Abstract
G protein-coupled receptors (GPCRs) transmit information to the cell interior by transducing external signals to heterotrimeric G protein subunits, Gα and Gβγ subunits, localized on the inner leaflet of the plasma membrane. Though the initial focus was mainly on Gα-mediated events, Gβγ subunits were later identified as major contributors to GPCR-G protein signalling. A broad functional array of Gβγ signalling has recently been attributed to Gβ and Gγ subtype diversity, comprising 5 Gβ and 12 Gγ subtypes, respectively. In addition to displaying selectivity towards each other to form the Gβγ dimer, numerous studies have identified preferences of distinct Gβγ combinations for specific GPCRs, Gα subtypes and effector molecules. Importantly, Gβ and Gγ subtype-dependent regulation of downstream effectors, representing a diverse range of signalling pathways and physiological functions have been found. Here, we review the literature on the repercussions of Gβ and Gγ subtype diversity on direct and indirect regulation of GPCR/G protein signalling events and their physiological outcomes. Our discussion additionally provides perspective in understanding the intricacies underlying molecular regulation of subtype-specific roles of Gβγ signalling and associated diseases.
Keywords: GPCRs, Heterotrimeric G proteins, Translocation, Signalling, Signal Transduction, Subcellular localization, Evolution, Human Disease
1. Introduction
1.1. GPCRs and G proteins
G protein-coupled receptors (GPCRs) transduce extracellular signals to the cell interior across the plasma membrane (PM) by activating heterotrimeric G proteins that consist of Gα, Gβ, and Gγ subunits. Structural conservation suggests that eukaryotic GPCRs are evolved from prokaryotic channelrhodopsins, and nearly 800 GPCRs are present in the human genome [1–3]. These receptors respond to a wide variety of extracellular ligands, including hormones, local mediators, neurotransmitters, odorants, photons (light), etc. Ligand binding-induced conformational changes in receptors trigger changes in the interacting heterotrimer at the cytosolic face of the receptor. Conformational changes in the heterotrimer promote GDP to GTP exchange in the Gα subunit, resulting in partial or complete dissociation of GαGTP from Gβγ where ligand-bound activated receptor stabilizes an ‘open’ conformation of Gα to release GDP, facilitating GDP to GTP exchange (Fig. 1). When the ligand is no longer bound to the GPCR, an inactive G protein heterotrimer is restored [4, 5]. While signalling of Gα isoforms has been studied extensively, despite the possible availability of 60 different combinations of Gβγ heterodimers, “Gβγ” remains primarily treated as an eponymous unitary signal transducer.
Figure 1. Activation of heterotrimeric G proteins by the activated G protein-coupled receptor (GPCR) upon ligand binding.
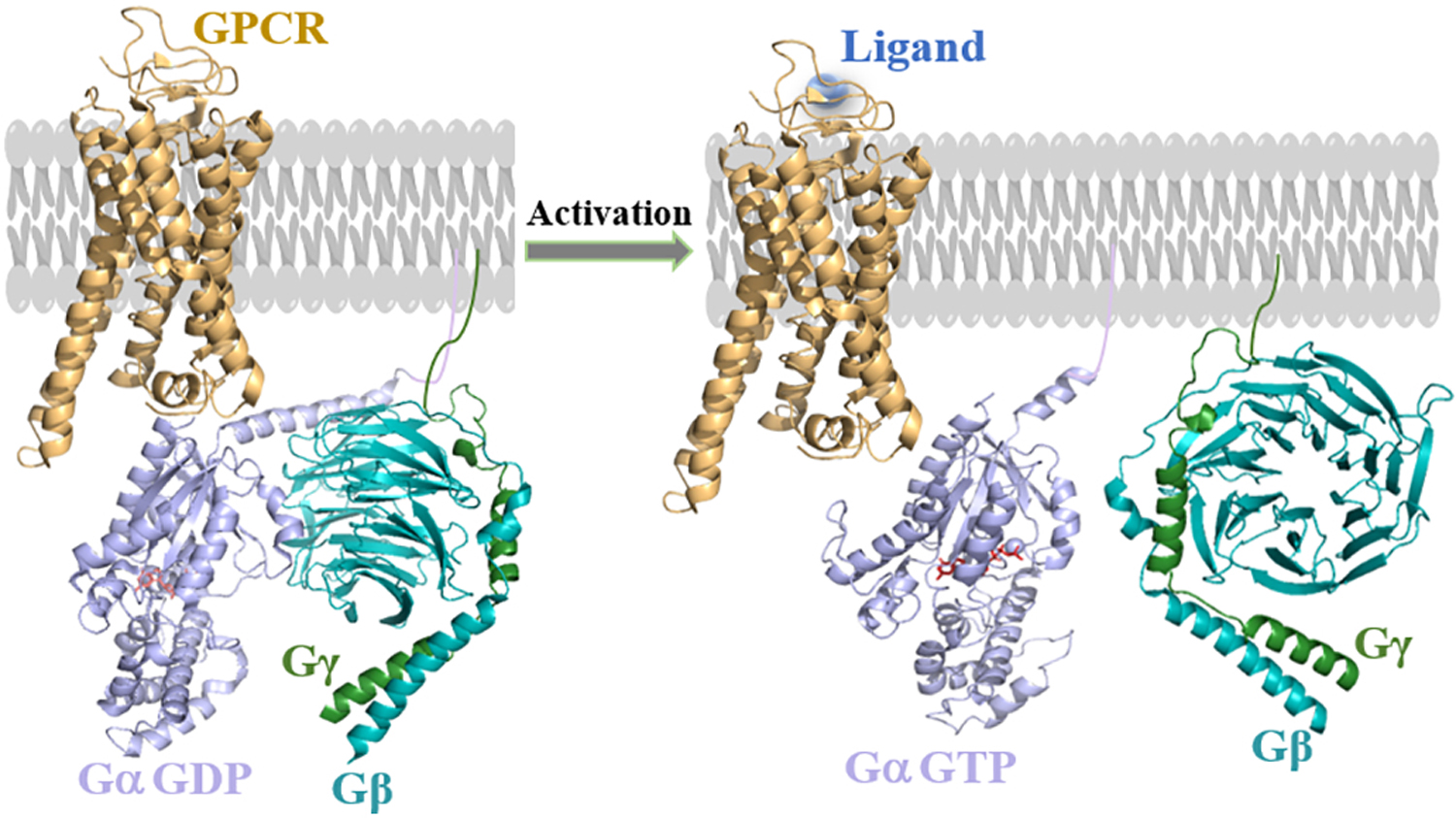
Adapted from the Protein Data Bank Identifier (PDB ID) 1GP2, 6R3Q and 6OY9. Ligand (blue) binding induces conformational changes in GPCR (brown), and promotes GDP to GTP exchange on the Gα (purple) subunit in the ‘open’ conformation along with structural changes in the binding site of Gβ (cyan blue). The heterotrimer complex dissociation into GαGTP and free Gβγ allows them to interact with respective downstream effectors.
1.2. Gβγ complexes
The human genome encodes 5 Gβ and 12 Gγ genes, resulting in significant potential structural and functional diversity in G protein heterotrimers. Among mammalian Gβ isoforms, Gβ1 to Gβ4 share more sequence homology than Gβ5 [6, 7]. Gβ1–4 are 36 kDa proteins while Gβ5 is a 40 kDa protein, with only 50% sequence similarity to other Gβ subunits. Comparatively smaller, Gγ subtypes are between 7–8.5 kDa in size [8]. Gγ subunits show greater sequence diversity than Gβ, indicating their possible roles in generating functional diversity of Gβγ signalling. Variation in amino acid sequence is prominent among the 12 Gγ subtypes, ranging from 20% to 80% [7]. Some Gγ subunits can undergo a number of post-translational modifications. Isoprenylation of the Cys residue in the CAAX motif at the carboxyl-terminal is one of the primary post-translational modifications in Gγ subunits. Prenylation allows membrane localization and likely controls the mobility of Gβγ subunits [9]. Many Gγ subunits contain a Leu residue in the CAAX sequence as X, suggested to promote geranylgeranylation of Gγ through a thioether linkage formation [10]. In several other Gγ types, X is a Ser residue, facilitating farnesylation of the protein [11, 12].
The Gβγ dimer that forms a stable structural unit is illustrated in Fig. 2. The Gβ subunit contains seven WD 40 repeats [13–15]. Comparatively shorter Gγ folds into two α helices. The C-terminal α-helix makes extensive contacts with the base of the Gβ propeller. Gβ and Gγ subunits are tightly associated with each other through hydrophobic interactions. As shown in Fig. 2, some interaction sites include Asp258 in Gβ that interacts with two residues linking the coiled-coil to the Gβ1 propeller; Arg22 in Gβ1 and Arg30 in Gγ1. Residues Phe40 and Phe64 in Gγ form further hydrophobic interactions with Gβ [16]. Unlike Gα, the Gβγ dimer does not undergo modification during activation of the G protein heterotrimer. Further, it is believed that association of Gβγ with the GDP-bound form of Gα generally prevents Gβγ from constitutively activating its effectors.
Figure 2. Assembly of Gβ1γ1 dimer crystal structure adapted from the PDB ID 1TBG.
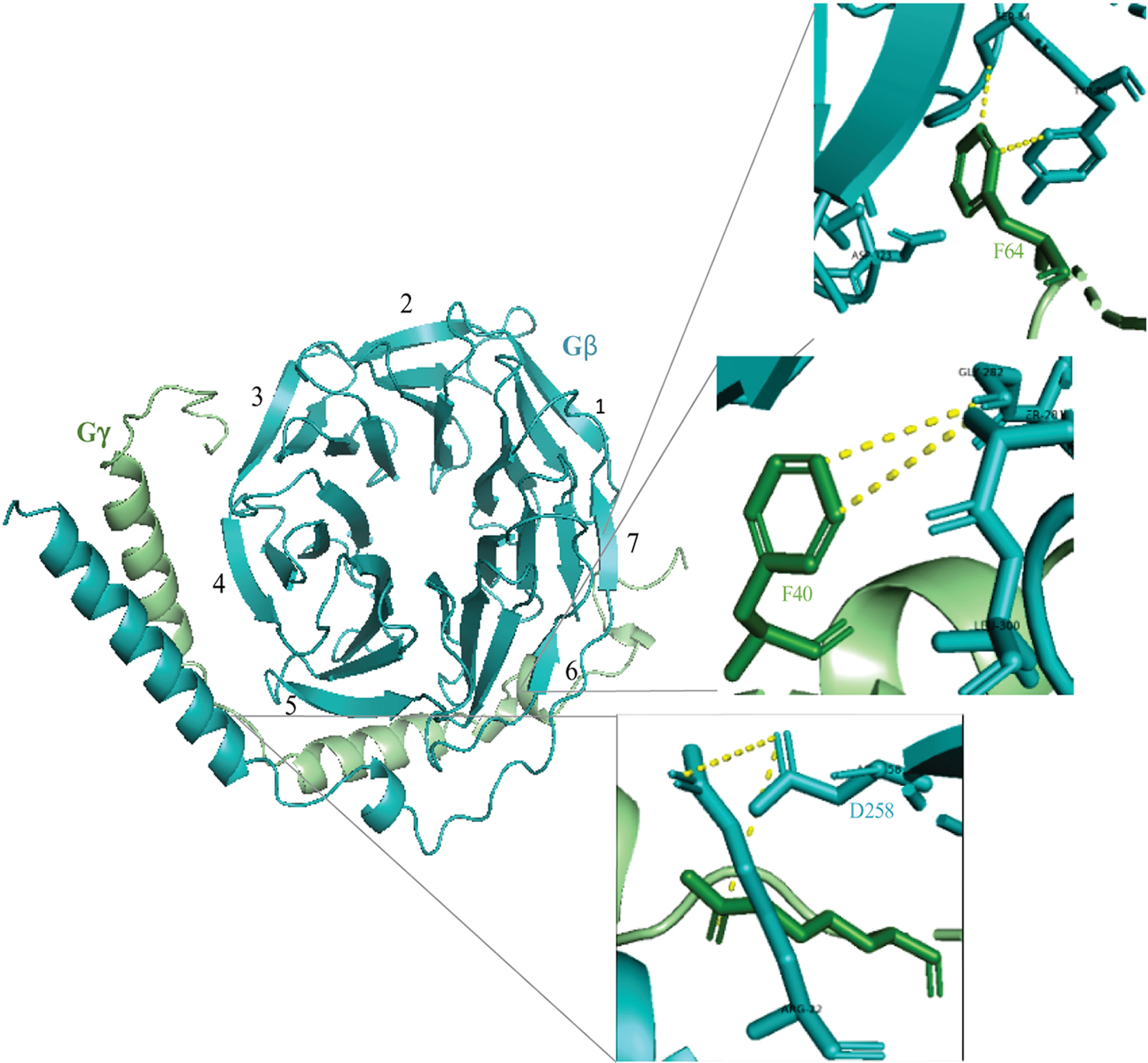
Gβ and Gγ subunits are shown in cyan, blue and green colors respectively. Black numerals indicate seven blades of the Gβ propeller. A ribbon representation of the dimer indicates some of the interaction sites between the subunits. The magnified diagram shows interaction of Gβ-Asp258 with residues on both the subunits. Hydrophobic interactions between Gγ residues including Phe40 and Phe64 with Gβ are also labeled.
1.3. Gβγ effectors
Active GαGTP and free Gβγ at the inner leaflet of the PM interact with numerous effector molecules. Gβγ dimers regulate a large cohort of effectors, including phospholipases, adenylyl cyclases (AC), G protein-coupled receptor kinases (GRKs), and ion channels [17]. The Gβγ dimer can activate phospholipase Cβ (PLCβ) isoforms, including PLCβ2 and PLCβ3. Although a crystal structure is not available, evidence suggests binding of the β-propeller of Gβ with the pleckstrin homology (PH) domain of PLCβ [18]. Further, Gβγ can regulate the activity of AC isoforms that generate the second messenger cAMP upon receptor activation. The effects of Gβγ on production of cAMP depend on the particular isoform of AC. Unlike with other effectors, Gβγ interacts with AC5 and AC6 through multiple interaction sites, reflecting the complex regulation of cAMP production by Gβγ [19]. Gβγ also interacts with ion channel proteins such as GIRK1 (Kir 3.1) and directly binds to both the N-terminal hydrophilic and C-terminal domains of GIRK1, and voltage-gated calcium channels [11] regulating neuronal and cardiovascular excitability [20, 21].
2. Evolution and subtype diversity of heterotrimeric G proteins
2.1. Gβ subtypes and their diversity across evolution
GPCR and G protein-mediated signalling controls numerous cellular functions in eukaryotes. This occurs at the level of the receptor, G protein, or downstream effectors to fine-tune signalling output [22–24]. Previously, it has been shown that receptors, G proteins, and downstream regulators are expanded through lineage-specific modifications, recurrent domain shuffling, gene duplication, and selection, offering additional levels of expression regulation [23]. Also, tissue-specific expression patterns of G protein subunits have been shown to control designated functions at specific locations in the human body. The functional diversity of G proteins is acquired through evolution and modulated by the particular location both within the cell and the tissue in question.
Based on the phylogenetic analysis performed using protein sequence data obtained from invertebrates, mammals, and plants, Gβ subunits are classified into five groups [25]. Except for G5β isoform, Gβ1–4 are highly conserved, sharing more than 80% sequence identity and form functional Gγβ heterodimers with Gγ subunits (Table 1) [26]. G5β is divergent from the rest of the G βsubunits, exhibiting only 50% sequence similarity [25, 26]. Therefore, it has been suggested that Gβ evolved from a common ancestor and then diverged into two super-families. While one superfamily contains Gβ1–4, the other subfamily consists of only Gβ5 [26], and distinct expression patterns of these subtypes can be seen in different organisms.
Table 1:
Identity matrix of mammalian Gβs
| β1 | β2 | β3 | β3S | β4 | β5 | β5L | |
|---|---|---|---|---|---|---|---|
| β1 | 100 | ||||||
| β2 | 87 | 100 | |||||
| β3 | 80 | 81 | 100 | ||||
| β3S | 74 | 73 | 88 | 100 | |||
| β4 | 86 | 88 | 78 | 71 | 100 | ||
| β5 | 51 | 52 | 52 | 44 | 53 | 100 | |
| β5L | 46 | 45 | 45 | 40 | 45 | 89 | 100 |
Gβ subunits in lower eukaryotes
Holozoan family animals and their closest single-cell relatives show an ancient duplication in their genomes, which may have given rise to Gβ1–4 and Gβ5 [1]. However, unicellular holozoans such as C. owczarzaki express only two Gβ subtypes, where one subtype is clustered with Gβ1–4 [27]. Data mining also shows that many pre-vertebrate metazoan genomes possessed Gβ subunits with properties resembling Gβ1–4 and Gβ5 before expanding into vertebrates [27]. Gβ subunits are well characterized in budding yeast, Saccharomyces cerevisiae, which is classified under kingdom fungi and domain Eukaryota. Both of their Gβγ types, beta subunit 1 (Gpb1) and 2 (Gpb2), exhibit sequence homology to vertebrate Gβ1 and Gβ3, respectively. Although it does not form a dimer with Gγ a noncanonical Gβ subunit Vsp5 in S. cerevisiae interacts with PI3K[28]. The fission yeast, Schizosaccharomyces pombe, also expresses a Gβ subunit, Gnr1, that shows sequence homology to the mammalian Gβ1 subunit but does not form a Gβγ dimer [29]. Most filamentous fungi and Dictyostelium sp. only express a single Gβ subunit [30], exhibiting a highly conserved sequence homology [31]. However, their Gβ subunit exhibits lower sequence homology to S. cerevisiae (38%) and S. pombe (45%) Gβ respectively [32]. The genome of another filamentous fungus, Neurospora crassa, also encodes a single Gβ subunit, which exhibits a 65% sequence identity to human Gβ1[32].
Gβ subunits in invertebrates
The Drosophila genome encodes three Gβ subunits, Gβ5, Gβ13F, and Gβ76C [33, 34]. The Gβ76C subunit in D. melanogaster is homologous to vertebrate Gβ1–4 [25]. Comparative analysis of invertebrate Gβ isoforms displays a common trait of Gβ subunit evolution between C. elegans and D. melanogaster [25]. GPB-2 from C. elegans and Gβ5 from D. melanogaster are homologous to vertebrate Gβ5. Additionally, this Gβ5 also dimerizes with RGS (R7) family proteins, thereby controlling the expression and stability of Gβ5 [35]. In the sea squirt, Ciona intestinalis, a similar evolutionary pattern of Gβ subunits to Drosophila is evident. The sea squirt expresses three Gβ subunits that are distinct from the Gβ subunits of other species. A cross genome phylogenetic analysis to identify the Gβ subunits in invertebrates showed that, other than Gβ1–4 and Gβ5, a separate Gβ cluster was found in arthropods, which is known as Gβe. Gβe was identified in all the insects and the crustacean D. pulex [27]. However, Gβe has not been found in protostomes, such as annelids and mollusks [27].
Gβ subunits in plants
Compared to the five different Gβ subtypes in vertebrates, plants are evolutionarily limited to one Gβ subunit [36, 37]. Based on the sequence analysis, Gβ type from AGB1 (Arabidopsis), NGB1 (Nicotiana), and RGB1 (O. sativa) are significantly homologous to each other while also showing similarity to vertebrate Gβ2 counterparts [25]. Maize Gβ, ZGB1 is homologous to Arabidopsis AGB1, with 76%. Their sequence homology to animal Gβ subunits is ~41% [37]. Based on the sequence alignment and secondary structure predictions, both ZGB1 and AGB1 consist of 7 WD repeats [37]. An important phylogenetic relationship exists between Gβ subunit in eudicots, monocots, and lower pants [38]. All the monocots exhibit more than 90% sequence similarity to RGB1. RGB1 also contains the canonical seven-β propeller architecture with six identified WD repeats [38]. N-myristoylation signals help RGB1 association with PM, thereby regulating signal transduction such as plant adaptation to high salt stress [39].
Gβ subunits in vertebrates
Using a cross genome phylogenetic analysis, it was found that in vertebrates, the Gβ1–4 cluster is common to all vertebrates except extant vertebrates such as lamprey [27]. The lamprey expresses two genes belonging to the Gβ1–4 cluster. Also, the Gβ5 cluster among mammals showed high sequence homology. Human Gβ5 only shows 50% homology to other Gβ subunits. On the other hand, human Gβ5 showed a 99% sequence similarity to mouse Gβ5. In mammals, Gβ subunits are tightly conserved regardless of their species type. Mammalian species such as mice (Mus musculus) and rats (Rattus norvegicus) have been widely used to study human GPCR, G protein functions since their G protein subtypes are conserved across species [25].
Structural differences in mammalian Gβ types
As discussed earlier, mammals express five different Gβ subunits. There are five different genes to encode Gβ subunits and their splice variants (β1, β2, β3, β3S, β4, β5, β5L) in the human genome [6, 40]. Splice variants in Gβ1, Gβ2, and Gβ4 subtypes have not been detected [41]. Instead of limiting to transcript variants, Gβ subtype-specific functions may have evolved through gene duplication followed by selection. GNB1 (Gβ1) is on chromosome 1 of the human genome. This transcript produces 12 exons, with 9 of them considered coding exons. The first two exons and the last exon of Gβ1 are noncoding. It has been shown that Ser at the second position of the Gβ1 can be N-acetylated or phosphorylated. Phosphorylation at His266 in Gβ1 also contributes to G protein activation. The information encoded in exons 6 and 7 includes the protein region of Gβ1 that forms the Gβγ interaction interface with Gα [17]. Based on the crystal structure of GIRK2 (Kir3.2) and Gβ1γ2, Arg52 in Gβ1 is involved in interactions between Gβ1 and GIRK [42]. GNB2 (Gβ2) is located on chromosome 7 in the human genome, and its transcript has 10 exons with 9 coding exons. Post-translational modifications such as N-acetylation and phosphorylation are predicted at Ser2 and Thr239, respectively. Earlier, it has been suggested that Gβ3 may be linked to either Gβ1 or Gβ2 since a protein corresponding to the Gβ3 gene was not identified. Discordancy analysis and in situ chromosome hybridization revealed that chromosomal localization of GNB3 is different from the locations of genes, GNB1 and GNB2 [43]. GNB3 was discovered on chromosome 12, and its transcription gives rise to 11 exons. The homology model of GNB3 generated after fitting GNB1 crystal structure data demonstrates several amino acid residues of Gβ3 are essential for the folding of β propeller. The Trp339 residue would affect the proper folding of β-propeller, and Ser67 would participate in hydrogen bonding to keep the top of the β barrel in the proper orientation as required for protein-protein interactions. A splice variant of GNB3 (GNB3S) is a result of an alternative splicing event taken place to remove exon 9 (123bp) of GNB3 [44]. Therefore, Gβ3S lacks one WD repeat domain (Fig. 3). Phosphorylation, acetylation, and ubiquitination signals were predicted in Gβ3. GNB4 (Gβ4) encodes 10 exons and shows molecular signatures for N-acetylation and phosphorylation. Gβ4 shares high sequence similarity to Gβ1 and Gβ2, at almost 86%. GNB5 is on chromosome 15 in the human genome with 12 exons. Gβ5 shows approximately 50% sequence homology to Gβ1–4, indicating likely functional differences from other Gβ subunits [45]. It has been shown that Gβ5 is functionally distinct from Gβ1–4, with comparably weak interactions with Gγ subunits [46]. Instead of irreversible binding to Gγ, Gβ5 interacts with R7 RGS family proteins. Gβ5L is identical to Gβ5, but Gβ5L has additional 126 bps due to an additional codon at the 5´ but lacks 5´ portions of its third exon, generating a 44-kDa protein (Fig. 4) [47]. This 126 bp-extension was not derived from the 5´ untranslated region of GNB5 but arose from retina-specific usage of a 5´ exon [40]. Gβ1–5 subunits are approximately 36 kDa in size, while Gβ5L is approximately 44 kDa. Free Gβγ subunits initiate canonical signalling pathways by activating multiple effectors [48–50]. Gβ subtypes (Gβ1–4) can dimerize with 12 different Gγ subtypes encoded in the human genome, resulting in 48 possible Gβγ combinations. A region of 14 amino acids in Gγ (36–49) was identified to control association with Gβ [51]. Gβ1 can form heterodimers with all Gγ subunits while Gβ2 fails to form heterodimers with Gγ1, showing restrictions in its Gγ partners [52, 53]. However, Gγ1 and 2 are unable to form heterodimers with Gβ3 [53]. Gβ3S was first identified as a gain of function mutant leading to enhanced activity of Gα and Gβγ [54]. Gβ3S showed less stability than other Gβ subunits and was incapable of forming functional heterodimers with different Gγ isoforms [55, 56]. However, a more sensitive yeast two-hybrid assay was used to assess Gβ and Gγ interactions. Here, the data showed that all Gγ isoforms could interact with Gβ1 and Gβ2 but poorly with Gβ3 and Gβ4 [57]. However, co-immunoprecipitation analysis of Gβ4 showed dimer formation with all known Gγ types [58]. A similar study also demonstrated that Gγ5 has more robust interactions with Gβ4 compared to Gγ12, 2, and 3[59].
Figure 3. Proposed structure of Gβ3 generated using coordinates obtained from Gβ1 crystal structure (PDB id: 3AH8).
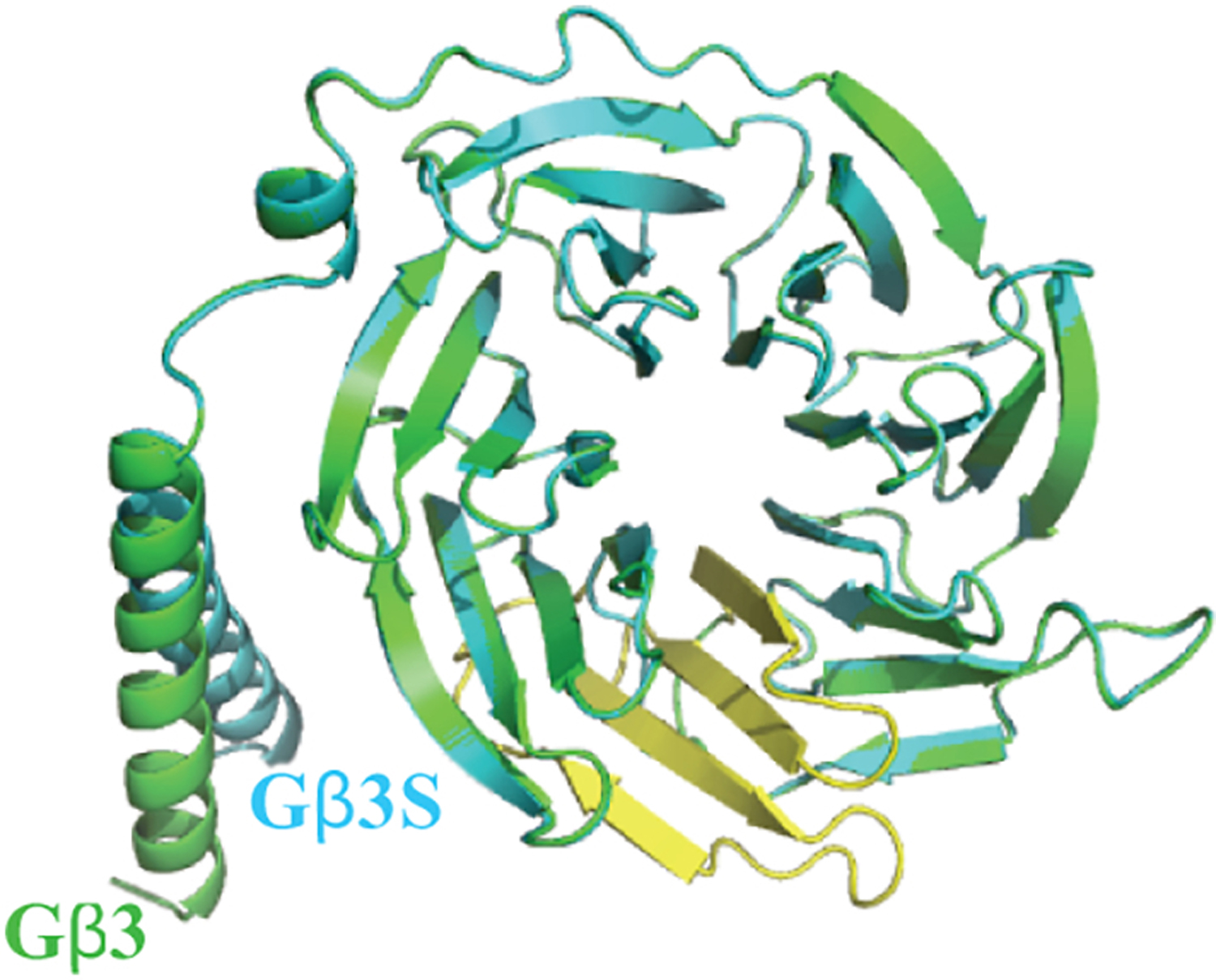
The homology model of Gβ3 (green) is superimposed with Gβ3S (blue) homology model generated using Phyre web portal (http://www.sbg.bio.ic.ac.uk) employing Gβ3 as the template. The yellow color indicates the 4th WD repeat of Gβ3 that absent in its splice variant, Gβ3S.
Figure 4. The molecular structure of Gβ5.
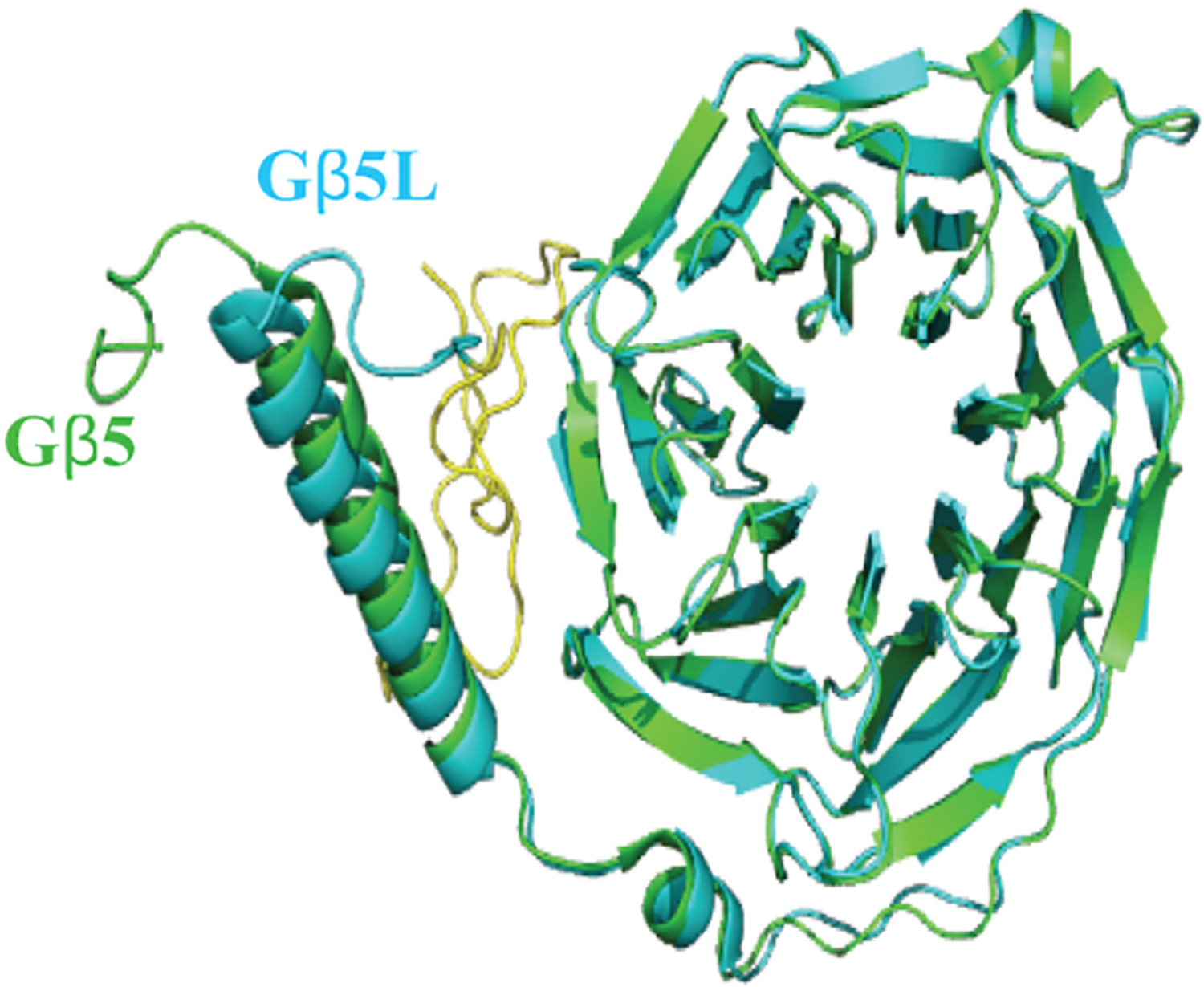
Gβ5 (Green) was modelled using coordinates obtained from PDB file 6N9G is superimposed with Gβ5L (Blue), homology model generated from Phyre web portal (http://www.sbg.bio.ic.ac.uk) using Gβ5 as template. The yellow colored region was encoded by the additional codon at the 5′ end.
Compared to Gβ1–4, due to its significant sequence differences, distinct tissue and even subcellular distributions, and the limited evidence for its ability form dimers with Gγ, Gβ5 has been characterized as unique. Gβ5 subunit dimerizes with R7 subfamily of RGS family proteins that contains a Gγ-like (GGL) domain. The R7 family consists of RGS6,7,9, and 11, and their structures are evolutionarily conserved in all animals from worm to human [60]. The Gβ5-R7 complex is widely expressed in the brain and nervous system. Comparison of Gβ5 and R7 family protein expression in brain is illustrated in Fig. 5. Gβ5-RGS7 is associated with motor control, reward behavior and nociception in mammals [61, 62]. In mammalian nervous systems, the R7 family proteins regulate key physiological functions such as synaptic transmission, memory formation, and light perception [62, 63]. The GGL domain of the R7 family is highly selective for Gβ5 and does not show association with other Gβ subunits. Evidence also shows that Gβ5-R7 complexes rapidly degrade in the absence of the other, indicating the mutual stabilization [64]. Though R7 mRNA levels remained intact, knocking out Gβ5 resulted in a reduction of the R7 family protein concentrations [65]. Interestingly, in vivo or cellular evidence for Gβ5-Gγ interactions is still not available [62]. Gβ5-R7 complexes form heterotrimers with the membrane anchoring R7 subunit, R7BP, which helps the expression and localization [63, 66]. There is also no in vivo evidence for Gα - Gβ5γ heterotrimers. However, recent evidence suggests that Gβ5-RGS7 can associate with several GPCRs, including the M3-muscarinic receptor [67] and orphan GPCRs [68]. Deletion of the third cytosolic loop of M3R reduced the sensitivity of mutant M3R to Gβ5-RGS7 complex. In reconstituted systems, Gβ5-RGS7 has been shown to attenuate Gi- and Gq-mediated signalling [67, 69], however, underlying molecular mechanisms are yet to be understood. Interestingly, a recent in vitro study showed that Gβ5 could associate with Gγ2, 3, 4, 5, 7, 8, and 12 subunits, independently of the Gα subunit [70]. Gβ5 and Gγ complexes also have been shown to activate effectors such as PLCβ2 [45] and AC [35], while they fail to activate ERK or JNK MAP kinases [71]. Gβ5L and Gγ2 overexpression in COS-7 cells showed PLCβ2 activation, indicating Gβ5L-Gγ2 interactions are possible [40]. However, no direct evidence has been shown to confirm Gβ5L-γ2 dimer formation in vivo. Further, interactions of Gβ5L with the other Gγ subtypes have not been reported.
Figure 5. Comparison of Gβ5 and R7 family protein expression in the brain.
