Abstract
Advances in stem cell cultures and human-induced pluripotent stem cells have inculcated interests in a rapidly evolving concept – ”organoids.” These are three-dimensional (3D) structures mimicking some of the phenomena of the real organs at anatomical, multicellular, and functional levels in vitro. Organoids have been proven to be better than two-dimensional cell culture in replicating the functionality, architectural, and geometrical features of tissues in vivo. Recent advancements have led to the generation of models for organ development and disease, finding applications in the drug discovery, screening of novel compounds, and personalized medicine. Since organoids follow the same natural pathway as the normal tissue or pathology, they can be used to study the expression of various genotypes and phenotypic variations across different species. In the light of these advancements, organoids are now being merged with bioengineering to come up with even better and reliable models to predict the disease progression and effectiveness of precision medicines, few of its important applications. This article discusses the various aspects of this emerging concept along with its uses, both in the present times and near future, with a special focus on pharmacological applications.
Keywords: Drug screening, in vitro, organoids, stem cells, three-dimensional
Introduction
There have been a lot of recent advancements in the field of cellular biology, tissue engineering, and microfabrication techniques. This has allowed the development of several three-dimensional (3D) cell culture methods such as multicellular spheroids, hydrogels, 3D bioprinting, organ-on-a-chip, and organoids. While these methods differ in their principles and protocols, they all converge in accelerating the implementation of 3D cell culture techniques in discovering novel drugs and further add to the ever-increasing productivity of the research and development sector of the pharmaceutical industry.[1] These models allow more flexibility and help in prediction of the efficacy and toxicity of newer drugs before they are considered for human trials, which in turn can help in lowering the rate of attrition of drugs under development.[2] These models are also advantageous in allowing researchers to check the efficacy and safety in a highly in vivo environment as compared to the traditional two-dimensional (2D) models. They also eliminate the issue of species difference which often impedes the outcomes of the preclinical studies.
This article is focused on introducing the concept of organoids to the reader and further exploring the types, characteristics, and advantages of these models over other conventional options available. It also gives an account of different types of organoid models as well as organs-on-a-chip system available vis-a-vis their development and utility, as well as proposed applications.
Organoids: The Concept
Organoids, also known as “organ buds,” are outcomes of a rapidly evolving type of dish-based technology with realistic microanatomy of the various organs of the body. They are 3D cultured structures which are derived from stem cells and contain different cell types developed from the stem cells or organ precursors. These cells, similar to the process in vivo, undergo cell sorting and spatially restricted lineage commitment to self-organize themselves. They can be classified as tissue and stem cell types depending on the development of the organ bud. Tissue organoids are basically stromal cell-free culture and mainly include the epithelial cells. Stem cell organoids are derivatives of “embryonic stem cells” or “induced pluripotent stem cells” (iPSCs). Till now, several organoid models have been developed to resemble and depict different tissues such as thyroid,[3] pancreas,[4] liver,[5,6] stomach,[7] intestine,[8] kidney,[9] lung,[10] and retina.[11]
Developing an Organoid
There are multiple methods described to develop an organoid. The first method is direct culture of a monolayer of cells on a bed of either feeder cells or a surface coated with extracellular matrix where the cells differentiate and organoids are formed. Another method is to allow further differentiation of the tissues using a mechanically supported culture. Human keratinocytes are an example, which can differentiate and self-assemble upon keeping the culture in contact with an air–liquid interface for a few weeks, and thus form a fully stratified tissue.[12] Embryoid bodies can also be developed using low-adhesion plates or hanging drop culture. Floating culture of aggregates can also be used to develop organoids; these are embryoid body like but are serum free and show fast re-aggregation in low-adhesion plates.
Organoids versus Organs: A Few Differences
Organoids can mimic some of the structures and functions of the real organs of the body, but they are different in certain key aspects. First, they do not have any vascular structure which is essential for nutrition and transport of waste from the body. Second, they do not have all the cell types that are present in vivo. Furthermore, few organoids mimic the early stages of organ development only. An example would be the retinal organoids which lack the outer segments, have photoreceptor cells that are not fully mature, and hence are not light sensitive. Similarly, the cerebral organoids lack the late developmental features such as the cortical plate layers.[13] There are certainly technical challenges in replicating the in vivo-like complexity and maturity, but the pace at which this field is advancing; solutions are expected sooner rather than later.
