Abstract
Astrogliopathy refers to alterations of astrocytes occurring in diseases of the nervous system, and it implies the involvement of astrocytes as key elements in the pathogenesis and pathology of diseases and injuries of the central nervous system. Reactive astrocytosis refers to the response of astrocytes to different insults to the nervous system, whereas astrocytopathy indicates hypertrophy, atrophy/degeneration and loss of function and pathological remodeling occurring as a primary cause of a disease or as a factor contributing to the development and progression of a particular disease. Reactive astrocytosis secondary to neuron loss and astrocytopathy due to intrinsic alterations of astrocytes occur in neurodegenerative diseases, overlap each other, and, together with astrocyte senescence, contribute to disease‐specific astrogliopathy in aging and neurodegenerative diseases with abnormal protein aggregates in old age. In addition to the well‐known increase in glial fibrillary acidic protein and other proteins in reactive astrocytes, astrocytopathy is evidenced by deposition of abnormal proteins such as β‐amyloid, hyper‐phosphorylated tau, abnormal α‐synuclein, mutated huntingtin, phosphorylated TDP‐43 and mutated SOD1, and PrPres, in Alzheimer's disease, tauopathies, Lewy body diseases, Huntington's disease, amyotrophic lateral sclerosis and Creutzfeldt‐Jakob disease, respectively. Astrocytopathy in these diseases can also be manifested by impaired glutamate transport; abnormal metabolism and release of neurotransmitters; altered potassium, calcium and water channels resulting in abnormal ion and water homeostasis; abnormal glucose metabolism; abnormal lipid and, particularly, cholesterol metabolism; increased oxidative damage and altered oxidative stress responses; increased production of cytokines and mediators of the inflammatory response; altered expression of connexins with deterioration of cell‐to‐cell networks and transfer of gliotransmitters; and worsening function of the blood brain barrier, among others. Increased knowledge of these aspects will permit a better understanding of brain aging and neurodegenerative diseases in old age as complex disorders in which neurons are not the only players.
Keywords: aging, astrocytes, astrocytic gliosis, astrocytopathy, astrogliopathy, neurodegenerative diseases with abnormal protein aggregates
Astrocytes: General Aspects
Astrocytes are neural stellate cells with long processes which are in contact with neurons and synapses, myelin sheaths, oligodendrocytes, microglia and blood vessels (capillaries, arterioles and venules); subpial, subventricular and perivascular astrocytes separate neurons from the cerebrospinal fluid and blood 172, 408. Astrocytes are arranged in non‐overlapping domains but form syncytial networks united by gap junctions 69, 164. Astrocytes are non‐excitable cells but they respond to various stimuli, they are enriched in potassium, calcium and water channels and participate in synaptic transmission through the control of neurotransmitters and transporters 219.
The coverage domain of a single astrocyte is estimated between 20,000 and 140,000 synapses in the rodent hippocampus, but between 250,000 and 2 million synapses in humans 69, 324, 325. Astrocytes in the white matter have more elongated processes; they are organized along nerve fibers and surround blood vessels by podocytes; it is not clear whether coverage domains of astrocytes in the grey matter have any counterpart in the white matter in connection with fiber tracts.
Glial fibrillary acidic protein (GFAP) is currently used as a marker of astrocytes, and GFAP immunostaining is particularly useful to label a subset of astrocytes under reactive conditions (Figure 1). However, not all astrocytes are GFAP‐immunoreactive and not all cells which express GFAP are astrocytes 218, 323, 417. Neural stem cells of the subventricular zone are GFAP‐immunoreactive but they are not considered astrocytes 323. It has been estimated that only about 15% of the total volume of astrocytes is revealed by GFAP immunohistochemistry in tissue sections of the rodent hippocampus 69. There are 10 isoforms/splice variants identified 217. The most abundant in the brain and spinal cord is GFAP‐α (isoform 1); GFAP‐δ (GFAP‐ɛ; isoform 2) is expressed by neurogenic astrocytes in the subventricular zone 207. GFAP‐α, GFAP‐ɛ and GFAP‐κ are the most well characterized isoforms of GFAP in the central nervous system. Other isoforms are expressed in the peripheral nervous system and other organs. Four isoforms of GFAP, collectively named GFAP + 1, are expressed in a subset of astrocytes throughout the brain. One GFAP + 1 is found in a subset of astrocytes in senile plaques in Alzheimer's disease 208.
Figure 1.
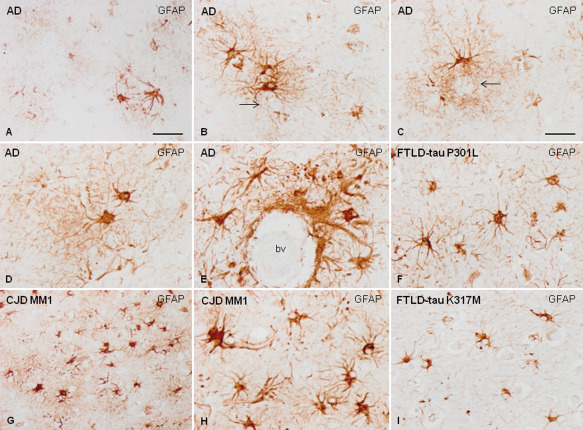
Reactive astrocytes as revealed with GFAP immunohistochemistry in AD (A–E), FTLD‐tau P301L (F), CJD subtype MM1 (G, H) and FTLD‐tau K317M. Reactive astrogliosis in AD tends to form clusters in the vicinity of β‐amyloid core deposits (A–C, B and C arrows), but they are also occasionally distributed with no apparent association with senile plaques (D). Reactive astrogliosis is also frequent in contact with the blood vessels (bv, E). In contrast, reactive astrogliosis in CJD and FTLD is more diffuse and does not form clusters (F–I). Reactive astrocytes in CJD are usually large with robust cellular processes strongly stained with GFAP antibodies (G, H). However, reactive astrocytes in FTLD‐tau are usually smaller in size with short, fine cellular processes which are more striking in FTLD‐tau K317M (I) than in FTLD‐tau P301L (F). This panel illustrates the diversity of GFAP‐reactive astrocytes in different neurodegenerative diseases, and highlights the atrophic morphology of astrocytes in FTLD‐tau K317M. Paraffin sections slightly counterstained with hematoxylin; B–F, H, I, bar in C = 35 μm; A and G, bar in A = 75 μm.
Vimentin, another intermediate filament of the cytoskeleton; the calcium‐binding protein S100β; aquaporin 4 water channel (AQ4); aldehyde dehydrogenase family 1, member 1 (ALDH1L1); glutamate transporters solute carrier family 1, member 1 (GLAST/EAAT1); solute carrier family 1, member 2 (GLT‐1/EAAT2); and bystin, connexin 43 and nestin are also molecular components of astrocytes 79 (Figure 2). YKL‐40 (CHI3L1) has recently been identified as a biomarker of reactive astrocytes linked to inflammation 44, 45, 263 (Figure 3). Other molecules are also expressed in astrocytes such as glutamine synthetase, N‐myc‐down‐regulated gene 2 (NDRG2) and the receptor for hialuronic acid CD44 7, 129. Sox9, a transcription factor, has been recently reported to be selectively expressed in the nuclei of astrocytes 430
Figure 2.
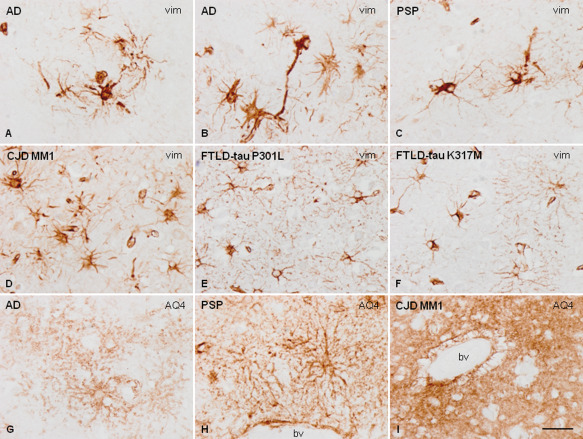
Vimentin (vim) immunohistochemistry shows that the morphology of cortical astrocytes varies in AD (A, B), PSP (C), CJD MM1 (D), FTLD‐tau P301L (E) and FTLD‐tau K317M (F). Astrocytes in FTLD, and particularly in FTLD‐tau K317M, have small, fine branches and fewer astrocyte processes. Aquaporin 4 (AQ4) antibodies stain astrocytic processes in the neuropil and in the vicinity of blood vessels (bv) in most neurodegenerative diseases with abnormal protein aggregates as in AD (G) and PSP (H). However, AQ4 immunoreactivity is stronger and more blurred in CJD, and vacuoles are formed at the peripery of blood vessels (bv, I). Paraffin sections, slightly counterstained with hematoxylin, bar = 35 μm.
Figure 3.
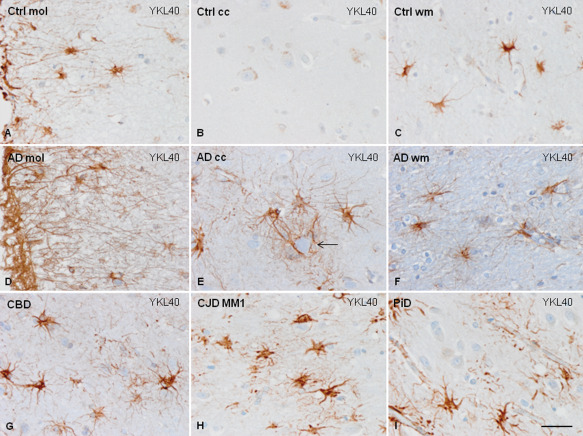
YKL40 immunoreactivity in astrocytes. YKL40 immunoreactivity in control brains is found in subpial astrocytes and in fibrous astrocytes in the white matter, but is almost absent in the cerebral cortex (A–C). However, strong YKL40 immunoreactivity is found, in addition, in cortical astrocytes in AD, mainly surrounding amyloid cores (arrow, D–F), and in reactive astrocytes in the cerebral cortex in CBD (G) CJD MM1 (H) and PiD (I). Paraffin sections, slightly counterstained with hematoxylin, bar = 35 μm.
Types of Astroglial Cells
Astrocytes, astroglial cells or astroglia were first categorized into protoplasmic and fibrous 15. However, the use of the Golgi method and other silver stains permitted the visualization and identification of a large variety of astrocytes 365. Subsequent studies classified the main types of astroglial cells in the central nervous system as protoplasmic astrocytes, fibrous astrocytes, radial glia, Bergmann glia, ependymal astrocytes, perivascular glia, marginal glia, tanycytes and velate glia 108. Two additional types are specific to human and other primates: interlaminar astrocytes and varicose projection astrocytes 89, 90, 324, 325. Recent classifications include protoplasmic astrocytes of the grey matter, interlaminar astrocytes of the cerebral cortex, subpial astrocytes of the cerebral cortex, fibrous astrocytes of the white matter, perivascular astrocytes, Bergmann glia, stem astrocytes of subventricular zones, radial glia of the developing brain, tanycytes of the hypothalamus, pituicytes and Müller glia of the retina 454.
In addition to morphological features which distinguish different astrocytes, increasing knowledge of the organization and functional properties of astrocytes envisages a more complex scenario with additional types. Astrocytes are heterogeneous with respect to their coverage domains, ion channels, calcium responses, glutamate transporters and expression of neurotransmitter receptors 24, 69, 70, 108, 160, 164, 166, 182, 183, 194, 260, 275, 302, 303, 326, 331, 366, 396, 402. Moreover, astrocytes from primates have characteristics which differ from those in rodents 285, 323, 324, 325. Single cell gene expression profiling is useful tool to identify different types of astrocytes in the developing and adult brain, and following injuries such as focal cerebral ischemia 383. Although our understanding of the diversified types and functions of astrocytes is at its inception, available information points to the possibility of diversified astrocytic responses depending on the cell type and the region in which a particular astrocyte is located, which in turn depends on its interaction with neurons, microglia and blood vessels. Gene expression studies of isolated neurons, astrocytes and oligodendrocytes help to improve understanding of cell diversity 72, 202, 261, 490, 491. However, identification of astrocyte sub‐types and recognition of their gene expression profiles has not been achieved.
Functions of Astrocytes
Astrocytes are key and unique regulators of multiple brain functions. They are sensitive to different stimuli, responding through complex calcium signaling 403. Although not able to propagate action potentials along their processes as neurons do, astrocytes express potassium and sodium channels, and exhibit evoked inward currents 314. The following are the major physiological functions of astrocytes:
Regulation of potassium, sodium and calcium homeostasis through specific channels and water by astrocyte‐specific AQ4 water channels 199, 220, 311, 341.
Cellular coupling through gap junctions mainly composed of connexins 314.
Regulation of blood flow through the release of mediators such as arachidonoic acid, prostaglandins and nitric oxide 152, 189.
Participation in the functioning of the blood brain barrier 1, 26, 152, 223.
Modulation of the synaptic transmission through the expression of glutamate, GABA and glycine transporters, thus facilitating turnover of neurotransmitters at the synapses 10, 314, 402.
Release of neurotransmitters such as glutamate, GABA, D‐serine, ATP and adenosine, thus functionally contributing to the tripartite synapse 17, 23, 162, 163, 164, 352.
Catabolisation of glutamate by glutamine synthetase and adenosine by adenosine kinase 198, 494.
Contribution to the formation, maintenance and pruning of synapses during development 29, 85, 423.
Providing energy through the storage of glycogen, production of lactate and transfer to neighboring neurons and synapses 59, 60, 351, 427.
Responsiveness to oxidative stress by producing glutathione, ascorbic acid and superoxide dismutases (SOD1, SOD2, SOD3) 106, 259, 411; up‐regulation of heme‐oxygenase 1 387, 388.
Astrogliopathy
This term refers to alterations of astrocytes occurring in diseases of the nervous system, and it implies the involvement of astrocytes as key elements in the pathogenesis and pathology of diseases and injuries of the central nervous system 335, 349, 351, 452, 454, 455. Astrogliopathy covers the seminal concept of gliodegeneration 93 and stresses the cardinal role of astrocytic dysfunction in the pathogenesis of neurological diseases 395.
Astrogliopathy includes reactive gliosis and astrocytopathy. Reactive astrogliosis is a reaction secondary to trauma and ischemic injuries, external toxins, metabolic disorders and neuron damage in neurodegenerative diseases. The term astrocytopathy is here used to include non‐reactive astrogliosis covering hypertrophy, atrophy and astroglial degeneration with loss of function manifested by variable and distinct molecular changes in astrocytes, and pathological remodeling 346. Senescent astrocytes can also be considered a particular form of astrocytophathy usually linked to old age. This classification is simplistic but it can be advantageous and instrumental to class molecular alterations in different settings and diseases.
Astrocytes can be the primary targets in rare genetic neurological diseases in which genetic alterations involve specific astrocytic genes. Astrocytes can also be vulnerable co‐primary targets in neurodegenerative diseases with abnormal protein aggregates such as Alzheimer's disease (AD), tauopathies, Parkinson's disease (PD), Huntington's disease (HD), amyotrophic lateral sclerosis (ALS) and Creutzfeldt‐Jakob disease (CJD).
Therefore, the term astrogliopathy points to a variety of alterations. Astrogliopathy following trauma and stroke is mainly manifested as reactive astrogliosis. Astrogliopathy in Alexander's disease is a typical genetic astrocytopathy. Astrogliopathies in neurodegenerative diseases are particularly complex as they occur in aged brains in which astrocytes may suffer from senescent changes, may have particular responses linked to reactive astrogliosis and, more importantly, may have disease‐specific alterations with particular manifestations of astrocytopathy depending on the neurodegenerative disease. The boundaries between reactive astrogliosis and the various alterations covered by the term astrocytopathy in neurodegenerative diseases are often blurred as for example in AD.
The weight of astrocytes in neurodegenerative diseases is exemplified by the fact that the majority of de‐regulated genes in mouse models of AD and ALS are expressed in astrocytes and microglia rather than in neurons, as revealed by the application of isolation techniques to analyze transcriptomes separately from neurons and glial cells 420.
Most neurodegenerative diseases have been considered cell autonomous meaning that damage of certain populations of neurons suffices to produce disease. However, convergence of damage of several cell types including astrocytes as well as damage to neurons may account for the selective vulnerability in many neurodegenerative diseases 193. The concept of non‐cell autonomous toxicity, first exemplified in ALS, is crucial to understand the pathogenesis of most neurodegenerative diseases 193.
Senescent Astrocytes
Astrocytes change with aging 371, 385. This is manifested by modifications in number, morphology of the cytoplasm and nucleus, accumulation of lipofuscin in the cytoplasm, hypertrophy of cytoplasmic filaments and increased expression of GFAP, S100β and vimentin 38, 92, 128, 188, 237, 274, 316, 345, 360, 385, 426, 441, 468. It is commonly considered that aging is accompanied by increased numbers of GFAP‐immunoreactive astrocytes. However, this is subject to species differences since no modifications in the number of astrocytes are observed with age in the occipital cortex of rhesus monkeys or in human neocortex 337, 353.
Senescence astrocytes exhibit increased oxidative damage and enhanced superoxide production 145 together with a senescence‐associated secretory phenotype similar to that seen in other cellular types 74, 91, 135 manifested by increased production of pro‐inflammatory cytokines 75, 240, 478. Isolated astrocytes from aged mice have increased inflammatory phenotype, increased zinc ion binding, decreased neuronal differentiation capacities and reduced hemoglobin synthesis when compared with astrocytes from young mice 334.
The senescence secretory phenotype can be triggered by different stimuli including oxidative stress, inflammation and inhibition of the ubiquitin‐protesome system 42. Increased inflammatory phenotype and decreased neuronal differentiation are also observed in human astrocytes subjected to oxidative stress‐induced senescence, although GFAP, and processing and presentation of antigens by major histocompatibility complex class II proteins, decrease in this cellular model 94. Reduction of GSH in glial cells induces neurotoxicity which may be relevant in aging and neurodegeneration 241.
Perivascular astrocyte senescence leads to altered blood brain barrier function in old age 1, 115, 298, 359. Aging in blood vessels is also accompanied by reduced expression of efflux transporters and increased expression of influx transporter receptor for advanced glycation end products which can participate in the abnormal transfer of β‐amyloid, leading to its accumulation in blood vessels 406, 407. Astrocyte senescence has been implicated in the pathogenesis of neurodegenerative diseases 40, 83, 371, 372, 449.
Reactive Astrogliosis
The term reactive gliosis is used for the response of astrocytes, microglia and NG2‐positive cells to different insults to the nervous system 67. Increased cytoplasmic size and robustness of branches (hypertrophy) accompanied or not by an increase in the number of astrocytes (hyperplasia) in response to external or intrinsic damage to the nervous system characterize reactive astrogliosis. Holzer and phosphotungstic acid hematoxylin stains, as well as several silver methods, were seminally used to identify astrogliosis. Increased expression of GFAP in astrocytes is considered a marker of reactive gliosis in histological sections 109, 481. However, GFAP immunohistochemistry does not permit the visualization of small astroglial processes as it is present only in the cytoplasm and proximal segments of radial branches; fine perisynaptic terminals and fine branches are not visualized by GFAP immunostaining. Moreover, subpopulations of reactive astrocytes have no substantial levels of GFAP.
