Abstract
Background
The bone-derived protein osteocalcin (OC), in its undercarboxylated (ucOC) form, has a beneficial effect on energy metabolism and may be a future therapeutic target for metabolic diseases. Increasing evidence suggests a link between ucOC and cardiovascular disease (CVD) development; however, the exact relationship is conflicting and unclear.
Scope of review
The aim of this review was to summarise the current research examining the interaction between OC and vascular dysfunction, the initiating stage in the development of atherosclerosis and CVD.
Major conclusions
In humans, the association between OC and vascular function is inconsistent. Several studies report that total OC (tOC) is associated with adverse function or beneficial function, whereas others report that tOC and ucOC has no effect on vascular function. The conflicting data are likely due to several methodological inconsistencies, in particular the lack of studies reporting circulating ucOC levels. In animal models, the direct administration of ucOC to isolated blood vessels ex vivo produced minimal changes in endothelial function, but importantly, no adverse responses. Finally, in human endothelial and vascular smooth muscle cells, ucOC treatment did not influence classical markers of cellular function, including endothelin-1, vascular adhesion molecule-1 and monocyte chemoattractant protein-1 after exposure to high glucose and inflammatory conditions. The lack of adverse effects in ex vivo and in vitro studies suggests that ucOC may be targeted as a future therapeutic for metabolic diseases, without the risk of detrimental effects in the vasculature. However, further studies are needed to confirm these findings and to investigate whether there is a direct beneficial influence of ucOC.
Keywords: Undercarboxylated osteocalcin, Endothelial function, Atherosclerosis, Bone-vascular crosstalk
Highlights
-
•
ucOC is implicated in the regulation of glucose homeostasis; but its role in the vasculature has been minimally reported.
-
•
Studies which examine the association between ucOC and vascular function in humans often report inconsistent outcomes.
-
•
In addition, ex vivo and in vitro studies have reported that ucOC likely does not directly regulate endothelial function.
-
•
ucOC may be targeted as a therapeutic treatment for metabolic diseases without a risk of adverse effects in the vasculature.
1. Introduction
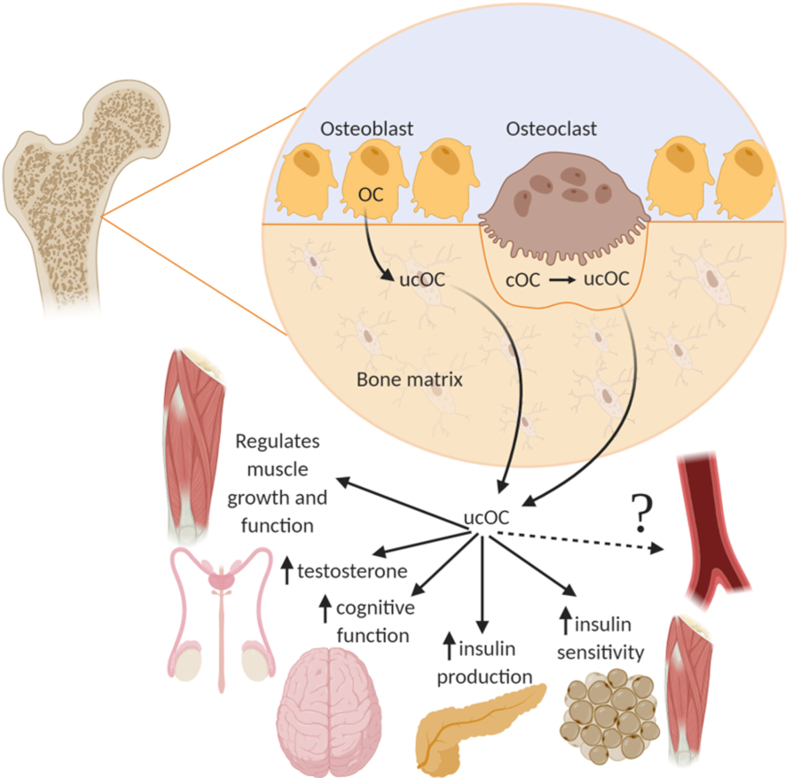
Osteocalcin (OC) is a vitamin K-dependent, osteoblast-derived protein that is commonly used as a marker of bone remodelling [1,2]. OC exists primarily in two forms; undercarboxylated osteocalcin (ucOC) and carboxylated osteocalcin (cOC), with each form suggested to have distinct functions [1]. Total OC (tOC), which is a combination of circulating ucOC and cOC, is more often reported because it is easier to measure. ucOC is thought to be the bioactive form of OC, involved in the regulation of glucose homeostasis and energy metabolism (Figure 1) [[3], [4], [5], [6], [7]]. This has led some to suggest that ucOC may be a future therapeutic target for metabolic diseases, such as insulin resistance and diabetes [[8], [9], [10]]. Despite this, several recent studies have challenged these findings and indicate that ucOC may not be as biologically active as initially proposed [11,12].
Figure 1.
Proposed biological functions of ucOC outside of the skeleton. ucOC is released into the circulation where it is thought to regulate biological processes in a number of tissues, such as skeletal muscle, testes, brain, pancreas and adipose tissue. Growing evidence also suggests it is involved in the vasculature. Created with BioRender.com.; Abbreviations - cOC: carboxylated osteocalcin, ucOC: undercarboxylated osteocalcin.
