Abstract
The human gastrointestinal tract (GI) harbors a diverse population of microbial life that continually shapes host pathophysiological responses. Despite readily available abundant metagenomic data, the functional dynamics of gut microbiota remain to be explored in various health and disease conditions. Microbiota generate a variety of metabolites from dietary products that influence host health and pathophysiological functions. Since gut microbial metabolites are produced in close proximity to gut epithelium, presumably they have significant impact on gut barrier function and immune responses. The goal of this review is to discuss recent advances on gut microbial metabolites in the regulation of intestinal barrier function. While the mechanisms of action of these metabolites are only beginning to emerge, they mainly point to a small group of shared pathways that control gut barrier functions. Amidst expanding technology and broadening knowledge, exploitation of beneficial microbiota and their metabolites to restore pathophysiological balance will likely prove to be an extremely useful remedial tool.
Keywords: Microbiota, Microbial Metabolites, Gut Barrier Function, Tight Junction Proteins, Permeability
Abbreviations used in this paper: AhR, aryl hydrocarbon receptor; AJ, adherens junction; ALD, alcoholic liver disease; CDCA, chenodeoxycholic acid; CLA, conjugated linoleic acid; CLDN, claudin; DCA, deoxycholic acid; DSS, dextran sulfate sodium; EA, ellagic acid; EGF, epidermal growth factor; ET, ellagitannin; FICZ, 6-formylindolo(3,2-b) carbazole; FMT, fecal microbiota transplantation; FXR, farnesoid X receptor; GF, germ free; GJ, gap junction; GLP, glucagon-like peptide; GP-BAR, G protein–coupled bile acid receptor; GPCR, G protein–coupled receptor; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; IEC, intestinal epithelial cell; IL, interleukin; LCA, lithocholic acid; LPS, lipopolysaccharide; MLCK, myosin light-chain kinase; MUC2, mucin 2; NOD, nucleotide-binding and oligomerization domain; NLR, nucleotide-binding and oligomerization domain-like receptor; OCLN, occludin; PKC, protein kinase C; SCFA, short-chain fatty acid; sEH, soluble epoxide hydrolase; TEER, transepithelial electrical resistance; TLR, Toll like receptor; TJ, tight junction; TNBS, 2,4,6-trinitrobenzene sulfonic acid; TNF-α, tumor necrosis factor alpha; TPE-CA, total phenolic extracts of Citrus aurantium L; UDCA, ursodeoxycholic acid; UroA, urolithin A; ZO, zonula occludens
Summary.
Gut microbial metabolites produced proximal to intestinal barrier regulate numerous host responsive activities. Current review article highlights recent advances in the area of gut metabolites and their impact on gut barrier function as well as their potential translational applications in regulating various disorders.
Humans and microbes have coevolved over millions of years, thereby contributing to the interdependency of host and microbiota physiological activities.1 Diverse interactive associations of host cells and microbes lead to mild to severe cellular and molecular responses depending on the status of host pathophysiological conditions. Deciphering the underlying relationship between the microbial community and human health provides a unique opportunity to utilize microbes and their metabolic products to prevent and treat numerous human disorders as well as maintain overall health. Increasing culture-independent omic-based technologies such as biomarker sequencing, metagenomics, metatranscriptomics, metaproteomics, and metabolomics have facilitated the discovery of novel functional dynamics of microbiota2, 3, 4, 5 and their relationship with host pathophysiology.6, 7, 8, 9
The human gut harbors abundant microbes (∼1012–1013) that play an important role in homeostatic mechanisms leading to the regulation of numerous physiological activities both in health and disease. Microbiota significantly influence the functions of the intestinal barrier that separates internal organs from harmful entities including microorganisms, luminal antigens, and luminal proinflammatory factors. Intestinal barrier function is compromised (barrier dysfunction) in several disease conditions leading to an increased translocation of bacteria, endotoxins, and other inflammatory mediators. Gut barrier dysfunction is associated with systemic inflammatory response resulting in aggravation of the pathophysiological status of underlying diseases. Recent studies have shown a significant correlation between gut barrier dysfunction with various gastrointestinal disease conditions such as inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and celiac disease. Gut barrier dysfunction is also strongly correlated with other autoimmune, inflammatory, and metabolic diseases such as diabetes, obesity, atherosclerosis, heart failure, hypertension, and food allergies, as well as with cancer.10,11 It has been reported that various external factors like alcohol, nonsteroidal anti-inflammatory drugs, and specific pathogens can directly alter gut barrier function contributing to the pathogenesis of various diseases.12, 13, 14 The imbalance of microbes in the gut (microbial dysbiosis) has been also linked with gut barrier dysfunction and an immature immune system, leading to wide spectrum of intestinal, hepatic, and neurological disorders.15, 16, 17, 18 Studies have revealed that fecal microbiota transplantation (FMT) from healthy hosts are positively correlated with disease reversal, especially those stemming from gut barrier dysfunction like IBD, IBS, celiac disease, and Clostridium difficile infections.19,20
One of the major functions of the gut microbiota is metabolism of dietary components leading to the generation of active metabolites that directly regulate human physiology including gut barrier function.21 Recent studies have explored the impact of microbial metabolites in restoration of healthy intestinal barrier function separate from their effects on immune cell functions.22, 23, 24 Detailed mechanisms are yet to be elucidated, but knowledge concerning the functional activities of metabolites on gut barrier function and the immune system is expanding gradually. Exploiting microbial metabolites to restore gut barrier integrity will prove to be an extremely useful remedial tool against several pathophysiological conditions. In this review, we discuss how the intestinal microbiota and their metabolites reinforce gut barrier function via bi-directional interactions with gut epithelial cells. Further, we will discuss potential treatment strategies to mitigate gut barrier dysfunction in various disorders.
Gut Barrier and Intestinal Epithelial Cell Junctions
The gut barrier is composed of 3 main interlinked/interdependent layers that provides a physical barrier against bacterial intrusion from the gut lumen. These include the luminal mucus layer, the gut epithelial layer formed by a continuous sheet of epithelial cells, and a third, internal layer that forms the mucosal immune system. The gut barrier acts as a physical and immunological defense against luminal microorganisms, viruses, food antigens, and environmental toxins. The barrier is selectively permeable to allow for translocation of essential dietary nutrients, electrolytes, amino acids, short-chain fatty acids (SCFAs), sugars, water, and select microbial metabolites from the intestinal lumen into the circulation. The gut epithelial barrier is composed of a single cell layer epithelial cells interspersed with functionally specialized differentiated epithelial cells. These include enterocytes, Paneth cells, goblet cells, tuft cells, enteroendocrine cells, and microfold cells, which together form a continuous and polarized monolayer leading to the separation of the lumen from lamina propria (Table 1). Among these, Paneth cells and microfold cells are only present in the small intestine, whereas enterocytes, goblet cells, and enteroendocrine and tuft cells are present in both the small intestine and colon, reviewed previously.25, 26, 27 The mucus layer28,29 and different types of intestinal cells play important and distinct roles in maintaining gut barrier function and homeostasis.
Table 1.
Cell Types in Intestinal Barrier and Their Role in Barrier Function
| Cell types | Role in gut barrier function |
|---|---|
| Enterocytes (small intestine, colon) |
|
| Paneth cells (small intestine) |
|
| Goblet cells (small intestine) |
|
| Tuft cells (small intestine, colon) |
|
| Enteroendocrine cells (small intestine, colon) |
|
| M cells (small intestine) |
|
AMP, antimicrobial peptide; GLP-2, glucagon-like peptide-2; M cell, microfold cell; MUC, mucin; TGF-β, transforming growth factor beta; TJ, tight junction; TNF-α, tumor necrosis factor alpha.
Intestinal epithelial cells (IECs) allow for selective penetration of nutrients, water, and electrolytes while simultaneously excluding microbial pathogen-associated molecular pattern, toxins, and foreign antigens.30 Enterocytes connect with each other in a continuous epithelial cell layer via adhesive junctional proteins that make up tight junction (TJ) proteins, adherens junction (AJ) proteins, gap junction proteins, and desmosomes (Table 2).31,32 These protein complexes not only mechanically secure extracellular cell to cell interactions, but also regulate intracellular adaptor protein–mediated interactions within gut epithelial cells. Epithelial cells tightly regulate paracellular (space between cells) and transcellular (through cell) permeability by posttranslational modifications of TJ proteins.33, 34, 35 TJ proteins are located on the apical side of the epithelial layer and form a continuous belt-like ring at the junction between the apical membrane and lateral membrane. TJ proteins consist of over 50 proteins that are crucial for maintaining cell-to-cell adhesion and gut barrier health.36 Some of the TJ proteins are composed of tetraspan or single-span transmembrane proteins that link to intracellular cytoskeletal proteins.37 TJ proteins such as occludin (OCLN), claudin (CLDN), and tricellulin are tetraspan transmembrane proteins, whereas junctional adhesion molecules are single-span transmembrane proteins.38, 39, 40, 41 Other TJ proteins such as zonula occludens (ZO) proteins (ZO-1, ZO-2, and ZO-3) consist of intracellular scaffold proteins. The postsynaptic density protein-95/Drosophila disc large tumor suppressor/ZO-1 protein (PDZ) binding domains of plaque proteins (ZO-1, ZO-2, ZO-3) link the cytoskeletal proteins (eg, F-actin) and TJ complex.42 TJ seals are generally formed when the extracellular domains of transmembrane TJ proteins anastomose with adjacent cells. TJ proteins regulate transport molecules based on their size/charge through the paracellular space. Adherence junctions and desmosomes primarily contribute mechanical contacts between adjacent cells. The physiological structures, properties, and functions of the gut epithelial junctional protein complexes have been extensively reviewed elsewhere.43, 44, 45, 46, 47, 48
Table 2.
Structural Components of Intestinal Epithelial Cells and Gut Barrier Function
| Structural components | Junctional proteins | Examples of junctional protein–mediated intestinal barrier dysfunction |
|---|---|---|
| Tight junction proteins | ZO, occludin, claudins, tricellulin, JAM |
|
| Adherens junction proteins | Cadherins, catenins |
|
| Desmosome | Desmoglein, desmocollins |
|
| Gap junctions | Connexin |
|
IFN-γ, interferon gamma; JAM, junctional adhesion molecules; MLCK, myosin light-chain kinase; TNF-α, tumor necrosis factor alpha; ZO, zonula occludens.
The functional or physical disruption of TJ, AJ, and gap junction proteins and desmosomes leads to increased gut permeability causing dysregulated translocation/transportation of inflammatory mediators, which potentially manifests as chronic gut inflammation. Increased inflammation further perpetuates the disruption of TJ proteins leading to increased permeability. The following sections will describe gut barrier dysfunction and impact of gut microbiota and their metabolites in the regulation of the gut barrier and its altered state in various inflammatory disorders.
Gut Barrier Dysfunction
Defective gut epithelial barrier function in combination with immune dysregulation is associated with several gastrointestinal tract–related disorders including but not limited to IBD, IBS, drug-induced toxicity, and colon cancer. Increased gut barrier permeability occurs through dysregulation of epithelial apoptosis/enterocyte death, mucus degradation, and increased paracellular permeability due to disruption of TJs. These pathways are independently regulated and functional consequences are distinct from each other. The following section describes the mechanisms and potential factors responsible for barrier dysfunction.
TJ-Mediated Paracellular Permeability
As described in previous sections, TJ proteins are responsible for paracellular transport and selective gut barrier permeability. It was reported that solutes and water cross TJs through 2 distinct pathways based on their size and charge selectivity: the pore pathway48, 49, 50 and the leak pathway.48,51 The pore pathway is exclusively size and charge selective and excludes molecules with a diameter ≤8 Å and is a high-conductance route. CLDN-2 as well as CLDNs 10a, 10b, 15, 16, and 17 were shown to play critical role in the pore pathway. In contrast to the pore pathway, the leak pathway allows macromolecule flux with an exclusion limit of ∼100 Å with lower conductance.51 The leak pathway is believed to be regulated by myosin light-chain kinase (MLCK), where constitutively active MLCK is sufficient to increase the leak pathway–dependent permeability both in vitro and in vivo.52,53 It was reported that MLCK, OCLN, and ZO-1 like TJ proteins play an important role in regulation of the leak pathway.54 Detailed discussions of paracellular permeability and potential mechanisms have been published.48,49,52,55 The gut microbiota and their metabolites significantly regulate these pathways leading to either enhancement or reduction of gut barrier function.
Unrestricted Paracellular Pathway: IEC Death
Dysregulated IEC death is commonly observed in IBD patients as well as in preclinical models of colitis. Increased IEC death is one of the confounding factors for increased barrier breach in gastrointestinal tract–related disorders. It was reported that apoptosis, necroptosis, and pyroptosis are major cell death pathways that influence IEC death and result in pathophysiological outcomes. The mechanisms of cell death in the gut epithelium and its implication for gut barrier function and chronic inflammation are reviewed elsewhere.56 For example, Gitter et al57 showed that tumor necrosis factor alpha (TNF-α) disrupts the gut barrier not only by degradation in TJs, but also through the induction of epithelial apoptosis. TNF-α induced loss of the epithelial integrity due to apoptosis accounted for about 56% of the damage and the rest was by disruption of TJs in nonapoptotic areas.57 Epithelial permeability is also modulated by interleukin (IL)-13 through regulation of Cldn-2. Upregulated IL-13 and Cldn-2 induce epithelial apoptosis, resulting in epithelial barrier disruption.58 The flux that occurs due to cell death (global barrier loss) is classified as “unrestricted pathway,” in which there is no size limit of molecules or antigens that can cross the membrane.48
Role of Microbial Metabolites in Gut Barrier Function
The gut microbiota is often referred to as an essential “organ” due to the vast density and richness of microbial life that exist in the gut lumen.5 Members of the gut microbiota influence host metabolic and immune status by modulating nutrient metabolism, xenobiotic and drug metabolism, and production of antimicrobial metabolites that limit numbers of competing microbes for the same niche. The following sections will discuss the role of gut microbial metabolites in regulating gut barrier function.