The intensity of reactive astrogliosis is variable and categorized as mild, moderate or severe, the latter implying marked hypertrophy and frequent proliferation. Severe astrogliosis may eventually form a scar in which cellular processes of reactive astrocytes densely overlap and pack together, forming a compact barrier which separates the preserved neural tissue from the local severely damaged area. Reactive astrocytes in glial scars interact with pericytes, meningotelial cells and fibroblasts, and form dense fibrous collagen‐rich barriers which are neuroprotective to potential external noxious agents 405. Reactive astrogliosis has been categorized into isomorphic/mild neuroprotective and anisomorphic/severe scar forming 350. This classification is also rather schematic as both forms may appear at different sites following a single injury, but again it is appropriate to separate polar forms of reactive astrogliosis.
Despite the apparent morphological similarities of reactive astrocytes, reactive astrogliosis is a very heterogeneous response that can be triggered by various stimuli, is mediated by different factors, has distinct functions, and leads to particular, even opposite, effects depending on diverse circumstances 67.
Reactive astrogliosis can be induced by molecules derived from external insults and from products of the metabolism of neurons, glial cells, pericytes, endothelial cells and other cell types. Mediators of the innate inflammatory response such as Toll receptors, neurotransmitters such as glutamate and noradrenaline, purines, steroid hormones, transforming growth factor α, ciliary neurotrophic factor, IL‐6 and other cytokines, oncostatin, leukemia inhibitory factor, serum proteins, nitric oxide, reactive oxygen species, ammonia, hypoxia and glucose deprivation, and peptides and proteins linked to particular neurodegenerative diseases such as β‐amyloid, among many others, can trigger reactive astrogliosis 25, 140, 142, 181, 221, 246, 347, 350, 363, 415, 472. Transduction depends on several signaling pathways which regulate anti‐ and pro‐inflammatory functions, expression of GFAP, vimentin and other cytoskeletal proteins, cell proliferation and chaperone function. Factors implicated in inter‐ and intracellular signaling pathways are signal transducer and activator of transcription 3 (STAT3), nuclear factor of kappa light polypeptide gene enhancer in B cells (NF‐κB), cyclooxygenase 2 (COX2), mitogen‐activated protein kinases (MAPKs), nuclear factor erythroid 2 (Nrf2), suppressor of cytokine signaling 3 (SOCS3), cAMP, ATP, Olig2, endotelin 1, peroxisome proliferator activated receptor alpha (PPAR), epidermal growth factor (EGF) and fibroblast growth factor (FGF), inter alia 36, 37, 179, 185, 190, 210, 292, 313, 327, 328, 348, 349, 350, 401, 415, 417, 421, 440, 467. Small non‐coding RNAs also participate in reactive astrogliosis 39, 187, 463. Resultant proteins are not uniformly expressed in the totality of reactive astrocytes but rather only in certain sub‐populations even within the same setting 112, 113, 350, 383, 415, 416, 417, 467.
Some reactive astrocytes have the capacity to proliferate locally 28, 66, 415, 417 and some of them may transform into multi‐potential cells 65, 368, 413. Astroglia may induce neurogenesis from adult stem cells 418. Inflammation‐induced NF‐κB activation promotes the conversion of astrocytes into neural progenitor cells 139. Proliferation results in an increase in the number of astrocytes which must not be mistaken for the mere enhancement of GFAP expression in already existent astrocytes. However, astrocyte proliferation in neurodegenerative diseases seems to be very limited 207, 209, 413, 426. Individual proteins and activation of certain pathways have distinct effects on particular settings 287. GFAP is a paradigm of potential dual opposite effects. Mice lacking GFAP are hypersensitive to traumatic cerebrospinal injury 312. Mice lacking GFAP and vimentin show reduced capacity for repair in the face of different noxious agents 248, 273, 346, 448, 469. Therefore, GFAP benefits the recovery of damaged nervous tissue. Yet excessive GFAP expression, as in scars, reduces the capacity for healing 271, 374. In this regard, astrocyte scar formation has been classically linked to reduced axonal regeneration 405. However, recent evidence indicates a more complex scenario in which early scar formation helps axon regeneration in the central nervous system 12.
The majority of signals and mediators have been identified in experimental models mimicking acute insults such as ischemia, excitotoxicity and spinal cord injury. Astroglial responses after ischemic stroke and focal traumatic lesions are classified into three phases: phase I, characterized by cell death and inflammation; phase II, recognized by cell proliferation and tissue replacement; and phase III, manifested by tissue remodeling 67.
However, it is worth stressing that comparative genomic analyses of reactive astrogliosis show marked stimulus‐dependent differences in gene expression in the face of relatively simple injuries such as trauma, focal ischemia, acute inflammation and acute exposure to toxins 11, 348, 488.
Potential functional effects of reactive astrogliosis
Reactive astrogliosis is interpreted as a beneficial reaction geared to protecting the nervous system from harmful internal and external stimuli. However, certain continuing astrocytic responses, for example those producing chronic inflammation, may increase neuronal damage 350. This aspect is relevant in long‐lasting neurodegenerative diseases in aging 461.
Reactive astrogliosis may protect neurons from excitotoxic damage, water and ionic imbalance, oxidative stress damage and infection 68, 82, 99, 114, 116, 179, 248, 295, 307, 362, 414, 415, 417, 431, 447, 451, 487.
However, reactive astrogliosis may also have adverse effects by increasing excitotoxic damage, increasing oxidative stress, facilitating the production of pro‐inflammatory cytokines and chronic inflammation, and increasing the permeability of the blood brain barrier 18, 53, 55, 168, 431, 432, 487, 488. Thus, decline of particular functions in reactive astrocytes facilitates neuronal damage 328, 376, 412, 422, 446, 450, 452, 454, 455.
Astrocytopathy
Decrease in the number of astrocytes, atrophy/degeneration and loss of function may occur as a primary cause of a disease or as a factor contributing to the development and progression of a particular disease 350.
Primary genetic astrocytopathies
Alexander's disease caused by mutations in GFAP is characterized by abnormal astrocytes and production of Rosenthal fibers composed of aggregates of filaments and dense inclusions in the cytoplasm of astrocytes. This results in impaired maintenance of myelin and axons, and leukodystrophy in infantile cases 56, 289, 361. Similar alterations are found in GFAP transgenic mice 288, 289. Megalencephalic leukoencephalopathy with subcortical cysts is caused by mutations in MLC1 gene the product of which is expressed in the distal processes of subpial, periventricular and perivascular astrocytes. The disease is manifested by myelin breakdown, vacuolization and formation of confluent cysts in the cerebral white matter 46, 179, 270, 390.
Secondary astrocytopathies to toxic and metabolic disturbances
Alzheimer II astrocytes, also known as naked reactive astrocytes, have a large, pale nucleus, often a prominent nucleolus and reduced GFAP immunoreactivity which is accompanied by increased numbers of mitochondria, enlargement of the endoplasmic reticulum and accumulation of glycogen. Alzheimer type II astrocytes are mainly observed in grey matter of the striatum, globus pallidus, substantia nigra and dentate nucleus following hyperammonemia linked to hepatoencephalopathy and related conditions as well as in experimental hyperammonemia 320, 321. Hyperammonemia in astrocytes alters potassium, sodium and calcium homeostasis, increases intracellular edema, interferes with glutamine synthetase and induces glutamate excitotoxicity 159, 200, 214, 250, 252, 297, 322.
Secondary astrocytopathy with inhibition of glutamate transport occurs in thiamine deficiency and Wernicke‐Korsakoff encephalopathy 173, 174, and after poisoning with heavy metals 451.
Astrocytopathy in neurodegenerative diseases in old age: Intracellular deposits of altered proteins
Combined neuronal damage and astrocytopathy is common in neurodegenerative diseases. The majority of neurodegenerative diseases with protein aggregates show accumulation of abnormal proteins in astrocytes and other glial cells 294.
Astrocytes containing hyper‐phosphorylated tau are major elements of the pathology in most tauopathies 149, 191, 226. Principal phenotypes are tufted astrocytes in PSP, astrocytic plaques in CBD and ramified astrocytes in PiD 16, 104, 117, 171, 197, 305, 317, 318, 479 (Figure 4A–C). It has been suggested that tufted astrocytes and astrocytic plaques do not coexist in PSP and CBD 225. However, association of tufted‐like astrocytes and astrocytic plaques is common in certain familial tauopathies linked to MAPT mutations 150.
Figure 4.
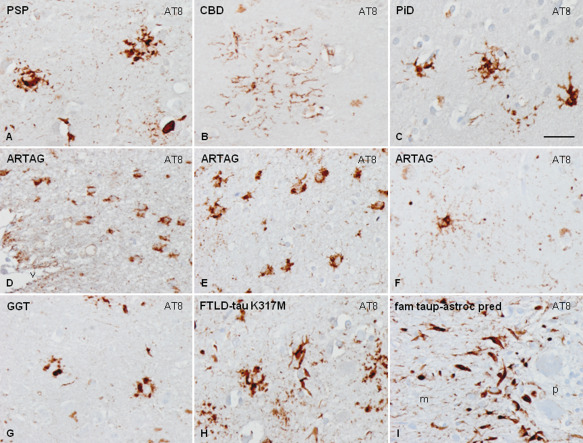
Morphology of astrocytes containing hyper‐phosphorylated tau in several tauopathies, tufted astrocytes in PSP (A), astrocytic plaque in CBD (B), ramified astrocytes in PiD (C), periventricular thorn‐like astrocytes (D) and clusters of thorn‐like astrocytes in the temporal lobe (E) in ARTAG, astrocyte with thin diffuse granular hyper‐phosphorylated tau in astrocytic processes in elderly (F), astrocytes with globular inclusions in GGT (G), tufted‐like polymorphous inclusions in astrocytes in FTLD‐tau K317M (H) and Bergmann glia in familial behavioral variant FTLD with astrocyte‐predominant tauopathy (I). Paraffin sections, processed for AT8 immunohistochemistry slightly counterstained with hematoxylin, bar = 35 μm.
Thorn‐like astrocytes occur in old age individuals frequently in association with other tauopathies including argyrophilic grain disease and particularly in aging‐related tau astrogliopathy (ARTAG) 126, 192, 228, 229, 230, 260, 267, 306, 319, 392, 438. Astrocytes with thin diffuse granular hyper‐phosphorylated tau in astrocytic processes are seen in the elderly 228, 230 (Figure 4D–F). Astrocytes with globular inclusions occur in GGT 6, 41 (Figure 4G). All these inclusions are composed of 4R tau isoforms but certain astrocytes in PiD and PSP contain 3R tau isoforms 124.
Various types of astrocytic inclusions are generated in familial FTLD‐tau, the morphology of which largely depends on the MAPT mutation 104, 149. Mutations in exons 1 and 10, and in introns following exons 9 and 10, are associated with neuronal and glial tau deposits 150. The varied morphology of intracytoplasmic tau‐immunoreactive inclusions in FTLD‐tau is represented by tufted‐like astrocytes, astrocytic plaques, ramified astrocytes, thorn‐like astrocytes and, rarely, astrocytes with globular inclusions 124, 150 (Figure 4H). Extensive astrocyte‐predominant tauopathy involving brain astrocytes and Bergmann glia has been reported in a rare familial variant of FTLD‐tau (Figure 4I). Familial PSP, CBD and GGT are suggested in some cases on the basis of the presence of “specific” astrocytic inclusions. However, it is premature to classify these disorders as genetic counterparts of common sporadic cases solely based on similar astrocytic pathology.
Tau phosphorylation, conformation and truncation in astrocytes have characteristics similar to their neuronal counterparts in tauopathies with equivalents to pre‐tangles and tangle stages 124. Active Tau‐kinases involved in tau phosphorylation are expressed in tau‐containing astrocytes in tauopathies 22, 119, 120, 121, 122, 125. Lack of epitopes derived from alternatively spliced exon 2 and 3 has been reported in glial tau when compared with neuronal tau in certain tauopathies 191. Tau acetylation is rarer in astrocytes when compared to neurons in tauopathies 194.
The molecular bases of the different types of tau‐bearing astrocytes in tauopathies are not known. Some differences are barely related to altered tau conformation and tau truncation 124. Redistribution of GFAP around the nucleus and surrounded by tufts of hyper‐phosphorylated tau is characteristic of tufted astrocytes in PSP (Figure 5) and FTLD‐tau/K317M (Figure 6). However, GFAP is disrupted by short segments or dots of hyper‐phosphorylated tau along the astrocytic processes in astrocytic plaques in CBD (Figure 7) and in astrocytes with proximal granular inclusions in FTLD‐tau/P301L 124. Displacement of GFAP by hyper‐phosphorylated tau is also observed in tau containing astrocytes in PiD (Figure 8). Although these differences may have functional implications, further study is needed to learn whether different types of astrocytes are selectively vulnerable to hyper‐phosphorylated tau and whether different species of tau have different effects on certain subpopulations of astrocytes 124. This possibility is supported by experimental studies showing that the intracerebral brain homogenates enriched in tau fibrils from PSP, CBD and AGD injected into ALZ17 mice induce astrocytic pathology resembling the inclusions characteristic of these human tauopathies 86. Moreover, certain tau prion strains propagate in cells and define different tauopathies 386, and certain tau prion strains have the capacity to induce tauopathy in astrocytes 211.
Figure 5.
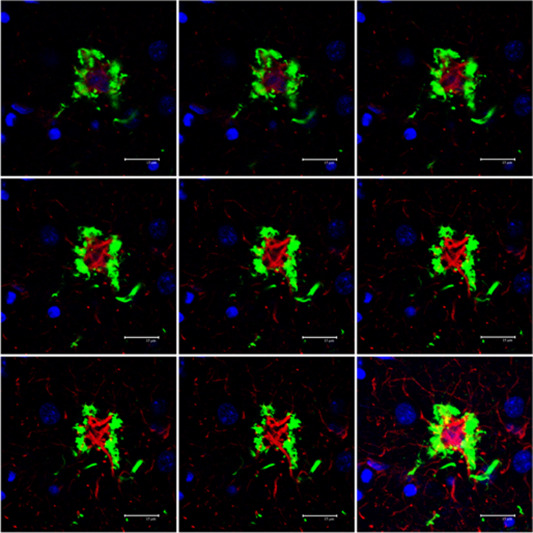
Serial reconstruction of a tufted astrocyte in PSP processed for double‐labeling immunofluorescence and confocal microscopy using antibodies to P‐tau (AT8, green) and GFAP (red). Note tufts of P‐tau at the periphery of the cytoplasm and proximal part of astrocytic branches, and redistribution of GFAP at the inner part of the cytoplasm with very poor GFAP immunostaining of astrocytic branches. Nuclei (blue) are stained with DRAQ5TM; bar = 15 μm.
Figure 6.
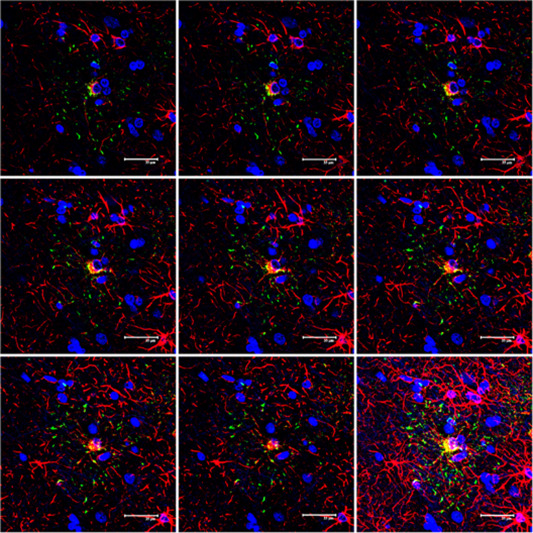
Serial reconstruction of an astrocytic plaque in CBD processed for double‐labeling immunofluorescence and confocal microscopy using antibodies to P‐tau (AT8, green) and GFAP (red). Note the localization of P‐tau in the distal regions of astrocytic branches and the irregular distribution of GFAP in the cytoplasm and astrocytic branches. Nuclei (blue) are stained with DRAQ5TM; bar = 35 μm.
Figure 7.
Double‐labeling immunofluorescence and confocal microscopy using antibodies to P‐tau (AT8, green) and GFAP (red) in FTLD‐tau/K317M. Note the peripheral distribution of P‐tau and the perinuclear localization of GFAP in tufted‐like astrocytes. Nuclei (blue) are stained with DRAQ5TM; bar = 20 μm.
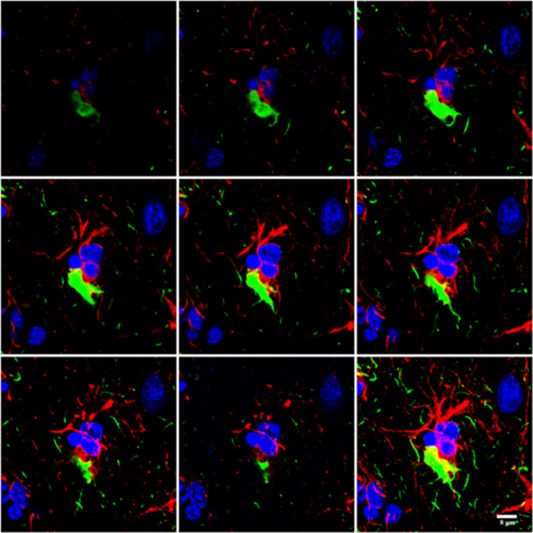
Figure 8.
Serial reconstruction of a hyper‐phosphorylated tau‐containing astrocyte in PiD processed for double‐labeling immunofluorescence and confocal microscopy using antibodies to P‐tau (AT8, green) and GFAP (red). Note the displacement of GFAP in the cytoplasm and the reduced number of GFAP‐positive branches. Nuclei (blue) are stained with DRAQ5TM; bar = 8 μm.
The distribution of astrocytes with abnormal protein deposits does not match the distribution of reactive astrogliosis as first recognized in progressive supranuclear palsy 437 but later extended to the majority of tauopathies (Figure 9). Double‐labeling immunofluorescence and confocal microscopy of astrocytic plaques, tufted astrocytes and astrocytes in the elderly reveal disruption of the astrocytic cytoskeleton manifested by reduction and re‐organization of GFAP immunoreactivity 124 in subpopulations of astrocytes without the appearance of any reactive astrocytes.
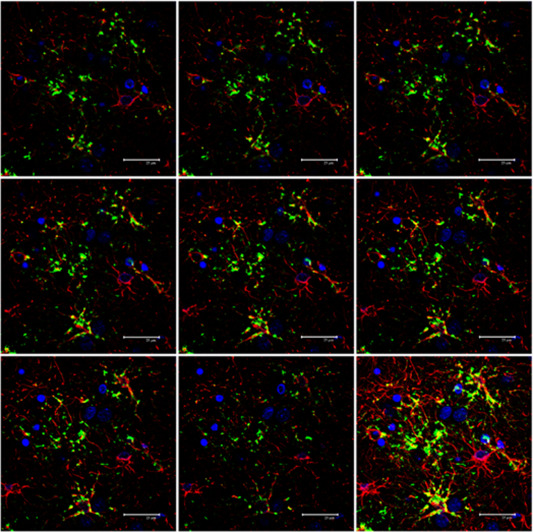
Figure 9.
