Abstract
Background
Endometriosis is a common gynecological condition in which stromal or glandular epithelium is implanted in extrauterine locations. Endometriosis causes detrimental effects on the granulosa cells, and phthalate interferes with the biological and reproductive function of endometrial cells at a molecular level.
Methods
This article retrospectively reviewed the studies on phthalate exposure and its relationship with endometriosis. A literature search was performed for scientific articles using the keywords “phthalate and endometriosis,” “endometriosis and granulosa cells,” “phthalate and granulosa cells,” and “phthalates and endometrial cells.”
Results
Endometriosis can affect cytokine production, steroidogenesis, cell cycle progression, expression of estrogen receptor‐α (ER‐α)/progesterone receptor (PR), and cause endoplasmic reticulum stress, senescence, apoptosis, autophagy, and oxidative stress in the granulosa cells. Mono‐n‐butyl phthalate (MnBP) alters the expression of cytokines, cell cycle‐associated genes, ovarian stimulation, steroidogenesis, and progesterone production. Several in vitro studies have demonstrated that phthalate caused inflammation, invasion, change in cytokines, increased oxidative stress, viability, resistance to hydrogen peroxide, and proliferation of endometrial cells.
Conclusion
This might provide new insights about the impact of phthalate on the pathogenesis of endometriosis and its consequences on the ovarian function.
Keywords: endometrial cells, endometriosis, granulosa cells, phthalate, reproductive function
1. INTRODUCTION
Endometriosis represents a common gynecological condition in which the stromal or glandular epithelium gets implanted in extrauterine locations. 1 , 2 , 3 The prevalence of endometriosis is estimated to be 10% among women of reproductive age, which is approximately 190 million women worldwide. 4 Some women with endometriosis are asymptomatic; however, most of them suffer from chronic pelvic pain, dysmenorrhea, deep dyspareunia, and infertility. 5 Although the hypotheses about the etiopathology of endometriosis have been reported for almost one century, the etiology remains unknown. 6 Several evidences suggested that environmental pollutants might be involved in the pathogenesis of endometriosis. 7 , 8 , 9 , 10 , 11 These endocrine‐disrupting chemicals interrupt hormonal homeostasis and result in estrogen signaling changes. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23
Phthalate is diesters of phthalate, which are commonly used in several plastic products, and solubilize other agents. 24 , 25 , 26 Ingestion and inhalation are the common routes of phthalate exposure. 27 , 28 , 29 After entering the body, phthalates are hydrolyzed by the digestive tract into monoesters, then absorbed, oxidized, and excreted in the urine. 28 , 30 , 31 , 32 Several reports have suggested a link between phthalates and human reproductive health through their influence on spermatogenesis in males. 33 Previous studies have demonstrated that sexually mature female rats exposed to di (2‐ethylhexyl) phthalate (DEHP) and its metabolite, mono (2‐ethylhexyl) phthalate (MEHP) showed a decrease in serum progesterone, delayed ovulation, and smaller preovulatory follicles with high levels of serum follicle‐stimulating hormone (FSH). 17 , 19 , 34 , 35 Many studies demonstrated that phthalate exposure is significantly associated with endometriosis. 10 , 36 , 37 , 38 , 39 , 40
Kim et al. demonstrated the effects of DEHP on endometrial cells, including cell invasion, viability, proliferation, and oxidative stress through mitogen‐activated protein kinase (MAPK)/ extracellular regulated protein kinase (Erk), and nuclear factor‐κB (NF‐κB) pathways. 10 , 41 Endometriosis is harmful to granulosa cells, because it affects steroidogenesis and cell cycle progression, lowers aromatase activity, and alters the mitochondrial gene expression in human granulosa cells. 38 , 42 , 43 , 44 , 45 , 46 , 47 This article retrospectively searched the keywords “phthalate and endometriosis,” “endometriosis and granulosa cells,” “phthalate and granulosa cells,” and “phthalates and endometrial cells” on PubMed. This review aims to evaluate the exposure of phthalate and the risk of endometriosis, and its impact on the granulosa cells based on current data.
2. PHTHALATE EXPOSURE AND THE RISK OF ENDOMETRIOSIS
Several studies and meta‐analyses have suggested an association between phthalate exposure and the risk of endometriosis (Table 1). In 2003, Cobellis et al. 48 found a positive correlation between plasma DEHP and endometriosis. DEHP and MEHP were detected in the peritoneal fluid. Reddy et al. published two papers about higher levels of butyl benzyl phthalate (BBP), DEHP, di‐n‐butyl phthalate (DnBP), and di‐n‐octyl phthalate (DnOP) in women with endometriosis compared to those in the control group, which were also significantly associated with stage I‐IV of endometriosis. 49 , 50 Nazri et al. also reported that DEHP was increased in the serum of women with endometriosis. 51 In Taiwan, two studies demonstrated an increased level of urinary mono‐n‐butyl phthalate (MnBP) in women with endometriosis. 38 , 52 In Korea, Kim et al. reported that women with endometriosis had higher levels of DEHP and MEHP in the plasma, and the presence of mono‐2‐ethyl‐5‐hydroxyhexyl phthalate (MEHHP), mono‐2‐ethyl‐5‐oxohexyl phthalate (MEOHP), Log mono‐2‐ethyl‐5‐carboxyphentyl phthalate (MECPP), Log MEHHP, Log MEOHP in the urine. 41 , 53 In the United States, 14 phthalate metabolites were analyzed in both, population cohort comprised women matched on age and residence (n = 131) and operative cohort comprised women undergoing laparoscopy (n = 495). The study found that MnBP, mono‐2‐carboxymethyl hexyl phthalate (MCMHP), MECPP, MEHP, MEHHP, MEOHP were higher in the population cohort in women with endometriosis. Moreover, MEHP and monooctyl phthalate (MOP) were increased in the operative cohort in women with endometriosis. 8 Upson et al. 54 showed an inverse association between MEHP and the risk of endometriosis. However, some studies have reported a reverse correlation or no association between endometriosis and some phthalate metabolites, which might be due to non‐adjustment for other covariates or small sample size. 54 , 55 , 56 , 57
TABLE 1.
