Abstract
Convection‐enhanced delivery (CED) describes a direct method of drug delivery to the brain through intraparenchymal microcatheters. By establishing a pressure gradient at the tip of the infusion catheter in order to exploit bulk flow through the interstitial spaces of the brain, CED offers a number of advantages over conventional drug delivery methods—bypass of the blood–brain barrier, targeted distribution through large brain volumes and minimization of systemic side effects. Despite showing early promise, CED is yet to fulfill its potential as a mainstream strategy for the treatment of neurological disease. Substantial research effort has been dedicated to optimize the technology for CED and identify the parameters, which govern successful drug distribution. It seems likely that successful clinical translation of CED will depend on suitable catheter technology being used in combination with drugs with optimal physicochemical characteristics, and on neuropathological analysis in appropriate preclinical models. In this review, we consider the factors most likely to influence the success or failure of CED, and review its application to the treatment of high‐grade glioma, Parkinson's disease (PD) and Alzheimer's disease (AD).
Keywords: Alzheimer's disease, blood–brain barrier, convection‐enhanced delivery, drug delivery, glioma, Parkinson's disease, perivascular spaces
Introduction
Convection‐enhanced drug delivery (CED) describes the direct infusion of drugs into the brain parenchyma through microcatheters 9. By using highly controlled flow rates to establish a pressure gradient at the tip of the infusion catheter, this technique allows drugs that do not cross the blood–brain barrier (BBB) to be delivered in therapeutic concentrations throughout large volumes of brain tissue, while minimizing systemic exposure. CED also has application for the delivery of drugs that cause intolerable or unwanted side effects when administered systemically at concentrations required to cross the BBB.
CED differs from traditional methods of intraparenchymal drug delivery, which usually involve the injection of agents into the brain and rely upon simple diffusion into adjacent tissue along a concentration gradient (Figure 1A). The “convective” nature of CED reflects the fact that infusates carried by a pressure gradient will reach distances approximately proportionate to the duration of infusion, whereas an agent carried by diffusion will reach distances proportional to the square root of the time elapsed.
Figure 1.
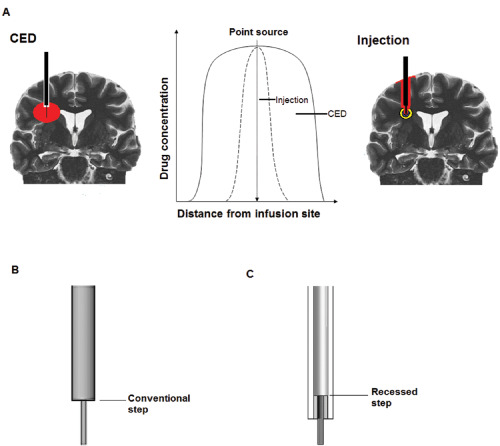
A. Convection‐enhanced delivery differs in both technique and technology from conventional methods of drug injection. By use of ultrafine microcatheters, minimally traumatic implantation techniques and highly controlled infusion rates, CED facilitates homogeneous drug distribution through large, clinically relevant brain volumes. In contrast, local injection methods are associated with significant reflux of drug, tissue trauma and inhomogeneous drug distribution, which is reliant on diffusion rather than bulk flow through the interstitial spaces of the brain. B. Conventional “stepped” catheter designs incorporate an abrupt change in catheter diameter proximal to catheter tip which acts to reduce reflux. C. We recently described a novel “recessed‐step” catheter in which the stepped element is held within an outer guide tube.
The concept of pressure‐mediated intraparenchymal drug infusion was first described in the early 1990s, but is yet to fulfill its potential as a mainstream therapeutic strategy for neuro‐oncological and neurodegenerative diseases. Extensive preclinical and early clinical studies have demonstrated that effective CED depends upon a number of parameters—the diameter of the catheter, the catheter implantation method, the rate of infusion, the physicochemical characteristics of the infusate, and the cytoarchitecture of the targeted brain tissue or structure.
Successful clinical translation of CED is likely to require an in‐depth understanding of all of these parameters, as well as the use of appropriate preclinical models and detailed neuropathological and neuroradiological analysis of drug effect and distribution. In this review, we consider the factors that are most likely to influence the success or failure of CED, and review its application to the treatment of high‐grade glioma, Parkinson's disease (PD) and Alzheimer's disease (AD).
Catheter Technology and Implantation Methods
One of the major barriers to effective clinical translation of CED is the incidence of infusate reflux or “backflow” along the catheter/brain interface. Reflux reduces the chances of achieving therapeutic drug concentrations in the target structure and increases the risk of off‐target side effects. The deleterious consequences of infusate reflux were clearly demonstrated in clinical trials of CED of paclitaxel for high grade brain tumors, which was associated with leakage of the drug into the subarachnoid spaces and chemical meningitis 45.
Preclinical studies have identified three parameters relating to catheter design and implantation method, which significantly increase the risk of reflux—catheter diameter, tissue trauma on catheter implantation and the speed of catheter insertion. Chen et al 16 were the first to analyze systematically the effect of catheter diameter on the incidence of reflux, and correlated smaller catheter diameter with reduced reflux. It seemed likely that larger bore catheters cause more tissue trauma on implantation, reducing the effectiveness of the seal at the catheter/brain interface. This hypothesis was tested and confirmed in subsequent preclinical and clinical studies, which found effective drug distribution in the target structure to be achievable with catheters less than 1 mm in diameter 56, 90. CED studies in agarose brain phantoms correlated reduced reflux with increased speed of catheter implantation—an effect also likely to be mediated by reduced “tissue” displacement on catheter insertion and consequently reduced trauma away from the catheter itself 15.
A further refinement was the introduction of “stepped” catheters, which facilitate high‐flow infusions without reflux 39, 92. A stepped catheter incorporates an abrupt change from a large to a small diameter proximal to the catheter tip (Figure 1B). A stepped catheter design was used to good effect in clinical trials delivering virally mediated gene therapy into the putamen for the treatment of PD 18. The mechanisms by which a step reduces the risk of reflux are not fully understood but are likely to be related to the complex relationship between focal compression of tissue to create a seal, and reductions in the hydrostatic pressure of infused fluids. We recently described a novel “recessed‐step” catheter, which incorporates a step internalized within an outer guide tube and has been used to deliver high‐volume, high‐flow rate infusions into a brain stem glioma without evidence of reflux 6 (Figure 1C). This “recessed‐step” design showed superior reflux resistance to a conventional stepped catheter, both in vitro and in vivo in a large animal (porcine) model 23.
Infusate Characteristics
The distribution of a drug delivered to the brain by CED is fundamentally reliant upon the physicochemical properties of the infused agent. By employing a pressure‐mediated infusion regime, CED aims to exploit bulk flow through the interstitial spaces of the brain. This potentially allows infused agents over a wide range of molecular weights, including particles and viral constructs, to be distributed effectively within the brain 9, 17, 41, 55.
