Abstract
Aims
Unlike in atrial fibrillation ablation, there is a lack of appropriately sized and properly designed studies regarding outflow tract (OT) premature ventricular complex (PVC) ablation outcomes with contact force sensing (CFS) catheters. We aimed to compare the acute success-, complication-, and long-term recurrence rates of manual CFS catheters with traditional irrigated catheters (T) in OT PVC ablation.
Methods and results
Single-centre, propensity-matched data of 75–75 patients ablated for right-sided OT (RVOT) or left-sided OT (LVOT) PVCs in 2015–17 with CFS or T catheters were compared. Acute success rate, peri-procedural complications, post-procedural daily PVC burden, and long-term recurrence rates were compared on intention-to-treat basis. Acute success rate equalled 80% in both groups, with no difference in force values in the CFS group comparing successful or failed cases [12.0 (8.75–17.0) vs. 16.0 (10.25–22.25) g, P = 0.21]. There were three cases of pseudo-aneurysm and one cardiac tamponade. PVC burden fell significantly from baseline 22 (15–30)% to 2 (0–10)% (P < 0.0001), with no difference between catheter types [CFS: 1 (0–7)% vs. T: 4 (1–12) %; P = 0.21]. There was no significant difference in recurrence-free survival of CFS and T catheters (58 vs. 59%, P = 0.29) during 12 months of follow-up, respectively. Recurrence in the CFS group did not differ either by the force exerted below or above the median value of 12 g (P = 0.66).
Conclusion
Both types of catheters can effectively reduce OT PVC burden with minimal serious complication rates. Ablation with CFS or T catheters gives similar acute- and long-term results.
Keywords: Contact force, Premature ventricular complexes, Catheter ablation, Outflow tract, Follow-up
What’s new?
Contact force catheters do not outperform traditional manual ablation catheters in outflow tract premature ventricular complex (PVC) ablation in a balanced, propensity-matched population.
Acute procedural success rates are the same with contact force and traditional catheters.
Long-term PVC recurrence rates do not differ significantly with contact force and with traditional catheters.
Premature ventricular complex recurrence rates do not differ significantly in subgroups divided by median contact force value either.
Introduction
Premature ventricular complexes (PVC) are one of the most frequent cardiac arrhythmias. The majority of them arise in the right- or left-ventricular outflow tract (OT) (RVOT, LVOT) regions, from the sinus of the aortic valve, or rarely from the epicardial surface of the OT region. The OT regions are the most frequent sources of idiopathic ventricular arrhythmias. While in the majority of cases, OT PVCs carry a benign long-term prognosis, they can be highly symptomatic and worsen quality of life. Catheter ablation of PVCs has been travelled a long journey over the past 25 years, when Professor Wellens wrote his thought-provoking editorial concerned about the appropriateness of this treatment for PVCs in 1995, and called for a randomized trial to compare outcomes of catheter ablation with antiarrhythmic drug therapy.1 After a quarter of a century this kind of a randomized study is still missing. Nevertheless, in a single-centre, retrospective study of 510 patients published in 2014 by Zhong et al.2 demonstrated that catheter ablation of PVCs significantly reduced PVC burden and improved LVEF compared with antiarrhythmic drug therapy. Drug therapy only moderately decreased PVC burden but did not improve LVEF at all compared with baseline. An average PVC burden of 27% can lead to PVC-induced cardiomyopathy, which can be reversed by catheter ablation.3 More recently, in 2019 Berruezo et al.4 gave proof in a prospective, multicentre study that in patients with reduced left ventricular systolic function successful ablation of PVCs with a reduction of at least 18% absolute points in post-ablation PVC burden significantly improved the composite clinical endpoint of cardiac mortality, transplant, or hospitalization for heart failure compared with those with less reduction in their burden. Ablation of OT PVCs is now a well-established treatment with acute success rates ∼80–90% and with variable long-term recurrence rates. Ventura et al.5 report a 52% long-term recurrence in their RVOT ablation cases with a mean time for recurrence of 6.28 years. In a cohort reported by Krittayaphong et al.,6 12% had a recurrence over a mean follow-up time of 72.2 months. Both studies included only acutely successful ablation cases in their survival analysis. Ablation in the OT region and the aortic sinus might be quite challenging and inherently carries a risk of serious cardiac complications. Contact force sensing (CFS) catheters give real-time feedback to the operator on the force exerted against the wall of the cardiac chambers. Effective force delivery correlates with freedom from atrial fibrillation.7 The use of CFS systems may confer better efficacy over traditional manual catheters without CFS capabilities (T) in atrial fibrillation ablation as Afzal et al.8 pointed it out in their meta-analysis of seven observational and two randomizes studies. Nevertheless, results of the TOUCH AF trial showed that awareness to force values was not translated to better outcomes when using a CFS system.9
The performance of CFS technology in the field of ventricular arrhythmia ablation is far less studied. Existing publications compare limited numbers of patients with heterogeneous origins of arrhythmias, with different ablation targets.10,11 Furthermore, they mainly focus on the comparison of three ablation methods: remote magnetic navigation (MNS), ablation with manual CFS catheters, and traditional manual ablation.
