Abstract
The age-associated decline in muscle mass has become synonymous with physical frailty among the elderly due to its major contribution in reduced muscle function. Alterations in protein and redox homeostasis along with chronic inflammation, denervation and hormonal dysregulation are all hallmarks of muscle wasting and lead to clinical sarcopenia in older adults. Reduction in skeletal muscle mass has been observed and reported in the scientific literature for nearly two centuries; however, identification and careful examination of molecular mediators of age-related muscle atrophy have only been possible for roughly three decades. Here we review molecular targets of recent interest in age-related muscle atrophy and briefly discuss emerging small molecule therapeutic treatments for muscle wasting in sarcopenic susceptible populations.
Keywords: inflammation, neuromuscular junction, proteostasis, sarcopenia, redox homeostasis
Introduction
One of the first interpretations in recorded history of frailty in older adults was made around ~400 B.C. by Hippocrates whose notions on aging reflect the current accepted concept of aging as increased frailty [1, 2]. Hippocrates viewed aging as a late stage of life in which there is an increased susceptibility to disease [1]. Around the fifth decade, humans will begin to lose on average ~1% of lean mass per year resulting in a 30-50% reduction in muscle mass by 80 years of age [3–7]. This age-related decline in muscle mass and associated muscle weakness, referred to as sarcopenia [8–10], has been clinically classified by several geriatrics organizations as two standard deviations below the mean for healthy young adults 20-30 years of age [11–14]. Based on the clinical definition, roughly 11-50% of adults 80 years of age and older are considered sarcopenic, although adults that are at least 50 years old typically exhibit age-related muscle atrophy of some degree [6, 14].
Promoting the maintenance of skeletal muscle in the elderly is vital to delay physical frailty as well as to preserve amino acid reservoirs in the body to reduce morbidity and mortality [15–19]. In the early twentieth century there was a gap in knowledge between the identification of muscle wasting in aging and the mechanisms involved. An early theory disproven by the Harvard Fatigue Laboratory in 1946 was that a loss of water content was the major contributor to the shrinking of muscle tissue [20]. More recent advances in cell physiology and molecular biology allowed the identification of numerous cellular signaling pathways and biological functions that are altered in association with age-associated muscle deterioration and influenced by a myriad of molecular mediators (Table 1) [21–27]. The mediators that have been most widely investigated in regards to muscle wasting during aging over the past few decades can be categorized in a few key areas: proteostasis, inflammation, denervation, redox homeostasis, and hormonal regulation. This review will focus on novel molecular mediators of age-related muscle atrophy within these areas that have been reported in the past decade and emerging drug treatments for sarcopenia.
Table 1.
Previous identified molecular targets of muscle wasting in aged mammals
| Classification | Molecular Targets | Age-related Atrophy Year Identification | Reference(s) |
|---|---|---|---|
| Anabolic Hormones | Testosterone | 1992 | [128] |
| DHEA | 1995 | [169] | |
| Growth Hormone | 1998 | [170, 171] | |
| IGF-1 | 1998 | [172] | |
| Proteostasis | mTOR signaling | 2004 | [173] |
| Caspases | 2004 | [174] | |
| FoxO* | 2004* | [175] | |
| Immune System | TNF-α | 2005 | [176, 177] |
| IL-6* | 1997* | [178, 179] | |
| Denervation-Induced Atrophy | AchR | 1984 | [85] |
| Redox Homeostasis | SOD1 | 2006 | [180] |
denotes targets or dates of only age-associated data in regards to muscle atrophy.
Abbreviations: AchR, acetylcholine receptor; DHEA, dehydroepiandrosterone; FoxO, forkhead family of transcription factors; IGF-1, insulin-like growth factor-1; IL-6, interleukin-6; mTOR, mammalian target of rapamycin; MuSK, muscle-specific kinase; SOD1, superoxide dismutase 1; TNF-α, tumor necrosis factor alpha.
Proteostasis and Age-related Muscle Atrophy
The maintenance of protein turnover through mediators of protein synthesis and degradation has been well documented for healthy aging and preservation of skeletal muscle tissue during aging in C. elegans, drosophila, and mammals [28–31]. Skeletal muscle comprises ~60% of proteins in the body [32]. Thus, the regulation of the Akt/mTOR pathway has been a major focus for maintaining skeletal muscle health [33, 34]. In the past few decades, exercise and calorie restriction interventions targeting protein homeostasis have produced improved lifespan and healthspan through maintaining muscle mass [35–38] demonstrating the importance of protein synthetic signaling in skeletal muscle during aging [33, 34]. Examples confirming the importance of mTOR in muscle maintenance have used a transgenic mouse model where the TOR agonist complex protein, TSC, is knocked out in the muscle leading to constitutive activation of mTORC1 [39, 40]. These mice display accelerated neuromuscular aging and autophagy dysfunction as early as 9 months of age and will typically perish within 12 months [39, 40].
Previous research investigating protein signaling upstream of Akt (e.g. IGF-1) has indicated that maintaining insulin signaling is necessary to preserve skeletal muscle mass in older individuals [41, 42]. As a specific illustration, induction of the upstream target of Akt, PI3k subunit p110α, has been reported to lead to increased muscle atrophy during aging [43]. M-p110αKO mice displayed decreased PI3k activity and Akt phosphorylation which led to muscle atrophy in most hind limb muscles by 24 months of age [43]. The intracellular enzyme, PI3k, is critical in the insulin signaling pathway and its relevance to the dietary restriction pathway may suggest its role to maintain proteostasis in older adults [44, 45].
The latest reports of molecular mediators of proteostasis as potential contributors to age-associated skeletal muscle atrophy show less reliance on regulating mTORC1 and more dependence on other mechanisms. For example, Activating Transcription Factor 4 (ATF4) has recently been identified as a molecular target for muscle wasting in aging [46], although its regulation within protein synthetic signaling has not been fully elucidated [46–48]. Support for the importance of ATF4 in regulating muscle mass during aging is provided by the observations of Ebert and colleagues that treatment with either ursolic acid or tomatidine, small molecules known to reduce age-related deficits in skeletal muscle mass. After 2 months of treatment with ursolic acid or tomatidine, a strong inverse relationship was found between ATF4-dependent mRNAs and either ursolic acid- or tomatidine-sensitive mRNAs, demonstrating a potential link between ATF4 and age-associated muscle atrophy. Moreover, 22-month-old muscle-specific Atf4 knockout mice had 8% larger skeletal muscle mass than wild-type controls, suggesting that ATF4 has a direct role in muscle wasting during aging [46].
Regulators of wingless type (WNT) signaling appear to play a role in maintaining muscle mass in aging muscle as well. Dkk3 is a secreted glycoprotein in skeletal muscle and non-canonical WNT signaling antagonist that induces muscle atrophy when upregulated [49–54]. Furthermore, Dkk3 level is increased in blood of older adults and is upregulated in senescent prostate epithelial cells [55, 56]. Conversely, Yin and colleagues found that Dkk3 is a key activator of Fbxo32 and Trim63 through recruitment of β-catenin, and Dkk3 is upregulated in muscles of 20-month old mice [56]. Moreover, reduction of Dkk3 through intramuscular injection of shRNA against Dkk3 restored skeletal muscle mass in aged mice [56].
The fibroblast growth factor FGF21 is a hormone secreted by fibroblasts and others cell types within skeletal muscle and is an important regulator of whole body metabolism [57–59]. FGF21 increases systemically with age in humans, and Tezze and colleagues found that muscle specific knockout of the autophagy gene, Opa1, increased levels of FGF21 and reduced muscle mass in slow, fast and mixed fiber type hind limb muscles of mice [57]. While Opa1 deficiency in muscle reduced muscle mass and increased FGF21, deletion of both Opa1 and FGF21 in mice partially restored skeletal muscle mass suggesting that FGF21 compensates for Opa1 and may be a key regulator of age-associated muscle wasting [57]. There are numerous other regulators of FGF21 involved with metabolism and energy homeostasis suggesting that FGF21 may play a central role in muscle maintenance in aging [57–59]. Altogether, evidence support that components of proteostasis including key signaling molecules in protein synthesis and autophagy are essential for the regulation of skeletal muscle maintenance in older adults.
Inflammation Induced Muscle Atrophy in Older Adults
The immune system plays a vital role in the protection against pathogens, in tissue and organ repair and remodeling, and in the maintenance of skeletal muscle tissue [60–63]. It has been reported that senescence of the immune system can be detected in middle aged mice (10-15 months old), earlier than many other organ systems [64, 65]. Specifically, substantial senescent changes in immune function precede skeletal muscle atrophy, which is not observed in C57BL/6 mice until they reach at least 18 months of age [22, 40, 41, 66–70]. These observations hint that proper regulation of the immune system may be necessary to maintain skeletal muscle mass. Due to physiological senescence within the immune system, older adults exhibit chronic low-grade systemic inflammation (Inflammaging) [26, 71–73]. Increased levels of pro-inflammatory cytokines (e.g. TNF, IL-6, CRP, etc.) are associated with declines in muscle mass and numerous studies have implied that excess pro-inflammatory cytokines systemically negatively impact skeletal muscle [60, 71, 73]. Hence, the systemic environment can contribute to age-related atrophy.
