Abstract
The identification of new therapeutic strategies is urgently needed for the management of patients affected by anaplastic thyroid cancer (ATC) due to their short survival and poor prognosis. Aim of the study was to determine the activity of the combination irinotecan/sunitinib on ATC cell growth in vitro and the antitumor effects in vivo. Proliferation assays were performed for 72 h on ATC cell lines exposed to the combination of SN-38, the active metabolite of irinotecan, and sunitinib. The simultaneous combination of sunitinib and SN-38, quantified by the combination index, determined a high synergism on ATC cells, increasing the intracellular concentrations of SN-38. Moreover, the synergistic combination greatly decreases the gene expression and the protein levels of vascular endothelial growth factor, colony stimulating factor 1 and ATP-binding cassette transporter G2 in ATC cells. A significant in vivo antitumor effect was observed in ATC xenografts with the simultaneous combination of irinotecan and sunitinib if compared to monotherapy. The simultaneous combination of irinotecan and sunitinib, in vitro and in vivo demonstrated a significant, synergistic ATC antitumor activity, suggesting a possible and rapid translation of this schedule into the clinics.
Keywords: Sunitinib, Irinotecan, Anaplastic thyroid cancer, Synergism, Tumor xenografts
Introduction
Anaplastic thyroid cancer (ATC) is among the most aggressive malignancies with extremely short survival, poor prognosis and a disease-specific mortality approaching 100% [1]. ATC accounts for approximately 5%–15% of primary malignant thyroid tumors that have been described to be resistant to standard radiotherapy and chemotherapy [2]. At present, surgery, radiotherapy and chemotherapy are not helpful in improving the survival time and the life quality of such patients [1], and the poor prognosis is attributed to its unlimited growth and invasive migration. Therefore, identifying new possible therapeutic strategies is critical for ATC management. In dated scientific literature, cytotoxic drugs have demonstrated limited or no activity in ATC when administered alone [1,3], whereas, the improvement in the recognition and comprehension of genetic and molecular alterations underlying the development of thyroid cancers [3], led to the development of new treatment options such as tyrosine kinase inhibitors (TKIs). While vandetanib, cabozantinib, sorafenib and lenvatinib have reached a phase III clinical trial with favorable results in medullary thyroid carcinoma and differentiated thyroid carcinoma, it is still unclear if ATC patients may benefit from this therapeutic strategy [3]. Despite several preclinical studies on TKIs, such as sunitinib [4] – approved for the treatment of patients with advanced renal cell carcinoma or advanced gastrointestinal stromal tumors after imatinib therapy – have shown some in vivo antitumor activity in ATC [5–8], TKIs monotherapy could be not effective in vitro, as in the case of pazopanib [9] or imatinib [10]. Indeed, Bible and colleagues reported that although some pazopanib-treated ATC patients in a phase 2 trial incurred transient disease regression, there were no RECIST responses [11]. One example of an effective preclinical drug combination in a murine orthotopic ATC model was represented by the association of irinotecan (a standard therapeutic choice for colorectal cancer) plus cetuximab [12]. To our knowledge, no preclinical data are available on the combination between irinotecan and sunitinib in ATC or other cancer types with the only exception of experiments performed in PC12 tumor-bearing mice, showing an enhanced in vivo activity of the combined schedule [13].
The aim of the present study is 1) to investigate whether different combined schedules of sunitinib and irinotecan based chemotherapy may synergize and 2) to reveal the underlying mechanism of these effects, challenging the dogma of untreatable ATC with chemotherapeutic drugs and tyrosine kinase inhibitors.
Materials and methods
Materials and drugs
Cell culture media RPMI, M-199 (used to isolate primary ATC cells) and DMEM, supplements and all other chemicals not listed in this section were obtained from Sigma Aldrich SRL (Milan, Italy). Quantitative real-time PCR reagents were from Applied Biosystems (Foster City, CA, USA). SN-38, the active metabolite of irinotecan, and sunitinib, were purchased from Selleckchem (DBA Italia, Milan, Italy), and dissolved in a stock solution of 10 mM in 100% dimethylsulfoxide (DMSO) for in vitro studies. DMSO concentration in the control’s media was the same used to make up the highest concentration of sunitinib and SN-38 in growth media for the same experiment.
Cell lines
Primary ATC cells were obtained as previously described [14–16], from thyroid biopsy at the moment of first surgery. The diagnosis was established on commonly accepted clinical, laboratory, and histological criteria [17]. Cells were maintained in DMEM supplemented with 10% FCS.
The human ATC cell line 8305C (BRAF V600E mutated) – established from undifferentiated thyroid carcinomas of a 67 year-old-female patient – was from DSMZ (Braunschweig, Germany, DSMZ no.: ACC 133) [18], whereas the human ATC cell line FB3 (HRas Q61R mutated) was obtained from Prof. Fulvio Basolo of the University of Pisa, Pisa, Italy [6]. Both cell lines were maintained in RPMI 1640 medium supplemented with 15% FBS and L-glutamine (2 mM). The cultures were free of mycoplasma species and cells were used for tests at the fourth passage.
Antiproliferative assay
In vitro chemosensitivity was tested on 8305C, FB3 cell lines, as previously described [6,19]. 8305C and FB3 cells were treated for 72 h (1 × 104 cells/well of cancer cells in 1 ml of medium) with sunitinib (0.01–100 μM) or SN-38 (0.01–100 μM) or with their vehicle.
In vitro assessment of synergism between sunitinib and SN-38 on ATC cells
The combination of sunitinib with SN-38 was explored on 8305C and FB3 cells with three different treatment schedules at a fixed molar concentration ratio (1:1), as follows: (A) simultaneous exposure: sunitinib plus SN-38 for 72 h; (B) sequential exposure: sunitinib alone for 24 h, sunitinib plus SN-38 for 24–72 h and SN-38 alone for 72–96 h; (C) reverse exposure: SN-38 alone for 24 h, SN-38 plus sunitinib for 24–72 h and sunitinib alone for 72–96 h. Therefore, the total exposure of cells to each drug was 72 h. To evaluate the level of interaction (synergistic, additive or antagonist) between SN-38 and sunitinib the Combination Index (CI) and Dose Reduction Index (DRI) method was followed [20], as previously described by our group [21,22].
Apoptosis measurement in primary ATC cells and FB3 cells
Primary ATC cells were incubated for 24 h with sunitinib (5, 10, 25 μM) in a humidified atmosphere (37 °C, 5% CO2). At the end of the experiment cells were stained with Hoechst 33342 as previously described [23]. The apoptosis index (ratio between apoptotic and total cells) × 100 was calculated. The Annexin V binding assay was used to further confirm the results of the cell apoptosis test as previously described [23]. Moreover, FB3 cells were treated for 72 h with SN-38 and sunitinib at a concentration corresponding to the experimental IC50 of cell proliferation, alone and in simultaneous combination. At the end of the experiment, the apoptosis analysis was performed using the Cell Death Detection ELISA Plus kit (Roche Diagnostics, Milan, Italy).
HPLC analysis of SN-38 intracellular concentrations in ATC cells
The quantitative analysis of irinotecan’s main metabolite SN-38 in cells was performed as previously described [21,24]. ATC cells, 8305C and FB3, were treated with vehicle alone, SN-38 (1 μM), sunitinib (1 μM), or a combination of the two (SN-38 1 μM + sunitinib 1 μM) for 2 h [25]. The analysis was performed using system LC Module I Plus (Waters, Milan, Italy) [21,24,26].
