Abstract
Background:
Type 2 diabetes increases risk of developing colorectal cancer (CRC), but the association of pre-existing diabetes with CRC survival remains unclear.
Methods:
We analyzed survival by diabetes status at cancer diagnosis among 4038 patients with CRC from two prospective U.S. cohorts. Cox proportional hazards regression was used to calculate HRs and 95% confidence intervals (CIs) for overall and cause-specific mortality, with adjustment for tumor characteristics and lifestyle factors.
Results:
In the first 5 years after CRC diagnosis, diabetes was associated with a modest increase in overall mortality in women (HR, 1.22; 95% CI, 1.00–1.49), but not in men (HR, 0.83; 95% CI, 0.62–1.12; P heterogeneity by sex = 0.04). Beyond 5 years, diabetes was associated with substantially increased overall mortality with no evidence of sex heterogeneity; in women and men combined, the HRs were 1.45 (95% CI, 1.09–1.93) during >5 to 10 years and 2.58 (95% CI, 1.91–3.50) during >10 years. Compared with those without diabetes, CRC patients with diabetes had increased mortality from other malignancies (HR, 1.78; 95% CI, 1.18–2.67) and cardiovascular disease (HR, 1.93; 95% CI, 1.29–2.91). Only women with diabetes for more than 10 years had increased mortality from CRC (HR, 1.33; 95% CI, 1.01–1.76).
Conclusions:
Among patients with CRC, pre-existing diabetes was associated with increased risk of long-term mortality, particularly from other malignancies and cardiovascular disease.
Impact:
Our findings highlight the importance of cardioprotection and cancer prevention to CRC survivors with diabetes.
Introduction
Type 2 diabetes (henceforth referred to as “diabetes”) is known to increase risk of developing colorectal cancer (CRC), the second leading cause of cancer death in the United States (1). In a meta-analysis of 15 studies, individuals with diabetes had a relative risk for CRC of 1.30 [95% confidence interval (CI), 1.20–1.40], compared with those without diabetes (2). Insulin resistance and hyperinsulinemia that occurs in early diabetes have been proposed as one of the major mechanisms. Insulin stimulates growth of colonic epithelial and carcinoma cells in vitro and increases bioactive insulin-like growth factor 1 that can promote cell proliferation and inhibit apoptosis (3).
In contrast to the ample evidence linking diabetes to CRC development, only a few prospective cohort studies have been conducted to examine the association of diabetes with survival among patients with CRC (4, 5), leaving several questions unresolved. First, studies found shorter survival for CRC patients with diabetes, but it is unclear whether these patients had increased mortality from the CRC itself or from other diseases associated with diabetes (e.g., other malignancies, cardiovascular disease). Second, with improving screening and treatment programs, a large fraction of patients with CRC are achieving long-term survival, with a 5-year survival rate of more than 60% (1). However, few studies have delineated the association of diabetes with survival by time period after CRC diagnosis. Third, the natural history of diabetes begins with insulin resistance and hyperinsulinemia, followed by hypoinsulinemia in later stages due to β cell dysfunction; the association of diabetes duration with CRC survival remains unclear.
To address these questions, we examined the association of diabetes with overall and cause-specific mortality among 4038 participants diagnosed with CRC from two prospective U.S. cohorts, the Nurses’ Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS).
Materials and Methods
Study population
NHS was initiated in 1976 when 121,700 U.S. female nurses aged 30–55 years completed a mailed questionnaire describing demographics, lifestyle choices, and medical history (6). HPFS was initiated in 1986 when 51,529 U.S. men aged 40–75 years working in health professions completed a mailed questionnaire on health-related behaviors and medical history (7). Participants have provided updated information through biennial follow-up questionnaires. A high follow-up rate of more than 90% has been achieved in both cohorts.
The present study included 4038 participants with pathologically confirmed incident CRC diagnosed between 1976 and 2014. When a participant (or next of kin for decedents) reported a diagnosis of CRC on a follow-up questionnaire, we asked permission to obtain hospital records and pathology reports. For nonrespondents, the National Death Index was used to ascertain any diagnosis of CRC that contributed to death; we then asked permission from next of kin for decedents to obtain medical records. Study physicians reviewed these records to confirm the diagnosis and record information on important tumor characteristics. We estimate that 96–97% of patients were identified through these methods (8, 9).
The study protocol was approved by the Institutional Review Boards of the Brigham and Women’s Hospital (Boston, MA) and the Harvard T.H. Chan School of Public Health (Boston, MA), and those of participating registries as required. All participants provided written informed consent for the researchers to access their medical records. The study was conducted in concordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects (Council for International Organizations of Medical Sciences).
Diabetes status at CRC diagnosis
On baseline and biennial follow-up questionnaires, participants were asked if they had ever been diagnosed with diabetes by a physician. To verify the diagnosis, a supplementary questionnaire was subsequently sent to obtain details on the date of diagnosis, symptoms, diagnostic tests, and treatment. In both cohorts, the validity of the supplementary questionnaire to confirm self-reported diabetes was established by review of medical records (10, 11).
In the present study, diabetes status at CRC diagnosis was determined from participant report on biennial questionnaires together with the supplementary questionnaire. For those without a reported date of diabetes diagnosis, we used the return date of the biennial questionnaire on which they first reported being told by a physician that they had diabetes. Patients with diabetes were defined as those diagnosed with diabetes before or in the same month as CRC diagnosis; those with diabetes after CRC diagnosis were considered patients without diabetes. Categories of diabetes treatment included metformin, other oral medication, insulin injection, and neither oral medication nor insulin injection. Participants receiving metformin included those who used both metformin and other oral medication. Participants receiving insulin injection included those who initially used oral medication and switched to insulin injection.
