Abstract
Refractive surgery refers to any procedure that corrects or minimizes refractive errors. Today, refractive surgery has evolved beyond the traditional laser refractive surgery, embodied by the popular laser in situ keratomileusis or ‘LASIK’. New keratorefractive techniques such as small incision lenticule extraction (SMILE) avoids corneal flap creation and uses a single laser device, while advances in surface ablation techniques have seen a resurgence in its popularity. Presbyopic treatment options have also expanded to include new ablation profiles, intracorneal implants, and phakic intraocular implants. With the improved safety and efficacy of refractive lens exchange, a wider variety of intraocular lens implants with advanced optics provide more options for refractive correction in carefully selected patients. In this review, we also discuss possible developments in refractive surgery beyond 2020, such as preoperative evaluation of refractive patients using machine learning and artificial intelligence, potential use of stromal lenticules harvested from SMILE for presbyopic treatments, and various advances in intraocular lens implants that may provide a closer to ‘physiological correction’ of refractive errors.
Subject terms: Surgery, Outcomes research
摘要
屈光手术是指矫正或减少屈光不正度数的任何手术。如今, 屈光手术已经超越了传统的手术方式, 以流行的激光原位角膜磨镶术或”LASIK”为代表(laser in-situ keratomileusis, LASIK)。新的角膜屈光技术如小切口角膜基质透镜取出术 (small incision lenticule extraction, SMILE) 避免了角膜瓣的产生和使用单一激光设备, 另外, 表面消融技术的进步使其的流行度回升。老花眼治疗方案也已扩展到涉及新的消融方式, 角膜内植入物和人工晶状体植入物等方面。随着屈光镜片更换的安全性和功效的提高, 具有更先进光学技术的人工晶状体植入物为精心挑选的患者提供了更多屈光矫正手术的选择。在这篇综述中, 讨论了2020年以后屈光手术可能的发展, 例如机器学习和人工智能用于屈光手术患者的术前评估, 从SMILE中获得的角膜基质透镜在老视治疗中的潜在应用, 以及人工晶状体植入物的各种进步可以提供更接近屈光不正的“生理性的矫正”。
Introduction
‘Refractive surgery’ encompasses any procedure that corrects refractive error, one of the leading causes of reversible visual impairment in the world [1]. It is now recognized that refractive surgery has signficant impact on quality of life and daily work, with benefits extending beyond spectacle independence [2]. Laser refractive surgery is recognized as an extremely effective and safe procedure for low to moderate levels of refractive error [3], with more than 99.5% achieving spectacle independence [4]. The US FDA run Patient-Reported Outcomes with laser in situ keratomileusis showed that, on average, 95% of patients were satisfied with their treatment [5].
Today, refractive surgery has evolved beyond the stereotypical ‘laser eye surgery’. Developments in femtosecond laser technology has led to the improvement of laser in situ keratomileusis (LASIK) and the birth of refractive lenticule extraction [6]. Novel refractive surgical implants have also been introduced, ranging from intracorneal to intraocular implants. However, with laser refractive surgery already achieving excellent clinical outcomes, it is often difficult to demonstrate that these newer procedures are superior to the established techniques [7].
Thus, the next frontier of refractive surgery challenges clinicians and scientists to achieve outcomes superior to the ‘traditional 20/20’, often used to depict ‘perfect’ uncorrected distance visual acuity (UDVA). Technologies have been developed to enhance preoperative assessments and imaging for better patient selection, there are now improved customized treatments to specifically correct ocular aberrations, and novel techniques to adapt to dynamic refractive changes in the eye such as presbyopia. In this review, we summarize the evolution of refractive surgery, which now ranges from keratorefractive procedures to refractive lens exchange. Each section will discuss historical development, recent advancements, and possible progress into the future beyond 2020.
Preoperative evaluation for refractive surgery
Traditionally, refractive surgery may be considered as two major sub-disciplines, which can be applied jointly in some cases to correct complex refractive errors: keratorefractive or intraocular lens (IOL)-based surgery. Keratorefractive surgery involves altering the corneal surface shape; while with IOL-based surgery, an IOL implant is added to the optical elements. Corneal topography provides an assessment of corneal surface shape, while wavefront analysis provides an assessment of image formation by the entire eye’s optical system. Conventionally, these investigations have been routinely used in preoperative evaluation for refractive surgery.
Placido-based curvature topographic systems are valuable tools in gauging the corneal curvature and refractive status, but do not directly portray the actual shape of the cornea [8]. Scheimpflug corneal tomography is a 3-D imaging technique that characterizes the anterior/posterior corneal surfaces, along with corneal thickness distribution. Preoperative assessment is important to exclude any contraindicated corneal conditions, while detection of subclinical keratoconus suspects is crucial to prevent iatrogenic postsurgical ectasia [9]. Integrating data derived from corneal topography, biometry, and wavefront analysis can also help clinicians validate decisions about customized refractive surgery treatments and IOL power selection.
Beyond corneal topography: biomechanics and high-resolution imaging
Recently, the addition of corneal biomechanics to corneal topography has been studied as a potential adjunct to preoperative evaluation for keratorefractive procedures. The corneal visualization tonometer (Corvis ST, Oculus Optikgeräte GmbH; Wetzlar, Germany) uses an ultra-high speed Scheimpflug camera that visualizes corneal changes during deformation to produce various parameters [10]. The Pentacam HR topography and Corvis ST biomechanical parameters were then analysed together using different artificial intelligence methods [11]. A tomographic and biomechanical index may provide greater accuracy for detecting subclinical keratoconus among eyes clinically deemed to have ‘normal topography’ [11].
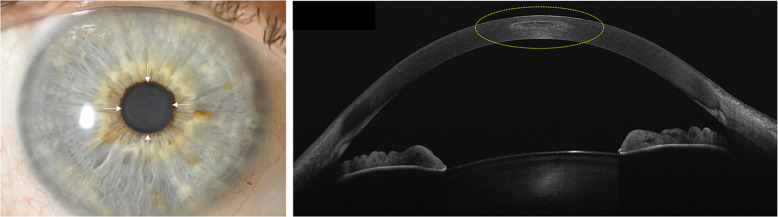
High-resolution swept-source optical coherence tomography (SS-OCT) provides anterior segment imaging and measurements in a single platform [12]. Newer OCT platforms capture corneal topography and tomography, anterior segment metrics, axial length measurement, and IOL calculation—Fig. 1 [13]. With OCT biometry, the ocular measurement can be combined with high-resolution macular scans for simultaneous screening for macular pathology [14]. In addition, corneal epithelial thickness measurements may have a role in planning for refractive surgery; or identification of early keratoconus through identification of focal epithelial thinning usually associated with areas of corneal steepening [15]. Very high-frequency ultrasound corneal analysis and high-resolution OCT can also generate epithelium thickness maps (Fig. 2) [16].
Fig. 1. High-resolution swept-source optical coherence tomography imaging of the anterior segment of a pseudophakic eye.
High-resolution swept-source OCT imaging of the anterior segment of a pseudophakic eye in which after piggy-back implantation of a sulcus lens to fix a refractive surprise after uneventful cataract surgery (ANTERION, Heidelberg Engineering GmbH, Heidelberg, Germany).
Fig. 2. Precision high-frequency ultrasound device (50 MHz) imaging of the cornea.
Ultrasound imaging can accurately image behind the iris allowing for improved ICL sizing. In addition to measurements of the anatomic structures comprising the anterior of the eye such as anterior chamber, the instrument can delineate the corneal epithelial layer thickness (ArcScan Insight 100, ArcScan Inc, Golden, USA).
Aberrometry and wavefront sensing
The development of new instrumentation to measure human optical aberrations and the recent refinements in the excimer laser delivery systems have opened a new era in vision correction: patient-customized, wavefront-guided treatment. Modern aberrometers are equipped with a corneal topographer system to compute the effect of the anterior and posterior corneal contribution to the ocular wavefront, and by subtraction, the effect of internal optics (the crystalline lens, or an IOL in pseudophakia). As described later, aberrometry data can be used to generate custom wavefront-guided ablation profiles procedures that aim to correct both the spherocylindrical refraction and higher-order aberrations (HOAs). Wavefront aberrometers measure only monochromatic aberrations whereas our eyes can see a polychromatic world. In the future, the discrepancy between the measured monochromatic wavefront and the actual polychromatic wavefront may help to find the precise amount of HOAs to correct. The ideal flat wavefront for high fidelity may be optimal for young patients with intact accommodative abilities, whereas adjusted-shape designed to increase the depth of focus may be preferable for some presbyopic patients [17]. The functional needs of the patient will have to be taken into consideration to truly optimize wavefront refractive surgical strategies, and adaptative optic capabilities will certainly have to be accessible to achieve these tasks [18]. A new aberration series has been proposed to better fit the low- and higher-order components of the wavefront. This new basis may quantify the aberrations more accurately and provide clinicians with coefficient magnitudes which better underline the impact of clinically significant aberration modes [19].
Cataract assessment using lens densitometry
Objective assessments to quantify cataract are becoming indispensable for modern cataract surgeons, especially to reliably assess early- to moderate-stage cataracts, and documenting cataract progression. Various clinical classifications, such as the subjective Lens Opacification Classification System (LOCS III), have limited use in assessing cataract density. Objective methods such as Scheimpflug imaging (Pentacam, Oculus Optikgeräte GmbH) and double-pass aberrometry (Optical Quality Analysis System, Visiometrics SL) have been shown to be repeatable and reliable methods to grade lens density [20]. Artal et al. have shown that an OSI of 1 or greater corresponds to lens opacification in the absence of ocular surface, corneal disease, or retinal disease [21]. Another device that can assess lens density is the IOLMaster 700 (Carl Zeiss Meditec AG), which is based on SS-OCT. Assessing cataract density using this SS-OCT technology has been reported to be reliable, using either a 2-dimensional (2-D) analysis based on a single B-scan centered on the vertex, or an automated global 3-D analysis (Fig. 3) [22].
Fig. 3. Average lens density (ALD) quantification with swept-source optical coherence tomography (SS-OCT).
Six radial B-scans including the lens were acquired three times at each meridian (0, 30, 60, 90, 120, and 150 degrees). An algorithm measured the density of the region of interest on a scale of 0–255 pixel intensity units. In a recent study, an SS-OCT ALD measurement of 73.8 pixel units or greater strongly suggested the presence of cataract, with a sensitivity of 96.2% and a specificity of 91.3%.
Beyond 2020: preoperative assessment for refractive surgery: machine learning
Machine learning is a general technique to find appropriate parameters or functions to classify input data from large amounts of training data. Many methodologies to implement machine learning, such as support vector machines, decision trees, or neural networks, have so far been advocated. Deep learning is one of the machine learning techniques dealing with the training of multilayer artificial neural networks. In recent years, multilayered neural networks, especially convolutional neural networks, have achieved impressive results in many types of image classifications in many scientific fields. Several studies have reported on the sensitivity and the specificity of keratoconus detection using machine learning [23–27]. In order to discriminate keratoconus from normal corneas these studies used either topographic numeric indices measured with a Placido disk-based corneal topographer, or tomographic numeric indices measured with a scanning slit tomographer and a rotating Scheimpflug camera, for machine learning. Deep learning using the whole image of six corneal color-coded maps (anterior elevation, anterior curvature, posterior elevation, posterior curvature, total refractive power, and pachymetry map) with the anterior segment OCT has also been performed to determine the diagnostic accuracy or the grade of keratoconus.
Cataract surgery is one of the most frequently performed surgeries in the world, and is increasingly a refractive as well as a rehabilitative procedure. IOL power calculations are undergoing continual improvements, with the latest generations’ formulas have shown promising precision and less refractive surprises compared with second and third generations’ formulas [28]. Accurate estimation of the effective lens position (ELP) is widely considered the main limiting factor in refractive predictability, and classical IOL power calculation formulas rely on a thin-lens model to calculate the IOL power with the use of 2–7 biometric parameters to predict ELP [29]. Most available formulas are based on optics, regression calculations or ray tracing. Supervised machine learning can create classification or regression models using algorithms trained from data without relying on prior assumptions. The Hill-Radial Basis Function and the Kane Formula belongs to this category. Currently, the results of these methods do not routinely exceed that of classic formulas [30], but a recent article showed that the combination of theoretical optics, regression, and artificial intelligence components could achieve better results [31].
Keratorefractive surgery
Keratorefractive essentially involves treating refractive errors by reshaping the cornea—traditionally with an excimer laser, but now possible using only a femtosecond laser via refractive lenticule extraction [32]. The evolution of keratorefractive surgery began with surface ablation techniques such as photorefractive keratectomy (PRK) that involves epithelial removal [33], or laser epithelial keratomileusis (LASEK) where 20% alcohol is used to displace the corneal epithelium [34]. The detached epithelial sheet was initially preserved to reduce inflammation and pain, but later techniques involved removal as the alcohol was found to affect its vitality [35]. More recently, excimer laser ablation has been used to remove the corneal epithelium directly i.e. transepithelial PRK [36]. One advantage of transepithelial PRK is that the epithelial layer removal and excimer is performed at the same time—although most reports suggest that healing time and visual outcomes results do not vary greatly amongst various techniques of epithelium removal [37, 38]. Surface ablation has regained popularity over the past few years due to the safety of the surgery and better biomechanics [39], especially in patients with high myopia and thin corneas [40, 41]. While the refractive predictability of surface ablation is comparable with LASIK, myopic regression may be more common after surface ablation [42]. Moreover, scarring and haze can occur from the healing response in the Bowman’s layer and anterior corneal stroma [43]. Low-dose topical mitomycin-C (0.02–0.04%) is usually applied after excimer laser to reduce haze formation [44, 45]. Nonetheless, patients may still experience more discomfort after surface ablation compared with LASIK, due to the healing of the epithelium [46].
