Abstract
BACKGROUND:
While genetic variation has a known impact on the risk for obsessive-compulsive disorder (OCD), there is also evidence that there are maternal components to this risk. Here, we partitioned sources of variation, including direct genetic and maternal effects, on risk for OCD.
METHODS:
The study population consisted of 822,843 individuals from the Swedish Medical Birth Register, born in Sweden between January 1, 1982, and December 31, 1990, and followed for a diagnosis of OCD through December 31, 2013. Diagnostic information about OCD was obtained using the Swedish National Patient Register.
RESULTS:
A total of 7184 individuals in the birth cohort (0.87%) were diagnosed with OCD. After exploring various generalized linear mixed models to fit the diagnostic data, genetic maternal effects accounted for 7.6% (95% credible interval: 6.9%–8.3%) of the total variance in risk for OCD for the best model, and direct additive genetics accounted for 35% (95% credible interval: 32.3%–36.9%). These findings were robust under alternative models.
CONCLUSIONS:
Our results establish genetic maternal effects as influencing risk for OCD in offspring. We also show that additive genetic effects in OCD are overestimated when maternal effects are not modeled.
Keywords: Assortative mating, Direct genetic effects, Heritability, Maternal effects, Obsessive-compulsive disorder, Population based
Obsessive-compulsive disorder (OCD) is a psychiatric condition characterized by unwanted recurring thoughts, urges or images (obsessions), and repetitive behaviors (compulsions) that neutralize distress brought on by obsessions (1–3). The prevalence of OCD is estimated at 0.75% to 2.5% of the general population (3–8). Extensive efforts have been made to enhance understanding of the neurobiological basis of OCD (9), yet the causes of OCD remain largely unknown (10). However, both genetic and environmental factors contribute to risk of developing OCD (11–17). The common single nucleotide polymorphism heritability of OCD has been estimated to be 28% in meta-analyses (18), whereas the overall heritability is reported to be 40% to 50% (19–24).
Some of the risk factors for OCD, such as preterm birth and low birth weight (16), involve both the mother and her offspring. Indeed, multiple studies have linked maternal conditions before and during pregnancy, such as maternal smoking and maternal history of autoimmune disease, to the risk of OCD (16,25). These factors could represent what geneticists call maternal effects. Maternal effects are influences on the offspring phenotype that result from maternal genotypes and from the maternal environment. These effects are distinct from the offspring’s genetics; instead, maternal effects arise from the genetic and environmental influences on a maternal phenotype, and in turn the maternal phenotype affects the phenotype of the child. For example, maternal effects would include maternal genotypes that alter the provision of critical messenger RNA or proteins to the developing embryo, genetic or environmental effects on the mother’s in utero environment, or the impact of maternal illness on offspring health (e.g., maternal infection could increase the risk of OCD in her offspring). Interestingly, maternal factors have been shown to increase risk for multiple psychiatric phenotypes in offspring [see, for example, (26–33)]. Potential transgenerational epigenetic changes in risk of neurodevelopmental disorders (34) could also represent maternal effects. Maternal effects can also have a protective role. In a study of the association between gestational vitamin D and risk of multiple sclerosis, it was shown that vitamin D may have a protective role in the etiology of multiple sclerosis (35).
Failure to include maternal effects in heritability models, when maternal effects are present, can lead to inflated estimates of direct additive genetic effects (36) and the formulation of an incomplete risk architecture. When estimating maternal effects, one can estimate variance components for the genetic maternal effect (GME) and for the environmental maternal effect (EME); the statistical model used in this work estimates only shared EME. GME captures the scenario where child phenotype is influenced by the genotype of the mother, independent of the genotype of the child. EME captures the environment affecting the phenotype of the mother (independent of her genotype), which subsequently influences the phenotype of interest in all of her children.
Here we used a large population-based, prospectively ascertained cohort of Swedish-born individuals and the relevant family data to examine GME, EME, and direct additive genetic effect (DG) on the causes for risk of OCD. We explored several models to adjust for potentially confounding factors such as sex, maternal age, paternal age, maternal psychiatric history, paternal psychiatric history, gestational age, and maternal smoking during pregnancy. In the Supplement, we determined the effect of assortative mating and the robustness of the estimates of direct additive genetics and maternal effects under different models.
METHODS AND MATERIALS
Study Population
At birth, all Swedish residents are assigned a unique personal number that is used in all the national registries. Since 1973, all children born in Sweden have been recorded in the national Medical Birth Register together with birth characteristics of the children and mothers (37). The study population consists of all live-born singleton children born in Sweden between January 1, 1982, and December 31, 1990, with known father and mother as defined by the Medical Birth Register. Prospective follow-up continued until December 2013, and emigrated individuals identified during the follow-up were excluded from the study. To define family relationships, we included information about all relatives of each child using the Swedish Multi-Generation Registry (38). The Multi-Generation Registry contains information for approximately 15 million individuals.