Epidemiological studies and meta‐analysis of the association between Phthalate or and endometriosis
| Author | Study design | No. of Endometriosis/control | Samples | Metabolites | Outcomes of endometriosis | Reference |
|---|---|---|---|---|---|---|
| Cobellis et al. 2003 | Case‐control | 35/24 | Blood | DEHP, MEHP | Higher plasma DEHP | 48 |
| Peritoneal fluid | Detectable peritoneal fluid DEHP and/or MEHP | |||||
| Reddy et al. 2006 | Case‐control | 49/38 | Blood | BBP, DEHP, DnBP, DnOP | Higher BBP, DEHP, DnBP, DnOP | 50 |
| Reddy et al. 2006 | Case‐control (stage I‐IV) | 85/135 | Blood | BBP, DEHP, DnBP, DnOP | Higher BBP, DEHP, DnBP, DnOP | 49 |
| Itoh et al. 2009 | Case‐control | 57/80 | Urine | MBzP, MEHHP, MEHP, MEOHP, MEP, MnBP | No significant association | 57 |
| Weuve et al. 2010 | Case‐sectional | 87/1020 | Urine | MnBP, MBzP, MEHHP, MEHP, MEOHP, MEP | Higher MnBP, Lower MEHP | 56 |
| Huang et al. 2010 | Case‐control | 28/29 | Urine | MMP, MEP, MnBP, MBzP, MEOHP, MEHHP | Higher MnBP | 52 |
| Kim et al. 2011 | Case‐control | 97/169 | Blood | DEHP, MEHP | Higher DEHP, MEHP | 53 |
| Buck Louis et al. 2013 | Cohort (Population) | 14/113 | Urine | MnBP, MBzP, MCHP, MCMHP, MCPP, MECPP, MEHHP, MEHP, MEOHP, MEP, MIBP, MMP, MNP, MOP | Two fold higher MnBP, MCMHP, MECPP, MEHP, MEHHP, MEOHP | 8 |
| Cohort (Operative) | 190/283 | Urine | Higher MEHP, MOP, | |||
| Upson et al. 2013 | Case‐control | 95/195 | Urine | MEHP, MEHHP,MEOHP, MECPP, DEHP, MBzP, BzBP, MEP, MiBP, MnBP, DBP | Lower MEHP | 54 |
| Kim et al. 2015 | Cohort | 55/33 | Urine | MBzP, MECPP, MEHHP, MEOHP, MnBP | Higher MEHHP, MEOHP, Log MECPP, Log MEHHP, Log MEOHP | 41 |
| Nazri et al. 2018 | Case‐control | 50/50 | Blood | DEHP | Higher DEHP | 51 |
| Cai et al. 2019 | Meta‐analysis | 8 studies | MEHHP, MEHP, MEP, MBzP, MEOHP | Higher MEHHP | 58 | |
| Wen et al. 2019 | Meta‐analysis | 6 studies | PAEs | Higher DEHP | 39 | |
| Moreira Fernandez et al. 2019 | Case‐control | 30/22 | Urine | MMP, MiBP, MnBP, MCHP, MiNP, MOP, MBzP, MEHP | No significant association | 55 |
| Chou et al. 2020 | Case‐control (Operative) | 123/82 | Urine | MnBP, MEHP, MBzP, MEOHP, MEHHP | Higher MnBP | 38 |
Abbreviations: BBP, butyl benzyl phthalate; DEHP, di‐2‐ethylhexyl phthalate; DnBP, di‐n‐butyl phthalate; DnOP, di‐n‐octyl phthalate; MBzP, monobenzyl phthalate; MCHP, monocyclohexyl phthalate; MCMHP, mono‐2‐carboxymethyl hexyl phthalate; MCPP, mono‐3‐carboxypropyl phthalate; MECPP, mono‐2‐ethyl‐5‐carboxyphentyl phthalate; MEHHP, mono‐2‐ethyl‐5‐hydroxyhexyl phthalate; MEHP, mono‐2‐ethylhexyl phthalate; MEOHP, mono‐2‐ethyl‐5‐oxohexyl phthalate; MEP, monoethyl phthalate; MIBP, mono‐2‐isobutyl phthalate; MMP, monomethyl phthalate;MNP, monoisonoyl phthalate; MnBP, mono‐n‐butyl phthalate; MOP, monooctyl phthalate; PAEs, phthalate esters.
Recently, two meta‐analyses reported about the association between endometriosis and phthalate metabolites. The most commonly used phthalate, DEHP, showed a significant risk of endometriosis (OR = 1.42; 95% CI: 1.19‐1.7). 39 Another meta‐analysis analyzed five phthalate metabolites from eight studies and reported that only MEHHP was associated with endometriosis in Asia but not in the USA. 58
These studies and meta‐analyses strengthen the evidence that phthalate metabolites might play an important role in the occurrence of endometriosis. More studies on urine samples of women with endometriosis should be conducted to prove the association between phthalate exposure and endometriosis.
3. GRANULOSA CELLS IN WOMEN WITH ENDOMETRIOSIS
Endometriosis might affect the granulosa cell steroidogenesis, change the cell cycle progression, cytokine expression (interleukin‐6 [IL6], interleukin‐8 [IL‐8], interleukin‐12 [IL‐12], and tumor necrosis factor‐α [TNF‐α]), alter the mitochondrial gene expression, decrease the aromatase activity, and vascular endothelial growth factor (VEGF) and growth differentiation factor‐9 (GDF‐9) in human granulosa cells. 42 , 43 , 44 , 45 , 46 , 63 These reports suggest that endometriosis might be harmful to granulosa cells making them less sensitive to luteinizing hormone stimulation. 47 , 64 Moreover, the expression of progesterone receptor (PR) and estrogen‐α in granulosa cells was higher in women with endometriosis than in those with tubal infertility. 65 Sreerangaraja Urs et al. 66 demonstrated that decrease in steroidogenic acute regulatory protein (StAR) and 3β‐hydroxysteroid dehydrogenase (3β‐HSD), mitochondrial dysfunction, and apoptosis were found in the granulosa cells of women with endometriosis. Sanchex et al. 67 reported that dysregulation of the wingless‐related integration site (WNT) pathway and down‐regulation of survivin were found in the granulosa cells of women with endometriosis.
Recently, Sirtuin 2 (silent information regulator proteins; SIRT2) and kisspeptin receptor (KISS1R) were found to be increased in the granulosa cells of women with endometriosis. 68 , 69 The granulosa cells from women with endometriosis had higher NF‐κB binding activity, increased expression of inhibitor of NF‐κB kinase subunit β (IKKβ) and NF‐κB inhibitor α (IκBα), which decreased the telomerase activity and human telomerase reverse transcriptase (hTERT). 70 Moreover, intrafollicular TNF‐α might decrease the telomerase activity and hTERT through NF‐κB activation. 70 Li et al. 71 found that down‐regulation of the long non‐coding RNA MALAT1 decreased the granulosa cell proliferation in women with endometriosis through an increase in the p21 expression via the MAPK/Erk activation pathway. In women with peritoneal endometriosis, the expression of bone morphogenetic protein 6 (BMP6) and mothers against decapentaplegic homolog 6 (SMAD6) were decreased in the granulosa cells. 72 Moreover, Ding et al. 73 demonstrated the increased Beclin‐1 (BECN1) and provoked autophagy in the late follicular progesterone elevation in the granulosa cells of women with ovarian endometriosis.
There are many evidences about increased oxidative stress in the granulosa cells of women with endometriosis, who when compared to women with normal ovaries showed higher 8‐hydroxydeoxyguanosine and lipid peroxidation (4‐hydroxy‐2‐nonenal). 74 , 75 , 76 Lin et al. reported that the granulosa cells from women with endometriosis had excessive reactive oxygen species, which provoked senescence through endoplasmic reticulum (ER) stress, decrease in mitochondrial membrane potential, and reduction in ATP production. 77 ER stress is significantly associated with oxidative stress. Treating the oxidative stress inducer caused upregulation of the unfold protein response (UPR)‐associated genes and apoptosis in human granulosa cells. 78 Moreover, the granulosa cells from women with endometriosis expressed several transcripts associated with UPR and increased the phosphorylation of ER stress sensor proteins, including inositol‐requiring enzyme 1 and double‐stranded RNA‐activated protein kinase‐like ER kinase (PERK). These results suggested that high oxidative stress in the granulosa cells in women with endometriosis provoked ER stress and apoptosis. 78 These studies illustrated that endometriosis is harmful to the granulosa cells and might lead to ovarian dysfunction (Figure 1).
FIGURE 1.