Laser in situ keratomileusis (LASIK)
While LASIK corneal flaps were previously created using an oscillating microkeratome [47], the addition of femtosecond lasers greatly reduced the risk of some of the more significant flap complications such as buttonhole, free cap, and irregular cuts [48]. One of the other main benefits of femtosecond lasers was the improved reproducibility of flap thickness, which enabled the use of thinner flaps with increased safety. Flap thickness reproducibility is an important factor for residual stromal thickness (RST) safety planning as a thicker than intended flap can lead to a lower than predicted RST and risk of ectasia—Fig. 4. The standard deviation of central flap thickness from older microkeratomes was reported to be in the range of 20–40 μm [47, 49], compared with current femtosecond flap thickness reproducibility of less than 5 µm [50, 51].
Fig. 4. Central flap thickness reproducibility comparing microkeratome and femtosecond laser.
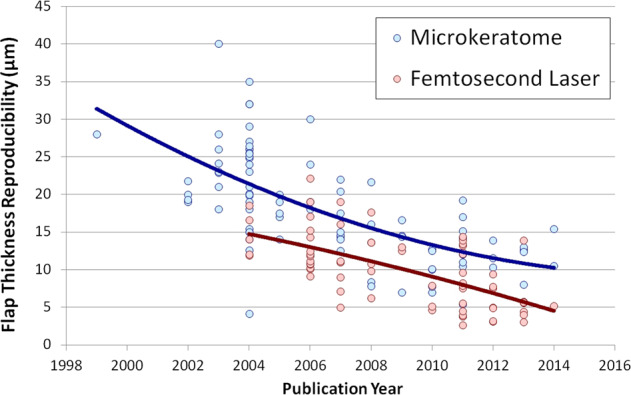
Graph showing the central flap thickness reproducibility for all studies published between 1998 and 2014, grouped by mechanical microkeratome (blue) and femtosecond laser (red).
A review of LASIK outcomes was reported on by Sandoval et al. [4] in 2016. The authors reviewed articles published between 2008 and 2015 representing more than 67,000 eyes. They found UDVA was 20/40 or better in 99.5% of eyes, spherical equivalent refraction was within ±1 diopter (D) of target in 98.6% of eyes, and loss of 2 or more lines of corrected distance visual acuity (CDVA) was 0.61%. Subjectively, patients were very satisfied with only 1.2% of patients reporting to be dissatisfied with the procedure. Within this analysis were treatments that were performed as far back as 2008. Therefore, some of the lasers that contributed to these outcomes would no longer fall into the “modern laser” category in 2020, so we can only expect future reviews to show even better outcomes.
Despite these excellent outcomes, it is still important to counsel patients on the occasional suboptimal effects, such as increased glare and haloes, residual refractive error or irregular astigmatism [52]. Dry eye is one of the most common side effects, which is usually temporary and may be managed with topical lubricants in most cases [53]. Postoperative flap-related complications include flap displacement, diffuse lamellar keratitis (DLK) [54], or epithelial ingrowth [55], all of which may be treated with topical eye drops or in some cases may require laser treatment or flap-lift [56]. Rarely, corneal ectasia can still occur, which has greatly reduced with the advent of more accurate preoperative imaging and assessments as already described [57].
Advances in excimer laser and wavefront-guided treatments
Both LASIK and surface ablation techniques rely on the excimer laser to reshape the cornea, which were initially based on a spherical shape as in the Munnerlyn formula. Aspheric profiles were first tested by Seiler et al. [58] who demonstrated significantly less induction of spherical aberration and glare. The introduction of flying spot lasers and a Gaussian beam profile further improved outcomes. Laser frequency has also been increasing over the years, which has reduced ablation time and the impact of corneal dehydration. O’Brart et al. [59] showed that increasing optical zone diameter decreased the impact of night vision disturbances, so modern lasers use large optical zones and improved transition zones [60].
As described above, another advance was the use of aberrometry measurements to treat naturally occurring HOAs [61–63]. However, wavefront-guided treatments did not eliminate residual HOAs, but did slightly reduce the induction of spherical aberration [64]. Wavefront-optimized treatments shifted the aim of the treatment to the control of spherical aberration, but can have variable effects on other HOAs [65, 66]. An alternative method for custom treatments is corneal topography-guided laser ablation [67], most useful where the refractive error of the eye matches its corneal topography i.e. most of the aberration is produced by the cornea [68]. Currently, excimer lasers with active eye tracking systems to compensate for cyclotorsion and micro-saccadic eye movements are already considered common standards of care for such treatments [69].
Beyond 2020: keratorefractive surgery for presbyopia correction
Traditionally, the principles used for monovision have been applied to keratorefractive surgery [70, 71], to provide patients with good distance and reading vision with high patient satisfaction [70, 72]. However, careful patient selection is required, with the loss of fusion and stereoacuity leading to poor acceptance as a potential outcome [73, 74].
Another option of keratorefractive surgery for presbyopia correction is to create a ‘multifocal cornea’. The majority of corneal multifocal treatments essentially creates a “central island” to provide near vision, while a hybrid combination of multifocality with some induced anisometropia may have improved safety [75, 76]. However, some studies using this hybrid protocol report an unacceptable rate of loss of two lines of CDVA [77]. Therefore, caution must be used when treating the cornea with any multifocal laser ablation profile because the change in optical quality can increase the risk of losing lines of CDVA in poorly selected candidates. Suggested selection criteria include low hyperopia (up to +3 D) or myopia (up to −4 D), low astigmatism, a maximum requirement of +2 near vision add and photopic pupillometry of less than 3.5 mm [78].
Recently, the application of extended depth of field in keratorefractive surgery has come from the research on the use of spherical aberration to increase the depth of field [79, 80]. Laser blended vision (LBV) is based on nonlinear changes in asphericity. LBV is tolerated by more than 95% of patients [81–83], compared with monovision which is tolerated by only between 59 and 67% of patients [84]. Because it is not a multifocal treatment, LBV has also been shown to provide good distance, intermediate, and near vision without the increased risk for losing lines of corrected visual acuity [81, 82, 85].
Small incision lenticule extraction (SMILE)
Following the introduction of the VisuMax femtosecond laser (Carl Zeiss Meditec, Jena, Germany), an all femtosecond laser, keyhole, flapless procedure was developed, referred to as SMILE. The SMILE procedure involves using a femtosecond laser to delineate a refractive lenticule within the stroma connected to the surface by a small incision—Figs. 5 and 6. The femtosecond interfaces are surgically separated and the refractive lenticule is removed through the small incision. SMILE brings two main advantages over LASIK: faster dry eye symptom recovery and better spherical aberration control [86–88]. Both of these advantages stem from the minimally invasive pocket incision that results in maximal retention of anterior corneal innervation as well as structural integrity. The evidence for reduced dry eye is supported by studies on corneal nerve regeneration [89], and recovery of corneal sensitivity [90].
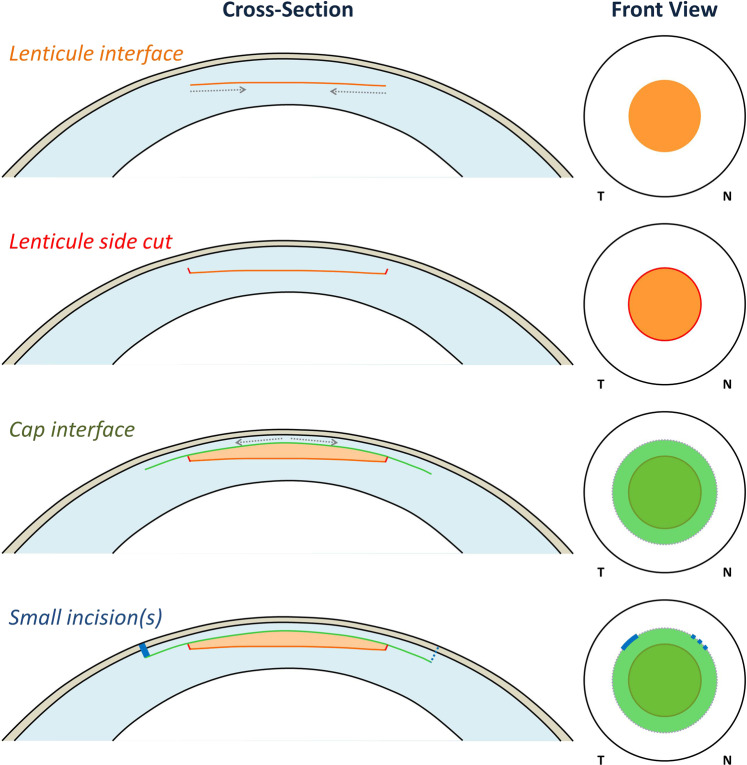
Fig. 5. Series of diagrams showing the femtosecond cutting sequence for a SMILE procedure.
(1) lenticule interface from out-to-in, (2) lenticule side cut, (3) cap interface from in-to-out, and (4) the small incision(s). Reprinted with permission from Reinstein et al. [117].
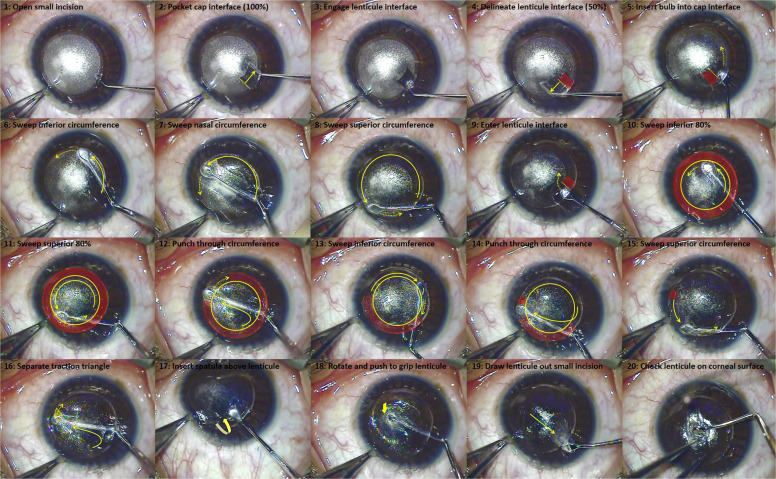
Fig. 6. Series of images showing the standard surgical technique for SMILE.
Reprinted with permission from Reinstein et al. [117].
Biomechanically, SMILE offers a theoretical advantage over LASIK by preservation of the stronger anterior stromal lamellae. Randleman et al. [91] and Scarcelli et al. [92] demonstrated that the strength of the stroma decreases from anterior to posterior within the central corneal region. Petsche et al. [93] found a similar result for transverse shear strength to decrease with stromal depth. Applying this knowledge to SMILE, since the anterior stroma remains uncut, the strongest part of the stroma continues to contribute to the strength of the cornea postoperatively. This has been evaluated using a theoretical [94], finite element modeling [95, 96], and laboratory experiments [97]. The clinical effect is less induction of spherical aberration compared with LASIK [98]. Therefore, it is possible to increase the optical zone diameter with SMILE, further reducing the spherical aberration induction, without compromising the corneal biomechanics compared with the equivalent LASIK treatment [99].
Refinements to SMILE
As SMILE has gained popularity, nearly every aspect of the treatment has been optimized. Initially, the main weakness was the slightly delayed visual recovery relative to the overnight ‘wow’ effect associated with LASIK. However, detailed research into the energy level and spot/track spacing has significantly improved visual recovery, without compromising the ease of lenticule separation [100–104]. Most published results suggest that SMILE is safe, effective, and predictable for treating moderate myopia and modest levels of astigmatism [105], with postoperative visual outcomes comparable with femtosecond LASIK [106, 107]. Vision-related quality of life has also been found to be comparable between SMILE and LASIK [108–110].
Suction loss is the most common complication for SMILE, with an incidence of about 0.50% [111–113]. However, there is a clear management protocol for this, further guided by a decision tree by Reinstein et al. [111, 112]. Thus, it is possible to complete the treatment on the same day (continuing with SMILE or converting to LASIK) without affecting the visual or refractive outcome [111, 112]. Postoperative complications of SMILE are essentially the same as LASIK, however there are two areas where some small differences have been identified. The first is with DLK where a unique appearing sterile multifocal inflammatory keratitis can present after SMILE, which needs to be aggressively treated [114]. The second area is epithelial ingrowth, which can be more common due to the instrument implanting epithelial cells within the interface by the instruments through the small 2-mm incision. This can be successfully treated by using a Nd:YAG laser or washing out the interface. Finally, a number of options for retreatment or enhancement after SMILE have been developed, including surface ablation, converting the cap in to a flap via side cut or Circle [115], and thin flap LASIK [116]. SMILE is now a mature and established procedure [117] that provides patients with a safe and effective outcome with current reports demonstrating that the visual and refractive outcomes are similar to LASIK [87, 118–120].
Beyond 2020: stromal lenticule implantation
The increasing popularity of SMILE is providing surgeons with thousands of human donor stromal lenticules that could potentially be used for the treatment of presbyopia [7, 121], hyperopia [9, 122, 123], and corneal ectatic diseases, such as keratoconus (Fig. 7) [124, 125]. The greater precision of the femtosecond laser allow for more accurate stromal lenticule creation, and may offer advantages over commercialized synthetic inlays in the aspect of biocompatibility, retaining nutrient flow within the stroma and reduced risk of extrusion. On the other hand, these biological inlays have a low but potential risk for rejection, while subject to eye banking and corneal transplantation regulations for donor quality and safety. The preoperative decellularization of these donor lenticules may reduce the risk of rejection [4, 124, 126, 127].
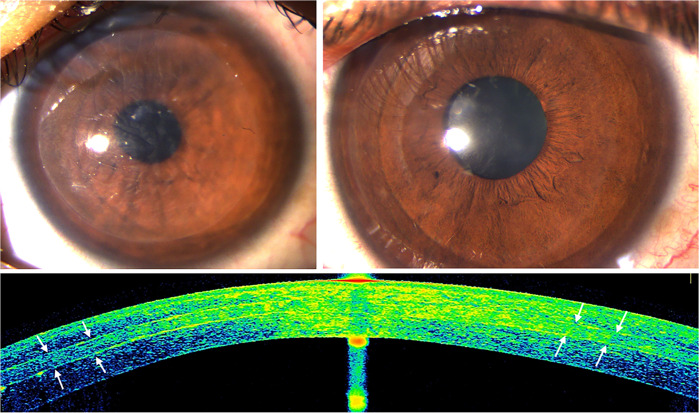
Fig. 7. Corneal stroma enhancement with a decellularized corneal stroma lenticule in a patient with advanced keratoconus.
