Abstract
The vessels of the central nervous system (CNS) have unique barrier properties. The endothelial cells (ECs) which comprise the CNS vessels contribute to the barrier via strong tight junctions, specific transporters, and limited endocytosis which combine to protect the brain from toxins and maintains brain homeostasis. Blood–brain barrier (BBB) leakage is a serious secondary injury in various CNS disorders like stroke, brain tumors, and neurodegenerative disorders. Currently, there are no drugs or therapeutics available to treat specifically BBB damage after a brain injury. Growing knowledge in the field of epigenetics can enhance the understanding of gene level of the BBB and has great potential for the development of novel therapeutic strategies or targets to repair a disrupted BBB. In this brief review, we summarize the epigenetic mechanisms or regulators that have a protective or disruptive role for components of BBB, along with the promising approaches to regain the integrity of BBB.
Keywords: Blood–brain barrier, Endothelial cells, Epigenetics, DNA methylation, Histone modifications
Introduction
Long-term gene expression programs during CNS development are directed by epigenetic mechanisms such as DNA methylation or hydroxymethylation and histone modifications [1]. Recent studies have identified additional epigenetic mechanisms like microRNAs, long non-coding RNAs, and histone variants [1, 2]. These epigenetic mechanisms are known to be influenced by the environment and experience [3].
The vascular network includes arteries and arterioles which distribute blood to the tissues, a fine network of capillary beds that supply essential nutrients and gases inside the tissue, and venules and veins which collect deoxygenated blood from tissues. Vascular properties differ, depending on the needs of the specific organs they vascularize. To meet the unique requirements of the CNS, specialized capillaries in the brain exhibits unique barrier characteristics which have been termed the blood–brain barrier (BBB). The BBB regulates the exchange of molecules between the blood and brain, thus managing the brain environment for vital functions. The CNS vessels are in contact with two immune cell populations one within the blood and the other in the CNS thought to regulate the BBB properties in response to an injury or infection. The CNS immune cell population includes macrophages and microglial cells [4]. Although it is protective, this selective barrier makes an obstacle for CNS drug delivery and significant research efforts have been made to create methods to open this barrier for drug delivery. Aging and conditions, such as hypertension and cerebrovascular ischemia, can aggravate the BBB, thereby changing the BBB components [5, 6], and can contribute to BBB disruption that predisposes the brain to neurological disease including Alzheimer's disease [7, 8]. Further, BBB disruption is a serious concern in many neurological diseases such as stroke and TBI [9–11]. This review covers the various ways in which epigenetic dysregulations contribute to BBB disruption and the epigenetic programs that are modified due to BBB disruption.
Epigenetic pathways of gene regulation
Epigenetic modifications are chemical modifications occurring in chromatin, DNA, or transcribed RNA that can influence gene expression or activity without changing the DNA sequence [12, 13]. Conventionally, these modifications affect histone proteins, DNA, and/or chromatin remodeling, but also the non-coding RNAs that can regulate the gene expression post-transcriptionally in response to various environmental cues belong to this group [14, 15]. Epigenetic modifications are heritable in nature, stage, and tissue-specific, and involved in global gene silencing to support the normal developmental processes.
DNA methylation
DNA, the basic unit of heredity is epigenetically modified by methylation. DNA methylation regulates the chromatin state and the accessibility of DNA to the transcription machinery. DNA methylation is carried out by a group of enzymes called DNA methyltransferases (DNMTs). DNMTs catalyze the covalent transfer of a methyl group from S-adenosyl methionine to the cytosine residue present in the CpG dinucleotides [16, 17]. DNMTs can be de novo methylase or maintenance methylase. De novo methylases are responsible for establishing the early methylation pattern during germ cell and embryo development [18]. De novo methylation is catalyzed by the redundant activities of DNMT3a and DNMT3b. Parallel to their redundant activity, each of these methyltransferases has unique targets. DNMT3a is required for the gene body methylation at Polycomb group (PcG) target developmental genes. DNMT3b has higher DNA methylation activity and hence a dominant role in the de novo methylation of X-chromosomes [19, 20]. Both DNMT3a and 3b function in conjunction with a third methyltransferase, DNMT3L. Although DNMT3L lacks methyltransferase activity, it acts as a cofactor regulating the activity of DNMT3a and b [21]. Maintenace methylase, DNMT1, maintains the methylation pattern set by the other two methyltransferases. DNMT1 maintains the methylation pattern through mitosis. After DNA replication, DNMT1 binds to the hemimethylated CpG sites and methylates the newly synthesized strand. Specific recruitment of DNMT1 to the hemimethylated sites is mediated through UHRF1, an E3 ubiquitin ligase [22]. Vertebrates have another DNMT, DNMT2, that shares high homology with other DNMTs. DNMT2 has a very poor/null methyltransferase activity on DNA templates. However, DNMT2 catalyzes tRNA methylation efficiently [23]. Methylation in the gene promoter represses transcription by 1) inhibiting the binding of different transcription factors (TFs) and/or RNA PolII to DNA, and 2) recruiting methyl binding proteins (MBPs), which bind to repressors and histone deacetylases [16, 24]. On the contrary, removal of the methyl group occurs passively during DNA replication, when the newly synthesized strand fails to add a methyl group [25]. Demethylation also occurs in an enzyme-mediated process. The methylated base is converted to a modified nucleotide by oxidation or deamination reaction catalyzed by ten-eleven translocations (TETs) and activation-induced deaminase (AID), respectively. The modified nucleotide is then recycled to generate cytosine by the base excision repair (BER) pathway [26].
Histone modifications
Histone proteins form the framework upon which the DNA is bound. Two units each of H2A, H2B, H3, and H4 histones associate to form a core histone octamer. The octamer is bound by 147 bases of DNA to form a nucleosome, the fundamental unit of chromatin compaction. Nucleosomes remain connected in a “beads-on-a-string” pattern by linker histone (H1) and associated DNA, allowing easy access for the transcriptional machinery and higher gene activity. Such open regions in the chromatin are referred to as euchromatin. Heterochromatin refers to the organization of nucleosomes into tight bundles, reducing the access of the transcriptional machinery. Histone tails extending from the nucleosome surface, as well as the ones, present within the body of the octamer, serve as the sites for chemical modification. Additionally, histones present in the octamer core can be substituted by a variant. This opens up associated DNA causing their activation [27, 28]. Modification of histones through chemical processes can be done through post-translational addition or removal of methyl, acetyl, sumoyl, and phosphate. The modifications also include ubiquitination, ADP-ribosylation, deamination, and proline isomerization [29, 30]. The addition of any of these groups alters the charge associated with the histone molecule and hence its interaction with the negatively charged DNA. Thus, these modifications change the accessibility of TFs and cofactors to the associated DNA [31, 32]. The addition/removal of acetyl or methyl groups is the most common histone modification and is discussed below.
Acetylation and deacetylation of histones
The addition of an acetyl group to the histone neutralizes the positive charge on the histones and reduces their attraction to the DNA molecules. This makes the DNA more accessible to the binding of TFs and other cofactor molecules, thereby positively affecting gene expression. Acetyl groups are added to the lysine residues present in the histone proteins. Acetylation is catalyzed by an enzyme called histone acetyltransferases (HATs/KATs) and is divided into two categories. Type a HATs are in the nucleus and they carry out the acetylation of nucleosomal histones and promote their transcription. Type b HATs are involved in the acetylation of newly synthesized histone molecules, before their incorporation into the nucleosome complex. They are distributed in the cytoplasm. Within the nucleus, histone acetylation can be reversed by HDACs. They remove the acetyl groups from the histone proteins, thus increasing the attraction of histone with the DNA molecule. This leads to the condensation of chromatin and hence gene repression. Eighteen HDACs identified in mammals have been classified into four different groups. Class I HDAC consists of the nuclear-localized HDAC1, 2, 3, and 8. HDACs shuttling between nucleus and cytoplasm constitute class II and include 4, 5, 6, 7, 9, and 10. Class III HDAC comprises NAD+ dependent proteins called sirtuins and class IV comprises HDAC11.
Methylation and demethylation of histones
Histone methylation within the nucleus is controlled by histone methyltransferases and histone demethylases. Methyl groups from S-adenosyl methionine are transferred to the lysine or arginine residue present in H3 and H4 histone by histone methyltransferases. Depending on the residue getting methylated and the degree of methylation, their effect on gene expression can vary. The important sites of methyl group addition to a lysine on H3 are 4, 9, 27, and 36, and on H4 is 20. Generally, H3 methylation on the 4th (K4) or 36th (K36) lysine residue activates transcription, whereas K9 and K27 methylation repress genes. H3K4 me1 is often associated with enhancer regions [25, 33]. Another histone methyltransferase called disruptor of telomeric silencing-like (DOT1L) catalyzes H3K79 methylation [34]. Histone methylation is reversed by demethylases. Histone demethylase, Jumonji domain-containing protein 3 (Jmjd3) antagonizes the repression caused by H3K27me3 methylation during hypoxic conditions [35]. Jmjd6 is a histone arginine demethylase catalyzing H3R2 and H4R3 demethylation (Flt1; [36]). H3K4 di/trimethylation is reversed by jumonji AT-rich interactive domain 1B (JARID1B) and Lysine Demethylase 5B (KDM5B). However, H3K4me1 and H3K4me2 are removed by another demethylase, lysine-specific demethylase 1 (LSD1) [37]. Plant homeodomain finger protein 8 (PHF8) is a histone demethylase catalyzing the removal of methyl groups from histone 3 lysine 9 (H3K9) and H4K20 [38].
Non-coding RNAs
Non-coding RNAs (ncRNAs) are a group of untranslated RNA molecules with regulatory functions. Based on the length of the RNA, ncRNAs are classified into small ncRNAs (sncRNAs) and long ncRNAs (lncRNAs). Small RNAs usually range in their size from 18 to 35 nucleotides, whereas the lnc RNAs are more than 200 nucleotides in length. SncRNAs show high functional variations and include transfer RNA (tRNA), ribosomal RNA (rRNA), small nuclear RNA (snRNA), small nucleolar RNA (snoRNA), Piwi-interacting RNA (piRNA). LncRNAs include intergenic ncRNAs, long intronic RNAs, telomeric ncRNA, pseudogene transcripts, enhancer RNA, and promoter-associated long RNA [39]. However, in the forthcoming sections, we will limit ourselves to ncRNAs involved in post-transcriptional regulation, directly by competing with functional RNAs. These include miRNAs and lncRNAs, specifically those binding to the target RNA and inhibiting translation. MiRNAs are single-stranded RNAs typically ranging in length from 20 to 24 nucleotides. They are located in the cytoplasm and stimulate the degradation of target RNA molecules by a pathway involving RNA induced silencing complex (RISC). The expression and function of miRNAs are closely associated with other epigenetic modifiers [39, 40]. LncRNAs show a greater variation in their sequence. They can also function as competitors to endogenous RNAs. LncRNA expressed from pseudogenes function as ‘antagomirs’ or ‘miR sponges’ by sequestering miRNAs [39, 41].
Epigenetics of the blood–brain barrier
BBB formation
During embryonic development, the mesoderm differentiates into angioblast and develops into a primitive blood vessel, this process is defined as vasculogenesis. Following this, new capillaries grow from this existing blood vessel by the process defined as angiogenesis. In mice, blood vessels invade the brain at embryonic day 9.5. It was reported that BBB genes including TJ proteins occludin and claudin-5 are expressed in the brain ECs at the initial stages of angiogenesis [42, 43]. However, a functional intact BBB is reported to be formed at E−15.5 in mice [44]. In humans, angiogenesis does not begin until fetal week 8, and the BBB is reported to be functional at an age of 4 months [45]. A detailed review of existing knowledge in the formation of BBB is available in [46, 47]. The details on vascular development in the brain and patterning are beyond the scope of this review, but it is relevant to the discussion in the review to provide a clear distinction between the neurovascular unit and BBB.