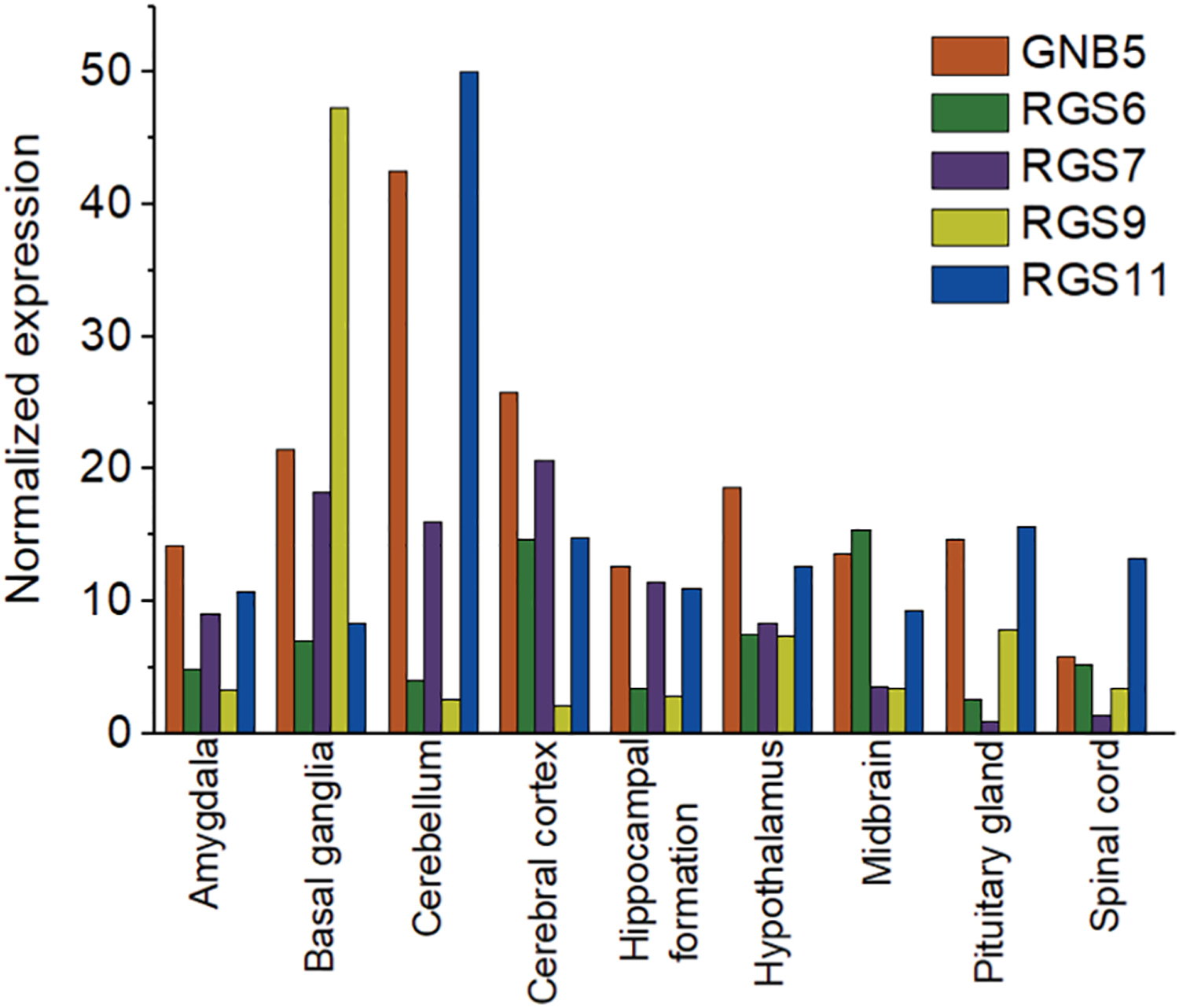
Consensus normalized RNA expression of Gβ5 and R7 family proteins in brain. RNA expression data were obtained from the FANTOM5 repository in the human protein atlas database.
The first crystal structure of Gβγ dimer was solved in 1996 [16]. Several crystal structures of Gβγ were solved thereafter, as complexes with different effectors [50, 72–74]. Based on the crystal structure of the transducin bound Gβγ dimer, the β subunit is primarily a seven-bladed β-propeller, and each propeller blade contains small four antiparallel strands spreading outwards from a central axis to generate the seven-fold symmetry [16]. Such symmetry has been found as the most favored arrangement in other proteins, including methylamine dehydrogenase and galactose oxidase [75]. This seven-fold symmetry of β strands also reflects in their amino acid sequence, which consists of seven structurally similar repeats and each contains approximately 40 amino acids with conserved core amino acids bounded by Trp-Asp (WD) [16] and Gly-His (GH) [76] and separated by a variable region. WD repeats are common to β subunits found in all organisms, including invertebrates [26]. The variable-length region is highly conserved within the family while differences have been noted in individual Gβ subunits. For example, the variable region between repeat two and three is different from repeat three and four while showing significant similarities in the same regions in evolutionarily distinct organisms [76]. Crystal structure data also indicates that WD repeats in the Gβ subunit initiate from the outermost strands of the β-sheet and terminate at similar positions in the adjacent β-sheet [16]. The outer β-stands of each blade in Gβ are made up of these variable regions in WD repeats. Therefore, each WD repeat generates four anti-parallel β-sheets, denoted by a-d [16]. Strand “a” is placed in the center of the tunnel, while strand “d” is found outside on the surface of the Gβ. This highly conserved Asp residue is also found in the loop, which connects strands “b” and “c” [16] (Fig. 6). However, the associated functions of these regions are still unknown.
Figure 6. Secondary structure prediction of Gβ2.
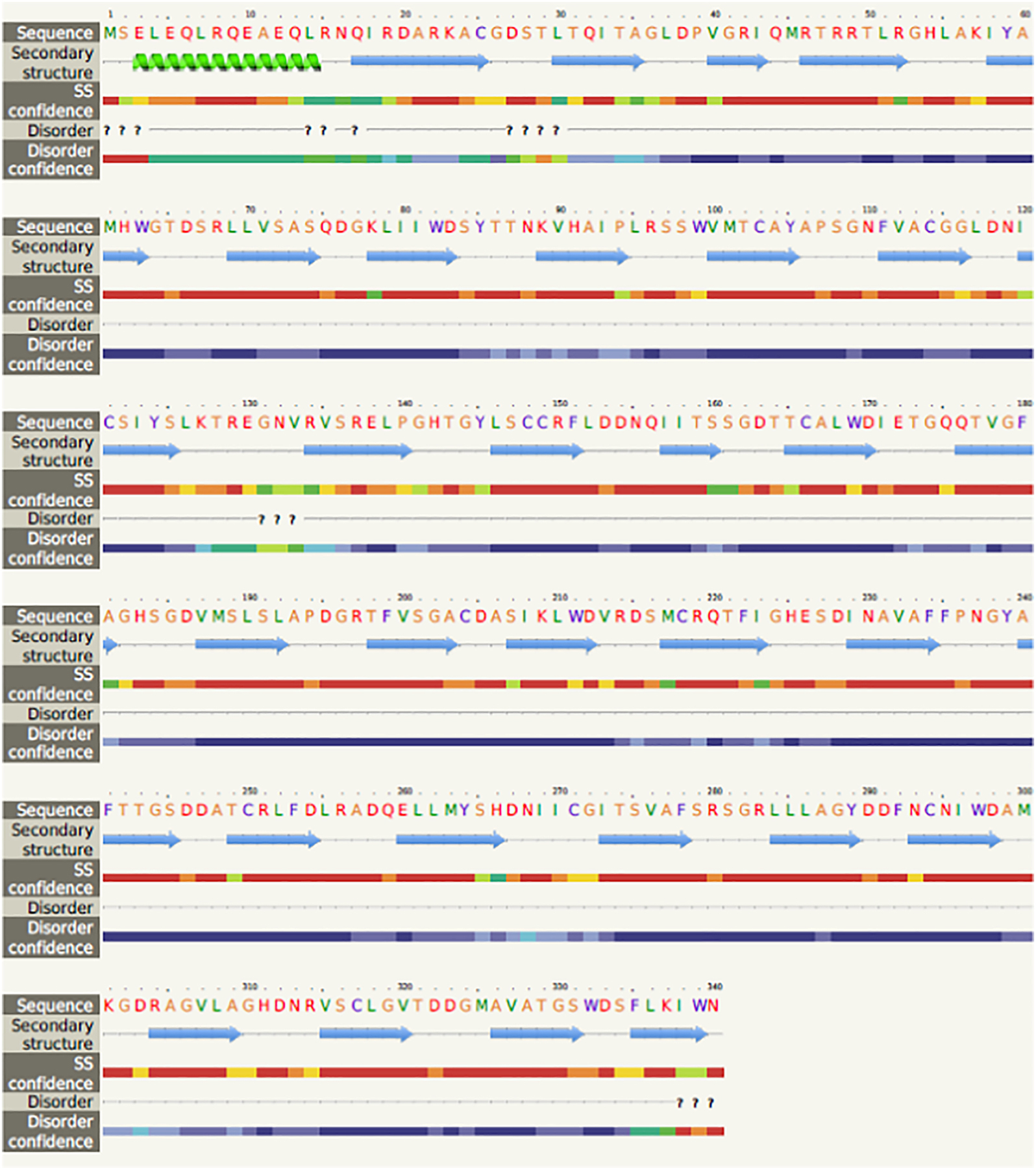
Gβ2 amino acid sequence (using Phyre2 web portal (http://www.sbg.bio.ic.ac.uk- Query sequence) is scanned through nr20 protein sequence database. The resultant multiple-sequence alignment is used to predict the secondary structure of the protein using PSIPRED (protein secondary structure prediction software). Gβ2 secondary structure was predicted using Gβ1 and other WD repeat containing proteins.
Furthermore, the Gβ subunit contains an N-terminal coiled-coil structure in the first 30 amino acids to help form three-stranded coiled-coil domains of G protein heterotrimers [77, 78]. Interactions between adjacent β sheets of Gβ are well characterized. Trp is a common residue found in WD repeats that interacts within the same repeat and Asp-His-Ser/Thr, resembling the catalytic triad of serine proteases buried in a non-polar environment between β-sheets [16]. The carboxylate group of Asp makes hydrogen bonds with main-chain amides in the positions of tight turns and adjacent conserved His in the loop, which connects the first and second strands of the same repeat. Consequently, such hydrogen bond arrangements stabilize the tight turn of one β-sheet to the outer strand of the β-sheet it follows [16].
Gβ subunits interact with Gα through two non-overlapping regions defined in the crystal structure of the G protein heterotrimer [17]. The Gβ residues in the switch interface (57, 59, 98, 99, 101, 117, 119, 143, 186, 228, and 332) and the N-terminal interface (55, 78, 80, and 89) interact with Gα. Ala mutants to replace the amino acids of Gβ interacting Gα were tested for their ability to form heterotrimers. Interestingly, Gβ mutants I80A, K89A, L117A, and W332A were defective in forming heterotrimers among all the mutants in this study, illustrating that these residues are critical determinants of Gα binding [17]. Gβ shares a common interface to interact with both Gα subunits and downstream effectors [17]. In the alanine scan, the N-terminal interface of Gβ1 exhibited a decreased ability to activate AC2. The Gβ1 mutants L117A, and N143A resulted in decreased association with GRK2 while W99A and D228A mutants could no longer activate PLCβ2 [17]. Ala mutations of Gβ1 residues 55, 78, 80, 89, 99, and 228 disrupted K+ currents via GIRK1/GIRK4 activation.
Tissue and cell type-specific Gβ distribution in mammals
Tissue and cell-type-specific G protein expression in mammals ranges from ubiquitous to restricted based on tissue type. Here, we examine Gβ transcript expression levels in 45 human tissues from the FANTOM5 repository in the human protein atlas database [79]. Consensus normalized expression levels of Gβ subunits (NX) in 45 human tissues are shown in Fig. 7.
Figure 7. Tissue specific distribution of all 5 Gβ subtypes.
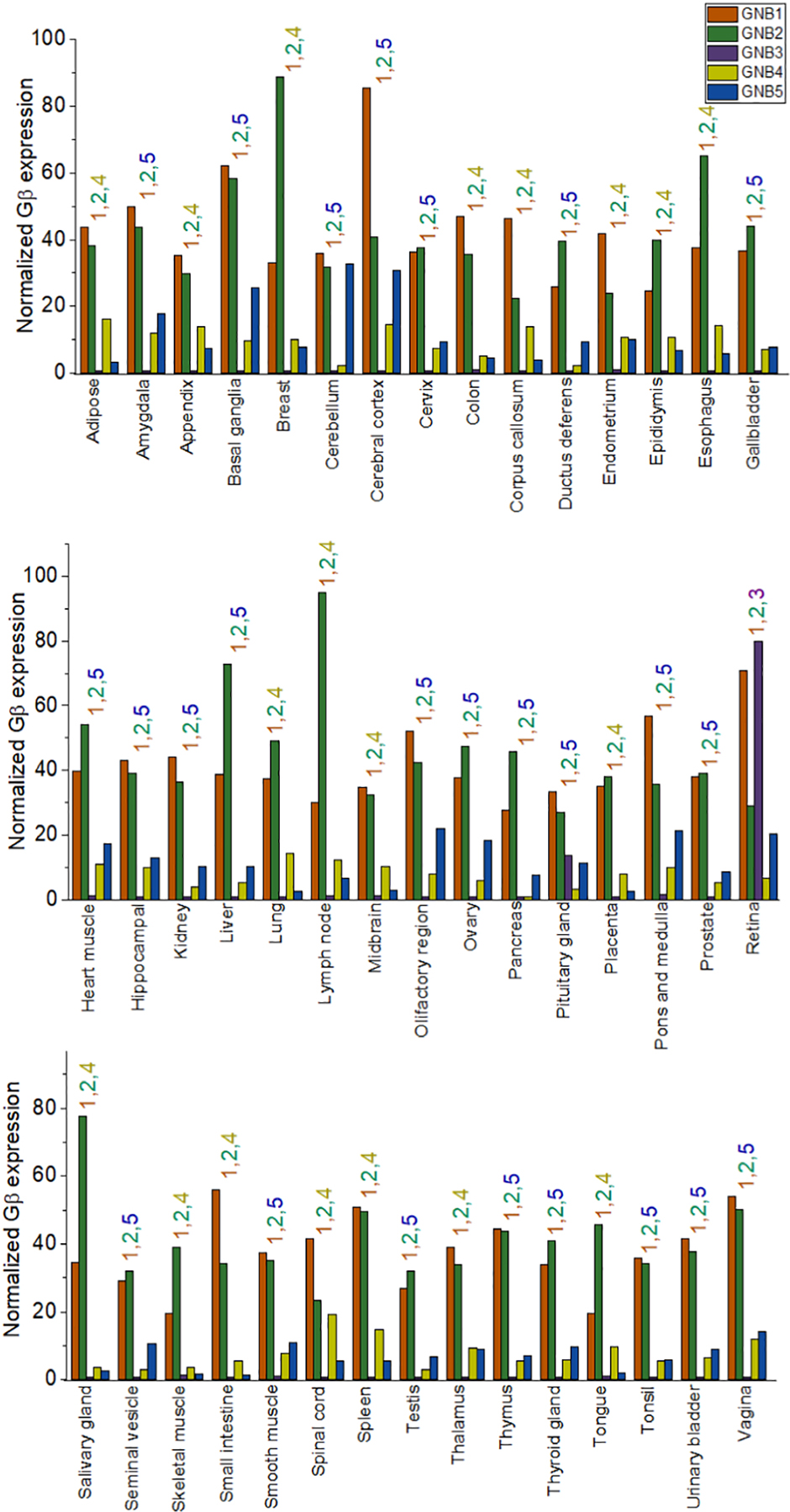
Consensus normalized RNA expression of Gβ subtypes in human tissues. RNA expression data for the distribution of Gβ subtypes in human tissues was obtained from the FANTOM5 repository in the human protein atlas database. Though Gβ subtypes exhibited higher sequence homology, they also show tissue type-specific distributions. The coloured-numbers above each tissue type represent the three most abundant Gβ types.
Gβ1 is ubiquitously expressed in all tissues in mammals [80–83]. According to protein atlas data, almost all tested human tissues have a higher expression of Gβ1 compared to other Gβ subunits. The highest expression of Gβ1 is observed in the human putamen and small intestine, whereas in mice, Gβ1 is abundant in the retina. Gβ3 is expressed at higher levels in the human retina along with Gβ1 and Gβ2 [84]. Several other reports also showed that Gβ3 is highly expressed in cone photoreceptors and bipolar cells of the retina [83, 85–88]. Gβ3S protein was found in lymphocytes and platelets of patients with essential hypertension [89]. From human atlas data, tissue-specific expression of Gβ4 exhibits a unique pattern in many cells. Compared to Gβ1 and Gβ2, Gβ4 expression widely varies in different tissues. Independent studies showed that Gβ4 is highly expressed in skin fibroblasts [90], brain, eye, heart, testis [54], lung, and placenta, while it is less abundant in the brain, spleen, and heart [91, 92]. Mouse and human Gβ5 are predominantly expressed in the brain [45], whereas human Gβ5 is also abundant in the pancreas, kidney, and heart [47]. In the human brain, Gβ5 is detectable in all regions but less abundant in the corpus callosum and spinal cord. Compared to other Gβ subunits, Gβ5 has a more restricted tissue expression pattern [45].
2.2. Gγ subtypes and their diversity in the animal kingdom
Gγ subtypes across evolution
Gβγ proteins play a crucial role in the pheromone response pathway in S. cerevisiae [93, 94]. This pathway promotes cell fusion and the generation of the diploid state [95]. Multiple types of Gγ subtypes are expressed in other fungal types [96]. S. pombe expresses a single Gβγ dimer with the Gγ protein, git11, which is involved in pheromone responses [97]. N. crassa expresses the GNG-1 subunit, which plays a role in fertility and asexual development and has a gene structure similar to the Gγ genes of mammals [98].
Like mammals, invertebrates express more than one Gβγ isoform, suggesting subunit evolution over time. For instance, C. elegans expresses two types of Gγ proteins, GPC-1 (guanine nucleotide-binding protein, gamma polypeptide-1) and GPC-2 (guanine nucleotide-binding protein, gamma polypeptide-2), which are similar to the vertebrate Gγ 1/9/11 subfamily and Gγ 13 respectively [25]. Gβγ in C. elegans controls spindle orientation during the early embryonic development stage [99–101]. The D. melanogaster genome encodes two Gγ proteins, Gγ1 and Gγ30, and these are again similar to vertebrate Gγ 1/9/11 subfamily and Gγ 13, respectively. Gγ proteins of D. melanogaster control cell division in neuronal and sensory organs along with three Gβ counterparts [102]. Additionally, Gβγ signalling controls wing expansion in these animals [103].
Fish and mammals have more Gγ subunits than fungi and invertebrates, indicating evolution of a broader signalling footprint. Studies also suggest that some fish species have more Gγ subunits than mammals [104]. Humans and mice have 12 Gγ subunit isoforms [7]. Genomic analyses have identified that there are at least 17 Gγ subunits in zebrafish. Most of these Gγ subtypes are orthologues of human Gγ subtypes. Four Gγ subunits in zebrafish, gng14, gng15, gng16, and gng17, do not have mammalian Gγ orthologues. An orthologue for human Gγ11 was not found in zebrafish; however, two Gγ paralogues, known as gngt2b and gng12b, have been identified. Their high sequence similarities showed that Gγ subunits found in fish species must have evolved from the same ancestor as in mammalian species [25]. This also suggests that the additional Gβγ subunits found in fish species might have evolved after divergence from the common ancestor or been lost in the evolution from fish to mammals [104]. Genomic data of the Atlantic cod, Gadus morhua, showed that Gγ1 and Gγ11 are redundant. It is not yet known whether these subunits are functionally redundant at the protein level as well [25].
Structural differences in mammalian Gγ types
The first Gγ subunit was identified by John Hildebrandt in 1983 in a study of human erythrocyte stimulatory and inhibitory regulatory G proteins [105]. The identified protein had a molecular weight of 5 kDa and complexed with the Gβ subunit [105]. All Gγ subunits are small proteins comprising two α-helical segments. Gγ subunits have no obvious tertiary structure. The N-terminal helix forms a coiled-coil structure with the N-terminal helix of Gβ, while the remainder interacts with the stem of the β-propeller domain of Gβ (Fig. 8). These interactions provide stability to the Gβγ dimer [16]. Gγ subunits are products of two exons that encode two domains of the protein, the N-terminal and C-terminal helices. The first exon encodes ~27–32 amino acids of the N-terminal helix. The second exon encodes ~40 amino acids that form the C-terminal helix. The first residue encoded by exon 2 is almost always a Val, which forms a hinge between the protein components formed by the two domains of the protein, and it is a primary site of Gβγ interaction. This Val residue associates with the N-terminal α-helix and the propeller domain of the Gβ subunit [106]. This residue is shown inside the red square in Fig. 9.
Figure 8. Molecular model structure of Gβγ dimer of Gβ1 and Gγ1, using coordinates obtained from PDB file 1TBG.
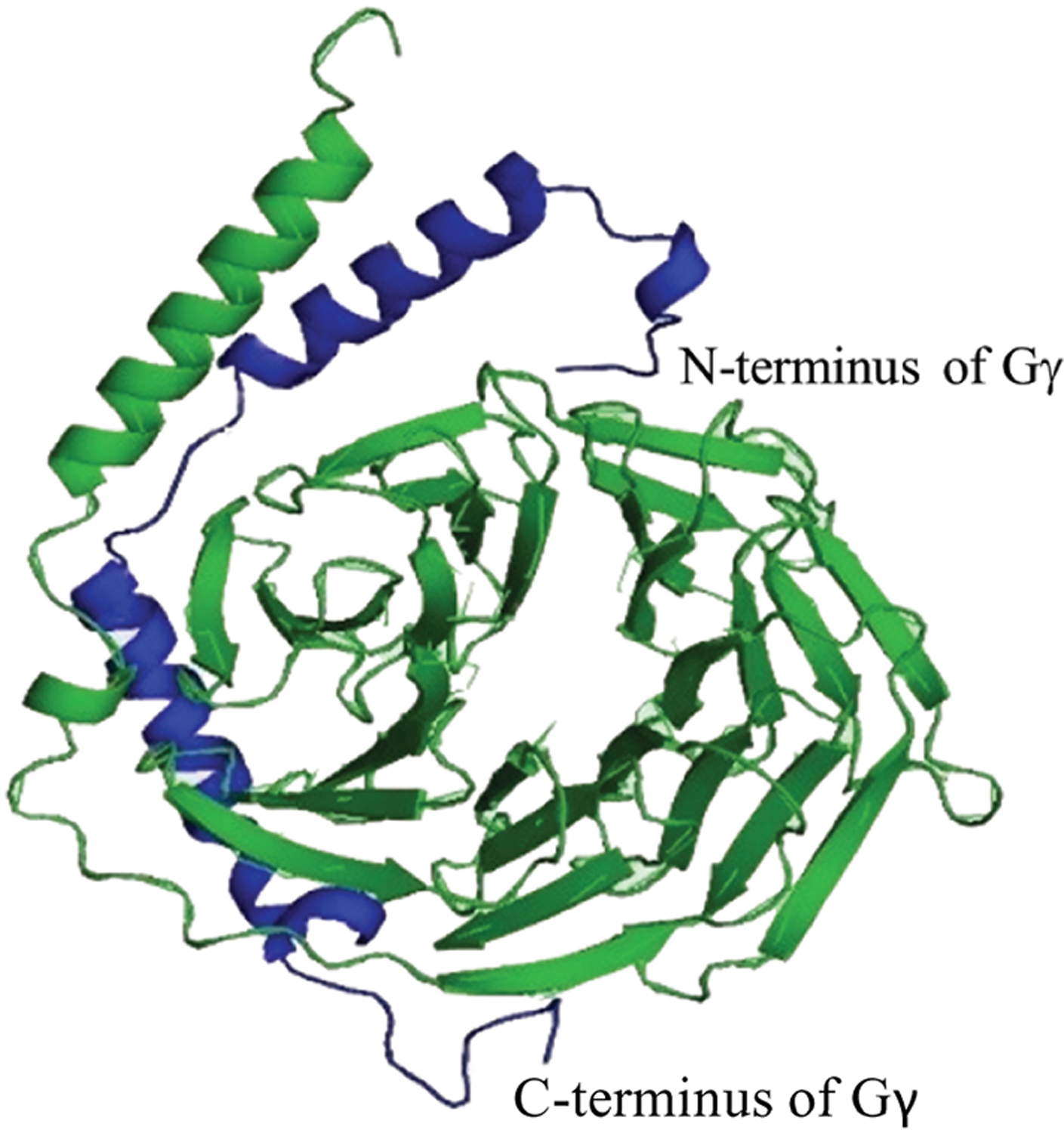
Gβ1 and Gγ1 are shown in green and blue respectively. The N-terminal helix of Gγ1 interacts with the N-terminal helix of Gβ1 to form a coiled-coiled structure. The C-terminal helix of Gγ1 shows interactions with the β-propeller domain of Gβ1.
Figure 9. Sequence alignment of N-terminal helices of Gγ subtypes.
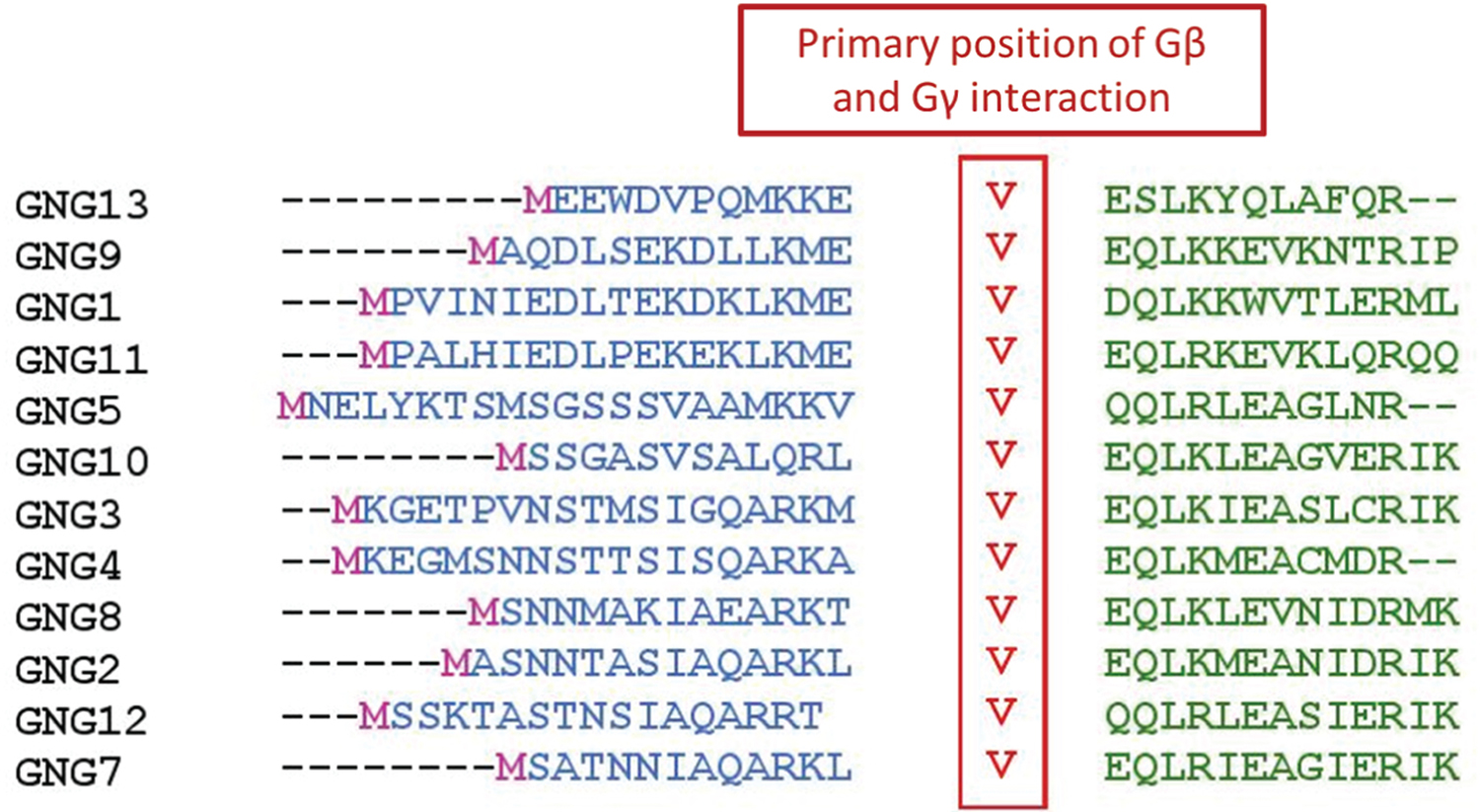
Primary point of interaction between Gγ and Gβ protein chains is a Val residue shown here in red. Amino acid sequences of Gγ subunits were obtained from the National Center for Biotechnology Information (NCBI) database for proteins. Sequence alignments were performed using the MUSCLE sequence alignment tool by EMBL-EBI.
Compared to Gβ subunits, Gγ subunits show significantly higher sequence diversity [107, 108]. Investigations into sequence diversity among Gγ subunits and their implications in cellular signalling remain at an early stage. However, recent findings show that the diversity among Gγ proteins in Gβγ dimer could be responsible for distinct signalling outcomes since cells can express distinct subsets of Gγ subtypes [48, 109]. Mammals, including humans, express 12 Gγ subunit subtypes, Gγ1 to Gγ13 (no Gγ6) [109]. Complete sequence analysis of the 12 human Gγ subtypes is shown in Table 2. Obtained sequence similarity scores show wide and discrete series of similarity scores ranging from 20–80 %. This range was much narrow for Gβ subunits, which indicate that the Gγ subunit is likely an important contributor to Gβγ signalling diversity
Table 2:
Sequence similarity scores of complete sequence analysis of human Gγ subunits.
| Gγ1 | Gγ2 | Gγ3 | Gγ4 | Gγ5 | Gγ7 | Gγ8 | Gγ9 | Gγ10 | Gγ11 | Gγ12 | Gγ13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gγ1 | 100 | |||||||||||
| Gγ2 | 35.1 | 100 | ||||||||||
| Gγ3 | 32.1 | 70.7 | 100 | |||||||||
| Gγ4 | 30.7 | 70.7 | 68.0 | 100 | ||||||||
| Gγ5 | 24.7 | 41.6 | 44.2 | 42.9 | 100 | |||||||
| Gγ7 | 37.8 | 65.2 | 58.0 | 58.0 | 48.5 | 100 | ||||||
| Gγ8 | 31.4 | 68.6 | 55.7 | 58.6 | 42.9 | 51.4 | 100 | |||||
| Gγ9 | 62.9 | 35.7 | 32.0 | 32.0 | 24.7 | 40.6 | 31.4 | 100 | ||||
| Gγ10 | 30.4 | 50.7 | 50.7 | 46.4 | 54.4 | 51.5 | 50.0 | 36.2 | 100 | |||
| Gγ11 | 75.7 | 33.8 | 32.4 | 29.7 | 27.6 | 41.1 | 29.7 | 60.3 | 28.8 | 100 | ||
| Gγ12 | 34.2 | 58.9 | 57.5 | 52.1 | 44.4 | 70.8 | 50.7 | 36.1 | 43.1 | 36.1 | 100 | |
| Gγ13 | 27.0 | 28.2 | 25.6 | 28.3 | 22.9 | 28.2 | 28.2 | 28.2 | 23.1 | 33.3 | 20.5 | 100 |
Since Gγ sequences show a wide range of similarities, a more extensive analysis of the similarity of the Gγ domains was undertaken. Tables 3 and 4 show sequence comparison of N-terminal and C-terminal helices of the 12 human Gγ subunits. As shown in Table 3, sequence similarity scores of N-terminal helices of human Gγ subunits are much lower than the whole sequence similarity scores. This shows that the N-terminal amino acid residues are less conserved among Gγ subunits. This could also suggest that the basis of differences among Gγ subtypes reflects N-terminal sequence differences.
Table 3:
Sequence similarity scores of the N-terminal helices of human Gγ subunits.
| Gγ1 | Gγ2 | Gγ3 | Gγ4 | Gγ5 | Gγ7 | Gγ8 | Gγ9 | Gγ10 | Gγ11 | Gγ12 | Gγ13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gγ1 | 100 | |||||||||||
| Gγ2 | 31.2 | 100 | ||||||||||
| Gγ3 | 27.8 | 54.5 | 100 | |||||||||
| Gγ4 | 22.6 | 61.3 | 61.3 | 100 | ||||||||
| Gγ5 | 24.2 | 27.3 | 33.3 | 33.3 | 100 | |||||||
| Gγ7 | 31.2 | 66.7 | 59.3 | 48.1 | 44.4 | 100 | ||||||
| Gγ8 | 28.6 | 71.4 | 46.4 | 46.4 | 28.6 | 46.4 | 100 | |||||
| Gγ9 | 60.7 | 28.6 | 27.3 | 27.3 | 19.4 | 28.6 | 32.1 | 100 | ||||
| Gγ10 | 29.6 | 48.1 | 44.4 | 40.7 | 48.1 | 51.9 | 42.9 | 39.3 | 100 | |||
| Gγ11 | 65.6 | 28.1 | 28.1 | 18.8 | 28.6 | 34.4 | 21.9 | 53.1 | 21.9 | 100 | ||
| Gγ12 | 19.4 | 51.6 | 51.6 | 41.9 | 35.5 | 58.8 | 51.6 | 22.6 | 41.9 | 19.4 | 100 | |
| Gγ13 | 23.3 | 29.2 | 29.2 | 29.0 | 27.3 | 25.0 | 25.0 | 29.2 | 25.0 | 33.3 | 16.7 | 100 |
Table 4:
Sequence similarity scores of the C-terminal helices of human Gγ subunits.
| Gγ1 | Gγ2 | Gγ3 | Gγ4 | Gγ5 | Gγ7 | Gγ8 | Gγ9 | Gγ10 | Gγ11 | Gγ12 | Gγ13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gγ1 | 100 | |||||||||||
| Gγ2 | 38.1 | 100 | ||||||||||
| Gγ3 | 35.7 | 83.3 | 100 | |||||||||
| Gγ4 | 36.4 | 75.0 | 70.5 | 100 | ||||||||
| Gγ5 | 25.0 | 50.0 | 50.0 | 50.0 | 100 | |||||||
| Gγ7 | 42.9 | 64.3 | 57.1 | 61.9 | 41.2 | 100 | ||||||
| Gγ8 | 33.3 | 66.7 | 61.9 | 64.3 | 50.0 | 54.8 | 100 | |||||
| Gγ9 | 64.3 | 40.5 | 35.7 | 35.7 | 29.3 | 48.8 | 31.0 | 100 | ||||
| Gγ10 | 31.0 | 52.4 | 54.8 | 47.6 | 56.1 | 51.2 | 54.8 | 34.1 | 100 | |||
| Gγ11 | 83.3 | 38.1 | 35.7 | 38.1 | 26.8 | 46.3 | 35.7 | 65.9 | 34.1 | 100 | ||
| Gγ12 | 45.2 | 64.3 | 61.9 | 57.1 | 48.8 | 80.5 | 50.0 | 46.3 | 43.9 | 48.8 | 100 | |
| Gγ13 | 29.5 | 31.8 | 27.3 | 34.1 | 20.9 | 32.6 | 29.5 | 34.9 | 25.6 | 32.6 | 30.2 | 100 |
As shown in Table 4, the C-terminal helices of human Gγ subtypes show higher sequence similarity than the N-terminal helices, congruent with previous studies [48, 106, 110]. This could indicate that functionally important core amino acid residues in Gγ subunits could be in the C-terminal helix. Sequence variation in the N-terminal domain might have functional consequences for distinct Gβγ signalling events or modes of regulation.
Except for Gγ13, all Gγ subtypes possess a conserved Phe residue in the C-terminal region (Fig. 10). This residue acts as the final contact point of Gγ with Gβ. It has also been shown that the amino acids from the above Phe to the prenylated Cys, termed hereafter pre-CAAX region, plays a prominent role in regulating the PM affinity of Gβγ [48]. The sequence comparison of pre-CAAX of the 12 Gγ subtypes is shown in Table 5. Sequence similarity scores of the pre-CAAX and CAAX regions of Gγ subunits are higher than the similarity scores obtained for the complete C-terminal helix. This shows that the conserved pre-CAAX and CAAX regions of Gγ again reflect the high sequence similarity of the C-terminal helices. This also indicates that the functionally conserved amino acid residues of Gγ are located in the pre-CAAX and CAAX regions (Fig. 11).
Figure 10. Sequence alignment of pre-CAAX and CAAX regions of Gγ subtypes.