Slit-lamp pictures 1 week (top-left) and 3 months after surgery (top-right; note the complete transparency recovery). Corneal OCT image (down): the implanted lenticule is easily identified 6 months after surgery (white arrows).
Preliminary human clinical results suggest biocompatibility, safety and long-term transparency of these implants in vivo [121–125]. However, one of the main limitations is the unpredictability of the refractive outcome, which is dependent on the stromal remodeling of both the inlay and the recipient stroma, leading to significant undercorrections [6, 123, 128, 129]. Further studies with larger samples, longer follow-ups, technique refinements, and treatment nomograms are required. On the other hand, encouraging results are being reported for advanced keratoconus, where a precise refractive outcome is not the target, but refractive stability may delay the need for corneal transplantation (Fig. 7) [124, 125]. Stromal lenticules (either plano or negative meniscus shape) and allogenic stromal ring segments have been used in clinical trials for keratoconus showing a moderate improvement in all visual, refractive and keratometric parameters [31, 37, 38].
Intracorneal implants
In 1949, José Ignacio Barraquer, described the “thickness law”, forming the basis for intracorneal implants leading to a hyperopic or myopic shift [130]. Keratophakia was described in 1964 as a lamellar refractive surgery procedure for the treatment of hyperopia and presbyopia, but abandoned due to interface scarring and unpredictable refractive results [131]. However, this led to the development of synthetic intracorneal implants known today as “inlays”. Early corneal inlays (made of polymethyl-methacrylate-PMMA or polysulfone) were associated with loss of transparency, corneal thinning or melting, and implant extrusion due to interruption of nutrient flow within the stroma [132, 133]. This critical limitation was partially overcome with the development of intracorneal ring segments (ICRS), new synthetic inlays with perforated designs, and new hydrogel biomaterials permitting the exchange of nutrients, such as glucose and oxygen within the corneal stroma [132, 134, 135]. Today, intracorneal implants for the treatment of myopia and astigmatism have been superseded by keratorefractive surgery. Intracorneal rings are made of inert, biocompatible synthetic materials that are implanted deep into the stroma to modify the corneal curvature and regularize its shape to reduce the refractive error—Fig. 8 [136]. Their capability to flatten the central cornea, reduce keratometric values, and corneal astigmatism, have made ICRS an important therapeutic approach for the visual rehabilitation of keratoconic eyes. However, the low refractive predictability and significant risk of losing corrected vision have caused ICRS to be abandoned as a purely refractive option in non-pathologic eyes [137].
Fig. 8. Slit-lamp photograph of an eye with intracorneal implants.
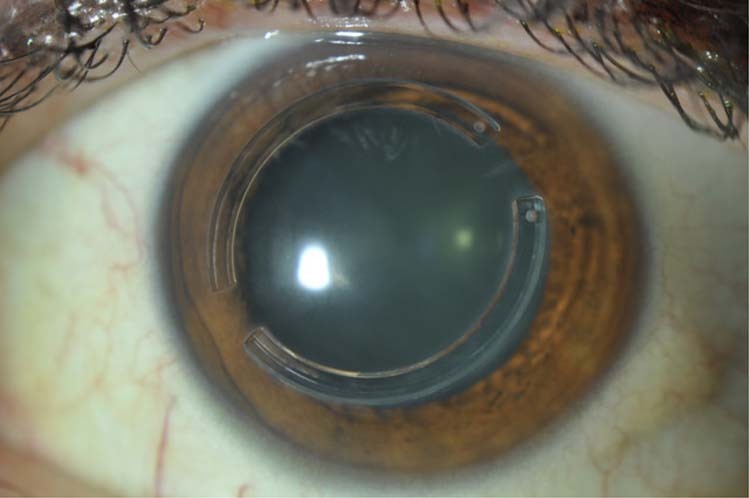
Intracorneal ring implanted in a patient with keratoconus.
Presbyopia corneal inlays
Corneal inlays have several theoretical advantages: there is no corneal tissue removal, it is minimally invasive, and can be explanted [138, 139]. There are three types of corneal inlays [138]: corneal reshaping inlays to reshape of the anterior corneal curvature, leaving a multifocal cornea; refractive inlays where there is a modification on the refractive index of the cornea with a bifocal optic; and small aperture inlay which improves the depth of focus. The technical specifications of these various inlays are summarized in Table 1. Presbyopia inlays are implanted in the nondominant eye, centered on the first Purkinje reflex within a corneal pocket or under a stromal flap [133]. The implantation depth depends on the inlay: those that alter the curvature of the cornea are implanted more superficially, while those with a small aperture or a different refraction index are implanted deeper to reduce anterior corneal curvature changes and to allow a proper diffusion of nutrients within the corneal stroma [133, 139]. Outcomes from various significant clinical trials of these corneal inlays are summarized in Table 2.
Table 1.
Technical specifications of commercially available presbyopia inlays.
| Inlay | Material | Type of inlay | Measurements | Mechanism of action |
|---|---|---|---|---|
| Raindrop |
Biocompatible hydrogel 80% water |
Corneal reshaping inlay |
Thickness of 10 µm at the periphery, and 32 µm at the center Diameter: 2 mm |
Reshapes the anterior cornea, creating a hyper-prolate region → multifocal cornea |
| Flexivue | Clear copolymer of hydroxyethyl methacrylate and methyl methacrylate with an ultraviolet blocker | Refractive inlay |
Thickness 15–20 µm Diameter: 3 mm |
The central 1.8-mm diameter of the disc is plano in power (for distance vision) and the peripheral zone has an add power which ranges from +1.25 D to 3.0 D in 0.25 D increments (for near vision) |
| Icolens | Copolymer of hydroxyethyl methacrylate and methyl methacrylate | Refractive inlay |
Thickness: 15 µm Diameter: 3 mm |
Central zone for distance and peripheral positive refractive zone for near |
| Kamra | Polyvinylidene fluoride, nanoparticles of carbon | Small aperture inlay | Thickness: 5 µm Diameter: 3.8 mm | Increases the depth of focus through the principle of small aperture optics |
Table 2.
Studies evaluating the clinical outcomes of the different available presbyopia inlays for the correction of presbyopia in emmetropic patients (those studies performing a concomitant myopic or hyperopic ablation have not been included).
| Author | Year | Inlay | Technique | Eyes | Follow-up (months) | Efficacy | Safety | Patient dissatisfaction | Explant rate | Financial interests |
|---|---|---|---|---|---|---|---|---|---|---|
| Garza et al. [132] | 2013 | Raindrop | Flap | 20 | 12 | 100% UNVA ≥ 20/40 | 42% lost 1 line of CDVA 68% lost 1 line of CNVA | 5% | 5% | Yes |
| Yoo et al. [142] | 2015 | Raindrop | Flap | 22 | 6 | Mean UNVA of 20/35 | 32% showed a decreased postop UDVA | 18% | Non reported | No |
| Whitman et al. [185] | 2016 | Raindrop | Flap | 373 | 12 | UNVA improved by 5.1 lines 93% UNVA ≥ 20/25 |
UDVA decreased by 1.2 lines CDVA decreased by half of 1 line |
8% | 3% | Yes |
| Malandrini et al. [133] | 2015 | Flexivue | 81 | 36 | UNVA improved by 6 lines | 8% lost >1 line of CDVA | 9% still required reading glasses | 7.4% | Yes | |
| Beer et al. [186] | 2019 | Flexivue | 30 | 36 | UNVA: 76.9% in J1 (from 87.1% at the 1-year postop) | 16.1% lost >3 lines of CDVA at 1 year (stable thereafter) | 26.7% (from 9.7% at the 1-year postop) | 13.3% | Yes | |
| Baily et al. [144] | 2014 | Icolens | 52 | 12 | 17% UNVA ≥ 20/40 | 77% lost ≥1 lines of CDVA | 10% | 21.1% | No | |
| Seyeddain et al. [187] | 2012 | Kamra | Flap | 32 | 36 | 97% UNVA ≥ 20/40a | 31.4% lost ≥1 lines of CDVA | 15.5% | 0% | Yes |
| Vukich et al. [145] | 2018 | Kamra | Flap/Pocket | 507 | 36 | UNVA improved by 3.3 lines 72.4% UNVA ≥ 20/32 | UDVA decreased by 0.4 lines CDVA was reduced by no more than 1 letter | 5.5 (1–7 scale; 7 being “very satisfied”) | 8.7% | Yes |
Efficacy is defined as mean postoperative monocular uncorrected near visual acuity (UNVA) in the implanted eye; safety is defined as percentage of eyes losing lines of monocular corrected distance visual acuity (CDVA) in the implanted eye.
UDVA uncorrected distance visual acuity, UDVA uncorrected distance visual acuity, J Jaeger.
aBinocular visual function, as monocular one was not available.
The RaindropTM (ReVision Optics Inc., Lake Forest, CA, USA) corneal reshaping inlay is made of a biocompatible hydrogel material with 80% water to allow the passage of nutrients within the corneal stroma (Fig. 9 left) [138–140]. It has no refractive power, formed by smoothly transitioning regions that provide near vision in the steepest central area, intermediate vision around this central area, and distance vision in the periphery that is marginally affected by the inlay [138–140]. Despite most patients being satisfied, 7.8% of eyes required inlay removal due to discontent with the visual outcome [141]. Other complications included marked glare (2.1%) or halos (4.1%) even one year after surgery; flap-related dry eye syndrome (4.7%), and inlay-related central corneal haze (14%)—Fig. 9 right. The Raindrop implant was discontinued from the market in January 2018 due to the evidence of late haze with loss of CDVA in clinical practice [132, 142].
Fig. 9. Raindrop inlay.
Slit lamp (left; white arrows point the edges of the inlay) and anterior segment optical coherence tomography (OCT) pictures (right). Observe how the thin inlay is seen in the OCT image and it appears surrounded by a mild stromal haze (yellow dashed line).
The Flexivue MicrolensTM (Presbia Cooperatief U.A., Amsterdam, Netherlands) and IcolensTM (Neoptics AG, Huenenberg, Switzerland) are bifocal inlays with a central 0.15 mm opening to facilitate the transfer of nutrients and oxygen through the cornea, implanted into a corneal pocket at 280–300 µm depth in the nondominant eye [133, 143, 144]. Light rays passing through the central zone of the inlay that does not have refractive power will be sharply focused for distance vision, while the refractive peripheral zone focus light rays on the retina for near vision (Table 1) [133, 143]. Available scientific evidence with these inlays is far more limited, with monocular reduction of UDVA, loss of contrast sensitivity and a significant frequent loss of CDVA reported [4, 16–18].
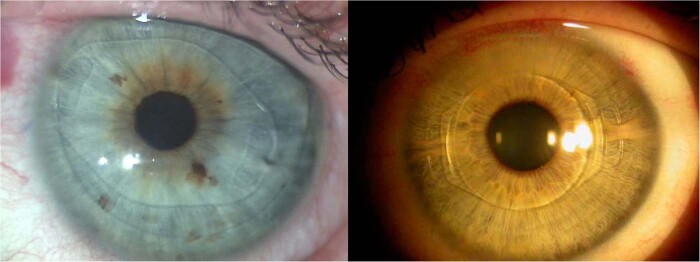
The Kamra VisionTM (Acufocus Inc., Irvine, CA, USA) is the most widely used corneal inlay, with nearly 20,000 inlays implanted worldwide [139, 140]. It has a central 1.6 mm aperture, and 8400 microperforations (5–11 µm in diameter) in the peripheral opaque ring to allow nutritional flow through the cornea (Fig. 10 left) [138–140]. However, as it is an opaque inlay it may be vey obvious in light-colored eyes. It improves near vision by increasing the depth of focus through the principle of small aperture optics (blockage of the peripheral unfocused rays of light) [139, 140]. It is usually implanted into a 6 × 6 mm diameter stomal pocket and 200–270 µm depth in the nondominant eye. A prospective, multicenter clinical trial (507 eyes with emmetropic presbyopia and 3-year follow-up) reported an average 3.3-line improvement in UNVA, 1-line improvement in UIVA, and 0.4-line reduction in UDVA on the implanted eye, while no loss in binocular distance vision was observed [19]. Despite the opaque nature of this inlay, no scotomas in the visual field have been observed, with a mean reduction of ~1 dB in contrast sensitivity [138, 145]. 8.7% of eyes required inlay removal due to dissatisfaction with the visual outcome. Other complications included significant glare (19%), halos (25%) night vision problems (30%), and inlay-related central corneal haze (2.8%)—Fig. 10, middle.
Fig. 10. Kamra inlay.
Slit-lamp pictures 3 months (left; observe the peripheral microperforations to allow corneal nutrition) and 3 years after implantation (middle). Note the progressive moderate haze associated with visual loss that justified inlay explantation, remaining a donut-shape central corneal scar still visible 4 years after inlay removal (right).
Beyond 2020: future of intracorneal implants and corneal inlays
Corneal inlays have proven to be an effective alternative for presbyopia management. However, the future of inlays beyond 2020 looks uncertain. Despite clinical investigation for more than 15 years, they have still not gained full popularity among refractive surgeons due to the frequent problems of centration, biological intolerance, and optical performance, causing a relatively high explantation rate over time secondary to late complications such as corneal stromal opacities, late hyperopic shift or inadequate visual performance [26]. The most promising of inlays remain the Kamra implant where it was observed in that UNVA, refractive stability, patient satisfaction, haze risk, and explantation rate significantly improved when the Kamra was implanted inside a lamellar pocket (and not a flap). This stromal pocket was created with a femtosecond laser using tight spot-line separation settings and with a depth ≥40% of the total corneal thickness. This could be due to a reduction in wound-healing response due to the reduced keratocyte density of the posterior stroma [19]. Similar outcomes were previously reported by other authors (Table 2), including eyes with previous cataract surgery with a monofocal IOL [21–23]. Perhaps more importantly, it has been shown that the procedure is reversible—Alió et al. demonstrated that Kamra inlay removal can be safely performed without permanently affecting corneal topography and aberrometry, with more than 60% of patients recovered preoperative visual acuity [24]. Certainly, more improvements are needed in the future as careful slit-lamp examination showed in most cases a mild haze, and occasionally, prominent donut-like scarring (Fig. 10 right) [24]. Corneal confocal microscopy demonstrated that the Kamra inlay had good intrastromal tolerance, although a low grade of keratocyte activation was found in all patients, and a stronger response was associated with a negative visual outcome [25].
