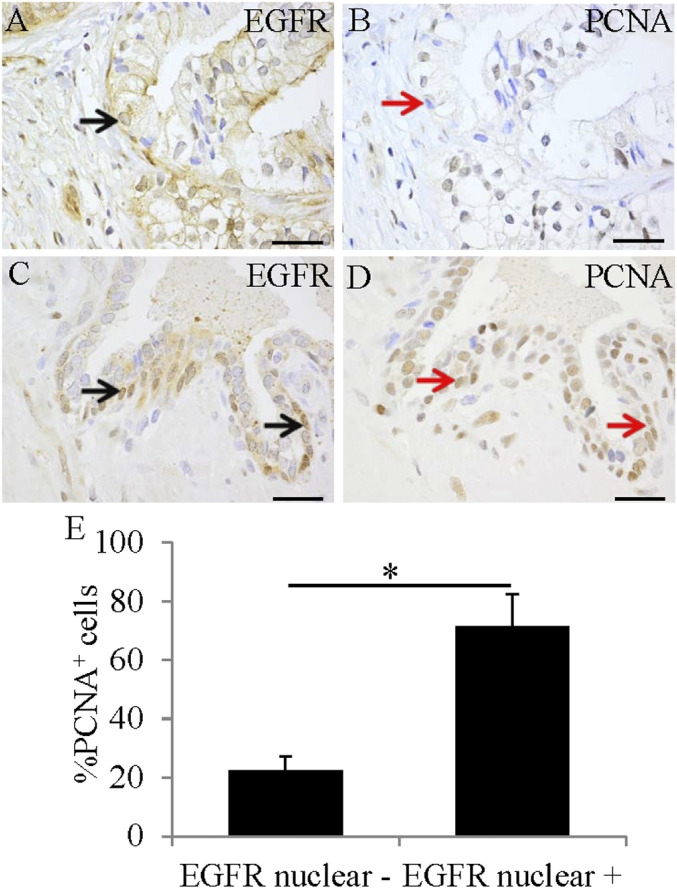
In addition, there were more PCNA-positive cells in the EGFR nuclear-positive patients (Fig. 4 B, D, and E) than in the EGFR nuclear-negative PCa patients (Fig. 4 A and C).
Fig. 4.
EGFR nuclear translocation was associated with increased expression of PCNA. Expression of EGFR was in the cell membrane (black arrow) (A) and in cell nuclei (black arrow) (C). Expression of PCNA in EGFR nuclear-positive cancers (red arrow) (D) was higher than that in EGFR nuclear-negative cancers (red arrow) (B). (Scale bars, 50 μm). PCNA expression was significantly higher in EGFR nuclear-positive cancers (*P < 0.05) (E).
Effect of Short-Term ADT+Abiraterone on ERβ Expression in the Prostate.
One strong argument against the clinical use of ERβ agonists to treat advanced PCa is the loss of ERβ expression in advanced PCa. To investigate whether duration of ADT+abiraterone influences ERβ expression, we analyzed ERβ expression in patients treated with abiraterone acetate for different durations of treatment (2 mo to 4 mo, 5 mo to 7 mo, 8 mo to 11 mo, and 12 mo). As shown in Table 4, short-term treatment with abiraterone increased ERβ expression. But, upon prolonged (>7 mo) ADT treatment, ERβ expression was lost from PCa.
Table 4.
Comparison of ERβ expression between different abiraterone time courses
| Time course | ERβ+ | ERβ- | Total | Percent ERβ+ |
| 2 to 4 m | 32 | 186 | 218 | 15 |
| 5 to 7 m | 10 | 53 | 63 | 16 |
| 8 to 11 m | 2 | 60 | 62 | 3 |
| 12 m | 0 | 71 | 71 | 0 |
| Total | 44 | 370 | 414 | 11 |
P value of the χ2 statistic between <7 m and >7 m is 0.002386.
Discussion
Since its discovery in 1996 (8), the role of ERβ in PCa prevention and treatment has been investigated in many laboratories. ERβ is a tumor suppressor whose expression is lost as PCa progresses. This loss limits the use of ERβ agonists for treatment of advanced PCa. In the present study, we show that the nuclear transport of EGFR and PTEN is ERβ regulated and could be targeted in the treatment of PCa. We have used PCa tissue arrays and ADT-treated PCa FFPE cancer samples to confirm that there is ERβ expression in PCa of low Gleason grades. We found that, in PCa, expression of ERβ was negatively correlated with EGFR nuclear location and that ADT+enzalutamide treatment promoted EGFR nuclear translocation. Furthermore, EGFR nuclear translocation was positively correlated with a higher rate of recurrence.