The host-microbiota relationship has been defined as the synergistic effect of microbial metabolites on host immunity, health and disease, energy metabolism, and cellular communication.59, 60, 61, 62, 63,64 Microbial metabolism contributes to the nutrient harvest from the diet. Gut microbiota metabolize dietary components and produce numerous active microbial metabolites based on the type of diet. Due to the fastidious relationship between the host immune system and microbiota, the production of helpful metabolites depends on toleration and existence of helpful microbes.64 Moreover, coevolution of host and the microbiota has apparently facilitated the production of structurally compatible microbial metabolites that bind to host receptors resulting in the modulation of host physiology.65 Recent developments in metagenomics, metatranscriptomics, and metabolomics have successfully discovered thousands of microbial metabolites and the associated genes for their synthesis. Thus far, it is estimated that ∼50,000 microbial metabolites are produced by microbes in the human gut. Among these, 22,500 compounds are estimated to have antibiotic properties. Nevertheless, only 150 compounds are in direct use in human and veterinary medicine and in agriculture, reviewed in.66 It is fascinating to observe that the number of bioactive fungal products at ∼8600 representing 38% of all microbial products. The description, properties, features, source, and structures of known bioactive metabolites are described elsewhere.66, 67, 68, 69, 70, 71 Here, we summarize how dietary microbial metabolites influence gut barrier function.
Microbial metabolites can be classified72 as dietary product-derived metabolites, such as compound K, or de novo synthesized metabolites such as SCFAs. A third category of metabolites include metabolites that were initially generated by the host and secreted into the lumen where they undergo modification by gut microbes such as secondary bile acids. Microbial metabolites produced in the gut directly interact with IECs and immune cells and impact host health.61,62 Further, they have been shown to significantly influence the maintenance of gut barrier integrity itself and intestinal homeostasis.73,74 The following section and Table 3 summarize the immunomodulatory activities of selected microbial metabolites and their role in gut barrier function.
Table 3.
List of Microbial Metabolites, Gut Microbiota, and Their Functions
| Metabolites | Gut Microbes | Functions |
|---|---|---|
| Short-chain fatty acids (acetate, propionate, butyrate, valerate, isobutyrate, isovalerate, 2-methylpropionate, hexanoate) | Bacteroidetes, Firmicutes, Campylobacter jejuni, Staphylococcus aureus, Bifidobacterium sp., Coprococcus, Clostridium, Roseburia, Faecalibacterium |
|
| Bacteriocin (nisin A, Mcc B17, MccJ25, colicin) | All major bacteria, archaea L. lactis, Klebsiella, Salmonella, Shigella, Pseudomonas, Enterobacteria (mostly Escherichia coli), S. enterica, B. cereus, S. aureus |
|
| Autoinducers (AI-2, AHL, PQS) | E. coli, S. aureus, P. aeruginosa, Clostridium perfringens |
|
| Vitamins (vitamin K2, menadione, vitamin B2, vitamin B6, vitamin B9) | Lactic acid bacteria, gram-positive organism, Bifidobacterium, S. aureus, L. monocytogenes, S. typhimurium |
|
| Microbial amino acids (lysine, D-aas, D-Ser) | V. cholera, E. coli, P. aeruginosa, S. aureus, enterohemorrhagic E. coli |
|
| Conjugated fatty acids (sphingomyelin, acylglycerol, phosphatidylcholine, cholesterol, phosphoethanolamine) | Bifidobacterium, Enterobacter, Clostridium, Citrobacter, Roseburia, Lactobacillus, Klebsiella |
|
| Indole derivatives (indole, indole-3-propionic acid, 5-hydroxyl indole, indoxyl sulfate, N-acetyltryptophan, indoxyl sulfate, serotonin, melatonin, melatonin 6-sulfate) | E. coli, C. sporogenes |
|
| Bile acid metabolites (cholic acid, deoxycholic acid, chenodeoxycholic acid, taurocholic acid, lithocholic acid, glycocholic acid, ursodeoxycholic acid) | Bifidobacterium, Bacteroides, Clostridium, Lactobacillus, Enterobacter |
|
| Choline metabolites (choline, methylamine, dimethylamine, trimethylamine, trimethylamine N-oxide, betaine, dimethylglycine) | Bifidobacterium, Firmicutes, Proteobacteria, Actinobacteria, Faecalibacterium prausnitzii |
|
| Phenolic, benzoyl, and phenyl derivatives (urolithins, enterolactone, 4-OH phenylacetic acid, equol, 8-prenylnaringenin, enterodiol) | Bifidobacterium, Lactobacillus, C. difficile, F. prausnitzii |
|
| Polyamines (putrescine, spermidine, cadaverine, spermine) | Campylobacter jejuni, C. saccharolyticum |
|
Short-Chain Fatty Acids
The human diet contains a large amount of dietary fiber and indigestible carbohydrates that escape proximal digestion and are fermented by gut commensals such as Bifidobacterium, Bacteroides, Enterobacter, Faecalibacterium, and Roseburia species into SCFAs. Propionate, butyrate, and acetate are SCFAs that not only are required for the nutritional demands of microbes, but also actively impact host immune cell differentiation and immunity and metabolism as well as regulating susceptibility to other pathogens.75, 76, 77, 78 It was shown that sodium butyrate at a concentration ranging between 1 and 10 mM significantly improved epithelial barrier function in E12 human colon cells by increasing the levels of mucin 2 (MUC2).79 However, at higher concentrations (50–100 mM), it showed no beneficial effects on MUC2 expression patterns,79 likely due to the induction of apoptosis at higher doses. Similarly, butyrate improved barrier function at lower doses, but reduced barrier function at higher doses in Caco-2 cells.80 It was shown that butyrate induces activation of AMPK (AMP-activated protein kinase) that protects intestinal barrier integrity by facilitating the assembly of TJ proteins.81 Treatment with sodium butyrate led to the upregulation of CLDN-1 transcription by increasing the interaction between the motif specific promoter region of CLDN-1 and transcription factor SP1.82 Furthermore, Feng et al83 reported that treatment with SCFA such as acetate, propionate, and butyrate induced expression of TJ proteins of IECs.83 SCFA treatment increases in paracellular transport, maintenance of ZO-1 and OCLN proteins, inhibition of NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) inflammasome activation, and autophagy. In addition, gastric infusion of SCFA in a pig gut barrier model enhanced OCLN and CLDN-1 expression. High local concentrations of SCFAs also increase anion release maintaining osmotic equilibrium in the intestinal lumen for resident microbes.84,85 Importantly, consumption of diets enriched in SCFA and butyrate production by human subjects with metabolic syndrome exhibited increased colonic expression of MUC2 and OCLN, suggesting beneficial action of butyrate in gut barrier function.86 Moreover, butyrate treatment resulted in downregulation of IL-1β levels, suggesting it may be beneficial for protection against inflammation-promoted barrier disruption.87
Indole Derivatives
A diet rich in red meat, fish, egg, cheese, cruciferous vegetables, and beans has high levels of the amino acid tryptophan.88 Dietary tryptophan is catabolized into indole by tryptophanase expressed by several microbes such as indole-positive Clostridium sporogenes. Indole and indole derivatives (such as indole-3-aldehyde, indole-3-pyruvate, indole-3-acetaldehyde, indole-3-acetamide, indole-3-propionic acid, indole-3-acetic acid, and indole-3-lactic acid) are essential for bacterial quorum sensing and intracellular signaling. Tryptophan microbial metabolites are also well-known ligands for the aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor that senses environmental toxins and endogenous ligands. Activation of AhR by tryptophan metabolites promotes immune cell maturation and decreased pathogen colonization.89 AhR signaling primarily influences the induction of immune responses via IL-22, which enhances gut barrier function (discussed in the following section). It was reported that indole levels in fecal samples of germ-free (GF) mice were 27-fold lower compared with conventionally raised mice.90 TJ and AJ proteins (Cldn7, Ocln, ZO-1, E-cadherin 1) are significantly decreased in GF mice compared with conventionally raised mice.90 Importantly, supplementation of indole capsules restored gut barrier integrity by elevating junctional protein expression in GF mice.90 FMT has resulted in enhanced metabolic functions of gut microbiota associated with indole alkaloid biosynthesis such as indole-3-acetic acid that in turn enhances gut barrier homeostasis.91
Activation of AhR by tryptophan ligands such as 6-formylindolo(3,2-b) carbazole (FICZ) enhances gut barrier function. AhR activation by FICZ was therapeutic in the dextran sodium sulfate (DSS)–induced mouse colitis model in which FICZ treatment upregulated TJ protein expression in inflamed mouse colons.92,93 Treatment with FICZ diminished MLCK expression and MLC in DSS-induced colitis mice. In addition, FICZ also prevented a TNF-α/interferon gamma–induced decrease in transepithelial electrical resistance (TEER) and disruption of TJ proteins in Caco-2 monolayers.92 FICZ was also shown to reverse hypoxia-induced barrier dysfunction.94 Thus, tryptophan agonists regulate multiple pathways in gut barrier integrity.
An impaired capacity of gut microbiota to metabolize tryptophan into multiple AhR agonists has been associated with metabolic syndrome in humans and mice.95 Dietary supplementation with synthetic AhR agonists or bacterial strains that naturally produce AhR ligands by Tryptophan metabolism enhanced gut barrier function via secretion of incretin hormone glucagon-like peptide-1.95 AhR–/– mice exhibit increased inflammation and severe colitis phenotypes compared with wild type mice in the 2,4,6-trinitrobenzene sulfonic acid (TNBS) or DSS-induced colitis models.22,96 Levels of TJ proteins in AhR–/– mice are also significantly reduced compared with wild type mice leading to increased gut leakiness.22 Additionally, AhR deficiency is associated with loss of intraepithelial lymphocytes, group 3 innate lymphoid cells and decreased IL-22 production.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99 It was demonstrated by the Stockinger group that deletion of AhR in IECs led to a defective barrier and uncontrolled proliferation of intestinal stem cells, leading to increased tumors in an azoxymethane-DSS–induced colon tumorigenesis model.100 Supplementation of dietary AhR ligands (like I3C) guarded the intestinal stem cell niche and maintained intestinal barrier function and decreased tumor burden in the azoxymethane-DSS–induced colon tumorigenesis model.100 In parallel to these observations in mice, low levels of naturally occurring AhR ligands were reported in IBD patients.100 Supplementation with both Lactobacillus strains and synthetic AhR agonists ameliorated intestinal inflammation in IBD patients.101
Bile Acid Metabolites
Bile acids that are synthesized in the liver from cholesterols in pericentral hepatocytes are referred to as primary bile acids. These primary bile acids, cholic acid and chenodeoxycholic acid (CDCA), are transported into the gut through the enterohepatic circulation system and are further metabolized by gut microbiota by dehydroxylation, dehydrogenation, and epimerization to generate secondary bile acids reviewed previously.102 Secondary bile acids include deoxycholic acid (DCA), ursocholic acid, ursodeoxycholic acid (UDCA), and lithocholic acid (LCA) in humans. cholic acid, CDCA, 6-hydroxylated bile acids, and muricholic acid are the primary bile acids in mice, which further converted into the secondary bile acids DCA, ursocholic acid, UDCA, murideoxycholic acid, and hyodeoxycholic acid. Secondary bile acids antagonize the nuclear membrane farnesoid X receptor (FXR) and the G protein–coupled receptor TGR5103 in the intestines. There are numerous studies published describing the impact of bile acids and their receptors on gut barrier action.104, 105, 106, 107, 108, 109 Some bile acid metabolites can both improve as well as exert negative effects on gut barrier TJ function.104 For example, it was shown that the primary bile acid CDCA and its 7α-dehydroxylated derivative LCA have opposite effects on epithelial integrity in human colonic T84 cells. Treatment with CDCA decreased the expression of Ocln and TEER values leading to increased permeability as well as an increase in the inflammatory cytokine IL-8. Although LCA by itself neither altered barrier function nor increased IL-8 production, LCA attenuated CDCA-induced barrier permeability and IL-8 production. It was shown that high fat diet-induced impairment of gut barrier function is associated with the proportion of UDCA vs DCA.105,107 Mice fed a diet containing DCA exhibited increased gut permeability (>1.5-fold) compared with mice fed a regular diet. In contrast, UDCA did not alter gut permeability but blocked the DCA-induced permeability in murine jejunal and colonic preparations. Mechanistically, DCA-induced tissue disruption is responsible for DCA-mediated increased permeability rather than DCA acting as an inflammatory mediator. Further, DCA exacerbated lipopolysaccharide (LPS)–induced barrier disruption.107 Using a Caco-2 cell model, Raimondi et al110 showed that DCA and CDCA, but not UDCA, treatment decreased TEER and increased permeability. This study concluded that DCA and CDCA modulated intestinal permeability via endothelial growth factor (EGF) receptor autophosphorylation, OCLN dephosphorylation, and rearrangement at the TJ level.110 The effects of these bile acids were mediated by the Src family kinases and was abolished by EGF treatment.110 A study on prematurely weaned piglets reported that administration of CDCA improves the gut barrier protection by inducing expression of ZO-1 and reducing expression of TNF-α, IL-6, and IL-10.111 It was showed that mice exposed to broad-spectrum antibiotics such as ampicillin, clindamycin and streptomycin resulted in reduced gut bacterial diversity and richness and impacted gut barrier integrity.112 The homeostasis of bile acids was significantly changed in these mice. Further, treatment with dietary flavanones and their glycosyl derivatives such as total phenolic extracts of Citrus aurantium L (TPE-CA) restored bacterial diversity and improved gut barrier integrity and homeostasis of bile acids. TPE-CA treatment induced the expression of TJ proteins such as ZO-1 and OCLN-associated proteins in these mice and improved the barrier integrity.112 Moreover, treatment with TPE-CA maintained the homeostasis of bile acid by regulating bile acids entry into the enterohepatic circulation through farnesoid X receptor (FXR)/fibroblast growth factor 15 pathway and FXR-targeted protein.112
Alcoholic liver disease (ALD) is associated with gut microbial dysbiosis,113, 114, 115 and ALD-associated microbiota changes lead to alterations in bile acid profiles (ie, increased amounts of unconjugated bile acids in the small intestine).116 Fexaramine, an intestine-restricted FXR agonist, restored intestinal FXR activity and gut barrier integrity in an ALD model. These studies suggested that targeted interventions can improve bile acid-FXR-fibroblast growth factor 15 signaling by modulation of hepatic Cyp7a1 and lipid metabolism, thereby reducing ethanol-induced liver disease in mice.116 The G protein–coupled bile acid receptor (GP-BAR1) (also called as TGR5) is highly expressed in the ileum and colon. The colons of GP-BAR1–/– mice exhibit altered subcellular distribution of ZO-1, leading to increased gut permeability. Importantly these mice were more susceptible to DSS- or TNBS-induced colitis.117 Mice treated with GP-BAR1 agonists (ciprofloxacin and oleanolic acid) abrogated the signs and symptoms of TNBS-induced colitis.117 In summary, bile acids have been shown to be a double-edged sword and an appropriate balance (ratio) of different bile acids is required to mediate gut barrier protective activity.
