Abstract
Cancer incidence varies among American Indian and Alaska Native (AI/AN) populations, as well as between AI/AN and White populations. This study examined trends for cancers with elevated incidence among AI/AN compared with non-Hispanic White populations and estimated potentially avoidable incident cases among AI/AN populations. Incident cases diagnosed during 2012–2016 were identified from population-based cancer registries and linked with the Indian Health Service patient registration databases to improve racial classification of AI/AN populations. Age-adjusted rates (per 100,000) and trends were calculated for cancers with elevated incidence among AI/AN compared with non-Hispanic White populations (rate ratio >1.0), by region. Trends were estimated using joinpoint regression analyses. Expected cancers were estimated by applying age-specific cancer incidence rates among non-Hispanic White populations to population estimates for AI/AN populations. Excess cancer cases among AI/AN populations were defined as observed minus expected cases. Liver, stomach, kidney, lung, colorectal and female breast cancers had higher incidence rate among AI/AN populations across most regions. Between 2012 and 2016, nearly 5,200 excess cancers were diagnosed among AI/AN populations, with the largest number of excess cancers (1,925) occurring in the Southern Plains region. Culturally informed efforts may reduce cancer disparities associated with these and other cancers among AI/AN populations.
Keywords: Cancer incidence, American Indian, Alaska Native, trends, health disparity
Previous data showed that cancer incidence rates among American Indian and Alaska Native (AI/AN) populations varied substantially from those of the general US population [1]. In addition, cancer incidence rates among AI/AN populations varied by geographic region and cancer type. Therefore, cancer incidence data, aggregated at the national level, is likely to mask substantial disparities and variations in cancer incidence rates among AI/AN populations and between AI/AN and non-Hispanic White populations.
The present study provides an overview of the leading cancer types with elevated incidence rates among AI/AN populations compared to the non-Hispanic White population during 2012–2016. We identified cancers with elevated incidence among AI/AN populations overall and by region and assessed the long-term trends of these cancers during 1999–2016. Prior studies have shown disparities in cancer incidence rates for common cancer types among AI/AN and non-Hispanic White populations; however, these studies did not specifically evaluate the cancers that disproportionately affect AI/AN populations. The purpose of this study is to highlight which cancers contribute to the largest relative disparities among AI/AN populations and to quantify the impact of these disparities (i.e., excess cases) between AI/AN and non-Hispanic White populations by geographic region. These data provide information that could be used to target public health interventions to reduce health inequities among AI/AN populations.
METHODS
Cancer incidence data came from the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention and the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute [2, 3]. During the period covered by this study (2012–2016 for rate and 1999–2016 for trends), tumor histology, tumor behavior, and primary cancer site were coded according to the Third Edition of the International Classification of Disease for Oncology (ICD-O-3) and classified according to SEER site categories [4].
To reduce racial misclassification of AI/AN populations, cancer registry data were linked with the IHS patient registration database by using previously established and validated techniques that improve accuracy of cancer incidence estimates among the AI/AN population [5, 6]. Each year, NPCR- and SEER-funded central registries submit data on cancers diagnosed during the most recent year to the respective program.
Incidence data from registries meeting rigorous quality control standards were combined into an analytic file U.S. Cancer Statistics [7]. By combining these registries, we have 100% coverage of the AI/AN population. To improve racial classification of AI/AN populations, we restricted analyses to purchased/referred care delivery area (PRCDA) counties, which contain, or are adjacent to, federally recognized tribal lands. Restricting to PRCDA counties provides more accurate correction for racial misclassification of the AI/AN population than other counties [5, 6]. Approximately 53% of the AI/AN population resides in these counties (Web Figure 1).
Population estimates that are used as denominators in the rate calculations are produced by the US Census Bureau. In a previous report, the updated, bridged, intercensal population estimates overestimated AI/AN populations of Hispanic origin [8]. In the present study, all analyses were limited to non-Hispanic AI/AN populations to avoid underestimation of incidence rates among the AI/AN population. Non-Hispanic White was chosen as the reference population. For conciseness, the term “non-Hispanic” was omitted when discussing both groups in this study.
Statistical Analysis
Cancer incidence rates for the 15 most common cancers were expressed per 100,000 population and were directly age-adjusted by using 19 age groups to the 2000 US standard population using SEER*Stat software version 8.3.2 [9]. Using the age-adjusted incidence rates, we calculated age-standardized rate-ratios (RRs) for the years 2012–2016 among the AI/AN population, with the White population as reference for each region. The leading cancer types with elevated incidence were identified for each region and sex separately. From the 15 most common cancers overall, the leading cancers with elevated incidence were identified as cancers with a rate ratio >1 (P<0.05) and ranked based on rate ratio. The six geographic regions and PRCDA counties have been described previously [1] and are shown in Web Figure 1. They include Alaska, the Northern Plains, Southern Plains, Pacific Coast, East, and Southwest. Rate ratios comparing males versus females were also calculated.
Long-term cancer incidence trends during 1999–2016 (average annual percent change, or AAPC) were estimated by joinpoint regression for the leading elevated cancers in each region. Trends for the entire period were estimated by using software developed by the NCI (Joinpoint Regression Program, Version 4.3.10) [10].
To estimate the number of excess cancers experienced among AI/AN populations, the 5-year, age-specific, cancer incidence rates among the White population were applied to the corresponding population estimates among the AI/AN populations, by sex, for the cancer types with elevated incidence in each region during 2012–2016. Excess cancers were calculated as observed minus expected cases for each cancer type, similar to previous studies that have examined elevated incidence and death [11, 12]. The observed to expected ratio was calculated to determine relative elevated incidence between the AI/AN and White populations.
RESULTS
Cancer incidence rates for the 15 most common cancers among AI/AN males and females compared to the White population, and their corresponding RRs, are shown in Web Tables 1 and 2. The leading cancers with elevated cancer incidence were selected on the basis of the RR comparing AI/AN versus White incidence rates by sex. Among AI/AN males, the leading cancer sites with elevated incidence were liver, stomach, kidney, colorectal, myeloma, and lung (Figure 1A, Web Table 1). The leading cancers with elevated incidence among AI/AN females included these sites, except for myeloma, and including cervical cancers (Figure 1B, Web Table 2). Rate ratios ranged from 1.09 (lung) to 2.37 (liver) among AI/AN males and 1.06 (lung) to 3.03 (liver) among AI/AN females. The incidence rates were higher among AI/AN males compared to females, ranging from 23% higher for lung cancer, to 129% higher for liver cancer (Figure 2).
Figure 1.
