Abstract
Background
Ageing is associated with an increased prevalence of comorbidities and sarcopenia as well as a decline of functional reserve of multiple organ systems, which may lead, in the context of the disease-related and/or treatment-related stress, to functional deconditioning. The multicomponent ‘Prehabilitation & Rehabilitation in Oncogeriatrics: Adaptation to Deconditioning risk and Accompaniment of Patients’ Trajectories (PROADAPT)’ intervention was developed multiprofessionally to implement prehabilitation in older patients with cancer.
Methods
The PROADAPT pilot study is an interventional, non-comparative, prospective, multicentre study. It will include 122 patients oriented to complex medical–surgical curative procedures (major surgery or radiation therapy with or without chemotherapy). After informed consent, patients will undergo a comprehensive geriatric assessment and will be offered a prehabilitation kit that includes an advice booklet with personalised objectives and respiratory rehabilitation devices. Patients will then be called weekly and monitored for physical and respiratory rehabilitation, preoperative renutrition, motivational counselling and iatrogenic prevention. Six outpatient visits will be planned: at inclusion, a few days before the procedure and at 1, 3, 6 and 12 months after the end of the procedure. The main outcome of the study is the feasibility of the intervention, defined as the ability to perform at least one of the components of the programme. Clinical data collected will include patient-specific and cancer-specific characteristics.
Ethics and dissemination
The study protocol was approved by the Ile de France 8 ethics committee on 5 June 2018. The results of the primary and secondary objectives will be published in peer-reviewed journals.
Trial registration number
NCT03659123. Pre-results of the trial.
Keywords: adult oncology, geriatric medicine, rehabilitation medicine, adult surgery
Strengths and limitations of this study.
The Prehabilitation & Rehabilitation in Oncogeriatrics: Adaptation to Deconditioning risk and Accompaniment of Patients’ Trajectories (PROADAPT) programme is a prehabilitation programme specifically tailored for older patients with cancer.
The programme was designed according to a multidisciplinary analysis of available evidence and according to a multistep validation process involving patients.
The PROADAPT pilot study is a prospective and multicentre trial designed to evaluate the feasibility of the intervention.
Different secondary outcomes, including quality of life, will be evaluated to better adapt the programme to patient specificities.
A specific attention will be paid to programme safety and patient compliance to the programme.
Introduction
Many oncological situations involve complex medical–surgical procedures that increase the risk of patient deconditioning in older and/or sarcopenic patients.1 This may lead to a disabling cascade, a ‘domino effect’, defined as the succession of adverse events in response to a primary stress.2 This is illustrated by increased morbidity and mortality3 and also a higher risk of unplanned hospitalisations for geriatric events, defined as immobilisation syndrome, acute confusion, undernutrition, falls, de novo urinary incontinence and adverse drug events.4 These generate frustration, appeals by patients and their families, additional hospital costs5 and, more importantly, a reduced duration of life without disability.6 One of the responses to this situation is enhanced rehabilitation after surgery.7
In order to reduce complications after surgery, preoperative rehabilitation (or prehabilitation) has often been considered for the general population.8 The majority of the programmes include nutrition, physical activity, motivational coaching and, for some, tobacco cessation8; the level of evidence is high for preoperative nutrition,9 but it is low for physical exercise due to heterogeneous programmes with often poor compliance10 and is deemed insufficient considering psychological preparation.11 Some programmes adapted interventions on nutrition, physical activity and motivational coaching to geriatric patients but conclusions as to the effectiveness of these are difficult to draw.12 It is also of note that Carli et al 13 did not report any significant difference in the efficacy of prehabilitation versus postoperative rehabilitation only in 110 frail patients aged 65 years or above operated on for colon cancer questioning the ability of standard prehabilitation to improve outcomes for frail older patients.
It would, therefore, potentially be of interest to widen the spectrum of interventions included in prehabilitation of older patients. To date, the other interventions known to prevent hospital-related geriatric deconditioning include comprehensive geriatric assessment,14 15 iatrogenic prevention (drug and care system related)16 17 and hospital-to-home transition to limit the risk of early readmission of patients.18 In addition, some degree of individualisation is also needed since cancer in the older patient is often associated with comorbidities, particularly cardiovascular disease,19 20 and the older population also has a higher risk of loss of autonomy and cognitive impairment, which can be increased with surgery.21–23
In this context, and after a systematic analysis of published data, we developed the Prehabilitation & Rehabilitation in Oncogeriatrics: Adaptation to Deconditioning risk and Accompaniment of Patients’ Trajectories (PROADAPT), a geriatric multiprofessional intervention programme. Such a multidomain intervention should be evaluated according the methodology of complex interventions evaluation.24 Hence, we designed the PROADAPT pilot study to evaluate the feasibility of such a complex intervention. This manuscript describes both PROADAPT multidomain intervention and PROADAPT pilot study.
Methods and analysis
Objectives
Primary objective
The primary objective of the PROADAPT pilot study is to assess the feasibility of the programme, defined as the achievement of at least one item of the programme during patient follow-up.
Secondary objectives
The secondary objectives of the study are:
To assess the achievement of each item of the programme independently of each other (rate of achievement of all or part of the instructions).
To assess patient satisfaction with the programme (online supplemental file 1).
To estimate the rate of compliance to items during the various visits.
To evaluate the functional status and quality of life (QoL) over 1 year following surgery (health-related QoL and other dimensions).
To assess post-treatment complications, their rates and their severity at 30 and 90 days according to the Clavien-Dindo and National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4 classification systems.25
To estimate the postoperative mortality at 30 and 90 days.
To estimate the costs of treatments (health system).
To study the therapeutic strategies (treatment completion rate).
To estimate the progression-free survival (PFS) rate at 1 year.
To estimate the overall survival (OS) at 1 year.
To estimate the tolerance of treatments.
To assess the change in geriatric covariates over 1 year.
bmjopen-2020-042960supp001.pdf (62.8KB, pdf)
Study design
PROADAPT pilot study is a prospective, non-comparative multicentre conducted in seven centres of the Auvergne-Rhône-Alpes region of France.
Study sites and participants
The study population will include older patients identified during multidisciplinary consultation meetings and oriented to complex medical–surgical curative procedures in the study centres (Lyon Sud Hospital, Croix Rousse Hospital and Edouard Herriot Hospital from the Hospices Civils de Lyon, Nord-Ouest Villefranche-sur-Saône Hospital, Annecy-Genevois Hospital, Chambery Hospital and Lyon-Villeurbanne Médipôle).