Of clinical importance is the increase in ERβ expression upon short-term abiraterone treatment and the finasteride-induced nuclear EGFR which could be prevented with isoflavones (phytoestrogens).
PCa is an AR-dependent disease. ADT, removal the synthesis of testicular androgens, is a reasonable treatment for advanced or metastatic PCa (29, 30). ADT is effective initially, but it eventually leads to CRPC. In the present study, immunochemical staining of sequential sections in tissue arrays showed that ERβ was expressed in both luminal and basal cells. But AR was only expressed in luminal epithelial cells and not in the basal cells. This localization pattern explains why proliferation and/or differentiation of basal cells is AR independent and why ADT is able to inhibit the proliferation of AR-positive cancer cells but has no effect on basal cells. Furthermore, ADT leads to the loss of DHT in the prostate. This means that there is also loss of 3βAdiol (5α-androstane-3β, 17β-diol), the endogenous ERβ ligand which is formed from DHT by 3β-hydroxysteroid dehydrogenase 6 (3β-HSD 6) in the prostate (31). During ADT, the ERβ endogenous ligand is depleted, and the ERβ is inactive. Under these conditions, a combination of an ERβ agonist with ADT should be useful for the treatment of PCa.
In the present study, we found that, as PCa advanced from normal tissue to low and then higher Gleason grades, ERβ expression decreased, EGFR moved from the cell surface to the nucleus, and there was increased expression of PCNA in epithelial cells. Nuclear localization of EGFR is highly associated with disease progression and decreased overall survival in many cancers (32–35). Nuclear localized EGFR also enhances resistance to chemotherapy, radiation, and anti-EGFR therapies (36–38). In several cancers, such as basal cell breast cancer (39), pleural mesothelioma (40) and triple-negative breast cancer (41), ERβ represses EGFR. However, few studies have reported on the role of ERβ in the nuclear translocation of EGFR. A previous study from our group found nuclear localization of EGFR in epithelial and stromal cells in uterus of ERβ−/− mice (23). To determine whether an ERβ agonist can prevent the migration of EGFR from the cell membrane to the nucleus, we investigated BPH patients treated with placebo, finasteride, or finasteride+isoflavone. Even though there were only eight patients in each group, we found that treatment with finasteride was associated with increased EGFR nuclear translocation, but, in men treated with finasteride plus the ERβ agonist, isoflavone, there was very little nuclear EGFR.
PTEN, a well-known tumor suppressor gene (42), is regulated by ERβ. PTEN was down-regulated in the VP of ERβ−/− mice, and, in WT mice, an ERβ agonist drove PTEN into the nucleus (12). In the present study, we confirmed that loss of ERβ is accompanied by the loss of PTEN.
ADT+enzalutamide increased EGFR nuclear translocation in PCa, and this EGFR nuclear localization was highly associated with a higher rate of recurrence. The mechanism through which ADT+enzalutamide is able to promote EGFR nuclear translocation remains to be investigated. One difference between treatment with finasteride and treatment with AR blockers is that inhibition of 5α-reductase with finasteride leads to loss of DHT but not loss of testosterone. Loss of DHT leads to loss of the endogenous ligand of ERβ, 3β-Adiol, and loss of ERβ function, while some AR remains because of the presence of testosterone. The effect of finasteride on prostate size is a slow process. The more immediate effects, EGFR translocation to the nucleus, may be due to loss of ERβ activity.
Treatment of men on ADT with abiraterone for 2 mo to 7 mo to inhibit 17-hydroxylase resulted in an increase in expression of ERβ. The increase in ERβ expression probably reflects the lack of effect of short-term abiraterone treatment on circulation levels of DHEA and DHEA sulfate. The likely explanation for this short-term effect is that, because of the very large reservoir of DHEA and DHEA sulfate in the body, inhibition of its synthesis does not cause an immediate loss of DHEA. In addition, abiraterone may have increased steroid sulfatases (43), changed the expression of HSD converting androstane-3α/β-diol to DHT (44), or blocked CYP7B1 to increase the level of 3βAdiol (45). On long-term abiraterone treatment, both DHEA and 3β-Adiol eventually are depleted, and ERβ is without ligand and becomes inactive and unstable.