Several novel immune mediators are currently being investigated for their role in age-associated skeletal muscle atrophy. TWEAK or TNF-related weak inducer of apoptosis was first characterized in 1997 and has been reported to be secreted by immune cells and lowly expressed in skeletal muscle [74–76]. TWEAK can signal through TNF receptor 1 (TNFR1) but has higher affinity to interact with fibroblast growth factor inducible molecule 14 (Fn14) [77, 78]. The Fn14 receptor has been reported to be upregulated in aged mice, which also exhibit a ~20% reduction in muscle fiber CSAs in comparison to young mice [76, 79]. Consistent with the association between Fn14 and muscle fiber atrophy, genetic knockout of Fn14 rescued TA muscle fiber CSA and enhanced levels of contractile proteins compared to wild-type littermates [79]. Kumar’s laboratory also found the downstream target of Fn14, NF-κB, had reduced activity in aged skeletal muscle tissue of Fn14-KO mice and suggested that the TWEAK-Fn14 pathway regulates multiple muscle atrophy pathways within aging skeletal muscle [79].
The anabolic muscle cytokine (myokine), IL-15, has been examined due to its direct association with declining mass with aging [80]. Calorie restricted diets that increase lifespan and preserve lean mass in elderly populations maintain IL-15 signaling in aged rats and subsequently reduce TNF-α secretion and NF-κB activity [81]. Thus, IL-15, an anti-apoptotic cytokine, has been proposed to act through the downregulation of TNF-α and induction of phosphorylation of the IκB to inhibit apoptosis [81]. Overall, various soluble mediators of the immune system have the capability to trigger skeletal muscle atrophy in aging.
Denervation-Induced Atrophy in Aging
The neuromuscular junction (NMJ) is an excitatory cholinergic synapse that constitutes the primary site of signaling between the nervous system and skeletal muscle. The primary cellular components of the NMJ are the presynaptic motor neuron, the postsynaptic muscle fiber and Schwann cells that cap the NMJ. Since the NMJ is critical for muscle contraction, any degenerative changes at the NMJ have the potential to impair muscle function contributing to physical frailty. With aging, NMJ fragmentation occurs as indicated by thinly dispersed acetylcholine receptor (AChR) clusters at the synapse [82] and is thought to be a result of continuous remodeling of NMJs throughout the lifespan that is reduced in effectiveness with aging. In parallel, extensive branching of motor nerve terminals at the NMJ is seen with aging [83–85]. Whether spinal motor neurons themselves are lost with age or remain intact is a controversial topic in the field. Nevertheless, progressive denervation is seen in late life in both humans [86] and rodents [87–89] and is likely contributing to muscle weakness and muscle fiber loss. Schwann cells, which maintain NMJs via trophic and antioxidant support, have recently been reported to reduce in number at the NMJ with age [90], however, their impact on NMJs with age is the least studied of the three NMJ cell types.
The most well studied and understood NMJ maintenance pathway is the Agrin-Lrp4-MuSK signaling pathway. Agrin, a proteoglycan that is secreted by motor neurons, acts by binding to its receptor low-density lipoprotein receptor-related protein (LRP4) located on muscle fibers, which then activates muscle specific kinase (MuSK). MuSK activation triggers a critical signal transduction cascade that leads to agrin-induced AChR clustering at the NMJ. Mice deficient for key proteins in this pathway (i.e. Agrin, Lrp4, and MuSK) are unable to assemble proper NMJs [91–93]. Recent studies in the literature have demonstrated the importance of the Agrin-Lrp4-MuSK signaling pathway in maintaining skeletal muscle mass. When conditionally knocking out agrin or LRP4, NMJ fragmentation similar to that seen in aging is observed [93, 94]. Furthermore, mice overexpressing neurotrypsin, which cleaves agrin, results in significant denervation and subsequent sarcopenic-like phenotype [95]. All components of the Agrin-LRP4-MuSK signaling pathway are critical for NMJ viability, however, only LRP4 protein levels are reduced in aged mice [96], suggesting that a deficiency in agrin signaling is not due to the nerve’s ability to synthesize agrin. In addition, reduced phosphorylation of LRP4 and MuSK was reported. Sarcoglycan α (SGα), a key component of the dystroglycoprotein complex and stabilizer of LRP4, was overexpressed in aged muscles and was found to be effective at preventing denervation, AChR fragmentation, and improve neuromuscular transmission in aged mice [96]. Hence, alterations to the Agrin-LRP4-MuSK signaling pathway, which are observed in the later stages of life, appear to contribute to muscle wasting in older adults.
Redox Homeostasis and Muscle Wasting
Progressive declines with aging in muscle mass and strength along with mitochondrial dysfunction [97–99] are accompanied by significant alterations in redox status in skeletal muscle resulting in part from increased reactive oxygen species (ROS) generation [100]. Both muscle and nerve contain regulatory systems to maintain intracellular ROS levels below a physiological threshold that would lead to cellular oxidative stress or aberrant ROS induced signaling. Clearance of ROS is performed in part by regulatory enzymes such as catalase, glutathione peroxidases, and superoxide dismutases (SOD). Although disagreement remains regarding whether the activity of these enzymes increases or decreases with aging, there is strong evidence supporting increases in biomarkers for DNA and mitochondrial DNA damage, protein oxidation, and lipid oxidation in skeletal muscle with aging [101–103]. Despite clear changes with aging in ROS generating and buffering systems, cause-effect relationships between alterations in redox regulation and sarcopenia have not been established.
Administration of exogenous antioxidants has yielded contradictory results in the field of exercise physiology and sarcopenia. Natural antioxidants function by inhibiting the oxidation of biomolecules. Vitamin C (ascorbic acid), for example, functions as an electron donor to lipid radicals to terminate lipid peroxidation [104]. In addition, vitamin C can interact with superoxide and hydroxyl-free radical and singlet oxygen. Vitamin C also has a role in regenerating vitamin E on cell membranes by reducing vitamin E radicals. Vitamin E is a fat-soluble antioxidant that is important for scavenging peroxide radicals [105] and vitamin E supplementation has been reported to be effective in lowering basal and post-exercise levels of lipid peroxides in older men [106]. A more recent study using combined supplementation of vitamin E and C showed reduced levels of oxidative stress (H2O2, total GSH, GSH/GSSG ratio, malondialdehyde and 8-OHdG) and increases in SOD1/2 and catalase activities in old rats, but no differences in muscle mass and maximal force production was seen [107].
Another non-specific antioxidant that has received much attention is N-Acetylcysteine (NAC), which functions by directly scavenging ROS and supplies cysteine for synthesis of glutathione. Glutathione is a substrate for glutathione peroxidase, an important enzyme required for removal of hydrogen peroxide [108]. Benefits of NAC have been reported in skeletal muscle [109, 110], however, it seems that these benefits are primarily observed in athletes that are capable of producing large amounts of ROS or in muscles that are in a pre-fatigued state [111]. However, a meta-analysis on NAC supplementation for sport performance and risk of adverse effects found no significant benefit or risk of side effects with NAC use [112]. Recent reports have found a phenotype improvement in mdx mice with treatment with Tempol, a superoxide dismutase mimetic [113, 114]. The investigators found improvements in force generating capacity and metabolic enzyme activity (citrate synthase and lactate dehydrogenase) in diaphragm muscles from mdx mice [114]. Hermes et al. also found improvements in grip strength as well as reduced markers of inflammation in dystrophic diaphragm and bicep brachii muscles in mdx mice treated with Tempol. Although promising, the safety and efficacy of Tempol needs further investigation in other conditions of frailty such as sarcopenia. In addition, other investigators have found no significant benefits with antioxidant vitamin supplementation on muscle function during aging [115–117]. The discrepancies between findings across studies could be owed to differences in dosage, administration timing, outcome measures, and muscles studied. Targeting specific antioxidant pathways may also provide greater therapeutic benefits than the administration of general antioxidants and may also explain the great variability of study results on antioxidant supplementation.
Despite contradictory data surrounding the utility of antioxidant supplementation, numerous studies using transgenic manipulations have shown that accumulation of ROS (and other free radicals) produce a pronounced neuromuscular degenerative phenotype in rodent models. Our group and collaborators have shown that mice deficient for CuZnSOD (SOD1) have high levels of oxidative damage, generate less muscle force, experience mitochondrial function deficits and show neuromuscular junction abnormalities and muscle fiber loss [98, 99]. Interestingly, the neurodegenerative phenotype is fully rescued in mice that express Sod1 solely in neurons, suggesting an important interaction between motor neurons and muscle fibers [22, 118, 119].