Further understanding the biological functions of ucOC is needed, particularly whether it may have off-target effects, if it is to be considered therapeutically. Increasing evidence suggests an association between tOC/ucOC and cardiovascular disease (CVD) development; however, the evidence is often conflicting and inconsistent, with some reports of adverse associations [8,13]. For example, in 2017, a systematic review and meta-analysis concluded that the association between tOC and atherosclerosis outcomes in humans was unclear [14]. In 2018, we published a review analysing the potential effect of tOC and ucOC on vascular outcomes in experimental studies and reported that while tOC treatment in vivo caused beneficial effects on CVD outcomes, this was often associated with simultaneous improvements in metabolic outcomes [15]. Therefore, whether the effect of tOC/ucOC on vascular function is indirect, i.e., the improvement in blood vessels function is due to lower glucose, or whether there is a direct effect of tOC/ucOC on the vasculature was unknown (Figure 1). This mini-review examines the most up-to-date literature on the direct interaction between OC (tOC and ucOC) and vascular function in cross-sectional, ex vivo and in vitro studies, discusses the potential limitations of these studies and identifies gaps for future research.
2. The association between OC and blood vessel function in humans
Endothelial dysfunction is the initial stage in the development of atherosclerosis and is a significant predictor of future adverse CVD events [16]. The majority of previous studies have reported the association of OC with the later stages of atherosclerotic plaque development, calcification and CVD events, often with conflicting findings [14,[17], [18], [19]]. However, given the importance of blood vessel dysfunction in the development of atherosclerosis, an increasing number of studies have begun to investigate the association of OC with endothelial and smooth muscle cell function (Table 1) [[20], [21], [22], [23], [24], [25], [26], [27]]. These studies will be discussed at length below.
Table 1.
Association of OC with blood vessel function and stiffness in humans.
| First Author, Year (Ref) | Participant characteristics (men/women) | Measurement of vascular function and OC | Results | Association of OC with blood vessel function |
|---|---|---|---|---|
| Chi, 2019 [27] |
n: 62 (21/41) Age: 56 Health Status: ESRD on peritoneal dialysis |
Vascular: PWV tOC: ELISA |
Higher tOC associated with higher PWV | ↑ |
| Kanazawa, 2009 [21] |
n: 328 (179/149 PM) Age: 65 - 67 Health status: T2DM |
Vascular: PWV tOC: RIA |
Higher tOC associated with lower PWV in men No association in women |
↓,↔ |
| Lin, 2020 [24] |
n: 68 (34/34) Age: 47 Health status: Kidney transplant |
Vascular: VRI tOC: ELISA |
Higher tOC associated with lower VRI | ↑ |
| Millar, 2020 [20] |
n: 48 (28/20) Age: 76 Health status: CKD and controls |
Vascular: PWV tOC: Multiplex assay |
No association between tOC and PWV in those with CKD or controls | ↔ |
| Sumino, 2007 [22] |
n: 85 PM women Age: 58 Health status: Community dwelling |
Vascular: BAFMD tOC: IRMA |
No association between tOC and BAFMD | ↔ |
| Tacey, 2020 [25] |
n: 38 (12/26 PM) Age: 73 Health status: Community dwelling |
Vascular: BAFMD, PWV tOC: ECLIA ucOC: hydroxyapatite binding |
Higher ucOC associated with lower PWV when unadjusted but not after adjustment for confounding variables No association between ucOC and BAFMD and no association of tOC with PWV or BAFMD |
↔ |
| Tacey, 2020 [26] |
n: 30 (20/10) Age: 62 Health status: Mild hypertension ∗Participants fed high vit K diet for 4-weeks |
Vascular: PWV tOC: ECLIA ucOC & cOC: hydroxyapatite binding |
The reduction in tOC and ucOC by the 4-week high vit K diet did not influence PWV | ↔ |
| Yun, 2016 [23] |
n: 3,604 (1,691/1,913) Age: 51 Health status: Community dwelling |
Vascular: PWV tOC: ECLIA |
Higher tOC associated with lower PWV in men Higher tOC associated with higher PWV in women when unadjusted but no association after adjustment for confounding variables |
↓,↔ |
Association of OC with endothelial function and atherosclerosis outcomes:↑: higher OC associated with adverse vascular function, ↓: higher OC associated with beneficial vascular function, ↔: no correlation between OC and vascular function.