Modulation of ABCG2, CSF-1 and VEGF gene expression
To evaluate the expression of the genes encoding human ABCG2, CSF-1, and VEGF proteins, 8305C and FB3 cells were grown in their respective media and treated with SN-38 and sunitinib or in combination in three different treatment schedules (simultaneous, sequential and reverse exposure) at a concentration corresponding to the experimental IC50 of cell proliferation or with vehicle alone for 72 h. Quantitative RT-PCR was performed with the Applied Biosystems 7900HT sequence detection system, as previously described [6,21]. Validated primers were purchased from Applied Biosystems (ABCG2, Assay ID Hs01053796_m1, CSF-1 Hs00174164_m1, VEGF Hs00170236_m1).
Quantification of ABCG2 and CSF-1 protein levels in ATC cells
To investigate the modulation of protein of ABCG2 and CSF-1 by the simultaneous combination of sunitinib and SN-38, 8305C and FB3 cells were treated for 72 h with sunitinib and SN-38 at the above mentioned concentrations or with vehicle alone. Tumor cell lysates were assayed as per the manufacturer’s instruction with the human ABCG2 and CSF-1 ELISA kit (Mybiosource, #MBS762174 – Sigma-Aldrich, #RAB0098–1KT). The optical density was determined using a Multiskan Spectrum microplate reader (Milan, Italy) set to 450 nm. The results were expressed as ng of ABCG2 and CSF-1 per mg of total protein.
Animals and treatments
Six-week-old CD nu/nu male mice, supplied by Envigo (Milan, Italy), were housed in microisolator cages on vented racks and manipulated using aseptic techniques. Housing and all procedures involving animals were performed according to the protocol approved by the Academic Organization Responsible for Animal Welfare (OPBA, Organismo Preposto per il Benessere Animale) at the University of Pisa, in accordance with the EU Directive 2010/63/EU for animal experiments and the Italian law D.lgs. 26/2014, and by the Italian Ministry of Health (authorization number 613/2015-PR).
On day 0, 2 × 106 ± 5% viable 8305C cells/mouse were inoculated subcutaneously and tumor volume were measured, as previously described [6,21]. Mice were treated with irinotecan (100 mg/kg/wk) and sunitinib (25 mg/kg/every two days) alone and in combination in three different schedules treatments – simultaneous and sequentials (i.e. irinotecan for two weeks followed by sunitinib for two weeks, or the reverse schedule). At the end of the experiment, mice were sacrificed by an anaesthetic overdose and tumors were excised and measured.
Immunohistochemistry
Briefly, tumor tissue samples from all the different treatment groups were fixed in 10% neutral-buffered formalin for 24 h and embedded in paraffin for histology and IHC. Sections (5-μM-thick) were incubated with anti-PECAM-1 antibody (M-20) (1:200, #sc1506, Santa Cruz Biotechnology) and anti-cleaved Caspase-3 (Asp175) (1:300, #sc7272, Santa Cruz Biotechnology) at 4 °C overnight. Negative controls were carried out by omitting the primary antibodies. Immunostaining was accomplished using a Benchmark immunostainer (Ventana, Tuscon, AZ). Immunoreaction was displayed using the avidin–biotin–peroxidase complex (ABC) method. Peroxidase activity was visualized with diaminobenzidine. Counterstaining was performed with haematoxylin. Quantification of caspase 3 positive cells and CD31 was performed as previously described [21,27].
Analysis of data
The analysis by ANOVA, followed by the Student-Newman–Keuls test, was used to assess the statistical differences of data in vitro and in vivo. P-values lower than 0.05 were considered significant. Statistical analyses were performed using the GraphPad Prism software package version 5.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
Sunitinib and SN-38 inhibit ATC cells proliferation in vitro
Both sunitinib and SN-38 inhibited in vitro the cell proliferation of thyroid cancer 8305C and FB3 cell lines in a concentration-dependent manner. The 72 h sunitinib exposure inhibited the 8305C (Fig. 1A) and FB3 (Fig. 1B) cell proliferation with an IC50 of 1.4 μM and 9 μM, respectively. A higher antiproliferative effect of SN-38 on 8305C (Fig. 1C) and FB3 (Fig. 1D) was found as demonstrated by the calculated IC50s (0.1863 μM and 0.252 μM, respectively).
Fig. 1.
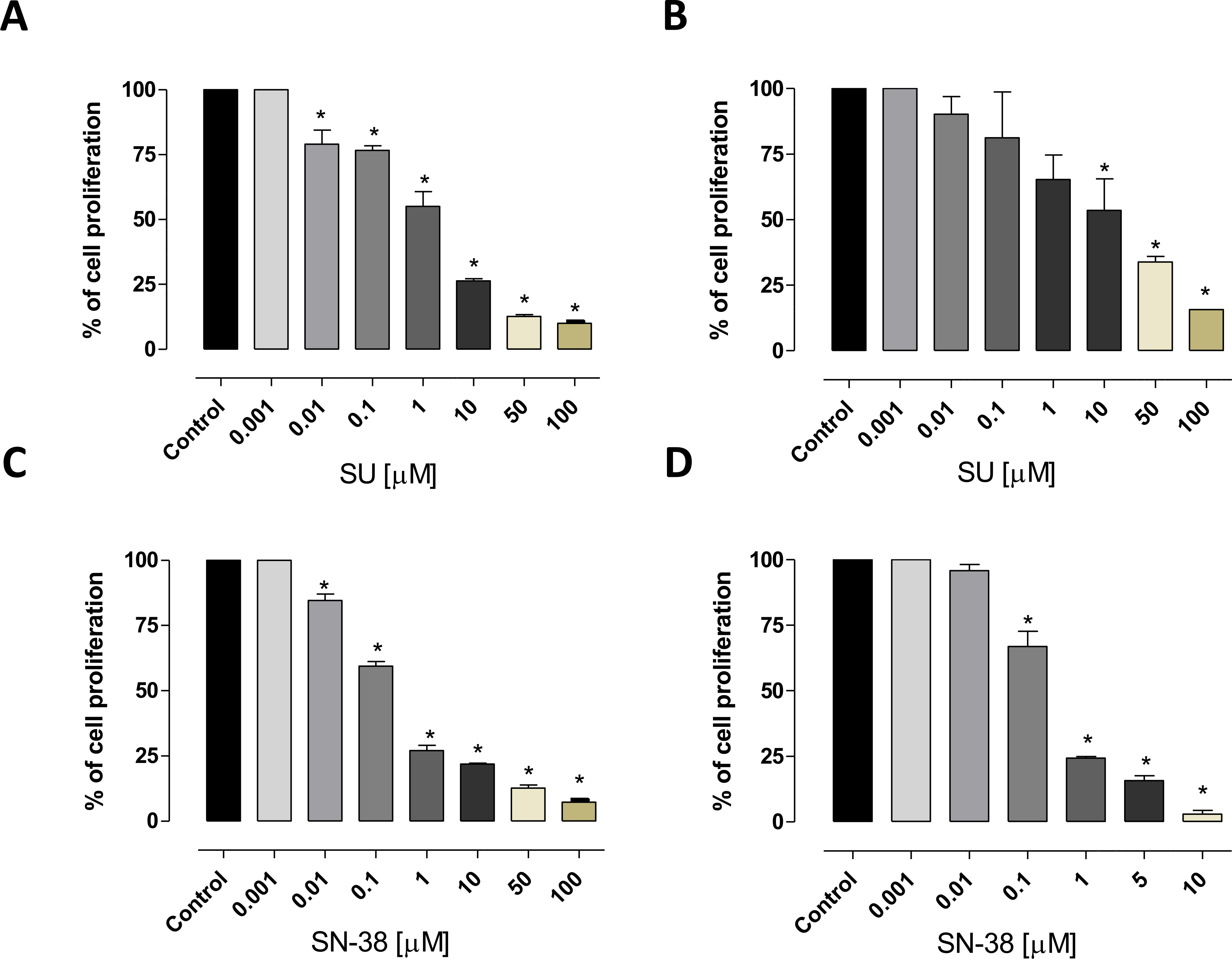
Antiproliferative effects of sunitinib (SU) and SN-38 in vitro on 8305C (A and C, respectively), and FB3 (B and D, respectively) cell lines. The antiproliferative effects of the drugs were studied after 72 h of exposure. The data are presented as percentage of vehicle-treated cells. The concentrations of drug that reduced cell proliferation by 50% (IC50) vs controls were calculated by a nonlinear regression fit of the mean values of the data obtained in triplicate experiments (i.e. at least 9 wells for each concentration). Columns and bars, mean values ± S.E., respectively. *, P < 0.001 vs. control.
