ABSTRACT
Background
In lower-middle-income settings, growth faltering in the first 6 mo of life occurs despite exclusive breastfeeding.
Objective
The aim was to test the efficacy of an approach to improve the dietary adequacy of mothers during lactation and thus improve the growth of their infants.
Methods
Eligible mother–infant dyads (infants ≤7 d of age) were randomly assigned to either intervention or control groups. Mothers in the intervention group received snacks that were to be consumed daily, which provided 600 kcal of energy—with 25–30% of energy derived from fats (150–180 kcal) and 13% of energy from protein (80 kcal). Micronutrients were supplemented as daily tablets. We provided counseling on breastfeeding and infant-care practices to mothers in both groups. The primary outcome was attained infant length-for-age z scores (LAZ) at 6 mo of age. Secondary outcomes included exclusive breastfeeding proportion reported by the mother, maternal BMI and midupper arm circumference (MUAC), hemoglobin concentrations in mothers and infants, and the proportion of anemic infants at 6 mo of age.
Results
We enrolled 816 mother–infant dyads. The intervention did not achieve a significant effect on LAZ at 6 mo (adjusted mean difference: 0.09; 95% CI: −0.03, 0.20). Exclusive breastfeeding at 5 mo was higher (45.1% vs. 34.5%; RR: 1.31; 95% CI: 1.04, 1.64) in the intervention group compared with the controls. There were no significant effects on mean hemoglobin concentration or the proportion of anemic infants at 6 mo of age compared with the control group. We noted significant effects on maternal nutritional status (BMI, MUAC, hemoglobin concentration, and proportion anemic).
Conclusions
Postnatal supplementation of 600 kcal energy, 20 g protein, and multiple micronutrients daily to lactating mothers did not affect infant LAZ at age 6 mo. Such supplementation may improve maternal nutritional status. This trial was registered at Clinical Trials Registry–India as CTRI/2018/04/013095.
Keywords: maternal nutrition, micronutrients, lactation, infant growth, maternal health, randomized controlled trial, India
Introduction
The first 6 mo of life epitomize a transition from the neonatal period to childhood—during which growth and neurologic and immunologic development occur rapidly (1). It is assumed that adequate nutrition is ensured during the first 6 mo of life by breastfeeding; however, recent evidence suggests that undernutrition occurs before this time and is associated with increased risk of mortality and growth failure in later life (2–4). High rates of undernutrition (20–30%) among 0- to 6-mo-old infants were also reported in the Indian National Family Health Survey-4 (2, 5).
Exclusive breastfeeding in the first 6 mo of life is beneficial for survival, a reduction in infections, and optimal neural development (6–8). However, a recent systematic review and meta-analysis of 35 published studies showed no significant beneficial effects of promoting breastfeeding on weight or length attained by children (9). A substantial proportion of infants experience growth faltering during the first 6 mo of life in India and other Southeast Asian countries (10). This faltering occurs even in infants who do not manifest low weight and length at birth, which indicates that environmental factors contribute to early growth failure—making a compelling case for considering interventions beyond the promotion of exclusive breastfeeding.
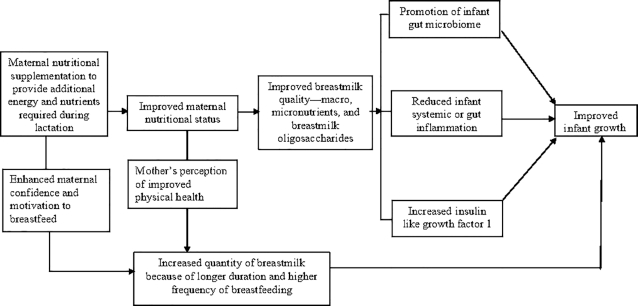
The contribution of maternal nutritional status and dietary intake to growth faltering may be far more important than is currently assumed. It is important to note that pre-existing deficiencies are common, as ∼20% of Indian mothers have a low BMI and ∼50% exhibit anemia and other micronutrient deficiencies (5, 11). Lactation increases nutritional demands, and this may further exacerbate the gaps in the nutrient adequacy of Indian mothers (12). Given the overwhelming advantages of exclusive breastfeeding, a strategic option to increase nutrient intake by infants during their first 6 mo of life would be to improve nutrient intake of the mothers. Studies have shown that, while breast-milk output is largely unaffected by maternal factors, macronutrient and micronutrient composition of breast milk beyond 3 mo of age might be affected by maternal nutritional status and dietary intake (13, 14). More specifically, while protein, lactose, and fat concentrations in breast milk remain relatively stable, maternal supplementation may influence fatty acid composition, especially of the PUFAs (e.g., DHA), as well as concentrations of thiamin; vitamin B-12; riboflavin; vitamins B-6, A, D, E, and K; iodine; and selenium (15). The fact that this possibility of altering breast-milk composition through maternal nutritional supplementation coincides with the timing of infant growth faltering raises an interesting point that high-quality nutritional supplements for the mothers may help in improving linear growth in infants. In addition to potentially improving breast-milk quality, supplementation may also improve maternal perception of physical health, and enhance a woman's confidence and motivation to breastfeed. In Figure 1, we present a conceptual framework that illustrates the pathways through which maternal supplementation during lactation might influence infant growth outcomes.
FIGURE 1.
Conceptual model illustrating pathways by which supplementation during lactation might influence infant growth.
The present study is primarily aimed at testing the efficacy of an approach designed to improve dietary adequacy by supplementing additional energy, protein, and micronutrients during lactation. Through the administration of high-quality nutritional supplements to lactating mothers in the first 6 mo postpartum, we hoped to improve the growth of the infants at 6 mo of life. Additionally, the effects on maternal BMI, hemoglobin concentration, and the proportion with anemia were assessed in mothers and infants after the 6-mo supplementation period.
Methods
Study setting, design, and participants
We conducted an individually randomized, controlled efficacy trial in low-resource settings of urban Delhi, India. Study subjects were mother–infant dyads, with infants initiated into breastfeeding and enrolled within 7 d of birth. Infants whose mothers had died or did not remain with the infant, those not initiated into breastfeeding by 7 d of age, those whose mothers exhibited chronic illness requiring prolonged medical treatment, infants requiring prolonged medical attention, those with major congenital malformations, and mother–infant dyads who were likely to move out of the study site within 6 mo were excluded from the study.
Screening and enrollment
A door-to-door survey was conducted to identify pregnant women and infants ≤7 d of age. The women identified were followed up until delivery, with more frequent contacts in the third trimester. For facility births, the screening and enrollment team visited the family once the infant arrived at home; while for those who delivered at home, the team visited as soon as possible after birth. During screening, the team member explained the study to the mother and family members and—for those who were willing—consent for screening was obtained from the mother. The mother and infant were assessed for eligibility and, if eligible, the screening and enrollment team requested group assignment through a Web-based system.
Randomization, allocation, and blinding
Eligible mother–infant dyads were randomly assigned to either the intervention or control group through a randomization list using blocks of variable length. The list was prepared offsite by a statistician based at the WHO, Geneva, Switzerland, who was not otherwise involved with the study. The allocation was via a Web-based system. A baseline form containing socioeconomic characteristics of the family was filled out for enrolled participants. The team obtained anthropometric measurements for infants [length, weight, midupper arm circumference (MUAC), and head circumference] and their mothers (height and weight). Only 1 mother–infant dyad was enrolled from a household. Although blinding of the study teams was not possible, the supplement delivery and counseling teams had limited interaction with the independent outcome-ascertainment team.