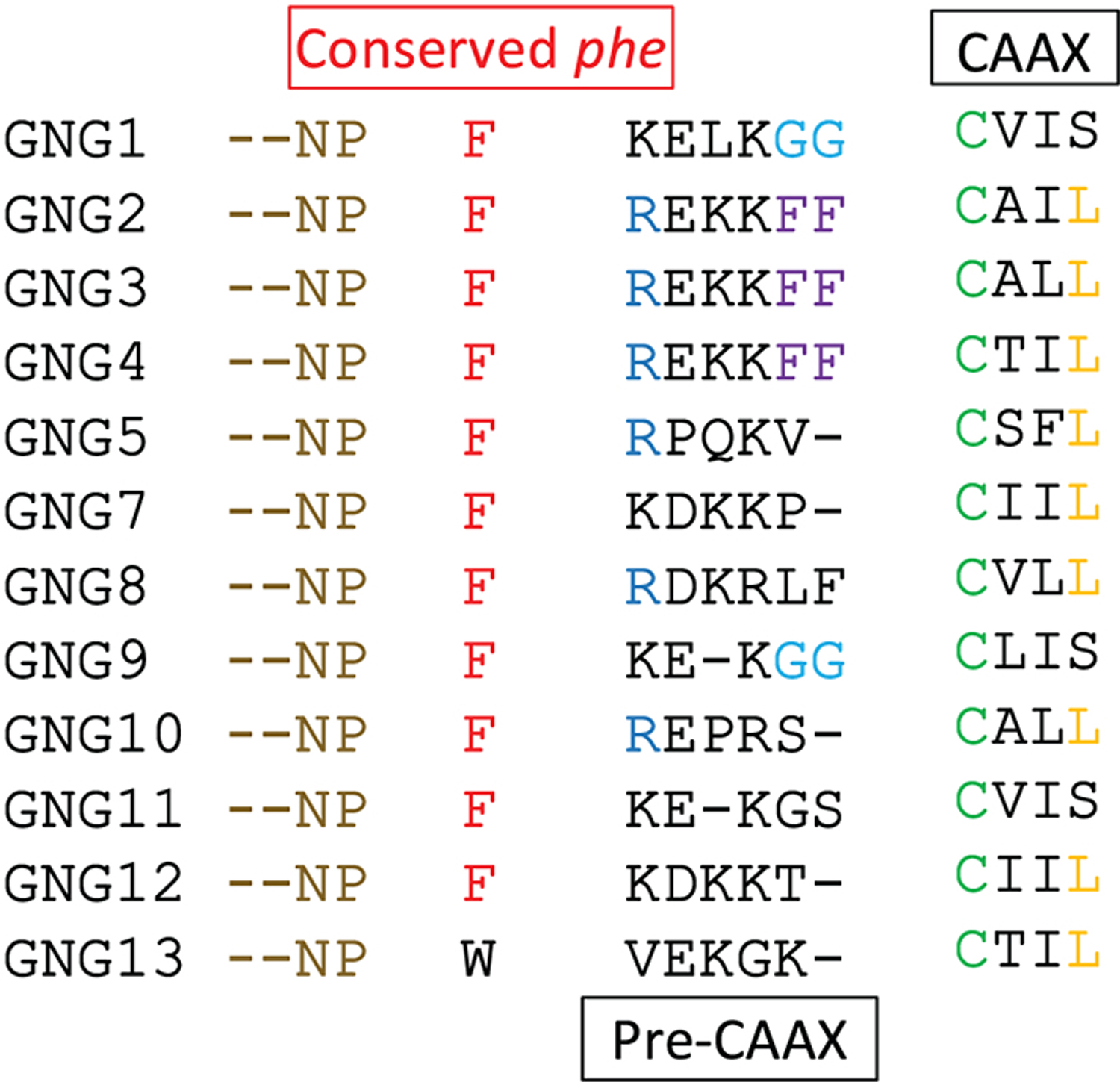
The point of prenylation, a Cys residue is shown in green. A Phe residue is conserved in the pre-CAAX region of all Gγ subtypes, except in Gγ13. The conserved Phe residue is shown in red. Sequence alignments were performed using the MUSCLE sequence alignment tool by EMBL-EBI.
Table 5:
Sequence similarity scores of the pre-CAAX and CAAX regions of human Gγ subunits.
| Gγ1 | Gγ2 | Gγ3 | Gγ4 | Gγ5 | Gγ7 | Gγ8 | Gγ9 | Gγ10 | Gγ11 | Gγ12 | Gγ13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gγ1 | 100 | |||||||||||
| Gγ2 | 45.5 | 100 | ||||||||||
| Gγ3 | 36.4 | 90.9 | 100 | |||||||||
| Gγ4 | 45.5 | 90.9 | 81.8 | 100 | ||||||||
| Gγ5 | 27.3 | 45.5 | 45.5 | 45.5 | 100 | |||||||
| Gγ7 | 45.5 | 54.5 | 45.5 | 54.5 | 40.0 | 100 | ||||||
| Gγ8 | 27.3 | 54.5 | 63.6 | 54.5 | 36.4 | 45.5 | 100 | |||||
| Gγ9 | 81.8 | 45.5 | 36.4 | 45.5 | 20.0 | 50.0 | 27.3 | 100 | ||||
| Gγ10 | 27.3 | 54.5 | 63.6 | 45.5 | 40.0 | 30.0 | 54.5 | 30.0 | 100 | |||
| Gγ11 | 81.8 | 45.5 | 36.4 | 45.5 | 20.0 | 50.0 | 36.4 | 80.0 | 40.0 | 100 | ||
| Gγ12 | 45.5 | 54.5 | 45.5 | 54.5 | 40.0 | 90.0 | 45.5 | 50.0 | 30.0 | 50.0 | 100 | |
| Gγ13 | 45.5 | 45.5 | 36.4 | 54.5 | 20.0 | 40.0 | 27.3 | 50.0 | 30.0 | 50.0 | 40.0 | 100 |
Figure 11. Sequence alignment of C-terminal helices of Gγ subtypes.

Pre-CAAX and CAAX regions of Gγ primarily govern Gβγ-PM interactions. Gγ types can be grouped based on their Pre-CAAX similarities. Sequence alignments were performed using the MUSCLE sequence alignment tool by EMBL-EBI.
Tissue and cell type-specific Gγ distribution in humans
mRNA profiling of human Gγ proteins generally shows ubiquitous distribution. However, each Gγ also shows distinct cell and tissue type-specific distribution patterns (Fig. 12), further suggesting that Gγ is a contributor to the functional diversity of Gβγ signalling. Transcript profiling data from the Human Protein Atlas (Fig. 12) shows that Gγ1 (GNG1) is hardly present in any tissue other than the retina. GNG1 expression in the retina showed the highest expression for any Gγ subtype out of all the tissues. Besides GNG5, GNG10, GNG11, and GNG12, other Gγ subtypes were present in trace amounts in most tissues. GNG3 was found to be abundant in the cerebellum and cerebral cortex. However, GNG3 is present only in trace amounts in the midbrain region. GNG5 was found to be the most abundant Gγ subtype in the human body. GNG5 shows exceptionally high amounts of expression in muscle tissues. GNG5 and GNG12 show high amounts of expression in organs in the digestive tract. GNG8 does not show significant expression in any of the tissues in the human body. GNG13 is also another Gγ subtype with reduced expression in most tissues. It only shows significant expression in the cerebellum, cerebral cortex, and retina.
Figure 12. Consensus normalized RNA expression levels of Gγ subtypes in human tissues.
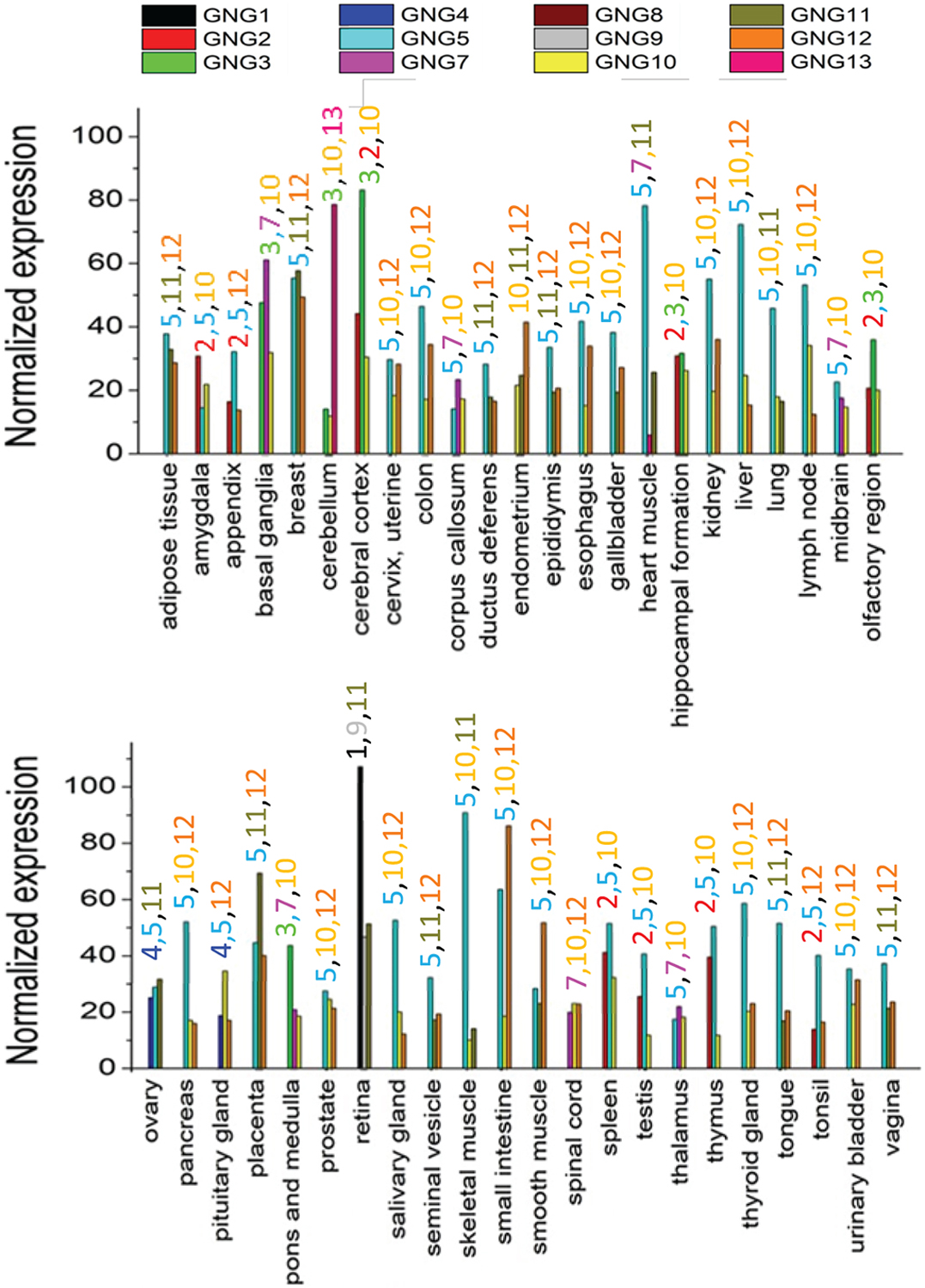
RNA expression data for the distribution of Gγ subtypes in human tissues was obtained from the FANTOM5 repository in the human protein atlas database. Three Gγ subtypes that are shows the highest expression in each tissue are given. At least at the RNA-level, Gγ shows a tissue type-specific distribution. The coloured-numbers labelled above each tissue type represent Gγ subtypes.
3. Gβ-subtype-specific regulation of signalling
GPCR and G protein activation initiate subsequent downstream cellular signalling events resulting in diverse physiological functions [111]. In this section, the determinants of signalling specificity by G protein subunits and specificity of heterotrimer combinations towards specific GPCRs are discussed.
GPCR activation regulates many signalling pathways, generally aligned with coupling to a specific Gα subunit in the heterotrimer including Gi/o, Gs, Gq, G12/13 and Gt, while the associated Gβγ subunits also regulate a large number of effectors [112]. G protein heterotrimers are comprised of 16 Gα, 7 Gβ (including known variants), and 12 Gγ have been identified in mammalian species that interact with GPCRs (Table 6) [41, 113, 114].
Table 6.
Isoforms of G proteins
| Subunit | Isoforms |
|---|---|
| α | s, olf, i1, i2, i3, oa, t1, t2, gus, z, q, 11, 14, 15, 12, 13 |
| β | 1, 2, 3, 3S, 4, 5, 5L |
| γ | 1, c (9), 11, 2, 3, 4, 5, 8, 10, 7, 12, 13 |
Numerous combinations of Gαβγ can form heterotrimers (Fig. 13 A, PDB Id: 1GOT[15]), and heterotrimer activation upon stimulation of GPCRs drives distinct cell- and tissue type-dependent signalling outcomes [41, 114]. Even though hundreds of (~960) heterotrimeric Gαβγ combinations (16Gα x 5Gβ x 12Gγ) and 60 heterodimeric Gβγ (5Gβ x 12Gγ) are theoretically possible, formation of all these different combinations is unlikely, and unique affinities among specific subunit types have been reported. Some examples include the affinity of all Gγ subunits to dimerize with Gβ1 and Gβ4, while Gβ2 and Gβ3 have show attenuated interactions with Gγ1/Gγ11[8, 115]. Additionally, weak affinities of Gβ5 for Gγ subunits have also been reported [8, 115]. In part because of specificity in dimer formation, Gβγ can interact with specific GPCRs [8]. Detailed discussion for different and specific heterotrimer interactions are in the subsequent sections below.
Figure 13. Structure of the Gαβγ transducin heterotrimer (PDB ID: 1GOT).
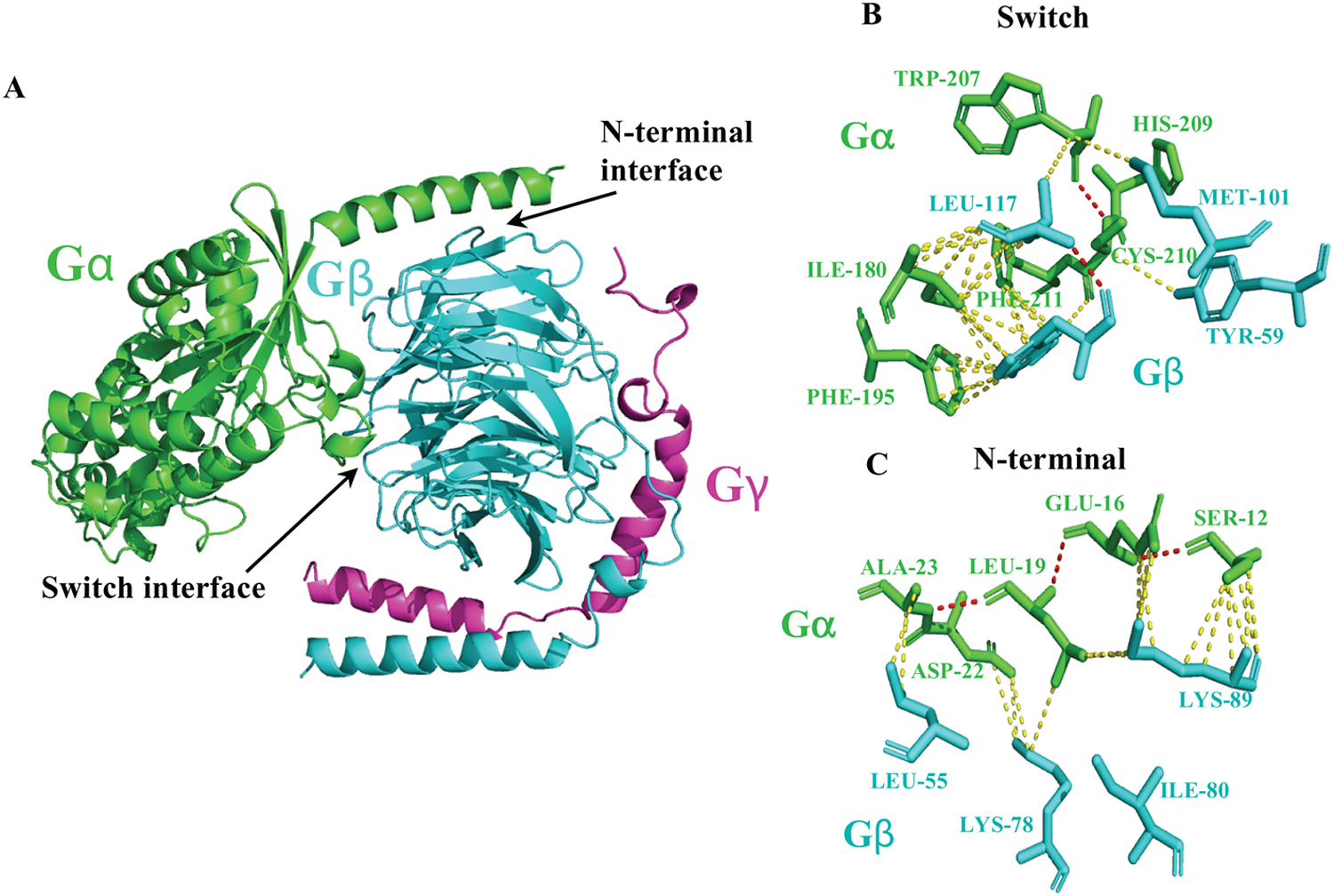
(A) Here, we indicate the G protein subunit interaction sites such as the helix at the N-terminus of Gα and the first blade of Gβ defined as the switch interface and the N-terminal interface, respectively. Determinants of polar interactions (red dashed lines) such as hydrogen bonds and ion pairs, and hydrophobic interactions (yellow dashed lines) between amino acids of Gα and Gβ subunits in the switch interface (B) and N-terminal interface (C) are shown. Hydrophobic residues such as Iso180, Phe195, Trp207, His209, Cys210, and Phe211 from Gα, interact with Tyr59, Trp99, Met101 and Leu117 residues of Gβ, in the switch interface. The N-terminal interface comprises of the Gα helix containing Ser12, Glu16, Leu19, Asp22, and Ala23, interacting with Leu55, Lys78, Iso80, and Lys89 on Gβ. In Addition, Lys 89 from Gβ is recognized as a conserved residue to facilitate dynamic ion-pair interactions with Gα.
3.1. The specificity of Gβ signalling
Changes in Gβ subunit signalling have been linked to pathophysiological effects such as abnormalities in brain morphology, eye electrophysiology, cardiac functions, vision, and embryonic defects in various animal models[116]. The crystal structure of heterotrimeric G protein transducin complex (Gαtβγ) shows that the β-propeller domain interacts with both Gα and Gγ subunits[15] (Fig. 1A). Key interactions occur between the switch I and II regions of Gα and Gβ (Fig. 1A, B). This region is important for conformational changes upon GDP to GTP exchange on Gα. Other interactions are identified between the helix at the N-terminus of Gα and the first blade of Gβ (Fig. 13C). These two regions are defined as the switch interface and the N-terminal interface [15] (Fig. 13). The switch interface includes hydrophobic residues from both Gα and Gβ; thus, it generates a network of hydrophobic interactions stabilized by hydrophilic interactions such as hydrogen bonds and ion pairs (Fig. 13B and Table 7). In the switch interface, hydrophobic residues such as Iso 180, Phe 195, Trp 207, His 209, Cys 210, and Phe 211 from Gα, interact with Tyr 59, Trp 99, Met 101 and Leu 117 residues of Gβ [15]. The N-terminal interface consists of the Gα helix containing Ser 12, Glu 16, Leu 19, Asp 22, and Ala 23, interacting with Leu 55, Lys 78, Iso 80, and Lys 89 on Gβ (Fig. 13C and Table 7). Additionally, Lys 89 from Gβ was identified as a conserved residue for facilitating dynamic ion-pair interactions with Gα [15]. Though these interacting residues are from the transducin heterotrimer, most residues are conserved across other Gα and β subunits as well. However, careful consideration should be given to other specific interactions between different Gαβ combinations in individual heterotrimers [117].
Table 7.
Interactions between G protein α and β subunits [15]
| G protein | Residues involved (PDB ID: 1GOT) | |
|---|---|---|
| Switch interface | N-terminal interface | |
| Gα | Iso180, Phe195, Trp207, His209, Cys210, Phe211 | Ser12, Glu16, Leu19, Asp22, Ala23 |
| Gβ | Tyr59, Trp99, Met101, Leu117 | Leu55, Lys78, Iso80, Lys89 |
3.2. Gβ-subtype-dependent Gβγ combinations and their control of cellular signalling
Specific roles for Gβ subunits in diverse signalling processes have been identified [25]. For example, Gβ subunit specificity in inhibiting voltage-gated N-type calcium channels has been shown previously [118]. Gβγ-mediated inhibition of N-type calcium channels by α2-adrenergic receptor (α2AR) activation was measured in rat superior cervical ganglion (SCG) neurons. Electrophysiological measurement of calcium currents via voltage-dependent calcium channels was performed. The injection of Gβ DNA into rat SCG neurons was performed to generate Gβ subtype levels in these cells. Among different Gβ subtypes, Gβ1 and Gβ2 exhibited greater inhibition of calcium currents. Additionally, compared to Gβ1 and Gβ2, Gβ5 showed a diminished effect while Gβ3 and Gβ4 were unable to regulate calcium currents [118]. In the same study, yeast two-hybrid screening was utilized to identify specific protein-protein interactions with Gβ subtypes and calcium channels. The interactions of a consensus sequence motif QXXER in calcium channels with Gβ has been suggested for specific regulatory effects on calcium currents [118]. For example, a QXXER peptide was identified from AC2 that inhibited the interactions between Gβγ and effectors, including PLCβ3, AC2, GIRK channels, and GRK2 [119]. Gβ subtype specificity was also investigated in other expression systems that incorporated effectors interacting with Gβ. Co-expression of Gβ1, together with AC5 or AC6, showed reduced cAMP generation in COS-7 cells, while co-expression of Gβ5 had no effect [120]. Gβ1, Gβ2, and Gβ4 showed enhanced activation of PLCε, while Gβ3 had a weaker effect [121]. Specific Gβγ combinations were shown to activate PI3K using purified recombinant proteins. There, Gβ1γ2, Gβ2γ2, and Gβ3γ2 induced comparable PI3K activity while Gβ5γ2 had no effect [122]. Another study demonstrated Gβ1γ2 and Gβ3γ2 induced activation of PKD and subsequent control of PKC-mediated cellular processes [123].
Different Gβ subtypes have been shown to involved in various physiological events. For example, Gβ2 has been shown to regulate GIRK channel function and is implicated in regulating heart rate and cardiac function [42]. Gβ3 is highly expressed in cone photoreceptors of the mammalian retina [86] and involved in light-induced photoreceptor activation and subsequent transducin signalling [85]. Additionally, Gβ3 also regulates signalling in bipolar cells in the mammalian retina. Gβ4 is involved in cardiac signalling through M2-muscarinic receptor activation, modulating GIRK channel activity and regulating heart rhythm [91]. Regulation of cardiac and neuronal signalling in mouse and zebrafish by Gβ5 has also been demonstrated [116, 124, 125].
3.3. Evidence for specific Gαβγ heterotrimer combinations and their interaction with preferred GPCRs
Preferential heterotrimer-GPCR interactions by specific Gβγ subtypes have been reported [126]. In this section, different experimental approaches and systems designed to investigate such interactions and corresponding evidence for specific Gβγ subtypes-GPCR interactions are described. Combinations of in vitro and in vivo biochemical assay systems including live cell imaging, electrophysiological measurements of ion currents in cultured cells, studies with purified Gβγ subtypes reconstituted with GPCRs/effectors, co-immunoprecipitation coupled with proteomic analysis have been used to probe heterotrimer-GPCR interactions and associated Gβγ subtypes specificity. In order to regulate expression of Gβγ subtypes in endogenous systems, techniques such as ribozyme suppression, injection of Gβγ subtype specific DNA into cells, use of Gβγ subtype-specific antisense suppression by injecting antisense oligomers into cells, and siRNA screening approaches have been used in conjunction with the earlier described experimental platforms. Thus, evidence from these experiments details the interaction of Gβγ subunits with receptor and the influence of Gβ subtypes in defining specific GPCR-heterotrimer preferences.
Combined in vivo and in vitro studies have been conducted in cultured cells to investigate specific GPCR-heterotrimer interactions. Specific Gβγ combinations have preferred interactions with α2AR to regulate signalling in in vitro systems such as cultured cells and in synapses in the central nervous system [115, 127, 128]. For instance, synaptosomes from transgenic mouse brain tissue have been isolated, followed by co-immunoprecipitation (co-IP) studies and proteomic analysis have demonstrated Gβ2γ2, Gβ2γ3, Gβ2γ4, and Gβ4γ12 are preferred heterotrimer partners for α2AR[115]. Plasma membrane preparations of Sf9 cells reconstituted with purified G protein subunits were used to examine the role of Gβγ dimers in signalling specificity at receptor level. When radioligand binding assays using the agonist [3H]-UK14304 was performed, the α2AR preferred heterodimers containing Gβ3 either with Gγ4, Gγ10, and Gγ11 and subsequent heterotrimer formation with Gαi [127]. This evidence suggests that specific G protein combinations in mice are involved in neuronal signalling and possibly implicated in neurological diseases.
Live cell imaging assays in cultured cells using transiently expressing fluorescently-tagged G protein subunits has been used to examine the selectivity of α2AR to activate Gαi and Gβ in HeLa cells [130]. Using FRET assays, proximity of proteins within 100 nm is regarded as a sensitive assay for measuring protein-protein interactions. FRET was measured between YFP-tagged Gαi and CFP-tagged Gβ upon the activation of α2AR [130]. Gαi1, Gαi2, and Gαi3 showed specific interactions with Gβ1, Gβ2, and Gβ4 upon activation of endogenous as well as overexpressed α2ARs in HeLa cells. Another live cell imaging assay was designed to probe β2-adrenergic receptor (β2AR) coupling using CFP-tagged Gαs and split-YFP tagged functional Gβ1γ7 dimer. Here the YFP-Gβ1γ7 dimer was designed using N-terminal YFP attached to Gβ1 (YFP-N-β1) and C-terminal YFP attached to Gγ7 (YFP-C-γ1), and YFP fluorescence was only observed when both Gβ1 and Gγ7 are co-expressed. Internalization of Gαsβ1γ7 upon β2AR stimulation was observed, indicating specific trafficking patterns for heterotrimers coupled to the β2AR [131].
Another approach to understand G protein subunit specificity with GPCRs is the use of antisense oligonucleotide or siRNA suppression system in cultured cells. Gβγ dimers containing Gβ4 and Gγ1 in M3-muscarinic receptor-mediated signalling has been observed in native HEK 293 cells [129]. Using a bioluminescence-based calcium assay combined with siRNA screening to identify potential Gβγ combinations, regulation of intracellular calcium and MAPK signalling by the M3-muscarinic receptor was examined [129]. Knocking down Gβ1 and Gβ4, as well as specific Gγ subunits using their respective siRNAs showed that the Gβ4γ4 combination was a key signalling driver, while knocking down Gβ1 disrupted non-canonical signalling events possibly suggesting a role in regulating gene expression. Antisense oligonucleotide injection into rat pituitary-derived GH3 cells to suppress expression of certain Gβ subtypes was used to assess selective Gβγ coupling with GPCRs in physiological conditions. M4-muscarinic receptors and somatostatin receptors required heterotrimers with specific Gβγ subunits to mediate inhibition of L-type Ca2+ channels [132]. Silencing Gβ (with Gβ-specific antisense oligonucleotides) and Gγ (with Gγ-specific antisense oligonucleotides) reduced somatostatin receptor activation-induced Ca2+ currents, suggesting a role for Gβγ subtype selectivity in somatostatin and M4 receptor signalling [25, 133]. Using electrophysiological measurements and antibody labeling in GH3 cells, the M4-muscarinic receptor interacted specifically with the heterotrimer Gαo1β3γ4, while somatostatin receptor preferred Gαo2β1γ3, inhibiting receptor-induced calcium currents. Ribozyme suppression has also been considered a useful method to identify physiologically relevant specific G protein heterotrimer-GPCR interactions. Specifically designed G protein subtype targeted ribozymes were used to selectively suppress the G protein expression. When tested in HEK 293 cells, this approach showed strong interactions between Gβ1 and Gγ7 suggesting Gβγ specificity for regulation of βAR and Gαs-mediated adenylyl cyclase signalling [134].
Studies with purified G protein subunits and GPCRs have also been reported. For example, baculoviral expression systems have been used in cells to express and purify G proteins with different radioisotope labels. A recent study conducted in Sf9 and HEK 293 cells showed subtype-specific Gβγ interactions with adenosine 1 and 2A receptors [135]. Here, Gβγ subunits in cells were enriched by stable isotope labeling using 13C6-Arg and 13C6-Lys and purified using receptor-Gα fusion proteins. Protein separations were performed by SDS-PAGE and HPLC, while tandem mass spectrometry was used to identify specific Gβγ dimers. Both fusion proteins, adenosine 1 receptors with Gαi and adenosine 2A receptors with Gαs, showed specific interactions with Gβ4 and Gγ5. Additionally, Gβ4 showed a higher affinity toward Gαs-fused adenosine 2A receptor over the adenosine 1-Gαi fusion [135]. In another study, Gβ was demonstrated as a determining factor for Gαs interaction in adenosine 2A receptor and β1AR-induced signalling [136]. There, the ability of adenosine 2A receptors and the β1AR to modulate adenylyl cyclase activity by measuring cAMP concentrations as well as G protein activation using GTP-γS assays in the presence of different combinations of purified Gβγ were demonstrated. Sf9 cells were used in combination with baculoviral expression to purify Gβγ and assays were performed using purified Gβγ reconstituted with Sf9 cell membranes expressing adenosine 2A receptors, β1AR and adenylyl cyclases; AC1 and AC2. Combinations of Gγ2 heterodimers with Gβ1–5 were tested to measure coupling efficiencies of Gαs with both β1AR, and adenosine 2A receptor, while AC2 stimulation and AC1 inhibition by G protein subtypes were also tested. Both receptors showed stronger interactions with the Gβ4γ2 dimer, while the Gβ5γ2 dimer showed attenuated interactions and signalling. Additionally, Gβ1γ2 showed a higher affinity towards for the β1AR [136]. In a recent study, in the presence of neurotensin 1 receptor, a higher than usual number of Gβγ combinations forming functional heterotrimers with Gαi and GαsL was shown [70]. Here, baculoviral expression of all three G protein subunits in a single vector compared to individual baculoviral vectors expressing only one G protein. Sf9 cells were used to express G proteins and GPCRs while co-immunoprecipitation was used to purify HA-tagged Gγ with its interacting G protein subunits and GPCRs. Except for Gβ5, nearly 120 Gαβγ combinations comprising Gβ1–4 and Gγ1–5, Gγ7–13 with either Gαi or GαsL were found to form functional heterotrimers with neurotensin 1 receptor. Additionally, with the exception of Gβ3, specific heterotrimer formation of Gαi with Gβ1, 2, 4 and Gγ1, 11 was noted [70].
Using baculoviral expression-aided purification of Gβγ from Sf9 and CHO-K1 cells, interactions between 5-HT1A, A1-adenosine, α2-adrenergic, and μ-opioid receptors and distinct Gβγ combinations was demonstrated [137]. In this study, Gβ1γ11 heterodimers showed a unique interaction with 5-HT1A and A1-adenosine receptors, while Gβ1γ7 heterodimer showed robust interactions with α2-adrenergic and μ-opioid receptors when co-expressed in Gαi in cellular systems [137].
Using co-immunoprecipitation of Gβγ subtypes, specific interactions with Gαq and Gβγ to regulate GqGTP/PLCβ1- or PLCβ2-mediated inositol triphosphate production was demonstrated in HEK293T cells [138]. Except for Gβ5γ2 and Gβ5γ13, Gβ1–4 dimers with γ2 and γ13 showed specific interactions with Gαq, regulating Gαq-induced PLCβ1 activation [138].
Additionally, preferred heterotrimer combinations were reported for rod and cone photoreceptors during phototransduction [88]. Using immunocytochemical labeling in retina sections isolated from dark-adapted monkeys, Gβ3, Gγ3, and Gγ2 were shown to interact with all three blue, green, and red cone photoreceptors. Additionally, Gβ3 showed rod bipolar cell expression. Enhanced localization of Gβ1, Gγ1, and Gγ3 signalling was observed primarily in rod photoreceptors implicating specific heterotrimer interactions for vision signalling [88].
Similarly, in olfactory receptors, Gα(olf) was shown to interact with Gβ1 and Gγ13 while in taste receptors, Gα(gustducin) specifically interacted with Gβ3, Gβ1, and Gγ13 [139, 140]. In those studies, olfactory receptor interactions and signalling with these specific Gαβγ combinations were tested using yeast-two-hybrid screening and cAMP measurements in HEK 293T cells [139]. Taste receptor activity in the presence of specific Gαβγ combinations was characterized using murine taste tissues by measuring IP3 levels induced by PLCβ2 [140]. Additionally, the specific heterotrimer combination Gα (olf), Gβ2 and Gγ7 with adenosine-A2A receptors was shown using Gγ7 knockout mice [141].
Although there are numerous investigations on specific GPCR-heterotrimer interactions and associated coupling, thorough analysis should be conducted to assess in vivo translatability of such experimental approaches since in celllulo and in vitro data do not always mimic physiological conditions found in vivo. Therefore, more physiologically relevant experimental approaches should be encouraged to draw conclusions regarding specific heterotrimer-GPCR interactions. Table 8 and Fig. 14 summarize what we know about Gαβγ combinations, their interacting GPCRs, and associated physiological functions.
Table 8.
Gα & Gβγ combinations, interacting GPCRs, and functions
| Gα & Gβγ combination | Interacting GPCR | Function |
|---|---|---|
| Gαi1 with Gβ3Gγ4, Gβ3Gγ10, Gβ3Gγ11 | α2-adrenergic | Anesthetic sparing, and working memory enhancement, nervous system [127] |
| Gαi, Gβ4, and Gγ5 | Adenosine A1 | Cardiac and neonatal physiology [135] |
| Gαi with Gβ1Gγ1, Gβ1Gγ11, Gβ2Gγ1, Gβ2Gγ11, Gβ4Gγ1, Gβ4Gγ11 | Neurotensin 1 | Hypotension, hyperglycemia, antinociception, hypothermia [70] |
| Gαi with Gβ1Gγ7 | μ-opioid | Pain and addiction [137] |
| Gαi with Gβ2Gγ2, Gβ2Gγ3, Gβ2Gγ4 | Auto-α2-adrenergic | Anesthetic sparing, and working memory enhancement, nervous system [115] |
| Gαi with Gβ4Gγ12 | Hetero-non-adrenergic | Anesthetic sparing, and working memory enhancement, nervous system [115] |
| Gαi with Gγ11 | 5-HT1A | Neurotransmission [137] |
| Gαi/o with Gγ11 | Adenosine A1 | Cardiac and neonatal physiology [137] |
| Gαo1 with Gβ3, and Gγ4 | M4-muscarinic | Inhibit voltage-sensitive Ca2+ channels [132] |
| Gαo2 with Gβ1, and Gγ3 | Somatostatin | Inhibit voltage-sensitive Ca2+ channels [132] |
| Gαt with Gβ3Gγ3 & Gβ3Gγ2 | Cone photoreceptors | Vision [88] |
| Gαt with Gβ1Gγ1 &Gβ1Gγ3 | Rod photoreceptors | Vision [88] |
| Gα(olf) with Gβ1Gγ13 | Olfactory receptors | Olfaction [139] |
| Gα(olf) with Gβ2Gγ7 | Adenosine A2A | Cardiac physiology, Parkinson’s disease [141] |
| Gα(gustducin) with Gβ3Gγ13 and Gβ1Gγ13 | Taste receptors | Taste [140] |
| Gαs with Gβ1, and Gγ7 | β-adrenergic | Cardiac physiology, heart rate [134] |
| Gαs with Gβ1Gγ2 | β1-adrenergic | cAMP signalling [136] |
| Gαs with Gβ4, and Gγ5 | Adenosine A2A | Cardiac physiology, Parkinson’s disease [135] |
| Gαq with Gβ1, Gβ2, Gβ3, Gβ4 and Gγ2, Gγ13 | N-type Ca2+ channels and endogenous GPCRs | Olfaction [138] |
| Gαq with Gβ4γ1 | M3-muscarinic | Calcium signalling, nervous system, muscle contraction [129] |
Figure 14: Gα and Gβγ combinations, their interacting GPCRs, and associated physiological functions.
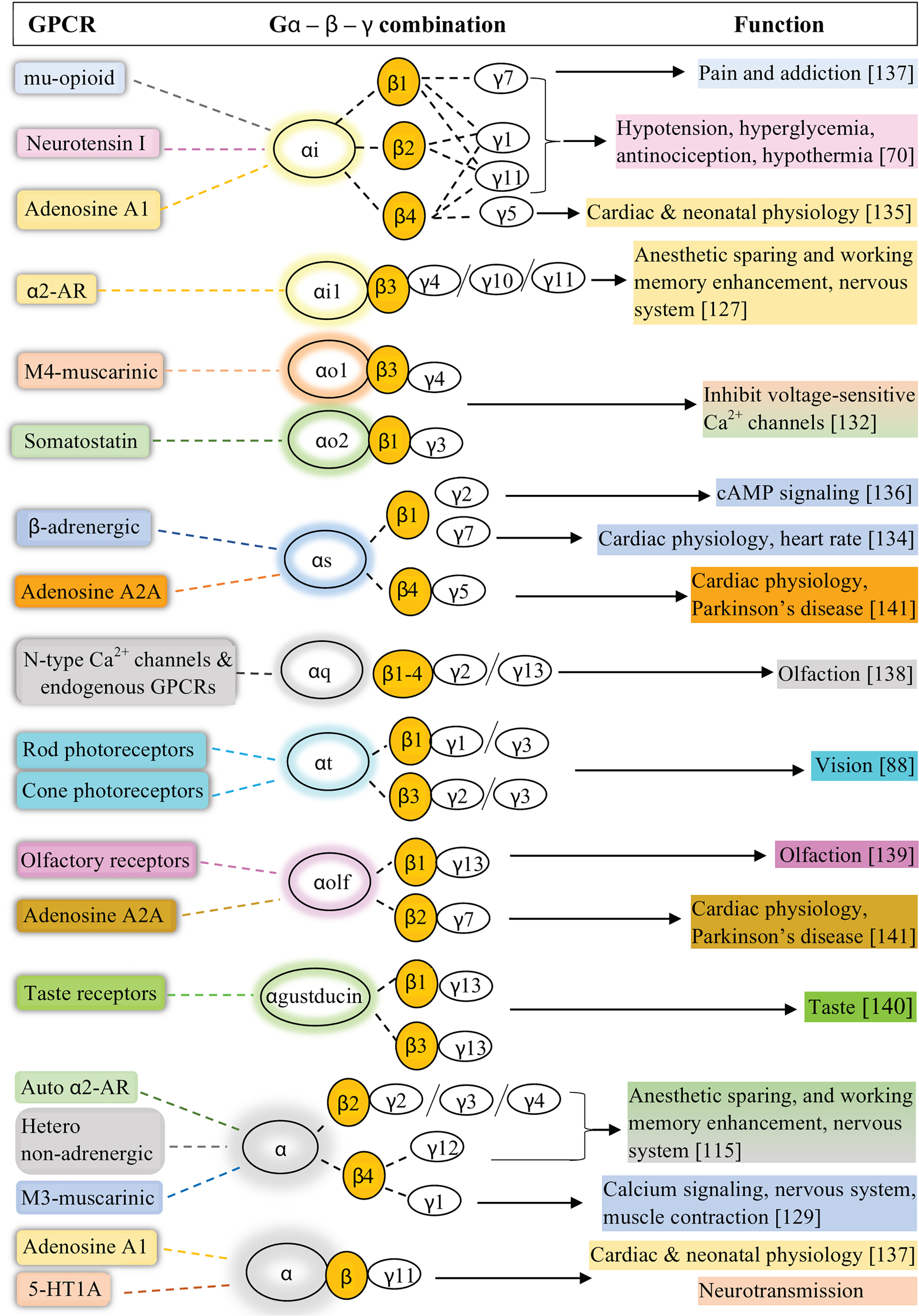
A summary of known combinations of G protein heterotrimers linked to particular GPCRs in different cells and tissues.