Ethics approval and waiver of informed consent were obtained from the Regional Ethical Review Board in Stockholm, Sweden. The requirement for informed consent was waived because the study was register based and data on the included individuals were de-identified.
Outcomes
Diagnostic information about OCD was obtained using the Swedish National Patient Register (NPR), which includes inpatient and outpatient specialist care. Sweden has a publicly financed health system, and all visits to a specialist clinician are recorded with a diagnosis code using the ICD. Since 1973, all psychiatric care admissions in Sweden have been recorded in the NPR. After 2001, outpatient specialist care has also been recorded. The NPR has reached full national coverage since 2005. Since 1997, ICD version 10 has been used to code all diagnoses. To identify cases, we used the earliest registered F42 ICD-10 OCD diagnosis code in the NPR because this has been shown to be most reliable (39). The youngest individuals in the cohort were 23 years at the end of follow-up, while the oldest individuals were 31 years.
Exposure Covariates
We evaluated the following covariates for their relationship with OCD: sex of the child, birth year of the child, maternal smoking collected at the first neonatal visit (no smoking, light smoking [smoking fewer than 10 cigarettes a day], or heavy smoking [smoking more than 10 cigarettes a day]), paternal and maternal ages at childbirth (years), gestational age (weeks), presence of maternal and/or paternal psychiatric history at the birth of the first child (yes/no for each). Maternal/paternal psychiatric history is defined as at least one psychiatric diagnosis for the mother/father (under ICD-7, -8, -9, or -10) at any time before the firstborn child (40). Additional description and analysis of the covariates can be found in the Supplement.
Statistical Analysis
We defined five relationship types based on the first-, second-, and third-degree relatives: full siblings (full sibs), paternal and maternal half-siblings (half-sibs), and three different cousin types depending on whether the two parents responsible for the cousin relationship are sisters (maternal parallel cousins), are brothers (paternal parallel cousins), or have another relationship (cross cousins). Individuals could contribute to multiple relationship types. We estimated relative recurrence risk (RRR) for all pairs of different relationship types using Cox proportional hazards regression, with attained age as the primary time scale and adjusted for sex, maternal and paternal age, maternal and paternal psychiatric history, gestational age, and maternal smoking. In the Cox regression, each individual was followed from 1997 until death, emigration from Sweden, diagnosis with OCD, or end of follow-up on December 31, 2013, whichever came first. We bootstrapped families 1000 times to get estimates of the confidence intervals (CIs) for the RRR (40).
Different approaches for estimating the heritability of binary disease have been proposed (41). Falconer’s liability threshold model (LTM) is based on regression of risk among certain relatives of diagnosed individuals divided by the risk in the general population for the different family types (42). Use of generalized linear mixed models (GLMMs) is a more flexible and general approach that can handle complex pedigrees of varying size and structures for analyzing maternal effects (41,43), and GLMMs have been frequently used for similar analyses (36,44–48). With either method, one can acquire an estimate of the proportion of total variance explained by genetic and environmental factors. These models assume that a binary outcome is derived from an underlying normally distributed trait with an observed threshold value.
The liability of OCD was partitioned into covariates, DG, GME, EME, and individual variation. In our primary analyses, we employed GLMMs to obtain the estimates of these components. Assume that y is the vector of binary outcomes, which are independent Bernoulli events with parameter p. The model that we used was
where
β is the vector of covariates with the incidence matrix X, d is the vector of random effects for DG with the incidence matrix Zd, m is the vector of random effects for GME with the design incidence Zm, me is the vector of EME with the incidence matrix , and , , and are the variances for DG, GME, and EME, respectively. The matrix A is the relationship matrix with the elements
Supplemental Table S17 explains the expected contribution of DG, GME, and EME to different relationship types. Comparison of maternal versus paternal half-sibs and cousins (maternal parallel cousins vs. other cousins) is informative to estimate maternal effects. GME contributes to full sibs, maternal half-sibs, and maternal parallel cousins. EME contributes to full sibs and maternal half-sibs, while it is assumed to be zero for paternal half-sibs and cousins (44).
We used a binary threshold–linear mixed model in a Bayesian framework with a noninformative prior to estimate the variance components and then calculated the proportions of phenotypic variance explained by direct additive genetics and maternal effects (49). We applied a Gibbs sampler implemented in thrgibbs1f90b—as a part of the family of programs Blupf90 (49)—to generate a sample size of 200,000, with 50,000 burn-in, from the posterior distribution of the variance components. Then, we calculated the mean of the posterior as the estimate of the variance components. The residual variance was fixed during the calculation. We reported the results with 95% credible intervals (CrIs) using Bayesian highest posterior density interval, which is analogous to two-sided 95% CIs in frequentist statistics (50). For the covariates, we reported the mean and standard deviation of the posterior to calculate the CrIs.