Advantages over Animal Models and Cell Cultures
There are numerous important advantages of organoids over the traditional methods of animal models and cell culture systems in disease modeling. A 3D cultured organoid can have multidirectional development, and thus, organogenesis as well as morphological features can be simulated in vivo. It can maintain its characteristics in vivo even after multiple generations without any significant changes genetically or physiologically. The gastrointestinal (GI) models can be made from the epithelial crypts in mouse GI tract or biopsy of human GI tract which can grow into crypt-villus structures within a few days.[14] The CRISPR/Cas9 is now allowing researchers to perform in vitro corrections or changes in the human genome while reducing the off-target effects during gene corrections in the organoids.[15]
The “Organ-on-a-Chip” Models
With the help of advancements in biotechnology and emergence of new methods, a variety of human tissue-derived in vitro models have been developed that can be adopted as individual tissue systems for testing any type of new chemical entities, drugs, or toxins. These systems can be used as disease-specific models by pairing genomics with tissue-engineering technologies. Techniques such as “microfabrication and microfluidic technology” can further help in the creation of “organ-on-a-chip” devices that allow cell and organoid culture, environmental sampling, high-throughput testing, fluid flow, and biosensing. The organoids are developed in vitro, much like the organs which grow and develop inside our body, whereas the organ-on-a-chip is a slightly modified approach. In this, cells from different organs are collected and allowed to grow in a fabricated device which has some of the organ’s functions and partly resembles the natural environment. It can be seen as a further advancement on the organoid models, immensely helpful in various pharmacological applications. While they are classified differently from the category of organoids in some of the literature, we have considered them together with organoids and the discussion entails both of them interchangeably. Such “organ-on-a-chip” represents a variety of tissues and is widely varying. They are currently being explored or implemented in the discovery of newer drugs.
Table 1 summarizes the different 3D cell culture techniques.
Table 1.
Various three-dimensional cell culture techniques with their pros and cons
| Cell culture technique | Pros | Cons |
|---|---|---|
| Spheroids | Easy handling and user-friendly | Unable to mimic complex architecture |
| Easy to transfer to different plates for subculture and for further studies | ||
| HTS can be performed | ||
| Can be co-cultured | ||
| Can be reproduced easily | ||
| Organoids and organ-on-a-chip | Specific for disease and patient | Can be variable |
| Mimics in vivo complexity and cellular architecture | Less susceptible for HTS | |
| Can provide in vivo like microenvironment | Difficult to achieve in vivo maturity | |
| Also provides chemical and physical gradients | ||
| Scaffolds/hydrogels | Can be done on microplates | Simple architecture |
| HTS can be performed | Can be greatly variable | |
| Can be easily reproduced | ||
| Can be co-cultured | ||
| 3D printing | Custom-made features and architecture | Deficiency of vasculature |
| Mimics chemical and physical gradients | Difficulty in procurement of raw materials for printing | |
| Can be easily produced on a large scale | Less susceptible to HTS | |
| Co-culture can be performed | Tissue maturity levels cannot be studied |
HTS=High-throughput screening, 3D=Three dimensional
Adipose tissue models
Vascularized adipose tissue (AT) “spheroids” have been developed from human stromal vascular fraction derived from AT obtained from nonobese individuals undergoing dermolipectomy. Modulation of AT vascularity is a possible tool for the treatment of obesity, although antiangiogenic drug actions may depend on age and obesity status. Endothelial cells (ECs) in the patient’s AT are crucial to predict outcomes in drug discovery. This model helps in studying AT in vitro, both in terms of physiology and disease pathology. It also paves the way for large-scale drug screening and opens research avenues for understanding possible links between adipogenesis and angiogenesis.[16]
In an attempt to overcome certain limitations with the existing 2D systems, a recent study devised a 3D tissue culture system equipped with magnetic nanoparticles (NPs) to enhance the development and growth of white AT.[17] AT samples obtained from normal individuals and those with type 2 diabetes mellitus have been studied using microfluidic devices for better understanding of glucose uptake and metabolism. Insulin resistance has also been studied by employing an automated method for administering doses and monitoring glucose activity in the cells over a period of 2 weeks.[18]
Cardiac organoids