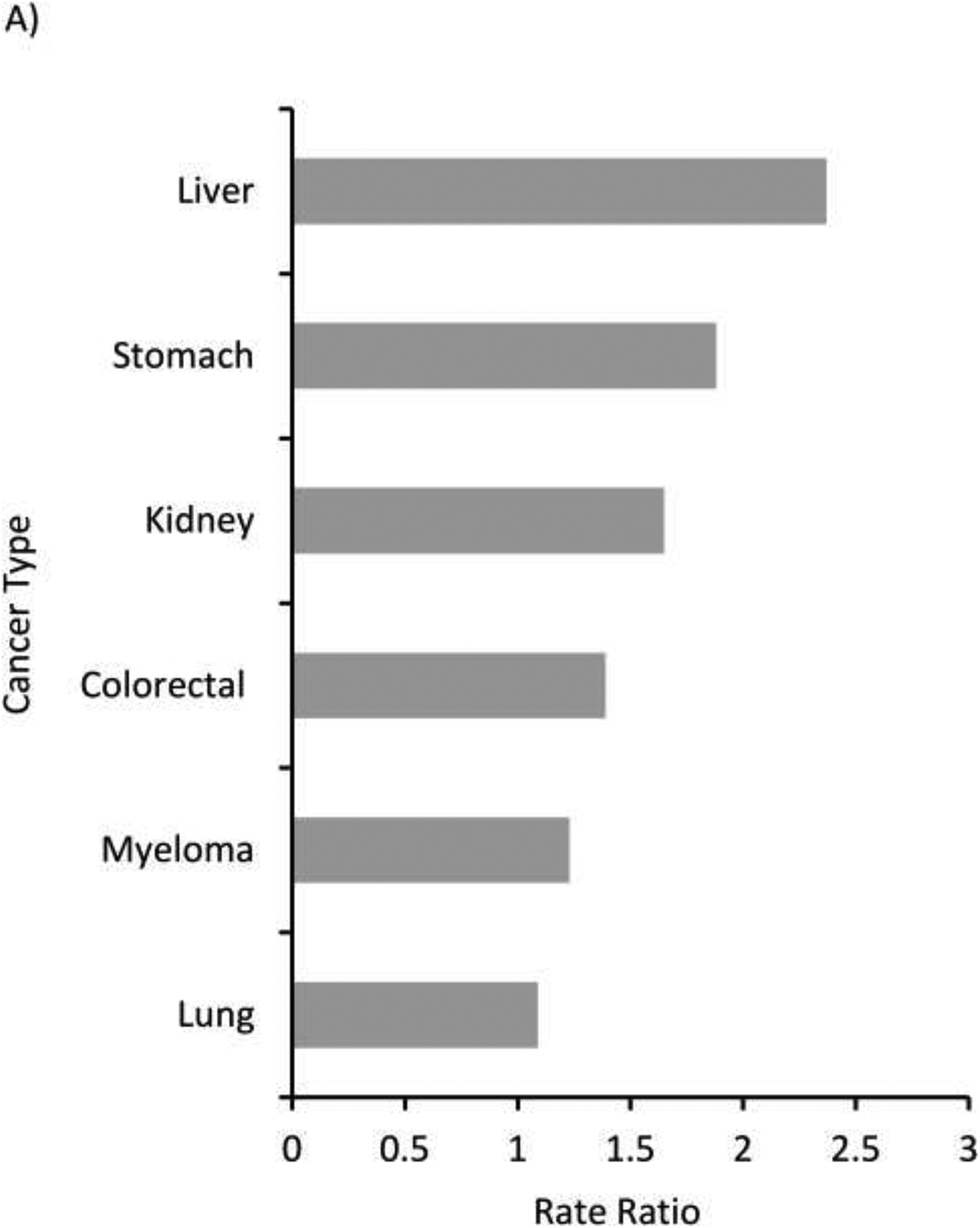
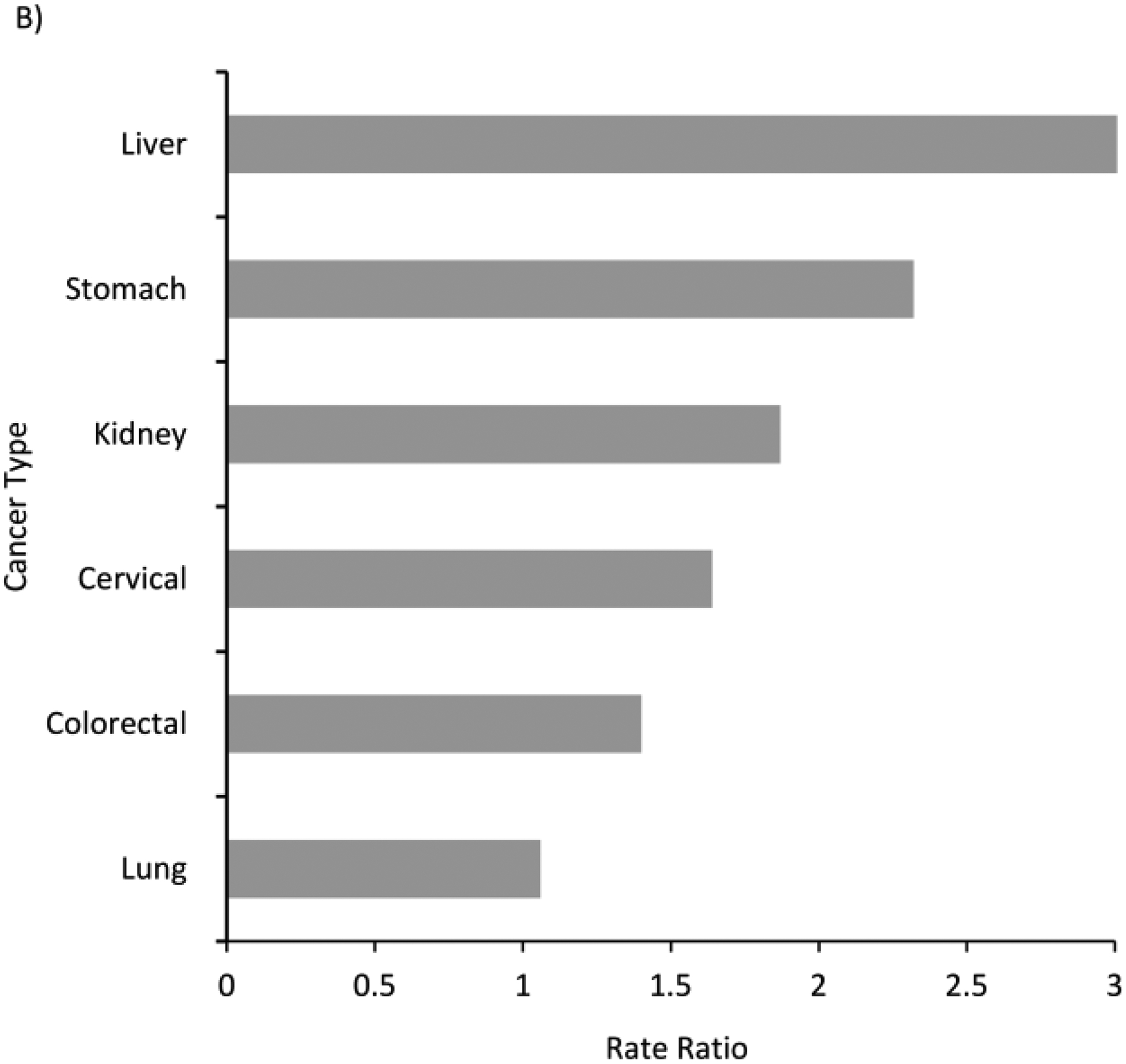
Leading cancers with elevated incidence ranked by rate ratio (AI/AN versus White) among AI/AN, Purchased/Referred Care Delivery Areas (PRCDA), overall United States, 2012–2016. A. Males, B. Females. PRCDA indicates Purchased/Referred Care Delivery Areas; AI/AN: American Indians/Alaska Natives. Includes only AI/AN of non-Hispanic origin. Leading cancers with elevated incidence selected from 15 most common cancers. See Web Tables 1 and 2. Source: Cancer registries in the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology and End Results Program (SEER). Rate ratios (RR) are AI/AN versus White and are calculated in SEER*Stat prior to rounding of rates and may note equal the RR calculated from the rates presented in the table. All excess burden cancers shown here are significantly higher in the AI/AN versus White populations (P<0.05)
Figure 2:
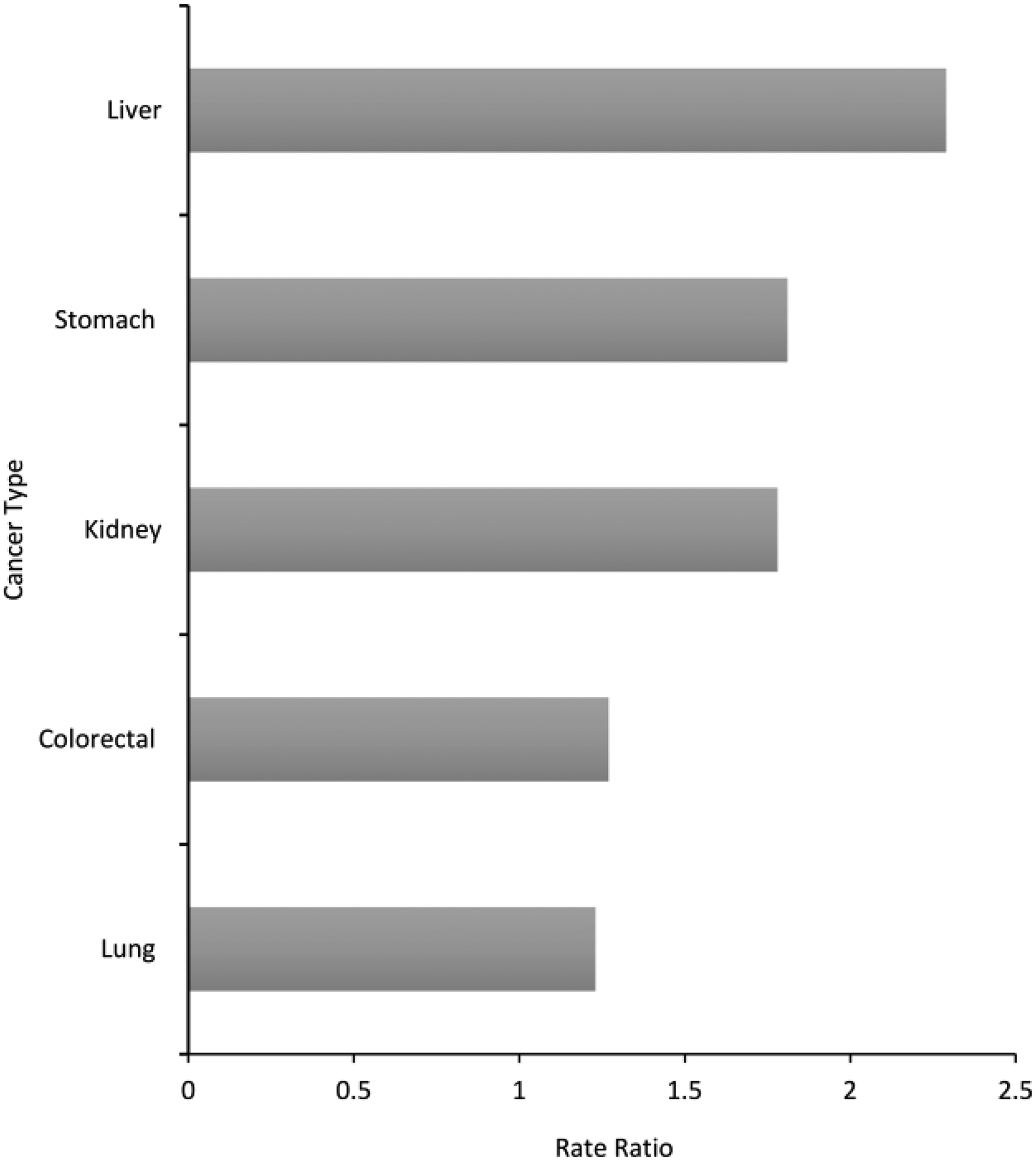
Male versus female rate ratio for leading cancers with elevated incidence among AI/AN populations, PRCDA, 2012–2016. Leading cancers with elevated incidence selected from 15 leading cancers. See Web Tables 1 and 2. Source: Cancer registries in the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology and End Results Program (SEER). Rate ratios (RR) are AI/AN versus White and are calculated in SEER*Stat prior to rounding of rates and may not equal the RR calculated from the rates presented in the table. Only leading causes of excess cancer burden that were common between males and females, and significantly greater than the White population are shown in this graph.
Among AI/AN males, the number and type of cancers with elevated incidence varied by region (Table 1). Alaska, the Southern Plains, Southwest, and the Northern Plains had the highest number of cancer types with elevated incidence among the AI/AN population. Rate ratios for cancers with elevated incidence ranged from 1.14 (colorectal cancer in the Southwest) to 4.36 (stomach cancer in Alaska). Liver, stomach, kidney, lung, and colorectal cancers were elevated in most regions, except for lung cancer in the Southwest and liver cancer in Alaska. In the East, only liver cancer was significantly higher among AI/AN males compared to White males. Liver cancer was the leading site with elevated incidence in four of six regions.