Sunitinib induces tumor cell apoptosis in primary ATC cells and FB3 cells
The percentage of apoptotic cells in primary ATC cultures increased in a concentration dependent manner (Fig. 2A): after 24 h treatment with sunitinib 5 μM, 54 ± 13.3% of the cells were apoptotic and this percentage increased up to 82 ± 10.2% and 95 ± 3.3% with sunitinib 10 μM or 25 μM, respectively (P < 0.001). Annexin V was used to further confirm the induced cell apoptosis (Fig. 2B). Fig. 2C shows the significant pro-apoptotic activity of sunitinib and SN-38 alone, and of their simultaneous combination on FB3 cells.
Fig. 2.
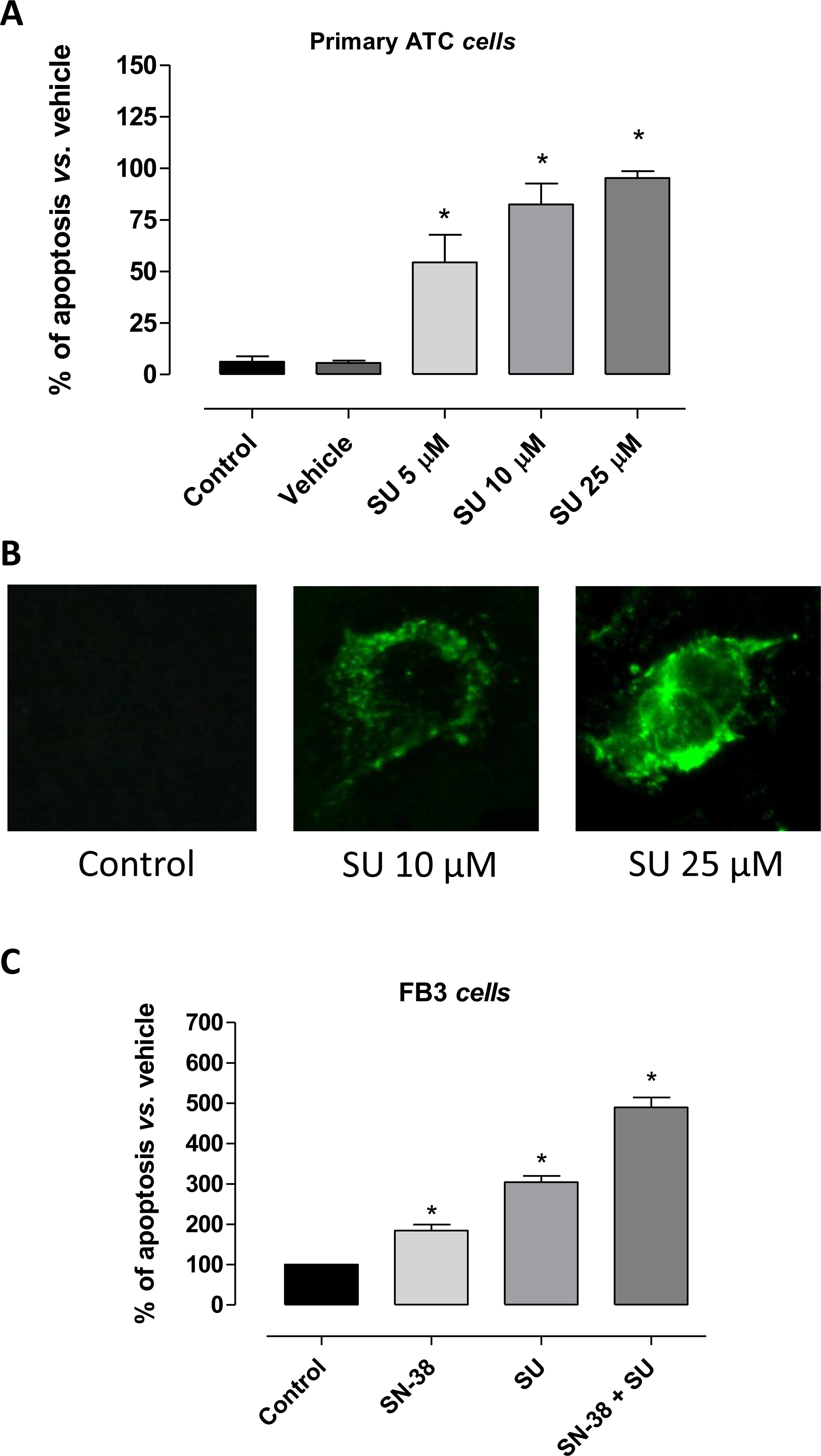
A) Apoptosis in primary ATC cells treated with sunitinib (SU) for 24 h (mean ± SD n = 5). Data were analyzed by one-way ANOVA with Newman–Keuls multiple comparisons test and with a test for linear trend (*P < 0.001 vs. control). The percentage of apoptotic cells after the treatment with vehicle (DMSO) was not significantly different from the one of control (not treated cells). B) Representative images of immunofluorescence Annexin V staining of sunitinib (SU) treated-cells. C) Pro-apoptotic effects of sunitinib (SU) and SN-38, alone or in combination, on proliferating FB3 cells treated for 72 h under hypoxic conditions (1% O2, 5% CO2, 95% humidity). All the absorbance values were plotted as a percentage of apoptosis relative to control cells (vehicle only), which is labelled as 100%. Columns and bars, mean values ± S.E., respectively. *P < 0.01 vs. vehicle-treated controls.
Synergistic effect of sunitinib and SN-38 on ATC cells proliferation
Simultaneous exposure of 8305C and FB3 cells to different concentrations of sunitinib and SN-38 for 72 h showed a strong synergism for fraction of affected cells higher than 30% (CI < 1 and DRI > 1;Table 1). Synergism corresponding to CI < 1 always yielded a favorable DRI > 1 for both drugs. Interestingly, the sequential and reverse exposure of sunitinib and SN-38 for 72 h in 8305C and FB3 cells were synergistically active only for fraction of affected cells ranging from 60% to 95% (CI < 1; Table 1), and then additive for fraction of affected cells ranging approximately from 30 to 50% (CI ~ 1; Table 1). However, these effects were lost for percentages lower than 25% (Table 1).
Table 1.