Taken together, our data suggest a key role of ERβ in inhibiting EGFR nuclear translocation and stimulating nuclear transport of PTEN. We suggest that, in advanced PCa, short-term abiraterone treatment increases ERβ expression, and thus there is a window in time when ERβ agonists may be useful in the treatment, even in advanced PCa.
Materials and Methods
Materials.
The details of a mouse line with a constitutive knockout of the ERβ gene using CRISPR-Cas9−mediated gene editing was previously reported by our group (13).
To determine whether expression of EGFR is related to PCa recurrence, 47 radical prostatectomy tissue specimens were analyzed from 46 deidentified patients treated at MDACC. These patients were enrolled in a preoperative study that assessed the effect of a neoadjuvant 6-mo treatment with abiraterone acetate (AA, androgen biosynthesis inhibitors) plus ADT versus AA combined with enzalutamide. The mechanism of action for enzalutamide is threefold. It is a potent, competitive binder of androgens at the level of the AR. It prevents the translocation of the AR from the cytoplasm to the nucleus. Within the nucleus, it inhibits AR binding to chromosomal DNA, which prevents further transcription of tumor genes and results in ADT in patients with newly diagnosed high-risk prostate cancer (clinical trial NCT01946165). The present study was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guideline, and all patients had consented to the processing of their tumor tissue samples in future research projects.
The median age of the patient population was 66 y (range: 47 y to 75 y). Thirty-four patients were White, three patients were Black, seven were Hispanic, one was Asian, and one was from another racial background. Staging upon diagnosis was as follows: 5 patients were stage cT1c, 3 were cT2a, 12 were cT2b, 4 were cT2c, 9 were cT3a, 12 were cT3b, and 1 was cT4. Pretreatment biopsy Gleason scores were as follows: 1 patient with GS 6 (3+3), 1 with GS 7 (3+4), 1 with GS 7 (4+3), 16 with GS 8 (4+4), 1 with GS 8 (5+3), 20 with GS 9 (4+5), 5 with GS 9 (5+4), and 1 with GS 10 (5+5). All patients received six cycles of treatment and subsequently underwent radical prostatectomy. Median prostate-specific antigen (PSA) at screening was 14.25 ng/mL (range: 0.5 ng/mL to 141 ng/mL) Thirty-one patients received treatment with AA+enzalutamide+ADT, and 15 patients were treated with AA+ADT. All patients except five achieved a PSA < 0.1 prior to surgery. Surgical staging was as follows: 14 patients were staged ypT2, 7 patients were staged ypT3a, and 25 patients were staged ypT3b. Sixteen patients had nodal involvement.
Samples with BPH from deidentified patients treated with placebo, finasteride, or finasteride+isoflavone were from Barmherzige Bruder Hospital and Danube Hospital, Vienna, Austria.
Immunohistochemistry.
Paraffin-embedded sections were deparaffinized, rehydrated, and heated in a Lab Vision PT module (Thermo Scientific) at 97 °C for 10 min to retrieve antigens. Sections were incubated with 0.05% Triton for 15 min. After washing with 1× phosphate-buffered saline, sections were blocked with buffer composed of 50% (vol/vol) methanol and 3% (vol/vol) H2O2 for 30 min and then with 3% (wt/vol) bovine serum albumin for 30 min. This was followed by overnight incubation at 4 °C with primary antibody: anti-ERβ (1:100; in house ERβ antibody mapping the C terminus part), anti-EGFR (1:200, Abcam), anti-AR (1:100; Abcam), anti-PTEN (1:100; Abcam), and anti-PCNA (1:10000; Abcam). Slides were incubated with secondary antibody followed by horseradish peroxidase polymer kit (Biocare Medical; GHP516) for 30 min. Finally, slides were developed with 3,3-diaminobenzidine tetrahydrochloride and counterstained with hematoxylin. Sections were covered with mounting medium after dehydration.
Immunofluorescence Double Staining.