The surface properties, and resultant tissue affinity, of the infused molecule appear to be far more important than molecular weight in influencing the extent of distribution in the brain 17. For example, cationic liposomes do not distribute effectively in the brain when delivered by CED because of their inherent affinity for the cell membrane 49, 69. Shielding the positive charge of cationic liposomes to confer either neutrality or anionic charge significantly improves distribution 37, 49, 69.
The presence of tissue‐binding moieties within the structure of an infused molecule is also a significant determinant of distribution. Both adeno‐associated virus serotype 2 (AAV2) and glial cell line‐derived neurotrophic factor (GDNF) contain heparin‐binding sites, which have an affinity for the heparan sulfates of the extracellular matrix within the brain. Co‐infusion of heparin was shown to improve the distribution of both AAV2 and GDNF in preclinical studies, by competitively binding the receptors 28, 59. The accumulation of amyloid‐β (Aβ) in the extracellular spaces of the brain in AD, en route from neurons to blood vessels, presumably reflects constraints similar to those preventing the distribution of molecules with high tissue affinity by CED 88, 89.
It seems likely that particulate therapies that are neutral or anionic, and hydrophilic molecules with low tissue affinity will be most effectively distributed in the brain by CED. However, these same characteristics may hamper their translational potential by resulting in rapid interstitial clearance from the brain—a problem we encountered in preclinical studies of CED of neprilysin (see below) 5. The tissue half‐life of drugs delivered to the brain by CED was reported to be as short as 7 h, which introduces a potential need for intermittent drug delivery and implantable CED catheter systems 2. We recently described the development of an implantable CED system, which facilitates chronic intermittent drug delivery to the brain at intervals determined by the tissue and biological half‐life of the infusate, through a transcutaneous bone‐anchored port 7 (Figure 2).
Figure 2.

Intermittent delivery of non‐gene therapies may be necessary for drugs with relatively short biological or tissue half‐lives. We recently developed an implantable CED catheter system, which facilitates chronic intermittent drug delivery to the brain through a transcutaneous bone‐anchored port.
Infusion Rates and Ramping Regimes
The ability to tightly control and titrate the rate of infusion is fundamentally important for successful CED. In two of the earliest analyses of the effect of infusion rate on distribution, higher flow rates (1 and 5 μL/minute) were associated with significantly greater reflux 16, 54. However, these early studies predated the use of stepped catheters, which have been effective in inhibiting reflux in large animal preclinical and clinical studies at flow rates of up to 10 μL/minute 6, 8, 23, 93. The facility to use relatively high flow rates is of paramount importance for clinical translation because high flow rates offer the potential to limit the duration of infusions to timescales, which are acceptable to patients, without compromising volumes of distribution. There is, however, a balance between using high infusion rates and increasing the risk of trauma because of excess pressure at the catheter tip. The maximum safe infusion rate for CED is currently under investigation and is likely to be dependent upon the diameter of the catheter used, as well as the nature of the target structure in the brain (gray matter, white matter or tumor).
The use of ramping regimes (slow, stepwise increases in infusion rate) has been postulated to reduce the risk of reflux and enhance distribution by inducing gradual expansion of the extracellular spaces of the brain 4, 76. Although no comprehensive comparison of ramped and nonramped infusion regimes has been reported, there is some anecdotal evidence from preclinical studies, which supports the use of ramping in clinical trials 8.
Influence of the Perivascular Spaces and Cytoarchitecture of Targeted Brain Regions
The relationship between the perivascular (Virchow–Robin) and subarachnoid spaces of the brain has been determined from histological, ultrastructural and tracer studies. The pia mater is reflected from the surface of the human brain on to the outer aspect of arteries and veins in the subarachnoid space, thereby separating the subarachnoid space from the subpial space and from perivascular spaces in the brain by a thin sheet of pial cells. A layer of pia mater coats arteries as they enter the brain and separates the artery wall from the surrounding brain 30, 31. The concept of “paravascular” fluid drainage through the brain arose from experimental evidence that demonstrated the rapid transit of tracers in cerebrospinal fluid (CSF) throughout the brain in parallel to the cerebral microvasculature 25, 66. By infusing horseradish peroxidase protein tracer into the CSF of cats and dogs, Rennels et al 67 were able to demonstrate consistent distribution throughout the perivascular space in association with large vessels, arterioles, capillaries and venules. The rapid transit of tracer could be completely abolished by occluding the aorta or brachiocephalic artery, which resulted in reduced cerebral artery pulsatility. Rennels et al 66 concluded that the apparently convective nature of fluid drainage was mediated by transmission of arterial pulsations through the perivascular space.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) offered an ultrastructural insight into the relationship of the perivascular spaces to the microvasculature 1, 64, 94. Pollock et al 64 compared the ultrastructural anatomy of the human anterior perforated substance and basal ganglia with that of the cerebral cortex, and identified differences in the relationship of the perivascular space to the vasculature in these regions of the brain. In the anterior perforated substance, the inner layer of the leptomeninges was closely related to the adventitia of the vessel wall, while the outer layer was continuous with the cortical pia mater. In contrast, arteries in the basal ganglia were surrounded by two leptomeningeal layers separated by a perivascular space, which is continuous with the perivascular space around arteries in the subarachnoid space, but not with the subarachnoid space itself 64.
To optimize therapeutic drug delivery by CED, it is necessary to understand fully the ultrastructural anatomy and physiology, which govern the dynamics of fluid flow through the interstitial and perivascular spaces. Early in vivo studies of CED attributed the distribution of macromolecules to interstitial flow and neuronal transport 40, 46, 95. However, as research progressed to include the delivery of liposomes and viral vectors, it became clear that much of the distribution of agents delivered by CED was in close association with blood vessels and perivascular spaces 38, 86.
To test the hypothesis that CED relies upon cerebrovascular pulsatility to drive infused agents through the perivascular space, Hadaczek et al 27 designed an experiment to assess whether manipulation of cardiovascular parameters would alter the volume of distribution of agents delivered by CED. In their study, a viral vector, liposomes and bovine serum albumin were delivered into the striatum in three groups of rats—a normotensive group, a hypertensive group treated with epinephrine and a no‐heartbeat third group in which the animals were euthanized immediately prior to infusion. The volume of distribution was up to 14 times higher in the hypertensive than the no‐heartbeat group. This study also concluded that the most informative cardiovascular parameter was pulse pressure, which correlated most significantly with the distribution of infused agents.
In vivo real‐time magnetic resonance imaging (MRI) of CED has offered further insight into the importance of the perivascular space in the distribution of molecules delivered by CED, particularly to the basal ganglia 38. Krauze et al delineated a distinct putaminal perivascular pathway by real‐time MRI of liposomal CED in primates 38, and postulated that variation in perivascular ultrastructure could explain the differences in drug distribution seen when CED is targeted to the putamen compared with targeting of white matter and cortex. Certainly, this variation in ultrastructure has been identified by previous studies 64. We found that intrastriatal CED of model solutes results in rapid and widespread perivascular distribution and uptake by perivascular macrophages, irrespective of molecular weight, co‐infusion of vasodilators and infusion regime 4 (Figure 3).
Figure 3.