Inclusion criteria are: age ≥70 years or ≥60 years with significant comorbidity (Cumulative Illness Rating Scale for Geriatrics ≥326) or disability (Activities of Daily Living (ADL) score <6/6 (27)), histologically or cytologically proven cancer, life expectancy >3 months and planned complex medical–surgical procedure with curative intent. Complex medical–surgical procedures are defined as major abdominal surgery (breast excluded), either minimally invasive or open.
Exclusion criteria are: other malignancy within the previous 5 years (except for adequately treated carcinoma in situ of the cervix or squamous carcinoma of the skin or adequately controlled limited basal cell skin cancer), unable to be regularly followed for any reason (geographic, familial, social and psychological) or with any mental or physical handicap at risk of interfering with the appropriate treatment.
A screening of older patients will be systematically performed during multidisciplinary meetings and described in the Consolidated Standards of Reporting Trials flow diagram of the article reporting the study.
Intervention
The PROADAPT intervention programme was developed on a multidimensional and multidisciplinary basis. From January 2016 to April 2018, nine regional meetings were organised, gathering 40 representatives of the following medical and paramedical specialties: geriatricians, nutritionists, surgeons (subspecialties: gynaecology, digestive surgery and urology), oncologists, anaesthesiologists, nurses, physiotherapists, occupational therapists andadapted physical activity monitors. A systematic review of published data were conducted in the following axes to provide a graded state-of-the-art nutrition, physical activity, patient education, medication rationalisation, cardiovascular optimisation, transition and standardisation of surgical procedures. Based on the qualitative grading of existing data, a modified Delphi method was used to covalidate the content of the standardised intervention checklist and the feasibility of the implementation of each point of this checklist (table 1).
Table 1.
PROADAPT programme: tasks according to the different domains and the successive chronological steps (before, during and after complex medical–surgical procedures)
| Nurse coaching & education bridging interventions | Coaching nurse self-presentation. Delivery of a personalised patient booklet care according best practice guidelines:
Weekly phone calls |
Coaching nurse visits / phone calls Communication of patient's preference to the staff | Coaching nurse visit in the rehabilitation ward communication of patient's preference and care difficulties to the staff (checklist):
|
Coaching nurse bi-weekly phone call communication of patient's care difficulties to the staff |
| Nutrition | W-4: nutritional evaluation Nutritional plan based on measured intake W-3: nutritional follow-up - weight W-2: nutritional follow up- weight W-1: nutritional follow up- weight+pre-operative immunonutrition |
If surgery: Care according best practice guidelines:
|
Nutritional plan based on:
Optimal management of:
|
Nutritional plan based on:
Optimal management of:
|
| Physical activity | W-4: physical performances evaluation Physical activity plan W-3, W-2, W-1: group physical activity + functionnal follow-up |
If surgery: care according best practice guidelines:
|
(to the discretion of the rehabilitation unit) | Pursuing of the pre-operative physical activity plan |
| Medication conciliation | Centralised medication conciliation and treatment optimisation (STOPP/START guidelines) | Centralised medication conciliation Advices for care according best practice guidelines:
|
Centralised medication conciliation | Centralised medication conciliation |
| Standardisation of surgical procedures | If surgery: consider antiseptic toothpaste If surgery: care according best practice Guidelines:
If surgery: consider IV iron supplementation |
PROADAPT, Prehabilitation & Rehabilitation in Oncogeriatrics: Adaptation to Deconditioning risk and Accompaniment of Patients’ Trajectories; STOPP/START, Screening Tool of Older Persons’ Prescriptions and Screening Tool to Alert to Right Treatment.
A PROADAPT booklet was developed; it is a standardised, adapted and evolutive tool designed to explain physical exercise and nutrition counselling and to ensure the collection of patients’ day-to-day achievements. The first version was tested by candidate patients before the validation of the current (version 3) of the booklet.
PROADAPT standardised geriatric intervention programme includes
Preoperative physical activity, including strength and endurance exercise±group activities over 4±2 weeks. Interventions with a high level of evidence were retained, according to an ongoing umbrella review of systematic reviews (http://www.crd.york.ac.uk/PROSPERO, Ref CRD4202010011027 28).
Nutrition: nutrition before and after physical activity, preoperative and postoperative immunonutrition±artificial nutrition (ie, enteral or parenteral nutrition) according to international guidelines.9
Patient (and caregiver) education and coaching (on nutrition and physical exercise) according to a weekly schedule with the activation of integrated supports.29
Standardised intervention procedures, according to a checklist established in consensus with surgeons.
Enhanced rehabilitation will be promoted according to international guidelines.30
Pharmaceutical medication conciliation and treatment optimisation according to a centralised process with pharmaceutical expertise.
Bridging interventions for hospital-to-home transition, according to a proposed standardised procedure including training of dedicated nurses and postdischarge phone calls follow-up over the 12 weeks after surgery. In practice, only two or three people from the coordination centre team are in charge of coaching for all patients. In the future, a nurse coach will be trained in each centre and will be responsible for patient coaching. Interventions with a high level of evidence were retained, according to an ongoing umbrella review of systematic reviews (http://www.crd.york.ac.uk/PROSPERO, Ref CRD42017055698).
The intervention is designed to be implemented at different moments of patient care (table 1).
During the prehabilitation period
A dedicated nurse, trained in patient education by the coordinating centre team (‘coaching nurse’), presents himself or herself to the patient for:
Presentation of the programme to the patient and his or her caregiver(s).
Personalisation of the PROADAPT booklet (see further) to the patient’s characteristics.
Collection of personal data, nutritional and functional habits.
Geriatric assessment using standardised scores (cognition using the Mini-Cog screening tool,31 depression using the geriatric depression scale in 4 and 15 items (GDS4/GDS15),32 nutrition using the Mini-Nutritional Assessment (MNA)33).
Collection of the information to be sent to the pharmacist: comorbidities and comedications.
Anticipation and organisation of the future appointments (anaesthetists, stomatherapists and others).
A weekly visit or phone call according to a structured interview for health education and transmission of nutritional and functional advice (see further).
Nutritional care is based on:
A personalised evaluation of nutritional balance and nutritional needs of the patient according to dietitian diagnosis based on measured intake and international recommendations.