Abbreviations - BAFMD: brachial artery flow-mediated dilation, CKD: chronic kidney disease, ECLIA: electrochemiluminescence immunoassay, ELISA: enzyme-linked immunosorbent assay, ESRD: end stage renal disease, IRMA: immunoradiometric assay, OC: osteocalcin, PM: post-menopausal, PWV: pulse wave velocity, RIA: radioimmunoassay, T2DM: type 2 diabetes mellitus, tOC: total osteocalcin, ucOC: undercarboxylated osteocalcin, Vit K: vitamin K, VRI: vascular reactivity index
Serum tOC, as opposed to ucOC, has been reported in the majority of studies examining the association between OC and vascular function. In older men with type 2 diabetes, but not older women with type 2 diabetes, higher levels of tOC were associated with lower pulse wave velocity (PWV) [21]. This indicates that higher tOC is associated with favourable vascular health, as lower PWV scores represent reduced vascular stiffness [28]. Similarly, in middle- and older-aged community-dwelling individuals, higher tOC was associated with lower PWV in men, but not women, after adjusting for confounding factors, including age, body mass index (BMI) and blood pressure (BP) [23]. In contrast, higher tOC was associated with higher PWV in men and women with end-stage renal disease on peritoneal dialysis [27]. Whereas, in both men and women with stage 3 and 4 chronic kidney disease (CKD), no association was reported between tOC and PWV [20]. Several studies have also examined the association of tOC with endothelial function (Table 1). In community-dwelling post-menopausal women, circulating tOC was not associated with endothelial function as measured by brachial artery flow mediated dilation (BAFMD) [22]. Conflictingly, in middle-aged men and women following a kidney transplant, a vascular reactivity index (VRI) assessment exhibited that those with poor vascular reactivity had significantly higher levels of tOC [24]. Importantly, because circulating OC is cleared by the kidneys, it is possible that OC clearance was reduced in those with altered kidney function. Yet, CKD is also associated with other co-morbidities, such as type 2 diabetes mellitus, which is known to influence circulating OC levels. Given the lack of studies on participants with altered kidney function, whether CKD influences endothelial dysfunction cannot be established, but should be explored in future studies. A major limitation of these studies is that they have reported tOC, but not ucOC, which is thought to be the bioactive form of OC. This may be due to the fact that ucOC is difficult to reliably measure, and the development of novel assays that directly measure ucOC may be important [29,30].
Several recent cross-sectional studies have examined the association of ucOC with measures of vascular function [25,26] (Table 1). Higher circulating levels of ucOC, but not tOC, were associated with lower PWV in older men and women, but when adjusted for BMI and age, no association was present [25]. Interestingly, when split by sex, the unadjusted model revealed that higher ucOC tended to be associated with lower PWV in women, but not men [25], conflicting with the sex differences that occurred in the previous studies which reported tOC. In addition, neither ucOC nor tOC were associated with BAFMD [25]. It is tempting to consider that the absolute circulating levels of OC and/or percentage of ucOC are responsible for these differences. However, as indicated, kidney clearance differences did not appear to affect the vascular responses. Similarly, glomerular filtration rate was not altered despite a reduction in ucOC following a diet of increased leafy green vegetables that are rich in vitamin K, known to reduce the percentage of ucOC in the circulation [31]. As such, it can be hypothesised that this type of diet may have an effect on vascular health. A 4-week leafy green diet intervention in middle-aged adults indeed reduced ucOC and tOC by 30% and 17%, respectively, but no parallel alteration in PWV was observed [26]. Furthermore, there was no correlation between the change in ucOC and the change in PWV, suggesting that ucOC may not be related to vascular stiffness in middle-aged adults [26]. Importantly, the same 4-week leafy green diet had previously been reported to increase circulating nitrate [32], which is likely beneficial to vascular function as nitrate increases nitric oxide bioavailability [33,34]. As such, the reduction in ucOC and the increase in nitrate may have counteracted each other to maintain vascular homeostasis. Additionally, the leafy green diet may have affected the levels of other vitamin K-dependent proteins, such as matrix Gla protein (MGP), which has previously been reported to regulate vascular calcification [35]. These hypotheses should be examined in the future.
Taken together, the most recent evidence in humans considered above is somewhat conflicting but suggests that OC is minimally associated with vascular function, particularly after adjusting for confounding variables. Importantly, differences appear to exist between men and women which must be explored in future studies. One potential cause of the sex-differences may be the increase in bone-remodelling that occurs post-menopause, which causes an increase in circulating OC levels [36]. Further, the number of studies reporting the concentration of ucOC are limited, but any association with tOC may be driven by the change in ucOC. As such, studies which have examined tOC alone must be interpreted with caution as the percentage of ucOC in circulation cannot be established. A limitation of the studies that reported ucOC in this review is that they measured ucOC with the hydroxyapatite binding method, which is an indirect method of measuring ucOC. Assays used to measure ucOC often differ between studies with some techniques not accurately measuring ucOC levels. As such, there is a need for more consistent and reliable assays to measure ucOC in circulation [29,30]. Several other limitations may have also influenced the inconsistent and conflicting findings. Firstly, different methods used to assess vascular function may account for some of the variability, as BAFMD and VRI directly measure endothelial function, whereas PWV is an indirect representation of vascular function and is representative of vascular stiffness and calcification [37,38]. Secondly, several studies were conducted in people with chronic conditions, which may have influenced circulating OC levels and/or vascular function. Overall, the number of studies is lacking and additional studies, in particular interventional studies, are needed to establish whether there is a direct effect of OC, particularly ucOC, on vascular function.
3. Ex vivo evidence for a role of OC in vascular disease
Until recently, limited research has examined the direct effect of OC in isolated vascular tissue ex vivo. Previous in vivo evidence demonstrated that OC administration produced improvements in BP, PWV and nitric oxide bioavailability in animal models [39,40]. However, the OC treatment caused simultaneous reductions in body weight and fasting plasma glucose levels and improved glucose tolerance, circulating lipids and markers of inflammation [39,40]. As such, it was not clear whether the improvements in vascular function were independent of, or dependent on, the metabolic alterations caused by OC [15]. As such, several studies have examined the effect of ucOC on the vasoactivity of isolated vascular tissue, free from the influence of other systems.
