Abstract
Background
The process of follicle development is tightly regulated by pituitary gonadotropins (follicle‐stimulating hormone [FSH] and luteinizing hormone [LH]) and intraovarian regulators (eg, steroids, growth factors, and cytokines).
Methods
This review outlines recent findings on the mechanisms of human follicle development, based on the research on animal models such as mice, rats, cows, and sheep.
Main findings
Phosphatidylinositol 3‐kinase/protein kinase B signaling pathway and anti‐Müllerian hormone are involved in primordial follicle activation during the gonadotropin‐independent phase. The intraovarian regulators, such as androgen, insulin‐like growth factor system, activin, oocyte‐derived factors (growth differentiation factor‐9 and bone morphogenetic protein 15), and gap junction membrane channel protein (connexin), play a central role in the acquisition of FSH dependence in preantral follicles during the gonadotropin‐responsive phase. Antral follicle development can be divided into FSH‐dependent growth and LH‐dependent maturation. The indispensable tetralogy for follicle selection and final maturation of antral follicles involves (a) acquisition of LH dependence, (b) greater capacity for E2 production, (c) activation of the IGF system, and (d) an antiapoptotic follicular microenvironment.
Conclusion
We reproductive endocrinologists should accumulate further knowledge from animal model studies to develop methods that promote early folliculogenesis and connect to subsequent gonadotropin therapy in infertile women.
Keywords: follicle development, follicle‐stimulating hormone, growth factor, luteinizing hormone, steroid
The process of follicle development is tightly regulated by pituitary gonadotropins (follicle‐stimulating hormone [FSH] and luteinizing hormone [LH]) and intraovarian regulators (eg, steroids, growth factors, and cytokines). This review outlines recent findings on the mechanisms of human follicle development, based on the research on animal models such as mice, rats, cows, and sheep.
1. INTRODUCTION
The ovarian follicle, consisting of an oocyte surrounded by two types of somatic cells (granulosa cells and thecal cells), represents the basic functional unit of the ovary. Follicle development involves activation of primordial follicles, continual growth through primary, secondary, preantral, and antral follicles, selection and maturation of a single dominant follicle, and ovulation (Figure 1). The process of follicle development is tightly regulated by pituitary gonadotropins (follicle‐stimulating hormone [FSH] and luteinizing hormone [LH]) and intraovarian regulators (eg, steroids, growth factors, and cytokines). 1 This review outlines recent findings on human follicle development mechanisms, based on research on animal models such as mice, rats, cows, and sheep. 2
FIGURE 1.
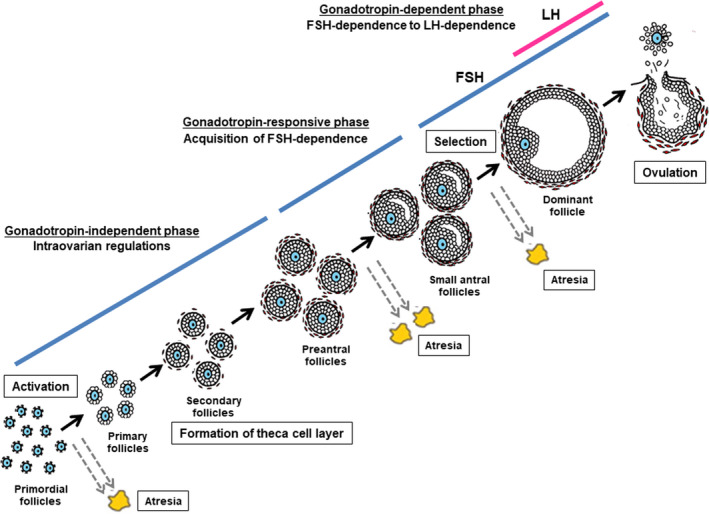
Follicle development, selection, and ovulation. FSH, follicle‐stimulating hormone; LH, luteinizing hormone
2. FOLLICULAR DEVELOPMENT STAGES
Follicle development can be classified into the following three phases according to their developmental stage and gonadotropin dependence 1 , 3 : (a) follicle growth through the primordial, primary, and secondary stages, which is entirely independent of FSH and LH (gonadotropin‐independent phase); (b) follicle transition from the preantral stage to the early antral stage, which, although primarily controlled by intraovarian regulators, 4 can be stimulated by FSH (gonadotropin‐responsive phase) 3 ; and (c) follicle growth and maturation beyond the early antral stage, which includes follicle recruitment, selection, and ovulation, and is dependent on FSH and LH (gonadotropin‐dependent phase). 5
3. GONADOTROPIN‐INDEPENDENT PHASE: ACTIVATION OF PRIMORDIAL FOLLICLES
Females are born with approximately 2 million primordial follicles in their ovaries. After birth, primordial follicles are dormant in the ovaries for a long time; however, eventually, a group of them begin to grow into primary follicles (primordial follicle activation). Although the activation mechanism of primordial follicles has not been clarified thus far, it has been suggested that some primordial follicles may proceed with growth by exiting from the inhibition of primordial follicle activation in the ovary. 2 According to recent animal studies, activation of the phosphatidylinositol 3‐kinase/protein kinase B (PI3K/Akt) signaling pathway 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 and inhibition of anti‐Müllerian hormone (AMH) 14 , 15 , 16 , 17 have been implicated in the activation of primordial follicles.
