Abstract
Background:
The absence of an FDA-approved medication for the treatment of cocaine use disorder (CUD) may, in part, reflect the varying conditions present when the decision to use cocaine is made, with one medication unlikely to work under all conditions. The objective of this double-blind, placebo-controlled, human laboratory study was to test the effects of modafinil, a medication with mixed efficacy for the treatment of CUD, using a novel self-administration procedure designed to model distinct clinical scenarios.
Methods:
During modafinil maintenance (0, 300 mg/day), participants chose to self-administer up to 7 doses of smoked cocaine (25 mg) under 9 conditions: immediately after exposure to: (a) cues associated with cocaine and a non-contingent cocaine administration, i.e. ‘prime’ (25 mg), (b) only cocaine cues, and (c) neither cues nor cocaine. Each condition was tested when self-administered cocaine cost $5, $10 and $ 15/dose.
Results:
Nontreatment-seeking cocaine smokers (3F,13M), spending $388 ± 218/week on cocaine and with no history of alcohol use disorder, completed the study. Relative to placebo, modafinil robustly attenuated self-administration when cocaine was expensive ($10,$ 15/dose) and when there was no ‘prime.’ Modafinil had no effect on self-administration when cocaine was inexpensive ($5/dose) or when participants received a ‘prime.’
Conclusions:
Modafinil’s effects on cocaine-taking varied substantially as a function of recent cocaine exposure and cost, which may help explain the mixed clinical findings. Modafinil may be most effective for preventing relapse in abstinent patients, particularly under conditions in which cocaine is costly, rather than initiating abstinence for those continuing to use cocaine.
Keywords: Cocaine use disorder, smoked cocaine, Modafinil, self-administration, relapse prevention, medications development
1. Introduction
Despite extensive preclinical and clinical study, there are no medications approved by the Food and Drug Administration (FDA) to facilitate treatment for cocaine use disorder (CUD: see Kampman, 2019). The failure to develop an effective cocaine pharmacotherapy may be, in part, because medications are typically tested in patients who are heterogeneous in their phase of drug use: some enter clinical trials already abstinent while others continue to use cocaine, and the response of these subpopulations to medication is distinct (e.g., Bisaga et al., 2010; Bisaga et al., 2006; Bisaga et al., 2005). As an example, Schmitz and colleagues (2014) demonstrated that levodopa-carbidopa and naltrexone were more efficacious in reducing cocaine use in ongoing cocaine users than in those already abstinent. It is unlikely that a single medication will both (1) initiate abstinence, i.e., interrupt ongoing cocaine use, and (2) prevent relapse, i.e., decrease the likelihood an abstinent patient will return to cocaine use.
In human laboratory models testing the effects of potential pharmacotherapies for CUD, the wake-promoting agent, modafinil, has been one of the only medications shown to reduce both the reinforcing and subjective effects of cocaine. Modafinil is a weak but selective dopamine transport inhibitor (Wisor, 2013), increasing extracellular dopamine levels but to a much smaller degree than cocaine (Madras et al., 2006; Mignot et al., 1994; see Minzenberg and Carter, 2008; Volkow et al., 2009). Modafinil has low abuse potential or stimulant-like effects in cocaine users (Jasinski, 2000; Rush et al., 2002; Vosburg et al., 2010), yet decreases i.v. and smoked cocaine’s abuse-related effects in controlled laboratory studies (Dackis et al., 2003; Hart et al., 2008; Malcolm et al., 2006; Verrico et al., 2014; but see Foltin et al., 2016). Importantly, modafinil (200, 400 mg/day) also decreased smoked cocaine self-administration (25, 50 mg; Hart et al., 2008) in non-treatment seeking cocaine users, a behavior extraordinarily difficult to disrupt (Haney and Spealman, 2008).
Despite these positive signals from the human laboratory, modafinil has shown mixed results in clinical trials. Some have shown that modafinil reduced cocaine use provided that patients were not also alcohol dependent (Anderson et al., 2009; Dackis et al., 2005; Kampman et al., 2015; Morgan et al., 2016), while others showed no effect even when those with alcohol dependence were excluded (Dackis et al., 2012; Schmitz et al., 2012), or when patients still using cocaine were considered separately from those who were drug-free at medication onset (Schmitz et al., 2014).
The objective of this study was to address the mixed clinical data by testing the effects of modafinil in a controlled laboratory setting, to begin to define the conditions in which modafinil alters cocaine self-administration. In preclinical studies, modafinil (or an analog of modafinil) reduced cocaine seeking following cocaine exposure (Mahler et al., 2014; Zhang et al., 2017), yet whether modafinil disrupts the impact of drug-paired cues or of cocaine exposure per se on human models of relapse has not been explicitly tested. The inpatient human laboratory setting offers an opportunity to investigate precisely how modafinil influences the decision to use cocaine by testing its self-administration under discrete experimental conditions that attempt to model decisions faced by cocaine users outside of the laboratory: in the presence or absence of contextual cues associated with cocaine use; abstinent or following cocaine exposure; high vs low cost cocaine conditions.
Specifically, smoked-cocaine users not seeking treatment for their drug use and having no history of alcohol dependence were maintained on both placebo and active (300 mg/day) modafinil under counter-balanced, double-blind conditions. Participants decided if they would initiate cocaine self-administration and, if so, how much cocaine they would self-administer after exposure to (a) cocaine-paired cues and noncontingent cocaine administration (i.e., ‘priming’), (b) cocaine-paired cues alone, or (c) neither cues nor cocaine. Participants had to purchase cocaine for self-administration using their study earnings. Cue/cocaine conditions were tested when the financial cost of each self-administered cocaine dose was low, moderate, and high. Pilot testing of these procedures (n=4), testing a range of costs, demonstrated that cocaine self-administration systematically varied as a function of cues, the prime and cocaine cost (Haney, 2009).