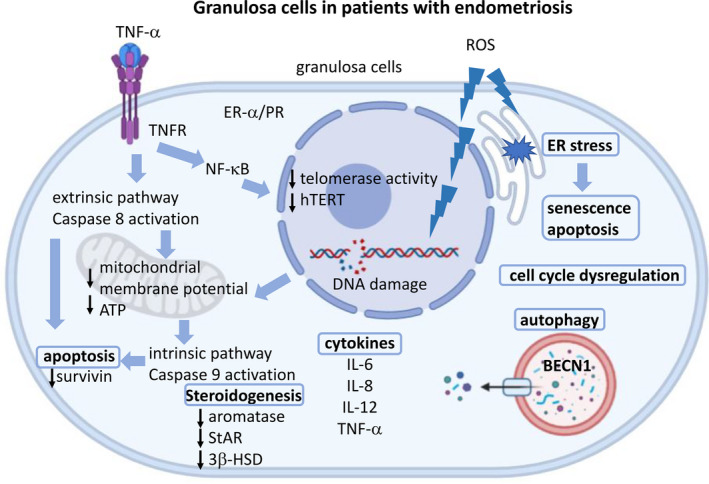
The potential effects of endometriosis on granulosa cells. Endometriosis might affect steroidogenesis (aromatase, StAR, 3β‐HSD), cytokine production (IL6, IL‐8, IL‐12, TNF‐ α), cell cycle progression, ER‐α/ PR, oxidative stress, ER stress, apoptosis, senescence, and autophagy in granulosa cells. The granulosa cells in women with endometriosis showed increased oxidative stress, which induced DNA damage, and decreased the mitochondrial membrane potential and ATP production and induced apoptosis. The increased TNF‐α activated NF‐κβ to decrease the telomerase activity and hTERT. TNF‐α also induced extrinsic and intrinsic apoptosis pathway and decreased survivin expression. The increased oxidative stress in the granulosa cells in women with endometriosis stimulated senescence and apoptosis through ER stress. StAR, steroidogenic acute regulatory protein; 3β‐HSD, 3β‐hydroxysteroid dehydrogenase; IL‐6, interleukin‐6; IL‐8, interleukin‐8; IL‐12, interleukin‐12; TNF‐α, tumor necrosis factor α; ER‐α, estrogen receptor‐ α ; PR, progesterone receptor; NF‐κB, nuclear factor‐κB.; hTERT, human telomerase reverse transcriptase, ER stress, endoplasmic reticulum stress; BECN1, beclin‐1. This figure was created with BioRender.com
4. EFFECTS OF PHTHALATE ON GRANULOSA CELLS
Phthalates are common synthetic chemicals with ubiquitous exposures in our daily life. Till now, several epidemiologic evidences have reported that phthalates might be toxic to the male and female reproductive system. 37 Studies from laboratory examinations showed that phthalates interacted with the female reproductive system in animal models; these findings support the potential hazardous effects of phthalates in women. Granulosa cells play an important role in the ovarian follicular growth and steroidogenesis. The first study from Treinen et al. 79 reported that MEHP decreased FSH‐induced cyclic adenosine monophosphate (cAMP) accumulation in the granulosa cells. Furthermore, MEHP also inhibited FSH‐induced progesterone production by a protein kinase‐C‐independent mechanism. 80 Davis et al. found that MEHP‐related decrease in estradiol concentration might be due to decreased aromatase independent of the cAMP‐stimulated pathway in granulosa cells. 17 , 19 , 81 Lovekamp‐Swan et al. demonstrated that MEHP stimulated peroxisome proliferator‐activated receptor‐α (PPAR‐α) and peroxisome proliferator‐activated receptor‐γ (PPAR‐γ) to inhibit aromatase and decreased cAMP stimulation to alter the metabolism‐ and differentiation‐associated genes. 82 , 83 MEHP stimulated basal steroidogenesis and StAR expression in primary cultures of Leydig cell progenitors and immature granulosa cells in rats. 84 It induced ovarian toxicity by inhibition of follicular development and abnormal steroid hormone synthesis in cultured rat ovarian follicles. 85 It also inhibited the rat granulosa cell viability, increased apoptosis through caspase‐3 activation and Bcl‐2‐associated x protein (BAX) expression, stimulated steroid hormone secretion, and induced the expression of key enzymes in progesterone expression and sex hormone receptors. 86 , 87 , 88
In DEHP‐fed rats, the estrous cycles were prolonged, delayed, or ovulation was suppressed; moreover, smaller preovulatory follicles were found, suggestive of polycystic ovaries and hypoestrogenic anovulatory cycles. 34 PPARs are the crucial regulators of cell differentiation and lipid metabolism. DEHP had a dual effect on the pituitary‐gonadal axis including stimulation of the hormonal effects of the pituitary gland and inhibition of steroidogenesis in granulosa cells at the same time. 89 The DEHP exposure induced apoptosis and increased the production of reactive oxygen species (ROS) in horse granulosa cells. 90 DEHP also provoked oxidative stress by increasing the ROS levels and mitochondrial membrane potential, and the levels of apoptotic markers (BAX and cytochrome c). 91 Furthermore, DEHP arrested the cell cycle progression in G0/G1 phases and increased the proportion of apoptosis in rat granulosa cells. 92 Chen et al. 93 found that benzyl butyl phthalate (BBP) stimulated necrosis through aryl hydrocarbon receptor (AhR) and cytochrome‐P450 (CYP)1B1 in HO23 cells (immortalized human granulosa cells). In KGN cells (human granulosa cell lines), DEHP reduced estradiol production and induced AhR expression to regulate the function of granulosa cells. 94 DEHP also induced several microRNAs (miRNAs), including let‐7b, miR‐17‐5p miR‐181a, and miR‐151, to inhibit the proliferation of follicular granulosa cells. Moreover, DEHP affected the anti‐apoptosis function of KIT ligand (KITL) and growth differentiation factor‐9 (GDF‐9) and increased the BAX/ BCL2 expression ratio to promote apoptosis of the granulosa cells. 95 Recently, Li et al. 88 demonstrated that quails fed on DEHP showed mitochondrial damage and decreased thickness of the ovarian granulosa cell layer, along with oxidative stress.
DBP is ubiquitous in our daily life and might affect the health in humans. Wang et al. 96 reported that DBP reduced FSH‐induced KIT ligand G (KITLG) expression and hypoxia‐inducible factor 1‐α (HIF1‐α) to suppress estradiol and progesterone production and proliferation of the granulosa cells. From global gene expression analysis, expression of the cell cycle, mitosis, Rho GTPases, polo‐like kinase‐1 (PLK1), Aurora B signaling pathways, and E2F‐mediated regulation of DNA replication, steroidogenic, angiogenic, and epidermal growth factor‐like growth factor genes, including CYP11A1, CYP19A1 (aromatase), VEGF‐A, betacellulin (BTC), StAR and epiregulin (EREG) were associated with DBP exposure in the granulosa cells. 97 , 98 Li et al. 99 reported that DBP reduced oocyte germinal vesicle breakdown (GVBD) and polar body extrusion (PBE) rate in mice, damaged oocyte cytoskeleton, and disrupted the cortical granule‐free domains (CGFDs), and induced early apoptosis of the oocyte and granulosa cells. In the human granulosa cell line KGN, treatment with DBP upregulated the expression of aromatase, estradiol, and FSH receptors. 100 Moreover, Mei et al. 101 found that dimethyl phthalate (DMP) increased the apoptotic rate of ovarian granulosa cells and interfered with the pituitary‐ovary axis. These studies proved that phthalate interferes with the biological and reproductive function.