The PI3K/Akt pathway is an intracellular signal transduction system that induces proliferation and survival of various cells and is believed to be involved in primordial follicle activation in the ovary. Phosphatase and tensin homolog (PTEN) deleted on chromosome 10 is an enzyme that suppresses the PI3k/Akt pathway. The inhibitory effect of PTEN maintains the dormancy of the primordial follicles in the ovary for a long time. 2 , 6 When the inhibitory effect of PTEN is removed and the PI3K/Akt pathway is activated in several primordial follicles, the oocyte transcription factor forkhead box O3 (FOXO3) is phosphorylated and exported from the nucleus and is degraded in the cytoplasm. 7 This loss of transcriptional activity of FOXO3 in oocytes possibly turns on the switch of primordial follicle activation (Figure 1). 2 , 6 In PTEN 10 and FOXO3 8 knockout (KO) mice, primordial follicles were activated globally and began to grow simultaneously, which resulted in early depletion of ovarian follicles. Although the exact mechanism of external stimulation that releases the inhibitory effect of PTEN and activates the PI3K/Akt pathway in the ovary has not been clarified, the involvement of the Kit ligand (KL) and its receptor c‐Kit has been speculated. 6 , 18 , 19
Anti‐Mullerian hormone is a growth factor belonging to the transforming growth factor‐β (TGF‐β) superfamily. In humans, AMH is expressed in the granulosa cells of preantral follicles and small antral follicles (~6 mm in diameter). However, its expression was not observed in large antral and preovulatory follicles. 20 , 21 , 22 In AMH KO mice, primordial follicles began to grow globally and were depleted faster, suggesting that AMH inhibited primordial follicle activation. 14 However, the exact mechanism of removing the inhibitory effect of AMH in the ovary to activate primordial follicles remains unclear. 2 Serum AMH levels reflect the number of preantral and small antral follicles and are used clinically to assess ovarian reserve and function.
Forkhead box L2 (FOXL2) is a granulosa‐specific transcription factor, and its expression begins in pregranulosa cells (granulosa progenitor cells) around the primordial follicles. 23 In FOXL2 KO mice, follicle growth was arrested between the primordial and primary stages, 24 , 25 and granulosa cells were transdifferentiated into Sertoli‐like cells, 26 suggesting that FOXL2 was involved in the activation of primordial follicles and maintenance of granulosa cell function.
4. GONADOTROPIN‐RESPONSIVE PHASE: ACQUISITION OF FSH DEPENDENCE IN PREANTRAL FOLLICLES
The developmental process of primordial, primary, and secondary follicles is not dependent on pituitary gonadotropins and is controlled by intraovarian regulators. Follicles acquire FSH dependence during the transitional stage from preantral to antral follicles, and the developmental mechanism begins to switch from intraovarian regulators to FSH. 1 , 3 Acquisition of FSH dependence is crucial in determining follicular fate (growth vs. atresia) beyond the preantral stage.
Follicle‐stimulating hormone is a glycoprotein hormone composed of heterodimers of α‐ and β‐subunits, and the β‐subunit characterizes the physiologic function of FSH. The specific receptor for FSH (FSHR) is expressed in granulosa cells of secondary and preantral follicles. 2 In KO mice of the FSHβ subunit or FSHR, primordial follicle activation and subsequent growth to preantral follicles were observed. Nevertheless, follicle growth was arrested at the preantral stage, and no antral follicles were formed in these KO mice. 5 , 27 These findings indicate that FSH is indispensable for follicle growth and antral formation during the preantral‐to‐antral transition.
Intraovarian regulators that play a central role in the acquisition of FSH dependence at the preantral stage include androgen, insulin‐like growth factor (IGF) system, activin, oocyte‐derived factors (growth differentiation factor‐9 [GDF‐9] and bone morphogenetic protein 15 [BMP 15]), and gap junction membrane channel protein (connexin). 2
Formation of the theca cell layer at the secondary and preantral stages is one of the key events for acquiring FSH dependence in preantral follicles (Figure 1). 1 The theca cell layers not only provide blood supply to follicles but also sensitize the follicles to gonadotropins. Granulosa cell factors (eg, IGF1 and KL) stimulate the recruitment of theca cells from ovarian cortical stromal cells, 28 , 29 , 30 whereas oocyte‐derived GDF‐9 is involved in the differentiation of theca cells during early follicle development. 31 , 32 , 33 , 34 When preantral follicles were formed, granulosa cells expressed FSHR and theca cells expressed LH receptor (LHR), thereby presenting the prototype of “2‐cell 2‐gonadotropin theory” (Figure 2). 1
FIGURE 2.