iPSCs have emerged as a novel approach in establishing and enhancing the efficacy of cardiac drugs. Derived from the patient’s somatic cells, the iPSCs are capable of transforming into any cell type including the cardiomyocytes. They harbor disease-specific genetic code and hence exhibit the unique phenotypic features of the cardiac disorder when cultured into 3D cardiac model. In addition, they mimic normal drug response, thereby serving as a reliable preclinical tool in assessing cardiac drug safety and efficacy. For example, a micro 3D “heart-on-chip” model of Barth syndrome, a mitochondrial cardiac myopathy caused by tafazzin gene mutation, has been developed. It reproduces poor cardiac contraction and structural abnormalities in the sarcomeres. Testing with isoproterenol, verapamil, metoprolol, and E4031 (hERG blocker) has been accomplished.[19]
The improved architecture of the cardiac organ-on-a-chip organoid, using microfabrications and bioprinting technologies, has been implemented for advanced drug discovery. These technologies offer a better and practical approach to 3D modeling compared to the 2D plating‐type models. These bioprinted hearts have demonstrated cardiac-like electrophysiology and similar response to drugs, as in vivo. A study based on 3D cardiomyocyte microfabrication has shown that they can replicate the Frank–Starling mechanics in cardiac muscle cells using the I-wire assay.[20] In addition, well‐developed sarcomeres have been found to be associated with them. Responses to stimulation by different b‐adrenergic compounds and proper auxotonic contractions were demonstrable in these cardiac organoid models.
Small airway-on-a-chip (asthma model)
To develop an organoid system to mimic asthma, primary “human airway epithelial cells” (hAECs) and primary human lung vascular ECs were cultured on either side of a porous matrix-coated polyester membrane with “air–liquid interface,” resulting in formation of a pseudostratified ciliated epithelium with functional cilia. On exposure to interleukin (IL)-13 (100 ng/ml) for 8 days, these “airways-on-a-chip” showed goblet cell hyperplasia, decreased ciliary function, increased granulocyte colony-stimulating factor (G-CSF), and granulocyte/macrophage colony-stimulating factor (GM-CSF) secretion into the endothelial channel. Asthma exacerbations to viral infections, which are not ethically possible in humans, were mimicked on these chips using polyinosinic-polycytidylic acid (poly[I: C]), resulting in a surge in IL-6, interferon-g-inducible protein-10, and other mediators. Tofacitinib, a JAK-STAT inhibitor used in rheumatoid arthritis, has been studied as a possible therapeutic tool for asthma using this model. High-dose (1–10 mM) tofacitinib showed a decrease in G-CSF, GM-CSF, goblet cell hyperplasia, and enhanced ciliary beating.[21]
Organoids of lung cancer
Recent advances in lung organoids aim at mimicking stiffness exhibited by lung tissue in vivo and models for lung cancer have also been developed. Human? non-small cell lung cancer (NSCLC) cells have been co-cultured along with human fetal lung fibroblasts on a poly-lactic-co-glycolic acid nanofiber scaffolding to estimate the toxicity of gefitinib, an epithelial growth factor receptor-targeted drug.[10] NSCLC models have been developed using lung microfluidic chip technology. Exposure of the H1975 human NSCLC cells in the microfluidic device to breathing lung alveolar cells has led to the creation of in vivo lung cancer models. These cells, resistant to the third-generation tyrosine kinase inhibitors, showed a significant drop in the tumor growth, when grown in a micro-aerated in vivo-like atmosphere, making this one of the first studies to incorporate the role of breathing mechanics in cancer resistance.[17,22]
Gastrointestinal models
Intestinal organoid models have helped in better understanding of GI functioning and bioavailability of oral drugs; have potential applications in finding novel drugs for intestinal disease conditions.[11] A recent study on “gut-on-a-chip” organoid demonstrated serious villous injury, mediated by pro-inflammatory cytokines such as IL‐1b, IL‐6, and IL‐8, mimicking the inflammatory milieu that is seen in inflammatory bowel disease (IBD). This allows the exploration of opportunities to develop drugs for IBD. Probiotic (VSL#3 cells) or antibiotic administration has demonstrated protection against this villous injury from cytokines.[23]
Hepatic organoids
The regenerative capacity of the liver has been utilized in developing liver organoids. Leucine-rich repeat-containing G-protein coupled receptor-5-positive progenitor cells in adult liver, especially around healthy bile ducts, activated by chemical damage, have been used to develop murine liver organoids. With advancing knowledge on these models, human adult and fetal liver samples (from partial hepatectomy) have now been used to develop hepatic organoids. 3D models of hepatic neoplasms have proved much superior to the conventional 2D liver cancer cell lines and “patient-derived xenografts (PDXs).” Accumulating cancer mutations and resulting clonal selection, loss of original heterogeneity, increased time and expenses, and incompatibility to large-scale drug testing are the drawbacks associated with PDX. The 3D tumor organoids, “tumoroids” in short, have emerged as a novel tool in personalized precision medicine. While retaining the original genotype and tumor phenotype, these models developed using the patients’ liver samples have been successfully able to establish all the three major phenotypic subtypes of primary liver cancer, thus paving the way for identification of novel biomarkers and potential therapeutic strategies including the extracellular signal-regulated kinase inhibitors.[24]
