Abstract
Prediabetes is an intermediate stage between normal glycemia and diabetes and is highly prevalent, especially in older age groups and obese individuals. Five different definitions of prediabetes are presently used in current practice, which are based on different cut points of HbA1C, fasting glucose, and 2-h glucose. A major challenge for the field is a lack of guidance on when one definition might be preferred over another. Risks of major complications in persons with prediabetes, including diabetes, cardiovascular disease, kidney disease, and death, also vary depending on the prediabetes definition used. Randomized clinical trials have demonstrated that lifestyle and pharmacologic interventions can be cost-effective, prevent diabetes, and improve cardiovascular risk factors in adults with prediabetes. However, the practical implementation of lifestyle modification or the use of metformin for treating prediabetes is inadequate and complicated by a lack of agreement on how to define the condition. Establishing consensus definitions for prediabetes should be a priority and will help inform expansion of insurance coverage for lifestyle modification and improve current screening and diagnostic practices.
Keywords: prediabetes, impaired glucose tolerance, impaired fasting glucose, epidemiology
INTRODUCTION
Prediabetes refers to an intermediate stage of dysglycemia along the continuum from normoglycemia to diabetes (3). Prediabetes is identified by laboratory measurement of fasting blood glucose (FBG), glycosylated hemoglobin (HbA1C), or 2-h postload blood glucose (2hBG) (3). The term prediabetes is used to identify those individuals who are at risk for future diabetes, but prediabetes is also associated with a high burden of cardiometabolic risk factors and is associated with poor outcomes (4). The increasing prevalence of prediabetes globally is a major public health concern and does not bode well for the growing epidemic of diabetes and its complications. The natural history of the condition is well documented, its detection can be straightforward, and evidence for its effective treatment has accumulated over the past two decades (56, 81, 86, 108). However, there is controversy regarding the optimal definition of prediabetes and active recognition and treatment of prediabetes has lagged, as clinicians may fail to see it as a disease state that needs addressing.
This review aims to describe the epidemiology of prediabetes and discusses current challenges for the field. We focus on evidence from surveys investigating the prevalence of prediabetes, observational studies of the association of prediabetes with major clinical outcomes, and intervention studies including randomized clinical trials of therapies for prediabetes and discuss current approaches to prediabetes in clinical practice. This summary should help inform the process of translating the current evidence into public health and clinical policies for diabetes prevention.
EPIDEMIOLOGY
Defining Prediabetes
The concept of prediabetes emerged in the late 1970s as a result of a better understanding of the natural history of diabetes (35, 46). The term was used to indicate the earliest identifiable stage of glucose dysregulation, characterized by plasma glucose levels that were intermediate between normal glucose tolerance and diabetes. In 1979, the National Diabetes Data Group used the term prediabetes to designate impaired glucose tolerance (IGT) (78), defined using 2-h post–glucose load values on an oral glucose tolerance test of 140 mg/dl to 199 mg/dl. The IGT definition was adopted by the American Diabetes Association (ADA) and the World Health Organization (WHO) (33, 78, 113). Subsequently, the ADA in 1997 (33) and the WHO in 1998 (5) introduced an additional category of impaired fasting glycemia (IFG) that was based on fasting blood glucose (FBG) values of 110–125 mg/dl. In 2003, the ADA issued new IFG diagnostic criteria, widening the FBG range from 110–125 mg/dl to 100–125 mg/dl (38). In 2010, a new hemoglobin A1C (HbA1C)-based definition of prediabetes was introduced by the ADA along with the first recommendations for the use of HbA1C for diagnosing diabetes (2).
These categories are used to identify individuals along the continuum of hyperglycemia who do not meet current thresholds for a diabetes diagnosis but who are at high risk of developing diabetes. Defining and identifying this intermediate risk group are important from a public health and clinical standpoint, as current evidence suggests that diabetes and cardiovascular prevention are most effective when implemented early in the disease process (31, 102). The importance of intervening in adults identified to have prediabetes has been reinforced by results from diabetes prevention trials (56, 81, 86, 108).
Currently, five definitions of prediabetes have been issued by professional societies, including the ADA (2), the WHO (112), and International Expert Committee (IEC) (75). These definitions identify phenotypes on the basis of the various tests of hyperglycemia (FBG, 2hBG, and HbA1C) (Table 1) (34, 66). These phenotypes are characterized by variable degrees of insulin resistance and beta-cell dysfunction, with near maximal insulin resistance and a loss of ≥80% of the beta-cell function in IGT (22). Because HbA1C is a measure of chronic hyperglycemia, phenotypes defined by HbA1C may reflect impairments in both fasting and 2-h glucose.
Table 1.
Current diagnostic criteria for prediabetes
| Tests | ADA | WHO | IEC |
|---|---|---|---|
| FPG | 100–125 mg/dl | 110–125 mg/dl | NA |
| 2hBG (75-g oral glucose tolerance test) | 140–199 mg/dl | 140–199 mg/dl | NA |
| HbA1C | 5.7–6.4% | NA | 6.0–6.4% |
Abbreviations: 2hBG, 2-hour postload blood glucose; ADA, American Diabetes Association; FPG, fasting plasma glucose; HbA1C, hemoglobin A1C; IEC, International Expert Committee; NA, not applicable; WHO, World Health Organization.
The IGT definition of prediabetes emerged from community-based studies showing that a 2hBG >140 mg/dl confers a higher risk for incident diabetes than do lower 2hBG values (78). The ADA-IFG definition was designed to be more comparable to IGT and to maximize the sensitivity for predicting incident diabetes (38). However, IFG defined using the 100 mg/dl FBG cut point identifies a lower-risk group, which exhibits a more favorable cardiovascular risk profile and a lower risk of developing diabetes compared with IFG based on the 110 mg/dl FBG cut point (112). Because of this lower-risk profile and the much higher prevalence of IFG based on the 100–125 mg/dl range, the WHO recommended that the lower cut point for IFG remain at 110 mg/dl (112). Consequently, two different definitions of IFG are currently in clinical use: 100–125 mg/dl recommended by the ADA and 110–125 mg/dl recommended by the WHO.
In 2009, the IEC recommended a new HbA1C-based prediabetes definition with a HbA1C range of 6.0% to 6.4% (75). In 2010, the ADA subsequently recommended an HbA1C of 5.7% to 6.4% (2) to define prediabetes. The WHO does not support the use of HbA1C for defining prediabetes (112).
Current criteria for IGT, IFG and HbA1C-based prediabetes and will identify different people (20, 67, 70). The ADA guidelines for the diagnosis of diabetes explicitly recommend that any single elevation of fasting glucose, 2-h glucose, or HbA1C be confirmed with a second test (a different test in the same blood sample or second test at a different time point) (3). No such recommendations presently exist for confirming a diagnosis of prediabetes. The reliance on a single measurement to identify prediabetes will result in some false-positive diagnoses (96).
Other glycemic markers such as glycated albumin and fructosamine have a potential for identifying prediabetes. These markers strongly correlate with HbA1C and FBG (51, 55), are associated with incident diabetes independent of HbA1C and FBG (51), predict macrovascular (99) and microvascular complications (98), and provide prognostic value similar to HbA1C with regard to the risk of cardiovascular disease, end-stage renal disease, and retinopathy (83). However, these biomarkers have not been incorporated into guidelines, and there is currently no consensus on the use of glycated albumin or fructosamine in clinical practice for defining glycemic status (100).
Challenges in Estimating the Burden of Prediabetes
That five definitions of prediabetes are currently in clinical use presents a challenge in the field (Table 1). Prevalence will vary widely depending on which definition is used and whether definitions are examined individually or combined (110). The various tests identify different people and have only moderate overlap, meaning that some people will be classified as having prediabetes by one definition but not by another. For example, among a sample of adults identified as having IGT, individuals in this group who also met criteria for having prediabetes defined by ADA-IFG, WHO-IFG, or HbA1C of 5.7–6.4% were 58.2%, 23.4%, and 32.3%, respectively (50). Categories of HbA1C used to define prediabetes (ADA 5.7–6.4% and IEC 5.5–6.4%) were chosen for their high specificity (20, 67, 70, 75) and will classify fewer individuals as having prediabetes and disproportionately capture those with higher fasting and 2-h glucose. An additional difficulty in estimating a population’s prediabetes burden is that it is a biochemically defined condition. In populations without systematic surveys with probability sampling that includes blood draws and laboratory measurements of glucose or HbA1C, it is challenging to accurately estimate the burden of prediabetes because most cases in the population are undiagnosed (65).