Immunofluorescence was performed as described before (46). Labeling with anti-ERβ (1:100; in house ERβ antibody mapping the C terminus part) and anti-PTEN (1:100; Abcam) was carried out. Primary antibodies were detected with donkey anti-chicken Cy3 (1:400; Jackson ImmunoResearch) and donkey anti-rabbit FITC (1:400; Santa Cruz Biotechnology). Samples were counterstained with DAPI (Vector) for cell nuclei visualization.
Data Analysis.
Data are expressed as mean ± SD; statistical comparisons were made by using a one-way ANOVA followed by a Bonferroni post hoc test or chi-square test. P < 0.05 was considered to indicate statistical significance. P < 0.01 was considered to indicate statistical significance for the BPH study, since there were only eight patients in each group.
Supplementary Material
Acknowledgments
This study was supported by grants from the Brockman Foundation (Grant G0500851), the Swedish Cancer Fund, and the Swedish Science Council. J.-A.G. is thankful to the Robert A. Welch Foundation (Grant E-0004).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2011269118/-/DCSupplemental.
Data Availability
All study data are included in the article and SI Appendix.
References
- 1.Bray F., et al., Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Karantanos T., et al., Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur. Urol. 67, 470–479 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tannock I. F.et al.; TAX 327 Investigators , Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 351, 1502–1512 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Gravis G., et al., Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet Oncol. 14, 149–158 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Cookson M. S., Lowrance W. T., Murad M. H., Kibel A. S.; American Urological Association , Castration-resistant prostate cancer: AUA guideline amendment. J. Urol. 193, 491–499 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Gillessen S., et al., Management of patients with advanced prostate cancer: The report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur. Urol. 73, 178–211 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Shore N. D., et al., Optimizing the role of androgen deprivation therapy in advanced prostate cancer: Challenges beyond the guidelines. Prostate 80, 527–544 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuiper G. G., Enmark E., Pelto-Huikko M., Nilsson S., Gustafsson J. A., Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U.S.A. 93, 5925–5930 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enmark E., et al., Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J. Clin. Endocrinol. Metab. 82, 4258–4265 (1997). [DOI] [PubMed] [Google Scholar]
- 10.Mak P., et al., ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: Implications for Gleason grading. Cancer Cell 17, 319–332 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dey P., Ström A., Gustafsson J. A., Estrogen receptor β upregulates FOXO3a and causes induction of apoptosis through PUMA in prostate cancer. Oncogene 33, 4213–4225 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Wu W. F., et al., Estrogen receptor β, a regulator of androgen receptor signaling in the mouse ventral prostate. Proc. Natl. Acad. Sci. U.S.A. 114, E3816–E3822 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warner M., et al., Ventral prostate and mammary gland phenotype in mice with complete deletion of the ERβ gene. Proc. Natl. Acad. Sci. U.S.A. 117, 4902–4909 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gehrig J., et al., Prospects of estrogen receptor β activation in the treatment of castration-resistant prostate cancer. Oncotarget 8, 34971–34979 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yarden Y., Sliwkowski M. X., Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2, 127–137 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Jorissen R. N., et al., Epidermal growth factor receptor: Mechanisms of activation and signalling. Exp. Cell Res. 284, 31–53 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Baek K.H., et al., Correlation of AR, EGFR, and HER2 expression levels in prostate cancer: Immunohistochemical analysis and chromogenic in situ hybridization. Cancer Res. Treat. 44, 50–56 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day K. C., et al., HER2 and EGFR overexpression support metastatic progression of prostate cancer to bone. Cancer Res. 77, 74–85 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sridhar S. S., et al., A multicenter phase II clinical trial of lapatinib (GW572016) in hormonally untreated advanced prostate cancer. Am. J. Clin. Oncol. 33, 609–613 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Brand T. M., et al., Nuclear EGFR as a molecular target in cancer. Radiother. Oncol. 108, 370–377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 21.Izumi K., Mizokami A., Lin W. J., Lai K. P., Chang C., Androgen receptor roles in the development of benign prostate hyperplasia. Am. J. Pathol. 