Mortality outcomes
Death ascertainment included reporting by family or postal authorities, and searching for names of persistent nonrespondents in the National Death Index, which has been shown to capture approximately 98% of deaths (12). Participants not found to be deceased were assumed to be alive. Cause of death was assigned by investigators blinded to other data according to the International Classification of Diseases, Revision 8. The primary outcome was overall mortality, and the secondary outcomes included mortality from CRC, other primary malignancies, and cardiovascular disease.
Covariates
Cancer stage, grade of tumor differentiation, location of primary tumor, and year of diagnosis (as a surrogate for treatment) were extracted from medical records. Race and height were asked on the baseline questionnaire. Body weight, physical activity, cigarette smoking, and medication use (including dose and frequency of aspirin use) were surveyed every two years. Participants received additional questionnaires every four years to report their usual diet (including alcohol intake). In the present study, data on body weight, physical activity, dietary intakes, and aspirin use were obtained from the questionnaire returned before CRC diagnosis. The Alternate Healthy Eating Index-2010 (AHEI-2010) (13) was computed to measure overall diet quality. Regular aspirin users were defined as those who used aspirin at least 2 times per week, including standard and low-dose aspirin.
Statistical analyses
Follow-up time was calculated from CRC diagnosis to death or the end of follow-up (June 2014 for NHS; January 2014 for HPFS), whichever came first. Patients without diabetes were the main reference group for all analyses. The Kaplan–Meier method was used to calculate the 5-year survival rate and generate survival curves, with statistical significance evaluated by the log-rank test. Cox proportional hazards regression was used to calculate HRs and 95% CIs for overall and cause-specific mortality. In analyses with cause-specific mortality as the outcome, death from other causes was censored. To examine whether diabetes status can predict survival independent of tumor characteristics, the models were stratified by sex and cancer stage and adjusted for age at cancer diagnosis, grade of tumor differentiation, location of primary tumor, and year of diagnosis. To control for the lifestyle factors that may affect survival, the models were additionally adjusted for body mass index, physical activity, smoking status, alcohol intake, AHEI-2010, and regular aspirin use.
We tested whether the association of diabetes with overall and cause-specific mortality changed over time, by evaluating the product of diabetes status and follow-up time as a continuous variable. The assumption was violated for overall mortality (P = 0.009 in women and 0.02 in men), which was addressed by including in the model interaction terms between diabetes status and follow-up periods including ≤5, >5 to 10, and >10 years (the assumption was satisfied within each follow-up period with P ≥ 0.46). In contrast, we did not find evidence that the association of diabetes with any cause-specific mortality changed over time (P ≥ 0.47).
Heterogeneity by sex was tested by Cochran’s Q statistic (14). We examined whether the association of diabetes with 5-year overall mortality and CRC-specific mortality differed by age at cancer diagnosis, body mass index, cancer stage, grade of tumor differentiation, and location of primary tumor. These analyses were conducted by entering the cross product of diabetes status and the potential effect modifier in the model, evaluated by the likelihood ratio test. All analyses were performed with SAS Software, version 9.4 (SAS Institute). All P values are two-sided.
Results
Baseline characteristics
Among 4038 patients with CRC (65.2% female), 429 (10.6%) had diabetes at cancer diagnosis. Baseline characteristics according to diabetes status are shown in Table 1. Compared with those without diabetes, CRC patients with diabetes were older, had a higher body mass index, were less physically active, consumed less alcohol, and were less likely to be current smokers (particularly in women) and more likely to use aspirin.