Phakic IOL implantation
The first phakic IOLs (PIOLs) were angle-supported and placed in the anterior chamber as early as 1953 by Dr. Strampelli [146]. In 1977, Prof. Worst developed the ‘iris-claw’ lens made from PMMA, reducing complications such as glaucoma [147]. It was only until 1986 when posterior chamber PIOLs were developed by Dr. Fyodorov, who’s designed was adapted to develop the implantable collamer lens (ICL)—made of a proprietary copolymer of hydroxyethyl methacrylate and porcine collagen. PIOL implants have the potential to confer better vision, achieve higher patient satisfaction compared with keratorefractive surgery in selected patients; and avoids the risk of corneal ectasia [148]. Another advantage is that the crystalline lens is retained, thus keeping natural accommodation in younger patients, with potentially less posterior segment complications such as retinal detachment [149]. However, careful patient selection is required for PIOL implantation as other complications such as glaucoma and cataract can occur [150]. Thus, such procedures are usually performed in patients who have contraindications to traditional laser refractive surgery or cannot achieve correction due to extremes of refractive error [149].
Current phakic IOLs
PIOLs come in two varieties: anterior chamber PIOLS and posterior chamber PIOLs. Anterior chamber can be further divided into angle-supported IOLs and iris-claw IOLs but only the iris-claw (Ophtec BV, the Netherlands and J&J, USA) is still available. The Artilens (as it is called now) is fixated in the eye to the iris. The lens is first centered in front of the pupil and then the iris tissue in the mid-periphery (which is immobile during pupillary movement) is enclosed between the claws to hold it in place. It comes in a rigid PMMA version (Fig. 11, left) and in a foldable version called artiflex (Fig. 11, right) and is made of polysiloxane. The Artilens can correct myopia, hyperopia, and astigmatism.
Fig. 11. Anterior chamber iris-claw intraocular lenses.
Left: rigid polymethyl-methacrylate (PMMA) lens. Right: foldable lens made of polysiloxane. Both from Ophtec BV, the Netherlands and J&J, USA.
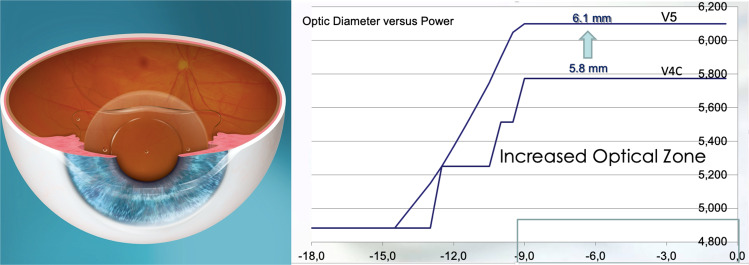
The archetypal posterior chamber PIOL is embodied by the Visian ICL (STAAR Surgical, USA) for the past two decades. The EVO ICL V4c (2011) is a single piece PIOL designed with a central port to eliminate the need for iridotomy or iridectomy that was required by earlier ICL models. The central port also allows aqueous flow from the posterior chamber to the anterior chamber to maintain normal physiology (Fig. 12) [151]. The main benefit of the EVO+ ICL (2016), which is available in powers from −0.50 to −14.00 D, is its extended optical zone (Fig. 12). A prospective, fellow eye-controlled study reported no difference in the safety, efficacy, predictability, and stability comparing the V4c and EVO+ ICL [152]. Through 5 years of postoperative follow-up, only one eye had developed an asymptomatic cataract in the conventional ICL group. There was no incidence of pigment dispersion glaucoma or pupillary block in any eye of either group [152].
Fig. 12. Example of implantable collamer lens (ICL) in situ.
The central port allows aqueous flow from the posterior chamber to the anterior chamber in order to maintain the normal physiology of the anterior segment of the eye. The EVO+ Visian ICL (STAAR Surgical, USA) introduced in 2016 now was an extended optical zone.
Beyond 2020: presbyopia-correcting PIOLs
The next potential frontier for PIOLs is to correct presbyopia using multifocal designs [153]. The Implantable Phakic Contact Lens (Presbyopia, Care Group) is made of hydrophilic acrylic material and has a trifocal diffractive optic (optical zone of 3.5 mm in diameter). It is available with additions between +1.5 and +3.5 D in steps of 0.5 D. Unfortunately no peer review article has been published. STAAR Surgical submitted their multi-site European clinical trial data for the EVO+ Visian ICL with an aspheric extended depth of focus (EDOF) optic, a lens that is designed to provide correction of myopia or hyperopia and presbyopia. Results from the clinical trial were submitted in July 2019 and if approved, this lens could be commercially available in the second quarter of 2020.
Refractive lens exchange
Refractive lens exchange or clear lens extraction is the removal of a clear crystalline lens and insertion of an IOL that replaces or augments the refractive ability of the eye [154]. This may be performed in selected patients where corneal laser surgery is not possible, or cannot achieve the desired refractive outcome [155]. Refractive lens exchange remains controversial in some clinical practices, as endophthalmitis can be more devastating compared with the risk of corneal infections that come with keratorefractive surgery [156]. Moreover, refractive lens exchange may have a higher risk of complications such as retinal detachment, compared with conventional cataract surgery if the patients are younger or more highly myopic [157]. Nonetheless, with the advances in surgical technology leading to better refractive outcomes, fast visual recovery and reduction in postoperative complications [158–160], the much improved risk-benefit ratio has led to this practice being performed more commonly in carefully selected patients [161]. The major advantage of refractive lens exchange is that all forms of refractive error can be treated based on the design of the IOL. Today, aspherical IOLs are widely used, as they match the optical quality of the eye’s natural lens and compensate for the positive spherical aberration of the cornea to provide sharper vision in eyes with larger pupils, or in situations with low lighting. Astigmatism may also be corrected with toric IOLs, which have different powers in opposite meridians of the lens. However, the surgeon needs to meticulously adjust the orientation of the IOL inside the eye for optimal astigmatism correction, because for every 3 degrees of misalignment 10% of the astigmatic correction is lost.
Presbyopia-correcting IOL implants
In recent years, new presbyopia-correcting IOL designs have rapidly developed using different refractive principles. However, proper patient counseling and setting realistic expectations are of the utmost importance, especially with regards to loss in contrast sensitivity, as well as the occurrence of glare and haloes. Moreover, precise IOL power calculation, preoperative evaluation, and treatment of ocular co-morbidities such as ocular surface disease, are key steps to ensure a satisfactory outcome. Today there is such a wide range of prebyopbia-correcting IOLs that the surgeon needs to discuss the most appropriate option based on the risk-benefits, balanced with patients’ expectations with visual requirements.
The first presbyopia-correcting IOLs were generally based on diffractive lens designs, either using apodization, in which a near-dominant central area is surrounded by concentric rings of decreasing height that result in diffraction of light at both distance and near [162]; or an aspheric anterior surface and a posterior surface with diffractive rings that focus both near and distance light regardless of pupil size [163]. Refractive segmented varifocal IOLs use an asymmetrical shape of the near-vision segment to transition between near- and far-vision zones [164]. Trifocal diffractive IOL attempts to improve intermediate vision by providing a third focus—Fig. 13, left [165]. The HOAs and visual quality have been reported to be similar to that of bifocal diffractive IOLs [166].
Fig. 13. Example of multifocal intraocular lens implants recently available.

Left: trifocal intraocular lens implant. Right: hybrid design extended depth of focus (EDOF) intraocular lens implant.
EDOF IOLs aim to give an elongated focus of vision by manipulating the induced spherical aberration of the IOL. However, the spherical aberration causes a degradation of visual quality and may affect distance visual acuity [167]. In order to negate this negative effect, some multifocal lenses incorporate limited amounts of negative spherical aberration. For example, the Tecnis Symfony IOL (J&J, USA) uses a biconvex anterior aspheric and posterior achromatic diffractive surface IOL with an echelette design [168]. Studies suggests that these IOLs mainly improve distance and intermediate vision with some loss in contrast sensitivity [169]. Other examples of hybrid multifocal IOLs that manipulate aberrations with multifocality is FineVision Triumf (Fig. 13, right). Another way to extend the depth of focus is to use the pinhole principle. The IC-8 (Acufocus, USA) utilizes a non-diffractive 3.23 mm opaque PVDF mask with a 1.36 mm central aperture that blocks unfocused paracentral light rays and permits paraxial light rays to enter (Fig. 14) [170]. This IOL may provide good distance, intermediate and near vision but decrease the peripheral visual field beyond 30 degrees of the fixation point. In particular this IOL may be effective in eyes with irregular corneal astigmatism.
Fig. 14. Photographs of a small aperture intraocular lens implant before and after implantation in situ.
Example of small aperture intraocular lens implant, which utilizes the pinhole principle to increase the depth of focus to about 3D.
Beyond 2020: accommodative IOL implants
The development of presbyopia-correcting IOL is challenging as accommodation is a dynamic process, and the above-mentioned designs do not directly address the mechanism of accommodation. Accomodative IOLs (AIOLs) attempt to imitate the mechanism of natural accommodation, with various theoretical assumptions. However, much more development is required to improve its clinical outcomes. AIOLs generally consist of either single-optic, dual-optic, or deformable surface designs [171]. They may be placed either in the sulcus or inside the capsular bag [172]. Single-optic AIOL have flexible supporting elements to transmit ciliary muscle contraction into an anterior displacement of the lens optic, resulting in increased dioptric power of the eye to improve near vision [173]. Experimental evidence suggests that beyond 6 months, the emptied capsular bag is unable to provide any significant dynamic force to an intracapsular located device [174], suggesting that sulcus placement of AIOLs may be needed [175–177]. A dual-optic lens design essentially consists of two separate optics: a high-powered ‘plus’ anterior optic of fixed dioptric power and a ‘minus’ posterior optic, coupled by spring haptics [178]. These lenses were designed to occupy the capsular bag completely, thus the capsular tension could theoretically change the distance between the anterior and posterior optic. Thus far, only the Lumina AIOL (AkkoLens International, Breda, The Netherlands) has published clinical evidence with positive clinical results. This acrylic hydrophilic polymer AIOL consisting of two mobile optical elements, which is implanted in the ciliary sulcus. The anterior element provides 5 D of correction, while the posterior provides 10–25 D of correction based on ocular biometry. Each optic has internal sinusoidal surfaces where its power increases linearly when one of the lenses slides onto the other due to ciliary body action. Therefore, when the eye accommodates and the ciliary muscle contracts, the two optics of the lens change their longitudinal position, passing one over the other thereby resulting in an increase of the dioptric power of the lens. Deformable surface AIOLs attempt to change the shape of its surface during accommodation, many of which are in the early research phase [175].
Summary and future developments in refractive surgery
The field of refractive surgery is rapidly evolving and cannot be comprehensively described in this review, with a wide variety of options to correct refractive error—Table 3. Rapid advances in technology and innovation has now increased the range of refractive surgical options available to patients. Preoperative assessments now allow for customized laser ablations to further achieve better visual quality. Developments in preoperative and intraoperative OCT imaging may also improve surgical planning and accuracy of incisions or placement of implants [12]. Keratorefractive surgery is now established as a safe and effective treatment option for refractive errors, with excellent visual outcomes, improvement in quality of life and achieves high patient satisfaction [179]. Keratorefractive surgery may also be combined with corneal collagen cross-linking, what may improve the safety profile in selected eyes with thinner corneas in the future [180–182]. Meanwhile, SMILE is a minimally invasive ‘flap-less’ procedure that is still being optimized, and gradually found to be comparable with traditional LASIK. Moreover, obtained stromal lenticules from SMILE procedures introduce a new field of potential surgical applications for the treatment of presbyopia [121], hyperopia [122, 123], and keratoconus, among others [124, 125]. These corneal stroma lenticule inlays may also be decellularised to improve biocompatibility [124, 126, 127]. On the other hand, significant improvement in efficacy, speed, and safety profiles of intraocular surgery has disrupted the traditional risk-benefit considerations associated with intraocular phakic implants and refractive lens exchange. Future improvements in femtosecond laser technology could also allow for customized capsulotomies to support special PCIOL implants during refractive lens exchange [183], or even induce refractive correction within the cornea or IOL without ablation using low energy levels [184]. With these emerging trends, it is important for ophthalmologists to be aware of the advantages and disadvantages of each refractive surgery option weighed against optical corrective options, while emphasizing on careful and appropriate patient selection.
Table 3.
Summary of refractive surgery techniques.
| Myopia, hyperopia, and astigmatism | Presbyopia | |
|---|---|---|
| Keratorefractive surgery |
Laser in situ keratomileuses (LASIK) Surface ablation including photorefractive keratectomy (PRK), laser epithelial keratomileusis (LASEK) |
Monovision treatment Multifocal treatment profiles EDOF treatment profiles e.g., ‘laser blended vision’b |
|
Small incision lenticule extraction (SMILE)—myopia and astigmatism SMILEa—hyperopia treatment |
SMILE—stromal lenticule implantation for presbyopia treatmenta | |
| Intracorneal implants | Intracorneal ring segments (currently not used for pure refractive correction) |
Kamra inlay Flexivue microlens implantb Icolensb |
| Phakic intraocular lens implants |
Implantable collamer lens (ICL) Iris-claw anterior chamber intraocular lens |
Implantable phakic contact lens (IPCL)b EDOF EVO+ Visian ICLa |
| Refractive lens exchange | Aspheric and toric IOLs |
Diffractive IOLs EDOF IOLs IC-8 IOLb Accommodative IOLs |
IOL Intraocular lens implants, EDOF extended depth of focus.
aEarly clinical data available, not yet approved for clinical use at time of publication.
bCE mark at time of publication.