Study interventions
Mothers in the intervention group were provided a food supplement in the form of a snack to be consumed daily that provided 600 kcal with 25–30% of energy (150–180 kcal) from fats and 13% of energy from proteins (80 kcal). The snack contained 20 g of protein from a mix of plant- and animal-source proteins, with ∼30% (5.4–6 g) of the protein coming from a dairy source. Micronutrient supplementation was provided for a period of 180 d as daily tablets that provided 80–100% of the RDA of vitamins A, D, E, C, B-6, B-12, and C; thiamin; riboflavin; niacin; folate; iron; zinc; iodine; selenium; and copper (16).
The food supplements were prepared by Hungry Foal (https://www.hungryfoal.com/), a for-profit organization located in Gurugram, Haryana, India, and provided in the form of locally acceptable snacks. The names and ingredients of the snacks are depicted in Table 1. The snacks were pretested for acceptability among women in the study population before study initiation, and ultimately 5 sweet and savory snacks were selected for use in the study. Mothers were given the snack of their choice, with an option to change their preference at the time of weekly replenishment. To minimize intrahousehold sharing, snacks were promoted to be used exclusively by lactating mothers, with labels portraying a lactating woman. Timing of the mother's meals was assessed, and the optimal time for the consumption of snacks was negotiated at enrollment such that they did not replace her regular meals. The composition of multiple micronutrient (MMN) tablets was similar to the UNICEF/WHO/United Nations University international MMN preparation (UNIMMAP), and tablets were donated by The Vitamin Angel Alliance, Inc. (Vitamin Angels), California (https://www.vitaminangels.org/) (17). The composition of the MMN tablets is shown in Supplemental Table 1.
TABLE 1.
Ingredients in the culturally acceptable snacks used in the present study1
| Snack name | Ingredients |
|---|---|
| Choco Energy Bites (Hungry Foal) | Oats, sugar, malt, vegetable oil, milk solids, liquid glucose, peanuts, almond, cocoa powder |
| Panjeeri (Hungry Foal)2 | Wheat flour, sugar, vegetable oil, milk solids, fox nuts, almond, peanuts |
| Jeera crackers (Hungry Foal)3 | Wheat flour, water, oats, vegetable oil, milk solids, sugar, cumin, salt |
| Nut mixture | Oats, peanuts, rice flakes, honey, raisins, soyabean oil, milk solids, almonds, salt, spices |
| Biscuit | All-purpose wheat flour, sugar, edible vegetable fat, starch, soya protein isolate, milk solids, inverted sugar syrup, cream powder, cocoa powder |
The shelf life of each of the snacks was 90 d.
Panjeeri: traditional snack considered as a nutritional supplement for lactating mothers.
Jeera crackers: name derived from the ingredient “cumin” (Jeera in the local language) because of its predominant taste in the snack.
During monthly visits, the counseling team visited the control- and intervention-group mothers and provided counseling on the importance of exclusive breastfeeding, infant-care practices that included early care-seeking for illness, maternal postpartum care, and optimal nutrition for the mother. This team also provided lactation-education support to mothers to promote exclusive breastfeeding. Mothers in both groups were counseled on the availability of iron–folic acid, calcium, and vitamin D through the national program (12, 18). Additionally, mothers in the intervention group were counseled monthly by nutritionists on the importance of supplement intake; and suboptimal intakes were discussed to identify any barriers to appropriate intake. The supplement-delivery team visited households to provide snack packets for 1 wk and MMN tablets for 1 mo to mothers in the intervention group. During the weekly visits, the empty snack packets were also collected. Data on compliance with respect to MMN tablets were collected each month through a tablet count.
Sample size
Assuming an SD of 0.20 (for a 0.53-cm length, 1 SD = 2.67 cm) (19) for mean differences in attained length-for-age z scores (LAZ) at 6 mo of infant age between the intervention and control groups, 80% power, a 1-sided 5% ɑ level, and 10% loss to follow-up, we required a total of 340 infants per group—i.e., a total of 680 mother–infant pairs. Based on the observation of a higher-than-assumed (∼15–20%) loss to follow-up due to outmigration, the investigators approached the Technical Advisory Group (TAG). The TAG then recommended increasing the sample size by 20% to ensure adequate statistical power, and the sample size was revised to 816—i.e., 408 each in the control and intervention groups.
Outcomes and their ascertainment
The primary outcome was attained LAZ at 6 mo of age. The secondary outcomes were attained infant weight-for-age z score (WAZ), weight-for-length z score (WLZ), MUAC z score (MUAC-Z), and head circumference z score at 6 mo of age; the proportion of infants showing stunting (LAZ < −2), wasting (WLZ < −2), and underweight (WAZ −2) at 6 mo of age; and the changes in LAZ, WLZ, and MUAC-Z at 0–3 and 4–6 mo of age.
Additional secondary outcomes were maternally reported exclusive breastfeeding proportion at 1, 3, and 5 mo of infant age; maternal BMI and MUAC at 6 mo of infant age; and hemoglobin concentration in mothers and infants and the proportion with anemia at 6 mo of infant age. Dietary intake by both the intervention- and control-group mothers was assessed using 24-h dietary recall at 3 mo of infant age.
Outcomes were assessed by an independent outcome-ascertainment team that was kept unaware of group allocation in order to reduce measurement bias. The team was trained and standardized in anthropometric measurements, and inter- and intraobserver standardization exercises were conducted at the beginning of the study and at 3-mo intervals thereafter. The team visited the households in pairs and performed anthropometric assessments at monthly intervals, from enrollment until 6 mo of infant age. Weights and lengths were taken by a pair of workers using digital weighing scales (model 354; Seca) and infantometers (model 417; Seca) to the nearest 10 g and 0.1 cm, respectively. Head circumference and MUAC were quantified using measuring tapes (model 212; Seca). The team also measured maternal height and weight at 6 mo of infant age using Seca-213 stadiometers to the nearest 0.1 cm and Salter 9509 weighing scales to the nearest 0.1 kg. Data on breastfeeding were determined by nutritionists through a 24-h recall using a structured questionnaire. Blood samples at the end of the 6-mo intervention period were collected from the mothers and their infants at home by trained phlebotomists. The anemia assessment was performed using capillary blood with a HemoCue Hb 201 Plus analyzer (20). All of the study staff received training in Good Clinical Practice guidelines.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Centre for Health Research and Development, Society for Applied Studies, India. Written informed consent was obtained in the local language from the caregivers before enrollment, and the study was registered in Clinical Trials Registry–India (CTRI/2018/04/013095).
Statistical analysis
We performed all of the analyses using STATA, version 16.0 (StataCorp). The mean ± SD or median (IQR) was calculated for continuous variables and proportions calculated for categorical variables. Comparisons of means, medians, and proportions by groups were used to assess whether the randomization scheme resulted in comparability between groups. For each participant, compliance with regard to snacks or MMN tablets was presented as a percentage (%) and was calculated as total packets or tablets of MMNs consumed divided by supplement packets or MMN tablets that should have been consumed, and multiplying the resulting fraction by 100. Mothers with hemoglobin <12 g/dL and infants with hemoglobin <11 g/dL were considered to have anemia (21). Exclusive breastfeeding was defined as the infants having access to no other food or drink (including water), except for breast milk (including expressed milk), but with allowances for the infants to receive medicines, vitamins, and minerals (22).