Table 1.
Baseline characteristics of patients with CRC by diabetes status at cancer diagnosis.
| Characteristic | Women | Men | Combined | |||
|---|---|---|---|---|---|---|
| No diabetes | Diabetes | No diabetes | Diabetes | No diabetes | Diabetes | |
| No. of patients | 2349 | 284 | 1260 | 145 | 3609 | 429 |
| Age at cancer diagnosis, mean (SD), years | 67.1 (10.2) | 71.4 (8.7) | 71.0 (9.6) | 74.8 (8.5) | 68.5 (10.1) | 72.5 (8.8) |
| Race, no. (%) | ||||||
| White | 2294 (97.7) | 274 (96.5) | 1145 (90.9) | 133 (91.7) | 3439 (95.3) | 407 (94.9) |
| Nonwhite | 55 (2.3) | 10 (3.5) | 46 (3.7) | 9 (6.2) | 101 (2.8) | 19 (4.4) |
| Unknown | 0 | 0 | 69 (5.5) | 3 (2.1) | 69 (1.9) | 3 (0.7) |
| Cancer stage, no. (%) | ||||||
| I | 504 (21.5) | 57 (20.1) | 336 (26.7) | 30 (20.7) | 840 (23.3) | 87 (20.3) |
| II | 595 (25.3) | 70 (24.6) | 228 (18.1) | 21 (14.5) | 823 (22.8) | 91 (21.2) |
| III | 555 (23.6) | 62 (21.8) | 227 (18.0) | 35 (24.1) | 782 (21.7) | 97 (22.6) |
| IV | 406 (17.3) | 47 (16.5) | 193 (15.3) | 14 (9.7) | 599 (16.6) | 61 (14.2) |
| Unknown | 289 (12.3) | 48 (16.9) | 276 (21.9) | 45 (31.0) | 565 (15.7) | 93 (21.7) |
| Grade of tumor differentiation, no. (%) | ||||||
| Well differentiated | 307 (13.1) | 25 (8.8) | 139 (11.0) | 16 (11.0) | 446 (12.4) | 41 (9.6) |
| Moderately differentiated | 1318 (56.1) | 166 (58.5) | 588 (46.7) | 61 (42.1) | 1906 (52.8) | 227 (52.9) |
| Poorly differentiated | 447 (19.0) | 59 (20.8) | 150 (11.9) | 22 (15.2) | 597 (16.5) | 81 (18.9) |
| Unknown | 277 (11.8) | 34 (12.0) | 383 (30.4) | 46 (31.7) | 660 (18.3) | 80 (18.6) |
| Location of primary tumor, no. (%) | ||||||
| Proximal colon | 1093 (46.5) | 147 (51.8) | 431 (34.2) | 56 (38.6) | 1524 (42.2) | 203 (47.3) |
| Distal colon | 693 (29.5) | 83 (29.2) | 366 (29.0) | 26 (17.9) | 1059 (29.3) | 109 (25.4) |
| Rectum | 504 (21.5) | 47 (16.5) | 265 (21.0) | 27 (18.6) | 769 (21.3) | 74 (17.2) |
| Unknown | 59 (2.5) | 7 (2.5) | 198 (15.7) | 36 (24.8) | 257 (7.1) | 43 (10.0) |
| Body mass index, mean (SD), kg/m2 | 25.9 (5.1) | 29.9 (5.8) | 26.1 (3.6) | 27.8 (4.3) | 26.0 (4.6) | 29.2 (5.4) |
| Physical activity, mean (SD), MET-hours/week | 16.5 (26.9) | 10.4 (14.5) | 27.5 (30.7) | 19.9 (22.8) | 20.8 (28.9) | 13.8 (18.5) |
| Current smoker, no. (%) | 356 (15.2) | 21 (7.4) | 76 (6.0) | 8 (5.5) | 432 (12.0) | 29 (6.8) |
| Alcohol intake, mean (SD), g/d | 6.4 (11.1) | 2.3 (5.6) | 13.5 (17.3) | 10.3 (14.6) | 9.0 (14.1) | 5.2 (10.5) |
| AHEI-2010, mean (SD) | 53.7 (11.2) | 54.4 (11.3) | 55.7 (12.1) | 55.7 (11.2) | 54.5 (11.6) | 54.8 (11.3) |
| Regular aspirin use, ≥2 times/week, no. (%) | 807 (34.4) | 120 (42.3) | 550 (43.7) | 81 (55.9) | 1357 (37.6) | 201 (46.9) |
| Median overall survival by cancer stage, years | ||||||
| I | 25.9 | 12.6 | 16.7 | 11.3 | 22.1 | 12.5 |
| II | 18.1 | 11.8 | 12.5 | 8.1 | 16.9 | 10.6 |
| III | 8.8 | 5.7 | 8.7 | 8.2 | 8.8 | 6.3 |
| IV | 0.9 | 0.8 | 0.9 | 1.1 | 0.9 | 0.8 |
| Unknown | 6.5 | 2.4 | 7.2 | 5.2 | 6.8 | 3.7 |
Abbreviation: MET, metabolic equivalent.
Causes of death
The median follow-up among patients who were alive at the end of follow-up was 12.6 years (interquartile range, 6.6–18.6 years). During the follow-up period, we documented 2440 deaths, 1450 (59.4%) of which were due to CRC. Non-CRC causes of death included other primary malignancies (n = 244), cardiovascular disease (n = 219), neurological disorders (n = 113), respiratory disease (n = 90), cerebrovascular disease (n = 78), and other or unknown reasons (n = 246). The 244 deaths from other malignancies included 59 deaths from lung cancer, 37 from hematologic cancers, 36 from other gastrointestinal cancers, 36 from genitourinary cancers, 28 from breast cancer, 8 from skin cancer, 5 from brain cancer, and 35 from cancers of other or unspecified sites. A summary of causes of death according to follow-up period is presented in Supplementary Table S1. During ≤5, >5 to 10, and >10 years after diagnosis, CRC accounted for 83.8%, 33.6%, and 11.1% of deaths, respectively.