The major G protein subunits in individual tissues were identified and categorized according to the human protein atlas database (RNA expression data available from http://www.proteinatlas.org) (Table 9). The expression levels of specific G proteins may contribute to the overall signalling specificities in these tissues. Some tissues show similar expression levels of G protein subunits, and thus the possible preferred heterotrimer formation and their contribution to signalling specificities need examination. Overall, the specificity of heterotrimeric G-proteins towards certain GPCRs and particular signalling events and cell and tissue-specific localization are likely to to be important in physiological and pathophysiological settings.
Table 9.
RNA expression level-based G protein subtype expression in different tissues
| Tissue | Gα | Gβ | Gγ | Tissue | Gα | Gβ | Gγ |
|---|---|---|---|---|---|---|---|
| adipose tissue | αi2 | β1 | γ5 | ovary | αi2 | β2 | γ11 |
| amygdala | αs | β1 | γ2 | pancreas | αs | β2 | γ5 |
| appendix | αi2 | β1 | γ5 | pituitary gland | αs | β1 | γ10 |
| basal ganglia | αL | β1 | γ7 | placenta | αi2 | β2 | γ11 |
| breast | αs | β2 | γ11 | pons and medulla | αs | β1 | γ3 |
| cerebellum | αs | β1 | γ13 | prostate | αi2 | β1 | γ5 |
| cerebral cortex | αs | β1 | γ3 | retina | αt1 | β3 | γt1 |
| cervix, uterine | αi2 | β2 | γ5 | salivary gland | αs | β2 | γ5 |
| colon | α11 | β1 | γ5 | seminal vesicle | αi2 | β2 | γ5 |
| corpus callosum | αi2 | β1 | γ7 | skeletal muscle | αs | β2 | γ5 |
| ductus deferens | αi2 | β2 | γ5 | small intestine | α11 | β1 | γ12 |
| endometrium | αi2 | β1 | γ12 | smooth muscle | αs | β1 | γ12 |
| epididymis | αi2 | β2 | γ5 | spinal cord | αs | β1 | γ10 |
| esophagus | αi2 | β2 | γ5 | spleen | αi2 | β2 | γ5 |
| gallbladder | αi2 | β2 | γ5 | testis | αi2 | β2 | γ5 |
| heart muscle | αs | β2 | γ5 | thalamus | αi2 | β1 | γ10 |
| hippocampus formation | αs | β1 | γ3 | thymus | αi2 | β1 | γ5 |
| kidney | αs | β1 | γ5 | thyroid gland | αs | β2 | γ5 |
| liver | αi2 | β2 | γ5 | tongue | αs | β2 | γ5 |
| lung | αi2 | β2 | γ5 | tonsil | αi2 | β1 | γ5 |
| lymph node | αi2 | β2 | γ5 | urinary bladder | αi2 | β1 | γ5 |
| midbrain | αi2 | β1 | γ5 | vagina | αi2 | β1 | γ5 |
| olfactory region | αs | β1 | γ2 |
4. Gγ subtype-specific regulation of GPCR and G protein signalling
4.1. Isoprenylation of Gγ subunits, PM composition, and Gβγ-PM interactions.
The plasma membrane (PM) is a semipermeable barrier that surrounds the cell interior, including the cytoplasm of the cell. The PM is composed of a lipid bilayer with embedded proteins, polysaccharides and lipids/lipoproteins. The lipid component is mainly composed of phospholipids, which are amphipathic molecules with a polar phosphate head group and non-polar two acyl lipid anchors. They arrange themselves in a bilayer in which lipid anchors are oriented towards the interior while polar head groups face outside. The PM is rich with five major types of phospholipids-phosphatidylcholine, phosphatidylinositol, phosphatidylserine, phosphatidylethanolamine, and sphingomyelin, where phosphatidylinositol, phosphatidylethanolamine, and phosphatidylserine are abundant in the inner leaflet of the PM [25, 142, 143].
Due to the net negative charge of the polar head groups of phospholipids, which are predominant in the inner leaflet (i.e., phosphatidylinositol, phosphatidylserine), the inner layer of the membrane has a net negative charge. With this composition (being hydrophobic and negatively charged), the PM plays a role in protein anchoring (i.e., the cytoskeleton), where electrostatic and hydrophobic interactions play a significant role. Anchorage to the PM and the proper localization of G proteins are crucial for their function. Gβγ subunits are anchored to the PM via a prenyl group attached to the C terminus of the Gγ subunit in the Gβγ dimer. Gγ is post-translationally modified with an isoprenyl lipid anchor, and there are two types of prenyl groups; 15 carbon farnesyl and 20 carbon geranylgeranyl[144].
Post-translationally lipidated proteins contain a common amino acid sequence known as the CAAX motif at their carboxy termini [144]. The CAAX motif is composed of a conserved cysteine residue (C) followed by two aliphatic amino acids (aa) and any amino acid (X), which varies depending on the protein [145]. As seen in Gγ9, Gγ1, and Gγ11, where X can be Met, Ser, Gln or Ala, the Gγ subunit is expected to be farnesylated. When the X residue is Leu, as seen in the other nine Gγ subtypes, Gγ geranylgeranylation is expected [146]. Out of the 12 Gγ isoforms, only Gγ1, Gγ9, and Gγ11 are known to be farnesylated, while the rest are geranylgeranylated [147]. Initially, it was believed that the final residue of the CAAX motif, ‘X’, which is variable, determines the type of isopropyl transferase/prenylation involved in Gγ prenylation [148]. However, recent studies have suggested that the other residues of the Gγ C terminus also play a role in this determination [146]. Though farnesylation or geranylgeranylation of most CAAX motif-containing proteins anticipated to follow the prenylation rules described above, there are deviations from this traditional paradigm[144]. Therefore, more studies are required to elucidate how the type of prenylation of Gγ is determined.
However, the prenyl group is attached to the carboxy terminus of Gγ by forming a thioether bond with the Cys residue of the CAAX motif. In farnesylation, the farnesyl group is transferred to the Cys residue of the CAAX motif from farnesyl diphosphate (FPP) by the enzyme farnesyltransferase, and during geranylgeranylation, the geranylgeranyl group is transferred from geranylgeranyl diphosphate (GGPP) by geranylgeranyl transferase (GGT-1)[145]. After prenyl group attachment, the last three amino acid residues (-aaX) are removed/cleaved by the proteolytic enzyme Ras-converting CAAX endopeptidase (RCE1), followed by methyl group addition to the new C terminus (carboxymethylation) by carboxymethyl transferase (Fig. 15) [149]. The hydrophobic nature of the isoprenyl group mediates anchorage of Gβγ with this lipid modification to the PM, which is also hydrophobic due to the presence of fatty acyl chains (Fig. 15). These two prenyl groups (15 C farnesyl and 20 C geranylgeranyl) have different PM affinities, and the geranylgeranyl group has relatively higher membrane affinity than the farnesyl group[48] (Fig. 16).
Figure 15. Prenylation and post prenylation processing at the CAAX motif in Gγ.
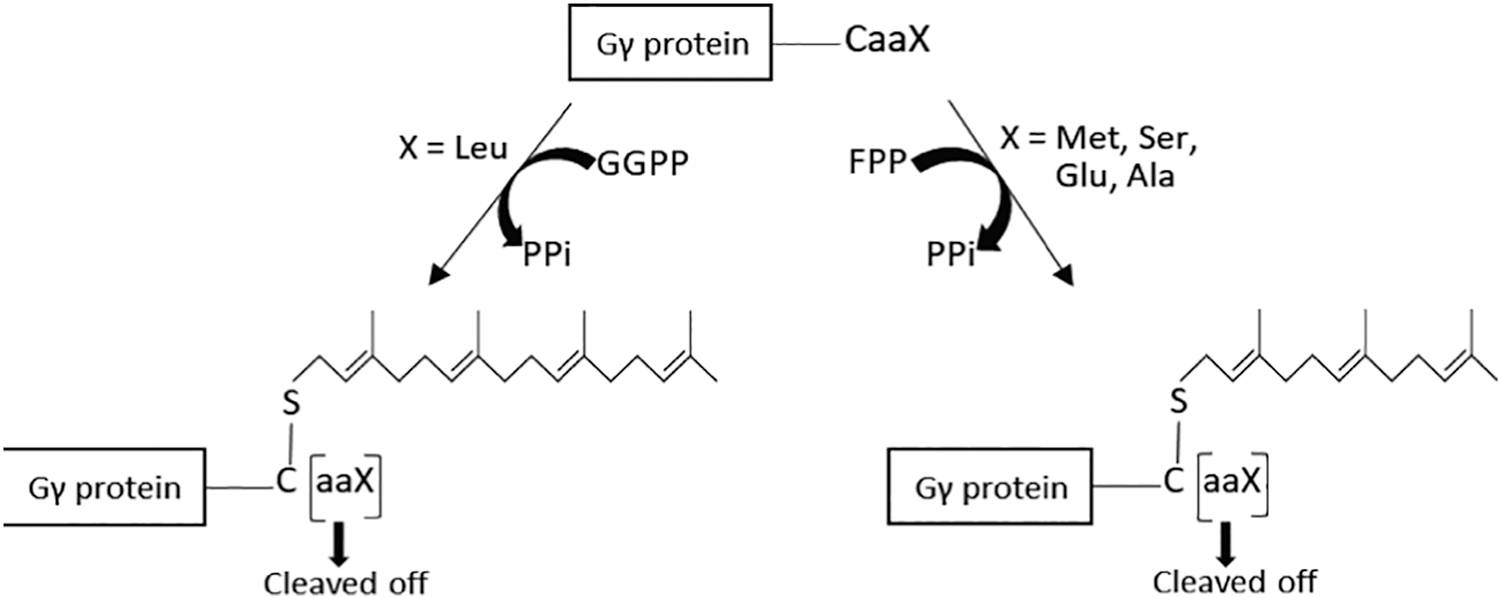
Farnesyl or geranylgeranyl moieties are transferred to the carboxy terminus of Gγ from FPP and GGPP with the aid of either farnesyl or geranylgeranyl transferases, respectively. The last three amino acids are subsequently cleaved off from the CAAX motif by RCE1 and is followed by a methyl group addition to the new C terminus. GGPP: Geranylgeranyl pyrophosphate, FPP: Farnesyl pyrophosphate, PPi: Pyrophosphate.
Figure 16. Farnesylated and geranylgeranylated membrane-anchored Gγ.

While prenyl groups (farnesyl and geranylgeranyl group in Gγ9 and Gγ3, respectively) anchor Gγ to the PM, positively charged and hydrophobic residues in the pre-CAAX region further strengthen this anchorage by controlling electrostatic and hydrophobic interactions, respectively with the negatively charged and hydrophobic PM.
4.2. Subtype-specific Gγ prenylation and PM affinity of Gβγ
G protein attachment to the inner leaflet of the PM is critical for G protein-mediated signal transduction since it allows G proteins to interact with their cognate receptors, undergo activation, and transduce signals through activation of PM-localized effector molecules. The crystal structure of the G protein heterotrimer provides information about the spatial orientation of the separate subunits in the heterotrimeric G protein complex[15]. The amino terminus of Gα subunits in G proteins is palmitoylated and/or myristoylated while the carboxy terminus of Gγ in Gβγ dimer is isoprenylated either with a 15C farnesyl or a 20C geranylgeranyl lipid anchor as discussed above [12]. It is suggested that a more hydrophobic geranylgeranyl group is sufficient to stably anchor a protein to membranes [150]. In previous studies, a high affinity, heat- and protease-sensitive binding sites have been identified for prenylated peptides in microsomal membrane preparations[151]. This binding site may play a role in targeting prenylated proteins to a membrane compartment where C-terminal proteolysis and carboxyl methylation occur [151]. Also, this carboxyl methylation neutralizes the negatively charged C terminus. It has been shown to increase the affinity of farnesylated peptides for lipid vesicles by ~10-fold and is considered a contributor to Gβγ membrane association [152].
Further, Gα lipid modifications have been shown to provide additional support required for PM targeting of Gβγ subunits [153]. The need of Gα for PM localization of Gβγ was demonstrated by overexpressing different combinations of Gβ and Gγ, without Gα, which resulted in limited localization of the Gβγ at the PM with the majority of Gβγ accumulating in intracellular structures. In contrast, co-expression of Gα resulted in strong PM localization of Gβγ [154]. This may also suggest that heterotrimer formation is a critical step on the correct folding or maturation of functional G proteins, especially those destined for the PM.
According to the crystal structure of Gβγ (PDB ID: 1TBG), Gγ interacts with the barrel surface of the Gβ propeller, however, without forming covalent interactions. The carboxyl terminus of the Gγ lies within ~18 Å of the amino terminus of the Gα. It is also closer to the carboxyl terminus of Gα. In the assembled Gβγ dimer, the amino terminus of the Gα and the prenylated carboxyl terminus of the Gγ lie parallel. The CAAX motif Cys in Gγ and amino terminus Gly and/or amino terminus Cys of Gα are the two domains in the heterotrimer that contain posttranslational lipid modifications, suggesting that these subunits play a major role in anchoring heterotrimers to the membrane[150]. The amino terminus of the Gα and the carboxyl terminus of the Gγ subunit lie near the inner surface of the PM while it interacts with the PM at the prenylated and carboxymethylated Cys[155].
Upon GPCR activation, heterotrimeric G proteins undergo activation and one fate is that they completely dissociate (there is some evidence that dissociation can also be partial, especially when they regulate the same effector protein) into active GαGTP and free Gβγ. Although Gβγ was initially considered as a PM-bound protein, recent work has shown that free Gβγ can detach from the PM and reversibly travel through the cytosol between the PM to internal membranes (IMs) until an equilibrium is reached, termed Gβγ translocation [48] (Fig. 17). Numerous studies have also shown that Gβγ signalling can occur in the nucleus and other organelles but it remains unclear how they are translocated to these compartments (reviewed in [156, 157]). The differences between the properties of the PM and IMs (i.e., membrane composition) and the higher surface area of IMs compared to the PM support the forward translocation from the PM to IMs as long as GPCRs are active [48].
Figure 17. GPCR-G protein activation and subsequent Gβγ translocation.
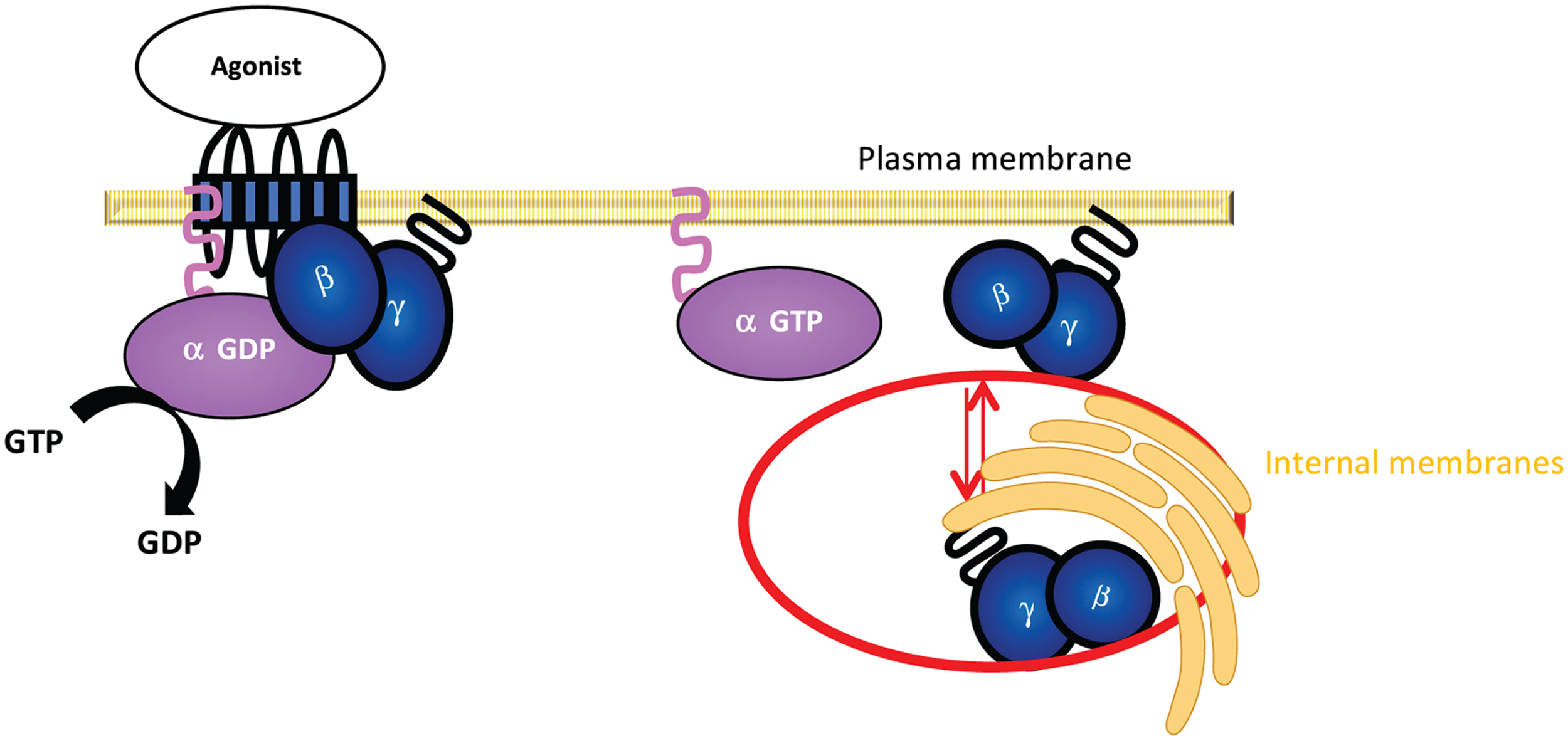
G protein αGDP and βγ subunits are associated at the PM as a heterotrimer and when a ligand activates the GPCR, GαGTP and Gβγ can dissociate from each other due to conformational changes in the Gα subunit. Gβγ then translocates from the PM to IMs in a reversible manner to regulate downstream signalling activities.
It has been found that Gβγ translocation is dependent on the particular Gγ subunit in the Gβγ dimer [48]-[11]. The translocation rates of different Gγ isoforms were measured using the time to reach the half-maximum translocation (Tt1/2). All 12 Gγ subunits showed distinct translocation rates and extents upon GPCR activation, where Gγ9 showed the fastest (Tt1/2 < 10 s) and Gγ3 showed the slowest Gβγ translocation. Gγ9, which has a farnesyl anchor and other Gγ subtypes, predicted to have farnesyl lipid modification (i.e., Gγ1 and Gγ11) showed faster translocation compared to the Gγ subunits with geranylgeranyl anchors [48]. Also, the rapidly translocating Gγ subunits showed a greater extent of translocation compared to slowly translocating ones [48]. This suggests that the PM affinity of Gβγ is Gγ subtype-dependent as Gγ provides the only PM anchor for Gβγ dimer. However, even though there are only two types of prenyl groups acting as membrane anchors for Gβγ subunits, the presence of 12 distinct translocation rates and differing degrees of translocation suggests that additional regulatory mechanisms also govern the PM affinity of Gβγ.
As shown in Table 2, a broader range of sequence homologies is exhibited by the Gγ isoforms, approximately ranging from 20 to 80% [110]. Sequence alignment of Gγ subunits shows that except for Gγ13, all other 11 Gγ types possess a conserved Phe residue (Phe59) at their C termini (Fig. 8). Based on the conserved domain analysis function at NCBI, the 5 to 6 residues between this conserved Phe, which demarcates the last contact point with Gβ and the CAAX motif (pre-CAAX region), also appear to interact with the PM, providing additional support to the prenyl anchor [158, 159].
A recent study with Gγ mutants comprised of high PM affinity Gγ with a substituted C terminus from a low PM affinity Gγ (i.e., Gγ3 with pre-CAAX + CAAX of Gγ9 (Gγ3-γ9CT) and vice-versa has shown that the mutants display translocation properties of the Gγ in which the introduced C termini are extracted [48]. However, when only the CAAX motifs were switched, the translocation properties did not entirely resemble that of the parental Gγ subtype, although a significant change was observed [48]. Also, a complete loss of PM localization was observed in Gγ9 upon Cys removal from the CAAX motif, and complete elimination of translocation was observed with incorporation of an additional Cys to Gγ3 CAAX motif.
Overall, these observations imply that the Gγ prenylation is essential for Gβγ anchorage to the PM, and that the pre-CAAX region fine-tunes Gβγ-PM interactions by controlling electrostatic and hydrophobic interactions, respectively [48]. This notion has been further supported by examining the amino acid composition of pre-CAAX regions of different Gγ types. As shown in Table 10, pre-CAAX regions of slowly translocating Gγ3 and Gγ2 (with high PM affinity) are composed of ∼80% positively charged and hydrophobic residues, which can form strong interactions with the negatively charged and hydrophobic PM. In comparison, only ∼50% hydrophobic residues are present in farnesylated Gγ subunits with low PM affinity (rapidly translocating) such as Gγ9 and Gγ1 [48].
Table 10:
Pre-CAAX regions of all 12 Gγ subunits, their translocation Tt½ times and PM affinities [48].
| Gγ | Pre-CAAX | Tt½ (s) | PM affinity |
|---|---|---|---|
| Gγ9 | NPFKE-KGGC-far | 5 ± 1 | Low affinity |
| Gγ1 | NPFKELKGGC-far | 13 ± 2 | |
| Gγ11 | NPFKE-KGSC-far | 38 ± 2 | |
| Gγ7 | NPFKDKKP-C-gerger | 41 ± 2 | |
| Gγ5 | NPFRPQKV-C-gerger | 71 ± 3 | Moderate affinity |
| Gγ12 | NPFKDKKT-C-gerger | 80 ± 2 | |
| Gγ10 | NPFREPRS-C-gerger | 97 ± 4 | |
| Gγ13 | NPWVE-KGKC-gerger | 100 ± 3 | |
| Gγ4 | NPFREKKFFC-gerger | 116 ± 2 | |
| Gγ8 | NPFRDKRLFC-gerger | 124 ± 1 | |
| Gγ2 | NPFREKKFFC-gerger | 181 ± 4 | High affinity |
| Gγ3 | NPFREKKFFC-gerger | 270 ± 4 |
4.3. Gγ subtype-dependent receptor selectivity of G protein heterotrimers
Since the general classification of GPCRs is based on their coupling to different Gα subtypes, selective coupling of Gα subunits to specific GPCRs has been the primary point of receptor definition [160, 161]. Nevertheless, emerging evidence from experiments conducted both in vivo and in vitro reports the interactions of Gγ subunits with the receptor and their influence in defining selectivity of heterotrimers for specific GPCRs [6]. Moreover, the Gβγ dimer collectively plays a vital role in facilitating Gα binding to receptors [162]. Therefore, in addition to Gα, Gβγ is also likely to be required for heterotrimer-GPCR interactions and subsequent signalling [163].
One study conducted in superior cervical ganglion (SCG) neurons using C-terminal prenylated peptides specific for different Gγ subtypes indicated the attenuation of signalling by M2- and M4-muscarinic receptors in the presence of Gγ5 peptide, while no significant effect was reported with Gγ7 or Gγ12 peptides [164]. This implied more favorable binding of Gγ5 heterotrimers to M2/M4-muscarinic receptors. The reported differential selectivity of Gγ5 and Gγ7 to interact with M2-muscarinic receptors led to another study to test ability to activate different G protein combinations of Gαo and Gαi with Gγ5 and Gγ7 [165]. This was achieved by examining the influence of different subunit compositions of heterotrimer on M2-muscarinic receptor interaction by using possible combinations of Gα and Gγ subtypes; αoβ1γ5, αoβ1γ7, αi2β1γ5, and αi2β1γ7. A significant difference between the receptor’s ability to activate αoβ1γ5 and αoβ1γ7 was observed, indicating a prominent interaction of αoβ1γ5 heterotrimer with the receptor. Further, selective interaction of different Gγ subtypes with the receptor was found to be due to the differential coupling of the specific Gγ subtypes to the M2 receptor, regardless of the type of Gβ isomer present in the Gβγ dimer [165]. A separate study conducted in HEK 293 cells also showed perturbation of β-adrenergic receptor signalling upon ribozyme-mediated suppression of Gγ7 subtype expression, indicating the selectivity of the β-adrenergic receptor for Gγ7 containing heterotrimers [166].
The C-terminus of Gγ has been shown to be predominantly involved in the Gβγ dimer-receptor interaction [167]. In addition to the highest amino acid sequence variability at the C-termini of Gγ subunits, the C-terminal CAAX sequence also determines the type of prenylation in a specific Gγ subtype. Therefore, the C-terminal sequence, together with the type of prenylation, can be important determinants of receptor selectivity for differential Gγ coupling [168, 169]. In an early study on identifying the effect of Gγ prenylation on Gβγ-GPCR interactions, specifically Gγ1 and Gγ2, which are post-translationally modified respectively with farnesyl and geranylgeranyl prenyl anchors [147]. In this study, the formation of the high-affinity agonist binding conformations of the A1 adenosine receptor and following exchange of GDP to GTP on the Gα subunit was used to measure the effectiveness of different Gβγ dimers on receptor interactions. In both assays, farnesylated Gβ1γ1 dimers were significantly less effective compared to geranylgeranylated Gβ1γ2 dimers. In order to ascertain the observed prenylation type-dependent affinity of Gβγ subtypes for A1 adenosine receptor, mutated versions of Gβγ dimers; Gγ1 with geranylgeranyl anchor (Gβ1γ1-S74L) and Gγ2 with a farnesyl anchor (Gβ1γ2-L71S) were used. Compared to WT Gβ1γ2, the Gβ1γ2 mutant was less effective while the Gβ1γ1 mutant was more effective in transducing receptor activity, indicating that the type of prenylation of Gγ subunits is an important determinant of its interaction with the receptor [147]. Consistent with these observations, a 10-fold difference between Gγ1 and Gγ2 subtypes in their affinities for bovine rhodopsin was reported [170, 171]. Investigation of the contribution of the primary structure and isoprenoid modification of Gγ on interaction with the receptor showed a differential affinity of farnesylated Gβ1γ1 and geranylgeranylated Gβ1γ2 for rhodopsin [172]. Also, the mutated version of Gγ2 with farnesylated lipid anchor exhibited a 2-fold decrease in affinity for the receptor compared to Gγ2 WT, while the geranylgeranylated Gγ1 exhibited an increased affinity for receptor compared to Gγ1 WT. Overall, the study suggested that C-terminal isoprenoid modification on Gγ subtypes contributes to their differential receptor selectivity.
4.4. Gγ subtype-dependent control of Gβγ-effector signalling
Upon activation of GPCRs in response to extracellular stimuli, signal transduction is initiated by activating Gα(GDP)βγ heterotrimers, converting them to Gα(GTP) and free Gβγ [173]. Activated Gα(GTP) and free Gβγ can independently interact with effectors, and in some cases the same effectors [153]. Supporting their interaction with Gβγ, multiple Gβγ-binding sites have been identified on numerous effectors including phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3Kγ), adenylyl cyclase (AC) isoforms, GIRK channels, PLC isoforms (PLCβ2, β3), GPCR kinases (GRKs), etc. [174–177]. By interacting with these effectors, Gβγ subunits modulate a broad array of cellular and physiological functions, including gene transcription, cellular secretion, contractility, and cell migration [25, 153]. Based on the subcellular localization of the effector, there are two general mechanisms of Gβγ-dependent effector regulation [153]. The first describes the recruitment of cytosolically distributed effector proteins to the PM to interact with their substrates located at the PM by binding to PM-anchored Gβγ. The second mechanism involves the direct interaction of activated Gβγ with PM-localized effectors [17, 153]. Although there are realistically over 50 possible combinations of different Gβγ dimers available [70], Gβγ-mediated effector activation has primarily been considered a single signalling event, considering Gβγ as a monolithic signalling actuator. The individual subtype-specific contributions of Gβ and Gγ subunits in Gβγ-effector interactions are just emerging.
Since the majority of the Gβγ effectors are near PM localized, interactions with effectors primarily occur at the PM, and therefore varying PM affinities of different Gβγ combinations should or likely contribute to differential Gβγ-effector interactions and signalling activation [178]. As previously mentioned, the conventional model of GPCR signalling describes Gα-mediated pathways as the central mediators of GPCR-G protein signal transduction [179–181]. However, the very first discovery of Gβγ mediated activation of acetylcholine-regulated GIRK channel in atrial myocytes, which subsequently govern muscarinic receptor-gated potassium channel activity [182], focused attention on the ability of Gβγ to directly activate downstream effectors. This led to an understanding that Gβγ activated several other effectors responsible for facilitating diverse signalling and cellular processes and cell physiology. The activation of the endogenous GIRK channel was observed in HL-1 cells upon activating endogenous Gi-coupled M2-muscarinic receptors [183]. In a study which investigated differential Gβγ translocation rates of regulated 12 Gγ isoforms, M2-muscarinic receptor activation of GIRK channels was reported to be dependent on the translocation rates of the accompanying Gγ type in Gβγ [184]. Here, the effect of slow translocating (high-PM-affinity) Gγ3 and fast translocating (low-PM-affinity) Gγ9 on the properties of muscarinic receptor-induced GIRK channel activation was measured in HL-1 cardiomyocytes. Interestingly, a higher amplitude of GIRK current was measured in Gγ3 expressing cells while control cells utilizing endogenous Gγ subunits of HL-1 cells and Gγ9 expressing cells reported a relatively lower GIRK current amplitude. This study suggested the dependence of translocation rates and relative abundance of Gγ isoforms on GIRK channel activation.
Among different isoforms of PI3K, PI3Kγ activity is directly induced by Gβγ [176, 185], and it has been clearly shown that Gβγ plays a crucial role in PI3Kγ-mediated migration of neutrophils in response to chemoattractants [186]. PI3Kγ-mediated PIP3 generation at the PM is a major regulator of the migratory activity of cells such as macrophages [48]. The accumulation of PIP3 at the leading edge of a migratory cell is essential for polarization of the cell, which in turn directs itself towards chemoattractants [187, 188]. Additionally, Gαi/o-coupled GPCR, blue opsin activation also has exhibited the accumulation of PIP3 at the leading edge of mouse macrophage cell line RAW 264.7, as a result of the generation of free Gβγ [189].
To catalyze PIP3 production by interacting with Gβγ, the catalytic subunit (p110) of PI3Kγ must be translocated to PM upon its activation [185, 190]. A recent study clearly demonstrated the inherent ability of GPCR-mediated PIP3 production in RAW 264.7 cells, which show predominant endogenous expression of high-PM-affinity Gγ3 and Gγ4 subunits [48]. However, HeLa cells, which show a significantly less abundant expression of these Gγ types, did not show PIP3 generation upon GPCR activation. Interestingly, the same study also showed basal levels of PIP3 generation, even without GPCR activation and robust production of PIP3 upon GPCR activation when Gγ3 was overexpressed in HeLa cells [48]. However, overexpression of low PM affinity Gγ9 in HeLa cells did not influence the ability of these cells to produce PIP3 upon GPCR activation.
Cytosolic Ca2+ plays a crucial role in inducing trailing edge retraction in migratory cells, and it is demonstrated that release of Ca2+ from intracellular stores is a Gβγ-mediated process during Gi-coupled GPCR-induced RAW 264.7 cell migration [191]. Accordingly, the Gγ subtype dependency of Ca2+ mobilization in RAW 264.7 cells upon endogenous Gi-coupled complement component 5a (C5a) receptor activation was examined [48]. This study showed a higher level of Ca2+ mobilization in RAW 264.7 cells utilizing endogenous Gβγ and overexpressed high PM affinity Gγ3. This data further confirmed the native expression of high PM affinity Gγ isoforms in these cells, consequently facilitating their characteristic cellular and physiological activities. Moreover, exogenous introduction of low PM affinity Gγ9 could not induce a significant level of Ca2+ mobilization. Interestingly, only a weak Ca2+ response was observed in cells expressing moderate PM affinity Gγ subunits such as Gγ4 and 12. Exhibiting the critical role of CT and CAAX motif sequence of Gγ subtypes in determining PM affinity and consequent effector interaction, the same study also showed loss of Ca2+ mobilization when mutant Gγ3 subunits wherein either CT or CAAX motif of Gγ3 WT was replaced by the respective Gγ9 sequence were introduced to RAW 264.7 cells [48].
Binding of cytosolic G protein-coupled receptor kinases (GRKs) to activated GPCRs at the PM and subsequent phosphorylation of receptors is a regulatory mechanism to prevent excessive GPCR signalling [192, 193]. Following GRK mediated phosphorylation, β-arrestin binds to GPCRs and facilitates clathrin-mediated receptor endocytosis [194]. Among the seven isoforms of GRKs (GRK1–7), GRK 2, 3, 5, and 6 are ubiquitously expressed. It was shown that Gαq, Gβγ, and phosphatidylinositol 4,5-bisphosphate (PIP2) play significant roles in specifically regulating GRK2 and GRK3 recruitment to active GPCRs [192, 195]. By employing a live cell confocal imaging approach, a recent study was able to identify the Gγ subtype specificity in regulating GRK2 recruitment [196]. A 3-fold higher GRK2 recruitment to activated M3-muscarinic receptor in low PM affinity Gγ9 expressing HeLa cells compared to high PM affinity Gγ3 expressing cells was observed. Considering the contrasting translocation kinetics and PM affinities of these two different Gγ subtypes, the observed differential magnitudes of GRK2 recruitment was explained. Here, it was suggested that heterotrimer dissociation upon GPCR activation facilitates exposure of Gαq-GTP and activated M3-muscarinic receptor for GRK2 interaction, allowing Gβγ to spatially re-orient and sandwich GRK2 between M3 receptor and Gβγ. Low PM affinity Gγ9 favors complete heterotrimer dissociation allowing a more permissive spatial orientation for GRK2 to interact in between the receptor and Gβγ. In contrast, the low mobility of high PM affinity Gγ3 may not sufficiently expose activated Gαq-GTP or GPCR for GRK2 to interact with, suggesting that differential PM affinities of Gγ subtypes play a crucial role in regulating GRK2 recruitment to activated GPCRs.
4.5. Gγ subtype-dependent regulation of cellular physiology
Golgi vesiculation
Even though the initial identification of GPCR-mediated G protein signalling is restricted to the PM, mounting evidence shows that the signalling ability of G proteins is also extended to subcellular locations such as the endoplasmic reticulum, the nucleus, and Golgi as well [11, 197, 198]. In addition to the role of G protein resident in IMs such as Golgi complexes [199, 200], it is also suggested that the GPCR-driven Gβγ dimer translocation from the PM to IMs [201–203] plays a specific role in signalling that occurs in IMs [204]. GPCR activation can stimulate Golgi fragmentation via a protein kinase D (PKD)- and PLCβ-driven pathway [204]. Considering the major role of PKD in Golgi vesiculation and their trafficking from trans-Golgi network to the PM [205, 206], it was later suggested that this is driven by GPCR-mediated Gβγ translocation from the PM to the Golgi complex [204]. Since different Gγ isoforms exhibit varying rates and extents of Gβγ translocation towards IMs based on their affinity for the PM [48, 109, 201, 203], this implies that Gβγ translocation-mediated Golgi vesiculation may be Gγ subtype dependent [204]. In the work mentioned above, M3-muscarinic receptor activation-induced Golgi fragmentation was demonstrated primarily in the presence of Gγ11, one of the rapidly translocating Gγ subtypes. In contrast, high-PM-affinity Gγ3 expressing cells failed to induce Golgi fragmentation upon M3R activation [204]. Consistent with this data, even in the absence of Gγ11 overexpression, M3-muscarinic receptor activation-induced Golgi vesiculation was observed in a cell line derived from lung tissue; A549 (human alveolar epithelial cells) [204], which endogenously expresses Gγ11 as a primary Gγ subtype [160]. The same cell line did not show Golgi fragmentation when slow translocating Gγ3 or Gγ4 were overexpressed [204]. This work established a paradigm where low-PM-affinity Gγ subtypes provide access for Gβγ to internal organelles and regulate effectors and signalling, following external receptor stimuli. This may have far ranging implications for Gβγ signalling inside the cell.
