Abstract
Purpose:
Clinical trials of venetoclax reported negligible rates of clinical tumor lysis syndrome (TLS) in patients with chronic lymphocytic leukemia (CLL) when using an extended dose escalation schedule. We aimed to understand TLS prophylaxis, rates of select adverse events (AEs), and impact of dosing modifications in routine clinical practice.
Experimental Design:
This retrospective cohort study included 297 CLL venetoclax treated patients outside of clinical trials in academic and community centers. Demographics, baseline disease characteristics, venetoclax dosing, TLS risk and prophylaxis, and AEs were collected.
Results:
The group was 69% male, 96% had relapsed/refractory CLL, 45% had deletion chromosome 17p, 84% had unmutated IGHV, 80% received venetoclax monotherapy, and median age was 67. TLS risk was categorized as low (40%), intermediate (32%), or high (28%), and 62% had imaging prior to venetoclax initiation. Clinical TLS occurred in 2.7% of patients and laboratory TLS occurred in 5.7%. Pre-venetoclax TLS risk group and creatinine clearance independently predict TLS development in multivariable analysis.
Grade 3/4 AEs included neutropenia (39.6%), thrombocytopenia (29.2%), infection (25%), neutropenic fever (7.9%), and diarrhea (6.9%). 22 patients (7.4%) discontinued venetoclax due to an AE. PFS was similar regardless of number of dose interruptions, length of dose interruption, and stable venetoclax dose.
Conclusion:
These data provide insights into current use of venetoclax in clinical practice, including TLS rates observed in clinical practice. We identified opportunities for improved adherence to TLS risk stratification and prophylaxis, which may improve safety.
Keywords: CLL, Venetoclax, Tumor lysis syndrome, Adverse events, Dosing
Introduction
Venetoclax inhibits BCL2 and induces apoptosis of chronic lymphocytic leukemia (CLL) cells, driving rapid tumor debulking and high response rates. Pivotal studies in relapsed and refractory (R/R) CLL have led to venetoclax approval in that setting either alone or in combination with rituximab.
In the initial phase I dose-escalation trial of venetoclax in CLL, this robust activity resulted in laboratory tumor lysis syndrome (TLS, defined by Howard criteria(2)) in 3 patients treated after a single initial starting dose of 100 or 200 mg. In the 56 patient dose-escalation cohort, a total of 3 cases of clinical TLS and 8 cases of laboratory TLS among 7 patients were observed. Of the 3 clinical cases, 1 experienced sudden death, 1 required hemodialysis, and another had transient acute kidney injury. Nine of 10 patients with TLS resumed treatment with venetoclax, and 8 did not have subsequent TLS recurrence. During the venetoclax monotherapy lead in of the phase I trial of venetoclax plus rituximab in relapsed/refractory CLL, two patients in the 50mg starting dose cohort experienced clinical TLS; one lead to death.(3) Based on this collective experience, an extended dose ramp-up schedule starting at 20 mg and TLS prophylaxis and monitoring strategy were devised. All 60 patients in the expansion cohort of the monotherapy trial were treated with this modification to venetoclax dosing, resulting in no episodes of clinical TLS.(4) Similarly, no additional clinical tumor lysis was experienced in the amended phase I venetoclax plus rituximab trial during the initial venetoclax dose ramp up. The phase II study of patients with R/R CLL harboring a chromosome 17p deletion (del(17p)) reported 5% laboratory TLS.(5) A phase II study of venetoclax in patients previously treated with ibrutinib reported 2% laboratory TLS.(6)
The U.S. Food and Drug Administration (FDA) package insert for venetoclax defines TLS risk category as well as the recommended minimal prophylaxis strategies by risk category.(7) Adherence to these recommendations outside of clinical trials is not known. Rates of TLS and other adverse events (AEs) of interest outside of the clinical trial setting have been less well described.(8,9)
We evaluated an international cohort of 297 patients treated with venetoclax, the largest cohort of patients treated outside of clinical trials to date, to describe clinical practice patterns for determination of TLS risk and prophylactic measures, as well as observed rates of TLS. We further characterized the AE profile for venetoclax outside of the clinical trial setting and outcomes for patients who require dose interruptions or dose reductions, data not readily available through clinical trial reports.
Methods
This international collaboration included 15 academic and 51 community centers across the United States and United Kingdom and was completed in partnership with the CLL Collaborative Study of Real World Evidence (CORE) and the United Kingdom CLL Forum. The CLL Collaborative Study of Real World Evidence (CORE) is a multicenter collaborative effort across academic and community centers aimed to pool retrospective data to study real world outcomes in CLL patients. The UK CLL forum is a collaborative work group of physicians and scientists with an interest in CLL who develop research, form guidelines, and share best practice. The UK CLL forum reached out to members of the broader CLL treating community in the UK to collect data from over 40 sites included in the manuscript. We have verified that no patients overlap between the contributions from US medical centers, CORE centers, and the UK CLL forum. The study was Institutional Review Board approved.