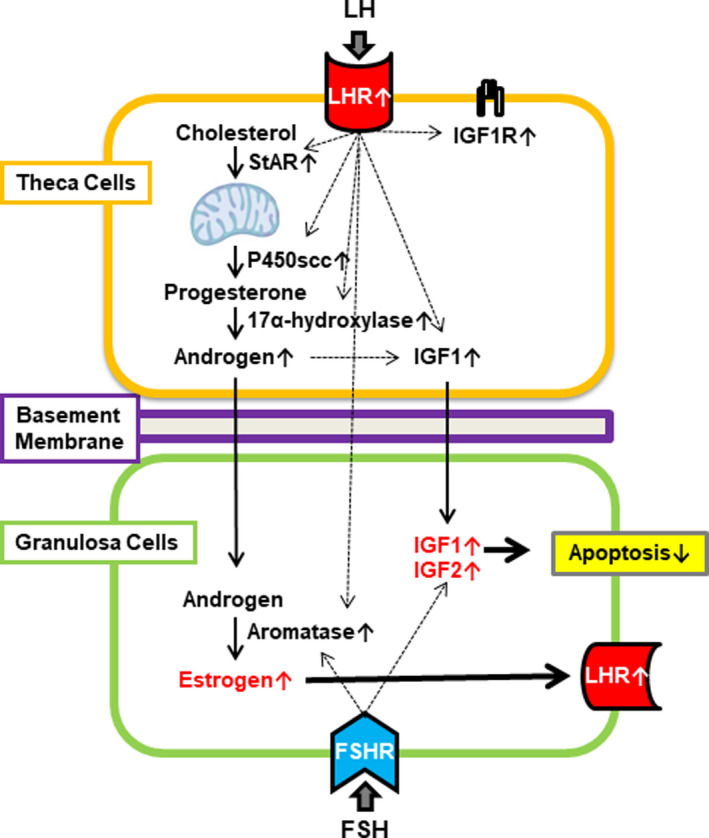
A hypothetical model illustrating a 2‐cell 2‐gonadotropin system around follicle selection. 76 FSH, follicle‐stimulating hormone; LH, luteinizing hormone; FSHR, FSH receptor; LHR, LH receptor; IGF, insulin‐like growth factor; IGF1R, IGF1 receptor
Theca‐derived androgens bind to androgen receptors (ARs) in granulosa cells, 35 thereby inducing FSHR expression and follicle growth during the preantral‐to‐antral transition. 34 , 36 , 37 , 38 AR deficiency in the mice ovary induces granulosa cell apoptosis, arrests antral follicle growth, and results in premature ovarian failure. 39 , 40 , 41 , 42 Thus, androgens play an important role in the growth, survival, and acquisition of FSH dependence in preantral follicles. 1 , 2
The IGF system includes two ligands (IGF1 and IGF2), two receptors (IGF1R and IGF2R), and some binding proteins (IGFBPs) that regulate IGF action in various cells. 43 IGF1 induces the expression of FSHR and aromatase (an enzyme that converts androgen to estrogen) in granulosa cells during the preantral‐to‐antral transition. 44 IGF1 KO mice exhibited preantral follicle blockage, suggesting that the activation of the IGF system in the ovary was essential for follicle growth beyond the preantral stage. 45 In the human ovary, IGF2 may be more functionally important than IGF1. 46
Activin, a growth factor of the TGF‐β superfamily, promotes FSH production and secretion in the pituitary gland. 47 , 48 , 49 , 50 , 51 Additionally, it induces the expression of FSHR and aromatase in granulosa cells. 52 , 53 , 54 , 55 In activin receptor KO mice, follicle growth was arrested at the early antral stage. 56
Both GDF‐9 and BMP 15 are oocyte‐derived growth factors belonging to the TGF‐β superfamily. GDF‐9 promotes androgen production in theca cells, and the androgen produced induces FSHR expression and proliferation of granulosa cells, thereby promoting follicle growth and acquisition of FSH dependence in rat preantral follicles. 34 , 57 In GDF‐9 KO mice, follicle growth was arrested at the secondary stage, and the theca cell layer was not formed. 31 , 58 Thus, GDF‐9 plays a central role in the crosstalk between oocyte‐granulosa‐theca cells during the preantral‐to‐antral transition. 1
Although GDF‐9 is involved in the early folliculogenesis of poly‐ovulatory animals (eg, mice and rats), BMP 15 may be more important in mono‐ovulatory animals (eg, sheep and humans). 2 In BMP 15 mutant sheep, follicle growth was arrested at the primary stage, similar to that in GDF9 KO mice. 59 , 60 , 61 Human BMP 15 mutations exhibited ovarian dysgenesis, early blockage in folliculogenesis, and premature ovarian failure. 62 , 63 , 64 Therefore, oocyte‐derived GDF‐9 and BMP 15 lead follicle development during the preantral‐to‐antral transition in both mono‐ovulatory and poly‐ovulatory animals.
The transmembrane protein, connexin, forms intercellular membrane channels of gap junctions that allow the exchange of ions, metabolites, and signaling molecules between adjacent cells. Connexin 37 KO mice exhibited follicle arrest during the preantral‐to‐antral transition, suggesting that the crosstalk between oocyte‐granulosa‐theca cells was essential for follicle growth beyond the transitional stage. 65
5. GONADOTROPIN‐DEPENDENT PHASE: FSH‐DEPENDENT GROWTH AND LH‐DEPENDENT MATURATION OF ANTRAL FOLLICLES
In humans, when antral follicles reach a diameter of 2‐5 mm, they are subjected to cyclic control by circulating FSH and LH (Figure 3). Approximately 5‐15 antral follicles begin FSH‐dependent growth each month, but only a single dominant follicle is selected and can eventually ovulate (Figure 1). 46 The primary function of follicles is to support the development of competent, mature oocytes. As antral follicles grow and mature, oocytes also grow, mature, and acquire capacitation.