Sensitivity Analysis
In addition to RRR, we used familial risk, the probability that an individual has an affected relative of a specific type, to compare risk among different categories of families. Then, we used an LTM to estimate the variance components and compared the results with the estimates from GLMMs, as described in the Supplement, as a simple check on the more complex GLMMs and with the expectation that the estimates would be similar.
RESULTS
The cohort contains 822,843 individuals, of which 7184 (0.87%) were diagnosed with OCD (60% female) using ICD-10 criteria (Table 1) followed from January 1997 through December 2013.
Table 1.
Study Cohort Including Individuals Born Between January 1, 1982, and December 31, 1990
| Total Population | Siblings | Cousins | ||||
|---|---|---|---|---|---|---|
| Full | mHS | pHS | mPC | oCS | ||
| Participants, n | 822,843 | 454,144 | 30,481 | 28,007 | 219,781 | 430,788 |
| Male, n (%) | 426,261 (52) | 235,540 (52) | 15,557 (51) | 14,292 (51) | 113,896 (52) | 223,175 (52) |
| Female, n (%) | 395,582 (48) | 218,604 (48) | 14,924 (49) | 13,715 (49) | 105,885 (48) | 207,613 (48) |
| Diagnosed With OCD, n | 7184 | 3709 | 300 | 292 | 1829 | 3577 |
| Male, n (%) | 2859 (40) | 1464 (39) | 112 (37) | 127 (44) | 718 (40) | 1399 (39) |
| Female, n (%) | 4325 (60) | 2245 (61) | 188 (62) | 165 (56) | 1111 (60) | 2178 (61) |
| Population Frequency | 0.0087 | 0.0081 | 0.0098 | 0.0104 | 0.0083 | 0.0083 |
| Male | 0.0067 | 0.0062 | 0.0072 | 0.0089 | 0.0063 | 0.0062 |
| Female | 0.0109 | 0.0103 | 0.0126 | 0.0120 | 0.0105 | 0.0105 |
| Incidence Ratea | 2.98 | 3.59 | 3.80 | 3.00 | 2.97 | 3.10 |
HS, maternal half-siblings; mPC, maternal parallel cousins; OCD, obsessive-compulsive disorder; oCS, other-cousins; pHS, paternal half-siblings.
Per 10,000 person-years.
RRR was calculated for different relation types (Figure 1) using Cox proportional hazards regression. These analyses showed higher point estimates for maternal half-sibs compared with paternal half-sibs as well as higher RRR for maternal cousins compared with other cousins. Analysis of familial risk exhibited a similar pattern between different relationship types (see Supplemental Table S16).
Figure 1.
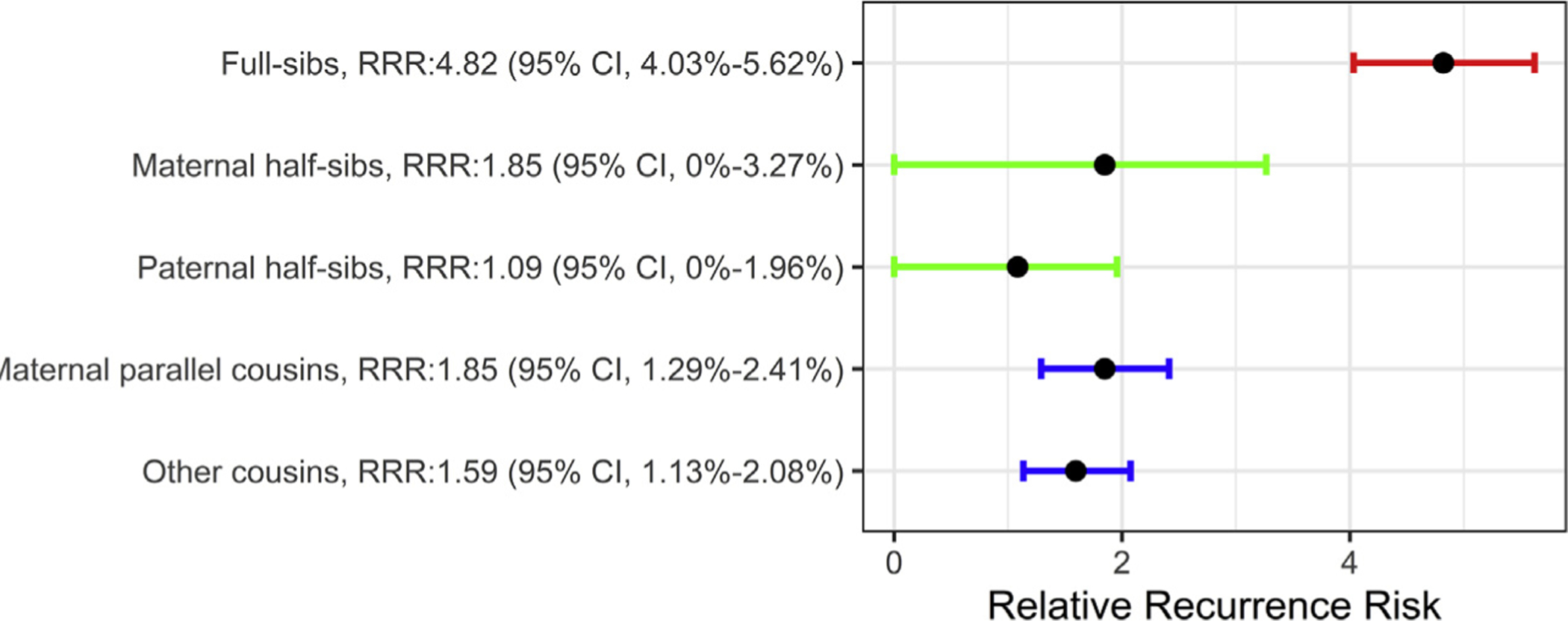
Relative recurrence risk (RRR) for different relation types. The confidence intervals (CI) for half-siblings started from zero because there are relatively few such families. Note that the result from other cousins is the mean of cousin pairs where two parents responsible for the cousin relationship are brothers, or a sister and a brother.