2. Material and Methods
2.1. Participants
The study was conducted from 2009-2014. Research volunteers were solicited through word-of-mouth referral and newspaper advertisement in New York, NY. Eligible volunteers were between 21-53 years of age, currently smoking cocaine (≥ twice/week, ≥ $70/week, testing positive for urinary benzoylecgonine) and explicitly not interested in treatment for their cocaine use. None met criteria for dependence on any other illicit drug or on alcohol, had a history of alcohol dependence or had any major psychiatric condition or medical disorders. Participants signed a consent form approved by The New York State Psychiatric Institute (NYSPI) Institutional Review Board which described the study, outlined possible risks, and indicated that varying doses of modafinil (including placebo) and smoked cocaine would be tested. Volunteers were compensated for their participation.
2.2. Study Schedule
Prior to study onset, participants were administered an active dose of modafinil (50 mg) and spent 1 hour in the presence of a research nurse to monitor vital signs and to confirm that the medication was well tolerated. As shown in Table 1, participants were then initiated on placebo or modafinil capsules in randomized, counter-balanced order. Dosing was initiated 3 days prior to hospital admission. Participants then moved into Columbia University’s Irving Institute for Clinical and Translational Research at New York-Presbyterian Hospital. Following completion of the first inpatient phase, participants had a minimum of 8 medication-free days to allow for medication clearance before starting the alternate medication dose and second inpatient phase.
TABLE 1:
Representative Modafinil Dosing Schedule
| Phase | Outpatient | Inpatient | Washout | Outpatient | Inpatient |
|---|---|---|---|---|---|
| Study Days | 1-3 | 4-21 | ≥ 8 days | 22-24 | 25-42 |
| Modafinil (mg/day) | 100-200* | 250-300* | NA | 0 | 0 |
Note:
Modafinil administration was initiated at 100 mg BID; dose increased by 50 mg each day until the maintenance dose was achieved (200 mg in AM and 100 mg in PM) on day 5. On the evening of the last study day, the PM dose was skipped. Medication administration was always observed by a research nurse except for the initial 3 outpatient days of each study phase (5 of the 41 dose administrations per phase were unobserved but compliance was confirmed by urinary riboflavin). The order in which active and placebo modafinil capsules was administered was counter-balanced.
2.3. Participant Instructions
Participants were instructed at study onset that two doses of cocaine were going to be tested (Dose A and Dose B), and that the nurse would always tell them which dose they were administering. Participants were told that at certain times, they would receive Dose A or Dose B at no cost to them and at other times they would be offered the opportunity to purchase Dose A or Dose B for self-administration (in actuality, only Dose A, i.e., 25 mg cocaine was made available for self-administration. Dose B, i.e., 0 mg cocaine, was inhaled as part of the contextual cue condition described below). Prior to the option to purchase cocaine, participants were told which dose was available and its cost.
At the end of each study day, participants were given $25 in faux money, representing a portion of their daily earnings. They kept this money in a lockbox in their room and brought the lockbox with them to each session. Participants always had enough money to purchase cocaine for self-administration. If a session was cancelled due to cardiovascular criteria, participants were refunded for doses not administered.
2.4. Cocaine Sessions
2.4.1. Experimenter-Administered, Cost-Free Cocaine Sessions:
Both inpatient phases began with 3 days of experimenter-administered cocaine sessions, where cocaine (Dose A: 25 mg) was administered at no cost seven times per session at 14-min intervals twice per day (9:00 AM; 3:00 PM). The purpose of this 3-day period was (a) to pair the contextual cues of the laboratory with active cocaine effects, and (b) to standardize cocaine exposure prior to self-administration sessions. Blood for plasma cocaine assays was assayed once per inpatient phase during an experimenter-administered cocaine session. Following antecubital vein catheter placement (Quik-Cath®, Travenol Laboratories, Deerfield, IL), blood was drawn at baseline, 4 min after the first cocaine administration, 10 minutes after the second cocaine administration, and 4 minutes after the seventh cocaine administration.
2.4.2. Self-Administered Cocaine Sessions:
Self-administration sessions (SA) began the following week. Three priming and cue conditions were tested (Table 2). In order to determine if the effects of priming and cues differed as a function of the cost of self-administered cocaine, each condition was tested when the financial cost of self-administered cocaine was low ($5), moderate ($10), and high ($15). The order of the 9 sessions was systematically varied between and within-subjects.
TABLE 2:
Self-administration Conditions
| Cost of Each Self-administered Cocaine Dose | ||
|---|---|---|
| Low ($5) | Moderate ($10) | High ($15) |
| No Stimuli | No Stimuli | No Stimuli |
| Cues | Cues | Cues |
| Cues+Prime | Cues+Prime | Cues+Prime |
Note: No Stimuli = Exposure to neither cues nor cocaine. Cue = Exposure to the contextual cues associated with cocaine administration. Cue+Prime = Exposure to the cues associated with cocaine and a non-contingent dose of cocaine (25 mg), i.e. ‘prime.’