A recent report from Hsieh and colleagues (2019), found that non-selenocysteine-containing phospholipid hydroperoxide glutathione peroxidase (GPx7), an oxidative stress sensor, inhibits O-GlcNAcylation to protect motor neurons from ROS accumulation with aging. The study found that an increase in ROS in motor neurons with aging leads to increased activity of GPx7 and subsequent inhibition of O-GlcNAcase (OGA), an enzyme responsible for the removal O-GlcNAc. O-GlycNAcylation is a posttranslational modification important in the stress response that confers cellular stress protection against ROS accumulation. O-GlcNAc levels are reduced in spinal cords of ALS mouse models [120, 121]. Mice deficient for GPx7 contain reduced O-GlcNAcylation and higher levels of ROS compared to wild type mice, and show spinal motor neuron death, paralysis, and muscle denervation [122]; however, pharmacological inhibition of OGA with Thiamet-G (TMG) increased O-GlycNAcylation and survival of spinal motor neurons during aging. Although this study offers an intriguing new pathway that has potential for pharmacological intervention, further studies are needed to understand the role of GPx7 in a more geriatric cohort of mice and to determine the efficacy of TMG in later timepoints of age. In summary, proper function of antioxidant enzymes SOD1, SOD2, and GPx7 are crucial to prevent oxidative stress-induced age-related skeletal muscle atrophy.
Pharmacological Sarcopenia Therapeutics
Over the past 20 years, much more attention has been focused on the treatment and even the prevention or delay of sarcopenia in older adults. In 2000, there were only two interventional studies for sarcopenia aimed at the amelioration of both muscle wasting and muscle weakness and now over 300 interventions have been completed or are ongoing through clinical trials for the treatment of sarcopenia (Figure 1). These interventional studies have utilized various approaches to combat age-related muscle wasting including nutraceuticals, meal modification, exercise intervention, and mechanical devices. In addition, roughly 30 small molecules or drugs have gone through clinical trials in past 20 years (Figure 2). Although pharmaceutical companies have investigated sarcopenia in clinical trials for 15 years, there have been substantial delays between the current research on novel targets of age-related atrophy and clinical trials for FDA approval, partially due to the fairly recent recognition of sarcopenia as an independent disease state in 2016 [123–125]. An example of this gap between basic research and translation to treatments for sarcopenia is the investigation of anabolic hormones and age associated muscle wasting. Growth hormone and testosterone were among the first researched targets in sarcopenia in the 1990’s and were the dominant drug intervention for clinical trials in the early 2000’s [126–130]. However, the majority of clinical trials investigating testosterone/androgen treatment failed to demonstrate clinically significant improvements in muscle mass or strengh, which shifted the focus to other targets to treat muscle wasting in aging.
Figure 1.
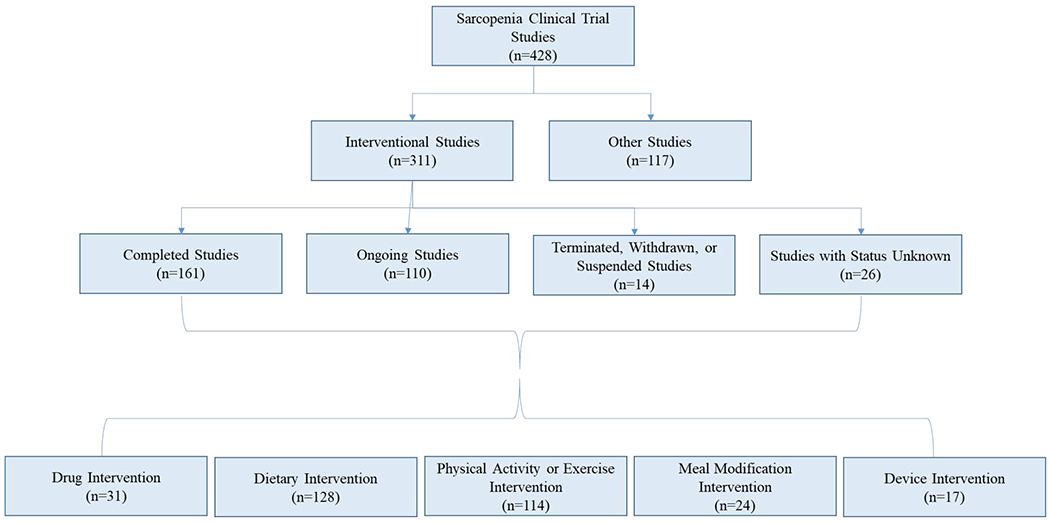
Flow chart of sarcopenia clinical trial studies since October 2019. Data received from clinicaltrials.gov. Roughly three quarters of clinical trials have an interventional approach to investigate treatments for sarcopenia. The interventional approaches to retain lean body mass in older adults have been focused more on lifestyle changes (dietary, exercise) and less on patented technologies (drugs, devices).
Figure 2.
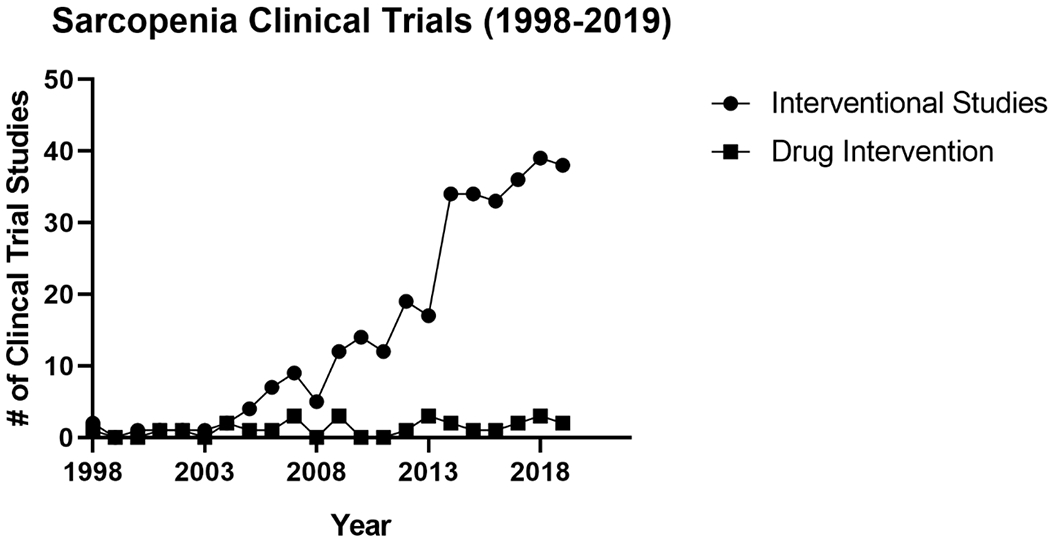
Timeline of interventional clinical trial sarcopenia studies. Sarcopenia studies have gradually increased within the first decade of interventional trials and have abruptly grown within the last decade. Although, production of interventional studies from both the industry and institutions have progressively increased in the past couple decades, drug interventions have remained stable even after the classification of sarcopenia as a disease state in 2016.
In the late 2000’s more promising drugs with fewer known side-effects targeting modulation of proteostasis and glucose metabolism (metformin, empagliflozin, pioglitazone), redox homeostasis (allopurinol), inflammation (ibuprofen) and other biological functions (BIO 101, potassium citrate) were introduced into clinical trials for sarcopenia[131–141]. Not to mention, multiple major pharmaceutical companies raced to bring the first small molecules to market that modify myostatin signaling and selective androgen receptor modulators to preserve muscle mass in sarcopenic patients (see review [125]).
Metabolic Therapeutics
Ghrelin is one satiety hormone that was selected by an investigator as a potential therapeutic for frailty due to its ability to stimulate growth hormone and improve appetite in older adults [142]. In phase 2 trials 16 subjects 70 years and older were given 7.5 mcg/kg of Ghrelin once a day subcutaneously for 12 weeks in addition to participation in resistance training (NCT01898611). The results obtained by Dual Energy X-ray Absorptiometry (DEXA) showed no differences in the changes in lean body mass between the placebo and Ghrelin treatment groups that participated in strength training.
Multiple drugs that have been used to treat type 2 diabetes have also been examined as potential treatments for sarcopenia. Metformin (Glucophage) is one promising drug with an expired patent that has been used extensively to lower blood sugar in type 2 diabetes mellitus [143, 144]. More recently, metformin has been examined for its role in longevity and muscle maintenance in C. elegans, fruit flies, and mice [145–147]. Metformin is an activator of AMPK and indirect mTOR antagonist that could be beneficial for both healthspan and lifespan in humans [147, 148]. Metformin went through phase 1/phase 2 clinical trials in 2014 to assess skeletal muscle mass, muscle function, and muscle characteristics over a 3-year period in adults 65 years of age and older (NCT01804049). Although there are no results reported, it has been suggested in the literature that metformin can delay muscle atrophy and improve muscle maintenance in aging mammals [149].
One ambitious phase 4 clinical trial performed by researchers at the Sticht Center on Aging attempted to improve body composition of older adults through the combination of Pioglitazone with hypocaloric diet and/or resistance exercise training. The major objective of these studies were to reduce fat free mass in older adults while preserving lean body mass (NCT00315146). Pioglitazone is a type 2 diabetes drug known to lower blood glucose levels that was selected based on its function as a PPAR-γ agonist that improves glucose and lipid metabolism in muscle. In these studies, 40 women and 48 men between 65-79 years of age with a BMI of at least 25 kg/m2 were randomly selected for a placebo or Pioglitazone with all groups being placed on a hypocaloric diet (calorie deficit of 500 kcal/day) and some groups participating in resistance exercise [138, 139]. The trials were partially successful because even though older adults on Pioglitazone and hypocaloric diet had reductions in fat and lean body mass, when resistance training was added to the intervention there was a reduction in the loss of skeletal muscle mass [138, 139].