Investigators at each US center collected data through chart review of CLL patients treated with venetoclax from the time of US commercial approval through 7/2018, and UK investigators collected data from 8/2015 through 7/2018. Data collected included demographics, disease characteristics, venetoclax dosing, TLS risk and prophylaxis, and AEs. To understand patterns of venetoclax use, we collected data on its administration as monotherapy and, if paired, which additional agent was given. To understand specific practices surrounding dosing schedules, we collected the maximum dose of venetoclax achieved during dose-escalation, the total number of dose reductions and/or interruptions (including duration) required while on therapy, and the stable venetoclax dose used following dose escalation.
To understand TLS risk, investigators assigned patients to low, intermediate, or high TLS risk categories as defined by the venetoclax package insert.(7,10) We also collected the absolute lymphocyte count (ALC) and largest lymph node measurement prior to venetoclax dosing (either by examination or CT imaging) to assess the accuracy of TLS risk assignment. The number of planned hospitalizations for venetoclax dose escalation and prophylaxis (allopurinol, rasburicase, normal saline, additional agents) were captured. Laboratory versus clinical TLS events were defined by the Howard criteria.(2) Selected AEs were collected and graded using the NCI Common Toxicity Terminology Criteria for Adverse Events v4.0 (CTCAE v4.0). Potential additional TLS risk factors were explored including age, sex, baseline renal function, cytogenetics, IGHV mutational status, number of prior therapies, exposure to prior ibrutinib, TLS risk classified by the investigator based on the FDA package insert,(7) and venetoclax administration as monotherapy or in combination with another agent.
We performed univariate analyses to evaluate the association between proposed risk factors and the development of TLS. Age and number of prior therapies were analyzed as continuous variables. Sex, baseline renal function, cytogenetics, IGHV mutational status, exposure to prior ibrutinib, TLS risk as defined by the investigator, and venetoclax monotherapy versus (vs.) combination were analyzed as categorical variables. Baseline renal function was stratified based on creatinine clearance <80 mL/min versus ≥80 mL/min as defined in the venetoclax package insert.(7) Cytogenetic abnormalities included this analysis were del(17p) and complex karyotype (3 or more chromosomal abnormalities(11)). IGHV mutational status was categorized as unmutated or mutated by standard criteria.(11) For all variables, odds ratios (OR) with 95% confidence intervals (CI) were estimated for association with TLS using logistic regression. All variables significantly associated with TLS to a level of p<0.05 in univariate analysis were included in a multiple logistic regression model. For independent predictors of TLS in this model, we report the area under the receiver operator characteristic curve (ROC). We used a bootstrap resampling method to evaluate and correct for any potential bias in our estimates and internally validated findings based on 100 bootstrap samples.
Progression free survival (PFS) was estimated with the Kaplan Meier method.(12) PFS was defined as the time from venetoclax initiation until progression or death from any cause, and patients were censored at the time of last follow up. Patients who went on to receive cellular therapy (stem cell transplant or CAR-T) but had not progressed were censored on the date of cellular therapy. The log-rank (LR) test was used to compare PFS among patients who receive 400 mg daily venetoclax vs. < 400 mg daily venetoclax, those with dose interruptions vs. those without, and length of dose interruption. Hazard ratios (HR) were estimated using Cox regression analyses.(13) All other analyses were descriptive. Tests were two-sided at the 5% level unless otherwise noted. Statistical analyses were performed using STATA 10.1 (Stata Statistical Software: Release 10, 2007; StatCorp LP, College Station, TX).
Results
Baseline Characteristics
Two hundred and ninety-seven patients from 15 academic (n=169) and 51 community (n=128) centers were treated with venetoclax outside of a clinical trial and included in this study. The group was 68% male and median age at venetoclax initiation was 67 years (range 37–91), 96% had relapsed or refractory (R/R) CLL, 45% had del(17p), and 84% had unmutated IGHV, and 47% had baseline renal dysfunction (creatinine clearance <80 mL/min) (Table 1). Venetoclax was given as monotherapy (80%, n=237) or paired with another agent (20%, n=59). Of those who received venetoclax paired, 75% (n=44) received anti-CD20 monoclonal antibody (Ab), 8.5% (n=5) received ibrutinib, and 16.5% (n=10) received another agent. Median time from venetoclax initiation to progression or last follow-up was 11 months (range 1 to 33 months).
Table 1.