Thus, under each cost condition, self-administration was tested when cocaine users had been exposed to: (a) the cues associated with cocaine and ‘primed’ with an experimenter-administered active cocaine dose (Dose A: 25 mg; +cue,+prime), (b) only the contextual cues associated with cocaine use (Dose B: 0 mg; +cues,−prime); these sessions were identical to a ‘prime’ session except that participants smoked 0 mg cocaine prior to their self-administration choice, and (c) neither cues or cocaine (−cues/−prime); for this condition, participants remained in their room and a staff member phoned to tell them the cost of self-administered cocaine that day; they had 2 min to indicate how many doses they would like to purchase, if any. If no doses were chosen, no session was run. This condition was designed to mimic the choice to seek cocaine in the absence of both cues (e.g., lab setting; ECG leads; blood pressure cuff; nurses associated with cocaine administration; inhaling on a cocaine pipe) associated with cocaine and the effects of cocaine itself. Note, there was no condition in which individuals were ‘primed’ in the absence of cues because cocaine had to be administered in a laboratory setting, which served as contextual cues for cocaine administration.
There were 7 opportunities to purchase cocaine within a session, so the maximum cost for self-administration was $ 105/session. Note, the cost of cocaine was considerably higher than ‘street’ cocaine (approximately $30/gram in NYC across the 5 years that the study was conducted) because pilot testing showed that inpatient, nontreatment-seeking volunteers were willing to spend large amounts of their own money to obtain cocaine; if costs were too low in the study, participants would not vary their self-administration as a function of the variables of interest (cues, cocaine exposure). The objective of this laboratory model was not to mimic behavior in the natural ecology but rather to assess medication effects on key behaviors in the laboratory to predict how the medication may function in the natural ecology (Haney and Spealman, 2008).
On days with self-administration sessions, participants were able to use their earnings to either purchase cocaine or purchase alternative, non-drug reinforcers. They could spend as much money on the alternative reinforcers as they could have spent if they purchased every cocaine dose instead, i.e., on days when cocaine cost $ 15/administration, they could spend $105 on alternatives, such as access to a portable PlayStation, non-alcoholic beer, personal hygiene items, or phone cards. None of these items were available unless purchased. Participants could also choose to not purchase any items and receive their earnings at study termination.
2.4.3. Session Procedures:
Research nurses monitored participants via a one-way mirror; an intercom system provided two-way communication. Electrocardiograms were continuously monitored via limb leads (MAC PC®, Marquette Electronics, Milwaukee, WI). Heart rate and systolic and diastolic blood pressure were recorded every two minutes (Sentry II-Model 6100 automated vital signs monitor, NBS Medical, Costa Mesa, CA) beginning 30 min prior to cocaine administration and ending 30-minutes following the last cocaine delivery. Participant monitoring was supervised on-site by the study psychiatrist, with a cardiology consultant immediately available if needed.
At time 0, cocaine was experimenter-administered in the +cue,+prime (Dose A: 25 mg) or +cue,-prime (Dose B: 0 mg) condition. Participants were told that they would smoke Dose A or Dose B at the start of the session at no cost to them. After that they would decide whether they would want to purchase additional doses of cocaine. Prior to each dosing, nurses would say “I am coming in with Dose A (or Dose B).” Four min later, participants were told that Dose A (25 mg) was available for self-administration. They were told the cost of self-administered cocaine for the day, and had 2 min to type in the number of doses they would like to purchase for the session (0-7); they were required to decide the total amount of cocaine to self-administer at the beginning of each session so that the effect of the conditions on choice could be dissociated from the effects of self-administered cocaine. The nurse collected the participant’s faux money for all of the cocaine purchased. The first dose, if purchased, was administered 8 min later. Subsequent cocaine doses were administered at 14 min intervals. For sessions with no cue or prime, participants who opted to purchase cocaine were brought to the laboratory, the nurse collected the money at the start of the session (−30 min), and baseline data was collected for 30 min. At time 0, the first purchased dose of cocaine was administered. Regardless of session type, a subjective-effects battery followed 4 min after each dose administration, and a final subjective-effects battery was completed 30 min after the last dose of cocaine was administered.
Cocaine was not given if cardiovascular activity exceeded vital signs criteria for at least 6 minutes [SP > 160 mmHg, DP > 100 mmHg, or HR > (220-participant’s age)*0.85], or if changes on the electrocardiogram were deemed unsafe [e.g., > 10 premature ventricular contractions (PVCs)/session, evidence of cardiac ischemia]. If two consecutive sessions were terminated due to abnormal vital signs, participants were counseled, referred to appropriate medical follow-up and discharged from the study.
2.4.4. Subjective-Effects Battery:
A computerized subjective-effects questionnaire, comprising a series of eighteen 100-mm visual analog scales (VAS) labeled “Not at all” (0 mm) at one end and “Extremely” at the other, was completed 5 times per session. Cluster analysis on the 18 mood ratings has yielded discrete clusters (Evans et al., 2002). Clusters analyzed were the Good Drug Effect cluster comprising “Good Drug Effect,” “High,” and “Stimulated,” and the Cocaine Quality cluster comprising “The choice was …”Good Quality,” “Potent,” and “I Liked the Choice”. Also analyzed was a VAS rating of cocaine craving, operationalized as “I want…” “Cocaine.”
2.4.5. Drugs:
Cocaine:
Cocaine base was derived from cocaine hydrochloride (Mallinckrodt) by NYSPI pharmacists. Participants were presented with cocaine in an 8 cm glass tube or ‘stem’ fitted with a fine metal screen. Participants held the stem while a nurse held a butane flame on the cocaine until all of it was inhaled. Participants put on eye masks immediately prior to each dosing in order to be blinded to visual cues such as the physical mass of the dose. The placebo condition was inhalation through an empty stem to which a butane flame was similarly applied. After each dose administration, the participant removed the eye mask.