Beyond 2020, refractive surgery may be guided by artificial intelligence (neural networks) in which multiple diagnostic tools will receive information about the eye and will guide the surgeon regarding the lens or the best corneal refractive surgery method to perform on a specific patient, to adequately correct the refractive error improving the quality of the retinal image to beyond normal levels. Most probably, pharmacology will have a role in the prevention of myopia, but not in the way we use it now but with the use of substances capable of stiffening the sclera and avoid further myopic progression. Presbyopia might be even prevented by eye drops capable of halting the hardening and thickening of the crystalline lens, and stimulating the ciliary body selectively. We might have a pharmacological prevention-treatment of cataracts, which would displace most cataract surgical procedures to be performed on patients at an older age than today. Moreover, therapeutic refractive surgery, emerging today, to restore pathological situations such as corneal irregular astigmatism secondary to medical or surgical causes (such as corneal graft surgery), might be managed not only with excimer laser ablations, but also with intracorneal ablations without excision of any type of tissue and no incisions, simply volatilizing tissue within the cornea (and old idea that has been pursued for long time). Moreover, intralenticular cataract surgery (by preserving the capsular bag and its subsequent “refill” with polymers with refractive properties) could provide an alternative to classical cataract surgery that preserves the elasticity of the lens (absent due to the unavoidable fibrosis after standard phaco techniques), and so enhance postoperative near visual function by a preservation of accommodation capacities.
Author contributions
All authors contributed to the overall concept, literature search, analysis, and interpretation of the literature and writing of the manuscript with designing of tables and figures.
Compliance with ethical standards
Conflict of interest
MA has no financial conflicts of interests to declare. DZR is a consultant for Carl Zeiss Meditec AG (Jena, Germany) DZR acknowledges a financial interest in Artemis Insight 100 VHF digital ultrasound (ArcScan Inc., Golden, CO)
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim TI, Alio Del Barrio JL, Wilkins M, Cochener B, Ang M. Refractive surgery. Lancet. 2019;393:2085–98. doi: 10.1016/S0140-6736(18)33209-4. [DOI] [PubMed] [Google Scholar]
- 2.Sugar A, Hood CT, Mian SI. Patient-reported outcomes following LASIK: quality of Life in the PROWL Studies. JAMA. 2017;317:204–5. doi: 10.1001/jama.2016.19323. [DOI] [PubMed] [Google Scholar]
- 3.Sugar A, Rapuano CJ, Culbertson WW, Huang D, Varley GA, Agapitos PJ, et al. Laser in situ keratomileusis for myopia and astigmatism: safety and efficacy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109:175–87. doi: 10.1016/s0161-6420(01)00966-6. [DOI] [PubMed] [Google Scholar]
- 4.Sandoval HP, Donnenfeld ED, Kohnen T, Lindstrom RL, Potvin R, Tremblay DM, et al. Modern laser in situ keratomileusis outcomes. J Cataract Refract Surg. 2016;42:1224–34. doi: 10.1016/j.jcrs.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Eydelman M, Hilmantel G, Tarver ME, Hofmeister EM, May J, Hammel K, et al. Symptoms and satisfaction of patients in the patient-reported outcomes with laser in situ keratomileusis (PROWL) studies. JAMA Ophthalmol. 2017;135:13–22. doi: 10.1001/jamaophthalmol.2016.4587. [DOI] [PubMed] [Google Scholar]
- 6.Ang M, Mehta JS, Chan C, Htoon HM, Koh JC, Tan DT. Refractive lenticule extraction: transition and comparison of 3 surgical techniques. J Cataract Refract Surg. 2014;40:1415–24. doi: 10.1016/j.jcrs.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Ang M, Tan D, Mehta JS. Small incision lenticule extraction (SMILE) versus laser in-situ keratomileusis (LASIK): study protocol for a randomized, non-inferiority trial. Trials. 2012;13:75. doi: 10.1186/1745-6215-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mi H, Tan N, Ang M, Htoon HM, Mehta JS. Comparison of anterior and posterior topographic analysis between 3 imaging systems. J Cataract Refract Surg. 2015;41:2533–45. doi: 10.1016/j.jcrs.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 9.Chan C, Ang M, Saad A, Chua D, Mejia M, Lim L, et al. Validation of an objective scoring system for forme fruste keratoconus detection and post-LASIK ectasia risk assessment in Asian eyes. Cornea. 2015;34:996–1004. doi: 10.1097/ICO.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 10.Roberts CJ, Mahmoud AM, Bons JP, Hossain A, Elsheikh A, Vinciguerra R, et al. Introduction of two novel stiffness parameters and interpretation of air puff-induced biomechanical deformation parameters with a dynamic Scheimpflug analyzer. J Refract Surg. 2017;33:266–73. doi: 10.3928/1081597X-20161221-03. [DOI] [PubMed] [Google Scholar]
- 11.Ambrosio R, Jr., Lopes BT, Faria-Correia F, Salomao MQ, Buhren J, Roberts CJ, et al. Integration of Scheimpflug-based corneal tomography and biomechanical assessments for enhancing ectasia detection. J Refract Surg. 2017;33:434–43. doi: 10.3928/1081597X-20170426-02. [DOI] [PubMed] [Google Scholar]
- 12.Ang M, Baskaran M, Werkmeister RM, Chua J, Schmidl D, Aranha Dos Santos V, et al. Anterior segment optical coherence tomography. Prog Retin Eye Res. 2018;66:132–56. doi: 10.1016/j.preteyeres.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Ang M, Chong W, Huang H, Tay WT, Wong TY, He MG, et al. Comparison of anterior segment optical tomography parameters measured using a semi-automatic software to standard clinical instruments. PLoS ONE. 2013;8:e65559. doi: 10.1371/journal.pone.0065559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sikorski BL, Suchon P. OCT biometry (B-OCT): a new method for measuring ocular axial dimensions. J Ophthalmol. 2019;2019:9192456. doi: 10.1155/2019/9192456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverman RH, Urs R, Roychoudhury A, Archer TJ, Gobbe M, Reinstein DZ. Epithelial remodeling as basis for machine-based identification of keratoconus. Investig Ophthalmol Vis Sci. 2014;55:1580–7. doi: 10.1167/iovs.13-12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinstein DZ, Srivannaboon S, Gobbe M, Archer TJ, Silverman RH, Sutton H, et al. Epithelial thickness profile changes induced by myopic LASIK as measured by Artemis very high-frequency digital ultrasound. J Refract Surg. 2009;25:444–50. doi: 10.3928/1081597x-20090422-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gatinel D, Azar DT, Dumas L, Malet J. Effect of anterior corneal surface asphericity modification on fourth-order zernike spherical aberrations. J Refract Surg. 2014;30:708–15. doi: 10.3928/1081597X-20140903-10. [DOI] [PubMed] [Google Scholar]
- 18.Amigo A, Martinez-Sorribes P, Recuerda M. Refractive changes induced by spherical aberration in laser correction procedures: an adaptive optics study. J Refract Surg. 2017;33:470–4. doi: 10.3928/1081597X-20170504-07. [DOI] [PubMed] [Google Scholar]
- 19.Gatinel D, Malet J, Dumas L. Polynomial decomposition method for ocular wavefront analysis. J Opt Soc Am A Opt Image Sci Vis. 2018;35:2035–45. doi: 10.1364/JOSAA.35.002035. [DOI] [PubMed] [Google Scholar]
- 20.Cabot F, Saad A, McAlinden C, Haddad NM, Grise-Dulac A, Gatinel D. Objective assessment of crystalline lens opacity level by measuring ocular light scattering with a double-pass system. Am J Ophthalmol. 2013;155:629–35. doi: 10.1016/j.ajo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Artal P, Benito A, Perez GM, Alcon E, De Casas A, Pujol J, et al. An objective scatter index based on double-pass retinal images of a point source to classify cataracts. PLoS ONE. 2011;6:e16823. doi: 10.1371/journal.pone.0016823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panthier C, de Wazieres A, Rouger H, Moran S, Saad A, Gatinel D. Average lens density quantification with swept-source optical coherence tomography: optimized, automated cataract grading technique. J Cataract Refract Surg. 2019;45:1746–52. doi: 10.1016/j.jcrs.2019.07.033. [DOI] [PubMed] [Google Scholar]
- 23.Accardo PA, Pensiero S. Neural network-based system for early keratoconus detection from corneal topography. J Biomed Inf. 2002;35:151–9. doi: 10.1016/s1532-0464(02)00513-0. [DOI] [PubMed] [Google Scholar]
- 24.Kovacs I, Mihaltz K, Kranitz K, Juhasz E, Takacs A, Dienes L, et al. Accuracy of machine learning classifiers using bilateral data from a Scheimpflug camera for identifying eyes with preclinical signs of keratoconus. J Cataract Refract Surg. 2016;42:275–83. doi: 10.1016/j.jcrs.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Arbelaez MC, Versaci F, Vestri G, Barboni P, Savini G. Use of a support vector machine for keratoconus and subclinical keratoconus detection by topographic and tomographic data. Ophthalmology. 2012;119:2231–8. doi: 10.1016/j.ophtha.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Smadja D, Touboul D, Cohen A, Doveh E, Santhiago MR, Mello GR, et al. Detection of subclinical keratoconus using an automated decision tree classification. Am J Ophthalmol. 2013;156:237–46 e231. doi: 10.1016/j.ajo.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 27.Yousefi S, Yousefi E, Takahashi H, Hayashi T, Tampo H, Inoda S, et al. Keratoconus severity identification using unsupervised machine learning. PLoS ONE. 2018;13:e0205998. doi: 10.1371/journal.pone.0205998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125:169–78. doi: 10.1016/j.ophtha.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Shammas HJ, Chan S. Precision of biometry, keratometry, and refractive measurements with a partial coherence interferometry-keratometry device. J Cataract Refract Surg. 2010;36:1474–8. doi: 10.1016/j.jcrs.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 30.Shajari M, Kolb CM, Petermann K, Bohm M, Herzog M, de’Lorenzo N, et al. Comparison of 9 modern intraocular lens power calculation formulas for a quadrifocal intraocular lens. J Cataract Refract Surg. 2018;44:942–8. doi: 10.1016/j.jcrs.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 31.Connell BJ, Kane JX. Comparison of the Kane formula with existing formulas for intraocular lens power selection. BMJ Open Ophthalmol. 2019;4:e000251. doi: 10.1136/bmjophth-2018-000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanellopoulos AJ, Pallikaris IG, Donnenfeld ED, Detorakis S, Koufala K, Perry HD. Comparison of corneal sensation following photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 1997;23:34–8. doi: 10.1016/s0886-3350(97)80148-4. [DOI] [PubMed] [Google Scholar]
- 33.Amm M, Wetzel W, Winter M, Uthoff D, Duncker GI. Histopathological comparison of photorefractive keratectomy and laser in situ keratomileusis in rabbits. J Refract Surg. 1996;12:758–66. doi: 10.3928/1081-597X-19961101-07. [DOI] [PubMed] [Google Scholar]
- 34.Azar DT, Ang RT, Lee JB, Kato T, Chen CC, Jain S, et al. Laser subepithelial keratomileusis: electron microscopy and visual outcomes of flap photorefractive keratectomy. Curr Opin Ophthalmol. 2001;12:323–8. doi: 10.1097/00055735-200108000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Chen CC, Chang JH, Lee JB, Javier J, Azar DT. Human corneal epithelial cell viability and morphology after dilute alcohol exposure. Investig Ophthalmol Vis Sci. 2002;43:2593–602. [PubMed] [Google Scholar]
- 36.Fadlallah A, Fahed D, Khalil K, Dunia I, Menassa J, El Rami H, et al. Transepithelial photorefractive keratectomy: clinical results. J Cataract Refract Surg. 2011;37:1852–7. doi: 10.1016/j.jcrs.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 37.Antonios R, Abdul Fattah M, Arba Mosquera S, Abiad BH, Sleiman K, Awwad ST. Single-step transepithelial versus alcohol-assisted photorefractive keratectomy in the treatment of high myopia: a comparative evaluation over 12 months. Br J Ophthalmol. 2017;101:1106–12. doi: 10.1136/bjophthalmol-2016-309409. [DOI] [PubMed] [Google Scholar]
- 38.Wen D, McAlinden C, Flitcroft I, Tu R, Wang Q, Alio J, et al. Postoperative efficacy, predictability, safety, and visual quality of laser corneal refractive surgery: a network meta-analysis. Am J Ophthalmol. 2017;178:65–78. doi: 10.1016/j.ajo.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez P, Moutsouris K, Pandolfi A. Biomechanical and optical behavior of human corneas before and after photorefractive keratectomy. J Cataract Refract Surg. 2014;40:905–17. doi: 10.1016/j.jcrs.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 40.Vestergaard AH. Past and present of corneal refractive surgery: a retrospective study of long-term results after photorefractive keratectomy and a prospective study of refractive lenticule extraction. Acta Ophthalmol. 2014;2:1–21. doi: 10.1111/aos.12385. [DOI] [PubMed] [Google Scholar]