Baseline Characteristics
| Total Cohort n=297 | Number with Available Data | |
|---|---|---|
| Age, median (range) | 67 (37–91) | 297 |
| Male, % | 68.0% | 297 |
| Baseline renal dysfunction (creatinine clearance < 80 mL/min), % | 46.8% | 188 |
| R/R, % | 95.6% | 297 |
| Unmutated IGHV, % | 84% | 94 |
| del(17p), % | 45% | 278 |
| Prior lines of therapy, median (range) | 3 (0–15) | 297 |
| Prior ibrutinib, % | 80% | 281 |
| Venetoclax monotherapy, % | 80% | 296 |
| TLS Risk | ||
| Low, % | 40% | 295 |
| Intermediate, % | 31.9% | 295 |
| High, % | 28.1% | 295 |
| TLS Rate | 8% | 296 |
TLS Risk, Prophylaxis, and Incidence
TLS risk classification by investigator at time of initiation of venetoclax was low in 40%, intermediate in 32%, and high in 28%. Sixty two percent of patients had a CT scan within 1 month prior to venetoclax initiation. CT scans were most commonly obtained in high risk patients (76%), with a lower proportion of intermediate (65%) and low risk (55%) having had CTs. The median ALC was 3000/μL in low risk, 27,000/μL in intermediate risk, and 35,100/μL in high risk. The proportion of patients in whom the largest lymph nodes was under 5 cm was 93% in the group at low risk for TLS, while only 30% of patients with high TLS risk had a lymph node under 5 cm. After review of patient level data of ALC and lymph node measurements, 168 patients had sufficient data available to determine TLS risk category. Based on available data, 12.5% of all patients were misclassified in terms of TLS risk. Of the misclassified cases, TLS risk appeared to be non-differential (under-classified in 43% (n=9) and over-classified in 57% (n=12)). Given only partial patient level data, subsequent analyses were based on investigator assigned TLS risk.
We examined strategies employed for TLS monitoring and prophylaxis. At least one planned hospitalization during dose escalation occurred in 88% of high TLS risk patients, 80% of intermediate TLS risk, and 56% of low TLS risk patients based on investigator classification. For patients at high TLS risk, 65% of patients had 2 or more planned hospitalizations during dose ramp up.
Data regarding use of TLS prophylactic agents were available for a subset of the total cohort (Table 2). Normal saline was administered in 85% (n=62/73) of low, 88% (n=49/56) of intermediate, and 97% (n=30/31) of high risk patients. Allopurinol was prescribed in 91% (n=68/75) of low risk patients, 93% (n=52/56) of intermediate, and 94% (n=29/31) of high risk patients. Twenty seven percent (n=28/102) of low risk, 42% (n=34/81) of intermediate risk, and 72% (n=57/79) of high risk patients received at least 1 dose of rasburicase. For those who received rasburicase, the median number of doses was 2 (range 1–17). For a small subset of patients (n=57), data regarding rasburicase dose was available with 23 receiving 3 mg flat dose, 28 receiving 6 mg flat dose, and 6 receiving standard weight-based dosing.
Table 2.
TLS Risk Stratification and Prophylaxis Strategy by TLS Risk
| Low Risk n=118 | Intermediate Risk n=94 | High Risk n=83 | Total Cohort n=297 | |||||
|---|---|---|---|---|---|---|---|---|
| Risk Stratification | % (unless noted) | Number with Available Data | % (unless noted) | Number with Available Data | % (unless noted) | Number with Available Data | % (unless noted) | Number with Available Data |
| CT Scan | 55% | 89 | 65% | 68 | 76% | 33 | 62%, | 190 |
| Largest Lymph Node | ||||||||
| < 5 cm | 93% | 90 | 58% | 66 | 30% | 33 | 71% | 189 |
| 5 to 10 cm | 7% | 90 | 41% | 66 | 28% | 33 | 22% | 189 |
| >10 cm | 0% | 90 | 2% | 66 | 42% | 33 | 8% | 189 |
| ALC, median (range) | 3000/μL (100–46,000/μL) | 76 | 27,000/μL (60–201,000/μL) | 56 | 35,100/μL (700–326,000/μL) | 31 | 9000/μL (60–326,600/μL) | 164 |
| Planned Hospitalizations | n=265 | |||||||
| 0 | 43% | 101 | 20% | 82 | 12% | 80 | 27% | 265 |
| 1 | 18% | 101 | 29% | 82 | 23% | 80 | 23% | 265 |
| 2 | 18% | 101 | 28% | 82 | 36% | 80 | 26% | 265 |
| 3 | 5% | 101 | 10% | 82 | 11% | 80 | 8% | 265 |
| 4 | 6% | 101 | 5% | 82 | 6% | 80 | 6% | 265 |
| 5 | 10% | 101 | 8% | 82 | 9% | 80 | 9% | 265 |
| 6 | 0% | 101 | 0% | 82 | 3% | 80 | 1% | 265 |
| Prophylaxis | ||||||||
| Allopurinol | 91% | 75 | 93% | 56 | 94%, | 31 | 92% | 163 |
| Rasburicase | 27% | 102 | 42% | 81 | 72% | 79 | 45% | 264 |
| Normal Saline | 85% | 73 | 88% | 56 | 97% | 31 | 88% | 161 |
| Outcomes | ||||||||
| Clinical TLS | n=2 | 117 | n=3 | 94 | n=3 | 83 | 2.7% | 296 |
| Laboratory TLS | n=3 | 117 | n=7 | 94 | n=7 | 83 | 5.7% | 296 |
| Requiring HD | n=0 | 117 | n=1 | 94 | n=0 | 83 | 0.3% | 296 |
| Deaths attributable to TLS | n=0 | 118 | n=0 | 94 | n=1 | 83 | 0.8% | 125 |
ALC = absolute lymphocyte count; TLS = tumor lysis syndrome; HD = hemodialysis
Clinical TLS occurred in 2.7% (n=8) of patients and laboratory occurred in 5.7% (n=17; Table 2). Of these 25 events (8.4% of total cohort), 5 total events (4.2%; 3 laboratory and 2 clinical) occurred in low risk patients, 10 events (10.7%; 7 laboratory and 3 clinical) occurred in intermediate risk patients, and 10 events (12.0%; 7 laboratory and 3 clinical) occurred in high risk patients. Only one patient required permanent venetoclax discontinuation because of TLS. Significant AEs related to TLS included requirement of hemodialysis for one patient (intermediate risk, baseline creatinine clearance <80 mL/min) and one death from TLS.(14) This patient had experienced rapid disease progression and treatment-related neutropenia requiring hospitalization. Following dose interruption, the patient was re-challenged with 400 mg of venetoclax without dose escalation. The rate of TLS was 3.4% in patients who received venetoclax paired with another agent and 9.3% for those who received venetoclax as monotherapy (p=0.15). Data regarding timing of paired agents were not available in this analysis.