To examine the direct biological effect of ucOC on endothelial function, New Zealand White rabbits were fed a 4-week atherogenic diet that has previously been shown to cause endothelial dysfunction [41]. The aortas of the rabbits were isolated and treated with ucOC (10 ng/ml), which caused a small, but significant improvement in endothelium-dependent relaxation in comparison to the control [42]. In a follow-up study, rabbits fed the same atherogenic diet exhibited an increase in circulating blood glucose and the aorta was exposed to high glucose conditions for 2 h to enhance endothelial dysfunction, prior to the administration of ucOC (10 ng/ml or 30 ng/ml) for 5 min [43]. The 10 ng/ml treatment of ucOC produced a trend for an improvement in potency of the endothelium-dependent vasodilator acetylcholine; however, the vasoactivity of the aorta was not significantly altered, either positively or negatively by ucOC [43]. Further, immunohistochemistry analysis of the rabbit aorta following the ex vivo experiments revealed that the ucOC treatment did not alter endothelial signalling molecules, endothelial nitric oxide synthase (eNOS), protein kinase B (Akt) or mammalian target of rapamycin (mTOR) [43]. However, a limitation of this study is that the atherogenic diet and acute high glucose incubation did not cause endothelial dysfunction in these sections of aorta, as was previously shown [41]. In a subsequent study, the vasoactive effect of ucOC dose response curves (0.3 ng/ml to 45 ng/ml) in the carotid arteries of New Zealand White rabbits was examined, following incubation in normal or high glucose media. The ucOC dose response curves did not alter endothelial function at any dose and did not affect the maximal relaxation to endothelium-dependent or endothelium-independent vasodilators [25]. A limitation of these studies is that recombinant mouse ucOC was used to treat the rabbit blood vessels, and there may differences in each species protein sequence, which may account for the lack of a biological effect. Overall, these results suggest that ucOC has no, or minimal, direct influence on endothelial and smooth muscle cell function in the conduit arteries of rabbits. While further research is needed, these findings suggest that the changes in vascular function previously reported in vivo occur indirectly via alterations in metabolic outcomes, not via a direct effect of OC on the vasculature.
4. In vitro evidence for the role of OC in vascular disease
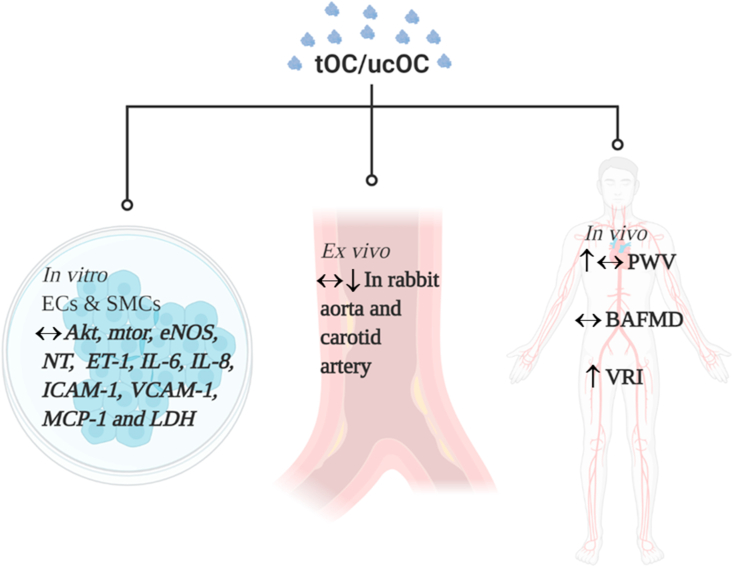
Blood vessel dysfunction is not only characterised by the alteration in vasodilation, but by a number of other pathological effects, including platelet adhesion, inflammation and thrombosis [44]. Following ucOC treatment (0.5–10 ng/ml) in a physiological environment, human aortic endothelial cells (HAECs) and human aortic smooth muscle cells (HASMCs) exhibited increased cell proliferation, but ucOC did not alter classical markers of vascular dysfunction, including cell permeability, adhesion (vascular adhesion molecule 1 (VCAM-1)), or vasoconstriction (endothelin 1 (ET-1)) [45]. Similarly, in HAECs cultured in high glucose (16 mM) media, ucOC (10 ng/ml) did not attenuate the increase in interleukin 6 (IL-6), ET-1, VCAM-1, monocyte chemoattractant protein 1 (MCP-1) or lactate dehydrogenase [43]. Moreover, ucOC (10 ng/ml) did not attenuate the increase in IL-6, interleukin 8 (IL-8), VCAM-1, intracellular adhesion molecule 1 (ICAM-1) or MCP-1 following exposure to acute (24 h) or chronic (144 h) inflammation in HAECs or HASMCs [46]. Together, these in vitro studies suggest that ucOC does not directly influence markers of dysfunction and inflammation in endothelial and smooth muscle cells (Figure 2). However, these findings are limited by the small number of studies and pathological conditions studied. Further investigation is required before definite conclusions can be established.
Figure 2.