FIGURE 3.
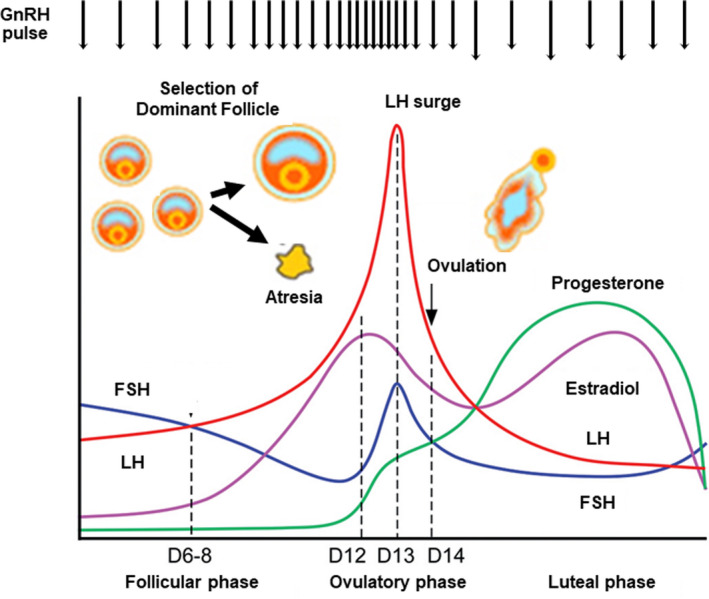
Hormonal dynamics of the hypothalamic‐pituitary‐ovarian axis during the menstrual cycle. FSH, follicle‐stimulating hormone; LH, luteinizing hormone; GnRH, gonadotropin‐releasing hormone; D, cycle date
Follicle‐stimulating hormone stimulates the proliferation and differentiation of granulosa cells of antral follicles through the induction of numerous genes via activation of the cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) pathway, mitogen‐activated protein kinase/extracellular signal‐regulated kinase (MAPK/ERK) pathway, and PI3K/Akt pathway. 66 FSH also strongly suppresses granulosa cell apoptosis of antral follicles by activating the PI3K‐Akt pathway. 66 In the absence of FSH, follicle atresia occurs through granulosa cell apoptosis. 3
In humans, FSH plays a central role in the regulation of follicle recruitment and growth, whereas LH is mandatory for further follicular growth (beyond 10 mm in diameter) and estradiol (E2) production in the antral stage. 67 , 68 FSHR expression is highest in granulosa cells of small antral follicles (2‐5 mm in diameter); however, its expression is strongly suppressed in granulosa cells during follicle selection (7‐9 mm in diameter). Conversely, LHR expression is significantly induced in granulosa cells during follicle selection. 69 Thus, antral follicle development can be classified into FSH‐dependent growth and LH‐dependent maturation (Figure 1). 70
6. GONADOTROPIN‐DEPENDENT PHASE: SELECTION OF A SINGLE DOMINANT FOLLICLE
The indispensable tetralogy for follicle selection and final maturation of antral follicles in bovine follicles involves (a) acquisition of LH dependence, (b) greater capacity for E2 production, (c) activation of the IGF system, and (d) an antiapoptotic follicular microenvironment. 71 , 72 , 73 , 74 , 75 , 76
In antral follicles, LHR is expressed only in theca cells and mural granulosa cells (outermost layer of granulosa cells lying next to the basal lamina) but not in cumulus cells (granulosa cells surrounding oocyte) nor oocytes. 77 LHR expression in mural granulosa cells is induced by FSH with various intraovarian factors (eg, E2, IGF1). 78 Conversely, LHR expression in cumulus cells is suppressed by oocyte‐derived factors (such as GDF‐9). 79 , 80 , 81 In KO mice of the LHβ subunit or LHR, antral follicle growth was arrested, and no preovulatory follicles or corpora lutea were found, indicating that LH was essential for antral follicle maturation and ovulation. 82 , 83 , 84
E2 stimulates proliferation, induces the expression of FSHR, LHR, aromatase, and IGF1, and suppresses cell apoptosis in granulosa cells. 2 In KO mice of estrogen receptor and aromatase, antral follicles could not reach the preovulatory stage and failed to ovulate. This resulted in atresia, suggesting that estrogen was necessary for follicle maturation, survival, and ovulation. 85 , 86 , 87 , 88 , 89
In humans, the traditional theory for follicle selection has been explained by the FSH‐E2‐inhibin axis. 72 , 90 E2 and inhibin‐B, secreted from granulosa cells of developing follicles, reduces circulating FSH levels during the early/mid‐follicular phase, which is essential in the orchestration of mono‐ovulation in women (Figure 3). 91 , 92 Only follicles with the highest FSH sensitivity (ie, the highest FSHR expression in granulosa cells) can survive and continue to develop as a single dominant follicle, even at reduced FSH levels. On the other hand, follicles with low‐FSH sensitivity (ie, lower FSHR expression in granulosa cells) cannot fully receive the antiapoptotic effect of FSH and induce granulosa cell apoptosis and follicle atresia under low‐FSH conditions. 75 , 90
Another recent theory for follicle selection is the acquisition of LH dependence in antral follicles. It is hypothesized that the first follicle that expresses LHR in mural granulosa cells and acquires LH dependence can survive and mature as a dominant follicle. 71 , 72 , 76 In bovine antral follicles, LH has been shown to induce LHR and aromatase expression, activate the IGF system, and suppress cell apoptosis in mural granulosa cells (tetralogy for follicle selection) via paracrine action from theca cells. 76 Thus, LH plays an important role in follicle selection and final maturation of antral follicles and preparation for subsequent ovulation (Figure 2).