Cellular senescence and autophagy
Cellular senescence is the irreversible cell cycle arrest of proliferating cells. During cellular senescence, upregulation of endogenous Gγ11 expression has been observed compared to other Gγ subtypes [207, 208]. Additionally, cellular senescence can be induced by overexpression of Gγ11 in normal human fibroblasts [208]. As one of the rapidly translocating Gγ subtypes from the PM to IMs upon GPCR-mediated G protein activation [201, 202] given its role in modulating Golgi structure [204], Gγ11 was also observed in cells undergoing senescence after being translocated to IMs [207]. Senescent cells secrete numerous factors such as cytokines, growth factors, metalloproteinases, and extracellular matrix proteins in increased amounts [209], and the Golgi complex facilitates these events. It was also reported that enhanced expression of Gγ11 in senescent cells is caused by reactive oxygen species (ROS) such as H2O2, suggesting a possible molecular mechanism linking the effects of ROS in cellular senescence [208, 210]. ERK 1/2 has been identified to promote cellular senescence [211]. Gγ11 mediated activation of ERK1/2 was also observed, further establishing its role in cellular senescence [208]. Reduction in transcription of GNG11 has been reported in several cancer tissues such as medullary thyroid carcinoma [212] and splenic marginal zone lymphoma [213], where cell proliferation is accelerated while cellular senescence is reduced.
Even though the involvement of Gα signalling in autophagy is better studied, the role of Gβγ is not well understood. Knockdown of Gαs, Gαq11, and Gα12/13 has been shown to induce autophagy [214, 215]. However, subsequent work reported Gγ7 as the first Gγ subtype to be identified as an autophagy inducer [216]. The molecular mechanism behind the ability of Gγ7 to induce autophagy my involve interaction of Gγ7 with mTOR, a central regulator for cell proliferation and autophagy. Gγ7 induces cell division inhibition, cell death, and autophagy by perturbing the mTOR pathway. Further, Gγ subtypes are also found to exhibit differential effects on regulation of the actin cytoskeleton. For instance, reduction of actin stress fibers was observed in HeLa and U2OS cell lines upon the overexpression of Gγ7 [216]. Gγ7 results in efficient Gβγ translocation, and molecules that trigger cell death and autophagy are either cytosolic or located in cell organelles.
Insulin secretion
Glucose serves as a major physiologic regulator, ultimately leading to insulin secretion via a cascade of signalling in pancreatic β cells [217]. Several GPCRs have been shown to provide distinct ‘on’ and ‘off’ signals regulating insulin secretion [218]. The M3-muscarinic receptor mediates insulin secretion in pancreatic β cells upon activation [219]. The mechanism that drives M3-stimulated insulin secretion is explained partly as a result of PKD-mediated insulin vesicle formation from the Golgi complex [220]. As discussed earlier, as vesicular formation is a Gβγ translocation-mediated and Gγ subtype dependent process [204], insulin secretion may also be Gγ subtype-dependent. M3 receptor activation in a mouse pancreatic β cell line, NIT-1, exhibited a considerable increase in insulin secretion, confirming direct involvement of M3 receptors in the process and showed endogenous expression of relatively efficient mediators of Gβγ translocation such as Gγ5, Gγ10, and Gγ13 [184, 204]. In agreement with this observation, overexpression of slowly translocating Gγ3 significantly reduced M3-muscarinic receptor-induced insulin secretion [204], suggesting that GPCR-induced insulin secretion is Gγ subtype-dependent.
SNARE regulation and neuromodulation
Neurotransmission is a critical function driven by GPCRs upon stimulation by neurotransmitters to maintain the proper functioning of neuronal circuits [221]. Specifically, the inhibitory activity of pre-synaptic Gi-coupled GPCRs plays a major role in controlling autoreceptors that reduce neurotransmission [222]. This modulation is achieved by inhibiting pre-synaptic exocytosis via three major membrane-delimited mechanisms by; i) inhibiting Gβγ regulation of voltage-dependent Ca2+ channels (VDCCs) [223, 224], ii) Gβγ-mediated activation of G protein-coupled inwardly-rectifying potassium (GIRK) channels [225], and iii) direct interaction of Gβγ with soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins which results in disruption of Ca2+ uptake and downstream exocytosis [222, 226]. To understand the molecular requirements for Gβγ-SNARE interaction, differential binding affinities of Gβγ isoforms to SNARE have been examined [227]. Compared to Gβ1γ1, Gβ1γ2 had a ~20 fold higher affinity for target membrane SNARE (t-SNARE) complex binding along with higher efficiency in inhibiting membrane fusion in permeabilized PC12 cells [227]. In peptide competition studies, it was later discovered that the N-terminal 2–7 residues of Gγ2 partly contribute to the tight binding of Gβ1γ2 to the t-SNARE complex [228]. It is conceivable that the enhanced Gβγ and t-SNARE interactions are governed by their mutual subcellular localization.
Cell migration
Cell migration is implicated in several physiological processes such as embryonic development and the immune response, where cell migration defects lead to complications in immune responses, wound healing, tissue repair, and cancer metastasis [229–232]. Although thought of as a Gi-coupled chemokine receptor-governed process, Gβγ plays a major role in modulating macrophage migration by inducing PI3K-PIP3 signalling at the leading edge of a migratory cell [191]. Further, another study also showed the necessity of Gβγ-mediated activation of PLCβ for macrophage migration [48]. As described above, PI3K-mediated PIP3 generation is driven by high PM affinity Gγ isoforms such as Gγ3 and Gγ4. Is cell migration also influenced by high PM affinity Gγ subtypes? Supporting this idea, the migratory mouse macrophage cell line, RAW264.7 showed a higher endogenous expression of high PM affinity Gγ3 and moderate PM affinity Gγ4. Interestingly, endogenous expression of low PM affinity Gγ subunits such as Gγ9, Gγ11, and Gγ1 in this cell line was comparatively lower. A complete loss of migratory ability of RAW264.7 cells was achieved by knocking down endogenous Gγ3, and interestingly this inhibitory effect was rescued by exogenously expressing high PM affinity Gγ2 in Gγ3 knockdown cells [48]. Since the PM affinity of a Gγ subtype is defined by the C terminal CAAX and pre-CAAX sequence of Gγ, which determine the type of prenylation, the influence of CAAX and CT sequences of Gγ isoforms on regulating RAW264.7 migration was also examined. Interestingly, mutant Gγ9 with Gγ3-CT was able to induce migration in RAW264.7 cells while cells expressing Gγ3 mutants containing either Gγ9 CAAX or CT sequences did not support GPCR induced migration [48]. An earlier study conducted using anti-peptide antibodies specific for Gγ isoforms known at the time (Gγ1, Gγ2, Gγ3, Gγ5, Gγ7) indicated that Gγ5 was co-localized with focal adhesions and actin stress fibers in neonatal cardiac fibroblasts [233]. Since focal adhesions are metabolically active regions associated with modulating adhesion, proliferation, and cell motility [234–236], and focal adhesion size also is correlated with predicting cell migration rate [237], Gγ5 may play a role in governing migration of embryonic cells [233].
4.6. Subcellular localization-specific Gβγ signalling and their Gγ subtype dependence
The long-standing norm that GPCR-G protein signalling was limited to the PM has been strongly challenged recently, and continuously emerging evidence reveals GPCR-G proteins also signal at and from IMs, including the endoplasmic reticulum (ER), the Golgi compartments, and the nucleus. After their synthesis, GPCRs and their associated signalling molecules are trafficked to the PM to execute their appropriate signalling functions. Upon prolonged exposure to their agonists, desensitization leads to the internalization of the receptor. The accepted notion was that internalized GPCRs also could activate arrestin-dependent signalling pathways, which may be either or both functionally and structurally independent of G proteins [238, 239]. Additionally, other cognate molecules associated with GPCR signalling such as heterotrimeric G proteins [240, 241], adenylyl cyclase isoforms [242], phospholipase A2 [243], phospholipase Cβ [244], RGS proteins [245], β-arrestin 1 [246], and GRKs [247] are also reported to be transported to IMs attesting to the importance of their signalling at IMs.
The involvement of G proteins in regulating critical cellular events associated with cell motility, migration, and development is highlighted by their direct interactions with cytoskeleton and cell adhesion elements [233, 248–250]. The interaction of G proteins with the actin cytoskeleton has been shown in several studies [233, 249, 250]. Here, the localization of both Gq/11 and Gβγ dimers with the actin cytoskeleton is highlighted. The localization of Gβγ to the actin cytoskeleton was again noted for dimers containing specific Gγ subtypes. One study reported the localization of Gβγ dimers with Gγ12 on F-actin [249]. As a result of heterologous Gβ1γ12 expression in NIH 3T3 cells, rounding of cells, disruption of stress fibers, and enhanced cell migration was observed [249, 250]. Another study reported prominent localization of Gγ5 containing Gβγ to focal adhesions [233]. However, the relevant physiological and biochemical significance associated with their specific localization remains incompletely characterized.
Many studies have revealed roles for heterotrimeric G proteins in asymmetric cell division in model organisms such as C. elegans and D. melanogaster but in mammals as well [251]. The involvement of Gβγ in regulating heterotrimer activity consequently reduces Gαi interaction with GPR1/2 (G protein regulator 1/2)/Pins/LGN (leucine–glycine–asparagine), which acts as a positive regulator of asymmetrical cell division [252]. According to the reclassification by Human Genome Organization using a standard nomenclature, the analogous vertebrate protein is named G-protein signalling modulator-2 (GPSM2) [253]. Multiple studies have demonstrated that cell division-associated subcellular translocation of GPSM2, which includes movement from the cytoplasm to the midbody [254], the spindle pole [253] or the cortex [255]. During cell division, Gβγ competes with GPSM2 and its related proteins for their common binding partner, Gαi/o. A study using live-cell imaging of fluorescently-tagged Gβ shows the dynamic trafficking of Gβγ between cell cortex and endosomes, wherein the presence of Gβγ is enhanced during mitosis [256]. Even though different relative binding affinities and guanine nucleotide dissociation (GDI) potencies of GPSM2 towards different isoforms of Gα were observed [257], further studies on the relative abilities of different subtypes of Gβ and Gγ to compete with GPSM2 for Gαi/o binding are required.
A growing body of evidence demonstrates that Gβγ subunits can function at the Golgi complex facilitating the trafficking of macromolecules from trans Golgi network (TGN) to PM [258]. It has been shown that Golgi vesiculation is driven by constitutive or inducible targeting of Gβγ to the Golgi while PM-localized Gβγ had no effect [186]. This was further confirmed by inhibition of protein transport in the presence of Gβγ sequestration using a Golgi-targeted GRK2ct [258]. One possible explanation for Gβγ targeting the IMs, including Golgi, is their translocation potential upon GPCR-mediated G protein activation [201, 202]. In that sense, perhaps Gγ subtypes signalling at Golgi are mostly translocation competent. In agreement with this notion, one study showed the ability of GPCR activation-mediated Gβγ translocation to induce Golgi fragmentation and regulate insulin secretion [204]. Golgi fragmentation was achieved by the overexpression of Gγ11, one of the rapidly translocating Gγ subtypes. We have discussed above the enhanced Golgi fragmentation in the A569 cell line, which shows an abundant endogenous expression of Gγ11 [204]. Cellular senescence is another cellular physiological function potentially regulated by Gγ signalling at the Golgi complex, and the increased expression of Gγ11 in senescent cells was reported in another study [207]. Consistent with all the above data, the downregulation of cellular functions driven by signalling at the Golgi complex was observed due to expression of low translocating/dominant-negative Gγ3 [204, 207].
In addition to conventional signalling of GPCR-mediated activation of inositol triphosphate 3 (IP3) receptors on ER by PLCβ, it has been shown that Gβγ also can induce Ca2+ release to the cytosol via a PLCβ-independent pathway by directly interacting with IP3 receptors. In this study, introduction of Gβγ into cells restored the ability to release Ca2+ in cells where IP3 binding to IP3 receptors was inhibited [259].
An earlier study showed G protein activity in the nucleus by demonstrating the regulatory effects of Gγ5 interactions with the transcriptional repressor adipocyte enhancer-binding protein 1 (AEBP1) [260]. Gγ5 was shown to prevent transcriptional repression activity of AEBP1 by forming a complex with AEBP1 in the nuclei of 3T3-L1 cells. Nevertheless, Gγ7 was not able to form a similar complex [260]. Other studies also reported Gβγ nuclear localization and their subsequent interaction with various transcription factors and mediation of their regulation [261, 262]. By interacting with glucocorticoid receptors (GRs) and co-migrating with it into the nucleus, Gβγ suppresses GR-induced transactivation of the GR, and associated gene expression [261]. Interestingly, it was also shown that the anti-glucocorticoid activity of Gβγ is exhibited only in Gβγ with unprenylated Gγ subunits [262]. This was further confirmed by treatment of cells with a prenylation inhibitor, lovastatin to increase nuclear localization of Gβγ, and increase anti-glucocorticoid activity.
5. Impact of Gβγ subtype-diversity in disease and their potential as therapeutic targets
It is evident that improper signalling by Gβγ is detrimental, and dysregulation of Gβγ-mediated signalling could impact common events in heart failure, inflammation, carcinogenesis, and morphine-dependent antinociception [263]. There are several issues to be dealt with in considering Gβγ as a potential therapeutic target. As Gβγ is ubiquitously expressed and subtype-specific Gβγ-effector interactions remain incompletely understood, blocking all Gβγ functions with the current tools we have available might lead to undesired side effects. Since Gβγ is required for interaction of G protein heterotrimers with GPCRs, genetic deletion of the native Gβγ completely disrupts GPCR-G protein signalling. Further, considering their propensity for translocation, Gβγ heterodimers plays key roles in controlling the activity of effectors at different subcellular locations [178]. This may also have to be considered when developing them as viable therapeutic targets, as potential pharmacokinetic constraints may make this more difficult.
Gβγ subunits control numerous cellular processes ranging from immune system function, visual responses, and heart rate control to cell migration and cancer metastasis [76, 153, 175, 177, 264–266]. Tissue-specific expression of both Gβ and Gγ subunits suggests a possible relationship between particular subunit combinations and designated functions in specific tissues and organs [46, 80, 267]. Abnormal G protein signalling either due to conditions involving dysregulated control of their expression and/or mutations in Gβ and Gγ subtypes, results in altered signalling in disease. To support this notion, here we discuss several mutants and variants of both Gβ and Gγ subunits that have been identified in association with diseases in the visual, nervous, and cardiovascular systems and that impact cancer. Investigation of molecular links between mutations and variants of Gβ and Gγ subtypes and resultant pathological outcomes in light of underlying signalling perturbation will be the first step of identifying and developing therapeutic targets.
Nervous system
Millions of people worldwide are affected by neurological, neurodevelopmental and neurodegenerative disorders (i.e. epilepsy, Alzheimer’s disease, Parkinson’s disease, brain tumors, etc.), which affect both central and peripheral nervous systems. Here, we discuss selected Gβ and Gγ variants, which have been reported to cause neurological disorders. Several pathogenic variants of heterozygous Gβ1 coding gene (GNB1) have been identified in autosomal dominant neurodevelopmental disorder, MRD42 (Mental Retardation, Autosomal Dominant 42, or GNB1 encephalopathy or GNB1 disorder) in humans. Germline mutations in the GNB1 gene causes a rare neurodevelopmental disorder characterized by global developmental delay, intellectual disability, hypotonia, and seizures [268]. Similarly, an individual with GDD, hypotonia, multiple congenital joint contractures, and multiple morbidities caused by a de novo GNB2 variant p.Gly77Arg has been described [269]. Although only one GNB2 variant has been identified, 58 individuals encompassing thirty different GNB1 missense variants have been described to date [268, 270–278]. While the individuals are heterozygous for these GNB1 variants, mosaicism has been noted in a patient with a milder phenotype [274]. Though most reported cases have occurred de novo, the disorder is inherited in an autosomal dominant fashion. To date, four individuals have been reported to have inherited a pathogenic GNB1 missense variant, three with the p.Arg96Leu [272] mutation, and one with the p.Thr243Ala mutation [270]. Most variants are seen in exons 6 and 7 and occur at the interaction surface between Gβ1 and Gα subunits or various downstream effectors. In fact, some of these mutations have been shown to disturb heterotrimer formation and coupling to the dopamine D1 receptor leading to a loss of function phenotype [272]. While the vast majority of GNB1 mutations have been reported to be missense variants, three splice site variants (c.268–1G>T, c.917–1G>T, c.700–1G>T) and three frameshift variants (c.272_275del, c.915_916del, c.987_988delAG) have also been reported [272, 279]. Interestingly, c.700–1G>T and c.987_988delAG have also been shown to impact heterotrimer formation and coupling to the dopamine D2 receptor [279].
Global knockout of Gβ1 in mice results in neural tube defects leading to embryonic or neonatal lethality. In addition, severe brain malformations such as reduced cortical thickness and reduced brain volume have also been observed in these animals. Furthermore, abnormal morphologic changes in neural progenitor cells and impaired neural progenitor cell proliferation were also noted with Gβ1 knockdown, indicating a key role for Gβ1 in neurogenesis in the embryonic state [25, 280]. Gβ1 silencing (with Gβ1-specific antisense oligonucleotides) reduced Ca2+ currents with somatostatin receptor activation, suggesting a role for Gβ1 as a mediator of somatostatin receptor activity [25, 133]. Since somatostatin receptors modulate neuronal activity [281], with a wide distribution in the central nervous system in the mammalian brain [282], Gβ1-specific signalling might be considered as a new therapeutic target for neurological disorders.
Mutations in GNB4 have been shown to cause an autosomal dominant hereditary form of Charcot-Marie Tooth disease (CMTDIF), characterized by progressive muscle atrophy and weakness, distal sensory impairment, decreased reflexes, and variable nerve conduction velocities. Eight individuals have been reported to date, the first described being a family of six affected individuals where two were heterozygous for the p.Gly53Asp mutation, and a de novo case where the individual harbored the p.Lys89Glu mutation. Both mutations were shown to impair bradykinin receptor signalling resulting in a reduction of PLCβ2 activation, lower IP3 production, and a reduction in cytosolic calcium [92]. Another de novo mutation, p.Lys57Glu, was seen in an individual presenting with muscle atrophy, pes cavus and scoliosis with an age of onset of 35 years old [283]. The p.Gln220Arg variant was seen in a Japanese male and his father whose disease severity was mild, age of onset was higher, and had axonal and demyelinating neuropathies respectively [284].
Mutations in GNB5 are associated with either intellectual developmental disorder with cardiac arrhythmia (IDDCA) or language delay or the milder attention deficit-hyperactivity disorder/cognitive impairment with or without cardiac arrhythmia (LADCI) [125, 285–288]. Clinical severity correlates with the genetic variant where individuals who are homozygous for the p.Ser81Leu missense variant exhibit the milder phenotype characterized by language delay, mild intellectual disability, ADHD, with or without arrhythmias [125, 288]. In contrast, individuals either homozygous or compound heterozygous for a null allele suffer from the more severe phenotype including severe intellectual disability, epilepsy, hypotonia, retinal disease, bradycardia, sick sinus syndrome, and premature sudden death [125, 285–287].
Gγ2 is the most abundant Gγ subunit in the brain [25, 46, 80], and is involved in nociception mediated through the peripheral and central nervous systems [289, 290]. Gγ2 silencing by specific antisense oligonucleotide injection followed by administration of morphine, DPDPE (δ-opioid receptor agonist), and the non-opioid receptor agonists (WIN 55212–2 and clonidine), resulted in significant reduction of analgesia in mice, compared to control mice with no Gγ2 silencing, indicating a role for Gγ2 in nociception [291, 292]. Also, antisense oligonucleotide-mediated Gγ2 silencing resulted in a reduction in galanin-induced inhibition of voltage-gated Ca2+ channels in rat pituitary-derived GH3 cells and RIN5mF rat insulinoma cells, suggesting that Gγ2-containing G protein heterotrimers are coupled to galanin receptors, which are found in the peripheral and central nervous systems as well as the endocrine system [293]. Additionally, this observation further suggests that Gγ2 has a potential role in regulating intracellular Ca2 homeostasis. Since Ca2+ homeostasis plays an important role in neurotransmitter release in the central nervous system [294], this could be a novel drug target for neurological disorders, including Alzheimeŕs and Parkinsońs diseases, in which the neurotransmission is reduced. Furthermore, Gγ3 knockout mice have been shown to be highly susceptible to seizures signifying a role for Gγ3 in regulating neuronal excitability [295].
Adenylyl cyclase (AC) activity plays an important role in memory, synaptic plasticity, as well as neurodegeneration [296], and altered AC activity has been shown in Alzheimer’s disease, where gradual and long-term memory loss is observed. With the loss of Gγ7 expression, reduced AC activity upon dopamine D1 receptor or adenosine A2A receptor activation has been observed in HEK 293 cells [141, 166, 297], implying the involvement of Gγ7 in both dopamine D1 and adenosine A2A receptor signalling. Since the adenosine A2A receptor plays an important role in regulating CNS neurotransmission [298], this would be a potential target for drug development for neurological disorders.
While human expression of GNG8 seems to be low in all tissues, it was one of many genes lost in 19q13.32 microdeletion syndrome, which presents as intellectual disability, dysmorphic features, and cardiac deficits [299]. In mice, GNG8 is expressed in the medial habenula (MHb) and interpeduncular nucleus (IPN), where knocking out GNG8 caused impairments in spatial and long-term memory, as well as cholinergic signalling [300]. Furthermore, Gγ3 knockout mice have been shown to be highly susceptible to seizures signifying a role for Gγ3 in regulating neuronal excitability. This phenotype was exacerbated in GNG3(−/−)GNG7 (−/−) double knockout mice which displayed a severe seizure phenotype that reduced their median life span to 75 days [301]. GNG13 is known for expression in the cerebellum; however, its expression in cerebellar Purkinje cells was reduced in Alzheimer’s patients compared to healthy control subjects [302], suggesting a possible role for GNG13 expression as a biomarker of cerebellar health. Additionally, a female patient with global developmental delay, cardiac defects, and failure of gonad development was seen to have a gain in copy number of the short arm of chromosome 16, which encompasses the GNG13 gene [303].
Visual system
Gα transducin (Gt) forms heterotrimers with Gβγ and plays a pivotal role in phototransduction [304–307]. Gγ1 was initially identified as the primary Gγ subtype present in transducin heterotrimer in rod cells [88, 307, 308]. Although Gγ1 shows a broad tissue expression; in the liver, kidney, placenta, uterus, etc., the majority of studies focusing on Gγ1 deal with signalling/phototransduction in the eye [25]. Photoreceptors in the retina undergo degeneration in several eye diseases, including macular degeneration, retinal detachment, and retinitis pigmentosa [309]. Disease is usually characterized by the loss of differentiated cells (i.e., rod cells) in the retina due to progressive cell death [310]. There are many causes of this degeneration, including genetic abnormalities (i.e., Stargardt disease), genetic and mechanistic diversity, so therapeutic development remains challenging [311]. Recent studies have also suggested that photoreceptor cell death may result primarily from non-apoptotic mechanisms, independent of caspase activity [312]. A study conducted in mice noted photoreceptor degeneration upon knock-out of Gγ1, suggesting a role of Gγ1 in photoreceptor function [304]. Since photoreceptors are not regenerated in mammals as in some other species (i.e., zebrafish) [313], scientists have recently tested if mammalian Müller glial cells; a source of retinal stem cells, could be stimulated to develop into rod photoreceptors in mammals [314]. However, since these mechanisms are not as robust as intrinsic repair mechanisms, development of novel methods to promote repair or regeneration of damaged photoreceptors is an essential goal in vision research, and one potential target gene might be Gγ1.
GNB3 has been identified as the cause of congenital stationary night blindness type 1H (CSNB1H), a unique retinal disorder with a dual anomaly in visual processing. It is characterized by varying degrees of dysfunction in the ON-class of bipolar cells and reduced cone sensitivity. CSNB1H was identified in a Lebanese-Armenian family where two brothers were compound heterozygous for the p.Lys57del deletion and the p.Trp339* nonsense mutation, while their aunt was homozygous for the p.Trp339∗ mutation [85]. A fourth sporadic case was identified in a woman homozygous for the p.Ser67Phe mutation [85]. A n additional GNB3 mutation was reported in a patient homozygous for the p.Arg42Ter nonsense mutation, who presented with an inherited retinal disease beginning in childhood characterized by nystagmus, mild disturbance of the central macula, and electroretinographic abnormalities [315]. In ON bipolar cells of the retina, mGluR6 activation leads to the closing of the TRPM1 channels. Gβ3 is part of the heterotrimeric G protein responsible for mGluR6 signalling. GNB3-knock out mice show that the absence of Gβ3 diminished light responses and mislocalized and reduced expression of other cascade partners, thereby altering synaptic organization [316]. In cones, the absence of Gβ3 resulted in reduced expression of G protein heterotrimer components and reduced light sensitivity [317]. In chicken, a three bp deletion (p.D153del) in GNB3 causes a retinopathy globe enlarged (rge) phenotype, categorized by retinal degeneration and embryonic mortality [318, 319].
Vision abnormalities, including vision impairment and abnormal eye movements are also seen in 60% of de novo GNB1 cases [278]. Abnormal eye movements such as nystagmus are most common, but others such as strabismus, and ophthalmoplegia have also been observed. This disease is also associated with cortical visual impairment, which is caused by disturbance of visual pathways such as optic nerve atrophy, retinal disease, etc. [116, 268, 320]. An abnormal morphology of retina with progressive degeneration has been noted in GNB1 heterozygous mice, further supporting the ophthalmic manifestations reported in affected patients with mutations in GNB1 [116].
Deactivation of G proteins induces the termination of light signalling responses in the mammalian eye. This process is catalyzed by a protein complex that includes Gαt, the regulator of G protein signalling 9 (RGS9), and a splice variant of Gβ5 subunit, Gβ5-L [25, 321, 322]. Gβ5-L is exclusively expressed in retinal photoreceptor cells and is involved in this process by interacting with G protein γ like domain (GGL domain) in RGS9–1 (Fig. 18) [25, 40, 45, 323]. With knockout of Gβ5L, G protein deactivation was decelerated in mice, indicating a role for Gβ5L in regulating heterotrimer inactivation [25, 321]. Individuals with mutations in the Gβ5 subunit have been shown to exhibit ophthalmologic abnormalities such as retinal disease and nystagmus [125, 286]. By measuring the optokinetic response, a GNB5-knockout zebrafish model showed that GNB5 is required for normal eye movement control [125]. GNB5-knockout mouse models have also been shown to display defective visual adaptation accompanied by an accelerated recovery of visual responses with altered development and function of retinal bipolar cells [116, 324–326].
Figure 18.
Crystal structure showing the Gβ5 (green) interaction with the GGL domain (maroon) of RGS9 (PDB ID: 2PBI) (light brown). The orientation of Gβ5 with the GGL domain of RGS9 is similar to Gγ1-Gβ1 interaction in the transducin heterotrimer, which is involved in vision transduction. RGS9 in mammalian eyes is involved in the propert termination phototransduction.
Cardiovascular system
Cardiovascular diseases remain a major cause of death and disability throughout the world. Here, we discuss the implication of certain GNB and GNG genes, and their variants as potential targets. An increased heart rate has been detected in GNB2 knockout mice, which reveals the involvement of Gβ2 in the regulation of cardiac muscle contractility [327]. Also, a heterozygous missense variant (Arg52Leu) of GNB2 has been identified in humans with autosomal dominant form of sinus node dysfunction (SND), which displays atrioventricular conduction dysfunction and atrial fibrillation without structural changes of the heart [42, 116]. Following the crystal structure of the mammalian G protein-coupled inwardly rectifying potassium channel 2/G protein complex (GIRK2)-β1γ2), reduced Gβ2-GIRK interactions could be anticipated with the Arg52 variant since this residue in Gβ2 is found in the Gβγ binding interface for GIRK [42, 116]. A functional study revealed involvement of this variant on GIRK channel activity alterations, which increases Ach-activated K+ currents [42]. Furthermore, a recent study with observations from sinus node dysfunction (SND) patients identified another Gβ2 variant (Trp101Cys), in addition to mutations in the KCNJ5 gene, which encodes the Kir3.4 subunit of the GIRK channel [328]. Since cardiac GIRK channels are directly activated by Gβγ dimers and are involved in controlling heart rate [182, 329], such Gβ variants may suggest strategies in potential drug design. Genome-wide association studies have mapped GNB2 and GNB4 to loci associated with variations in heart rate [330, 331]. While a role for GNB2 in control of heart rate has been investigated, a role for GNB4 has yet to be established. Further, a C825T polymorphism of GNB3 has been linked with increased atrial inward rectifying currents, which may reduce the risk of developing atrial fibrillation, characterized by irregular heartbeat [332, 333]. Furthermore, Gβ3 knockout mice have been shown to display mild bradycardia [56]. This may indicate that Gβ3 also plays a distinct role in regulating cardiac signalling by controlling the heart rate [116]. Although the exact molecular mechanism underlying these observations remains to be investigated further, the C825T polymorphism in Gβ3 is reported to favor alternative splicing resulting in two different splice variants; Gβ3s and Gβ3s225, altering their signalling ability [334–336]. Several studies have reported enhanced coronary vasoconstriction [337] and impaired vasodilation [338] with the C825T polymorphism in Gβ3, supporting this notion [334].
As previously mentioned, LADCI and IDDCA patients also exhibit cardiovascular phenotypes including bradycardia and sick sinus syndrome in addition to the neurological phenotypes described above, suggesting a role for GNB5 in cardiovascular disease. In addition, when GNB5-knockout zebrafish larvae were treated with carbachol, a strong decrease in heart rate was observed suggesting that GNB5 normally plays an inhibitory role in GIRK activity mediated by the parasympathetic nervous system [125]. Conversely, when the sympathetic agonist isoproterenol was used, heart rate did not differ from wild-type animals implying GNB5 is not crucial for sympathetic control [125].
Gene expression profiling analysis indicated that GNG2 was differentially expressed in children with vasovagal syncope type 1 [339], a transient loss of consciousness often presented as a rapid decline in heart rate and a drop in blood pressure. Copy number variations resulting in deletions in the GNB1 or GNG7 genes have been associated with the risk of atrial fibrillation-related thromboembolism in the general Taiwanese population [340]. In contrast, in the Polish population, the rs13093 GNG5 mutation is a risk factor for essential hypertension [341]. A cardiovascular role for GNG5 is further emphasized by the phenotypes observed in GNG5 knockout mice. GNG5(−/−) mice exhibit severe cardiac defects such as un-looped hearts containing a single ventricle, abnormal headfolds, and hypoplastic pharyngeal arches, ultimately resulting in embryonic lethality [342].
Cancer
Many studies have also linked Gβγ signalling and mutations in individual Gβ and Gγ subunits to progression and outcomes in different types of cancer. Only one patient with a germline GNB1 mutation discussed above has been reported to have cancer in addition to the neurodevelopmental phenotype. The patient was heterozygous for the p.Gly77Ala mutation, developed acute lymphoblastic leukemia (ALL), which was successfully treated at an early age [273]. Somatic mutations in the GNB1 and GNB2 genes have been shown to occur in multiple cancers often conferring cytokine-independent growth. Interestingly, 11 GNB1 K57 mutations were seen in myeloid neoplasms, while I80 mutations were seen in 7 of 8 B cell neoplasms, suggesting lineage-based clustering. As in the de novo GNB1 mutations, these GNB1 mutations have been shown to reduce binding to most Gα subunits but not Gγ subunits. This also led to increased phosphorylation of phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinase (MAPK), and mTOR substrates [343]. GNB1 mutations were shown to occur with other oncogenic alterations such as BCR-ABL in B cell acute lymphoblastic leukemia and JAK2 mutations in myeloid neoplasms, resulting in resistance to their respective inhibitors; similarly, the GNB2 mutation K78E was identified in BRAF-mutated melanoma, also resulting in resistance to BRAF inhibition [343]. The K89M GNB1 mutation, in particular, was identified as the cause of tyrosine kinase inhibitor resistance in ETV6-ABL1-positive leukemic cells by restoring the inhibited PI3K/Akt/mTOR and MAPK signalling pathways [344].
The previously mentioned C825T polymorphism in the GNB3 gene has also been shown to be implicated in the progression of various cancers such as breast cancer [345], bladder cancer [346], thyroid carcinomas [347, 348], head and neck squamous cell carcinoma [349] and glioblastoma multiforme [350]. Additional GNB3 somatic mutations have also been found in malignant melanoma samples [351]. GNB4 was identified as a target silenced by DNA methylation in anti-estrogen-resistant breast cancer cell lines, where its overexpression and knockdown indicated a role for GNB4 in breast cancer growth [352]. GNB4 was also shown to promote gastric cancer cell proliferation and migration, where its expression was associated with the long-term survival rate of gastric cancer patients [353], indicating the possibility of using GNB4 as a predictive marker. Using GNB4 as a predictor has also been suggested in other cancers as two haplotype blocks have been identified in intron 1 of the GNB4 gene, where the first is associated with survival in bladder cancer patients [354], and the second with survival in colorectal cancer patients [355].
While Gβ5 is known to be predominantly expressed in the brain, Gβ5 has also been shown to be implicated in colorectal cancer. Whole-exome sequencing and gene expression analysis have suggested that mutations in GNB5 contribute to lung metastasis in colorectal cancer, possibly through phospholipase C signalling [356]. Gβ5 has been shown to regulate colorectal cancer cell apoptosis induced by TRAIL by reducing the surface expression of the TRAIL-R2 receptor, increasing expression of the anti-apoptotic protein XIAP, and activating the NF-κB signalling pathway [357]. In addition, Gβ5 antagonism has been shown to overcome TRAIL-mediated apoptotic resistance and cetuximab resistance in both wild-type and mutant KRAS cells, indicating a role for Gβ5 in colorectal cancer cell therapy [357, 358].
Malignant melanoma is a highly aggressive melanocytic tumor. Evidence exists encouraging investigation of GNG2 as a potential therapeutic target for the metastasis of malignant melanoma. GNG2 expression is reduced in malignant melanoma [359], leading to increased cell migration and invasion, and augmented proliferation, while GNG2 overexpression inhibits metastasis in human malignant melanoma cells [360]. Gγ10 may also serve a role in melanoma metastasis as somatic mutations in GNG10 were seen in 8.75% of metastases [351]. GNG3 was a key differentially expressed gene in glioblastoma multiforme (GBM) [361, 362]. In addition, GNG4 was shown to be hypermethylated and downregulated in GBM; Gγ4 is a potential tumor suppressor as overexpression in vitro reduced proliferation, colony formation, and transformation of immortalized astrocytes [363]. A tumor suppressor role for GNG4 has been previously suggested in renal cell carcinoma [364]. In colon cancer, GNG4 may serve as a diagnostic indicator since expression was observed to be higher in left-sided colon cancer than in right-sided colon cancer, and a prognostic indicator as high GNG4 expression showed higher disease stage and lower survival rate [365]. GNG5 was identified as one of 10 genes that correlate to lower grade glioma tumor purity and patient prognosis [366]. Gγ5 was shown to be an unfavorable prognostic indicator as glioma patients with GNG5 overexpression had shorter overall survival times [367].
In classical Hodgkin’s lymphoma, screening for homozygous deletions in four cell lines determined one of the genes deleted was GNG7, indicating a potential tumor suppressor role [368]. GNG7 expression levels verified in osteosarcoma, cervical carcinoma, breast cancer, colon cancer, and nasopharyngeal carcinoma cells lines all displayed a reduction in Gγ7 expression [216]. Reduced Gγ7 expression was also observed in clear cell renal cell carcinoma [369], head and neck squamous cell carcinoma (HNSCC) [370], esophageal cancer [371], intrahepatic cholangiocarcinoma [372], and pancreatic cancer [373]. Though the precise reason for differing GNG7 expression levels in cancer have not been fully elucidated, the GNG7 gene promoter was heavily methylated in clear cell renal cell carcinoma tissues [369], in HNSCC [370, 374], and in esophageal cancer [371]. GNG7 promoter hypermethylation, as well as GNG7 gene mutations in clear cell renal cell carcinoma, and loss of heterozygosity in esophageal cancer, may serve as a reason for decreased GNG7 expression observed in various cancers. GNG7 expression correlated with poor overall survival as well as tumor grade, size, and invasion [369–372]. GNG7 was also identified as a differentially expressed and prognosis-related gene in lung adenocarcinoma, proposed to be used for diagnosis and predicting prognosis both on its own and as part of a four-gene panel [375, 376]. Together evidence suggests a tumor suppressor role for GNG7 in multiple types of cancer.
GNG10 expression levels in peripheral blood mononuclear cells correlated with prognosis in head and neck squamous cell carcinoma and radiotherapy response in nasopharyngeal carcinoma [377]. GNG11 was identified as a gene hub in lung squamous cell carcinoma, where it is associated with tumor size, the maximum standardized uptake value, and recurrence-free survival [378]. GNG11 was also shown to act as a hub gene in lung adenocarcinoma where high expression correlated with better survival outcomes, suggesting a potential tumor suppressor role [379, 380]. In female non-smokers with lung adenocarcinoma, low GNG11 expression was observed and associated with poor patient survival rates [381, 382], further highlighting a potential role for Gγ11 and tumor suppression. GNG11 was also shown to be downregulated in acute myeloid leukemia, B-lineage acute lymphoblastic leukemia, and T-lineage acute lymphoblastic leukemia [383]. GNG12 expression was observed to be higher in pancreatic ductal adenocarcinoma (PDAC) patient specimens and was accompanied by a poor prognosis of pancreatic cancer; Elevated GNG12 expression promoted PDAC tumor growth both in vitro and in vivo, suggesting a role for GNG12 in PDAC treatment or as a marker of unfavorable prognosis [384]. In gastrointestinal stromal tumors, high GNG13 expression may also serve as a poor prognosis-related biomarker [385].