Global Prevalence of Prediabetes
Comprehensive global prevalence data on prediabetes are lacking. In 2019, the International Diabetes Federation (IDF) estimated the global IGT prevalence at 7.5% in both men and women (92). The latter estimate corresponds to approximately 374 million adults aged 18–99 years, with about half (48.1%) below the age of 50 years and about one-third (28.3%) in the age group of 20–39 years (who are thus likely to spend many years at high risk of developing adverse outcomes) (92). The vast majority of individuals with prediabetes (72.2%) reside in low- and middle-income countries (LMICs), the North American and Caribbean regions had the highest IGT prevalence (13.8%), and the European region has the lowest prevalence (5.1%) (92).
The 2019 IDF estimates do not include data on IFG or HbA1C and thus underestimate the extent of prediabetes as compared with estimates that combine all glycemic measures. Relying on IGT only, as done by IDF, leaves out an important fraction of individuals with prediabetes states defined by other tests (101). Nonetheless, estimates of prediabetes prevalence are not available for many countries, and global data on prediabetes rely on statistical extrapolations and substantial assumptions, with corresponding uncertainty.
Large surveys in Chinese adults using all three glycemic tests (HbA1C, FBG, or 2hBG) have described a prevalence of prediabetes on any one of the three tests ranging from 36% (109) in one study to as high as 50.1% in another study (114).
In the United States, national data on prevalence of prediabetes are available from the National Health and Nutrition Examination Survey (NHANES). NHANES is a serial, population-based cross-sectional survey designed to produce national estimates, generalizable to the US population. NHANES includes standardized assessments of FBG, 2hBG, and HbA1C, allowing for comparisons across different definitions of prediabetes. In our analysis for this report of the most recent NHANES cycle (2015–2016), the prevalence of prediabetes in the US adult population aged 20 or older varied substantially depending on the definition used, from 4.3% (IEC-HbA1C) to 43.5% (ADA-IFG) (Figure 1). If a combination of HbA1C 5.7–6.4%, FPG 100–125 mg/dl, and 2hBG 140–199 mg/dl was used—meaning all three criteria were satisfied—the prevalence was 2.5%. If any of the three definitions were used to define prediabetes, the prevalence was 51.3% (any of one of the criteria was satisfied). These data demonstrate the challenge of arriving at a single best estimate of the burden of prediabetes in the population. A combined definition—based on elevations in HbA1C and fasting glucose and/or 2-h glucose—has frequently been used in reports of national prevalence (19, 69). However, it would be unusual to use this combined definition of any elevation in one of three tests to diagnose prediabetes in clinical practice. Thus, there is presently a problematic disconnect between how prediabetes is defined in clinical practice and how prevalence is estimated in epidemiologic studies.
Figure 1.
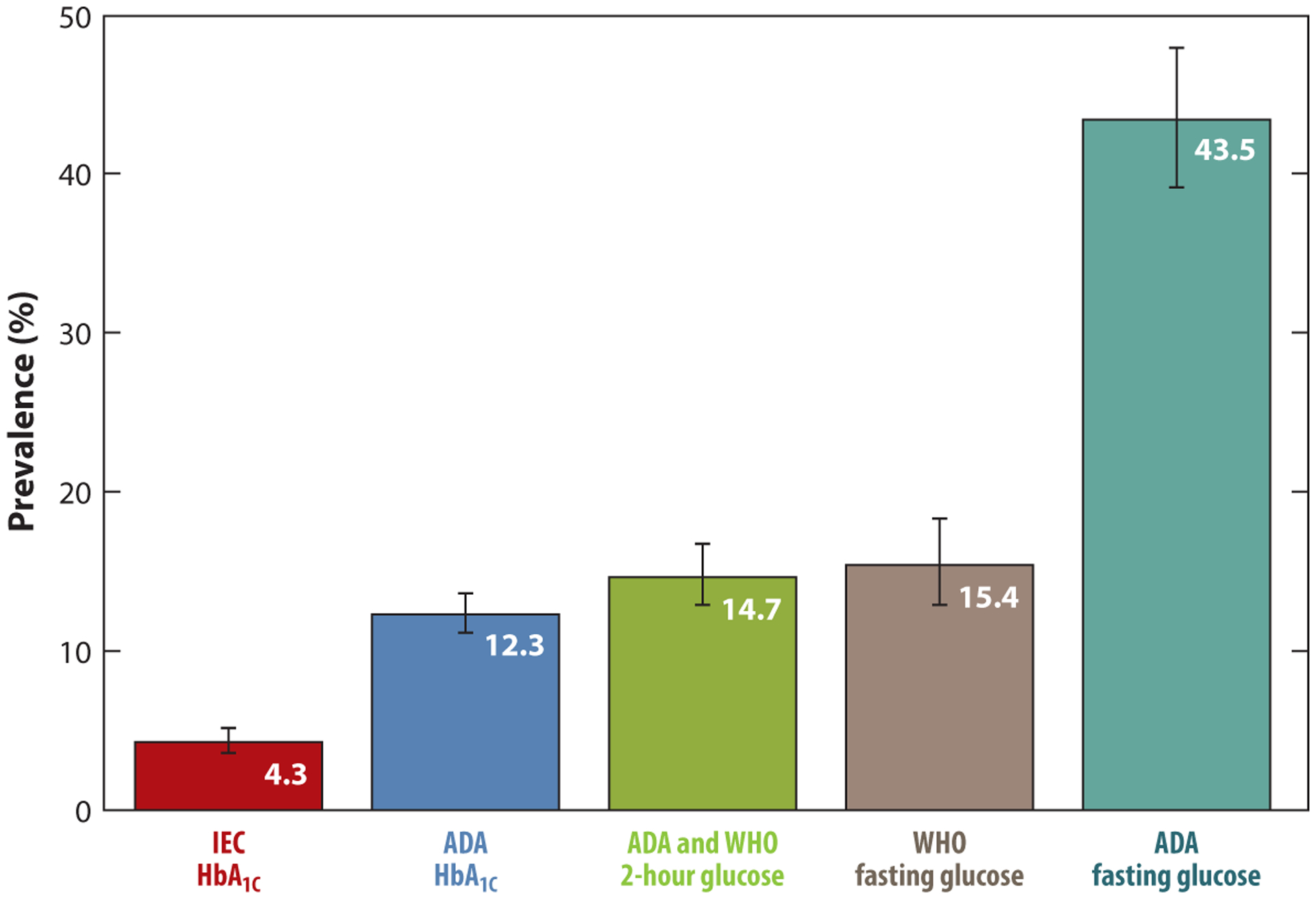
Prevalence of prediabetes in US adults aged 20 or older according to clinical definitions of prediabetes. Definitions of prediabetes: International Expert Committee (IEC) HbA1C 6.0–6.4%; American Diabetes Association (ADA) HbA1C 5.7–6.4%; ADA and World Health Organization (WHO) 2-h glucose 140–199 mg/dl; WHO fasting glucose 110–125 mg/dl; ADA fasting glucose 100–125 mg/dl. Data from National Health and Nutrition Examination Survey (NHANES) 2015–2016.
Demographic Differences in Prevalence of Prediabetes
Age and body mass index (BMI) are two of the strongest risk factors for prediabetes; evidence has demonstrated a strong age-related increase in prediabetes. In an analysis of the 2011–2012 NHANES, the prediabetes prevalence (using any elevation in 2hBG, FPG, or HbA1C) was 28.2% in adults aged 20–44 years and 49.5% in adults ≥65 years (69). The prevalence of prediabetes is substantially higher in obese individuals compared with normal-weight adults. Indeed, more than 80% of individuals with self-reported prediabetes are overweight or obese (65).
Racial disparities in prediabetes prevalence mirror those seen for diabetes. In the United States, there is a higher prevalence of prediabetes among non-Hispanic blacks and Hispanics than among non-Hispanic Asian participants (69). Although the prevalence of prediabetes among Asians is lower than that of their white counterparts, they had a substantially lower BMI (69). The higher prevalence of prediabetes and higher risk of cardiometabolic outcomes at BMI cut points in Asians and Asian Americans is a source of controversy; thus, recent recommendations advocate using lower BMI cut points in this population (48).
Analyses of NHANES and also of the Atherosclerosis Risk in Communities (ARIC) study demonstrate that demographic profiles differ, depending on the definition of prediabetes used. For example, individuals with prediabetes defined by HbA1C tended to be older, more likely to be female, more likely to be black (compared with white), and more likely to be obese than individuals meeting ADA-IFG or ADA/WHO-IGT definitions of prediabetes (6, 89, 110).