182, 1942–1949 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim E. H., Larson J. A., Andriole G. L., Management of benign prostatic hyperplasia. Annu. Rev. Med. 67, 137–151 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Wada-Hiraike O., et al., Role of estrogen receptor beta in uterine stroma and epithelium: Insights from estrogen receptor beta-/- mice. Proc. Natl. Acad. Sci. U.S.A. 103, 18350–18355 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alonso-Magdalena P., et al., A role for epithelial-mesenchymal transition in the etiology of benign prostatic hyperplasia. Proc. Natl. Acad. Sci. U.S.A. 106, 2859–2863 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu X., et al., Dynamic regulation of estrogen receptor-beta expression by DNA methylation during prostate cancer development and metastasis. Am. J. Pathol. 164, 2003–2012 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Psyrri A., et al., Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin. Cancer Res. 11, 5856–5862 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Brand T. M., Iida M., Li C., Wheeler D. L., The nuclear epidermal growth factor receptor signaling network and its role in cancer. Discov. Med. 12, 419–432 (2011). [PMC free article] [PubMed] [Google Scholar]
- 28.Lindberg K., Helguero L. A., Omoto Y., Gustafsson J. Å., Haldosén L. A., Estrogen receptor β represses Akt signaling in breast cancer cells via downregulation of HER2/HER3 and upregulation of PTEN: Implications for tamoxifen sensitivity. Breast Cancer Res. 13, R43 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aoun F., et al., Androgen deprivation therapy in the treatment of locally advanced, nonmetastatic prostate cancer: Practical experience and a review of the clinical trial evidence. Ther. Adv. Urol. 9, 73–80 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pagliarulo V., Androgen deprivation therapy for prostate cancer. Adv. Exp. Med. Biol. 1096, 1–30 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Weihua Z., Lathe R., Warner M., Gustafsson J. A., An endocrine pathway in the prostate, ERbeta, AR, 5alpha-androstane-3beta,17beta-diol, and CYP7B1, regulates prostate growth. Proc. Natl. Acad. Sci. U.S.A. 99, 13589–13594 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Traynor A. M., et al., Nuclear EGFR protein expression predicts poor survival in early stage non-small cell lung cancer. Lung Cancer 81, 138–141 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharip A., et al., Analysis of origin and protein-protein interaction maps suggests distinct oncogenic role of nuclear EGFR during cancer evolution. J. Cancer 8, 903–912 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alsahafi E., et al., Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 10, 540 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang C. C., et al., Higher nuclear EGFR expression is a better predictor of survival in rectal cancer patients following neoadjuvant chemoradiotherapy than cytoplasmic EGFR expression. Oncol. Lett. 17, 1551–1558 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liccardi G., Hartley J. A., Hochhauser D., EGFR nuclear translocation modulates DNA repair following cisplatin and ionizing radiation treatment. Cancer Res. 71, 1103–1114 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dittmann K., Mayer C., Czemmel S., Huber S. M., Rodemann H. P., New roles for nuclear EGFR in regulating the stability and translation of mRNAs associated with VEGF signaling. PLoS One 12, e0189087 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi Y., et al., Nuclear EGFR-PKM2 axis induces cancer stem cell-like characteristics in irradiation-resistant cells. Cancer Lett. 422, 81–93 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Thomas C., et al., ERbeta1 represses basal breast cancer epithelial to mesenchymal transition by destabilizing EGFR. Breast Cancer Res. 14, R148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinton G., et al., Estrogen receptor β exerts tumor repressive functions in human malignant pleural mesothelioma via EGFR inactivation and affects response to gefitinib. PLoS One 5, e14110 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samanta S., Sharma V. M., Khan A., Mercurio A. M., Regulation of IMP3 by EGFR signaling and repression by ERβ: Implications for triple-negative breast cancer. Oncogene 31, 4689–4697 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masson G. R., Williams R. L., Structural mechanisms of PTEN regulation. Cold Spring Harb. Perspect. Med. 10, a036152 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penning T. M., Dehydroepiandrosterone (DHEA)-SO4 depot and castration-resistant prostate cancer. Vitam. Horm. 108, 309–331 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ando T., et al., Dihydrotestosterone synthesis pathways from inactive androgen 5α-androstane-3β,17β-diol in prostate cancer cells: Inhibition of intratumoural 3β-hydroxysteroid dehydrogenase activities by abiraterone. Sci. Rep. 6, 32198 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang W., Norlin M., Regulation of steroid hydroxylase CYP7B1 by androgens and estrogens in prostate cancer LNCaP cells. Biochem. Biophys. Res. Commun. 344, 540–546 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Wu W. F., et al., Targeting estrogen receptor β in microglia and T cells to treat experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U.S.A. 110, 3543–3548 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.