Conclusion
As we move away from thinking about Gβγ signalling as if it were mediated by a single protein, we come to see that a comprehension of the impact of subunit diversity is critical to our understanding of their vast functions in health and disease. Targeting these events pharmacologically will be contingent on a clearer understanding of the myriad mechanistic nuances involved.
Highlights.
Gβγ subunits are major contributors to GPCR-G protein signalling.
A broad functional array of Gβγ signalling has recently been attributed to Gβ and Gγ subtype diversity,
We review the literature on the repercussions of Gβ and Gγ subtype diversity on direct and indirect regulation of GPCR/G protein signalling events and their physiological outcomes.
We provide perspective in understanding the roles of subtype-specific roles of Gβγ signalling and associated diseases
Funding
This work was supported by NIH-NIGMS, grant number 1R15GM126455-01A1 to AK and by a grant from the Canadian Institutes of Health Research (CIHR) to T.E.H. (PJT-159687). T.E.H. holds the Canadian Pacific Chair in Biotechnology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- [1].de Mendoza A, Sebe-Pedros A, Ruiz-Trillo I, The evolution of the GPCR signaling system in eukaryotes: modularity, conservation, and the transition to metazoan multicellularity, Genome Biology and Evolution 6(3) (2014) 606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kobilka BK, G protein coupled receptor structure and activation, BBA-Biomembranes 1768(4) (2007) 794–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shalaeva DN, Galperin MY, Mulkidjanian AY, Eukaryotic G protein-coupled receptors as descendants of prokaryotic sodium-translocating rhodopsins, Biol Direct 10 (2015) 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mahoney JP, Sunahara RK, Mechanistic insights into GPCR-G protein interactions, Curr Opin Struc Biol 41 (2016) 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Duc NM, Kim HR, Chung KY, Recent Progress in Understanding the Conformational Mechanism of Heterotrimeric G Protein Activation, Biomol Ther (Seoul) 25(1) (2017) 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gautam N, Downes GB, Yan K, Kisselev O, The G-protein βγ complex, Cell Signal 10(7) (1998) 447–55. [DOI] [PubMed] [Google Scholar]
- [7].Hurowitz EH, Melnyk JM, Chen YJ, Kouros-Mehr H, Simon MI, Shizuya H, Genomic characterization of the human heterotrimeric G protein α, β, and γ subunit genes, DNA Research 7(2) (2000) 111–20. [DOI] [PubMed] [Google Scholar]
- [8].McIntire WE, Structural determinants involved in the formation and activation of G protein βγ dimers, Neurosignals 17(1) (2009) 82–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fogg VC, Azpiazu I, Linder ME, Smrcka A, Scarlata S, Gautam N, Role of the γ subunit prenyl moiety in G protein βγ complex interaction with phospholipase Cβ, J Biol Chem 276(45) (2001) 41797–802. [DOI] [PubMed] [Google Scholar]
- [10].Lindorfer MA, Sherman NE, Woodfork KA, Fletcher JE, Hunt DF, Garrison JC, G protein γ subunits with altered prenylation sequences are properly modified when expressed in Sf9 cells, J Biol Chem 271(31) (1996) 18582–7. [DOI] [PubMed] [Google Scholar]
- [11].Dupré DJ, Robitaille M, Rebois RV, Hébert TE, The role of Gβγ subunits in the organization, assembly, and function of GPCR signaling complexes, Annual Review of Pharmacology and Toxicology 49(1) (2009) 31–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wedegaertner PB, G protein trafficking, Subcell Biochem 63 (2012) 193–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Neer EJ, Schmidt CJ, Nambudripad R, Smith TF, The ancient regulatory-protein family of WD-repeat proteins, Nature 371(6495) (1994) 297–300. [DOI] [PubMed] [Google Scholar]
- [14].McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS, G-protein signaling: back to the future, Cellular and Molecular Life Sciences : CMLS 62(5) (2005) 551–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB, The 2.0 A crystal structure of a heterotrimeric G protein, Nature 379(6563) (1996) 311–9. [DOI] [PubMed] [Google Scholar]
- [16].Sondek J, Bohm A, Lambright DG, Hamm HE, Sigler PB, Crystal structure of a G-protein βγ dimer at 2.1A resolution, Nature 379(6563) (1996) 369–74. [DOI] [PubMed] [Google Scholar]
- [17].Ford CE, Skiba NP, Bae H, Daaka Y, Reuveny E, Shekter LR, Rosal R, Weng G, Yang CS, Iyengar R, Miller RJ, Jan LY, Lefkowitz RJ, Hamm HE, Molecular basis for interactions of G protein βγ subunits with effectors, Science 280(5367) (1998) 1271–4. [DOI] [PubMed] [Google Scholar]
- [18].Li Y, Sternweis PM, Charnecki S, Smith TF, Gilman AG, Neer EJ, Kozasa T, Sites for Gα binding on the G protein β subunit overlap with sites for regulation of phospholipase Cβ and adenylyl cyclase, J Biol Chem 273(26) (1998) 16265–72. [DOI] [PubMed] [Google Scholar]
- [19].Brand CS, Sadana R, Malik S, Smrcka AV, Dessauer CW, Adenylyl Cyclase 5 Regulation by Gβγ Involves Isoform-Specific Use of Multiple Interaction Sites, Molecular Pharmacology 88(4) (2015) 758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Huang C-L, Slesinger PA, Casey PJ, Jan YN, Jan LY, Evidence that direct binding of Gβγ to the GIRK1 G protein-gated inwardly rectifying K+ channel is important for channel activation, Neuron 15(5) (1995) 1133–1143. [DOI] [PubMed] [Google Scholar]
- [21].Berlin S, Keren-Raifman T, Castel R, Rubinstein M, Dessauer CW, Ivanina T, Dascal N, G α(i) and Gβγ jointly regulate the conformations of a Gβγ effector, the neuronal G protein-activated K+ channel (GIRK), J Biol Chem 285(9) (2010) 6179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bockaert J, Marin P, Dumuis A, Fagni L, The ‘magic tail’ of G protein-coupled receptors: an anchorage for functional protein networks, FEBS Lett 546(1) (2003) 65–72. [DOI] [PubMed] [Google Scholar]
- [23].Bockaert J, Roussignol G, Becamel C, Gavarini S, Joubert L, Dumuis A, Fagni L, Marin P, GPCR-interacting proteins (GIPs): nature and functions, Biochem Soc Trans 32(Pt 5) (2004) 851–5. [DOI] [PubMed] [Google Scholar]
- [24].Rebois RV, Robitaille M, Galés C, Dupré DJ, Baragli A, Trieu P, Ethier N, Bouvier M, Hébert TE, Heterotrimeric G proteins form stable complexes with adenylyl cyclase and Kir3.1 channels in living cells, J Cell Sci 119 (2006) 2807–18. [DOI] [PubMed] [Google Scholar]
- [25].Khan SM, Sleno R, Gora S, Zylbergold P, Laverdure JP, Labbe JC, Miller GJ, Hébert TE, The expanding roles of Gβγ subunits in G protein-coupled receptor signaling and drug action, Pharmacol Rev 65(2) (2013) 545–77. [DOI] [PubMed] [Google Scholar]
- [26].Simon MI, Strathmann MP, Gautam N, Diversity of G proteins in signal transduction, Science 252(5007) (1991) 802–8. [DOI] [PubMed] [Google Scholar]
- [27].Krishnan A, Mustafa A, Almen MS, Fredriksson R, Williams MJ, Schioth HB, Evolutionary hierarchy of vertebrate-like heterotrimeric G protein families, Mol Phylogenet Evol 91 (2015) 27–40. [DOI] [PubMed] [Google Scholar]
- [28].Slessareva JE, Dohlman HG, G protein signaling in yeast: new components, new connections, new compartments, Science 314(5804) (2006) 1412–3. [DOI] [PubMed] [Google Scholar]
- [29].Goddard A, Ladds G, Forfar R, Davey J, Identification of Gnr1p, a negative regulator of Gα signalling in Schizosaccharomyces pombe, and its complementation by human Gβ subunits, Fungal Genet Biol 43(12) (2006) 840–51. [DOI] [PubMed] [Google Scholar]
- [30].Shpakov AO, Pertseva MN, Signaling systems of lower eukaryotes and their evolution, International Review of Cell and Molecular Biology 269 (2008) 151–282. [DOI] [PubMed] [Google Scholar]
- [31].Li L, Wright SJ, Krystofova S, Park G, Borkovich KA, Heterotrimeric G protein signaling in filamentous fungi, Annual Review of Microbiology 61 (2007) 423–52. [DOI] [PubMed] [Google Scholar]
- [32].Yang Q, Poole SI, Borkovich KA, A G-protein β subunit required for sexual and vegetative development and maintenance of normal Gα protein levels in Neurospora crassa, Eukaryotic cell 1(3) (2002) 378–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Boto T, Gomez-Diaz C, Alcorta E, Expression analysis of the 3 G-protein subunits, Gα, Gβ, and Gγ, in the olfactory receptor organs of adult Drosophila melanogaster, Chem Senses 35(3) (2010) 183–93. [DOI] [PubMed] [Google Scholar]
- [34].Yarfitz S, Provost NM, Hurley JB, Cloning of a Drosophila melanogaster guanine nucleotide regulatory protein β-subunit gene and characterization of its expression during development, Proceedings of the National Academy of Sciences USA 85(19) (1988) 7134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chase DL, Patikoglou GA, Koelle MR, Two RGS proteins that inhibit Gα(o) and Gα(q) signaling in C. elegans neurons require a Gβ(5)-like subunit for function, Current Biology 11(4) (2001) 222–31. [DOI] [PubMed] [Google Scholar]
- [36].Ma H, Yanofsky MF, Meyerowitz EM, Molecular cloning and characterization of GPA1, a G protein α subunit gene from Arabidopsis thaliana, Proceedings of the National Academy of Sciences USA 87(10) (1990) 3821–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Weiss CA, Garnaat CW, Mukai K, Hu Y, Ma H, Isolation of cDNAs encoding guanine nucleotide-binding protein β-subunit homologues from maize (ZGB1) and Arabidopsis (AGB1), Proceedings of the National Academy of Sciences USA 91(20) (1994) 9554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yadav DK, Shukla D, Tuteja N, Isolation, in silico characterization, localization and expression analysis of abiotic stress-responsive rice G-protein β subunit (RGB1), Plant Signal Behav 9(5) (2014) e28890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].de Jonge HR, Hogema B, Tilly BC, Protein N-myristoylation: critical role in apoptosis and salt tolerance, Sci STKE 2000(63) (2000) pe1. [DOI] [PubMed] [Google Scholar]
- [40].Watson AJ, Aragay AM, Slepak VZ, Simon MI, A novel form of the G protein β subunit Gβ5 is specifically expressed in the vertebrate retina, J Biol Chem 271(45) (1996) 28154–60. [DOI] [PubMed] [Google Scholar]
- [41].Downes GB, Gautam N, The G protein subunit gene families, Genomics 62(3) (1999) 544–52. [DOI] [PubMed] [Google Scholar]
- [42].Stallmeyer B, Kuss J, Kotthoff S, Zumhagen S, Vowinkel K, Rinne S, Matschke LA, Friedrich C, Schulze-Bahr E, Rust S, Seebohm G, Decher N, Schulze-Bahr E, A Mutation in the G-Protein Gene GNB2 Causes Familial Sinus Node and Atrioventricular Conduction Dysfunction, Circ Res 120(10) (2017) e33–e44. [DOI] [PubMed] [Google Scholar]
- [43].Levine MA, Modi WS, O’Brien SJ, Chromosomal localization of the genes encoding two forms of the G protein β polypeptide, β1 and β3, in man, Genomics 8(2) (1990) 380–6. [DOI] [PubMed] [Google Scholar]
- [44].Sondek J, Siderovski DP, Gγ-like (GGL) domains: new frontiers in G-protein signaling and β-propeller scaffolding, Biochemical Pharmacology 61(11) (2001) 1329–37. [DOI] [PubMed] [Google Scholar]
- [45].Watson AJ, Katz A, Simon MI, A fifth member of the mammalian G-protein β-subunit family. Expression in brain and activation of the β2 isotype of phospholipase C, J Biol Chem 269(35) (1994) 22150–6. [PubMed] [Google Scholar]
- [46].Wettschureck N, Offermanns S, Mammalian G proteins and their cell type specific functions, Physiol Rev 85(4) (2005) 1159–204. [DOI] [PubMed] [Google Scholar]
- [47].Jones PG, Lombardi SJ, Cockett MI, Cloning and tissue distribution of the human G protein β5 cDNA, BBA-Biomembranes 1402(3) (1998) 288–91. [DOI] [PubMed] [Google Scholar]
- [48].Senarath K, Payton JL, Kankanamge D, Siripurapu P, Tennakoon M, Karunarathne A, Gγ identity dictates efficacy of Gβγ signaling and macrophage migration, J Biol Chem 293(8) (2018) 2974–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Giri L, Patel AK, Karunarathne WK, Kalyanaraman V, Venkatesh KV, Gautam N, A G-protein subunit translocation embedded network motif underlies GPCR regulation of calcium oscillations, Biophysical Journal 107(1) (2014) 242–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lin Y, Smrcka AV, Understanding molecular recognition by G protein βγ subunits on the path to pharmacological targeting, Molecular Pharmacology 80(4) (2011) 551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Spring DJ, Neer EJ, A 14-amino acid region of the G protein γ subunit is sufficient to confer selectivity of γ binding to the β subunit, J Biol Chem 269(36) (1994) 22882–6. [PubMed] [Google Scholar]
- [52].Iniguez-Lluhi JA, Simon MI, Robishaw JD, Gilman AG, G protein βγ subunits synthesized in Sf9 cells. Functional characterization and the significance of prenylation of γ, J Biol Chem 267(32) (1992) 23409–17. [PubMed] [Google Scholar]
- [53].Schmidt CJ, Thomas TC, Levine MA, Neer EJ, Specificity of G protein β and γ subunit interactions, J Biol Chem 267(20) (1992) 13807–10. [PubMed] [Google Scholar]
- [54].Rosskopf D, Koch K, Habich C, Geerdes J, Ludwig A, Wilhelms S, Jakobs KH, Siffert W, Interaction of Gβ3s, a splice variant of the G-protein Gβ3, with Gγ- and Gα-proteins, Cell Signal 15(5) (2003) 479–88. [DOI] [PubMed] [Google Scholar]
- [55].Ruiz-Velasco V, Ikeda SR, A splice variant of the G protein β3-subunit implicated in disease states does not modulate ion channels, Physiological Genomics 13(2) (2003) 85–95. [DOI] [PubMed] [Google Scholar]
- [56].Ye Y, Sun Z, Guo A, Song LS, Grobe JL, Chen S, Ablation of the GNB3 gene in mice does not affect body weight, metabolism or blood pressure, but causes bradycardia, Cell Signal 26(11) (2014) 2514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yan K, Kalyanaraman V, Gautam N, Differential ability to form the G protein βγ complex among members of the β and γ subunit families, J Biol Chem 271(12) (1996) 7141–6. [DOI] [PubMed] [Google Scholar]
- [58].Rosskopf D, Nikula C, Manthey I, Joisten M, Frey U, Kohnen S, Siffert W, The human G protein β4 subunit: gene structure, expression, Gγ and effector interaction, FEBS Lett 544(1–3) (2003) 27–32. [DOI] [PubMed] [Google Scholar]
- [59].Asano T, Morishita R, Ueda H, Kato K, Selective association of G protein β(4) with γ(5) and γ(12) subunits in bovine tissues, J Biol Chem 274(30) (1999) 21425–9. [DOI] [PubMed] [Google Scholar]
- [60].Patil DN, Rangarajan ES, Novick SJ, Pascal BD, Kojetin DJ, Griffin PR, Izard T, Martemyanov KA, Structural organization of a major neuronal G protein regulator, the RGS7-Gβ5-R7BP complex, Elife 7 (2018) e42150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ostrovskaya O, Xie K, Masuho I, Fajardo-Serrano A, Lujan R, Wickman K, Martemyanov KA, RGS7/Gβ5/R7BP complex regulates synaptic plasticity and memory by modulating hippocampal GABABR-GIRK signaling, Elife 3 (2014) e02053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Slepak VZ, Structure, function, and localization of Gβ5-RGS complexes, Prog Mol Biol Transl Sci 86 (2009) 157–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Anderson GR, Posokhova E, Martemyanov KA, The R7 RGS protein family: multi-subunit regulators of neuronal G protein signaling, Cell Biochemistry and Biophysics 54(1–3) (2009) 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Witherow DS, Wang Q, Levay K, Cabrera JL, Chen J, Willars GB, Slepak VZ, Complexes of the G protein subunit Gβ5 with the regulators of G protein signaling RGS7 and RGS9. Characterization in native tissues and in transfected cells, J Biol Chem 275(32) (2000) 24872–80. [DOI] [PubMed] [Google Scholar]
- [65].Chen CK, Eversole-Cire P, Zhang H, Mancino V, Chen YJ, He W, Wensel TG, Simon MI, Instability of GGL domain-containing RGS proteins in mice lacking the G protein β-subunit Gβ5, Proceedings of the National Academy of Sciences USA 100(11) (2003) 6604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Jayaraman M, Zhou H, Jia L, Cain MD, Blumer KJ, R9AP and R7BP: traffic cops for the RGS7 family in phototransduction and neuronal GPCR signaling, Trends Pharmacol Sci 30(1) (2009) 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sandiford SL, Slepak VZ, The Gβ5-RGS7 complex selectively inhibits muscarinic M3 receptor signaling via the interaction between the third intracellular loop of the receptor and the DEP domain of RGS7, Biochemistry 48(10) (2009) 2282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Orlandi C, Posokhova E, Masuho I, Ray TA, Hasan N, Gregg RG, Martemyanov KA, GPR158/179 regulate G protein signaling by controlling localization and activity of the RGS7 complexes, The Journal of Cell Biology 197(6) (2012) 711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Karpinsky-Semper D, Volmar CH, Brothers SP, Slepak VZ, Differential effects of the Gβ5-RGS7 complex on muscarinic M3 receptor-induced Ca2+ influx and release, Molecular Pharmacology 85(5) (2014) 758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hillenbrand M, Schori C, Schoppe J, Pluckthun A, Comprehensive analysis of heterotrimeric G-protein complex diversity and their interactions with GPCRs in solution, Proceedings of the National Academy of Sciences USA 112(11) (2015) E1181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zhang S, Coso OA, Lee C, Gutkind JS, Simonds WF, Selective activation of effector pathways by brain-specific G protein β5, J Biol Chem 271(52) (1996) 33575–9. [DOI] [PubMed] [Google Scholar]
- [72].Kozasa T, The structure of GRK2-Gβγ complex: intimate association of G-protein signaling modules, Trends Pharmacol Sci 25(2) (2004) 61–3. [DOI] [PubMed] [Google Scholar]
- [73].Gaudet R, Savage JR, McLaughlin JN, Willardson BM, Sigler PB, A molecular mechanism for the phosphorylation-dependent regulation of heterotrimeric G proteins by phosducin, Mol Cell 3(5) (1999) 649–60. [DOI] [PubMed] [Google Scholar]
- [74].Whorton MR, MacKinnon R, Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium, Cell 147(1) (2011) 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Murzin AG, Structural principles for the propeller assembly of β-sheets: the preference for seven-fold symmetry, Proteins 14(2) (1992) 191–201. [DOI] [PubMed] [Google Scholar]
- [76].Clapham DE, Neer EJ, G protein βγ subunits, Annual review of pharmacology and toxicology 37(1) (1997) 167–203. [DOI] [PubMed] [Google Scholar]
- [77].Garritsen A, van Galen PJ, Simonds WF, The N-terminal coiled-coil domain of β is essential for γ association: a model for G-protein βγ subunit interaction, Proceedings of the National Academy USA 90(16) (1993) 7706–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Gudermann T, Nurnberg B, Schultz G, Receptors and G proteins as primary components of transmembrane signal transduction. Part 1. G-protein-coupled receptors: structure and function, Journal of Molecular Medicine (Berlin, Germany) 73(2) (1995) 51–63. [DOI] [PubMed] [Google Scholar]
- [79].Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F, Proteomics. Tissue-based map of the human proteome, Science 347(6220) (2015) 1260419. [DOI] [PubMed] [Google Scholar]
- [80].Betty M, Harnish SW, Rhodes KJ, Cockett MI, Distribution of heterotrimeric G-protein β and γ subunits in the rat brain, Neuroscience 85(2) (1998) 475–86. [DOI] [PubMed] [Google Scholar]
- [81].Fong HK, Amatruda TT 3rd, Birren BW, Simon MI, Distinct forms of the β subunit of GTP-binding regulatory proteins identified by molecular cloning, Proceedings of the National Academy of Sciences USA 84(11) (1987) 3792–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Largent BL, Jones DT, Reed RR, Pearson RC, Snyder SH, G protein mRNA mapped in rat brain by in situ hybridization, Proceedings of the National Academy of Sciences USA 85(8) (1988) 2864–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Liang JJ, Cockett M, Khawaja XZ, Immunohistochemical localization of G protein β1, β2, β3, β4, β5, and γ3 subunits in the adult rat brain, Journal of Neurochemistry 71(1) (1998) 345–55. [PubMed] [Google Scholar]
- [84].Levine MA, Smallwood PM, Moen PT Jr., Helman LJ, Ahn TG, Molecular cloning of β3 subunit, a third form of the G protein β-subunit polypeptide, Proceedings of the National Academy USA 87(6) (1990) 2329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Vincent A, Audo I, Tavares E, Maynes JT, Tumber A, Wright T, Li S, Michiels C, Consortium GNB, Condroyer C, MacDonald H, Verdet R, Sahel JA, Hamel CP, Zeitz C, Heon E, Biallelic Mutations in GNB3 Cause a Unique Form of Autosomal-Recessive Congenital Stationary Night Blindness, Am J Hum Genet 98(5) (2016) 1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ritchey ER, Bongini RE, Code KA, Zelinka C, Petersen-Jones S, Fischer AJ, The pattern of expression of guanine nucleotide-binding protein β3 in the retina is conserved across vertebrate species, Neuroscience 169(3) (2010) 1376–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lee RH, Lieberman BS, Yamane HK, Bok D, Fung BK, A third form of the G protein β subunit. 1. Immunochemical identification and localization to cone photoreceptors, J Biol Chem 267(34) (1992) 24776–81. [PubMed] [Google Scholar]
- [88].Peng YW, Robishaw JD, Levine MA, Yau KW, Retinal rods and cones have distinct G protein β and γ subunits, Proceedings of the National Academy of Sciences USA 89(22) (1992) 10882–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Siffert W, Rosskopf D, Siffert G, Busch S, Moritz A, Erbel R, Sharma AM, Ritz E, Wichmann HE, Jakobs KH, Horsthemke B, Association of a human G-protein β3 subunit variant with hypertension, Nat Genet 18(1) (1998) 45–8. [DOI] [PubMed] [Google Scholar]
- [90].von Weizsäcker E, Strathmann MP, Simon MI, Diversity among the β subunits of heterotrimeric GTP-binding proteins: Characterization of a novel β-subunit cDNA, Biochemical and Biophysical Research Communications 183(1) (1992) 350–356. [DOI] [PubMed] [Google Scholar]
- [91].Ruiz-Velasco V, Ikeda SR, Puhl HL, Cloning, tissue distribution, and functional expression of the human G protein β4-subunit, Physiological Genomics 8(1) (2002) 41–50. [DOI] [PubMed] [Google Scholar]
- [92].Soong BW, Huang YH, Tsai PC, Huang CC, Pan HC, Lu YC, Chien HJ, Liu TT, Chang MH, Lin KP, Tu PH, Kao LS, Lee YC, Exome sequencing identifies GNB4 mutations as a cause of dominant intermediate Charcot-Marie-Tooth disease, Am J Hum Genet 92(3) (2013) 422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Grishin AV, Weiner JL, Blumer KJ, Biochemical and genetic analysis of dominant-negative mutations affecting a yeast G-protein γ subunit, Mol Cell Biol 14(7) (1994) 4571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Whiteway M, Hougan L, Dignard D, Thomas DY, Bell L, Saari GC, Grant FJ, O’Hara P, MacKay VL, The STE4 and STE18 genes of yeast encode potential β and γ subunits of the mating factor receptor-coupled G protein, Cell 56(3) (1989) 467–77. [DOI] [PubMed] [Google Scholar]
- [95].Slessareva JE, Routt SM, Temple B, Bankaitis VA, Dohlman HG, Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein α subunit at the endosome, Cell 126(1) (2006) 191–203. [DOI] [PubMed] [Google Scholar]
- [96].Kim DU, Park SK, Chung KS, Choi MU, Yoo HS, The G protein β subunit Gpb1 of Schizosaccharomyces pombe is a negative regulator of sexual development, Mol Gen Genet 252(1–2) (1996) 20–32. [DOI] [PubMed] [Google Scholar]
- [97].Landry S, Hoffman C, The git5 Gβ and git11 Gγ Form an Atypical Gβγ Dimer Acting in the Fission Yeast Glucose/cAMP Pathway, Genetics (2001) 157: 1159–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Krystofova S, Borkovich KA, The heterotrimeric G-protein subunits GNG-1 and GNB-1 form a Gβγ dimer required for normal female fertility, asexual development, and Gα protein levels in Neurospora crassa, Eukaryotic Cell 4(2) (2005) 365–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zwaal RR, Ahringer J, van Luenen HG, Rushforth A, Anderson P, Plasterk RH, G proteins are required for spatial orientation of early cell cleavages in C. elegans embryos, Cell 86(4) (1996) 619–29. [DOI] [PubMed] [Google Scholar]
- [100].Gotta M, Ahringer J, Distinct roles for Gα and Gβγ in regulating spindle position and orientation in Caenorhabditis elegans embryos, Nat Cell Biol 3(3) (2001) 297–300. [DOI] [PubMed] [Google Scholar]
- [101].Tsou MF, Hayashi A, Rose LS, LET-99 opposes Gα/GPR signaling to generate asymmetry for spindle positioning in response to PAR and MES-1/SRC-1 signaling, Development 130(23) (2003) 5717–30. [DOI] [PubMed] [Google Scholar]
- [102].Izumi Y, Ohta N, Itoh-Furuya A, Fuse N, Matsuzaki F, Differential functions of G protein and Baz-aPKC signaling pathways in Drosophila neuroblast asymmetric division, J Cell Biol 164(5) (2004) 729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].K.D. Katanayeva N, Portmann R, Hess D, Katanaev VL Competing Activities of Heterotrimeric G Proteins in Drosophila Wing Maturation., PLoS ONE 5(8) ((2010)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Mulligan T, Blaser H, Raz E, Farber SA, Prenylation-deficient G protein γ subunits disrupt GPCR signaling in the zebrafish, Cell Signal 22(2) (2010) 221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hildebrandt JD, Codina J, Risinger R, Birnbaumer L, Identification of a γ-Subunit Associated with the Adenylyl Cyclase Regulatory Protein-Ns and Protein-Ni, Journal of Biological Chemistry 259(4) (1984) 2039–2042. [PubMed] [Google Scholar]
- [106].Yang W, Hildebrandt JD, Genomic analysis of G protein γ subunits in human and mouse - the relationship between conserved gene structure and G protein βγ dimer formation, Cell Signal 18(2) (2006) 194–201. [DOI] [PubMed] [Google Scholar]
- [107].Senarath K, Kankanamge D, Samaradivakara S, Ratnayake K, Tennakoon M, Karunarathne A, Chapter Five - Regulation of G Protein βγ Signaling, in: Shukla AK (Ed.), International Review of Cell and Molecular Biology, Academic Press; 2018, pp. 133–191. [DOI] [PubMed] [Google Scholar]
- [108].Higgins JB, Casey PJ, The role of prenylation in G-protein assembly and function, Cell Signal 8(6) (1996) 433–7. [DOI] [PubMed] [Google Scholar]
- [109].Senarath K, Ratnayake K, Siripurapu P, Payton JL, Karunarathne A, Reversible G Protein βγ9 Distribution-Based Assay Reveals Molecular Underpinnings in Subcellular, Single-Cell, and Multicellular GPCR and G Protein Activity, Anal Chem 88(23) (2016) 11450–11459. [DOI] [PubMed] [Google Scholar]
- [110].Cook LA, Schey KL, Cleator JH, Wilcox MD, Dingus J, Hildebrandt JD, Identification of a region in G protein γ subunits conserved across species but hypervariable among subunit isoforms, Protein Sci 10(12) (2001) 2548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Rosenbaum DM, Rasmussen SG, Kobilka BK, The structure and function of G-protein-coupled receptors, Nature 459(7245) (2009) 356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Gilman AG, G proteins: transducers of receptor-generated signals, Annual Review of Biochemistry 56 (1987) 615–49. [DOI] [PubMed] [Google Scholar]
- [113].Dingus J, Hildebrandt JD, Synthesis and Assembly of G Protein βγ Dimers: Comparison of In Vitro and In Vivo Studies, in: Dupré DJ, Hébert TE, Jockers R (Eds.), GPCR Signalling Complexes – Synthesis, Assembly, Trafficking and Specificity, Springer; Netherlands, Dordrecht, 2012, pp. 155–180. [Google Scholar]
- [114].Hildebrandt JD, Role of subunit diversity in signaling by heterotrimeric G proteins, Biochemical Pharmacology 54(3) (1997) 325–39. [DOI] [PubMed] [Google Scholar]
- [115].Yim YY, Betke KM, McDonald WH, Gilsbach R, Chen Y, Hyde K, Wang Q, Hein L, Hamm HE, The in vivo specificity of synaptic Gβ and Gγ subunits to the α2A adrenergic receptor at CNS synapses, Sci Rep 9(1) (2019) 1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Malerba N, De Nittis P, Merla G, The Emerging Role of Gβ Subunits in Human Genetic Diseases, Cells 8(12) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Krishna Kumar K, Shalev-Benami M, Robertson MJ, Hu H, Banister SD, Hollingsworth SA, Latorraca NR, Kato HE, Hilger D, Maeda S, Weis WI, Farrens DL, Dror RO, Malhotra SV, Kobilka BK, Skiniotis G, Structure of a Signaling Cannabinoid Receptor 1-G Protein Complex, Cell 176(3) (2019) 448–458 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Garcia DE, Li B, Garcia-Ferreiro RE, Hernandez-Ochoa EO, Yan K, Gautam N, Catterall WA, Mackie K, Hille B, G-protein β-subunit specificity in the fast membrane-delimited inhibition of Ca2+ channels, J Neurosci 18(22) (1998) 9163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Chen J, DeVivo M, Dingus J, Harry A, Li J, Sui J, Carty DJ, Blank JL, Exton JH, Stoffel RH, et al. , A region of adenylyl cyclase 2 critical for regulation by G protein βγ subunits, Science 268(5214) (1995) 1166–9. [DOI] [PubMed] [Google Scholar]
- [120].Bayewitch ML, Avidor-Reiss T, Levy R, Pfeuffer T, Nevo I, Simonds WF, Vogel Z, Inhibition of adenylyl cyclase isoforms V and VI by various Gβγ subunits, FASEB journal 12(11) (1998) 1019–25. [DOI] [PubMed] [Google Scholar]
- [121].Wing MR, Houston D, Kelley GG, Der CJ, Siderovski DP, Harden TK, Activation of phospholipase Cε by heterotrimeric G protein βγ-subunits, J Biol Chem 276(51) (2001) 48257–61. [DOI] [PubMed] [Google Scholar]
- [122].Maier U, Babich A, Macrez N, Leopoldt D, Gierschik P, Illenberger D, Nurnberg B, Gβ5γ2 is a highly selective activator of phospholipid-dependent enzymes, J Biol Chem 275(18) (2000) 13746–54. [DOI] [PubMed] [Google Scholar]
- [123].Diaz Anel AM, Malhotra V, PKCε is required for β1γ2/ β3γ2- and PKD-mediated transport to the cell surface and the organization of the Golgi apparatus, J Cell Biol 169(1) (2005) 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Zhang JH, Pandey M, Seigneur EM, Panicker LM, Koo L, Schwartz OM, Chen W, Chen CK, Simonds WF, Knockout of G protein β5 impairs brain development and causes multiple neurologic abnormalities in mice, Journal of Neurochemistry 119(3) (2011) 544–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Lodder EM, De Nittis P, Koopman CD, Wiszniewski W, Moura de Souza CF, Lahrouchi N, Guex N, Napolioni V, Tessadori F, Beekman L, Nannenberg EA, Boualla L, Blom NA, de Graaff W, Kamermans M, Cocciadiferro D, Malerba N, Mandriani B, Akdemir ZHC, Fish RJ, Eldomery MK, Ratbi I, Wilde AAM, de Boer T, Simonds WF, Neerman-Arbez M, Sutton VR, Kok F, Lupski JR, Reymond A, Bezzina CR, Bakkers J, Merla G, GNB5 Mutations Cause an Autosomal-Recessive Multisystem Syndrome with Sinus Bradycardia and Cognitive Disability, Am J Hum Genet 99(3) (2016) 704–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Robishaw JD, Preferential Assembly of G-αβγ Complexes Directed by the γ Subunits, in: Dupré DJ, Hébert TE, Jockers R (Eds.), GPCR Signalling Complexes – Synthesis, Assembly, Trafficking and Specificity, Springer Netherlands, Dordrecht, 2012, pp. 181–191. [Google Scholar]
- [127].Richardson M, Robishaw JD, The α2A-adrenergic receptor discriminates between Gi heterotrimers of different βγ subunit composition in Sf9 insect cell membranes, J Biol Chem 274(19) (1999) 13525–33. [DOI] [PubMed] [Google Scholar]
- [128].Krumins AM, Gilman AG, Targeted knockdown of G protein subunits selectively prevents receptor-mediated modulation of effectors and reveals complex changes in non-targeted signaling proteins, J Biol Chem 281(15) (2006) 10250–62. [DOI] [PubMed] [Google Scholar]
- [129].Khan SM, Min A, Gora S, Houranieh GM, Campden R, Robitaille M, Trieu P, Petrin D, Jacobi AM, Behlke MA, Angers S, Hébert TE, Gβ4γ1 as a modulator of M3 muscarinic receptor signalling and novel roles of Gβ1 subunits in the modulation of cellular signalling, Cell Signal 27(8) (2015) 1597–608. [DOI] [PubMed] [Google Scholar]
- [130].Gibson SK, Gilman AG, Giα and Gβ subunits both define selectivity of G protein activation by α2-adrenergic receptors, Proceedings of the National Academy of Sciences USA 103(1) (2006) 212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Hynes TR, Mervine SM, Yost EA, Sabo JL, Berlot CH, Live cell imaging of Gs and the β2-adrenergic receptor demonstrates that both αs and β1γ7 internalize upon stimulation and exhibit similar trafficking patterns that differ from that of the β2-adrenergic receptor, J Biol Chem 279(42) (2004) 44101–12. [DOI] [PubMed] [Google Scholar]
- [132].Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B, Selectivity in signal transduction determined by γ subunits of heterotrimeric G proteins, Science 259(5096) (1993) 832–4. [DOI] [PubMed] [Google Scholar]
- [133].Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B, Different β-subunits determine G-protein interaction with transmembrane receptors, Nature 358(6385) (1992) 424–6. [DOI] [PubMed] [Google Scholar]
- [134].Wang Q, Mullah BK, Robishaw JD, Ribozyme approach identifies a functional association between the G protein β1γ7 subunits in the β-adrenergic receptor signaling pathway, J Biol Chem 274(24) (1999) 17365–71. [DOI] [PubMed] [Google Scholar]
- [135].Bigler Wang D, Sherman NE, Shannon JD, Leonhardt SA, Mayeenuddin LH, Yeager M, McIntire WE, Binding of β4γ5 by adenosine A1 and A2A receptors determined by stable isotope labeling with amino acids in cell culture and mass spectrometry, Biochemistry 50(2) (2011) 207–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].McIntire WE, MacCleery G, Garrison JC, The G protein β subunit is a determinant in the coupling of Gs to the β1-adrenergic and A2a adenosine receptors, J Biol Chem 276(19) (2001) 15801–9. [DOI] [PubMed] [Google Scholar]
- [137].Lim WK, Myung CS, Garrison JC, Neubig RR, Receptor-G protein γ specificity: γ11 shows unique potency for A(1) adenosine and 5-HT(1A) receptors, Biochemistry 40(35) (2001) 10532–41. [DOI] [PubMed] [Google Scholar]
- [138].Blake BL, Wing MR, Zhou JY, Lei Q, Hillmann JR, Behe CI, Morris RA, Harden TK, Bayliss DA, Miller RJ, Siderovski DP, Gβ association and effector interaction selectivities of the divergent Gγ subunit Gγ(13), J Biol Chem 276(52) (2001) 49267–74. [DOI] [PubMed] [Google Scholar]
- [139].Kerr DS, Von Dannecker LE, Davalos M, Michaloski JS, Malnic B, Ric-8B interacts with Gαolf and Gγ13 and co-localizes with Gαolf, Gβ1 and Gγ13 in the cilia of olfactory sensory neurons, Mol Cell Neurosci 38(3) (2008) 341–8. [DOI] [PubMed] [Google Scholar]