The effects of MnBP on granulosa cells are shown in Figure 2. With a low dose of MnBP, the expression of progesterone, vimentin, and phosphorylated p65 was significantly increased in the mouse granulosa cells. Further experiments found that MnBP stimulated the binding of p65 to vimentin promoter to induce progesterone production. 102 Recently, Chou et al. demonstrated that MnBP attenuated the ratio of the mitochondrial membrane potential and affected the gene expression levels of baculoviral inhibitor of apoptosis repeat‐containing 5 (BIRC3), budding uninhibited by benzimidazoles 1 homolog beta, mitotic checkpoint serine/threonine kinase beta (BUB1B), cell division cycle 20 (CDC20), cyclin B1, IL‐1β, and TNF‐α in human granulosa cells. Moreover, MnBP also decreased the steroidogenesis genes and hormones, including anti‐Mullerian hormone (AMH), inhibin B, StAR, and cytochrome cholesterol side‐chain cleavage enzyme (P450ssc), and expression of human granulosa cells. 38
FIGURE 2.
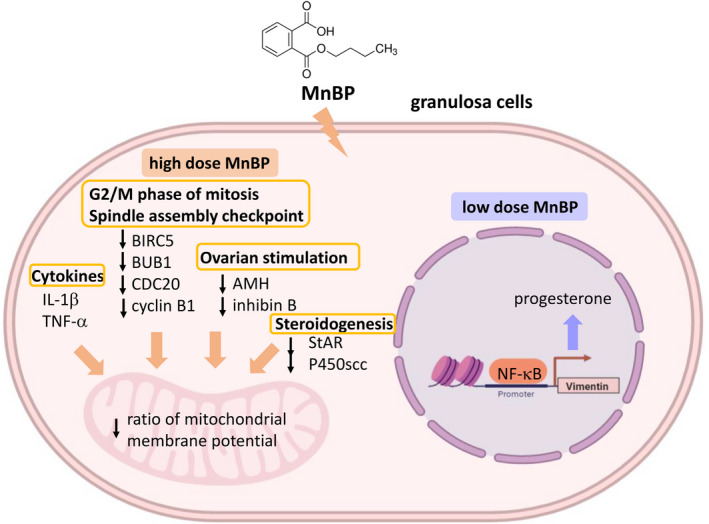
Potential mechanisms of MnBP on human granulosa cells. A high dose of MnBP, it stimulates IL‐1β and TNF‐α cytokine expression. MnBP also affects the G2/M phase of mitosis and spindle assembly checkpoint, including BIRC5, BUB1, CDC20, and cyclin B1 gene expression. These changes cause decrease in AMH, inhibin B, StAR, and P450scc, which affect ovarian stimulation and steroidogenesis. The affected gene expressions result in poor health of the cells. A low dose of MnBP stimulated NF‐κB binding to vimentin promoter and induced progesterone production. MnBP, Mono‐n‐butyl phthalate; IL‐1β, interleukin‐1β; TNF‐α, tumor necrosis factor α; BIRC5, baculoviral inhibitor of apoptosis repeat‐containing 5; BUB1B, budding uninhibited by benzimidazoles 1 homolog beta, mitotic checkpoint serine/threonine kinase beta; CDC20, cell division cycle 20; AMH, anti‐Mullerian hormone; StAR, steroidogenic acute regulatory protein; P450ssc, cytochrome cholesterol side‐chain cleavage enzyme; NF‐κB, nuclear factor‐κB. This figure was created with BioRender.com
5. EFFECTS OF PHTHALATE METABOLITES ON ENDOMETRIAL CELLS
The first report of the effects of phthalate on endometrial cells showed that DEHP and MEHP stimulated the secretion of prostaglandin F2‐α (PGF2‐α) and inhibited the secretion of prostaglandin E2 (PGE2). 103 Kim et al. 104 also found that DEHP results in increased viability of the endometrial stromal cells in the serum‐free condition with exposure to hydrogen peroxide. Another study demonstrated that DEHP induced the expression of IL‐1β, IL‐8, matrix metalloproteinase‐2 (MMP2), intercellular cell adhesion molecule‐1 (ICAM‐1), cyclooxygenase‐2 (COX2), and PPARγ to stimulate the inflammatory response, and it might be mediated by PPARγ. 105
The effects of DEHP on human endometrial cells include increased ROS generation and decreased expression of superoxide dismutase (SOD), glutathione peroxidase (GPX), heme oxygenase (HO), and catalase (CAT), phosphorylated‐Erk/ phosphorylated‐p38 and NF‐κB‐mediated transcription, and estrogen receptor‐α (ER‐α) expression. 106 In DEHP‐treated mice, the volume of peritoneal endometriotic lesion increased, with higher expression of MMP‐2, MMP‐9, and p21‐activated kinase‐4 (Pak‐4). Increased cell invasion and phosphorylation of Erk were observed in DEHP‐treated endometrial cells. 41 Human endometrial cells from the eutopic endometrium of endometriosis showed upregulation of aldo‐keto reductase (AKR) 1C1, AKR1C2, AKR1C3, and AKR1B10 after DEHP exposure, while AKR1C3 continuously increased in the endometrial cells of the ectopic endometrium in patients of endometriosis both before and after DEHP exposure. 107 Under conditions of hypoxia, DEHP decreased the ER‐α protein and VEGF secretion in Ishikawa endometrial adenocarcinoma cells. 108 In chronic, low‐dose DEHP feeding mice, the endometrial stromal cells were significantly increased and changed the localization of steroid hormone receptors. 109 These studies are illustrated in Figure 3.
FIGURE 3.
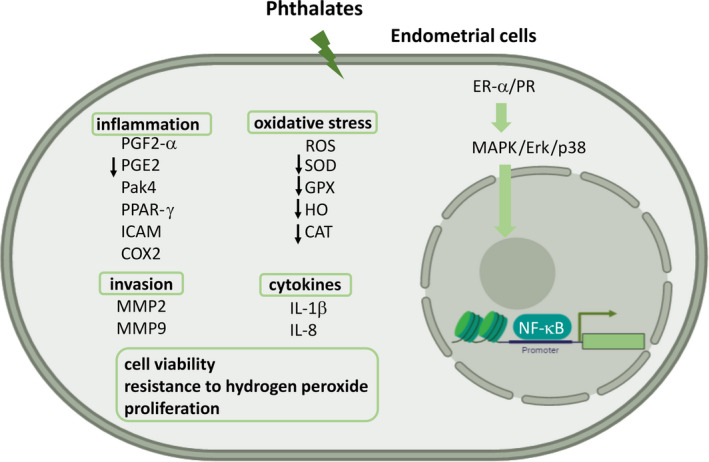
The effect of phthalates on endometrial cells. After phthalate stimulation, the endometrial cells showed inflammation, invasion, change of cytokines, increased oxidative stress, cell viability, resistance to hydrogen peroxide, and proliferation. The inflammatory effects stimulated the secretion of PGF2‐α, Pak‐4, PPARγ, ICAM‐1, COX2, cytokine (IL‐1β and IL‐8), and inhibited the secretion of PGE2. Phthalate also increased ROS generation and decreased the expression of SOD, GPX, HO, and CAT. In DEHP‐treated mice, the endometrial cell might show increased migration through MMP‐2 and MMP‐9. Increased ER‐α/PR activated p‐ERK/p‐p38 and NF‐κB. Exposure to phthalate induced endometrial cell viability, resistance to hydrogen peroxide and proliferation. PGF2‐α, prostaglandin F2‐α; IL‐1β, interleukin‐1β; IL‐8, interleukin‐8; Pak‐4, p21‐acticvated kinase‐4; PPARγ, peroxisome proliferator‐activated receptor‐γ; ICAM‐1, intercellular cell adhesion molecule‐1; COX2, cyclooxygenase‐2; prostaglandin E2, PGE2. ROS, reactive oxygen species; SOD, superoxide dismutase, GPX, glutathione peroxidase; HO, heme oxygenase; CAT, catalase; MMP2, matrix metalloproteinase‐2; MMP9, matrix metalloproteinase‐9; ER‐α, estrogen receptor‐α; PR, progesterone receptor; NF‐κB, nuclear factor‐κB. This figure was created with BioRender.com
From these studies, it is evident that phthalate exposure might affect gene regulation, invasion, cell viability, and proliferation of endometrial cells to influence the development of endometriosis.