Conjugated Fatty Acids
Intestinal bacteria such as Bifidobacterium, Butyrivibrio, Enterobacter, Lactobacillus, Clostridium, Citrobacter, Roseburia, Klebsiella, and Megasphaera can produce conjugated fatty acid metabolites from fat-enriched diets.118 Conjugated fatty acids, such as conjugated linoleic acid (CLA), are known to influence gut barrier function. Treatment with the trans-10 CLA isomer caused a redistribution of ZO-1 and OCLN and increased paracellular permeability in Caco-2 colon epithelial cells.119 Dietary supplementation of CLA ameliorated DSS-induced colitis in mice.120 Importantly, CLA treatment reduced the prevalence of Bacteroides and increased Bifidobacterium and Odoribacter in this model. Additionally, treatment with CLA significantly increased the expression of the TJ proteins ZO-1, OCLN, and CLDN-3 in the DSS-induced colitis mouse model.120 Ren et al reported that the probiotic L. plantarum ZS2058 produced isomers of conjugated fatty acids, α-linolenic acid (conjugated linolenic acid (CLNA) 1 and CLNA2) mitigated DSS-induced colitis by enhancing gut barrier function through upregulation of ZO-1, OCLN, CLDN-3, and E-cadherin 1 in colonic tissues. Additionally, treatment with CLNA1 and CLNA2 significantly reduced inflammatory mediators (TNF-α, IL-1β, and IL-6) while upregulating the expression of the colonic anti-inflammatory cytokine IL-10 and nuclear receptor peroxisome-activated receptor-γ. These treatments also rebalanced the intestinal microbial composition in the colons of DSS-treated mice, including increasing the α-diversity.121
Polyamines
The polyamines such as spermine, spermidine, and putrescine have important implications in human health, mainly in the intestinal maturation and in the differentiation and development of immune system. The gut microbiota metabolize polyamine rich foods (eg, wheat germ, citrus fruits, mushrooms, soybeans) to generate polyamines in the lower intestine. Polyamines are also produced in the cytoplasm of human cells either by the enzyme ornithine decarboxylase or by AdoMetDC (S-adenosyl-methionine decarboxylase) and a transferase in the upper intestine.
Polyamines exhibit numerous beneficial effects including increased longevity, recovery of injured mucosa, as well as favorable effects on cognitive function.122, 123, 124 Here, we briefly discuss the polyamine regulation of intestinal barrier integrity. Gut microbes such as E. coli, Bacteroides spp. and Fusobacterium spp. can synthesize polyamines.122 Bacterial polyamines include spermidine, homospermidine, norspermidine, putrescine, cadaverine, and 1,3-diaminopropane with putrescine and spermidine.125 Polyamines enhance gut barrier integrity through inducing E-cadherin, the cell-cell adhesion protein in c-Myc–dependent manner.126 It was shown that depletion of cellular polyamines by α-difluoromethylornithine reduced intracellular free Ca2+ concentrations in normal IECs (IEC-6 cell line),127 which leads to decreased expression of E-cadherin and increased paracellular permeability. Interestingly, polyamine depletion does not alter expression of TJ proteins such as ZO-1, ZO-2, and junctional adhesion molecule-1. However, exogenous supplementation of the spermidine reversed the effects of α-difluoromethylornithine on intracellular Ca2+ and E-cadherin expression and restored paracellular permeability to near normal levels.128 These studies suggested that polyamines regulate gut barrier function through upregulating E-cadherin in an intracellular Ca2+-dependent manner.127
Polyphenolic Derivatives
Dietary polyphenols, which have limited bioavailability, are most abundantly present in berries, walnuts, and pomegranate. Polyphenolic compounds (eg, tannins, flavanols, flavanones, flavones, isoflavones, flavan-3-ols, lignans, chlorogenic acids, anthocyanidin) are metabolized by gut microbiota as indicated in Table 3 and converted to active phenolic derivatives responsible for numerous physiological activities. Removal of sugars by microbes leads to breakdown of glycosylated derivatives of polyphenols, which are inactive components of the human diet. Limitations in bioavailability of polyphenolic compounds are exploited by microbes via conversion into microbial-derived secondary metabolites. For example, dietary ellagic acid (EA) derivatives such as ellagitannins (ETs) are metabolized into various bioactive metabolites including urolithins (Urolithin A, B, C, D, and iso-urolithin A) by human gut microflora.129 The health benefits of consumption of pomegranate and pomegranate juice are attributed to the activities of these urolithins.130,131 Compared with ETs or EA, urolithins have a much higher rate of absorption most likely due to increased lipophilicity.132 Among urolithins, urolithin A (UroA) displays potent anti-inflammatory, antioxidative, and antiaging properties.130,133, 134, 135 However, UroA can only be detected in ∼ 40%–50% of human subjects upon consumption of EA/ET-containing diets.131,136, 137, 138, 139, 140, 141, 142, 143 Two factors are critical for the generation of UroA in humans (1) presence of UroA producing bacteria and (2) consumption of EA/ET-containing foods (eg, pomegranate, berries, walnuts). The concentration of serum urolithins can reach up to micromolar levels without displaying toxicity in certain populations.132,134,138,144,145 For example, upon consumption of 1L of pomegranate juice daily for 5 days by healthy volunteers, urolithin levels ranged from 0.5 to 18.6 μM in the plasma.136 It was reported that 2 species of human intestinal bacteria, Gordonibacter urolithinfaciens, sp. nov. and G. pamelaeae DSM 19378(T), convert EA to Urolithin M-5, M-6 and C.146 Recently, B. pseudocatenulatum strain INIA P815 was shown to be responsible for the production of UroA and UroB by metabolizing EA.147 However, functional activities and production of UroA in vivo yet to be directly determined using gnotobiotic animals. Numerous studies have shown the beneficial activities of UroA in various pathophysiological conditions due to its anti-inflammatory, antioxidative, anticancer, and antiaging properties.134,148,149 Our group demonstrated that treatment with UroA significantly enhanced gut barrier integrity through upregulation of TJ proteins via an AhR-Nrf2–dependent pathway.22 UroA failed to upregulate TJ proteins such as Cldn4, ZO-1 and Ocln in AhR–/– and Nrf2–/– mice. Importantly, treatment with UroA mitigated DSS- or TNBS-induced colitis in mice through enhancing gut barrier function and blocking the production of inflammatory cytokines.22 A human phase I clinical trial showed that a polyphenol-rich diet modulates gut microbiota and reduces intestinal permeability, including decreased transport of pathogenic microbes into the bloodstream.150 Johnson et al151 reported that dietary polyphenols such as isoflavones and lignans and corresponding microbial metabolites protected the gut epithelium by reducing permeability and inhibited LPS-induced inflammation. Lignan derived microbial metabolites such as equol and enterolactone protected against nitric oxide–, TNF-α–, and IL-6–induced barrier dysfunction.151 Moreover, anthocyanins and proanthocyanidins, polyphenols form blueberries, ameliorated E. coli–induced gut barrier dysfunction.152
In summary, numerous microbial metabolites discussed previously shown to exhibit direct impact on gut epithelium and play an important role in regulating gut barrier function thereby mucosal immunity and vice versa.
Role of Bacterial Structural Components in Gut Barrier Function
Numerous bacterial products regulate gut barrier function by activating Toll-like receptors (TLR) and nucleotide-binding and oligomerization domain (NOD)-like receptor (NLR) pathways.153, 154, 155, 156 Here, we highlight some of the bacterial components and their receptors that participate in regulating gut barrier integrity. IECs express TLR2 that recognizes bacterial lipopeptides, lipoteichoic acid and yeast zymosan, and forms heterodimers with TLR1 or TLR 6.157 Ligand-induced TLR2 activation leads to the specific activation of protein kinase C α (PKC-α) and PKC-δ and is responsible for increased TEER as well as increased apical tightening and sealing of the TJs. PKC phosphorylation by TLR2 activation leads to stabilization of ZO-1, which is a target of PKC. It was shown that the treatment with the TLR2 agonist Pam3CSK4 (PCSK [proprotein convertase subtilisin/kexin]) increased the gap junctional intercellular communication through connexin-43 during IEC injury.158 Furthermore, Cario et al159 demonstrated using both in vitro and ex vivo models that TLR2 stimulation effectively preserves TJ-associated barrier assembly against stress-induced damage through promotion of PI3K/Akt-mediated cell survival in an MyD88-dependent manner. In this study, the authors showed that oral treatment with the TLR2 ligand, PCSK significantly suppressed mucosal inflammation and apoptosis by efficiently restoring TJ-associated integrity of the intestinal epithelium in a mouse colitis model.159 Bacterial LPS mediates proinflammatory signaling through TLR4 and the adapter molecule MyD88. Numerous studies including ours showed that LPS disrupts junctional protein complexes and increases inflammatory cytokines leading to increased gut permeability. Blocking LPS-mediated signaling has been shown to protect against gut barrier disruption. For example, wogonin suppresses the inflammatory response and maintains intestinal barrier function via the TLR4-MyD88-TAK1 (TGF-β activated kinase 1)-NF-κB (nuclear factor-kappa B) pathway in Caco-2 cells.160 It was also shown that the TLR4/NF-κB signaling pathway may be critical to the mechanism underlying hypoxia-induced damage to intestinal barrier function and bacterial translocation.161 Importantly, treatment with a TLR4 antagonist (CRX-526) inhibited the development of disease in an acute DSS colitis model.108 Although blocking TLR4 with antibodies limited acute inflammation in the DSS model, subsequent tissue repair was hampered, suggesting an important role for TLR4 in the resolution of inflammation and restoration of tissue integrity.162 TLR4–/– mice exhibited less intestinal permeability (FITC-dextran permeability assay) compared with wild type mice in a burn injury model due to unaltered expression of the TJ protein OCLN.163 Overall, these studies suggest that TLR4 plays an important role in modulating gut barrier function.