- [140].Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF, Gγ13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium, Nat Neurosci 2(12) (1999) 1055–62. [DOI] [PubMed] [Google Scholar]
- [141].Schwindinger WF, Mihalcik LJ, Giger KE, Betz KS, Stauffer AM, Linden J, Herve D, Robishaw JD, Adenosine A2A receptor signaling and Golf assembly show a specific requirement for the γ7 subtype in the striatum, J Biol Chem 285(39) (2010) 29787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Hessel E, Heck M, Muller P, Herrmann A, Hofmann KP, Signal transduction in the visual cascade involves specific lipid-protein interactions, J Biol Chem 278(25) (2003) 22853–60. [DOI] [PubMed] [Google Scholar]
- [143].Jastrzebska B, Debinski A, Filipek S, Palczewski K, Role of membrane integrity on G protein-coupled receptors: Rhodopsin stability and function, Prog Lipid Res 50(3) (2011) 267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Gelb MH, Brunsveld L, Hrycyna CA, Michaelis S, Tamanoi F, Van Voorhis WC, Waldmann H, Therapeutic intervention based on protein prenylation and associated modifications, Nat Chem Biol 2(10) (2006) 518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Maurer-Stroh S, Eisenhaber F, Refinement and prediction of protein prenylation motifs, Genome Biol 6(6) (2005) R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Kalman VK, Erdman RA, Maltese WA, Robishaw JD, Regions outside of the CAAX motif influence the specificity of prenylation of G protein γ subunits, J Biol Chem 270(24) (1995) 14835–41. [DOI] [PubMed] [Google Scholar]
- [147].Yasuda H, Lindorfer MA, Woodfork KA, Fletcher JE, Garrison JC, Role of the prenyl group on the G protein γ subunit in coupling trimeric G proteins to A1 adenosine receptors, J Biol Chem 271(31) (1996) 18588–95. [DOI] [PubMed] [Google Scholar]
- [148].Stickney JT, Buss JE, Murine guanylate-binding protein: incomplete geranylgeranyl isoprenoid modification of an interferon-γ-inducible guanosine triphosphate-binding protein, Mol Biol Cell 11(7) (2000) 2191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Wang M, Casey PJ, Protein prenylation: unique fats make their mark on biology, Nat Rev Mol Cell Biol 17(2) (2016) 110–22. [DOI] [PubMed] [Google Scholar]
- [150].Resh MD, Covalent lipid modifications of proteins, Current Biology 23(10) (2013) R431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Thissen JA, Casey PJ, Microsomal membranes contain a high affinity binding site for prenylated peptides, J Biol Chem 268(19) (1993) 13780–3. [PubMed] [Google Scholar]
- [152].Wedegaertner PB, Wilson PT, Bourne HR, Lipid modifications of trimeric G proteins, J Biol Chem 270(2) (1995) 503–6. [DOI] [PubMed] [Google Scholar]
- [153].Smrcka AV, G protein βγ subunits: central mediators of G protein-coupled receptor signaling, Cellular and molecular life sciences : CMLS 65(14) (2008) 2191–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Marrari Y, Crouthamel M, Irannejad R, Wedegaertner PB, Assembly and trafficking of heterotrimeric G proteins, Biochemistry 46(26) (2007) 7665–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Jiang H, Zhang X, Chen X, Aramsangtienchai P, Tong Z, Lin H, Protein Lipidation: Occurrence, Mechanisms, Biological Functions, and Enabling Technologies, Chem Rev 118(3) (2018) 919–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Khan SM, Sung JY, Hébert TE, Gβγ subunits-Different spaces, different faces, Pharmacological research 111 (2016) 434–441. [DOI] [PubMed] [Google Scholar]
- [157].Martin RD, Bouazza CA, Hébert TE, Organellar Gβγ signaling—GPCR signaling beyond the cell surface, in: Jastrzebska B, Park PSH (Eds.), GPCRs, Academic Press; 2020, pp. 257–267. [Google Scholar]
- [158].Akgoz M, Kalyanaraman V, Gautam N, G protein βγ complex translocation from plasma membrane to Golgi complex is influenced by receptor γ subunit interaction, Cell Signal 18(10) (2006) 1758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Marchler-Bauer A, Bo Y, Han L, He J, Lanczycki CJ, Lu S, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Geer LY, Bryant SH, CDD/SPARCLE: functional classification of proteins via subfamily domain architectures, Nucleic Acids Res 45(D1) (2017) D200–D203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Morishita R, Ueda H, Kato K, Asano T, Identification of two forms of the γ subunit of G protein, γ10 and γ11, in bovine lung and their tissue distribution in the rat, FEBS Lett 428(1–2) (1998) 85–8. [DOI] [PubMed] [Google Scholar]
- [161].Kostenis E, Zeng FY, Wess J, Structure-function analysis of muscarinic receptors and their associated G proteins, Life Sci 64(6–7) (1999) 355–62. [DOI] [PubMed] [Google Scholar]
- [162].Fung BK, Characterization of transducin from bovine retinal rod outer segments. I. Separation and reconstitution of the subunits, J Biol Chem 258(17) (1983) 10495–502. [PubMed] [Google Scholar]
- [163].Shinozawa T, Uchida S, Martin E, Cafiso D, Hubbell W, Bitensky M, Additional component required for activity and reconstitution of light-activated vertebrate photoreceptor GTPase, Proceedings of the National Academy of Sciences USA 77(3) (1980) 1408–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Azpiazu I, Cruzblanca H, Li P, Linder M, Zhuo M, Gautam N, A G protein γ subunit-specific peptide inhibits muscarinic receptor signaling, J Biol Chem 274(50) (1999) 35305–8. [DOI] [PubMed] [Google Scholar]
- [165].Hou Y, Azpiazu I, Smrcka A, Gautam N, Selective role of G protein γ subunits in receptor interaction, J Biol Chem 275(50) (2000) 38961–4. [DOI] [PubMed] [Google Scholar]
- [166].Wang Q, Jolly JP, Surmeier JD, Mullah BM, Lidow MS, Bergson CM, Robishaw JD, Differential dependence of the D1 and D5 dopamine receptors on the G protein γ7 subunit for activation of adenylylcyclase, J Biol Chem 276(42) (2001) 39386–93. [DOI] [PubMed] [Google Scholar]
- [167].Kisselev O, Pronin A, Ermolaeva M, Gautam N, Receptor-G protein coupling is established by a potential conformational switch in the βγ complex, Proceedings of the National Academy of Sciences USA 92(20) (1995) 9102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Kisselev OG, Ermolaeva MV, Gautam N, A Farnesylated Domain in the G-Protein γ-Subunit Is a Specific Determinant of Receptor Coupling, Journal of Biological Chemistry 269(34) (1994) 21399–21402. [PubMed] [Google Scholar]
- [169].Kisselev O, Ermolaeva M, Gautam N, Efficient interaction with a receptor requires a specific type of prenyl group on the G protein γ subunit, J Biol Chem 270(43) (1995) 25356–8. [DOI] [PubMed] [Google Scholar]
- [170].Fawzi AB, Fay DS, Murphy EA, Tamir H, Erdos JJ, Northup JK, Rhodopsin and the Retinal G-Protein Distinguish among G-Protein βγ-Subunit Forms, Journal of Biological Chemistry 266(19) (1991) 12194–12200. [PubMed] [Google Scholar]
- [171].Wildman DE, Tamir H, Leberer E, Northup JK, Dennis M, Prenyl modification of guanine nucleotide regulatory protein γ2 subunits is not required for interaction with the transducin α subunit or rhodopsin, Proceedings of the National Academy of Sciences USA 90(3) (1993) 794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Jian X, Clark WA, Kowalak J, Markey SP, Simonds WF, Northup JK, Gβγ affinity for bovine rhodopsin is determined by the carboxyl-terminal sequences of the γ subunit, J Biol Chem 276(51) (2001) 48518–25. [DOI] [PubMed] [Google Scholar]
- [173].Galés C, Van Durm JJ, Schaak S, Pontier S, Percherancier Y, Audet M, Paris H, Bouvier M, Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes, Nat Struct Mol Biol 13(9) (2006) 778–86. [DOI] [PubMed] [Google Scholar]
- [174].Scott JK, Huang SF, Gangadhar BP, Samoriski GM, Clapp P, Gross RA, Taussig R, Smrcka AV, Evidence that a protein-protein interaction ‘hot spot’ on heterotrimeric G protein βγ subunits is used for recognition of a subclass of effectors, EMBO J 20(4) (2001) 767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Tang WJ, Gilman AG, Type-specific regulation of adenylyl cyclase by G protein βγ subunits, Science 254(5037) (1991) 1500–3. [DOI] [PubMed] [Google Scholar]
- [176].Murga C, Laguinge L, Wetzker R, Cuadrado A, Gutkind JS, Activation of Akt/protein kinase B by G protein-coupled receptors. A role for α and βγ subunits of heterotrimeric G proteins acting through phosphatidylinositol-3-OH kinase γ, J Biol Chem 273(30) (1998) 19080–5. [DOI] [PubMed] [Google Scholar]
- [177].Ikeda SR, Voltage-dependent modulation of N-type calcium channels by G-protein bβγ subunits, Nature 380(6571) (1996) 255–8. [DOI] [PubMed] [Google Scholar]
- [178].O’Neill PR, Karunarathne WK, Kalyanaraman V, Silvius JR, Gautam N, G-protein signaling leverages subunit-dependent membrane affinity to differentially control βγ translocation to intracellular membranes, Proceedings of the National Academy of Sciences USA 109(51) (2012) E3568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Gauthier C, Rozec B, Manoury B, Balligand JL, β3 adrenoceptors as new therapeutic targets for cardiovascular pathologies, Curr Heart Fail Rep 8(3) (2011) 184–92. [DOI] [PubMed] [Google Scholar]
- [180].Talan MI, Ahmet I, Xiao RP, Lakatta EG, β(2)AR agonists in treatment of chronic heart failure: long path to translation, J Mol Cell Cardiol 51(4) (2011) 529–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J, The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications, J Am Coll Cardiol 54(19) (2009) 1747–62. [DOI] [PubMed] [Google Scholar]
- [182].Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE, The βγ subunits of GTP-binding proteins activate the muscarinic K+ channel in heart, Nature 325(6102) (1987) 321–6. [DOI] [PubMed] [Google Scholar]
- [183].Nobles M, Sebastian S, Tinker A, HL-1 cells express an inwardly rectifying K+ current activated via muscarinic receptors comparable to that in mouse atrial myocytes, Pflug Arch Eur J Phy 460(1) (2010) 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Ajith Karunarathne WK, O’Neill PR, Martinez-Espinosa PL, Kalyanaraman V, Gautam N, All G protein βγ complexes are capable of translocation on receptor activation, Biochem Biophys Res Commun 421(3) (2012) 605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Brock C, Schaefer M, Reusch HP, Czupalla C, Michalke M, Spicher K, Schultz G, Nurnberg B, Roles of Gγ in membrane recruitment and activation of p110γ/p101 phosphoinositide 3-kinase γ, J Cell Biol 160(1) (2003) 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Lehmann DM, Seneviratne AM, Smrcka AV, Small molecule disruption of G protein βγ subunit signaling inhibits neutrophil chemotaxis and inflammation, Molecular Pharmacology 73(2) (2008) 410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR, Polarization of chemoattractant receptor signaling during neutrophil chemotaxis, Science 287(5455) (2000) 1037–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Wang F, Herzmark P, Weiner OD, Srinivasan S, Servant G, Bourne HR, Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils, Nat Cell Biol 4(7) (2002) 513–8. [DOI] [PubMed] [Google Scholar]
- [189].Karunarathne WK, Giri L, Kalyanaraman V, Gautam N, Optically triggering spatiotemporally confined GPCR activity in a cell and programming neurite initiation and extension, Proceedings of the National Academy of Sciences USA 110(17) (2013) E1565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Braselmann S, Palmer TM, Cook SJ, Signalling enzymes: bursting with potential, Current Biology 7(8) (1997) R470–3. [DOI] [PubMed] [Google Scholar]
- [191].Siripurapu P, Kankanamge D, Ratnayake K, Senarath K, Karunarathne A, Two independent but synchronized Gβγ subunit-controlled pathways are essential for trailing-edge retraction during macrophage migration, J Biol Chem 292(42) (2017) 17482–17495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F Jr., The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling, BBA-Biomembranes 1768(4) (2007) 913–22. [DOI] [PubMed] [Google Scholar]
- [193].Tobin AB, G-protein-coupled receptor phosphorylation: where, when and by whom, Br J Pharmacol 153 Suppl 1(Suppl 1) (2008) S167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Cahill TJ 3rd, Thomsen AR, Tarrasch JT, Plouffe B, Nguyen AH, Yang F, Huang LY, Kahsai AW, Bassoni DL, Gavino BJ, Lamerdin JE, Triest S, Shukla AK, Berger B, Little J.t., Antar A, Blanc A, Qu CX, Chen X, Kawakami K, Inoue A, Aoki J, Steyaert J, Sun JP, Bouvier M, Skiniotis G, Lefkowitz RJ, Distinct conformations of GPCR-β-arrestin complexes mediate desensitization, signaling, and endocytosis, Proceedings of the National Academy of Sciences USA 114(10) (2017) 2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Wolters V, Krasel C, Brockmann J, Bunemann M, Influence of Gαq on the dynamics of m3-acetylcholine receptor-G-protein-coupled receptor kinase 2 interaction, Molecular Pharmacology 87(1) (2015) 9–17. [DOI] [PubMed] [Google Scholar]
- [196].Samaradivakara S, Kankanamge D, Senarath K, Ratnayake K, Karunarathne A, G protein γ (Gγ) subtype dependent targeting of GRK2 to M3 receptor by Gβγ, Biochem Biophys Res Commun 503(1) (2018) 165–170. [DOI] [PubMed] [Google Scholar]
- [197].Boivin B, Vaniotis G, Allen BG, Hébert TE, G protein-coupled receptors in and on the cell nucleus: a new signaling paradigm? Journal of Receptor and Signal Transduction Research 28(1–2) (2008) 15–28. [DOI] [PubMed] [Google Scholar]
- [198].Denker SP, McCaffery JM, Palade GE, Insel PA, Farquhar MG, Differential distribution of α subunits and βγ subunits of heterotrimeric G proteins on Golgi membranes of the exocrine pancreas, J Cell Biol 133(5) (1996) 1027–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Wilson BS, Komuro M, Farquhar MG, Cellular variations in heterotrimeric G protein localization and expression in rat pituitary, Endocrinology 134(1) (1994) 233–44. [DOI] [PubMed] [Google Scholar]
- [200].Gleeson PA, Lock JG, Luke MR, Stow JL, Domains of the TGN: coats, tethers and G proteins, Traffic 5(5) (2004) 315–26. [DOI] [PubMed] [Google Scholar]
- [201].Saini DK, Kalyanaraman V, Chisari M, Gautam N, A family of G protein βγ subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation, J Biol Chem 282(33) (2007) 24099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Akgoz M, Kalyanaraman V, Gautam N, Receptor-mediated reversible translocation of the G protein βγ complex from the plasma membrane to the Golgi complex, J Biol Chem 279(49) (2004) 51541–4. [DOI] [PubMed] [Google Scholar]
- [203].Saini DK, Chisari M, Gautam N, Shuttling and translocation of heterotrimeric G proteins and Ras, Trends Pharmacol Sci 30(6) (2009) 278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Saini DK, Karunarathne WK, Angaswamy N, Saini D, Cho JH, Kalyanaraman V, Gautam N, Regulation of Golgi structure and secretion by receptor-induced G protein βγ complex translocation, Proceedings of the National Academy of Sciences USA 107(25) (2010) 11417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Bard F, Malhotra V, The formation of TGN-to-plasma-membrane transport carriers, Annu Rev Cell Dev Bi 22 (2006) 439–55. [DOI] [PubMed] [Google Scholar]
- [206].Bossard C, Bresson D, Polishchuk RS, Malhotra V, Dimeric PKD regulates membrane fission to form transport carriers at the TGN, J Cell Biol 179(6) (2007) 1123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Cho JH, Saini DK, Karunarathne WK, Kalyanaraman V, Gautam N, Alteration of Golgi structure in senescent cells and its regulation by a G protein γ subunit, Cell Signal 23(5) (2011) 785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Hossain MN, Sakemura R, Fujii M, Ayusawa D, G-protein γ subunit GNG11 strongly regulates cellular senescence, Biochem Biophys Res Commun 351(3) (2006) 645–50. [DOI] [PubMed] [Google Scholar]
- [209].Coppe JP, Desprez PY, Krtolica A, Campisi J, The senescence-associated secretory phenotype: the dark side of tumor suppression, Annual Review of Pathology 5 (2010) 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Takauji Y, Kudo I, En A, Matsuo R, Hossain MN, Nakabayashi K, Miki K, Fujii M, Ayusawa D, GNG11 (G-protein subunit γ11) suppresses cell growth with induction of reactive oxygen species and abnormal nuclear morphology in human SUSM-1 cells, Biochemistry and Cell Biology = Biochimie et Biologie Cellulaire 95(4) (2017) 517–523. [DOI] [PubMed] [Google Scholar]
- [211].Rybakova Y, Akkuratov E, Kulebyakin K, Brodskaya O, Dizhevskaya A, Boldyrev A, Receptor-mediated oxidative stress in murine cerebellar neurons is accompanied by phosphorylation of MAP (ERK 1/2) kinase, Current Aging Science 5(3) (2012) 225–30. [DOI] [PubMed] [Google Scholar]
- [212].Musholt TJ, Hanack J, Brehm C, von Wasielewski R, Musholt PB, Searching for non-RET molecular alterations in medullary thyroid carcinoma: expression analysis by mRNA differential display, World Journal of Surgery 29(4) (2005) 472–82. [DOI] [PubMed] [Google Scholar]
- [213].Ruiz-Ballesteros E, Mollejo M, Rodriguez A, Camacho FI, Algara P, Martinez N, Pollan M, Sanchez-Aguilera A, Menarguez J, Campo E, Martinez P, Mateo M, Piris MA, Splenic marginal zone lymphoma: proposal of new diagnostic and prognostic markers identified after tissue and cDNA microarray analysis, Blood 106(5) (2005) 1831–8. [DOI] [PubMed] [Google Scholar]
- [214].Petiot A, Ogier-Denis E, Bauvy C, Cluzeaud F, Vandewalle A, Codogno P, Subcellular localization of the Gαi3 protein and Gα interacting protein, two proteins involved in the control of macroautophagy in human colon cancer HT-29 cells, The Biochemical Journal 337 ( Pt 2) (1999) 289–95. [PMC free article] [PubMed] [Google Scholar]
- [215].Ogier-Denis E, Pattingre S, El Benna J, Codogno P, Erk1/2-dependent phosphorylation of Gα-interacting protein stimulates its GTPase accelerating activity and autophagy in human colon cancer cells, J Biol Chem 275(50) (2000) 39090–5. [DOI] [PubMed] [Google Scholar]
- [216].Liu J, Ji X, Li Z, Yang X, Wang W, Zhang X, G protein γ subunit 7 induces autophagy and inhibits cell division, Oncotarget 7(17) (2016) 24832–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Ashcroft FM, Rorsman P, Electrophysiology of the pancreatic β-cell, Progress in Biophysics and Molecular Biology 54(2) (1989) 87–143. [DOI] [PubMed] [Google Scholar]
- [218].Amisten S, Salehi A, Rorsman P, Jones PM, Persaud SJ, An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans, Pharmacol Ther 139(3) (2013) 359–91. [DOI] [PubMed] [Google Scholar]
- [219].Gautam D, Jeon J, Li JH, Han SJ, Hamdan FF, Cui Y, Lu H, Deng C, Gavrilova O, Wess J, Metabolic roles of the M3 muscarinic acetylcholine receptor studied with M3 receptor mutant mice: a review, Journal of Receptor and Signal Transduction Research 28(1–2) (2008) 93–108. [DOI] [PubMed] [Google Scholar]
- [220].Sumara G, Formentini I, Collins S, Sumara I, Windak R, Bodenmiller B, Ramracheya R, Caille D, Jiang H, Platt KA, Meda P, Aebersold R, Rorsman P, Ricci R, Regulation of PKD by the MAPK p38δ in insulin secretion and glucose homeostasis, Cell 136(2) (2009) 235–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Leenders AG, Sheng ZH, Modulation of neurotransmitter release by the second messenger-activated protein kinases: implications for presynaptic plasticity, Pharmacol Ther 105(1) (2005) 69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Blackmer T, Larsen EC, Takahashi M, Martin TF, Alford S, Hamm HE, G protein βγ subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry, Science 292(5515) (2001) 293–7. [DOI] [PubMed] [Google Scholar]
- [223].Dunlap K, Fischbach GD, Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones, J Physiol 317(1) (1981) 519–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Dolphin AC, Mechanisms of modulation of voltage-dependent calcium channels by G proteins, J Physiol 506 ( Pt 1) (1998) 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Wickman KD, Iniguez-Lluhl JA, Davenport PA, Taussig R, Krapivinsky GB, Linder ME, Gilman AG, Clapham DE, Recombinant G-protein βγ-subunits activate the muscarinic-gated atrial potassium channel, Nature 368(6468) (1994) 255–7. [DOI] [PubMed] [Google Scholar]
- [226].Betke KM, Wells CA, Hamm HE, GPCR mediated regulation of synaptic transmission, Progress in Neurobiology 96(3) (2012) 304–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Blackmer T, Larsen EC, Bartleson C, Kowalchyk JA, Yoon EJ, Preininger AM, Alford S, Hamm HE, Martin TF, G protein βγ directly regulates SNARE protein fusion machinery for secretory granule exocytosis, Nat Neurosci 8(4) (2005) 421–5. [DOI] [PubMed] [Google Scholar]
- [228].Zurawski Z, Page B, Chicka MC, Brindley RL, Wells CA, Preininger AM, Hyde K, Gilbert JA, Cruz-Rodriguez O, Currie KPM, Chapman ER, Alford S, Hamm HE, Gβγ directly modulates vesicle fusion by competing with synaptotagmin for binding to neuronal SNARE proteins embedded in membranes, J Biol Chem 292(29) (2017) 12165–12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Keller R, Cell migration during gastrulation, Current Opinion in Cell Biology 17(5) (2005) 533–41. [DOI] [PubMed] [Google Scholar]
- [230].Locascio A, Nieto MA, Cell movements during vertebrate development: integrated tissue behaviour versus individual cell migration, Current Opinion in Genetics & Development 11(4) (2001) 464–9. [DOI] [PubMed] [Google Scholar]
- [231].Luster AD, Alon R, von Andrian UH, Immune cell migration in inflammation: present and future therapeutic targets, Nat Immunol 6(12) (2005) 1182–90. [DOI] [PubMed] [Google Scholar]
- [232].Wang W, Goswami S, Sahai E, Wyckoff JB, Segall JE, Condeelis JS, Tumor cells caught in the act of invading: their strategy for enhanced cell motility, Trends Cell Biol 15(3) (2005) 138–45. [DOI] [PubMed] [Google Scholar]
- [233].Hansen CA, Schroering AG, Carey DJ, Robishaw JD, Localization of a heterotrimeric G protein γ subunit to focal adhesions and associated stress fibers, J Cell Biol 126(3) (1994) 811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Zachary I, Rozengurt E, Focal adhesion kinase (p125FAK): a point of convergence in the action of neuropeptides, integrins, and oncogenes, Cell 71(6) (1992) 891–4. [DOI] [PubMed] [Google Scholar]
- [235].Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT, pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions, Proceedings of the National Academy of Sciences USA 89(11) (1992) 5192–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Burridge K, Turner CE, Romer LH, Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly, J Cell Biol 119(4) (1992) 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Kim DH, Wirtz D, Focal adhesion size uniquely predicts cell migration, FASEB J. 27(4) (2013) 1351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Lefkowitz RJ, Rajagopal K, Whalen EJ, New roles for β-arrestins in cell signaling: not just for seven-transmembrane receptors, Mol Cell 24(5) (2006) 643–652. [DOI] [PubMed] [Google Scholar]
- [239].Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ, Distinct β-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation, J Biol Chem 281(16) (2006) 10856–64. [DOI] [PubMed] [Google Scholar]
- [240].Zhang JH, Barr VA, Mo Y, Rojkova AM, Liu S, Simonds WF, Nuclear localization of G protein β5 and regulator of G protein signaling 7 in neurons and brain, J Biol Chem 276(13) (2001) 10284–9. [DOI] [PubMed] [Google Scholar]
- [241].Boivin B, Villeneuve LR, Farhat N, Chevalier D, Allen BG, Sub-cellular distribution of endothelin signaling pathway components in ventricular myocytes and heart: lack of preformed caveolar signalosomes, J Mol Cell Cardiol 38(4) (2005) 665–76. [DOI] [PubMed] [Google Scholar]
- [242].Yamamoto S, Kawamura K, James TN, Intracellular distribution of adenylate cyclase in human cardiocytes determined by electron microscopic cytochemistry, Microscopy Research and Technique 40(6) (1998) 479–87. [DOI] [PubMed] [Google Scholar]
- [243].Schievella AR, Regier MK, Smith WL, Lin LL, Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum, J Biol Chem 270(51) (1995) 30749–54. [DOI] [PubMed] [Google Scholar]
- [244].Kim CG, Park D, Rhee SG, The role of carboxyl-terminal basic amino acids in Gqα-dependent activation, particulate association, and nuclear localization of phospholipase C-β1, J Biol Chem 271(35) (1996) 21187–92. [DOI] [PubMed] [Google Scholar]
- [245].Burchett SA, In through the out door: nuclear localization of the regulators of G protein signaling, Journal of Neurochemistry 87(3) (2003) 551–9. [DOI] [PubMed] [Google Scholar]
- [246].Scott MG, Le Rouzic E, Perianin A, Pierotti V, Enslen H, Benichou S, Marullo S, Benmerah A, Differential nucleocytoplasmic shuttling of β-arrestins. Characterization of a leucine-rich nuclear export signal in β-arrestin2, J Biol Chem 277(40) (2002) 37693–701. [DOI] [PubMed] [Google Scholar]
- [247].Johnson LR, Scott MG, Pitcher JA, G protein-coupled receptor kinase 5 contains a DNA-binding nuclear localization sequence, Mol Cell Biol 24(23) (2004) 10169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Dave RH, Saengsawang W, Yu JZ, Donati R, Rasenick MM, Heterotrimeric G-proteins interact directly with cytoskeletal components to modify microtubule-dependent cellular processes, Neurosignals 17(1) (2009) 100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Ueda H, Yamauchi J, Itoh H, Morishita R, Kaziro Y, Kato K, Asano T, Phosphorylation of F-actin-associating G protein γ12 subunit enhances fibroblast motility, J Biol Chem 274(17) (1999) 12124–8. [DOI] [PubMed] [Google Scholar]
- [250].Ueda H, Saga S, Shinohara H, Morishita R, Kato K, Asano T, Association of the γ12 subunit of G proteins with actin filaments, J Cell Sci 110 ( Pt 13) (1997) 1503–11. [DOI] [PubMed] [Google Scholar]
- [251].Hewavitharana T, Wedegaertner PB, Non-canonical signaling and localizations of heterotrimeric G proteins, Cell Signal 24(1) (2012) 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Knust E, G protein signaling and asymmetric cell division, Cell 107(2) (2001) 125–8. [DOI] [PubMed] [Google Scholar]
- [253].Du Q, Stukenberg PT, Macara IG, A mammalian Partner of inscuteable binds NuMA and regulates mitotic spindle organization, Nat Cell Biol 3(12) (2001) 1069–75. [DOI] [PubMed] [Google Scholar]
- [254].Blumer JB, Chandler LJ, Lanier SM, Expression analysis and subcellular distribution of the two G-protein regulators AGS3 and LGN indicate distinct functionality. Localization of LGN to the midbody during cytokinesis, J Biol Chem 277(18) (2002) 15897–903. [DOI] [PubMed] [Google Scholar]
- [255].Kaushik R, Yu F, Chia W, Yang X, Bahri S, Subcellular localization of LGN during mitosis: evidence for its cortical localization in mitotic cell culture systems and its requirement for normal cell cycle progression, Mol Biol Cell 14(8) (2003) 3144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Thyagarajan K, Afshar K, Gonczy P, Polarity mediates asymmetric trafficking of the Gβ heterotrimeric G-protein subunit GPB-1 in C. elegans embryos, Development 138(13) (2011) 2773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].McCudden CR, Willard FS, Kimple RJ, Johnston CA, Hains MD, Jones MB, Siderovski DP, Gα selectivity and inhibitor function of the multiple GoLoco motif protein GPSM2/LGN, BBA-Biomembranes 1745(2) (2005) 254–64. [DOI] [PubMed] [Google Scholar]
- [258].Irannejad R, Wedegaertner PB, Regulation of constitutive cargo transport from the trans-Golgi network to plasma membrane by Golgi-localized G protein βγ subunits, J Biol Chem 285(42) (2010) 32393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Zeng W, Mak DO, Li Q, Shin DM, Foskett JK, Muallem S, A new mode of Ca2+ signaling by G protein-coupled receptors: gating of IP3 receptor Ca2+ release channels by Gβγ, Current Biology 13(10) (2003) 872–6. [DOI] [PubMed] [Google Scholar]
- [260].Park JG, Muise A, He GP, Kim SW, Ro HS, Transcriptional regulation by the γ5 subunit of a heterotrimeric G protein during adipogenesis, EMBO J 18(14) (1999) 4004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Kino T, Tiulpakov A, Ichijo T, Chheng L, Kozasa T, Chrousos GP, G protein β interacts with the glucocorticoid receptor and suppresses its transcriptional activity in the nucleus, J Cell Biol 169(6) (2005) 885–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Kino T, Kozasa T, Chrousos GP, Statin-induced blockade of prenylation alters nucleocytoplasmic shuttling of GTP-binding proteins γ2 and β2 and enhances their suppressive effect on glucocorticoid receptor transcriptional activity, European Journal of Clinical Investigation 35(8) (2005) 508–13. [DOI] [PubMed] [Google Scholar]
- [263].Smrcka AV, Lehmann DM, Dessal AL, G protein βγ subunits as targets for small molecule therapeutic development, Comb Chem High Throughput Screen 11(5) (2008) 382–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Camps M, Carozzi A, Schnabel P, Scheer A, Parker PJ, Gierschik P, Isozyme-selective stimulation of phospholipase C-β2 by G protein βγ-subunits, Nature 360(6405) (1992) 684–6. [DOI] [PubMed] [Google Scholar]
- [265].Stephens L, Smrcka A, Cooke FT, Jackson TR, Sternweis PC, Hawkins PT, A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein βγ subunits, Cell 77(1) (1994) 83–93. [DOI] [PubMed] [Google Scholar]
- [266].Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA, Modulation of Ca2+ channels by G-protein βγ subunits, Nature 380(6571) (1996) 258–62. [DOI] [PubMed] [Google Scholar]
- [267].Cali JJ, Balcueva EA, Rybalkin I, Robishaw JD, Selective tissue distribution of G protein γ subunits, including a new form of the γ subunits identified by cDNA cloning, J Biol Chem 267(33) (1992) 24023–7. [PubMed] [Google Scholar]
- [268].Petrovski S, Kury S, Myers CT, Anyane-Yeboa K, Cogne B, Bialer M, Xia F, Hemati P, Riviello J, Mehaffey M, Besnard T, Becraft E, Wadley A, Politi AR, Colombo S, Zhu X, Ren Z, Andrews I, Dudding-Byth T, Schneider AL, Wallace G, G. University of Washington Center for Mendelian, Rosen ABI, Schelley S, Enns GM, Corre P, Dalton J, Mercier S, Latypova X, Schmitt S, Guzman E, Moore C, Bier L, Heinzen EL, Karachunski P, Shur N, Grebe T, Basinger A, Nguyen JM, Bezieau S, Wierenga K, Bernstein JA, Scheffer IE, Rosenfeld JA, Mefford HC, Isidor B, Goldstein DB, Germline De Novo Mutations in GNB1 Cause Severe Neurodevelopmental Disability, Hypotonia, and Seizures, Am J Hum Genet 98(5) (2016) 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Fukuda T, Hiraide T, Yamoto K, Nakashima M, Kawai T, Yanagi K, Ogata T, Saitsu H, Exome reports A de novo GNB2 variant associated with global developmental delay, intellectual disability, and dysmorphic features, European Journal of Medical Genetics 63(4) (2020) 103804. [DOI] [PubMed] [Google Scholar]
- [270].Firth HV, Richards SM, Bevan AP, Clayton S, Corpas M, Rajan D, Van Vooren S, Moreau Y, Pettett RM, Carter NP, DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources, Am J Hum Genet 84(4) (2009) 524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Steinrucke S, Lohmann K, Domingo A, Rolfs A, Baumer T, Spiegler J, Hartmann C, Munchau A, Novel GNB1 missense mutation in a patient with generalized dystonia, hypotonia, and intellectual disability, Neurology. Genetics 2(5) (2016) e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Lohmann K, Masuho I, Patil DN, Baumann H, Hébert E, Steinrucke S, Trujillano D, Skamangas NK, Dobricic V, Huning I, Gillessen-Kaesbach G, Westenberger A, Savic-Pavicevic D, Munchau A, Oprea G, Klein C, Rolfs A, Martemyanov KA, Novel GNB1 mutations disrupt assembly and function of G protein heterotrimers and cause global developmental delay in humans, Human molecular genetics 26(6) (2017) 1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Brett M, Lai AH, Ting TW, Tan AM, Foo R, Jamuar S, Tan EC, Acute lymphoblastic leukemia in a child with a de novo germline gnb1 mutation, American journal of medical genetics. Part A 173(2) (2017) 550–552. [DOI] [PubMed] [Google Scholar]
- [274].Hemati P, Revah-Politi A, Bassan H, Petrovski S, Bilancia CG, Ramsey K, Griffin NG, Bier L, Cho MT, Rosello M, Lynch SA, Colombo S, Weber A, Haug M, Heinzen EL, Sands TT, Narayanan V, Primiano M, Aggarwal VS, Millan F, Sattler-Holtrop SG, Caro-Llopis A, Pillar N, Baker J, Freedman R, Kroes HY, Sacharow S, Stong N, Lapunzina P, Schneider MC, Mendelsohn NJ, Singleton A, Loik Ramey V, Wou K, Kuzminsky A, Monfort S, Weiss M, Doyle S, Iglesias A, Martinez F, McKenzie F, Orellana C, van Gassen KLI, Palomares M, Bazak L, Lee A, Bircher A, Basel-Vanagaite L, Hafstrom M, Houge G, Group CRR, study DDD, Goldstein DB, Anyane-Yeboa K, Refining the phenotype associated with GNB1 mutations: Clinical data on 18 newly identified patients and review of the literature, American Journal of Medical Genetics. Part A 176(11) (2018) 2259–2275. [DOI] [PubMed] [Google Scholar]
- [275].Szczaluba K, Biernacka A, Szymanska K, Gasperowicz P, Kosinska J, Rydzanicz M, Ploski R, Novel GNB1 de novo mutation in a patient with neurodevelopmental disorder and cutaneous mastocytosis: Clinical report and literature review, European Journal of Medical Genetics 61(3) (2018) 157–160. [DOI] [PubMed] [Google Scholar]
- [276].Jones HF, Morales-Briceno H, Barwick K, Lewis J, Sanchis-Juan A, Raymond FL, Stewart K, Waugh MC, Mahant N, Kurian MA, Dale RC, Mohammad SS, Myoclonus-dystonia caused by GNB1 mutation responsive to deep brain stimulation, Movement Disorders 34(7) (2019) 1079–1080. [DOI] [PubMed] [Google Scholar]
- [277].Endo W, Ikemoto S, Togashi N, Miyabayashi T, Nakajima E, Hamano SI, Shibuya M, Sato R, Takezawa Y, Okubo Y, Inui T, Kato M, Sengoku T, Ogata K, Hamanaka K, Mizuguchi T, Miyatake S, Nakashima M, Matsumoto N, Haginoya K, Phenotype-genotype correlations in patients with GNB1 gene variants, including the first three reported Japanese patients to exhibit spastic diplegia, dyskinetic quadriplegia, and infantile spasms, Brain & Development 42(2) (2020) 199–204. [DOI] [PubMed] [Google Scholar]