3D liver models have also proven far better than their 2D counterparts when it comes to studies on hepatotoxicity, owing to the instability of hepatocytes in the 2D cell culture and impaired cytochrome P450 function. With the hepatic organoids expressing phase 1 and phase 2 CYP enzymes, studies comparing their efficacy in predicting drug toxicity with cell cultures have been done. It has been found that the toxic dose of trovafloxacin is 50 times higher in 2D hepatocyte model, thereby establishing the 3D organoids as a valuable tool in studying potential drug-induced hepatotoxicity.[24] Similarly, organoid models with bile duct and sinusoid‐like structures have been created. They show glucose uptake and gluconeogenesis almost identical to that in vivo. Recently developed scaffold-free 3D bioprinting created liver showed unique longevity, thus allowing the long-term study of drug metabolism.[5] Organoids developed from biopsy samples of patients with intrahepatic cholangiocarcinoma have been able to prove that the hepatocytes, and not the cholangiocytes as it was previously believed to, are the source for the tumor cells.[6]
Organoids in renal diseases
Patient-specific 3D renal organoids have been developed from differentiated whole kidney cells either from renal biopsies, resection specimens, or from cells in the urinary sediments. “Renal cell therapy,” though in early research, seems to be promising in the treatment of chronic kidney disease or end-stage renal disease (ESRD). The renal organoids can act as an autologous source for renal cell therapy. These are developed using “hanging drop plates,” involving various renal cells along with the extracellular matrix, growth factors establishing cell-cell communication, and cell-specific gene expression, thereby recreating the original architecture of the kidney in a much better way than the 2D renal cell models. The nephrotoxic potential of aspirin, penicillin-G, and cisplatin has been evaluated using this model.[25]
Renal organoids with more selective filtering have been developed by recapitulating mature and functional glomerular podocytes. The nephrotoxicity of Adriamycin has been studied using the “glomerulus-on-a-chip” model, which mimics the function, developmental steps, and pathogenetic process of the capillaries in the glomerulus.[9] Diabetic nephropathy, which is one of the major causes of ESRD, has also been studied. Glomerular microenvironment has been reconstituted within a microfluidic device with parallel channels lined by primary glomerular microtissue, 3D basement membrane, and a microfluidic perfusion system to mimic glomerular filtration barrier (GFB).[26] ? It has been shown that hyperglycemia due to diabetes induces apoptosis of glomerular cells, increases the permeability of GFB to albumin, causes destruction of the tight junctions between the glomerular ECs, and increased expression of GLUT-1. This opens up avenues for further research on nephropathy including better treatment options using the glomerulus models. Models for assessing prognosis in polycystic kidney disease have been made based on the expression of genes – polycystin-1 (PC)-1 and PC-2.[27]
Placental organoids in preeclampsia
The impact of extracellular placental basement membrane proteins on cytotrophoblast invasion has been studied using bioprinting technology. Placental extracellular matrix (pECM) was successfully bioprinted to design the cytotrophoblast invasion of placental basement membrane.[28] To understand the effect on rates of invasion, cells were printed at different concentrations at the edge of the model. Placental models loaded with pECM showed a significant increase in cytotrophoblast invasion rates, and PI3K inhibitors reduced rates of invasion. Invasion rates were critically lower in the absence of BM proteins, which is associated with preeclampsia. This could be further studied for possible therapeutics for preeclampsia in the future. The effect of NPs on the human placenta, which can induce stress reactions such as reactive oxygen species, inflammation, and apoptosis, has been studied using 3D co-culture microtissue models designed using “scaffold-free hanging drop technology” with trophoblast cell layers having placental fibroblasts within them. NP toxicity studies have shown that cadmium? telluride and copper oxide affect the viability of the microtissue and decrease the secretion of human chorionic gonadotropin. These 3D models help in better understanding the toxicity associated with NP and potential of their safe usage in pregnancy.