In the Supplement, we explain in detail how we chose a subset of covariates to include in the model. Here, we summarize the most significant and relevant results. First, we analyzed each covariate separately. We observed an odds ratio of 1.60 for female versus male individuals diagnosed with OCD (Supplemental Table S1). Analysis of the results did not indicate a clear trend in population frequency of OCD for birth year over the time period used in the study (Supplemental Figure S1). The population frequency of OCD was higher for children with older parents. For ease of modeling, we created two categories for paternal age: younger than 35 years (population frequency of 0.0118) and older than 35 years (population frequency of 0.0134); we created the same split for maternal age, yielding population frequencies of 0.0120 and 0.0143, respectively (Supplemental Tables S2–S5). We observed that the population frequency of OCD increased substantially when the parents had a psychiatric history, likely due to the high correlation of parental psychiatric history and DG (Supplemental Tables S6–S9). The odds ratio for children having OCD given that either their mother or their father had OCD (using ICD-9) was 4.92 (95% CI = 3.92–6.10, p = 3.93 × 10−32) or 5.11 (95% CI = 3.81–6.75, p = 1.46 × 10−20), respectively (Supplemental Tables S8 and S9). These estimates are close to one another and with the RRR for full sibs (4.82) (Table 1). This similarity is consistent with roughly equal DG (i.e., additive effects) on OCD risk from both mother and father and suggests that that OCD status of the mother is not confounded with any potential maternal effects on OCD. Information for parental psychiatric history was available using ICD-7, -8, -9, and -10 codes. The odds ratio for OCD was 2.18 (95% CI = 1.96–2.42, p = 5.82 × 10−40) or 1.88 (95%CI = 1.68–2.10, p = 2.06 × 10−25) in children with maternal or paternal psychiatric disorder, respectively (Supplemental Tables S6 and S7). We analyzed the association between maternal smoking during pregnancy and maternal psychiatric history. Mothers of children with OCD and mothers of children without OCD had similar rates of heavy smoking as determined at the first neonatal visit (Supplemental Tables S10 and S11). The population frequency of OCD was higher for gestational age under 37 weeks (Supplemental Tables S12 and S13). To determine a parsimonious logistic model that has the best fit to the outcome, we used forward selection with a penalty for including a covariate (Bayesian information criterion) to choose among these possible covariates (see Supplemental Tables S14 and S15). The model sex + age of mother (maternal age) was most parsimonious.
We used GLMMs to estimate the contribution of DG and maternal effects on OCD. The best GLMM that explained the data included DG + GME, as determined by Bayes factor analyses (Table 2), and yielded an estimate that 35% (95% CrI = 32.3%–36.9%) of the liability for OCD was due to DG and 7.6% was due to GME (for comparison of different models, see Supplemental Tables S21–S25). To evaluate the sensitivity of the results to the underlying assumption of GLMMs, we also made use of liability threshold modeling. The best LTM estimated DG and GME to be 31.9% and 5.8%, respectively, quite similar to the CrI of the GLMM estimates, and did not provide evidence for paternal effects (Supplemental Table S18).
Table 2.
Proportions of Phenotypic Variance Explained by Different Models
| Model | Method | DG (95% CrI) | GME (95% CrI) | R (95% CrI) | Sex (95% CrI) | AOM (95% CrI) |
|---|---|---|---|---|---|---|
| DG + GME | GLMM | 35% (32.3–36.9) | 7.6% (6.9–8.3) | 57.4% Fixed | 1.26 (1.21–1.31) | 1.14 (1.05–1.23) |
| LTM | 31.9% | 5.8% | 62.3% | – | – | |
| DG | GLMM | 48.2% (43.1–52.6) | – | 51.8% Fixed | 1.27 (1.20–1.35) | 1.17 (1.06–1.29) |
| LTM | 43.6% | – | 56.7% | – | – |
AOM, age of mother; CrI, credible interval; DG, direct additive genetic effect; EME, environmental maternal effect; GLMM, generalized linear mixed model; GME, genetic maternal effect; LTM, liability threshold model; R, individual variation (residual).