Trends in Prediabetes Prevalence
Over the past three decades in the United States, prevalence of prediabetes has increased (14, 68, 97) across all ethnic subgroups (14, 16) and all definitions of prediabetes (14, 16). Data from other parts of the world and based on IFG also suggest a growing prevalence of prediabetes over time (21, 79). IDF projections indicate that, by 2045, the number of adults with IGT will be 548 million, corresponding to 8.4% of the world’s adult population (92). The global epidemic of obesity and the rising global prevalence of prediabetes are of major concern. These trends do not bode well for the future outlook of diabetes and its complications across the world.
COMPLICATIONS IN PERSONS WITH PREDIABETES
Risk of Diabetes or Regression to Normal Glucose Tolerance
A significant proportion of individuals with prediabetes will develop diabetes over time, though the magnitude of this risk depends substantially on the prediabetes definition used. The risk of diabetes among persons with prediabetes is a central question, but it is also somewhat tautological. Diabetes is defined by elevated fasting glucose, 2-h glucose, or HbA1C. Thus, those individuals with the highest fasting glucose, 2-h glucose, or HbA1C within the prediabetic range will, by definition, be at the highest risk for developing diabetes. Nonetheless, many individuals with prediabetes do not progress rapidly or do not progress at all to diabetes. Some individuals, especially those with glycemic values at the lower end of the prediabetes range, will revert to normal glucose tolerance or the normal fasting state.
A 2007 meta-analysis of community-based cohort studies reported an absolute annual incidence of diabetes among individuals with WHO-IFG or IGT of 5–10% (39), with a relative risk for diabetes versus normoglycemia of 6.35 [95% confidence interval (CI) 4.87–7.82] for IGT; 5.52 (3.13–7.91) for isolated IGT; 4.66 (2.47–6.85) for IFG; 7.54 (4.63–10.45) for isolated IFG; and 12.13 (4.27–20.00) for both IFG and IGT (39). In a 2010 meta-analysis, the IEC-HbA1C prediabetes state (6.0–6.5%) was associated with a relative risk of approximately 20 compared with HbA1C <5%, with a 5-year cumulative incidence of diabetes ranging from 25% to 50% (115). A large 2018 meta-analysis (103 prospective cohort studies with up to 24 years of follow-up) found relative risks for diabetes of 4.32 for ADA-IFG, 5.47 for WHO-IFG, 3.61 for IGT, 6.90 for IFG and IGT, 5.55 for HbA1C >5.7%, and 10.10 for HbA1C >6.0% (90). Regardless of the definition, prediabetes identifies individuals at high risk for progression to diabetes, although absolute and relative risks vary depending on the definition used. IFG and IGT definitions tend to be associated with similar risks of future diabetes (with a higher risk if IFG and IGT are combined), whereas HbA1C definitions have the highest risk. As mentioned earlier, HbA1C cut points for prediabetes are more specific than those for FBG or 2hBG. Thus, HbA1C-defined prediabetes identifies fewer but higher-risk individuals, as borne out in recent individual epidemiologic studies and meta-analyses.
There are fewer data on rates of regression to normoglycemia among individuals with prediabetes. In a meta-analysis, the relative risk of regression from IGT to normoglycemia (compared with people who remained normoglycemic) was 0.33 (95% CI 0.23–0.43) over a 1-year follow-up period (39), suggesting low but not insubstantial rates of regression. In a different study of IFG, the reported cumulative proportion of individuals who reverted to normoglycemia by 10 years of follow-up was 55% (36). A meta-analysis of prospective studies (n = 18 studies involving 11,287 participants), which defined prediabetes by HbA1C using either the ADA (HbA1C of 5.7–6.4%) or the IEC (HbA1C 6.0–6.4%) definitions of prediabetes, reported cumulative incidence of regression ranging from 14% to 39% within 1–5 years of follow-up and from 17% to 31% for 6–11 years of follow-up (90). Some degree of regression might be expected in populations receiving lifestyle interventions to mitigate prediabetes risk; however, some of this regression undoubtedly reflects the known variability in tests of glycemia, which are highest for 2-h glucose, lowest for HbA1C, and intermediate for fasting glucose (96).
Cardiovascular Risk Factors, Morbidity, and Mortality
Individuals with prediabetes have a high burden of cardiovascular risk factors. In an analysis of data from NHANES 2011–2014, adults with prediabetes (defined using ADA-FPG or HbA1C) had a high prevalence of hypertension (36.6%), dyslipidemia (51.2%), albuminuria (7.7%), or reduced estimated glomerular filtration rate (4.6%). Overall, 24.3% were current smokers and had an elevated estimated 10-year cardiovascular event risk of approximately 7% (6).
In terms of cardiovascular outcomes, a large meta-analysis of prospective studies (53 studies, 1.6 million individuals, median follow-up duration 9.5 years) examined the risks of cardiovascular disease and death in persons with prediabetes as compared with normal glycemia (49). In this study, prediabetes (IGT or IFG by ADA or WHO criteria) was associated with an increased risk of cardiovascular disease (relative risks ranging from 1.13 to 1.30) and all-cause mortality (relative risks: 1.13–1.32).
Prediabetes states defined by HbA1C values of 5.7–6.4% or 6.1–6.4% were associated with risk of cardiovascular disease (relative risks: 1.21 and 1.25, respectively) (49). The strongest associations with cardiovascular disease, of 6.1–6.4%, were observed for WHO-IFG (relative risk: 1.30) and HbA1C (relative risk: 1.25) (49). Several cohort studies have shown an elevated risk of all-cause mortality among individuals with prediabetes (IFG or IGT) as compared with those with normoglycemia (10, 11, 17, 59, 74, 94, 103). Similarly, a high risk of hospitalization has been described among individuals with prediabetes (95). In a community-based population of US adults, we compared the prognostic value of the three tests of glycemia for all five prediabetes definitions (by ADA, WHO, and IEC) (110). All definitions were associated with a risk of adverse outcomes, including cardiovascular disease, kidney disease, and all-cause mortality, but, consistent with prior studies, the magnitude differed depending on the definition used (Figure 2) (110). HbA1C-based definitions identified the fewest number of individuals but were associated with the highest risks of complications.
Figure 2.
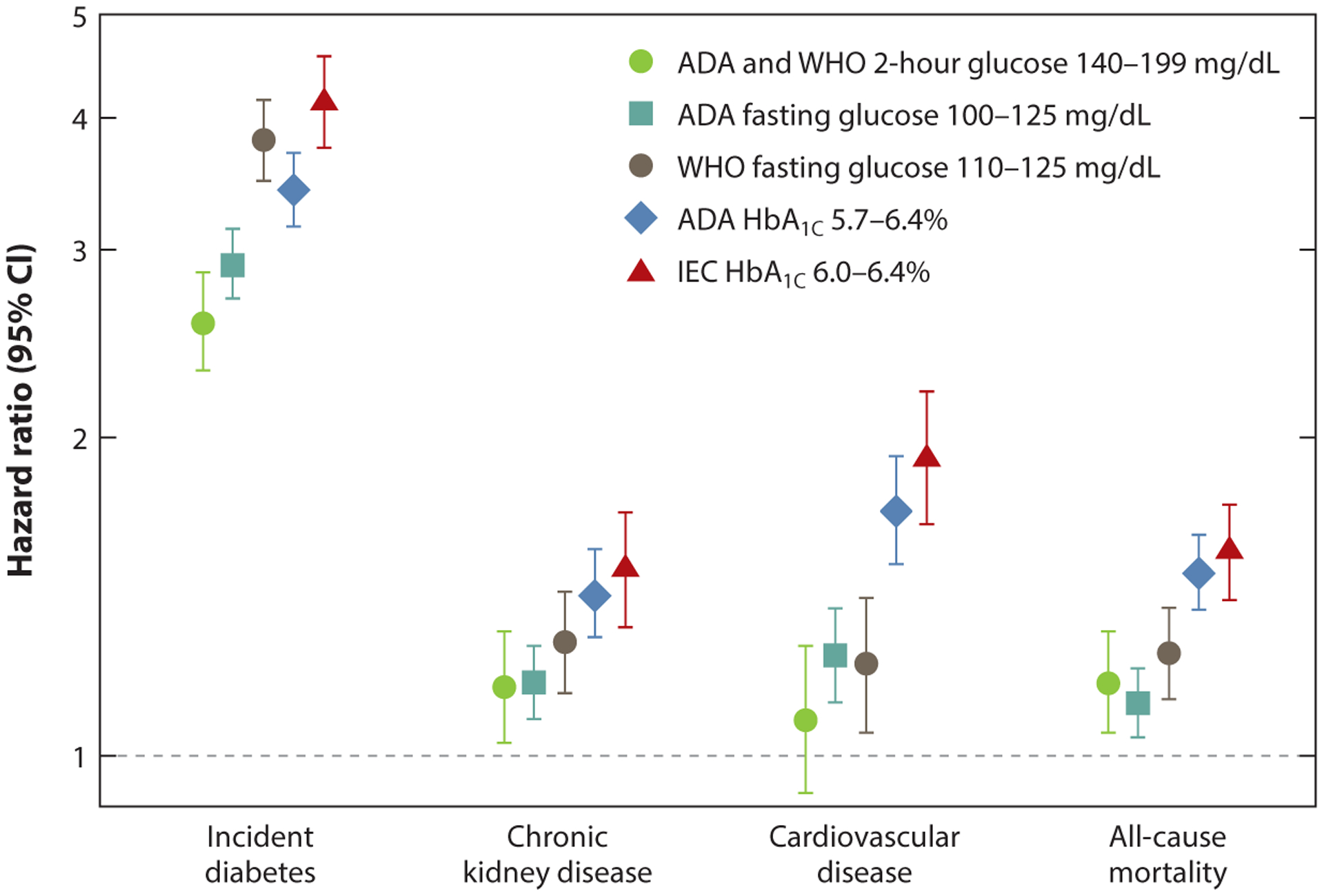
Hazard ratios (95% confidence intervals) for different definitions of prediabetes with incident diabetes, chronic kidney disease, cardiovascular disease, and all-cause mortality in the community-based ARIC study. Abbreviations: ADA, American Diabetes Association; ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; IEC, International Expert Committee; WHO, World Health Organization. Figure based on data from Reference 106.