An anti-hyperglycemic medication used for type 2 diabetes patients, Empagliflozin, is currently in phase 4 trials to determine whether this small molecule can delay the progression of age-related skeletal muscle atrophy [137]. Over 120 participants will be randomly selected to participate in 10 weeks of circuit resistance training (CRT), a Vegetarian-Mediterranean diet, or given a daily treatment of 10 mg Empagliflozin (NCT03560375). All three groups will follow-up with an additional 10 weeks of CRT along with their previous diet intervention. Changes in lean body mass will be measured using a body composition analyzer and completed results should be reported after November 2020.
Other Therapeutics
Therapeutics in several other categories have gone through clinical trials in the past decade and some of these drugs may contribute to treating sarcopenia in the near future. For example, the non-steroidal anti-inflammatory drug (NSAID) Ibuprofen was examined because chronic low grade inflammation and muscle atrophy are associated with aging [26, 72, 73, 150]. In this 9-month study, post-menopausal women were either given a placebo or 400 mg of Ibuprofen after resistance exercise 3 days a week (NCT01886196). The clinical trial was unsuccessful in showing that either ibuprofen or ibuprofen with the addition of resistance exercise training could improve lean body mass in aging [141]. Resistance training alone however, maintained skeletal muscle mass in older women thus suggesting that NSAIDs may not be beneficial in sarcopenic adults.
Allopurinol is another drug that has gone through clinical trials and may partially remedy age-related skeletal muscle atrophy in humans [151]. Allopurinol has been used in the past to treat gout and kidney stones and has completed phase 4 trials for sarcopenia [152, 153]. Allopurinol activates the antioxidant enzyme xanthine oxidase to salvage redox homeostasis in older adults and thus is proposed to have potential to rescue lean body mass in the elderly [151]. Limited reports by investigators from the University of Dundee have shown that 5 months of treatment with allopurinol improves muscle energetics (NCT01550107). Whether oral ingestion of allopurinol can aid in muscle maintenance longitudinally, has yet to be examined.
Potassium citrate is a urinary alkalinizing medication that has been explored in clinical trials to determine if the substance can be repurposed to treat sarcopenia. The premise for the usage of this substance is that potassium citrate will neutralize the excess acid production that is observed in individuals that consume a Western diet, thus improving skeletal muscle mass in older adults (NCT00509405). Phase 3 trials were completed in over 200 participants in ages 65-80 years old with 2 years of treatment; however, results were never reported for this clinical trial.
Sarconeos (BIOL101) is a novel small molecule that is currently going through phase 2 trials. It is suggested that BIOL activates the MAS receptor (angiotensin agonist G protein-coupled transmembrane receptor) in muscle cells potentially through the drug’s active ingredient, 20-hydroxyecdysone [130]. Over 200 subjects 65 years of age and older are being administered either a placebo or BIOL orally to determine the efficacy of the 6-month treatment (NCT03452488). Clinical trials for this study should wrap up by the end of 2020. Overall, small molecules display promise to offset, delay, or at the very least reduce the decline of muscle mass that is attributed with aging. Moreover, there are new delivery systems for sarcopenic drugs (e.g. chewing gum [US20140294915A1]. whey protein micelles [EP2768322A1]) and numerous novels targets yet to be explored by pharmaceutical companies (Table 3), which leaves the area of sarcopenic treatment full of opportunity to improve the quality of life in the elderly community.
Table 3.
Selected sarcopenia drugs in clinical trials
| Drug Name | Drug Target | Phase | Status | Date | Result | Sponsor Company/Institution | NCT ID |
|---|---|---|---|---|---|---|---|
| Anabolic Hormone | |||||||
| Testosterone Undecanoate | AR | N/A | Ongoing | Jul-19 | Institute of Liver and Biliary Sciences | NCT03995251 | |
| Topical Testosterone | AR | Phase 2 | Completed | Sep-02 | ↑ LBM | National Institute on Aging (NIA) | NCT00183040 |
| Testosterone Gel | AR | Phase 4 | Completed | Oct-04 | Manchester University NHS Foundation | NCT00190060 | |
| Testosterone Gel 1% | AR | Phase 3 | Ongoing | Sep-17 | Washington University School of Medicine | NCT02938923 | |
| MK-0773 | AR | Phase 2 | Completed | Oct-07 | ↑ LBM | Merck Sharp & Dohme Corp. | NCT00529659 |
| Enobosarm | AR | Phase 2 | Completed | Sep-18 | GTx (Merged with Oncternal Therapeutics) | NCT03241342 | |
| Recombinant HGH | GHR | Phase 2 | Completed | Sep-02 | *↑ LBM | National Institute on Aging (NIA) | NCT00183040 |
| Anamorelin HCL | GHS-R | Phase 1 | Ongoing | Aug-19 | Tufts University | NCT04021706 | |
| MK-677 | GHS-R | Phase 1,2 | Completed | Jul-98 | ↑ LBM | University of Virginia | NCT00474279 |
| DHEA | NMDA, GABA, σ1-R | Phase 3 | Completed | Apr-01 | Washington University School of Medicine | NCT00205686 | |
| Oxytocin nasal spray | Oxytocin-R | Phase 1,2 | Ongoing | Sep-17 | University of Texas Health Science Center | NCT03119610 | |
| Metabolic | |||||||
| Ghrelin | GHS-R | Phase 2 | Completed | Jul-13 | University of Pennsylvania | NCT01898611 | |
| Metformin | PKB | Phase 1,2 | Completed | Apr-14 | VA Office of Research and Development | NCT01804049 | |
| Empagliflozin | SGLT2 | Phase 4 | Ongoing | May-18 | Tel-Aviv Sourasky Medical Center | NCT03560375 | |
| Pioglitazone | PPAR-y | Phase 4 | Completed | Apr-06 | ↓ LBM | Wake Forest University Health Sciences | NCT00315146 |
| Anti-inflammatory | |||||||
| Ibuprofen (NSAIDs) | Prostaglandins | N/A | Completed | Apr-13 | No diff | University of Saskatchewan | NCT01886196 |
| Redox Homeostasis | |||||||
| Allopurinol | Xanthine Oxidase | Phase 4 | Completed | Feb-15 | University of Dundee | NCT01550107 | |
| Other | |||||||
| BIO101 | MAS-R | Phase 2 | Onqoinq | May-18 | Biophytis | NCT03452488 | |
| Bimaqrumab | ActRII | Phase 2 | Completed | Jul-15 | = LBM | Novartis | NCT02468674 |
| Landoqrozumab | Myo statin | Phase 2 | Completed | May-12 | ↑ LBM | Eli Lilly | NCT01604408 |
| Trevoqrumab | Myo statin | Phase 2 | Completed | Nov-13 | ↑ LBM | Reqeneron | NCT01963598 |
| Garetosmab | ActivinRIlA | Phase 1 | Completed | Apr-13 | Reqeneron | NCT02943239 | |
| Potassium citrate | Acidic Compounds | Phase 3 | Completed | Jul-07 | Kantonsspital Baseband Bruderholz | NCT00509405 | |
Sarcopenia clinical trial studies that have at least one lean body mass outcome measure in older adults were included.
Increase in lean body mass was observed in combination to topical testosterone.
Date (Study start date). Abbreviations: AR, androgen receptor; DHEA, dehydroepiandrosterone; GABA, gamma aminobutyric acid; GHR growth hormone receptor; GHS, growth hormone secretagogue; LBM, lean body mass; N/A, not available; NMDA, N-methyl-D-aspartate; NSAIDs, nonsteroidal anti-inflammatory drugs; PKB, protein kinase B; PPAR-γ, peroxisome proliferator-activated receptor gamma; -R, receptor; SGLT2 sodium glucose co-transporter 2.
Perspectives & Conclusion
Herein we have discussed the primary pathways and biological functions implicated in age-related skeletal muscle atrophy stemming from both longevity and muscle biology research. Moreover, these pathways likely interplay with multiple processes to delay or offset skeletal muscle atrophy in aging as well as intersect with each other. Novel mediators that influence proteostasis, including pi 10α PI3K, UCP1, and heat shock proteins (HSPs) have demonstrated dual benefits in several organisms for enhancement of lifespan and healthspan [28, 30, 31, 154, 155] and should be further investigated for their role in age-associated muscle atrophy and treatment of sarcopenia in humans.