- [278].Revah-Politi A, Sands TT, Colombo S, Goldstein DB, Anyane-Yeboa K, GNB1 Encephalopathy, in: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (Eds.), GeneReviews((R)), University of Washington, Seattle (WA), 1993. [Google Scholar]
- [279].Schultz-Rogers L, Masuho I, Pinto EVF, Schmitz CT, Schwab TL, Clark KJ, Gunderson L, Pichurin PN, Wierenga K, Martemyanov KA, Klee EW, Haploinsufficiency as a disease mechanism in GNB1-associated neurodevelopmental disorder, Molecular Genetics & Genomic Medicine 8(11) (2020) e1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Hurst JH, Mumaw J, Machacek DW, Sturkie C, Callihan P, Stice SL, Hooks SB, Human neural progenitors express functional lysophospholipid receptors that regulate cell growth and morphology, BMC Neurosci 9(1) (2008) 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Liguz-Lecznar M, Urban-Ciecko J, Kossut M, Somatostatin and Somatostatin-Containing Neurons in Shaping Neuronal Activity and Plasticity, Front Neural Circuit 10 (2016) 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Martel G, Dutar P, Epelbaum J, Viollet C, Somatostatinergic systems: an update on brain functions in normal and pathological aging, Front Endocrinol (Lausanne) 3(154) (2012) 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Lassuthova P, Safka Brozkova D, Neupauerova J, Krutova M, Mazanec R, Seeman P, Confirmation of the GNB4 gene as causal for Charcot-Marie-Tooth disease by a novel de novo mutation in a Czech patient, Neuromuscular disorders : NMD 27(1) (2017) 57–60. [DOI] [PubMed] [Google Scholar]
- [284].Miura S, Morikawa T, Fujioka R, Noda K, Kosaka K, Taniwaki T, Shibata H, A novel missense variant (Gln220Arg) of GNB4 encoding guanine nucleotide-binding protein, subunit β4 in a Japanese family with autosomal dominant motor and sensory neuropathy, European journal of medical genetics 60(9) (2017) 474–478. [DOI] [PubMed] [Google Scholar]
- [285].Poke G, King C, Muir A, de Valles-Ibanez G, Germano M, Moura de Souza CF, Fung J, Chung B, Fung CW, Mignot C, Ilea A, Keren B, Vermersch AI, Davis S, Stanley T, Moharir M, Kannu P, Shao Z, Malerba N, Merla G, Mefford HC, Scheffer IE, Sadleir LG, The epileptology of GNB5 encephalopathy, Epilepsia 60(11) (2019) e121–e127. [DOI] [PubMed] [Google Scholar]
- [286].Malerba N, Towner S, Keating K, Squeo GM, Wilson W, Merla G, A NGS-Targeted Autism/ID Panel Reveals Compound Heterozygous GNB5 Variants in a Novel Patient, Frontiers in Genetics 9 (2018) 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Turkdogan D, Usluer S, Akalin F, Agyuz U, Aslan ES, Familial early infantile epileptic encephalopathy and cardiac conduction disorder: A rare cause of SUDEP in infancy, Seizure 50 (2017) 171–172. [DOI] [PubMed] [Google Scholar]
- [288].Shamseldin HE, Masuho I, Alenizi A, Alyamani S, Patil DN, Ibrahim N, Martemyanov KA, Alkuraya FS, GNB5 mutation causes a novel neuropsychiatric disorder featuring attention deficit hyperactivity disorder, severely impaired language development and normal cognition, Genome Biol 17(1) (2016) 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Renthal W, Pain Genetics, in: Rosenberg RN, Pascual JM (Eds.), Rosenberg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease, Academic Press, Boston, 2015, pp. 1089–1100. [Google Scholar]
- [290].King CD, Keil A, Sibille KT, Chronic Pain and Perceived Stress, in: Fink G (Ed.), Stress: Concepts, Cognition, Emotion, and Behavior, Academic Press, San Diego, 2016, pp. 413–421. [Google Scholar]
- [291].Hosohata K, Logan JK, Varga E, Burkey TH, Vanderah TW, Porreca F, Hruby VJ, Roeske WR, Yamamura HI, The role of the G protein γ(2) subunit in opioid antinociception in mice, Eur J Pharmacol 392(3) (2000) R9–R11. [DOI] [PubMed] [Google Scholar]
- [292].Varga EV, Hosohata K, Borys D, Navratilova E, Nylen A, Vanderah TW, Porreca F, Roeske WR, Yamamura HI, Antinociception depends on the presence of G protein γ2-subunits in brain, Eur J Pharmacol 508(1–3) (2005) 93–8. [DOI] [PubMed] [Google Scholar]
- [293].Kalkbrenner F, Degtiar VE, Schenker M, Brendel S, Zobel A, Heschler J, Wittig B, Schultz G, Subunit composition of G(o) proteins functionally coupling galanin receptors to voltage-gated calcium channels, EMBO J 14(19) (1995) 4728–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Bergantin LB, Caricati-Neto A, Challenges for the pharmacological treatment of neurological and psychiatric disorders: Implications of the Ca(2+)/cAMP intracellular signalling interaction, Eur J Pharmacol 788 (2016) 255–260. [DOI] [PubMed] [Google Scholar]
- [295].Schwindinger WF, Giger KE, Betz KS, Stauffer AM, Sunderlin EM, Sim-Selley LJ, Selley DE, Bronson SK, Robishaw JD, Mice with deficiency of G protein γ3 are lean and have seizures, Mol Cell Biol 24(17) (2004) 7758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Dasgupta A, Genetic Markers of Alcohol Use Disorder, in: Dasgupta A (Ed.), Alcohol and its Biomarkers, Elsevier, San Diego, 2015, pp. 245–288. [Google Scholar]
- [297].Schwindinger WF, Betz KS, Giger KE, Sabol A, Bronson SK, Robishaw JD, Loss of G protein γ7 alters behavior and reduces striatal α(olf) level and cAMP production, J Biol Chem 278(8) (2003) 6575–9. [DOI] [PubMed] [Google Scholar]
- [298].Pinna A, Wardas J, Domenici MR, Popoli P, Cossu G, Morelli M, Control of Motor Function by Adenosine A2A Receptors in Parkinson’s and Huntington’s Disease, in: Blum D, Lopes LV (Eds.), Adenosine Receptors in Neurodegenerative Diseases, Academic Press, San Diego, 2017, pp. 187–213. [Google Scholar]
- [299].Castillo A, Kramer N, Schwartz CE, Miles JH, DuPont BR, Rosenfeld JA, Graham JM Jr., 19q13.32 microdeletion syndrome: three new cases, European Journal of Medical Genetics 57(11–12) (2014) 654–8. [DOI] [PubMed] [Google Scholar]
- [300].Lee HJ, Choi TI, Kim YM, Lee S, Han B, Bak IS, Moon SA, Yu DY, Shin KS, Kwon YK, Moon C, Ryu JH, Hoe HS, Kim CH, Shim I, Regulation of habenular G-protein γ8 on learning and memory via modulation of the central acetylcholine system, Molecular Psychiatry (2020). [DOI] [PubMed]
- [301].Schwindinger WF, Mirshahi UL, Baylor KA, Sheridan KM, Stauffer AM, Usefof S, Stecker MM, Mirshahi T, Robishaw JD, Synergistic roles for G-protein γ3 and γ7 subtypes in seizure susceptibility as revealed in double knock-out mice, J Biol Chem 287(10) (2012) 7121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Sanfilippo C, Musumeci G, Kazakova M, Mazzone V, Castrogiovanni P, Imbesi R, Di Rosa M, GNG13 Is a Potential Marker of the State of Health of Alzheimer’s Disease Patients’ Cerebellum, Journal of Molecular Neuroscience (2020). [DOI] [PubMed] [Google Scholar]
- [303].Erickson RP, Yatsenko SA, Larson K, Cheung SW, A Case of Agonadism, Skeletal Malformations, Bicuspid Aortic Valve, and Delayed Development with a 16p13.3 Duplication Including GNG13 and SOX8 Upstream Enhancers: Are Either, Both or Neither Involved in the Phenotype?, Molecular Syndromology 1(4) (2011) 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Lobanova ES, Finkelstein S, Herrmann R, Chen YM, Kessler C, Michaud NA, Trieu LH, Strissel KJ, Burns ME, Arshavsky VY, Transducin γ-subunit sets expression levels of α- and β-subunits and is crucial for rod viability, J Neurosci 28(13) (2008) 3510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Burns ME, Baylor DA, Activation, deactivation, and adaptation in vertebrate photoreceptor cells, Annu Rev Neurosci 24 (2001) 779–805. [DOI] [PubMed] [Google Scholar]
- [306].Fain GL, Matthews HR, Cornwall MC, Koutalos Y, Adaptation in vertebrate photoreceptors, Physiol Rev 81(1) (2001) 117–151. [DOI] [PubMed] [Google Scholar]
- [307].Arshavsky VY, Lamb TD, Pugh EN Jr., G proteins and phototransduction, Annual Review of Physiology 64 (2002) 153–87. [DOI] [PubMed] [Google Scholar]
- [308].Scherer SW, Feinstein DS, Oliveira L, Tsui LC, Pittler SJ, Gene structure and chromosome localization to 7q21.3 of the human rod photoreceptor transducin γ-subunit gene (GNGT1), Genomics 35(1) (1996) 241–3. [DOI] [PubMed] [Google Scholar]
- [309].Horton JC, Parker AB, Botelho JV, Duncan JL, Spontaneous Regeneration of Human Photoreceptor Outer Segments, Sci Rep 5(1) (2015) 12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Schwartz SD, Pan CK, Klimanskaya I, Lanza R, Retinal Degeneration, in: Lanza R, Langer R, Vacanti J (Eds.), Principles of Tissue Engineering, Academic Press, Boston, 2014, pp. 1427–1440. [Google Scholar]
- [311].Chen Y, Palczewska G, Mustafi D, Golczak M, Dong Z, Sawada O, Maeda T, Maeda A, Palczewski K, Systems pharmacology identifies drug targets for Stargardt disease-associated retinal degeneration, J Clin Invest 123(12) (2013) 5119–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Sancho-Pelluz J, Arango-Gonzalez B, Kustermann S, Romero FJ, van Veen T, Zrenner E, Ekstrom P, Paquet-Durand F, Photoreceptor cell death mechanisms in inherited retinal degeneration, Molecular Neurobiology 38(3) (2008) 253–69. [DOI] [PubMed] [Google Scholar]
- [313].Wang YJ, Cai SJ, Cui JL, Chen Y, Tang X, Li YH, Correlation between photoreceptor injury-regeneration and behavior in a zebrafish model, Neural Regen Res 12(5) (2017) 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Yao K, Qiu S, Wang YV, Park SJH, Mohns EJ, Mehta B, Liu X, Chang B, Zenisek D, Crair MC, Demb JB, Chen B, Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas, Nature 560(7719) (2018) 484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Arno G, Holder GE, Chakarova C, Kohl S, Pontikos N, Fiorentino A, Plagnol V, Cheetham ME, Hardcastle AJ, Webster AR, Michaelides M, Consortium UKIRD, Recessive Retinopathy Consequent on Mutant G-Protein β Subunit 3 (GNB3), JAMA Ophthalmology 134(8) (2016) 924–7. [DOI] [PubMed] [Google Scholar]
- [316].Dhingra A, Ramakrishnan H, Neinstein A, Fina ME, Xu Y, Li J, Chung DC, Lyubarsky A, Vardi N, Gβ3 is required for normal light ON responses and synaptic maintenance, J Neurosci 32(33) (2012) 11343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Nikonov SS, Lyubarsky A, Fina ME, Nikonova ES, Sengupta A, Chinniah C, Ding XQ, Smith RG, Pugh EN Jr., Vardi N, Dhingra A, Cones respond to light in the absence of transducin β subunit, J Neurosci 33(12) (2013) 5182–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Tummala H, Ali M, Getty P, Hocking PM, Burt DW, Inglehearn CF, Lester DH, Mutation in the guanine nucleotide-binding protein β−3 causes retinal degeneration and embryonic mortality in chickens, Investigative Ophthalmology & Visual Science 47(11) (2006) 4714–8. [DOI] [PubMed] [Google Scholar]
- [319].Ritchey ER, Zelinka C, Tang J, Liu J, Code KA, Petersen-Jones S, Fischer AJ, Vision-guided ocular growth in a mutant chicken model with diminished visual acuity, Experimental Eye Research 102 (2012) 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Huo R, Burden SK, Hoyt CS, Good WV, Chronic cortical visual impairment in children: aetiology, prognosis, and associated neurological deficits, Br J Ophthalmol 83(6) (1999) 670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Krispel CM, Chen CK, Simon MI, Burns ME, Prolonged photoresponses and defective adaptation in rods of Gβ5−/− mice, J Neurosci 23(18) (2003) 6965–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Burns ME, Pugh EN Jr., Lessons from photoreceptors: turning off G-protein signaling in living cells, Physiology 25(2) (2010) 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Chen CK, Burns ME, He W, Wensel TG, Baylor DA, Simon MI, Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9–1, Nature 403(6769) (2000) 557–60. [DOI] [PubMed] [Google Scholar]
- [324].Krispel CM, Chen CK, Simon MI, Burns ME, Novel form of adaptation in mouse retinal rods speeds recovery of phototransduction, J Gen Physiol 122(6) (2003) 703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Tian M, Zallocchi M, Wang W, Chen CK, Palczewski K, Delimont D, Cosgrove D, Peng YW, Light-induced translocation of RGS9–1 and Gβ5L in mouse rod photoreceptors, PLoS One 8(3) (2013) e58832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].Rao A, Dallman R, Henderson S, Chen CK, Gβ5 is required for normal light responses and morphology of retinal ON-bipolar cells, J Neurosci 27(51) (2007) 14199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Dickinson ME, Flenniken AM, Ji X, Teboul L, Wong MD, White JK, Meehan TF, Weninger WJ, Westerberg H, Adissu H, Baker CN, Bower L, Brown JM, Caddle LB, Chiani F, Clary D, Cleak J, Daly MJ, Denegre JM, Doe B, Dolan ME, Edie SM, Fuchs H, Gailus-Durner V, Galli A, Gambadoro A, Gallegos J, Guo S, Horner NR, Hsu CW, Johnson SJ, Kalaga S, Keith LC, Lanoue L, Lawson TN, Lek M, Mark M, Marschall S, Mason J, McElwee ML, Newbigging S, Nutter LM, Peterson KA, Ramirez-Solis R, Rowland DJ, Ryder E, Samocha KE, Seavitt JR, Selloum M, Szoke-Kovacs Z, Tamura M, Trainor AG, Tudose I, Wakana S, Warren J, Wendling O, West DB, Wong L, Yoshiki A, C. International Mouse Phenotyping, Jackson L, I.C.d.l.S. Infrastructure Nationale Phenomin, Charles River L, Harwell MRC, P. Toronto Centre for, I. Wellcome Trust Sanger, Center RB, MacArthur DG, Tocchini-Valentini GP, Gao X, Flicek P, Bradley A, Skarnes WC, Justice MJ, Parkinson HE, Moore M, Wells S, Braun RE, Svenson KL, de Angelis MH, Herault Y, Mohun T, Mallon AM, Henkelman RM, Brown SD, Adams DJ, Lloyd KC, McKerlie C, Beaudet AL, Bucan M, Murray SA, High-throughput discovery of novel developmental phenotypes, Nature 537(7621) (2016) 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Kuss J, Stallmeyer B, Goldstein M, Rinne S, Pees C, Zumhagen S, Seebohm G, Decher N, Pott L, Kienitz MC, Schulze-Bahr E, Familial Sinus Node Disease Caused by a Gain of GIRK (G-Protein Activated Inwardly Rectifying K(+) Channel) Channel Function, Circulation. Genomic and Precision Medicine 12(1) (2019) e002238. [DOI] [PubMed] [Google Scholar]
- [329].Gehrmann J, Meister M, Maguire CT, Martins DC, Hammer PE, Neer EJ, Berul CI, Mende U, Impaired parasympathetic heart rate control in mice with a reduction of functional G protein βγ-subunits, American Journal of Physiology. Heart and Circulatory Physiology 282(2) (2002) H445–56. [DOI] [PubMed] [Google Scholar]
- [330].den Hoed M, Eijgelsheim M, Esko T, Brundel BJ, Peal DS, Evans DM, Nolte IM, Segre AV, Holm H, Handsaker RE, Westra HJ, Johnson T, Isaacs A, Yang J, Lundby A, Zhao JH, Kim YJ, Go MJ, Almgren P, Bochud M, Boucher G, Cornelis MC, Gudbjartsson D, Hadley D, van der Harst P, Hayward C, den Heijer M, Igl W, Jackson AU, Kutalik Z, Luan J, Kemp JP, Kristiansson K, Ladenvall C, Lorentzon M, Montasser ME, Njajou OT, O’Reilly PF, Padmanabhan S, St Pourcain B, Rankinen T, Salo P, Tanaka T, Timpson NJ, Vitart V, Waite L, Wheeler W, Zhang W, Draisma HH, Feitosa MF, Kerr KF, Lind PA, Mihailov E, Onland-Moret NC, Song C, Weedon MN, Xie W, Yengo L, Absher D, Albert CM, Alonso A, Arking DE, de Bakker PI, Balkau B, Barlassina C, Benaglio P, Bis JC, Bouatia-Naji N, Brage S, Chanock SJ, Chines PS, Chung M, Darbar D, Dina C, Dorr M, Elliott P, Felix SB, Fischer K, Fuchsberger C, de Geus EJ, Goyette P, Gudnason V, Harris TB, Hartikainen AL, Havulinna AS, Heckbert SR, Hicks AA, Hofman A, Holewijn S, Hoogstra-Berends F, Hottenga JJ, Jensen MK, Johansson A, Junttila J, Kaab S, Kanon B, Ketkar S, Khaw KT, Knowles JW, Kooner AS, Kors JA, Kumari M, Milani L, Laiho P, Lakatta EG, Langenberg C, Leusink M, Liu Y, Luben RN, Lunetta KL, Lynch SN, Markus MR, Marques-Vidal P, Mateo Leach I, McArdle WL, McCarroll SA, Medland SE, Miller KA, Montgomery GW, Morrison AC, Muller-Nurasyid M, Navarro P, Nelis M, O’Connell JR, O’Donnell CJ, Ong KK, Newman AB, Peters A, Polasek O, Pouta A, Pramstaller PP, Psaty BM, Rao DC, Ring SM, Rossin EJ, Rudan D, Sanna S, Scott RA, Sehmi JS, Sharp S, Shin JT, Singleton AB, Smith AV, Soranzo N, Spector TD, Stewart C, Stringham HM, Tarasov KV, Uitterlinden AG, Vandenput L, Hwang SJ, Whitfield JB, Wijmenga C, Wild SH, Willemsen G, Wilson JF, Witteman JC, Wong A, Wong Q, Jamshidi Y, Zitting P, Boer JM, Boomsma DI, Borecki IB, van Duijn CM, Ekelund U, Forouhi NG, Froguel P, Hingorani A, Ingelsson E, Kivimaki M, Kronmal RA, Kuh D, Lind L, Martin NG, Oostra BA, Pedersen NL, Quertermous T, Rotter JI, van der Schouw YT, Verschuren WM, Walker M, Albanes D, Arnar DO, Assimes TL, Bandinelli S, Boehnke M, de Boer RA, Bouchard C, Caulfield WL, Chambers JC, Curhan G, Cusi D, Eriksson J, Ferrucci L, van Gilst WH, Glorioso N, de Graaf J, Groop L, Gyllensten U, Hsueh WC, Hu FB, Huikuri HV, Hunter DJ, Iribarren C, Isomaa B, Jarvelin MR, Jula A, Kahonen M, Kiemeney LA, van der Klauw MM, Kooner JS, Kraft P, Iacoviello L, Lehtimaki T, Lokki ML, Mitchell BD, Navis G, Nieminen MS, Ohlsson C, Poulter NR, Qi L, Raitakari OT, Rimm EB, Rioux JD, Rizzi F, Rudan I, Salomaa V, Sever PS, Shields DC, Shuldiner AR, Sinisalo J, Stanton AV, Stolk RP, Strachan DP, Tardif JC, Thorsteinsdottir U, Tuomilehto J, van Veldhuisen DJ, Virtamo J, Viikari J, Vollenweider P, Waeber G, Widen E, Cho YS, Olsen JV, Visscher PM, Willer C, Franke L, Global BC, Consortium CA, Erdmann J, Thompson JR, Consortium PG, Pfeufer A, Consortium QG, Sotoodehnia N, Consortium Q-I, Newton-Cheh C, Consortium C-A, Ellinor PT, Stricker BH, Metspalu A, Perola M, Beckmann JS, Smith GD, Stefansson K, Wareham NJ, Munroe PB, Sibon OC, Milan DJ, Snieder H, Samani NJ, Loos RJ, Identification of heart rate-associated loci and their effects on cardiac conduction and rhythm disorders, Nat Genet 45(6) (2013) 621–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Smolock EM, Ilyushkina IA, Ghazalpour A, Gerloff J, Murashev AN, Lusis AJ, Korshunov VA, Genetic locus on mouse chromosome 7 controls elevated heart rate, Physiological Genomics 44(13) (2012) 689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Dobrev D, Wettwer E, Himmel HM, Kortner A, Kuhlisch E, Schuler S, Siffert W, Ravens U, G-Protein β(3)-subunit 825T allele is associated with enhanced human atrial inward rectifier potassium currents, Circulation 102(6) (2000) 692–7. [DOI] [PubMed] [Google Scholar]
- [333].Schreieck J, Dostal S, von Beckerath N, Wacker A, Flory M, Weyerbrock S, Koch W, Schomig A, Schmitt C, C825T polymorphism of the G-protein β3 subunit gene and atrial fibrillation: association of the TT genotype with a reduced risk for atrial fibrillation, American Heart Journal 148(3) (2004) 545–50. [DOI] [PubMed] [Google Scholar]
- [334].Dorr M, Schmidt CO, Spielhagen T, Bornhorst A, Hentschel K, Franz C, Empen K, Kocher T, Diehl SR, Kroemer HK, Volzke H, Ewert R, Felix SB, Rosskopf D, β-blocker therapy and heart rate control during exercise testing in the general population: role of a common G-protein β−3 subunit variant, Pharmacogenomics 11(9) (2010) 1209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Rosskopf D, Manthey I, Habich C, Kielbik M, Eisenhardt A, Nikula C, Urban M, Kohnen S, Graf E, Ravens U, Siffert W, Identification and characterization of G β3s2, a novel splice variant of the G-protein β3 subunit, The Biochemical Journal 371(Pt 1) (2003) 223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Nurnberger J, Dammer S, Mitchell A, Siffert W, Wenzel RR, Gossl M, Philipp T, Michel MC, Schafers RF, Effect of the C825T polymorphism of the G protein β3 subunit on the systolic blood pressure-lowering effect of clonidine in young, healthy male subjects, Clinical Pharmacology and Therapeutics 74(1) (2003) 53–60. [DOI] [PubMed] [Google Scholar]
- [337].Baumgart D, Naber C, Haude M, Oldenburg O, Erbel R, Heusch G, Siffert W, G protein β3 subunit 825T allele and enhanced coronary vasoconstriction on α(2)-adrenoceptor activation, Circ Res 85(10) (1999) 965–9. [DOI] [PubMed] [Google Scholar]
- [338].Mitchell A, Pace M, Nurnberger J, Wenzel RR, Siffert W, Philipp T, Schafers RF, Insulin-mediated venodilation is impaired in young, healthy carriers of the 825T allele of the G-protein β3 subunit gene (GNB3), Clinical Pharmacology and Therapeutics 77(6) (2005) 495–502. [DOI] [PubMed] [Google Scholar]
- [339].Huang YJ, Zhou ZW, Xu M, Ma QW, Yan JB, Wang JY, Zhang QQ, Huang M, Bao L, Alteration of gene expression profiling including GPR174 and GNG2 is associated with vasovagal syncope, Pediatric Cardiology 36(3) (2015) 475–80. [DOI] [PubMed] [Google Scholar]
- [340].Hsieh CS, Huang PS, Chang SN, Wu CK, Hwang JJ, Chuang EY, Tsai CT, Genome-Wide Copy Number Variation Association Study of Atrial Fibrillation Related Thromboembolic Stroke, Journal of Clinical Medicine 8(3) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Hejduk P, Sakowicz AM, Mikolajczyk M, Sliwinska A, Drzewoski J, Pietrucha T, Association between single nucleotide polymorphisms of the G-protein γ5 subunit and the risk of essential hypertension in the population of Poland, Polskie Archiwum Medycyny Wewnetrznej 125(12) (2015) 903–9. [DOI] [PubMed] [Google Scholar]
- [342].Moon AM, Stauffer AM, Schwindinger WF, Sheridan K, Firment A, Robishaw JD, Disruption of G-protein γ5 subtype causes embryonic lethality in mice, PLoS One 9(3) (2014) e90970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [343].Yoda A, Adelmant G, Tamburini J, Chapuy B, Shindoh N, Yoda Y, Weigert O, Kopp N, Wu SC, Kim SS, Liu H, Tivey T, Christie AL, Elpek KG, Card J, Gritsman K, Gotlib J, Deininger MW, Makishima H, Turley SJ, Javidi-Sharifi N, Maciejewski JP, Jaiswal S, Ebert BL, Rodig SJ, Tyner JW, Marto JA, Weinstock DM, Lane AA, Mutations in G protein β subunits promote transformation and kinase inhibitor resistance, Nature Medicine 21(1) (2015) 71–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [344].Zimmermannova O, Doktorova E, Stuchly J, Kanderova V, Kuzilkova D, Strnad H, Starkova J, Alberich-Jorda M, Falkenburg JHF, Trka J, Petrak J, Zuna J, Zaliova M, An activating mutation of GNB1 is associated with resistance to tyrosine kinase inhibitors in ETV6-ABL1-positive leukemia, Oncogene 36(43) (2017) 5985–5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Clar H, Langsenlehner U, Krippl P, Renner W, Leithner A, Gruber G, Hofmann G, Yazdani-Biuki B, Langsenlehner T, Windhager R, A polymorphism in the G protein β3-subunit gene is associated with bone metastasis risk in breast cancer patients, Breast Cancer Research and Treatment 111(3) (2008) 449–52. [DOI] [PubMed] [Google Scholar]
- [346].Eisenhardt A, Siffert W, Rosskopf D, Musch M, Mosters M, Roggenbuck U, Jockel KH, Rubben H, Lummen G, Association study of the G-protein β3 subunit C825T polymorphism with disease progression in patients with bladder cancer, World Journal of Urology 23(4) (2005) 279–86. [DOI] [PubMed] [Google Scholar]
- [347].Sheu SY, Gorges R, Ensinger C, Ofner D, Farid NR, Siffert W, Schmid KW, Different genotype distribution of the GNB3 C825T polymorphism of the G protein β3 subunit in adenomas and differentiated thyroid carcinomas of follicular cell origin, The Journal of pathology 207(4) (2005) 430–5. [DOI] [PubMed] [Google Scholar]
- [348].Sheu SY, Handke S, Brocker-Preuss M, Gorges R, Frey UH, Ensinger C, Ofner D, Farid NR, Siffert W, Schmid KW, The C allele of the GNB3 C825T polymorphism of the G protein β3-subunit is associated with an increased risk for the development of oncocytic thyroid tumours, The Journal of Pathology 211(1) (2007) 60–6. [DOI] [PubMed] [Google Scholar]
- [349].Lehnerdt GF, Franz P, Bankfalvi A, Grehl S, Jahnke K, Lang S, Schmid KW, Siffert W, Frey UH, Association study of the G-protein β3 subunit C825T polymorphism with disease progression an overall survival in patients with head and neck squamous cell carcinoma, Cancer Epidemiology, Biomarkers & Prevention 17(11) (2008) 3203–7. [DOI] [PubMed] [Google Scholar]
- [350].El Hindy N, Adamzik M, Lambertz N, Bachmann HS, Worm K, Egensperger R, Frey UH, Asgari S, Sure U, Siffert W, Sandalcioglu IE, Association of the GNB3 825T-allele with better survival in patients with glioblastoma multiforme, Journal of Cancer Research and Clinical Oncology 136(9) (2010) 1423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Cardenas-Navia LI, Cruz P, Lin JC, Program NCS, Rosenberg SA, Samuels Y, Novel somatic mutations in heterotrimeric G proteins in melanoma, Cancer Biology & Therapy 10(1) (2010) 33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [352].Wang B, Li D, Rodriguez-Juarez R, Farfus A, Storozynsky Q, Malach M, Carpenter E, Filkowski J, Lykkesfeldt AE, Kovalchuk O, A suppressive role of guanine nucleotide-binding protein subunit β4 inhibited by DNA methylation in the growth of anti-estrogen resistant breast cancer cells, BMC Cancer 18(1) (2018) 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [353].Gao J, Pan H, Zhu Z, Yu T, Huang B, Zhou Y, Guanine nucleotide-binding protein subunit β−4 promotes gastric cancer progression via activating Erk1/2, Acta Biochimica et Biophysica Sinica 52(9) (2020) 975–987. [DOI] [PubMed] [Google Scholar]
- [354].Riemann K, Struwe H, Eisenhardt A, Obermaier B, Schmid KW, Siffert W, Characterization of intron-1 haplotypes of the G protein β4 subunit gene--association with survival and progression in patients with urothelial bladder carcinoma, Pharmacogenetics and Genomics 18(11) (2008) 999–1008. [DOI] [PubMed] [Google Scholar]
- [355].Riemann K, Struwe H, Alakus H, Obermaier B, Schmitz KJ, Schmid KW, Siffert W, Association of GNB4 intron-1 haplotypes with survival in patients with UICC stage III and IV colorectal carcinoma, Anticancer Research 29(4) (2009) 1271–4. [PubMed] [Google Scholar]
- [356].Fang LT, Lee S, Choi H, Kim HK, Jew G, Kang HC, Chen L, Jablons D, Kim IJ, Comprehensive genomic analyses of a metastatic colon cancer to the lung by whole exome sequencing and gene expression analysis, International Journal of Oncology 44(1) (2014) 211–21. [DOI] [PubMed] [Google Scholar]
- [357].Fuchs D, Metzig M, Bickeboller M, Brandel C, Roth W, The Gβ5 protein regulates sensitivity to TRAIL-induced cell death in colon carcinoma, Oncogene 34(21) (2015) 2753–63. [DOI] [PubMed] [Google Scholar]
- [358].Park SM, Hwang CY, Cho SH, Lee D, Gong JR, Lee S, Nam S, Cho KH, Systems analysis identifies potential target genes to overcome cetuximab resistance in colorectal cancer cells, The FEBS Journal 286(7) (2019) 1305–1318. [DOI] [PubMed] [Google Scholar]
- [359].Yajima I, Kumasaka MY, Naito Y, Yoshikawa T, Takahashi H, Funasaka Y, Suzuki T, Kato M, Reduced GNG2 expression levels in mouse malignant melanomas and human melanoma cell lines, American Journal of Cancer Research 2(3) (2012) 322–9. [PMC free article] [PubMed] [Google Scholar]
- [360].Yajima I, Kumasaka MY, Yamanoshita O, Zou C, Li X, Ohgami N, Kato M, GNG2 inhibits invasion of human malignant melanoma cells with decreased FAK activity, American Journal of Cancer Research 4(2) (2014) 182–8. [PMC free article] [PubMed] [Google Scholar]
- [361].Zhou Y, Yang L, Zhang X, Chen R, Chen X, Tang W, Zhang M, Identification of Potential Biomarkers in Glioblastoma through Bioinformatic Analysis and Evaluating Their Prognostic Value, BioMed Research International 2019 (2019) 6581576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Chen X, Pan Y, Yan M, Bao G, Sun X, Identification of potential crucial genes and molecular mechanisms in glioblastoma multiforme by bioinformatics analysis, Molecular Medicine Reports 22(2) (2020) 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Shukla S, Pia Patric IR, Thinagararjan S, Srinivasan S, Mondal B, Hegde AS, Chandramouli BA, Santosh V, Arivazhagan A, Somasundaram K, A DNA methylation prognostic signature of glioblastoma: identification of NPTX2-PTEN-NF-kappaB nexus, Cancer Research 73(22) (2013) 6563–73. [DOI] [PubMed] [Google Scholar]
- [364].Maina EN, Morris MR, Zatyka M, Raval RR, Banks RE, Richards FM, Johnson CM, Maher ER, Identification of novel VHL target genes and relationship to hypoxic response pathways, Oncogene 24(28) (2005) 4549–58. [DOI] [PubMed] [Google Scholar]
- [365].Song J, Yang J, Lin R, Cai X, Zheng L, Chen Y, Molecular heterogeneity of guanine nucleotide binding-protein γ subunit 4 in left- and right-sided colon cancer, Oncology Letters 20(6) (2020) 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [366].Xiong Z, Xiong Y, Liu H, Li C, Li X, Identification of purity and prognosis-related gene signature by network analysis and survival analysis in brain lower grade glioma, Journal of Cellular and Molecular Medicine 24(19) (2020) 11607–11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [367].Yang B, Han ZY, Wang WJ, Ma YB, Chu SH, GNG5 is an unfavourable independent prognostic indicator of gliomas, Journal of Cellular and Molecular Medicine 24(21) (2020) 12873–12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].Giefing M, Arnemann J, Martin-Subero JI, Nielander I, Bug S, Hartmann S, Arnold N, Tiacci E, Frank M, Hansmann ML, Kuppers R, Siebert R, Identification of candidate tumour suppressor gene loci for Hodgkin and Reed-Sternberg cells by characterisation of homozygous deletions in classical Hodgkin lymphoma cell lines, British Journal of Haematology 142(6) (2008) 916–24. [DOI] [PubMed] [Google Scholar]
- [369].Xu S, Zhang H, Liu T, Chen Y, He D, Li L, G Protein γ subunit 7 loss contributes to progression of clear cell renal cell carcinoma, Journal of Cellular Physiology 234(11) (2019) 20002–20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [370].Hartmann S, Szaumkessel M, Salaverria I, Simon R, Sauter G, Kiwerska K, Gawecki W, Bodnar M, Marszalek A, Richter J, Brauze D, Zemke N, Jarmuz M, Hansmann ML, Siebert R, Szyfter K, Giefing M, Loss of protein expression and recurrent DNA hypermethylation of the GNG7 gene in squamous cell carcinoma of the head and neck, Journal of Applied Genetics 53(2) (2012) 167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].Ohta M, Mimori K, Fukuyoshi Y, Kita Y, Motoyama K, Yamashita K, Ishii H, Inoue H, Mori M, Clinical significance of the reduced expression of G protein γ7 (GNG7) in oesophageal cancer, British Journal of Cancer 98(2) (2008) 410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [372].Utsunomiya T, Inoue H, Taguchi K, Shimada M, Sugimachi K, Mori M, G protein γ7 expression as a new clinicopathological marker in patients with intrahepatic cholangiocarcinoma, Archives of Surgery (Chicago, Ill. : 1960) 137(2) (2002) 181–5. [DOI] [PubMed] [Google Scholar]
- [373].Shibata K, Mori M, Tanaka S, Kitano S, Akiyoshi T, Identification and cloning of human G-protein γ7, down-regulated in pancreatic cancer, Biochem Biophys Res Commun 246(1) (1998) 205–9. [DOI] [PubMed] [Google Scholar]
- [374].Demokan S, Chuang AY, Chang X, Khan T, Smith IM, Pattani KM, Dasgupta S, Begum S, Khan Z, Liegeois NJ, Westra WH, Sidransky D, Koch W, Califano JA, Identification of guanine nucleotide-binding protein γ7 as an epigenetically silenced gene in head and neck cancer by gene expression profiling, International Journal of Oncology 42(4) (2013) 1427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [375].Gao LW, Wang GL, Comprehensive bioinformatics analysis identifies several potential diagnostic markers and potential roles of cyclin family members in lung adenocarcinoma, OncoTargets and Therapy 11 (2018) 7407–7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [376].Li C, Long Q, Zhang D, Li J, Zhang X, Identification of a four-gene panel predicting overall survival for lung adenocarcinoma, BMC Cancer 20(1) (2020) 1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [377].Liu G, Zeng X, Wu B, Zhao J, Pan Y, RNA-Seq analysis of peripheral blood mononuclear cells reveals unique transcriptional signatures associated with radiotherapy response of nasopharyngeal carcinoma and prognosis of head and neck cancer, Cancer Biology & Therapy 21(2) (2020) 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [378].Niemira M, Collin F, Szalkowska A, Bielska A, Chwialkowska K, Reszec J, Niklinski J, Kwasniewski M, Kretowski A, Molecular Signature of Subtypes of Non-Small-Cell Lung Cancer by Large-Scale Transcriptional Profiling: Identification of Key Modules and Genes by Weighted Gene Co-Expression Network Analysis (WGCNA), Cancers 12(1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [379].Hua P, Zhang Y, Jin C, Zhang G, Wang B, Integration of gene profile to explore the hub genes of lung adenocarcinoma: A quasi-experimental study, Medicine 99(43) (2020) e22727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [380].Hsu YL, Hung JY, Lee YL, Chen FW, Chang KF, Chang WA, Tsai YM, Chong IW, Kuo PL, Identification of novel gene expression signature in lung adenocarcinoma by using next-generation sequencing data and bioinformatics analysis, Oncotarget 8(62) (2017) 104831–104854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [381].Shi K, Li N, Yang M, Li W, Identification of Key Genes and Pathways in Female Lung Cancer Patients Who Never Smoked by a Bioinformatics Analysis, Journal of Cancer 10(1) (2019) 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [382].Yang G, Chen Q, Xiao J, Zhang H, Wang Z, Lin X, Identification of genes and analysis of prognostic values in nonsmoking females with non-small cell lung carcinoma by bioinformatics analyses, Cancer Management and Research 10 (2018) 4287–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [383].Haouas H, Haouas S, Uzan G, Hafsia A, Identification of new markers discriminating between myeloid and lymphoid acute leukemia, Hematology (Amsterdam, Netherlands) 15(4) (2010) 193–203. [DOI] [PubMed] [Google Scholar]
- [384].Li J, Jin C, Zou C, Qiao X, Ma P, Hu D, Li W, Jin J, Jin X, Fan P, GNG12 regulates PD-L1 expression by activating NF-κB signaling in pancreatic ductal adenocarcinoma, FEBS Open Biol 10(2) (2020) 278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [385].Ju KC, Zhang B, Hu YL, Feng Y, Li XH, Liu YF, Li P, Mao QS, Xue WJ, High expression of G protein subunit γ13 is associated with poor prognosis of gastrointestinal stromal tumor, Pathology, Research and Practice 216(10) (2020) 153143. [DOI] [PubMed] [Google Scholar]