Our primary analysis consisted of the comparison of outcomes between study groups and was based on the intention-to-treat principle. For binary outcomes, we used a generalized linear model (GLM) of the binomial family with a log-link function to calculate the effect size (RR ratio and 95% CI). For continuous outcomes, GLMs of the Gaussian family with an identity-link function were used to calculate the effect size (difference in means and 95% CIs). Purposive selection of variables for adjustments to the model was performed—that is, those variables that brought at least a 15% change in the univariate effect size between exposure (study groups) and outcome were considered for adjustment (23, 24). We included covariates in the model to narrow the CIs for the effect estimates. Although not prespecified, we evaluated effects of the intervention in subgroups in which supplementation might exert larger effects on infant growth. An exploratory analysis relating to the primary outcome (i.e., attained LAZ at 6 mo of infant age) was conducted with maternal height (<150 cm or ≥150 cm) and BMI categories (in kg/m2; <18.5, 18.5–24.9, or ≥25.0) and subgroups based on infant weight at enrollment (<2500 g or ≥2500 g) and stunting (LAZ < −2), wasting (WLZ < −2), and underweight (WAZ < −2) status at birth.
We also built generalized estimating equation (GEE) models for changes in anthropometric indices from enrollment to 3 mo and from 3 to 6 mo. This approach accounted for interdependence between multiple measurements in the same infant. We used GEE models of the Gaussian family with an identity-link function, an exchangeable correlation structure with intervention-by-time as interaction term. We also estimated mean 3-monthly changes of anthropometric indices as intervention-by-time interaction terms were not significant (P < 0.05). For all of the models, we specified a robust estimator of variance and an exchangeable correlation structure. In addition, for all of the analyses, effect sizes were reported with a 95% CI and a 2-sided P value <0.05 was considered to show statistical significance.
Results
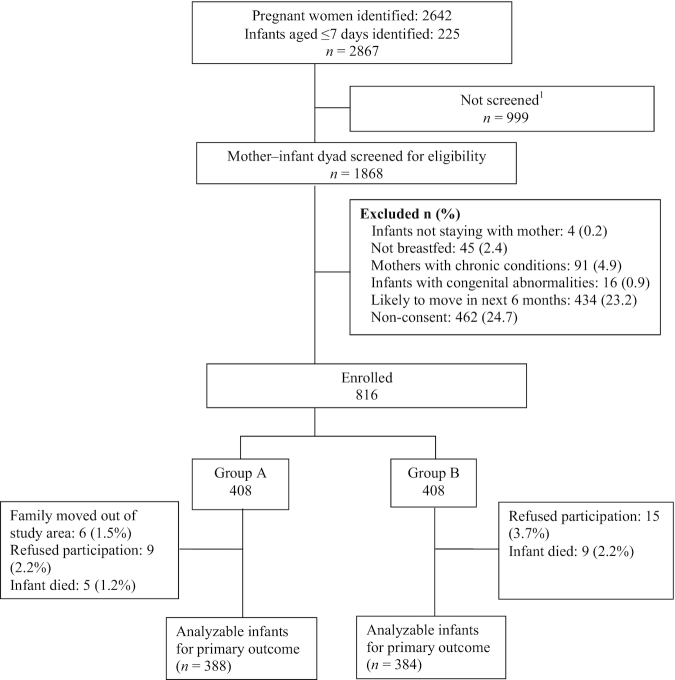
Between 9 May 2018 and 31 May 2019, a total of 2642 pregnant mothers and 225 infants <7 d of age were identified through a household survey. A total of 1868 mother–infant dyads were screened for eligibility (1705 from the 2642 pregnant mothers and 163 from the 225 infants aged <7 d who were identified). We excluded 1052 mother–infant dyads because they did not meet our eligibility criteria or the family did not provide consent to participate, and thus 816 were ultimately enrolled. These mother–infant dyads were randomly assigned to either the intervention (n = 408) or control (n = 408) group (Figure 2). Table 2 shows the baseline characteristics of the infants, their parents, and families. In the intervention and control groups, the mean ± SD infant age at enrollment was 4.76 ± 1.37 d versus 4.69 ± 1.39 d, and the mean LAZ was −1.24 ± 1.07 versus −1.27 ± 1.09, respectively. The maternal duration of schooling [median years (IQR): 8 (0, 10) vs. 8 (2, 10)] and BMI (mean ± SD: 22.70 ± 3.63 vs. 22.56 ± 3.56) were similar for both groups. Table 3 shows the data on compliance with the maternal snacks and micronutrient supplements among the intervention-group mothers. The mean ± SD days that 1 full packet of snacks was consumed over the 6-mo intervention period was 155.41 ± 35.19, and the proportion of mothers who consumed packets on >75% of the days was 83.8%. For MMNs, the mean ± SD number of days that 1 tablet was consumed was 146.54 ± 34.53 and the proportion of mothers who consumed a tablet for >75% of the days was 78.7%.
FIGURE 2.
Trial profile. 1Delivered outside Delhi (n = 487), infant died (n = 74), stillbirth (n = 16), mother and infant hospitalized during first 7 d of age (n = 140), not visited for screening as the required 816 mother–infant dyads were enrolled (n = 19), family not available (n = 226), refused participation (n = 37).
TABLE 2.