6. CONCLUSION
In this review, the interaction between phthalate exposure and granulosa cells in women with endometriosis has been discussed based on the evidence from several studies. A thorough understanding of the effects of phthalate on granulosa cells and endometrial cells might provide new insights into the pathogenesis of endometriosis and its biological effects on ovarian function. More studies are necessary to understand the detailed mechanisms of the interplay between phthalate, granulosa cells, and endometriosis.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human and Animal Rights: This article does not contain any study with human or animal participants that have been performed by any of the authors.
ACKNOWLEDGEMENTS
This work was supported by the Ministry of Science and Technology (grant number 104‐2314‐B‐038‐063‐MY2, grant number 106‐2314‐B‐038‐072, grant number 107‐2314‐B‐038‐006), Academia Sinica (grant number BM10501010036, grant number BM10601010024, grant number BM10701010027), National Health Research Institute (grant number MG‐105‐SP‐07, grant number MG‐106‐SP‐07, grant number MG‐107‐SP‐07; CRT), and Ministry of Science and Technology (grant number 107‐2314‐B‐009‐006; YCC). This work was also supported by the Center for Intelligent Drug Systems and Smart Bio‐devices (IDS2B) from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. The figures were created with Biorender.com.
Chou Y‐C, Tzeng C‐R. The impact of phthalate on reproductive function in women with endometriosis. Reprod Med Biol. 2021;20:159–168. 10.1002/rmb2.12364
REFERENCES
- 1. Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann N Y Acad Sci. 2002;955:89‐100; discussion 118, 396–406. [DOI] [PubMed] [Google Scholar]
- 2. Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P. Endometriosis. Nat Rev Dis Primers. 2018;4(1):9. [DOI] [PubMed] [Google Scholar]
- 3. Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382(13):1244‐1256. [DOI] [PubMed] [Google Scholar]
- 4. Shafrir AL, Farland LV, Shah DK, et al. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. 2018;51:1‐15. [DOI] [PubMed] [Google Scholar]
- 5. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789‐1799. [DOI] [PubMed] [Google Scholar]
- 6. Olive DL, Schwartz LB. Endometriosis. N Engl J Med. 1993;328(24):1759‐1769. [DOI] [PubMed] [Google Scholar]
- 7. Lai GL, Yeh CC, Yeh CY, et al. Decreased zinc and increased lead blood levels are associated with endometriosis in Asian Women. Reprod Toxicol. 2017;74:77‐84. [DOI] [PubMed] [Google Scholar]
- 8. Buck Louis GM, Peterson CM, Chen Z, et al. Bisphenol A and phthalates and endometriosis: the Endometriosis: Natural History, Diagnosis and Outcomes Study. Fertil Steril. 2013;100(1):162‐169 e161–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smarr MM, Kannan K, Buck Louis GM. Endocrine disrupting chemicals and endometriosis. Fertil Steril. 2016;106(4):959‐966. [DOI] [PubMed] [Google Scholar]
- 10. Kim JH, Kim SH. Exposure to phthalate esters and the risk of endometriosis. Dev Reprod. 2020;24(2):71‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L. Endocrine‐disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol. 2020;8(8):703‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agarwal DK, Lawrence WH, Autian J. Antifertility and mutagenic effects in mice from parenteral administration of di‐2‐ethylhexyl phthalate (DEHP). J Toxicol Environ Health. 1985;16(1):71‐84. [DOI] [PubMed] [Google Scholar]
- 13. Lamb JCT, Chapin RE, Teague J, Lawton AD, Reel JR. Reproductive effects of four phthalic acid esters in the mouse. Toxicol Appl Pharmacol. 1987;88(2):255‐269. [DOI] [PubMed] [Google Scholar]
- 14. Agarwal DK, Lawrence WH, Turner JE, Autian J. Effects of parenteral di‐(2‐ethylhexyl)phthalate (DEHP) on gonadal biochemistry, pathology, and reproductive performance of mice. J Toxicol Environ Health. 1989;26(1):39‐59. [DOI] [PubMed] [Google Scholar]
- 15. Huggert L, Morgan PB. Description and biology of Trichopria painteri n.sp. (Hymenoptera: Diapriidae), a solitary parasitoid of Stomoxys calcitrans (Diptera: Muscidae) from Harare, Zimbabwe. Med Vet Entomol. 1993;7(4):358‐362. [DOI] [PubMed] [Google Scholar]
- 16. Laskey JW, Berman E. Steroidogenic assessment using ovary culture in cycling rats: effects of bis(2‐diethylhexyl)phthalate on ovarian steroid production. Reprod Toxicol. 1993;7(1):25‐33. [DOI] [PubMed] [Google Scholar]
- 17. Davis BJ, Weaver R, Gaines LJ, Heindel JJ. Mono‐(2‐ethylhexyl) phthalate suppresses estradiol production independent of FSH‐cAMP stimulation in rat granulosa cells. Toxicol Appl Pharmacol. 1994;128(2):224‐228. [DOI] [PubMed] [Google Scholar]
- 18. Arcadi FA, Costa C, Imperatore C, et al. Oral toxicity of bis(2‐ethylhexyl) phthalate during pregnancy and suckling in the Long‐Evans rat. Food Chem Toxicol. 1998;36(11):963‐970. [DOI] [PubMed] [Google Scholar]
- 19. Lovekamp TN, Davis BJ. Mono‐(2‐ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol Appl Pharmacol. 2001;172(3):217‐224. [DOI] [PubMed] [Google Scholar]
- 20. Crews D, McLachlan JA. Epigenetics, evolution, endocrine disruption, health, and disease. Endocrinology. 2006;147(6 Suppl):S4‐S10. [DOI] [PubMed] [Google Scholar]
- 21. Diamanti‐Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine‐disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Upson K. Environmental risk factors for endometriosis: a critical evaluation of studies and recommendations from the epidemiologic perspective. Curr Epidemiol Rep. 2020;7(3):149‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheon YP. Di‐(2‐ethylhexyl) phthalate (DEHP) and uterine histological characteristics. Dev Reprod. 2020;24(1):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heudorf U, Mersch‐Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. 2007;210(5):623‐634. [DOI] [PubMed] [Google Scholar]
- 25. Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29(1):134‐139. discussion 181–135. [DOI] [PubMed] [Google Scholar]
- 26. Kelley KE, Hernandez‐Diaz S, Chaplin EL, Hauser R, Mitchell AA. Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environ Health Perspect. 2012;120(3):379‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006;26(3):803‐824. [DOI] [PubMed] [Google Scholar]
- 28. Wittassek M, Koch HM, Angerer J, Bruning T. Assessing exposure to phthalates ‐ the human biomonitoring approach. Mol Nutr Food Res. 2011;55(1):7‐31. [DOI] [PubMed] [Google Scholar]