Table 1:
Age-adjusted Rates and Average Annual Percent Change of Leading Cancers with Elevated Incidencea for American Indians/Alaska Nativesb compared to Whites, By Region, Males, United States, PRCDA Counties, 2012–2016
| Region | AI/AN Rate | AI/AN Count | White Rate | White Count | Rate Ratioc | 95% CI for Rate Ratiod | AI/AN AAPCf | White AAPCf |
|---|---|---|---|---|---|---|---|---|
| Northern Plains | ||||||||
| Liver | 27.5 | 142 | 8.3 | 2,193 | 3.32 | 2.72–4.00 | 4.5g | 3.8g |
| Stomach | 16.5 | 73 | 7.4 | 1,821 | 2.25 | 1.70–2.91 | −1.2 | −1.2 |
| Kidney | 45.9 | 238 | 22.6 | 5,524 | 2.03 | 1.76–2.34 | 2.9g | 2.0g |
| Colorectal | 74.9 | 358 | 42.1 | 10,165 | 1.78 | 1.57–2.00 | −1.7g | −2.7g |
| Lung | 109.3 | 457 | 66.9 | 16,838 | 1.63 | 1.47–1.81 | −1.3 | −1.7g |
| Alaska | ||||||||
| Stomach | 28.1 | 64 | 6.4 | 79 | 4.36 | 3.00–6.32 | −1.8 | −0.8 |
| Colorectal | 90.1 | 180 | 36.5 | 466 | 2.47 | 2.03–2.99 | −1.6 | −3.9g |
| Esophageal | 15.8 | 31 | 7.7 | 100 | 2.06 | 1.26–3.24 | NA | −1.2 |
| Lung | 104.9 | 195 | 58.9 | 692 | 1.78 | 1.49–2.12 | −0.9 | −2.9g |
| Oropharyngeal | 25.7 | 61 | 15.1 | 229 | 1.70 | 1.22–2.33 | −1 | −0.5 |
| Pancreas | 19.5 | 38 | 12.1 | 149 | 1.61 | 1.05–2.39 | 1.2 | 1.7 |
| Kidney | 29.5 | 69 | 20.9 | 281 | 1.41 | 1.05–1.88 | 0.9 | 0.7 |
| Southern Plains | ||||||||
| Liver | 25.7 | 198 | 10.5 | 1,027 | 2.45 | 2.08–2.88 | 6.2g | 4.0g |
| Kidney | 46.5 | 342 | 23.8 | 2,133 | 1.96 | 1.73–2.21 | 3.1g | 2.3g |
| Stomach | 12.0 | 75 | 6.9 | 628 | 1.74 | 1.32–2.24 | −1.0 | −0.1 |
| Myeloma | 12.3 | 77 | 7.5 | 692 | 1.63 | 1.24–2.10 | 2.4 | 1.6g |
| Colorectal | 75.7 | 520 | 46.7 | 4,193 | 1.62 | 1.47–1.79 | −0.9 | −2.4g |
| Lung | 112.0 | 730 | 82.5 | 7,719 | 1.36 | 1.25–1.47 | −0.2 | −2.2g |
| Esophageal | 10.6 | 76 | 8 | 753 | 1.33 | 1.02–1.71 | 1.4 | 0.2 |
| Pacific Coast | ||||||||
| Liver | 29.5 | 234 | 11.4 | 6,226 | 2.59 | 2.25–2.98 | 4.8g | 3.8g |
| Kidney | 25.7 | 184 | 21.1 | 10,349 | 1.22 | 1.04–1.42 | 1.2 | 2.1g |
| Lung | 69.8 | 431 | 59 | 29,260 | 1.18 | 1.06–1.31 | −0.5 | −2.8g |
| Colorectal | 46.6 | 296 | 40 | 19,339 | 1.16 | 1.02–1.32 | −2.0g | −2.8g |
| East | ||||||||
| Liver | 19.4 | 60 | 11.3 | 6,216 | 1.72 | 1.29–2.25 | 8.1g | 3.5g |
| Southwest | ||||||||
| Stomach | 15.5 | 128 | 5.8 | 1,430 | 2.66 | 2.18–3.21 | −1.2 | −1.6g |
| Liver | 22.4 | 221 | 8.7 | 2,251 | 2.58 | 2.22–2.98 | 3.9g | 2.5g |
| Kidney | 36.5 | 346 | 18.9 | 4,523 | 1.93 | 1.72–2.17 | 1.7g | 1.9g |
| Myeloma | 9.5 | 80 | 5.8 | 1,450 | 1.63 | 1.26–2.06 | 1.1 | 0.5 |
| Colorectal | 43.4 | 401 | 38.1 | 9,098 | 1.14 | 1.02–1.27 | 2.2g | −3.0g |
PRCDA indicates Purchased/Referred Care Delivery Areas; AI/AN: American Indians/Alaska Natives; W: non-Hispanic White; RR: Rate Ratio, NA; Not Applicable, indicates trend could not be calculated.
Leading cancers with elevated incidence selected from 15 most common cancers. See Web Table 1. Source: Cancer registries in the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology and End Results Program (SEER).
AI/AN race is reported by NPCR and SEER registries or through linkage with the IHS patient registration database. Includes only AI/AN of non-Hispanic origin.
Rates are per 100,000 persons and are age-adjusted to the 2000 U.S. standard population (19 age groups - Census P25–1130).
Rate ratios (RR) are AI/AN versus White and are calculated in SEER*Stat prior to rounding of rates and may not equal RR calculated from rates presented in table.
Indicates significant RR, P<0.05
AAPC: Average Annual Percent Change. Trends estimated for the years 1999–2016, calculated using joinpoint regression analysis
Indicates significant AAPC, P<0.05
Among AI/AN males, the incidence rate of several cancers increased during the study period (Table 1), with some of the largest increases occurring in liver cancers in the East (AAPC 8.1), Southern Plains (AAPC: 6.2), Pacific Coast (AAPC: 4.8) and Northern Plains (AAPC 4.5), and kidney cancers in the Northern Plains (AAPC: 2.9) and Southern Plains (AAPC: 3.1). Significant decreases were observed in colorectal cancer incidence rates in the Northern Plains (AAPC: −1.7) and Pacific Coast (AAPC −2.0).
Among AI/AN females, the Southern Plains, Northern Plains, Alaska, and the Pacific Coast had the most types of cancer with elevated incidence (Table 2). Rate ratios for elevated incidence cancers ranged from 1.15 (corpus and uterus in the Pacific Coast) to 4.07 (stomach in Alaska). Rate ratios for liver cancer ranged from 2.17 in the East and Alaska to 3.63 in the Southwest. Stomach cancer was elevated in every region. Kidney cancer was elevated in 5 of 6 regions (except the East); the highest RR occurred in the Northern Plains (RR = 2.12).