Neurovascular unit
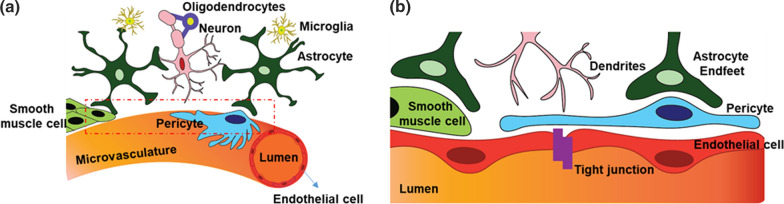
As the blood vessel invades the brain, the ECs and perivascular cells, called pericytes, come to close contact with both neuronal and glial cells to form a neurovascular unit (NVU, Fig. 1) [48, 49]. The vascular components (ECs, pericytes, and vascular smooth muscle cells) and neuroglial components (neurons, oligodendrocytes, microglia, astrocytes, and astrocyte-derived basement membrane) of the NVU interact dynamically and communicate to regulate proper angiogenesis, BBB formation, and maintenance in the brain and the blood-retinal barrier (BRB) in the eye. This unique communication program likely provides important cues to modify the epigenetic programs in BBB cell types and thus support the formation and maintenance of BBB, until now these interactions have not been studied in any detail.
Fig. 1.
Diagrammatic representation of a neurovascular unit (NVU). NVU consisting of vascular cells, glial cells and neurons are shown in a, and the enlarged view of the inset is given in b. Endothelial cells (red), pericytes (blue), and smooth muscle cells (pale green) represent the vascular cells. The glia cells are given in dark green (astrocytes), and blue (oligodendrocytes), yellow (microglia) and the neurons in pink color. Tight junctions connecting the endothelial cells are also shown in b
Structural Components of the BBB
The specialized multicellular BBB structure contributes to the barrier properties including limiting or regulating the transport of molecules (influx and efflux), protection from toxic materials and pathogens (Fig. 1). CNS blood capillaries formed by ECs are the primary functional component of the BBB. Perivascular cells pericytes and ECs share a common basement membrane with attachment points to extracellular matrix components mediated by integrins and less ubiquitous cell-ECM receptors such as dystroglycan. The BBB is composed of a basement membrane (comprised of e.g. type IV collagen, laminin, and fibronectin), surrounded by astrocyte end-feet ensheathing the vessels and pericytes (PCs) [50]. In CNS vessels, ECs are held together with the help of strong tight junctions (TJs), which limit the paracellular flux of solutes, and are characterized by specific transporters, which,deliver molecules across the barrier and securely controls the brain homeostasis. The structural components of the BBB are comprehensively reviewed in [51–53]. Emerging experimental techniques in genomics and proteomics are now providing a larger picture of the molecular components of the BBB. Current understanding of the unique transcriptome and proteins in CNS ECs was obtained mainly by comparing isolated CNS ECs with peripheral and other brain cells [42, 43, 54–57].
Tight junctions
Highly expressed TJ proteins are unique to CNS endothelial cells. It is the TJs between adjacent endothelial cells that confer the low paracellular permeability and high electrical resistance of the barrier, making it able to function 50–100 times tighter than peripheral microvessels [58–60]. TJs consist of complexes of claudins, occludins, and other transmembrane proteins scaffolded to the actin cytoskeleton by zona occludens (ZO) proteins [59, 61]. Together, these elements function to maintain homeostasis in the changing milieu of the CNS.
DNA methylation
Currently, no data exist to explain the DNA methylation events that directly regulate the expression of TJ proteins. This study is only an example of indirect regulation of TJ localization/degradation via TIMP2 and MMPs, and no direct DNA-methylation regulation for TJ proteins has been reported until now. Following middle cerebral artery occlusion (MCAO) in mice, a global DNA methylation event was reported as an increase in global DNA methylation in the mouse brain. The study shows that these global methylation changes after MCAO increased the methylation in the promoter of tissue inhibitors of metalloproteinase 2 (TIMP2). TIMP2 functions to inhibit matrix metalloproteinase (MMPs) which are a group of enzymes involved in protein degradation of the extracellular matrix and the non-covalent binding of TIMPs will inhibit MMP activity. MMP-mediated disruption of TJ proteins is well documented [62, 63]. Increased MMP activity/secretion can affect BBB permeability, with MMP-2 and -9 being associated with BBB breakdown following stroke. Consequently, decreased TIMP2 expression/activity can be related to increased MMP-9 activity and degradation of the basal lamina [64] and tight junction components [65] resulting in BBB damage. Together, the MCAO-induced hypermethylation of the TIMP2 promoter contributes to a decreased activity of TIMP2 and an increased MMP activity that contributes to TJ protein degradation. Consistent with these findings, the pharmacological and genetic inhibition of DNMT was effective in attenuating the stroke symptoms [66, 67].
Conversely, a leaky BBB can contribute to changes in DNA methylation events leading to gene expression changes. Using in vitro and in vivo BBB disruption models, it was shown that BBB leakage can influence the expression of the DNA methyltransferase enzyme DNMT3b [68]. The study reported that BBB disruption influenced the DNA methylation events via increased expression of noncoding RNA miRNA29b. The increased miRNA29b affects the expression of DNMT3b and MMP expression. Supporting these findings, DNA methyltransferase inhibitor treatment (5-Azacytidine) ameliorates the BBB damage via reducing the expression of miRNA29b [68]
Future studies are required to investigate the role of DNA methylation changes in diseases where the BBB is compromised. Further studies are also warranted to elucidate how BBB leakage-induced stress contributes to DNA methylation events in other cell types of the neurovascular unit.
HDACs and their inhibitors
Histone acetylation and deacetylation play a critical role in chromatin remodeling and epigenetics. HDACs have been identified as potential therapeutic targets in different neurological diseases [69]. The availability of different specific HDAC inhibitors has augmented our understanding of HDAC functions, mechanism of actions, and genomic profile influenced by this mechanism. Several compounds that inhibit HDAC activity have now been developed and characterized. Clinically HDAC inhibitors are successful in causing cell growth arrest, differentiation and/or apoptosis, and tumor growth restriction. Promisingly, the clinical efficacy of HDAC inhibitors extends beyond cancer treatments, and they have now been explored for their therapeutic potential in all top 10 leading causes of death in the US [70].
The most notable secondary effect after stroke is BBB damage. Recent efforts were made to target the HDACs to ameliorate TJ protein degradation. HDAC inhibitors, valproic acid, and sodium butyrate (class I, IIA, and III inhibitors) were used to treat an ischemic stroke rat model, and these effects on protecting the BBB were studied. It was reported that this treatment was beneficial in decreasing the degradation of TJ proteins such as Claudin-5 and ZO-1. The associated mechanism was through suppression of NF-κB activation and MMP-9 induction [71, 72]. In another recent study, class IIA HDAC inhibitor—TMP269 treatment for mice subjected to cerebral ischemia/reperfusion injury leads to an increased expression of the tight-junction proteins, ZO-1, Occludin, and Claudin-5 in ECs, and thereby stabilize the BBB [73].
In parallel to this, recent research investigates the epigenetic changes after stroke. An increased HDAC expression was reported after an ischemic stroke. The author claims that this can contribute to BBB injury, and inhibiting HDAC can protect BBB [71, 74]. Conversely, an increased expression of HDAC4 after ischemic stroke was reported to protect the BBB break down via elevation of TJ proteins like claudin-5, occludin, and ZO-1, and through reducing the expression of NADPH oxidase and MMP-9 [75]. In an in vitro ischemic model using oxygen–glucose deprivation (OGD) in CNS endothelial cells an upregulation of endothelial HDAC9 expression was shown and this was associated with a decreased expression of TJ proteins, like ZO-1, claudin-5, and occludin. Supporting these findings, genetically targeting HDAC9 pre-OGD re-established the TJ protein expression in ECs [74]. Another in vitro study, using cultured primary human brain microvascular ECs undergoing OGD and reoxygenation, reported increased transendothelial cell permeability and downregulation of junctions proteins. These changes were correlated with increased HDAC3 activity and decreased PPARγ activity. Supporting the role of HDAC3 in regulating the TJ protein expression, treatment with a selective HDAC3 inhibitor RGFP966 reduced the paracellular permeability and increased the expression of TJ protein Claudin-5 via PPARγ receptor [76]. The same group also reported an increased HDAC3 expression in the hippocampus and cortex of diabetic mice accompanied by BBB leakage and was rescued via HDAC3 inhibitor treatment through miR-200a/Keap1/Nrf2 signaling pathway [77].
Histone methylation
Another important and prevalent chromatin modification that is known to influence TJ protein expression is a post-translational modification of histones by methylation. A recent study showed that the glucocorticoid dexamethasone (glucocorticoids are currently used clinically for preventing tumor-associated brain edema [78]) can suppress the expression of JMJD3, a histone H3K27 demethylase in TNFα treated BBB disruption model on mouse brain microvascular endothelial cell line (bEnd.3). This happens via the recruitment of glucocorticoid receptor α (GRα) and nuclear receptor co-repressor (N-CoR) to the negative glucocorticoid response element in the upstream region of the JMJD3 gene. Further, the decreased expression of JMJD3 is correlated with a decreased activation of MMP-2, MMP-3, and MMP-9, and increased expression of claudin-5 and occludin [79–81]. Polycomb repressive complex proteins catalyze the methylation of H3K27 leading to repressive histone modification H3K27me3 in the gene promoter. A study to understand the role of vascular endothelial cadherins (VEC) in vascular stabilization, showed that VEC overexpression increases the expression of the claudin-5 gene by preventing the binding of PRC2 to the CLDN5 gene. VEC mediates this by forming a complex with Wnt transducer β-catenin and PRC2 subunit EZH2 [82]. Caveolin-1 a key protein involved in the BBB [83–85] was repressed in Influenza-associated encephalopathy (IAE). The repression was mediated by the SET domain bifurcated 2 (Setdb2) by methylation of histone H3 lysine 9 [86].
From the above-discussed studies, it is evident that histone modifications have a critical role in controlling the expression of TJ proteins. However, more in-depth attempts are needed to understand the epigenetic mechanisms and signaling pathways that contribute to the formation and maintenance of TJs.
Adherens junctions
The BBB is characterized by the high expression of TJs and low expression of adherens junctions (AJs) when compared to non-CNS EC barriers [87]. The basic molecular structure of adherens junctions (AJs) resemble TJs. A major AJ reported to be present in the CNS ECs is CDH5 along with a low expression of N- and E-cadherins [88]. It is reported that during CNS angiogenesis ECs have a relatively high expression of cadherin-10 compared to CDH5 [89, 90]. Stable AJs are key to the formation of TJs. It was reported that TJ protein CLDN5 expression was upregulated by CDH5 by inducing the phosphorylation of forkhead box factor FoxO1 through Akt activation and by limiting the translocation of β-catenin to the nucleus [91]. Furthermore, the same group reported the epigenetic link for these findings (described in the TJs histone methylation section of this review).