Assortative mating has been reported among individuals with a diagnosis of OCD (51). Assortative mating can impact estimates of heritability, albeit modestly (52); therefore, in the Supplement, we analyzed the impact of assortative mating on the estimate of DGs using an LTM. We observed evidence for substantial assortative mating among individuals with OCD, meaning that individuals with OCD chose a partner with OCD more frequently than expected under a random mating pattern. We observed that assortative mating inflated the estimate of DG by 4% to 5% (Supplemental Table S20). We also determined how the omission of maternal effects affected the estimate of DG. In a model without maternal effects, 43.6% to 48.2% of the phenotypic variation was estimated as due to direct DG, in contrast to 31.9% to 35.0% if maternal effects were included in the model. In total, our model explained 37.8% to 42.6% of the liability of OCD based on DG and GME.
DISCUSSION
In this cohort study of Swedish children born between January 1, 1982. and December 31, 1990, we found that genetic maternal effects contribute significantly to causes of risk for OCD. This is, to our knowledge, the first study to estimate this effect on risk for OCD and the first quantitative genetic study to identify a role for maternal effects in risk for any psychiatric disorder. Our results also demonstrate an association between parental factors and risk for OCD such as parental age, parental psychiatric history, maternal smoking during pregnancy, and gestational age, in accordance with previous studies (16). Intriguingly, some of these factors have their own genetic influences, and it is possible that a portion of their genetic basis explains a portion of the genetically based maternal effects.
The analysis of RRR of OCD is consistent with maternal effects in OCD risk architecture. The RRR for paternal half-sibs was 1.084, while the RRR for maternal half-sibs was 1.849; likewise, maternal parallel cousins carried somewhat higher risk, as compared to other cousins (1.85 vs. 1.595). However, the CIs for RRRs overlapped, so GLMMs were needed for an accurate estimate of maternal effects. Using GLMMs, and under the liability threshold assumption, we estimated that 7.6% (95% CrI = 6.9%–8.3%) of the variance in risk is explained by GME and 35% (95% CrI = 32.3%–36.9%) is explained by DG while adjusting for the sex of the individual and the age of the mother. Female individuals were at 1.26 times higher risk relative to male individuals (95% CrI = 1.21–1.31), and offspring of older mothers were at 1.14 times higher risk for OCD (95% CrI = 1.05–1.23). Interestingly, we observed a somewhat larger effect for maternal age when fitting a model with only DG, hinting that maternal age could be correlated with maternal genetic effects. Our result strongly favors the DG + GME model, suggesting that shared EME have little or no effect on risk. However, it is important to note that the model we are using is capable of estimating only shared EME. To estimate unshared EME—that is, EME impacting only some of the children in a family, such as infection during one pregnancy—individual information for that effect would need to be available.
We observed that the RRR for half-sibs, in particular for paternal half-sibs, is lower than that for cousins (Figure 1), which can potentially affect the estimate of maternal effects. However, by using a weighted LTM, we generated weights for each family type and showed that the lower number of half-sibs, in comparison with the number of full sibs and cousins, does not have a substantial effect on the estimates of the variance components (see Supplement), and it is indeed handled appropriately by the GLMMs. In addition, using the complete birth cohort between 1982 and 1990 and including all different family types, instead of a sample of the population, made the exposed and unexposed groups for different family types more comparable and minimized the selection bias.
Our observation that GME contributes significantly to risk for OCD provides a justification for directly or indirectly assessing the role of specific maternal genes and loci in OCD risk, as has been recently carried out for other phenotypes (53,54). The evidence that unmodeled GME and assortative mating inflate the estimates of DG provides important insights into ongoing studies on DG loci in OCD. Finally, the current findings provide an interesting contrast to our previous study on autism spectrum disorder, where we observed little or no evidence for maternal effects (44). While maternal, prenatal, and perinatal factors have been shown to have associations with many neurodevelopmental outcomes (16,25), the nature of such associations is often obscure. From our studies, we can conclude that some of the maternal factors contributing to risk for OCD in offspring may reflect maternal genetic influences on maternal phenotypes, which in turn affect the phenotype of the child.