Hyperglycemia-related microvascular complications, including retinopathy (77), neuropathy (53), and nephropathy (85), are frequently present among individuals with prediabetes. In the US Diabetes Prevention Program (DPP) study, which included individuals with prediabetes defined by IGT or IFG, 7.9% of participants had signs of retinopathy (77). In the 2009–2014 NHANES survey, between 7.5% and 16% of those with prediabetes had peripheral neuropathy, depending on how the latter was defined (53). Data from the 1999–2006 NHANES showed that approximately 18% of US adults with prediabetes have some form of chronic kidney disease (85).
Depending on the definition of prediabetes used, associations with major clinical outcomes differ. Nonetheless, the literature demonstrates a high burden of cardiometabolic risk factors in adults with prediabetes, a concerning high prevalence of microvascular disease in persons with prediabetes, and that all prediabetes definitions are associated with a high risk of diabetes and excess risk of major complications and death.
TREATMENT FOR PREDIABETES
Lifestyle Modifications: Universally Recommended
Landmark clinical trials have demonstrated that diabetes can be prevented with intensive lifestyle modification among individuals with prediabetes (Table 2) (56, 81, 86, 108). These trials, conducted in various settings including China (81), Finland (108), the United States (56), and India (86), showed that over a 3–6-year period, lifestyle interventions (dietary changes plus increased physical activity) reduced the incidence of diabetes by 28–58% compared with the placebo or minimal intervention (standard of care) groups. In all these trials, except the Indian study (86), the effects of lifestyle modification were mediated primarily by weight loss (43).
Table 2.
Landmark diabetes prevention trials
| Study | Country | Years of study | Prediabetes phenotypes | Age of participants (in years) | Study arms (n) | Weight target | Mean follow-up (in years) | Risk reduction for diabetes (intervention versus control) |
|---|---|---|---|---|---|---|---|---|
| Chinese Da Qing (81) | China | 1986–1992 | IGT | ≥25 | Diet (130) Exercise (141) Diet and exercise (126) Control (133) |
No specific weight target | 6 | Diet (31.5%) Exercise (46%) Diet and exercise (42%) |
| Finnish DPS (108) | Finland | 1993–2001 | IGT | 40–65 | Diet and exercise (265) Control (257) |
>5% weight loss | 4 | Diet and exercise (58%) |
| American DPP (56) | United States | 1996–2001 | IGT and ADA-IFG | ≥25 | Diet and exercise (1,079) Metformin(1,073) Control (1,082) |
7% weight loss | 2.8 | Diet and exercise (58%) Metformin (31%) |
| Indian DPP (86) | India | IGT | 33–55 | Diet and exercise (133) Metformin (133) Diet, exercise, and metformin (136) Control (136) |
No specific target | 3 | Diet and exercise (28.5%) Metformin (26.4%) Diet, exercise, and metformin (28.2%) |
Abbreviations: DPP, Diabetes Prevention Program; DPS, Finnish Diabetes Prevention Study; IFG, impaired fasting glycemia; IGT, impaired glucose tolerance.
In most of the major trials, the effects of lifestyle modification on diabetes incidence persisted for several years after discontinuation of the active intervention. Indeed, in the extended follow-up reports from the Finnish Diabetes Prevention Study (DPS), Chinese Da Qing, and US DPP studies (42, 58, 62–64, 76), over a 10- to 30-year period, incidence rates of diabetes in the intervention group were persistently lower.
The DPP trial ended after three years, but the investigators have continued long-term follow-up in the Diabetes Prevention Program Observational Study (DPPOS); they have conducted several detailed investigations into the long-term posttrial effects of the lifestyle intervention. In addition to a long-term reduction in incident diabetes at 10- and 15-year follow-up (58, 76), the intervention led to a long-term improvement in the cardiovascular disease risk factors, including systolic and diastolic blood pressure, low-density lipoprotein cholesterol, and triglycerides (80). However, the lifestyle intervention did not result in a significant reduction in risk of microvascular disease (nephropathy, retinopathy, or neuropathy) at 15-year follow-up (76). The lifestyle modification also did not significantly affect subclinical atherosclerosis as assessed by coronary artery calcium (40). Longer-term data and analyses of cardiovascular events are pending.
In the Da Qing study, which was conducted from 1986 to 1992 and enrolled individuals with IGT in China, diabetes prevention was associated with a significant reduction in diabetes during the 6-year trial period (81) and in the posttrial period after 20, 23, and 30 years of follow-up (42, 62, 63). In addition, the lifestyle arm was associated with a decreased number of deaths from cardiovascular disease and a reduction in all-cause mortality after 23 years (62). In the Da Qing study, after 30 years of follow-up, in addition to the reduction in deaths from cardiovascular disease, there was also a significant 36% reduction in cardiovascular disease events associated with lifestyle modification (42). The Da Qing study also showed that diabetes prevention through lifestyle modification can affect microvascular complications, with a 47% lower risk of severe retinopathy in the intervention group than in the control group over a 20-year period (41).
The persistent benefit of the in-trial effect in these lifestyle intervention studies has been termed the “legacy effect” or the result of “metabolic memory” (71). Indeed, accruing evidence suggests that interventions to achieve normoglycemia early in the disease course translate into robust and longer-lasting effects as compared with interventions implemented later in the life course through mechanisms that are yet to be fully elucidated.
Most of the major diabetes prevention trials enrolled individuals with IGT [except the US DPP, which also included IFG individuals (93)] and did not include individuals identified using HbA1C (56, 81, 86, 108) because major diabetes prevention studies were initiated at a time when the definitions of prediabetes did not include HbA1C. Current guidelines have reasonably assumed that the results of diabetes prevention trials can be extended to individuals with prediabetes identified using FBG or HbA1C. The absolute and relative effectiveness of lifestyle interventions to prevent diabetes in adults meeting IFG or HbA1C definitions of prediabetes have not been directly demonstrated (104, 111). However, analyses of data from the DPP trial examining baseline HbA1C indicated that values in the prediabetes range were robust predictors of the incidence of diabetes (57). Current guidelines for diabetes prevention recommend lifestyle modification as the first-line approach in persons with prediabetes in clinical practice regardless of the definition used to identify the person as having prediabetes (4).
Drug Therapies
A number of randomized, controlled trials have been conducted to evaluate the benefits of various pharmaceutical interventions for diabetes prevention (Table 3). These trials have shown diabetes risk reductions ranging from 25% to 70% as compared with placebo, depending on the drug used and the duration of follow-up. Metformin was assessed in the DPP trial (31% diabetes risk reduction versus placebo over 3 years) (56) and the Indian DPP (26.4% risk reduction versus placebo over 2.5 years) (86). Thiazolidinediones were investigated in the DPP trial (75% by troglitazone over 1 year versus placebo) (26), the DREAM trial (62% reduction by rosiglitazone over 3 years versus placebo) (30), and the Actos Now trial (72% reduction by pioglitazone over 3 years versus placebo) (23). Alpha glucosidase inhibitors were assessed in the STOP-NIDDM trial (25% risk reduction by acarbose over 3 years versus placebo) (18) and in a Japanese study (40% risk reduction by voglibose over 1 year versus placebo) (54). Glucagon-like peptide-1 analogues also have a significant effect on diabetes incidence (79% risk reduction by liraglutide over 3 years versus placebo) (61, 84).
Table 3.