For those with baseline creatinine clearance ≥80 mL/min, the rate of TLS was 5.0% while the rate was 14.7% in patients with creatinine clearance <80 mL/min (p=0.02). Of the 8 patients who developed clinical TLS, data regarding prophylaxis strategy were available for 7 patients. All 7 (2 low, 2 intermediate, and 2 high risk) had planned hospitalizations for dose escalation (median 3 hospitalizations, range 2–4) and all received allopurinol and normal saline prophylaxis. Two of 7 also received rasburicase for prophylaxis.
Univariate analyses examining risk of TLS by baseline variables including sex, age at venetoclax initiation, number of prior therapies, prior ibrutinib, cytogenetics, IGHV mutational status, creatinine clearance, venetoclax monotherapy vs. paired, and TLS risk group as defined by the FDA package insert were performed (Supplemental Table 1). Variables associated with increased TLS risk were (1) creatinine clearance (<80 vs ≥80 mL/min OR 3.3, 95% CI 1.1–9.5, p=0.031) and (2) FDA package insert defined TLS risk group prior to initiation of venetoclax (high risk OR 3.1 95% CI 1.007–9.3, p=0.048).
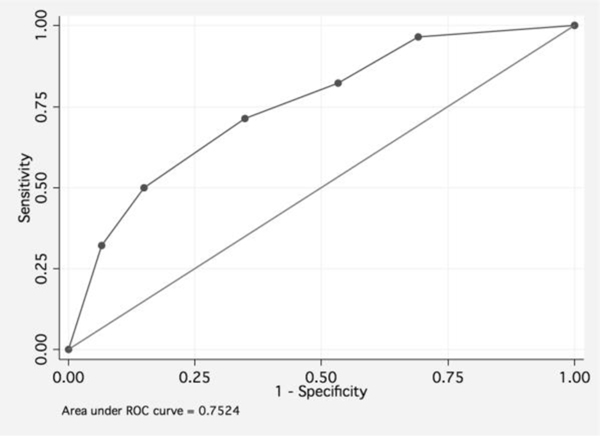
In a multivariable analysis adjusted for patient age at start of venetoclax, TLS risk group and creatinine clearance (categorized by <80 and ≥80 mL/min) independently predict TLS development (high risk vs. low risk OR 3.0, 95% CI 1.7–5.4, p<0.001; creatinine clearance <80 vs ≥80 mL/min OR 2.6, 95% CI 1.1–6.2, p=0.031). The area under the ROC curve for this model including TLS risk group and creatinine clearance was estimated to be 75.2% (Figure 1). As a comparison, the area under the ROC curve for TLS risk as per the FDA package insert was 64.7%. Both variables remained significant in multivariable bootstrap analysis (creatinine clearance <80 vs ≥80 mL/min OR 2.6, p<0.001; high vs. low risk OR 3.0, p=0.021).
Figure 1.
Receiver-operating characteristic curve of sensitivity vs. 1–specificity for a multivariate model including TLS risk group (low, medium, high) and creatinine clearance (< 80 mL/min vs ≥ 80 mL/min). The area under the ROC curve is 79.6%
Other Adverse Events
Data regarding AEs of interest were captured and described in Table 3. In this cohort, grade 3/4 neutropenia (absolute neutrophil count <1000/μL) was observed in 39.6% with neutropenic fevers in 7.9%, thrombocytopenia (platelets <50/μL) in 29.2%, and diarrhea (≥7 stools/day) in 6.9%. AEs were similar for those who received venetoclax paired with another agent. Specifically, for patients who received venetoclax in combination with another agent, AEs included grade 3/4 neutropenia in 35%, thrombocytopenia in 29%, neutropenic fever in 6.3%, and diarrhea in 6.4%.