Baseline characteristics of enrolled infants and their families, by study group1
| Variables | Intervention (n = 408) | Control (n = 408) |
|---|---|---|
| Infant characteristics at enrollment (age 0–7 d) | ||
| Age in days at enrollment | 4.76 ± 1.37 | 4.69 ± 1.39 |
| Birth weight, reported or documented,2 kg | 2.77 ± 0.42 | 2.74 ± 0.44 |
| Gestational age,3 wk | 38.8 ± 1.66 | 38.7 ± 1.81 |
| Prematurity, n (%) | 30 (7.4) | 48 (11.8) |
| Low birth weight as <2500 g at enrollment, n (%) | 114 (28.0) | 111 (27.2) |
| Delivered at home, n (%) | 87 (21.3) | 70 (17.2) |
| Number of male infants, n (%) | 207 (50.7) | 217 (53.2) |
| Breastfeeding initiated <1 h of birth, n (%) | 117 (28.7) | 115 (28.2) |
| Age at initiation of breastfeeding, median (IQR), h | 2 (0, 6) | 2 (0, 5) |
| Weight,4 kg | 2.74 ± 0.43 | 2.74 ± 0.44 |
| Length,4 cm | 48.03 ± 2.07 | 47.94 ± 2.25 |
| LAZ using WHO Growth Standards | −1.24 ± 1.07 | −1.27 ± 1.09 |
| Stunting as <2 LAZ, n (%) | 93 (22.9) | 96 (23.5) |
| WLZ score using WHO Growth Standards5 | −1.00 ± 1.03 | −0.97 ± 1.06 |
| Wasting as <2 WLZ,5n (%) | 59 (15.7) | 51 (13.8) |
| WAZ score using WHO Growth Standards | −1.40 ± 1.03 | −1.40 ± 1.02 |
| Underweight as <2 WAZ, n (%) | 102 (25.1) | 104 (25.5) |
| BMI-Z using WHO Growth Standards | −1.24 ± 1.02 | −1.20 ± 1.03 |
| Midupper arm circumference, cm | 9.43 ± 0.84 | 9.41 ± 0.81 |
| Head circumference, cm | 33.16 ± 1.31 | 33.15 ± 1.54 |
| Sociodemographic characteristics | ||
| Wealth quintile, n (%) | ||
| Poorest | 87 (21.3) | 77 (18.8) |
| Very poor | 84 (20.6) | 79 (19.4) |
| Poor | 88 (21.6) | 75 (18.4) |
| Less poor | 75 (18.4) | 88 (21.6) |
| Least poor | 74 (18.1) | 89 (21.8) |
| Annual family income, median (IQR), US$ | 1580 (1580, 3160) | 1580 (1422, 3160) |
| Nuclear family, n (%) | 189 (46.3) | 167 (40.9) |
| Religion | ||
| Hindu, n (%) | 324 (79.4) | 326 (79.9) |
| Maternal characteristics | ||
| Age, y | 24.43 ± 3.74 | 24.54 ± 3.72 |
| Duration of schooling, median (IQR), y | 8 (0, 10) | 8 (2, 10) |
| Never been to school, n (%) | 118 (28.9) | 101 (24.8) |
| Homemakers, n (%) | 406 (99.5) | 404 (99.0) |
| BMI, kg/m2 | 22.70 ± 3.63 | 22.56 ± 3.56 |
| BMI <18.5 kg/m2, n (%) | 34 (8.4) | 43 (10.5) |
| BMI ≥25.0 kg/m2, n (%) | 99 (24.3) | 95 (23.3) |
| MUAC, cm | 24.4 ± 2.8 | 24.2 ± 2.8 |
| Height, cm | 151.25 ± 5.36 | 151.38 ± 5.99 |
| Height <150 cm, n (%) | 158 (38.7) | 162 (39.7) |
| Paternal characteristics | ||
| Age, y | 28.02 ± 4.32 | 28.21 ± 4.33 |
| Duration of schooling, median (IQR), y | 9 (5, 10.5) | 8 (5, 12) |
| Unemployed, n (%) | 21 (5.2) | 23 (5.6) |
Data are reported as means ± SDs unless stated otherwise. BMI-Z, BMI z score; LAZ, length-for-age z score; MUAC, midupper arm circumference; WAZ, weight-for-age z score; WLZ, weight-for-length z score.
Data available for 319 infants in the intervention group and 336 infants in the control group.
Documented gestational age (ultrasound/antenatal card/reported months of gestation at birth).
One mother–infant dyad in the intervention group refused anthropometric measurements after providing consent.
WLZ scores could not be calculated for 32 and 38 infants in the intervention and control groups, respectively, as length at enrollment was <45 cm. The WHO anthropometric z-score calculator has no provision to estimate WLZ for length <45 cm.
TABLE 3.
Compliance with maternal snacks and multiple micronutrients1
| Values | |
|---|---|
| One full supplement packet consumed by the mother in days, mean ± SD | 155.4 ± 35.1 |
| Number (%) of days where a full-packet supplement was consumed | |
| >75% | 342 (83.8) |
| 51–75% | 47 (11.5) |
| 26–50% | 12 (2.9) |
| ≤25% | 7 (1.7) |
| Daily micronutrient tablet consumed by the mother in days, mean ± SD | 146.5 ± 34.5 |
| Number (%) of days where 1 tablet of the micronutrient was consumed | |
| >75% | 321 (78.6) |
| 51–75% | 64 (15.6) |
| 26–50% | 8 (1.9) |
| ≤25% | 15 (3.6) |
n = 408 enrolled mother–infant dyads.
The mean ± SD LAZ at 6 mo in the intervention and control groups was −0.89 ± 1.12 and −1.0 ± 1.01, respectively, with no significant difference in effect on LAZ (adjusted mean difference: 0.09; 95% CI: −0.03, 0.20). We noted no significant effect of the intervention on other anthropometric outcomes at 6 mo of age. The changes in LAZ between enrollment to 3 mo (adjusted mean difference: 0.03; 95% CI: −0.07, 0.12) and 3 to 6 mo of age (adjusted mean difference: 0.06; 95% CI: −0.04, 0.16) were also similar (Table 4). Maternally reported exclusive breastfeeding was higher in the intervention group relative to the control group at 5 mo (45.1% vs. 34.5%; RR: 1.31; 95% CI: 1.04, 1.64; P = 0.02) (Table 5). There were no significant effects of the intervention on mean hemoglobin concentration or proportion with anemia among infants in the 2 study groups.
TABLE 4.
Effect of maternal nutritional supplementation during lactation on infant growth1
| Measure | Unadjusted risk ratio or unadjusted mean difference (95% CI) | Adjusted risk ratio or adjusted mean difference (95% CI)2 | ||
|---|---|---|---|---|
| Intervention (n = 408) | Control (n = 408) | |||
| Attained anthropometric measures at 6 mo of infant age3 | ||||
| Primary outcome | ||||
| LAZ at 6 mo (n = 388, 384) | −0.89 ± 1.12 | −1.0 ± 1.01 | 0.10 (−0.05, 0.25) | 0.09 (−0.03, 0.20) |
| Secondary outcomes | ||||
| WAZ at 6 mo (n = 388, 384) | −1.08 ± 1.16 | −1.17 ± 1.07 | 0.08 (−0.08, 0.24) | 0.08 (−0.05, 0.21) |
| WLZ at 6 mo (n = 388, 384) | −0.63 ± 1.16 | −0.66 ± 1.06 | 0.03 (−0.12, 0.19) | 0.04 (−0.11, 0.20) |
| Showed stunting at 6 mo (n = 388, 384) | 59 (15.2) | 58 (15.1) | 1.00 (0.70,1.45) | 1.09 (0.75,1.59) |
| Showed wasting at 6 mo (n = 388, 384) | 47 (12.1) | 36 (9.4) | 1.29 (0.84, 1.99) | 1.29 (0.83, 1.99) |
| Underweight at 6 mo (n = 388, 384) | 73 (18.8) | 77 (20.1) | 0.94 (0.68, 1.29) | 0.96 (0.70, 1.33) |
| MUAC z score at 6 mo (n = 388, 384) | −0.53 ± 1.06 | −0.56 ± 0.99 | 0.03 (−0.12, 0.17) | 0.03 (−0.11, 0.16) |
| Head circumference z score at 6 mo in cm, (n = 388, 384) | −1.39 ± 1.01 | −1.45 ± 1.06 | 0.06 (−0.09, 0.20) | 0.04 (−0.09, 0.17) |
| Change in anthropometric measures over the 6-mo period4 | ||||
| Change in LAZ at 0–3 mo (n = 386, 367) | 0.16 ± 0.72 | 0.15 ± 0.74 | 0.01 (−0.09, 0.11) | 0.03 (−0.07, 0.12) |
| Change in LAZ at 3–6 mo (n = 378, 361) | 0.16 ± 0.66 | 0.11 ± 0.66 | 0.04 (−0.06, 0.14) | 0.06 (−0.04, 0.16) |
| Mean 3-monthly change in LAZ | 0.03 (−0.04, 0.09) | 0.04 (−0.02, 0.10) | ||
| Change in WLZ at 0–3 mo (n = 358, 334) | 0.48 ± 1.21 | 0.45 ± 1.38 | 0.03 (−0.13, 0.19) | 0.04 (−0.10, 0.19) |
| Change in WLZ at 3–6 mo (n = 378, 361) | −0.18 ± 0.82 | −0.14 ± 0.89 | −0.03 (−0.19, 0.13) | −0.05 (−0.19, 0.10) |
| Mean 3-monthly change in WLZ | 0.00 (−0.10, 0.10) | 0.00 (−0.08, 0.08) | ||
| Change in MUAC at 0–3 mo (n = 386, 367) | 3.29 ± 1.07 | 3.24 ± 1.09 | 0.05 (−0.08,0.18) | 0.06 (−0.07,0.19) |
| Change in MUAC at 3–6 mo (n = 378, 361) | 0.76 ± 0.74 | 0.80 ± 0.74 | −0.05 (−0.18, 0.08) | −0.04 (−0.17, 0.09) |
| Mean 3-monthly change in MUAC | 0.00 (−0.08,0.08) | 0.01 (−0.07, 0.09) | ||
Data are n (%) or mean ± SD, with outcome measures of adjusted risk ratios for the numbers of infants showing stunting, wasting, or underweight, and adjusted mean differences for other growth parameters. None of the P values (2-sided) were significant. BMI-Z, BMI z score; GEE, generalized estimating equation; GLM, generalized linear model; MUAC, midupper arm circumference; LAZ, length-for-age z score; WAZ, weight-for-age z score; WLZ, weight-for-length z score.