In any epidemiological study, biases cannot be ruled out. Our study includes individuals who sought health care and had a diagnosis of OCD in the NPR. Those who were diagnosed only as outpatients before 2001 or were manifesting milder forms of OCD, or those who did not seek health services, might not be captured in our study design. Therefore, while etiological discovery is often evaluated in more severe cases, there may be qualitatively or genetically different sources of risk for milder OCD. In addition, the data are likely right censored, in particular for individuals born in the later years of the study. This can contribute to underdiagnoses of OCD and decreases the population frequency. At the same time, by using the Swedish Medical Birth Register as our sampling frame, we created a genetically homogeneous sample, minimizing the risk of confounding due to population stratification. Importantly, our sample is based on clinical diagnoses of OCD by a specialist, which would also be expected to reduce biases and case misclassification. However, OCD is an etiologically heterogeneous disorder consisting of multiple potentially overlapping symptom dimensions. Ignoring the symptom dimensions of OCD and modeling the diagnosis as dichotomous outcomes can potentially bias the results.
Conclusions
This is the first detailed analysis of maternal effects in OCD risk architecture. Our results show that genetically based maternal effects contribute to offspring risk for OCD, and we conclude that such maternal effects contribute to a significant portion of the total genetic architecture of OCD, in addition to directly inherited, additive genetic effects. Our results also make it likely that direct genetic effects on OCD risk were overestimated in prior studies. These results, while needing to be replicated in an independent sample, provide new insights into the causes of risk for OCD and provide a rationale for assessing the role of specific maternal genes and loci in OCD risk.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This study was supported by grants from the Mindich Child Health and Development Institute (to DEG and SS), the Friedman Brain Institute (to DEG), and the Beatrice and Samuel A. Seaver Foundation (to DEG, SS, JDB, and BM), Icahn School of Medicine at Mount Sinai; the Mindworks Charitable Lead Trust (to DEG); the Stanley Center for Psychiatric Research (to DEG and JDB); and the National Institute of Mental Health (Grant No. R37MH057881 [to BD], Grant Nos. R01MH097849 and R01MH097849-S1 [to JDB]). Computational analyses were performed on resources provided by SNIC through Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX) under Projects SNIC 2016/1-359, SNIC 2017/7-113, and SNIC 2017/3-75.
BM had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Other author responsibilities were as follows. Study concept and design: JDB, BD, DEG, LK, BM, and SS. Acquisition, analysis, or interpretation of data: JDB, BD, DEG, LK, BM, and SS. Drafting of the manuscript: JDB, BD, DEG, LK, and BM. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: BD, LK, and BM. Obtained funding: JDB, BD, DEG, and SS. Study supervision: JDB, BD, DEG, and SS.
We acknowledge the guidance and support provided by Yudi Pawitan during this project.
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report or in the decision to submit the manuscript for publication. The responsible authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2020.01.006.
REFERENCES
- 1.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, VA: American Psychiatric Association. [Google Scholar]
- 2.Fogel J (2003): An epidemiological perspective of obsessive-compulsive disorder in children and adolescents. Can Child Adolesc Psychiatr Rev 12:33–36. [PMC free article] [PubMed] [Google Scholar]
- 3.Ruscio AM, Stein DJ, Chiu WT, Kessler RC (2010): The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 15:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karno M, Golding JM, Sorenson SB, Burnam MA (1988): The epidemiology of obsessive-compulsive disorder in five US communities. Arch Gen Psychiatry 45:1094–1099. [DOI] [PubMed] [Google Scholar]
- 5.Osland S, Arnold PD, Pringsheim T (2018): The prevalence of diagnosed obsessive compulsive disorder and associated comorbidities: A population-based Canadian study. Psychiatry Res 268:137–142. [DOI] [PubMed] [Google Scholar]
- 6.Weissman M, Bland R, Canino G (1994): The cross national epidemiology of obsessive compulsive disorder. J Clin Psychiatry 55(suppl):5–10. [PubMed] [Google Scholar]
- 7.Fontenelle LF, Mendlowicz MV, Versiani M (2006): The descriptive epidemiology of obsessive-compulsive disorder. Prog Neuro-psychopharmacol Biol Psychiatry 30:327–337. [DOI] [PubMed] [Google Scholar]
- 8.Torres AR, Prince MJ, Bebbington P, Bhugra D, Brugha TS, Farrell M, et al. (2006): Obsessive-compulsive disorder: Prevalence, comorbidity, impact, and help-seeking in the British National Psychiatric Morbidity Survey of 2000. Am J Psychiatry 15:1978–1985. [DOI] [PubMed] [Google Scholar]