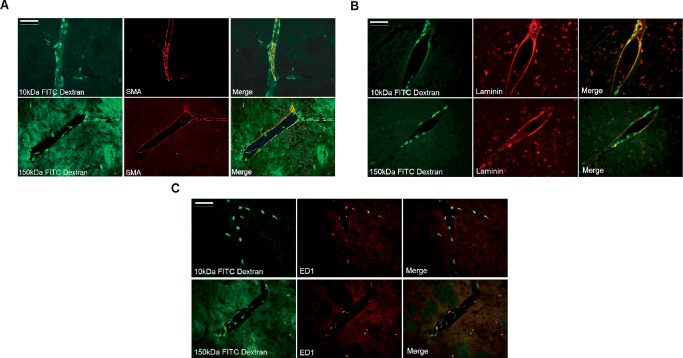
Intrastriatal CED of a model solute (FITC dextran, green) in rat brain resulted in widespread perivascular distribution irrespective of molecular weight. Colocalization with smooth muscle (SMA, red, A) in the walls of arteries and arterioles, laminin (red, B) and ED1‐positive perivascular cells (red, C) was detectable at very early time points after infusion (scale bar = 100 μm). Reprinted from Barua et al 4. Copyright held by the authors (2012).
These studies add to previous findings that indicate the importance of assessing the consequences of perivascular drug distribution and uptake when considering the translational potential of molecules for CED. The precise perivascular location of drugs delivered to the brain by CED varies according to the physicochemical characteristics of the infusate: interstitial fluid and low molecular weight solutes drain from the interstitial spaces of the brain along the basement membranes of capillaries and arteries 82, whereas infusates of particles with diameters exceeding 20 nm accumulate in the potential space between the outer wall of arteries and the glia limitans of the surrounding brain 13.
While an understanding of the ultrastructural anatomy of the perivascular and interstitial spaces of the brain is clearly relevant to achieving effective CED, the macroscopic anatomy of the targeted brain structure and adjacent structures is also important. High‐volume CED infusions in a canine model of spontaneous glioma were associated with ventricular compression and leakage of infusate into the ventricles—events that could have serious adverse effects in a clinical setting 84.
Experience in Neuro‐Oncology
The majority of preclinical and clinical CED studies have focused on the treatment of high‐grade glioma. CED has a number of potential advantages for application in neuro‐oncology, where inadequate penetration of the BBB is implicated in the poor response to systemically administered chemotherapies 91. Although increasing the concentration of a systemically administered chemotherapy can result in improved penetration of the BBB, this strategy risks intolerable side effects and systemic toxicity.
CED trials in neuro‐oncology have used a wide range of therapies including conventional chemotherapies (paclitaxel, topotecan, nimustine), targeted toxins (IL13‐PE38QRR, TP‐38, PRX321), oligonucleotides and TGF‐β2 inhibitors 10, 11, 14, 42, 45, 65, 70. The results of these clinical trials, with a few notable exceptions, have been somewhat disappointing—a situation largely attributed to poor drug distribution and reflux, resulting in off‐target side effects and subtherapeutic drug concentrations within the tumor target 42, 45, 58, 74. A major problem in many of these early clinical studies was an inability to visualize the distribution of the infused therapy in relation to the tumor target or along the catheter track. A number of studies also reported unacceptable device‐related adverse events 10.
The lessons from these studies are clear—for CED to be effective in neuro‐oncology, it is necessary to combine optimized catheter technology with an infusate that has physicochemical characteristics suitable for CED and is delivered by an effective infusion regime, to achieve reflux‐free infusions restricted to the desired structural target.
Experience in PD
A number of clinical studies have been undertaken with the aim of delivering GDNF directly to the posterodorsal putamen, which is known to be the site of greatest dopaminergic neuronal depletion in PD. Gill et al 22 enrolled five patients with symptoms poorly controlled by medical therapy into the first open‐label study of continuous intraputaminal delivery of GDNF via microcatheters attached to a subcutaneous infusion pump in the anterior abdominal wall. All five patients demonstrated improvement in clinical and 18F‐dopa positron emission tomography (PET) imaging parameters. Patients entered into a 12‐month extension study showed sustained improvement without serious adverse effects 60. One patient receiving unilateral infusion of GDNF died of causes unrelated to the study, and post‐mortem examination confirmed that infusion of GDNF into the posterodorsal putamen resulted in a marked increase in tyrosine hydroxylase‐positive nerve fibers in the putamen and an increase in growth‐associated protein 43 in the ipsilateral substantia nigra 47.
After a second successful open‐label study by Slevin et al 79 enrolling 10 patients, a multicenter randomized controlled trial was commenced in the UK and United States. Thirty‐four patients were randomized to receive either bilateral infusions of GDNF into the putamen or placebo 43. After 6 months, patients receiving GDNF had failed to demonstrate sufficient clinical improvement to achieve statistical significance despite improvements in PET imaging parameters. The disappointing results of this randomized study led to withdrawal of GDNF and cessation of clinical trials.
Technical variations in catheter design and drug delivery were suspected to have contributed to the failure of the multicenter trial following the success of the open‐label studies. To investigate this hypothesis, Salvatore et al 71 analyzed the distribution of 125I‐radiolabelled GDNF in the putamen of nonhuman primates after infusion using the same delivery system as in the multicenter study. Radiographic analysis of GDNF distribution within the putamen revealed significant variability, with most of the GDNF restricted to the immediate vicinity of the catheter tip. The authors concluded that when translated to the human putamen, the bioavailability of GDNF would have been limited to 2–9% of the putaminal volume.
Significant research has subsequently been dedicated to applying CED to the treatment of PD with the aim of overcoming poor drug distribution. Intraputaminal CED of adeno‐associated virus serotype 2 (AAV2) expressing human aromatic L‐amino acid decarboxylase (hAADC) was successfully performed in 10 patients with PD 85, and a phase II study is currently underway. A randomized double‐blind phase II study of CED of GDNF using an intermittent infusion paradigm has also recently commenced, which has been designed to address the pitfalls of prior studies.
Potential in Alzheimer's Disease
In contrast to PD and high‐grade glioma, there is no easily identifiable surgical target for CED in AD. Neuronal damage and degeneration in multiple brain regions including the medial temporal lobe and associated memory circuits, basal forebrain and cerebral cortex have been implicated in the progression of cognitive decline. As such, it is likely that for a therapeutic agent delivered intraparenchymally to be clinically effective in AD, it must be administered to multiple brain regions.
The primary abnormality in AD is thought to be the abnormal accumulation of Aβ within the brain. The main pathways for Aβ removal are enzymatic degradation, microglial uptake and degradation, receptor‐mediated transport across the cerebrovascular endothelium, and bulk flow within the interstitial fluid through the perivascular extracellular matrix. Recent interest in amyloid‐degrading enzymes as potential therapeutic agents has raised their profile in Alzheimer's research 53. To investigate the potential of using CED to promote Aβ degradation and clearance, we recently undertook preclinical studies of CED of neprilysin (NEP).