A weekly follow-up of weight and nutritional intake. If the coaching nurse identifies an unfavourable nutritional trend, he or she reports it to the referring physician and nutritionist.
Artificial nutrition if needed according European Society for Clinical Nutrition and Metabolism recommendations.9 34 35
Preoperative immunonutrition during 7 days before surgery.35
Total body rehabilitation:
Two to three times a week: strength exercise (each time with dedicated exercises for upper and lower limbs, as well as abdominal muscles; 20–45 min each sequence).
Two to three times a week: endurance exercise (walking or cycle ergometer), 20–45 min each sequence.
Three times a day: respiratory physiotherapy.
Once a week (if possible): group activities (according to the centre organisation and home–hospital distance).
Pharmaceutical conciliation and optimisation according to Screening Tool of Older Persons’ Prescriptions and Screening Tool to Alert to Right Treatment criteria version 236 and international recommendations concerning perioperative care7: to be transmitted to the surgical and anaesthesia team without any obligation to change patient care.
During perioperative period
The coaching nurse contacts the surgical team for transmission of:
Patient’s personal data.
Physical (nutritional, functional and/or comorbidities) as well as psychological difficulties.
Results of medication conciliation.
During rehabilitation period
The coaching nurse contacts the rehabilitation team for transmission of:
Patient’s personal data and care course.
Physical (nutritional, functional and/or comorbidities) as well as psychological difficulties.
Results of medication conciliation.
The rehabilitation programme is left to the discretion of the rehabilitation team (standard care and local habits).
A weekly phone call from the coaching nurse to the rehabilitation team for nutritional and functional follow-up, as well as medication conciliation.
During hospital–home transition period
The coaching nurse contacts the patient’s general practitioner for transmission of:
Patient’s personal data and care course.
Physical (nutritional, functional and/or comorbidities) as well as psychological difficulties.
Results of medication conciliation.
Biweekly phone call of the coaching nurse to the patient for nutritional and functional follow-up.
Advice for optimisation of symptom management: abdominal pain, nausea, vomiting and so on.
Participant timeline
Six successive evaluations are planned for the participants.
The inclusion visit is planned during a geriatric consultation planned at least 7 days before the start day of the complex medical–surgical procedure. If the complex medical–surgical procedure is delayed for any reason or the patient receives a neoadjuvant treatment, the prehabilitation period may be prolonged up to 9 months. In such cases, the frequency of the phone calls is decreased (from 1/week to 1/month) after 4 weeks. During the inclusion visit, lasting about 1 hour, the following data are collected:
Clinical (blood pressure, heart rate, Eastern Cooperative Oncology Group (ECOG) score37 and comorbidities), laboratory (albumin, prealbumin and C reactive protein) and paraclinic (year of birth, sex, weight, height, body mass index and change in weight over the last 3 and 6 months).
All concomitant treatments and drug conciliation.
The history of the disease (primitive site, metastases, histology of the initial tumour and presence of tumour markers).
Radiological disease assessments (date and nature).
Standardised geriatric assessment using validated questionnaires with a particular attention on physical activity and nutrition (ADL38/instrumental ADL (IADL),39 G8,40 Rapid Assessment of Physical Activity,41 daily physical instrumental activities (Physical Instrumental Activities of Daily Living (AIPVQ)),42 European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30)43 and Elderly specific questionnaire in 14 items, (QLQ-ELD14),44 the EUROQOL evaluation of quality of life (EUROQOL EQ-5D-3L) evaluating five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression in three levels,45 fatigue short form inventory (SF-36),46 short physical performance battery (SPPB),47 Timed Up and Go (TUG) test,48 nine-item Fatigue Severity Scale,49 MNA,33 GDS4/GDS15,32 Mini-Cog,31 Tinetti test,50 Borg Scale,51 Pain Scale52 and Nutrition Scale53) (tables 2 and 3).
-
Delivery of the ‘PROADAPT kit’ during a meeting with a dedicated paramedic (nurse, physiotherapist, ergotherapist and so on) to:
Provide to the patient Voldyne (Hudson RCI, Temecula, California, USA) and Triflo II (Tyco Healthcare, Mansfield, Massachusetts, USA) incentive spirometry devices for inspiratory muscle training.
-
Present the PROADAPT booklet that includes a battery of exercises and nutritional counselling specifically designed for this older population:
Muscle strengthening of the upper limbs (six exercises, three difficulty levels), lower limbs (six exercises, three difficulty levels), abdominal wall (four exercises, three difficulty levels); objective: two to three sessions per day for a total duration of between 20 and 45 min.
Endurance/aerobic activities (seven exercises, three difficulty levels with three duration objectives); objective: every day.
Inspiratory muscle training with Voldyne and Triflo II devices; objective: three sessions per day for a total duration of 30 min.
General nutritional counselling adapted to the older population: food enrichment, intermeal collations and oral nutritional supplements.
Fulfil a 3-day food statement that allows, in the 7 days after inclusion, to deliver a dietitian-driven personalised nutritional counselling. If needed, in case of unfavourable nutritional parameters, artificial nutrition is introduced.
-
Prescription, if needed:
Of home physiotherapy according to the PROADAPT programme for respiratory training sessions and physical activity training sessions.
Of oral nutritional supplements.
Of usual medicines, adapted following pharmaceutical review.
For patients requiring inpatient follow-up, hospital admission for a few days in a rehabilitation unit for a physiotherapeutic programme and/or artificial nutrition (enteral preferred).
Table 2.
PROADAPT pilot trial: questionnaires and screening tests
| Domain | |
| Autonomy | ADL and IADL |
| Geriatric screening | G8 |
| Physical activity | RAPA and AIPVQ |
| Quality of life | QLQ-C30, QLQ-ED14, EQ-5D-3L and SF-36 |
| Locomotion and balance | TUG test and SPPB |
| Pain | Pain Scale |
| Nutrition | Nutrition Scale |
| Tiredness severity | FSS |
| Depression/anxiety | MNA and GDS4/GDS15 |
| Cognitive assessment | Mini-Cog |
| Fall risk assessment | Tinetti test |
| Breathlessness | Borg Scale |
ADL, Activities of Daily Living; AIPVQ, Physical Instrumental Activities of Daily Living (in French: Activités Instrumentales Physiques de la Vie Quotidienne); EQ-5D-3L, EUROQOL evaluation of quality of life in five dimensions and three levels; FSS, Fatigue Severity Scale; GDS, Geriatric Depression Scale; IADL, Instrumental Activities of Daily Living; MNA, Mini-Nutritional Assessment; PROADAPT, Prehabilitation & Rehabilitation in Oncogeriatrics: Adaptation to Deconditioning risk and Accompaniment of Patients’ Trajectories; QLQ-C30, quality of life questionnaire core 30 of the European Organisation for Research and Treatment of Cancer (EORTC); QLQ-ELD14, Older patients-specific quality of life questionnaire in 14 items of the EORTC; RAPA, Rapid Assessment of Physical Activity; SF-36, Short Form 36 Health Survey Questionnaire; SPPB, Short Physical Performance Battery.