Association between pre-existing diabetes and overall mortality
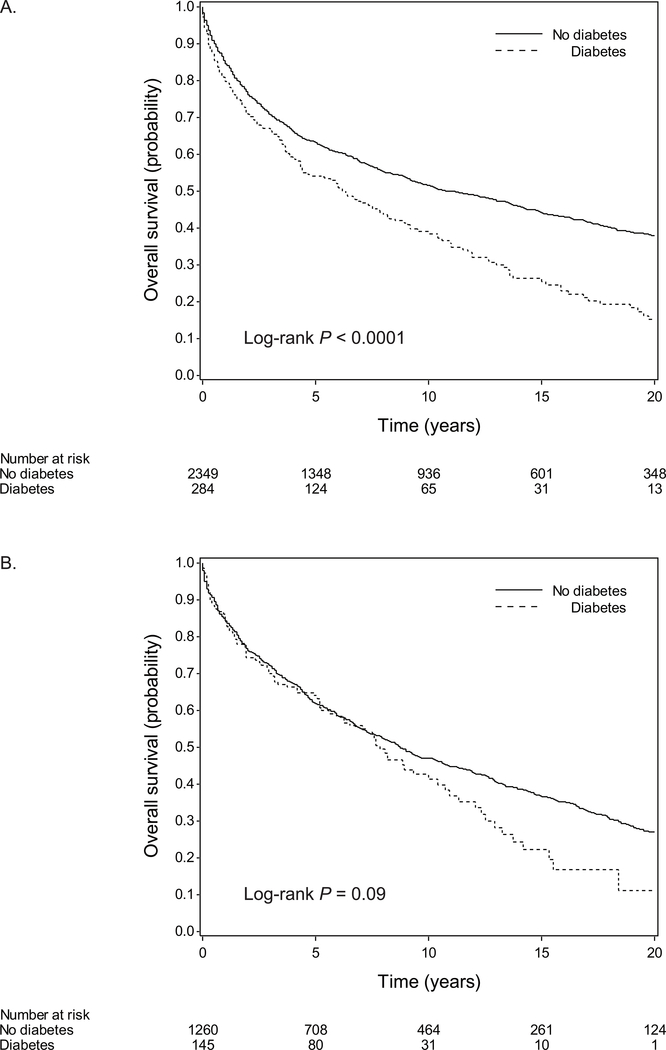
The overall survival curves by diabetes status are shown in Figure 1 (log-rank P < 0.0001 in women and = 0.09 in men). The median overall survival for patients with and without diabetes was 6.3 and 11.1 years in women, and 7.8 and 8.8 years in men, respectively (Table 2). In the first 5 years after CRC diagnosis, diabetes was associated with increased overall mortality in women (HR, 1.22; 95% CI, 1.00–1.49), but not in men (HR, 0.83; 95% CI, 0.62–1.12; P heterogeneity by sex = 0.04) (Table 2). The association between diabetes and 5-year overall mortality did not differ significantly by age at cancer diagnosis, cancer stage, grade of tumor differentiation, location of primary tumor, or body mass index (Supplementary Table S2). Beyond 5 years, diabetes was associated with increased overall mortality with no evidence of sex heterogeneity (P heterogeneity ≥ 0.50); in women and men combined, the HRs were 1.45 (95% CI, 1.09–1.93) during >5 to 10 years and 2.58 (95% CI, 1.91–3.50) during >10 years (Table 2).
Figure 1.
Overall survival by diabetes status at CRC diagnosis in (A) women and (B) men.
Table 2.
HRs for overall mortality by diabetes status at CRC diagnosis, stratified by follow-up period after diagnosis.
| Overall mortality | Women | Men | Combined | |||
|---|---|---|---|---|---|---|
| No diabetes | Diabetes | No diabetes | Diabetes | No diabetes | Diabetes | |
| No. of patients | 2349 | 284 | 1260 | 145 | 3609 | 429 |
| Median overall survival, years | 11.1 | 6.3 | 8.8 | 7.8 | 10.0 | 7.0 |
| % alive at 5 years | 63.0 | 54.2 | 61.8 | 64.0 | 62.6 | 57.6 |
| Follow-up period | ||||||
| ≤5 years | ||||||
| No. of deaths | 845 | 122 | 467 | 51 | 1312 | 173 |
| Age-adjusted HR (95% CI) | 1 | 1.19 (0.99–1.44) | 1 | 0.78 (0.58–1.04) | 1 | NAc |
| Multivariate HR (95% CI)a | 1 | 1.24 (1.03–1.51) | 1 | 0.86 (0.64–1.15) | 1 | NAc |
| Multivariate HR (95% CI)b | 1 | 1.22 (1.00–1.49) | 1 | 0.83 (0.62–1.12) | 1 | NAc |
| >5 to 10 years | ||||||
| No. of deaths | 229 | 31 | 159 | 24 | 388 | 55 |
| Age-adjusted HR (95% CI) | 1 | 1.57 (1.08–2.28) | 1 | 1.31 (0.85–2.02) | 1 | 1.48 (1.11–1.96) |
| Multivariate HR (95% CI)a | 1 | 1.58 (1.08–2.30) | 1 | 1.30 (0.84–2.01) | 1 | 1.49 (1.12–1.98) |
| Multivariate HR (95% CI)b | 1 | 1.56 (1.06–2.28) | 1 | 1.28 (0.82–1.98) | 1 | 1.45 (1.09–1.93)d |
| >10 years | ||||||
| No. of deaths | 286 | 35 | 177 | 14 | 463 | 49 |
| Age-adjusted HR (95% CI) | 1 | 2.67 (1.87–3.80) | 1 | 2.23 (1.28–3.86) | 1 | 2.39 (1.78–3.22) |
| Multivariate HR (95% CI)a | 1 | 2.71 (1.90–3.88) | 1 | 2.27 (1.29–3.99) | 1 | 2.57 (1.90–3.47) |
| Multivariate HR (95% CI)b | 1 | 2.77 (1.93–3.98) | 1 | 2.22 (1.26–3.91) | 1 | 2.58 (1.91–3.50)d |
Abbreviation: NA, not available.
Stratified by sex (in women and men combined) and cancer stage (I to IV or unknown) and adjusted for age at cancer diagnosis (continuous), race (White, nonwhite, or unknown), grade of tumor differentiation (well differentiated, moderately differentiated, poorly differentiated, or unknown), location of primary tumor (proximal colon, distal colon, rectum, or unknown), and year of diagnosis (continuous).
Additionally adjusted for body mass index (continuous), physical activity (continuous), smoking status (never, past, or current), alcohol intake (continuous), AHEI-2010 (continuous), and regular aspirin use (yes or no).
HRs not shown due to sex heterogeneity (P heterogeneity by sex = 0.04 in the full model).
P heterogeneity by sex = 0.50 and 0.52 during >5 to 10 and >10 years, respectively.