- 29. Parlett LE, Calafat AM, Swan SH. Women's exposure to phthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol. 2013;23(2):197‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kay VR, Chambers C, Foster WG. Reproductive and developmental effects of phthalate diesters in females. Crit Rev Toxicol. 2013;43(3):200‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Specht IO, Toft G, Hougaard KS, et al. Associations between serum phthalates and biomarkers of reproductive function in 589 adult men. Environ Int. 2014;66:146‐156. [DOI] [PubMed] [Google Scholar]
- 32. Frederiksen H, Skakkebaek NE, Andersson AM. Metabolism of phthalates in humans. Mol Nutr Food Res. 2007;51(7):899‐911. [DOI] [PubMed] [Google Scholar]
- 33. Hauser R, Calafat AM. Phthalates and human health. Occup Environ Med. 2005;62(11):806‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davis BJ, Maronpot RR, Heindel JJ. Di‐(2‐ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol Appl Pharmacol. 1994;128(2):216‐223. [DOI] [PubMed] [Google Scholar]
- 35. Craig ZR, Wang W, Flaws JA. Endocrine‐disrupting chemicals in ovarian function: effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction. 2011;142(5):633‐646. [DOI] [PubMed] [Google Scholar]
- 36. Sirohi D, Al Ramadhani R, Knibbs LD. Environmental exposures to endocrine disrupting chemicals (EDCs) and their role in endometriosis: a systematic literature review. Rev Environ Health. 2020. 10.1515/reveh-2020-0046 [DOI] [PubMed] [Google Scholar]
- 37. Garcia‐Penarrubia P, Ruiz‐Alcaraz AJ, Martinez‐Esparza M, Marin P, Machado‐Linde F. Hypothetical roadmap towards endometriosis: prenatal endocrine‐disrupting chemical pollutant exposure, anogenital distance, gut‐genital microbiota and subclinical infections. Hum Reprod Update. 2020;26(2):214‐246. [DOI] [PubMed] [Google Scholar]
- 38. Chou YC, Chen YC, Chen MJ, Chang CW, Lai GL, Tzeng CR. Exposure to mono‐n‐butyl phthalate in women with endometriosis and its association with the biological effects on human granulosa cells. Int J Mol Sci. 2020;21(5):1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wen X, Xiong Y, Qu X, et al. The risk of endometriosis after exposure to endocrine‐disrupting chemicals: a meta‐analysis of 30 epidemiology studies. Gynecol Endocrinol. 2019;35(8):645‐650. [DOI] [PubMed] [Google Scholar]
- 40. Vabre P, Gatimel N, Moreau J, et al. Environmental pollutants, a possible etiology for premature ovarian insufficiency: a narrative review of animal and human data. Environ Health. 2017;16(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim SH, Cho S, Ihm HJ, et al. Possible role of phthalate in the pathogenesis of endometriosis. in vitro, animal, and human data. J Clin Endocrinol Metab. 2015;100(12):E1502‐1511. [DOI] [PubMed] [Google Scholar]
- 42. Harlow CR, Cahill DJ, Maile LA, et al. Reduced preovulatory granulosa cell steroidogenesis in women with endometriosis. J Clin Endocrinol Metab. 1996;81(1):426‐429. [DOI] [PubMed] [Google Scholar]
- 43. Toya M, Saito H, Ohta N, Saito T, Kaneko T, Hiroi M. Moderate and severe endometriosis is associated with alterations in the cell cycle of granulosa cells in patients undergoing in vitro fertilization and embryo transfer. Fertil Steril. 2000;73(2):344‐350. [DOI] [PubMed] [Google Scholar]
- 44. de Abreu LG, Romao GS, Dos Reis RM, Ferriani RA, De Sa MF, De Moura MD. Reduced aromatase activity in granulosa cells of women with endometriosis undergoing assisted reproduction techniques. Gynecol Endocrinol. 2006;22(8):432‐436. [DOI] [PubMed] [Google Scholar]
- 45. Yamashita Y, Asano M, Morishima S, Fujino K, Terai Y, Ohmichi M. Mitochondrial gene expression in granulosa cells of severe endometriosis with in vitro fertilization and embryo transfer. Fertil Steril. 2007;88(6):1703‐1705. [DOI] [PubMed] [Google Scholar]
- 46. Carlberg M, Nejaty J, Froysa B, Guan Y, Soder O, Bergqvist A. Elevated expression of tumour necrosis factor alpha in cultured granulosa cells from women with endometriosis. Hum Reprod. 2000;15(6):1250‐1255. [DOI] [PubMed] [Google Scholar]
- 47. Sanchez AM, Somigliana E, Vercellini P, Pagliardini L, Candiani M, Vigano P. Endometriosis as a detrimental condition for granulosa cell steroidogenesis and development: From molecular alterations to clinical impact. J Steroid Biochem Mol Biol. 2016;155(Pt A):35‐46. [DOI] [PubMed] [Google Scholar]
- 48. Cobellis L, Latini G, De Felice C, et al. High plasma concentrations of di‐(2‐ethylhexyl)‐phthalate in women with endometriosis. Hum Reprod. 2003;18(7):1512‐1515. [DOI] [PubMed] [Google Scholar]
- 49. Reddy BS, Rozati R, Reddy S, Kodampur S, Reddy P, Reddy R. High plasma concentrations of polychlorinated biphenyls and phthalate esters in women with endometriosis: a prospective case control study. Fertil Steril. 2006;85(3):775‐779. [DOI] [PubMed] [Google Scholar]
- 50. Reddy BS, Rozati R, Reddy BV, Raman NV. Association of phthalate esters with endometriosis in Indian women. BJOG. 2006;113(5):515‐520. [DOI] [PubMed] [Google Scholar]
- 51. Nazir S, Usman Z, Imran M, Lone KP, Ahmad G. Women diagnosed with endometriosis show high serum levels of diethyl hexyl phthalate. J Hum Reprod Sci. 2018;11(2):131‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huang PC, Tsai EM, Li WF, et al. Association between phthalate exposure and glutathione S‐transferase M1 polymorphism in adenomyosis, leiomyoma and endometriosis. Hum Reprod. 2010;25(4):986‐994. [DOI] [PubMed] [Google Scholar]
- 53. Kim SH, Chun S, Jang JY, Chae HD, Kim CH, Kang BM. Increased plasma levels of phthalate esters in women with advanced‐stage endometriosis: a prospective case‐control study. Fertil Steril. 2011;95(1):357‐359. [DOI] [PubMed] [Google Scholar]
- 54. Upson K, Sathyanarayana S, De Roos AJ, et al. Phthalates and risk of endometriosis. Environ Res. 2013;126:91‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moreira Fernandez MA, Cardeal ZL, Carneiro MM, Andre LC. Study of possible association between endometriosis and phthalate and bisphenol A by biomarkers analysis. J Pharm Biomed Anal. 2019;172:238‐242. [DOI] [PubMed] [Google Scholar]
- 56. Weuve J, Hauser R, Calafat AM, Missmer SA, Wise LA. Association of exposure to phthalates with endometriosis and uterine leiomyomata: findings from NHANES, 1999–2004. Environ Health Perspect. 2010;118(6):825‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Itoh H, Iwasaki M, Hanaoka T, Sasaki H, Tanaka T, Tsugane S. Urinary phthalate monoesters and endometriosis in infertile Japanese women. Sci Total Environ. 2009;408(1):37‐42. [DOI] [PubMed] [Google Scholar]