CI (Combination Index) and DRI (Dose Reduction Index) values for the drug combinations at 25%, 50%, and 75% levels of inhibition of 8305C and FB3 cell proliferation. SN-38, active metabolite of irinotecan; SU, sunitinib.
| Simultaneous treatment | CI values SN-38 + SU | |||||||
| 25% (SU + SN-38) | 50% (SU + SN-38) | 75% (SU + SN-38) | ||||||
| 8305C | 4.467 | 0.844 | 0.153 | |||||
| FB3 | 0.044 | 0.104 | 0.270 | |||||
| Sequential treatment | SU → SN-38 | |||||||
| 8305C | 1.073 | 0.910 | 0.442 | |||||
| FB3 | 11.432 | 1.121 | 0.266 | |||||
| Reverse treatment | SN-38 → SU | |||||||
| 8305C | 2.589 | 1.069 | 0.442 | |||||
| FB3 | 11.826 | 1.236 | 0.313 | |||||
| 25% | DRI values 50% | 75% | ||||||
| Simultaneous treatment | SN-38 + SU | |||||||
| SU | SN-38 | SU | SN-38 | SU | SN-38 | |||
| 8305C | 0.666 | 0.318 | 3.886 | 1.705 | 22.69 | 9.141 | ||
| FB3 | 200.36 | 25.68 | 601.87 | 9.76 | 1808 | 3.71 | ||
| Sequential treatment | SU → SN-38 | |||||||
| SU | SN-38 | SU | SN-38 | SU | SN-38 | |||
| 8305C | 1.194 | 0.571 | 3.099 | 1.340 | 8.038 | 3.145 | ||
| FB3 | 0.094 | 1.209 | 1.442 | 2.339 | 22.04 | 4.523 | ||
| Reverse treatment | SN-38 → SU | |||||||
| SU | SN-38 | SU | SN-38 | SU | SN-38 | |||
| 8305C | 2.882 | 1.378 | 2.515 | 1.088 | 2.195 | 0.859 | ||
| FB3 | 0.091 | 1.168 | 1.308 | 2.121 | 18.76 | 3.851 |
Sunitinib increases intracellular SN-38 concentrations
To investigate in more detail the bases of the synergistic effect, a modulation of intracellular drug concentrations was hypothesized, and thus SN-38 levels on treated cells were measured. Significantly higher SN-38 intracellular concentrations (approximately 30%) were found in 8305C (Fig. 3A) and FB3 (Fig. 3B) cells exposed to the drug in combination with sunitinib, when compared with SN-38 alone.
Fig. 3.
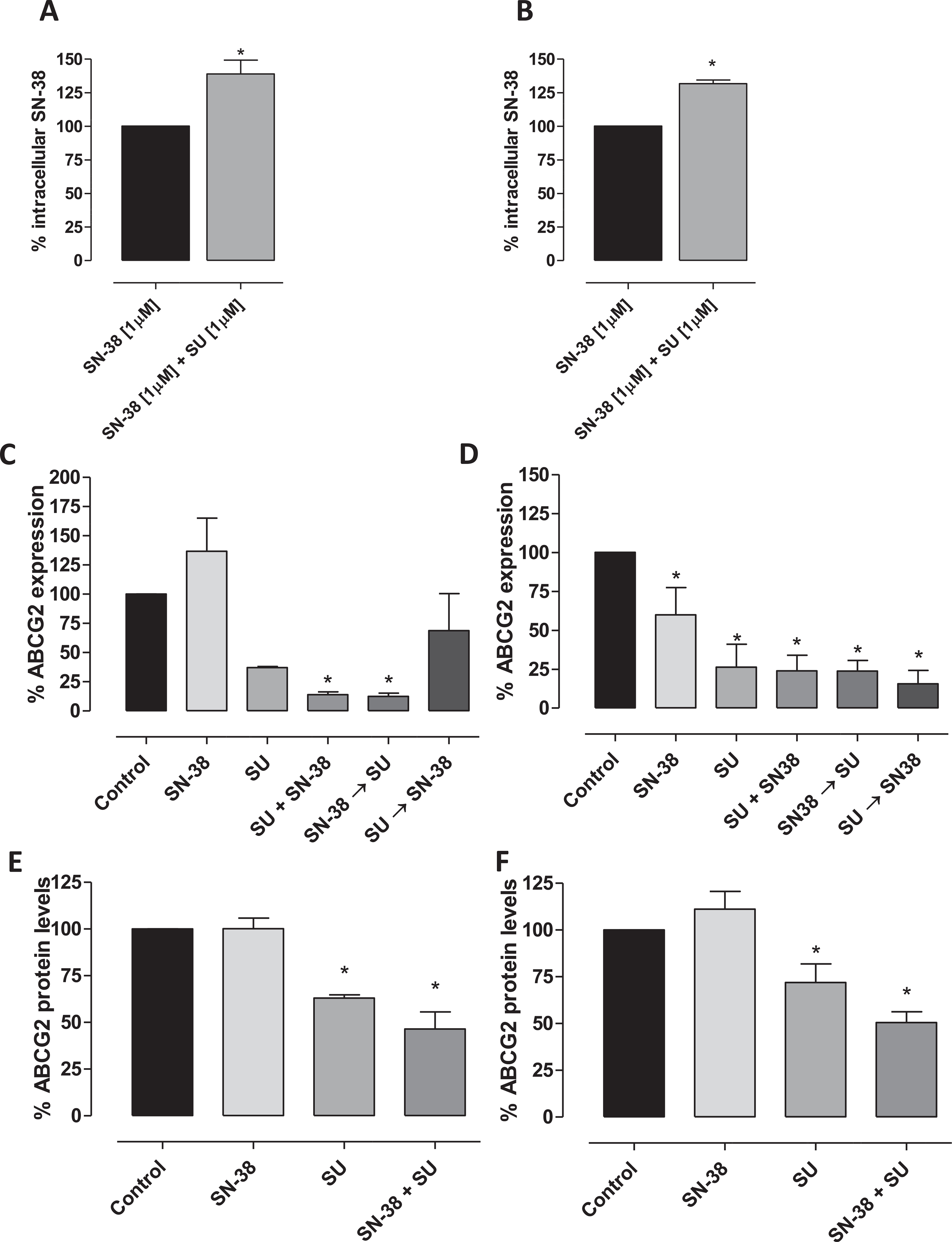
Accumulation of SN-38 (ng · mg−1 protein) in 8305C (A) and FB3 (B) cell lines after exposure to 1 μM SN-38 alone and in combination with sunitinib (SU). Columns and bars indicate the mean percentage values (±S.D.) vs. treated cells with SN-38 alone. ABCG2 gene expression (2−ΔΔCt) and ABCG2 (ng · mg−1 protein) protein levels in 8305C (C and E, respectively) and FB3 (D and F, respectively) cells exposed to sunitinib or with vehicle alone for 72 h. Data are expressed as percentage of vehicle-treated cells. Columns and bars, mean values ± S.D., respectively. *P < 0.05 vs. vehicle-treated controls. The quantitation of gene expression was performed using the ΔΔCt calculation, where Ct is the threshold cycle; the amount of target, normalized to the endogenous control, glyceraldehyde 3-phosphate dehydrogenase, and relative to the calibrator (vehicle treated control cells), is given as 2−ΔΔCt. The quantitation of protein levels was performed by ELISA. The optical density was determined using a Multiskan Spectrum microplate reader set to 450 nm. The results were expressed as nanograms of ABCG2 per milligram of total protein. All experiments were repeated, independently, three times.
Based on these findings, the modulation of ABCG2 expression was investigated to deepen the mechanisms underlying the synergistic interaction between sunitinib and SN-38. ABCG2 is a transporter involved in the biodisposition of chemotherapeutic drugs such as SN-38 [28], that is one of its substrate. The gene expression and the protein levels of this transporter were quantified in 8305C and FB3 cell lines exposed to SN-38, sunitinib and their combination at the experimental IC50. Fig. 3C and D show a significant decrease of ABCG2 gene expression in 8305C and FB3 cells, respectively, by sunitinib and the simultaneous combination of sunitinib and SN-38 (combination drugs exposure in 8305C 13.9 ± 2.45% and in FB3 24 ± 17.4% vs. 100% of control expression; P < 0.001). Moreover, the combination of the two drugs showed a decrease of ABCG2 protein levels (Fig. 3E and F) when compared to the single drugs alone (combination drugs exposure in 8305C 46.4 ± 9.18% and in FB3 50.5 ± 9.99% vs. 100% of control; P < 0.001).