The aim of our study was to compare acute success rates and 12-month follow-up results of CFS guided vs. traditional manual endocardial ablation on an intention-to-treat basis in a large, balanced patient cohort treated exclusively for OT PVCs.
Methods
Patients
In this observational study, we analysed data of our single-centre retrospective cohort of 205 consecutive adult patients who underwent their first PVC ablation in 2015–17. Symptomatic palpitations with a high (>15%) daily PVC burden refractory to antiarrhythmic medication or patients unwilling to take antiarrhythmic drugs were criteria for inclusion. After exclusion of 28 patients with non-OT PVC ablation, data of 177 patients underwent propensity matching to create balanced cohorts. All patients gave informed consent to the procedure and the study. Patients discontinued antiarrhythmic medication 48 h prior to ablation. The V2S/V3R amplitude ratio of the OT PVC on the surface ECG described in detail by Yoshida et al.12 was used for lateralization of the source of origin. The study protocol was reviewed and approved by the Local Research Ethical Committee and was in accordance with the Declarations of Helsinki.
Procedure and follow-up
Procedures were performed under local anaesthesia using a transfemoral venous and/or arterial vascular access. There was neither a trans-septal approach for left-sided ablation nor a direct subxyphoid access for epicardial ablation. Ablation in the coronary sinus was performed in two occasional cases. Electroanatomical mapping (EAM) was performed in all patients to map the site of earliest endocardial activation and to guide ablation using a CARTO 3 (Biosense Webster Inc., Diamond Bar, CA, USA) or EnSite (St Jude Medical, St. Paul, MN, USA) platform. Activation mapping identified sites for ablation with earliest local activation advancing the onset of PVC QRS by at least 30 ms. During pace-mapping, a site with a minimum match of 11/12 was considered a target to energy delivery. When spontaneous, clinically relevant PVCs were absent during the procedure we used isoproterenol induction in 10 µg boluses. Commercially available 3.5 mm open-irrigated tip radiofrequency (RF) catheters were used for all mapping and ablation procedures. We used the following catheters in the T group: Navistar Thermocool (Biosense Webster Inc., Diamond Bar, CA, USA), AlCath Flux Blue (Biotronik SE & Co., KG. Berlin, Germany), Blazer Open-Irrigated (Boston Scientific Inc., Marlborough, MA, USA), and FlexAbility (Abbott Inc., Abbott Park, IL, USA). Thermocool SmartTouch (Biosense Webster Inc., Diamond Bar, CA, USA) or TactiCath (St Jude Medical, St. Paul, MN, USA) were the catheter models used in the CFS group. A force range of 10–40 g was set as target. Radiofrequency energy was delivered in 60 s duration impulses with an energy setting of 30–40 W, in power-controlled mode with a temperature limit of 43°C. Acute success was defined as complete elimination of the clinical PVC during the post-ablation waiting period with isoproterenol challenge. We defined a >5% daily PVC burden on a 24 h Holter-ECG recording as recurrence during follow-up. Visits and Holters were recorded at 3 months, and then every 6 months on, or in between these periods when the patient felt symptomatic. Left-sided ablations included PVCs originating from the LVOT region or the left and right sinuses of Valsalva.