Pharmaceutical trials for diabetes prevention
| Study | Country | Year | Prediabetes phenotypes | Trial drugs | Study size | Mean follow-up (in years) | Relative risk reduction for diabetes intervention versus placebo (95% confidence interval) |
|---|---|---|---|---|---|---|---|
| TRIPOD (13) | United States | 2002 | IGT (women with a history of GDM) | Troglitazone versus placebo | 266 | 2.5 | 55% (17, 75) |
| STOP-NIDDM (18) | International | 2002 | IGT and IFG | Acarbose versus placebo | 1,429 | 3.3 | 25% (10, 37) |
| DPP (56) | United States | 2002 | IGT and IFG | Metformin versus placebo | 3,234 | 2.8 | 31% (17, 43) |
| US-DPP (26) | United States | 2005 | IGT | Troglitazone versus placebo | 585 | 0.9 | 75% (NR) |
| XENDOS study (105) | International | 2006 | IGT | Orlistat versus placebo | 3,305 | 4 | 37% (14, 54) |
| Indian DPP (86) | India | 2006 | IGT | Metformin versus placebo | 531 | 2.5 | 26.4% (19.1, 35.1) |
| DREAM trial (30) | International | 2006 | IGT and IFG | Rosiglitazone versus placebo | 5,269 | 3 | 62% (56, 67) |
| DREAM trial (12) | International | 2006 | IGT and IFG | Ramipril versus placebo | 5,269 | 3 | 9% (−3, 20) |
| Voglibose trial (54) | Japan | 2009 | IGT | Voglibose versus placebo | 1,780 | 0.9 | 40% (18, 57) |
| NAVIGATOR (47) | International | 2010 | IGT and IFG | Nateglinide versus placebo | 9,306 | 5 | −7% (−15, 0)a (favors placebo) |
| NAVIGATOR (47) | International | 2010 | IGT and IFG | Valsartan versus placebo | 9,306 | 5 | 14% (8, 20) |
| ACT NOW trial (23) | United States | 2011 | IGT | Pioglitazone versus placebo | 602 | 2.4 | 72% (51, 84) |
| SCALE (61, 84) | International | 2017 | IGT and IFG | Liraglutide versus placebo | 2,254 | 3 | 79% (66, 87) |
Abbreviations: ACT NOW, ACTOS Now for Prevention of Diabetes; DPP, Diabetes Prevention Program; DREAM, Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication; GDM, gestational diabetes mellitus; IFG, impaired fasting glycemia; IGT, impaired glucose tolerance; NAVIGATOR, Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research; NR, not reported; SCALE, Satiety and Clinical Adiposity–Liraglutide Evidence; STOP-NIDDM, Study To Prevent Non-Insulin-Dependent Diabetes Mellitus; TRIPOD, Troglitazone Prevention of Diabetes; XENDOS, XENical in the Prevention of Diabetes in Obese Subjects.
Denotes a lack of risk reduction.
In the DPP trial, although metformin was overall less effective than lifestyle modification, it was as effective as lifestyle modification in prediabetic participants with BMI ≥35 kg/m2 (56) but less effective than placebo in those aged ≥60 years (44% diabetes risk reduction among participants aged 25–44 years versus 11% in those ≥60 years of age) (56). It is worth noting that analyses in these subgroups (obesity, younger age) were post hoc and not prespecified. Subsequent analyses also demonstrated that, among prediabetic women with a history of gestational diabetes mellitus (GDM), metformin and intensive lifestyle modification had equivalent effects on the incidence of diabetes (50% risk reduction) (9, 88). After 15 years of follow-up in the DPPOS, the effect of metformin (versus placebo) was greater among women with a history of GDM (41% risk reduction) as compared with women without a history of GDM (6% risk reduction) (29).
The observed effects of medications for the prevention of diabetes have generally been lower than those seen for intensive lifestyle modification, which has more sustainable effects. The drug effects have tended to wear out after a washout period (44); their withdrawal frequently leads to a glycemic rebound (25, 106). However, this rebound effect may not be specific to pharmaceutical interventions because the initial effects of the lifestyle intervention on HbA1C in DPP have tended to wane over time in the posttrial period (28). In trials that assessed the combined effect of lifestyle modification and a pharmacologic intervention (metformin or pioglitazone), no additional benefit beyond lifestyle modification was found (86, 87). Given the relatively short duration of most diabetes prevention trials, evidence on the long-term benefits of pharmaceutical therapies on outcomes such as cardiovascular disease and mortality is limited.
Translating the Evidence on Diabetes Prevention into Practice
Current ADA recommendations for the management of prediabetes include (a) a referral of individuals with prediabetes to an intensive behavioral lifestyle intervention program modeled on the DPP trial to achieve and maintain 7% loss of initial body weight and increase moderate-intensity physical activity (e.g., brisk walking) to at least 150 minutes per week; (b) annual monitoring for the development of diabetes; and (c) use of metformin among individuals with BMI ≥35 kg/m2, those aged less than 60 years, and women with prior GDM (4).
Despite the robust evidence from trials such as the DPP, which demonstrated that resource-intensive lifestyle support interventions to achieve modest weight loss can yield health benefits, the effort to translate and implement diabetes prevention programs in the United States and globally have lagged. The detection and treatment rates of prediabetes remain low. Data from the 2013–2014 NHANES show that only 7.4% of US adults report a history of prediabetes. Of these, 80% were overweight or obese, but only half reported actively trying to lose weight or met physical activity guidelines (65). Other data demonstrate that only one-third of people with prediabetes have received recommendations for diet or exercise from their health care providers (52). A national survey among primary care physicians showed that only 36% of those surveyed refer patients to a diabetes prevention lifestyle change program (107). Another study using data from the more recent 2016–2017 National Health Interview Survey reported that only 5% of individuals with prediabetes had been told by their physician to participate in a diabetes prevention program (8).
Metformin is seldom used in daily practice, despite the ADA’s recommendation to use it for prediabetes treatment. In 2010–2012, only 3.7% of prediabetes patients with United Healthcare insurance (one the largest private insurers in the United States) were prescribed the medication (72). In the 2013–2014 NHANES, metformin use among those with self-reported prediabetes was reported in only 8% of US adults (65).
The lifestyle interventions implemented in landmark trials were resource intensive. For example, the lifestyle intervention tested in the DPP trial consisted of 16 individual sessions taught by case managers (trained nutritionists, exercise physiologists, or behavioral psychologists) during the first six months of the intervention (24). These core sessions were followed by twice-monthly in-person maintenance sessions, with telephone contact between sessions (24). The translation of such interventions in practice is challenging. A number of US-based studies have assessed whether there are acceptable and low-cost alternatives to the resource-intensive DPP lifestyle interventions. These studies retained the core principles of the DPP intervention and tested adaptations of DPP delivery in clinics and communities; these DPP-like lifestyle interventions resulted in weight loss of approximately 4%, on average, over 12 months (7). DPP-like interventions in real-world settings also led to improvement in cardiovascular risk factors (73). In parts of the world other than the United States, a number of controlled and uncontrolled translation studies have also shown the feasibility and acceptability of diabetes prevention (37).
Economic considerations are important and have implications for various stakeholders (policy makers, public health agencies, insurers, and health care providers, and consumers), but few real-life studies have assessed the cost-effectiveness of prediabetes screening and treatment strategies. Simulations have shown that diabetes prevention using lifestyle modification is cost-effective (32, 45); this finding is corroborated by actual cost data from the DPP study (27). Patients may now be referred to National DPP lifestyle change programs, and the coverage of these programs, including through the Centers for Medicare & Medicaid Services and commercial insurers, is expanding (1, 15). However, insurance coverage for prediabetes in the United States remains limited.
A number of obstacles impede progress in the management of prediabetes in the United States and globally. The first obstacle is the lack of a standardized approach to identifying individuals with prediabetes and limited screening for prediabetes both in the community and in clinics. The second obstacle is the cost of interventions, especially intensive lifestyle interventions, which can be expensive and complicated to implement, maintain, and reimburse, especially in the context of the fragmented US health care and insurance system. A third obstacle involves the challenges posed by the effective real-world implementation of behavioral interventions in daily practice. The DPP and other trials have demonstrated that even modest weight loss can reduce the risk of diabetes. However, sustaining weight loss and making long-lasting improvements in diet and lifestyle are challenging for most individuals.
CONCLUSION
Prediabetes is common and a major public health issue globally. Individuals with prediabetes have a high risk of progression to diabetes and elevated risks of cardiovascular disease, kidney disease, and death. Lifestyle modification is the first-line therapeutic approach to prediabetes but is often difficult to sustain in practice. A lifestyle approach has a number of advantages, including potential cost-effectiveness and the adaptability to various settings worldwide. However, several challenges have limited cogent prediabetes treatment strategies, including the lack of a standardized clinical and public health approach for individuals with prediabetes as well as issues related to cost and reimbursement.