Organoid models in ocular pathology
Retinal organoids have been synthesized using the rotating-wall vessel bioreactor,[11] which addresses the issue of imbalance in supply of nutrients and oxygen between the periphery and the center, thereby preventing early degeneration. This could potentially be used therapeutically to replace cells in conditions such as retinitis pigmentosa, glaucoma, and age-related macular edema. A major challenge in treating advanced cases of retinoblastoma is the chemo-resistance exhibited by vitreal and subretinal deposits or “seeds” associated with the primary tumor. This could be due to poor vascularity of the vitreous cavity and the subretinal space, resulting in inadequate chemotherapeutic concentrations of drugs such as melphalan, methotrexate, and topotecan at these sites.
3D, self-organizing organoid models of advanced retinoblastoma have been generated, mimicking cellular features and retaining genetic alterations of the original tumor tissue. They have been used to compare the penetration and effectiveness of chemotherapeutic agents. Melphalan and topotecan, administered in combination, were found to be superior to melphalan alone in terms of number of chemotherapy cycles and rapid control of “seeds.”[29]
Table 2 summarizes various organoid model systems with a few examples of each.
Table 2.
Important organoid models based on different tissue and cell types
| Tissue types | Major Cell types | Organoid models |
|---|---|---|
| Brain | Neuron, oligodendrocyte, microglia, astrocyte, ECs | Huntington’s disease[30] |
| Neuroectoderm[31] | ||
| Eye | Rod, cone, retinal ganglion, retinal epithelium, bipolar cells | Retina-on-a-chip |
| Retinal organoid | ||
| Ear | Type I hair cell, Type II hair cell, Inner cochlear hair cell | Cochlear hair cells[32] |
| Vestibular tissue[33] | ||
| Skin | Melanocyte, keratinocyte | Keratinocyte[34] |
| Fibroblast[35] | ||
| Lung | Lung epithelium, type I pneumocyte, type II pneumocyte, alveolar macrophages, ECs | Lung epithelium |
| fibrotic lung disease | ||
| Heart | Cardiomyocyte, cardiac fibroblast, ECs, smooth muscles | Embryotoxicity screening Cardiomyocyte |
| Blood | Lymphocytes, monocytes, granulocytes | Endothelium[36] |
| T cell | ||
| Stomach | Mucous cell, parietal cell, chief cell, gastrin cell, neuroendocrine cell | Gastric cells |
| Esophagus[37] | ||
| Pancreas | Alpha-cell, beta-cell, delta-cell, gamma-cell, stellate cell | Pancreatic cancer[38] |
| Ductal organoid[39] | ||
| Liver | Hepatocyte, stellate cell, Kupffer cell, oval cell, EC | Hepatobiliary |
| Liver buds | ||
| Kidney | Podocyte, ECs, parietal cells, brush border cells, mesangial cells | Nephron |
| Parietal cells | ||
| Intestine | Enterocyte, goblet cells, enterochromaffin cells, Paneth cells, tuft cell | Enterocytes |
| Intestinal epithelium |
ECs=Endothelial cells
Multi-Organs-On-a-Chip
Otherwise referred to as the “human-on-a-chip,” this involves simultaneous integration of multiple constructs onto an individual chip with continuous circulation of media and inter-organ interaction, so as to mimic complex organ function. The intestines and the liver have been the first organs to be integrated, following which “two-organs,” “three-organs,” “four-organs” and “ten-organs-on-a-chip” have been developed. Classified into “static,” “semi-static,” and “flexible” approaches in multi-organ connections and interactions, it is a concept expected to materialize into a major tool for drug discovery.