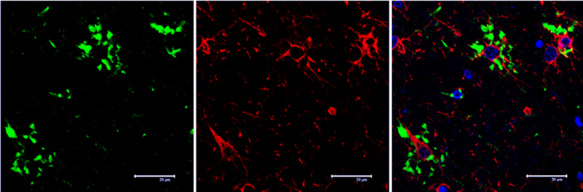
Serial reconstruction of thorn‐like astrocytes in ARTAG processed for double‐labeling immunofluorescence and confocal microscopy using antibodies to P‐tau (AT8, green) and GFAP (red). Note the presence of P‐tau in some astrocytes whereas P‐tau is absent in other GFAP‐positive astrocytes. Nuclei (blue) are stained with DRAQ5TM; bar = 25 μm.
Small heat shock proteins in glial cells are expressed in astrocytes with and without hyper‐phosphorylated tau deposition in various tauopathies 266.
Loss of function in astrocytopathies of neurodegenerative diseases with abnormal protein aggregates
Impaired glutamate transport is postulated in several diseases as a consequence of decreased expression of glutamate transporters in astroglia. Reduced EAAT2/GLT‐1 expression is found in ALS and related transgenic models 57, 132, 158, 184, 258, 276, 291, 340, 379, 380, 381, in certain tauopathies 96, 123, murine models of HD 34, 111, 254, 293, in some transgenic models of Alzheimer's disease 77, 283, and in A53T α‐synuclein transgenic mice 156.
Release of other neurotransmitters by astrocytes is also selectively altered in certain neurodegenerative diseases. GABA release is increased in AD 201, 475 but reduced in the striatum in HD 473.
Altered astrocyte‐dependent potassium homeostasis occurs in AD 333, HD 19, 439, PD 424, 466 and ALS 205.
Altered calcium signaling is found in models of AD 2, 3, 101, 153, 233, 358, 375, 432, 458, PD 48 and ALS 212, 281. Glucose metabolism is altered in AD and HD and in other neurodegenerative diseases. Whether these changes are partly due to altered glucose metabolism in astrocytes is controversial, depending on the disease and animal models used 36.
Cholesterol metabolism is impaired in several neurodegenerative diseases. Cholesterol is largely synthesized in astrocytes and is transported by ApoE 4 to neurons. ApoE 4 is a major risk factor for late onset AD and the substrates of this effect are related to decline cholesterol metabolism, abnormal APP processing and disrupted β‐amyloid clearance 63. Cholesterol metabolism is altered in transgenic models of AD 333; cholesterol biosynthesis is also low in transgenic models of HD 399, 443, 444, 445.
Expression of astrocyte connexins is abnormal in several neurodegenerative diseases 95, 151, 286, 310, 459 thus sustaining possible modifications in gap junctions, and deteriorated function of cell‐to‐cell networks, ion homeostasis and transfer of gliotransmitters 47.
Worsening function of the blood brain barrier in several neurodegenerative diseases is thoroughly documented 493.
Oxidative stress and responses in astrocytes in neurodegenerative diseases with abnormal protein aggregates
Astrocytes in neurodegenerative diseases may be a source of reactive oxygen and reactive nitrogen species occurring in parallel with the generation of certain peptides and proteins such as β‐amyloid, superoxide dismutase 1 (SOD1), TAR DNA‐binding protein 43 (TDP‐43) and prion protein scrapie (PrPSc) 4, 9, 78, 133, 176, 373. Reactive astrocytes may also sustain oxidative stress responses, increasing glutathione, SOD2 and Nrf2 protein levels 3, 73, 81, 447.
Redox proteomic studies have identified several proteins which are oxidatively damaged in neurodegenerative diseases (71, 280, 428, 429 for review). Some of them are expressed mainly in neurons and their damage has been associated with loss of function. However, a large number of oxidized proteins are enriched in astrocytes. This is particularly important regarding proteins linked to glycolysis and energy metabolism, oxidative stress responses and cytoskeleton 280.
Although its significance is not known, GFAP is consistently oxidized in most neurodegenerative diseases assessed to date 279, 280, 304, 338. GLT‐1 is oxidatively modified by 4‐hydroxy‐2‐nonenal in AD 238.
DNA and mRNA are also oxidatively damaged in neurodegenerative diseases. However, the percentage of damage corresponding to astrocytes has not been determined in most of them. Astrocyte DNA oxidative damage has been reported in AD 409.
Astrocytes and inflammation in neurodegenerative diseases with abnormal protein aggregates
Aging is accompanied by low levels of activated innate inflammatory responses 265, 269, 272, 482. Microglia and astrocytes, together with neurons, participate in the process of activation of inflammatory responses in aging and in neurodegenerative diseases with abnormal protein aggregates 175, 355. Moreover, microglia has the capacity to transform a subset of reactive astrocytes through the combination of IL‐1α, TNF and C1q 253. These astrocytes lose the ability to promote neuronal survival, outgrowth, synaptogenesis and phagocytosis, and induce the death of neurons and oligodendrocytes 253. They are common in AD, PD, HD and ALS.
Inflammatory responses are disease‐, region‐ and stage‐dependent, thus largely differing in AD, tauopathies, CJD, PD, DLB and ALS 14, 143, 144, 262, 265, 268, 269. Several cytokines and mediators are expressed in reactive astrocytes in these diseases, although the precise localization of individual inflammatory mediators is largely unknown. No particular attention has been devoted so far to learning about specific inflammatory responses in the different types of abnormal astrocytes with abnormal hyper‐phosphorylated tau in tauopathies.
Regular use of non‐steroidal anti‐inflammatory therapy reduces the number of astrocytes (and active microglia) in AD 8.
Astrogliopathy in Alzheimer's Disease
Reactive astrogliosis
Astrocytes are key players in the cellular phase of AD involving β‐amyloid turnover, calcium homeostasis, tripartite synaptic function, neuroinflammation and oxidative stress responses, among others 148, 382, 425.
Astrogliosis is a relatively early event in AD and related mouse models 32, 76, 177, 236, 328, 370, 454, 457, 484. Reactive astrocytes are mainly found around β‐amyloid deposits in plaques and blood vessels in humans and AD models 308, 328, 370, 410, 450. Nevertheless, reactive astrocytes are also found in areas without plaques 31, 208, 410 (Figures 1A–E and 2A,B).
The majority of studies dealing with reactive astrogliosis in AD involve β‐amyloid. Reactive astrocytes contain β‐amyloid 137, 157, 309, 315 and N‐terminal truncated β‐amyloid 435. Reactive astrocytes cluster around β‐amyloid plaques and perivascular β‐amyloid deposits where they internalize and degrade β‐amyloid fibrils 224, 476. Apolipoprotein E promotes aggregation and degradation of β‐amyloid in astrocytes as this function is attenuated in ApoE KO mice 224. Low density lipoprotein receptor‐related protein 1 (LRP1) is involved in the uptake of β‐amyloid and it is also a receptor of ApoE 30, 217. All these observations point to that astrocytes are involved in plaque formation.
Astrocytes transplanted into the brain of a transgenic AD model migrate to the site of β‐amyloid deposition, internalize β‐amyloid and clear β‐amyloid plaques 356, 357. The mechanisms used by reactive astrocytes to degrade β‐amyloid are multiple and complementary, including activation of metalloproteinases 480, 485 and lysosomal degradation 30, 249, 477.
β‐amyloid can also be generated in glial cells, as β‐secretase (BACE) is expressed in astrocytes under appropriate conditions 177, 377, 378, 492.
The characteristics of reactive astrogliosis and the role played by reactive astrocytes depend on the region and local environment at a particular stage of the disease 32, 206, 222, 232, 236, 328, 450, 484. It has been suggested that astrocytes around plaques may be protective 284. The protective role of subpopulations of astrocytes is supported by the observation of increased plaque load and increased dystrophic neurites surrounding β‐amyloid plaques in APP/PS1 GFAP‐/‐Vim‐/‐ transgenic mice 232. Yet these observations were not validated in another study 206. Evidence of possible harmful effects of activated astrocytes on neurons come from in vitro studies showing that astrocytes in contact with β‐amyloid increase neuronal vulnerability in several co‐culture paradigms 2, 3, 4, 9, 147, 339. β‐amyloid‐induced glutamate release by astrocytes might be a contributory factor 433. De‐regulation of specific metabotropic glutamate receptors in astroglia is also a putative harmful effect of β‐amyloid 255. Acquisition of pro‐inflammatory profile and activation of the β‐amyloidogenic pathway further potentiates toxicity 493.
Astrocytes themselves show fragmentation of calpain‐immunoreactive processes 146, and increased caspase 3 and CD95 immunoreactivity and increased apoptosis 230.
In addition to the relation with plaques, astrocytes also respond to neurofibrillary tangles; glial responses correlate positively with tangle burden and they increase linearly around existing plaques and in the vicinity of tangles 398.
Astrocytopathy
Astrocytes in AD‐related transgenic mice show atrophy and reduced branching 33, 236, 484. Astrogliosis and astrocyte atrophy are region‐dependent and occur in AD and transgenic models of AD 328, 372, 452. Reduced branching largely contributes to decreased astrocytic domain coverage in AD 350. In animal models, atrophy of astrocytes may precede reactive astrogliosis, thus suggesting that astrocytes are vulnerable at very early stages of the pathology 32, 236, 328, 452, 454.
Reduced glucose metabolism in AD can be sustained, at least in part, by decreased astrocyte metabolism linked to β‐amyloid toxicity 9 but also to reduced metabolism of atrophic astrocytes.
Altered mRNA and/or protein expression of glutamate transporters and altered glutamate homeostasis have been reported in transgenic models of AD 77, 283. Expression levels of GLT‐1 and glutamate homeostasis in prefrontal cortex are controversial in AD 235, 282. Another study shows inverse relation between GFAP (increase) and EAAT2 (reduction) expression with disease progression as defined by Braak stage of neurofibrillary tangle degeneration 410, 411.
Indirect data point to functional alterations of glutamate transporters in AD as a result of oxidative damage, splice variants and altered solubility of EAAT2 107, 238, 394, 474. Glutamine synthase is reduced in reactive astrocytes in AD and related models 245, 329 thus resulting in decreased neuronal GABA‐mediated inhibition 336.
Astrocytes bearing β‐amyloid show abnormal calcium homeostasis 2, 3, 4, 153, 233, 234, 256, 375, 453. Connexin 43 expression is altered in AD and animal models 282, 306 which may compromise the extent of coverage domain and synaptic function in neighboring neurons 234. β‐amyloid peptides also induce mitochondrial dysfunction and oxidative stress in astrocytes 4.
Astrocytes surrounding plaques show increased expression of cytokines and mediators of the immune response in AD and related models 247, 299, 332, 333, 355. Transcriptional studies of isolated astrocytes from the cortex of aged controls and APPswe/PS1dE9 transgenic mice show increased expression of inflammatory genes, and reduced expression of neuronal support genes and genes involved in neuronal communication 333. This phenomenon appears to be secondary to β‐amyloid deposition, as the expression of neuroinflammatory markers increases in cultured astrocytes exposed to β‐amyloid fibrils 244.Transcriptomics of laser‐captured microdissection using GFAP as a marker revealed marked dysregulation of insulin, phosphatidylinositol 3‐kinase (PI3K)/Akt, and mitogen‐activated protein kinase (MAPK) signaling pathways at advanced Braak stages of the disease; minor and different alterations were found at earlier stages thus indicating different responses of astrocytes along with disease progression and impaired key signals in astrocytes in AD 411.
Disruption of the immune/inflammatory calcineurin/nuclear factor of activated T‐cells, using a GFAP promoter, reduces glial activation and β‐amyloid burden, and improves cognitive and synaptic function in APP/PS1 transgenic mice 138.
Abnormal astrocytes in AD may contribute to the alterations in the function of the blood brain barrier 35, 493, partly due to the reduction in GLT‐1 expression 471.
Finally, whether astrocytes contribute to the spreading of β‐amyloid and hyperphosphorylated tau and facilitate disease progression in AD is a hot question 98.
Astrogliopathy in Tauopathies
Tauopathies are a heterogeneous group of diseases having in common the deposition of hyper‐phosphorylated tau in neurons and glial cells with specific characteristics with reference to regional vulnerability, morphology of deposits in different cell types, and biochemical traits of abnormal tau 105, 226.
Reactive astrogliosis
Reactive astrogliosis is common in all tauopathies and its distribution correlates with the degree of regional vulnerability to neuronal degeneration and neuronal loss.
In PSP, reactive astrogliosis is marked in the subthalamic nucleus, substantia nigra and colliculus and, to a lesser extent in the globus pallidus; astrogliosis is mild or moderate in the striatum and cerebral cortex (Figure 2C). This correlates with the presence of neurofibrillary tangles and neuron loss rather than with the presence of tufted astrocytes 437.
In CBD, reactive astrogliosis is marked in the cerebral cortex mainly in the upper cortical layers, the immediate subcortical white matter and the substantia nigra. Reactive gliosis is mild or moderate in the striatum, pallidum and subthalamic nucleus. Fibrillary astrogliosis is found in the white matter. Double‐labeling immunofluorescence and confocal microscopy show that only some astrocytes in the cerebral cortex contain hyper‐phosphorylated tau‐conforming astrocytic plaques, whereas others do not. Reactive astrocytes in the white matter do not contain abnormal tau, excepting clusters of thorn‐like astrocytes when present 104.
In PiD, marked reactive astrogliosis involves the cerebral cortex of the frontal and temporal lobes, followed by the hippocampus. Moderate or severe reactive astrogliosis also occurs in the striatum and thalamus. Involvement of other regions parallels neuron loss. Reactive astrogliosis in PiD does not match with the presence of tau accumulation in a subpopulation of astrocytes, although many reactive astrocytes in the temporal cortex contain hyper‐phosphorylated tau (305.
Astrogliosis and astrocyte degeneration occur in the cerebral cortex in frontotemporal lobar degeneration 58, 215. However, the extent and distribution of reactive astrogliosis varies depending on the MAPT mutation and the degree of neuronal damage. For example, reactive astrogliosis is moderate in most cases bearing the P301L mutation, whereas reactive astrogliosis is severe in FTLD‐tau linked to K317M mutation 489. Interestingly, reactive astrocytes in FTLD‐tau K317M, and to a lesser extent in some cases of FTLD‐tau P301L, show atrophy and reduced numbers and predominance of thin astrocytic processes as revealed by GFAP and vimentin immunohistochemistry (Figures 1F,I and 2E,F).
In contrast to other tauopathies, the majority of astrocytes in FTLD‐tau K317M contain hyper‐phosphorylated tau as revealed by double‐labeling immunofluorescence and confocal microscopy (Figure 6). Hyperphosphorylated tau and GFAP also occur in most astrocytes in the rare familial behavioral variant FTLD associated with astrocyte‐predominant tauopathy (Figure 10).
Figure 10.
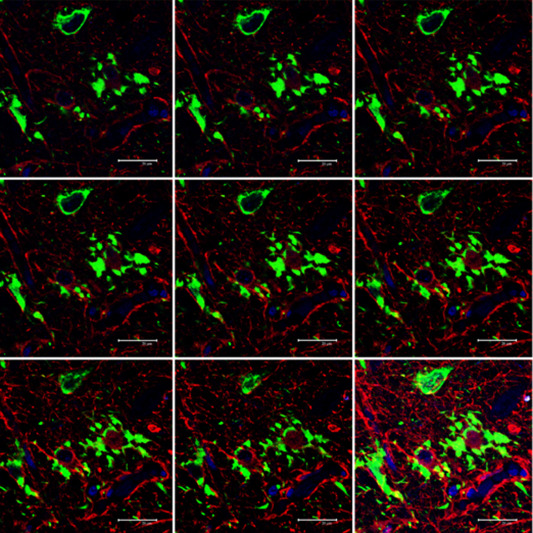
Serial reconstruction of hyper‐phosphorylated tau‐containing astrocytes in familial behavioral variant FTLD associated with astrocyte‐predominant tauopathy, processed for double‐labeling immunofluorescence and confocal microscopy using antibodies to P‐tau (AT8, green) and GFAP (red). Note the tufted‐like cytoplasmic morphology and podocytes at the surface of a blood vessel. A tau‐positive neuron is seen in the upper part of the figure. Nuclei (blue) are stained with DRAQ5TM; bar = 20 μm.
Reactive astrogliosis also occurs in transgenic mouse models; the hippocampus is mainly affected in mice bearing the P301S mutation 265.
A characteristic response of reactive astrocytes in most tauopathies, including AD, is the expression of small heat shock proteins (HSP25/27 and αB‐crystallin) which are rarely co‐expressed in glial cells bearing hyper‐phosphorylated tau 97, 266, 367, 393, 470. It is of note that tau in neurons induces HSP27 overexpression in astrocytes 127, and the production of small HSPs, together with peroxiredoxin, apolipoprotein E and latexin; in FTLD‐tau astrocytes procure neuroprotection against tau toxicity 483.
Nevertheless, astrocytes in PiD and FTLD are vulnerable to degeneration and death; beaded processes and apoptosis as revealed by combined several markers are not uncommon 58, 278.
Astrocytopathy
In spite of the evident astrocytopathy, little is known about the functional effects of hyper‐phosphorylated tau in tau‐containing astrocytes in tauopathies 204. Overexpression of tau in cultured astrocytes leads to degenerative changes in the Golgi complex and cytoskeleton, and to cell death 486. Tau expression in astrocytes might putatively affect nuclear function and DNA transcription as postulated for tau‐containing neurons in tauopathies and fly models 88, 136, 178, 213. FTD astrocytes derived from neural stem cells carrying the N279K MAPT mutation show increased vulnerability to oxidative stress and altered transcriptome profile. Moreover, co‐cultures of mutant astrocytes with healthy neurons increase oxidative stress and produce marked modifications in the genomic expression of neurons 166.
It remains a challenge to learn the reasons for and the functional consequences of the various types, often disease‐dependent, of astrocytes accumulating hyper‐phosphorylated tau species. Little is known about tufted astrocytes in PSP, astrocytic plaques in CBD and globose astrocytes in GGT except that they differ from reactive astrocytes by their different localization and GFAP distribution in the cytoskeleton.
Transgenic mice selectively expressing abnormal hyper‐phosphorylated tau in astrocytes produce neurodegeneration which is accompanied by decreased expression of GLT‐1 and GLAST in astrocytes 96, 130. GLT‐1 expression is markedly reduced in most astrocytes bearing hyper‐phosphorylated tau in a rare familial behavioral variant of frontotemporal dementia associated with astrocyte‐predominant tauopathy not linked to mutations in MAPT 123.