Table 3.
Rates of Selected Grade 3/4 Adverse Events
| Total Cohort n=297 | Number with available data | |
|---|---|---|
| Total TLS | 8.1% | 296 |
| Neutropenia,1 % | 39.6% | 189 |
| Thrombocytopenia,2 % | 29.2% | 188 |
| Febrile neutropenia, % | 7.9% | 188 |
| Infections, % | 25% | 189 |
| Diarrhea,3 % | 6.9% | 186 |
Neutropenia defined as absolute neutrophil count < 1000/μL
Thrombocytopenia defined as platelet count < 50,000/μL
Diarrhea defined as > 7 stools per day
Prior chemoimmunotherapy was a significant predictor of neutropenia, even after adjusting for number of therapies prior to venetoclax (OR 2.0, p=0.021).
Venetoclax Dosing
Data regarding venetoclax dosing, including rates of dose reduction and interruption are included in Table 4. During dose escalation, 81% completed the full dose escalation to a maximum dose of 400 mg. The stable dose achieved was 400 mg for 65%, 200 mg for 9%, 100 mg for 17%, 50 mg for 6%, 20 mg for 3%. Data regarding concomitant medications and dose reductions for concurrent CYP3A4 inhibitors were not available. PFS was similar for those whose final venetoclax dose was 400 mg and < 400 mg (Supplemental Figure 1A). At least one dose reduction was required for 29% of patients, and 32% of patients required at least one dose interruption. The median length of dose interruption was 7 days (range 1 – 132). Dose interruption did not impact PFS (Supplemental Figure 1B). Dose reductions (≥8 days vs. < 8 days) did not impact PFS (Supplemental Figure 1C).
Table 4.
Venetoclax Dosing and Discontinuation
| Rate, % (proportion) | |
|---|---|
| Achieved 400 mg daily | 81% (137/169) |
| Maintained 400 mg daily | 65% (108/166) |
| Required Dose Reduction | 29% (51/177) |
| Required Dose Interruption Duration median (range) |
32% (58/181) 7 days (1–132) |
| Venetoclax Discontinuation Rate | 40% (119/297) |
| Venetoclax Discontinued for AE | 7.7% (23/297) |
With a median follow up of 12 months (range 1–33 months), 40% (119/297) of patients discontinued venetoclax. The most common reason for discontinuation was CLL progression (35.3%; 42/119 discontinuations, 38/42 with prior ibrutinib exposures), followed by toxicity (19.3%; 23/119), planned cellular therapy (14.3%; 17/119), and Richter’s transformation (10.9%; 13/119). The most common toxicities leading to discontinuation in descending order were thrombocytopenia, infection, and neutropenia.
Discussion
Although data available from several clinical trials clearly demonstrate significant activity, durable remissions, and manageable side effects of venetoclax in the R/R setting, non-trial experience is limited. These data from 297 patients treated with venetoclax outside of the clinical trial setting allow for a richer understanding of TLS classification and prophylaxis strategies employed, rates of clinical and laboratory TLS and other adverse events, and predictors of TLS development. The observations from this dataset can help to shape clinical management strategies, particularly from a patient safety perspective.
Though the package insert for venetoclax defines TLS risk based on tumor burden and provides guidance on prophylaxis strategy, setting of administration, and frequency of assessments, our data suggest variable adherence to these recommendations outside of the clinical trial setting. First, we identified limited use of CT scans to determine lymph node size as only 62% of the cohort had a CT to inform risk stratification. We found that 12.5% of patients who had sufficient data to verify their assigned TLS risk category were misclassified based on the FDA package insert.(7) However, this does not account for possible misclassifications based on non-palpable large lymph nodes unidentified because of lack of radiographic evaluation. These data demonstrate an educational need as imaging is recommended to inform TLS risk, and thus TLS prophylaxis, monitoring, and need for planned hospitalizations. Regardless of TLS risk, adherence to measures such as intravenous fluids and uric acid lowering agents were universally high.
For patients categorized as low risk, the vast majority (91%) were appropriately prescribed allopurinol. Interestingly, 85% received normal saline and 57% had at least one planned hospitalization during dose ramp up. The package insert guidance for patients at low TLS risk is to employ oral hydration and perform the dose ramp up in the outpatient setting. These discrepancies may reflect burden of comorbidities, impaired renal function, or functional status of patients receiving venetoclax. Further, these practices may represent an abundance of caution on the part of the treating physician using a new agent with a novel AE profile or local logistics in which admission is more practical than outpatient monitoring. It is interesting that, despite very conservative TLS prophylactic measures, a small number of clinical events were noted in low risk patients (laboratory TLS 2.5%, clinical TLS 1.7%). Data regarding prophylaxis strategy was available for 4 of 5 low risk patients who developed TLS; 100% were hospitalized for dose escalation (median number of hospitalizations was 4, range 3–5), 100% received allopurinol, and 50% received rasburicase. These data suggest that less intensive TLS prophylaxis and monitoring strategies than recommended by the package insert should not be considered at this time. Additionally, future studies should aim to understand which other factors contribute to the risk for TLS despite standard classification as low risk.