Convection‐enhanced delivery of NEP
NEP, the prototypic Aβ‐degrading metalloendopeptidase, is one of the most efficient of a range of thiorphan‐ and phosphoramidon‐sensitive endopeptidases in degrading Aβ in vitro 53, 78. NEP was initially identified as a regulator of Aβ level when infusion of thiorphan—a NEP inhibitor—into rat striatum elevated the level of exogenously administered radiolabelled Aβ 32. Genetic ablation of the NEP gene in hAPP mice confirmed the importance of NEP in preventing Aβ accumulation: endogenous Aβ increased, and clearance of exogenously administered Aβ was impaired although not completely inhibited 33.
Inactivation of NEP in hAPP mice not only increased Aβ, including oligomeric Aβ 29, causing plaque‐like deposits to form in the brain, but also impaired synaptic plasticity, caused behavioral and cognitive abnormalities 29, 51 and cerebral amyloid angiopathy 21. The severity of CAA was greater in NEP −/− than NEP +/− mice 21. Infusion of thiorphan in rats was also associated with impairment of cognitive performance 57, 96.
Overexpression of human NEP (approximately eightfold increase) in hAPP (Swe/Ind) transgenic mice markedly reduced cerebral Aβ, largely preventing plaque formation, and significantly improved life expectancy 44, 63. The combined data from in vitro and in vivo studies suggest that increasing the levels of NEP in the brain may have therapeutic potential for the treatment of AD.
Injection of lentivirus or adenovirus encoding human NEP into the hippocampus of hAPP mice reduced Aβ load and significantly improved performance in memory tasks 34, 52. However, this approach is associated with systemic and local immune responses, which can neutralize the therapeutic effects 48. Long‐term gene transfer of human NEP in hAPP mice reduced intracellular and extracellular Aβ and improved behavior and memory 20, 80. Mice also showed evidence of reduced oxidative stress and inflammation 20 and less synaptic and dendritic damage 80.
Although viral vector‐mediated NEP gene delivery has proven effective in transgenic mouse models, there are significant ethical and practical barriers to the translation of this approach to clinical trials. Many of these problems might be overcome by delivery of NEP directly to the brain by CED. In our studies, intrastriatal CED of human recombinant NEP in adult Wistar rats resulted in widespread interstitial and perivascular distribution (Figure 4), a 20‐fold increase in NEP protein level, a corresponding increase in NEP enzyme activity, and no evidence of toxicity. Furthermore, in normal‐aged rats, CED of NEP produced a significant reduction in endogenous Aβ40 within 24 h of a single infusion.
Figure 4.
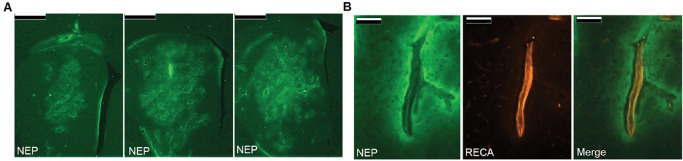
A. Intrastriatal CED of NEP (green) resulted in widespread interstitial distribution through large volumes of the striatum (scale bar = 1 mm). B. CED was also associated with perivascular distribution with accumulation of NEP external to the endothelium marked with RECA (red, scale bar = 100 μm). Reprinted from Barua et al 5. Copyright (2012), with permission from IOS Press.
By using relatively high flow rates (2.5 μL/minute), it was possible to distribute NEP throughout a large volume of the striatal interstitium. The use of high flow rates is of importance for clinical translation, and with a volume of infusion (Vi) to volume of distribution (Vd) ratio of 4.6, NEP has significant potential for application to the clinical setting.
The gray matter volume of the basal forebrain cholinergic system (including the nucleus basalis of Meynert) has been estimated at approximately 350 mm3 in patients with mild AD 26. With a single CED catheter targeted to the human basal forebrain, it may be possible to distribute NEP throughout this structure with a 30‐minute infusion time at a flow rate of 2.5 μL/minute, based on a Vi : Vd ratio of 4.6. This extrapolation is based on the assumption that the Vi : Vd ratio demonstrated in rat striatum 5 is directly translatable to the human basal forebrain.
However, the clearance of NEP from the brain following CED was found to be unexpectedly rapid, with levels returning to baseline within 24 h of infusion. The short half‐life of exogenously administered NEP introduces the requirement for intermittent CED if this approach is to be effective. Although the short half‐life might be thought to limit the therapeutic potential of this Aβ‐degrading strategy, significant advantages are conferred by the rapid clearance from the brain. The rapidity of clearance allows close and dynamic control of dosing, and immediate cessation of treatment in the event of unforeseen adverse effects—such fine control is not possible with viral vector‐mediated gene therapy. And although the short half‐life of NEP imposes the need for repeated drug administration, advances in CED technology are already meeting this challenge. We recently described the use of an implantable CED catheter and transcutaneous port system, which facilitate chronic intermittent drug delivery 7, 8.
Therapeutic strategies applicable to CED
CED is potentially applicable to a number of AD therapies in addition to enhancing Aβ degradation and clearance. Nerve growth factor (NGF) is important for the survival and maintenance of the cholinergic basal forebrain and hippocampal cells, and is produced in the cerebral cortex and hippocampus, which are targets of these projection neurons. See Allen et al 3 for an in‐depth review of the therapeutic potential of NGF in AD. The targeted intracerebral injection of NGF protein and cell‐based gene therapies were previously trialed as neurorestorative therapies in AD 83, 87. However, the therapeutic potential of both of these strategies may be hampered by limited drug distribution, a problem that could be overcome by CED of neurotrophin‐based treatments in AD.
The application of nanotechnology to CED also offers further opportunities for the treatment of AD. Nanoparticles are formulations of synthetic chemical components that self‐assemble into particles of less than 1000 nm in diameter. Their size makes them a potential vehicle for CED, as the effective pore size of the extracellular matrix is on a nanometer scale. By altering nanoparticle formulations, it may be possible to enhance the distribution characteristics of infusates, to improve or inhibit cellular uptake and to facilitate slow release of therapeutic agents 49, 50, 62.
There is also significant interest in developing viral vector‐mediated gene therapies for CED 12, 19. In addition to enhancing expression of a desired transgene, CED of small‐interfering RNAs (siRNAs) could offer a means of knocking down genes encoding enzymes involved in Aβ production, such as β‐ and γ‐secretases 61, 81.
Recent preclinical studies have demonstrated the potential of combining CED with axonal transport for delivering viral vector‐mediated gene therapies to specific regions of the cerebral cortex. This has potential in a wide range of neurological diseases, including AD and several other forms of dementia. Convection‐enhanced delivery of adeno‐associated virus (AAV)‐based vectors to the thalamus was shown to result in transgene expression in widespread cortical areas as a consequence of both anterograde and retrograde transport of AAV vectors 36.