Glutamate metabolism is also impaired in the hippocampus in transgenic mice bearing the P301L in Mapt 317.
Finally, an important aspect is the plausible role of astrocytes in seeding and progression of tauopathy. Tau obtained from several tauopathies inoculated into the mouse brain can spread to resident astrocytes 43, 86. Certain tau prion strains have the capacity to induce tauopathy in astrocytes 211.
Astrogliopathy in Parkinson's Disease
Reactive astrogliosis
Reactive astrogliosis is mild in the substantia nigra and other brain regions in PD even at advanced stages of the disease 131, 296. Different responses occur in experimental models of parkinsonism as marked reactive astrogliosis occurs in transgenic mice expressing A53T α‐synuclein 156 and in 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP)‐induced parkinsonism 169. Reactive astrocytes in an MPTP‐induced model of parkinsonism in mice favor neuronal survival 169 modulated via β‐catenin 243.
Astrocytopathy
Small amounts of α‐synuclein composed of filaments of about 25–40 nm in diameter are found in protoplasmic astrocytes in PD, even in regions in which Lewy bodies and neurites are seldom observed, such as the striatum 49, 165, 419, 462. The use of the novel anti‐α‐synuclein 5G4 antibody, which strongly binds to the high molecular weight fraction of β‐sheet rich oligomers, has shown α‐synuclein immunoreactive thin threads associate with glial reaction and astrocytic α‐synuclein as an important component of the pathology 227.
Astrocytes bearing abnormal α‐synuclein generate abnormal mitochondria 52, 389, produce pro‐inflammatory cytokines and other inflammatory mediators in vitro 240, which in turn can activate astrocytes and microglia 27. The number of inclusions correlates with the severity of nigral neuronal loss 462. Star‐like astrocytes containing α‐synuclein have also been reported in Lewy Body Disease; immunoreactivity of these inclusions is enhanced following formic acid pretreatment and using an antibody against the non‐Aβ component portion of α‐synuclein 434.
Astrocytes expressing mutant A53T α‐synuclein in transgenic mice have reduced expression of GLT‐1 and GLAST, abnormal re‐distribution of GLT‐1 in blood vessels, and altered expression of AQ4 in the soma and proximal astrocyte processes 156. All these changes may increase excitotoxic neuronal damage and impair regulation of the blood brain barrier in mutant mice. On the other hand, astrocytes may have neuroprotective functions, as Nrf2 over‐expression in astrocytes increases neuron survival in the same transgenic model of α‐synucleinopathy 141. Nrf2 expression in astrocytes also confers neuroprotection in the MPTP model of parkinsonism 81. Whether these mechanisms apply to PD is not known. Together, these observations suggest that astrocytes participate in the pathogenesis of PD linked to α‐synuclein accumulation 61, 80.
The specific roles of astrocytes linked to other proteins whose mutations are causative of PD are poorly understood. Astrocytes lacking DJ‐1 have reduced capacities to protect neurons from mitochondrial and oxidative stress insults 300, 301. Nurr1/coREST in cultured astrocytes and microglia protects dopaminergic neurons from inflammation‐induced cell death following lipopolysaccharide injection in mice 384. Although Nurr1 is an important regulatory factor in the generation of dopaminergic neurons, it is still too early to implicate alterations of this pathway in the pathogenesis of PD.
Parkin is induced in cultured astrocytes following unfolded protein stress, thus facilitating ubiquitination of substrates to be degraded by the ubiquitin‐proteasome system (UPS) 239. This observation favors a potential role of astrocytes over neurons in the removal of parkin substrates via the UPS. This observation has been used to suggest that the expression of parkin mutations in astrocytes might reduce the UPS function in PD cases bearing parkin mutations 239.
Regarding capacities of neurons and astrocytes linked to seeding, α‐synuclein can be transferred from neurons to astrocytes 240. Whether abnormal α‐synuclein in astrocytes can be transferred to neurons is a matter of study.
Astrogliopathy in Huntingtons's Disease
Reactive astrogliosis
Marked reactive astrogliosis is found mainly in the striatum but also in the frontal cortex and other brain regions in HD; its intensity increases with disease severity 111, 460. Reactive astrogliosis is remarkably less marked in several transgenic mouse models of HD 37, 154, 155, 257, 439.
Astrocytopathy
Reduction of GLT‐1 mRNA accompanied by decreased glutamate uptake has been reported in the striatum and cerebral cortex in two transgenic mouse models of HD 34, 111, 254, 293. Studies in humans have shown reduced EAAT2 mRNA and protein expression in the striatum 21, 111. Glutamate transport is also reduced in prefrontal cortex in HD 170.
Glutamine synthase is reduced in animal models of HD thus decreasing glutamine levels and the putative production of GABA 254. Reduced astrocytic GABA increases cellular damage in the striatum in a mouse model of HD 473.
Abnormal huntingtin accumulates in the nuclei of astrocytes in rodent models and in HD, lending support to the hypothesis that astrocytes actively participate in the pathogenesis of the disease 51, 111, 404. Toxic capacities of astrocytes depend on the size of polyglutamine repeats 50, 51. This is further supported by the fact that astrocytes generated from induced pluripotent stem cells from HD patients show degenerating characteristics which are dependent on the number of CAG repeats 203.
Increased calcium‐dependent release of glutamate by astrocytes 242 and deficient Kir4.1 potassium ion channel in astrocytes observed in different HD mouse models 216, 439 may contribute to neuronal damage in HD as well.
Astrocytes participate in the inflammatory response in HD through NF‐κB 185. Moreover, the astrocytic response reduces the survival of pericytes through an IκB kinase‐dependent pathway thus impairing vascular reactivity in HD 186.
Astrocytopathy in HD is also manifested by decreased production of brain derived neurotrophic factor (BDNF) and transforming growth factor‐β (TGF‐β) 20, 465. Other alterations of HD astrocytes compromise mitochondrial function 330, 391, cholesterol metabolism 399, 443, 444, 445 and connexin expression 459.
Astrogliopathy in Amyotrophic Lateral Sclerosis
Reactive astrogliosis
Marked reactive astrocytosis is observed in the pyramidal tracts and anterior spinal roots of the spinal cord and affected nerves of the brain stem in parallel with severity and long‐term involvement of the damaged tracts in ALS. Reactive GFAP astrocytes are present in the anterior horn of the spinal cord and motor nuclei of the brain stem, and less markedly in primary motor cortex. Astrocytic gliosis in the posterior columns and spinocerebellar tracts in certain sporadic and familial cases depends on the myelin and axonal damage of these tracts.
A small percentage of familial ALS cases are linked to mutations in SOD1. Astrocytosis is also observed in SOD1 transgenic mouse models 62. Marked neuronal alterations accompanied by mild astrogliosis are found in the anterior thalamus of SOD1 (G93A) ALS mice 100. Astroglial alterations precede clinical symptoms in ALS models 62, 184.
Astrocytopathy
A major alteration in ALS is reduced expression of glutamate transporters in astrocytes and the production of truncated EAAT2/GLT‐1 protein with altered subcellular localization and impaired function resulting from aberrant EAAT2 mRNA processing including intron‐retention and exon‐skipping 57, 132, 258, 276, 290, 379, 381. Loss of EAAT2/GLT‐1 may increase excitotoxic motor neuron damage and is considered a primary factor in the pathogenesis of ALS. However, truncated EAAT2/GLT‐1 forms can be found in normal individuals, and their role in the pathogenesis of ALS is uncertain. Loss of EAAT2 is also observed in SOD1 transgenic mice bearing the G85R mutation 184. The implication of glutamate transporters in ALS is further supported by analyzing the consequences of their manipulation in animal models. Over‐expression of EAAT2 in astrocytes increases neuron survival in SOD1 transgenic mice 158, whereas loss of the glutamate transporter modifies disease progression in ALS mice 340.
Another important point is the damaging effects of astrocytes on motor neurons in familial ALS cases bearing SOD1 mutations. Mutant SOD1 expression in neurons is not sufficient to cause cell death, and wild‐type non‐neuronal cells increase motor neuron survival in ALS mice, thus suggesting that astrocytes are crucial players in familial ALS due to mutations in SOD1 5, 87, 264. The key role of astrocytes is further supported by observations in primary co‐cultures of mutant SOD1 astrocytes with motor neurons in which mutant astrocytes decrease motor neuron survival when compared with co‐cultures using wild astrocytes 102, 308. Therefore, mutant SOD1 in astrocytes is crucial in the homeostasis of motor neurons in SOD1 transgenic mice. Proteinaceous aggregates are found in spinal cord astrocytes in SOD1 transgenic mice 342. Whether abnormal SOD1 species are present in sporadic ALS is under study.
Mutant mouse astrocytes and human astrocyte‐like cells obtained from sporadic and familial ALS cases are toxic for neurons 102, 103, 161, 277, 290, 308.
Astrocytes from ALS patients carrying mutations in TARDBP show intracytoplasmic TDP‐43 inclusions and reduced cell survival, but they are not toxic for control and TARDBP mutant neurons 397.
Other proposed astrocyte‐linked mechanisms leading to motor neuron demise in ALS are activation of the Fas cell surface death receptor (FAS), nitric oxide pathways and nerve grNGF/p75 signaling in astrocytes 118, 343, 364, disruption of the astrocytic tumor necrosis factor receptor superfamily 1‐glial derived neurotrophic factor (TNFR1‐GDNF) axis 54, and impairment of lactate transport 118, among other elements 78, 281, 344, 376. As in other situations, reactive astrocytes in ALS activate STAT3 pathway 400, 401. Astrocytes in ALS may also present altered respiratory function and impaired anti‐oxidant responses 78, 277, 447. No less important is the participation of astrocytes in the inflammatory response 5, 14, 102, 354. The convergence of damaged neurons and accompanying alterations of non‐neuronal partners in ALS supports the idea of non‐cell autonomous toxicity in the pathogenesis of this disease and many other neurodegenerative disorders 193.
A final point is the putative involvement of astrocytes in mutant SOD1 seeding and cell‐to‐cell progression; increased chromogranin‐mediated secretion of mutant SOD1 in ALS might facilitate both mechanisms 110, 442.
Astrogliopathy in Creutzfeldt‐Jakob Disease
Reactive astrogliosis
Marked astrogliosis is a characteristic feature in CJD which parallels spongiform change, microgliosis and neuron loss 64, 251. Reactive gliosis may replace neurons in cerebral cortex in long‐lasting diseases with massive neuron loss. Reactive astrocytes are often large and show strong GFAP (Figure 1G,H), vimentin (Figure 2D) and YKL40 (Figure 3H) immunoreactivity. CJD is characterized by a severe inflammatory response, with up‐regulation of pro‐ and anti‐inflammatory cytokines, receptors, chemokines and other mediators of the inflammatory response; astrocytes express several intermediates including IL‐6 (262).
Reactive astrocytes in CJD, as in other prion diseases, contain αB‐crystallin 464.
What triggers reactive astrogliosis in CJD is not fully understood, but the PrP 106–126 peptide obtained from the amyloidogenic region of the prion protein induces proliferation of cortical astrocytes 436.
Reactive astrocytes in CJD are enriched in aquaporin 1 and AQ4 196, 369. Immunohistochemistry to AQ4 decorates the astrocytic processes at stages with moderate lesions; however, AQ4 immunoreactivity is blurred in areas with extensive spongiform change (Figure 2I).
Oxidation, glycoxidation, lipoxidation and nitration are found in CJD 133. Astrocytes are targets of oxidative damage, as 4‐hydroxynonenal adducts accumulate in astrocytes in CJD and murine scrapie 13. Oxidative stress responses are also marked in reactive astrocytes in CJD as illustrated by increased SOD2 expression 133.
Astrocytopathy
GLT‐1 immunoreactivity decreases in parallel with spongiform change in the cerebral cortex in CJD (Figure 11).
Figure 11.
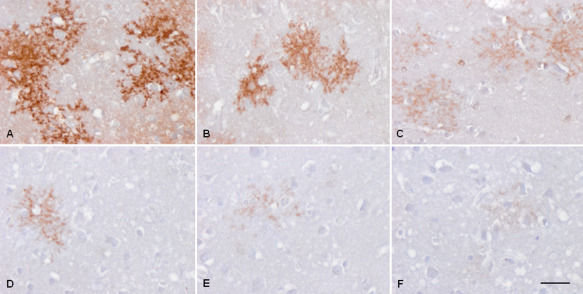
GLT‐1 immunoreactivity in the cerebral cortex in control (A) and three CJD MM1 cases (B–F). Reduced immunoreactivity to almost absence is observed in parallel with spongiform change. Paraffin sections slightly counterstained with hematoxylin; bar = 40 μm.
Clusterin, a heterodimeric glycoprotein, is up‐regulated in CJD and expressed in astrocytes in association with punctate‐type PrPSc and in PrPSc plaques. Clusterin in CJD has abnormal solubility and forms co‐aggregates with PrPSc, thereby probably contributing to PrPSc pathogenesis 134.
Current immunohistochemical methods discriminate different patterns of PrPSc deposition in CJD—principally synaptic‐like, perineuronal, plaque‐like, kuru‐plaque‐like and globoid, depending on the PRP type and codon129 genotype. However, more precise methods show the presence of disease‐associated prion protein linked to synapses, neuronal bodies, dendrites, axons, astrocytes and microglia 231. Moreover, in vitro studies show that human astrocytes have the capacity to take up and degrade normal and protease‐resistant prion protein 84. These data suggest that astrocytes can contribute to the turnover of normal and abnormal prion protein. In this line, recent studies have shown that astrocytes form intercellular connections and nanotubes which drive the transfer of PrPSc from astrocytes to neurons, thereby contributing to prion disease progression 456.
Concluding Remarks
Astrogliopathy is a key element in the pathogenesis and pathology of several neurological diseases including degenerative diseases of the central nervous system. Astrogliopathy includes reactive astrocytosis and astrocytopathy (covering hypertrophy, atrophy/degeneration and loss of function and pathological remodeling) which overlap each other, and, together with astrocyte senescence, contribute to disease‐specific astrogliopathy in aging and neurodegenerative diseases with abnormal protein aggregates in old age. In addition to deposition of abnormal proteins in astrocytes, impaired glutamate transport; abnormal metabolism and release of neurotransmitters; abnormal ion and water homeostasis; abnormal glucose and cholesterol metabolism; increased oxidative damage accompanied by altered oxidative stress responses; increased production of cytokines and mediators of the inflammatory response; altered expression of connexins with deterioration of cell‐to‐cell networks and transfer of gliotransmitters; and worsening function of the blood brain barrier, among others, actively participate in neuron and glial cell degeneration. Finally, although preliminary, recent observations suggest a role of astrocytes in the seeding and transfer of abnormal proteins, and in the progression of neurodegenerative diseases.
Acknowledgments
This study was supported by grants from CIBERNED and Instituto de Salud Carlos III (Ministerio de Economía y Competitividad) co‐funded by FEDER funds/European Regional Development Fund (ERDF) – a way to build Europe: PIE14/00034 and PI14/00757, and by the Seventh Framework Programme of the European Commission (grant agreement 278486: DEVELAGE). I wish to thank T. Yohannan for editorial assistance.