Table 2:
Age-adjusted Rates and Average Annual Percent Change of Leading Cancers with Elevated Incidencea for American Indians/Alaska Nativesb compared to Whites, By Region, Females, United States, PRCDA Counties, 2012–2016
| Region | AI/AN Rate | AI/AN Count | White Rate | White Count | Rate Ratioe | 95% CI for Rate Ratio | AI/AN AAPCf | White AAPCf |
|---|---|---|---|---|---|---|---|---|
| Northern Plains | ||||||||
| Liver | 10.5 | 64 | 3.2 | 912 | 3.27 | 2.45–4.28 | 2.4 | 2.5g |
| Kidney | 23.5 | 143 | 11.1 | 2,941 | 2.12 | 1.76–2.53 | 0.7 | 1.4g |
| Lung | 102.0 | 551 | 53.3 | 15,266 | 1.92 | 1.75–2.09 | 0.3 | −0.3 |
| Stomach | 5.9 | 33 | 3.1 | 863 | 1.92 | 1.29–2.74 | −4.3g | −0.9 |
| Cervical | 12.0 | 73 | 6.3 | 1,294 | 1.90 | 1.47–2.42 | −0.5 | −1.6g |
| Colorectal | 51.3 | 299 | 33 | 9,157 | 1.55 | 1.37–1.75 | −1.5 | −2.6g |
| Oropharyngeal | 10.1 | 58 | 6.7 | 1,834 | 1.51 | 1.12–1.98 | 2.5 | 0.4 |
| Alaska | ||||||||
| Stomach | 14.5 | 33 | 3.6 | 43 | 4.07 | 2.44–6.71 | −3.4 | −3.0 |
| Colorectal | 96.3 | 216 | 32.6 | 376 | 2.96 | 2.47–3.53 | −1.3 | −2.1g |
| Oropharyngeal | 13.4 | 32 | 5.1 | 63 | 2.61 | 1.61–4.13 | −0.9 | −0.3 |
| Liver | 7.7 | 19 | 3.5 | 45 | 2.17 | 1.15–3.88 | 1.4 | 2 |
| Kidney | 21.3 | 54 | 11.9 | 145 | 1.79 | 1.26–2.49 | 2.1 | 2.1 |
| Cervical | 10.9 | 26 | 6.8 | 79 | 1.60 | 1.00–2.56 | 1.1 | −0.5 |
| Lung | 71.9 | 164 | 47.8 | 546 | 1.51 | 1.25–1.81 | −0.2 | −2.3g |
| Female Breast | 151.0 | 373.0 | 121.0 | 1488 | 1.25 | 1.11–1.41 | 0.3 | −1.3g |
| Southern Plains | ||||||||
| Liver | 10.3 | 88 | 3.5 | 381 | 2.93e | 2.27–3.73 | 4.8g | 2.2g |
| Stomach | 6.8 | 56 | 3.0 | 319 | 2.27e | 1.66–3.05 | −1.5 | −0.7 |
| Kidney | 27.7 | 235 | 13.2 | 1,335 | 2.10e | 1.81–2.42 | 2.7g | 2.9g |
| Cervical | 13.8 | 118.0 | 8.5 | 644 | 1.63 | 1.32–2.00 | −0.8 | −0.7 |
| Pancreas | 16.3 | 129.0 | 10.2 | 1,112 | 1.60 | 1.31–1.93 | 2.9g | 1.4g |
| Colorectal | 55.7 | 452.0 | 35.3 | 3,683 | 1.58 | 1.42–1.75 | −0.4 | −1.8g |
| Lung | 84.6 | 697.0 | 56.6 | 6,224 | 1.49 | 1.38–1.62 | −0.1 | −0.8g |
| Corpus and Uterus, NOS | 29.9 | 261.0 | 21.9 | 2,265 | 1.37 | 1.19–1.56 | 1.6g | 0.9g |
| Female Breast | 158.7 | 1337.0 | 119 | 11,905 | 1.33 | 1.26–1.41 | 1.0g | −0.4 |
| Ovary | 15.5 | 132.0 | 11.6 | 1,167 | 1.33 | 1.10–1.61 | −0.5 | −1.5g |
| Non-Hodgkin Lymphoma | 19.9 | 158.0 | 15.1 | 1,582 | 1.32 | 1.11–1.56 | −1.0 | 0.1 |
| Thyroid | 23.6 | 207.0 | 20.1 | 1,636 | 1.18 | 1.01–1.36 | 6.6g | 5.0g |
| Pacific Coast | ||||||||
| Liver | 11.2 | 94 | 3.9 | 2284 | 2.85 | 2.26–3.55 | 3.7 | 3.2g |
| Stomach | 6.1 | 43 | 3.0 | 1687 | 2.03 | 1.44–2.78 | 1.2 | −1.2g |
| Cervical | 13.8 | 105 | 6.9 | 2,802 | 2.00 | 1.62–2.44 | 1.8 | −0.9g |
| Kidney | 16.5 | 134 | 10.4 | 5,566 | 1.59 | 1.32–1.90 | 1.5 | 1.9g |
| Colorectal | 43.2 | 317 | 32 | 17,641 | 1.35 | 1.20–1.51 | −0.6 | −2.2g |
| Lung | 60.7 | 456 | 51.5 | 29,686 | 1.18 | 1.07–1.30 | −0.2 | −1.6g |
| Corpus and Uterus, NOS | 30.8 | 262 | 26.7 | 15,011 | 1.15 | 1.01–1.31 | 1.8 | −0.9g |
| East | ||||||||
| Liver | 7.4 | 22 | 3.4 | 2,138 | 2.17 | 1.33–3.35 | NA | −2.5 |
| Stomach | 8.2 | 25 | 4.0 | 2,435 | 2.05 | 1.30–3.07 | 4.4g | −0.7 |
| Southwest | ||||||||
| Liver | 12.1 | 138 | 3.3 | 918 | 3.63 | 3.00–4.37 | 3.6g | 2.9g |
| Stomach | 8.5 | 93 | 2.7 | 717 | 3.16 | 2.50–3.94 | −0.9 | −0.8g |
| Kidney | 17.2 | 208 | 9.3 | 2,378 | 1.84 | 1.58–2.13 | 2.1g | 1.2g |
| Corpus and Uterus, NOS | 28.2 | 353 | 22.5 | 5,912 | 1.25 | 1.12–1.40 | −1.7 | −1.1g |
PRCDA indicates Purchased/Referred Care Delivery Areas; AI/AN: American Indians/Alaska Natives; W: non-Hispanic White; LCL: lower confidence level; UCL: upper confidence level; NA: Not Applicable, indicates trend could not be calculated; NOS: Not otherwise specified
Leading cancers with elevated incidence selected from 15 most common cancers. See Web Table 2. Source: Cancer registries in the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology and End Results Program (SEER).
AI/AN race is reported by NPCR and SEER registries or through linkage with the IHS patient registration database. Includes only AI/AN of non-Hispanic origin.
Rates are per 100,000 persons and are age-adjusted to the 2000 U.S. standard population (19 age groups - Census P25–1130).
Rate ratios (RR) are AI/AN versus White and are calculated in SEER*Stat prior to rounding of rates and may not equal RR calculated from rates presented in table.
Indicates significant RR, P<0.05
AAPC: Average Annual Percent Change. Trends estimated for the years 1999–2016, calculated using joinpoint regression analysis
Indicates significant AAPC, P<0.05
During 2012–2016, stomach cancer incidence rates decreased significantly among AI/AN females in the Northern Plains (AAPC −4.3) (Table 2). Incidence rates of liver cancer increased significantly in the Southern Plains (AAPC: 4.8) and Southwest (AAPC: 3.6). Rates of kidney cancer also increased significantly in the Southern Plains (AAPC: 2.7) and Southwest (AAPC: 2.1). Cancers of the pancreas (AAPC: 2.9), corpus and uterus (AAPC: 1.6), and thyroid (AAPC: 6.6) increased significantly in the Southern Plains; and stomach cancer increased in the East (AAPC: 4.4). Rates of breast cancer also increased in the Southern Plains (AAPC: 1.0).
For the leading sites with elevated incidence among AI/AN males (Table 3), the observed to expected ratios ranged from 1.14 for colorectal cancer in the Pacific Coast to 5.33 for stomach cancer in Alaska. An estimated 2,450 excess cancers were diagnosed among AI/AN males. The largest number of excess cancers among AI/AN males occurred in Southern Plains (727) and some of the highest numbers of excess cancers are attributable to colorectal, lung, and liver cancers.