The direct effect of tOC/ucOC on vascular function in in vitro and ex vivo and the association of OC with vascular function in humans. ↔ No effect of tOC/ucOC on vascular function, ↓ Beneficial effect of tOC/ucOC on vascular function, ↑ Adverse effect of tOC/ucOC on vascular function. Created with BioRender.com; Abbreviations - Akt: protein kinase b, BAFMD: brachial artery flow-mediated dilation, ECs: endothelial cells, eNOS: endothelial nitric oxide synthase, ET-1: endothelin 1, ICAM-1: intracellular adhesion molecular 1, IL-6: interleukin 6, IL-8: interleukin 8, LDH: lactate dehydrogenase, MCP-1: monocyte chemoattractant protein 1, mTOR: mammalian target of rapamycin, NT: nitrotyrosine, PWV: pulse wave velocity, SMCs: smooth muscle cells, ucOC: undercarboxylated osteocalcin, VCAM-1: vascular cell adhesion molecule 1, VRI: vascular reactivity index.
5. GPRC6A; the putative OC receptor
G-protein-coupled receptors are a diverse family of plasma membrane receptors that mediate a large proportion of cellular responses [47]. G protein-coupled receptor class C group 6 member A (GPRC6A) is suggested as the putative receptor for OC in several tissues, including the testes, β-cells and skeletal muscle [[48], [49], [50], [51], [52]]. Recent studies using genetically modified animal models support this theory [53,54]. In addition, another receptor, G protein-coupled receptor 158 (GPR158), has been identified as the OC receptor in the brain [[55], [56], [57]], suggesting that OC may have multiple receptors. In the vasculature, a study has reported the presence of GPRC6A in HAECs and HASMCs [45]. Further, immunohistochemistry analysis has identified that GPRC6A is present in the endothelium of human and rabbit arteries [42]. However, whether OC interacts with this receptor in the vasculature is presently unknown. Future studies should examine whether GPRC6A is the receptor for OC, whether it is an additional receptor or whether OC does not interact with any receptor in the vasculature [58].
6. Recommendations for future research
As the recent evidence suggests that ucOC has no, or only a small beneficial effect on vascular function, additional studies are needed to confirm these findings, and several suggestions for future research are outlined below. Firstly, as each form of OC mediates diverse biological functions, it is crucial that studies in humans report the circulating concentration of ucOC using a standardised and reliable assay. Secondly, further interventional studies are needed in humans to examine whether changes in circulating OC influence changes in vascular outcomes. As OC currently cannot be administered directly to humans, studies may use vitamin K, corticosteroids, exercise or other factors known to influence the circulating levels of OC [[59], [60], [61]]. Thirdly, mechanistic ex vivo and in vitro studies in both animal models and human tissue need to investigate the effect of longer term ucOC administration in models of disease and dysfunction. Additionally, vascular calcification is a critical later stage of atherosclerosis development and the differentiation of vascular smooth muscle cells into an osteoblast-like phenotype expressing OC is characteristic of this stage. As such, investigating the role of OC, in particular cOC, the isoform of OC most abundant in mineralised tissue, at this stage of atherosclerotic development is of interest and may further elucidate reasons for the differences observed in the in vivo compared to the ex vivo and in vitro studies (Figure 2).
In conclusion, despite conflicting associations in humans, in the studies discussed in this review OC has no, or minimal, direct influence on endothelial and smooth muscle cell function. While the reported beneficial effects of OC on blood vessel function are limited, there have been no reports of adverse effects in in vitro or ex vivo studies. The lack of adverse effects is important if ucOC may be targeted as a future pharmaceutical therapeutic for metabolic diseases, as treatment could be implemented without the risk of detrimental effects to vascular function. Whether there is a direct beneficial influence requires further investigation.
Author contributions
Alexander Tacey: Writing - original draft, Writing - reviewing and editing, preparation of figures. Alan Hayes: Writing - reviewing and editing. Anthony Zulli: Writing - reviewing and editing. Itamar Levinger: Writing - reviewing and editing. All authors approved the final version of manuscript.
Sources of funding
No funding was received for this article.
CONFLICT OF INTEREST
None.