- 41.Munnerlyn CR, Koons SJ, Marshall J. Photorefractive keratectomy: a technique for laser refractive surgery. J Cataract Refract Surg. 1988;14:46–52. doi: 10.1016/s0886-3350(88)80063-4. [DOI] [PubMed] [Google Scholar]
- 42.Na KS, Chung SH, Kim JK, Jang EJ, Lee NR, Joo CK. Comparison of LASIK and surface ablation by using propensity score analysis: a multicenter study in Korea. Investig Ophthalmol Vis Sci. 2012;53:7116–21. doi: 10.1167/iovs.12-9826. [DOI] [PubMed] [Google Scholar]
- 43.Wachtlin J, Langenbeck K, Schrunder S, Zhang EP, Hoffmann F. Immunohistology of corneal wound healing after photorefractive keratectomy and laser in situ keratomileusis. J Refract Surg. 1999;15:451–8. doi: 10.3928/1081-597X-19990701-11. [DOI] [PubMed] [Google Scholar]
- 44.Kim TI, Pak JH, Lee SY, Tchah H. Mitomycin C-induced reduction of keratocytes and fibroblasts after photorefractive keratectomy. Investig Ophthalmol Vis Sci. 2004;45:2978–84. doi: 10.1167/iovs.04-0070. [DOI] [PubMed] [Google Scholar]
- 45.Lee DH, Chung HS, Jeon YC, Boo SD, Yoon YD, Kim JG. Photorefractive keratectomy with intraoperative mitomycin-C application. J Cataract Refract Surg. 2005;31:2293–8. doi: 10.1016/j.jcrs.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 46.Faktorovich EG, Melwani K. Efficacy and safety of pain relief medications after photorefractive keratectomy: review of prospective randomized trials. J Cataract Refract Surg. 2014;40:1716–30. doi: 10.1016/j.jcrs.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Shemesh G, Dotan G, Lipshitz I. Predictability of corneal flap thickness in laser in situ keratomileusis using three different microkeratomes. J Refract Sur. 2002;18:S347–351. doi: 10.3928/1081-597X-20020502-13. [DOI] [PubMed] [Google Scholar]
- 48.Santhiago MR, Kara-Junior N, Waring GOt. Microkeratome versus femtosecond flaps: accuracy and complications. Curr Opin Ophthalmol. 2014;25:270–4. doi: 10.1097/ICU.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 49.Solomon KD, Donnenfeld E, Sandoval HP, Al Sarraf O, Kasper TJ, Holzer MP, et al. Flap thickness accuracy: comparison of 6 microkeratome models. J Cataract Refract Surg. 2004;30:964–77. doi: 10.1016/j.jcrs.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 50.Reinstein DZ, Archer TJ, Gobbe M. Accuracy and reproducibility of Cap thickness in small incision lenticule extraction. J Refract Surg. 2013;29:810–5. doi: 10.3928/1081597X-20131023-02. [DOI] [PubMed] [Google Scholar]
- 51.Zhai CB, Tian L, Zhou YH, Zhang QW, Zhang J. Comparison of the flaps made by femtosecond laser and automated keratomes for sub-bowman keratomileusis. Chin Med J. 2013;126:2440–4. [PubMed] [Google Scholar]
- 52.Zhao LQ, Wei RL, Cheng JW, Li Y, Cai JP, Ma XY. Meta-analysis: clinical outcomes of laser-assisted subepithelial keratectomy and photorefractive keratectomy in myopia. Ophthalmology. 2010;117:1912–22. doi: 10.1016/j.ophtha.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 53.Wallerstein A, Jackson WB, Chambers J, Moezzi AM, Lin H, Simmons PA. Management of post-LASIK dry eye: a multicenter randomized comparison of a new multi-ingredient artificial tear to carboxymethylcellulose. Clin Ophthalmol. 2018;12:839–48. doi: 10.2147/OPTH.S163744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segev F, Mimouni M, Sela T, Munzer G, Kaiserman I. Risk factors for sporadic diffuse lamellar keratitis after microkeratome laser-assisted in situ keratomileusis: a retrospective large database analysis. Cornea. 2018;37:1124–9. doi: 10.1097/ICO.0000000000001674. [DOI] [PubMed] [Google Scholar]
- 55.Yesilirmak N, Chhadva P, Cabot F, Galor A, Yoo SH. Post-laser in situ keratomileusis epithelial ingrowth: treatment, recurrence, and long-term results. Cornea. 2018;37:1517–21. doi: 10.1097/ICO.0000000000001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ayala MJ, Alio JL, Mulet ME, De La Hoz F. Treatment of laser in situ keratomileusis interface epithelial ingrowth with neodymium:yytrium-aluminum-garnet laser. Am J Ophthalmol. 2008;145:630–4. doi: 10.1016/j.ajo.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 57.Bohac M, Koncarevic M, Pasalic A, Biscevic A, Merlak M, Gabric N, et al. Incidence and clinical characteristics of post LASIK ectasia: a review of over 30,000 LASIK cases. Semin Ophthalmol. 2018;33:869–77. doi: 10.1080/08820538.2018.1539183. [DOI] [PubMed] [Google Scholar]
- 58.Seiler T, Genth U, Holschbach A, Derse M. Aspheric photorefractive keratectomy with excimer laser. Refract Corneal Surg. 1993;9:166–72. [PubMed] [Google Scholar]
- 59.O’Brart DP, Corbett MC, Lohmann CP, Kerr Muir MG, Marshall J. The effects of ablation diameter on the outcome of excimer laser photorefractive keratectomy. A prospective, randomized, double-blind study. Arch Ophthalmol. 1995;113:438–43. doi: 10.1001/archopht.1995.01100040054026. [DOI] [PubMed] [Google Scholar]
- 60.Kalski RS, Sutton G, Bin Y, Lawless MA, Rogers C. Comparison of 5-mm and 6-mm ablation zones in photorefractive keratectomy for myopia. J Refract Surg. 1996;12:61–7. doi: 10.3928/1081-597X-19960101-13. [DOI] [PubMed] [Google Scholar]
- 61.Buhren J, Pesudovs K, Martin T, Strenger A, Yoon G, Kohnen T. Comparison of optical quality metrics to predict subjective quality of vision after laser in situ keratomileusis. J Cataract Refract Surg. 2009;35:846–5. doi: 10.1016/j.jcrs.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 62.Drum BA. Aberration analyses needed for FDA evaluation of safety and effectiveness of wavefront-guided refractive surgical devices. J Refract Surg. 2003;19:S588–91. doi: 10.3928/1081-597X-20030901-16. [DOI] [PubMed] [Google Scholar]
- 63.Pesudovs K. Wavefront aberration outcomes of LASIK for high myopia and high hyperopia. J Refract Surg. 2005;21:S508–12. doi: 10.3928/1081-597X-20050901-18. [DOI] [PubMed] [Google Scholar]
- 64.Myrowitz EH, Chuck RS. A comparison of wavefront-optimized and wavefront-guided ablations. Curr Opin Ophthalmol. 2009;20:247–50. doi: 10.1097/icu.0b013e32832a2336. [DOI] [PubMed] [Google Scholar]
- 65.Jun I, Kang DS, Tan J, Choi JY, Heo W, Kim JY, et al. Comparison of clinical outcomes between wavefront-optimized versus corneal wavefront-guided transepithelial photorefractive keratectomy for myopic astigmatism. J Cataract Refract Surg. 2017;43:174–82. doi: 10.1016/j.jcrs.2016.11.045. [DOI] [PubMed] [Google Scholar]
- 66.Lee WS, Manche EE. Comparison of simulated keratometric changes following wavefront-guided and wavefront-optimized myopic laser-assisted in situ keratomileusis. Clin Ophthalmol. 2018;12:613–9. doi: 10.2147/OPTH.S161387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moshirfar M, Shah TJ, Skanchy DF, Linn SH, Kang P, Durrie DS. Comparison and analysis of FDA reported visual outcomes of the three latest platforms for LASIK: wavefront guided Visx iDesign, topography guided WaveLight Allegro Contoura, and topography guided Nidek EC-5000 CATz. Clin Ophthalmol. 2017;11:135–47. doi: 10.2147/OPTH.S115270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schallhorn SC, Farjo AA, Huang D, Boxer Wachler BS, Trattler WB, Tanzer DJ, et al. Wavefront-guided LASIK for the correction of primary myopia and astigmatism a report by the American Academy of Ophthalmology. Ophthalmology. 2008;115:1249–61. doi: 10.1016/j.ophtha.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 69.Smadja D, Reggiani-Mello G, Santhiago MR, Krueger RR. Wavefront ablation profiles in refractive surgery: description, results, and limitations. J Refract Surg. 2012;28:224–32. doi: 10.3928/1081597X-20120217-01. [DOI] [PubMed] [Google Scholar]
- 70.Goldberg DB. Laser in situ keratomileusis monovision. J Cataract Refract Surg. 2001;27:1449–55. doi: 10.1016/s0886-3350(01)01001-x. [DOI] [PubMed] [Google Scholar]
- 71.Miranda D, Krueger RR. Monovision laser in situ keratomileusis for pre-presbyopic and presbyopic patients. J Refract Surg. 2004;20:325–8. doi: 10.3928/1081-597X-20040701-04. [DOI] [PubMed] [Google Scholar]
- 72.Ayoubi MG, Leccisotti A, Goodall EA, McGilligan VE, Moore TC. Femtosecond laser in situ keratomileusis versus conductive keratoplasty to obtain monovision in patients with emmetropic presbyopia. J Cataract Refract Surg. 2010;36:997–1002. doi: 10.1016/j.jcrs.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 73.Fawcett SL, Herman WK, Alfieri CD, Castleberry KA, Parks MM, Birch EE. Stereoacuity and foveal fusion in adults with long-standing surgical monovision. J Aapos. 2001;5:342–7. doi: 10.1067/mpa.2001.119785. [DOI] [PubMed] [Google Scholar]
- 74.Kato S, Ito M, Shimizu K, Kamiya K. Etiology and outcomes of secondary surgical intervention for dissatisfied patients after pseudophakic monovision. Int Ophthalmol. 2018;38:1003–9. doi: 10.1007/s10792-017-0551-1. [DOI] [PubMed] [Google Scholar]
- 75.Holland D. PresbyLASIK treatment for simultaneous correction of presbyopia and ametropia: development to PresbyMAX hybrid at the Augenklinik Bellevue. ESCRS. 2014.
- 76.Chan TC, Kwok PS, Jhanji V, Woo VC, Ng AL. Presbyopic correction using monocular Bi-aspheric ablation profile (PresbyMAX) in hyperopic eyes: 1-year outcomes. J Refract Surg. 2017;33:37–43. doi: 10.3928/1081597X-20161006-03. [DOI] [PubMed] [Google Scholar]
- 77.Luger MH, McAlinden C, Buckhurst PJ, Wolffsohn JS, Verma S, Arba Mosquera S. Presbyopic LASIK using hybrid bi-aspheric micro-monovision ablation profile for presbyopic corneal treatments. Am J Ophthalmol. 2015;160:493–505. doi: 10.1016/j.ajo.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 78.Vargas-Fragoso V, Alio JL. Corneal compensation of presbyopia: PresbyLASIK: an updated review. Eye Vis. 2017;4:11. doi: 10.1186/s40662-017-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rocha KM, Vabre L, Chateau N, Krueger RR. Expanding depth of focus by modifying higher-order aberrations induced by an adaptive optics visual simulator. J Cataract Refract Surg. 2009;35:1885–92. doi: 10.1016/j.jcrs.2009.05.059. [DOI] [PubMed] [Google Scholar]
- 80.Benard Y, Lopez-Gil N, Legras R. Optimizing the subjective depth-of-focus with combinations of fourth- and sixth-order spherical aberration. Vis Res. 2011;51:2471–7. doi: 10.1016/j.visres.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 81.Reinstein DZ, Archer TJ, Gobbe M. Laser blended vision for presbyopia correction. In: Pallikaris IG, Plainis S, Charman WN, editors. Presbyopia: origins, effects and treatment. Thorofare, NJ: SLACK Incorporated; 2012. [Google Scholar]
- 82.Reinstein DZ, Archer TJ, Gobbe M. LASIK for myopic astigmatism and presbyopia using non-linear aspheric micro-monovision with the Carl Zeiss Meditec MEL 80 platform. J Refract Surg. 2011;27:23–37. doi: 10.3928/1081597X-20100212-04. [DOI] [PubMed] [Google Scholar]
- 83.Reinstein DZ, Couch DG, Archer TJ. LASIK for hyperopic astigmatism and presbyopia using Micro-monovision With the Carl Zeiss Meditec MEL80. J Refract Surg. 2009;25:37–58. doi: 10.3928/1081597X-20090101-07. [DOI] [PubMed] [Google Scholar]
- 84.Evans BJ. Monovision: a review. Ophthalmic Physiol Opt. 2007;27:417–39. doi: 10.1111/j.1475-1313.2007.00488.x. [DOI] [PubMed] [Google Scholar]
- 85.Reinstein DZ, Carp GI, Archer TJ, Gobbe M. LASIK for the correction of presbyopia in emmetropic patients using aspheric ablation profiles and a micro-monovision protocol with the Carl Zeiss Meditec MEL80 and VisuMax. J Refract Surg. 2012;28:531–41. doi: 10.3928/1081597X-20120723-01. [DOI] [PubMed] [Google Scholar]
- 86.Vestergaard AH, Grauslund J, Ivarsen AR, Hjortdal JO. Efficacy, safety, predictability, contrast sensitivity, and aberrations after femtosecond laser lenticule extraction. J Cataract Refract Surg. 2014;40:403–11. doi: 10.1016/j.jcrs.2013.07.053. [DOI] [PubMed] [Google Scholar]
- 87.Sekundo W, Gertnere J, Bertelmann T, Solomatin I. One-year refractive results, contrast sensitivity, high-order aberrations and complications after myopic small-incision lenticule extraction (ReLEx SMILE) Graefes Arch Clin Exp Ophthalmol. 2014;252:837–43. doi: 10.1007/s00417-014-2608-4. [DOI] [PubMed] [Google Scholar]
- 88.Moshirfar M, McCaughey MV, Reinstein DZ, Shah R, Santiago-Caban L, Fenzl CR. Small-incision lenticule extraction. J Cataract Refract Surg. 2015;41:652–65. doi: 10.1016/j.jcrs.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 89.Denoyer A, Landman E, Trinh L, Faure JF, Auclin F, Baudouin C. Dry eye disease after refractive surgery: comparative outcomes of small incision lenticule extraction versus LASIK. Ophthalmology. 2015;122:669–76. doi: 10.1016/j.ophtha.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 90.Reinstein DZ, Archer TJ, Gobbe M, Bartoli E. Corneal sensitivity after small-incision lenticule extraction and laser in situ keratomileusis. J Cataract Refract Surg. 2015;41:1580–7. doi: 10.1016/j.jcrs.2014.12.055. [DOI] [PubMed] [Google Scholar]