Clinical Translation—Real‐Time Imaging
The experience gained from clinical trials in neuro‐oncology and PD is strong evidence of the critical importance of understanding and controlling drug distribution within the desired target. Much research interest has focused on real‐time magnetic resonance tracking of infusate distribution. Co‐infusion of gadolinium and gadolinium surrogates, such as encapsulating liposomes, facilitates T1‐weighted tracking of distribution 24, 70, 75. However, this approach assumes that the distribution of contrast agent is equivalent to that of the therapeutic agent. Although gadolinium‐based contrast agents are generally considered to be stable complexes, it seems prudent to determine whether they alter the therapeutic effect of co‐infused drugs as exchange reactions with metal ions are known to occur at physiological conditions 77. T2‐weighted MRI signal change has also been used as a surrogate marker of infusate distribution 5, 68. Yet, it seems likely that T2‐weighted imaging significantly underestimates the volume of distribution of the infused therapy 35.
The use of T2 imaging for real‐time tracking may be of more practical use in patients without preexisting T2 abnormalities on MRI. Co‐infusion of radiolabelled infusates or infusion of radiolabelled therapeutic agents are alternative strategies that have been used to good effect in clinical trials, particularly in neuro‐oncology. 123I‐labelled human serum albumin was co‐infused with cintredekin besudotox, and single‐photon emission computerized tomography (SPECT) matched with co‐registered MR images 73. In a second study, 123I‐labelled therapeutic antitenascin monoclonal antibodies were administered by CED in patients with glioblastoma and the volume of distribution was analyzed by SPECT 72. However, it has proven difficult to correlate T2 signal change induced by CED in patients with preexisting T2 hyperintensities with volumes of distribution on SPECT 73.
Despite these flaws, real‐time tracking of infusions is fundamentally important for ensuring successful clinical trials by providing the investigator with confidence that the therapy is reaching (and limited to) the desired target. However, interpretation of the data obtained by MRI should ideally be based on information from combined neuropathological, biochemical and imaging studies using the same infusates and CED equipment in animal models as in clinical trials.
Conclusions
CED has the potential to improve the treatment of a wide range of neurological conditions. The theoretical advantages of CED are multiple and include bypass of the BBB, limitation of systemic side effects, delivery of therapeutic drug concentrations and distribution through clinically relevant brain volumes. However, most preclinical and clinical CED research to date has focused on drug delivery for diseases characterized by localized pathology, such as brain tumors, or diseases in which a localized surgical target can be identified, such as PD.
The widespread pathology associated with neurodegenerative disease states such as AD adds an additional layer of complexity, as it is likely that an effective treatment for AD would require delivery to multiple brain regions including the basal forebrain, medial temporal lobe and cerebral cortex. The facility to distribute a drug through very large volumes of brain tissue depends on both the physicochemical characteristics of the infused therapy and the technology used to deliver it.
The interstitial distribution of liposomal drugs delivered by CED is dependent on the infused agent having low tissue affinity 49. Further investigation is required to determine whether the most widely distributed soluble agents are low molecular weight hydrophilic and/or anionic compounds 69. Further study is also required to determine whether infused agents with low tissue affinity are likely to be rapidly cleared from the brain, as suggested by the CED of NEP. This possibility introduces the requirement for either repeated intermittent drug infusions or, in the case of proteins, gene therapy.
The design and development of a chronically implantable catheter and transcutaneous bone‐anchored port makes it now feasible to perform intermittent CED via multiple catheters 7. This technology is currently being used for the treatment of recurrent glioblastoma and could be applied to a CED trial in AD.
Effective clinical translation of CED will be dependent on the adoption of suitable catheter technology and implantation methods, used in combination with therapeutic agents that have appropriate physicochemical properties. Extensive preclinical studies correlating infusate distribution with neuropathological and neuroradiological findings are also required to maximize the chances of success in a clinical setting.
Disclosures
NB is a consultant advisor to Renishaw Plc, a manufacturer of drug delivery catheter systems. SG is Renishaw's clinical director.
Acknowledgments
This work was supported by grants from the Engineering and Physical Sciences Research Council, The Royal College of Surgeons of England, The Dunhill Medical Trust and the Alzheimer's Research UK.
References
- 1. Alcolado R, Weller RO, Parrish EP, Garrod D (1988) The cranial arachnoid and pia mater in man: anatomical and ultrastructural observations. Neuropathol Appl Neurobiol 14:1–17. [DOI] [PubMed] [Google Scholar]
- 2. Allard E, Hindre F, Passirani C, Lemaire L, Lepareur N, Noiret N et al (2008) 188Re‐loaded lipid nanocapsules as a promising radiopharmaceutical carrier for internal radiotherapy of malignant gliomas. Eur J Nucl Med Mol Imaging 35:1838–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK (2013) GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther 138:155–175. [DOI] [PubMed] [Google Scholar]
- 4. Barua NU, Bienemann AS, Hesketh S, Wyatt MJ, Castrique E, Love S, Gill SS (2012) Intrastriatal convection‐enhanced delivery results in widespread perivascular distribution in a pre‐clinical model. Fluids Barriers CNS 9:2. doi: 10.1186/2045-8118-9-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barua NU, Miners JS, Bienemann AS, Wyatt MJ, Welser K, Tabor AB et al (2012) Convection‐enhanced delivery of neprilysin: a novel amyloid‐β‐degrading therapeutic strategy. J Alzheimers Dis 32:43–56. [DOI] [PubMed] [Google Scholar]
- 6. Barua NU, Lowis SP, Woolley M, O'Sullivan S, Harrison R, Gill SS (2013) Robot‐guided convection‐enhanced delivery of carboplatin for advanced brainstem glioma. Acta Neurochir (Wien) 155:1459–1465. [DOI] [PubMed] [Google Scholar]
- 7. Barua NU, Woolley M, Bienemann AS, Johnson DE, Lewis O, Wyatt MJ et al (2013) Intermittent convection‐enhanced delivery to the brain through a novel transcutaneous bone‐anchored port. J Neurosci Methods 15:223–232. [DOI] [PubMed] [Google Scholar]
- 8. Bienemann A, White E, Woolley M, Castrique E, Johnson DE, Wyatt M et al (2012) The development of an implantable catheter system for chronic or intermittent convection‐enhanced delivery. J Neurosci Methods 203:284–291. [DOI] [PubMed] [Google Scholar]