Adjusted for wealth quintile, gestational age, and LAZ and BMI-Z at baseline.
Analysis using GLM.
Analysis using GEE with Gaussian family, identity link, exchangeable correlation; adjusted for wealth quintile, BMI-Z at baseline, and gestational age for LAZ changes; adjusted for wealth quintile, LAZ scores, BMI-Z at baseline and gestational age for WLZ changes; adjusted for wealth quintile, LAZ, and BMI-Z at baseline and gestational age for MUAC changes.
TABLE 5.
Effect of maternal nutritional supplementation during lactation on breastfeeding practices, maternal BMI, and biochemical outcomes1
| Measure | Adjusted risk ratio or adjusted mean difference (95% CI)2 | ||
|---|---|---|---|
| Intervention (n = 408) | Control (n = 408) | ||
| Breastfeeding practices | |||
| Exclusively breastfed (maternally reported) | |||
| At 1 mo (n = 390, 392) | 325 (83.3) | 319 (81.4) | 1.02 (0.88, 1.19) |
| At 3 mo (n = 392, 383) | 274 (69.9) | 244 (63.7) | 1.10 (0.92, 1.30) |
| At 5 mo (n = 388, 374) | 175 (45.1) | 129 (34.5) | 1.31 (1.04, 1.64)3 |
| Maternal anthropometry at 6 mo | |||
| BMI in kg/m2 (n = 386, 381) | 22.71 ± 3.92 | 22.13 ± 4.03 | 0.37 (0.09, 0.64)3 |
| MUAC in cm (n = 386, 381) | 24.98 ± 2.89 | 24.47 ± 3.0 | 0.36 (0.12, 0.60)3 |
| BMI <18.5 (n = 386, 381) | 45 (11.7) | 63 (16.5) | 0.76 (0.50, 1.15) |
| BMI ≥25 (n = 386, 381) | 100 (25.9) | 91 (23.9) | 0.96 (0.71, 1.28) |
| Mothers’ Hb concentration and proportion with anemia at 6 mo | |||
| Hb in g/dL (n = 371, 351) | 11.99 ± 1.16 | 11.62 ± 1.38 | 0.37 (0.19, 0.56)3 |
| Proportion anemic with Hb <12 g/dL (n = 371, 351) | 147 (39.6) | 195 (55.6) | 0.71 (0.58, 0.88)3 |
| Infants’ Hb concentration and proportion with anemia at 6 mo | |||
| Hb in g/dL (n = 368, 351) | 10.50 ± 1.22 | 10.37 ± 1.15 | 0.13 (−0.05, 0.30) |
| Proportion anemic with Hb <11 g/dL (n = 368, 351) | 242 (65.8) | 242 (68.9) | 0.95 (0.80, 1.14) |
Data are n (%) or mean ± SD, with outcome measures of adjusted risk ratio for proportion with breastfeeding outcomes, maternal BMI <18.5 kg/m2, and proportion of infants and mother with anemia; adjusted mean differences for maternal BMI, maternal MUAC, and hemoglobin concentration in infants and mothers. Hb, hemoglobin; MUAC, midupper arm circumference.
For breastfeeding proportion/hemoglobin concentration/proportion with anemia of both mother and infants, no adjustments were made; maternal BMI was adjusted for wasting at enrollment, maternal BMI at enrollment, and maternal MUAC at baseline; maternal MUAC was adjusted for maternal BMI at enrollment and maternal MUAC at baseline.
Denotes significant 2-sided P value (<0.05).
There were some effects of the intervention on the nutritional status of the mothers. In the intervention group, the mean ± SD maternal BMI remained the same during the period of supplementation—that is, 22.70 ± 3.63 at baseline and 22.71 ± 3.92 at an infant age of 6 mo. However, we noted a decline in BMI in the control-group mothers from 22.56 ± 3.56 at baseline to 22.13 ± 4.03 at 6 mo of infant age. The adjusted mean difference in BMI between the 2 groups at the end of 6 mo of supplementation was 0.37 (95% CI: 0.09, 0.64; P = 0.009). The mean ± SD maternal MUAC in the intervention and control groups was 24.98 ± 2.89 cm and 24.47 ± 3.0 cm, respectively, with an adjusted mean difference of 0.36 cm (95% CI: 0.12, 0.60 cm; P = 0.003) (Table 5). Mothers in the intervention group had a higher hemoglobin concentration (mean ± SD: 11.99 ± 1.16 vs. 11.62 ± 1.38 g/dL) than the control group, with an adjusted mean difference of 0.37 g/dL (95% CI: 0.19, 0.56 g/dL; P < 0.001). The anemia (hemoglobin <12 g/dL) rates in the intervention-group mothers were lower than in the control group at 6 mo of infant age (39.6% vs. 55.6%; RR: 0.71; 95% CI: 0.58, 0.88; P = 0.002) (Table 5).
In a post hoc exploratory analysis, there was no evidence of a significant effect of the intervention on LAZ at 6 mo of age within the maternal height and BMI categories, or within subgroups based on infant weight at enrollment, or on stunting, wasting, or underweight status at birth.
Table 6 depicts the findings of the maternal 24-h dietary recalls in both the intervention and control groups at 3 mo of infant age. The mean ± SD intake of calories in kilocalories (2247 ± 775 vs. 2088 ± 874), carbohydrates in grams (304 ± 115 vs. 284 ± 130), proteins in grams (70 ± 26 vs. 64 ± 27), and fats in grams (72 ± 37 vs. 69 ± 41) was slightly higher in the intervention-group mothers compared with the control group; all differences except for fats were statistically significant.
TABLE 6.
Twenty-four-hour dietary recalls of mothers enrolled in the study at 3 mo of infant age1
| Intervention2 | Control | |
|---|---|---|
| Number of mothers for whom dietary recalls conducted | 395 | 393 |
| Total calories in kilocalories consumed3 | 2247 ± 775 | 2088 ± 874 |
| Total carbohydrate in grams consumed3 | 304 ± 115 | 284 ± 130 |
| Total protein in grams consumed3 | 70 ± 26 | 64 ± 27 |
| Total fat in grams consumed | 72 ± 37 | 69 ± 41 |
Data are presented as mean ± SD.