Statistical analysis
The following variables were included in the logistic regression model for propensity score matching: age, sex, hypertension, left ventricular ejection fraction (LVEF), daily PVC burden, left-sided PVC origin, and use of antiarrhythmic medication. The algorithm using a 1 : 1 ratio and nearest neighbour method created a propensity-matched cohort. Maximum allowed difference in matching was set to 0.1. Continuous variables were expressed as median and interquartile ranges (IQR), whereas categorical ones as numbers and percentages. Dichotomous variables were compared with Fisher’s exact test. We compared continuous variables with normal distribution using unpaired t-test, and we used a Mann–Whitney U-test for the ones with non-normal distribution. Normality of distribution was assessed with a Shapiro–Wilk test. We computed recurrence-free survival by using the Kaplan–Meier method and differences in recurrence rates were calculated with log-rank test. We evaluated recurrence-free survival rates on an intention-to-treat basis between the CFS and T group, hence not only acutely successful cases were included in the analysis. We accepted a P-value of <0.05 as statistically significant. Prism Plus5 (GraphPad Software, La Jolla, CA, USA) and SPSS Statistics 25 (IBM Corp., Armonk, NY, USA) software were used for statistical analysis.
Results
Baseline characteristics, procedural parameters
Out of the 177 OT PVC patients (97 CFS and 80T cases) propensity matching algorithm created a cohort of 150 paired patients for further analysis, and in 27 cases matching was ineffective. 75 cases underwent CFS-guided manual ablation and 75 matched patients had a procedure with a T catheter. The CFS and T groups created by matching were balanced and there was no significant difference in baseline clinical variables of the studied 150 patients (Table 1). The median age of the entire cohort was 58 years (48–69), including 77 (51%) females. Median LVEF was 55 (44–60)%, and 37% of the cohort had LVEF <50%, evenly distributed between groups (P = 0.80). The ablation target side was well-balanced with 79 (53%) localized in the RVOT and 71 (47%) in the LVOT. No significant difference could be found in baseline antiarrhythmic medications (Table 2). β-Blockers were the most frequently used drugs in 55% of the cases. Procedure-related parameters showed non-significant differences, as well (Table 3). Median procedure time was 60 (54–86) min. A bilateral ablation approach, when the successful ablation spot to eliminate the clinical PVC was finally on the contralateral side of the initial access was needed in about one-fifth of the cases in both groups. The median force and force-time integral (FTI) exerted during ablation were 12.0 (9.5–18.5) grams and 699 (407–1270) gs, respectively.
Table 1.
Baseline clinical characteristics
| All patients (n = 150) | T (n = 75) | CFS (n = 75) | P | |
|---|---|---|---|---|
| Age (year) | 58 (42–69) | 55 (42–68) | 60 (43–69) | 0.30 |
| Female | 77 (51%) | 43 (57%) | 34 (45%) | 0.19 |
| Hypertension | 79 (52%) | 41 (55%) | 38 (51%) | 0.74 |
| Diabetes | 20 (13%) | 10 (13%) | 10 (13%) | 1.00 |
| Ischaemic heart disease | 23 (15%) | 15 (20%) | 8 (11%) | 0.17 |
| eGFR (mL/m2) | 74 (60–90) | 71 (57–90) | 80 (64–90) | 0.31 |
| Daily PVC burden (%) | 22 (15–30) | 22 (15–30) | 21 (15–30) | 0.90 |
| LVEF (%) | 55 (44–60) | 55 (43–60) | 52 (44–60) | 0.94 |
| PVC QRS width (ms) | 160 (120–180) | 160 (148–180) | 160 (140–169) | 0.36 |
| Antiarrhythmic usea | 107 (71%) | 53 (71%) | 54 (72%) | 0.74 |
| LVOT originb | 71 (47%) | 35 (47%) | 36 (47%) | 1.00 |
Continuous variables are expressed as median and interquartile range.
CFS, irrigated contact force sensing manual ablation catheters; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; PVC, premature ventricular complex; T, traditional irrigated manual ablation catheters without contact force sensing capability.
Including β-blockers.
Including all left-sided sites of origin.
Table 2.