Sunitinib and SN-38 combination inhibits CSF-1 and VEGF gene expression and decrease CSF-1 protein levels in ATC cells
To study the effects of sunitinib and SN-38 combination on the angiogenesis-related genes CSF-1 and VEGF in 8305C and FB3 cell lines. mRNA levels of both genes were evaluated. Fig. 4 show the significant inhibition of VEGF (Fig. 4A) gene expression in 8305C and FB3 cells and CSF-1 (Fig. 4B) in FB3 cells, by sunitinib plus SN-38. Of note, the decreased expression of these genes was obtained also in the sequential and reverse treatment schedule (Supplementary Fig. 1S A and B). Based on these findings, the intracellular CSF-1 protein levels were measured in treated and vehicle-treated cells. Significant lower CSF-1 concentrations were found in 8305C and FB3 cells (Fig. 4C) exposed to the combination of the two drugs if compared to single drugs.
Fig. 4.
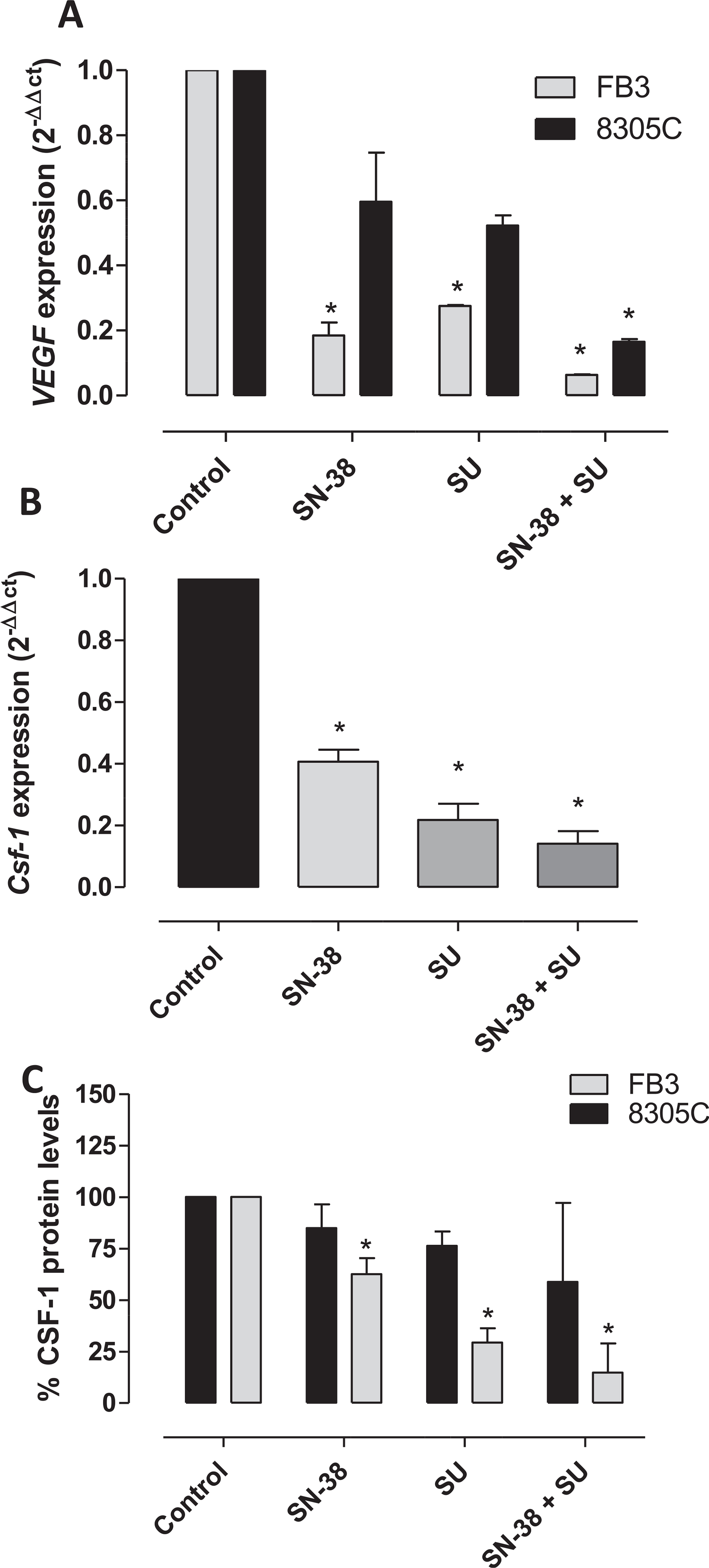
VEGF (A) and CSF-1 (B) gene expression (2−ΔΔCt) in 8305C and FB3 cells and (C) CSF-1 (ng · mg−1 protein) protein levels in FB3 cells exposed to sunitinib (SU) or with vehicle alone for 72 h. Columns and bars, mean values ± S.D., respectively. *P < 0.05 vs. vehicle-treated controls. Amplifications were normalized to glyceraldehyde 3-phosphate dehydrogenase, and the quantitation of gene expression was performed using the ΔΔCt calculation, where Ct is the threshold cycle; the amount of target, normalized to the endogenous control and relative to the calibrator (vehicle-treated control cells), is given as 2−ΔΔCt. The quantitation of protein levels was performed by ELISA. The optical density was determined using a Multiskan Spectrum microplate reader set to 450 nm. The results were expressed as nanograms of CSF-1 per milligram of total protein. All experiments were repeated, independently, three times.
Sunitinib and irinotecan combination exerts its antitumor activity in vivo
Fig. 5A reports the results of the in vivo experiment. Tumors in control animals showed an exponential enlargement in their dimensions, after 20 days from the implant. Tumor growth was assessed in six different treatment groups: vehicle control, irinotecan, sunitinib, the simultaneous combination of irinotecan and sunitinib and the two consecutive schedules (i.e. the sequential combination of sunitinib for two weeks followed by irinotecan for two weeks, and the reverse schedule of irinotecan followed by sunitinib). Single-agent irinotecan and sunitinib had a moderate but significant antitumor effect vs. control. In contrast, the simultaneous combination of irinotecan and sunitinib caused a significant strong tumor growth delay if compared to the single drugs (e.g. at day 21, 420.72 mm3 vs. 1974.7 mm3 of sunitinib treated group, P < 0.05), and a greater effect than that observed with the other two consecutive combination schedules (e.g. at day 21, 1129 mm3 of irinotecan → sunitinib and 1209 mm3 of sunitinib → irinotecan vs. 420.72 mm3 of simultaneous irinotecan + sunitinib treated group, P < 0.05). Supplementary Fig. 2S shows the calculated tumors weights at day 14 after the beginning of therapy. Mice treated with single agents and the three different combination schedules maintained a stable body weight throughout the course of the experiment.
Fig. 5.