The supplement is included in the totals for the intervention group.
Statistically significant at 2-sided P < 0.05.
Discussion
In this study, we found that maternal supplementation in the first 6 mo postpartum led to a small, nonsignificant improvement in infant linear growth at 6 mo of age. However, supplementation resulted in improvements in maternal health outcomes: BMI, MUAC, hemoglobin concentration, and a lower rate of anemia.
Investigators have over the last 3 decades examined the effect of nutritional supplementation of lactating mothers with respect to breast-milk volume, composition, and infant growth, but these studies have not been conclusive (25–28). Previous studies have shown an effect of maternal supplementation during pregnancy and in the first 6 mo of postnatal life on infant growth outcomes (29–32). The findings from these studies suggested an improvement in birth size/length but did not show a large improvement in infant linear growth or other anthropometric outcomes at 6 mo of age. In a recent network meta-analysis, it was shown that in comparison to standard-of-care, fortified lipid-based nutrient supplementation during pregnancy and the first 6 mo of postnatal life resulted in a mean difference of 0.08 (95% CI: −0.12, 0.29) in LAZ at 6 mo (33). This pooled estimate is similar to the findings of the current study in which we focused only on maternal supplementation during the first 6 mo of lactation.
Evidence from the extant literature suggests an improvement in birth length due to maternal supplementation during pregnancy; however, no such effect of continued maternal supplementation in the lactation period on infant growth in the first 6 mo of life has been noted. We posit the following potential explanations for this. First, the supplementation may not alter breast-milk composition significantly. Although maternal supplementation can increase breast-milk thiamin, riboflavin, iodine, selenium, and vitamin B-6, A, D, E, and K concentrations, most of these are type I nutrients. Other key growth-limiting micronutrients, such as zinc, phosphorus, and magnesium, in breast milk are stable or refractory to maternal intake or supplementation (15, 34, 35). Type I nutrients are those that are required to maintain normal bodily functions and consequently their deficiencies lead to characteristic clinical symptoms associated with dysfunction of a particular biochemical pathway. On the other hand, type II nutrients are those that are required for optimal growth of lean tissues (36, 37).
The available literature also suggests that the protein concentrations are stable from 2 to 6 mo of infant age and unaffected by maternal nutritional status (15). Similarly, the lactose concentrations are fairly consistent, with a small CV (2–4%), and they are independent of maternal diet and nutritional status (15). Fat concentrations also remain stable in mature milk. However, the fatty acid composition—especially of the PUFAs (e.g., DHA)—depends upon the nutritional intake and status of the mother (15). The concentrations of PUFAs in breast milk have also been shown to be positively associated with infant growth (both weight and length) during the first 6 mo of life (38). Second, there may be a need for additional interventions that focus on the prevention of infections and the promotion of optimal infant health care. Third, the lack of any observed effects of supplementation on linear growth may be due to gut infection leading to both local and systemic inflammation, as this study was performed in a setting with poor environmental hygiene, food, and water quality (39, 40). Furthermore, ∼40% of the mothers were of short height (<150 cm), and therefore null effects of supplementation due to intergenerational adversity cannot be ruled out.
Lactation leading to increased nutrient demands and modest improvements in maternal BMI, MUAC, and hemoglobin concentrations in mothers in the intervention group call for focused nutritional programs for lactating mothers. A higher proportion of exclusive breastfeeding due to maternal supplementation has also been shown in previous randomized controlled trials (41, 42). One of the common reasons for introducing food other than breast milk to low-middle-income settings is the perception of inadequate maternal milk production (43–45). It is thus possible that maternal supplementation leads to increased confidence in the mother regarding her milk production. This finding is of particular importance in India—where breastfeeding practices remain suboptimal—and from a public health perspective, it underscores the relevance of coupling nutritional programs for lactating mothers with programs aimed at the promotion of exclusive breastfeeding. An important issue to consider is that infants who are predominantly or exclusively breastfed have a different metabolic profile and micronutrient status relative to those who are either not breastfed or breastfed less frequently. Also, morbidity could be reduced in infants with greater exclusive breastfeeding. In our study, the possibility that the increase in the proportion of infants who were exclusively breastfed in mothers who received nutrient supplementation might influence infant growth outcomes cannot therefore be ruled out.
We did not in the present study find an effect of maternal supplementation on infant hemoglobin status or the proportion of infants with anemia after 6 mo of supplementation. A recent review suggested that breast-milk concentrations of micronutrients and minerals related to iron metabolism and erythropoiesis (such as iron, folate, copper, and zinc) were unaltered despite maternal supplementation (15). Additionally, concentrations of thiamin and vitamin B-12 were increased in breast milk upon maternal supplementation only in the case of maternal dietary insufficiency (15). These findings could possibly explain the lack of an observed effect on infant hemoglobin and anemia status despite an improvement in maternal hemoglobin and reduction in anemia status.
The strengths of our study included a rigorous study design and low loss-to-follow-up rates. Outcomes were assessed by a trained and standardized team. Although it was difficult to ensure complete blinding due to the nature of the intervention, there was minimal contact between supplement delivery and independent outcome-ascertainment teams. Our study entailed a few limitations. First, while bias due to lack of blinding was, to some extent, alleviated by having different teams measuring the outcomes and delivering the interventions, this did not remove any bias introduced before the outcomes were measured. The possibility of behavioral modification(s)—such as those related to exclusive breastfeeding—could therefore not be discounted. Such behavioral modifications might have potentially introduced other actions that could have influenced child growth in either direction. In addition, the proportion of infants who were exclusively breastfed might have been overreported, as these were ascertained through maternal recall. Second, while direct observation of the consumption of snacks and MMNs would have been ideal, the assessment of compliance with respect to snacks and MMNs was reported. Third, this study had limited power to detect a small effect size. For the 0.10-SD difference in the mean LAZ that we found in our study, the current sample size provided ∼40% power for detecting a difference. Finally, the 24-h dietary recalls indicated some displacement of the regular meals; however, compliance with the supplement was high—84% of the mothers consumed a full-supplement packet for >75% of days.
In conclusion, the present study does not support our hypothesis that additional supplementation of energy, protein, and micronutrients during lactation has a modest impact on the growth of infants during their first 6 mo of life. We, however, noted a trend towards improved linear growth that failed to reach statistical significance. The findings support the provision of nutritional supplementation to lactating mothers in order to improve infant growth.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the core support provided by the Department of Maternal, Newborn, Child, and Adolescent Health, and the WHO, Geneva (WHO Collaborating Centre IND-158). We also acknowledge the support extended by the Knowledge Integration and Technology Platform (KnIT), a Grand Challenges Initiative of the Department of Biotechnology and Biotechnology Industry Research Assistance Council (BIRAC) of the Government of India, and the Bill & Melinda Gates Foundation. We thank Dr. Parul Christian for her technical guidance and support throughout this study. We acknowledge the support of Ms. Kalpana Beesabathuni from Sight and Life for her technical input in finalizing the nutritional content of maternal snacks. We are also thankful to Hungry Foal, based at Gurugram, Haryana, India, for manufacturing the snacks used in this study. The authors’ responsibilities were as follows—ST, NB, RPU, RC, and RB: designed the research; ST, HB, TK, and GK: conducted the research; AVK, PD, and SD: provided technical inputs in designing the supplements and provided training to the study team; RPU, RC, ST, BK, and RB: analyzed the data or performed statistical analysis; ST, RPU, RC, and NB: prepared the manuscript; and all authors: were responsible for the final content of this manuscript and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
The present study received funding from the Bill & Melinda Gates Foundation through the Biotechnology Industry Research Assistance Council (BIRAC) of the Department of Biotechnology, Government of India (OPP1177843). We acknowledge the support of the Vitamin Angel Alliance, Inc. (Vitamin Angels), California, for donating the micronutrient supplements for use in the study. The funders had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: GEE, generalized estimating equation; GLM, generalized linear model; LAZ, length-for-age z score; MMN, multiple micronutrient; MUAC, midupper arm circumference; MUAC-Z, midupper arm circumference z score; TAG, Technical Advisory Group; WAZ, weight-for-age z score; WLZ, weight-for-length z score.