Baseline medications
| All patients (n = 150) | T (n = 75) | CFS (n = 75) | P | |
|---|---|---|---|---|
| β-Blockers | 83 (55%) | 40 (53%) | 43 (57%) | 0.74 |
| Verapamil | 10 (7%) | 5 (7%) | 5 (7%) | 1.00 |
| Amiodarone | 10 (7%) | 6 (8%) | 4 (5%) | 0.75 |
| Sotalol | 1 (0.7%) | 1 (1.3%) | 0 | 1.00 |
| Propafenon | 3 (2%) | 1 (1.3%) | 2 (2.7%) | 1.00 |
| ACEI/ARB | 67 (45%) | 33 (44%) | 34 (45%) | 1.00 |
| MRA | 16 (11%) | 10 (13%) | 6 (8%) | 0.43 |
| VKA | 10 (7%) | 8 (11%) | 2 (3%) | 0.10 |
| NOAC | 3 (2%) | 1 (1.3%) | 2 (2.7%) | 1.00 |
| ASA | 43 (29%) | 20 (27%) | 23 (31%) | 0.72 |
| Clopidogrel | 16 (11%) | 6 (8%) | 10 (13%) | 0.43 |
ACEI, angiotensin converting enzyme; ARB, angiotensin receptor inhibitor; ASA, acetyl-salicylic acid; MRA, mineralocorticoid receptor antagonist; NOAC, non-vitamin-K oral anticoagulant; VKA, vitamin-K antagonist.
Table 3.
Procedural parameters and complications
| All patients (n = 150) | T (n = 75) | CFS (n = 75) | P | |
|---|---|---|---|---|
| Procedural parameters | ||||
| Procedure time (min) | 60 (54–86) | 60 (50–80) | 61 (55–90) | 0.53 |
| Fluoro-time (min) | 2.3 (1.5–4.0) | 2.6 (1.6–4.2) | 2.2 (1.4–3.9) | 0.42 |
| RF time (s) | 360 (200–767) | 325 (180–661) | 393 (240–785) | 0.36 |
| RF applications (N) | 6.0 (3.0–13.0) | 7.0 (3.0–12.0) | 6.0 (4.0–13.0) | 0.80 |
| Bilateral ablation | 32 (21%) | 14 (19%) | 18 (24%) | 0.55 |
| RF above leafleta | 19 (10%) | 12 (12%) | 7 (8%) | 0.42 |
| Acute success rate | 120 (80%) | 60 (80%) | 60 (80%) | 1.00 |
| Force (g) | – | – | 12.0 (9.5–18.5) | N/A |
| FTI (gs) | – | – | 699 (407–1270) | N/A |
| Complications | ||||
| Groin haematoma | 6 (4.0%) | 2 (2.7%) | 4 (5.3%) | 0.68 |
| Pseudo-aneurysm | 3 (2.0%) | 1 (1.3%) | 2 (2.7%) | 1.00 |
| Cardiac tamponade | 1 (0.7%) | 0 | 1 (1.3%) | 1.00 |
| Pericardial effusion | 2 (1.3%) | 1 (1.3%) | 1 (1.3%) | 1.00 |
| Stroke/TIA | 0 (0%) | 0 (0%) | 0 (0%) | NA |
Continuous variables are expressed as median and interquartile range.
FTI, force-time integral; N/A, not applicable; RF, radiofrequency; TIA, transitory ischaemic attack.
Ablation target above the valvular planes.
Complications
Access-site vascular complications included 6 (4%) groin haematomas and 3 (2%) pseudo-aneurysms with two of them requiring thrombin injection or surgical closure (Table 3). One patient in the CFS group developed cardiac tamponade requiring urgent percutaneous drainage, when a maximum force of 70 g reached in the RVOT accidentally. A haemodynamically insignificant pericardial effusion occurred in two cases post-ablation. There was no procedure-related death.
Acute success
Acute success rates were the same in the CFS and T group with 60–60 (80–80%) successful cases. The 67% of ablation pulses were in the force range. The percentages in range in acutely successful and unsuccessful cases were 66% and 73%, respectively (P = 0.52). Median force values did not differ significantly between acutely successful and failed cases [12.0 (8.75–17.0) vs. 16.0 (10.25–22.25) g, P = 0.21]. This held true for FTI values, as well [726 (336–1258) vs. 670 (465–1590) gs, P = 0.44].
Clinical follow-up
Follow-up time was 12 months. Five patients (3%) were lost to follow-up right after their discharge from our hospital. These patients were alive on telephone interview but declined to take part in any follow-up visits, therefore they were excluded from the analysis of recurrence. The 24 h PVC burden plunged significantly from a baseline median value of 22% (15–30%) to 2% (0–10%), P < 0.0001. The magnitude of reduction was similar in the CFS [21% (15–30%) vs. 1% (0–7%); P < 0.001] and T group [22% (15–30%) vs. 4% (1–12%); P < 0.01] without a significant between-group difference (P = 0.21).