- 9. Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH (1994) Convection‐enhanced delivery of macromolecules in the brain. PNAS 91:2076–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bogdahn U, Hau P, Stockhammer G, Venkataramana NK, Mahapatra AK, Suri A et al (2011) Targeted therapy for high‐grade glioma with the TGF‐β2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro‐oncol 13:132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bruce JN, Fine RL, Canoll P, Yun J, Kennedy BC, Rosenfeld SS et al (2011) Regression of recurrent malignant gliomas with convection‐enhanced delivery of topotecan. Neurosurgery 69:1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cachon‐Gonzalez MB, Wang SZ, McNair R, Bradley J, Lunn D, Ziegler R et al (2012) Gene transfer corrects acute GM2 gangliosidosis—potential therapeutic contribution of perivascular enzyme flow. Mol Ther 20:1489–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carare RO, Bernardes‐Silva M, Newman TA, Page AM, Nicoll JAR, Perry VH et al (2008) Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries. Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol and Appl Neurobiol 34:131–144. [DOI] [PubMed] [Google Scholar]
- 14. Carpentier A, Metellus P, Ursu R, Zohar S, Lafitte F, Barrie M et al (2010) Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study. Neuro‐oncol 12:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Casanova F, Carney PR, Sarntinoranont M (2012) Influence of needle insertion speed on backflow for convection‐enhanced delivery. J Biomech Eng 134:041006. doi: 10.1115/1.4006404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen MY, Lonser RR, Morrison PF, Governale LS, Oldfield EH (1999) Variables affecting convection‐enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue‐cannula sealing time. J Neurosurg 90:315–320. [DOI] [PubMed] [Google Scholar]
- 17. Chen MY, Hoffer A, Morrison PF, Hamilton JF, Hughes J, Schlageter KS et al (2005) Surface properties, more than size, limiting convective distribution of virus‐sized particles and viruses in the central nervous system. J Neurosurg 103:311–319. [DOI] [PubMed] [Google Scholar]
- 18. Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA et al (2009) Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology 73:1662–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Debinski W, Tatter SB (2010) Convection‐enhanced delivery to achieve widespread distribution of viral vectors: predicting clinical implementation. Curr Opin Mol Ther 12:647–653. [PubMed] [Google Scholar]
- 20. El‐Amouri SS, Zhu H, Yu J, Marr R, Verma IM, Kindy MS (2008) Neprilysin: an enzyme candidate to slow the progression of Alzheimer's disease. Am J Pathol 172:1342–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farris W, Schutz SG, Cirrito JR, Shankar GM, Sun X, George A et al (2007) Loss of neprilysin function promotes amyloid plaque formation and causes cerebral amyloid angiopathy. Am J Pathol 171:241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gill SS, Patel NK, Hotton GR, O'Sullivan K, McCarter R, Bunnage M et al (2003) Direct brain infusion of glial cell line‐derived neurotrophic factor in Parkinson disease. Nat Med 9:589–595. [DOI] [PubMed] [Google Scholar]
- 23. Gill T, Barua NU, Woolley M, Bienemann AS, Johnson DE, O'Sullivan S et al (2013) In vitro and in vivo testing of a novel recessed‐step catheter for reflux‐free convection‐enhanced drug delivery to the brain. J Neurosci Methods 219:1–9. [DOI] [PubMed] [Google Scholar]
- 24. Gimenez F, Krauze MT, Valles F, Hadaczek P, Bringas J, Sharma N et al (2011) Image‐guided convection‐enhanced delivery of GDNF protein into monkey putamen. Neuroimage 54(Suppl 1):S189–S195. [DOI] [PubMed] [Google Scholar]
- 25. Gregory TF, Rennels ML, Blaumanis OR, Fujimoto K (1985) A method for microscopic studies of cerebral angioarchitecture and vascular‐parenchymal relationships, based on the demonstration of “paravascular” fluid pathways in the mammalian central nervous system. J Neurosci Methods 14:5–14. [DOI] [PubMed] [Google Scholar]
- 26. Grothe M, Heinsen H, Teipel SJ (2011) Atrophy of the cholinergic basal forebrain over the adult age range and in early stages of Alzheimer's disease. Biol Psychiatry 71:805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hadaczek P, Yamashita Y, Mirek H, Tamas L, Bohn MC, Noble C et al (2006) The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther 14:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hamilton JF, Morrison PF, Chen MY, Harvey‐White J, Pernaute RS, Phillips H et al (2001) Heparin coinfusion during convection‐enhanced delivery (CED) increases the distribution of the glial‐derived neurotrophic factor (GDNF) ligand family in rat striatum and enhances the pharmacological activity of neurturin. Exp Neurol 168:155–161. [DOI] [PubMed] [Google Scholar]
- 29. Huang SM, Mouri A, Kokubo H, Nakajima R, Suemoto T, Higuchi M et al (2006) Neprilysin‐sensitive synapse‐associated amyloid‐beta peptide oligomers impair neuronal plasticity and cognitive function. J Biol Chem 281:17941–17951. [DOI] [PubMed] [Google Scholar]
- 30. Hutchings M, Weller RO (1986) Anatomical relationships of the pia mater to cerebral blood vessels in man. J Neurosurg 65:316–325. [DOI] [PubMed] [Google Scholar]
- 31. Ichimura T, Fraser PA, Cserr HF (1991) Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res 545:103–113. [DOI] [PubMed] [Google Scholar]
- 32. Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E et al (2000) Identification of the major Aβ1‐42‐degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med 6:143–150. [DOI] [PubMed] [Google Scholar]
- 33. Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP et al (2001) Metabolic regulation of brain Aβ by neprilysin. Science 292:1550–1552. [DOI] [PubMed] [Google Scholar]
- 34. Iwata N, Mizukami H, Shirotani K, Takaki Y, Muramatsu S, Lu B et al (2004) Presynaptic localization of neprilysin contributes to efficient clearance of amyloid‐beta peptide in mouse brain. J Neurosci 24:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iyer RR, Butman JA, Walbridge S, Gai ND, Heiss JD, Lonser RR (2011) Tracking accuracy of T2‐ and diffusion‐weighted magnetic resonance imaging for infusate distribution by convection‐enhanced delivery. J Neurosurg 115:474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kells AP, Hadaczek P, Yin D, Bringas J, Varenika V, Forsayeth J, Bankiewicz KS (2009) Efficient gene therapy‐based method for the delivery of therapeutics to primate cortex. PNAS 106:2407–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kikuchi T, Saito R, Sugiyama S, Yamashita Y, Kumabe T, Krauze M et al (2008) Convection‐enhanced delivery of polyethylene glycol‐coated liposomal doxorubicin: characterization and efficacy in rat intracranial glioma models. J Neurosurg 109:867–873. [DOI] [PubMed] [Google Scholar]