Contributor Information
Sunita Taneja, Center for Health Research and Development, Society for Applied Studies, New Delhi, India.
Ravi Prakash Upadhyay, Center for Health Research and Development, Society for Applied Studies, New Delhi, India.
Ranadip Chowdhury, Center for Health Research and Development, Society for Applied Studies, New Delhi, India.
Anura V Kurpad, Department of Physiology, St John's Medical College, Bengaluru, India.
Himani Bhardwaj, Center for Health Research and Development, Society for Applied Studies, New Delhi, India.
Tivendra Kumar, Center for Health Research and Development, Society for Applied Studies, New Delhi, India.
Pratibha Dwarkanath, Department of Physiology, St John's Medical College, Bengaluru, India.
Beena Bose, Department of Physiology, St John's Medical College, Bengaluru, India.
Sarita Devi, Department of Physiology, St John's Medical College, Bengaluru, India.
Gunjan Kumar, Center for Health Research and Development, Society for Applied Studies, New Delhi, India.
Baljeet Kaur, Center for Health Research and Development, Society for Applied Studies, New Delhi, India.
Rajiv Bahl, Department of Maternal, Newborn, Child, and Adolescent Health, World Health Organization, Geneva, Switzerland.
Nita Bhandari, Center for Health Research and Development, Society for Applied Studies, New Delhi, India.
Data Availability
The data described in the manuscript, code book, and analytic code will not be made available. The organization conducting the trial (Society for Applied Studies, India) is a collaborator in the Healthy Birth, Growth, and Development Knowledge Integration (HBGDKi) initiative launched by the Bill & Melinda Gates Foundation, and the data generated from the study will be shared as part of the HBGDKi repository (https://github.com/HBGDki). However, individual requests can be considered on a case-by-case basis. The request for data should be accompanied by a detailed proposal describing the intended scientific question(s) to be addressed. Proposals should be submitted to Dr. Sunita Taneja (sunita.taneja@sas.org.in).
References
- 1. Mwangome M, Ngari M, Fegan G, Mturi N, Shebe M, Bauni E, Berkley JA. Diagnostic criteria for severe acute malnutrition among infants aged under 6 mo. Am J Clin Nutr. 2017;105(6):1415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choudhary TS, Srivastava A, Chowdhury R, Taneja S, Bahl R, Martines J, Bhan MK, Bhandari N. Severe wasting among Indian infants <6 months: findings from the National Family Health Survey 4. Matern Child Nutr. 2019;15(4):e12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mwangome MK, Fegan G, Fulford T, Prentice AM, Berkley JA. Midupper arm circumference at age of routine infant vaccination to identify infants at elevated risk of death: a retrospective cohort study in the Gambia. Bull World Health Organ. 2012;90:887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vygen SB, Roberfroid D, Captier V, Kolsteren P. Treatment of severe acute malnutrition in infants aged <6 months in Niger. J Pediatr. 2013;162(3):515–21, e3. [DOI] [PubMed] [Google Scholar]
- 5. International Institute for Population Sciences . National Family Health Survey (NFHS-4) 2012-14. [Internet].Mumbai (India): International Institute for Population Sciences (IIPS) and Macro International; 2009. [cited 2020 May 10]. Available from: http://www.rchiips.org/nfhs/nfhs4.shtml. [Google Scholar]
- 6. Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;8:CD003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sankar MJ, Sinha B, Chowdhury R, Bhandari N, Taneja S, Martines J, Bahl R. Optimal breastfeeding practices and infant and child mortality: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):3–13. [DOI] [PubMed] [Google Scholar]
- 8. Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):14–19. [DOI] [PubMed] [Google Scholar]
- 9. Giugliani ER, Horta BL, Loret de Mola C, Lisboa BO, Victora CG. Effect of breastfeeding promotion interventions on child growth: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):20. [DOI] [PubMed] [Google Scholar]
- 10. Victora CG, de Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125(3):e473. [DOI] [PubMed] [Google Scholar]
- 11. Gonmei Z, Toteja GS. Micronutrient status of Indian population. Indian J Med Res. 2018;148(5):511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Indian Council of Medical Research . Nutrient requirements and Recommended Dietary Allowances for Indians. A report of the Expert Group of the Indian Council of Medical Research (2009); National Institute of Nutrition. [Internet]. Available from: http://icmr.nic.in/final/rda-2010.pdf. [Google Scholar]
- 13. Dewey KG, Heinig MJ, Nommsen LA, Lonnerdal B. Maternal versus infant factors related to breast milk intake and residual milk volume: the DARLING study. Pediatrics. 1991;87(6):829–37. [PubMed] [Google Scholar]
- 14. Nommsen LA, Lovelady CA, Heinig MJ, Lönnerdal B, Dewey KG. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: the DARLING study. Am J Clin Nutr. 1991;53(2):457–65. [DOI] [PubMed] [Google Scholar]
- 15. Dror DK, Allen LH. Overview of nutrients in human milk. Adv Nutr. 2018;9(Suppl 1):278S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. WHO/FAO . Vitamin and mineral requirement in human nutrition. 2nd ed[Internet]. Geneva (Switzerland): World Health Organization; 2004. Available from: https://apps.who.int/iris/bitstream/handle/10665/42716/9241546123.pdf. [Google Scholar]
- 17. UNICEF/UNU/WHO . Composition of a multi-micronutrient supplement to be used in pilot programmes among pregnant women in developing countries. New York: UNICEF; 1999. [Google Scholar]
- 18. Maternal Health Division; Ministry of Health and Family Welfare, Government of India . National guidelines for calcium supplementation during pregnancy and lactation. [Internet]. December 2014. Available from: http://www.nrhmhp.gov.in/sites/default/files/files/NG_calcium.pdf. [Google Scholar]
- 19. Bhandari N, Mazumder S, Bahl R, Martines J, Black RE, Bhan MK; Infant Feeding Study Group . An educational intervention to promote appropriate complementary feeding practices and physical growth in infants and young children in rural Haryana, India. J Nutr. 2004;134(9):2342. [DOI] [PubMed] [Google Scholar]
- 20. Hemocue Hb 201+ System . [cited 2020 16 Sep][Internet]. Available from: https://www.hemocue.com/-/media/hemocue-images/hemocuedotcom-images/product-images/hb/pdf-folders-etc/web-update-01092015.pdf. [Google Scholar]
- 21. World Health Organization . Hemoglobin concentrations for the diagnosis of anemia and assessment of severity. Vitamin and Mineral Nutrition Information System. [Internet]. Geneva (Switzerland): World Health Organization; 2011. Report No.: WHO/NMH/NHD/MNM/11.1. Available from: https://www.who.int/vmnis/indicators/haemoglobin.pdf. [Google Scholar]