Flagellin, a structural component of bacterial flagellum, is exerted activities through TLR5 and has been shown to play an essential and nonredundant role in protecting the gut from enteric microbes.164 It was demonstrated in numerous reports from Dr Gewirtz’s group that expression and activation of TLR5 on IECs regulates the composition and localization of microbiota and prevents disease associated intestinal inflammation.165, 166, 167, 168 Activation of TLR5 by flagellin in ileal tissues of SAMP1/YitFc (SAMP) mice (a spontaneous model of Crohn's disease–like ileitis) led to decreased epithelial barrier resistance and altered expression of the TJ proteins CLDN-3, OCLN, and ZO-1.169 Further, TLR5–/– mice developed spontaneous colitis due to increased bacterial burdens and reduced anti-inflammatory cytokine secretion.166,170
Hypomethylated CpG (cytosine-guanine dinucleotide DNA) DNA motifs (microbial CpG-DNA are recognized by TLR9. TLR9–/– mice are more susceptible to DSS-induced colitis compared with wild types mice, suggesting that activation of TLR9 is important for protection against intestinal damage through induction of vascular EGF and intestinal progenitor cell differentiation as well as being critical for wound repair mechanisms.171
NLRs proteins are also responsible for maintaining gut barrier integrity by sensing the presence of commensal microbiota and the regulation of intestinal inflammation.172,173 NLRs like NOD1 and NOD2 are expressed both on immune and epithelial cells and detect the bacterial cell wall component peptidoglycan. Activation of NLRs results in inflammatory responses mediated by NF-κB, mitogen-activated protein kinase, or caspase-1 activation. It was reported that Nod1–/– mice have increased levels of intestinal permeability and inflammation in the murine DSS-induced colitis model compared with wild type mice,174 suggesting an important role in intestinal barrier function. Furthermore, muramyl dipeptide or peptidoglycan mediated activation of NOD2 is reported to negatively regulate TLR signaling and provide protection of wildtype mice against colitis.175,176 The absence of NOD protein–mediated microbial sensing leads to a loss of gut barrier homeostasis and leads to increased susceptibility to gut inflammatory diseases.176 NOD1 and NOD2 knockout mice exhibit increased paracellular permeability and are reported to be more susceptible to DSS colitis mediated intestinal injury.177 Couturier-Millard et al178 reported that NOD2 deficiency leads to microbial dysbiosis leading to increased risk of colitis.
Additionally, the mucosal immune system profoundly impacts gut barrier functions both in health and disease conditions, reviewed previously.48,179, 180, 181, 182, 183 As discussed previously, microbial metabolites regulate host immunity by exploiting metabolite-specific immune cell receptors such as AhR, FXR, pregnane X receptor, membrane bile acid receptor (M-BAR/TGR5), purinergic receptor (P2X7), and G protein–coupled receptors (GPR41, GPR43, GPR109A). These receptors, which play crucial roles in host-microbiota interactions, are expressed at various levels in different cell types such as IECs, innate lymphoid cells, macrophages, T cells, and dendritic cells.184 Functional immune responses often modulate gut barrier integrity; hence, the fine tuning of gut microbiota to evoke necessary immune responses to alter intestinal barrier function is critical.
In summary, bacterial structural components either directly or indirectly (through increasing inflammatory mediators and regulating immune system) impact and modulate the gut barrier functions both in health and disease conditions.
Treatment Strategies Against Gut Barrier Dysfunction
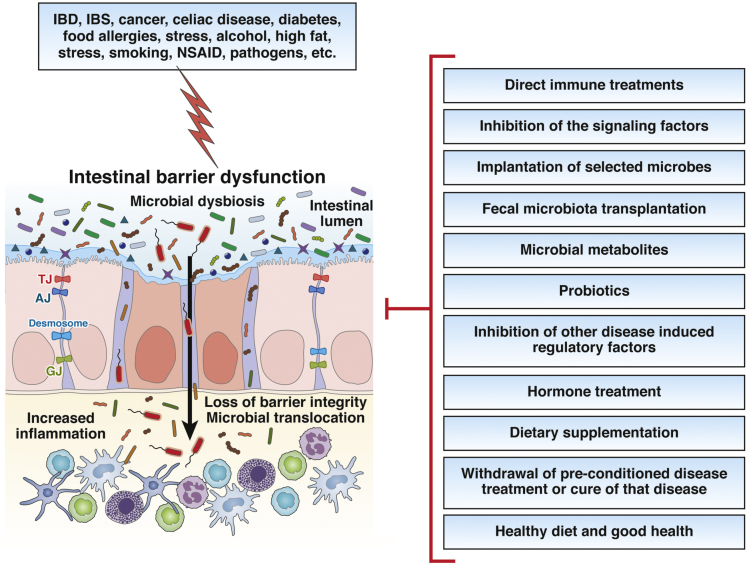
Microbial manipulations and supplementation of microbial metabolites are being considered as emerging preventive and therapeutic medicines for numerous disorders185, 186, 187 including those with gut barrier dysfunction and dysbiosis. Though beneficial activities of microbial metabolites in regulating gut barrier function have been shown in animal models, thus far, no microbial metabolites or their analogues are in clinical use. The following section reviews experimental approaches that could be efficacious in improving gut barrier function (Figure 1).
Figure 1.
Potential treatment strategies against gut barrier dysfunction. Host-microbiota interactions and external stimuli drive changes in gut barrier integrity and lead to gut barrier dysfunction. Impaired junctional proteins and increased intestinal permeability lead to pathogen-mediated mucosal inflammation and subsequent uncontrolled immune responses mediated by macrophages, T cells, B cells, mast cells, neutrophils, and dendritic cells. Potential preventive/therapeutic interventions are shown. Inhibition of disease induced regulatory factors, hormone treatment, dietary supplementation, and healthy diets and habits can potentially restore intestinal epithelial barrier function. GJ, gap junction protein; NSAID, nonsteroidal anti-inflammatory drugs.
One approach would entail modulation of immune cell function. Conventional treatment strategies include the use of immunosuppressants such as azathioprine and mesalamine that are used in the clinic to treat ulcerative colitis and Crohn's disease.188 Another therapeutic approach includes blocking Th1 responses or using neutralizing antibodies such as infliximab (anti-TNF) antibody.189,190 Altering the signaling pathways involved in gut barrier dysfunction are reported as potential therapeutics. It was shown by the Turner group that a domain binding small molecule called “divertin” blocks the recruitment of MLCK1 without inhibiting enzyme function.191 Further, they showed that treatment with divertin blocked acute interferon gamma–primed TNF-induced MLC phosphorylation and barrier loss in Caco-2 monolayers model system. Importantly, they provided the evidence that treatment with divertin regulate paracellular transport rather than transcellular transport across the membranes. Further, they demonstrated that treatment with divertin corrected increased intestinal permeability in IL-10–/– mice (spontaneous colitis model) without significant systemic, mucosal, or epithelial toxicity.191 Treatment with divertin also reduced the progression and severity of experimental chronic colitis of T cell transfer model (CD4+ effector T cells in immunodeficient mice) by correcting gut permeability.191 Recent advances in defining the functional role of specific commensal bacteria has led to novel approaches to attain gut homeostasis and improve barrier function. Implantation of 17 pre-selected clostridial bacterial strains that are capable of enhancing Treg cell abundance and inducing anti-inflammatory mediators, IL-10 and inducible T-cell co-stimulator, into GF mice attenuated TNBS-induced colitis and enhanced gut barrier function.192 Similarly, implantation with IL-10 producing Lactococcus lactis reduced inflammation in a mouse colitis disease model.193,194 FMT is another emerging tool for treatment of gut dysbiosis as it can effectively repopulate the gut with a more favorable microflora. FMT was reported to decrease antibiotic, chemotherapy, and injury-induced gut permeability and increased the prevalence of specific microbiota strains exhibiting anti-inflammatory properties.195,196
Several microbial metabolites are being considered as promising therapeutics against gut barrier dysfunction. As discussed above, the EA-derived microbial metabolite UroA is reported to increase intestinal barrier integrity and illustrated the opportunity for the use of microbial metabolites to treat inflammatory intestinal diseases.22 Our group showed that UroA and its synthetic structural analogue UAS03 enhanced gut barrier function via upregulation of TJ proteins such as CLDN-4, OCLN, and ZO-1. Additionally, UroA and UAS03 also blocked unwarranted inflammation and mitigated colitis in pre-clinical models.22
Oxyberberine, another microbial metabolite derived from berberine ameliorated gut barrier dysfunction via inhibition of the TLR4, MyD88, and NF-κB signaling pathways197 and downregulated inflammatory cytokines such as TNFα, IL-6, and IL-1β. Probiotics such as L. plantarum are reported to modulate the normal gut microbiome and maintain gut barrier homeostasis and regulate permeability by strengthening the intestinal epithelium. Furthermore, probiotic strains such as L. plantarum also elevate the levels of host defense peptides such as pBD2, PG1-5 and pBD2 and improve gut barrier function.83 It was shown that a bioactive compound from Astragalus membranaceus, Astragaloside IV (AS-IV) induced TJ protein expression by suppressing RhoA/NLRP3 inflammasome signaling pathways in a sepsis model.197 Activation of mitochondrial dynamin-related protein (Drp1) regulates gut barrier function through regulating SCFA-producing gut microbiota in hemorrhagic shock mouse models.198 It was shown that activated Drp1 led to distribution of ZO1 protein and increased intestinal permeability in a ROS-specific manner. Further, blocking Drp1-induced ROS accumulation by N-acetylcysteine in IECs protected TJs and improved intestinal barrier function after hypoxia.198 Studies have shown that soluble epoxide hydrolase (sEH) and its metabolites (as dihydroxyeicosatrienoic acids) were upregulated in the colon tissues of diet-induced obese mice. It was demonstrated that increased expression of sEH led to enhanced gut permeability in diet-induced obesity models.199 Further, genetic deletion of sEH (sEH knockout mice) abolished obesity-induced gut leakage, endotoxin lipopolysaccharide and bacterial translocation.199 Pharmacological inhibition of sEH (trans-4-(4-[3-(4-trifluoromethoxyphenyl) ureido] cyclohexyloxy) benzoic acid (t-TUCB), prevented obesity-induced gut barrier dysfunction suggesting sEH inhibitors may be promising agents for gut barrier dysfunction treatment in obesity conditions.199
A variety of evidence also suggests that hormone treatment can be a potential therapeutic strategy to combat barrier dysfunction. For example, sex hormones such as estrogen and progesterone induced expression of TJ proteins and inhibited gut permeability in inflammatory organoid models from IBD patients and 2D cell line models.200 Zhou et al201 reported that progesterone upregulated OCLN expression in the gut and inhibited NF-κB activation to maintain gut barrier homeostasis. Irisin, a newly identified exercise hormone, was also reported to ameliorate gut barrier dysfunction via activation of the AMPK-UCP 2 pathway and binding to the integrin αVβ5 receptor.202
Dietary component such as curcumin supplementation reduced high fat diet induced hepatic steatosis by modulating the gut-liver axis and by enhancing ZO-1 and OCLN expression in the intestines.203 Dietary supplements have also provided evidence for treatment of gut barrier dysfunction. Supplementation of dietary garcinol inhibited intestinal barrier dysfunction via elevated ZO-1, CLDN-1, and OCLN expression.204 Dietary cellulose supplements also can ameliorate LPS-mediated intestinal barrier damage and protect against gut epithelial apoptosis.205 However, growing evidences also suggests that certain treatments related to diseases such as sepsis, systemic inflammatory response syndrome, small bowel ischemia, hypoxia, and cancer lead to loss of gut barrier function as the result of enterocyte damage. Hence, withdrawal of treatment in some cases can also reverse gut barrier dysfunction.206
Last, but definitely not the least, dietary habits and a healthy lifestyle are of the foremost importance in maintaining healthy intestinal barrier functions.207 Early-stage disease development as well as early detection of an imbalance of gut permeability due to external stimuli or dietary habits can be reversed. A healthy life without stress, obesity, and diabetes and accompanied by an alcohol-free diet can reverse gut barrier dysfunction over time. Emerging knowledge of host-microbiota interactions allows for the development of new therapeutic strategies to restore a healthy gut ecosystem and prevent a variety of intestinal diseases.
Conclusions
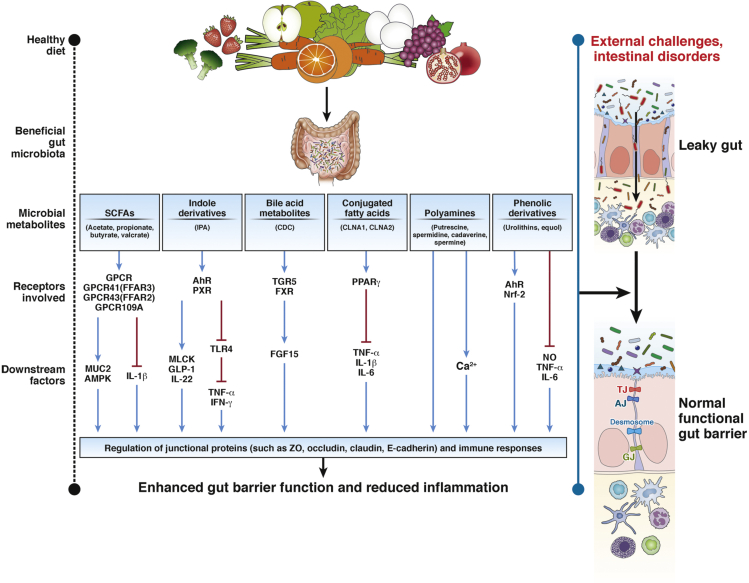
The current review provides a summary of current understanding of the role of gut microbial metabolites in maintenance of gut barrier integrity. The review also discusses potential therapeutic targets and mechanisms of action (Figure 2). In addition, we outlined potential strategies to improve gut barrier integrity. The influence of gut microbiota on intestinal barrier permeability and subsequent host cellular homeostasis is indisputable. Intestinal microbial homeostasis is required for maintenance of good health and disease prevention. Emerging technologies have given insight into microbial dynamics and composition, stimulating the development of new hypotheses concerning microbiota function and host-microbe interactions. Thus, an in-depth understanding of the relationship between a host and its microbiota helps to elucidate detailed mechanistic understanding of pathogenesis and reveals pathways that can be modulated for disease prevention. Reversal of gut dysbiosis is the key factor for any treatment utilizing gut microbiota. In fact, studies have shown that microbial-derived metabolites largely impact pathogen colonization, gut barrier integrity, and host metabolism. Moreover, increasing recognition of the association between gut microbes, gut barrier dysfunction, and intestinal diseases highlights the importance of understanding microbial metabolite production and function. Hence, defining a “healthy” microbiota and microbial metabolites and the development of de novo multiomic technologies as biomarkers and therapeutic tools will significantly advance microbiota research. Furthermore, a deeper understanding of intestinal microbiota dynamics with respect to gut barrier function provides translational opportunities for therapeutic discovery, including drug design, microbe transplantation, and a clearer perspective of host pathophysiology. Additionally, the advantages of extensive longitudinal studies highlight a direction for future investigation, which will unequivocally show the impact of external environmental factors on host physiology and on shaping the gut microbiome population in the human intestine. In a nutshell, there is hope that proper utilization of beneficial gut microbes and gut metabolites will maintain human health devoid of intestinal diseases.