Transporters or solute carriers
The barrier to paracellular diffusion contributed by TJs potentially isolates the brain from many essential polar nutrients such as glucose and amino acids necessary for metabolism and therefore the CNS endothelium forming the BBB express a large number of specific transporters, including solute carriers and ABC (ATP-binding cassette) transporter proteins, for a wide variety of solutes and nutrients, mediating flux into and out of the brain [92–101]. Classification and roles of BBB transporters can be found in [102–104].
Glucose is the primary metabolic fuel for the mammalian brain and a continuous supply is required to maintain normal CNS function. The BBB regulates glucose transport into the brain via specific glucose transporters. A study to understand the prioritized glucose supply into the brain during fasting reports that fasting‐induced the production of ketone body β‐hydroxybutyrate (β‐OHB) which enhances expression of the glucose transporter gene GLUT-1 via histone modifications. Brain microvascular endothelial cells treated with β‐OHB upregulated the expression of GLUT-1 via inhibiting HDAC2 and elevation of acetylation in H3K9 at the critical cis‐regulatory region [105]. The multidrug resistance protein 1 (MDR1, ABCB1, P-glycoprotein) is a major efflux transporter located on the surface of capillary endothelial cells that restricts the accumulation of xenobiotics in the brain. Immortalized human brain capillary endothelial (hCMEC/D3) cells treated with HDAC inhibitors valproic acid (VPA), apicidin, and suberoylanilide hydroxamic acid (SAHA) increased the mRNA and protein levels of MDR1 by 30–200% via increased acetylation in H3K9/K14 [106].
Apart from the above few studies, no other research was found reporting on the role of epigenetic programs in regulating the expression of BBB transporters.
Other cell types in the BBB
Pericytes
The abluminal surface of the CNS vessel shows the highest coverage of pericytes that invest and support the endothelial layer throughout the vasculature. Cerebral vessels have a high pericyte to EC ratio [107, 108] pointing to their significant role in the neurovascular unit, controlling BBB integrity and function, supporting the stability of vessels, contributing to the elasticity of vessels regulating the blood flow, and protecting endothelial cells from potentially harmful substances [109–111]. Crosstalk between pericyte and endothelial cells enhances the EC TJ formation and decreases transcytosis and leukocyte adhesion molecule expression in the developing BBB [43]. Considering the aforementioned functions, dysfunction or apoptosis of blood–brain barrier pericytes is a vital factor in the pathogenesis of several diseases that are associated with microvascular instability.
HDACs and their inhibitors
An in vitro study was conducted to investigate the effect of HDAC inhibitors on pericyte proliferation, cell viability, migration, and differentiation. The results showed that HDAC inhibitors valproic acid and trichostatin A inhibited the proliferation and migration of pericytes with no effect on cell viability. Further, HDAC inhibitor treatment in pericytes increased the transcription of angiogenesis-related genes such as angiopoietin-like 4, transforming growth factor beta2, and TIMP2 [112]. BBB is also reported to be compromised during HIV infection. An attempt to investigate the role of occludin on BBB breakdown after HIV infection and its impact on pericytes reports that occludin levels control the metabolic responses of pericytes after HIV infection. HIV infection in pericytes reduces the occludin level and this is correlated with a decreased expression and activation of the class III histone deacetylase sirtuin (SIRT)-1 along with elevated nuclear localization of gene repressor C-terminal-binding protein (CtBP)-1 and NFκB-p65 activation. Further, this study demonstrates occludin as a NADH oxidase and showed that cellular levels of NADH inversely correlated with the cellular content of occludin that controls the expression and activation of SIRT-1 [113]. In another study, overexpression of SIRT3 was shown to increase the pericyte density and improved the pericyte EC coverage in the lungs of LPS treated mice [114]. The overexpression of SIRT3 could also be promising for brain pericytes and to support BBB repair after an injury.
Astrocytes
Astrocytes serve as a bridge that connects neuronal signaling to the CNS vasculature. Astrocyte structure includes specialized processes called astrocyte endfeet that extend from the astrocyte cell body and attach to the basement membrane that surrounds the endothelial cells and pericytes [115–117]. Astrocytes regulate the BBB through its synaptic glutamate levels, via scavenging free radicals and producing neurotrophic factors to communicate with other cell types in the BBB [118, 119]. In an in-vitro co-culture experiment with ECs cultured alone, ECs co-cultured with astrocytes or astrocyte-conditioned media enhanced the endothelial cell barrier properties including the transporter expression and increased TJ formation thus supporting the astrocyte interaction with endothelial cells supporting the formation of the BBB [120].
DNA methylation
Astrocyte end-feet have orthogonal arrays of intramembranous particles (OAPs) consisting of the most abundant water channel aquaporin-4 (AQP4) and the ATP-sensitive inward rectifier potassium channel Kir4.1 [121]. It is reported that DNA methylation is an important process in the development of astrocytes since demethylation of astrocyte-specific genes such as GFAP, S100β, and AQP4 in neural stem cells (NSCs) promotes the switch from neurogenesis to astrogenesis [122–125]. In astrocytes, changes in global DNA methylation patterns have been shown to occur in psychiatric disorders [126] and alcohol abuse [127]. However, DNA methylation events in astrocytes that contribute to BBB formation or damage are not known.
HDACs and their inhibitors
The GLUT1 transporter has an important role in astrocyte metabolism and supporting neuronal energy metabolism [128]. It is reported that cerebral astrocyte culture incubated with Pan-HDAC inhibitor valproic acid could increase histone acetylation at the SLC2A1 promotor thereby facilitating glucose uptake in the astrocytes [129]. This is important as maintaining the astrocyte metabolism after an injury can ensure BBB maintenance. Addressing the expression pattern of HDACs in the cortex and hippocampus of mice after photothrombotic infarction showed that HDAC1 was expressed in the nuclei and cytoplasm of GFAP(+) astrocytes in the hippocampus. Expression was also observed, to some extent, in astrocyte end-feet [130]. In another study, the activity of HDAC2 and HDAC8 in neurons and astrocytes was reported to be elevated 7 days after ischemia. The study also reports that HDAC2 was predominantly localized in the nuclei, and HDAC8 was predominantly observed in the cytoplasm. Together the above research can be read as the increased expression of HDACs have some critical role in BBB damage and HDAC inhibitor can potentially minimize this effect after ischemic stroke [71, 74, 131].
BBB and non-coding RNA
MicroRNAs (miR) which cause degradation or translational repression of mRNA play an important role in the development and progression of BBB dysfunction. Knowledge of miRNAs function in the BBB came from the early work identifying miR-125a-5p and other miRNAs in regulating brain endothelial tightness [132]. The increasing number of research articles connecting different miRNAs and BBB disruption highlights the significance of targeting miRs for BBB repair in various neurological conditions including TBI and stroke [133, 134]. Keeping in mind that small nucleotide-based drugs are easy to develop and targeting miR has great success, drugs targeting and based on miRs have a pronounced potential in treating BBB damage in neurological diseases [133].
Table 1 shows different miRs and their mechanism of action which are reported to be involved in the BBB disruption after different pathological conditions including stroke. Our impression from the literature is that miRs and BBB disruption are studied mostly in stroke conditions. Although miRNAs are inhibitory, depending on their target mRNA, an increase or decrease of miRNA expression is relevant. MiRs can directly or indirectly cause the degradation of BBB proteins or translational repression of BBB mRNA. For example, it is reported that miR-132 can directly target MMP-9 and after stroke, the reduced miR-132 expression increases MMP-9 activity which degrades TJ proteins or components of the basal lamina. Further TJ proteins like ZO-1, occludin, and claudin-5 were reported to be positively regulated by miR-126, miR-107, and miR-127 and negatively regulated by miR-98 and miR-150 in different disease models. A comprehensive, systematic review of miRNA regulation of TJ proteins has been conducted [135], and more miRNA have been found involved in TJ protein regulation summarized in Table 1.
Table 1.
MicroRNA candidates which are reported being involved in BBB disruption
| miRNA | Disease model | Species | miRNA Expression | BBB dysfunction | Mechanism |
|---|---|---|---|---|---|
| miR-107 [160] | Alzheimer's disease | In vitro human brain ECs(HBMEC) | Decreased | amyloid-β incubation down-regulates the expression of tight junction proteins ZO-1, Occludin, and Claudin-5 | Overexpression protects BBB Via its direct target endophilin-1 |
| miR-126-3P [161] | Intracerebral hemorrhage | Rat | Decreased | Cerebral edema, BBB leakage | Administration of miR-126 protects BBB via targeting PIK3R2 and the Akt signaling pathway |
| miR-126-3P, 5P [162] | Stroke | Mice | Decreased | Brain edema, BBB leakage, decreased ZO-1 and Occludin | miR-126-3p, 5p overexpression reduced the expression of proinflammatory cytokines IL-1β and TNF-α and adhesion molecules VCAM-1 and E-selectin and attenuated BBB disruption |
| miR-1303 [163] | Coxsackievirus A16 (CA16) infection model | In vitro and Monkey | Decrease | Degradation of junctional complexes Claudin4, Claudin5, CDH5, and ZO-1 | Infection downregulates miR-1303, which directly targets MMP9 |
| miR-130a [164] | Stroke | Rat | Increase | Brain edema, BBB leakage | Increased miR-130a expression after stroke directly inhibit Homeobox A5 expression, which down-regulates occludin |
| miR-132 [165] | Stroke | Mice | Decreased | Brain edema, BBB leakage | miR-132 overexpression decreased the degradation of tight junction proteins CDH5 and β-Catenin via targeting MMP9 |
| miR-132 [166] | Stroke | Mice | Increased | Brain edema, BBB leakage, decreased expression of CDH5 and β-catenin | Targets MMP-9 and suppress the expression |
| miR-143 [167] | methamphetamine-induced BBB dysfunction model | Mice/in vitro HBMEC | Increased | The decreased expression of TJ proteins including Claudin-5, ZO-1, and Occludin | miR-143 targets p53 upregulated modulator of apoptosis (PUMA) to induce BBB dysfunction |
| miR-149-5p [168] | Stroke | Mice | Decreased | BBB leakage, Pericyte migration | Targeting sphingosine-1-phosphate receptor (S1PR)2 expressed in pericytes |
| miR-150 [169] | Stroke | Rat | Increased | BBB leakage and decreased claudin-5 expression | miR-150 could regulate the claudin-5 expression and endothelial cell survival by targeting Tie-2 |
| miR-155 [170] | Experimental autoimmune encephalomyelitis (EAE) | Mice/ in vitro HBMEC | Decrease | BBB leakage | Negative regulator of BBB targeting cell adhesion components annexin-2 and claudin-1 and focal adhesion components DOCK-1 and syntenin-1 |
| miR-21 [171] | Stroke | Rat | Increase | Brain edema, BBB leakage | Target MAPK signaling |
| miR-210 [172] | neonatal hypoxic-ischemic encephalopathy | Rat | Increased | cerebral edema, BBB leakage, reduced expression of tight junction protein occludin and adherens junction protein β-catenin | Increased level of miR-210 in the model negatively regulates BBB integrity |
| MiR-212/132 [173] | Hypoxia | Mice/ in vitro HBMEC | increased | Increased expression of MiR-212/132 in hypoxic HBMEC decreased mRNA and protein expression of Cldn1, Jam3, and Tjap1 | By targeting Cldn1, Jam3, and Tjap1 |
| miR-29b [174] | Stroke | Mice | Decrease | BBB leakage, Decreased expression of ZO-1, and occludin | Via targeting AQP4 |
| miR29b [68] | BBB dysfunction model | Mice/in vitro | increased | Homocysteine induced BBB leakage | miR29b-mediate BBB dysfunction via targeting DNMT3b and MMP9 |
| miR-501-3p [175] | Vascular dementia | Mice | Increase | Decreased expression of claudin-5, ZO-1, and occludin | TNFα upregulates the miR-501-3p that directly targets ZO-1 |
| miR-539 [176] | Stroke | Rat | Decrease | BBB leakage | miR-539 targets MMP-9 |
| miR-98 [177] | Stroke | Mice | Decrease | BBB leakage, | Reduced leukocytes infiltration and diminished microglia activation |
| miRNA‐9‐5p [178] | TBI | Rat/In vitro | Increased | Decreased expression of claudin-5, ZO-1, and occludin | Via activating Hedgehog pathway and inhibiting NF‐κB/MMP‐9 pathway |
In an effort to investigate the differences in miRNAs expression in rat cortical pericyte during hypoxic stress showed differential regulation of miRNAs with 27 miRNAs upregulated and 31 miRNAs downregulated. These differentially regulated miRNAs are capable of targeting important signaling factors such as HIF-1α (miR-322 [136] increased and miR-199a [137] decreased in pericytes after hypoxic stress), TGF-β (miR-376b-3p [138] increased miR-140[139], miR-145 [140] decreased in pericytes after hypoxic stress) and VEGF (miR-126a [141] increased, and miR-297[142], miR-16[143], miR-17-5p[144] decreased in pericytes after hypoxic stress). Let-7 miRNA expression in pericytes is reported as involved in pericyte differentiation in response to hypoxic stress [145]. As an example of endothelial-pericyte cross talk, an in-vitro study reports that pericytes could uptake miR-503 originated from endothelial cells exposed to high glucose (hyperglycemia) [146, 147]. Under diabetic-induced microvascular dysfunction, inhibition of lncRNA-myocardial infarction-associated transcript (MIAT) or lncRNA-metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is shown to reduce the pericyte loss [148].