For high risk patients, the FDA package insert recommends use of oral and IV hydration, allopurinol with consideration of rasburicase, and hospitalization for the first 2 dose levels. Use of normal saline (97%) and allopurinol (94%) was nearly ubiquitous, and rasburicase was employed frequently (72%). Not consistent with the FDA package insert, 12% of this group were never hospitalized and 23% were hospitalized once for dose escalation. With these management strategies, the rate of laboratory TLS was 8.4% and clinical TLS was 3.6%, rates that are higher than may be expected based on clinical trial experience.(15) Of the 10 high-risk patients who experienced TLS, 2 patients (both with laboratory TLS) were not hospitalized ≥2 times for dose escalation, 1 did not receive allopurinol given history of Stevens Johnsons Syndrome and instead received rasburicase, and 4 (2 clinical, 2 laboratory) did not receive rasburicase. All of these patients received normal saline. This observation highlights the opportunity for improvement in adherence to the FDA/EMA package insert based on risk, which may allow for lower rates of TLS as have been observed in clinical trials.
TLS high risk group and impaired renal function (creatinine clearance <80 mL/min) independently predict development of TLS. Modeling using these variables performs better than using TLS risk group alone (ROC area 75.2% vs. 64.7%). These data suggest that decision making regarding prophylaxis strategy and setting for dose escalation should consider a patient’s renal function in addition to their TLS risk.
Regarding AE profile, a comprehensive safety analysis of venetoclax monotherapy in CLL patients treated on three early phase clinical trials described rates of grade 3/4 toxicities including neutropenia in 37%, thrombocytopenia in 14%, and diarrhea in 2.5% (9/350).(15) Our observed rate of grade 3/4 neutropenia was similar at 39.6%, though our observed rates of thrombocytopenia (29.2%) and diarrhea (6.9%) were higher than this pooled clinical trial experience. Our data suggest that the toxicity profile for patients treated with venetoclax monotherapy versus venetoclax paired with another agent is similar. While combination therapy appears to be well tolerated, these data highlight the need for prospective data examining monotherapy versus combination therapy to assess comparative efficacy.(16) This analysis was limited by heterogenous population of combination therapy, though the majority (75%) were in combination with anti-CD20 Ab.
Compared to the real-world experiences with ibrutinib where most discontinuations are due to toxicity (rather than progression), toxicity drives discontinuation in a smaller proportion of venetoclax treated patients.(17,18) In our cohort, 29% of patients required dose reduction, 32% required dose interruption for median length of 7 days (range 1–132 days), and 40% discontinued venetoclax with 7.7% having discontinued for AEs. As a comparison, in a composite analysis of safety of venetoclax in the clinical trial setting that included 350 patients, 13% required dose reduction, 34% required dose interruption, and 10% discontinued venetoclax due to AEs.(15) This suggests that, in clinical practice as compared to clinical trials, more patients require dose reductions, although rates of dose interruption and discontinuation for AEs were similar. Notably, we found that dose reductions and interruptions did not adversely impact PFS, and duration of interruption (<8 vs. ≥8 days, a cutoff used by Barr, et al. to examine outcomes with ibrutinib therapy dose interruptions(19)) did not significantly impact PFS in Cox regression analysis. This observation is consistent with data suggesting that outcomes are not adversely impacted by dose interruptions or reductions in patients treated with ibrutinib.(20–22) These hypothesis generating data are the first to examine outcomes stratified by dose reduction or interruption of venetoclax as venetoclax clinical trials have not reported outcomes stratified by these features. These observations are consistent with pharmacodynamic data suggesting that lower doses of venetoclax yield similar responses in lymphocyte count and lymph node size. These data will need confirmation in future studies but provide reassurance that, if clinically required, venetoclax dose interruption and dose reductions do not appear to negatively impact outcomes.
This study is limited by its retrospective design, which required data collection by multiple providers. Although investigators were directed to characterize TLS risk based on the venetoclax package insert and development of TLS based on Howard criteria, central review for consistency of data collection was not possible and we cannot rule out ascertainment bias in these data. The toxicity data presented here were collected based on retrospective chart review, which is not as closely controlled as data collected through prospective trials. However, while many retrospective studies only capture AEs that lead to drug discontinuation, we collected AEs in all patients to provide a more comprehensive AE profile in clinical practice. Given that we do not have data on timing of dose reductions and interruptions, survival data presented here have the potential for survivor bias and need to be confirmed in future studies. Further, given lack of data on concurrent medications and drug-drug interactions, some dose reductions reported here may reflect appropriate dose reduction strategy in the setting of a CYP3A4 inhibitor. Many retrospective series are limited by predominance of patients treated in academic centers. By including both academic and community centers in this study, we have attempted to overcome this limitation.