7. CONCLUSION
Women are responsible for species conservation and are destined to explore the possibility of pregnancy through follicle development and ovulation every month. The selection of a single dominant follicle and massive oocyte loss by follicle atresia in each cycle may be a biological intent to inherit the best genomic information within the selected oocytes to the next generation. On the other hand, infertility treatment requires as many oocytes as possible to increase pregnancy rates, and increasing the number of developing follicles by ovarian stimulation is one of the most important starting points of treatment. However, we can currently control only gonadotropin‐dependent antral follicle growth; the activation of primordial follicles; and gonadotropin‐independent growth until preantral follicles remain uncontrollable. We should study these aspects further from animal model studies to develop methods that promote early folliculogenesis, as well as subsequent gonadotropin therapy in infertile women.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
HUMAN /ANIMAL RIGHTS
This article does not contain any studies with human and animal subjects performed by any of the authors.
Orisaka M, Miyazaki Y, Shirafuji A, et al. The role of pituitary gonadotropins and intraovarian regulators in follicle development: A mini‐review. Reprod Med Biol. 2021;20:169–175. 10.1002/rmb2.12371
REFERENCES
- 1. Orisaka M, Tajima K, Tsang BK, Kotsuji F. Oocyte‐granulosa‐theca cell interactions during preantral follicular development. J Ovarian Res. 2009;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pangas SA, Rajkovic A. Follicular Development: Mouse, Sheep; and Human Models. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill's Physiology of Reproduction. London: Elsevier; 2014:947‐996. [Google Scholar]
- 3. McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200‐214. [DOI] [PubMed] [Google Scholar]
- 4. Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G. Gonadotrophin‐releasing hormone deficiency in a mutant mouse with hypogonadism. Nature. 1977;269:338‐340. [DOI] [PubMed] [Google Scholar]
- 5. Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201‐204. [DOI] [PubMed] [Google Scholar]
- 6. Hsueh AJ, Kawamura K, Cheng Y, Fauser BC. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36:1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K‐dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321:197‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215‐218. [DOI] [PubMed] [Google Scholar]
- 9. Gallardo TD, John GB, Bradshaw K, et al. Sequence variation at the human FOXO3 locus: a study of premature ovarian failure and primary amenorrhea. Hum Reprod. 2008;23:216‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reddy P, Liu L, Adhikari D, et al. Oocyte‐specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611‐613. [DOI] [PubMed] [Google Scholar]
- 11. Zheng W, Nagaraju G, Liu Z, Liu K. Functional roles of the phosphatidylinositol 3‐kinases (PI3Ks) signaling in the mammalian ovary. Mol Cell Endocrinol. 2012;356:24‐30. [DOI] [PubMed] [Google Scholar]
- 12. Jagarlamudi K, Liu L, Adhikari D, et al. Oocyte‐specific deletion of Pten in mice reveals a stage‐specific function of PTEN/PI3K signaling in oocytes in controlling follicular activation. PLoS One. 2009;4:e6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li J, Kawamura K, Cheng Y, et al. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci USA. 2010;107:10280‐10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Durlinger AL, Kramer P, Karels B, et al. Control of primordial follicle recruitment by anti‐Müllerian hormone in the mouse ovary. Endocrinology. 1999;140:5789‐5796. [DOI] [PubMed] [Google Scholar]
- 15. Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti‐Müllerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21:2223‐2227. [DOI] [PubMed] [Google Scholar]
- 16. Durlinger AL, Gruijters MJ, Kramer P, et al. Anti‐Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891‐4899. [DOI] [PubMed] [Google Scholar]
- 17. Durlinger AL, Gruijters MJ, Kramer P, et al. Anti‐Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076‐1084. [DOI] [PubMed] [Google Scholar]
- 18. Huang EJ, Manova K, Packer AI, Sanchez S, Bachvarova RF, Besmer P. The murine steel panda mutation affects kit ligand expression and growth of early ovarian follicles. Dev Biol. 1993;157:100‐109. [DOI] [PubMed] [Google Scholar]
- 19. Kuroda H, Terada N, Nakayama H, Matsumoto K, Kitamura Y. Infertility due to growth arrest of ovarian follicles in Sl/Slt mice. Dev Biol. 1988;126:71‐79. [DOI] [PubMed] [Google Scholar]