- 91.Randleman JB, Dawson DG, Grossniklaus HE, McCarey BE, Edelhauser HF. Depth-dependent cohesive tensile strength in human donor corneas: implications for refractive surgery. J Refract Surg. 2008;24:S85–9. doi: 10.3928/1081597X-20080101-15. [DOI] [PubMed] [Google Scholar]
- 92.Scarcelli G, Pineda R, Yun SH. Brillouin optical microscopy for corneal biomechanics. Investig Ophthalmol Vis Sci. 2012;53:185–90. doi: 10.1167/iovs.11-8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Petsche SJ, Chernyak D, Martiz J, Levenston ME, Pinsky PM. Depth-dependent transverse shear properties of the human corneal stroma. Investig Ophthalmol Vis Sci. 2012;53:873–80. doi: 10.1167/iovs.11-8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reinstein DZ, Archer TJ, Randleman JB. Mathematical model to compare the relative tensile strength of the cornea after PRK, LASIK, and small incision lenticule extraction. J Refract Surg. 2013;29:454–60. doi: 10.3928/1081597X-20130617-03. [DOI] [PubMed] [Google Scholar]
- 95.Seven I, Vahdati A, Pedersen IB, Vestergaard A, Hjortdal J, Roberts CJ, et al. Contralateral eye comparison of SMILE and Flap-Based corneal refractive surgery: computational analysis of biomechanical impact. J Refract Surg. 2017;33:444–53. doi: 10.3928/1081597X-20170504-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sinha Roy A, Dupps WJ, Jr., Roberts CJ. Comparison of biomechanical effects of small-incision lenticule extraction and laser in situ keratomileusis: finite-element analysis. J Cataract Refract Surg. 2014;40:971–80. doi: 10.1016/j.jcrs.2013.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spiru B, Kling S, Hafezi F, Sekundo W. Biomechanical differences between femtosecond lenticule extraction (FLEx) and small incision lenticule extraction (SmILE) tested by 2D-extensometry in ex vivo porcine eyes. Investig Ophthalmol Vis Sci. 2017;58:2591–5. doi: 10.1167/iovs.16-20211. [DOI] [PubMed] [Google Scholar]
- 98.Pedersen IB, Ivarsen A, Hjortdal J. Changes in astigmatism, densitometry, and aberrations after SMILE for low to high myopic astigmatism: a 12-month prospective study. J Refract Surg. 2017;33:11–7. doi: 10.3928/1081597X-20161006-04. [DOI] [PubMed] [Google Scholar]
- 99.Damgaard IB, Ang M, Mahmoud AM, Farook M, Roberts CJ, Mehta JS. Functional optical zone and centration following SMILE and LASIK: a prospective, randomized, contralateral Eye Study. J Refract Surg. 2019;35:230–7. doi: 10.3928/1081597X-20190313-01. [DOI] [PubMed] [Google Scholar]
- 100.Han T, Shang J, Zhou X, Xu Y, Ang M, Zhou X. Refractive outcomes comparing small-incision lenticule extraction and femtosecond laser-assisted laser in situ keratomileusis for high myopia. J Cataract Refract Surg. 2020;46:419–27. [DOI] [PubMed]
- 101.Ji YW, Kang DSY, Reinstein DZ, Archer TJ, Choi JY, Kim EK, et al. Effect of lowering laser energy on the surface roughness of human corneal lenticules in small-incision lenticule extraction. J Refract Surg. 2017;33:617–24. doi: 10.3928/1081597X-20170620-02. [DOI] [PubMed] [Google Scholar]
- 102.Ji YW, Kim M, Yong Kang DS, Reinstein D, Archer T, Choi JY, et al. Lower laser energy levels lead to better visual recovery after small-incision lenticule extraction: prospective, randomized clinical trial. Am J Ophthalmol. 2017;179:159–70. doi: 10.1016/j.ajo.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 103.Donate D, Thaeron R. Lower energy levels improve visual recovery in small incision lenticule extraction (SMILE) J Refract Surg. 2016;32:636–42. doi: 10.3928/1081597X-20160602-01. [DOI] [PubMed] [Google Scholar]
- 104.Li L, Schallhorn JM, Ma J, Cui T, Wang Y. Energy setting and visual outcomes in SMILE: a retrospective Cohort Study. J Refract Surg. 2018;34:11–6. doi: 10.3928/1081597X-20171115-01. [DOI] [PubMed] [Google Scholar]
- 105.Shen Z, Shi K, Yu Y, Yu X, Lin Y, Yao K. Small incision lenticule extraction (SMILE) versus femtosecond laser-assisted in situ keratomileusis (FS-LASIK) for myopia: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0158176. doi: 10.1371/journal.pone.0158176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y, Shen Q, Jia Y, Zhou D, Zhou J. Clinical outcomes of SMILE and FS-LASIK used to treat myopia: a meta-analysis. J Refract Surg. 2016;32:256–65. doi: 10.3928/1081597X-20151111-06. [DOI] [PubMed] [Google Scholar]
- 107.Ang M, Farook M, Htoon HM, Mehta JS. Randomized clinical trial comparing femtosecond LASIK and small-incision lenticule extraction. Ophthalmology. 2020;127:724–30. [DOI] [PubMed]
- 108.Ang M, Ho H, Fenwick E, Lamoureux E, Htoon HM, Koh J, et al. Vision-related quality of life and visual outcomes after small-incision lenticule extraction and laser in situ keratomileusis. J Cataract Refract Surg. 2015;41:2136–44. doi: 10.1016/j.jcrs.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 109.Ang M, Farook M, Htoon HM, Tan D, Mehta JS. Simulated night vision after small-incision lenticule extraction. J Cataract Refract Surg. 2016;42:1173–80. doi: 10.1016/j.jcrs.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 110.Damgaard IB, Ang M, Farook M, Htoon HM, Mehta JS. Intraoperative patient experience and postoperative visual quality after SMILE and LASIK in a randomized, paired-eye, controlled study. J Refract Surg. 2018;34:92–9. doi: 10.3928/1081597X-20171218-01. [DOI] [PubMed] [Google Scholar]
- 111.Reinstein DZ, Archer TJ, Vida RS, Carp GI. Suction stability management in small incision lenticule extraction: incidence and outcomes of suction loss in 4000 consecutive procedures. Acta Ophthalmol. 2020;98:e72–e80. [DOI] [PubMed]
- 112.Reinstein DZ, Archer TJ, Vida RS, Carp GI. Suction stability management in SMILE: development of a decision tree for managing eye movements and suction loss. J Refract Surg. 2018;34:809–16. doi: 10.3928/1081597X-20181023-01. [DOI] [PubMed] [Google Scholar]
- 113.Ang M, Chaurasia SS, Angunawela RI, Poh R, Riau A, Tan D, et al. Femtosecond lenticule extraction (FLEx): clinical results, interface evaluation, and intraocular pressure variation. Investig Ophthalmol Vis Sci. 2012;53:1414–21. doi: 10.1167/iovs.11-8808. [DOI] [PubMed] [Google Scholar]
- 114.Reinstein DZ, Stuart AJ, Vida RS, Archer TJ, Carp GI. Incidence and outcomes of sterile multifocal inflammatory keratitis and diffuse lamellar keratitis after SMILE. J Refract Surg. 2018;34:751–9. doi: 10.3928/1081597X-20181001-02. [DOI] [PubMed] [Google Scholar]
- 115.Siedlecki J, Luft N, Mayer WJ, Siedlecki M, Kook D, Meyer B, et al. CIRCLE enhancement after myopic SMILE. J Refract Surg. 2018;34:304–9. doi: 10.3928/1081597X-20180308-02. [DOI] [PubMed] [Google Scholar]
- 116.Reinstein DZ, Carp GI, Archer TJ, Vida RS. Outcomes of re-treatment by LASIK After SMILE. J Refract Surg. 2018;34:578–88. doi: 10.3928/1081597X-20180717-02. [DOI] [PubMed] [Google Scholar]
- 117.Reinstein DZ, Archer TJ, Carp GI. The surgeon’s guide to small incision lenticule extraction (SMILE) Thorofare, New Jersey: SLACK Incorporated; 2018. [Google Scholar]
- 118.Ganesh S, Gupta R. Comparison of visual and refractive outcomes following femtosecond laser- assisted lasik with smile in patients with myopia or myopic astigmatism. J Refract Surg. 2014;30:590–6. doi: 10.3928/1081597X-20140814-02. [DOI] [PubMed] [Google Scholar]
- 119.Reinstein DZ, Carp GI, Archer TJ, Gobbe M. Outcomes of small incision lenticule extraction (SMILE) in low myopia. J Refract Surg. 2014;30:812–8. doi: 10.3928/1081597X-20141113-07. [DOI] [PubMed] [Google Scholar]
- 120.Pradhan KR, Reinstein DZ, Carp GI, Archer TJ, Gobbe M, Dhungana P. Quality control outcomes analysis of small-incision lenticule extraction for myopia by a novice surgeon at the first refractive surgery unit in Nepal during the first 2 years of operation. J Cataract Refract Surg. 2016;42:267–74. doi: 10.1016/j.jcrs.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 121.Jacob S, Kumar DA, Agarwal A, Agarwal A, Aravind R, Saijimol AI. Preliminary evidence of successful near vision enhancement with a new technique: PrEsbyopic Allogenic Refractive Lenticule (PEARL) Corneal Inlay Using a SMILE Lenticule. J Refract Surg. 2017;33:224–9. doi: 10.3928/1081597X-20170111-03. [DOI] [PubMed] [Google Scholar]
- 122.Li M, Li M, Sun L, Ni K, Zhou X. Predictive formula for refraction of autologous lenticule implantation for hyperopia correction. J Refract Surg. 2017;33:827–33. doi: 10.3928/1081597X-20171016-01. [DOI] [PubMed] [Google Scholar]
- 123.Pradhan KR, Reinstein DZ, Carp GI, Archer TJ, Gobbe M, Gurung R. Femtosecond laser-assisted keyhole endokeratophakia: correction of hyperopia by implantation of an allogeneic lenticule obtained by SMILE from a myopic donor. J Refractive Surg. 2013;29:777–82. doi: 10.3928/1081597X-20131021-07. [DOI] [PubMed] [Google Scholar]
- 124.Alio Del Barrio JL, El Zarif M, Azaar A, Makdissy N, Khalil C, Harb W, et al. Corneal stroma enhancement with decellularized stromal laminas with or without stem cell recellularization for advanced keratoconus. Am J Ophthalmol. 2018;186:47–58. doi: 10.1016/j.ajo.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 125.Mastropasqua L, Nubile M, Salgari N, Mastropasqua R. Femtosecond laser-assisted stromal lenticule addition keratoplasty for the treatment of advanced keratoconus: a Preliminary Study. J Refract Surg. 2018;34:36–44. doi: 10.3928/1081597X-20171004-04. [DOI] [PubMed] [Google Scholar]
- 126.Alio del Barrio JL, Chiesa M, Garagorri N, Garcia-Urquia N, Fernandez-Delgado J, Bataille L, et al. Acellular human corneal matrix sheets seeded with human adipose-derived mesenchymal stem cells integrate functionally in an experimental animal model. Exp Eye Res. 2015;132:91–100. doi: 10.1016/j.exer.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 127.Liu YC, Teo EPW, Ang HP, Seah XY, Lwin NC, Yam GHF, et al. Biological corneal inlay for presbyopia derived from small incision lenticule extraction (SMILE) Sci Rep. 2018;8:1831. doi: 10.1038/s41598-018-20267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu R, Zhao J, Xu Y, Li M, Niu L, Liu H, et al. Femtosecond laser-assisted corneal small incision allogenic intrastromal lenticule implantation in monkeys: a Pilot Study. Investig Ophthalmol Vis Sci. 2015;56:3715–20. doi: 10.1167/iovs.14-15296. [DOI] [PubMed] [Google Scholar]
- 129.Damgaard IB, Ivarsen A, Hjortdal J. Biological lenticule implantation for correction of hyperopia: an ex vivo study in human corneas. J Refract Surg. 2018;34:245–52. doi: 10.3928/1081597X-20180206-01. [DOI] [PubMed] [Google Scholar]
- 130.Barraquer JI. Modification of refraction by means of intracorneal inclusions. Int Ophthalmol Clin. 1966;6:53–78. [PubMed] [Google Scholar]
- 131.Barraquer JI. Keratophakia. Trans ophthalmological societies U Kingd. 1972;92:499–516. [PubMed] [Google Scholar]
- 132.Garza EB, Gomez S, Chayet A, Dishler J. One-year safety and efficacy results of a hydrogel inlay to improve near vision in patients with emmetropic presbyopia. J Refract Surg. 2013;29:166–72. doi: 10.3928/1081597X-20130129-01. [DOI] [PubMed] [Google Scholar]
- 133.Malandrini A, Martone G, Menabuoni L, Catanese AM, Tosi GM, Balestrazzi A, et al. Bifocal refractive corneal inlay implantation to improve near vision in emmetropic presbyopic patients. J Cataract Refract Surg. 2015;41:1962–72. doi: 10.1016/j.jcrs.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 134.Yilmaz OF, Bayraktar S, Agca A, Yilmaz B, McDonald MB, van de Pol C. Intracorneal inlay for the surgical correction of presbyopia. J Cataract Refract Surg. 2008;34:1921–7. doi: 10.1016/j.jcrs.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 135.Mulet ME, Alio JL, Knorz MC. Hydrogel intracorneal inlays for the correction of hyperopia: outcomes and complications after 5 years of follow-up. Ophthalmology. 2009;116:1455–60. doi: 10.1016/j.ophtha.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 136.Vega-Estrada A, Alio JL. The use of intracorneal ring segments in keratoconus. Eye Vis. 2016;3:8. doi: 10.1186/s40662-016-0040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vega-Estrada A, Alio JL, Brenner LF, Javaloy J, Plaza Puche AB, Barraquer RI, et al. Outcome analysis of intracorneal ring segments for the treatment of keratoconus based on visual, refractive, and aberrometric impairment. Am J Ophthalmol. 2013;155:575–84.e571. doi: 10.1016/j.ajo.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 138.Lindstrom RL, Macrae SM, Pepose JS, Hoopes PC., Sr Corneal inlays for presbyopia correction. Curr Opin Ophthalmol. 2013;24:281–7. doi: 10.1097/ICU.0b013e328362293e. [DOI] [PubMed] [Google Scholar]