The role of inflammation in relation to muscle wasting and aging is being re-evaluated highlighting the source and location of immune cells in response to the microenvironment. Historically, gerontology studies were focused predominately on low-grade, chronic, systemic inflammation in aging, in the absence of overt infection (termed inflammaging) [26, 71, 156]. More recent investigations of the contribution of inflammation to aging skeletal muscle have shifted focus towards inflammatory mediators released by the myofibers themselves with alterations in so called “myokines” tending to favor muscle deterioration with aging [59, 157– 159]. Nonetheless, general inflammaging and studies of myokines do not consider cytokines secreted by other cell types localized in skeletal muscle tissue. These additional sources of soluble immune mediators should not be overlooked because altered secretion of cytokines from macrophages, fibroblasts, and fibro/adipogenic progenitors within skeletal muscle have potential to negatively impact skeletal muscle maintenance during aging [160, 161].
Prevention of NMJ decline and preservation of synaptic transmission with age may provide therapeutic benefits. Although no current drug exists that specifically targets neuromuscular degeneration, life-long high intensity exercise has been shown to be an effective at preventing motor unit loss in humans [162]. In addition, caloric restriction, considered the most effective non-genetic intervention of aging and life-span extension, is effective at preserving NMJs and reducing muscle fiber turnover in the tibialis anterior muscle in aged mice [163]. These studies suggest that critical preservation pathways exist that facilitate NMJ maintenance in aged animals and could potentially be enhanced for therapeutic utility.
Furthermore, determining the important ROS mediated pathways that lead to muscle denervation and fiber loss will likely produce novel therapeutics. While the field has mainly focused on oxidative damage (i.e. DNA, lipid, protein oxidation) future studies are needed to understand the dual roles of ROS; cell signaling and redox homeostasis. Since sarcopenia is accompanied by multifactorial changes in both muscle and nerve it is likely that both tissue-specific ROS mediated signaling and redox homeostatic changes are involved and therefore simultaneously targeting specific mediators in the antioxidant network will provide the most effective therapeutic potential.
Although rodent and cell culture models have been predominately used to identify novel molecules as targets for age-related skeletal muscle atrophy, the contributions of human studies to the identification of key mediators of muscle wasting should not be overlooked. Molecular analysis of tissues from older adults are indeed necessary to confirm whether certain proteins are involved in muscle wasting in humans and to what degree specific mediators and signaling pathways influence skeletal muscle maintenance. Research studies using human tissues from older adults also give insight for future directions that need to be further explored and characterized in rodent models (e.g. myokines, metabolome) [164, 165]. As an example, in the early 2000’s Welle and colleagues revealed that over 1100 genes in older women and over 700 genes in older men have differential gene expression in comparison to their younger counterparts, and a gene involved with DNA repair, Gadd45a may be involved in muscle wasting in humans [166, 167]. Since then more compelling evidence has shown a direct link of Gadd45a and muscle atrophy [47, 168], but whether there is direct contribution to sarcopenic muscle atrophy remains unknown.
Finally, because the Food & Drug Administration only oversees the approval of drugs that treat a disease state, clinical trials of pharmaceutical approaches for the prevention of sarcopenia are hampered somewhat. Continued expansion of the list of potential targets emerging from basic research along with recent consensus on the classification and diagnosis of sarcopenia will enhance the development of pharmaceuticals approaches to treat age-related skeletal muscle atrophy and physical frailty in elderly populations [123, 124].
Table 2.
Summary of novel targets of age-related skeletal muscle atrophy (2009-2019)
| Classification | Molecular Targets | Atrophy-related Mediated Targets | Age-related Atrophy Year Identification | Reference(s) |
|---|---|---|---|---|
| Proteostasis | TSC | Fox03, mTORC1 | 2014 | [39] |
| dkk3 | Fox03-p-catenin, WNT signaling | 2018 | [56] | |
| ATF4 | 4E-BP1? | 2015 | [46] | |
| FGF21 | FoxOs | 2017 | [57] | |
| p110α PI3K | mTORCl | 2019 | [43] | |
| Immune System | TWEAK-Fn14 | NF-κB | 2014 | [79] |
| IL-15 | NF-κB, IL-15Ra, TNF-R1 | 2009 | [81] | |
| IL-10* | Unknown | 2016 | [181] | |
| Denervation-Induced Atrophy | Sarcoglycan α | LRP4, MuSK | 2018 | [96] |
| LRP4 | MuSK | 2014 | [93] | |
| Agrin | LRP4 | 2012 | [94] | |
| Redox Homeostasis | GPx7 | OGA | 2019 | [122] |
denotes target with no evidence from muscle weight data.
Abbreviations: ATF4, activating transcription factor 4; dkk3, dickkopf 3; FoxO, forkhead family of transcription factors; FGF21, fibroblast growth factor 21; GPx7, non-selenocysteine-containing phospholipid hydroperoxide glutathione peroxidase; IL, interleukin; mTORC1, mammalian target of rapamycin complex 1; LRP4, low-density lipoprotein receptor-related protein 4; MuSK, muscle-specific kinase; NF-κB, necrosis factor kappa-light-chain-enhancer of activated B cells; OGA, o-GlcNAcylation; TNF-R1, tumor necrosis factor receptor 1; TWEAK, TNF-related weak inducer of apoptosis; WNT, wingless-INT.
Acknowledgements
This project was supported by P01 AG-051442 and NIA training grant AG000114. We confirm that all authors have read the journal’s authorship agreement and that the manuscript has been reviewed by and approved by all named authors. We also confirm that all authors have read the journal’s policy on disclosure of potential conflicts of interest and declare that they have no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ritch A History of geriatric medicine: from Hippocrates to Marjory Warren. J R Coll Physicians Edinb. 2012;42:368–74. [DOI] [PubMed] [Google Scholar]
- [2].Kirkwood TB, Ritter MA. The interface between ageing and health in man. Age Ageing. 1997;26 Suppl 4:9–14. [DOI] [PubMed] [Google Scholar]
- [3].Morley JE, Anker SD, von Haehling S. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology-update 2014. J Cachexia Sarcopenia Muscle. 2014;5:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cesari M, Leeuwenburgh C, Lauretani F, et al. Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr. 2006;83:1142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Visser M Epidemiology of muscle mass loss with age. Sarcopenia edn. 2012. [Google Scholar]
- [6].Mitchell WK, Atherton PJ, Williams J, Larvin M, Lund JN, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bell K, Von Allmen M, Devries M, Phillips S. Muscle disuse as a pivotal problem in sarcopenia-related muscle loss and dysfunction. J Frailty Aging. 2016;5:33–41. [DOI] [PubMed] [Google Scholar]
- [8].Rosenberg IH. Summary comments. Am J Clin Nutr. 1989;50:1231–3. [Google Scholar]
- [9].Lynch GS. Age-related muscle wasting and weakness: mechanisms and treatments. Sarcopenia; 2010. [Google Scholar]
- [10].Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr. 1993;123:465–8. [DOI] [PubMed] [Google Scholar]
- [11].Morley JE, Cruz-Jentoft AJ. Definitions of Sarcopenia. Sarcopenia, 2012. [Google Scholar]
- [12].Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis report of the european working group on sarcopenia in older people. Age Ageing. 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Welle S Basic mechanisms of protein turnover. In Human Protein Metabolism. Springer. 2012:11–28. [Google Scholar]
- [16].Espinoza S, Walston JD. Frailty in older adults: insights and interventions. Cleve Clin J Med. 2005;72:1105–12. [DOI] [PubMed] [Google Scholar]
- [17].Lutomski J, Baars M, Boter H, et al. Frailty, disability and multi-morbidity: the relationship with quality of life and healthcare costs in elderly people. Ned Tijdschr Geneeskd. 2014;158:A7297–A. [PubMed] [Google Scholar]
- [18].Kulmala J, Nykänen I, Hartikainen S. Frailty as a predictor of all-cause mortality in older men and women. Geriatr Gerontol Int. 2014;14:899–905. [DOI] [PubMed] [Google Scholar]
- [19].Pasini E, Corsetti G, Aquilani R, et al. Protein-amino acid metabolism disarrangements: The hidden enemy of chronic age-related conditions. Nutrients. 2018;10:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Horvath SM. The influence of the aging process on the distribution of certain components of the blood and the gastrocnemius muscle of the albino rat. J Gerontol. 1946;1:213–23. [DOI] [PubMed] [Google Scholar]
- [21].Sakuma K, Yamaguchi A. Molecular mechanisms in aging and current strategies to counteract sarcopenia. Curr Aging Sci. 2010;3:90–101. [DOI] [PubMed] [Google Scholar]
- [22].Sakellariou GK, McDonagh B. Redox homeostasis in age-related muscle atrophy. Adv Exp Med Biol. 2018;1088:281–306. [DOI] [PubMed] [Google Scholar]
- [23].Bottoni A, dos Anjos Garnes S, Lasakosvitsch F, Bottoni A. Sarcopenia: an overview and analysis of molecular mechanisms. Nutrire. 2019;44:6. [Google Scholar]