- 20. Rajpert‐De Meyts E, Jørgensen N, Graem N, Müller J, Cate RL, Skakkebaek NE. Expression of anti‐Müllerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab. 1999;84:3836‐3844. [DOI] [PubMed] [Google Scholar]
- 21. Weenen C, Laven JS, Von Bergh AR, et al. Anti‐Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77‐83. [DOI] [PubMed] [Google Scholar]
- 22. Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti‐Müllerian hormone. Reproduction. 2002;124:601‐609. [DOI] [PubMed] [Google Scholar]
- 23. Crisponi L, Deiana M, Loi A, et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet. 2001;27:159‐166. [DOI] [PubMed] [Google Scholar]
- 24. Uda M, Ottolenghi C, Crisponi L, et al. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet. 2004;13:1171‐1181. [DOI] [PubMed] [Google Scholar]
- 25. Schmidt D, Ovitt CE, Anlag K, et al. The murine winged‐helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933‐942. [DOI] [PubMed] [Google Scholar]
- 26. Uhlenhaut NH, Jakob S, Anlag K, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130‐1142. [DOI] [PubMed] [Google Scholar]
- 27. Dierich A, Sairam MR, Monaco L, et al. Impairing follicle‐stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA. 1998;95:13612‐13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang CT, Weitsman SR, Dykes BN, Magoffin DA. Stem cell factor and insulin‐like growth factor‐I stimulate luteinizing hormone‐independent differentiation of rat ovarian theca cells. Biol Reprod. 2001;64:451‐456. [DOI] [PubMed] [Google Scholar]
- 29. Orisaka M, Tajima K, Mizutani T, et al. Granulosa cells promote differentiation of cortical stromal cells into theca cells in the bovine ovary. Biol Reprod. 2006;75:734‐740. [DOI] [PubMed] [Google Scholar]
- 30. Honda A, Hirose M, Hara K, et al. Isolation, characterization, and in vitro and in vivo differentiation of putative thecal stem cells. Proc Natl Acad Sci USA. 2007;104:12389‐12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. Molecular characterization of the follicle defects in the growth differentiation factor 9‐deficient ovary. Mol Endocrinol. 1999;13:1018‐1034. [DOI] [PubMed] [Google Scholar]
- 32. Wu X, Chen L, Brown CA, Yan C, Matzuk MM. Interrelationship of growth differentiation factor 9 and inhibin in early folliculogenesis and ovarian tumorigenesis in mice. Mol Endocrinol. 2004;18:1509‐1519. [DOI] [PubMed] [Google Scholar]
- 33. Solovyeva EV, Hayashi M, Margi K, et al. Growth differentiation factor‐9 stimulates rat theca‐interstitial cell androgen biosynthesis. Biol Reprod. 2000;63:1214‐1218. [DOI] [PubMed] [Google Scholar]
- 34. Orisaka M, Jiang JY, Orisaka S, Kotsuji F, Tsang BK. Growth differentiation factor 9 promotes rat preantral follicle growth by up‐regulating follicular androgen biosynthesis. Endocrinology. 2009;150:2740‐2748. [DOI] [PubMed] [Google Scholar]
- 35. Tetsuka M, Whitelaw PF, Bremner WJ, Millar MR, Smyth CD, Hillier SG. Developmental regulation of androgen receptor in rat ovary. J Endocrinol. 1995;145:535‐543. [DOI] [PubMed] [Google Scholar]
- 36. Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle‐stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84:2951‐2956. [DOI] [PubMed] [Google Scholar]
- 38. Wang H, Andoh K, Hagiwara H, et al. Effect of adrenal and ovarian androgens on type 4 follicles unresponsive to FSH in immature mice. Endocrinology. 2001;142:4930‐4936. [DOI] [PubMed] [Google Scholar]
- 39. Shiina H, Matsumoto T, Sato T, et al. Premature ovarian failure in androgen receptor‐deficient mice. Proc Natl Acad Sci USA. 2006;103:224‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hu YC, Wang PH, Yeh S, et al. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc Natl Acad Sci USA. 2004;101:11209‐11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sen A, Hammes SR. Granulosa cell‐specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24:1393‐1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walters KA, Middleton LJ, Joseph SR, et al. Targeted loss of androgen receptor signaling in murine granulosa cells of preantral and antral follicles causes female subfertility. Biol Reprod. 2012;87:151. [DOI] [PubMed] [Google Scholar]
- 43. Monget P, Bondy C. Importance of the IGF system in early folliculogenesis. Mol Cell Endocrinol. 2000;163:89‐93. [DOI] [PubMed] [Google Scholar]
- 44. Zhou J, Kumar TR, Matzuk MM, Bondy C. Insulin‐like growth factor I regulates gonadotropin responsiveness in the murine ovary. Mol Endocrinol. 1997;11:1924‐1933. [DOI] [PubMed] [Google Scholar]
- 45. Baker J, Hardy MP, Zhou J, et al. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10:903‐918. [DOI] [PubMed] [Google Scholar]