Association between pre-existing diabetes and cause-specific mortality
The association between diabetes and cause-specific mortality did not differ significantly by sex (P heterogeneity = 0.10, 0.28, and 0.88 for mortality from CRC, other malignancies, and cardiovascular disease, respectively; Table 3). In women and men combined, diabetes was not associated with CRC-specific mortality (HR, 1.09; 95% CI, 0.91–1.30), but associated with increased mortality from other malignancies (HR, 1.78; 95% CI, 1.18–2.67) and cardiovascular disease (HR, 1.93; 95% CI, 1.29–2.91). Nonetheless, a non-significant increase in CRC-specific mortality was noted for women with diabetes (HR, 1.21; 95% CI, 0.98–1.49). The association between diabetes and CRC-specific mortality did not differ significantly by age at cancer diagnosis, cancer stage, grade of tumor differentiation, location of primary tumor, or body mass index (Supplementary Table S3).
Table 3.
HRs for cause-specific mortality by diabetes status at CRC diagnosis.
| Cause of death | Women | Men | Combined | |||
|---|---|---|---|---|---|---|
| No diabetes | Diabetes | No diabetes | Diabetes | No diabetes | Diabetes | |
| No. of patients | 2349 | 284 | 1260 | 145 | 3609 | 429 |
| CRC | ||||||
| No. of deaths | 865 | 107 | 433 | 45 | 1298 | 152 |
| Age-adjusted HR (95% CI) | 1 | 1.19 (0.97–1.45) | 1 | 0.85 (0.63–1.16) | 1 | 1.07 (0.90–1.27) |
| Multivariate HR (95% CI)a | 1 | 1.24 (1.01–1.52) | 1 | 0.89 (0.65–1.23) | 1 | 1.11 (0.94–1.32) |
| Multivariate HR (95% CI)b | 1 | 1.21 (0.98–1.49) | 1 | 0.88 (0.64–1.21) | 1 | 1.09 (0.91–1.30)c |
| Other malignancies | ||||||
| No. of deaths | 142 | 23 | 72 | 7 | 214 | 30 |
| Age-adjusted HR (95% CI) | 1 | 2.02 (1.29–3.15) | 1 | 1.06 (0.49–2.34) | 1 | 1.67 (1.13–2.45) |
| Multivariate HR (95% CI)a | 1 | 2.00 (1.27–3.13) | 1 | 1.18 (0.52–2.63) | 1 | 1.69 (1.14–2.50) |
| Multivariate HR (95% CI)b | 1 | 2.15 (1.34–3.46) | 1 | 1.28 (0.56–2.91) | 1 | 1.78 (1.18–2.67)c |
| Cardiovascular disease | ||||||
| No. of deaths | 62 | 13 | 126 | 18 | 188 | 31 |
| Age-adjusted HR (95% CI) | 1 | 2.61 (1.42–4.81) | 1 | 1.60 (0.96–2.66) | 1 | 1.88 (1.27–2.77) |
| Multivariate HR (95% CI)a | 1 | 2.70 (1.46–4.99) | 1 | 1.88 (1.10–3.21) | 1 | 2.19 (1.46–3.26) |
| Multivariate HR (95% CI)b | 1 | 1.94 (1.01–3.73) | 1 | 1.82 (1.07–3.12) | 1 | 1.93 (1.29–2.91)c |
Stratified by sex (in women and men combined) and cancer stage (I to IV or unknown) and adjusted for age at cancer diagnosis (continuous), race (White, nonwhite, or unknown), grade of tumor differentiation (well differentiated, moderately differentiated, poorly differentiated, or unknown), location of primary tumor (proximal colon, distal colon, rectum, or unknown), and year of diagnosis (continuous).
Additionally adjusted for body mass index (continuous), physical activity (continuous), smoking status (never, past, or current), alcohol intake (continuous), AHEI-2010 (continuous), and regular aspirin use (yes or no).
P heterogeneity by sex = 0.10, 0.28, and 0.88 for mortality from CRC, other malignancies, and cardiovascular disease, respectively.
Association with duration of pre-existing diabetes
We analyzed overall and cause-specific mortality by diabetes duration at CRC diagnosis (≤10 or >10 years) in women and men separately, and found increased mortality with longer diabetes duration in women. Compared with those without diabetes, women with diabetes for more than 10 years had increased overall mortality during all follow-up periods, with HRs of 1.39 (95% CI, 1.08–1.78) during ≤5 years, 2.30 (95% CI, 1.39–3.78) during >5 to 10 years, and 4.21 (95% CI, 2.27–7.79) during >10 years (Table 4). These women also had increased mortality from CRC (HR, 1.33; 95% CI, 1.01–1.76), other malignancies (HR, 2.66; 95% CI, 1.39–5.09), and cardiovascular disease (HR, 2.54; 95% CI, 1.09–5.89; Supplementary Table S4).
Table 4.
HRs for overall mortality by diabetes duration at CRC diagnosis, stratified by follow-up period after diagnosis.