Figure 2.
Potential pathways responsible for microbial mediated enhancement of gut barrier function. Healthy diets get metabolized by beneficial gut commensal microbiota in the human gut to produce various types of microbial metabolites such as SCFAs, indole derivatives, bile acid metabolites, conjugated fatty acids, polyamines, and phenolic derivatives. Each microbial metabolite activates various pathways responsible for modulation of gut barrier and inflammation through the activation of membrane or nuclear receptors as shown.
Acknowledgments
The authors would like to thank Drs Juhi A. Bagaitkar and Fred Ausbel for poof reading and insightful discussions.
Footnotes
Conflicts of interest This author disclose the following: Venkatakrishna Rao Jala is one of the scientific co-founders of Artus Therapeutics. The remaining authors disclose no conflicts.
Funding Venkatakrishna Rao Jala is supported by National Institutes of Health (NIH)/National Cancer Institute (R21CA216090), NIH/National Cancer Institute (CA191683), NIH/National Institute of General Medical Sciences CoBRE grant (P20GM125504-01), P30ES030283 (NIH/National Institute of Environmental Health Sciences), and the Jewish Heritage Fund for Excellence Research Enhancement Grant and James Graham Brown Cancer Center.
References
- 1.Li M., Wang B., Zhang M., Rantalainen M., Wang S., Zhou H., Zhang Y., Shen J., Pang X., Zhang M., Wei H., Chen Y., Lu H., Zuo J., Su M., Qiu Y., Jia W., Xiao C., Smith L.M., Yang S., Holmes E., Tang H., Zhao G., Nicholson J.K., Li L., Zhao L. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A. 2008;105:2117–2122. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh J., Byrd A.L., Deming C., Conlan S., Program N.C.S., Kong H.H., Segre J.A. Biogeography and individuality shape function in the human skin metagenome. Nature. 2014;514:59–64. doi: 10.1038/nature13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch S.V., Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 4.Human Microbiome Project C Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M., Gill S.R., Nelson K.E., Relman D.A. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamada N., Chen G.Y., Inohara N., Nunez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ha C.W., Lam Y.Y., Holmes A.J. Mechanistic links between gut microbial community dynamics, microbial functions and metabolic health. World J Gastroenterol. 2014;20:16498–16517. doi: 10.3748/wjg.v20.i44.16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu H., Tremaroli V., Backhed F. Linking microbiota to human diseases: a systems biology perspective. Trends Endocrinol Metab. 2015;26:758–770. doi: 10.1016/j.tem.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Claesson M.J., Jeffery I.B., Conde S., Power S.E., O'Connor E.M., Cusack S., Harris H.M., Coakley M., Lakshminarayanan B., O'Sullivan O., Fitzgerald G.F., Deane J., O'Connor M., Harnedy N., O'Connor K., O'Mahony D., van Sinderen D., Wallace M., Brennan L., Stanton C., Marchesi J.R., Fitzgerald A.P., Shanahan F., Hill C., Ross R.P., O'Toole P.W. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 10.Gopalakrishnan V., Helmink B.A., Spencer C.N., Reuben A., Wargo J.A. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33:570–580. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martini E., Krug S.M., Siegmund B., Neurath M.F., Becker C. Mend your fences: the epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2017;4:33–46. doi: 10.1016/j.jcmgh.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elamin E., Masclee A., Troost F., Pieters H.J., Keszthelyi D., Aleksa K., Dekker J., Jonkers D. Ethanol impairs intestinal barrier function in humans through mitogen activated protein kinase signaling: a combined in vivo and in vitro approach. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Utzeri E., Usai P. Role of non-steroidal anti-inflammatory drugs on intestinal permeability and nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:3954–3963. doi: 10.3748/wjg.v23.i22.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkes J., Viswanathan V.K., Savkovic S.D., Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52:439–451. doi: 10.1136/gut.52.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjarnason I., MacPherson A., Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 16.Leclercq S., Matamoros S., Cani P.D., Neyrinck A.M., Jamar F., Starkel P., Windey K., Tremaroli V., Backhed F., Verbeke K., de Timary P., Delzenne N.M. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111:E4485–E4493. doi: 10.1073/pnas.1415174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Gonzalez M., Diaz-Zepeda C., Eyzaguirre-Velasquez J., Gonzalez-Arancibia C., Bravo J.A., Julio-Pieper M. Investigating gut permeability in animal models of disease. Front Physiol. 2018;9:1962. doi: 10.3389/fphys.2018.01962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank D.N., St Amand A.L., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moayyedi P., Surette M.G., Kim P.T., Libertucci J., Wolfe M., Onischi C., Armstrong D., Marshall J.K., Kassam Z., Reinisch W., Lee C.H. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149:102–109.e6. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Kim P., Gadani A., Abdul-Baki H., Mitre R., Mitre M. Fecal microbiota transplantation in recurrent Clostridium difficile infection: a retrospective single-center chart review. JGH Open. 2019;3:4–9. doi: 10.1002/jgh3.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maurice C.F., Haiser H.J., Turnbaugh P.J. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39–50. doi: 10.1016/j.cell.2012.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh R., Chandrashekharappa S., Bodduluri S.R., Baby B.V., Hegde B., Kotla N.G., Hiwale A.A., Saiyed T., Patel P., Vijay-Kumar M., Langille M.G.I., Douglas G.M., Cheng X., Rouchka E.C., Waigel S.J., Dryden G.W., Alatassi H., Zhang H.G., Haribabu B., Vemula P.K., Jala V.R. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun. 2019;10:89. doi: 10.1038/s41467-018-07859-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ewaschuk J.B., Diaz H., Meddings L., Diederichs B., Dmytrash A., Backer J., Looijer-van Langen M., Madsen K.L. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1025–G1034. doi: 10.1152/ajpgi.90227.2008. [DOI] [PubMed] [Google Scholar]
- 24.Anderson R.C., Cookson A.L., McNabb W.C., Park Z., McCann M.J., Kelly W.J., Roy N.C. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010;10:316. doi: 10.1186/1471-2180-10-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 26.Okumura R., Takeda K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp Mol Med. 2017;49:e338. doi: 10.1038/emm.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allaire J.M., Crowley S.M., Law H.T., Chang S.-Y., Ko H.-J., Vallance B.A. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 2018;39:677–696. doi: 10.1016/j.it.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Corfield A.P. The interaction of the gut microbiota with the mucus barrier in health and disease in human. Microorganisms. 2018;6:78. doi: 10.3390/microorganisms6030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corfield A.P., Carroll D., Myerscough N., Probert C.S. Mucins in the gastrointestinal tract in health and disease. Front Biosci. 2001;6:D1321–D1357. doi: 10.2741/corfield. [DOI] [PubMed] [Google Scholar]
- 30.Kunzelmann K., Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- 31.Niessen C.M. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2007;127:2525–2532. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- 32.Groschwitz K.R., Hogan S.P. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3–20. doi: 10.1016/j.jaci.2009.05.038. quiz 21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Itallie C.M., Anderson J.M. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 34.Fung K.Y.Y., Fairn G.D., Lee W.L. Transcellular vesicular transport in epithelial and endothelial cells: challenges and opportunities. Traffic. 2018;19:5–18. doi: 10.1111/tra.12533. [DOI] [PubMed] [Google Scholar]
- 35.Shigetomi K., Ikenouchi J. Regulation of the epithelial barrier by post-translational modifications of tight junction membrane proteins. J Biochem. 2018;163:265–272. doi: 10.1093/jb/mvx077. [DOI] [PubMed] [Google Scholar]
- 36.Chiba H., Osanai M., Murata M., Kojima T., Sawada N. Transmembrane proteins of tight junctions. Biochim Biophys Acta. 2008;1778:588–600. doi: 10.1016/j.bbamem.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 37.Schulzke J.D., Fromm M. Tight junctions: molecular structure meets function. Ann N Y Acad Sci. 2009;1165:1–6. doi: 10.1111/j.1749-6632.2009.04925.x. [DOI] [PubMed] [Google Scholar]
- 38.Furuse M., Hirase T., Itoh M., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furuse M., Fujita K., Hiiragi T., Fujimoto K., Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikenouchi J., Furuse M., Furuse K., Sasaki H., Tsukita S., Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garrido-Urbani S., Bradfield P.F., Imhof B.A. Tight junction dynamics: the role of junctional adhesion molecules (JAMs) Cell Tissue Res. 2014;355:701–715. doi: 10.1007/s00441-014-1820-1. [DOI] [PubMed] [Google Scholar]
- 42.Odenwald M.A., Choi W., Kuo W.T., Singh G., Sailer A., Wang Y., Shen L., Fanning A.S., Turner J.R. The scaffolding protein ZO-1 coordinates actomyosin and epithelial apical specializations in vitro and in vivo. J Biol Chem. 2018;293:17317–17335. doi: 10.1074/jbc.RA118.003908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Hernandez V., Quiros M., Nusrat A. Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation. Ann N Y Acad Sci. 2017;1397:66–79. doi: 10.1111/nyas.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laukoetter M.G., Bruewer M., Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr Opin Gastroenterol. 2006;22:85–89. doi: 10.1097/01.mog.0000203864.48255.4f. [DOI] [PubMed] [Google Scholar]
- 45.Buckley A., Turner J.R. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol. 2018;10:A02931. doi: 10.1101/cshperspect.a029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farkas A.E., Nusrat A. Pharmacological targeting of the inflamed intestinal barrier. Curr Pharm Des. 2016;22:5400–5414. doi: 10.2174/1381612822666160726123857. [DOI] [PubMed] [Google Scholar]
- 47.Choi W., Yeruva S., Turner J.R. Contributions of intestinal epithelial barriers to health and disease. Exp Cell Res. 2017;358:71–77. doi: 10.1016/j.yexcr.2017.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuo L., Kuo W.T., Turner J.R. Tight junctions as targets and effectors of mucosal immune homeostasis. Cell Mol Gastroenterol Hepatol. 2020;10:327–340. doi: 10.1016/j.jcmgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turner J.R. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 50.Anderson J.M., Van Itallie C.M. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buschmann M.M., Shen L., Rajapakse H., Raleigh D.R., Wang Y., Wang Y., Lingaraju A., Zha J., Abbott E., McAuley E.M., Breskin L.A., Wu L., Anderson K., Turner J.R., Weber C.R. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol Biol Cell. 2013;24:3056–3068. doi: 10.1091/mbc.E12-09-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen L., Black E.D., Witkowski E.D., Lencer W.I., Guerriero V., Schneeberger E.E., Turner J.R. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci. 2006;119:2095–2106. doi: 10.1242/jcs.02915. [DOI] [PubMed] [Google Scholar]
- 53.Su L., Shen L., Clayburgh D.R., Nalle S.C., Sullivan E.A., Meddings J.B., Abraham C., Turner J.R. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136:551–563. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu A.S., McCarthy K.M., Francis S.A., McCormack J.M., Lai J., Rogers R.A., Lynch R.D., Schneeberger E.E. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005;288:C1231–C1241. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- 55.Odenwald M.A., Turner J.R. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14:9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patankar J.V., Becker C. Cell death in the gut epithelium and implications for chronic inflammation. Nat Rev Gastroenterol Hepatol. 2020;17:543–556. doi: 10.1038/s41575-020-0326-4. [DOI] [PubMed] [Google Scholar]
- 57.Gitter A.H., Bendfeldt K., Schulzke J.D., Fromm M. Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis. FASEB J. 2000;14:1749–1753. doi: 10.1096/fj.99-0898com. [DOI] [PubMed] [Google Scholar]
- 58.Heller F., Fromm A., Gitter A.H., Mankertz J., Schulzke J.D. Epithelial apoptosis is a prominent feature of the epithelial barrier disturbance in intestinal inflammation: effect of pro-inflammatory interleukin-13 on epithelial cell function. Mucosal Immunol. 2008;1 Suppl 1:S58–S61. doi: 10.1038/mi.2008.46. [DOI] [PubMed] [Google Scholar]