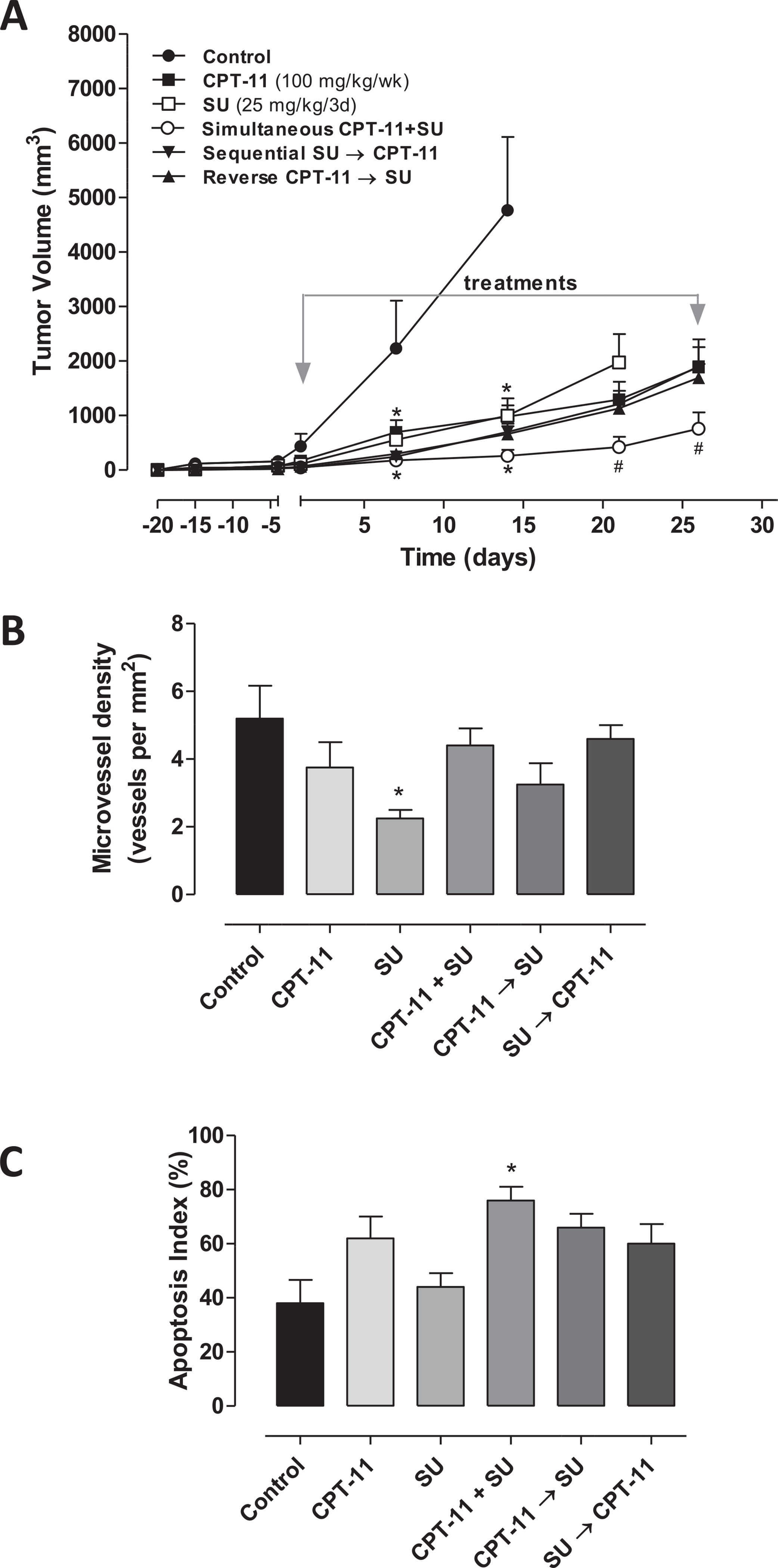
In vivo antitumor effects of the single drugs and three different combination schedules of sunitinib (SU) and irinotecan (CPT-11) on 8305C tumors xenotransplanted in mice (A); Immunohistochemistry quantification of CD31 (B) and Capase-3 positive cells (C) in 8305C tumor xenografts administered with vehicle, SU at 25 mg/kg every 3 days, CPT-11 100 mg/kg every week through i.p. injection, and their combinations. Symbols/columns and bars, mean values ± S.D., respectively.*P < 0.001 vs. vehicle-treated controls. #P < 0.001 vs. sunitinib-treated group.
CD31 staining in tumor sections from control, irinotecan and all three combination schedules treated animals showed a similar presence of number of vessels, which instead were statistically decreased in mice treated with sunitinib alone (Fig. 5B). Conversely, basal levels of caspase-3 activity detected in tumors samples from control mice were not modified by sunitinib treatment, but they were significantly increased in the group treated with the simultaneous irinotecan plus sunitinib (Fig. 5C).
Discussion
ATC accounts for only 3% of all thyroid cancers, but with its aggressive nature and high mortality rate it accounts for the majority of deaths from thyroid cancer [29]. Despite the poor prognosis is attributed to its unlimited growth and invasive migration, ATC is not sensitive to any current systemic therapies including radiation or chemotherapy [1,30]. Clearly, identifying novel therapeutic strategies are desperately needed for the ATC management. Phase II and small retrospective studies did not identify highly effective drugs or regimens, in part by failing to recruit sufficient numbers of patients [31]. Although chemotherapy alone can be considered for ATC patients with unresectable or metastatic disease, cytotoxic drugs administered alone are not very effective [1,32]. Indeed, ATC have exhibited limited or modest responses to traditional chemotherapeutic agents such as doxorubicin, paclitaxel, cisplatin, carboplatin, methotrexate and mitoxantrone [31,33]. Even though with rare complete responses and heavy toxicity, doxorubicin remains the single most effective cytotoxic chemotherapy for the treatment of metastatic disease [31]. The improvement in the discovery of genetic and molecular alterations underlying the development of thyroid cancers [3] led to the study of the potential usefulness of TKIs in the clinical treatment of advanced thyroid cancer [33,34]. The potential benefits on survival of a combined approach of intensity-modulated radiotherapy with chemotherapy – docetaxel – and sunitinib was described in a patient with ATC [35]. Sunitinib showed evidence of activity in a phase I trial in many tumors, including thyroid cancers [36]. However, TKIs are usually more effective in combination with standard chemotherapy [21,26]. Sunitinib have shown to be active in vitro and in vivo against activated endothelial and ATC cell lines via the inhibition of Akt and ERK1/2 phosphorylation and through the down-regulation of cyclin-D1 [6]. Moreover, our present findings corroborate the antiproliferative effect of sunitinib even on primary ATC cells (data not shown). In clinics, the off-label use of sunitinib and sorafenib was evaluated in 33 patients with widely progressive metastatic differentiated thyroid cancer, and both drugs appeared to be effective [36]. Moreover, Carr et al. conducted a phase II study to assess the efficacy of sunitinib, reporting its effectiveness as a continuous administration in patients with iodine-refractory, well-differentiated thyroid carcinoma and medullary thyroid cancer [37]. Among chemotherapeutic drugs, preclinical studies using irinotecan have demonstrated a promising activity in undifferentiated thyroid carcinomas [22]. Moreover, a combination therapy including irinotecan with cetuximab have shown to inhibit the growth and progression of orthotopic ATC xenografts in nude mice [12].
Based on these premises, our group decided to evaluate different schedules of treatment with sunitinib in combination with irinotecan, and investigate the mechanisms of action involved in the antitumor activity of this treatment combination.
Our study clearly demonstrated, for the first time, that the simultaneous combination of sunitinib and irinotecan was highly synergistic on ATC cells. Currently, there are no data about the in vitro antiproliferative effect of SN-38 in combination with sunitinib on ATC cells to compare our findings. However, SN-38 in combination with other anti-VEGFR2 TKIs such as semaxinib [27] or CLM3 [22] revealed a synergistic effect on replicating human endothelial cells suggesting that this combined approach may really give a significant therapeutic advantage in various preclinical settings.