- 139.Konstantopoulos A, Mehta JS. Surgical compensation of presbyopia with corneal inlays. Expert Rev Med Devices. 2015;12:341–52. doi: 10.1586/17434440.2015.1007124. [DOI] [PubMed] [Google Scholar]
- 140.Arlt E, Krall E, Moussa S, Grabner G, Dexl A. Implantable inlay devices for presbyopia: the evidence to date. Clin Ophthalmol. 2015;9:129–37. doi: 10.2147/OPTH.S57056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Whitman J, Dougherty PJ, Parkhurst GD, Olkowski J, Slade SG, Hovanesian J, et al. Treatment of presbyopia in emmetropes using a shape-changing corneal inlay: one-year clinical outcomes. Ophthalmology. 2016;123:466–75. doi: 10.1016/j.ophtha.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 142.Yoo A, Kim JY, Kim MJ, Tchah H. Hydrogel inlay for presbyopia: objective and subjective visual outcomes. J Refract Surg. 2015;31:454–60. doi: 10.3928/1081597X-20150623-03. [DOI] [PubMed] [Google Scholar]
- 143.Limnopoulou AN, Bouzoukis DI, Kymionis GD, Panagopoulou SI, Plainis S, Pallikaris AI, et al. Visual outcomes and safety of a refractive corneal inlay for presbyopia using femtosecond laser. J Refract Surg. 2013;29:12–8. doi: 10.3928/1081597X-20121210-01. [DOI] [PubMed] [Google Scholar]
- 144.Baily C, Kohnen T, O’Keefe M. Preloaded refractive-addition corneal inlay to compensate for presbyopia implanted using a femtosecond laser: one-year visual outcomes and safety. J Cataract Refract Surg. 2014;40:1341–8. doi: 10.1016/j.jcrs.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 145.Vukich JA, Durrie DS, Pepose JS, Thompson V, van de Pol C, Lin L. Evaluation of the small-aperture intracorneal inlay: Three-year results from the cohort of the U.S. Food and Drug Administration clinical trial. J Cataract Refract Surg. 2018;44:541–56. [DOI] [PubMed]
- 146.Chen LJ, Chang YJ, Kuo JC, Rajagopal R, Azar DT. Metaanalysis of cataract development after phakic intraocular lens surgery. J Cataract Refract Surg. 2008;34:1181–200. doi: 10.1016/j.jcrs.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 147.Huang D, Schallhorn SC, Sugar A, Farjo AA, Majmudar PA, Trattler WB, et al. Phakic intraocular lens implantation for the correction of myopia: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116:2244–58. doi: 10.1016/j.ophtha.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 148.Barsam A, Allan BD. Excimer laser refractive surgery versus phakic intraocular lenses for the correction of moderate to high myopia. Cochrane Database Syst Rev. 2014:CD007679. 10.1002/14651858.CD007679.pub4. [DOI] [PMC free article] [PubMed]
- 149.Nanavaty MA, Daya SM. Refractive lens exchange versus phakic intraocular lenses. Curr Opin Ophthalmol. 2012;23:54–61. doi: 10.1097/ICU.0b013e32834cd5d1. [DOI] [PubMed] [Google Scholar]
- 150.Pop M, Payette Y. Refractive lens exchange versus iris-claw Artisan phakic intraocular lens for hyperopia. J Refract Surg. 2004;20:20–4. doi: 10.3928/1081-597X-20040101-04. [DOI] [PubMed] [Google Scholar]
- 151.Packer M. Meta-analysis and review: effectiveness, safety, and central port design of the intraocular collamer lens. Clin Ophthalmol. 2016;10:1059–77. doi: 10.2147/OPTH.S111620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shimizu K, Kamiya K, Igarashi A, Kobashi H. Long-term comparison of posterior chamber phakic intraocular lens with and without a central hole (Hole ICL and Conventional ICL) implantation for moderate to high myopia and myopic astigmatism: consort-compliant article. Medicine. 2016;95:e3270. doi: 10.1097/MD.0000000000003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Alio JL, Mulet ME. Presbyopia correction with an anterior chamber phakic multifocal intraocular lens. Ophthalmology. 2005;112:1368–74. doi: 10.1016/j.ophtha.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 154.Alio JL, Grzybowski A, El Aswad A, Romaniuk D. Refractive lens exchange. Surv Ophthalmol. 2014;59:579–98. doi: 10.1016/j.survophthal.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 155.Alio JL, Grzybowski A, Romaniuk D. Refractive lens exchange in modern practice: when and when not to do it? Eye Vis. 2014;1:10. doi: 10.1186/s40662-014-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu YC, Wilkins M, Kim T, Malyugin B, Mehta JS. Cataracts. Lancet. 2017;390:600–12. doi: 10.1016/S0140-6736(17)30544-5. [DOI] [PubMed] [Google Scholar]
- 157.Westin O, Koskela T, Behndig A. Epidemiology and outcomes in refractive lens exchange surgery. Acta Ophthalmol. 2015;93:41–5. doi: 10.1111/aos.12460. [DOI] [PubMed] [Google Scholar]
- 158.Creuzot-Garcher C, Benzenine E, Mariet AS, de Lazzer A, Chiquet C, Bron AM, et al. Incidence of acute postoperative endophthalmitis after cataract surgery: a nationwide study in France from 2005 to 2014. Ophthalmology. 2016;123:1414–20. doi: 10.1016/j.ophtha.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 159.Jabbarvand M, Hashemian H, Khodaparast M, Jouhari M, Tabatabaei A, Rezaei S. Endophthalmitis occurring after cataract surgery: outcomes of more than 480 000 cataract surgeries, epidemiologic features, and risk factors. Ophthalmology. 2016;123:295–301. doi: 10.1016/j.ophtha.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 160.Behndig A, Montan P, Stenevi U, Kugelberg M, Lundstrom M. One million cataract surgeries: Swedish National Cataract Register 1992-2009. J Cataract Refract Surg. 2011;37:1539–45. doi: 10.1016/j.jcrs.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 161.Jaycock P, Johnston RL, Taylor H, Adams M, Tole DM, Galloway P, et al. The Cataract National Dataset electronic multi-centre audit of 55,567 operations: updating benchmark standards of care in the United Kingdom and internationally. Eye. 2009;23:38–49. doi: 10.1038/sj.eye.6703015. [DOI] [PubMed] [Google Scholar]
- 162.Lichtinger A, Rootman DS. Intraocular lenses for presbyopia correction: past, present, and future. Curr Opin Ophthalmol. 2012;23:40–6. doi: 10.1097/ICU.0b013e32834cd5be. [DOI] [PubMed] [Google Scholar]
- 163.Montes-Mico R, Ferrer-Blasco T, Charman WN, Cervino A, Alfonso JF, Fernandez-Vega L. Optical quality of the eye after lens replacement with a pseudoaccommodating intraocular lens. J Cataract Refract Surg. 2008;34:763–8. doi: 10.1016/j.jcrs.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 164.Alio JL, Pinero DP, Plaza-Puche AB, Chan MJ. Visual outcomes and optical performance of a monofocal intraocular lens and a new-generation multifocal intraocular lens. J Cataract Refract Surg. 2011;37:241–50. doi: 10.1016/j.jcrs.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 165.Yoon CH, Shin IS, Kim MK. Trifocal versus bifocal diffractive intraocular lens implantation after cataract surgery or refractive lens exchange: a meta-analysis. J Korean Med Sci. 2018;33:e275. doi: 10.3346/jkms.2018.33.e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rosen E, Alio JL, Dick HB, Dell S, Slade S. Efficacy and safety of multifocal intraocular lenses following cataract and refractive lens exchange: metaanalysis of peer-reviewed publications. J Cataract Refract Surg. 2016;42:310–28. doi: 10.1016/j.jcrs.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 167.Alio JL. Presbyopic lenses: evidence, masquerade news, and fake news. Asia Pac J Ophthalmol. 2019;8:273–4. doi: 10.1097/01.APO.0000577792.28242.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rocha KM. Extended depth of focus IOLs: the next chapter in refractive technology? J Refract Surg. 2017;33:146–9. doi: 10.3928/1081597X-20170217-01. [DOI] [PubMed] [Google Scholar]
- 169.Tarib I, Diakonis VF, Breyer D, Hohn F, Hahn U, Kretz FTA. Outcomes of combining a trifocal and a low-addition bifocal intraocular lens in patients seeking spectacle independence at all distances. J Cataract Refract Surg. 2019;45:620–9. doi: 10.1016/j.jcrs.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 170.Dick HB, Piovella M, Vukich J, Vilupuru S, Lin L, Clinical I. Prospective multicenter trial of a small-aperture intraocular lens in cataract surgery. J Cataract Refract Surg. 2017;43:956–68. doi: 10.1016/j.jcrs.2017.04.038. [DOI] [PubMed] [Google Scholar]
- 171.Liang YL, Jia SB. Clinical application of accommodating intraocular lens. Int J Ophthalmol. 2018;11:1028–37. doi: 10.18240/ijo.2018.06.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Alio JL, Alio Del Barrio JL, Vega-Estrada A. Accommodative intraocular lenses: where are we and where we are going. Eye Vis. 2017;4:16. doi: 10.1186/s40662-017-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ale JB, Manns F, Ho A. Paraxial analysis of the depth of field of a pseudophakic eye with accommodating intraocular lens. Optom Vis Sci. 2011;88:789–94. doi: 10.1097/OPX.0b013e318219c155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Alio JL, Ben-Nun J. Study of the force dynamics at the capsular interface related to ciliary body stimulation in a primate model. J Refract Surg. 2015;31:124–8. doi: 10.3928/1081597X-20150122-08. [DOI] [PubMed] [Google Scholar]
- 175.Alio JL, Ben-nun J, Rodriguez-Prats JL, Plaza AB. Visual and accommodative outcomes 1 year after implantation of an accommodating intraocular lens based on a new concept. J Cataract Refract Surg. 2009;35:1671–8. doi: 10.1016/j.jcrs.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 176.Alio JL, Simonov AN, Romero D, Angelov A, Angelov Y, van Lawick W, et al. Analysis of accommodative performance of a new accommodative intraocular lens. J Refract Surg. 2018;34:78–83. doi: 10.3928/1081597X-20171205-01. [DOI] [PubMed] [Google Scholar]
- 177.Alio JL, Simonov A, Plaza-Puche AB, Angelov A, Angelov Y, van Lawick W, et al. Visual outcomes and accommodative response of the lumina accommodative intraocular lens. Am J Ophthalmol. 2016;164:37–48. doi: 10.1016/j.ajo.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 178.Sheppard AL, Bashir A, Wolffsohn JS, Davies LN. Accommodating intraocular lenses: a review of design concepts, usage and assessment methods. Clin Exp Optom. 2010;93:441–52. doi: 10.1111/j.1444-0938.2010.00532.x. [DOI] [PubMed] [Google Scholar]
- 179.Solomon KD, Fernandez de Castro LE, Sandoval HP, Biber JM, Groat B, Neff KD, et al. LASIK world literature review: quality of life and patient satisfaction. Ophthalmology. 2009;116:691–701. doi: 10.1016/j.ophtha.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 180.Kanellopoulos AJ, Asimellis G. Combined laser in situ keratomileusis and prophylactic high-fluence corneal collagen crosslinking for high myopia: two-year safety and efficacy. J Cataract Refract Surg. 2015;41:1426–33. doi: 10.1016/j.jcrs.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 181.Tomita M, Yoshida Y, Yamamoto Y, Mita M, Waring Gt. In vivo confocal laser microscopy of morphologic changes after simultaneous LASIK and accelerated collagen crosslinking for myopia: one-year results. J Cataract Refract Surg. 2014;40:981–90. doi: 10.1016/j.jcrs.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 182.Cheema AS, Mozayan A, Channa P. Corneal collagen crosslinking in refractive surgery. Curr Opin Ophthalmol. 2012;23:251–6. doi: 10.1097/ICU.0b013e3283543cbd. [DOI] [PubMed] [Google Scholar]
- 183.Ewe SY, Abell RG, Vote BJ. Femtosecond laser-assisted versus phacoemulsification for cataract extraction and intraocular lens implantation: clinical outcomes review. Curr Opin Ophthalmol. 2018;29:54–60. doi: 10.1097/ICU.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 184.Ding L, Knox WH, Buhren J, Nagy LJ, Huxlin KR. Intratissue refractive index shaping (IRIS) of the cornea and lens using a low-pulse-energy femtosecond laser oscillator. Investig Ophthalmol Vis Sci. 2008;49:5332–9. doi: 10.1167/iovs.08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Whitman J, Hovanesian J, Steinert RF, Koch D, Potvin R. Through-focus performance with a corneal shape-changing inlay: One-year results. J Cataract Refract Surg. 2016;42:965–71. doi: 10.1016/j.jcrs.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 186.Beer SMC, Werner L, Nakano EM, Santos RT, Hirai F, Nitschke EJ, et al. A 3-year follow-up study of a new corneal inlay: clinical results and outcomes. Br J Ophthalmol. 2020;104:723–8. doi: 10.1136/bjophthalmol-2019-314314. [DOI] [PubMed] [Google Scholar]
- 187.Seyeddain O, Bachernegg A, Riha W, Rückl T, Reitsamer H, Grabner G, et al. Femtosecond laser-assisted small-aperture corneal inlay implantation for corneal compensation of presbyopia: two-year follow-up. J Cataract Refract Surg. 2013;39:234–41. doi: 10.1016/j.jcrs.2012.09.018. [DOI] [PubMed] [Google Scholar]