References
- 1. Abbott NJ, Rönnbäck L, Hansson E (2006) Astrocyte‐endothelial interactions at the blood‐brain barrier. Nat Rev Neurosci 7:41–53. [DOI] [PubMed] [Google Scholar]
- 2. Abramov AY, Canevari L, Duchen MR (2003) Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. J Neurosci 23:5088–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abramov AY, Canevari L, Duchen MR (2004) Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta 1742:81–87. [DOI] [PubMed] [Google Scholar]
- 4. Abramov AY, Canevari L, Duchen MR (2004) Beta‐amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci 24:565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aebischer J, Cassina P, Otsmane B, Moumen A, Seilhean D, Meininger V et al (2011) IFNγ triggers a LIGHT‐dependent selective death of motoneurons contributing to the non‐cell‐autonomous effects of mutant SOD1. Cell Death Differ 18:754–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahmed Z, Bigio EH, Budka H, Dickson DW, Ferrer I, Ghetti B et al Globular glial tauopathies (GGT): consensus recommendations. Acta Neuropathol 126:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akiyama H, Tooyama I, Kawamata T, Ikeda K, McGeer PL (1993) Morphological diversities of CD44 positive astrocytes in the cerebral cortex of normal subjects and patients with Alzheimer's disease. Brain Res 632:249–259. [DOI] [PubMed] [Google Scholar]
- 8. Alafuzoff I, Overmyer M, Helisalmi S, Soininen H (2000) Lower counts of astroglia and activated microglia in patients with Alzheimer's disease with regular use of non‐steroidal anti‐inflammatory drugs. J Alzheimer's Dis 2:37–46. [DOI] [PubMed] [Google Scholar]
- 9. Allaman I, Gavillet M, Bélanger M, Laroche T, Viertl D, Lashuel HA, Magistretti PJ (2010) Amyloid‐beta aggregates cause alterations of astrocytic metabolic phenotype: impact on neuronal viability. J Neurosci 30:3326–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anderson CM, Swanson RA (2000) Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32:1–14. [PubMed] [Google Scholar]
- 11. Anderson MA, Ao Y, Sofroniew MV (2014) Heterogeneity of reactive astrocytes. Neurosci Lett 565:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R et al (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andreoletti O, Levavasseur E, Uro‐Coste E, Tabouret G, Sarradin P, Delisle MB et al (2002) Astrocytes accumulate 4‐hydroxynonenal adducts in murine scrapie and human Creutzfeldt‐Jakob disease. Neurobiol Dis 11:386–393. [DOI] [PubMed] [Google Scholar]
- 14. Andrés‐Benito P, Moreno J, Aso E, Povedano M, Ferrer I (2017) Amyotrophic lateral sclerosis, gene deregulation in the anterior horn of the spinal cord and frontal cortex area 8: implications in frontotemporal lobar degeneration. Aging 9:823–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andriezen WL (1893) The neuroglia elements of the brain. BMJ 2:227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arai T, Ikeda K, Akiyama H, Tsuchiya K, Yagishita S, Takamatsu J (2001) Intracellular processing of aggregated tau differs between corticobasal degeneration and progressive supranuclear palsy. Neuroreport 12:935–938. [DOI] [PubMed] [Google Scholar]
- 17. Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999) Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22:208–215. [DOI] [PubMed] [Google Scholar]
- 18. Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR (2009) VEGF‐mediated disruption of endothelial CLN‐5 promotes blood‐brain barrier breakdown. Proc Natl Acad Sci USA 106:1977–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ariano MA, Cepeda C, Calvert CR, Flores‐Hernández J, Hernández‐Echeagaray E, Klapstein GJ et al (2005) Striatal potassium channel dysfunction in Huntington's disease transgenic mice. J Neurophysiol 93:2565–2574. [DOI] [PubMed] [Google Scholar]
- 20. Arregui L, Benítez JA, Razgado LF, Vergara P, Segovia J (2011) Adenoviral astrocyte‐specific expression of BDNF in the striata of mice transgenic for Huntington's disease delays the onset of the motor phenotype. Cell Mol Neurobiol 31:1229–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arzberger T, Krampfl K, Leimgruber S, Weindl A (1997) Changes of NMDA receptor subunit (NR1, NR2B) and glutamate transporter (GLT1) mRNA expression in Huntington's disease–an in situ hybridization study. J Neuropathol Exp Neurol 56:440–454. [DOI] [PubMed] [Google Scholar]
- 22. Atzori C, Ghetti B, Piva R, Srinivasan AN, Zolo P, Delisle MB et al (2001) Activation of the JNK/p38 pathway occurs in diseases characterized by tau protein pathology and is related to tau phosphorylation but not to apoptosis. J Neuropathol Exp Neurol 60:1190–1197. [DOI] [PubMed] [Google Scholar]
- 23. Auld DS, Robitaille R (2003) Glial cells and neurotransmission: an inclusive view of synaptic function. Neuron 40:389–400. [DOI] [PubMed] [Google Scholar]
- 24. Bachoo RM, Kim RS, Ligon KL, Maher EA, Brennan C, Billings N et al (2004) Molecular diversity of astrocytes with implications for neurological disorders. Proc Natl Acad Sci USA 101:8384–8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Balasingam V, Tejada‐Berges T, Wright E, Bouckova R, Yong VW (1994) Reactive astrogliosis in the neonatal mouse brain and its modulation by cytokines. J Neurosci 14:846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ballabh P, Braun A, Nedergaard M (2004) The blood‐brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 16:1–13. [DOI] [PubMed] [Google Scholar]
- 27. Barcia C, Ros CM, Annese V, Gómez A, Ros‐Bernal F, Aguado‐Llera D et al (2012) IFN‐γ signaling, with the synergistic contribution of TNF‐α, mediates cell specific microglial and astroglial activation in experimental models of Parkinson's disease. Cell Death Dis 3:e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bardehle S, Krüger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H et al (2013) Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci 16:580–586. [DOI] [PubMed] [Google Scholar]
- 29. Barres BA (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60:430–440. [DOI] [PubMed] [Google Scholar]
- 30. Basak JM, Verghese PB, Yoon H, Kim J, Holtzman DM (2012) Low‐density lipoprotein receptor represents an apolipoprotein E‐independent pathway of Aβ uptake and degradation by astrocytes. J Biol Chem 287:13959–13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beach TG, McGeer EG (1988) Lamina‐specific arrangement of astrocytic gliosis and senile plaques in Alzheimer's disease visual cortex. Brain Res 463:357–361. [DOI] [PubMed] [Google Scholar]
- 32. Beauquis J, Pavía P, Pomilio C, Vinuesa A, Podlutskaya N, Galvan V, Saravia F (2013) Environmental enrichment prevents astroglial pathological changes in the hippocampus of APP transgenic mice, model of Alzheimer's disease. Exp Neurol 239:28–37. [DOI] [PubMed] [Google Scholar]
- 33. Beauquis J, Vinuesa A, Pomilio C, Pavía P, Galván V, Saravia F (2014) Neuronal and glial alterations, increased anxiety, and cognitive impairment before hippocampal amyloid deposition in PDAPP mice, model of Alzheimer's disease. Hippocampus 24:257–269. [DOI] [PubMed] [Google Scholar]
- 34. Behrens PF, Franz P, Woodman B, Lindenberg KS, Landwehrmeyer GB (2002) Impaired glutamate transport and glutamate‐glutamine cycling: downstream effects of the Huntington mutation. Brain 125:1908–1922. [DOI] [PubMed] [Google Scholar]
- 35. Bell RD, Zlokovic BV (2009) Neurovascular mechanisms and blood‐brain barrier disorder in Alzheimer's disease. Acta Neuropathol 118:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ben‐Haim L, Carrillo‐de Sauvage MA, Ceyzériat K, Escartin C (2015) Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci 9:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ben Haim L, Ceyzériat K, Carrillo‐de Sauvage MA, Aubry F, Auregan G, Guillermier M et al (2015) The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer's and Huntington's diseases. J Neurosci 35:2817–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Berciano MT, Andres MA, Calle E, Lafarga M (1995) Age‐induced hypertrophy of astrocytes in rat supraoptic nucleus: a cytological, morphometric, and immunocytochemical study. Anat Rec 243:129–144. [DOI] [PubMed] [Google Scholar]
- 39. Bhalala OG, Pan L, Sahni V, McGuire TL, Gruner K, Tourtellotte WG, Kessler JA (2012) microRNA‐21 regulates astrocytic response following spinal cord injury. J Neurosci 32:17935–17947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU et al (2012) Astrocyte senescence as a component of Alzheimer's disease. PLoS One 7:e45069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bigio EH, Lipton AM, Yen SH, Hutton ML, Baker M, Nacharaju P et al (2001) Frontal lobe dementia with novel tauopathy: sporadic multiple system tauopathy with dementia. J Neuropathol Exp Neurol 60:328–341. [DOI] [PubMed] [Google Scholar]
- 42. Bitto A, Sell C, Crowe E, Lorenzini A, Malaguti M, Hrelia S, Torres C (2010) Stress‐induced senescence in human and rodent astrocytes. Exp Cell Res 316:2961–2968. [DOI] [PubMed] [Google Scholar]
- 43. Boluda S, Iba M, Zhang B, Raible KM, Lee VM, Trojanowski JQ (2015) Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer's disease or corticobasal degeneration brains. Acta Neuropathol 129:221–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bonneh‐Barkay D, Bissel SJ, Kofler J, Starkey A, Wang G, Wiley CA (2012) Astrocyte and macrophage regulation of YKL‐40 expression and cellular response in neuroinflammation. Brain Pathol 22:530–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bonneh‐Barkay D, Wang G, Starkey A, Hamilton RL, Wiley CA (2010) In vivo CHI3L1 (YKL‐40) expression in astrocytes in acute and chronic neurological diseases. J Neuroinflammation 7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boor PK, de Groot K, Waisfisz Q, Kamphorst W, Oudejans CB, Powers JM et al (2005) MLC1: a novel protein in distal astroglial processes. J Neuropathol Exp Neurol 64:412–419. [DOI] [PubMed] [Google Scholar]
- 47. Bosch M, Kielian T (2014) Hemichannels in neurodegenerative diseases: is there a link to pathology?. Front Cell Neurosci 8:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bosson A, Boisseau S, Buisson A, Savasta M, Albrieux M (2015) Disruption of dopaminergic transmission remodels tripartite synapse morphology and astrocytic calcium activity within substantia nigra pars reticulata. Glia 63:673–683. [DOI] [PubMed] [Google Scholar]
- 49. Braak H, Sastre M, Del Tredici K (2007) Development of alpha‐synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson's disease. Acta Neuropathol 114:231–241. [DOI] [PubMed] [Google Scholar]
- 50. Bradford J, Shin JY, Roberts M, Wang CE, Li XJ, Li S (2009) Expression of mutant huntingtin in mouse brain astrocytes causes age‐dependent neurological symptoms. Proc Natl Acad Sci USA 106:22480–22485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bradford J, Shin JY, Roberts M, Wang CE, Sheng G, Li S, Li XJ (2010) Mutant huntingtin in glial cells exacerbates neurological symptoms of Huntington disease mice. J Biol Chem 285:10653–10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Braidy N, Gai WP, Xu YH, Sachdev P, Guillemin GJ, Jiang XM et al (2013) Uptake and mitochondrial dysfunction of alpha‐synuclein in human astrocytes, cortical neurons and fibroblasts. Transl Neurodegener 2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brambilla R, Bracchi‐Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S et al (2005) Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med 202:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brambilla L, Guidotti G, Martorana F, Iyer AM, Aronica E, Valori CF, Rossi D (2016) Disruption of the astrocytic TNFR1‐GDNF axis accelerates motor neuron degeneration and disease progression in amyotrophic lateral sclerosis. Hum Mol Genet 25:3080–3095. [DOI] [PubMed] [Google Scholar]
- 55. Brambilla R, Persaud T, Hu X, Karmally S, Shestopalov VI, Dvoriantchikova G et al (2009) Transgenic inhibition of astroglial NF‐kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol 182:2628–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brenner M, Johnson AB, Boespflug‐Tanguy O, Rodriguez D, Goldman JE, Messing A (2001) Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat Genet 27:117–120. [DOI] [PubMed] [Google Scholar]
- 57. Bristol LA, Rothstein JD (1996) Glutamate transporter gene expression in amyotrophic lateral sclerosis motor cortex. Ann Neurol 39:676–679. [DOI] [PubMed] [Google Scholar]
- 58. Broe M, Kril J, Halliday GM (2004) Astrocytic degeneration relates to the severity of disease in frontotemporal dementia. Brain 127:2214–2220. [DOI] [PubMed] [Google Scholar]
- 59. Brown AM, Ransom BR (2015) Astrocyte glycogen as an emergency fuel under conditions of glucose deprivation or intense neural activity. Metab Brain Dis 30:233–239. [DOI] [PubMed] [Google Scholar]
- 60. Brown AM, Ransom BR (2007) Astrocyte glycogen and brain energy metabolism. Glia 55:1263–1271. [DOI] [PubMed] [Google Scholar]
- 61. Brück D, Wenning GK, Stefanova N, Fellner L (2016) Glia and alpha‐synuclein in neurodegeneration: a complex interaction. Neurobiol Dis 85:262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG et al (1997) ALS‐linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1‐containing inclusions. Neuron 18:327–338. [DOI] [PubMed] [Google Scholar]
- 63. Bu G (2009) Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci 10:333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Budka H, Head MW, Ironside JW, Gambetti P, Poarchi P, Tagliavini F Sporadic Creutzfledt‐Jakob disease. In: Neurodegeneration, The Molecular Pathology of Dementia and Molecular Disorders. Dickson DW, Weller RO(eds), pp. 322–335. Wiley‐Blackwell: Oxford. [Google Scholar]
- 65. Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP et al (2008) Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. Proc Natl Acad Sci USA 105:3581–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Buffo A, Rolando C, Ceruti S (2010) Astrocytes in the damaged brain: molecular and cellular insights into their reactive response and healing potential. Biochem Pharmacol 79:77–89. [DOI] [PubMed] [Google Scholar]
- 67. Burda JE, Sofroniew MV (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81:229–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN et al (1999) Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar‐forming, reactive astrocytes in adult transgenic mice. Neuron 23:297–308. [DOI] [PubMed] [Google Scholar]
- 69. Bushong EA, Martone ME, Jones YZ, Ellisman MH (2002) Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Butt AM, Kalsi A (2006) Inwardly rectifying potassium channels (Kir) in central nervous system glia: a special role for Kir4.1 in glial functions. J Cell Mol Med 10:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Butterfield DA, Sultana R (2008) Redox proteomics: understanding oxidative stress in the progression of age‐related neurodegenerative disorders. Expert Rev Proteomics 5:157–160. [DOI] [PubMed] [Google Scholar]
- 72. Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS et al (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28:264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Calkins MJ, Vargas MR, Johnson DA, Johnson JA (2010) Astrocyte‐specific overexpression of Nrf2 protects striatal neurons from mitochondrial complex II inhibition. Toxicol Sci 115:557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Campisi J (2005) Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120:513–522. [DOI] [PubMed] [Google Scholar]
- 75. Campuzano O, Castillo‐Ruiz MM, Acarin L, Castellano B, Gonzalez B (2009) Increased levels of proinflammatory cytokines in the aged rat brain attenuate injury‐induced cytokine response after excitotoxic damage. J Neurosci Res 87:2484–2497. [DOI] [PubMed] [Google Scholar]
- 76. Carter SF, Schöll M, Almkvist O, Wall A, Engler H, Långström B, Nordberg A (2012) Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C‐deuterium‐L‐deprenyl: a multitracer PET paradigm combining 11C‐Pittsburgh compound B and 18F‐FDG. J Nucl Med 53:37–46. [DOI] [PubMed] [Google Scholar]
- 77. Cassano T, Serviddio G, Gaetani S, Romano A, Dipasquale P, Cianci S et al (2012) Glutamatergic alterations and mitochondrial impairment in a murine model of Alzheimer disease. Neurobiol Aging 33:1121. [DOI] [PubMed] [Google Scholar]
- 78. Cassina P, Cassina A, Pehar M, Castellanos R, Gandelman M, de León A et al (2008) Mitochondrial dysfunction in SOD1G93A‐bearing astrocytes promotes motor neuron degeneration: prevention by mitochondrial‐targeted antioxidants. J Neurosci 28:4115–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chandrasekaran A, Avci HX, Leist M, Kobolák J, Dinnyés A (2016) Astrocyte differentiation of human pluripotent stem cells: new tools for neurological disorder research. Front Cell Neurosci 10:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Charron G, Doudnikoff E, Canron MH, Li Q, Véga C, Marais S et al (2014) Astrocytosis in parkinsonism: considering tripartite striatal synapses in physiopathology?. Front Aging Neurosci 6:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA (2009) Nrf2‐mediated neuroprotection in the MPTP mouse model of Parkinson's disease: Critical role for the astrocyte. Proc Natl Acad Sci USA 106:2933–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chen Y, Vartiainen NE, Ying W, Chan PH, Koistinaho J, Swanson RA (2001) Astrocytes protect neurons from nitric oxide toxicity by a glutathione‐dependent mechanism. J Neurochem 77:1601–1610. [DOI] [PubMed] [Google Scholar]
- 83. Chinta SJ, Lieu CA, Demaria M, Laberge RM, Campisi J, Andersen JK (2013) Environmental stress, ageing and glial cell senescence: a novel mechanistic link to Parkinson's disease?. J Intern Med 273:429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Choi YP, Head MW, Ironside JW, Priola SA (2014) Uptake and degradation of protease‐sensitive and ‐resistant forms of abnormal human prion protein aggregates by human astrocytes. Am J Pathol 184:3299–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A et al (2005) Thrombospondins are astrocyte‐secreted proteins that promote CNS synaptogenesis. Cell 120:421–433. [DOI] [PubMed] [Google Scholar]
- 86. Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J et al (2013) Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci USA 110:9535–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillée S, Rule M et al (2003) Wild‐type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 302:113–117. [DOI] [PubMed] [Google Scholar]
- 88. Colodner KJ, Feany MB (2010) Glial fibrillary tangles and JAK/STAT‐mediated glial and neuronal cell death in a Drosophila model of glial tauopathy. J Neurosci 30:16102–16113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Colombo JA, Yáñez A, Lipina SJ (1997) Interlaminar astroglial processes in the cerebral cortex of non human primates: response to injury. J Hirnforsch 38:503–512. [PubMed] [Google Scholar]
- 90. Colombo JA, Yáñez A, Puissant V, Lipina S (1995) Long, interlaminar astroglial cell processes in the cortex of adult monkeys. J Neurosci Res 40:551–556. [DOI] [PubMed] [Google Scholar]