- 38. Krauze MT, Saito R, Noble C, Bringas J, Forsayeth J, McKnight TR et al (2005) Effects of the perivascular space on convection‐enhanced delivery of liposomes in primate putamen. Exp Neurol 196:104–111. [DOI] [PubMed] [Google Scholar]
- 39. Krauze MT, Saito R, Noble C, Tamas M, Bringas J, Park JW et al (2005) Reflux‐free cannula for convection‐enhanced high‐speed delivery of therapeutic agents. J Neurosurg 103:923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kroll RA, Pagel MA, Muldoon LL, Roman‐Goldstein S, Neuwelt EA (1996) Increasing volume of distribution to the brain with interstitial infusion: dose, rather than convection, might be the most important factor. Neurosurgery 38:746–752. [PubMed] [Google Scholar]
- 41. Ksendzovsky A, Walbridge S, Saunders RC, Asthagiri AR, Heiss JD, Lonser RR (2012) Convection‐enhanced delivery of M13 bacteriophage to the brain. J Neurosurg 117:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G et al (2010) Phase III randomized trial of CED of IL13‐PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro‐oncol 12:871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R et al (2006) Randomized controlled trial of intraputamenal glial cell line‐derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 59:459–466. [DOI] [PubMed] [Google Scholar]
- 44. Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X et al (2003) Enhanced proteolysis of β‐amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40:1087–1093. [DOI] [PubMed] [Google Scholar]
- 45. Lidar Z, Mardor Y, Jonas T, Pfeffer R, Faibel M, Nass D et al (2004) Convection‐enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg 100:472–479. [DOI] [PubMed] [Google Scholar]
- 46. Lieberman DM, Laske DW, Morrison PF, Bankiewicz KS, Oldfield EH (1995) Convection‐enhanced distribution of large molecules in gray matter during interstitial drug infusion. J Neurosurg 82:1021–1029. [DOI] [PubMed] [Google Scholar]
- 47. Love S, Plaha P, Patel NK, Hotton GR, Brooks DJ, Gill SS (2005) Glial cell line‐derived neurotrophic factor induces neuronal sprouting in human brain. Nat Med 11:703–704. [DOI] [PubMed] [Google Scholar]
- 48. Lowenstein PR, Mandel RJ, Xiong WD, Kroeger K, Castro MG (2007) Immune responses to adenovirus and adeno‐associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions. Curr Gene Ther 7:347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. MacKay JA, Deen DF, Szoka FC, Jr (2005) Distribution in brain of liposomes after convection enhanced delivery; modulation by particle charge, particle diameter, and presence of steric coating. Brain Res 1035:139–153. [DOI] [PubMed] [Google Scholar]
- 50. MacKay JA, Li W, Huang Z, Dy EE, Huynh G, Tihan T et al (2008) HIV TAT peptide modifies the distribution of DNA nanolipoparticles following convection‐enhanced delivery. Mol Ther 16:893–900. [DOI] [PubMed] [Google Scholar]
- 51. Madani R, Poirier R, Wolfer DP, Welzl H, Groscurth P, Lipp HP et al (2006) Lack of neprilysin suffices to generate murine amyloid‐like deposits in the brain and behavioral deficit in vivo . J Neurosci Res 84:1871–1878. [DOI] [PubMed] [Google Scholar]
- 52. Marr RA, Rockenstein E, Mukherjee A, Kindy MS, Hersh LB, Gage FH et al (2003) Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J Neurosci 23:1992–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Miners JS, Barua N, Kehoe PG, Gill S, Love S (2011) Aβ‐degrading enzymes: potential for treatment of Alzheimer disease. J Neuropathol Exp Neurol 70:944–959. [DOI] [PubMed] [Google Scholar]
- 54. Morrison PF, Chen MY, Chadwick RS, Lonser RR, Oldfield EH (1999) Focal delivery during direct infusion to brain: role of flow rate, catheter diameter, and tissue mechanics. Am J Physiol 277:1218–1229. [DOI] [PubMed] [Google Scholar]
- 55. Morrison PF, Laske DW, Bobo H, Oldfield EH, Dedrick RL (1994) High‐flow microinfusion: tissue penetration and pharmacodynamics. Am J Physiol 266:292–305. [DOI] [PubMed] [Google Scholar]
- 56. Morrison PF, Lonser RR, Oldfield EH (2007) Convective delivery of glial cell line‐derived neurotrophic factor in the human putamen. J Neurosurg 107:74–83. [DOI] [PubMed] [Google Scholar]
- 57. Mouri A, Zou LB, Iwata N, Saido TC, Wang D, Wang MW et al (2006) Inhibition of neprilysin by thiorphan (i.c.v.) causes an accumulation of amyloid β and impairment of learning and memory. Behav Brain Res 168:83–91. [DOI] [PubMed] [Google Scholar]
- 58. Mueller S, Polley MY, Lee B, Kunwar S, Pedain C, Wembacher‐Schroder E et al (2011) Effect of imaging and catheter characteristics on clinical outcome for patients in the PRECISE study. J Neurooncol 101:267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nguyen JB, Sanchez‐Pernaute R, Cunningham J, Bankiewicz KS (2001) Convection‐enhanced delivery of AAV‐2 combined with heparin increases TK gene transfer in the rat brain. Neuroreport 12:1961–1964. [DOI] [PubMed] [Google Scholar]
- 60. Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS (2005) Intraputamenal infusion of glial cell line‐derived neurotrophic factor in PD: a two‐year outcome study. Ann Neurol 57:298–302. [DOI] [PubMed] [Google Scholar]
- 61. Peng KA, Masliah E (2010) Lentivirus‐expressed siRNA vectors against Alzheimer disease. Methods Mol Biol 614:215–224. [DOI] [PubMed] [Google Scholar]
- 62. Perlstein B, Ram Z, Daniels D, Ocherashvilli A, Roth Y, Margel S, Mardor Y (2008) Convection‐enhanced delivery of maghemite nanoparticles: increased efficacy and MRI monitoring. Neuro Oncol 10:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Poirier R, Wolfer DP, Welzl H, Tracy J, Galsworthy MJ, Nitsch RM, Mohajeri MH (2006) Neuronal neprilysin overexpression is associated with attenuation of Aβ‐related spatial memory deficit. Neurobiol Dis 24:475–483. [DOI] [PubMed] [Google Scholar]
- 64. Pollock H, Hutchings M, Weller RO, Zhang ET (1997) Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J Anat 191:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rand RW, Kreitman RJ, Patronas N, Varricchio F, Pastan I, Puri RK (2000) Intratumoral administration of recombinant circularly permuted interleukin‐4‐Pseudomonas exotoxin in patients with high‐grade glioma. Clin Cancer Res 6:2157–2165. [PubMed] [Google Scholar]
- 66. Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA (1985) Evidence for a “paravascular” fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res 326:47–63. [DOI] [PubMed] [Google Scholar]
- 67. Rennels ML, Blaumanis OR, Grady PA (1990) Rapid solute transport throughout the brain via paravascular fluid pathways. Adv Neurol 52:431–439. [PubMed] [Google Scholar]
- 68. Richardson RM, Gimenez F, Salegio EA, Su X, Bringas J, Berger MS, Bankiewicz KS (2011) T2 imaging in monitoring of intraparenchymal real‐time convection enhanced delivery. Neurosurgery 69:154–163. [DOI] [PubMed] [Google Scholar]