Table 3.
PROADAPT pilot study: flow diagram
| Baseline | Pre therapeutic visit (0–5 days before intervention) | M1, M3 and M6 | M12 | End of study visit | |
| Comprehensive geriatric assessment | |||||
| G8 | × | × | × | ||
| ADL/IADL | × | × | × | ||
| GDS4/GDS15 | × | × | × | ||
| Mini-Cog | × | × | × | ||
| MNA | × | × | × | ||
| QLQ-C30 | × | × | × | × | |
| QLQ-ELD14 | × | × | × | × | |
| EQ-5D-3L | × | × | × | × | |
| Pain scale | × | × | × | × | × |
| Nutrition scale | × | × | × | × | × |
| Socioeconomic evaluation | × | ||||
| Physical and respiratory assessments | |||||
| FSS | × | × | × | × | |
| SF-36 | × | × | × | × | |
| Timed Up and Go | × | × | × | ||
| SPPB | × | × | × | × | |
| Borg Scale | × | × | × | ||
| RAPA questionnaire | × | × | × | × | |
| AIPVQ Scale | × | × | × | × | |
| Tinetti test | × | × | × | ||
| Equimog evaluation | × | × | × | ||
| Triflo II | × | × | |||
| Voldyne | × | × | |||
| Physical activity data collection | × | × | × | × | |
| Patient satisfaction | |||||
| Standardised questionnaire | × | ||||
ADL, Activities of Daily Living; AIPVQ, Physical Instrumental activities of daily living (in French: Activités Instrumentales Physiques de la Vie Quotidienne); EQ-5D-3L, EUROQOL evaluation of quality of life in five dimensions and three levels; FSS, Fatigue Severity Scale; GDS, Geriatric Depression Scale; IADL, Instrumental Activities of Daily Living; MNA, Mini Nutritional Assessment; QLQ-C30, quality of life questionnaire core 30 of the European Organisation for Research and Treatment of Cancer (EORTC); QLQ-ELD14, Older patients-specific quality of life questionnaire in 14 items of the EORTC; RAPA, Rapid Assessment of Physical Activity; SF-36, Short Form 36 Health Survey Questionnaire; SPPB, Short Physical Performance Battery.
During the preintervention period, phone calls by a dedicated paramedic are planned (once a week for the first 4 weeks and then once a month until the intervention). Calls are semidirected interviews focused on the patient’s autonomy, physical activity, appetite and sleep over the last period (week/month). A special attention is paid to encouraging patient motivation and compliance to the programme.
The pretherapeutic visit is scheduled when possible within the 5 days before the day of the intervention. This visit is performed in the surgery or radiotherapy unit only if the visit is necessary before the intervention and does not modify standard therapeutic care; it collects:
Clinical, laboratory and paraclinic data.
All concomitant treatments and drug conciliation.
Data concerning pain, nutrition, fitness and physical tests (tables 2 and 3).
During the postintervention period, paramedics trained in clinical research will resume follow-up phone calls as before the intervention once a week for 12 weeks after day 0 (D0), and once a month up to 12 months after D0. D0 is defined as the last day of surgery (day of the last resumption of surgery in the limit of 30 days after the first intervention) or the last day of radiotherapy. For weekly calls, a margin of ±2 days is allowed, and for monthly calls, a margin of ±7 days is allowed.
Visits at 1, 3 and 6 months after the intervention (±7 days): the patient may have started an antineoplastic treatment according to standard of care. The visit could be with the surgeon, the radiotherapist or the oncologist according local habits. The data to be collected are:
Clinical (blood pressure, heart rate and ECOG scale37), laboratory (albumin, prealbumin and C reactive protein) and patient characteristics (weight and body mass index).
All concomitant treatments and drug conciliation.
Patient care (surgery and complications and treatment for the cancer).
Radiological disease assessments (date and nature).
QoL, pain, nutrition, fitness, physical performance through questionnaires and tests regarding (tables 2 and 3).
Hospital costs related to complications.
The end of study visit is planned at 12 months (per-protocol) or at the date of trial premature discontinuation (±7 days) for the collection of the data listed previously. In addition, and in case of omission of one or more of the intermediate visits, data relating to complications occurring during the post-therapeutic period are collected.
Outcomes and measurements
Primary outcome
The main outcome measure will be the proportion of patients who have completed at least one item in the PROADAPT programme after 12 months after D0. The workshops of the programme are:
Physical and respiratory rehabilitation.
Renutrition session.
Telephone nurse follow-up.
Secondary outcomes
The secondary outcomes of the study are:
The feasibility of each stage of the programme independently of each other (rate of achievement of all or part of the instructions).
-
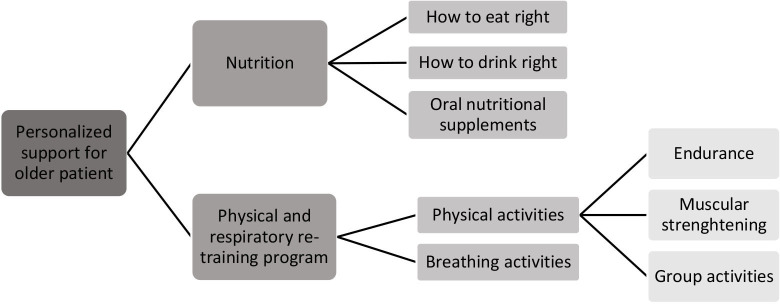
Preoperative physical rehabilitation including (figure 1):
Muscle strengthening.
Respiratory rehabilitation.
Endurance work.
Preoperative nutrition counselling (figure 1).
Drug reconciliation/iatrogenic prevention.
Pretherapeutic follow-up calls.
Postsurgery or postradiotherapy follow-up calls.
Figure 1.