- [24].Picca A, Calvani R, Bossola M, et al. Update on mitochondria and muscle aging: all wrong roads lead to sarcopenia. Biol Chem. 2018;399:421–36. [DOI] [PubMed] [Google Scholar]
- [25].Furrer R, Handschin C. Muscle wasting diseases: novel targets and treatments. Annu Rev Pharmacol and Toxicol. 2019;59:315–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Salminen A, Kaarniranta K, Kauppinen A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging. 2012;4:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lynch GS. Sarcopenia–age-related muscle wasting and weakness: mechanisms and treatments: Springer. 2010. [Google Scholar]
- [28].Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143:813–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Antonucci TC. Annual review of gerontology and geriatrics. Annu Rev Gerontol Geriatr. 2014. [Google Scholar]
- [30].Ben-Zvi A, Miller EA, Morimoto RI. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci. 2009;106:14914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem. 2015;84:435–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Poortmans JR, Carpentier A, Pereira-Lancha LO, Lancha A Jr., Protein turnover, amino acid requirements and recommendations for athletes and active populations. Braz J Med Biol Res. 2012;45:875–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–8. [DOI] [PubMed] [Google Scholar]
- [34].Blagosklonny MV, Hall MN. Growth and aging: a common molecular mechanism. Aging. 2009;1:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Parise G, Yarasheski KE. The utility of resistance exercise training and amino acid supplementation for reversing age-associated decrements in muscle protein mass and function. Curr Opin Clin Nutr Metab Care. 2000;3:489–95. [DOI] [PubMed] [Google Scholar]
- [36].Yarasheski KE. Exercise, aging, and muscle protein metabolism. J Gerontol Series A Biol Sci Med Sci. 2003;58:M918–M22. [DOI] [PubMed] [Google Scholar]
- [37].Yarasheski K Managing sarcopenia with progressive resistance exercise training. J Nutr Health Aging. 2002;6:349–56. [PubMed] [Google Scholar]
- [38].Sharples AP, Hughes DC, Deane CS, Saini A, Selman C, Stewart CE. Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell. 2015;14:511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Castets P, Lin S, Rion N, et al. Sustained activation of mTORC1 in skeletal muscle inhibits constitutive and starvation-induced autophagy and causes a severe, late-onset myopathy. Cell Metab. 2013;17:731–44. [DOI] [PubMed] [Google Scholar]
- [40].Tang H, Inoki K, Brooks SV, et al. mTORC1 underlies age-related muscle fiber damage and loss by inducing oxidative stress and catabolism. Aging Cell. 2019;18:e12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Clavel S, Coldefy AS, Kurkdjian E, Salles J, Margaritis I, Derijard B. Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat tibialis anterior muscle. Mech Ageing Dev. 2006;127:794–801. [DOI] [PubMed] [Google Scholar]
- [42].O’Neill ED, Wilding JP, Kahn CR, et al. Absence of insulin signalling in skeletal muscle is associated with reduced muscle mass and function: evidence for decreased protein synthesis and not increased degradation. Age (Dordr). 2010;32:209–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Li ME, Lauritzen HP, O’Neill BT, et al. Role of p110α subunit of PI3-kinase in skeletal muscle mitochondrial homeostasis and metabolism. Nat Commun. 2019;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. [DOI] [PubMed] [Google Scholar]
- [45].Taylor RC, Dillin A. Aging as an event of proteostasis collapse. Cold Spring Harb Perspect Biol. 2011;3:a004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ebert SM, Dyle MC, Bullard SA, et al. Identification and Small Molecule Inhibition of an Activating Transcription Factor 4 (ATF4)-dependent Pathway to Age-related Skeletal Muscle Weakness and Atrophy. J Biol Chem. 2015;290:25497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bongers KS, Fox DK, Ebert SM, et al. Skeletal muscle denervation causes skeletal muscle atrophy through a pathway that involves both Gadd45a and HDAC4. Am J Physiol Endocrinol Metab. 2013;305:E907–E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ebert SM, Monteys AM, Fox DK, et al. The transcription factor ATF4 promotes skeletal myofiber atrophy during fasting. Mol Endocrinol. 2010;24:790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469. [DOI] [PubMed] [Google Scholar]
- [50].Semënov MV, Tamai K, Brott BK, Kühl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–61. [DOI] [PubMed] [Google Scholar]
- [51].Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signaling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683. [DOI] [PubMed] [Google Scholar]
- [52].Mao B, Wu W, Li Y, et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321. [DOI] [PubMed] [Google Scholar]
- [53].Brott BK, Sokol SY. Regulation of Wnt/LRP signaling by distinct domains of Dickkopf proteins. Mol Cell Biol. 2002;22:6100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mao B, Wu W, Davidson G, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/β-catenin signalling. Nature. 2002;417:664. [DOI] [PubMed] [Google Scholar]
- [55].Zenzmaier C, Sklepos L, Berger P. Increase of Dkk-3 blood plasma levels in the elderly. Exp Gerontol. 2008;43:867–70. [DOI] [PubMed] [Google Scholar]
- [56].Yin J, Yang L, Xie Y, et al. Dkk3 dependent transcriptional regulation controls age related skeletal muscle atrophy. Nat Commum. 2018;9:1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tezze C, Romanello V, Desbats MA, et al. Age-associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metab. 2017;25:1374–89. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tezze C, Romanello V, Sandri M. FGF21 as Modulator of Metabolism in Health and Disease. Front Physiol. 2019;10:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Oost LJ, Kustermann M, Armani A, Blaauw B, Romanello V. Fibroblast growth factor 21 controls mitophagy and muscle mass. J Cachexia Sarcopenia Muscle. 2019;10:630–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Brunelli S, Rovere-Querini P. The immune system and the repair of skeletal muscle. Pharmacol Res. 2008;58:117–21. [DOI] [PubMed] [Google Scholar]
- [61].McLennan IS, Koishi K. The transforming growth factor-betas: multifaceted regulators of the development and maintenance of skeletal muscles, motoneurons and Schwann cells. Int J Dev Biol. 2004;46:559–67. [PubMed] [Google Scholar]
- [62].Casadevall A Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 1998;6:102–7. [DOI] [PubMed] [Google Scholar]
- [63].Randow F, MacMicking JD, James LC. Cellular self-defense: how cell-autonomous immunity protects against pathogens. Science. 2013;340:701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Flurkey K, Currer JM, Harrison D. The Mouse in biomedical research (second edition: mouse models in aging research. Academic Press; 2007;637–72. [Google Scholar]
- [65].Pawelec G Immunosenescence: impact in the young as well as the old? Mech Ageing Dev. 1999;108:1. [DOI] [PubMed] [Google Scholar]
- [66].Altun M, Besche HC, Overkleeft HS, et al. Muscle wasting in aged, sarcopenic rats is associated with enhanced activity of the ubiquitin proteasome pathway. J Biol Chem. 2010;285:39597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Carlson BM, Borisov AB, Dedkov EI, et al. Effects of Long-Term Denervation on Skeletal Muscle in Old Rats. J Gerontol A Biol Sci Med Sci. 2002;57:B366–B74. [DOI] [PubMed] [Google Scholar]
- [68].Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT. Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS One. 2012;7:e29082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Castelo-Branco C, Soveral I. The immune system and aging: a review. Gynecol Endocrinol. 2014;30:16–22. [DOI] [PubMed] [Google Scholar]
- [70].Khan SS, Singer BD, Vaughan DE. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell. 2017;16:624–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69 Suppl 1:S4–9. [DOI] [PubMed] [Google Scholar]
- [72].Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–99. [DOI] [PubMed] [Google Scholar]
- [73].Wang J, Leung KS, Chow SK, Cheung WH. Inflammation and age-associated skeletal muscle deterioration (sarcopaenia). J Orthop Translat. 2017;10:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chicheportiche Y, Bourdon PR, Xu H, et al. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997;272:32401–10. [DOI] [PubMed] [Google Scholar]
- [75].Maecker H, Varfolomeev E, Kischkel F, et al. TWEAK attenuates the transition from innate to adaptive immunity. Cell. 2005;123:931–44. [DOI] [PubMed] [Google Scholar]
- [76].Mittal A, Bhatnagar S, Kumar A, et al. The TWEAK–Fn14 system is a critical regulator of denervation-induced skeletal muscle atrophy in mice. J Cell Biol. 2010;188:833–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Liu Q, Xiao S, Xia Y. TWEAK/Fn14 activation participates in skin inflammation. Mediators Inflamm. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wiley SR, Cassiano L, Lofton T, et al. A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity. 2001;15:837–46. [DOI] [PubMed] [Google Scholar]