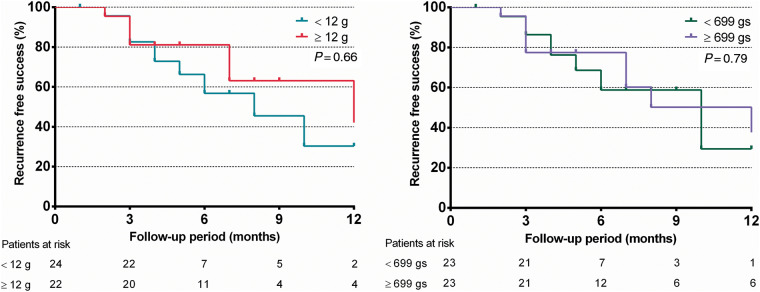
On an intention-to-treat basis, 21 (28%) and 23 (31%) patients experienced an OT PVC recurrence during 12 months in the CFS and T groups, respectively. There was no significant difference in recurrence-free survival rates between the CFS (58%) and T (59%) groups (log-rank P = 0.29) over 12 months of follow-up, as depicted on Figure 1. If we grouped recurrence by the force exerted below or above the median value and median FTI, there were still no significant differences in recurrence rates either (P = 0.66 and 0.79, respectively), as shown in Figure 2. Moreover, 71% of the recurrent cases was in the target force range whereas in recurrence-free cases this figure was 65%, the difference being insignificant (P = 0.49).
Figure 1.
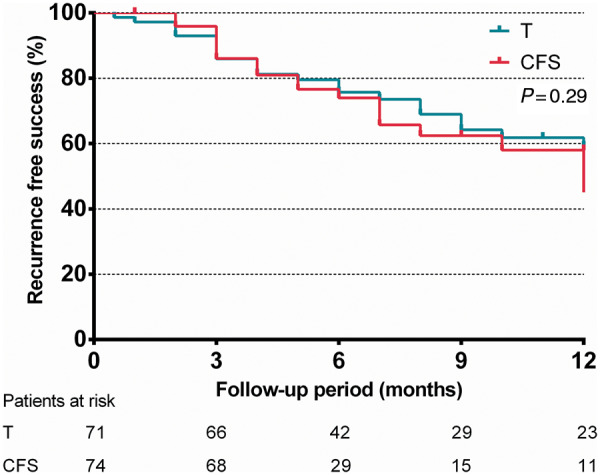
Intention-to-treat-based comparison of arrhythmia recurrence-free survival rates of patients who underwent outflow tract PVC ablation with CFS and traditional manual ablation catheters (T) using the Kaplan–Meier method. P represents log-rank test result. CFS, contact force sensing; PVC, premature ventricular complex.
Figure 2.
Intention-to-treat-based comparison of arrhythmia recurrence-free survival rates of patients who underwent outflow tract PVC ablation with CFS ablation catheters stratified by the median contact force value of 12 g (left panel), and the median FTI of 699 gs (right panel). P represents log-rank test result. CFS, contact force sensing; FTI, force–time integral; PVC, premature ventricular complex.
There was also no difference in the 12-month recurrence-free survival of the CFS group on a year-by-year basis (71%, 54%, and 59% in 2015, 2016, and 2017, respectively; P value for trend: 0.72). This held true for the T group, as well (68%, 48%, and 44% in 2015, 2016, and 2017, respectively; P for trend 0.75).
Discussion
Durable ablation lesion formation is regarded as one of the key factors in successful catheter ablation and sufficient and continuous catheter tip-tissue contact expressed by contact force and FTI values is regarded as the main determinant of it.7 Comparison of results with CFS vs. T catheters gave hope that by controlling force during lesion creation a 37% reduction in recurrence could be reached after atrial fibrillation ablation.8 Data from the randomized multicentre TOUCH-AF trial in persistent atrial fibrillation casted some doubt on the superiority of CFS feedback, because neither the forces exerted (14 vs. 12 g) nor the arrhythmia-free survival rates (60% vs. 63%) differed significantly between the force-guided or blinded arms.9 It is important to note that no T catheters were used in this study; only CFS catheters were compared with and without direct force feedback.