| Overall mortality | Women | Men | ||||
|---|---|---|---|---|---|---|
| No diabetes | ≤10-year diabetes | >10-year diabetes | No diabetes | ≤10-year diabetes | >10-year diabetes | |
| No. of patients | 2349 | 148 | 136 | 1260 | 82 | 63 |
| Median overall survival, years | 11.1 | 10.0 | 4.3 | 8.8 | 10.4 | 5.2 |
| % alive at 5 years | 63.0 | 62.1 | 45.4 | 61.8 | 71.6 | 53.3 |
| Follow-up period | ||||||
| ≤5 years | ||||||
| No. of deaths | 845 | 53 | 69 | 467 | 23 | 28 |
| Age-adjusted HR (95% CI) | 1 | 1.00 (0.75–1.31) | 1.42 (1.11–1.81) | 1 | 0.63 (0.42–0.96) | 0.97 (0.66–1.43) |
| Multivariate HR (95% CI)a | 1 | 1.10 (0.83–1.45) | 1.39 (1.09–1.78) | 1 | 0.74 (0.48–1.12) | 1.00 (0.67–1.47) |
| Multivariate HR (95% CI)b | 1 | 1.06 (0.79–1.40) | 1.39 (1.08–1.78) | 1 | 0.72 (0.47–1.10) | 0.96 (0.65–1.41) |
| >5 to 10 years | ||||||
| No. of deaths | 229 | 14 | 17 | 159 | 14 | 10 |
| Age-adjusted HR (95% CI) | 1 | 1.11 (0.65–1.91) | 2.36 (1.44–3.87) | 1 | 1.15 (0.67–1.99) | 1.63 (0.86–3.10) |
| Multivariate HR (95% CI)a | 1 | 1.12 (0.65–1.92) | 2.41 (1.47–3.95) | 1 | 1.19 (0.69–2.07) | 1.51 (0.78–2.91) |
| Multivariate HR (95% CI)b | 1 | 1.12 (0.65–1.93) | 2.30 (1.39–3.78) | 1 | 1.17 (0.67–2.03) | 1.48 (0.77–2.86) |
| >10 years | ||||||
| No. of deaths | 286 | 24 | 11 | 177 | 13 | 1 |
| Age-adjusted HR (95% CI) | 1 | 2.32 (1.52–3.52) | 4.04 (2.20–7.42) | 1 | 2.41 (1.37–4.26) | NAc |
| Multivariate HR (95% CI)a | 1 | 2.37 (1.55–3.61) | 4.01 (2.18–7.40) | 1 | 2.46 (1.37–4.41) | NAc |
| Multivariate HR (95% CI)b | 1 | 2.40 (1.57–3.67) | 4.21 (2.27–7.79) | 1 | 2.41 (1.34–4.32) | NAc |
Abbreviation: NA, not available.
Stratified by cancer stage (I to IV or unknown) and adjusted for age at cancer diagnosis (continuous), race (White, nonwhite, or unknown), grade of tumor differentiation (well differentiated, moderately differentiated, poorly differentiated, or unknown), location of primary tumor (proximal colon, distal colon, rectum, or unknown), and year of diagnosis (continuous).
Additionally adjusted for body mass index (continuous), physical activity (continuous), smoking status (never, past, or current), alcohol intake (continuous), AHEI-2010 (continuous), and regular aspirin use (yes or no).
HRs not shown due to the small number of patients in the risk set (n = 6).
In men with CRC, a clear association with diabetes duration was not evident. Men with diabetes for more than 10 years did not have a significant increase in overall or any cause-specific mortality, compared with those without diabetes (Table 4 and Supplementary Table S4).
Association with treatment of pre-existing diabetes
Although somewhat limited by sample size, we analyzed 5-year overall and CRC-specific mortality by diabetes treatment in women and men combined (Supplementary Table S5). Compared with those without diabetes, those with diabetes receiving metformin had decreased 5-year overall mortality (HR, 0.52; 95% CI, 0.30–0.91), and those receiving neither oral medication nor insulin injection had increased 5-year overall mortality (HR, 1.68; 95% CI, 1.17–2.41). Similar associations with CRC-specific mortality were noted.
Discussion
Among the 4038 patients with CRC from two prospective U.S. cohorts, pre-existing diabetes was associated with a modest increase in overall mortality in women, but not in men, during the first 5 years after cancer diagnosis. Beyond 5 years, diabetes was associated with substantially increased overall mortality with no evidence of sex heterogeneity. The excess mortality was largely caused by cardiovascular disease and malignancies other than CRC. Only women with diabetes for more than 10 years had increased mortality from CRC.
To date, many studies have evaluated the association between pre-existing diabetes and survival among patients with CRC. The vast majority of these studies were hospital-based and enrolled a small number of patients. Only a few population-based studies have been conducted (15), most of which identified patients with CRC from cancer registries or primary care data. These studies generally lacked information on lifestyle factors that have been associated with CRC survival, such as excess body weight, cigarette smoking, alcohol intake, and aspirin use (16). Given the link between unhealthy lifestyle behaviors and diabetes, multivariate modeling is necessary to evaluate the independent association between diabetes and patient survival. Another issue in these studies relates to the completeness and accuracy of the linked databases to ascertain diabetes status, such as the Medicare claims data that were shown to capture only 69% of people with diabetes (17).
To our knowledge, only two prospective cohort studies have been conducted to examine the association between diabetes and survival among patients with all stages of CRC with adjustment for lifestyle factors (4, 5). Among 2278 patients with CRC from the Cancer Prevention Study-II Nutrition Cohort, those with diabetes had increased risk of overall mortality (HR, 1.53; 95% CI, 1.23–1.83), CRC-specific mortality (HR, 1.29; 95% CI, 0.98–1.70), and cardiovascular disease-specific mortality (HR, 2.16; 95% CI, 1.44–3.24) (4). Among 3913 patients within the Multiethnic Cohort Study in California and Hawaii, only diabetes that existed for at least 10 years was associated with overall (HR, 1.49; 95% CI, 1.22–1.82) and CRC-specific mortality (HR, 1.48; 95% CI, 1.06–2.07) (5).
The present study found a time-varying association between diabetes and overall mortality after CRC diagnosis. In the first 5 years, women with CRC and diabetes had an estimated 22% higher risk of overall mortality, but the mortality was not elevated in men. Beyond 5 years, diabetes was associated with a much greater increase in overall mortality with no evidence of sex heterogeneity. One explanation is that diabetes was more strongly associated with mortality from non-CRC diseases, such as other malignancies and cardiovascular disease, which accounted for the majority of deaths beyond 5 years. Another factor is diabetes duration that increased with follow-up and was positively associated with mortality in the present study.