- 91. Coppé JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence‐associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cotrina ML, Nedergaard M (2002) Astrocytes in the aging brain. J Neurosci Res 67:1–10. [DOI] [PubMed] [Google Scholar]
- 93. Croisier E, Graeber MB (2006) Glial degeneration and reactive gliosis in alpha‐synucleinopathies: the emerging concept of primary gliodegeneration. Acta Neuropathol 112:517–530. [DOI] [PubMed] [Google Scholar]
- 94. Crowe EP, Tuzer F, Gregory BD, Donahue G, Gosai SJ, Cohen J et al (2016) Changes in the tanscriptome of human astrocytes accompanying oxidative stress‐induced senescence. Front Aging Neurosci 8:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cui Y, Masaki K, Yamasaki R, Imamura S, Suzuki SO, Hayashi S et al (2014) Extensive dysregulations of oligodendrocytic and astrocytic connexins are associated with disease progression in an amyotrophic lateral sclerosis mouse model. J Neuroinflammation 11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dabir DV, Robinson MB, Swanson E, Zhang B, Trojanowski JQ, Lee VM, Forman MS (2006) Impaired glutamate transport in a mouse model of tau pathology in astrocytes. J Neurosci 26:644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dabir DV, Trojanowski JQ, Richter‐Landsberg C, Lee VM, Forman MS (2004) Expression of the small heat‐shock protein alphaB‐crystallin in tauopathies with glial pathology. Am J Pathol 164:155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dal Prà I, Chiarini A, Gui L, Chakravarthy B, Pacchiana R, Gardenal E et al (2015) Do astrocytes collaborate with neurons in spreading the “infectious” aβ and Tau drivers of Alzheimer's disease?. Neuroscientist 21:9–29. [DOI] [PubMed] [Google Scholar]
- 99. Dallerac G, Rouach N (2016) Astrocytes as new targets to improve cognitive functions. Prog Neurobiol 144:48–67. [DOI] [PubMed] [Google Scholar]
- 100. Debye B, Schmülling L, Zhou L, Rune G, Beyer C, Johann S Neurodegeneration and NLRP3 inflammasome expression in the anterior thalamus of SOD1 (G93A) ALS mice. Brain Pathol (in press); doi: 10.1111/bpa.12467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Delekate A, Füchtemeier M, Schumacher T, Ulbrich C, Foddis M, Petzold GC (2014) Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer's disease mouse model. Nat Commun 5:5422. [DOI] [PubMed] [Google Scholar]
- 102. Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC (2008) Human embryonic stem cell‐derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS‐causing mutation. Cell Stem Cell 3:637–648. [DOI] [PubMed] [Google Scholar]
- 103. Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K (2007) Non‐cell autonomous effect of glia on motor neurons in an embryonic stem cell‐based ALS model. Nat Neurosci 10:608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dickson DW, Hauw JJ, Agid Y, Litvan I (2011) Progressive supranuclear palsy and corticobasal degeneration. In: Neurodegeneration, the Molecular Pathology of Dementia and Molecular Disorders. Dickson DW, Weller RO (eds), pp. 135–155. Wiley‐Blackwell: Oxford. [Google Scholar]
- 105. Dickson DW, Kouri N, Murray ME, Josephs KA (2011) Neuropathology of frontotemporal lobar degeneration‐tau (FTLD‐tau). J Mol Neurosci 45:384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dringen R (2000) Metabolism and functions of glutathione in brain. Prog Neurobiol 62:649–671. [DOI] [PubMed] [Google Scholar]
- 107. Duerson K, Woltjer RL, Mookherjee P, Leverenz JB, Montine TJ, Bird TD et al (2009) Detergent‐insoluble EAAC1/EAAT3 aberrantly accumulates in hippocampal neurons of Alzheimer's disease patients. Brain Pathol 19:267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Emsley JG, Macklis JD (2006) Astroglial heterogeneity closely reflects the neuronal‐defined anatomy of the adult murine CNS. Neuron Glia Biol 2:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Eng LF, Ghirnikar RS, Lee YL (2000) Glial fibrillary acidic protein: GFAP‐thirty‐one years (1969–2000). Neurochem Res 25:1439–1451. [DOI] [PubMed] [Google Scholar]
- 110. Ezzi SA, Larivière R, Urushitani M, Julien JP (2010) Neuronal over‐expression of chromogranin A accelerates disease onset in a mouse model of ALS. J Neurochem 115:1102–1111. [DOI] [PubMed] [Google Scholar]
- 111. Faideau M, Kim J, Cormier K, Gilmore R, Welch M, Auregan G et al (2010) In vivo expression of polyglutamine‐expanded huntingtin by mouse striatal astrocytes impairs glutamate transport: a correlation with Huntington's disease subjects. Hum Mol Genet 19:3053–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Falsig J, Pörzgen P, Lund S, Schrattenholz A, Leist M (2006) The inflammatory transcriptome of reactive murine astrocytes and implications for their innate immune function. J Neurochem 96:893–907. [DOI] [PubMed] [Google Scholar]
- 113. Fang D, Li Z, Zhong‐ming Q, Mei WX, Ho YW, Yuan XW, Ya K (2008) Expression of bystin in reactive astrocytes induced by ischemia/reperfusion and chemical hypoxia in vitro. Biochim Biophys Acta 1782:658–663. [DOI] [PubMed] [Google Scholar]
- 114. Farina C, Aloisi F, Meinl E (2007) Astrocytes are active players in cerebral innate immunity. Trends Immunol 28:138–145. [DOI] [PubMed] [Google Scholar]
- 115. Farrall AJ, Wardlaw JM (2009) Blood‐brain barrier: ageing and microvascular disease–systematic review and meta‐analysis. Neurobiol Aging 30:337–352. [DOI] [PubMed] [Google Scholar]
- 116. Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24:2143–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Feany MB, Dickson DW (1995) Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 146:1388–1396. [PMC free article] [PubMed] [Google Scholar]
- 118. Ferraiuolo L, Higginbottom A, Heath PR, Barber S, Greenald D, Kirby J, Shaw PJ (2011) Dysregulation of astrocyte‐motoneuron cross‐talk in mutant superoxide dismutase 1‐related amyotrophic lateral sclerosis. Brain 134:2627–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ferrer I, Barrachina M, Tolnay M, Rey MJ, Vidal N, Carmona M et al (2003) Phosphorylated protein kinases associated with neuronal and glial tau deposits in argyrophilic grain disease. Brain Pathol 13:62–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ferrer I, Barrachina M, Puig B (2002) Glycogen synthase kinase‐3 is associated with neuronal and glial hyperphosphorylated tau deposits in Alzheimer's disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol 104:583–591. [DOI] [PubMed] [Google Scholar]
- 121. Ferrer I, Blanco R, Carmona M, Puig B (2001) Phosphorylated mitogen‐activated protein kinase (MAPK/ERK‐P), protein kinase of 38 kDa (p38‐P), stress‐activated protein kinase (SAPK/JNK‐P), and calcium/calmodulin‐dependent kinase II (CaM kinase II) are differentially expressed in tau deposits in neurons and glial cells in tauopathies. J. Neural Transm 108:1397–1415. [DOI] [PubMed] [Google Scholar]
- 122. Ferrer I, Blanco R, Carmona M, Ribera R, Goutan E, Puig B et al (2001) Phosphorylated map kinase (ERK1, ERK2) expression is associated with early tau deposition in neurones and glial cells, but not with increased nuclear DNA vulnerability and cell death, in Alzheimer disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration. Brain Pathol 11:144–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ferrer I, Legati A, García‐Monco JC, Gomez‐Beldarrain M, Carmona M, Blanco R et al (2015) Familial behavioral variant frontotemporal dementia associated with astrocyte‐predominant tauopathy. J Neuropathol Exp Neurol 74:370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ferrer I, López‐González I, Carmona M, Arregui L, Dalfó E, Torrejón‐Escribano B et al (2014) Glial and neuronal tau pathology in tauopathies: characterization of disease‐specific phenotypes and tau pathology progression. J Neuropathol Exp Neurol 73:81–97. [DOI] [PubMed] [Google Scholar]
- 125. Ferrer I, Pastor P, Rey MJ, Muñoz E, Puig B, Pastor E et al (2003) Tau phosphorylation and kinase activation in familial tauopathy linked to deln296 mutation. Neuropathol Appl Neurobiol 29:23–34. [DOI] [PubMed] [Google Scholar]
- 126. Ferrer I, Santpere G, van Leeuwen FW (2008) Argyrophilic grain disease. Brain 131:1416–1432. [DOI] [PubMed] [Google Scholar]
- 127. Filipcik P, Cente M, Zilka N, Smolek T, Hanes J, Kucerak J et al (2015) intraneuronal accumulation of misfolded tau protein induces overexpression of Hsp27 in activated astrocytes. Biochim Biophys Acta 1852:1219–1229. [DOI] [PubMed] [Google Scholar]
- 128. Finch CE (2003) Neurons, glia, and plasticity in normal brain aging. Neurobiol Aging 24:S123–S127. [DOI] [PubMed] [Google Scholar]
- 129. Flügge G, Araya‐Callis C, Garea‐Rodriguez E, Stadelmann‐Nessler C, Fuchs E (2014) NDRG2 as a marker protein for brain astrocytes. Cell Tissue Res 357:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Forman MS, Lal D, Zhang B, Dabir DV, Swanson E, Lee VM, Trojanowski JQ (2005) Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration. J Neurosci 25:3539–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Forno LS, DeLanney LE, Irwin I, Di Monte D, Langston JW (1992) Astrocytes and Parkinson's disease. Prog Brain Res 94:429–436. [DOI] [PubMed] [Google Scholar]
- 132. Fray AE, Ince PG, Banner SJ, Milton ID, Usher PA, Cookson MR, Shaw PJ (1998) The expression of the glial glutamate transporter protein EAAT2 in motor neuron disease: an immunohistochemical study. Eur J Neurosci 10:2481–2489. [DOI] [PubMed] [Google Scholar]
- 133. Freixes M, Rodríguez A, Dalfó E, Ferrer I (2006) Oxidation, glycoxidation, lipoxidation, nitration, and responses to oxidative stress in the cerebral cortex in Creutzfeldt‐Jakob disease. Neurobiol Aging 27:1807–1815. [DOI] [PubMed] [Google Scholar]
- 134. Freixes M, Puig B, Rodríguez A, Torrejón‐Escribano B, Blanco R, Ferrer I (2004) Clusterin solubility and aggregation in Creutzfeldt‐Jakob disease. Acta Neuropathol 108:295–301. [DOI] [PubMed] [Google Scholar]
- 135. Freund A, Orjalo AV, Desprez PY, Campisi J (2010) Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med 16:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Frost B, Hemberg M, Lewis J, Feany MB (2014) Tau promotes neurodegeneration through global chromatin relaxation. Nat Neurosci 17:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Funato H, Yoshimura M, Yamazaki T, Saido TC, Ito Y, Yokofujita J et al (1998) Astrocytes containing amyloid beta‐protein (Abeta)‐positive granules are associated with Abeta40‐positive diffuse plaques in the aged human brain. Am J Pathol 152:983–992. [PMC free article] [PubMed] [Google Scholar]
- 138. Furman JL, Sama DM, Gant JC, Beckett TL, Murphy MP, Bachstetter AD et al (2012) Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer's disease. J Neurosci 32:16129–16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gabel S, Koncina E, Dorban G, Heurtaux T, Birck C, Glaab E et al (2016) Inflammation promotes a conversion of astrocytes into neural progenitor cells via NF‐κB activation. Mol Neurobiol 53:5041–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gadea A, Schinelli S, Gallo V (2008) Endothelin‐1 regulates astrocyte proliferation and reactive gliosis via a JNK/c‐Jun signaling pathway. J Neurosci 28:2394–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Gan L, Vargas MR, Johnson DA, Johnson JA (2012) Astrocyte‐specific overexpression of Nrf2 delays motor pathology and synuclein aggregation throughout the CNS in the alpha‐synuclein mutant (A53T) mouse model. J Neurosci 32:17775–177787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Gao K, Wang CR, Jiang F, Wong AY, Su N, Jiang JH et al (2013) Traumatic scratch injury in astrocytes triggers calcium influx to activate the JNK/c‐Jun/AP‐1 pathway and switch on GFAP expression. Glia 61:2063–2077. [DOI] [PubMed] [Google Scholar]
- 143. Garcia‐Esparcia P, López Gonzalez I, Grau‐Rivera O, García‐Garrido MF, Konetti A, Llorens F et al (2017) Dementia with Lewy bodies: molecular pathology in the frontal cortex in typical and rapidly progressive forms. Front Neurol 8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Garcia‐Esparcia P, Llorens F, Carmona M, Ferrer I (2014) Complex deregulation and expression of cytokines and mediators of the immune response in Parkinson's disease brain is region dependent. Brain Pathol 24:584–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. García‐Matas S, Gutierrez‐Cuesta J, Coto‐Montes A, Rubio‐Acero R, Díez‐Vives C, Camins A et al (2008) Dysfunction of astrocytes in senescence‐accelerated mice SAMP8 reduces their neuroprotective capacity. Aging Cell 7:630–640. [DOI] [PubMed] [Google Scholar]
- 146. Garwood C, Faizullabhoy A, Wharton SB, Ince PG, Heath PR, Shaw PJ, MRC Cognitive Function and Ageing Neuropathology Study Group et al (2013) Calcium dysregulation in relation to Alzheimer‐type pathology in the ageing brain. Neuropathol Appl Neurobiol 39:788–799. [DOI] [PubMed] [Google Scholar]
- 147. Garwood CJ, Pooler AM, Atherton J, Hanger DP, Noble W (2011) Astrocytes are important mediators of Aβ‐induced neurotoxicity and tau phosphorylation in primary culture. Cell Death Dis 2:e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Garwood CJ, Radcliffe LE, Simpson JE, Heath PR, Ince PG, Wharton SB (2017) Review: astrocytes in Alzheimer's disease and other age‐associated dementias: the supporting player with a central role. Neuropathol Appl Neurobiol 43:281–298. [DOI] [PubMed] [Google Scholar]
- 149. Ghetti B, Oblak AL, Boeve BF, Johnson KA, Dickerson BC, Goedert M (2015) Invited review: Frontotemporal dementia caused by microtubule‐associated protein tau gene (MAPT) mutations: a chameleon for neuropathology and neuroimaging. Neuropathol Appl Neurobiol 41:24–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ghetti B, Wszolek ZK, Boeve BF, Spina S, Goedert M (2011) Frontotemporal dementia and Parkinsonism linked to chromosome. In: Neurodegeneration, The Molecular Pathology of Dementia and Molecular Disorders. Dickson DW, Weller RO (eds), pp. 110–134. Wiley‐Blackwell, Oxford. [Google Scholar]
- 151. Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N (2010) Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci 11:87–99. [DOI] [PubMed] [Google Scholar]
- 152. Gordon GR, Mulligan SJ, MacVicar BA (2007) Astrocyte control of the cerebrovasculature. Glia 55:1214–1221. [DOI] [PubMed] [Google Scholar]
- 153. Grolla AA, Sim JA, Lim D, Rodriguez JJ, Genazzani AA, Verkhratsky A (2013) Amyloid‐β and Alzheimer's disease type pathology differentially affects the calcium signalling toolkit in astrocytes from different brain regions. Cell Death Dis 4:e623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Gu X, André VM, Cepeda C, Li SH, Li XJ, Levine MS, Yang XW (2007) Pathological cell‐cell interactions are necessary for striatal pathogenesis in a conditional mouse model of Huntington's disease. Mol Neurodegener 2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Gu X, Li C, Wei W, Lo V, Gong S, Li SH et al (2005) Pathological cell‐cell interactions elicited by a neuropathogenic form of mutant Huntingtin contribute to cortical pathogenesis in HD mice. Neuron 46:433–444. [DOI] [PubMed] [Google Scholar]
- 156. Gu XL, Long CX, Sun L, Xie C, Lin X, Cai H (2010) Astrocytic expression of Parkinson's disease‐related A53T alpha‐synuclein causes neurodegeneration in mice. Mol Brain 3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Guénette SY (2003) Astrocytes: a cellular player in Abeta clearance and degradation. Trends Mol Med 9:279–280. [DOI] [PubMed] [Google Scholar]
- 158. Guo H, Lai L, Butchbach ME, Stockinger MP, Shan X, Bishop GA, Lin CL (2003) Increased expression of the glial glutamate transporter EAAT2 modulates excitotoxicity and delays the onset but not the outcome of ALS in mice. Hum Mol Genet 12:2519–2532. [DOI] [PubMed] [Google Scholar]
- 159. Haack N, Dublin P, Rose CR (2014) Dysbalance of astrocyte calcium under hyperammonemic conditions. PLoS One 9:e105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Haas B, Schipke CG, Peters O, Söhl G, Willecke K, Kettenmann H (2006) Activity‐dependent ATP‐waves in the mouse neocortex are independent from astrocytic calcium waves. Cereb Cortex 16:237–246. [DOI] [PubMed] [Google Scholar]
- 161. Haidet‐Phillips AM, Gross SK, Williams T, Tuteja A, Sherman A, Ko M et al (2013) Altered astrocytic expression of TDP‐43 does not influence motor neuron survival. Exp Neurol 250:250–259. [DOI] [PubMed] [Google Scholar]
- 162. Halassa MM, Fellin T, Haydon PG (2009) Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology 57:343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Halassa MM, Fellin T, Haydon PG (2007a) The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 13:54–63. [DOI] [PubMed] [Google Scholar]
- 164. Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG (2007b) Synaptic islands defined by the territory of a single astrocyte. J Neurosci 27:6473–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Halliday GM, Stevens CH (2011) Glia: initiators and progressors of pathology in Parkinson's disease. Mov Disord 26:6–17. [DOI] [PubMed] [Google Scholar]
- 166. Hallmann AL, Araúzo‐Bravo MJ, Mavrommatis L, Ehrlich M, Röpke A, Brockhaus J et al (2017) Astrocyte pathology in a human neural stem cell model of frontotemporal dementia caused by mutant TAU protein. Sci Rep 7:42991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Hamby ME, Coppola G, Ao Y, Geschwind DH, Khakh BS, Sofroniew MV (2012) Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple G‐protein‐coupled receptors. J Neurosci 32:14489–14510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hamby ME, Hewett JA, Hewett SJ (2006) TGF‐beta1 potentiates astrocytic nitric oxide production by expanding the population of astrocytes that express NOS‐2. Glia 54:566–577. [DOI] [PubMed] [Google Scholar]
- 169. Hashida K, Kitao Y, Sudo H, Awa Y, Maeda S, Mori K et al (2012) ATF6alpha promotes astroglial activation and neuronal survival in a chronic mouse model of Parkinson's disease. PLoS One 7:e47950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Hassel B, Tessler S, Faull RL, Emson PC (2008) Glutamate uptake is reduced in prefrontal cortex in Huntington's disease. Neurochem Res 33:232–237. [DOI] [PubMed] [Google Scholar]
- 171. Hattori M, Hashizume Y, Yoshida M, Iwasaki Y, Hishikawa N, Ueda R, Ojika K (2003) Distribution of astrocytic plaques in the corticobasal degeneration brain and comparison with tuft‐shaped astrocytes in the progressive supranuclear palsy brain. Acta Neuropathol 106:143–149. [DOI] [PubMed] [Google Scholar]
- 172. Haydon PG, Carmignoto G (2006) Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86:1009–1031. [DOI] [PubMed] [Google Scholar]
- 173. Hazell AS (2009) Astrocytes are a major target in thiamine deficiency and Wernicke's encephalopathy. Neurochem Int 55:129–135. [DOI] [PubMed] [Google Scholar]
- 174. Hazell AS, Sheedy D, Oanea R, Aghourian M, Sun S, Jung JY et al (2010) Loss of astrocytic glutamate transporters in Wernicke encephalopathy. Glia 58:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL et al (2015) Neuroinflammation in Alzheimer's disease. Lancet Neurol 14:388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Heneka MT, Kummer MP, Latz E (2014) Innate immune activation in neurodegenerative disease. Nat Rev Immunol 14:463–477. [DOI] [PubMed] [Google Scholar]