Astrocytes are known to express several miRNAs and these miRNAs can affect various functions of astrocytes [149–154]. Astrocytes-derived factors such as vascular endothelial growth factors, matrix metalloproteinases, nitric oxide, and endothelin-1 can affect the vascular tone and BBB permeability [155–159]. Considering this astrocytic miRNAs are a potential therapeutic target for BBB damage however this axis is yet to be explored.
Epigenetic strategies for the treatment of blood–brain barrier damage
Currently, there are no treatment modalities available to directly treat BBB dysfunction. The most common drugs to treat BBB dysfunction are glucocorticoids which help to stabilize TJ proteins [179–182]. In multiple sclerosis, interferon-beta treatment is one of the most promising immunomodulatory for reducing inflammatory damage. Few pre-clinical and clinical evidence support that interferon-beta could also be effective in treating BBB [183–185]. However, these drugs are not specific to the BBB, are mainly used as anti-inflammation, affect numerous physiologic processes, and increase the risk of complications, including infection and hyperglycemia [186]. The gene expression profile of humans and mice that contributes to the BBB function is now available [187–192]. This dataset was acquired by comparing the brain EC transcriptome to peripheral ECs [187, 193]. A deep understanding of the epigenetic mechanisms that regulate the transcription of BBB genes in brain ECs and other BBB cell types is warranted so that epigenetic drugs can be repurposed or developed to manipulate the BBB gene expression to treat BBB dysfunction in various neurological diseases. Epigenetic markings of DNA and histones are introduced and removed by enzymes, and they are therefore potentially reversible, paving the way for potential therapeutic targets. Promisingly small compounds that target epigenetic mechanisms including HDAC inhibitors have been FDA approved for the treatment of certain cancers [194]. Although many epigenetic drugs are in clinical trials for cancer treatments (Table 2), there is still room for improvement, as they are relatively unstable, can have toxic side effects, and are not available for oral administration. Table 2 mentions the available compounds that target epigenetic mechanisms. Information on epigenetic drugs' clinical availability and mechanism of action can be found in [194–198]. Promisingly, apart from its use as cancer drugs, clinically HDAC inhibitors are now used in psychiatry and neurology as mood stabilizers and anti-epileptics [199].
Table 2.
Details of the drug targeting epigenetic modifiers in endothelial cells
| Name of the drug | Epigenetic enzyme altered by the drug | Status of the clinical study/disease or condition |
|---|---|---|
| Trichostatin A | Inhibits class I and II HDAC enzymes | Clinical trial-completed (NCT03838926)/Relapsed or Refractory Hematologic Malignancies |
| Valproic acid/VPA | Inhibits class I and class IIa HDACs | Clinical trial-completed (NCT01233609)/ Retinitis Pigmentosa |
| Sodium butyrate/NaB | Inhibits class I and class IIa HDACs | Clinical trial-completed NCT00800930/ Shigellosis |
| Suberoylanilide hydroxamic acid/SAHA/Vorinostat | Inhibits class I and II HDACs | Clinical trial-completed (NCT00106626)/ Advanced Cancer |
| 3-Deazaneplanocin-A /DZNep | Inhibits EZH2 methyltransferase | – |
| Methylthioadenosine/MTA | Inhibits H3K4 methylase | Clinical trial-completed (NCT03083015)/ Necrotic Pulp |
| Morpholino | Inhibits histone acetyltransferase 7/KAT7 | Clinical trial-completed (NCT03375255)/ Muscular Dystrophy, Duchenne |
| Resveratrol/RV | Activates sirtuin1/Sirt1 | Clinical trial-completed (NCT01010009)/ Cognitive and Cerebral Blood Flow Effects of Resveratrol |
| Panobinostat/LBH589 | Inhibits ClssI, II, and IV HDACs | Clinical trial-completed (NCT00840346)/ Acute Myeloblastic Leukaemia |
| Entinostat/SNDX-275/MS 27–275 | Inhibits HDAC1/3 | Clinical trial- completed (NCT02897778)/Cardiac Safety Study With Advanced Solid Tumors |
| Mocetinostat | Inhibits HDAC1 and HDAC2 | Clinical trial-completed (NCT02303262)/ Metastatic Leiomyosarcoma |
| Lithium | Inhibits GSK3b | Clinical trial-completed (NCT01259388)/ Progressive Multiple Sclerosis |
| 5-Aza-2′-deoxycytidine (5-dAzaC) | Inhibits DNMT | Clinical trial-completed (NCT00744757)/ Myelodysplastic Syndrome |
| GSK2879552 | Inhibits LSD1 | Terminated clinical trials Relapsed/Refractory Small Cell Lung Carcinoma |
| Tazemetostat | Inhibits EZH2 (PRC2 subunit) | Clinical trial-completed (NCT02860286)/ Relapsed or Refractory Malignant Mesothelioma |
Emerging questions related to the prognostic and diagnostic value of epigenetic modifications in the BBB genes for predicting neurodegenerative processes and cognitive decline now exist. To learn the gene expression programs in CNS ECs that contribute to BBB dysfunction, the gene expression profile of brain ECs from different mice disease models which shows BBB dysfunction such as stroke, TBI, multiple sclerosis were compared. Interestingly, though the trigger for BBB dysfunction in each disease differs, a similar change in EC gene expression pattern contributes to BBB dysfunction [188]. This is a very significant finding as we can correlate the gene expression changes to changes in the epigenetic marks on those BBB genes. Furthermore, by manipulating the epigenetic regulation of those genes, it is possible to manipulate the expression of BBB genes to repair the BBB damage. Numerous studies have shown that AD brain endothelium expresses low levels of GLUT1, a BBB-specific glucose transporter, which then leads to reduced transport of glucose into the brain [200]. Identifying these epigenetic changes and reversing them may offer a promising therapeutic opportunity to target BBB dysfunction in AD.
For human BBB studies currently, MRI imaging is the most commonly used technique. Limited availability of human brain vessels for BBB studies makes it almost impossible to understand the BBB rupture mechanism in humans. Advances in stem cell technology now allow developing in vitro human BBB models from patients with different neurodegenerative disorders carrying genetic mutations [201, 202]. These human studies will enhance the knowledge of epigenetic variations in several neurological diseases that leads to BBB damage by comparing it to in vitro BBB models from healthy or isogenic controls.
Conclusion and future direction
BBB leakage is a major factor that determines disease progression, outcome, and therapeutic response. Management and prevention of BBB leakage pose a notable challenge to the medical community. Currently, no readily available clinical agent exists that can effectively prevent leakage or repair BBB. Novel therapeutic strategies that can meet this challenge might emerge from understanding the epigenetics of BBB development and damage. From our above review of literature, it is clear that most mechanistic insights on epigenetics and BBB breakdown have been gained from animal models of stroke. We have limited knowledge about the epigenetic mechanisms underlying breakdown in neurodegenerative disorders such as Alzheimer’s disease and multiple sclerosis. More research is warranted to investigate the epigenetic changes in BBB genes of CNS ECs, pericytes, astrocytes, and neurons after a BBB breakdown. We hope the development of advanced sequencing techniques, availability of ChiP antibodies and advanced methods to purify the brain cell types will allow comprehensive research in this area. Furthermore, there is a huge lack of human data to support the preclinical findings. Considering species differences affecting BBB permeability, it is also very relevant to investigate that the existing animal data are translatable to the human situation.
Acknowledgements
This work was supported by the University of Texas Health science center Houston (PKT, DWM, SLB), American Heart Association and Stroke (PKT), and the National Institute of Neurological Disorders and Stroke (SLB).