We found increased CRC-specific mortality for women with diabetes for more than 10 years. If hyperinsulinemia that occurs in the early stage of diabetes accounts for this association, we would expect a similar or greater increase in the mortality among those with diabetes for a shorter period, which we did not find. Moreover, a previous study conducted in the same cohorts found no association between diabetes duration and risk for CRC development (18). Together, these data do not support a strong role of hyperinsulinemia in the relationship between diabetes and colorectal carcinogenesis.
The somewhat stronger association between diabetes and mortality in female patients with CRC is a novel finding and requires confirmation in future studies. One possible explanation is that glycemic control for diabetics is generally worse in women than in men (19). Studies also found that women were more likely to receive less aggressive treatment for diabetes (20) and had a lower adherence to glucose-lowering medication (21) than men. Hyperglycemia can contribute to cancer progression by affecting cancer cell behaviors, including proliferation, apoptosis, migration, and invasion, and by enhancing chemotherapy resistance and intolerance (22, 23). The negative influence of hyperglycemia on CRC survival was supported by our observation that CRC patients with diabetes who used neither insulin injection nor oral medication for glycemic control had the worst survival.
Our observation that CRC survivors with diabetes had increased mortality from other malignancies has not been previously reported but is biologically plausible. Diabetes has been associated with increased risk (24, 25) and decreased survival (26–28) of several cancers (e.g., liver, pancreas, endometrium, breast, prostate). Notably, the nearly 1.8 times higher risk of mortality from other malignancies is more pronounced than the association between diabetes and cancer mortality in the general population with no CRC (typically <1.3 times) (29–31). CRC development is known to be associated with multiple diet and lifestyle factors, which also contribute to morbidity and mortality of other cancers (32) and may account for the greater association between diabetes and cancer mortality among patients with CRC. Regardless of the underlying mechanisms, our findings suggest that prevention of other malignancies should be an important concern for CRC survivors with diabetes.
Consistent with previous reports (4, 33), we found that CRC patients with diabetes had substantially increased mortality from cardiovascular disease. Diabetes and cardiovascular disease are closely linked, as several of the risk factors for cardiovascular disease (e.g., obesity, hypertension, hyperglycemia, dyslipidemia) are common in people with diabetes (34). In the general population, cardiovascular disease is the major cause of morbidity and mortality among people with diabetes, so reducing risk factors for cardiovascular disease is a critical part of diabetes management. While the present study included patients diagnosed with CRC as early as the 1970s, recent reports demonstrate that owing to effective treatment for diabetes, there has been a substantial decline in cardiovascular disease mortality among people with diabetes over the past decades (35, 36).
This study has several strengths, including the prospective design, long follow-up time, high follow-up rates, biennial self-report on diabetes status and subsequent confirmation, as well as detailed information on tumor characteristics and lifestyle factors. Limitations of this study also require consideration. We did not control for differences in CRC treatment, because this information was not systematically collected in our cohorts. We cannot exclude that CRC patients with diabetes may have been treated less aggressively for CRC (37) and therefore had worse survival. Our study participants were predominantly White, and studies in other racial populations are warranted.
In conclusion, our study suggests that among patients with CRC, pre-existing diabetes is associated with increased risk of long-term mortality, particularly from other malignancies and cardiovascular disease. These findings highlight the importance of cardioprotection and prevention of other malignancies to CRC survivors with diabetes.
Supplementary Material
Acknowledgments:
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-Up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Financial Support: The Nurses’ Health Study is supported by the National Institutes of Health (NIH) grants UM1 CA186107 and P01 CA87969. The Health Professionals Follow-Up Study is supported by the NIH grant U01 CA167552. This work was additionally supported by the Pussycat Foundation Helen Gurley Brown Presidential Initiative to C.Y. and K.N.; by the NIH grant K07 CA188126 and American Cancer Society Research Scholar Grant RSG NEC-130476 to X.Z.; by the NIH grants R01 CA137178 and K24 DK098311 and the Damon Runyon Cancer Research Foundation to A.T.C.; by the NIH grant P50 CA127003 to C.S.F.; by the NIH grant R35 CA197735 to S.O.; by the NIH grants K07 CA148894 and R01 CA205406 and the Project P Fund to K.N.; and by the Entertainment Industry Foundation’s National Colorectal Cancer Research Alliance (NCCRA).