- 22. Butte NF, Lopez-Alarcon MG, Garza C. Nutrient adequacy of exclusive breastfeeding for the term infant during the first six months of life. [Internet]. Geneva (Switzerland): World Health Organization; 2002. Available from: https://apps.who.int/iris/bitstream/handle/10665/42519/9241562110.pdf?ua=1. [Google Scholar]
- 23. Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. New York: Wiley; 2013. [Google Scholar]
- 25. Sosa R, Klaus M, Urrutia JJ. Feed the nursing mother, thereby the infant. J Pediatr. 1976;88(4 Pt 1):668–70. [DOI] [PubMed] [Google Scholar]
- 26. Whitehead RG, editor. Maternal diet, breast-feeding capacity, and lactational infertility. Report of a joint UNU/WHO workshop held in Cambridge, United Kingdom, 9–11 March 1981. Food Nutrition Bulletin, Suppl 6. Tokyo: United Nations University, World Health Organization; 1983. [Google Scholar]
- 27. Abe SK, Balogun OO, Ota E, Takahashi K, Mori R. Supplementation with multiple micronutrients for breastfeeding women for improving outcomes for the mother and baby. Cochrane Database Syst Rev. 2016;2:CD010647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Delgado-Noguera MF, Calvache JA, Bonfill Cosp X, Kotanidou EP, Galli-Tsinopoulou A. Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. Cochrane Database Syst Rev. 2015;7:CD007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ashorn P, Alho L, Ashorn U, Cheung YB, Dewey KG, Harjunmaa U, Lartey A, Nkhoma M, Phiri N, Phuka Jet al. . The impact of lipid-based nutrient supplement provision to pregnant women on newborn size in rural Malawi: a randomized controlled trial. Am J Clin Nutr. 2015;101(2):387. [DOI] [PubMed] [Google Scholar]
- 30. Ashorn P, Alho L, Ashorn U, Cheung YB, Dewey KG, Gondwe A, Harjunmaa U, Lartey A, Phiri N, Phiri TEet al. . Supplementation of maternal diets during pregnancy and for 6 months postpartum and infant diets thereafter with small-quantity lipid-based nutrient supplements does not promote child growth by 18 months of age in rural Malawi: a randomized controlled trial. J Nutr. 2015;145(6):1345. [DOI] [PubMed] [Google Scholar]
- 31. Mridha MK, Matias SL, Chaparro CM, Paul RR, Hussain S, Vosti SA, Harding KL, Cummins JR, Day LT, Saha SLet al. . Lipid-based nutrient supplements for pregnant women reduce newborn stunting in a cluster-randomized controlled effectiveness trial in Bangladesh. Am J Clin Nutr. 2016;103(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adu-Afarwuah S, Lartey A, Okronipa H, Ashorn P, Peerson JM, Arimond M, Ashorn U, Zeilani M, Vosti S, Dewey KG. Small-quantity, lipid-based nutrient supplements provided to women during pregnancy and 6 mo postpartum and to their infants from 6 mo of age increase the mean attained length of 18-mo-old children in semi-urban Ghana: a randomized controlled trial. Am J Clin Nutr. 2016;104(3):797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park JJH, Fang ML, Harari O, Dron L, Siden EG, Majzoub R, Jeziorska V, Thorlund K, Mills EJ, Bhutta ZA. Association of early interventions with birth outcomes and child linear growth in low-income and middle-income countries: Bayesian network meta-analyses of randomized clinical trials. JAMA Netw Open. 2019;2(7):e197871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jorgensen JM, Arnold C, Ashorn P, Ashorn U, Chaima D, Cheung YB, Davis JC, Fan YM, Goonatilleke E, Kortekangas Eet al. . Lipid-based nutrient supplements during pregnancy and lactation did not affect human milk oligosaccharides and bioactive proteins in a randomized trial. J Nutr. 2017;147(10):1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bravi F, Wiens F, Decarli A, Dal Pont A, Agostoni C, Ferraroni M. Impact of maternal nutrition on breast-milk composition: a systematic review. Am J Clin Nutr. 2016;104(3):646. [DOI] [PubMed] [Google Scholar]
- 36. Golden MH. Specific deficiencies versus growth failure: type I and type II nutrients. SCN News. 1995;(12):10–14. [PubMed] [Google Scholar]
- 37. Golden MH. Proposed recommended nutrient densities for moderately malnourished children. Food Nutr Bull. 2009 Sep;30(3 Suppl):S267–342. [DOI] [PubMed] [Google Scholar]
- 38. Xiang M, Zetterström R. Relation between polyunsaturated fatty acids and growth. Acta Paediatr Suppl. 1999;88(430):78–82. [DOI] [PubMed] [Google Scholar]
- 39. Kosek MN, MAL-ED Network Investigators . Causal pathways from enteropathogens to environmental enteropathy: findings from the MAL-ED birth cohort study. EBioMedicine. 2017;18:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Keusch GT, Rosenberg IH, Denno DM, Duggan C, Guerrant RL, Lavery JV, Tarr PI, Ward HD, Black RE, Nataro JPet al. . Implications of acquired environmental enteric dysfunction for growth and stunting in infants and children living in low- and middle-income countries. Food Nutr Bull. 2013;34:357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Z, Tran NT, Nguyen TS, Nguyen LT, Berde Y, Tey SL, Low YL, Huynh DTT. Impact of maternal nutritional supplementation in conjunction with a breastfeeding support program during the last trimester to 12 weeks postpartum on breastfeeding practices and child development at 30 months old. PLoS One. 2018;13(7):e0200519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. González-Cossío T, Habicht JP, Rasmussen KM, Delgado HL. Impact of food supplementation during lactation on infant breast-milk intake and on the proportion of infants exclusively breast-fed. J Nutr. 1998;128(10):1692. [DOI] [PubMed] [Google Scholar]
- 43. Yaqub A, Gul S. Reasons for failure of exclusive breastfeeding in children less than six months of age. J Ayub Med Coll Abbottabad. 2013;25(1-2):165–7. [PubMed] [Google Scholar]
- 44. Gatti L. Maternal perceptions of insufficient milk supply in breastfeeding. J Nurs Scholarsh. 2008;40(4):355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nishimura H, Krupp K, Gowda S, Srinivas V, Arun A, Madhivanan P. Determinants of exclusive breastfeeding in rural South India. Int Breastfeed J. 2018;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data described in the manuscript, code book, and analytic code will not be made available. The organization conducting the trial (Society for Applied Studies, India) is a collaborator in the Healthy Birth, Growth, and Development Knowledge Integration (HBGDKi) initiative launched by the Bill & Melinda Gates Foundation, and the data generated from the study will be shared as part of the HBGDKi repository (https://github.com/HBGDki). However, individual requests can be considered on a case-by-case basis. The request for data should be accompanied by a detailed proposal describing the intended scientific question(s) to be addressed. Proposals should be submitted to Dr. Sunita Taneja (sunita.taneja@sas.org.in).