- 177. Heneka MT, Sastre M, Dumitrescu‐Ozimek L, Dewachter I, Walter J, Klockgether T, Van Leuven F (2005) Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J Neuroinflammation 2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Hernández‐Ortega K, Garcia‐Esparcia P, Gil L, Lucas JJ, Ferrer I (2016) Altered machinery of protein synthesis in Alzheimer's: from the nucleolus to the ribosome. Brain Pathol 26:593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK et al (2008) STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci 28:7231–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Hoegg‐Beiler MB, Sirisi S, Orozco IJ, Ferrer I, Hohensee S, Auberson M et al (2014) Disrupting MLC1 and GlialCAM and ClC‐2 interactions in leukodystrophy entails glial chloride channel dysfunction. Nat Commun 5:3475. [DOI] [PubMed] [Google Scholar]
- 181. Hostenbach S, Cambron M, D'haeseleer M, Kooijman R, De Keyser J (2014) Astrocyte loss and astrogliosis in neuroinflammatory disorders. Neurosci Lett 565:39–41. [DOI] [PubMed] [Google Scholar]
- 182. Houades V, Koulakoff A, Ezan P, Seif I, Giaume C (2008) Gap junction‐mediated astrocytic networks in the mouse barrel cortex. J Neurosci 28:5207–5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Houades V, Rouach N, Ezan P, Kirchhoff F, Koulakoff A, Giaume C (2006) Shapes of astrocyte networks in the juvenile brain. Neuron Glia Biol 2:3–14. [DOI] [PubMed] [Google Scholar]
- 184. Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B et al (2002) Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant‐mediated amyotrophic lateral sclerosis (ALS). Proc Natl Acad Sci USA 99:1604–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Hsiao HY, Chen YC, Chen HM, Tu PH, Chern Y (2013) A critical role of astrocyte‐mediated nuclear factor‐κB‐dependent inflammation in Huntington's disease. Hum Mol Genet 22:1826–1842. [DOI] [PubMed] [Google Scholar]
- 186. Hsiao HY, Chen YC, Huang CH, Chen CC, Hsu YH, Chen HM et al (2015) Aberrant astrocytes impair vascular reactivity in Huntington disease. Ann Neurol 78:178–192. [DOI] [PubMed] [Google Scholar]
- 187. Hutchison ER, Kawamoto EM, Taub DD, Lal A, Abdelmohsen K, Zhang Y et al (2013) Evidence for miR‐181 involvement in neuroinflammatory responses of astrocytes. Glia 61:1018–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Hwang ES, Yoon G, Kang HT (2009) A comparative analysis of the cell biology of senescence and aging. Cell Mol Life Sci 66:2503–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Iadecola C, Nedergaard M (2007) Glial regulation of the cerebral microvasculature. Nat Neurosci 10:1369–1376. [DOI] [PubMed] [Google Scholar]
- 190. Iglesias J, Morales L, Barretro GE (2017) Metabolic and inflammatory adaptation of reactive astrocytes: role of PPARs. Mol Neurobiol 54:2518–2538. [DOI] [PubMed] [Google Scholar]
- 191. Ikeda K, Akiyama H, Arai T, Nishimura T (1998) Glial tau pathology in neurodegenerative diseases: their nature and comparison with neuronal tangles. Neurobiol Aging 19:S85–S91. [DOI] [PubMed] [Google Scholar]
- 192. Ikeda K, Akiyama H, Kondo H, Haga C, Tanno E, Tokuda T, Ikeda S (1995) Thorn‐shaped astrocytes: possibly secondarily induced tau‐positive glial fibrillary tangles. Acta Neuropathol 90:620–625. [DOI] [PubMed] [Google Scholar]
- 193. Ilieva H, Polymenidou M, Cleveland DW (2009) Non–cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol 187:761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Irwin DJ, Cohen TJ, Grossman M, Arnold SE, McCarty‐Wood E, Van Deerlin VM et al (2013) Acetylated tau neuropathology in sporadic and hereditary tauopathies. Am J Pathol 183:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Israel JM, Schipke CG, Ohlemeyer C, Theodosis DT, Kettenmann H (2003) GABAA receptor‐expressing astrocytes in the supraoptic nucleus lack glutamate uptake and receptor currents. Glia 44:102–110. [DOI] [PubMed] [Google Scholar]
- 196. Iwasaki Y, Mimuro M, Yoshida M, Hashizume Y, Ito M, Kitamoto T et al (2007) Enhanced Aquaporin‐4 immunoreactivity in sporadic Creutzfeldt‐Jakob disease. Neuropathology 27:314–323. [DOI] [PubMed] [Google Scholar]
- 197. Iwasaki Y, Yoshida M, Hattori M, Goto A, Aiba I, Hashizume Y, Sobue G (2004) Distribution of tuft‐shaped astrocytes in the cerebral cortex in progressive supranuclear palsy. Acta Neuropathol 108:399–405. [DOI] [PubMed] [Google Scholar]
- 198. Jayakumar AR, Norenberg MD (2016) Glutamine synthetase: role in neurological disorders. Adv Neurobiol 13:327–350. [DOI] [PubMed] [Google Scholar]
- 199. Jayakumar AR, Panickar KS, Curtis KM, Tong XY, Moriyama M, Norenberg MD (2011) Na‐K‐Cl cotransporter‐1 in the mechanism of cell swelling in cultured astrocytes after fluid percussion injury. J Neurochem 117:437–448. [DOI] [PubMed] [Google Scholar]
- 200. Jayakumar AR, Rama Rao KV, Tong XY, Norenberg MD (2009) Calcium in the mechanism of ammonia‐induced astrocyte swelling. J Neurochem 109 (1 suppl.):252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH et al (2014) GABA from reactive astrocytes impairs memory in mouse models of Alzheimer's disease. Nat Med 20:886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. John Lin CC, Yu K, Hatcher A, Huang TW, Lee HK, Carlson J et al (2017) Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci 20:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Juopperi TA, Kim WR, Chiang CH, Yu H, Margolis RL, Ross CA et al (2012) Astrocytes generated from patient induced pluripotent stem cells recapitulate features of Huntington's disease patient cells. Mol Brain 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Kahlson MA, Colodner KJ (2015) Glial tau pathology in tauopathies: functional consequences. J Exp Neurosci 9:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Kaiser M, Maletzki I, Hülsmann S, Holtmann B, Schulz‐Schaeffer W, Kirchhoff F et al (2006) Progressive loss of a glial potassium channel (KCNJ10) in the spinal cord of the SOD1 (G93A) transgenic mouse model of amyotrophic lateral sclerosis. J Neurochem 99:900–912. [DOI] [PubMed] [Google Scholar]
- 206. Kamphuis W, Kooijman L, Orre M, Stassen O, Pekny M, Hol EM (2015) GFAP and vimentin deficiency alters gene expression in astrocytes and microglia in wild‐type mice and changes the transcriptional response of reactive glia in mouse model for Alzheimer's disease. Glia 63:1036–1056. [DOI] [PubMed] [Google Scholar]
- 207. Kamphuis W, Mamber C, Moeton M, Kooijman L, Sluijs JA, Jansen AH et al (2012) GFAP isoforms in adult mouse brain with a focus on neurogenic astrocytes and reactive astrogliosis in mouse models of Alzheimer disease. PLoS One 7:e42823–e42823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Kamphuis W, Middeldorp J, Kooijman L, Sluijs JA, Kooi EJ, Moeton M et al (2014) Glial fibrillary acidic protein isoform expression in plaque related astrogliosis in Alzheimer's disease. Neurobiol Aging 35:492–510. [DOI] [PubMed] [Google Scholar]
- 209. Kamphuis W, Orre M, Kooijman L, Dahmen M, Hol EM (2012) Differential cell proliferation in the cortex of the APPswePS1dE9 Alzheimer's disease mouse model. Glia 60:615–629. [DOI] [PubMed] [Google Scholar]
- 210. Kang W, Hébert JM (2011) Signaling pathways in reactive astrocytes, a genetic perspective. Mol Neurobiol 43:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Kaufman SK, Sanders DW, Thomas TL, Ruchinskas AJ, Vaquer‐Alicea J, Sharma AM et al (2016) Tau prion strains dictate patterns of cell pathology, progression rate, and regional vulnerability in vivo. Neuron 92:796–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Kawamata H, Ng SK, Diaz N, Burstein S, Morel L, Osgood A et al (2014) Abnormal intracellular calcium signaling and SNARE‐dependent exocytosis contributes to SOD1G93A astrocyte‐mediated toxicity in amyotrophic lateral sclerosis. J Neurosci 34:2331–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Ke YD, Dramiga J, Schütz U, Kril JJ, Ittner LM, Schröder H, Götz J (2012) Tau‐mediated nuclear depletion and cytoplasmic accumulation of SFPQ in Alzheimer's and Pick's disease. PLoS One 7:e35678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Kelly T, Kafitz KW, Roderigo C, Rose CR (2009) Ammonium‐evoked alterations in intracellular sodium and pH reduce glial glutamate transport activity. Glia 57:921–934. [DOI] [PubMed] [Google Scholar]
- 215. Kersaitis C, Halliday GM, Kril JJ (2004) Regional and cellular pathology in frontotemporal dementia: relationship to stage of disease in cases with and without Pick bodies. Acta Neuropathol 108:515–523. [DOI] [PubMed] [Google Scholar]
- 216. Khakh BS, Sofroniew MV (2014) Astrocytes and Huntington's disease. ACS Chem Neurosci 5:494–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Kim J, Castellano JM, Jiang H, Basak JM, Parsadanian M, Pham V et al (2009) Overexpression of low‐density lipoprotein receptor in the brain markedly inhibits amyloid deposition and increases extracellular A beta clearance. Neuron 64:632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Kimmelberg HK (2004) The problem odf astrocyte identity. Neurochem Int 45:191–202. [DOI] [PubMed] [Google Scholar]
- 219. Kimelberg HK (2010) Functions of mature mammalian astrocytes: a current view. Neuroscientist 16:79–106. [DOI] [PubMed] [Google Scholar]
- 220. Kirischuk S, Parpura V, Verkhratsky A (2012) Sodium dynamics: another key to astroglial excitability?. Trends Neurosci 35:497–506. [DOI] [PubMed] [Google Scholar]
- 221. Klein MA, Möller JC, Jones LL, Bluethmann H, Kreutzberg GW, Raivich G (1997) Impaired neuroglial activation in interleukin‐6 deficient mice. Glia 19:227–233. [DOI] [PubMed] [Google Scholar]
- 222. Kobayashi K, Hayashi M, Nakano H, Shimazaki M, Sugimori K, Koshino Y (2004) Correlation between astrocyte apoptosis and Alzheimer changes in gray matter lesions in Alzheimer's disease. J Alzheimers Dis 6:623–632. [DOI] [PubMed] [Google Scholar]
- 223. Koehler RC, Roman RJ, Harder DR (2009) Astrocytes and the regulation of cerebral blood flow. Trends Neurosci 32:160–169. [DOI] [PubMed] [Google Scholar]
- 224. Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J et al (2004) Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid‐beta peptides. Nat Med 10:719–726. [DOI] [PubMed] [Google Scholar]
- 225. Komori T, Arai N, Oda M, Nakayama H, Mori H, Yagishita S et al (1998) Astrocytic plaques and tufts of abnormal fibers do not coexist in corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol 96:401–408. [DOI] [PubMed] [Google Scholar]
- 226. Kovacs GG (2015) Invited review: Neuropathology of tauopathies: principles and practice. Neuropathol Appl Neurobiol 41:3–23. [DOI] [PubMed] [Google Scholar]
- 227. Kovacs GG, Breydo L, Green R, Kis V, Puska G, Lőrincz P et al (2014) Intracellular processing of disease‐associated α‐synuclein in the human brain suggests prion‐like cell‐to‐cell spread. Neurobiol Dis 69:76–92. [DOI] [PubMed] [Google Scholar]
- 228. Kovacs GG, Ferrer I, Grinberg LT, Alafuzoff I, Attems J, Budka H et al Aging‐related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol 131:87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Kovacs GG, Milenkovic I, Wöhrer A, Höftberger R, Gelpi E, Haberler C et al (2013) Non‐Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community‐based autopsy series. Acta Neuropathol 126:365–384. [DOI] [PubMed] [Google Scholar]
- 230. Kovacs GG, Molnár K, László L, Ströbel T, Botond G, Hönigschnabl S et al (2011) A peculiar constellation of tau pathology defines a subset of dementia in the elderly. Acta Neuropathol 122:205–222. [DOI] [PubMed] [Google Scholar]
- 231. Kovács GG, Preusser M, Strohschneider M, Budka H (2005) Subcellular localization of disease‐associated prion protein in the human brain. Am J Pathol 166:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Kraft AW, Hu X, Yoon H, Yan P, Xiao Q, Wang Y et al (2013) Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. FASEB J 27:187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ (2009) Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 323:1211–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ (2008) Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 59:214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Kulijewicz‐Nawrot M, Syková E, Chvátal A, Verkhratsky A, Rodríguez JJ (2013) Astrocytes and glutamate homoeostasis in Alzheimer's disease: a decrease in glutamine synthetase, but not in glutamate transporter‐1, in the prefrontal cortex. ASN Neuro 5:273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Kulijewicz‐Nawrot M, Verkhratsky A, Chvátal A, Syková E, Rodríguez JJ (2012) Astrocytic cytoskeletal atrophy in the medial prefrontal cortex of a triple transgenic mouse model of Alzheimer's disease. J Anat 221:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Landfield PW, Braun LD, Pitler TA, Lindsey JD, Lynch G (1981) Hippocampal aging in rats: a morphometric study of multiple variables in semithin sections. Neurobiol Aging 2:265–275. [DOI] [PubMed] [Google Scholar]
- 238. Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA (2001) The glial glutamate transporter, GLT‐1, is oxidatively modified by 4‐hydroxy‐2‐nonenal in the Alzheimer's disease brain: the role of Abeta1–42. J Neurochem 78:413–416. [DOI] [PubMed] [Google Scholar]
- 239. Ledesma MD, Galvan C, Hellias B, Dotti C, Jensen PH (2002) Astrocytic but not neuronal increased expression and redistribution of parkin during unfolded protein stress. J Neurochem 83:1431–1440. [DOI] [PubMed] [Google Scholar]
- 240. Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S et al (2010) Direct transfer of alpha‐synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem 285:9262–9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Lee M, Cho T, Jantaratnotai N, Wang YT, McGeer E, McGeer PL (2010) Depletion of GSH in glial cells induces neurotoxicity: relevance to aging and degenerative neurological diseases. FASEB J 24:2533–2545. [DOI] [PubMed] [Google Scholar]
- 242. Lee W, Reyes RC, Gottipati MK, Lewis K, Lesort M, Parpura V, Gray M (2013) Enhanced Ca(2+)‐dependent glutamate release from astrocytes of the BACHD Huntington's disease mouse model. Neurobiol Dis 58:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. L'Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Serapide MF et al (2014) Wnt/β‐catenin signaling is required to rescue midbrain dopaminergic progenitors and promote neurorepair in ageing mouse model of Parkinson's disease. Stem Cells 32:2147–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Lepore AC, Dejea C, Carmen J, Rauck B, Kerr DA, Sofroniew MV, Maragakis NJ (2008) Selective ablation of proliferating astrocytes does not affect disease outcome in either acute or chronic models of motor neuron degeneration. Exp Neurol 211:423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Le Prince G, Delaere P, Fages C, Lefrançois T, Touret M, Salanon M, Tardy M (1995) Glutamine synthetase (GS) expression is reduced in senile dementia of the Alzheimer type. Neurochem Res 20:859–862. [DOI] [PubMed] [Google Scholar]
- 246. Levison SW, Jiang FJ, Stoltzfus OK, Ducceschi MH (2000) IL‐6‐type cytokines enhance epidermal growth factor‐stimulated astrocyte proliferation. Glia 32:328–337. [DOI] [PubMed] [Google Scholar]
- 247. Li C, Zhao R, Gao K, Wei Z, Yin MY, Lau LT et al (2011) Astrocytes: implications for neuroinflammatory pathogenesis of Alzheimer's disease. Curr Alzheimer Res 8:67–80. [DOI] [PubMed] [Google Scholar]
- 248. Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC et al (2008) Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab 28:468–481. [DOI] [PubMed] [Google Scholar]
- 249. Li Y, Cheng D, Cheng R, Zhu X, Wan T, Liu J, Zhang R (2014) Mechanisms of U87 astrocytoma cell uptake and trafficking of monomeric versus protofibril Alzheimer's disease amyloid‐β proteins. PLoS One 9:e99939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Liang C, Du T, Zhou J, Verkhratsky A, Peng L (2014) Ammonium increases Ca(2+) signalling and up‐regulates expression of TRPC1 gene in astrocytes in primary cultures and in the in vivo brain. Neurochem Res 39:2127–2235. [DOI] [PubMed] [Google Scholar]
- 251. Liberski PP, Bratosiewicz‐Wasik J, Gajdusek DC, Brown P (2002) Ultrastructural studies of experimental scrapie and Creutzfeldt‐Jakob disease in hamsters. II. Astrocytic and macrophage reaction towards axonal destruction. Acta Neurobiol Exp (Wars) 62:131–139. [DOI] [PubMed] [Google Scholar]
- 252. Lichter‐Konecki U, Mangin JM, Gordish‐Dressman H, Hoffman EP, Gallo V (2008) Gene expression profiling of astrocytes from hyperammonemic mice reveals altered pathways for water and potassium homeostasis in vivo. Glia 56:365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Liévens JC, Woodman B, Mahal A, Spasic‐Boscovic O, Samuel D, Kerkerian‐Le Goff L, Bates GP (2001) Impaired glutamate uptake in the R6 Huntington's disease transgenic mice. Neurobiol Dis 8:807–821. [DOI] [PubMed] [Google Scholar]
- 255. Lim D, Iyer A, Ronco V, Grolla AA, Canonico PL, Aronica E, Genazzani AA (2013) Amyloid beta deregulates astroglial mGluR5‐mediated calcium signaling via calcineurin and Nf‐kB. Glia 61:1134–1145. [DOI] [PubMed] [Google Scholar]
- 256. Lim D, Ronco V, Grolla AA, Verkhratsky A, Genazzani AA (2014) Glial calcium signalling in Alzheimer's disease. Rev Physiol Biochem Pharmacol 167:45–65. [DOI] [PubMed] [Google Scholar]
- 257. Lin CH, Tallaksen GS, Chien WM, Cearley JA, Jackson WS, Crouse AB et al (2001) Neurological abnormalities in a knock‐in mouse model of Huntington's disease. Hum Mol Genet 10:137–144. [DOI] [PubMed] [Google Scholar]
- 258. Lin CL, Bristol LA, Jin L, Dykes‐Hoberg M, Crawford T, Clawson L, Rothstein JD (1998) Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron 20:589–602. [DOI] [PubMed] [Google Scholar]
- 259. Lindenau J, Noack H, Possel H, Asayama K, Wolf G (2000) Cellular distribution of superoxide dismutases in the rat CNS. Glia 29:25–34. [DOI] [PubMed] [Google Scholar]
- 260. Liu AK, Goldfinger MH, Questari HE, Pearce RK, Gentleman SM (2016) ARTAG in the basal forebrain: widening the constellation of astrocytic tau pathology. Acta Neuropathol Commun 4:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA et al (2007) Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450:56–62. [DOI] [PubMed] [Google Scholar]
- 262. Llorens F, López‐González I, Thüne K, Carmona M, Zafar S, Andréoletti O et al (2014) Subtype and regional‐specific neuroinflammation in sporadic Creutzfeldt‐Jakob disease. Front Aging Neurosci 6:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Llorens F, Thüne K, Tahir W, Schmitz M, Kanata E, Kovaci L, et al (2017) YKL‐40 in the brain and cerebrospinal fluid in neurodegenerative dementias. Mol Neurodeg. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Lobsiger CS, Cleveland DW (2007) Glial cells as intrinsic components of non‐cell‐autonomous neurodegenerative diseases. Nature Neurosci 10:1355–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. López‐González I, Aso E, Carmona M, Armand‐Ugon M, Blanco R, Naudí A et al (2015) Neuroinflammatory gene regulation, mitochondrial function, oxidative stress, and brain lipid modifications with disease progression in Tau P301S transgenic mice as a model of frontotemporal lobar degeneration‐Tau. J Neuropathol Exp Neurol 74:975–999. [DOI] [PubMed] [Google Scholar]