Table 3:
Expected and Observed Number of Cancers and Observed to Expected Ratio for Leading Cancers with Elevated Incidenceb by Region, AI/AN Males, PRCDA Counties 2012–2016
| Observed Casesc | Expected Casesd | Excess Cancers | Observed to Expected Ratio | |
|---|---|---|---|---|
| Northern Plains | ||||
| Liver | 142 | 42 | 100 | 3.38 |
| Stomach | 73 | 32 | 41 | 2.28 |
| Kidney | 238 | 112 | 126 | 2.13 |
| Colorectal | 358 | 192 | 166 | 1.86 |
| Lung | 457 | 286 | 171 | 1.60 |
| Total Excess Cancer Cases | 604 | |||
| Alaska | ||||
| Stomach | 64 | 12 | 52 | 5.33 |
| Colorectal | 180 | 74 | 106 | 2.43 |
| Esophageal | 31 | 15 | 16 | 2.07 |
| Lung | 195 | 107 | 88 | 1.82 |
| Oral Cavity and Pharynx | 61 | 36 | 25 | 1.69 |
| Pancreas | 38 | 23 | 15 | 1.65 |
| Kidney | 69 | 45 | 24 | 1.53 |
| Total Excess Cancer Cases | 326 | |||
| Southern Plains | ||||
| Liver | 138 | 40 | 98 | 3.45 |
| Kidney | 342 | 174 | 168 | 1.97 |
| Stomach | 75 | 46 | 29 | 1.63 |
| Myeloma | 77 | 48 | 29 | 1.60 |
| Colorectal | 520 | 321 | 199 | 1.62 |
| Lung | 730 | 544 | 186 | 1.34 |
| Esophageal | 76 | 58 | 18 | 1.31 |
| Total Excess Cancer Cases | 727 | |||
| Pacific Coast | ||||
| Liver | 234 | 87 | 147 | 2.69 |
| Kidney | 184 | 144 | 40 | 1.28 |
| Lung | 431 | 358 | 73 | 1.20 |
| Colorectal | 296 | 259 | 37 | 1.14 |
| Total Excess Cancer Cases | 297 | |||
| East | ||||
| Liver | 60 | 32 | 28 | 1.88 |
| Total Excess Cancer Cases | 28 | |||
| Southwest | ||||
| Stomach | 128 | 50 | 78 | 2.56 |
| Liver | 221 | 87 | 134 | 2.54 |
| Kidney | 346 | 179 | 167 | 1.93 |
| Myeloma | 80 | 50 | 30 | 1.60 |
| Colorectal | 401 | 342 | 59 | 1.17 |
| Total Excess Cancer Cases | 468 |
AI/AN race is reported by NPCR and SEER registries or through linkage with the IHS patient registration database. Includes only AI/AN of non-Hispanic origin. Source: Cancer registries in the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology and End Results Program (SEER)
Leading cancers with elevated incidence selected from 15 most common cancers. See Web Table 1
Observed counts are the number of cancer cases observed in the dataset
Expected counts are calculated by applying age specific cancer incidence rates from the White population to the AI/AN population
Among AI/AN females, the observed to expected ratios for the leading causes of elevated cancer incidence (Table 4) ranged from 1.14 (corpus and uterus in the Pacific Coast) to 4.13 (stomach in Alaska). Overall, 2,732 excess cancers were diagnosed among AI/AN females in these 6 regions. The largest number of excess cancers among AI/AN females occurred in the Southern Plains (1198).
Table 4:
Expected and Observed Number of Cancers and Observed to Expected Ratio for Leading Cancers with Elevated Incidenceb by Region AI/AN Females, PRCDA Counties 20122016
| Observed Casesc | Expected Casesd | Excess Cancers | Observed to Expected Ratio | |
|---|---|---|---|---|
| Northern Plains | ||||
| Liver | 64 | 18 | 46 | 3.56 |
| Kidney | 143 | 66 | 77 | 2.17 |
| Lung | 551 | 296 | 255 | 1.86 |
| Stomach | 33 | 17 | 16 | 1.94 |
| Cervical | 73 | 40 | 33 | 1.83 |
| Colorectal | 299 | 177 | 122 | 1.69 |
| Oropharyngeal | 58 | 40 | 18 | 1.45 |
| Total Excess Cancer Cases | 567 | |||
| Alaska | ||||
| Stomach | 33 | 8 | 25 | 4.13 |
| Colorectal | 216 | 74 | 142 | 2.92 |
| Oropharyngeal | 32 | 12 | 20 | 2.67 |
| Liver | 19 | 9 | 10 | 2.11 |
| Kidney | 54 | 28 | 26 | 1.93 |
| Cervical | 26 | 16 | 10 | 1.63 |
| Lung | 164 | 105 | 59 | 1.56 |
| Total Excess Cancer Cases | 292 | |||
| Southern Plains | ||||
| Liver | 88 | 30 | 58 | 2.93 |
| Stomach | 56 | 24 | 32 | 2.33 |
| Kidney | 235 | 114 | 121 | 2.06 |
| Cervical | 118 | 71 | 47 | 1.66 |
| Pancreas | 129 | 81 | 48 | 1.59 |
| Colorectal | 452 | 286 | 166 | 1.58 |
| Lung | 697 | 467 | 230 | 1.49 |
| Corpus and Uterus, NOS | 261 | 190 | 71 | 1.37 |
| Female Breast | 1337 | 1012 | 325 | 1.32 |
| Ovary | 132 | 99 | 33 | 1.33 |
| Non-Hodgkin Lymphoma | I58 | 122 | 36 | 1.30 |
| Thyroid | 207 | 176 | 31 | 1.18 |
| Total Excess Cancer Cases | 1198 | |||
| Pacific Coast | ||||
| Liver | 94 | 32 | 62 | 2.94 |
| Stomach | 43 | 22 | 21 | 1.95 |
| Cervical | 105 | 52 | 53 | 2.02 |
| Colorectal | 317 | 235 | 82 | 1.35 |
| Lung | 456 | 377 | 79 | 1.21 |
| Corpus and Uterus, NOS | 253 | 222 | 31 | 1.14 |
| Total Excess Cancer Cases | 328 | |||
| East | ||||
| Liver | 22 | 11 | 11 | 2.00 |
| Stomach | 25 | 12 | 13 | 2.08 |
| Total Excess Cancer Cases | 24 | |||
| Southwest | ||||
| Liver | 138 | 40 | 98 | 3.45 |
| Stomach | 93 | 30 | 63 | 3.10 |
| Kidney | 208 | 111 | 97 | 1.87 |
| Corpus and Uterus, NOS | 337 | 272 | 65 | 1.24 |
| Total Excess Cancer Cases | 323 |
AI/AN race is reported by NPCR and SEER registries or through linkage with the IHS patient registration database. Includes only AI/AN of non-Hispanic origin. Source: Cancer registries in the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology and End Results Program (SEER)
Leading cancers with elevated incidence selected from 15 most common cancers. See Web Table 2
Observed counts are the number of cancer cases observed in the dataset
Expected counts are calculated by applying age specific cancer incidence rates from the White population to the AI/AN population
DISCUSSION
This study provides a comprehensive overview of the leading cancer types with elevated incidence among the AI/AN population, by region, for the years 2012–2016. The cancers identified as excess cancers in this study are driving cancer disparities in AI/AN populations and have been shown to contribute to cancer related disparities in other underrepresented populations [13–15]. The present study confirms prior findings that showed substantial regional variation in cancer incidence rates among the AI/AN population [1, 16]. These findings provide further evidence that data aggregated across cancer sites or by region mask important differences in cancer incidence, both within the AI/AN population and between the AI/AN and White population. Liver, stomach, kidney, lung, and colorectal cancers were among the cancers consistently elevated across several regions among the AI/AN population. This study estimated that nearly 5,200 cases of cancer in the AI/AN population were potentially avoidable during 2012–2016 among AI/AN populations in the 6 study areas if racial disparities had not existed in cancer incidence.