- 69. Saito R, Krauze MT, Noble CO, Tamas M, Drummond DC, Kirpotin DB et al (2006) Tissue affinity of the infusate affects the distribution volume during convection‐enhanced delivery into rodent brains: implications for local drug delivery. J Neurosci Methods 154:225–232. [DOI] [PubMed] [Google Scholar]
- 70. Saito R, Sonoda Y, Kumabe T, Nagamatsu K, Watanabe M, Tominaga T (2011) Regression of recurrent glioblastoma infiltrating the brainstem after convection‐enhanced delivery of nimustine hydrochloride. J Neurosurg 7:522–526. [DOI] [PubMed] [Google Scholar]
- 71. Salvatore MF, Ai Y, Fischer B, Zhang AM, Grondin RC, Zhang Z et al (2006) Point source concentration of GDNF may explain failure of phase II clinical trial. Exp Neurol 202:497–505. [DOI] [PubMed] [Google Scholar]
- 72. Sampson JH, Akabani G, Friedman AH, Bigner D, Kunwar S et al (2006) Comparison of intratumoral bolus injection and convection‐enhanced delivery of radiolabeled antitenascin monoclonal antibodies. Neurosurg Focus 20:E14. [DOI] [PubMed] [Google Scholar]
- 73. Sampson JH, Raghavan R, Provenzale JM, Croteau D, Reardon DA et al (2007) Induction of hyperintense signal on T2‐weighted MR images correlates with infusion distribution from intracerebral convection‐enhanced delivery of a tumor‐targeted cytotoxin. AJR Am J Roentgenol 188:703–709. [DOI] [PubMed] [Google Scholar]
- 74. Sampson JH, Archer G, Pedain C, Wembacher‐Schroder E, Westphal M, Kunwar S et al (2010) Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg 113:301–309. [DOI] [PubMed] [Google Scholar]
- 75. Sampson JH, Brady M, Raghavan R, Mehta AI, Friedman AH, Reardon DA et al (2011) Co‐localization of gadolinium‐DTPA with high molecular weight molecules after intracerebral convection‐enhanced delivery in man. Neurosurgery 69:668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sanftner LM, Sommer JM, Suzuki BM, Smith PH, Vijay S, Vargas JA et al (2005) AAV2‐mediated gene delivery to monkey putamen: evaluation of an infusion device and delivery parameters. Exp Neurol 194:476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sarka L, Burai L, Brücher E (2000) The rates of the exchange reactions between [Gd(DTPA)]2− and the endogenous ions Cu2+ and Zn2+: a kinetic model for the prediction of the in vivo stability of [Gd(DTPA)]2‐, used as a contrast agent in magnetic resonance imaging. Chemistry 6:719–724. [DOI] [PubMed] [Google Scholar]
- 78. Shirotani K, Tsubuki S, Iwata N, Takaki Y, Harigaya W, Maruyama K et al (2001) Neprilysin degrades both amyloid β peptides 1‐40 and 1‐42 most rapidly and efficiently among thiorphan‐ and phosphoramidon‐sensitive endopeptidases. J Biol Chem 276:21895–21901. [DOI] [PubMed] [Google Scholar]
- 79. Slevin JT, Gash DM, Smith CD, Gerhardt GA, Kryscio R, Chebrolu H et al (2006) Unilateral intraputaminal glial cell line‐derived neurotrophic factor in patients with Parkinson disease: response to 1 year each of treatment and withdrawal. Neurosurg Focus 20:E1. [DOI] [PubMed] [Google Scholar]
- 80. Spencer B, Marr RA, Rockenstein E, Crews L, Adame A, Potkar R et al (2008) Long‐term neprilysin gene transfer is associated with reduced levels of intracellular Aβ and behavioral improvement in APP transgenic mice. BMC Neurosci 9:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Stiles DK, Zhang Z, Ge P, Nelson B, Grondin R, Ai Y et al (2012) Widespread suppression of huntingtin with convection‐enhanced delivery of siRNA. Exp Neurol 233:463–471. [DOI] [PubMed] [Google Scholar]
- 82. Szentistvanyi I, Patlak CS, Ellis RA, Cserr HF (1984) Drainage of interstitial fluid from different regions of rat brain. Am J Physiol 246:F835–F844. [DOI] [PubMed] [Google Scholar]
- 83. Tuszynski MH, Thal L, Pay M, Salmon DP, U HS, Bakay R et al (2005) A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med 11:551–555. [DOI] [PubMed] [Google Scholar]
- 84. Valles F, Fiandaca MS, Bringas J, Dickinson P, LeCouteur R, Higgins R et al (2009) Anatomic compression caused by high‐volume convection‐enhanced delivery to the brain. Neurosurgery 65:579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Valles F, Fiandaca MS, Eberling JL, Starr PA, Larson PS, Christine CW et al (2010) Qualitative imaging of adeno‐associated virus serotype 2‐human aromatic L‐amino acid decarboxylase gene therapy in a phase I study for the treatment of Parkinson disease. Neurosurgery 67:1377–1385. [DOI] [PubMed] [Google Scholar]
- 86. Varenika V, Kells AP, Valles F, Hadaczek P, Forsayeth J, Bankiewicz KS (2009) Controlled dissemination of AAV vectors in the primate brain. Prog Brain Res 175:163–172. [DOI] [PubMed] [Google Scholar]
- 87. Wahlberg LU, Lind G, Almqvist PM, Kusk P, Tornoe J, Juliusson B et al (2012) Targeted delivery of nerve growth factor via encapsulated cell biodelivery in Alzheimer disease: a technology platform for restorative neurosurgery. J Neurosurg 117:340–347. [DOI] [PubMed] [Google Scholar]
- 88. Weller RO, Massey A, Newman TA, Hutchings M, Kuo YM, Roher AE (1998) Cerebral amyloid angiopathy: amyloid β accumulates in putative interstitial fluid drainage pathways in Alzheimer's disease. Am J Pathol 153:725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Weller RO, Subash M, Preston SD, Mazanti I, Carare RO (2008) Perivascular drainage of amyloid‐β peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease. Brain Pathol 18:253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. White E, Bienemann A, Malone J, Megraw L, Bunnun C, Wyatt M, Gill S (2011) An evaluation of the relationships between catheter design and tissue mechanics in achieving high‐flow convection‐enhanced delivery. J Neurosci Methods 199:87–97. [DOI] [PubMed] [Google Scholar]
- 91. Whittle IR, Malcolm G, Jodrell DI, Reid M (1999) Platinum distribution in malignant glioma following intraoperative intravenous infusion of carboplatin. Br J Neurosurg 13:132–137. [DOI] [PubMed] [Google Scholar]
- 92. Yin D, Forsayeth J, Bankiewicz KS (2010) Optimized cannula design and placement for convection‐enhanced delivery in rat striatum. J Neurosci Methods 187:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yin D, Valles FE, Fiandaca MS, Bringas J, Gimenez F, Berger MS et al (2011) Optimal region of the putamen for image‐guided convection‐enhanced delivery of therapeutics in human and non‐human primates. Neuroimage 54:S196–S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang ET, Inman CB, Weller RO (1990) Interrelationships of the pia mater and the perivascular (Virchow‐Robin) spaces in the human cerebrum. J Anat 170:111–123. [PMC free article] [PubMed] [Google Scholar]
- 95. Zirzow GC, Sanchez OA, Murray GJ, Brady RO, Oldfield EH (1999) Delivery, distribution, and neuronal uptake of exogenous mannose‐terminal glucocerebrosidase in the intact rat brain. Neurochem Res 24:301–305. [DOI] [PubMed] [Google Scholar]
- 96. Zou LB, Mouri A, Iwata N, Saido TC, Wang D, Wang MW et al (2006) Inhibition of neprilysin by infusion of thiorphan into the hippocampus causes an accumulation of amyloid β and impairment of learning and memory. J Pharmacol Exp Ther 317:334–340. [DOI] [PubMed] [Google Scholar]