Potential Competing Interests:
B.M.W. declares research funding from Celgene and Eli Lilly and Company and consulting for BioLineRx, Celgene, G1 Therapeutics, and GRAIL. J.A.M. declares research funding from Boston Biomedical and consulting for Cota Healthcare, Ignyta, and Taiho Pharmaceutical. A.T.C. declares research funding from Bayer and consulting for Bayer and Pfizer. C.S.F. declares consulting for Agios, Bain Capital, Bayer, Celgene, Dicerna Pharmaceuticals, Eli Lilly and Company, Entrinsic Health Solutions, Five Prime Therapeutics, Genentech, Gilead Sciences, KEW, Merck & Co., Merrimack Pharmaceuticals, Pfizer, Sanofi, Taiho Pharmaceutical, and Unum Therapeutics. He also serves as a Director for CytomX Therapeutics and owns unexercised stock options for CytomX Therapeutics and Entrinsic Health Solutions. K.N. declares research funding from Evergrande Group, Genentech, Gilead Sciences, Pharmavite, Revolution Medicines, Tarrex Biopharma, and Trovagene; advisory board participation for Array Biopharma, Bayer, Eli Lilly and Company, Genentech, and Seattle Genetics; and consulting for Tarrex Biopharma. Other authors declare no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: a cancer journal for clinicians. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. Journal of the National Cancer Institute. 2005;97:1679–87. [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E Insulin, insulin-like growth factors and colon cancer: a review of the evidence. The Journal of nutrition. 2001;131:3109S–20S. [DOI] [PubMed] [Google Scholar]
- 4.Dehal AN, Newton CC, Jacobs EJ, Patel AV, Gapstur SM, Campbell PT. Impact of diabetes mellitus and insulin use on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:53–9. [DOI] [PubMed] [Google Scholar]
- 5.Amshoff Y, Maskarinec G, Shvetsov YB, Raquinio PH, Grandinetti A, Setiawan VW, et al. Type 2 diabetes and colorectal cancer survival: The multiethnic cohort. International journal of cancer Journal international du cancer. 2018;143:263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. Journal of women’s health. 1997;6:49–62. [DOI] [PubMed] [Google Scholar]
- 7.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–8. [DOI] [PubMed] [Google Scholar]
- 8.Giovannucci E, Colditz GA, Stampfer MJ, Hunter D, Rosner BA, Willett WC, et al. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. women. Journal of the National Cancer Institute. 1994;86:192–9. [DOI] [PubMed] [Google Scholar]
- 9.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Kearney J, et al. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. men. Journal of the National Cancer Institute. 1994;86:183–91. [DOI] [PubMed] [Google Scholar]
- 10.Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338:774–8. [DOI] [PubMed] [Google Scholar]
- 11.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Archives of internal medicine. 2001;161:1542–8. [DOI] [PubMed] [Google Scholar]
- 12.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. American journal of epidemiology. 1994;140:1016–9. [DOI] [PubMed] [Google Scholar]
- 13.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 15.Zhu B, Wu X, Wu B, Pei D, Zhang L, Wei L. The relationship between diabetes and colorectal cancer prognosis: A meta-analysis based on the cohort studies. PloS one. 2017;12:e0176068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Zutphen M, Kampman E, Giovannucci EL, van Duijnhoven FJB. Lifestyle after Colorectal Cancer Diagnosis in Relation to Survival and Recurrence: A Review of the Literature. Curr Colorectal Cancer Rep. 2017;13:370–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorina Y, Kramarow EA. Identifying chronic conditions in Medicare claims data: evaluating the Chronic Condition Data Warehouse algorithm. Health Serv Res. 2011;46:1610–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Y, Yang W, Song M, Smith-Warner SA, Yang J, Li Y, et al. Type 2 diabetes and risk of colorectal cancer in two large U.S. prospective cohorts. British journal of cancer. 2018;119:1436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Legato MJ, Gelzer A, Goland R, Ebner SA, Rajan S, Villagra V, et al. Gender-specific care of the patient with diabetes: review and recommendations. Gender medicine. 2006;3:131–58. [DOI] [PubMed] [Google Scholar]
- 20.Kautzky-Willer A, Kamyar MR, Gerhat D, Handisurya A, Stemer G, Hudson S, et al. Sex-specific differences in metabolic control, cardiovascular risk, and interventions in patients with type 2 diabetes mellitus. Gender medicine. 2010;7:571–83. [DOI] [PubMed] [Google Scholar]
- 21.Kirkman MS, Rowan-Martin MT, Levin R, Fonseca VA, Schmittdiel JA, Herman WH, et al. Determinants of adherence to diabetes medications: findings from a large pharmacy claims database. Diabetes care. 2015;38:604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryu TY, Park J, Scherer PE. Hyperglycemia as a risk factor for cancer progression. Diabetes & metabolism journal. 2014;38:330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan W, Shen X, Lei J, Xu Q, Yu Y, Li R, et al. Hyperglycemia, a neglected factor during cancer progression. BioMed research international. 2014;2014:461917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes care. 2010;33:1674–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Y, Zhang X, Ma Y, Yuan C, Wang M, Wu K, et al. Incident type 2 diabetes duration and cancer risk: A prospective study in two US cohorts. Journal of the National Cancer Institute. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes care. 2012;35:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan C, Rubinson DA, Qian ZR, Wu C, Kraft P, Bao Y, et al. Survival among patients with pancreatic cancer and long-standing or recent-onset diabetes mellitus. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. Jama. 2008;300:2754–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Wu F, Saito E, Lin Y, Song M, Luu HN, et al. Association between type 2 diabetes and risk of cancer mortality: a pooled analysis of over 771,000 individuals in the Asia Cohort Consortium. Diabetologia. 2017;60:1022–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. The New England journal of medicine. 2011;364:829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes care. 2012;35:1835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA: a cancer journal for clinicians. 2018;68:31–54. [DOI] [PubMed] [Google Scholar]
- 33.Walker JJ, Brewster DH, Colhoun HM, Fischbacher CM, Lindsay RS, Wild SH. Cause-specific mortality in Scottish patients with colorectal cancer with and without type 2 diabetes (2000–2007). Diabetologia. 2013;56:1531–41. [DOI] [PubMed] [Google Scholar]
- 34.Leon BM, Maddox TM. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World journal of diabetes. 2015;6:1246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rawshani A, Franzen S, Eliasson B, Svensson AM, Miftaraj M, McGuire DK, et al. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. The New England journal of medicine. 2017;376:1407–18. [DOI] [PubMed] [Google Scholar]
- 36.Preis SR, Hwang SJ, Coady S, Pencina MJ, D’Agostino RB Sr., Savage PJ, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009;119:1728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. International journal of cancer. 2007;120:1986–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.