Modafinil:
Participants were maintained on placebo or modafinil (0, 300 mg/d; Teva Pharmaceuticals) in counter-balanced order. Medication administration began 3 days prior to each inpatient phase. The first capsule was administered by the research nurse on Friday AM and participants took the next 5 capsule administrations as outpatients. On Monday AM, medication compliance was determined by the presence of riboflavin (compounded in the study capsules) in urine samples, assessed using ultraviolet detection, and the nurse administered the Monday AM capsule. During the inpatient study phases, a nurse administered modafinil in the morning (9:00AM) and evening (5:00PM). Modafinil dose started at 50 mg BID and was increased by 50 mg/day until the maintenance dose (AM: 200 mg; PM: 100 mg) was achieved on day 5. On the evening of the last study day, the PM dose was skipped.
2.5. Data Analysis
Data were analyzed using planned comparisons generated using a repeated measures Analysis of Variance. The effects of modafinil maintenance on the number of choices to smoke cocaine within a session were analyzed using within-subject factors: modafinil dose (0, 300 mg/day), cocaine cost ($5, 10, 15), and cue/prime condition (none, cue, prime). Nine planned comparisons were completed to compare placebo to modafinil for each cost and cue condition (Table 1).
The effects of modafinil on plasma cocaine levels, subjective and cardiovascular effects were measured during the third day of experimenter-administered cocaine sessions, when modafinil had achieved steady-state and when a controlled amount of cocaine had been administered. For analysis of plasma cocaine, there were two within-subject factors: modafinil dose and time within session (baseline, t4, t10, t88). The planned comparisons were single degree of freedom comparisons that used the error term for the modafinil dose x cost x condition interaction. P values less than 0.01 were considered statistically significant. Huynh-Feldt corrections were used, when appropriate.
3. Results
3.1. Participant Characteristics
Table 3 portrays demographic data for the 16 research volunteers who completed the study. An additional 18 participants were enrolled but did not complete the study, primarily due to the long and intensive study design and stringent medical criteria for cocaine administration. Initially, we tested a lower dose of cocaine (12 mg) which was not self-administered (n=3 enrolled; 2 medically discharged; 1 completed) so these data were not included in the analysis. Fourteen participants were medically discharged during the study. Of these, 10 exceeded cardiovascular limits during sessions for ongoing cocaine administration (n=6 on placebo; n=4 on modafinil); 1 participant (on modafinil) was transferred to the hospital emergency room when he experienced a brief run of ventricular tachycardia following cocaine administration (asymptomatic; cleared on cardiology follow up); 1 (on placebo) was found to be taking clonidine; 1 (on placebo) gained 19 pounds after his first inpatient phase and thus exceeded weight criteria for re-enrollment; 1 (on placebo) had a mechanical fall outdoors due to wintery conditions and required medical attention. Three participants left the study for personal reasons (n=1 on placebo; n=2 on modafinil).
TABLE 3:
Demographic characteristics of participants
| Number of participants | 16 (13M; 3F) |
| Race (Black/Asian) | 15/1 |
| Age (years) | 44.8 ± 5.3 |
| Cocaine use (#days/wk) | 5.2 ± 1.6 |
| Cocaine use ($$/wk) | $388 ± 218 |
| Daily Cigarette Smokers (#) | 13 |
| Cigarettes/day (#) | 7.3 ± 2.2 |
| Alcohol drinkers (#) | 9 |
| Alcohol drinks/week (#) | 13.1 ± 15.4 |
Note: Data are presented as means (± standard deviation) or as frequency. Alcohol drinkers defined as ≥ 1 drink/week.
3.2. Self-administration
Figure 1 (top), portraying the number of cocaine doses self-administered as a function of modafinil dose, cost per cocaine administration, and cue/prime condition (n=16 participants), demonstrates that modafinil’s effects varied as a function of cost and condition. When cocaine was inexpensive ($5/dose), modafinil did not significantly reduce the number of doses purchased. When cocaine cost $10/dose, modafinil significantly decreased cocaine self-administration when no cues or prime were present [F(l,60 for all conditions)=31.01, p < 0.0001] and when the cue alone was present [F=10.55, p < 0.002], When cocaine cost $ 15/dose, modafinil significantly decreased cocaine self-administration only when cues alone were presented [F=9.57, p < 0.003].
Figure 1.