- [79].Tajrishi MM, Sato S, Shin J, et al. The TWEAK–Fn14 dyad is involved in age-associated pathological changes in skeletal muscle. Biochem Biophys Res Commun. 2014;446:1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Pistilli EE, Alway SE. Systemic elevation of interleukin-15 in vivo promotes apoptosis in skeletal muscles of young adult and aged rats. Biochem Biophys Res Commun. 2008;373:20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Marzetti E, Carter CS, Wohlgemuth SE, et al. Changes in IL-15 expression and deathreceptor apoptotic signaling in rat gastrocnemius muscle with aging and life-long calorie restriction. Mech Ageing Dev. 2009;130:272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Robbins N, Fahim M. Progression of age changes in mature mouse motor nerve terminals and its relation to locomotor activity. J Neurocytol. 1985;14:1019–36. [DOI] [PubMed] [Google Scholar]
- [83].Barker DJP, Ip M. Sprouting and degeneration of mammalian motor axons in normal and de-afferentated skeletal muscle. Proc R Soc Lond B Biol Sci. 1966;163:538–54. [DOI] [PubMed] [Google Scholar]
- [84].Wokke J, Jennekens F, Van den Oord C, Veldman H, Smit L, Leppink G. Morphological changes in the human end plate with age. J Neurol Sci. 1990;95:291–310. [DOI] [PubMed] [Google Scholar]
- [85].Oda K Age changes of motor innervation and acetylcholine receptor distribution on human skeletal muscle fibres. J Neurol Sci. 1984;66:327–38. [DOI] [PubMed] [Google Scholar]
- [86].Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. [DOI] [PubMed] [Google Scholar]
- [87].Chai RJ, Vukovic J, Dunlop S, Grounds MD, Shavlakadze T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLoS One. 2011;6:e28090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Valdez G, Tapia JC, Kang H, et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci U S A. 2010;107:14863–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Valdez G, Tapia JC, Lichtman JW, Fox MA, Sanes JR. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PLoS One. 2012;7:e34640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Snyder-Warwick AK, Satoh A, Santosa KB, Imai Si, Jablonka-Shariff A. Hypothalamic Sirt1 protects terminal Schwann cells and neuromuscular junctions from age-related morphological changes. Aging Cell. 2018;17:e12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].DeChiara TM, Bowen DC, Valenzuela DM, et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–12. [DOI] [PubMed] [Google Scholar]
- [92].Gautam M, Noakes PG, Moscoso L, et al. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–35. [DOI] [PubMed] [Google Scholar]
- [93].Barik A, Lu Y, Sathyamurthy A, et al. LRP4 is critical for neuromuscular junction maintenance. J Neurosci. 2014;34:13892–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Samuel MA, Valdez G, Tapia JC, Lichtman JW, Sanes JR. Agrin and synaptic laminin are required to maintain adult neuromuscular junctions. PLoS One. 2012;7:e46663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Bütikofer L, Zurlinden A, Bolliger MF, Kunz B, Sonderegger P. Destabilization of the neuromuscular junction by proteolytic cleavage of agrin results in precocious sarcopenia. FASEB J. 2011;25:4378–93. [DOI] [PubMed] [Google Scholar]
- [96].Zhao K, Shen C, Li L, et al. Sarcoglycan Alpha Mitigates Neuromuscular Junction Decline in Aged Mice by Stabilizing LRP4. J Neurosci. 2018;38:8860–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy?: Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15-to 83-year-old men. J Neurol Sci. 1988;84:275–94. [DOI] [PubMed] [Google Scholar]
- [98].Larkin LM, Davis CS, Sims-Robinson C, et al. Skeletal muscle weakness due to deficiency of CuZn-superoxide dismutase is associated with loss of functional innervation. Am J Physiol Regul, Integr Comp Physiol. 2011;301:R1400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Jang YC, Lustgarten MS, Liu Y, et al. Increased superoxide in vivo accelerates geassociated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010;24:1376–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Jackson MJ, McArdle A. Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. J Physiol. 2011;589:2139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Mecocci P, Fano G, Fulle S, et al. Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic Biol Med. 1999;26:303–8. [DOI] [PubMed] [Google Scholar]
- [102].Gianni P, Jan KJ, Douglas MJ, Stuart PM, Tarnopolsky MA. Oxidative stress and the mitochondrial theory of aging in human skeletal muscle. Exp Gerontol. 2004;39:1391–400. [DOI] [PubMed] [Google Scholar]
- [103].Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Nimse SB, Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–8006. [Google Scholar]
- [105].Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Meydani M, Evans W, Handelman G, et al. Protective effect of vitamin E on exerciseinduced oxidative damage in young and older adults. Am J Physiol. 1993;264:R992–R8. [DOI] [PubMed] [Google Scholar]
- [107].Ryan MJ, Dudash HJ, Docherty M, et al. Vitamin E and C supplementation reduces oxidative stress, improves antioxidant enzymes and positive muscle work in chronically loaded muscles of aged rats. Exp Gerontol. 2010;45:882–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Zafarullah M, Li W, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].McKenna MJ, Medved I, Goodman CA, et al. N-acetylcysteine attenuates the decline in muscle Na+, K+-pump activity and delays fatigue during prolonged exercise in humans. J Physiol. 2006;576:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, et al. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol (1985). 2004;97:1477–85. [DOI] [PubMed] [Google Scholar]
- [111].Šalamon Š, Kramar B, Marolt TP, Poljšak B, Milisav I. Medical and Dietary Uses of N-Acetylcysteine. Antioxidants. 2019;8:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Rhodes K, Braakhuis A. Performance and side effects of supplementation with N-acetylcysteine: a systematic review and meta-analysis. Sports Med. 2017;47:1619–36. [DOI] [PubMed] [Google Scholar]
- [113].de Almeida Hermes T, Mâncio RD, Macedo AB, et al. Tempol treatment shows phenotype improvement in mdx mice. PLoS One. 2019;14:e0215590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Burns D, Ali I, Rieux C, Healy J, Jasionek G, O’Halloran K. Tempol supplementation restores diaphragm force and metabolic enzyme activities in mdx mice. Antioxidants. 2017;6:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Bailey DM, Williams C, Betts JA, Thompson D, Hurst TL. Oxidative stress, inflammation and recovery of muscle function after damaging exercise: effect of 6-week mixed antioxidant supplementation. Eur J Appl Physiol. 2011;111:925–36. [DOI] [PubMed] [Google Scholar]
- [116].Close GL, Ashton T, Cable T, et al. Ascorbic acid supplementation does not attenuate post-exercise muscle soreness following muscle-damaging exercise but may delay the recovery process. Br J Nutr. 2006;95:976–81. [DOI] [PubMed] [Google Scholar]
- [117].Petersen EW, Ostrowski K, Ibfelt T, et al. Effect of vitamin supplementation on cytokine response and on muscle damage after strenuous exercise. Am J Physiol Cell Physiol. 2001;280:C1570–C5. [DOI] [PubMed] [Google Scholar]
- [118].Sakellariou GK, McDonagh B, Porter H, et al. Comparison of Whole Body SOD1 Knockout with Muscle-Specific SOD1 Knockout Mice Reveals a Role for Nerve Redox Signaling in Regulation of Degenerative Pathways in Skeletal Muscle. Antioxid Redox Signal. 2018;28:275–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Sakellariou GK, Davis CS, Shi Y, et al. Neuron-specific expression of CuZnSOD prevents the loss of muscle mass and function that occurs in homozygous CuZnSODknockout mice. FASEB J. 2014;28:1666–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Lüdemann N, Clement A, Hans VH, Leschik J, Behl C, Brandt R. O-glycosylation of the tail domain of neurofilament protein M in human neurons and in spinal cord tissue of a rat model of amyotrophic lateral sclerosis (ALS). J Biol Chem. 2005;280:31648–58. [DOI] [PubMed] [Google Scholar]
- [121].Shan X, Vocadlo DJ, Krieger C. Reduced protein O-glycosylation in the nervous system of the mutant SOD1 transgenic mouse model of amyotrophic lateral sclerosis. Neurosci Lett. 2012;516:296–301. [DOI] [PubMed] [Google Scholar]
- [122].Hsieh Y-L, Su F-Y, Tsai L-K, et al. NPGPx-Mediated Adaptation to Oxidative Stress Protects Motor Neurons from Degeneration in Aging by Directly Modulating O-GlcNAcase. Cell Rep. 2019;29:2134–43. [DOI] [PubMed] [Google Scholar]
- [123].Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7:512–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Correa-de-Araujo R Sarcopenia in the Context of Skeletal Muscle Function Deficit (SMFD). IntechOpen. 2017. [Google Scholar]
- [125].Hardee JP, Lynch GS. Current pharmacotherapies for sarcopenia. Expert Opin Pharmacother. 2019;20:1645–57. [DOI] [PubMed] [Google Scholar]
- [126].van den Beld AW, Lamberts SW. Endocrine aspects of healthy ageing in men. Novartis Found Symp. 2002;242:3–16. [PubMed] [Google Scholar]
- [127].Tenover JS. Androgen replacement therapy to reverse and/or prevent age-associated sarcopenia in men. Baillieres Clin Endocrinol Metab. 1998;12:419–25. [DOI] [PubMed] [Google Scholar]
- [128].Tenover JS. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab. 1992;75:1092–8. [DOI] [PubMed] [Google Scholar]
- [129].Kaiser FE, Silver AJ, Morley JE. The effect of recombinant human growth hormone on malnourished older individuals. J Am Geriatr Soc. 1991;39:235–40. [DOI] [PubMed] [Google Scholar]
- [130].Rooks D, Roubenoff R. Development of pharmacotherapies for the treatment of sarcopenia. J Frailty Aging.2019;8:120–30. [DOI] [PubMed] [Google Scholar]