- 9.Mas S, Gassó P, Morer A, Calvo A, Bargalló N, Lafuente A, et al. (2016): Integrating genetic, neuropsychological and neuroimaging data to model early-onset obsessive compulsive disorder severity. PLoS One 11:e153846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pittenger C (2017): The neurobiology of tic disorders and obsessive-compulsive disoder: Human and animal models. In: Charney DS, Nestler EJ, Sklar P, Buxbaum JD, editors. Charney & Nestler’s Neurobiology of Mental Illness, 5th ed. New York: Oxford University Press, 879. [Google Scholar]
- 11.Browne HA, Gair SL, Scharf JM, Grice DE (2014): Genetics of obsessive-compulsive disorder and related disorders. Psychiatr Clin North Am 37:319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nestadt G, Grados M, Samuels J (2011): Genetics of OCD. Psychiatr Clin North Am 33:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cappi C, Brentani H, Lima L, Sanders SJ, Zai G, Diniz BJ, et al. (2016): Whole-exome sequencing in obsessive-compulsive disorder identifies rare mutations in immunological and neurodevelopmental pathways. Transl Psychiatry 6:e764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gazzellone MJ, Zarrei M, Burton CL, Walker S, Uddin M, Shaheen SM, et al. (2016): Uncovering obsessive-compulsive disorder risk genes in a pediatric cohort by high-resolution analysis of copy number variation. J Neurodev Disord 8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGrath LM, Yu D, Marshall C, Davis LK, Thiruvahindrapuram B, Li B, et al. (2014): Copy number variation in obsessive-compulsive disorder and tourette syndrome: A cross-disorder study. J Am Acad Child Adolesc Psychiatry 53:910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brander G, Rydell M, Kuja-Halkola R, Fernández de la Cruz L, Lichtenstein P, Serlachius E, et al. (2016): Association of perinatal risk factors with obsessive-compulsive disorder: A population-based birth cohort, sibling control study. JAMA Psychiatry 73:1135–1144. [DOI] [PubMed] [Google Scholar]
- 17.Browne HA, Hansen SN, Buxbaum JD, Gair SL, Nissen JB, Nikolajsen KH, et al. (2015): Familial clustering of tic disorders and obsessive-compulsive disorder. JAMA Psychiatry 72:359–366. [DOI] [PubMed] [Google Scholar]
- 18.Arnold PD, Askland KD, Barlassina C, Bellodi L, Bienvenu OJ, Black D, et al. (2018): Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry 23:1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clifford CA, Murray RM, Fulker DW (1984): Genetic and environmental influences on obsessional traits and symptoms. Psychol Med 14:791–800. [DOI] [PubMed] [Google Scholar]
- 20.Jonnal AH, Gardner CO, Prescott CA, Kendler KS (2000): Obsessive and compulsive symptoms in a general population sample of female twins. Am J Med Genet 96:791–796. [DOI] [PubMed] [Google Scholar]
- 21.Eley TC, Bolton D, O’Connor TG, Perrin S, Smith P, Plomin R (2003): A twin study of anxiety-related behaviours in pre-school children. J Child Psychol Psychiatry 44:945–960. [DOI] [PubMed] [Google Scholar]
- 22.Hudziak JJ, Van Beijsterveldt CEM, Althoff RR, Stanger C, Rettew DC, Nelson EC, et al. (2004): Genetic and environmental contributions to the Child Behavior Checklist Obsessive-Compulsive Scale: A cross-cultural twin study. Arch Gen Psychiatry 61:608–616. [DOI] [PubMed] [Google Scholar]
- 23.Taylor S (2011): Etiology of obsessions and compulsions: A meta-analysis and narrative review of twin studies. Clin Psychol Rev 31:1361–1372. [DOI] [PubMed] [Google Scholar]
- 24.Monzani B, Rijsdijk F, Harris J, Mataix-Cols D (2014): The structure of genetic and environmental risk factors for dimensional representations of DSM-5 obsessive-compulsive spectrum disorders. JAMA Psychiatry 71:182–189. [DOI] [PubMed] [Google Scholar]
- 25.Murphy TK, Storch EA, Turner A, Reid JM, Tan J, Lewin AB (2010): Maternal history of autoimmune disease in children presenting with tics and/or obsessive-compulsive disorder. J Neuroimmunol 229:243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, et al. (2014): Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol 10:643–660. [DOI] [PubMed] [Google Scholar]
- 27.Estes ML, McAllister AK (2015): Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci 16:469–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mataix-Cols D, Frans E, Pérez-Vigil A, Kuja-Halkola R, Gromark C, Isomura K, et al. (2018): A total-population multigenerational family clustering study of autoimmune diseases in obsessive–compulsive disorder and Tourette’s/chronic tic disorders. Mol Psychiatry 23:1652–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breen MS, Wingo AP, Koen N, Donald KA, Nicol M, Zar HJ, et al. (2018): Gene expression in cord blood links genetic risk for neurodevelopmental disorders with maternal psychological distress and adverse childhood outcomes. Brain Behav Immun 73:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serati M, Barkin JL, Orsenigo G, Altamura AC, Buoli M (2017): The role of obstetric and neonatal complications in childhood attention deficit and hyperactivity disorder - a systematic review. J Child Psychol Psychiatry 58:1290–1300. [DOI] [PubMed] [Google Scholar]