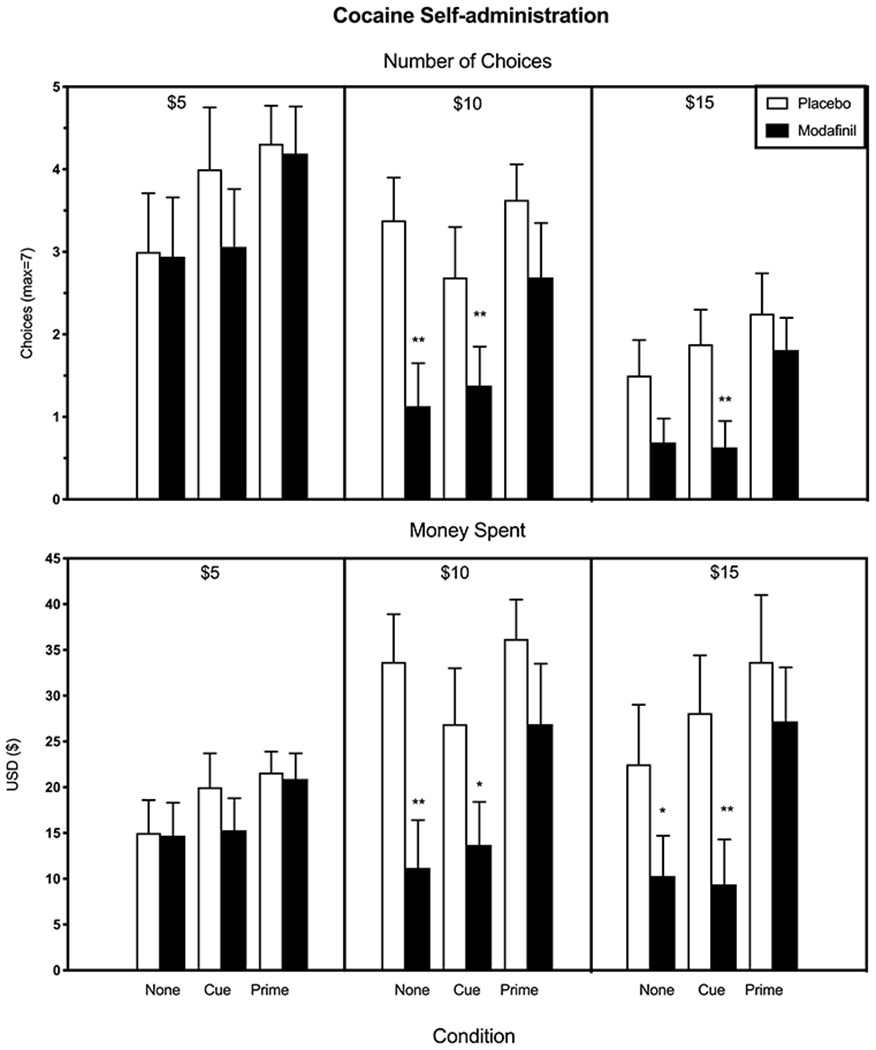
Mean number of cocaine choices (top) and amount of money spent on self-administration (bottom) as a function of modafinil dose, cost per cocaine administration, and cue/prime condition; each bar reflects data from all participants (n=16). Error bars represent + SEM. Asterisks denote a significant difference between active and placebo modafinil for each cue/prime and cost condition (*p < 0.01, ** p < 0.005). Note, chosen doses that were chosen and paid for were administered.
Figure 1 (bottom) portrays self-administration data in terms of the amount of money spent for cocaine as a function of modafinil dose, cost per cocaine administration, and cue/prime condition. Under the $5 cost condition, modafinil had no effect on the amount of money spent for cocaine relative to placebo. When cocaine cost either $10 or $ 15/dose, modafinil significantly decreased the amount of money spent on cocaine when no cues or prime were present [$10, F=25.01, p<0.0001; $15, F=7.34, p<0.01] and when cues alone were present [$10, F=8.51, p<0.008; F=17.37, p<0.0003], but had no effect if a noncontingent dose of cocaine had been administered.
3.3. Subjective Effects Measures
Figure 2 portrays cocaine’s abuse-related effects when cocaine (25 mg) was experimenter-administered 7 times. Modafinil had no significant effect on peak ratings of ‘Good Effect,’ cocaine craving or quality relative to placebo.
Figure 2.
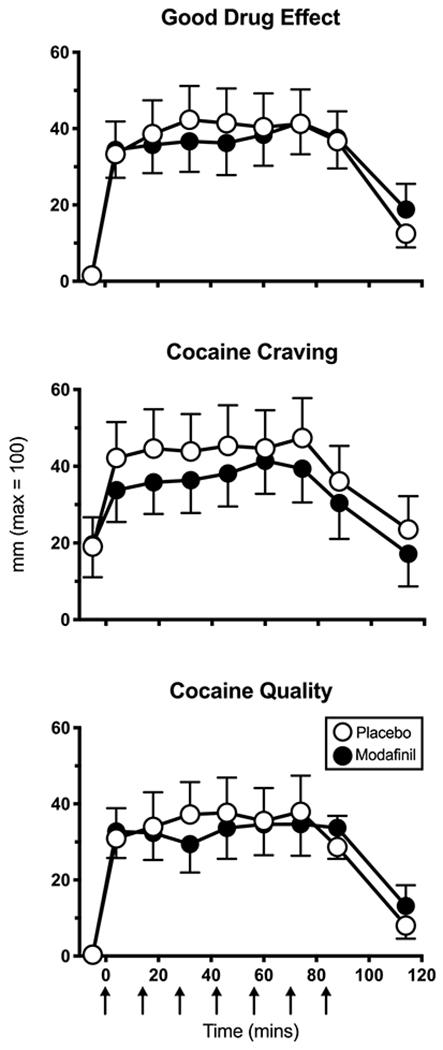
Mean visual analog scale ratings as a function of modafinil dose and time within session (n=16 participants). Arrows indicate time at which cocaine (25 mg) was administered.
3.4. Cardiovascular Measures
Relative to placebo, modafinil had no significant effect on peak heart rate, diastolic or systolic blood pressure during a session in which cocaine (25 mg) was experimenter-administered 7 times. During placebo maintenance, peak heart rate (bpm), systolic and diastolic pressure (mmHg) were, respectively: 101.5 ± 3.8, 146.4 ± 4.4, and 92.6 ± 3.9 (Mean ± SEM). During modafinil maintenance, peak heart rate, systolic and diastolic pressure were: 98.7 ± 3.2, 14.5.4 ± 5.3, and 88.1 ± 2.3.
3.5. Plasma Cocaine
Modafinil had no significant effect on cocaine plasma levels compared to placebo. During placebo maintenance, mean plasma cocaine levels were: 3.4 ± 0.8, 115.7 ± 12.0, 197.5 ± 20 and 447.7 ± 49.3 ng/ml at baseline, t4, t10 and t88 following the first cocaine administration. During modafinil maintenance, plasma cocaine levels were 2.7 ± 0.7, 77.3 ± 9.2, 163.3 ± 16.5 and 435.4 ± 48.8 ng/ml at these time points. Note, we were unable to reliably draw blood from 2 participants so plasma analysis was conducted in n=14.