Authors' contributions
All authors, SAI, IEM, DWM, ACD, SLB, and PKT, contributed to the writing, illustration, and reviewing of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the University of Texas Health Science Center, AHA career development grant to Peeyush Kumar T #18CDA34110036, and a National Institutes of Health K23 Grant # NS106054-01A1 to Dr. Blackburn.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare there is no conflict of interest for this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Stephanie A. Ihezie and Iny Elizabeth Mathew—shared the first author
References
- 1.Qureshi IA, Mehler MF. Epigenetic mechanisms underlying nervous system diseases. Handb Clin Neurol. 2018;147:43–58. doi: 10.1016/B978-0-444-63233-3.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang J-Y, Aromolaran KA, Zukin RS. The emerging field of epigenetics in neurodegeneration and neuroprotection. Nat Rev Neurosci [Internet]. 2017 [cited 2017 Jul 14];18:347–61. Available from: http://www.nature.com.ezproxyhost.library.tmc.edu/nrn/journal/v18/n6/pdf/nrn.2017.46.pdf [DOI] [PMC free article] [PubMed]
- 3.Cushing BS, Kramer KM. Mechanisms underlying epigenetic effects of early social experience: the role of neuropeptides and steroids. Neurosci Biobehav Rev. 2005;29:1089–105. doi: 10.1016/j.neubiorev.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Hudson LC, Bragg DC, Tompkins MB, Meeker RB. Astrocytes and microglia differentially regulate trafficking of lymphocyte subsets across brain endothelial cells. Brain Res. 2005;1058:148–160. doi: 10.1016/j.brainres.2005.07.071. [DOI] [PubMed] [Google Scholar]
- 5.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, et al. Blood–brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson MA, Banks WA. Age-associated changes in the immune system and blood–brain barrier functions. Int J Mol Sci. 2019;20:1632. doi: 10.3390/ijms20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mooradian AD. Effect of aging on the blood-brain barrier. Neurobiol Aging. 1988;9:31–39. doi: 10.1016/S0197-4580(88)80013-7. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, et al. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol. 2018;163:144–71. doi: 10.1016/j.pneurobio.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury [Internet]. Nat. Rev. Neurol. 2010 [cited 2018 Feb 14]. p. 393–403. Available from: http://www.nature.com.ezproxyhost.library.tmc.edu/articles/nrneurol.2010.74.pdf [DOI] [PMC free article] [PubMed]
- 11.Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood-Brain Barrier Pathophysiology in Traumatic Brain Injury. Transl. Stroke Res. 2011. p. 492–516. [DOI] [PMC free article] [PubMed]
- 12.Jablonka EVA, Raz GAL. Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Q Rev Biol. 2009;84:131–176. doi: 10.1086/598822. [DOI] [PubMed] [Google Scholar]
- 13.Kilvitis HJ, Alvarez M, Foust CM, Schrey AW, Robertson M, Richards CL. Ecological epigenetics. Adv Exp Med Biol. 2014;781:191–210. doi: 10.1007/978-94-007-7347-9_10. [DOI] [PubMed] [Google Scholar]
- 14.Wei JW, Huang K, Yang C, Kang CS. Non-coding RNAs as regulators in epigenetics (Review) Oncol Rep. 2017;37:3–9. doi: 10.3892/or.2016.5236. [DOI] [PubMed] [Google Scholar]
- 15.Kaikkonen MU, Lam MTY, Glass CK. Non-coding RNAs as regulators of gene expression and epigenetics. Cardiovasc Res. 2011;90:430–40. doi: 10.1093/cvr/cvr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miranda TB, Jones PA. DNA methylation: the nuts and bolts of repression. J Cell Physiol. 2007;213:384–390. doi: 10.1002/jcp.21224. [DOI] [PubMed] [Google Scholar]
- 17.Narayanan S, Loganathan G, Mokshagundam SP, Hughes MG, Williams SK, Balamurugan AN. Endothelial cell regulation through epigenetic mechanisms: depicting parallels and its clinical application within an intra-islet microenvironment. Diabetes Res Clin Pract. 2018;143:120–33. doi: 10.1016/j.diabres.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Jones PA, Liang G. Rethinking how DNA methylation patterns are maintained. Nat Rev Genet. 2009;10:805–811. doi: 10.1038/nrg2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao L, Emperle M, Guo Y, Grimm SA, Ren W, Adam S, et al. Comprehensive structure-function characterization of DNMT3B and DNMT3A reveals distinctive de novo DNA methylation mechanisms. Nat Commun. 2020;11:1–14. doi: 10.1038/s41467-020-17109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yagi M, Kabata M, Tanaka A, Ukai T, Ohta S, Nakabayashi K, et al. Identification of distinct loci for de novo DNA methylation by DNMT3A and DNMT3B during mammalian development. Nat Commun. 2020;11:1–14. doi: 10.1038/s41467-020-16989-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veland N, Lu Y, Hardikar S, Gaddis S, Zeng Y, Liu B, et al. DNMT3L facilitates DNA methylation partly by maintaining DNMT3A stability in mouse embryonic stem cells. Nucleic Acids Res. 2019;47:152–167. doi: 10.1093/nar/gky947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishiyama A, Mulholland CB, Bultmann S, Kori S, Endo A, Saeki Y, et al. Two distinct modes of DNMT1 recruitment ensure stable maintenance DNA methylation. Nat Commun. 2020;11:1–7. doi: 10.1038/s41467-020-15006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaiser S, Jurkowski TP, Kellner S, Schneider D, Jeltsch A, Helm M. The RNA methyltransferase Dnmt2 methylates DNA in the structural context of a tRNA. RNA Biol. 2017;14:1241–1251. doi: 10.1080/15476286.2016.1236170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neri F, Rapelli S, Krepelova A, Incarnato D, Parlato C, Basile G, et al. Intragenic DNA methylation prevents spurious transcription initiation. Nature. 2017;543:72–77. doi: 10.1038/nature21373. [DOI] [PubMed] [Google Scholar]
- 25.Chen ZX, Riggs AD. DNA methylation and demethylation in mammal. J. Biol. Chem. 2011. p. 18347–53. [DOI] [PMC free article] [PubMed]
- 26.Bochtler M, Kolano A, Xu GL. DNA demethylation pathways: Additional players and regulators. BioEssays. 2017;39:1–13. doi: 10.1002/bies.201600178. [DOI] [PubMed] [Google Scholar]
- 27.Felsenfeld G, Groudine M. Controlling the double helix. Nature. 2003;421:448–453. doi: 10.1038/nature01411. [DOI] [PubMed] [Google Scholar]
- 28.Chen S, Yang J, Wei Y, Wei X. Epigenetic regulation of macrophages: from homeostasis maintenance to host defense. Cell Mol Immunol. 2020;17:36–49. doi: 10.1038/s41423-019-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venkatesh S, Workman JL. Histone exchange, chromatin structure and the regulation of transcription. Nat Rev Mol Cell Biol. 2015;16:178–189. doi: 10.1038/nrm3941. [DOI] [PubMed] [Google Scholar]
- 31.Fraineau S, Palii CG, McNeill B, Ritso M, Shelley WC, Prasain N, et al. Epigenetic activation of pro-angiogenic signaling pathways in human endothelial progenitors increases vasculogenesis. Stem Cell Rep. 2017;9:1573–1587. doi: 10.1016/j.stemcr.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He H, Hu Z, Xiao H, Zhou F, Yang B. The tale of histone modifications and its role in multiple sclerosis. Hum. Genomics. 2018;12:1–12. doi: 10.1186/s40246-018-0163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, et al. High-Resolution Profiling of Histone Methylations in the Human Genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Duan Y, Wu X, Zhao Q, Gao J, Huo D, Liu X, et al. DOT1L promotes angiogenesis through cooperative regulation of VEGFR2 with ETS-1. Oncotarget. 2016;7:69674–69687. doi: 10.18632/oncotarget.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohtani K, Vlachojannis GJ, Koyanagi M, Boeckel JN, Urbich C, Farcas R, et al. Epigenetic regulation of endothelial lineage committed genes in pro-angiogenic hematopoietic and endothelial progenitor cells. Circ Res. 2011;109:1219–1229. doi: 10.1161/CIRCRESAHA.111.247304. [DOI] [PubMed] [Google Scholar]
- 36.Boeckel JN, Guarani V, Koyanagi M, Roexe T, Lengeling A, Schermuly RT, et al. Jumonji domain-containing protein 6 (Jmjd6) is required for angiogenic sprouting and regulates splicing of VEGF-receptor 1. Proc Natl Acad Sci USA. 2011;108:3276–3281. doi: 10.1073/pnas.1008098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fraineau S, Palii CG, Allan DS, Brand M. Epigenetic regulation of endothelial-cell-mediated vascular repair. FEBS J. 2015;282:1605–1629. doi: 10.1111/febs.13183. [DOI] [PubMed] [Google Scholar]
- 38.Gu L, Hitzel J, Moll F, Kruse C, Malik RA, Preussner J, et al. The histone demethylase PHF8 Is essential for endothelial cell migration. PLoS ONE. 2016;11:1–15. doi: 10.1371/journal.pone.0146645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stamatovic SM, Phillips CM, Martinez-Revollar G, Keep RF, Andjelkovic AV. Involvement of epigenetic mechanisms and non-coding RNAs in blood-brain barrier and neurovascular unit injury and recovery after stroke. Front. Neurosci. 2019;13:1–15. doi: 10.3389/fnins.2019.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quan Z, Zheng D, Qing H. Regulatory roles of long non-coding rnas in the central nervous system and associated neurodegenerative diseases. Front Cell Neurosci. 2017;11:175. doi: 10.3389/fncel.2017.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ebert MS, Sharp PA. Emerging roles for natural microRNA sponges. Curr Biol. 2010;20:R858. doi: 10.1016/j.cub.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/β-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for bloodĝ€"brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marín-Padilla M. The human brain intracerebral microvascular system: Development and structure. Front Neuroanat. 2012;6:38. doi: 10.3389/fnana.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanchette M, Daneman R. Formation and maintenance of the BBB. Mech Dev. 2015;138:8–16. doi: 10.1016/j.mod.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Saili KS, Zurlinden TJ, Schwab AJ, Silvin A, Baker NC, Hunter ES, et al. Blood-brain barrier development: systems modeling and predictive toxicology. Birth Defects Res. 2017;109:1680–1710. doi: 10.1002/bdr2.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown LS, Foster CG, Courtney JM, King NE, Howells DW, Sutherland BA. Pericytes and neurovascular function in the healthy and diseased brain. Front Cell Neurosci. 2019;13:282. doi: 10.3389/fncel.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lok J, Gupta P, Guo S, Kim WJ, Whalen MJ, Van Leyen K, et al. Cell-cell signaling in the neurovascular unit. Neurochem Res. 2007;32:2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- 50.Price TO, Sheibani N, Shah GN. Regulation of high glucose-induced apoptosis of brain pericytes by mitochondrial CA VA: A specific target for prevention of diabetic cerebrovascular pathology. Biochim Biophys Acta - Mol Basis Dis. 2017;1863:929–935. doi: 10.1016/j.bbadis.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chow BW, Gu C. The Molecular Constituents of the Blood-Brain Barrier [Internet]. Trends Neurosci. NIH Public Access; 2015 [cited 2019 Nov 11]. p. 598–608. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26442694 [DOI] [PMC free article] [PubMed]