PROADAPT programme: interventions at the patient’s level. PROADAPT, Prehabilitation & Rehabilitation in Oncogeriatrics: Adaptation to Deconditioning risk and Accompaniment of Patients’ Trajectories.
Patient satisfaction with the overall programme at the end of the study (end of follow-up or study discontinuation) estimated using a questionnaire (online supplemental file 1).
To assess the change over time before surgery of: physical parameters and exercises, inspiratory parameters and exercises (Voldyne and Triflo), as well as weight and food intake (qualitative and quantitative assessments).
-
To assess the change over 1 year of:
Physical performance (SPPB, gate speed and TUG test) and functional independence on ADL,38 IADL39 and AIPVQ.42
Nutritional parameters of the patient (weight, albuminaemia and appetite).
Health-related QoL for patients based on QLQ-C30 and ELD14 (five dimensions: mobility, disease burden, emotional and physical functioning, and tiredness).54 55
Pharmaceutical conciliation.
Estimate the rate and nature of postoperative complications at 30 days (NCI-CTCAE).
Estimate the rate and nature of postoperative complications according to the CCI (Comprehensive Complication Index) at 30 and 90 days.
Estimate postoperative mortality at 30 and 90 days.
Estimate the 1 year OS rate.
Estimate the 1 year PFS rate.
Estimate the longitudinal change of QoL according to QLQ C30, ELD14 and EQ-5D.
Estimate treatment costs (health system).
Study therapeutic strategies (treatment completion rate).
Estimate the change of geriatric covariates over 1 year.
Sample size calculation
The programme will be considered feasible, at the patient level, if all or part of the programme is implemented in at least 50% of the included patients (=alternative hypothesis). This threshold was defined in line with previous studies on prehabilitation for older cancer patients that reported compliance rates between 16% and 95%.56 57 Considering that the PROADAPT programme is complex even if tailored to older patients, we anticipate modest compliance rates.
To reject the null hypothesis of programme feasibility in less than 35% of patients, with an alpha risk of 5% and a power of 90% (beta=10%, bilateral test), the number of subjects required is 111; accounting for 10% non-treatable patients, a total of 122 patients should be included. The included patients will be analysed according to the intention-to-treat principle.
Data management and statistical analyses
Data are monitored by a clinical research assistant (CRA). Inconsistencies will be reported to the study investigators in order to decide whether the data should be corrected or considered as missing. Any changes in the data will be reported.
Data analyses will be performed by the data management and analysis centre. The analyses will be carried out by an independent statistician with the latest version of the R software environment (R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/). All of the characteristics collected will be subjected to a descriptive analysis.
Descriptive analyses
A flow diagram will describe the data available for the patient population at baseline and during each follow-up visit. Eligibility criteria for treated patients will be verified, as well as follow-up and end of study visits. Reasons for premature end of study will be provided.
Characteristics of the study population, numbers and proportions of missing values will be reported. Patient characteristics will be described using mean and SD or median and IQR for quantitative variables, and frequencies and distribution for categorical variables. A comparison of baseline characteristics between patients with complete follow-up and those with attrition will be performed. If needed, methods for handling missing data (multiple imputation, mixed model or auxiliary variable) will be used when appropriate.
Primary analysis
The proportion of patients who have completed at least one PROADAPT programme activity 12 months after the start of treatment will be estimated using mean and SD.
Secondary analyses
Time-to-event variables: follow-up, OS and PFS
The probabilities of events at specific measurement times will be estimated according to the Kaplan-Meier method. Medians of event-free survival will be reported by treatment arm with its 95% CI, if the number of events allows estimation of the median.
OS and PFS probabilities at 12 months (after the day of the last revision of surgery or the last day of radiotherapy) will be provided with 95% CI.
Quality of life
Analyses of the QoL data will be performed according to the modified intention-to-treat principle: all included patients, regardless of compliance with the eligibility criteria and whether they were followed-up and for whom the QoL scores are available at inclusion will be included in the analysis. Patient QoL, linked to health, will be analysed after 3 months through five dimensions: mobility, disease burden, emotional and physical functioning, and fatigue.
Data monitoring
The successful completion of the database is ensured by the hospital CRA. The hospital CRA also ensures compliance with the study protocol. The sponsor CRA verifies that the rights of the participants are respected.
End of study
Patients leave the study either on a per-protocol basis during the ‘end of study visit’ on month 12 after the intervention or at any time during the conduct of the study if they no longer wish to participate. However, as indicated in the information letter to the patients/caregivers, the data collected before exclusion may be used as part of the study.
Confidentiality
Correspondence tables will be kept in a separate file that does not contain clinical data. The access to the nominative information is protected by a password, and confidentiality is guaranteed by the study.
Protocol amendments
Any important modification requiring a new ethics committee approval will be communicated in future publications. Any potential impact of protocol modifications on the results will be discussed as appropriate.
Trial status
Patient enrolment began on 3 July 2018. Data are currently being collected.
Patient and public involvement
Patients were involved at different steps of the trial: during the development of the PROADAPT booklet, several (n=30) patients were asked to answer an anonymous questionnaire in order to improve its ergonomics; the information letter and consent form for the study were reviewed by the patients committee of the Ligue Nationale de Lutte contre le Cancer (a French association of cancer patients).
Discussion
Discussion of the intervention
Prehabilitation has long been conceptualised as an effective means of improving the functional capacity of the individual to enable them to resist various stressors. Originally developed in the military as the association of physical training to improve strength and endurance, improvement of nutritional intake and general education,58 it has been transposed into medicine and major surgery—initially when an ICU admission is planned—at the beginning of the century.59
Despite a growing interest in the medical community for prehabilitation, and particularly cancer prehabilitation, the level of evidence for specific interventions remains too low for it to be implemented in everyday practice. Among the main limitations include the heterogeneity of programmes, sometimes poor patient compliance and the fact that most studies were small pilot studies developed for patients fitter and younger than those who are likely to benefit the most from prehabilitation. Another point to emphasise is that most programmes include only one intervention—physical, nutritional or psychological rehabilitation—while multimodal interventions are often considered to be more effective in older populations.