4. Discussion
This study demonstrates that the effects of modafinil on cocaine self-administration consistently varied as a function of the conditions present when the decision to initiate or continue to use cocaine was made. Modafinil produced substantial (up to 70%) reductions in cocaine self-administration when cocaine was costly ($10, $ 15/dose), whether participants had been exposed to cues associated with cocaine or not. By contrast, modafinil had no significant effect on the choice to initiate or continue cocaine use relative to placebo when modafinil was relatively inexpensive ($5/dose) or if participants had received a single, noncontingent dose of cocaine before the decision to purchase more drug was made.
These findings parallel those from another study we conducted testing the same doses of modafinil and cocaine but using different strategies to model the real-world decisions cocaine users face (Foltin et al., 2016). There we asked if modafinil shifted cocaine self-administration when the response effort (i.e., keyboard presses) required to obtain cocaine was low (500 responses/dose) and participants had the opportunity to choose a low-value alternative to cocaine (2 opportunities to play a game for money) or when the response effort required for cocaine was large (2500 responses/dose) and the alternative to cocaine was more valuable (4 game plays for money). As in the present study, modafinil did not alter cocaine self-administration when the choice was ‘low cost,’ but decreased cocaine choice when the response effort for cocaine and the alternative value was comparatively high (Foltin et al., 2016).
These human laboratory findings may help to explain the mixed clinical findings to date. Modafinil was most effective at reducing cocaine use when participants had not yet used cocaine that day, suggesting that the medication might be more effective at reducing the likelihood that abstinent smokers will relapse to cocaine use, rather than in reducing cocaine use in patients who have not achieved abstinence, particularly under conditions in which cocaine is costly. In most clinical trials (e.g., Dackis et al., 2012), the majority of patients continue to use cocaine when medication is introduced. Although Schmitz and colleagues (2014) did not find that modafinil’s effects (400 mg/day) varied as a function of abstinence versus ongoing use, the authors acknowledge that the number of participants enrolled was small for a between-groups comparison, and medication compliance was estimated at 73%. Thus, the question of whether modafinil would best be used clinically as a medication to prevent relapse remains unanswered.
Variation in medication compliance is another factor almost certainly contributing to the mixed clinical effects of modafinil. In the human laboratory, where medication compliance is ensured, modafinil has consistently shown a positive signal. Similarly, in a clinical trial that showed positive outcome, Kampman and colleagues (2015) used contingency management procedures to achieve high medication compliance in patients, and they concluded that good compliance contributed to their positive outcome with modafinil relative to other clinical studies (e.g., Dackis et al., 2012; Schmitz et al., 2012).
In terms of potential mechanisms, modafinil reduced cocaine self-administration without modifying any other effects relative to placebo, e.g., cocaine craving or abuse-related subjective effects, e.g., ‘good drug effect,’ ratings of drug quality, cardiovascular outcomes, consistent with our earlier study (Foltin et al., 2016). Modafinil effects on these measures have varied, both in our laboratory and elsewhere, with some studies showing modafinil reduces certain abuse-related effects (see Introduction) while others do not. In this study, we assessed modafinil’s influence on abuse liability measures at an early phase of the study, shortly after achieving medication steady-state, and cocaine self-administration was measured in the subsequent two weeks. It may be that the temporal offset between the measurement of cocaine’s reinforcing and subjective effects explains why modafinil reduced cocaine self-administration without reducing cocaine’s abuse-related effects.
Modafinil has been shown to improve cognitive performance (working memory, attention, learning) and to decrease impulsivity in stimulant users (Canavan et al., 2014; Dean et al., 2011; Ghahremani et al., 2011; Kalechstein et al., 2013). Acute modafinil pretreatment (200 mg dose) has also been shown to decrease cocaine cue-induced activation of brain regions associated with motivation to self-administer drugs of abuse (ventral tegmental area) while increasing activation of areas associated with cognitive control (anterior cingulate cortex), suggesting that modafinil may facilitate treatment outcome by reducing drug cue reactivity and by improving decision-making about drug taking (Goudriaan et al., 2013). Although contextual cues did not increase cocaine self-administration relative to no cues in our model, modafinil may have improved decision making overall about drug taking, provided that a priming dose of cocaine was not administered prior to the decision to use cocaine. Cocaine may mitigate the effects of modafinil on decision-making regarding drug use.
Finally, another mechanism by which modafinil may have reduced cocaine self-administration is by normalizing cocaine-induced sleep abnormalities. Sleep was not measured in this study but cocaine abstinence has been associated with decreases in total sleep time, alterations in REM sleep and increased sleep latency, and modafinil (400 mg) administration in the morning has been shown to normalize sleep architecture and reduce daytime sleepiness (Morgan et al., 2010). Modafinil-induced improvements in slow-wave sleep mediated a higher rate of cocaine-free urines and were associated with more consecutive days abstinent in a clinical study, supporting improved sleep as a possible mechanism by which modafinil reduces cocaine use (Morgan et al., 2016).
The current study has several limitations. In clinical trials, modafinil appears more efficacious in reducing cocaine use in men than in women (Dackis et al., 2012). Few women were enrolled herein, a frequent problem for studies with lengthy inpatient phases, so our conclusions are limited to male cocaine users. Another issue related to the study design was the number of participants who did not complete the study, which did not reflect medication tolerability but rather the demanding procedures: Participants had to commit to two, 3-week inpatient phases, and medical and cardiovascular guidelines were strictly followed so the majority enrolled did not complete the study. Thus, our findings are limited to a physically healthier population of cocaine users than may be enrolled in a clinical trial (Foltin et al., 2016).