- 58. Cai W, Yang J, Liu Y, Bi Y, Wang H. Association between phthalate metabolites and risk of endometriosis: a meta‐analysis. Int J Environ Res Public Health. 2019;16(19):3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yamashita Y, Ueda M, Takehara M, et al. Influence of severe endometriosis on gene expression of vascular endothelial growth factor and interleukin‐6 in granulosa cells from patients undergoing controlled ovarian hyperstimulation for in vitro fertilization‐embryo transfer. Fertil Steril. 2002;78(4):865‐871. [DOI] [PubMed] [Google Scholar]
- 60. Lu X, Wu Y, Gao XH, Wang YW, Wang L, Sun XX. Effect of letrozole on estradiol production and P450 aromatase messenger RNA expression of cultured luteinized granulosa cells from women with and without endometriosis. Fertil Steril. 2012;98(1):131‐135. [DOI] [PubMed] [Google Scholar]
- 61. Kawabe S, Yamashita Y, Saito N, et al. The effect of moderate to severe endometriosis on expression of growth differentiation factor‐9 mRNA in human granulosa cells under controlled ovarian hyperstimulation. Reprod Med Biol. 2015;14(4):179‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Da Broi MG, Giorgi VSI, Wang F, Keefe DL, Albertini D, Navarro PA. Influence of follicular fluid and cumulus cells on oocyte quality: clinical implications. J Assist Reprod Genet. 2018;35(5):735‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Prins JR, Marissen LM, Scherjon SA, Hoek A, Cantineau AEP. Is there an immune modulating role for follicular fluid in endometriosis? A narrative review. Reproduction. 2020;159(1):R45‐R54. [DOI] [PubMed] [Google Scholar]
- 64. Cahill DJ, Harlow CR, Wardle PG. Pre‐ovulatory granulosa cells of infertile women with endometriosis are less sensitive to luteinizing hormone. Am J Reprod Immunol. 2003;49(2):66‐69. [DOI] [PubMed] [Google Scholar]
- 65. Karita M, Yamashita Y, Hayashi A, et al. Does advanced‐stage endometriosis affect the gene expression of estrogen and progesterone receptors in granulosa cells? Fertil Steril. 2011;95(3):889‐894. [DOI] [PubMed] [Google Scholar]
- 66. Sreerangaraja Urs DB, Wu WH, Komrskova K, et al. Mitochondrial function in modulating human granulosa cell steroidogenesis and female fertility. Int J Mol Sci. 2020;21(10):3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sanchez AM, Vigano P, Quattrone F, et al. The WNT/beta‐catenin signaling pathway and expression of survival promoting genes in luteinized granulosa cells: endometriosis as a paradigm for a dysregulated apoptosis pathway. Fertil Steril. 2014;101(6):1688‐1696. [DOI] [PubMed] [Google Scholar]
- 68. Gonzalez‐Fernandez R, Martin‐Ramirez R, Rotoli D, et al. Granulosa‐lutein cell sirtuin gene expression profiles differ between normal donors and infertile women. Int J Mol Sci. 2019;21(1):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Blasco V, Pinto FM, Fernandez‐Atucha A, Gonzalez‐Ravina C, Fernandez‐Sanchez M, Candenas L. Female infertility is associated with an altered expression of the neurokinin B/neurokinin B receptor and kisspeptin/kisspeptin receptor systems in ovarian granulosa and cumulus cells. Fertil Steril. 2020;114(4):869‐878. [DOI] [PubMed] [Google Scholar]
- 70. Li Y, Li R, Ouyang N, et al. Investigating the impact of local inflammation on granulosa cells and follicular development in women with ovarian endometriosis. Fertil Steril. 2019;112(5):882‐891 e881. [DOI] [PubMed] [Google Scholar]
- 71. Li Y, Liu YD, Chen SL, et al. Down‐regulation of long non‐coding RNA MALAT1 inhibits granulosa cell proliferation in endometriosis by up‐regulating P21 via activation of the ERK/MAPK pathway. Mol Hum Reprod. 2019;25(1):17‐29. [DOI] [PubMed] [Google Scholar]
- 72. De Conto E, Matte U, Cunha‐Filho JS. BMP‐6 and SMAD4 gene expression is altered in cumulus cells from women with endometriosis‐associated infertility. Acta Obstet Gynecol Scand. 2020. 10.1111/aogs.13931 [DOI] [PubMed] [Google Scholar]
- 73. Ding Y, Zhu Q, He Y, et al. Induction of autophagy by Beclin‐1 in granulosa cells contributes to follicular progesterone elevation in ovarian endometriosis. Transl Res. 2021;227:15–29. [DOI] [PubMed] [Google Scholar]
- 74. Karuputhula NB, Chattopadhyay R, Chakravarty B, Chaudhury K. Oxidative status in granulosa cells of infertile women undergoing IVF. Syst Biol Reprod Med. 2013;59(2):91‐98. [DOI] [PubMed] [Google Scholar]
- 75. Seino T, Saito H, Kaneko T, Takahashi T, Kawachiya S, Kurachi H. Eight‐hydroxy‐2'‐deoxyguanosine in granulosa cells is correlated with the quality of oocytes and embryos in an in vitro fertilization‐embryo transfer program. Fertil Steril. 2002;77(6):1184‐1190. [DOI] [PubMed] [Google Scholar]
- 76. Avila J, Gonzalez‐Fernandez R, Rotoli D, Hernandez J, Palumbo A. Oxidative stress in granulosa‐lutein cells from in vitro fertilization patients. Reprod Sci. 2016;23(12):1656‐1661. [DOI] [PubMed] [Google Scholar]
- 77. Lin X, Dai Y, Tong X, et al. Excessive oxidative stress in cumulus granulosa cells induced cell senescence contributes to endometriosis‐associated infertility. Redox Biol. 2020;30:101431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kunitomi C, Harada M, Takahashi N, et al. Activation of endoplasmic reticulum stress mediates oxidative stress‐induced apoptosis of granulosa cells in ovaries affected by endometrioma. Mol Hum Reprod. 2020;26(1):40‐52. [DOI] [PubMed] [Google Scholar]
- 79. Treinen KA, Dodson WC, Heindel JJ. Inhibition of FSH‐stimulated cAMP accumulation and progesterone production by mono(2‐ethylhexyl) phthalate in rat granulosa cell cultures. Toxicol Appl Pharmacol. 1990;106(2):334‐340. [DOI] [PubMed] [Google Scholar]
- 80. Treinen KA, Heindel JJ. Evidence that MEHP inhibits rat granulosa cell function by a protein kinase C‐independent mechanism. Reprod Toxicol. 1992;6(2):143‐148. [DOI] [PubMed] [Google Scholar]
- 81. Reinsberg J, Wegener‐Toper P, van der Ven K, van der Ven H, Klingmueller D. Effect of mono‐(2‐ethylhexyl) phthalate on steroid production of human granulosa cells. Toxicol Appl Pharmacol. 2009;239(1):116‐123. [DOI] [PubMed] [Google Scholar]
- 82. Lovekamp‐Swan T, Jetten AM, Davis BJ. Dual activation of PPARalpha and PPARgamma by mono‐(2‐ethylhexyl) phthalate in rat ovarian granulosa cells. Mol Cell Endocrinol. 2003;201(1–2):133‐141. [DOI] [PubMed] [Google Scholar]