References
- 1.Li J., Zhang H., Yang C., Li Y., Dai Z. An overview of osteocalcin progress. J Bone Miner Metab. Jul 2016;34(4):367–379. doi: 10.1007/s00774-015-0734-7. [DOI] [PubMed] [Google Scholar]
- 2.Zoch M.L., Clemens T.L., Riddle R.C. New insights into the biology of osteocalcin. Bone. Jan 2016;82:42–49. doi: 10.1016/j.bone.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin X., Onda D.A., Yang C.H., Lewis J.R., Levinger I., Loh K. Roles of bone-derived hormones in type 2 diabetes and cardiovascular pathophysiology. Mol Metab. Jun 13 2020;40:101040. doi: 10.1016/j.molmet.2020.101040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dirckx N., Moorer M.C., Clemens T.L., Riddle R.C. The role of osteoblasts in energy homeostasis. Nature Reviews Endocrinology. Nov 2019;15(11):651–665. doi: 10.1038/s41574-019-0246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossi M., Battafarano G., Pepe J., Minisola S., Del Fattore A. The endocrine function of osteocalcin regulated by bone resorption: a lesson from reduced and increased bone mass diseases. Int J Mol Sci. Sep 11 2019;20(18) doi: 10.3390/ijms20184502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferron M., Wei J., Yoshizawa T., Del Fattore A., DePinho R.A., Teti A. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. Jul 23 2010;142(2):296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee N.K., Sowa H., Hinoi E., Ferron M., Ahn J.D., Confavreux C. Endocrine regulation of energy metabolism by the skeleton. Cell. Aug 10 2007;130(3):456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Toni L., Jawich K., De Rocco Ponce M., Di Nisio A., Foresta C. Protein Pept Lett; May 5 2020. Osteocalcin: a protein hormone connecting metabolism, bone and testis function. [DOI] [PubMed] [Google Scholar]
- 9.Ferron M., Hinoi E., Karsenty G., Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. Apr 01 2008;105(13):5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferron M., McKee M.D., Levine R.L., Ducy P., Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. Feb 2012;50(2):568–575. doi: 10.1016/j.bone.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diegel C.R., Hann S., Ayturk U.M., Hu J.C.W., Lim K.E., Droscha C.J. An osteocalcin-deficient mouse strain without endocrine abnormalities. PLoS Genetics. May 2020;16(5) doi: 10.1371/journal.pgen.1008361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moriishi T., Ozasa R., Ishimoto T., Nakano T., Hasegawa T., Miyazaki T. Osteocalcin is necessary for the alignment of apatite crystallites, but not glucose metabolism, testosterone synthesis, or muscle mass. PLoS Genetics. May 2020;16(5) doi: 10.1371/journal.pgen.1008586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Onofrio L., Maddaloni E., Buzzetti R. Diabetes Metab Res Rev; Oct 10 2019. Osteocalcin and sclerostin: background characters or main actors in cardiovascular disease? [DOI] [PubMed] [Google Scholar]
- 14.Millar S.A., Patel H., Anderson S.I., England T.J., O'Sullivan S.E. Osteocalcin, vascular calcification, and atherosclerosis: a systematic review and meta-analysis. Frontiers in Endocrinology. 2017;8:183. doi: 10.3389/fendo.2017.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tacey A., Qaradakhi T., Brennan-Speranza T., Hayes A., Zulli A., Levinger I. Potential role for osteocalcin in the development of atherosclerosis and blood vessel disease. Nutrients. Oct 4 2018;10(10) doi: 10.3390/nu10101426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanhoutte P.M., Shimokawa H., Feletou M., Tang E.H. Endothelial dysfunction and vascular disease - a 30th anniversary update. Acta Physiologica. Jan 2017;219(1):22–96. doi: 10.1111/apha.12646. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y., Ma X., Xiong Q., Zhang X., Shen Y., Bao Y. Osteocalcin value to identify subclinical atherosclerosis over atherosclerotic cardiovascular disease (ASCVD) risk score in middle-aged and elderly Chinese asymptomatic men. Clinical Chemistry and Laboratory Medicine. Oct 25 2018;56(11):1962–1969. doi: 10.1515/cclm-2018-0320. [DOI] [PubMed] [Google Scholar]
- 18.Ling Y., Wang Z., Wu B., Gao X. Association of bone metabolism markers with coronary atherosclerosis and coronary artery disease in postmenopausal women. J Bone Miner Metab. May 2018;36(3):352–363. doi: 10.1007/s00774-017-0841-8. [DOI] [PubMed] [Google Scholar]
- 19.Liu D., Chen L., Dong S., Peng Z., Yang H., Chen Y. Bone mass density and bone metabolism marker are associated with progression of carotid and cardiac calcified plaque in Chinese elderly population. Osteoporosis International. Sep 2019;30(9):1807–1815. doi: 10.1007/s00198-019-05031-5. [DOI] [PubMed] [Google Scholar]
- 20.Millar S.A., John S.G., McIntyre C.W., Ralevic V., Anderson S.I., O'Sullivan S.E. An investigation into the role of osteocalcin in human arterial smooth muscle cell calcification. Frontiers in Endocrinology. 2020;11:369. doi: 10.3389/fendo.2020.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanazawa I., Yamaguchi T., Yamamoto M., Yamauchi M., Kurioka S., Yano S. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab. Jan 2009;94(1):45–49. doi: 10.1210/jc.2008-1455. [DOI] [PubMed] [Google Scholar]
- 22.Sumino H., Ichikawa S., Kasama S., Takahashi T., Sakamoto H., Kumakura H. Relationship between brachial arterial endothelial function and lumbar spine bone mineral density in postmenopausal women. Circulation Journal. Oct 2007;71(10):1555–1559. doi: 10.1253/circj.71.1555. [DOI] [PubMed] [Google Scholar]