To conclude, self-administration of smoked cocaine dose with robust reinforcing effects (e.g., Haney et al., 2001, 2006, 2011) by non-treatment seeking, cocaine users is an exceedingly difficult behavior to disrupt (Foltin et al., 2016), and modafinil produced a larger magnitude effect on self-administration in this study than any other medication to our knowledge. The decision to use cocaine occurs in a complex environment containing both drug and nondrug reinforcers, and a recently published ‘postmortem’ on a large, failed clinical trial concluded that the predictive validity of preclinical and human laboratory models testing potential CUD pharmacotherapies would be improved if the choices faced by drug users were modelled (Negus and Banks, 2020). We suggest that our novel human laboratory design, which does model the complex choices faced by cocaine users, elucidates the conditions in which modafinil would be most efficacious clinically.
Clearly, developing an effective medication for CUD is urgent and challenging. There is a marked increase in cocaine overdose rates since 2010 (SAMHSA, 2018), and in the midst of the opioid crisis, more Black Americans die from cocaine overdose than opioid overdose (Shiels et al., 2018). Re-visiting medications that have shown mixed efficacy and considering their effects under carefully defined conditions is one way to address this problem (Brandt et al., In press). The FDA (2019) established strategies for reducing inter-patient variability in clinical trials in order to increase the likelihood that a medication can be shown to work for at least a subset of patients. Kampman (2019) suggested ensuring medication adherence and not enrolling patients with comorbid alcohol use disorder would improve modafinil’s efficacy. To this we add an additional factor: the conditions present when the decision to use cocaine is made. Maintenance on modafinil significantly reduced cocaine use if: (1) participants had not recently used cocaine, and (2) cocaine was available at a moderate to high financial cost. These results suggest that modafinil should be studied in a well-powered clinical trial as a relapse prevention approach, perhaps in concert with contingency management procedures (Schierenberg et al., 2012), in which the cost of drug use is integral to the treatment provided.
Highlights.
Modafinil has had mixed efficacy for treating cocaine use disorder
This study tested modafinil’s effects on cocaine self-administration under a range of conditions
Modafinil robustly reduced self-administration when cocaine was costly and no cocaine was ‘on board.’
Modafinil had little effect if cocaine was recently used or could be self-administered at low cost.
Modafinil may be most effective for preventing relapse rather than initiating abstinence
Acknowledgements:
The authors would also like to thank the nurses, physicians and research assistants working in the Marian W. Fischman Cocaine Research Laboratory, and appreciate the contributions of their fellow investigators: Drs. Suzette Evans, Stephanie Collins Reed, Gillinder Bedi, and Nehal Vadhan.
Role of Funding Source: This research was supported by NIDA RO1 DA023650: Haney. The research was also supported in part by Columbia University Medical Center’s CTSA grant no. UL1 RR024156 from NCATS-NCRR/NIH. Nothing to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No conflict declared.
References
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC 3rd, Elkashef AM, 2009. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend 104(1-2), 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Cheng WY, Levin FR, Mariani JJ, Raby WN, Nunes EV, 2010. A placebo-controlled trial of memantine for cocaine dependence with high-value voucher incentives during a pre-randomization lead-in period. Drug Alcohol Depend 111(1-2), 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, Nunes EV, 2006. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alcohol Depend 81(3), 267–274. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, Vosburg SK, Nunes EV, 2005. Utility of lead-in period in cocaine dependence pharmacotherapy trials. Drug Alcohol Depend 77(1), 7–11. [DOI] [PubMed] [Google Scholar]
- Brandt L, Chao T, Comer SD, Levin FR, In press. Pharmacotherapeutic Strategies for Treating Cocaine Use Disorder - What Do We Have to Offer? Addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavan SV, Forselius EL, Bessette AJ, Morgan PT, 2014. Preliminary evidence for normalization of risk taking by modafinil in chronic cocaine users. Addict Behav 39(6), 1057–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP, 2005. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology 30(1), 205–211. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Plebani JG, Pettinati HM, Sparkman T, O’Brien CP, 2012. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Journal of Substance Abuse Treatment 43(3), 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW, Rowan A, Poole S, White L, O’Brien CP, 2003. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend 70(1), 29–37. [DOI] [PubMed] [Google Scholar]
- Dean AC, Sevak RJ, Monterosso JR, Hellemann G, Sugar CA, London ED, 2011. Acute modafinil effects on attention and inhibitory control in methamphetamine-dependent humans. J Stud Alcohol Drugs 72(6), 943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW, 2002. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 159(4), 397–406. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M, Bedi G, Evans SM, 2016. Modafinil decreases cocaine choice in human cocaine smokers only when the response requirement and the alternative reinforcer magnitude are large. Pharmacology Biochemistry and Behavior 150-151, 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration Center for Drug Evaluation and Research, C.f.B.E.a.R., 2019. Guidance for industry. Enrichment strategies for clinical trials to support determination of effectiveness of human drugs and biological products. [Google Scholar]
- Ghahremani DG, Tabibnia G, Monterosso J, Hellemann G, Poldrack RA, London ED, 2011. Effect of modafinil on learning and task-related brain activity in methamphetamine-dependent and healthy individuals. Neuropsychopharmacology 36(5), 950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, Veltman DJ, van den Brink W, Dom G, Schmaal L, 2013. Neurophysiological effects of modafinil on cue-exposure in cocaine dependence: a randomized placebo-controlled cross-over study using pharmacological fMRI. Addict Behav 38(2), 1509–1517. [DOI] [PubMed] [Google Scholar]