Clinical performance of contact force-guided ablation in the outflow tracts
To the best of our knowledge, this was the first study to directly compare the clinical outcomes of CFS and T catheter ablation techniques only in PVC ablation with an ablation target situated exclusively in the OT region. In our propensity-matched population of 150 patients, there was no additional value of the use of CFS ablation catheters in OT PVC ablation over traditional manual catheters. Data exist already on the non-superior results of CFS ablation in ventricular arrhythmias in general.10 Ventricular arrhythmias form a quite heterogeneous group regarding baseline patient condition, arrhythmia substrate, and long-term prognosis. In our opinion, it is important to focus on the distinct and clinically most frequent subtype, OT PVCs. It is important to note, that de Vries et al.11 already conducted a moderate-sized study containing 17 CFS, 25 MNS, and 31 traditional manual ablation cases with 79% of RVOT origin, and a nearly 10-year long inclusion period in the field of OT PVC and ventricular tachycardia (VT) ablation with similarly neutral results. Unfortunately, they did not report their actual numerical force values in their publication, only a pursued target range of 10–20 g was mentioned. What makes the strength of our work besides reporting exact force and FTI values is the balanced inclusion of a higher number of patients with homogenous baseline characteristics between the CFS and T group. Noteworthy, the representation of right- and left-sided cases was also equal. Moreover, our centre performed these 150 ablations in the relatively short time-frame of 3 years without any substantial change in mapping strategy.
Factors probably affecting efficacy of ablation in the outflow tract region
One should not forget about other fundamental determinants of successful ablation in complex arrhythmias such as appropriate indication considering age and co-morbidities, precise mapping protocol, catheter delivery to a substrate localized in difficult-to-reach regions via steerable sheaths or magnetic navigation, use of open-irrigation systems, and operator experience. Based on the results we can hypothesize that sufficient manual contact force is not a key to long-term clinical success in the OT regions, unlike in the left atrium. One should keep in mind that during atrial fibrillation ablation the creation of a rather extensive lesion set is anatomically guided against a thin atrial wall suggesting a kind of uniformity in transmural lesions created above a certain force-threshold.7 On the contrary, ablation in the OT region is limited in space, the target is activation-guided, and lesions are performed against a wall of tissue with rather variable depth and anatomy, where perpendicular tip-tissue contact cannot be achieved every time. Difficult-to-reach epicardial foci and deep preferential conduction described by Shirai et al.13 exist in this region and can complicate procedure and decrease success rates. These targets are most probably not destroyable even with the best RF lesion with optimal contact delivered in the traditional unipolar mode. Improved and stable catheter tip delivery to the ablation target area via remote MNS did not improve success rates in ventricular arrhythmia ablation.10,11 Nevertheless, there are encouraging results in improving recurrence-free survival after ischaemic VT ablation with integrating a novel contact feedback module into an MNS system.14
Role of the learning curve
The existence of a learning curve is a non-negligible factor when adopting a new technique. Even with T catheters, indirect signs of contact must be recognized such as tactile feedback, catheter tip motion on imaging, and signal properties of unipolar mapping. The higher possibility of tenting and distortion caused by stiff CFS catheters on the EAM should also be considered when one changes platform from T catheters. We started regular CFS ablations in 2014, and the reason we started data collection in 2015 was to allow sufficient time for the learning curve to be completed. This was mirrored in our year-by-year results showing no significant annual differences.
Force values in the ventricles
The force value cut-offs to create an effective and durable ablation lesion in the ventricles are less well studied than in atrial fibrillation ablation, where a force value of >10 g and an FTI of > 392 gs were established.7,15 Values over 9 and 8 g are regarded as the cut-offs for stable catheter contact in the right- and left-ventricular endocardial surface based on the work of Mizuno et al.16 The average force reached in their 5920 mapped points was 12 ± 9.0 g. Of note, 84% of our force values were above 8 g and the mean force value of our CFS cohort was 14.2 ± 7.4 g suggesting adequate force generation against the ventricular walls. Capulzini et al.17 report that the mean force value reached in their 61 patients ablated for PVCs was 18.3 ± 6.4 g. Their impressively high acute success rate of 96.7% and 1-year recurrence-free rate of 93.4% suggest that besides their accurate mapping strategy, these force values should be enough to create durable lesions. Of note, their study had no comparator arm; all cases were ablated with CFS catheters.