- 59.Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thursby E., Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Z., Quan G., Jiang X., Yang Y., Ding X., Zhang D., Wang X., Hardwidge P.R., Ren W., Zhu G. Effects of metabolites derived from gut microbiota and hosts on pathogens. Front Cell Infect Microbiol. 2018;8:314. doi: 10.3389/fcimb.2018.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Araki S., Suzuki M., Fujimoto M., Kimura M. Enhancement of resistance to bacterial infection in mice by vitamin B2. J Vet Med Sci. 1995;57:599–602. doi: 10.1292/jvms.57.599. [DOI] [PubMed] [Google Scholar]
- 63.Donia M.S., Fischbach M.A. Human microbiota. Small molecules from the human microbiota. Science. 2015;349:1254766. doi: 10.1126/science.1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 65.Cohen L.J., Esterhazy D., Kim S.H., Lemetre C., Aguilar R.R., Gordon E.A., Pickard A.J., Cross J.R., Emiliano A.B., Han S.M., Chu J., Vila-Farres X., Kaplitt J., Rogoz A., Calle P.Y., Hunter C., Bitok J.K., Brady S.F. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature. 2017;549:48–53. doi: 10.1038/nature23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berdy J. Bioactive microbial metabolites. J Antibiot (Tokyo) 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 67.Bethal V. Mode of action of microbial bioactive metabolites. Folia Microbiol (Praha) 2006;51:359–369. doi: 10.1007/BF02931577. [DOI] [PubMed] [Google Scholar]
- 68.Fernandez-Navarro T., Salazar N., Gutierrez-Diaz I., Sanchez B., Ruas-Madiedo P., de Los Reyes-Gavilan C.G., Margolles A., Gueimonde M., Gonzalez S. Bioactive compounds from regular diet and faecal microbial metabolites. Eur J Nutr. 2018;57:487–497. doi: 10.1007/s00394-016-1332-8. [DOI] [PubMed] [Google Scholar]
- 69.Omura S. Thom Award Lecture. Trends in the search for bioactive microbial metabolites. J Ind Microbiol. 1992;10:135–156. doi: 10.1007/BF01569759. [DOI] [PubMed] [Google Scholar]
- 70.Umezawa H. Recent advances in bioactive microbial secondary metabolites. Jpn J Antibiot. 1977;30:138–163. [PubMed] [Google Scholar]
- 71.Zhang L.S., Davies S.S. Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome Med. 2016;8:46. doi: 10.1186/s13073-016-0296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kho Z.Y., Lal S.K. The human gut microbiome - a potential controller of wellness and disease. Front Microbiol. 2018;9:1835. doi: 10.3389/fmicb.2018.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alam A., Neish A. Role of gut microbiota in intestinal wound healing and barrier function. Tissue Barriers. 2018;6:1539595. doi: 10.1080/21688370.2018.1539595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prochazkova P., Roubalova R., Dvorak J., Tlaskalova-Hogenova H., Cermakova M., Tomasova P., Sediva B., Kuzma M., Bulant J., Bilej M., Hrabak P., Meisnerova E., Lambertova A., Papezova H. Microbiota, microbial metabolites, and barrier function in a patient with anorexia nervosa after fecal microbiota transplantation. Microorganisms. 2019;7:338. doi: 10.3390/microorganisms7090338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wong J.M., de Souza R., Kendall C.W., Emam A., Jenkins D.J. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 76.Hernández-Chirlaque C., Aranda C.J., Ocón B., Capitán-Cañadas F., Ortega-González M., Carrero J.J., Suárez M.D., Zarzuelo A., Sánchez de Medina F., Martínez-Augustin O. Germ-free and antibiotic-treated mice are highly susceptible to epithelial injury in DSS Colitis. J Crohns Colitis. 2016;10:1324–1335. doi: 10.1093/ecco-jcc/jjw096. [DOI] [PubMed] [Google Scholar]
- 77.Wu W., Sun M., Chen F., Cao A.T., Liu H., Zhao Y., Huang X., Xiao Y., Yao S., Zhao Q., Liu Z., Cong Y. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017;10:946–956. doi: 10.1038/mi.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Macfarlane S., Macfarlane G.T. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 79.Nielsen D.S.G., Jensen B.B., Theil P.K., Nielsen T.S., Knudsen K.E.B., Purup S. Effect of butyrate and fermentation products on epithelial integrity in a mucus-secreting human colon cell line. Journal of Functional Foods. 2018;40:9–17. [Google Scholar]
- 80.Peng L., He Z., Chen W., Holzman I.R., Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res. 2007;61:37–41. doi: 10.1203/01.pdr.0000250014.92242.f3. [DOI] [PubMed] [Google Scholar]
- 81.Peng L., Li Z.R., Green R.S., Holzman I.R., Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang H.B., Wang P.Y., Wang X., Wan Y.L., Liu Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein claudin-1 transcription. Dig Dis Sci. 2012;57:3126–3135. doi: 10.1007/s10620-012-2259-4. [DOI] [PubMed] [Google Scholar]
- 83.Feng Y., Wang Y., Wang P., Huang Y., Wang F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell Physiol Biochem. 2018;49:190–205. doi: 10.1159/000492853. [DOI] [PubMed] [Google Scholar]
- 84.Roe A.J., McLaggan D., Davidson I., O'Byrne C., Booth I.R. Perturbation of anion balance during inhibition of growth of Escherichia coli by weak acids. J Bacteriol. 1998;180:767–772. doi: 10.1128/jb.180.4.767-772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sivaprakasam S., Bhutia Y.D., Yang S., Ganapathy V. Short-chain fatty acid transporters: role in colonic homeostasis. Compr Physiol. 2017;8:299–314. doi: 10.1002/cphy.c170014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hald S. Aarhus University Hospital, Aarhus, Denmark; Aarhus, Denmark: 2015. Effects of dietary fibres on gut microbiota, faecal short-chain fatty acids and intestinal inflammation [PhD thesis] [Google Scholar]
- 87.Diao H., Jiao A.R., Yu B., Mao X.B., Chen D.W. Gastric infusion of short-chain fatty acids can improve intestinal barrier function in weaned piglets. Genes Nutr. 2019;14:4. doi: 10.1186/s12263-019-0626-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hendrikx T., Schnabl B. Indoles: metabolites produced by intestinal bacteria capable of controlling liver disease manifestation. J Intern Med. 2019;286:32–40. doi: 10.1111/joim.12892. [DOI] [PubMed] [Google Scholar]
- 89.Zelante T., Iannitti R.G., Cunha C., De Luca A., Giovannini G., Pieraccini G., Zecchi R., D'Angelo C., Massi-Benedetti C., Fallarino F., Carvalho A., Puccetti P., Romani L. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 90.Shimada Y., Kinoshita M., Harada K., Mizutani M., Masahata K., Kayama H., Takeda K. Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geng S., Cheng S., Li Y., Wen Z., Ma X., Jiang X., Wang Y., Han X. Faecal microbiota transplantation reduces susceptibility to epithelial injury and modulates tryptophan metabolism of the microbial community in a piglet model. J Crohns Colitis. 2018;12:1359–1374. doi: 10.1093/ecco-jcc/jjy103. [DOI] [PubMed] [Google Scholar]
- 92.Yu M., Wang Q., Ma Y., Li L., Yu K., Zhang Z., Chen G., Li X., Xiao W., Xu P., Yang H. Aryl hydrocarbon receptor activation modulates intestinal epithelial barrier function by maintaining tight junction integrity. Int J Biol Sci. 2018;14:69–77. doi: 10.7150/ijbs.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Teng Y., Ren Y., Sayed M., Hu X., Lei C., Kumar A., Hutchins E., Mu J., Deng Z., Luo C., Sundaram K., Sriwastva M.K., Zhang L., Hsieh M., Reiman R., Haribabu B., Yan J., Jala V.R., Miller D.M., Van Keuren-Jensen K., Merchant M.L., McClain C.J., Park J.W., Egilmez N.K., Zhang H.G. Plant-derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe. 2018;24:637–652.e8. doi: 10.1016/j.chom.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han B., Sheng B., Zhang Z., Pu A., Yin J., Wang Q., Yang K., Sun L., Yu M., Qiu Y., Xiao W., Yang H. Aryl hydrocarbon receptor activation in intestinal obstruction ameliorates intestinal barrier dysfunction via suppression of MLCK-MLC phosphorylation pathway. Shock. 2016;46:319–328. doi: 10.1097/SHK.0000000000000594. [DOI] [PubMed] [Google Scholar]
- 95.Natividad J.M., Agus A., Planchais J., Lamas B., Jarry A.C., Martin R., Michel M.L., Chong-Nguyen C., Roussel R., Straube M., Jegou S., McQuitty C., Le Gall M., da Costa G., Lecornet E., Michaudel C., Modoux M., Glodt J., Bridonneau C., Sovran B., Dupraz L., Bado A., Richard M.L., Langella P., Hansel B., Launay J.M., Xavier R.J., Duboc H., Sokol H. Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab. 2018;28:737–749.e4. doi: 10.1016/j.cmet.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 96.Shao T., Zhao C., Li F., Gu Z., Liu L., Zhang L., Wang Y., He L., Liu Y., Liu Q., Chen Y., Donde H., Wang R., Jala V.R., Barve S., Chen S.Y., Zhang X., Chen Y., McClain C.J., Feng W. Intestinal HIF-1alpha deletion exacerbates alcoholic liver disease by inducing intestinal dysbiosis and barrier dysfunction. J Hepatol. 2018;69:886–895. doi: 10.1016/j.jhep.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kiss E.A., Vonarbourg C., Kopfmann S., Hobeika E., Finke D., Esser C., Diefenbach A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 98.Lee J.S., Cella M., McDonald K.G., Garlanda C., Kennedy G.D., Nukaya M., Mantovani A., Kopan R., Bradfield C.A., Newberry R.D., Colonna M. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2011;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Y., Innocentin S., Withers D.R., Roberts N.A., Gallagher A.R., Grigorieva E.F., Wilhelm C., Veldhoen M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 100.Metidji A., Omenetti S., Crotta S., Li Y., Nye E., Ross E., Li V., Maradana M.R., Schiering C., Stockinger B. The environmental sensor AHR protects from inflammatory damage by maintaining intestinal stem cell homeostasis and barrier integrity. Immunity. 2019;50:1542. doi: 10.1016/j.immuni.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Russo E., Giudici F., Fiorindi C., Ficari F., Scaringi S., Amedei A. Immunomodulating activity and therapeutic effects of short chain fatty acids and tryptophan post-biotics in inflammatory bowel disease. Front Immunol. 2019;10:2754. doi: 10.3389/fimmu.2019.02754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dawson P.A., Karpen S.J. Intestinal transport and metabolism of bile acids. J Lipid Res. 2015;56:1085–1099. doi: 10.1194/jlr.R054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li T., Chiang J.Y. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66:948–983. doi: 10.1124/pr.113.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sarathy J., Detloff S.J., Ao M., Khan N., French S., Sirajuddin H., Nair T., Rao M.C. The Yin and Yang of bile acid action on tight junctions in a model colonic epithelium. Physiol Rep. 2017;5 doi: 10.14814/phy2.13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stenman L.K., Holma R., Korpela R. High-fat-induced intestinal permeability dysfunction associated with altered fecal bile acids. World J Gastroenterol. 2012;18:923–929. doi: 10.3748/wjg.v18.i9.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pavlidis P., Powell N., Vincent R.P., Ehrlich D., Bjarnason I., Hayee B. Systematic review: bile acids and intestinal inflammation-luminal aggressors or regulators of mucosal defence? Aliment Pharmacol Ther. 2015;42:802–817. doi: 10.1111/apt.13333. [DOI] [PubMed] [Google Scholar]
- 107.Stenman L.K., Holma R., Eggert A., Korpela R. A novel mechanism for gut barrier dysfunction by dietary fat: epithelial disruption by hydrophobic bile acids. Am J Physiol Gastrointest Liver Physiol. 2013;304:G227–G234. doi: 10.1152/ajpgi.00267.2012. [DOI] [PubMed] [Google Scholar]
- 108.Fort M.M., Mozaffarian A., Stöver A.G., Correia Jda S., Johnson D.A., Crane R.T., Ulevitch R.J., Persing D.H., Bielefeldt-Ohmann H., Probst P., Jeffery E., Fling S.P., Hershberg R.M. A synthetic TLR4 antagonist has anti-inflammatory effects in two murine models of inflammatory bowel disease. J Immunol. 2005;174:6416–6423. doi: 10.4049/jimmunol.174.10.6416. [DOI] [PubMed] [Google Scholar]
- 109.Ticho A.L., Malhotra P., Dudeja P.K., Gill R.K., Alrefai W.A. Bile acid receptors and gastrointestinal functions. Liver Res. 2019;3:31–39. doi: 10.1016/j.livres.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raimondi F., Santoro P., Barone M.V., Pappacoda S., Barretta M.L., Nanayakkara M., Apicella C., Capasso L., Paludetto R. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G906–G913. doi: 10.1152/ajpgi.00043.2007. [DOI] [PubMed] [Google Scholar]