- Haney M, 2009. Self-administration of cocaine, cannabis and heroin in the human laboratory: benefits and pitfalls. Addict Biol 14(1), 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Foltin RW, 2006. Effects of baclofen on cocaine self-administration: opioid- and nonopioid-dependent volunteers. Neuropsychopharmacology 31(8), 1814–1821. [DOI] [PubMed] [Google Scholar]
- Haney M, Rubin E, Foltin RW, 2011. Aripiprazole maintenance increases smoked cocaine self-administration in humans. Psychopharmacology (Berl) 216(3), 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Spealman R, 2008. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 199(3), 403–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW, 2001. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology (Berl) 155(4), 330–337. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW, 2008. Smoked Cocaine Self-Administration is Decreased by Modafinil. Neuropsychopharmacology 33(4), 761–768. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, 2000. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacology 14(1), 53–60. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Mahoney JJ 3rd, Yoon JH, Bennett R, De la Garza R 2nd, 2013. Modafinil, but not escitalopram, improves working memory and sustained attention in long-term, high-dose cocaine users. Neuropharmacology 64, 472–478. [DOI] [PubMed] [Google Scholar]
- Kampman KM, 2019. The treatment of cocaine use disorder. Science Advances 5(10), eaax1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman KM, Lynch KG, Pettinati HM, Spratt K, Wierzbicki MR, Dackis C, O’Brien CP, 2015. A double blind, placebo controlled trial of modafinil for the treatment of cocaine dependence without co-morbid alcohol dependence. Drug and Alcohol Dependence 155, 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miller GM, Fischman AJ, 2006. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther 319(2), 561–569. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Hensley-Simon M, Tahsili-Fahadan P, LaLumiere RT, Thomas C, Fallon RV, Kalivas PW, Aston-Jones G, 2014. Modafinil attenuates reinstatement of cocaine seeking: role for cystine-glutamate exchange and metabotropic glutamate receptors. Addict Biol 19(1), 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm R, Swayngim K, Donovan JL, DeVane CL, Elkashef A, Chiang N, Khan R, Mojsiak J, Myrick DL, Hedden S, Cochran K, Woolson RF, 2006. Modafinil and cocaine interactions. Am J Drug Alcohol Abuse 32(4), 577–587. [DOI] [PubMed] [Google Scholar]
- Mignot E, Nishino S, Guilleminault C, Dement WC, 1994. Modafinil binds to the dopamine uptake carrier site with low affinity. Sleep 17(5), 436–437. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS, 2008. Modafinil: A Review of Neurochemical Actions and Effects on Cognition. Neuropsychopharmacology 33(7), 1477–1502. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Angarita GA, Canavan S, Pittman B, Oberleitner L, Malison RT, Mohsenin V, Hodges S, Easton C, McKee S, Bessette A, Forselius E, 2016. Modafinil and sleep architecture in an inpatient–outpatient treatment study of cocaine dependence. Drug and Alcohol Dependence 160, 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott E, Pittman B, Stickgold R, Malison RT, 2010. Normalizing effects of modafinil on sleep in chronic cocaine users. Am J Psychiatry 167(3), 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Banks ML, 2020. Learning from lorcaserin: lessons from the negative clinical trial of lorcaserin to treat cocaine use disorder. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush CR, Kelly TH, Hays LR, Baker RW, Wooten AF, 2002. Acute behavioral and physiological effects of modafinil in drug abusers. Behav Pharmacol 13(2), 105–115. [DOI] [PubMed] [Google Scholar]
- Schierenberg A, van Amsterdam J, van den Brink W, Goudriaan AE, 2012. Efficacy of contingency management for cocaine dependence treatment: a review of the evidence. Curr Drug Abuse Rev 5(4), 320–331. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Rathnayaka N, Green C, Moeller FG, Dougherty A, Grabowski J, 2012. Combination of Modafinil and d-amphetamine for the Treatment of Cocaine Dependence: A Preliminary Investigation. Frontiers in Psychiatry 3(77). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Green CE, Stotts AL, Lindsay JA, Rathnayaka NS, Grabowski J, Moeller FG, 2014. A two-phased screening paradigm for evaluating candidate medications for cocaine cessation or relapse prevention: modafinil, levodopa-carbidopa, naltrexone. Drug Alcohol Depend 136, 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels MS, Freedman ND, Thomas D, Berrington de Gonzalez A, 2018. Trends in U.S. Drug Overdose Deaths in Non-Hispanic Black, Hispanic, and Non-Hispanic White Persons, 2000-2015. Ann Intern Med 168(6), 453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2018. Key substance use and mental health indicators in the United States: Results from the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 18-5068, NSDUH Series H-53). Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Verrico CD, Haile CN, Mahoney Iii JJ, Thompson-Lake DGY, Newton TF, De La Garza Ii R, 2014. Treatment with modafinil and escitalopram, alone and in combination, on cocaine-induced effects: A randomized, double blind, placebo-controlled human laboratory study. Drug and Alcohol Dependence 141, 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang GJ, Jayne M, Hooker JM, Wong C, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog-Torres K, 2009. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. Jama 301(11), 1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosburg SK, Hart CL, Haney M, Rubin E, Foltin RW, 2010. Modafinil does not serve as a reinforcer in cocaine abusers. Drug Alcohol Depend 106(2-3), 233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor J, 2013. Modafinil as a catecholaminergic agent: empirical evidence and unanswered questions. Front Neurol 4, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Bi GH, Yang HJ, He Y, Xue G, Cao J, Tanda G, Gardner EL, Newman AH, Xi ZX, 2017. The Novel Modafinil Analog, JJC8-016, as a Potential Cocaine Abuse Pharmacotherapeutic. Neuropsychopharmacology 42(9), 1871–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]