- 52.Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC. The blood-brain barrier: an engineering perspective. Front Neuroeng. 2013;6:7. doi: 10.3389/fneng.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He Q, Liu J, Liang J, Liu X, Li W, Liu Z, et al. Towards Improvements for Penetrating the Blood-Brain Barrier—Recent Progress from a Material and Pharmaceutical Perspective. Cells. 2018;7:24. doi: 10.3390/cells7040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohtsuki S, Hirayama M, Ito S, Uchida Y, Tachikawa M, Terasaki T. Quantitative targeted proteomics for understanding the blood-brain barrier: towards pharmacoproteomics. Expert Rev Proteomics. 2014;11:303–313. doi: 10.1586/14789450.2014.893830. [DOI] [PubMed] [Google Scholar]
- 55.Li JY, Boado RJ, Pardridge WM. Blood-brain barrier genomics. J Cereb Blood Flow Metab. 2001;21:61–68. doi: 10.1097/00004647-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 56.Enerson BE, Drewes LR. The rat blood-brain barrier transcriptome. J Cereb Blood Flow Metab. 2006;26:959–973. doi: 10.1038/sj.jcbfm.9600249. [DOI] [PubMed] [Google Scholar]
- 57.Cayrol R, Wosik K, Berard JL, Dodelet-Devillers A, Ifergan I, Kebir H, et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9:137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- 58.Romero IA, Radewicz K, Jubin E, Michel CC, Greenwood J, Couraud PO, et al. Changes in cytoskeletal and tight junctional proteins correlate with decreased permeability induced by dexamethasone in cultured rat brain endothelial cells. Neurosci Lett. 2003;344:112–116. doi: 10.1016/S0304-3940(03)00348-3. [DOI] [PubMed] [Google Scholar]
- 59.Feng J, Mantesso A, De Bari C, Nishiyama A, Sharp PT. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci U S A. 2011;108:6503–6508. doi: 10.1073/pnas.1015449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann N Y Acad Sci. 2009;1165:113–120. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 63.Wang Z, Meng CJ, Shen XM, Shu Z, Ma C, Zhu GQ, et al. Potential contribution of hypoxia-inducible factor-1α, aquaporin-4, and matrix metalloproteinase-9 to blood-brain barrier disruption and brain edema after experimental subarachnoid hemorrhage. J Mol Neurosci. 2012;48:273–280. doi: 10.1007/s12031-012-9769-6. [DOI] [PubMed] [Google Scholar]
- 64.Mun-Bryce S, Rosenberg GA. Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. Am J Physiol - Regul Integr Comp Physiol. 1998;274:43–45. doi: 10.1152/ajpregu.1998.274.5.R1203. [DOI] [PubMed] [Google Scholar]
- 65.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Endres M, Meisel A, Biniszkiewicz D, Namura S, Prass K, Ruscher K, et al. DNA methyltransferase contributes to delayed ischemic brain injury. J Neurosci. 2000;20:3175–3181. doi: 10.1523/JNEUROSCI.20-09-03175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mondal NK, Behera J, Kelly KE, George AK, Tyagi PK, Tyagi N. Tetrahydrocurcumin epigenetically mitigates mitochondrial dysfunction in brain vasculature during ischemic stroke. Neurochem Int. 2019;122:120–138. doi: 10.1016/j.neuint.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalani A, Kamat PK, Familtseva A, Chaturvedi P, Muradashvili N, Narayanan N, et al. Role of microRNA29b in blood-brain barrier dysfunction during hyperhomocysteinemia: an epigenetic mechanism. J Cereb Blood Flow Metab. 2014;34:1212–1222. doi: 10.1038/jcbfm.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gräff J, Tsai LH. The potential of HDAC inhibitors as cognitive enhancers. Annu. Rev. Pharmacol. Toxicol. 2013;53:311–30. doi: 10.1146/annurev-pharmtox-011112-140216. [DOI] [PubMed] [Google Scholar]
- 70.Hull EE, Montgomery MR, Leyva KJ. HDAC Inhibitors as Epigenetic Regulators of the Immune System: Impacts on Cancer Therapy and Inflammatory Diseases. Biomed Res Int. 2016;2016:8797206. doi: 10.1155/2016/8797206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park MJ, Sohrabji F. The histone deacetylase inhibitor, sodium butyrate, exhibits neuroprotective effects for ischemic stroke in middle-aged female rats. J Neuroinflammation. 2016;13:1–4. doi: 10.1186/s12974-015-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: The roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab. 2011;31:52–57. doi: 10.1038/jcbfm.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su L, Liang D, Kuang SY, Dong Q, Han X, Wang Z. Neuroprotective mechanism of TMP269, a selective class IIA histone deacetylase inhibitor, after cerebral ischemia/reperfusion injury. Neural Regen Res. 2020;15:277–284. doi: 10.4103/1673-5374.265562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi W, Wei X, Wang Z, Han H, Fu Y, Liu J, et al. HDAC9 exacerbates endothelial injury in cerebral ischaemia/reperfusion injury. J Cell Mol Med. 2016;20:1139–1149. doi: 10.1111/jcmm.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Hu DN, Zhu Y, Sun H, Gu P, Zhu D, et al. Regulation of Matrix Metalloproteinase-2 Secretion from Scleral Fibroblasts and Retinal Pigment Epithelial Cells by miR-29a. Biomed Res Int. 2017;2017. [DOI] [PMC free article] [PubMed]
- 76.Zhao J, Krystofiak ES, Ballesteros A, Cui R, Van Itallie CM, Anderson JM, et al. Multiple claudin–claudin cis interfaces are required for tight junction strand formation and inherent flexibility. Commun Biol. 2018;1. [DOI] [PMC free article] [PubMed]
- 77.Zhao Q, Zhang F, Yu Z, Guo S, Liu N, Jiang Y, et al. HDAC3 inhibition prevents blood-brain barrier permeability through Nrf2 activation in type 2 diabetes male mice. J Neuroinflammation. 2019;16:103. doi: 10.1186/s12974-019-1495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schroeder T, Bittrich P, Noebel C, Kuhne JF, Schroeder J, Schoen G, et al. Efficiency of Dexamethasone for Treatment of Vasogenic Edema in Brain Metastasis Patients: A Radiographic Approach. Front Oncol. 2019;9. [DOI] [PMC free article] [PubMed]
- 79.Na W, Shin JY, Lee JY, Jeong S, Kim WS, Yune TY, et al. Dexamethasone suppresses JMJD3 gene activation via a putative negative glucocorticoid response element and maintains integrity of tight junctions in brain microvascular endothelial cells. J Cereb Blood Flow Metab. 2017;37:3695–3708. doi: 10.1177/0271678X17701156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hedley-Whyte ET, Hsu DW. Effect of dexamethasone on blood-brain barrier in the normal mouse. Ann Neurol. 1986;19:373–377. doi: 10.1002/ana.410190411. [DOI] [PubMed] [Google Scholar]
- 81.Salvador E, Shityakov S, Förster C. Glucocorticoids and endothelial cell barrier function. Cell Tissue Res. 2014;355:597–605. doi: 10.1007/s00441-013-1762-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morini MF, Giampietro C, Corada M, Pisati F, Lavarone E, Cunha SI, et al. VE-cadherin-mediated epigenetic regulation of endothelial gene expression. Circ Res. 2018;122:231–245. doi: 10.1161/CIRCRESAHA.117.312392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, et al. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron. 2017;94:581–594. doi: 10.1016/j.neuron.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deng J, Huang Q, Wang F, Liu Y, Wang Z, Wang Z, et al. The role of caveolin-1 in blood-brain barrier disruption induced by focused ultrasound combined with microbubbles. J Mol Neurosci. 2012;46:677–87. doi: 10.1007/s12031-011-9629-9. [DOI] [PubMed] [Google Scholar]
- 85.Zhao YL, Song JN, Zhang M. Role of caveolin-1 in the biology of the blood-brain barrier. Rev Neurosci. 2014;25:247–54. doi: 10.1515/revneuro-2013-0039. [DOI] [PubMed] [Google Scholar]
- 86.Imakita N, Kitabatake M, Ouji-Sageshima N, Hara A, Morita-Takemura S, Kasahara K, et al. Abrogated Caveolin-1 expression via histone modification enzyme Setdb2 regulates brain edema in a mouse model of influenza-associated encephalopathy. Sci Rep. 2019;9:1–2. doi: 10.1038/s41598-018-36489-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tietz S, Engelhardt B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions [Internet] J. Cell Biol. 2015;209:493–506. doi: 10.1083/jcb.201412147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li W, Chen Z, Chin I, Chen Z, Dai H. The role of VE-cadherin in blood-brain barrier integrity under central nervous system pathological conditions. Curr Neuropharmacol. 2018;16:1375–1384. doi: 10.2174/1570159X16666180222164809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Breier G, Breviario F, Caveda L, Berthier R, Schnürch H, Gotsch U, et al. Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Blood. 1996;87:630–641. doi: 10.1182/blood.V87.2.630.bloodjournal872630. [DOI] [PubMed] [Google Scholar]
- 90.Williams MJ, Lowrie MB, Bennett JP, Firth JA, Clark P. Cadherin-10 is a novel blood-brain barrier adhesion molecule in human and mouse. Brain Res. 2005;1058:62–72. doi: 10.1016/j.brainres.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 91.Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, et al. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 92.Nag S, Begley DJ. Blood-brain barrier, exchange of metabolites and gases. Pathol Genet Cerebrovasc Dis. 2005. p. 22–9.
- 93.Betz AL, Firth JA, Goldstein GW. Polarity of the blood-brain barrier: Distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res. 1980;192:17–28. doi: 10.1016/0006-8993(80)91004-5. [DOI] [PubMed] [Google Scholar]
- 94.Mertsch K, Maas J. Blood-brain barrier penetration and drug development from an industrial point of view. Curr Med Chem - Cent Nerv Syst Agents. 2002;2:187–201. doi: 10.2174/1568015023358067. [DOI] [Google Scholar]
- 95.Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 96.Roberts LM, Black DS, Raman C, Woodford K, Zhou M, Haggerty JE, et al. Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience. 2008;155:423–438. doi: 10.1016/j.neuroscience.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 97.Bernacki J, Dobrowołska A, Nerwińska K, Małecki A. Physiology and pharmacological role of the blood-brain barrier. Pharmacol Rep. 2008;60:600–22. [PubMed] [Google Scholar]
- 98.Ohtsuki S, Sato S, Yamaguchi H, Kamoi M, Asashima T, Terasaki T. Exogenous expression of claudin-5 induces barrier properties in cultured rat brain capillary endothelial cells. J Cell Physiol. 2007;210:81–86. doi: 10.1002/jcp.20823. [DOI] [PubMed] [Google Scholar]
- 99.Begley DJ. The blood-brain barrier: Principles for targeting peptides and drugs to the central nervous system. J Pharm Pharmacol. 1996;48:136–146. doi: 10.1111/j.2042-7158.1996.tb07112.x. [DOI] [PubMed] [Google Scholar]
- 100.Begley DJ, Brightman MW. Structural and functional aspects of the blood-brain barrier. Prog Drug Res. 2003;61:39–78. doi: 10.1007/978-3-0348-8049-7_2. [DOI] [PubMed] [Google Scholar]
- 101.Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200:629–38. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Urquhart BL, Kim RB. Blood-brain barrier transporters and response to CNS-active drugs. Eur J Clin Pharmacol. 2009;65:1063–70. doi: 10.1007/s00228-009-0714-8. [DOI] [PubMed] [Google Scholar]
- 103.Makrides V, Dolgodilina E. Virgintino D. Blood-Brain Barrier Transporters and Neuroinflammation: Partners in Neuroprotection and in Pathology. Blood Brain Barrier Inflamm; 2017. pp. 103–151. [Google Scholar]
- 104.Barar J, Rafi MA, Pourseif MM, Omidi Y. Blood-brain barrier transport machineries and targeted therapy of brain diseases. BioImpacts. 2016;6:225–248. doi: 10.15171/bi.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tanegashima K, Sato-Miyata Y, Funakoshi M, Nishito Y, Aigaki T, Hara T. Epigenetic regulation of the glucose transporter gene Slc2a1 by β-hydroxybutyrate underlies preferential glucose supply to the brain of fasted mice. Genes Cells. 2017;22:71–83. doi: 10.1111/gtc.12456. [DOI] [PubMed] [Google Scholar]