- 111.de Diego-Cabero N., Mereu A., Menoyo D., Holst J.J., Ipharraguerre I.R. Bile acid mediated effects on gut integrity and performance of early weaned piglets. BMC Vet Res. 2015;11:111. doi: 10.1186/s12917-015-0425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu L., Liu Z., Li H., Cao Z., Li W., Song Z., Li X., Lu A., Lu C., Liu Y. Naturally occurring TPE-CA maintains gut microbiota and bile acids homeostasis via FXR signaling modulation of the liver-gut axis. Front Pharmacol. 2020;11:12. doi: 10.3389/fphar.2020.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maccioni L., Gao B., Leclercq S., Pirlot B., Horsmans Y., De Timary P., Leclercq I., Fouts D., Schnabl B., Stärkel P. Intestinal permeability, microbial translocation, changes in duodenal and fecal microbiota, and their associations with alcoholic liver disease progression in humans. Gut Microbes. 2020;12:1782157. doi: 10.1080/19490976.2020.1782157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Meroni M., Longo M., Dongiovanni P. Alcohol or gut microbiota: who is the guilty? Int J Mol Sci. 2019;20:4568. doi: 10.3390/ijms20184568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cassard A.M., Ciocan D. Microbiota, a key player in alcoholic liver disease. Clin Mol Hepatol. 2018;24:100–107. doi: 10.3350/cmh.2017.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hartmann P., Hochrath K., Horvath A., Chen P., Seebauer C.T., Llorente C., Wang L., Alnouti Y., Fouts D.E., Stärkel P., Loomba R., Coulter S., Liddle C., Yu R.T., Ling L., Rossi S.J., DePaoli A.M., Downes M., Evans R.M., Brenner D.A., Schnabl B. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology. 2018;67:2150–2166. doi: 10.1002/hep.29676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cipriani S., Mencarelli A., Chini M.G., Distrutti E., Renga B., Bifulco G., Baldelli F., Donini A., Fiorucci S. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schoeler M., Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord. 2019;20:461–472. doi: 10.1007/s11154-019-09512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roche H.M., Terres A.M., Black I.B., Gibney M.J., Kelleher D. Fatty acids and epithelial permeability: effect of conjugated linoleic acid in Caco-2 cells. Gut. 2001;48:797–802. doi: 10.1136/gut.48.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen Y., Yang B., Ross R.P., Jin Y., Stanton C., Zhao J., Zhang H., Chen W. Orally administered CLA ameliorates DSS-induced colitis in mice via intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokine and gut microbiota modulation. J Agric Food Chem. 2019;67:13282–13298. doi: 10.1021/acs.jafc.9b05744. [DOI] [PubMed] [Google Scholar]
- 121.Ren Q., Yang B., Zhang H., Ross R.P., Stanton C., Chen H., Chen W. c9, t11, c15-CLNA and t9, t11, c15-CLNA from Lactobacillus plantarum ZS2058 ameliorate dextran sodium sulfate-induced colitis in mice. J Agric Food Chem. 2020;68:3758–3769. doi: 10.1021/acs.jafc.0c00573. [DOI] [PubMed] [Google Scholar]
- 122.Tofalo R., Cocchi S., Suzzi G. Polyamines and gut microbiota. Front Nutr. 2019;6:16. doi: 10.3389/fnut.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Timmons J., Chang E.T., Wang J.Y., Rao J.N. Polyamines and gut mucosal homeostasis. J Gastrointest Dig Syst. 2012;2 Suppl 7:001. [PMC free article] [PubMed] [Google Scholar]
- 124.Matsumoto M., Kurihara S., Kibe R., Ashida H., Benno Y. Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PLoS One. 2011;6 doi: 10.1371/journal.pone.0023652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Michael A.J. Biosynthesis of polyamines and polyamine-containing molecules. Biochem J. 2016;473:2315–2329. doi: 10.1042/BCJ20160185. [DOI] [PubMed] [Google Scholar]
- 126.Liu L., Guo X., Rao J.N., Zou T., Xiao L., Yu T., Timmons J.A., Turner D.J., Wang J.Y. Polyamines regulate E-cadherin transcription through c-Myc modulating intestinal epithelial barrier function. Am J Physiol Cell Physiol. 2009;296:C801–C810. doi: 10.1152/ajpcell.00620.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guo X., Rao J.N., Liu L., Zou T.T., Turner D.J., Bass B.L., Wang J.Y. Regulation of adherens junctions and epithelial paracellular permeability: a novel function for polyamines. Am J Physiol Cell Physiol. 2003;285:C1174–C1187. doi: 10.1152/ajpcell.00015.2003. [DOI] [PubMed] [Google Scholar]
- 128.Guo X., Rao J.N., Liu L., Zou T., Keledjian K.M., Boneva D., Marasa B.S., Wang J.Y. Polyamines are necessary for synthesis and stability of occludin protein in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1159–G1169. doi: 10.1152/ajpgi.00407.2004. [DOI] [PubMed] [Google Scholar]
- 129.Heber D. Multitargeted therapy of cancer by ellagitannins. Cancer LEtt. 2008;269:262–268. doi: 10.1016/j.canlet.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 130.Espin J.C., Larrosa M., Garcia-Conesa M.T., Tomas-Barberan F. Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: the evidence so far. Evid Based Complement Alternat Med. 2013;2013:270418. doi: 10.1155/2013/270418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cerda B., Periago P., Espin J.C., Tomas-Barberan F.A. Identification of urolithin a as a metabolite produced by human colon microflora from ellagic acid and related compounds. J Agric Food Chem. 2005;53:5571–5576. doi: 10.1021/jf050384i. [DOI] [PubMed] [Google Scholar]
- 132.Espin J.C., Gonzalez-Barrio R., Cerda B., Lopez-Bote C., Rey A.I., Tomas-Barberan F.A. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J Agric Food Chem. 2007;55:10476–10485. doi: 10.1021/jf0723864. [DOI] [PubMed] [Google Scholar]
- 133.Tomas-Barberan F.A., Gonzalez-Sarrias A., Garcia-Villalba R., Nunez-Sanchez M.A., Selma M.V., Garcia-Conesa M.T., Espin J.C. Urolithins, the rescue of 'old' metabolites to understand a 'new' concept: metabotypes as a nexus between phenolic metabolism, microbiota dysbiosis and host health status. Mol Nutr Food Res. 2017;61 doi: 10.1002/mnfr.201500901. mnfr.201500901. [DOI] [PubMed] [Google Scholar]
- 134.Ryu D., Mouchiroud L., Andreux P.A., Katsyuba E., Moullan N., Nicolet-Dit-Felix A.A., Williams E.G., Jha P., Lo Sasso G., Huzard D., Aebischer P., Sandi C., Rinsch C., Auwerx J. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016;22:879–888. doi: 10.1038/nm.4132. [DOI] [PubMed] [Google Scholar]
- 135.Saha P., Yeoh B.S., Singh R., Chandrasekar B., Vemula P.K., Haribabu B., Vijay-Kumar M., Jala V.R. Gut microbiota conversion of dietary ellagic acid into bioactive phytoceutical urolithin A inhibits heme peroxidases. PLoS One. 2016;11 doi: 10.1371/journal.pone.0156811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cerda B., Espin J.C., Parra S., Martinez P., Tomas-Barberan F.A. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur J Nutr. 2004;43:205–220. doi: 10.1007/s00394-004-0461-7. [DOI] [PubMed] [Google Scholar]
- 137.Garcia-Villalba R., Beltran D., Espin J.C., Selma M.V., Tomas-Barberan F.A. Time course production of urolithins from ellagic acid by human gut microbiota. J Agric Food Chem. 2013;61:8797–8806. doi: 10.1021/jf402498b. [DOI] [PubMed] [Google Scholar]
- 138.Gonzalez-Sarrias A., Gimenez-Bastida J.A., Garcia-Conesa M.T., Gomez-Sanchez M.B., Garcia-Talavera N.V., Gil-Izquierdo A., Sanchez-Alvarez C., Fontana-Compiano L.O., Morga-Egea J.P., Pastor-Quirante F.A., Martinez-Diaz F., Tomas-Barberan F.A., Espin J.C. Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol Nutr Food Res. 2010;54:311–322. doi: 10.1002/mnfr.200900152. [DOI] [PubMed] [Google Scholar]
- 139.Tomas-Barberan F.A., Garcia-Villalba R., Gonzalez-Sarrias A., Selma M.V., Espin J.C. Ellagic acid metabolism by human gut microbiota: consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J Agric Food Chem. 2014;62:6535–6538. doi: 10.1021/jf5024615. [DOI] [PubMed] [Google Scholar]
- 140.Cerda B., Tomas-Barberan F.A., Espin J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability. J Agric Food Chem. 2005;53:227–235. doi: 10.1021/jf049144d. [DOI] [PubMed] [Google Scholar]
- 141.Seeram N.P., Zhang Y., McKeever R., Henning S.M., Lee R.P., Suchard M.A., Li Z., Chen S., Thames G., Zerlin A., Nguyen M., Wang D., Dreher M., Heber D. Pomegranate juice and extracts provide similar levels of plasma and urinary ellagitannin metabolites in human subjects. J Med Food. 2008;11:390–394. doi: 10.1089/jmf.2007.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Seeram N.P., Henning S.M., Zhang Y., Suchard M., Li Z., Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr. 2006;136:2481–2485. doi: 10.1093/jn/136.10.2481. [DOI] [PubMed] [Google Scholar]
- 143.Nunez-Sanchez M.A., Garcia-Villalba R., Monedero-Saiz T., Garcia-Talavera N.V., Gomez-Sanchez M.B., Sanchez-Alvarez C., Garcia-Albert A.M., Rodriguez-Gil F.J., Ruiz-Marin M., Pastor-Quirante F.A., Martinez-Diaz F., Yanez-Gascon M.J., Gonzalez-Sarrias A., Tomas-Barberan F.A., Espin J.C. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol Nutr Food Res. 2014;58:1199–1211. doi: 10.1002/mnfr.201300931. [DOI] [PubMed] [Google Scholar]
- 144.Larrosa M., Gonzalez-Sarrias A., Yanez-Gascon M.J., Selma M.V., Azorin-Ortuno M., Toti S., Tomas-Barberan F., Dolara P., Espin J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem. 2010;21:717–725. doi: 10.1016/j.jnutbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 145.Heilman J., Andreux P., Tran N., Rinsch C., Blanco-Bose W. Safety assessment of Urolithin A, a metabolite produced by the human gut microbiota upon dietary intake of plant derived ellagitannins and ellagic acid. Food Chem Toxicol. 2017;108:289–297. doi: 10.1016/j.fct.2017.07.050. [DOI] [PubMed] [Google Scholar]
- 146.Selma M.V., Beltran D., Garcia-Villalba R., Espin J.C., Tomas-Barberan F.A. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct. 2014;5:1779–1784. doi: 10.1039/c4fo00092g. [DOI] [PubMed] [Google Scholar]
- 147.Gaya P., Peiroténa A., Medinaa M., Álvarezb I., Landete J.M. Bifidobacterium pseudocatenulatum INIA P815: The first bacterium abl e to produce urolithins A and B from ellagic acid. J Funct Foods. 2018;45:95–99. [Google Scholar]
- 148.García-Niño W.R., Zazueta C. Ellagic acid: Pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol Res. 2015;97:84–103. doi: 10.1016/j.phrs.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 149.Kang I., Buckner T., Shay N.F., Gu L., Chung S. Improvements in metabolic health with consumption of ellagic acid and subsequent conversion into urolithins: evidence and mechanisms. Adv Nutr. 2016;7:961–972. doi: 10.3945/an.116.012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guglielmetti S., Bernardi S., Del Bo C., Cherubini A., Porrini M., Gargari G., Hidalgo-Liberona N., Gonzalez-Dominguez R., Peron G., Zamora-Ros R., Winterbone M.S., Kirkup B., Kroon P.A., Andres-Lacueva C., Riso P. Effect of a polyphenol-rich dietary pattern on intestinal permeability and gut and blood microbiomics in older subjects: study protocol of the MaPLE randomised controlled trial. BMC Geriatr. 2020;20:77. doi: 10.1186/s12877-020-1472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Johnson S.L., Kirk R.D., DaSilva N.A., Ma H., Seeram N.P., Bertin M.J. Polyphenol microbial metabolites exhibit gut and blood(-)brain barrier permeability and protect murine microglia against LPS-induced inflammation. Metabolites. 2019;9:78. doi: 10.3390/metabo9040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Polewski M.A., Esquivel-Alvarado D., Wedde N.S., Kruger C.G., Reed J.D. Isolation and characterization of blueberry polyphenolic components and their effects on gut barrier dysfunction. J Agric Food Chem. 2020;68:2940–2947. doi: 10.1021/acs.jafc.9b01689. [DOI] [PubMed] [Google Scholar]
- 153.Ong H.S., Yim H.C.H. Microbial factors in inflammatory diseases and cancers. Adv Exp Med Biol. 2017;1024:153–174. doi: 10.1007/978-981-10-5987-2_7. [DOI] [PubMed] [Google Scholar]
- 154.Mu C., Yang Y., Zhu W. Crosstalk between the immune receptors and gut microbiota. Curr Protein Pept Sci. 2015;16:622–631. doi: 10.2174/1389203716666150630134356. [DOI] [PubMed] [Google Scholar]
- 155.Marques R., Boneca I.G. Expression and functional importance of innate immune receptors by intestinal epithelial cells. Cell Mol Life Sci. 2011;68:3661–3673. doi: 10.1007/s00018-011-0829-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wells J.M., Rossi O., Meijerink M., van Baarlen P. Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4607–4614. doi: 10.1073/pnas.1000092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Creagh E.M., O'Neill L.A. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352–357. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 158.Ey B., Eyking A., Gerken G., Podolsky D.K., Cario E. TLR2 mediates gap junctional intercellular communication through connexin-43 in intestinal epithelial barrier injury. J Biol Chem. 2009;284:22332–22343. doi: 10.1074/jbc.M901619200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cario E., Gerken G., Podolsky D.K. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 160.Wang W., Xia T., Yu X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-kappaB pathway in vitro. Inflamm Res. 2015;64:423–431. doi: 10.1007/s00011-015-0822-0. [DOI] [PubMed] [Google Scholar]