- 46. Zeleznik AJ, Plant TM. Control of the Menstrual Cycle. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill's Physiology of Reproduction. London: Elsevier; 2014:1307‐1362. [Google Scholar]
- 47. Suszko MI, Lo DJ, Suh H, Camper SA, Woodruff TK. Regulation of the rat follicle‐stimulating hormone beta‐subunit promoter by activin. Mol Endocrinol. 2003;17:318‐332. [DOI] [PubMed] [Google Scholar]
- 48. Weiss J, Crowley WF Jr, Halvorson LM, Jameson JL. Perifusion of rat pituitary cells with gonadotropin‐releasing hormone, activin, and inhibin reveals distinct effects on gonadotropin gene expression and secretion. Endocrinology. 1993;132:2307‐2311. [DOI] [PubMed] [Google Scholar]
- 49. Weiss J, Guendner MJ, Halvorson LM, Jameson JL. Transcriptional activation of the follicle‐stimulating hormone beta‐subunit gene by activin. Endocrinology. 1995;136:1885‐1891. [DOI] [PubMed] [Google Scholar]
- 50. Besecke LM, Guendner MJ, Schneyer AL, Bauer‐Dantoin AC, Jameson JL, Weiss J. Gonadotropin‐releasing hormone regulates follicle‐stimulating hormone‐beta gene expression through an activin/follistatin autocrine or paracrine loop. Endocrinology. 1996;137:3667‐3673. [DOI] [PubMed] [Google Scholar]
- 51. DePaolo LV, Bald LN, Fendly BM. Passive immunoneutralization with a monoclonal antibody reveals a role for endogenous activin‐B in mediating FSH hypersecretion during estrus and following ovariectomy of hypophysectomized, pituitary‐grafted rats. Endocrinology. 1992;130:1741‐1743. [DOI] [PubMed] [Google Scholar]
- 52. Yokota H, Yamada K, Liu X, et al. Paradoxical action of activin A on folliculogenesis in immature and adult mice. Endocrinology. 1997;138:4572‐4576. [DOI] [PubMed] [Google Scholar]
- 53. Zhao J, Taverne MA, van der Weijden GC, Bevers MM, van den Hurk R. Effect of activin A on in vitro development of rat preantral follicles and localization of activin A and activin receptor II. Biol Reprod. 2001;65:967‐977. [DOI] [PubMed] [Google Scholar]
- 54. Nakamura M, Nakamura K, Igarashi S, et al. Interaction between activin A and cAMP in the induction of FSH receptor in cultured rat granulosa cells. J Endocrinol. 1995;147:103‐110. [DOI] [PubMed] [Google Scholar]
- 55. Miró F, Smyth CD, Hillier SG. Development‐related effects of recombinant activin on steroid synthesis in rat granulosa cells. Endocrinology. 1991;129:3388‐3394. [DOI] [PubMed] [Google Scholar]
- 56. Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995;374:356‐360. [DOI] [PubMed] [Google Scholar]
- 57. Orisaka M, Orisaka S, Jiang JY, et al. Growth differentiation factor 9 is antiapoptotic during follicular development from preantral to early antral stage. Mol Endocrinol. 2006;20:2456‐2468. [DOI] [PubMed] [Google Scholar]
- 58. Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor‐9 is required during early ovarian folliculogenesis. Nature. 1996;383:531‐535. [DOI] [PubMed] [Google Scholar]
- 59. McNatty KP, Juengel JL, Wilson T, Galloway SM, Davis GH. Genetic mutations influencing ovulation rate in sheep. Reprod Fertil Dev. 2001;13:549‐555. [DOI] [PubMed] [Google Scholar]
- 60. Braw‐Tal R, McNatty KP, Smith P, et al. Ovaries of ewes homozygous for the X‐linked Inverdale gene (FecXI) are devoid of secondary and tertiary follicles but contain many abnormal structures. Biol Reprod. 1993;49:895‐907. [DOI] [PubMed] [Google Scholar]
- 61. Smith P, O W‐S, Corrigan KA, et al. Ovarian morphology and endocrine characteristics of female sheep fetuses that are heterozygous or homozygous for the inverdale prolificacy gene (fecX1). Biol Reprod. 1997;57:1183‐1192. [DOI] [PubMed] [Google Scholar]
- 62. Di Pasquale E, Beck‐Peccoz P, Persani L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein‐15 (BMP15) gene. Am J Hum Genet. 2004;75:106‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tiotiu D, Alvaro Mercadal B, Imbert R, et al. Variants of the BMP15 gene in a cohort of patients with premature ovarian failure. Hum Reprod. 2010;25:1581‐1587. [DOI] [PubMed] [Google Scholar]
- 64. Lakhal B, Laissue P, Braham R, et al. A novel BMP15 variant, potentially affecting the signal peptide, in a familial case of premature ovarian failure. Clin Endocrinol. 2009;71:752‐753. [DOI] [PubMed] [Google Scholar]
- 65. Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature. 1997;385:525‐529. [DOI] [PubMed] [Google Scholar]
- 66. Hunzicker‐Dunn M, Mayo K. Gonadotropin Signaling in the Ovary. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill's Physiology of Reproduction. London: Elsevier; 2014:895‐1022. [Google Scholar]
- 67. The European Recombinant Human LH Study Group . Recombinant human luteinizing hormone (LH) to support recombinant human follicle‐stimulating hormone (FSH)‐induced follicular development in LH‐ and FSH‐deficient anovulatory women: a dose‐finding study. J Clin Endocrinol Metab. 1998;83:1507‐1514. [DOI] [PubMed] [Google Scholar]