The basis of the significant synergistic activity was due to the significant increase of SN-38 intracellular levels mediated by sunitinib activity in ATC cells, that greatly enhanced the antitumor effects of the simultaneous exposure to the two drugs. In this regard, our group previously suggested that the combination of different TKIs – such as axitinib, pazopanib and sunitinib – with SN-38 or topotecan, increased the intracellular accumulation of camptothecins, by TKIs-mediated downregulation of ABCG2, a transporter involved in the resistance to irinotecan and other chemotherapeutic drugs [21,26]. Thus, our present findings strengthen these previous experiences and suggest that the simultaneous combination with sunitinib affects the bioavailability of SN-38, increasing its intracellular concentration in ATC cells.
Thyroid cancers are heavily infiltrated with macrophages and 95% of anaplastic thyroid cancer cases showed high tumor-associated macrophages (TAMs) infiltration, which correlated with poor survival rate [38]. Recently, Weinberger and colleagues have identified a set of consistently altered biological processes and pathways in ATC [29]. They found several genes associated with TAMs, lymphocytic infiltration and phagocytosis by macrophages, including the CSF1R highly upregulated in ATC samples. Macrophages depend on CSF-1 for differentiation and survival and, at high density, they may influence the overall gene expression profile in ATC [29]. Several clinical trials of CSF1/CSF1R inhibitors have been completed or are currently ongoing on different malignancies [39]. TKIs, such as imatinib [40], dasatinib [41], and sunitinib [42], have been proposed to regulate the tumorigenic and immunosuppressive functions of TAMs [43]. In our study, we found a significantly decrease of CSF-1 gene expression and protein levels in particular due to the simultaneous schedules with sunitinib and SN-38 in ATC cells. Our results may suggest that the decline of CSF-1 levels, in ATC, could decrease the macrophages stimulation induced by this myelopoietic growth factor in vivo. Moreover, CSF-1 induces VEGF production and the angiogenic activity from human monocytes [44]. In this regard, the well known anti-VEGF activity of sunitinib was confirmed in our in vivo experiments, and also the decrease of VEGF gene expression was observed in particular after the simultaneous combined treatment in ATC cells.
To translate the in vitro findings into the in vivo setting, we have evaluated the antitumor activity of irinotecan and sunitinib and the three different combined schedules in 8305C tumor xenograft models. All treatments were very well tolerated. The results demonstrated an improved efficacy of the simultaneous combined regimen, as expected by the in vitro data. No in vivo data are available to make an analogy between the results of our association with other similar combined approaches in ATC. However, irinotecan has revealed a stronger in vivo antitumor effects if administered in combination with anti-VEGFR-2 TKIs such as semaxinib in colon cancer models [27], axitinib in pancreatic cancer [21], or CEP-7055 in glioblastoma and colon cancer [45]. Moreover, in our in vitro experiments, we were able to show that sunitinib triggers apoptosis in primary ATC cells. In agreement with these findings, the combination treatments were able to significantly increase the activity of caspase-3 expression compared to controls, as noted in the in vivo model.
In conclusion, in our study we report, for the first time to our knowledge, the remarkable efficacy of the simultaneous combination of sunitinib and irinotecan, in vitro and in vivo, demonstrating a highly significant, synergistic antitumor activity in ATC cells. These findings may suggest a possible translation of this schedule into the clinics.
Supplementary Material
Acknowledgements
The study has been supported by a grant from Associazione Italiana per la Ricerca sul Cancro (IG-17672) to GB. TDD is the recipient of the 2015 SIF (Italian Society of Pharmacology)-MSD Fellowship. The authors thank Giada Salvia for her suggestions.
Footnotes
Conflict of interest
None.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.canlet.2017.09.032.
References
- [1].Haddad RI, Lydiatt WM, Ball DW, Busaidy NL, Byrd D, Callender G, et al. , Anaplastic thyroid carcinoma, version 2.2015, J. Natl. Compr. Canc Netw 13 (2015) 1140–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Smallridge RC, Marlow LA, Copland JA, Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies, Endocr. Relat. Cancer 16 (2009) 17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alonso-Gordoa T, Diez JJ, Duran M, Grande E, Advances in thyroid cancer treatment: latest evidence and clinical potential, Ther. Adv. Med. Oncol 7 (2015) 22–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chow LQ, Eckhardt SG, Sunitinib: from rational design to clinical efficacy, J. Clin. Oncol 25 (2007) 884–896. [DOI] [PubMed] [Google Scholar]
- [5].Kim S, Yazici YD, Calzada G, Wang ZY, Younes MN, Jasser SA, et al. , Sorafenib inhibits the angiogenesis and growth of orthotopic anaplastic thyroid carcinoma xenografts in nude mice, Mol. Cancer Ther 6 (2007) 1785–1792. [DOI] [PubMed] [Google Scholar]
- [6].Di Desidero T, Fioravanti A, Orlandi P, Canu B, Giannini R, Borrelli N, et al. , Antiproliferative and proapoptotic activity of sunitinib on endothelial and anaplastic thyroid cancer cells via inhibition of Akt and ERK1/2 phosphorylation and by down-regulation of cyclin-D1, J. Clin. Endocrinol. Metab 98 (2013) E1465–E1473. [DOI] [PubMed] [Google Scholar]
- [7].Gule MK, Chen Y, Sano D, Frederick MJ, Zhou G, Zhao M, et al. , Targeted therapy of VEGFR2 and EGFR significantly inhibits growth of anaplastic thyroid cancer in an orthotopic murine model, Clin. Cancer Res 17 (2011) 2281–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang L, Gaskins K, Yu Z, Xiong Y, Merino MJ, Kebebew E, An in vivo mouse model of metastatic human thyroid cancer, Thyroid 24 (2014) 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McLarnon A, Thyroid cancer: pazopanib alone is not effective against anaplastic thyroid cancer, Nat. Rev. Endocrinol 8 (2012) 565. [DOI] [PubMed] [Google Scholar]
- [10].Dziba JM, Ain KB, Imatinib mesylate (gleevec; STI571) monotherapy is ineffective in suppressing human anaplastic thyroid carcinoma cell growth in vitro, J. Clin. Endocrinol. Metab 89 (2004) 2127–2135. [DOI] [PubMed] [Google Scholar]
- [11].Bible KC, Suman VJ, Menefee ME, Smallridge RC, Molina JR, Maples WJ, et al. , A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer, J. Clin. Endocrinol. Metab 97 (2012) 3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim S, Prichard CN, Younes MN, Yazici YD, Jasser SA, Bekele BN, et al. , Cetuximab and irinotecan interact synergistically to inhibit the growth of orthotopic anaplastic thyroid carcinoma xenografts in nude mice, Clin. Cancer Res 12 (2006) 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maitani Y, Saito H, Seishi Y, Iwase Y, Yamauchi T, Higashiyama K, et al. , A combination of liposomal sunitinib plus liposomal irinotecan and liposome co-loaded with two drugs enhanced antitumor activity in PC12-bearing mouse, J. Drug Target 20 (2012) 873–882. [DOI] [PubMed] [Google Scholar]