- 23.Yun S.H., Kim M.J., Choi B.H., Park K.C., Park K.S., Kim Y.S. Low level of osteocalcin is related with arterial stiffness in Korean adults: an inverse J-shaped relationship. J Clin Endocrinol Metab. Jan 2016;101(1):96–102. doi: 10.1210/jc.2015-2847. [DOI] [PubMed] [Google Scholar]
- 24.Lin L., Chiu L.T., Lee M.C., Hsu B.G. Serum osteocalcin level is negatively associated with vascular reactivity index by digital thermal monitoring in kidney transplant recipients. Medicina (Kaunas) Aug 9 2020;56(8) doi: 10.3390/medicina56080400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tacey A., Smith C., Woessner M.N., Chubb P., Neil C., Duque G. Undercarboxylated osteocalcin is associated with vascular function in female older adults but does not influence vascular function in male rabbit carotid artery ex vivo. PloS One. 2020;15(11) doi: 10.1371/journal.pone.0242774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tacey A., Sim M., Smith C., Woessner M.N., Byrnes E., Lewis J.R. Association between circulating osteocalcin and cardiometabolic risk factors following a 4-week leafy green vitamin K-rich diet. Annals of Nutrition & Metabolism. Nov 24 2020;76(5):1–7. doi: 10.1159/000511660. [DOI] [PubMed] [Google Scholar]
- 27.Chi P.J., Lin Y.L., Tasi J.P., Wang C.H., Hou J.S., Lee C.J. Osteocalcin and carotid-femoral pulse wave velocity in patients on peritoneal dialysis. Ci Ji Yi Xue Za Zhi. Jan-Mar 2019;31(1):23–28. doi: 10.4103/tcmj.tcmj_12_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H.L., Kim S.H. Pulse wave velocity in atherosclerosis. Front Cardiovasc Med. 2019;6:41. doi: 10.3389/fcvm.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacombe J., Al Rifai O., Loter L., Moran T., Turcotte A.F., Grenier-Larouche T. Am J Physiol Endocrinol Metab; Jan 14 2020. Measurement of bioactive osteocalcin in humans using a novel immunoassay reveals association with glucose metabolism and beta-cell function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonneau J., Ferland G., Karelis A.D., Doucet E., Faraj M., Rabasa-Lhoret R. Association between osteocalcin gamma-carboxylation and insulin resistance in overweight and obese postmenopausal women. Journal of Diabetic Complications. Jun 2017;31(6):1027–1034. doi: 10.1016/j.jdiacomp.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 31.Sim M., Lewis J.R., Prince R.L., Levinger I., Brennan-Speranza T.C., Palmer C. The effects of vitamin K-rich green leafy vegetables on bone metabolism: a 4-week randomised controlled trial in middle-aged and older individuals. BoneKEy Reports. Jun 2020;12:100274. doi: 10.1016/j.bonr.2020.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blekkenhorst L.C., Lewis J.R., Prince R.L., Devine A., Bondonno N.P., Bondonno C.P. Nitrate-rich vegetables do not lower blood pressure in individuals with mildly elevated blood pressure: a 4-wk randomized controlled crossover trial. American Journal of Clinical Nutrition. Jun 1 2018;107(6):894–908. doi: 10.1093/ajcn/nqy061. [DOI] [PubMed] [Google Scholar]
- 33.Blekkenhorst L.C., Prince R.L., Ward N.C., Croft K.D., Lewis J.R., Devine A. Development of a reference database for assessing dietary nitrate in vegetables. Molecular Nutrition & Food Research. Aug 2017;61(8) doi: 10.1002/mnfr.201600982. [DOI] [PubMed] [Google Scholar]
- 34.Bondonno C.P., Blekkenhorst L.C., Liu A.H., Bondonno N.P., Ward N.C., Croft K.D. Vegetable-derived bioactive nitrate and cardiovascular health. Mol Aspects Med. Jun 2018;61:83–91. doi: 10.1016/j.mam.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Danziger J. Vitamin K-dependent proteins, warfarin, and vascular calcification. Clinical Journal of the American Society of Nephrology. Sep 2008;3(5):1504–1510. doi: 10.2215/CJN.00770208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu W.W., Zhang Z., He J.W., Fu W.Z., Wang C., Zhang H. Establishing reference intervals for bone turnover markers in the healthy shanghai population and the relationship with bone mineral density in postmenopausal women. Int J Endocrinol. 2013;2013:513925. doi: 10.1155/2013/513925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frolow M., Drozdz A., Kowalewska A., Nizankowski R., Chlopicki S. Comprehensive assessment of vascular health in patients; towards endothelium-guided therapy. Pharmacological Reports. Aug 2015;67(4):786–792. doi: 10.1016/j.pharep.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Haydar A.A., Covic A., Colhoun H., Rubens M., Goldsmith D.J. Coronary artery calcification and aortic pulse wave velocity in chronic kidney disease patients. Kidney International. May 2004;65(5):1790–1794. doi: 10.1111/j.1523-1755.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- 39.Dou J., Li H., Ma X., Zhang M., Fang Q., Nie M. Osteocalcin attenuates high fat diet-induced impairment of endothelium-dependent relaxation through Akt/eNOS-dependent pathway. Cardiovascular Diabetology. Apr 07 2014;13:74. doi: 10.1186/1475-2840-13-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang L., Yang L., Luo L., Wu P., Yan S. Osteocalcin improves metabolic profiles, body composition and arterial stiffening in an induced diabetic rat model. Experimental and Clinical Endocrinology & Diabetes. Apr 2017;125(4):234–240. doi: 10.1055/s-0042-122138. [DOI] [PubMed] [Google Scholar]