Complications
Outflow tract regions are regarded among the most vulnerable ones in the human heart. Ablation-related complications were rare as they occurred in 8% of the cases and only three patients (2%) had major complications requiring intervention. There was no statistical difference in complication rates as shown in Table 3. Notably, our only tamponade case was in the CFS group. The stiffer design of CFS catheters might also pose some additional risk. de Vries et al.11 reported a similarly 10% overall and 3% major complication rate related to ablation of OT arrhythmias. Major complications reported by Hendriks et al.10 were also similar, reaching only 3.3%. Although Akca et al.18 reported a significantly lower rate of perforation in their CFS group, this difference was led by atrial fibrillation ablations. In their subset of 190 ventricular arrhythmia ablation cases, there was no significant difference in the rate of major complications and this held true for cardiac perforation, as well. The three-fold additional cost of manual CFS catheters over conventional irrigated ones and the lack of studies to convincingly demonstrate their better safety profile in PVC ablation somewhat question the routine use of manual CFS ablation catheters in our institute in this indication.
Limitations
A significant limitation of our work lies in its non-randomized nature, therefore an unmeasured confounder may exist. Nevertheless, the balanced proportion of 11 baseline clinical variables, 6 procedural parameters, and the equal use of 6 types of antiarrhythmic drugs probably minimalize this bias. Furthermore, only 13% of our patients were scanned with cardiac magnetic resonance imaging, the most sensitive non-invasive imaging modality to detect structural heart disease including ventricular scar tissue, so subtle structural disease could be left undetected.
It is important to emphasize that our sample size was not enough to draw any firm and definitive conclusion regarding so sparsely occurring major complications comparing manual CFS and T ablation in the OT region.
Force applied in the T group is inherently unknown and some bias might arise with pursuing a force range as the operator tended to stop energy delivery outside the range. Because this was a retrospective study, no arm with a CFS catheter with force values blinded to the operator is available for comparison with an operator being aware of these values. Radiofrequency lesion times were relatively longer than usual in both groups, implying higher cumulative energy delivery to the OT regions which may have contributed to our neutral results. Although catheter tip orientation has an impact on the vector of contact force in the OT regions, force vector analysis was not performed in our work.
Catheters of five different manufacturers were used, each is differing slightly in mechanical properties and handling that could affect flexibility, manoeuvrability, and steerability. These properties could in turn affect RF lesion quality. No comparison was made within the CFS or T group regarding this issue, which can be a major confounding factor.
Conclusions
Our propensity-matched data of 150 patients point towards that there is no additional value of using manual CFS ablation catheters over traditional, irrigated ones in improving the outcomes of OT PVC ablation regardless of the force and FTI values achieved with CFS catheters.
Acknowledgements
Authors express their sincere gratitude to Tünde Bettenbuch, Marianna Srej, Dániel Kira, Károly Ladunga, and Dávid Tasnádi for their continuous and unconditional help in realising the project.
Funding
none declared
Conflict of interest: G.S. reports personal fees from Johnson and Johnson Medical and Abbott, not related to the present study. All remaining authors have declared no conflicts of interest.
Data availability
The data underlying this article cannot be shared publicly due to patient privacy reasons as data related to an Olympic-level top performance athlete is also included. The data will be shared on a reasonable request to the corresponding author.