- 106.You D, Wen X, Gorczyca L, Morris A, Richardson JR, Aleksunes LM. Increased MDR1 transporter expression in human brain endothelial cells through enhanced histone acetylation and activation of aryl hydrocarbon receptor signaling. Mol Neurobiol. 2019;56:6986–7002. doi: 10.1007/s12035-019-1565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 108.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat Neurosci. 2016;19:771–783. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- 110.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 111.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011;14:1398–405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karén J, Rodriguez A, Friman T, Dencker L, Sundberg C, Scholz B. Effects of the histone deacetylase inhibitor valproic acid on human pericytes In Vitro. PLoS One. 2011;6:e24954. doi: 10.1371/journal.pone.0024954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Castro V, Bertrand L, Luethen M, Dabrowski S, Lombardi J, Morgan L, et al. Occludin controls HIV transcription in brain pericytes via regulation of SIRT-1 activation. FASEB J. 2016;30:1234–1246. doi: 10.1096/fj.15-277673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zeng H, He X, Tuo QH, Liao DF, Zhang GQ, Chen JX. LPS causes pericyte loss and microvascular dysfunction via disruption of Sirt3/angiopoietins/Tie-2 and HIF-2α/Notch3 pathways. Sci Rep. 2016;6:1–3. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 116.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 118.Liu CY, Yang Y, Ju WN, Wang X, Zhang HL. Emerging roles of astrocytes in neuro-vascular unit and the tripartite synapse with emphasis on reactive gliosis in the context of Alzheimer’s disease. Front Cell Neurosci. 2018;12:193. doi: 10.3389/fncel.2018.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cabezas R, Ávila M, Gonzalez J, El-Bachá RS, Báez E, García-Segura LM, et al. Astrocytic modulation of blood brain barrier: perspectives on Parkinson’s disease. Front Cell Neurosci. 2014;8:211. doi: 10.3389/fncel.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hayashi Y, Nomura M, Yamagishi SI, Harada SI, Yamashita J, Yamamoto H. Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia. 1997;19:13–26. doi: 10.1002/(SICI)1098-1136(199701)19:1<13::AID-GLIA2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 121.Neuhaus J. Orthogonal arrays of particles in astroglial cells: Quantitative analysis of their density, size, and correlation with intramembranous particles. Glia. 1990;3:241–251. doi: 10.1002/glia.440030403. [DOI] [PubMed] [Google Scholar]
- 122.Takouda J, Katada S, Nakashima K. Emerging mechanisms underlying astrogenesis in the developing mammalian brain. Proc Japan Acad Ser B Phys Biol Sci. 2017;93:386–398. doi: 10.2183/pjab.93.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Namihira M, Nakashima K, Taga T. Developmental stage dependent regulation of DNA methylation and chromatin modification in a immature astrocyte specific gene promoter. FEBS Lett. 2004;572:184–188. doi: 10.1016/j.febslet.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 124.Namihira M, Kohyama J, Semi K, Sanosaka T, Deneen B, Taga T, et al. Committed neuronal precursors confer astrocytic potential on residual neural precursor cells. Dev Cell. 2009;16:245–255. doi: 10.1016/j.devcel.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 125.Hatada I, Namihira M, Morita S, Kimura M, Horii T, Nakashima K. Astrocyte-specific genes are generally demethylated in neural precursor cells prior to astrocytic differentiation. PLoS ONE. 2008;3:e3189. doi: 10.1371/journal.pone.0003189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nagy C, Suderman M, Yang J, Szyf M, Mechawar N, Ernst C, et al. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry. 2015;20:320–328. doi: 10.1038/mp.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Miguel-Hidalgo JJ. Molecular neuropathology of astrocytes and oligodendrocytes in alcohol use disorders. Front Mol Neurosci. 2018;11:78. doi: 10.3389/fnmol.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morgello S, Uson RR, Schwartz EJ, Haber RS. The human blood-brain barrier glucose transporter (GLUT1) is a glucose transporter of gray matter astrocytes. Glia. 1995;14:43–54. doi: 10.1002/glia.440140107. [DOI] [PubMed] [Google Scholar]
- 129.Kim SK, Yang H, Pascual JM, De Vivo DC. Valproic acid enhances glucose transport in the cultured brain astrocytes of glucose transporter 1 heterozygous mice. J Child Neurol. 2013;28:70–76. doi: 10.1177/0883073812440044. [DOI] [PubMed] [Google Scholar]
- 130.Baltan S, Bachleda A, Morrison RS, Murphy SP. Expression of histone deacetylases in cellular compartments of the mouse brain and the effects of ischemia. Transl Stroke Res. 2011;2:411–423. doi: 10.1007/s12975-011-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Staszewski O, Prinz M. Glial epigenetics in neuroinflammation and neurodegeneration. Cell Tissue Res. 2014;356:609–16. doi: 10.1007/s00441-014-1815-y. [DOI] [PubMed] [Google Scholar]
- 132.Reijerkerk A, Alejandro Lopez-Ramirez M, van het Hof B, Drexhage JAR, Kamphuis WW, Kooij G, et al. MicroRNAs regulate human brain endothelial cell-barrier function in inflammation: Implications for multiple sclerosis. J Neurosci. 2013;33:6857–6863. doi: 10.1523/JNEUROSCI.3965-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25:270–276. doi: 10.1038/s41591-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goodall EF, Leach V, Wang C, Cooper-Knock J, Heath PR, Baker D, et al. Age-associated mRNA and miRNA expression changes in the blood-brain barrier. Int J Mol Sci. 2019;20:3097. doi: 10.3390/ijms20123097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cichon C, Sabharwal H, Rüter C, Schmidt MA. MicroRNAs regulate tight junction proteins and modulate epithelial/endothelial barrier functions. Tissue Barriers. 2014;2:e944446. doi: 10.4161/21688362.2014.944446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ghosh G, Subramanian IV, Adhikari N, Zhang X, Joshi HP, Basi D, et al. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-α isoforms and promotes angiogenesis. J Clin Invest. 2010;120:4141–4154. doi: 10.1172/JCI42980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gu S, Chan WY. Flexible and versatile as a Chameleon-Sophisticated functions of microRNA-199a. Int J Mol Sci. 2012;13:8449–66. doi: 10.3390/ijms13078449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bakhshandeh B, Soleimani M, Paylakhi SH, Ghaemi N. A microRNA signature associated with chondrogenic lineage commitment. J Genet. 2012;91:171–182. doi: 10.1007/s12041-012-0168-0. [DOI] [PubMed] [Google Scholar]
- 139.Pais H, Nicolas FE, Soond SM, Swingler TE, Clark IM, Chantry A, et al. Analyzing mRNA expression identifies Smad3 as a microRNA-140 target regulated only at protein level. RNA. 2010;16:489–494. doi: 10.1261/rna.1701210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Davis-Dusenbery BN, Chan MC, Reno KE, Weisman AS, Layne MD, Lagna G, et al. Down-regulation of Krüppel-like Factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-β and bone morphogenetic protein 4. J Biol Chem. 2011;286:28097–28110. doi: 10.1074/jbc.M111.236950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhu N, Zhang D, Xie H, Zhou Z, Chen H, Hu T, et al. Endothelial-specific intron-derived miR-126 is down-regulated in human breast cancer and targets both VEGFA and PIK3R2. Mol Cell Biochem. 2011;351:157–164. doi: 10.1007/s11010-011-0723-7. [DOI] [PubMed] [Google Scholar]
- 142.Jafarifar F, Yao P, Eswarappa SM, Fox PL. Repression of VEGFA by CA-rich element-binding microRNAs is modulated by hnRNP L. EMBO J. 2011;30:1324–1334. doi: 10.1038/emboj.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chamorro-Jorganes A, Araldi E, Penalva LOF, Sandhu D, Fernández-Hernando C, Suárez Y. MicroRNA-16 and MicroRNA-424 regulate cell-autonomous angiogenic functions in endothelial cells via targeting vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1. Arterioscler Thromb Vasc Biol. 2011;31:2595–2606. doi: 10.1161/ATVBAHA.111.236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lichner Z, Mejia-Guerrero S, Ignacak M, Krizova A, Bao TT, Girgis AHF, et al. Pleiotropic action of renal cell carcinoma-dysregulated miRNAs on hypoxia-related signaling pathways. Am J Pathol. 2012;180:1675–1687. doi: 10.1016/j.ajpath.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 145.Truettner JS, Katyshev V, Esen-Bilgin N, Dietrich WD, Dore-Duffy P. Hypoxia alters microRNA expression in rat cortical pericytes. MicroRNA. 2013;2:32–45. doi: 10.2174/2211536611302010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Caporali A, Emanueli C. MicroRNA-503 and the Extended MicroRNA-16 Family in Angiogenesis. Trends Cardiovasc Med. 2011;21:162–6. doi: 10.1016/j.tcm.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Caporali A, Meloni M, Nailor A, Mitić T, Shantikumar S, Riu F, et al. P75NTR-dependent activation of NF-κB regulates microRNA-503 transcription and pericyte-endothelial crosstalk in diabetes after limb ischaemia. Nat Commun. 2015;6:1–3. doi: 10.1038/ncomms9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yan B, Yao J, Liu JY, Li XM, Wang XQ, Li YJ, et al. LncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res. 2015;116:1143–1156. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 149.Meares GP, Rajbhandari R, Gerigk M, Tien CL, Chang C, Fehling SC, et al. MicroRNA-31 is required for astrocyte specification. Glia. 2018;66:987–998. doi: 10.1002/glia.23296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hong P, Jiang M, Li H. Functional requirement of dicer1 and miR-17-5p in reactive astrocyte proliferation after spinal cord injury in the mouse. Glia. 2014;62:2044–2060. doi: 10.1002/glia.22725. [DOI] [PubMed] [Google Scholar]
- 151.Korotkov A, Broekaart DWM, van Scheppingen J, Anink JJ, Baayen JC, Idema S, et al. Increased expression of matrix metalloproteinase 3 can be attenuated by inhibition of microRNA-155 in cultured human astrocytes. J Neuroinflammation. 2018;15:211. doi: 10.1186/s12974-018-1245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bhalala OG, Pan L, Sahni V, McGuire TL, Gruner K, Tourtellotte WG, et al. microRNA-21 regulates astrocytic response following spinal cord injury. J Neurosci. 2012;32:17935–17947. doi: 10.1523/JNEUROSCI.3860-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang CY, Yang SH, Tzeng SF. MicroRNA-145 as one negative regulator of astrogliosis. Glia. 2015;63:194–205. doi: 10.1002/glia.22743. [DOI] [PubMed] [Google Scholar]
- 154.Ouyang YB, Xu L, Lu Y, Sun X, Yue S, Xiong XX, et al. Astrocyte-enriched miR-29a targets PUMA and reduces neuronal vulnerability to forebrain ischemia. Glia. 2013;61:1784–1794. doi: 10.1002/glia.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Michinaga S, Koyama Y. Dual roles of astrocyte-derived factors in regulation of blood-brain barrier function after brain damage. Int J Mol Sci. 2019;20:571. doi: 10.3390/ijms20030571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lo ACY, Chen AYS, Hung VKL, Yaw LP, Fung MKL, Ho MCY, et al. Endothelin-1 overexpression leads to further water accumulation and brain edema after middle cerebral artery occlusion via aquaporin 4 expression in astrocytic end-feet. J Cereb Blood Flow Metab. 2005;25:998–1011. doi: 10.1038/sj.jcbfm.9600108. [DOI] [PubMed] [Google Scholar]