Liver cancer was a leading cancer with elevated incidence among AI/AN populations in nearly every region. Incidence rates were as much as four times higher than rates among the White population among both AI/AN males and females, confirming previous findings [17]. The prevalence of hepatitis C virus (HCV) infection, a known risk factor of liver cancer, is higher among the AI/AN population than among the non-Hispanic White population [17, 18]. To increase screening for HCV among the AI/AN population in the Southern Plains, the Cherokee Nation Health Services implemented a clinical decision support tool within the health records for primary care physicians, targeting individuals at high risk of liver cancer. This effort resulted in a nearly 14% increase in the number of eligible individuals receiving HCV screening during 2012–2015, and nearly 90% of the individuals subsequently treated after HCV diagnosis achieved a cure [19]. Other efforts include collaborations between IHS and Project ECHO (Extension for Community Healthcare Outcomes) aimed at improving HCV-related care for the AI/AN population through teleconsulting and “telementoring” partnerships between specialists and providers in rural and underserved communities [20].
This study also confirmed previous findings about the elevated incidence of kidney cancer among the AI/AN population [21]. Increases in kidney cancer incidence have been linked with rising rates of obesity [21–23]. Previous studies suggested that smoking and hypertension may also play roles in elevated kidney cancer rates [24]. These few known risk factors for kidney cancer are unlikely to fully explain the observed geographic variation in kidney cancer incidence, or the elevated incidence rates among the AI/AN population. Additional studies can help better understand the factors driving differences in kidney cancer incidence between the AI/AN and White populations.
Despite ongoing efforts to reduce the high prevalence of commercial tobacco use among AI/AN populations, the present study suggests that lung cancer incidence rates have largely remained stagnant among the AI/AN population. A new Government Performance and Results Act measure was established in 2006 to track tobacco cessation service delivery among current smokers within the IHS and tribal programs [25]. Although this measure has progressively improved each year, from the baseline of 12% in 2006 to over 50% in 2016 [25], the present study suggests that expanding culturally competent tobacco control strategies for the use of commercial tobacco remains an important aspect of cancer prevention among the AI/AN population.
This study confirms persistent disparities in colorectal cancer incidence rates among the AI/AN population. Although decreasing colorectal cancer incidence rates were observed among AI/AN males in the Northern Plains and Pacific Coast regions, rates actually increased among AI/AN males in the Southwest region. There were no significant declines in colorectal cancer incidence rates among AI/AN females in regions with elevated colorectal cancer incidence, suggesting the need to increase screening for this disease. In addition, inequities in colorectal cancer risk factors could be addressed, such as higher diabetes prevalence, lower dietary intake of fruits and vegetables, and higher consumption of sugar sweetened beverages, alcohol, and tobacco products. Collaborative efforts to increase colorectal cancer screening in regions with the highest incidence of disease show promise, but alone they may not be sufficient to eliminate disparities in incidence [26–29].
Although stomach cancer incidence rates for the overall US population are low, rates of stomach cancer among the AI/AN population are high, specifically among the Alaska Native population. The association between stomach cancer and Helicobactor Pylori infection can likely account for a large portion of these increased rates of stomach cancer, specifically in Alaska where the prevalence of infection among the AI/AN population can range from 64% to 81%, and reinfection rates after treatment are as high as 16% [30, 31]. Although Helicobactor Pylori is an important risk factor, infection is not a sufficient cause of stomach cancer [32]. Other environmental and behavioral factors linked with the development of stomach cancer include high intake of salt, nitrites, and nitrates; family history; smoking; and obesity [33–36]. Screening and early detection of stomach cancer may be beneficial to high-risk AI/AN individuals, such as first-degree relatives of persons with stomach cancer, because survival rates after treatment of advanced stage disease are poor [37]. Although screening high-risk populations for stomach cancer is appropriate among countries with relatively high incidence rates, screening is generally thought to be costly and unwarranted because of the low overall burden among countries with low rates of stomach cancer, such as the United States [32]. Studies have shown that data on exposure to risk factors associated with stomach cancer can aid in the identification of high-risk subgroups for more targeted screening and intervention [38, 39]; these strategies might also be effective in Indian country [37].
This study has limitations. To reduce racial misclassification of AI/AN populations, the IHS patient registration database was linked with data from the central cancer registries. However, these linkages only address the racial misclassification for individuals that have previously accessed services through the IHS; thus, AI/AN individuals who are not members of federally recognized tribes are not included. In addition, individuals living in urban, non-PRCDA areas are not represented in these data. Results based on these data may not be generalizable to all AI/AN individuals in the United States. Finally, restriction of the analyses to non-Hispanic AI/AN populations may not accurately represent all AI/AN populations. Although this exclusion reduced overall AI/AN incidence rates by less than 5%, this exclusion may disproportionally affect cancer incidence rates in certain regions. Finally, this study uses data from central cancer registries and does not consider social determinants of health that may affect cancer incidence.
The present study highlights cancers with elevated incidence among the AI/AN compared to the White populations. Elevated incidence in liver, stomach, kidney, lung, and colorectal cancers represent important health inequities between AI/AN and White populations. Areas for improvement for cancer prevention and control among the AI/AN population include efforts to promote healthy environments and address the underlying social determinants of cancer risk. Culturally informed, community-based interventions to support healthy behaviors, reduce exposure to carcinogens, promote recommended screening for cancer or its risk factors, and increase access to preventive health services may reduce these persistent disparities in cancer incidence among the AI/AN population.
Supplementary Material
Acknowledgments
This study was supported by the Centers for Disease Control and Prevention. Centers for Disease Control and Prevention coauthors participated as a part of their official duties.
Abbreviations:
- AI/AN
American Indian and Alaska Native
- IHS
Indian Health Service
- AAPC
Average Annual Percent Change
- HCV
hepatitis C virus
- PRCDA
Purchased/Referred Care Delivery Area
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflicts of Interest: None Declared
REFERENCES
- 1.Melkonian SC, Jim MA, Haverkamp D, Wiggins CL, McCollum J, White MC, et al. Disparities in Cancer Incidence and Trends among American Indians and Alaska Natives in the United States, 2010–2015. 2019;28:1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8:1117–21. [PubMed] [Google Scholar]
- 3.Thoburn KK, German RR, Lewis M, Nichols PJ, Ahmed F, Jackson-Thompson J. Case completeness and data accuracy in the Centers for Disease Control and Prevention’s National Program of Cancer Registries. Cancer. 2007;109:1607–16. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Site Recode. Available at https://seer.cancer.gov/siterecode/. Accessed 3/1/2020
- 5.Jim MA, Arias E, Seneca DS, Hoopes MJ, Jim CC, Johnson NJ, et al. Racial misclassification of American Indians and Alaska Natives by Indian Health Service Contract Health Service Delivery Area. Am J Public Health. 2014;104 Suppl 3:S295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espey DK, Wiggins CL, Jim MA, Miller BA, Johnson CJ, Becker TM. Methods for improving cancer surveillance data in American Indian and Alaska Native populations. Cancer. 2008;113:1120–30. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Cancer Statistics; Data Visualizations Tool. Technical Notes. Diagnosis Years 1999–2016. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. November 2018 Submission. https://www.cdc.gov/cancer/uscs/technical_notes/index.htm. Accessed 1/1/2020. [Google Scholar]
- 8.Arias E, Schauman WS, Eschbach K, Sorlie PD, Backlund E. The validity of race and Hispanic origin reporting on death certificates in the United States. Vital Health Stat 2. 2008:1–23. [PubMed] [Google Scholar]
- 9.Surveillance Research Program, National Cancer Institute. SEER*Stat Software, Latest Release: 8.3.2. 2016. Available at http://seer.cancer.gov/seerstat. Accessed October 2016.