- 83. Lovekamp‐Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect. 2003;111(2):139‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Svechnikova K, Svechnikova I, Soder O. Gender‐specific adverse effects of mono‐ethylhexyl phthalate on steroidogenesis in immature granulosa cells and rat leydig cell progenitors in vitro. Front Endocrinol (Lausanne). 2011;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Inada H, Chihara K, Yamashita A, et al. Evaluation of ovarian toxicity of mono‐(2‐ethylhexyl) phthalate (MEHP) using cultured rat ovarian follicles. J Toxicol Sci. 2012;37(3):483‐490. [DOI] [PubMed] [Google Scholar]
- 86. Li N, Liu K, Yuan H, et al. The effect of mono‐(2‐ethylhexyl) phthalate on apoptosis of rat ovarian granulosa cells in vitro. Environ Toxicol Pharmacol. 2015;39(2):643‐650. [DOI] [PubMed] [Google Scholar]
- 87. Li N, Liu T, Guo K, et al. Effect of mono‐(2‐ethylhexyl) phthalate (MEHP) on proliferation of and steroid hormone synthesis in rat ovarian granulosa cells in vitro. J Cell Physiol. 2018;233(4):3629‐3637. [DOI] [PubMed] [Google Scholar]
- 88. Li XN, Li HX, Yang TN, et al. Di‐(2‐ethylhexyl) phthalate induced developmental abnormalities of the ovary in quail (Coturnix japonica) via disruption of the hypothalamic‐pituitary‐ovarian axis. Sci Total Environ. 2020;741:140293. [DOI] [PubMed] [Google Scholar]
- 89. Svechnikova I, Svechnikov K, Soder O. The influence of di‐(2‐ethylhexyl) phthalate on steroidogenesis by the ovarian granulosa cells of immature female rats. J Endocrinol. 2007;194(3):603‐609. [DOI] [PubMed] [Google Scholar]
- 90. Ambruosi B, Uranio MF, Sardanelli AM, et al. In vitro acute exposure to DEHP affects oocyte meiotic maturation, energy and oxidative stress parameters in a large animal model. PLoS ONE. 2011;6(11):e27452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tripathi A, Pandey V, Sahu AN, Singh AK, Dubey PK. Encircling granulosa cells protects against di‐(2‐ethylhexyl)phthalate‐induced apoptosis in rat oocytes cultured in vitro. Zygote. 2019;27(4):203‐213. [DOI] [PubMed] [Google Scholar]
- 92. Li N, Liu T, Zhou L, He J, Ye L. Di‐(2‐ethylhcxyl) phthalate reduces progesterone levels and induces apoptosis of ovarian granulosa cell in adult female ICR mice. Environ Toxicol Pharmacol. 2012;34(3):869‐875. [DOI] [PubMed] [Google Scholar]
- 93. Chen HS, Chiang PH, Wang YC, et al. Benzyl butyl phthalate induces necrosis by AhR mediation of CYP1B1 expression in human granulosa cells. Reprod Toxicol. 2012;33(1):67‐75. [DOI] [PubMed] [Google Scholar]
- 94. Ernst J, Jann JC, Biemann R, Koch HM, Fischer B. Effects of the environmental contaminants DEHP and TCDD on estradiol synthesis and aryl hydrocarbon receptor and peroxisome proliferator‐activated receptor signalling in the human granulosa cell line KGN. Mol Hum Reprod. 2014;20(9):919‐928. [DOI] [PubMed] [Google Scholar]
- 95. Liu J, Wang W, Zhu J, et al. Di(2‐ethylhexyl) phthalate (DEHP) influences follicular development in mice between the weaning period and maturity by interfering with ovarian development factors and microRNAs. Environ Toxicol. 2018;33(5):535‐544. [DOI] [PubMed] [Google Scholar]
- 96. Wang XJ, Xiong GP, Luo XM, et al. Dibutyl phthalate inhibits the effects of follicle‐stimulating hormone on rat granulosa cells through down‐regulation of follicle‐stimulating hormone receptor. Biol Reprod. 2016;94(6):144. [DOI] [PubMed] [Google Scholar]
- 97. Adir M, Salmon‐Divon M, Combelles CMH, Mansur A, Cohen Y, Machtinger R. In vitro exposure of human luteinized mural granulosa cells to dibutyl phthalate affects global gene expression. Toxicol Sci. 2017;160(1):180‐188. [DOI] [PubMed] [Google Scholar]
- 98. Adir M, Combelles CMH, Mansur A, et al. Dibutyl phthalate impairs steroidogenesis and a subset of LH‐dependent genes in cultured human mural granulosa cell in vitro. Reprod Toxicol. 2017;69:13‐18. [DOI] [PubMed] [Google Scholar]
- 99. Li FP, Zhou JL, Guo AW, et al. Di(n‐butyl) phthalate exposure impairs meiotic competence and development of mouse oocyte. Environ Pollut. 2019;246:597‐607. [DOI] [PubMed] [Google Scholar]
- 100. Ma Y, Zhang J, Zeng R, et al. Effects of the dibutyl phthalate (DBP) on the expression and activity of aromatase in human granulosa cell line KGN. Ann Clin Lab Sci. 2019;49(2):175‐182. [PubMed] [Google Scholar]
- 101. Mei Y, Rongshuang M, Ruizhi Z, Hongyuan H, Qiyue T, Shuhua Z. Effects of dimethyl phthalate (DMP) on serum sex hormone levels and apoptosis in C57 female mice. Int J Endocrinol Metab. 2019;17(2):e82882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhang C, Gong P, Ye Y, et al. NF‐kappaB‐vimentin is involved in steroidogenesis stimulated by mono‐butyl phthalate in primary cultured ovarian granulosa cells. Toxicol In Vitro. 2017;45(Pt 1):25‐30. [DOI] [PubMed] [Google Scholar]
- 103. Wang X, Shang L, Wang J, Wu N, Wang S. Effect of phthalate esters on the secretion of prostaglandins (F2alpha and E2) and oxytocin in cultured bovine ovarian and endometrial cells. Domest Anim Endocrinol. 2010;39(2):131‐136. [DOI] [PubMed] [Google Scholar]
- 104. Kim YH, Kim SH, Lee HW, Chae HD, Kim CH, Kang BM. Increased viability of endometrial cells by in vitro treatment with di‐(2‐ethylhexyl) phthalate. Fertil Steril. 2010;94(6):2413‐2416. [DOI] [PubMed] [Google Scholar]
- 105. Huang Q, Zhang H, Chen YJ, Chi YL, Dong S. The inflammation response to DEHP through PPARgamma in endometrial cells. Int J Environ Res Public Health. 2016;13(3):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cho YJ, Park SB, Han M. Di‐(2‐ethylhexyl)‐phthalate induces oxidative stress in human endometrial stromal cells in vitro. Mol Cell Endocrinol. 2015;407:9‐17. [DOI] [PubMed] [Google Scholar]
- 107. Kim Y, Kim MR, Kim JH, Cho HH. Aldo‐keto reductase activity after diethylhexyl phthalate exposure in eutopic and ectopic endometrial cells. Eur J Obstet Gynecol Reprod Biol. 2017;215:215‐219. [DOI] [PubMed] [Google Scholar]
- 108. Park C, Lee J, Kong B, et al. The effects of bisphenol A, benzyl butyl phthalate, and di(2‐ethylhexyl) phthalate on estrogen receptor alpha in estrogen receptor‐positive cells under hypoxia. Environ Pollut. 2019;248:774‐781. [DOI] [PubMed] [Google Scholar]
- 109. Kim J, Cha S, Lee MY, et al. Chronic and low dose exposure to nonlyphenol or Di(2‐Ethylhexyl) phthalate alters cell proliferation and the localization of steroid hormone receptors in uterine endometria in mice. Dev Reprod. 2019;23(3):263‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]