- 157.Liu B, Neufeld AH. Expression of nitric oxide synthase-2 (NOS-2) in reactive astrocytes of the human glaucomatous optic nerve head. Glia. 2000;30:178–186. doi: 10.1002/(SICI)1098-1136(200004)30:2<178::AID-GLIA7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 158.Yin KJ, Cirrito JR, Yan P, Hu X, Xiao Q, Pan X, et al. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-β peptide catabolism. J Neurosci. 2006;26:10939–10948. doi: 10.1523/JNEUROSCI.2085-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chapouly C, Argaw AT, Horng S, Castro K, Zhang J, Asp L, et al. Astrocytic TYMP and VEGFA drive blood-brain barrier opening in inflammatory central nervous system lesions. Brain. 2015;138:1548–1567. doi: 10.1093/brain/awv077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu W, Cai H, Lin M, Zhu L, Gao L, Zhong R, et al. MicroRNA-107 prevents amyloid-beta induced blood-brain barrier disruption and endothelial cell dysfunction by targeting Endophilin-1. Exp Cell Res. 2016;343:248–257. doi: 10.1016/j.yexcr.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 161.Xi T, Jin F, Zhu Y, Wang J, Tang L, Wang Y, et al. MicroRNA-126-3p attenuates blood-brain barrier disruption, cerebral edema and neuronal injury following intracerebral hemorrhage by regulating PIK3R2 and Akt. Biochem Biophys Res Commun. 2017;494:144–151. doi: 10.1016/j.bbrc.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 162.Pan J, Qu M, Li Y, Wang L, Zhang L, Wang Y, et al. MicroRNA-126-3p/-5p Overexpression Attenuates Blood-Brain Barrier Disruption in a Mouse Model of Middle Cerebral Artery Occlusion. Stroke. 2020;51:619–627. doi: 10.1161/STROKEAHA.119.027531. [DOI] [PubMed] [Google Scholar]
- 163.Song J, Hu Y, Li H, Huang X, Zheng H, Hu Y, et al. miR-1303 regulates BBB permeability and promotes CNS lesions following CA16 infections by directly targeting MMP9. Emerg Microbes Infect. 2018;7:1–5. doi: 10.1038/s41426-018-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang Y, Wang MD, Xia YP, Gao Y, Zhu YY, Chen SC, et al. MicroRNA-130a regulates cerebral ischemia-induced blood-brain barrier permeability by targeting Homeobox A5. FASEB J. 2018;32:935–944. doi: 10.1096/fj.201700139RRR. [DOI] [PubMed] [Google Scholar]
- 165.Zuo X, Lu J, Manaenko A, Qi X, Tang J, Mei Q, et al. MicroRNA-132 attenuates cerebral injury by protecting blood-brain-barrier in MCAO mice. Exp Neurol. 2019;316:12–19. doi: 10.1016/j.expneurol.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 166.Wanet A, Tacheny A, Arnould T, Renard P. MiR-212/132 expression and functions: Within and beyond the neuronal compartment. Nucleic Acids Res. 2012;40:4742–4753. doi: 10.1093/nar/gks151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bai Y, Zhang Y, Hua J, Yang X, Zhang X, Duan M, et al. Silencing microRNA-143 protects the integrity of the blood-brain barrier: Implications for methamphetamine abuse. Sci Rep. 2016;6:1–5. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wan Y, Jin HJ, Zhu YY, Fang Z, Mao L, He Q, et al. MicroRNA-149-5p regulates blood-brain barrier permeability after transient middle cerebral artery occlusion in rats by targeting S1PR2 of pericytes. FASEB J. 2018;32:3133–3148. doi: 10.1096/fj.201701121R. [DOI] [PubMed] [Google Scholar]
- 169.Fang Z, He QW, Li Q, Chen XL, Baral S, Jin HJ, et al. MicroRNA-150 regulates blood-brain barrier permeability via Tie-2 after permanent middle cerebral artery occlusion in rats. FASEB J. 2016;30:2097–2107. doi: 10.1096/fj.201500126. [DOI] [PubMed] [Google Scholar]
- 170.Lopez-Ramirez MA, Wu D, Pryce G, Simpson JE, Reijerkerk A, King-Robson J, et al. MicroRNA-155 negatively affects blood-brain barrier function during neuroinflammation. FASEB J. 2014;28:2551–2565. doi: 10.1096/fj.13-248880. [DOI] [PubMed] [Google Scholar]
- 171.Liu F, Zheng S, Liu T, Liu Q, Liang M, Li X, et al. MicroRNA-21 promotes the proliferation and inhibits apoptosis in Eca109 via activating ERK1/2/MAPK pathway. Mol Cell Biochem. 2013;381:115–125. doi: 10.1007/s11010-013-1693-8. [DOI] [PubMed] [Google Scholar]
- 172.Ma Q, Dasgupta C, Li Y, Huang L, Zhang L. MicroRNA-210 suppresses junction proteins and disrupts blood-brain barrier integrity in neonatal rat hypoxic-ischemic brain injury. Int J Mol Sci. 2017;18:1356. doi: 10.3390/ijms18071356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Burek M, König A, Lang M, Fiedler J, Oerter S, Roewer N, et al. Hypoxia-induced microRNA-212/132 alter blood-brain barrier integrity through inhibition of tight junction-associated proteins in human and mouse brain microvascular endothelial cells. Transl Stroke Res. 2019;10:672–683. doi: 10.1007/s12975-018-0683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang Y, Huang J, Ma Y, Tang G, Liu Y, Chen X, et al. MicroRNA-29b is a therapeutic target in cerebral ischemia associated with aquaporin 4. J Cereb Blood Flow Metab. 2015;35:1977–1984. doi: 10.1038/jcbfm.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Toyama K, Spin JM, Deng AC, Huang TT, Wei K, Wagenhäuser MU, et al. MicroRNA-mediated therapy modulating blood-brain barrier disruption improves vascular cognitive impairment. Arterioscler Thromb Vasc Biol. 2018;38:1392–1406. doi: 10.1161/ATVBAHA.118.310822. [DOI] [PubMed] [Google Scholar]
- 176.Fan F, Yang J, Xu Y, Guan S. MiR-539 targets MMP-9 to regulate the permeability of blood-brain barrier in ischemia/reperfusion injury of brain. Neurochem Res. 2018;43:2260–2267. doi: 10.1007/s11064-018-2646-0. [DOI] [PubMed] [Google Scholar]
- 177.Bernstein DL, Zuluaga-Ramirez V, Gajghate S, Reichenbach NL, Polyak B, Persidsky Y, et al. miR-98 reduces endothelial dysfunction by protecting blood–brain barrier (BBB) and improves neurological outcomes in mouse ischemia/reperfusion stroke model. J Cereb Blood Flow Metab. 2020;40:1953–1965. doi: 10.1177/0271678X19882264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu J, He J, Tian X, Luo Y, Zhong J, Zhang H, et al. microRNA-9-5p alleviates blood–brain barrier damage and neuroinflammation after traumatic brain injury. J Neurochem. 2020;153:710–726. doi: 10.1111/jnc.14963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Duh EJ. A novel mechanism for glucocorticoid-induced tightening of endothelial barriers. Investig. Ophthalmol. Vis. Sci. 2013. p. 4016. [DOI] [PubMed]
- 180.Förster C, Burek M, Romero IA, Weksler B, Couraud PO, Drenckhahn D. Differential effects of hydrocortisone and TNFα on tight junction proteins in an in vitro model of the human blood-brain barrier. J Physiol. 2008;586:1937–1949. doi: 10.1113/jphysiol.2007.146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hue CD, Cho FS, Cao S, Dale Bass CR, Meaney DF, Morrison B. Dexamethasone potentiates in vitro blood-brain barrier recovery after primary blast injury by glucocorticoid receptor-mediated upregulation of ZO-1 tight junction protein. J Cereb Blood Flow Metab. 2015;35:1191–1198. doi: 10.1038/jcbfm.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Keil JM, Liu X, Antonetti DA. Glucocorticoid induction of occludin expression and endothelial barrier requires transcription factor p54 NONO. Investig Ophthalmol Vis Sci. 2013;54:4007–4015. doi: 10.1167/iovs.13-11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stone LA, Frank JA, Albert PS, Bash C, Smith ME, Maloni H, et al. The effect of interferon-β on blood—brain barrier disruptions demonstrated by constrast-enhanced magnetic resonance imaging in relapsing—remitting multiple sclerosis. Ann Neurol. 1995;37:611–619. doi: 10.1002/ana.410370511. [DOI] [PubMed] [Google Scholar]
- 184.Rg Kraus J, Oschmann P. The impact of interferon-b treatment on the blood-brain barrier. Drug Discov Today [Internet]. 2006 [cited 2021 Mar 11];11. Available from: www.drugdiscoverytoday.com [DOI] [PubMed]
- 185.Dhib-Jalbut S, Marks S. Interferon-β mechanisms of action in multiple sclerosis. Neurology. 2010;574:S17–24. doi: 10.1212/WNL.0b013e3181c97d99. [DOI] [PubMed] [Google Scholar]
- 186.Suh S, Park MK. Glucocorticoid-induced diabetes mellitus: an important but overlooked problem. Endocrinol Metab. 2017;32:180–9. doi: 10.3803/EnM.2017.32.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: A new resource for understanding the development and function of brain endothelial cells. PLoS ONE. 2010;5:e13741. doi: 10.1371/journal.pone.0013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Munji RN, Soung AL, Weiner GA, Sohet F, Semple BD, Trivedi A, et al. Profiling the mouse brain endothelial transcriptome in health and disease models reveals a core blood–brain barrier dysfunction module. Nat Neurosci. 2019;22:1892–1902. doi: 10.1038/s41593-019-0497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Urich E, Lazic SE, Molnos J, Wells I, Freskgård PO. Transcriptional profiling of human brain endothelial cells reveals key properties crucial for predictive in vitro blood-brain barrier models. PLoS ONE. 2012 doi: 10.1371/journal.pone.0038149&type=printable. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kallmann BA, Wagner S, Hummel V, Buttmann M, Bayas A, Tonn JC, et al. Characteristic gene expression profile of primary human cerebral endothelial cells. FASEB J. 2002;16:589–591. doi: 10.1096/fj.01-0594fje. [DOI] [PubMed] [Google Scholar]
- 191.Kalari KR, Thompson KJ, Nair AA, Tang X, Bockol MA, Jhawar N, et al. BBBomics-human blood brain barrier transcriptomics hub. Front Neurosci. 2016;10:71. doi: 10.3389/fnins.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Song HW, Foreman KL, Gastfriend BD, Kuo JS, Palecek SP, Shusta EV. Transcriptomic comparison of human and mouse brain microvessels. Sci Rep. 2020;10:1–4. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hupe M, Li MX, Kneitz S, Davydova D, Yokota C, Kele-olovsson J, et al. Gene expression profiles of brain endothelial cells during embryonic development at bulk and single-cell levels. Sci Signal. 2017;10:1–13. doi: 10.1126/scisignal.aag2476. [DOI] [PubMed] [Google Scholar]
- 194.Kelly TK, De Carvalho DD, Jones PA. Epigenetic modifications as therapeutic targets. Nat Biotechnol. 2010;10:1069–78. doi: 10.1038/nbt.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bates SE. Epigenetic therapies for cancer. N Engl J Med. 2020;383:650–663. doi: 10.1056/NEJMra1805035. [DOI] [PubMed] [Google Scholar]
- 196.Jones PA, Issa JPJ, Baylin S. Targeting the cancer epigenome for therapy. Nat. Rev. Genet. 2016;17:630–41. doi: 10.1038/nrg.2016.93. [DOI] [PubMed] [Google Scholar]
- 197.Cheng Y, He C, Wang M, Ma X, Mo F, Yang S, et al. Targeting epigenetic regulators for cancer therapy: Mechanisms and advances in clinical trials. Signal Transduct Target Ther. 2019;4:1–39. doi: 10.1038/s41392-019-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ganesan A, Arimondo PB, Rots MG, Jeronimo C, Berdasco M. The timeline of epigenetic drug discovery from reality to dreams. Clin Epigenet. 2019;11:1–7. doi: 10.1186/s13148-019-0776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mottamal M, Zheng S, Huang TL, Wang G. Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules. 2015;20:3898–941. doi: 10.3390/molecules20033898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci. 2015;18:521–530. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Workman MJ, Svendsen CN. Recent advances in human iPSC-derived models of the blood-brain barrier. Fluids Barriers CNS. 2020;17:1. doi: 10.1186/s12987-020-00191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lee S, Huang EJ. Modeling ALS and FTD with iPSC-derived neurons. Brain Res. 2017;1656:88–97. doi: 10.1016/j.brainres.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.