- 161.Luo H., Guo P., Zhou Q. Role of TLR4/NF-kappaB in damage to intestinal mucosa barrier function and bacterial translocation in rats exposed to hypoxia. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ungaro R., Fukata M., Hsu D., Hernandez Y., Breglio K., Chen A., Xu R., Sotolongo J., Espana C., Zaias J., Elson G., Mayer L., Kosco-Vilbois M., Abreu M.T. A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1167–G1179. doi: 10.1152/ajpgi.90496.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Peterson C.Y., Costantini T.W., Loomis W.H., Putnam J.G., Wolf P., Bansal V., Eliceiri B.P., Baird A., Coimbra R. Toll-like receptor-4 mediates intestinal barrier breakdown after thermal injury. Surg Infect (Larchmt) 2010;11:137–144. doi: 10.1089/sur.2009.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Steiner T.S. How flagellin and toll-like receptor 5 contribute to enteric infection. Infect Immun. 2007;75:545–552. doi: 10.1128/IAI.01506-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chassaing B., Ley R.E., Gewirtz A.T. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology. 2014;147:1363–1377.e17. doi: 10.1053/j.gastro.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vijay-Kumar M., Sanders C.J., Taylor R.T., Kumar A., Aitken J.D., Sitaraman S.V., Neish A.S., Uematsu S., Akira S., Williams I.R., Gewirtz A.T. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Leifer C.A., McConkey C., Li S., Chassaing B., Gewirtz A.T., Ley R.E. Linking genetic variation in human Toll-like receptor 5 genes to the gut microbiome's potential to cause inflammation. Immunol Lett. 2014;162:3–9. doi: 10.1016/j.imlet.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vijay-Kumar M., Aitken J.D., Gewirtz A.T. Toll like receptor-5: protecting the gut from enteric microbes. Semin Immunopathol. 2008;30:11–21. doi: 10.1007/s00281-007-0100-5. [DOI] [PubMed] [Google Scholar]
- 169.Lopetuso L.R., Jia R., Wang X.M., Jia L.G., Petito V., Goodman W.A., Meddings J.B., Cominelli F., Reuter B.K., Pizarro T.T. Epithelial-specific Toll-like receptor (TLR)5 activation mediates barrier dysfunction in experimental ileitis. Inflamm Bowel Dis. 2017;23:392–403. doi: 10.1097/MIB.0000000000001035. [DOI] [PubMed] [Google Scholar]
- 170.Carvalho F.A., Aitken J.D., Gewirtz A.T., Vijay-Kumar M. TLR5 activation induces secretory interleukin-1 receptor antagonist (sIL-1Ra) and reduces inflammasome-associated tissue damage. Mucosal Immunol. 2011;4:102–111. doi: 10.1038/mi.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rose W.A., 2nd, Sakamoto K., Leifer C.A. TLR9 is important for protection against intestinal damage and for intestinal repair. Sci Rep. 2012;2:574. doi: 10.1038/srep00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Parlato M., Yeretssian G. NOD-like receptors in intestinal homeostasis and epithelial tissue repair. Int J Mol Sci. 2014;15:9594–9627. doi: 10.3390/ijms15069594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yeretssian G. Effector functions of NLRs in the intestine: innate sensing, cell death, and disease. Immunol Res. 2012;54:25–36. doi: 10.1007/s12026-012-8317-3. [DOI] [PubMed] [Google Scholar]
- 174.Chen G.Y., Shaw M.H., Redondo G., Núñez G. The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 2008;68:10060–10067. doi: 10.1158/0008-5472.CAN-08-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Watanabe T., Asano N., Murray P.J., Ozato K., Tailor P., Fuss I.J., Kitani A., Strober W. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118:545–559. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Macho Fernandez E., Valenti V., Rockel C., Hermann C., Pot B., Boneca I.G., Grangette C. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut. 2011;60:1050–1059. doi: 10.1136/gut.2010.232918. [DOI] [PubMed] [Google Scholar]
- 177.Natividad J.M., Petit V., Huang X., de Palma G., Jury J., Sanz Y., Philpott D., Garcia Rodenas C.L., McCoy K.D., Verdu E.F. Commensal and probiotic bacteria influence intestinal barrier function and susceptibility to colitis in Nod1–/–; Nod2–/– mice. Inflamm Bowel Dis. 2012;18:1434–1446. doi: 10.1002/ibd.22848. [DOI] [PubMed] [Google Scholar]
- 178.Couturier-Maillard A., Secher T., Rehman A., Normand S., De Arcangelis A., Haesler R., Huot L., Grandjean T., Bressenot A., Delanoye-Crespin A., Gaillot O., Schreiber S., Lemoine Y., Ryffel B., Hot D., Nùñez G., Chen G., Rosenstiel P., Chamaillard M. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest. 2013;123:700–711. doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Arpaia N., Campbell C., Fan X., Dikiy S., van der Veeken J., deRoos P., Liu H., Cross J.R., Pfeffer K., Coffer P.J., Rudensky A.Y. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Konieczna P., Ferstl R., Ziegler M., Frei R., Nehrbass D., Lauener R.P., Akdis C.A., O'Mahony L. Immunomodulation by Bifidobacterium infantis 35624 in the murine lamina propria requires retinoic acid-dependent and independent mechanisms. PLoS One. 2013;8 doi: 10.1371/journal.pone.0062617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mazmanian S.K., Liu C.H., Tzianabos A.O., Kasper D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 182.Kayama H., Takeda K. Manipulation of epithelial integrity and mucosal immunity by host and microbiota-derived metabolites. Eur J Immunol. 2020;50:921–931. doi: 10.1002/eji.201948478. [DOI] [PubMed] [Google Scholar]
- 183.Allaire J.M., Crowley S.M., Law H.T., Chang S.Y., Ko H.J., Vallance B.A. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 2018;39:677–696. doi: 10.1016/j.it.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 184.Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T., Takahashi M., Fukuda N.N., Murakami S., Miyauchi E., Hino S., Atarashi K., Onawa S., Fujimura Y., Lockett T., Clarke J.M., Topping D.L., Tomita M., Hori S., Ohara O., Morita T., Koseki H., Kikuchi J., Honda K., Hase K., Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 185.Ahern P.P., Faith J.J., Gordon J.I. Mining the human gut microbiota for effector strains that shape the immune system. Immunity. 2014;40:815–823. doi: 10.1016/j.immuni.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Duan F.F., Liu J.H., March J.C. Engineered commensal bacteria reprogram intestinal cells into glucose-responsive insulin-secreting cells for the treatment of diabetes. Diabetes. 2015;64:1794–1803. doi: 10.2337/db14-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Duan F., Curtis K.L., March J.C. Secretion of insulinotropic proteins by commensal bacteria: rewiring the gut to treat diabetes. Appl Environ Microbiol. 2008;74:7437–7438. doi: 10.1128/AEM.01019-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Khare V., Krnjic A., Frick A., Gmainer C., Asboth M., Jimenez K., Lang M., Baumgartner M., Evstatiev R., Gasche C. Mesalamine and azathioprine modulate junctional complexes and restore epithelial barrier function in intestinal inflammation. Sci Rep. 2019;9:2842. doi: 10.1038/s41598-019-39401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Powrie F., Leach M.W., Mauze S., Menon S., Caddle L.B., Coffman R.L. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 190.Noth R., Stuber E., Hasler R., Nikolaus S., Kuhbacher T., Hampe J., Bewig B., Schreiber S., Arlt A. Anti-TNF-alpha antibodies improve intestinal barrier function in Crohn's disease. J Crohns Colitis. 2012;6:464–469. doi: 10.1016/j.crohns.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 191.Graham W.V., He W., Marchiando A.M., Zha J., Singh G., Li H.-S., Biswas A., Ong M.L.D.M., Jiang Z.-H., Choi W., Zuccola H., Wang Y., Griffith J., Wu J., Rosenberg H.J., Wang Y., Snapper S.B., Ostrov D., Meredith S.C., Miller L.W., Turner J.R. Intracellular MLCK1 diversion reverses barrier loss to restore mucosal homeostasis. Nat Med. 2019;25:690–700. doi: 10.1038/s41591-019-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Atarashi K., Tanoue T., Oshima K., Suda W., Nagano Y., Nishikawa H., Fukuda S., Saito T., Narushima S., Hase K., Kim S., Fritz J.V., Wilmes P., Ueha S., Matsushima K., Ohno H., Olle B., Sakaguchi S., Taniguchi T., Morita H., Hattori M., Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 193.Braat H., Rottiers P., Hommes D.W., Huyghebaert N., Remaut E., Remon J.P., van Deventer S.J., Neirynck S., Peppelenbosch M.P., Steidler L. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn's disease. Clin Gastroenterol Hepatol. 2006;4:754–759. doi: 10.1016/j.cgh.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 194.Steidler L., Hans W., Schotte L., Neirynck S., Obermeier F., Falk W., Fiers W., Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–1355. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 195.Le Bastard Q., Ward T., Sidiropoulos D., Hillmann B.M., Chun C.L., Sadowsky M.J., Knights D., Montassier E. Fecal microbiota transplantation reverses antibiotic and chemotherapy-induced gut dysbiosis in mice. Sci Rep. 2018;8:6219. doi: 10.1038/s41598-018-24342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schmidt E.K.A., Torres-Espin A., Raposo P.J.F., Madsen K.L., Kigerl K.A., Popovich P.G., Fenrich K.K., Fouad K. Fecal transplant prevents gut dysbiosis and anxiety-like behaviour after spinal cord injury in rats. PLoS One. 2020;15 doi: 10.1371/journal.pone.0226128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li C., Ai G., Wang Y., Lu Q., Luo C., Tan L., Lin G., Liu Y., Li Y., Zeng H., Chen J., Lin Z., Xian Y., Huang X., Xie J., Su Z. Oxyberberine, a novel gut microbiota-mediated metabolite of berberine, possesses superior anti-colitis effect: Impact on intestinal epithelial barrier, gut microbiota profile and TLR4-MyD88-NF-kappaB pathway. Pharmacol Res. 2020;152:104603. doi: 10.1016/j.phrs.2019.104603. [DOI] [PubMed] [Google Scholar]
- 198.Duan C., Kuang L., Xiang X., Zhang J., Zhu Y., Wu Y., Yan Q., Liu L., Li T. Activated Drp1-mediated mitochondrial ROS influence the gut microbiome and intestinal barrier after hemorrhagic shock. Aging (Albany NY) 2020;12:1397–1416. doi: 10.18632/aging.102690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang Y., Yang J., Wang W., Sanidad K.Z., Cinelli M.A., Wan D., Hwang S.H., Kim D., Lee K.S.S., Xiao H., Hammock B.D., Zhang G. Soluble epoxide hydrolase is an endogenous regulator of obesity-induced intestinal barrier dysfunction and bacterial translocation. Proc Natl Acad Sci U S A. 2020;117:8431–8436. doi: 10.1073/pnas.1916189117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.van der Giessen J., van der Woude C.J., Peppelenbosch M.P., Fuhler G.M. A direct effect of sex hormones on epithelial barrier function in inflammatory bowel disease models. Cells. 2019;8:261. doi: 10.3390/cells8030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhou Z., Bian C., Luo Z., Guille C., Ogunrinde E., Wu J., Zhao M., Fitting S., Kamen D.L., Oates J.C., Gilkeson G., Jiang W. Progesterone decreases gut permeability through upregulating occludin expression in primary human gut tissues and Caco-2 cells. Sci Rep. 2019;9:8367. doi: 10.1038/s41598-019-44448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bi J., Zhang J., Ren Y., Du Z., Li T., Wang T., Zhang L., Wang M., Wu Z., Lv Y., Wu R. Irisin reverses intestinal epithelial barrier dysfunction during intestinal injury via binding to the integrin alphaVbeta5 receptor. J Cell Mol Med. 2020;24:996–1009. doi: 10.1111/jcmm.14811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yang J., Guo Y., Henning S., Chan B., Long J., Zhong J., Acin-Perez R., Petcherski A., Shirihai O., Heber D., Li Z. Ellagic acid and its microbial metabolite urolithin a alleviate diet-induced insulin resistance in mice (OR24-03-19) Curr Dev Nutr. 2019;3 doi: 10.1002/mnfr.202000091. nzz031.OR24-03-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang T., Yao W., Li J., Shao Y., He Q., Xia J., Huang F. Dietary garcinol supplementation improves diarrhea and intestinal barrier function associated with its modulation of gut microbiota in weaned piglets. J Anim Sci Biotechnol. 2020;11:12. doi: 10.1186/s40104-020-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 205.Di Caro V., Alcamo A.M., Cummings J.L., Clark R.S.B., Novak E.A., Mollen K.P., Morowitz M.J., Aneja R.K. Effect of dietary cellulose supplementation on gut barrier function and apoptosis in a murine model of endotoxemia. PLoS One. 2019;14 doi: 10.1371/journal.pone.0224838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Piton G., Capellier G. Biomarkers of gut barrier failure in the ICU. Curr Opin Crit Care. 2016;22:152–160. doi: 10.1097/MCC.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 207.Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68:1516–1526. doi: 10.1136/gutjnl-2019-318427. [DOI] [PMC free article] [PubMed] [Google Scholar]