A major challenge in the field is a lack of consensus about how to define prediabetes, which has led to disparate prevalence estimates and a lack of consistency in approaches to screening and diagnosis. Using HbA1C to define prediabetes has a number of advantages over glucose-based definitions. First, HbA1C is strongly associated with adverse outcomes. Second, HbA1C testing has a number of practical advantages: Elevated levels are highly specific for long-term hyperglycemia, it is a nonfasting test with less preanalytical variability (fewer factors that can influence the test results), and it has low intraindividual variability compared with glucose (91, 96). Third, HbA1C is central to decision-making regarding treatment, particularly pharmacotherapy, in prediabetes and diabetes. Nonetheless, despite major advantages associated with the use of HbA1C, the WHO does not recommend using HbA1C to identify prediabetes. That the WHO recommends using HbA1C for the diagnosis of diabetes but not for prediabetes is problematic and has contributed to confusion in the field.
Going forward, guideline organizations need to come together to reach consensus on recommendations for the use of HbA1C and on standard definitions for prediabetes. Not everyone with prediabetes will develop diabetes; some 5-year risk estimates range from as low as 7% to as high as 50%. The variability in risk estimates is directly related to heterogeneity in definitions. In some settings, glucose testing may be preferred over HbA1C for prediabetes screening, especially if HbA1C testing is too costly or not available. Point-of-care assays for HbA1C are not widely recommended for the diagnosis of diabetes because there are concerns regarding proficiency testing and some assays do not meet existing quality criteria. However, certain point-of-care assays are certified by the National Glycohemoglobin Standardization Program and demonstrate excellent comparability to traditional laboratory assays (60). Thus, rapid point-of-care tests may provide an opportunity for broader HbA1C-based screening and identification of prediabetes and diabetes in some populations, especially in settings where obtaining traditional fasting venous samples might not be feasible. There is also compelling evidence for a role for glycated albumin and fructosamine as useful complementary or alternative tests when HbA1C testing is problematic or when glucose and HbA1C test results conflict (82, 98, 100). To date, however, no clinical organizations have provided guidance on how these tests might be used in practice.
When implementing screening programs, inherent trade-offs exist between using more sensitive versus more specific criteria for prediabetes. For example, fasting glucose-based definitions—particularly ADA-IFG—will identify many more people, but this population will be lower risk than individuals identified by other clinical definitions. Cost-effectiveness studies of different detection strategies are also needed to inform the trade-offs between broader versus narrower prediabetes definitions.
The lack of consensus on definitions has created dissonance between how the burden of prediabetes is estimated in epidemiologic studies and how prediabetes criteria are used to identify high-risk individuals in clinical practice. Epidemiologic studies have typically relied on combined definitions, using broad criteria, resulting in extremely high prevalence estimates, which is inconsistent with how prediabetes would be defined in clinical practice. Clear guidance for when certain definitions are preferred in public health practice and in clinical settings is sorely needed.
Evidence has increasingly shown that early intervention provides the greatest long-term benefit. Achieving effective diabetes prevention in daily practice requires a modification of the current clinical workflow to enhance referral of individuals with prediabetes to appropriate prevention programs. Expansion of insurance coverage is also needed, ideally by mandating diabetes prevention coverage by health care plans.
Ultimately, the lack of consensus regarding a single best definition of prediabetes continues to present a major challenge for the field and for clinical practice. Prediabetes, by any definition, is clearly associated with substantial excess risk of major clinical outcomes (49, 110), and even modest weight loss can have a major effect on reducing risk (56). However, disagreement on defining prediabetes complicates treatment decisions, insurance coverage, and our understanding of the true burden and risks of the condition. Thus, the field urgently needs to reach an agreement on prediabetes definitions to establish optimal approaches to screening, diagnosis, and treatment.
ACKNOWLEDGMENTS
E.T. was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) grant K23 HL153774. E.S. was supported by NIH/NHLBI grant K24 HL152440. The authors thank Bethany Warren and Dan Wang for assistance in generating the figures included in this review.
Glossary
- FBG
fasting blood glucose
- HbA1C
glycosylated hemoglobin
- 2hBG
2-hour postload blood glucose
- IGT
impaired glucose tolerance
- ADA
American Diabetes Association
- WHO
World Health Organization
- IFG
impaired fasting glycemia
- FBG
fasting blood glucose
- IEC
International Expert Committee
- IDF
International Diabetes Federation
- NHANES
National Health and Nutrition Examination Survey
- DPP
Diabetes Prevention Program
- DREAM
Diabetes Reduction Assessment With Ramipril and Rosiglitazone Medication
- STOP-NIDDM
Study to Prevent Non-Insulin-Dependent Diabetes Mellitus
- GDM
gestational diabetes mellitus
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Ackermann RT, O’Brien MJ. 2020. Evidence and challenges for translation and population impact of the Diabetes Prevention Program. Curr. Diab. Rep 20(3):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ADA (Am. Diabetes Assoc.). 2010. Standards of medical care in diabetes–2010. Diabetes Care 33(Suppl.1):S11–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ADA (Am. Diabetes Assoc.). 2020. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes–2020. Diabetes Care 43(Suppl. 1):S14–31 [DOI] [PubMed] [Google Scholar]
- 4.ADA (Am. Diabetes Assoc.). 2020. 3. Prevention or delay of type 2 diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 43(Suppl. 1):S32–36 [DOI] [PubMed] [Google Scholar]
- 5.Alberti KG, Zimmet PZ. 1998. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med 15(7):539–53 [DOI] [PubMed] [Google Scholar]
- 6.Ali MK, Bullard KMK, Saydah S, Imperatore G, Gregg EW. 2018. Cardiovascular and renal burdens of prediabetes in the USA: analysis of data from serial cross-sectional surveys, 1988–2014. Lancet Diabetes Endocrinol. 6(5):392–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali MK, Echouffo-Tcheugui J, Williamson DF. 2012. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff. 31(1):67–75 [DOI] [PubMed] [Google Scholar]
- 8.Ali MK, McKeever Bullard K, Imperatore G, Benoit SR, Rolka DB, et al. 2019. Reach and use of diabetes prevention services in the United States, 2016–2017. JAMA Netw. Open 2(5):e193160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aroda VR, Christophi CA, Edelstein SL, Zhang P, Herman WH, et al. 2015. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J. Clin. Endocrinol. Metab 100(4):1646–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barr ELM, Boyko EJ, Zimmet PZ, Wolfe R, Tonkin AM, Shaw JE. 2009. Continuous relationships between non-diabetic hyperglycaemia and both cardiovascular disease and all-cause mortality: the Australian Diabetes, Obesity, and Lifestyle (AusDiab) study. Diabetologia 52(3):415–24 [DOI] [PubMed] [Google Scholar]
- 11.Barr ELM, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, et al. 2007. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 116(2):151–57 [DOI] [PubMed] [Google Scholar]
- 12.Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, et al. 2006. Effect of ramipril on the incidence of diabetes. N. Engl. J. Med 355:1551–52 [DOI] [PubMed] [Google Scholar]
- 13.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, et al. 2002. Preservation of pancreatic β-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 51(9):2796–803 [DOI] [PubMed] [Google Scholar]
- 14.Bullard KM, Saydah SH, Imperatore G, Cowie CC, Gregg EW, et al. 2013. Secular changes in U.S. prediabetes prevalence defined by hemoglobin A1c and fasting plasma glucose: National Health and Nutrition Examination Surveys, 1999–2010. Diabetes Care 36:2286–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burd C, Gruss S, Albright A, Zina A, Schumacher P, Alley D. 2020. Translating knowledge into action to prevent type 2 diabetes: Medicare expansion of the National Diabetes Prevention Program lifestyle intervention. Milbank Q. 98(1):172–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caspersen CJ, Thomas GD, Beckles GLA, Bullard KM. 2015. Secular changes in prediabetes indicators among older-adult Americans, 1999–2010. Am. J. Prev. Med 48(3):253–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chi PW, Cheng TYD, Tsai SP, Hsu HL, Wang SL. 2005. Increased mortality risks of pre-diabetes (impaired fasting glucose) in Taiwan. Diabetes Care 28(11):2756–61 [DOI] [PubMed] [Google Scholar]
- 18.Chiasson J-L, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. 2002. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 359(9323):2072–77 [DOI] [PubMed] [Google Scholar]