- [131].Gao W, Reiser PJ, Coss CC, et al. Selective androgen receptor modulator treatment improves muscle strength and body composition and prevents bone loss in orchidectomized rats. Endocrinology. 2005;146:4887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Papanicolaou DA, Ather S, Zhu H, et al. A phase IIA randomized, placebo-controlled clinical trial to study the efficacy and safety of the selective androgen receptor modulator (SARM), MK-0773 in female participants with sarcopenia. J Nutr Health Aging. 2013;17:533–43. [DOI] [PubMed] [Google Scholar]
- [133].Becker C, Lord SR, Studenski SA, et al. Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015;3:948–57. [DOI] [PubMed] [Google Scholar]
- [134].Murphy KT, Koopman R, Naim T, et al. Antibody-directed myostatin inhibition in 21-mo-old mice reveals novel roles for myostatin signaling in skeletal muscle structure and function. FASEB J. 2010;24:4433–42. [DOI] [PubMed] [Google Scholar]
- [135].Latres E, Pangilinan J, Miloscio L, et al. Myostatin blockade with a fully human monoclonal antibody induces muscle hypertrophy and reverses muscle atrophy in young and aged mice. Skelet Muscle. 2015;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Ebner N, von Haehling S. Unlocking the wasting enigma: highlights from the 8th Cachexia Conference. J Cachexia Sarcopenia Muscle. 2016;7:90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Buch A, Eldor R, Kis O, et al. The effect of circuit resistance training, empagliflozin or “vegeterranean diet” on physical and metabolic function in older subjects with type 2 diabetes: a study protocol for a randomized control trial (CEV-65 trial). BMC Geriatr. 2019;19:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Shea MK, Nicklas BJ, Marsh AP, et al. The effect of pioglitazone and resistance training on body composition in older men and women undergoing hypocaloric weight loss. Obesity (Silver Spring). 2011;19:1636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Marsh AP, Kyla Shea M, et al. Resistance training and pioglitazone lead to improvements in muscle power during voluntary weight loss in older adults. J Gerontol A Biol Sci Med Sci. 2013;68:828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Duff W, Chilibeck PD, Candow DG, et al. Effects of ibuprofen and resistance training on bone and muscle. Med Sci Sports Exerc. 2017;49:633–40. [DOI] [PubMed] [Google Scholar]
- [141].Duff WR, Kontulainen SA, Candow DG, et al. Effects of low-dose ibuprofen supplementation and resistance training on bone and muscle in postmenopausal women: A randomized controlled trial. Bone Rep. 2016;5:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Garin MC, Burns CM, Kaul S, Cappola AR. Clinical review: The human experience with ghrelin administration. J Clinical Endocrinol Metab. 2013;98:1826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–9. [DOI] [PubMed] [Google Scholar]
- [144].Rojas LBA, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetology & metabolic syndrome. Diabetol Metab Syndr. 2013;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Martin-Montalvo A, Mercken EM, Mitchell et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Cabreiro F, Au C, Leung K-Y, et al. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem. 2008;77:727–54. [DOI] [PubMed] [Google Scholar]
- [148].Soliman GA, Steenson SM, Etekpo AH. Effects of metformin and a mammalian target of rapamycin (mTOR) ATP-competitive inhibitor on targeted metabolomics in pancreatic cancer cell line. Metabolomics (Los Angel). 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Kolosova N, Vitovtov A, Stefanova N. Metformin reduces the signs of sarcopenia in old OXYS rats. Adv Gerontol. 2016;6:70–4. [PubMed] [Google Scholar]
- [150].Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69:S4–S9. [DOI] [PubMed] [Google Scholar]
- [151].Ferrando B, Olaso-Gonzalez G, Sebastia V, Viosca E, Gomez-Cabrera MC, Vina J. [Allopurinol and its role in the treatment of sarcopenia]. Rev Esp Geriatr Gerontol. 2014;49:292–8. [DOI] [PubMed] [Google Scholar]
- [152].Rundles RW, Metz EN, Silberman HR. Allopurinol in the treatment of gout. Ann Intern Med. 1966;64:229–58. [DOI] [PubMed] [Google Scholar]
- [153].Maschio G, Tessitore N, D’Angelo A, et al. Prevention of calcium nephrolithiasis with low-dose thiazide, amiloride and allopurinol. Am J Med. 1981;71:623–6. [DOI] [PubMed] [Google Scholar]
- [154].Gates AC, Bernal-Mizrachi C, Chinault SL, et al. Respiratory uncoupling in skeletal muscle delays death and diminishes age-related disease. Cell Metab. 2007;6:497–505. [DOI] [PubMed] [Google Scholar]
- [155].Murshid A, Eguchi T, Calderwood SK. Stress proteins in aging and life span. Int J Hyperthermia. 2013;29:442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54. [DOI] [PubMed] [Google Scholar]
- [157].Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J App Phisol (1985). 2005;98:1154–62. [DOI] [PubMed] [Google Scholar]
- [158].Pillon NJ, Bilan PJ, Fink LN, Klip A. Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. Am J Phyisol Endocrinol Metab. 2013;304:E453–65. [DOI] [PubMed] [Google Scholar]
- [159].Demontis F, Piccirillo R, Goldberg AL, Perrimon N. The influence of skeletal muscle on systemic aging and lifespan. Aging Cell. 2013;12:943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Sloboda DD, Brown LA, Brooks SV. Myeloid cell responses to contraction-induced injury differ in muscles of young and old mice. J Gerontol A Biol Sci Med Sci. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Biferali B, Proietti D, Mozzetta C, Madaro L. Fibro–Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network. Front Phyisol. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Power GA, Allen MD, Gilmore KJ, et al. Motor unit number and transmission stability in octogenarian world class athletes: Can age-related deficits be outrun? J App Physiol (1985). 2016;121:1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Valdez G, Tapia JC, Kang H, et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci U S A. 2010;107:14863–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Hamada K, Vannier E, Sacheck JM, Witsell AL, Roubenoff RJ. Senescence of human skeletal muscle impairs the local inflammatory cytokine response to acute eccentric exercise. FASEB J. 2005;19:264–6. [DOI] [PubMed] [Google Scholar]
- [165].Fazelzadeh P, Hangelbroek RW, Tieland M, et al. The muscle metabolome differs between healthy and frail older adults. J Proteome Res. 2016;15:499–509. [DOI] [PubMed] [Google Scholar]
- [166].Welle S, Brooks AI, Delehanty JM, Needler N, Thornton CA. Gene expression profile of aging in human muscle. Physiol Genomics. 2003;14:149–59. [DOI] [PubMed] [Google Scholar]
- [167].Welle S, Brooks AI, Delehanty JM, et al. Skeletal muscle gene expression profiles in 20–29 year old and 65–71 year old women. Exp Gerontol. 2004;39:369–77. [DOI] [PubMed] [Google Scholar]
- [168].Bullard SA, Seo S, Schilling B, et al. Gadd45a Protein Promotes Skeletal Muscle Atrophy by Forming a Complex with the Protein Kinase MEKK4. J Biol Chem. 2016;291:17496–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Yen SS, Morales AJ, Khorram O. Replacement of DHEA in aging men and women. Potential remedial effects. Ann N Y Acad Sci. 1995;774:128–42. [DOI] [PubMed] [Google Scholar]
- [170].Roubenoff R, Rall LC, Veldhuis JD, et al. The relationship between growth hormone kinetics and sarcopenia in postmenopausal women: the role of fat mass and leptin. J Clin Endocrinol Metab. 1998;83:1502–6. [DOI] [PubMed] [Google Scholar]
- [171].Brioche T, Kireev RA, Cuesta S, et al. Growth hormone replacement therapy prevents sarcopenia by a dual mechanism: improvement of protein balance and of antioxidant defenses. J Gerontol A Biol Sci Med Sci. 2014;69:1186–98. [DOI] [PubMed] [Google Scholar]
- [172].Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci U S A. 1998;95:15603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Parkington JD, LeBrasseur NK, Siebert AP, Fielding RA. Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J Appl Physiol (1985). 2004;97:243–8. [DOI] [PubMed] [Google Scholar]
- [174].Dirks AJ, Leeuwenburgh C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radic Biol Med. 2004;36:27–39. [DOI] [PubMed] [Google Scholar]
- [175].Machida S, Booth FW. Increased nuclear proteins in muscle satellite cells in aged animals as compared to young growing animals. Exp Gerontol. 2004;39:1521–5. [DOI] [PubMed] [Google Scholar]
- [176].Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19:668–70. [DOI] [PubMed] [Google Scholar]
- [177].Dirks AJ, Leeuwenburgh C. Tumor necrosis factor α signaling in skeletal muscle: effects of age and caloric restriction. J Nutr Biochem. 2006;17:501–8. [DOI] [PubMed] [Google Scholar]
- [178].Inflammatory Roubenoff R. and hormonal mediators of cachexia. J Nutr.b 1997;127:1014s–6s. [DOI] [PubMed] [Google Scholar]
- [179].Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–46. [DOI] [PubMed] [Google Scholar]
- [180].Muller FL, Song W, Liu Y, et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. [DOI] [PubMed] [Google Scholar]
- [181].Ko F, Abadir P, Marx R, et al. Impaired mitochondrial degradation by autophagy in the skeletal muscle of the aged female interleukin 10 null mouse. Exp Gerontol. 2016;73:23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