Considering these points, the PROADAPT intervention was developed according to an innovative management strategy since it started in 2016 by multiprofessional meetings conceived as brainstorming sessions in order to develop a multidisciplinary programme dedicated to prehabilitation and follow-up of older patients. The multidisciplinary conception of the intervention, the particular attention paid to older patients’ specificities and the previous experience of the participants in various fields, including patient education, cognition and physiotherapy, are hopefully the warrants of the most tailored approach to the target population. For example, a large font was used in the booklet and the illustrations are highly schematic and highly contrasted, and furthermore, each sentence was verified by a panel of patients in order to ensure correct understanding. This resulted in high rate of satisfaction regarding the booklet that was evaluated by 30 patients (unpublished data).
This pilot study is the first step towards an ambitious programme, since the PROADAPT programme will be evaluated in the future in two randomised studies, PROADAPT-ovary/EWOC-2 (NCT04284969) and PROADAPT sus-mesocolic, designed to evaluate the impact of the PROADAPT programme on post-treatment complications versus usual practice. In order to favour patient compliance and follow-up, an eHealth tool, ID-PROADAPT, has been developed that will help supervise the course of patients’ care.
Discussion of the study design
In line with the previous points, this pilot study was designed to answer the critical question: is a multidomain prehabilitation programme feasible in an older cancer population? This question encompasses several points: is the programme physically adapted to an older population? Is such a programme applicable in ambulatory care? How to build pedagogical tools adapted for such ambulatory use? Are such pedagogical tools understandable? What is the compliance of the patients to each domain of the intervention programme?… Another point is to understand is whether the patient’s care team accepts such intervention; however, this point was previously evaluated by Ghignone et al, 60 who demonstrated through an international survey that surgeons are generally in favour for such programmes since 71% of them would accept to prehabilitate their elderly patients 4 weeks before surgery, if such intervention is proven to be effective. Nevertheless, the participation of surgeons and anaesthetists during initial brainstorming sessions was of major interest since they greatly enriched the structure of the programme.
Thus, the construct of this trial may appear as highly complex with overabundant secondary endpoints, but this design encompasses as much as possible the complexity of preventive care in an older population, which has to mix adaptation to the target population and the ability to maintain compliance over time.
Ethics and dissemination
The study sponsor is the Hospices Civils de Lyon, responsible for study insurance and pharmacovigilance. The study protocol (V2) was approved by the Ile de France eight ethics committee on 5 June 2018 and cover all sites involved in this study. The amended versions were as follows: V3 dated 23 October 2018 (change in the recruitment period and addition of new investigation centres), V4 dated 17 May 2019 (request for an additional 12-month extension, update with the General Data Protection Regulations (GDPR) and update of the patient booklet) and V5 dated 17 July 2020 (addition of a cohort of 30 patients to test the follow-up programme with the ID-PROADAPT eHealth tool (online supplemental file 2), request for an additional 8-month extension). The current version is the V5 dated 17 July 2020, authorised on 10 September 2020. The research will be carried out in accordance with the Helsinki Declaration and International Conference on Harmonisation-Good Clinical Practice Guidelines. The trial protocol fulfils the SPIRIT 2013 checklist (online supplemental table 1) and WHO trial registration data set (online supplemental table 2). The study complies with the principles of the data protection act in France and with the GDPR in force in Europe. Each investigator must collect a written informed consent at the beginning of the procedure. This consent is retained in the patient’s medical chart. The patient can stop participation in the study at any time with an oral instruction given to the investigator or CRA. Patients will be informed of additional amendments according to the law in force.
bmjopen-2020-042960supp002.pdf (116.8KB, pdf)
bmjopen-2020-042960supp003.pdf (68.8KB, pdf)
The results of the primary and secondary objectives will be published in peer-reviewed journals. All authors of future publications will have to meet the criteria for authorship stated in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals by the International Committee of Medical Journal Editors.
Supplementary Material
Acknowledgments
This pilot protocol is part of the Prehabilitation & Rehabilitation in Oncogeriatrics: Adaptation to Deconditioning risk and Accompaniment of Patients’ Trajectories (PROADAPT) working group initiative for improving care for older people with cancer. The authors also acknowledge the teams of Lyon Sud Hospital, Edouard Herriot Hospital of Lyon, Croix Rousse Hospital of Lyon, Annecy Genevois Hospital Center, Hôpital Nord Ouest Hospital Center, Chambéry Hospital Center and Médipôle Lyon-Villeurbanne who contribute to patient enrolment in this study. They also thank Philip Robinson (DRCI, Hospices Civils de Lyon) for help in manuscript preparation. The authors would like to thank the patients’ review committee of the Ligue Nationale de Lutte contre le Cancer for the valuable editing of the information note and the consent form.
Footnotes
Collaborators: PROADAPT working group: physicians: G Albrand, D Barnoud, D Benayoun, B Billod, J Bonhomme, A-L Bres, C Brunengo, A Chanelière, Y Chauleur, G Copaescu, H Curé, A-M Dascalita, B De La Vigerie, S Ducoulombier, O Ganne, F Gervais, T Gilbert, M Giroud, R Guyot, M Haïne, N Jomard, C Lecardonnel, B Leroy, J-A Long, A Marion, E Nony, S Parent, A Pelisset-Vanhersecke, A Pirollet, J-E Terrier, J Trautmann and M-A Vincent; pharmacist: D Charlety; paramedics: A Laurent and M Tomatis; clinical research teams: C El Khoury, E Gadéa-Deschamps and S Goutte.
Contributors: All authors participated to the PROADAPT intervention conception. Study protocol was conceived by DD, CF, CG, SP-B, OLS, AM, VP and CR. DD and CF assumed fundraising and grant follow-up. MR led the drafting of the manuscript. All authors critically reviewed and approved the final version of the protocol.