- 19.Cowie CC, Casagrande SS, Geiss LS. 2018. Prevalence and incidence of type 2 diabetes and prediabetes. In Diabetes in America, pp. 1–25. Bethesda, MD: Natl. Inst. Diabetes Dig. Kidney Dis. 3rd ed. [PubMed] [Google Scholar]
- 20.Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, et al. 2010. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care 33(3):562–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, et al. 2011. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 378(9785):31–40 [DOI] [PubMed] [Google Scholar]
- 22.DeFronzo RA. 2009. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58(4):773–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, et al. 2011. Pioglitazone for diabetes prevention in impaired glucose tolerance. N. Engl. J. Med 364(12):1104–15 [DOI] [PubMed] [Google Scholar]
- 24.Diabetes Prev. Progr. Res. Group. 2002. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 25:2165–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diabetes Prev. Progr. Res. Group. 2003. Effects of withdrawal from metformin on the development of diabetes in the Diabetes Prevention Program. Diabetes Care 26(4):977–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diabetes Prev. Progr. Res. Group. 2005. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes 54(4):1150–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diabetes Prev. Progr. Res. Group. 2012. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care 35(4):723–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diabetes Prev. Progr. Res. Group. 2015. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 3(11):866–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diabetes Prev. Progr. Res. Group. 2019. Long-term effects of metformin on diabetes prevention: identification of subgroups that benefited most in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care 42(4):601–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DREAM Trial Invest., Gerstein HC, Yusuf S, Bosch J, Pogue J, et al. 2006. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 368(9541):1096–105 [DOI] [PubMed] [Google Scholar]
- 31.Echouffo-Tcheugui JB, Ali MK, Griffin SJ, Narayan KMV. 2011. Screening for type 2 diabetes and dysglycemia. Epidemiol. Rev 33(1):63–87 [DOI] [PubMed] [Google Scholar]
- 32.Eddy DM, Schlessinger L, Kahn R. 2005. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann. Intern. Med 143(4):251–64 [DOI] [PubMed] [Google Scholar]
- 33.Expert Comm. Diagnosis Classif. Diabetes Mellitus. 1997. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20(7):1183–97 [DOI] [PubMed] [Google Scholar]
- 34.Færch K, Johansen NB, Witte DR, Lauritzen T, Jørgensen ME, Vistisen D. 2015. Relationship between insulin resistance and β-cell dysfunction in subphenotypes of prediabetes and type 2 diabetes. J. Clin. Endocrinol. Metab 100(2):707–16 [DOI] [PubMed] [Google Scholar]
- 35.Fajans SS. 1973. I. Identification of chemical diabetes. The definition of chemical diabetes. Metabolism 22(2):211–17 [DOI] [PubMed] [Google Scholar]
- 36.Forouhi NG, Luan J, Hennings S, Wareham NJ. 2007. Incidence of type 2 diabetes in England and its association with baseline impaired fasting glucose: the Ely study 1990–2000. Diabet. Med 24(2):200–7 [DOI] [PubMed] [Google Scholar]
- 37.Galaviz KI, Weber MB, Straus A, Haw JS, Narayan KMV, Ali MK. 2018. Global diabetes prevention interventions: a systematic review and network meta-analysis of the real-world impact on incidence, weight, and glucose. Diabetes Care 41(7):1526–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Genuth S, Alberti KGMM, Bennett P, Buse J, Defronzo R, et al. 2003. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26(11):3160–67 [DOI] [PubMed] [Google Scholar]
- 39.Gerstein HC, Santaguida P, Raina P, Morrison KM, Balion C, et al. 2007. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res. Clin. Pract 78(3):305–12 [DOI] [PubMed] [Google Scholar]
- 40.Goldberg RB, Aroda VR, Bluemke DA, Barrett-Connor E, Budoff M, et al. 2017. Effect of long-term metformin and lifestyle in the Diabetes Prevention Program and its outcome study on coronary artery calcium. Circulation 136(1):52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong Q, Gregg EW, Wang J, An Y, Zhang P, et al. 2011. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: the China da Qing Diabetes Prevention Outcome Study. Diabetologia 54(2):300–7 [DOI] [PubMed] [Google Scholar]
- 42.Gong Q, Zhang P, Wang J, Ma J, An Y, et al. 2019. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 7(6):452–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, et al. 2006. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 29(9):2102–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haw JS, Galaviz KI, Straus AN, Kowalski AJ, Magee MJ, et al. 2017. Long-term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern. Med 177(12):1808–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herman WH, Hoerger TJ, Brandle M, Hicks K, Sorensen S, et al. 2005. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann. Intern. Med 142(5):323–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herron CA. 1979. Screening in diabetes mellitus: report of the Atlanta workshop. Diabetes Care 2(4):357–62 [DOI] [PubMed] [Google Scholar]
- 47.Holman RR, Haffner SM, McMurray JJ, Bethel MA, Holzhauer B, et al. 2010. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N. Engl. J. Med 362(16):1463–76 [DOI] [PubMed] [Google Scholar]
- 48.Hsu WC, Araneta MRG, Kanaya AM, Chiang JL, Fujimoto W. 2015. BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care 38(1):150–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang Y, Cai X, Mai W, Li M, Hu Y. 2016. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ 355:i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.James C, Bullard KM, Rolka DB, Geiss LS, Williams DE, et al. 2011. Implications of alternative definitions of prediabetes for prevalence in U.S. adults. Diabetes Care 34(2):387–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Juraschek SP, Steffes MW, Selvin E. 2012. Associations of alternative markers of glycemia with hemoglobin A(1c) and fasting glucose. Clin. Chem 58(12):1648–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karve A, Hayward RA. 2010. Prevalence, diagnosis, and treatment of impaired fasting glucose and impaired glucose tolerance in nondiabetic U.S. adults. Diabetes Care 33(11):2355–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katon JG, Reiber GE, Nelson KM. 2013. Peripheral neuropathy defined by monofilament insensitivity and diabetes status: NHANES 1999–2004. Diabetes Care 36(6):1604–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K. 2009. Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet 373(9675):1607–14 [DOI] [PubMed] [Google Scholar]
- 55.Kengne AP, Erasmus RT, Levitt NS, Matsha TE. 2017. Alternative indices of glucose homeostasis as biochemical diagnostic tests for abnormal glucose tolerance in an African setting. Prim. Care Diabetes 11(2):119–31 [DOI] [PubMed] [Google Scholar]
- 56.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, et al. 2002. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med 346(6):393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knowler WC, Edelstein SL, Goldberg RB, Ackermann RT, Crandall JP, et al. 2015. HbA1C as a predictor of diabetes and as an outcome in the Diabetes Prevention Program: a randomized clinical trial. Diabetes Care 38(1):51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, et al. 2009. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 374(9702):1677–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lancet. 1999. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes epidemiology: collaborative analysis of diagnostic criteria in Europe. Lancet 354(9179):617–21 [PubMed] [Google Scholar]
- 60.Lenters-Westra E, Slingerland RJ. 2014. Three of 7 hemoglobin A1c point-of-care instruments do not meet generally accepted analytical performance criteria. Clin. Chem 60(8):1062–72 [DOI] [PubMed] [Google Scholar]
- 61.Le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DCW, et al. 2017. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet 389(10077):1399–409 [DOI] [PubMed] [Google Scholar]
- 62.Li G, Zhang P, Wang J, An Y, Gong Q, et al. 2014. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2(6):474–80 [DOI] [PubMed] [Google Scholar]
- 63.Li G, Zhang P, Wang J, Gregg EW, Yang W, et al. 2008. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 371(9626):1783–89 [DOI] [PubMed] [Google Scholar]
- 64.Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, et al. 2006. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 368(9548):1673–79 [DOI] [PubMed] [Google Scholar]
- 65.Liu C, Foti K, Grams ME, Shin J-I, Selvin E. 2020. Trends in self-reported prediabetes and metformin use in the USA: NHANES 2005–2014. J. Gen. Intern. Med 35(1):95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lorenzo C, Wagenknecht LE, Hanley AJG, Rewers MJ, Karter AJ, Haffner SM. 2010. A1C between 5.7 and 6.4% as a marker for identifying pre-diabetes, insulin sensitivity and secretion, and cardiovascular risk factors: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 33(9):2104–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mann DM, Carson AP, Shimbo D, Fonseca V, Fox CS, Muntner P. 2010. Impact of A1C screening criterion on the diagnosis of pre-diabetes among U.S. adults. Diabetes Care 33(10):2190–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Menke A, Casagrande S, Cowie CC. 2016. Prevalence of diabetes in adolescents aged 12 to 19 years in the United States, 2005–2014. JAMA 316(3):344–45 [DOI] [PubMed] [Google Scholar]