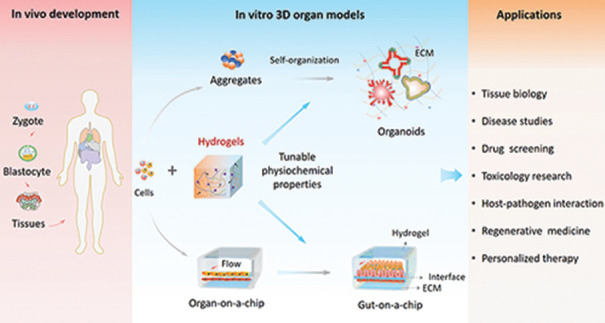
Figure 1 illustrates the organoids and organ-on-a-chip and their applications.
Figure 1.
Illustration of organoids and organ-on-a-chip with applications (Image courtesy: Wang Yaqing, Dalian Institute of Chemical Physics, Chinese Academy of Sciences)
Specific Applications in Pharmacology: A Brief Summary
Drug discovery
Novel therapies have their own limitations such as inter-patient variations in clinical response, uncertainties in predicting outcomes, and longer time consumed in drug testing and trials. Patient- and disease-specific organoids are likely to evolve into powerful tools aimed at precision therapy. Malignancies, infections, and developmental disorders can be replicated with biopsy specimens from patients, which might be useful for drug testing, gene editing, or evaluating the prognosis. In addition to new drug discovery, drug screening using organoids can help in assessing inter-individual variations in drug efficacy of existing drugs. Testing of potential drugs on the organoids can lead to newer leads for pharmaceutical research and development. Organoids may complement animal testing in toxicity studies also. For example, intestinal organoids may serve as a model for in vitro testing of drugs and toxins that damage the intestinal epithelium and also for understanding the cellular death pathways.
Personalized medicine
In vitro organoids can grow rapidly, and differentiate stably, and are hence amenable to genetic manipulations. They can be powerful tools for research on human diseases and personalized or tailored therapy. For example, organoids were developed from the rectal biopsy of two patients of cystic fibrosis and a positive response to ivacaftor was identified.[40] Similarly, organoids developed from cancer patients can help understand individual treatment regimens. In the context of exploring novel drugs, the organoids from primary human pancreatic ductal adenocarcinoma are an important example. Organoids have proven to be useful in drug screening platform-based preclinical models for profiling of tumors.
Conclusion
This ex vivo technology of organoids is an immense breakthrough in the field of basic and clinical researches. Organoids are already playing important roles in developmental and stem cell biology, as well as disease modeling. In pharmacology, they have made an immediate impact on the fields of drug discovery, screening, toxicity, and personalized medicine. Their application deserves further exploration through systematic studies. Overall, organoids or the “organs-on-a-chip” hold great promise as a tool in medical research, particularly pharmacology.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, et al. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9:203–14. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- 2.Fang Y, Eglen RM. Three-dimensional cell cultures in drug discovery and development. SLAS Discov. 2017;22:456–72. doi: 10.1177/1087057117696795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonica F, Kasprzyk DF, Schiavo AA, Romitti M, Costagliola S. Generation of functional thyroid tissue using 3D-based culture of embryonic stem cells. Methods Mol Biol. 2017;1597:85–95. doi: 10.1007/978-1-4939-6949-4_7. [DOI] [PubMed] [Google Scholar]
- 4.Greggio C, De Franceschi F, Figueiredo-Larsen M, Gobaa S, Ranga A, Semb H, et al. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development. 2013;140:4452–62. doi: 10.1242/dev.096628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kizawa H, Nagao E, Shimamura M, Zhang G, Torii H. Scaffold-free 3D bio-printed human liver tissue stably maintains metabolic functions useful for drug discovery. Biochem Biophys Rep. 2017;10:186–91. doi: 10.1016/j.bbrep.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito Y, Nakaoka T, Muramatsu T, Ojima H, Sukeda A, Sugiyama Y, et al. Induction of differentiation of intrahepatic cholangiocarcinoma cells to functional hepatocytes using an organoid culture system. Sci Rep. 2018;8:2821. doi: 10.1038/s41598-018-21121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, et al. Differentiated Troy+chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–68. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallach TE, Bayrer JR. Intestinal organoids: New frontiers in the study of intestinal disease and physiology. J Pediatr Gastroenterol Nutr. 2017;64:180–5. doi: 10.1097/MPG.0000000000001411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musah S, Mammoto A, Ferrante TC, Jeanty SSF, Hirano-Kobayashi M, Mammoto T, et al. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng. 2017;1:0069. doi: 10.1038/s41551-017-0069. (doi: 101038/s41551-017-0069 Epub 2017 May 10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Li K, Zhang X, Liu C, Guo B, Wen W, et al. Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Lab Chip. 2018;18:486–95. doi: 10.1039/c7lc01224a. [DOI] [PubMed] [Google Scholar]
- 11.DiStefano T, Chen HY, Panebianco C, Kaya KD, Brooks MJ, Gieser L, et al. Accelerated and improved differentiation of retinal organoids from pluripotent stem cells in rotating-wall vessel bioreactors. Stem Cell Reports. 2018;10:300–13. doi: 10.1016/j.stemcr.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalabis J, Wong GS, Vega ME, Natsuizaka M, Robertson ES, Herlyn M, et al. Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nat Protoc. 2012;7:235–46. doi: 10.1038/nprot.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancaster MA, Knoblich JA. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science. 2014;345:1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 14.Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109:466–71. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drost J, van Boxtel R, Blokzijl F, Mizutani T, Sasaki N, Sasselli V, et al. Use of CRISPR-modified human stem cell organoids to study the origin of mutational signatures in cancer. Science. 2017;358:234–8. doi: 10.1126/science.aao3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller S, Ader I, Creff J, Leménager H, Achard P, Casteilla L, et al. Human adipose stromal-vascular fraction self-organizes to form vascularized adipose tissue in 3D cultures. Sci Rep. 2019;9:7250. doi: 10.1038/s41598-019-43624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittal R, Woo FW, Castro CS, Cohen MA, Karanxha J, Mittal J, et al. Organ-on-chip models: Implications in drug discovery and clinical applications. J Cell Physiol. 2019;234:8352–80. doi: 10.1002/jcp.27729. [DOI] [PubMed] [Google Scholar]
- 18.Zambon A, Zoso A, Gagliano O, Magrofuoco E, Fadini GP, Avogaro A, et al. High temporal resolution detection of patient-specific glucose uptake from human ex vivo adipose tissue on-chip. Anal Chem. 2015;87:6535–43. doi: 10.1021/ac504730r. [DOI] [PubMed] [Google Scholar]
- 19.Tzatzalos E, Abilez OJ, Shukla P, Wu JC. Engineered heart tissues and induced pluripotent stem cells: Macro And microstructures for disease modeling, drug screening, and translational studies. Adv Drug Deliv Rev. 2016;96:234–44. doi: 10.1016/j.addr.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sidorov VY, Samson PC, Sidorova TN, Davidson JM, Lim CC, Wikswo JP. I-Wire Heart-on-a-Chip I: Three-dimensional cardiac tissue constructs for physiology and pharmacology. Acta Biomater. 2017;48:68–78. doi: 10.1016/j.actbio.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benam KH, Villenave R, Lucchesi C, Varone A, Hubeau C, Lee HH, et al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat Methods. 2016;13:151–7. doi: 10.1038/nmeth.3697. [DOI] [PubMed] [Google Scholar]
- 22.Hassell BA, Goyal G, Lee E, Sontheimer-Phelps A, Levy O, Chen CS, et al. Human organ chip models recapitulate orthotopic lung cancer growth, therapeutic responses, and tumor dormancy in vitro. Cell Rep. 2017;21:508–16. doi: 10.1016/j.celrep.2017.09.043. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Choi JH, Kim HJ. Human gut-on-a-chip technology: Will this revolutionize our understanding of IBD and future treatments? Expert Rev Gastroenterol Hepatol. 2016;10:883–5. doi: 10.1080/17474124.2016.1200466. [DOI] [PubMed] [Google Scholar]