Despite these limitations, we believe that these data provide important insights into the current use of venetoclax in clinical practice and help inform future use and study design. We have identified both TLS risk classification and TLS prophylaxis strategies that differ from the FDA/EMA package insert, as well as rates of TLS that are higher than those reported in clinical trials of venetoclax. With improved attention to accurate risk classification and adherence to recommended TLS prophylaxis strategies, rates of TLS may be further reduced, likely translating to better patient outcomes. We have validated that the FDA recommended TLS risk strata are predictive of TLS events in clinical practice and that baseline renal function is an important independent predictor of TLS, which should be considered across all TLS risk categories. For patients with a creatinine clearance of <80 mL/min, additional attention to prophylaxis strategies and more conservative monitoring may be warranted. Finally, we found that PFS is not impacted by dose reductions, dose interruptions, or duration of dose interruptions. Further prospective data examining this question will further inform practice. In summary, these data provide important insights into venetoclax use which may allow for improved safety of this effective agent in CLL.
Supplementary Material
Translational Relevance:
Venetoclax, a potent inhibitor of BCL-2, induces apoptosis and is approved for relapsed/refractory CLL (400 mg daily). We examined the adverse event profile of venetoclax and report current approaches to tumor lysis syndrome (TLS) risk stratification and prophylaxis in clinical practice. We identified factors associated with TLS and neutropenia. Pooled pharmacodynamic data (PD) for exposure–efficacy analyses suggest that steady state concentrations of 0.008 μg/mL and 0.146 μg/mL decrease lymphocyte counts and lymph node size by 50% respectively.(1) PD modelling suggests similar responses at 200 mg or 400 mg/day after 6 months of venetoclax exposure (80.9% vs. 84.8%).(1) Impact of dose modifications on outcomes in practice has not been studied. In this large, multicenter cohort of venetoclax-treated CLL patients, we stratified outcomes based on stable venetoclax dose and dose interruptions. Our data suggest that, when clinically indicated, venetoclax dose reduction and/or interruptions do not appear to adversely impact outcomes.
Acknowledgements:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748
Conflict of Interest Statement: LER Abbvie travel grant; CPF Abbvie: Consultancy and speaker work. Travel support. Research support. Roche: Consultancy and speaker work. Travel support. Research support; TAE none; DMB AbbVie: consultant, scientific advisory board, site PI clinical trial (grant paid to institution) Genentech: consultant, scientific advisory board, site PI clinical trial (grant paid to institution) AZ: consultant, site PI clinical trial (grant paid to institution) Pharmacyclics: consultant TG therapeutics: scientific advisory board, site PI clinical trial (grant paid to institution) Teva: consultant, scientific advisory board Novartis: consulting BeiGene: site PI clinical trial (grant paid to institution) DTRM: site PI clinical trial (grant paid to institution); JNA Advisory board/consulting, Genentech, Abbvie, PCYC, Honoraria Janssen, Acerta; SJS Research support and honoraria for Abbvie, Genentech, Pharmacyclics, Janssen, TG therapeutics, DTRM, Gilead; CN Employment Cardinal Health; BTH Genentech/Abbvie, Advisory boards and research funding; NNS none; FL none; MY Abbvie consultancy; BDC Consulting: Abbvie, TG Therapeutics, Roche-Genentech, Pharmacyclics, GIlead, Epizyme, Celgene, Morhphosys, Astellas, Astra Zeneca, Bayer. Research funding (to institution): Abbvie, TG Therapeutics, Roche-Genentech, Pharmacyclics, GIlead, Epizyme, Celgene, Trillium, Astra Zeneca; NL Abbvie: research funding to institution; participation in advisory boards Acerta: research funding to institution Astra Zeneca: research funding to institution; participation in advisory boards Beigene: research funding to institution Celgene: member scientific advisory board Genentech: research funding to institution; participation in advisory boards Gilead: research funding to institution; participation in advisory boards Janssen: participation in advisory boards Juno: research funding to institution; participation in advisory board Oncternal: research funding to institution TG therapeutics: research funding to institution Pharmacyclics: participation in advisory boards Verastem: research funding to institution; AKS none; CCC Honoraria from Pharmacyclics and Abbvie, Consulting for Abbvie; PMB Consulting for Abbvie/PCYC, Gilead, Verastem, Genentech; APS none; MS none; CSU Consultant Genentech, Pharmacyclics, Research Abbvie; HHT none; AMW none; JR none; CD none; HM none; CK none; JMP consulting for Gilead and Pharmacyclics; AMW none; RJ Honoraria from Genentech, AbbVie; AG none; SM none; LP none; AS none; NB none; AS Consultancy: Abbvie, Pharmacyclics, Genentech, Jazz, Pharmaceuticals Advisory Board: Pharmacyclics, Abbvie, Genentech Speakers bureau: Abbvie, Pharmacyclics, Genentech, Jazz Pharmaceuticals, Gilead Sciences, Verastem, Kite, Seattle Genetics; AAK none; ARM Research funding TG Therapeutics, Abbvie, Sunesis, LOXO, Pharmacyclics / J&J, Regeneron, DTRM BioPharma Consultancy TG Therapeutics, Abbvie, Acerta, Sunesis, LOXO, Pharmacyclics / J&J, Regeneron, Celgene, Prime Oncology.