- 41.Tacey A., Qaradakhi T., Smith C., Pittappillil C., Hayes A., Zulli A. The effect of an atherogenic diet and acute hyperglycaemia on endothelial function in rabbits is artery specific. Nutrients. 2020;12(7):2108. doi: 10.3390/nu12072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qaradakhi T., Gadenec L.K., Tacey A.B., Hare D.L., Buxton B.F., Apostolopoulos V. The effect of recombinant undercarboxylated osteocalcin on endothelial dysfunction. Calcified Tissue International. Nov 2019;105(5):546–556. doi: 10.1007/s00223-019-00600-6. [DOI] [PubMed] [Google Scholar]
- 43.Tacey A., Millar S., Qaradakhi T., Smith C., Hayes A., Anderson S. Undercarboxylated osteocalcin has no adverse effect on endothelial function in rabbit aorta or human vascular cells. J Cell Physiol. Sep 16 2020;236(4) doi: 10.1002/jcp.30048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esper R.J., Nordaby R.A., Vilarino J.O., Paragano A., Cacharron J.L., Machado R.A. Endothelial dysfunction: a comprehensive appraisal. Cardiovascular Diabetology. Feb 23 2006;5(4) doi: 10.1186/1475-2840-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Millar S.A., Anderson S.I., O'Sullivan S E. Human vascular cell responses to the circulating bone hormone osteocalcin. J Cell Physiol. Nov 2019;234(11):21039–21048. doi: 10.1002/jcp.28707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Millar S.A., Zala I., Anderson S.I., O'Sullivan S.E. Osteocalcin does not influence acute or chronic inflammation in human vascular cells. J Cell Physiol. Sep 24 2019;235(4) doi: 10.1002/jcp.29231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenbaum D.M., Rasmussen S.G., Kobilka B.K. The structure and function of G-protein-coupled receptors. Nature. May 21 2009;459(7245):356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pi M., Kapoor K., Ye R., Nishimoto S.K., Smith J.C., Baudry J. Evidence for osteocalcin binding and activation of GPRC6A in beta-cells. Endocrinology. May 2016;157(5):1866–1880. doi: 10.1210/en.2015-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pi M., Nishimoto S.K., Quarles L.D. GPRC6A: jack of all metabolism (or master of none) Mol Metab. Feb 2017;6(2):185–193. doi: 10.1016/j.molmet.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mera P., Laue K., Wei J., Berger J.M., Karsenty G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol Metab. Oct 2016;5(10):1042–1047. doi: 10.1016/j.molmet.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oury F., Khrimian L., Denny C.A., Gardin A., Chamouni A., Goeden N. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. Sep 26 2013;155(1):228–241. doi: 10.1016/j.cell.2013.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oury F., Sumara G., Sumara O., Ferron M., Chang H., Smith C.E. Endocrine regulation of male fertility by the skeleton. Cell. Mar 4 2011;144(5):796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pi M., Xu F., Ye R., Nishimoto S.K., Kesterson R.A., Williams R.W. Humanized GPRC6A(KGKY) is a gain-of-function polymorphism in mice. Scientific Reports. Jul 7 2020;10(1):11143. doi: 10.1038/s41598-020-68113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pi M., Xu F., Ye R., Nishimoto S.K., Williams R.W., Lu L. Role of GPRC6A in regulating hepatic energy metabolism in mice. Scientific Reports. Apr 29 2020;10(1):7216. doi: 10.1038/s41598-020-64384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khrimian L., Obri A., Karsenty G. Modulation of cognition and anxiety-like behavior by bone remodeling. Mol Metab. Dec 2017;6(12):1610–1615. doi: 10.1016/j.molmet.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khrimian L., Obri A., Ramos-Brossier M., Rousseaud A., Moriceau S., Nicot A.S. Gpr158 mediates osteocalcin's regulation of cognition. Journal of Experimental Medicine. Oct 2 2017;214(10):2859–2873. doi: 10.1084/jem.20171320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kosmidis S., Polyzos A., Harvey L., Youssef M., Denny C.A., Dranovsky A. RbAp48 protein is a critical component of GPR158/OCN signaling and ameliorates age-related memory loss. Cell Reports. Oct 23 2018;25(4):959–973. doi: 10.1016/j.celrep.2018.09.077. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jorgensen C.V., Brauner-Osborne H. Pharmacology and physiological function of the orphan GPRC6A receptor. Basic and Clinical Pharmacology and Toxicology. Feb 14 2020;126 doi: 10.1111/bcpt.13397. [DOI] [PubMed] [Google Scholar]
- 59.Binkley N., Harke J., Krueger D., Engelke J., Vallarta-Ast N., Gemar D. Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density, or geometry in healthy postmenopausal North American women. Journal of Bone and Mineral Research. Jun 2009;24(6):983–991. doi: 10.1359/JBMR.081254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levinger I., Jerums G., Stepto N.K., Parker L., Serpiello F.R., McConnell G.K. The effect of acute exercise on undercarboxylated osteocalcin and insulin sensitivity in obese men. Journal of Bone and Mineral Research. Dec 2014;29(12):2571–2576. doi: 10.1002/jbmr.2285. [DOI] [PubMed] [Google Scholar]
- 61.Parker L., Lin, Garnham A., McConnell G., Stepto N.K., Hare D.L. Glucocorticoid-induced insulin resistance in men is associated with suppressed undercarboxylated osteocalcin. Journal of Bone and Mineral Research. Aug 23 2019;34(1) doi: 10.1002/jbmr.3574. [DOI] [PubMed] [Google Scholar]