References
- 1. Wellens HJJ. Radiofrequency catheter ablation of benign ventricular ectopic beats: a therapy in search of a disease? J Am Coll Cardiol 1995;26:850–1. [DOI] [PubMed] [Google Scholar]
- 2. Zhong L, Lee YH, Huang XM, Asirvatham SJ, Shen WK, Friedman PA et al. Relative efficacy of catheter ablation vs antiarrhythmic drugs in treating premature ventricular contractions: a single-center retrospective study. Heart Rhythm 2014;11:187–93. [DOI] [PubMed] [Google Scholar]
- 3. Penela D, Fernández-Armenta J, Aguinaga L, Tercedor L, Ordonez A, Bisbal F et al. Clinical recognition of pure premature ventricular complex-induced cardiomyopathy at presentation. Heart Rhythm 2017;14:1864–70. [DOI] [PubMed] [Google Scholar]
- 4. Berruezo A, Penela D, Jáuregui B, Soto-Iglesias D, Aguinaga L, Ordóñez A et al. Mortality and morbidity reduction after frequent premature ventricular complexes ablation in patients with left ventricular systolic dysfunction. Europace 2019;21:1079–87. [DOI] [PubMed] [Google Scholar]
- 5. Ventura R, Steven D, Klemm HU, Lutomsky B, Mullerleile K, Rostock T et al. Decennial follow-up in patients with recurrent tachycardia originating from the right ventricular outflow tract: electrophysiologic characteristics and response to treatment. Eur Heart J 2007;28:2338–45. [DOI] [PubMed] [Google Scholar]
- 6. Krittayaphong R, Sriratanasathavorn C, Dumavibhat C, Pumprueg S, Boonyapisit W, Pooranawattanakul S et al. Electrocardiographic predictors of long-term outcomes after radiofrequency ablation in patients with right-ventricular outflow tract tachycardia. Europace 2006;8:601–6. [DOI] [PubMed] [Google Scholar]
- 7. Reddy VY, Shah D, Kautzner J, Schmidt B, Saoudi N, Herrera C et al. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm 2012;9:1789–95. [DOI] [PubMed] [Google Scholar]
- 8. Afzal MR, Chatta J, Samanta A, Waheed S, Mahmoudi M, Vukas R et al. Use of contact force sensing technology during radiofrequency ablation reduces recurrence of atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm 2015;12:1990–6. [DOI] [PubMed] [Google Scholar]
- 9. Conti S, Weerasooriya R, Novak P, Champagne J, Lim HE, Macle L et al. Contact force sensing for ablation of persistent atrial fibrillation: a randomized, multicenter trial. Heart Rhythm 2018;15:201–8. [DOI] [PubMed] [Google Scholar]
- 10. Hendriks AA, Akca F, Dabiri Abkenari L, Khan M, Bhagwandien R, Yap SC et al. Safety and clinical outcome of catheter ablation of ventricular arrhythmias using contact force sensing: consecutive case series. J Cardiovasc Electrophysiol 2015;26:1224–9. [DOI] [PubMed] [Google Scholar]
- 11. de Vries LJ, Hendriks AA, Yap SC, Theuns DAMJ, van Domburg RT, Szili-Torok T. Procedural and long-term outcome after catheter ablation of idiopathic outflow tract ventricular arrhythmias: comparing manual, contact force, and magnetic navigated ablation. Europace 2018;20:ii22–7. [DOI] [PubMed] [Google Scholar]
- 12. Yoshida N, Yamada T, McElderry HT, Inden Y, Shimano M, Murohara T et al. A novel electrocardiographic criterion for differentiating a left from right ventricular outflow tract tachycardia origin: the V2S/V3R index. J Cardiovasc Electrophysiol 2014;25:747–53. [DOI] [PubMed] [Google Scholar]
- 13. Shirai Y, Goya M, Isobe M, Hirao K. Preferential pathway pacing within the aortic sinus of valsalva: strong evidence for the existence of preferential conduction with different exit sites traversing the ventricular septum. J Cardiovasc Electrophysiol 2015;26:805–8. [DOI] [PubMed] [Google Scholar]
- 14. Noten AME, Hendriks AA, Yap SC, Mol D, Bhagwandien R, Wijchers S et al. Contact feedback improves 1-year outcomes of remote magnetic navigation-guided ischemic ventricular tachycardia ablation. Int J Cardiol 2020;315:36–44. [DOI] [PubMed] [Google Scholar]
- 15. Squara F, Latcu DG, Massaad Y, Mahjoub M, Bun SS, Saoudi N. Contact force and force-time integral in atrial radiofrequency ablation predict transmurality of lesions. Europace 2014;16:660–7. [DOI] [PubMed] [Google Scholar]
- 16. Mizuno H, Vergara P, Maccabelli G, Trevisi N, Eng SC, Brombin C et al. Contact force monitoring for cardiac mapping in patients with ventricular tachycardia. J Cardiovasc Electrophysiol 2013;24:519–24. [DOI] [PubMed] [Google Scholar]
- 17. Capulzini L, Vergara P, Mugnai G, Salghetti F, Abugattas JP, El Bouchaibi S et al. Acute and one year outcome of premature ventricular contraction ablation guided by contact force and automated pacemapping software. J Arrhythmia 2019;35:542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akca F, Janse P, Theuns DA, Szili-Torok T. A prospective study on safety of catheter ablation procedures: contact force guided ablation could reduce the risk of cardiac perforation. Int J Cardiol 2015;179:441–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article cannot be shared publicly due to patient privacy reasons as data related to an Olympic-level top performance athlete is also included. The data will be shared on a reasonable request to the corresponding author.