- 69.Menke A, Casagrande S, Geiss L, Cowie CC. 2015. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 314(10):1021–29 [DOI] [PubMed] [Google Scholar]
- 70.Menke A, Rust KF, Cowie CC. 2018. Diabetes based on 2-h plasma glucose among those classified as having prediabetes based on fasting plasma glucose or A1c. Diabetes Vasc. Dis. Res 15(1):46–54 [DOI] [PubMed] [Google Scholar]
- 71.Miller RG, Orchard TJ. 2020. Understanding metabolic memory: a tale of two studies. Diabetes 69(3):291–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moin T, Li J, Duru OK, Ettner S, Turk N, et al. 2015. Metformin prescription for insured adults with prediabetes from 2010 to 2012: a retrospective cohort study. Ann. Intern. Med 162(8):542–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mudaliar U, Zabetian A, Goodman M, Echouffo-Tcheugui JB, Albright AL, et al. 2016. Cardiometabolic risk factor changes observed in Diabetes Prevention Programs in US settings: a systematic review and meta-analysis. PLOS Med. 13(7):e1002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakagami T 2004. Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia 47(3):385–94 [DOI] [PubMed] [Google Scholar]
- 75.Nathan DM, Balkau B, Bonora E, Borch-Johnsen K, Buse JB, et al. 2009. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32(7):1327–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nathan DM, Barrett-Connor E, Crandall JP, Edelstein SL, Goldberg RB, et al. 2015. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the DPP Outcomes Study. Lancet Diabetes Endocrinol. 3(11):866–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nathan DM, Chew E, Christophi CA, Davis MD, Fowler S, et al. 2007. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet. Med 24(2):137–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Natl. Diabetes Data Group. 1979. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 28(12):1039–57 [DOI] [PubMed] [Google Scholar]
- 79.NCD Risk Factor Collab. (NCD-RisC). 2015. Effects of diabetes definition on global surveillance of diabetes prevalence and diagnosis: a pooled analysis of 96 population-based studies with 331,288 participants. Lancet Diabetes Endocrinol. 3(8):624–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Orchard TJ, Temprosa M, Barrett-Connor E, Fowler SE, Goldberg RB, et al. 2013. Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet. Med 30(1):46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pan X-R, Li G-W, Hu Y-H, Wang J-X, Yang W-Y, et al. 1997. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care 20(4):537–44 [DOI] [PubMed] [Google Scholar]
- 82.Parrinello CM, Selvin E. 2014. Beyond HbA1C and glucose: the role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr. Diab. Rep 14(11):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parrinello CM, Sharrett AR, Maruthur NM, Bergenstal RM, Grams ME, et al. 2016. Racial differences in and prognostic value of biomarkers of hyperglycemia. Diabetes Care 39(4):589–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, et al. 2015. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med 373(1):11–22 [DOI] [PubMed] [Google Scholar]
- 85.Plantinga LC, Crews DC, Coresh J, Miller ER 3rd, Saran R, et al. 2010. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin. J. Am. Soc. Nephrol 5(4):673–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. 2006. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 49(2):289–97 [DOI] [PubMed] [Google Scholar]
- 87.Ramachandran A, Snehalatha C, Mary S, Selvam S, Kumar CKS, et al. 2009. Pioglitazone does not enhance the effectiveness of lifestyle modification in preventing conversion of impaired glucose tolerance to diabetes in Asian Indians: results of the Indian Diabetes Prevention Programme-2 (IDPP-2). Diabetologia 52(6):1019–26 [DOI] [PubMed] [Google Scholar]
- 88.Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, et al. 2008. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J. Clin. Endocrinol. Metab 93(12):4774–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rhee MK, Herrick K, Ziemer DC, Vaccarino V, Weintraub WS, et al. 2010. Many Americans have pre-diabetes and should be considered for metformin therapy. Diabetes Care 33(1):49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Richter B, Hemmingsen B, Metzendorf MI, Takwoingi Y. 2018. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst. Rev 10(10):CD012661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sacks DB. 2011. A1C versus glucose testing: a comparison. Diabetes Care 34(2):518–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, et al. 2019. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract 157:107843. [DOI] [PubMed] [Google Scholar]
- 93.Saito T, Watanabe M, Nishida J, Izumi T, Omura M, et al. 2011. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch. Intern. Med 171(15):1352–60 [DOI] [PubMed] [Google Scholar]
- 94.Saydah SH, Loria CM, Eberhardt MS, Brancati FL. 2001. Subclinical states of glucose intolerance and risk of death in the U.S. Diabetes Care 24(3):447–53 [DOI] [PubMed] [Google Scholar]
- 95.Schneider ALC, Kalyani RR, Golden S, Stearns SC, Wruck L, et al. 2016. Diabetes and prediabetes and risk of hospitalization: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care 39(5):772–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Selvin E, Crainiceanu CM, Brancati FL, Coresh J. 2007. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch. Intern. Med 167(14):1545–51 [DOI] [PubMed] [Google Scholar]
- 97.Selvin E, Parrinello CM, Sacks DB, Coresh J. 2014. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann. Intern. Med 160(8):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Selvin E, Rawlings AM, Grams M, Klein R, Sharrett AR, et al. 2014. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2(4):279–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Selvin E, Rawlings AM, Lutsey PL, Maruthur N, Pankow JS, et al. 2015. Fructosamine and glycated albumin and the risk of cardiovascular outcomes and death. Circulation 132(4):269–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Selvin E, Warren B, He X, Sacks DB, Saenger AK. 2018. Establishment of community-based reference intervals for fructosamine, glycated albumin, and 1,5-anhydroglucitol. Clin. Chem 64(5):843–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shen J, Kondal D, Rubinstein A, Irazola V, Gutierrez L, et al. 2016. A multiethnic study of pre-diabetes and diabetes in LMIC. Glob. Heart 11(1):61–70 [DOI] [PubMed] [Google Scholar]
- 102.Siu AL. 2015. Screening for abnormal blood glucose and type 2 diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med 163(11):861–68 [DOI] [PubMed] [Google Scholar]
- 103.Sorkin JD, Muller DC, Fleg JL, Andres R. 2005. The relation of fasting and 2-h postchallenge plasma glucose concentrations to mortality: data from the Baltimore Longitudinal Study of Aging with a critical review of the literature. Diabetes Care 28(11):2626–32 [DOI] [PubMed] [Google Scholar]
- 104.Thankappan KR, Sathish T, Tapp RJ, Shaw JE, Lotfaliany M, et al. 2018. A peer-support lifestyle intervention for preventing type 2 diabetes in India: a cluster-randomized controlled trial of the Kerala Diabetes Prevention Program. PLOS Med. 15(6):e1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. 2004. XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 27(1):155–61 [DOI] [PubMed] [Google Scholar]
- 106.Tripathy D, Schwenke DC, Banerji MA, Bray GA, Buchanan TA, et al. 2016. Diabetes incidence and glucose tolerance after termination of pioglitazone therapy: results from ACT NOW. J. Clin. Endocrinol. Metab 101(5):2056–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tseng E, Greer RC, O’Rourke P, Yeh H-C, McGuire MM, et al. 2019. National survey of primary care physicians’ knowledge, practices, and perceptions of prediabetes. J. Gen. Intern. Med 34(11):2475–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, et al. 2001. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med 344(18):1343–50 [DOI] [PubMed] [Google Scholar]
- 109.Wang L, Gao P, Zhang M, Huang Z, Zhang D, et al. 2017. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 317(24):2515–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Warren B, Pankow JS, Matsushita K, Punjabi NM, Daya NR, et al. 2017. Comparative prognostic performance of definitions of prediabetes: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 5(1):34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weber MB, Ranjani H, Staimez LR, Anjana RM, Ali MK, et al. 2016. The stepwise approach to diabetes prevention: results from the D-CLIP randomized controlled trial. Diabetes Care 39(10):1760–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.WHO (World Health Organ.). 2006. Definition and diagnosis of diabetes mellitus and intermediate. Rep, WHO, Geneva. https://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf [Google Scholar]
- 113.WHO (World Health Organ.) Expert Comm. Diabetes Mellitus. 1980. WHO Expert Committee on Diabetes Mellitus. Tech. Rep. 646, WHO, Geneva. https://apps.who.int/iris/bitstream/handle/10665/41399/WHO_TRS_646.pdf?sequence=1&isAllowed=y [Google Scholar]
- 114.Xu Y, Wang L, He J, Bi Y, Li M, et al. 2013. Prevalence and control of diabetes in Chinese adults. JAMA 310(9):948–59 [DOI] [PubMed] [Google Scholar]
- 115.Zhang X, Gregg EW, Williamson DF, Barker LE, Thomas W, et al. 2010. A1C level and future risk of diabetes: a systematic review. Diabetes Care 33(7):1665–73 [DOI] [PMC free article] [PubMed] [Google Scholar]