- 24.Akbari S, Arslan N, Senturk S, Erdal E. Next-generation liver medicine using organoid models. Front Cell Dev Biol. 2019;7:345. doi: 10.3389/fcell.2019.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding B, Sun G, Liu S, Peng E, Wan M, Chen L, et al. Three-dimensional renal organoids from whole kidney cells: Generation, optimization, and potential application in nephrotoxicology in vitro. Cell Transplant. 2020;29:963689719897066. doi: 10.1177/0963689719897066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Tao T, Su W, Yu H, Yu Y, Qin J. A disease model of diabetic nephropathy in a glomerulus-on-a-chip microdevice. Lab Chip. 2017;17:1749–60. doi: 10.1039/c7lc00134g. [DOI] [PubMed] [Google Scholar]
- 27.Cruz NM, Song X, Czerniecki SM, Gulieva RE, Churchill AJ, Kim YK, et al. Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nat Mater. 2017;16:1112–9. doi: 10.1038/nmat4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo CY, Guo T, Cabrera-Luque J, Arumugasaamy N, Bracaglia L, Garcia-Vivas A, et al. Placental basement membrane proteins are required for effective cytotrophoblast invasion in a three-dimensional bioprinted placenta model. J Biomed Mater Res A. 2018;106:1476–87. doi: 10.1002/jbm.a.36350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saengwimol D, Rojanaporn D, Chaitankar V, Chittavanich P, Aroonroch R, Boontawon T, et al. A three-dimensional organoid model recapitulates tumorigenic aspects and drug responses of advanced human retinoblastoma. Sci Rep. 2018;8:15664. doi: 10.1038/s41598-018-34037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haremaki T, Metzger JJ, Rito T, Ozair MZ, Etoc F, Brivanlou AH. Self-organizing neuruloids model developmental aspects of Huntington’s disease in the ectodermal compartment. Nat Biotechnol. 2019;37:1198–208. doi: 10.1038/s41587-019-0237-5. [DOI] [PubMed] [Google Scholar]
- 31.Kanton S, Boyle MJ, He Z, Santel M, Weigert A, Sanchís-Calleja F, et al. Organoid single-cell genomic atlas uncovers human-specific features of brain development. Nature. 2019;574:418–22. doi: 10.1038/s41586-019-1654-9. [DOI] [PubMed] [Google Scholar]
- 32.Jeong M, O’Reilly M, Kirkwood NK, Al-Aama J, Lako M, Kros CJ, et al. Generating inner ear organoids containing putative cochlear hair cells from human pluripotent stem cells. Cell Death Dis. 2018;9:922. doi: 10.1038/s41419-018-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattei C, Lim R, Drury H, Nasr B, Li Z, Tadros MA, et al. Generation of vestibular tissue-like organoids from human pluripotent stem cells using the rotary cell culture system. Front Cell Dev Biol. 2019;7:25. doi: 10.3389/fcell.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y, Park N, Rim YA, Nam Y, Jung H, Lee K, et al. Establishment of a complex skin structure via layered co-culture of keratinocytes and fibroblasts derived from induced pluripotent stem cells. Stem Cell Res Ther. 2018;9:217. doi: 10.1186/s13287-018-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim Y, Ju JH. Generation of 3D skin organoid from cord blood-derived induced pluripotent stem cells? J Vis Exp. 2019;146 doi: 10.3791/59297. doi: 103791/59297. [DOI] [PubMed] [Google Scholar]
- 36.Motazedian A, Bruveris FF, Kumar SV, Schiesser JV, Chen T, Ng ES, et al. Multipotent RAG1+progenitors emerge directly from haemogenic endothelium in human pluripotent stem cell-derived haematopoietic organoids. Nat Cell Biol. 2020;22:60–73. doi: 10.1038/s41556-019-0445-8. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Yang Y, Jiang M, Huang SX, Zhang W, Al Alam D, et al. 3D modeling of esophageal development using human PSC-derived basal progenitors reveals a critical role for notch signaling. Cell Stem Cell. 2018;23:516–29e5. doi: 10.1016/j.stem.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang L, Holtzinger A, Jagan I, BeGora M, Lohse I, Ngai N, et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med. 2015;21:1364–71. doi: 10.1038/nm.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hohwieler M, Illing A, Hermann PC, Mayer T, Stockmann M, Perkhofer L, et al. Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut. 2017;66:473–86. doi: 10.1136/gutjnl-2016-312423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartfeld S, Clevers H. Stem cell-derived organoids and their application for medical research and patient treatment. J Mol Med (Berl) 2017;95:729–38. doi: 10.1007/s00109-017-1531-7. [DOI] [PubMed] [Google Scholar]