REFERENCES
- 1.Freise KJ, Jones AK, Eckert D, Mensing S, Wong SL, Humerickhouse RA, et al. Impact of Venetoclax Exposure on Clinical Efficacy and Safety in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia. Clin Pharmacokinet 2017;56(5):515–23 doi 10.1007/s40262-016-0453-9. [DOI] [PubMed] [Google Scholar]
- 2.Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med 2011;364(19):1844–54 doi 10.1056/NEJMra0904569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seymour JF, Ma S, Brander DM, Choi MY, Barrientos J, Davids MS, et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol 2017;18(2):230–40 doi 10.1016/S1470-2045(17)30012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med 2016;374(4):311–22 doi 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour J, Munir T, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol 2016;17(6):768–78. [DOI] [PubMed] [Google Scholar]
- 6.Jones J, Mato A, Wierda W, Davids M, Choi M, Cheson B, et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol 2018;19(1):65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA. Highlights of Prescribing Information: Venclexta. 2018. [Google Scholar]
- 8.Nabhan C, Klink A, Samp J, Pauff J, Nielsen J, Meissner B, et al. Management, adverse events, and outcomes of 282 CLL pateints treated with venetoclax in the real world. EHA 2018;214816. [Google Scholar]
- 9.Mato AR, Roeker LE, Allan JN, Pagel JM, Brander DM, Hill BT, et al. Outcomes of front-line ibrutinib treated CLL patients excluded from landmark clinical trial. Am J Hematol 2018;93(11):1394–401 doi 10.1002/ajh.25261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European_Medicines_Agency. European public assessment report: Venclyxto. 2016. [Google Scholar]
- 11.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018;131(25):2745–60 doi 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
- 12.Bland JM, Altman DG. Survival probabilities (the Kaplan-Meier method). BMJ 1998;317(7172):1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson P, Gill R. Cox’s regression model for counting processes: a large sample study. Ann Statist 1982;10(4):1100–20. [Google Scholar]
- 14.Mato AR, Thompson M, Allan JN, Brander DM, Pagel JM, Ujjani CS, et al. Real-world outcomes and management strategies for venetoclax-treated chronic lymphocytic leukemia patients in the United States. Haematologica 2018;103(9):1511–7 doi 10.3324/haematol.2018.193615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davids MS, Hallek M, Wierda W, Roberts AW, Stilgenbauer S, Jones JA, et al. Comprehensive Safety Analysis of Venetoclax Monotherapy for Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia. Clin Cancer Res 2018;24(18):4371–9 doi 10.1158/1078-0432.CCR-17-3761. [DOI] [PubMed] [Google Scholar]
- 16.Sarraf Yazdy M, Mato AR, Cheson BD. Combinations or sequences of targeted agents in CLL: is the whole greater than the sum of its parts (Aristotle, 360 BC)? Blood 2019;133(2):121–9 doi 10.1182/blood-2018-08-869503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mato AR, Nabhan C, Thompson MC, Lamanna N, Brander DM, Hill B, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica 2018;103(5):874–9 doi 10.3324/haematol.2017.182907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forum UC. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica 2016;101(12):1563–72 doi 10.3324/haematol.2016.147900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barr PM, Brown JR, Hillmen P, O’Brien S, Barrientos JC, Reddy NM, et al. Impact of ibrutinib dose adherence on therapeutic efficacy in patients with previously treated CLL/SLL. Blood 2017;129(19):2612–5 doi 10.1182/blood-2016-12-737346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mato AR, Timlin C, Ujjani C, Skarbnik A, Howlett C, Banerjee R, et al. Comparable outcomes in chronic lymphocytic leukaemia (CLL) patients treated with reduced-dose ibrutinib: results from a multi-centre study. Br J Haematol 2018;181(2):259–61 doi 10.1111/bjh.14540. [DOI] [PubMed] [Google Scholar]
- 21.Akhtar OS, Attwood K, Lund I, Hare R, Hernandez-Ilizaliturri FJ, Torka P. Dose reductions in ibrutinib therapy are not associated with inferior outcomes in patients with chronic lymphocytic leukemia (CLL). Leuk Lymphoma 2019:1–6 doi 10.1080/10428194.2018.1554862. [DOI] [PubMed] [Google Scholar]
- 22.Chen LS, Bose P, Cruz ND, Jiang Y, Wu Q, Thompson PA, et al. A pilot study of lower doses of ibrutinib in patients with chronic lymphocytic leukemia. Blood 2018;132(21):2249–59 doi 10.1182/blood-2018-06-860593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.