- 31.Rivera HM, Christiansen KJ, Sullivan EL (2015): The role of maternal obesity in the risk of neuropsychiatric disorders. Front Neurosci 9:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ornoy A, Weinstein-Fudim L, Ergaz Z (2015): Prenatal factors associated with autism spectrum disorder (ASD). Reprod Toxicol 56:155–169. [DOI] [PubMed] [Google Scholar]
- 33.Liu CH, Keshavan MS, Tronick E, Seidman LJ (2015): Perinatal risks and childhood premorbid indicators of later psychosis: Next steps for early psychosocial interventions. Schizophr Bull 41:801–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xavier MJ, Roman SD, Aitken RJ, Nixon B (2019): Transgenerational inheritance: How impacts to the epigenetic and genetic information of parents affect offspring health. Hum Reprod Update 25:519–541. [DOI] [PubMed] [Google Scholar]
- 35.Mirzaei F, Michels KB, Munger K, O’Reilly E, Chitnis T, Forman MR, et al. (2011): Gestational vitamin D and the risk of multiple sclerosis in offspring. Ann Neurol 70:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devlin B, Daniels M, Roeder K (1997): The heritability of IQ. Nature 388:468–471. [DOI] [PubMed] [Google Scholar]
- 37.Cnattingius S, Ericson A, Gunnarskog J, Kallen B (1990): A quality study of a medical birth registry. Scand J Soc Med 18:143–148. [DOI] [PubMed] [Google Scholar]
- 38.Ekbom A (2011): The Swedish Multi-Generation Register. Methods Mol Biol 675:215–220. [DOI] [PubMed] [Google Scholar]
- 39.Rück C, Larsson KJ, Lind K, Perez-Vigil A, Isomura K, Sariaslan A, et al. (2015): Validity and reliability of chronic tic disorder and obsessive-compulsive disorder diagnoses in the Swedish National Patient Register. BMJ Open 5:e7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A (2014): The familial risk of autism. JAMA 311:1770–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tenesa A, Haley CS (2013): The heritability of human disease: Estimation, uses and abuses. Nat Rev Genet 14:139–149. [DOI] [PubMed] [Google Scholar]
- 42.Falconer DS (1965): The inheritance of liablity to certain diseases, estimated from the incidence among relatives. Ann Hum Genet 29:51–75. [Google Scholar]
- 43.Knott SA, Sibly RM, Smith RH, Moller H (1995): Maximum likelihood estimation of genetic parameters in life-history studies using the “animal model.” Funct Ecol 9:122–126. [Google Scholar]
- 44.Yip BHK, Bai D, Mahjani B, Klei L, Pawitan Y, Hultman CM, et al. (2018): Heritable variation, with little or no maternal effect, accounts for recurrence risk to autism spectrum disorder in Sweden. Biol Psychiatry 83:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolf JB, Wade MJ (2009): What are maternal effects (and what are they not)? Philos Trans R Soc Lond B Biol Sci 364:1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holand AM, Steinsland I (2016): Is my study system good enough? A case study for identifying maternal effects. Ecol Evol 6:3486–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindström L, Pawitan Y, Reilly M, Hemminki K, Lichtenstein P, Czene K (2006): Estimation of genetic and environmental factors for melanoma onset using population-based family data. Stat Med 25:3110–3123. [DOI] [PubMed] [Google Scholar]
- 48.Pawitan Y, Reilly M, Nilsson E, Cnattingius S, Lichtenstein P (2004): Estimation of genetic and environmental factors for binary traits using family data. Stat Med 23:449–465. [DOI] [PubMed] [Google Scholar]
- 49.Misztal I, Tsuruta S, Lourenco D, Masuda Y, Aguilar I, Legarra A, Vitezica Z (2016): BLUPF90 Family of Programs. Athens, GA: University of Georgia. [Google Scholar]
- 50.Lee PM (2012): Bayesian Statistics: An Introduction, 4th ed. Hoboken, NJ: John Wiley. [Google Scholar]
- 51.Nordsletten AE, Larsson H, Crowley JJ, Almqvist C, Lichtenstein P, Mataix-Cols D (2016): Patterns of nonrandom mating within and across 11 major psychiatric disorders. JAMA Psychiatry 73:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peyrot WJ, Robinson MR, Penninx BWJH, Wray NR (2016): Exploring boundaries for the genetic consequences of assortative mating for psychiatric traits. JAMA Psychiatry 73:1189–1195. [DOI] [PubMed] [Google Scholar]
- 53.Warrington NM, Freathy RM, Neale MC, Evans DM (2018): Using structural equation modelling to jointly estimate maternal and fetal effects on birthweight in the UK Biobank. Int J Epidemiol 47:1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beaumont RN, Warrington NM, Cavadino A, Tyrrell J, Nodzenski M, Horikoshi M, et al. (2018): Genome-wide association study of offspring birth weight in 86 577 women identifies five novel loci and highlights maternal genetic effects that are independent of fetal genetics. Hum Mol Genet 27:742–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