- 68. Filicori M, Cognigni GE, Tabarelli C, et al. Stimulation and growth of antral ovarian follicles by selective LH activity administration in women. J Clin Endocrinol Metab. 2002;87:1156‐1161. [DOI] [PubMed] [Google Scholar]
- 69. Jeppesen JV, Kristensen SG, Nielsen ME, et al. LH‐receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab. 2012;97:E1524‐E1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hillier SG. Gonadotropic control of ovarian follicular growth and development. Mol Cell Endocrinol. 2001;179:39‐46. [DOI] [PubMed] [Google Scholar]
- 71. Fortune JE. Ovarian follicular growth and development in mammals. Biol Reprod. 1994;50:225‐232. [DOI] [PubMed] [Google Scholar]
- 72. Ginther OJ, Beg MA, Bergfelt DR, Donadeu FX, Kot K. Follicle selection in monovular species. Biol Reprod. 2001;65:638‐647. [DOI] [PubMed] [Google Scholar]
- 73. Beg MA, Ginther OJ. Follicle selection in cattle and horses: role of intrafollicular factors. Reproduction. 2006;132:365‐377. [DOI] [PubMed] [Google Scholar]
- 74. Thierry van Dessel HJ, Chandrasekher Y, Yap OW, et al. Serum and follicular fluid levels of insulin‐like growth factor I (IGF‐I), IGF‐II, and IGF‐binding protein‐1 and ‐3 during the normal menstrual cycle. J Clin Endocrinol Metab. 1996;81:1224‐1231. [DOI] [PubMed] [Google Scholar]
- 75. Jolly PD, Tisdall DJ, Heath DA, Lun S, McNatty KP. Apoptosis in bovine granulosa cells in relation to steroid synthesis, cyclic adenosine 3',5'‐monophosphate response to follicle‐stimulating hormone and luteinizing hormone, and follicular atresia. Biol Reprod. 1994;51:934‐944. [DOI] [PubMed] [Google Scholar]
- 76. Hattori K, Orisaka M, Fukuda S, et al. Luteinizing hormone facilitates antral follicular maturation and survival via thecal paracrine signaling in cattle. Endocrinology. 2018;159:2337‐2347. [DOI] [PubMed] [Google Scholar]
- 77. Richards JS, Ascoli M. Endocrine, paracrine, and autocrine signaling pathways that regulate ovulation. Trends Endocrinol Metab. 2018;29:313‐325. [DOI] [PubMed] [Google Scholar]
- 78. Kishi H, Kitahara Y, Imai F, Nakao K, Suwa H. Expression of the gonadotropin receptors during follicular development. Reprod Med Biol. 2018;17:11‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Eppig JJ, Wigglesworth K, Pendola F, Hirao Y. Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod. 1997;56:976‐984. [DOI] [PubMed] [Google Scholar]
- 80. Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor‐9 in the mammalian ovary. Mol Endocrinol. 1999;13:1035‐1048. [DOI] [PubMed] [Google Scholar]
- 81. Chang HM, Qiao J, Leung PC. Oocyte‐somatic cell interactions in the human ovary‐novel role of bone morphogenetic proteins and growth differentiation factors. Hum Reprod Update. 2016;23:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone beta‐subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci USA. 2004;101:17294‐17299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lei ZM, Mishra S, Zou W, et al. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol Endocrinol. 2001;15:184‐200. [DOI] [PubMed] [Google Scholar]
- 84. Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15:172‐183. [DOI] [PubMed] [Google Scholar]
- 85. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162‐11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Couse JF, Hewitt SC, Bunch DO, et al. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science. 1999;286:2328‐2331. [DOI] [PubMed] [Google Scholar]
- 87. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277‐4291. [DOI] [PubMed] [Google Scholar]
- 88. Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA. 1998;95:6965‐6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Britt KL, Drummond AE, Cox VA, et al. An age‐related ovarian phenotype in mice with targeted disruption of the Cyp 19 (aromatase) gene. Endocrinology. 2000;141:2614‐2623. [DOI] [PubMed] [Google Scholar]
- 90. Yding Andersen C. Inhibin‐B secretion and FSH isoform distribution may play an integral part of follicular selection in the natural menstrual cycle. Mol Hum Reprod. 2017;23:16‐24. [DOI] [PubMed] [Google Scholar]
- 91. Hillier SG. Current concepts of the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum Reprod. 1994;9:188‐191. [DOI] [PubMed] [Google Scholar]
- 92. Yding Andersen C, Bungum L, Nyboe Andersen A, Humaidan P. Preovulatory progesterone concentration associates significantly to follicle number and LH concentration but not to pregnancy rate. Reprod Biomed Online. 2011;23:187‐195. [DOI] [PubMed] [Google Scholar]