- [14].Antonelli A, Ferrari SM, Fallahi P, Berti P, Materazzi G, Barani L, et al. , Primary cell cultures from anaplastic thyroid cancer obtained by fine-needle aspiration used for chemosensitivity tests, Clin. Endocrinol. (Oxf) 69 (2008) 148–152. [DOI] [PubMed] [Google Scholar]
- [15].Antonelli A, Ferrari SM, Fallahi P, Berti P, Materazzi G, Marchetti I, et al. , Evaluation of the sensitivity to chemotherapeutics or thiazolidinediones of primary anaplastic thyroid cancer cells obtained by fine-needle aspiration, Eur. J. Endocrinol 159 (2008) 283–291. [DOI] [PubMed] [Google Scholar]
- [16].Antonelli A, Ferrari SM, Fallahi P, Berti P, Materazzi G, Minuto M, et al. , Thiazolidinediones and antiblastics in primary human anaplastic thyroid cancer cells, Clin. Endocrinol. (Oxf) 70 (2009) 946–953. [DOI] [PubMed] [Google Scholar]
- [17].Sherman SI, Thyroid carcinoma, Lancet 361 (2003) 501–511. [DOI] [PubMed] [Google Scholar]
- [18].Ito T, Seyama T, Hayashi Y, Hayashi T, Dohi K, Mizuno T, et al. , Establishment of 2 human thyroid-carcinoma cell-lines (8305c, 8505c) bearing p53 gene-mutations, Int. J. Oncol 4 (1994) 583–586. [DOI] [PubMed] [Google Scholar]
- [19].Antonelli A, Bocci G, La Motta C, Ferrari SM, Fallahi P, Ruffilli I, et al. , CLM94, a novel cyclic amide with anti-VEGFR-2 and antiangiogenic properties, is active against primary anaplastic thyroid cancer in vitro and in vivo, J. Clin. Endocrinol. Metab 97 (2012) E528–E536. [DOI] [PubMed] [Google Scholar]
- [20].Chou TC, Drug combination studies and their synergy quantification using the Chou-Talalay method, Cancer Res. 70 (2010) 440–446. [DOI] [PubMed] [Google Scholar]
- [21].Canu B, Fioravanti A, Orlandi P, Di Desidero T, Ali G, Fontanini G, et al. , Irinotecan synergistically enhances the antiproliferative and proapoptotic effects of axitinib in vitro and improves its anticancer activity in vivo, Neoplasia 13 (2011) 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bocci G, Fioravanti A, La Motta C, Orlandi P, Canu B, Di Desidero T, et al. , Antiproliferative and proapoptotic activity of CLM3, a novel multiple tyrosine kinase inhibitor, alone and in combination with SN-38 on endothelial and cancer cells, Biochem. Pharmacol 81 (2011) 1309–1316. [DOI] [PubMed] [Google Scholar]
- [23].Antonelli A, Ferrari SM, Fallahi P, Frascerra S, Piaggi S, Gelmini S, et al. , Dysregulation of secretion of CXC alpha-chemokine CXCL10 in papillary thyroid cancer: modulation by peroxisome proliferator-activated receptor-gamma agonists, Endocr. Relat. Cancer 16 (2009) 1299–1311. [DOI] [PubMed] [Google Scholar]
- [24].Allegrini G, Falcone A, Fioravanti A, Barletta MT, Orlandi P, Loupakis F, et al. , A pharmacokinetic and pharmacodynamic study on metronomic irinotecan in metastatic colorectal cancer patients, Br. J. Cancer 98 (2008) 1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Perry J, Ghazaly E, Kitromilidou C, McGrowder EH, Joel S, Powles T, A synergistic interaction between lapatinib and chemotherapy agents in a panel of cell lines is due to the inhibition of the efflux pump BCRP, Mol. Cancer Ther 9 (2010) 3322–3329. [DOI] [PubMed] [Google Scholar]
- [26].Di Desidero T, Xu P, Man S, Bocci G, Kerbel RS, Potent efficacy of metronomic topotecan and pazopanib combination therapy in preclinical models of primary or late stage metastatic triple-negative breast cancer, Oncotarget 6 (2015) 42396–42410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bocci G, Falcone A, Fioravanti A, Orlandi P, Di Paolo A, Fanelli G, et al. , Antiangiogenic and anticolorectal cancer effects of metronomic irinotecan chemotherapy alone and in combination with semaxinib, Br. J. Cancer 98 (2008) 1619–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shukla S, Ohnuma S, Ambudkar SV, Improving cancer chemotherapy with modulators of ABC drug transporters, Curr. Drug Targets 12 (2011) 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Weinberger P, Ponny SR, Xu H, Bai S, Smallridge R, Copland J, et al. , Cell cycle m-phase genes are highly upregulated in anaplastic thyroid carcinoma, Thyroid 27 (2017) 236–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wein RO, Weber RS, Anaplastic thyroid carcinoma: palliation or treatment? Curr. Opin. Otolaryngol. Head. Neck Surg 19 (2011) 113–118. [DOI] [PubMed] [Google Scholar]
- [31].Sherman SI, Cytotoxic chemotherapy for differentiated thyroid carcinoma, Clin. Oncol. R. Coll. Radiol 22 (2010) 464–468. [DOI] [PubMed] [Google Scholar]
- [32].Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, et al. , American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer, Thyroid 22 (2012) 1104–1139. [DOI] [PubMed] [Google Scholar]
- [33].Bernet V, Smallridge R, New therapeutic options for advanced forms of thyroid cancer, Expert Opin. Emerg. Drugs 19 (2014) 225–241. [DOI] [PubMed] [Google Scholar]
- [34].Begum S, Rosenbaum E, Henrique R, Cohen Y, Sidransky D, Westra WH, BRAF mutations in anaplastic thyroid carcinoma: implications for tumor origin, diagnosis and treatment, Mod. Pathol 17 (2004) 1359–1363. [DOI] [PubMed] [Google Scholar]
- [35].Schoenfeld JD, Odejide OO, Wirth LJ, Chan AW, Survival of a patient with anaplastic thyroid cancer following intensity-modulated radiotherapy and sunitinib–a case report, Anticancer Res. 32 (2012) 1743–1746. [PubMed] [Google Scholar]
- [36].Cabanillas ME, Waguespack SG, Bronstein Y, Williams MD, Feng L, Hernandez M, et al. , Treatment with tyrosine kinase inhibitors for patients with differentiated thyroid cancer: the M. D. Anderson experience, J. Clin. Endocrinol. Metab 95 (2010) 2588–2595. [DOI] [PubMed] [Google Scholar]
- [37].Carr LL, Mankoff DA, Goulart BH, Eaton KD, Capell PT, Kell EM, et al. , Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation, Clin. Cancer Res 16 (2010) 5260–5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA, Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer, Endocr. Relat. Cancer 15 (2008) 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yang L, Zhang Y, Tumor-associated macrophages: from basic research to clinical application, J. Hematol. Oncol 10 (2017) 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dewar AL, Cambareri AC, Zannettino AC, Miller BL, Doherty KV, Hughes TP, et al. , Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib, Blood 105 (2005) 3127–3132. [DOI] [PubMed] [Google Scholar]
- [41].Brownlow N, Mol C, Hayford C, Ghaem-Maghami S, Dibb NJ, Dasatinib is a potent inhibitor of tumour-associated macrophages, osteoclasts and the FMS receptor, Leukemia 23 (2009) 590–594. [DOI] [PubMed] [Google Scholar]
- [42].Faivre S, Demetri G, Sargent W, Raymond E, Molecular basis for sunitinib efficacy and future clinical development, Nat. Rev. Drug Discov 6 (2007) 734–745. [DOI] [PubMed] [Google Scholar]
- [43].Cui R, Yue W, Lattime EC, Stein MN, Xu Q, Tan XL, Targeting tumor-associated macrophages to combat pancreatic cancer, Oncotarget 7 (2016) 50735–0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Eubank TD, Galloway M, Montague CM, Waldman WJ, Marsh CB, M-CSF induces vascular endothelial growth factor production and angiogenic activity from human monocytes, J. Immunol 171 (2003) 2637–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jones-Bolin S, Zhao H, Hunter K, Klein-Szanto A, Ruggeri B, The effects of the oral, pan-VEGF-R kinase inhibitor CEP-7055 and chemotherapy in orthotopic models of glioblastoma and colon carcinoma in mice, Mol. Cancer Ther 5 (2006) 1744–1753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