- 10.National Cancer Institute. Joinpoint regression program, Version 4.3.1.0 (2016) Bethesda, MD: National Cancer Institute. http://surveillance.cancer.gov/joinpoint. Accessed 11/1/2019. [Google Scholar]
- 11.Robbins HA, Pfeiffer RM, Shiels MS, Li J, Hall HI, Engels EA. Excess cancers among HIV-infected people in the United States. J Natl Cancer Inst. 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weir HK, Li C, Henley SJ, Joseph D. Years of Life and Productivity Loss from Potentially Avoidable Colorectal Cancer Deaths in U.S. Counties with Lower Educational Attainment (2008–2012). Cancer Epidemiol Biomarkers Prev. 2017;26:736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinheiro PS, Callahan KE, Gomez SL, Marcos-Gragera R, Cobb TR, Roca-Barcelo A, et al. High cancer mortality for US-born Latinos: evidence from California and Texas. BMC Cancer. 2017;17:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller KD, Goding Sauer A, Ortiz AP, Fedewa SA, Pinheiro PS, Tortolero-Luna G, et al. Cancer Statistics for Hispanics/Latinos, 2018. CA Cancer J Clin. 2018;68:425–45. [DOI] [PubMed] [Google Scholar]
- 15.Pinheiro PS, Callahan KE, Jones PD, Morris C, Ransdell JM, Kwon D, et al. Liver cancer: A leading cause of cancer death in the United States and the role of the 1945–1965 birth cohort by ethnicity. JHEP Rep. 2019;1:162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White MC, Espey DK, Swan J, Wiggins CL, Eheman C, Kaur JS. Disparities in cancer mortality and incidence among American Indians and Alaska Natives in the United States. Am J Public Health. 2014;104 Suppl 3:S377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melkonian SC, Jim MA, Reilley B, Erdrich J, Berkowitz Z, Wiggins CL, et al. Incidence of primary liver cancer in American Indians and Alaska Natives, US, 1999–2009. Cancer Causes Control. 2018;29:833–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rempel JD, Uhanova J. Hepatitis C virus in American Indian/Alaskan Native and Aboriginal peoples of North America. Viruses. 2012;4:3912–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mera J, Vellozzi C, Hariri S, Carabin H, Drevets DA, Miller A, et al. Identification and Clinical Management of Persons with Chronic Hepatitis C Virus Infection - Cherokee Nation, 2012–2015. MMWR Morb Mortal Wkly Rep. 2016;65:461–6. [DOI] [PubMed] [Google Scholar]
- 20.Arora S, Kalishman S, Thornton K, Komaromy M, Katzman J, Struminger B, et al. Project ECHO (Project Extension for Community Healthcare Outcomes): A National and Global Model for Continuing Professional Development. J Contin Educ Health Prof. 2016;36 Suppl 1:S48–9. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Weir HK, Jim MA, King SM, Wilson R, Master VA. Kidney cancer incidence and mortality among American Indians and Alaska Natives in the United States, 1990–2009. Am J Public Health. 2014;104 Suppl 3:S396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanfilippo KM, McTigue KM, Fidler CJ, Neaton JD, Chang Y, Fried LF, et al. Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension. 2014;63:934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scelo G, Larose TL. Epidemiology and Risk Factors for Kidney Cancer. J Clin Oncol. 2018:JCO2018791905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Indian Health Service. Quality of IHS Health Care: Performance Measures. Available at: http://www.ihs.gov/qualityofcare/index.cfm?module=chart&rpt_type=gpra&measure=18. Accessed Feb 12, 2020.
- 26.Nadeau M, Walaszek A, Perdue DG, Rhodes KL, Haverkamp D, Forster J. Influences and Practices in Colorectal Cancer Screening Among Health Care Providers Serving Northern Plains American Indians, 2011–2012. Prev Chronic Dis. 2016;13:E167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redwood D, Provost E, Asay E, Roberts D, Haverkamp D, Perdue D, et al. Comparison of fecal occult blood tests for colorectal cancer screening in an Alaska Native population with high prevalence of Helicobacter pylori infection, 2008–2012. Prev Chronic Dis. 2014;11:E56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haverkamp D, Perdue DG, Espey D, Cobb N. A survey of Indian Health Service and tribal health providers’ colorectal cancer screening knowledge, perceptions, and practices. J Health Care Poor Underserved. 2011;22:243–57. [DOI] [PubMed] [Google Scholar]
- 29.Redwood D, Suryaprasad A, Haverkamp D, Wong C, Provost E, Espey D. Evaluating an Electronic Measure of Colorectal Cancer Screening at Indian Health Service Facilities, 2008–2010. IHS Prim Care Provid. 2014;39:86–93. [PMC free article] [PubMed] [Google Scholar]
- 30.McMahon BJ, Bruce MG, Hennessy TW, Bruden DL, Sacco F, Peters H, et al. Reinfection after successful eradication of Helicobacter pylori: a 2-year prospective study in Alaska Natives. Aliment Pharmacol Ther. 2006;23:1215–23. [DOI] [PubMed] [Google Scholar]
- 31.McMahon BJ, Bruce MG, Koch A, Goodman KJ, Tsukanov V, Mulvad G, et al. The diagnosis and treatment of Helicobacter pylori infection in Arctic regions with a high prevalence of infection: Expert Commentary. Epidemiol Infect. 2016;144:225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernini M, Barbi S, Roviello F, Scarpa A, Moore P, Pedrazzani C, et al. Family history of gastric cancer: a correlation between epidemiologic findings and clinical data. Gastric Cancer. 2006;9:9–13. [DOI] [PubMed] [Google Scholar]
- 34.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–77. [DOI] [PubMed] [Google Scholar]
- 35.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Elia L, Galletti F, Strazzullo P. Dietary salt intake and risk of gastric cancer. Cancer Treat Res. 2014;159:83–95. [DOI] [PubMed] [Google Scholar]
- 37.Nolen LD, Vindigni SM, Parsonnet J, Symposium L, Bruce MG, Martinson HA, et al. Combating gastric cancer in Alaska Native people: An expert and community symposium: Alaska Native Gastric Cancer Symposium. Gastroenterology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eom BW, Joo J, Kim S, Shin A, Yang HR, Park J, et al. Prediction Model for Gastric Cancer Incidence in Korean Population. PLoS One. 2015;10:e0132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sekiguchi M, Oda I, Taniguchi H, Suzuki H, Morita S, Fukagawa T, et al. Risk stratification and predictive risk-scoring model for lymph node metastasis in early gastric cancer. J Gastroenterol. 2016;51:961–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