Funding: This work was supported by the Agence Régionale de Santé (ARS) Auvergne Rhône Alpes, through the call for projects 'Innovate in health for the Auvergne Rhône Alpes region' jointly organised by the ARS, Bpifrance, the Auvergne Rhône Alpes region, URIOPASS, ORAIDA and Cluster I-care and the decision of the selection committee on 27 June 2016 (Grant N°2016-149 (63)). It provided funding for nurses, dieticians, physiotherapists and clinical research assistants.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
PROADAPT working group:
G. Albrand, D. Barnoud, D. Benayoun, B Billod, J. Bonhomme, A.-L. Bres, C. Brunengo, A. Chanelière, Y. Chauleur, G. Copaescu, H. Curé, A-M. Dascalita, B. De La Vigerie, S. Ducoulombier, O. Ganne, F. Gervais, T. Gilbert, M. Giroud, R. Guyot, M. Haïne, N. Jomard, C. Lecardonnel, B. Leroy, J-A. Long, A. Marion, E. Nony, S. Parent, A. Pelisset-Vanhersecke, A. Pirollet, J-E. Terrier, J. Trautmann, and M-A. Vincent
References
- 1. Wagner D, DeMarco MM, Amini N, et al. Role of frailty and sarcopenia in predicting outcomes among patients undergoing gastrointestinal surgery. World J Gastrointest Surg 2016;8:27. 10.4240/wjgs.v8.i1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heppenstall CP, Wilkinson TJ, Hanger HC, et al. Frailty: dominos or deliberation? N Z Med J 2009;122:42–53. [PubMed] [Google Scholar]
- 3. Lopez-Lopez V, Gómez-Ruiz AJ, Eshmuminov D, et al. Surgical oncology in patients aged 80 years and older is associated with increased postoperative morbidity and mortality: a systematic review and meta-analysis of literature over 25 years. Surg Oncol 2020;33:81–95. 10.1016/j.suronc.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 4. Tan H-J, Saliba D, Kwan L, et al. Burden of geriatric events among older adults undergoing major cancer surgery. J Clin Oncol 2016;34:1231–8. 10.1200/JCO.2015.63.4592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akushevich I, Kravchenko J, Akushevich L. Medical cost trajectories and Onsets of cancer and noncancer diseases in US elderly population, medical cost trajectories and Onsets of cancer and noncancer diseases in US elderly population. Comput Math Method Med 2011;2011:e857892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Roo AC, Li Y, Abrahamse PH, et al. Long-term functional decline after high-risk elective colorectal surgery in older adults. Dis Colon Rectum 2020;63:75–83. 10.1097/DCR.0000000000001541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mohanty S, Rosenthal RA, Russell MM, et al. Optimal perioperative management of the geriatric patient: a best practices guideline from the American College of surgeons NSQIP and the American geriatrics Society. J Am Coll Surg 2016;222:930–47. 10.1016/j.jamcollsurg.2015.12.026 [DOI] [PubMed] [Google Scholar]
- 8. Treanor C, Kyaw T, Donnelly M. An international review and meta-analysis of prehabilitation compared to usual care for cancer patients. J Cancer Surviv 2018;12:64–73. 10.1007/s11764-017-0645-9 [DOI] [PubMed] [Google Scholar]
- 9. Arends J, Bachmann P, Baracos V. ESPEN guidelines on nutrition in cancer patients. Clin Nutr [online] 2016. http://www.clinicalnutritionjournal.com/article/S0261-5614(16)30181-9/abstract [DOI] [PubMed] [Google Scholar]
- 10. Singh F, Newton RU, Galvão DA, et al. A systematic review of pre-surgical exercise intervention studies with cancer patients. Surg Oncol 2013;22:92–104. 10.1016/j.suronc.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 11. Powell R, Scott NW, Manyande A. Psychological preparation and postoperative outcomes for adults undergoing surgery under general anaesthesia. Cochrane anaesthesia, critical and emergency care group, editor. Cochrane Database Syst Rev [online] 2016. http://doi.wiley.com/10.1002/14651858.CD008646.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santa Mina D, Alibhai SMH. Prehabilitation in geriatric oncology. J Geriatr Oncol 2020;11:731–4. 10.1016/j.jgo.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 13. Carli F, Bousquet-Dion G, Fiore JF. Prehabilitation vs postoperative rehabilitation for frail patients. JAMA Surg 2020;155:899–900. 10.1001/jamasurg.2020.1816 [DOI] [PubMed] [Google Scholar]
- 14. Ellis G, Whitehead MA, O’Neill D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2011;7:CD006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ellis G, Gardner M, Tsiachristas A. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2017;12:CD006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meid AD, Lampert A, Burnett A, et al. The impact of pharmaceutical care interventions for medication underuse in older people: a systematic review and meta-analysis. Br J Clin Pharmacol 2015;80:768–76. 10.1111/bcp.12657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Royal S, Smeaton L, Avery AJ, et al. Interventions in primary care to reduce medication related adverse events and hospital admissions: systematic review and meta-analysis. Qual Saf Health Care 2006;15:23–31. 10.1136/qshc.2004.012153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rennke S, Nguyen OK, Shoeb MH, et al. Hospital-initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med 2013;158:433–40. 10.7326/0003-4819-158-5-201303051-00011 [DOI] [PubMed] [Google Scholar]
- 19. Ferlay J, Soerjomataram I, Ervik M. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [online]. Lyon, France: International Agency for Research on Cancer, 2013. http://globocan.iarc.fr [Google Scholar]
- 20. Koene RJ, Prizment AE, Blaes A, et al. Shared risk factors in cardiovascular disease and cancer. Circulation 2016;133:1104–14. 10.1161/CIRCULATIONAHA.115.020406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sjölund B-M, Wimo A, Qiu C, et al. Time trends in prevalence of activities of daily living (ADL) disability and survival: comparing two populations (aged 78+ years) living in a rural area in Sweden. Arch Gerontol Geriatr 2014;58:370–5. 10.1016/j.archger.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 22. Sjölund B-M, Wimo A, Engström M, et al. Incidence of ADL disability in older persons, physical activities as a protective factor and the need for informal and formal care – results from the SNAC-N project. PLoS One 2015;10:e0138901 https://www-ncbi-nlm-nih-gov.gate2.inist.fr/pmc/articles/PMC4583409/ 10.1371/journal.pone.0138901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jagger C, Gillies C, Moscone F, et al. Inequalities in healthy life years in the 25 countries of the European Union in 2005: a cross-national meta-regression analysis. Lancet 2008;372:2124–31. 10.1016/S0140-6736(08)61594-9 [DOI] [PubMed] [Google Scholar]
- 24. Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new medical research council guidance. BMJ 2008;337:a1655. 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Criteres de Toxicite. Available: http://www.cepd.fr/CUSTOM/CEPD_toxicite.pdf
- 26. Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc 1968;16:622–6. 10.1111/j.1532-5415.1968.tb02103.x [DOI] [PubMed] [Google Scholar]
- 27. Falandry C, Stefani L, Granger M. Interventions to improve physical performances of older people with cancer before major surgery: an umbrella review. J Geriatr Oncol. 2018;9:S94–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Falandry C, Stefani L, Andre L, et al. Interventions to improve physical performances of older people with cancer before complex medico-surgical procedures: protocol for an umbrella review of systematic reviews and meta-analyses. Medicine 2020;99:e21780. 10.1097/MD.0000000000021780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Glasgow RE, Emmons KM. How can we increase translation of research into practice? types of evidence needed. Annu Rev Public Health 2007;28:413–33. 10.1146/annurev.publhealth.28.021406.144145 [DOI] [PubMed] [Google Scholar]
- 30. Colburn JL, Mohanty S, Burton JR. Surgical guidelines for perioperative management of older adults: what geriatricians need to know. J Am Geriatr Soc 2017;65:1339–46. 10.1111/jgs.14877 [DOI] [PubMed] [Google Scholar]
- 31. McCarten JR, Anderson P, Kuskowski MA, et al. Screening for cognitive impairment in an elderly veteran population: acceptability and results using different versions of the Mini-Cog. J Am Geriatr Soc 2011;59:309–13. 10.1111/j.1532-5415.2010.03249.x [DOI] [PubMed] [Google Scholar]
- 32. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982;17:37–49. 10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- 33. Guigoz Y, Vellas B, Garry PJ. Assessing the nutritional status of the elderly: the mini nutritional assessment as part of the geriatric evaluation. Nutr Rev 2009;54:S59–65. 10.1111/j.1753-4887.1996.tb03793.x [DOI] [PubMed] [Google Scholar]
- 34. Arends J, Bodoky G, Bozzetti F, et al. ESPEN guidelines on enteral nutrition: non-surgical oncology. Clin Nutr 2006;25:245–59. 10.1016/j.clnu.2006.01.020 [DOI] [PubMed] [Google Scholar]
- 35. Weimann A, Braga M, Harsanyi L, et al. ESPEN guidelines on enteral nutrition: surgery including organ transplantation. Clin Nutr 2006;25:224–44. 10.1016/j.clnu.2006.01.015 [DOI] [PubMed] [Google Scholar]
- 36. O'Mahony D, O'Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015;44:213–8. 10.1093/ageing/afu145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the eastern cooperative Oncology Group. Am J Clin Oncol 1982;5:649–56. 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 38. Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963;185:914–9. 10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- 39. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86. 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- 40. Bellera CA, Rainfray M, Mathoulin-Pélissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol 2012;23:2166–72. 10.1093/annonc/mdr587 [DOI] [PubMed] [Google Scholar]
- 41. Topolski TD, LoGerfo J, Patrick DL, et al. The rapid assessment of physical activity (rapa) among older adults. Prev Chronic Dis 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- 42. de Souto Barreto P, Ferrandez A-M. Activités instrumentales physiques de la vie quotidienne chez les personnes âgées: validation d’une échelle. In: Chapuis-Lucciani N, Guihard-Costa AM, Boëtsch G, eds. L’anthropologie du vivant: objets et méthodes, 2010: 83–7. [Google Scholar]
- 43. Quinten C, Coens C, Ghislain I, et al. The effects of age on health-related quality of life in cancer populations: a pooled analysis of randomized controlled trials using the European organisation for research and treatment of cancer (EORTC) QLQ-C30 involving 6024 cancer patients. Eur J Cancer 2015;51:2808–19. 10.1016/j.ejca.2015.08.027 [DOI] [PubMed] [Google Scholar]
- 44. Wheelwright S, Darlington A-S, Fitzsimmons D, et al. International validation of the EORTC QLQ-ELD14 questionnaire for assessment of health-related quality of life elderly patients with cancer. Br J Cancer 2013;109:852–8. 10.1038/bjc.2013.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Janssen MF, Pickard AS, Golicki D, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717–27. 10.1007/s11136-012-0322-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ware JE, Sherbourne CD. The mos 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 47. Fish J. Short physical performance battery. : Kreutzer JS, DeLuca J, Caplan B, . Encyclopedia of clinical neuropsychology [online]. New York: Springer New York, 2011: 2289–91. http://link.springer.com/10.1007/978-0-387-79948-3_1832 [Google Scholar]
- 48. Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 49. Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46:1121–3. 10.1001/archneur.1989.00520460115022 [DOI] [PubMed] [Google Scholar]
- 50. Tinetti ME, Baker DI, McAvay G, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med 1994;331:821–7. 10.1056/NEJM199409293311301 [DOI] [PubMed] [Google Scholar]
- 51. Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 1970;2:92–8. [PubMed] [Google Scholar]
- 52. Agence Nationale d’Accréditation et d’Evaluation en Santé (ANAES) . Assessment and follow-up of chronic pain in adults in ambulatory medicine, 1999. [Google Scholar]
- 53. Société Francophone Nutrition Clinique et Métabolisme (SFNCM) . Evaluation of food intake.
- 54. Aaronson NK, Ahmedzai S, Bergman B, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 55. Wheelwright S, Darlington A-S, Fitzsimmons D, et al. International validation of the EORTC QLQ-ELD14 questionnaire for assessment of health-related quality of life elderly patients with cancer. Br J Cancer 2013;109:852–8. 10.1038/bjc.2013.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Boereboom C, Doleman B, Lund JN, et al. Systematic review of pre-operative exercise in colorectal cancer patients. Tech Coloproctol 2016;20:81–9. 10.1007/s10151-015-1407-1 [DOI] [PubMed] [Google Scholar]
- 57. Sun V, Raz DJ, Kim JY, et al. Barriers and facilitators of adherence to a perioperative physical activity intervention for older adults with cancer and their family caregivers. J Geriatr Oncol 2020;11:256–62. 10.1016/j.jgo.2019.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Prehabilitation, rehabilitation, and revocation in the army. Br Med J 1946;1:192–7. [PubMed] [Google Scholar]
- 59. Topp R, Ditmyer M, King K, et al. The effect of bed rest and potential of prehabilitation on patients in the intensive care unit. AACN Clin Issues 2002;13:263–76. 10.1097/00044067-200205000-00011 [DOI] [PubMed] [Google Scholar]
- 60. Ghignone F, van Leeuwen BL, Montroni I, et al. The assessment and management of older cancer patients: a SIOG surgical task force survey on surgeons’ attitudes. Eur J Surg Oncol 2016;42:297–302. 10.1016/j.ejso.2015.12.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-042960supp001.pdf (62.8KB, pdf)
bmjopen-2020-042960supp002.pdf (116.8KB, pdf)
bmjopen-2020-042960supp003.pdf (68.8KB, pdf)