- 266. López‐González I, Carmona M, Arregui L, Kovacs GG, Ferrer I (2014) αB‐crystallin and HSP27 in glial cells in tauopathies. Neuropathology 34:517–526. [DOI] [PubMed] [Google Scholar]
- 267. López‐González I, Carmona M, Blanco R, Luna‐Muñoz J, Martínez‐Mandonado A, Mena R, Ferrer I (2013) Characterization of thorn‐shaped astrocytes in white matter of temporal lobe in Alzheimer's disease brains. Brain Pathol 23:144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. López González I, Garcia‐Esparcia P, Llorens F, Ferrer I (2016) Genetic and transcriptomic profiles of inflammation in neurodegenerative diseases: Alzheimer, Parkinson, Creutzfeldt‐Jakob and tauopathies. Int J Mol Sci 17:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. López‐González I, Schlüter A, Aso E, Garcia‐Esparcia P, Ansoleaga B, LLorens F et al (2015) Neuroinflammatory signals in Alzheimer disease and APP/PS1 transgenic mice: correlations with plaques, tangles, and oligomeric species. J Neuropathol Exp Neurol 74:319–344. [DOI] [PubMed] [Google Scholar]
- 270. López‐Hernández T, Sirisi S, Capdevila‐Nortes X, Montolio M, Fernández‐Dueñas V, Scheper GC et al (2011) Molecular mechanisms of MLC1 and GLIALCAM mutations in megalencephalic leukoencephalopathy with subcortical cysts. Hum Mol Genet 20:3266–3277. [DOI] [PubMed] [Google Scholar]
- 271. Lu YB, Iandiev I, Hollborn M, Körber N, Ulbricht E, Hirrlinger PG et al (2011) Reactive glial cells: increased stiffness correlates with increased intermediate filament expression. FASEB J 25:624–631. [DOI] [PubMed] [Google Scholar]
- 272. Lucin KM, Wyss‐Coray T (2009) Immune activation in brain aging and neurodegeneration: too much or too little?. Neuron 64:110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Lundkvist A, Reichenbach A, Betsholtz C, Carmeliet P, Wolburg H, Pekny M (2004) Under stress, the absence of intermediate filaments from Müller cells in the retina has structural and functional consequences. J Cell Sci 117:3481–3488. [DOI] [PubMed] [Google Scholar]
- 274. Lynch AM, Murphy KJ, Deighan BF, O'Reilly JA, Gun'ko YK, Cowley TR et al (2010) The impact of glial activation in the aging brain. Aging Dis 1:262–278. [PMC free article] [PubMed] [Google Scholar]
- 275. Macnab LT, Pow DV (2007) Expression of the exon 9‐skipping form of EAAT2 in astrocytes of rats. Neuroscience 150:705–711. [DOI] [PubMed] [Google Scholar]
- 276. Maragakis NJ, Rothstein JD (2006) Mechanisms of disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol 2:679–689. [DOI] [PubMed] [Google Scholar]
- 277. Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH (2008) Non‐cell‐autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell 3:649–657. [DOI] [PubMed] [Google Scholar]
- 278. Martin JA, Craft DK, Su JH, Kim RC, Cotman CW (2001) Astrocytes degenerate in frontotemporal dementia: possible relation to hypoperfusion. Neurobiol Aging 22:195–207. [DOI] [PubMed] [Google Scholar]
- 279. Martínez A, Carmona M, Portero‐Otin M, Naudí A, Pamplona R, Ferrer I (2008) Type‐dependent oxidative damage in frontotemporal lobar degeneration: cortical astrocytes are targets of oxidative damage. J Neuropathol Exp Neurol 67:1122–1136. [DOI] [PubMed] [Google Scholar]
- 280. Martínez A, Portero‐Otin M, Pamplona R, Ferrer I (2010) Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates. Brain Pathol 20:281–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Martorana F, Brambilla L, Valori CF, Bergamaschi C, Roncoroni C, Aronica E et al (2012) The BH4 domain of Bcl‐X(L) rescues astrocyte degeneration in amyotrophic lateral sclerosis by modulating intracellular calcium signals. Hum Mol Genet 21:826–840. [DOI] [PubMed] [Google Scholar]
- 282. Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L (1996) Deficient glutamate transport is associated with neurodegeneration in Alzheimer's disease. Ann Neurol 40:759–766. [DOI] [PubMed] [Google Scholar]
- 283. Masliah E, Alford M, Mallory M, Rockenstein E, Moechars D, Van Leuven F (2000) Abnormal glutamate transport function in mutant amyloid precursor protein transgenic mice. Exp Neurol 163:381–387. [DOI] [PubMed] [Google Scholar]
- 284. Mathur R, Ince PG, Minett T, Garwood CJ, Shaw PJ, Matthews FE, MRC Cognitive Function and Ageing Neuropathology Study Group et al (2015) A reduced astrocyte response to β‐amyloid plaques in the ageing brain associates with cognitive impairment. PLoS One 10:e0118463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Matyash V, Kettenmann H (2010) Heterogeneity in astrocyte morphology and physiology. Brain Res Rev 63:2–10. [DOI] [PubMed] [Google Scholar]
- 286. Mei X, Ezan P, Giaume C, Koulakoff A (2010) Astroglial connexin immunoreactivity is specifically altered at β‐amyloid plaques in β‐amyloid precursor protein/presenilin1 mice. Neuroscience 171:92–105. [DOI] [PubMed] [Google Scholar]
- 287. Messing A, Brenner M (2003) GFAP: functional implications gleaned from studies of genetically engineered mice. Glia 43:87–90. [DOI] [PubMed] [Google Scholar]
- 288. Messing A, Brenner M, Feany MB, Nedergaard M, Goldman JE (2012) Alexander disease. J Neurosci 32:5017–5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Messing A, Head MW, Galles K, Galbreath EJ, Goldman JE, Brenner M (1998) Fatal encephalopathy with astrocyte inclusions in GFAP transgenic mice. Am J Pathol 152:391–398. [PMC free article] [PubMed] [Google Scholar]
- 290. Meyer K, Ferraiuolo L, Miranda CJ, Likhite S, McElroy S, Renusch S et al (2014) Direct conversion of patient fibroblasts demonstrates non‐cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc Natl Acad Sci USA 111:829–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Meyer T, Fromm A, Münch C, Schwalenstöcker B, Fray AE, Ince PG et al (1999) The RNA of the glutamate transporter EAAT2 is variably spliced in amyotrophic lateral sclerosis and normal individuals. J Neurol Sci 170:45–50. [DOI] [PubMed] [Google Scholar]
- 292. Migheli A, Piva R, Atzori C, Troost D, Schiffer D (1997) c‐Jun, JNK/SAPK kinases and transcription factor NF‐kappa B are selectively activated in astrocytes, but not motor neurons, in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 56:1314–1322. [DOI] [PubMed] [Google Scholar]
- 293. Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR et al (2008) Up‐regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington's disease phenotype in the R6/2 mouse. Neuroscience 153:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Miller DW, Cookson MR, Dickson DW (2004) Glial cell inclusions and the pathogenesis of neurodegenerative diseases. Neuron Glia Biol 1:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Min KJ, Yang MS, Kim SU, Jou I, Joe EH (2006) Astrocytes induce hemeoxygenase‐1 expression in microglia: a feasible mechanism for preventing excessive brain inflammation. J Neurosci 26:1880–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Mirza B, Hadberg H, Thomsen P, Moos T (2000) The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson's disease. Neuroscience 95:425–432. [DOI] [PubMed] [Google Scholar]
- 297. Montana V, Verkhratsky A, Parpura V (2014) Pathological role for exocytotic glutamate release from astrocytes in hepatic encephalopathy. Curr Neuropharmacol 12:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Morita T, Mizutani Y, Sawada M, Shimada A (2005) Immunohistochemical and ultrastructural findings related to the blood–brain barrier in the blood vessels of the cerebral white matter in aged dogs. J Comp Pathol 133:14–22. [DOI] [PubMed] [Google Scholar]
- 299. Mrak RE, Griffinbc WS (2001) The role of activated astrocytes and of the neurotrophic cytokine S100B in the pathogenesis of Alzheimer's disease. Neurobiol Aging 22:915–922. [DOI] [PubMed] [Google Scholar]
- 300. Mullett SJ, Di Maio R, Greenamyre JT, Hinkle DA (2013) DJ‐1 expression modulates astrocyte‐mediated protection against neuronal oxidative stress. J Mol Neurosci 49:507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Mullett SJ, Hinkle DA (2011) DJ‐1 deficiency in astrocytes selectively enhances mitochondrial Complex I inhibitor‐induced neurotoxicity. J Neurochem 117:375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Müller T, Fritschy JM, Grosche J, Pratt GD, Möhler H, Kettenmann H (1994) Developmental regulation of voltage‐gated K+ channel and GABAA receptor expression in Bergmann glial cells. J Neurosci 14:2503–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Müller T, Möller T, Neuhaus J, Kettenmann H (1996) Electrical coupling among Bergmann glial cells and its modulation by glutamate receptor activation. Glia 17:274–284. [DOI] [PubMed] [Google Scholar]
- 304. Muntané G, Dalfó E, Martínez A, Rey MJ, Avila J, Pérez M et al (2006) Glial fibrillary acidic protein is a major target of glycoxidative and lipoxidative damage in Pick's disease. J Neurochem 99:177–185. [DOI] [PubMed] [Google Scholar]
- 305. Muñoz DG, Morris HR, Rossor M (2011) Pick's disease. In: Neurodegeneration, The Molecular Pathology of Dementia and Molecular Disorders. Dickson DW, Weller RO (eds), pp. 156–164, Wiley‐Blackwell, Oxford. [Google Scholar]
- 306. Muñoz DG, Woulfe J, Kertesz A (2007) Argyrophilic thorny astrocyte clusters in association with Alzheimer's disease pathology in possible primary progressive aphasia. Acta Neuropathol 114:347–357. [DOI] [PubMed] [Google Scholar]
- 307. Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV (2006) Essential protective roles of reactive astrocytes in traumatic brain injury. Brain 129:2761–2772. [DOI] [PubMed] [Google Scholar]
- 308. Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S (2007) Astrocytes expressing ALS‐linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci 10:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang KC, Wegiel J (2004) Contribution of glial cells to the development of amyloid plaques in Alzheimer's disease. Neurobiol Aging 25:663–674. [DOI] [PubMed] [Google Scholar]
- 310. Nagy JI, Li W, Hertzberg EL, Marotta CA (1996) Elevated connexin43 immunoreactivity at sites of amyloid plaques in Alzheimer's disease. Brain Res 717:173–178. [DOI] [PubMed] [Google Scholar]
- 311. Navarrete M, Perea G, Maglio L, Pastor J, García de Sola R, Araque A (2013) Astrocyte calcium signal and gliotransmission in human brain tissue. Cereb Cortex 23:1240–1246. [DOI] [PubMed] [Google Scholar]
- 312. Nawashiro H, Messing A, Azzam N, Brenner M (1998) Mice lacking GFAP are hypersensitive to traumatic cerebrospinal injury. Neuroreport 9:1691–1696. [DOI] [PubMed] [Google Scholar]
- 313. Neary JT, Zimmermann H (2009) Trophic functions of nucleotides in the central nervous system. Trends Neurosci 32:189–198. [DOI] [PubMed] [Google Scholar]
- 314. Nedergaard M, Ransom B, Goldman SA (2003) New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 26:523–530. [DOI] [PubMed] [Google Scholar]
- 315. Nicoll JA, Weller RO (2003) A new role for astrocytes: beta‐amyloid homeostasis and degradation. Trends Mol Med 9:281–282. [DOI] [PubMed] [Google Scholar]
- 316. Nichols NR, Day JR, Laping NJ, Johnson SA, Finch CE (1993) GFAP mRNA increases with age in rat and human brain. Neurobiol Aging 14:421–429. [DOI] [PubMed] [Google Scholar]
- 317. Nilsen LH, Rae C, Ittner LM, Götz J, Sonnewald U (2013) Glutamate metabolism is impaired in transgenic mice with tau hyperphosphorylation. J Cereb Blood Flow Metab 33:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Nishimura M, Namba Y, Ikeda K, Oda M (1992) Glial fibrillary tangles with straight tubules in the brains of patients with progressive supranuclear palsy. Neurosci Lett 143:35–38. [DOI] [PubMed] [Google Scholar]
- 319. Nishimura T, Ikeda K, Akiyama H, Kondo H, Kato M, Li F et al (1995) Immunohistochemical investigation of tau‐positive structures in the cerebral cortex of patients with progressive supranuclear palsy. Neurosci Lett 201:123–126. [DOI] [PubMed] [Google Scholar]
- 320. Norenberg MD (1994) Astrocyte responses to CNS injury. J Neuropathol Exp Neurol 53:213–219. [DOI] [PubMed] [Google Scholar]
- 321. Norenberg MD, Jayakumar AR, Rama Rao KV, Panickar KS (2007) New concepts in the mechanism of ammonia‐induced astrocyte swelling. Metab Brain Dis 22:219–234. [DOI] [PubMed] [Google Scholar]
- 322. Norenberg MD, Rama Rao KV, Jayakumar AR (2004) Ammonia neurotoxicity and the mitochondrial permeability transition. J Bioenerg Biomembr 36:303–307. [DOI] [PubMed] [Google Scholar]
- 323. Oberheim NA, Goldman SA, Nedergaard M (2012) Heterogeneity of astrocytic form and function. Methods Mol Biol 814:23–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F et al (2009) Uniquely hominid features of adult human astrocytes. J Neurosci 29:3276–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Oberheim NA, Wang X, Goldman S, Nedergaard M (2006) Astrocytic complexity distinguishes the human brain. Trends Neurosci 29:547–553. [DOI] [PubMed] [Google Scholar]
- 326. Ogata K, Kosaka T (2002) Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience 113:221–233. [DOI] [PubMed] [Google Scholar]
- 327. Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K et al (2006) Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 12:829–834. [DOI] [PubMed] [Google Scholar]
- 328. Olabarria M, Noristani HN, Verkhratsky A, Rodríguez JJ (2010) Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer's disease. Glia 58:831–838. [DOI] [PubMed] [Google Scholar]
- 329. Olabarria M, Noristani HN, Verkhratsky A, Rodríguez JJ (2011) Age‐dependent decrease in glutamine synthetase expression in the hippocampal astroglia of the triple transgenic Alzheimer's disease mouse model: mechanism for deficient glutamatergic transmission?. Mol Neurodegener 6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Oliveira JM (2010) Mitochondrial bioenergetics and dynamics in Huntington's disease: tripartite synapses and selective striatal degeneration. J Bioenerg Biomembr 42:227–234. [DOI] [PubMed] [Google Scholar]
- 331. Olsen ML, Campbell SL, Sontheimer H (2007) Differential distribution of Kir4.1 in spinal cord astrocytes suggests regional differences in K+ homeostasis. J Neurophysiol 98:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Orre M, Kamphuis W, Dooves S, Kooijman L, Chan ET, Kirk CJ et al (2013) Reactive glia show increased immunoproteasome activity in Alzheimer's disease. Brain 136:1415–1431. [DOI] [PubMed] [Google Scholar]
- 333. Orre M, Kamphuis W, Osborn LM, Jansen AH, Kooijman L, Bossers K, Hol EM (2014) Isolation of glia from Alzheimer's mice reveals inflammation and dysfunction. Neurobiol Aging 35:2746–2760. [DOI] [PubMed] [Google Scholar]
- 334. Orre M, Kamphuis W, Osborn LM, Melief J, Kooijman L, Huitinga I et al (2014) Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol Aging 35:1–14. [DOI] [PubMed] [Google Scholar]
- 335. Osborn LM, Kamphuis W, Wadman WJ, Hol EM (2016) Astrogliosis: an integral player in the pathogenesis of Alzheimer's disease. Prog Neurobiol 144:121–141. [DOI] [PubMed] [Google Scholar]
- 336. Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ et al (2010) Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci 13:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Pakkenberg B, Pelvig D, Marner L, Bundgaard MJ, Gundersen HJ, Nyengaard JR, Regeur L (2003) Aging and the human neocortex. Exp Gerontol 38:95–99. [DOI] [PubMed] [Google Scholar]
- 338. Pamplona R, Dalfó E, Ayala V, Bellmunt MJ, Prat J, Ferrer I, Portero‐Otín M (2005) Proteins in human brain cortex are modified by oxidation, glycoxidation, and lipoxidation. Effects of Alzheimer disease and identification of lipoxidation targets. J Biol Chem 280:21522–21530. [DOI] [PubMed] [Google Scholar]
- 339. Paradisi S, Sacchetti B, Balduzzi M, Gaudi S, Malchiodi‐Albedi F (2004) Astrocyte modulation of in vitro beta‐amyloid neurotoxicity. Glia 46:252–260. [DOI] [PubMed] [Google Scholar]
- 340. Pardo AC, Wong V, Benson LM, Dykes M, Tanaka K, Rothstein JD, Maragakis NJ (2006) Loss of the astrocyte glutamate transporter GLT1 modifies disease in SOD1(G93A) mice. Exp Neurol 201:120–130. [DOI] [PubMed] [Google Scholar]
- 341. Parpura V, Verkhratsky A (2012) Homeostatic function of astrocytes: Ca(2+) and Na(+) signalling. Transl Neurosci 3:334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Patel SA, Maragakis NJ (2002) Amyotrophic lateral sclerosis: pathogenesis, differential diagnoses, and potential interventions. J Spinal Cord Med 25:262–273. [DOI] [PubMed] [Google Scholar]
- 343. Pehar M, Cassina P, Vargas MR, Castellanos R, Viera L, Beckman JS et al (2004) Astrocytic production of nerve growth factor in motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J Neurochem 89:464–473. [DOI] [PubMed] [Google Scholar]
- 344. Pehar M, Vargas MR, Cassina P, Barbeito AG, Beckman JS, Barbeito L (2005) Complexity of astrocyte‐motor neuron interactions in amyotrophic lateral sclerosis. Neurodegener Dis 2:139–146. [DOI] [PubMed] [Google Scholar]
- 345. Peinado MA, Quesada A, Pedrosa JA, Torres MI, Martinez M, Esteban FJ et al (1998) Quantitative and ultrastructural changes in glia and pericytes in the parietal cortex of the aging rat. Microsc Res Tech 43:34–42. [DOI] [PubMed] [Google Scholar]
- 346. Pekny M, Eliasson C, Siushansian R, Ding M, Dixon SJ, Pekna M et al (1999) The impact of genetic removal of GFAP and/or vimentin on glutamine levels and transport of glucose and ascorbate in astrocytes. Neurochem Res 24:1357–1362. [DOI] [PubMed] [Google Scholar]
- 347. Pekny M, Nilsson M (2005) Astrocyte activation and reactive gliosis. Glia 50:427–434. [DOI] [PubMed] [Google Scholar]
- 348. Pekny M, Pekna M (2016) Reactive gliosis in the pathogenesis of CNS diseases. Biochim Biophys Acta 1862:483–491. [DOI] [PubMed] [Google Scholar]
- 349. Pekny M, Pekna M (2014) Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev 94:1077–1098. [DOI] [PubMed] [Google Scholar]
- 350. Pekny M, Pekna M, Messing A, Steinhäuser C, Lee JM, Parpura V et al (2016) Astrocytes: a central element in neurological diseases. Acta Neuropathol 131:323–345. [DOI] [PubMed] [Google Scholar]
- 351. Pellerin L, Bouzier‐Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ (2007) Activity‐dependent regulation of energy metabolism by astrocytes: an update. Glia 55:1251–1262. [DOI] [PubMed] [Google Scholar]
- 352. Perea G, Navarrete M, Araque A (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32:421–431. [DOI] [PubMed] [Google Scholar]
- 353. Peters A, Verderosa A, Sethares C (2008) The neuroglial population in the primary visual cortex of the aging rhesus monkey. Glia 56:1151–1161. [DOI] [PubMed] [Google Scholar]
- 354. Phatnani HP, Guarnieri P, Friedman BA, Carrasco MA, Muratet M, O'Keeffe S et al (2013) Intricate interplay between astrocytes and motor neurons in ALS. Proc Natl Acad Sci USA 110:E756–E765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Phillips EC, Croft CL, Kurbatskaya K, O'Neill MJ, Hutton ML, Hanger DP et al (2014) Astrocytes and neuroinflammation in Alzheimer's disease. Biochem Soc Trans 42:1321–1325. [DOI] [PubMed] [Google Scholar]
- 356. Pihlaja R, Koistinaho J, Kauppinen R, Sandholm J, Tanila H, Koistinaho M (2011) Multiple cellular and molecular mechanisms are involved in human Aβ clearance by transplanted adult astrocytes. Glia 59:1643–1657. [DOI] [PubMed] [Google Scholar]
- 357. Pihlaja R, Koistinaho J, Malm T, Sikkilä H, Vainio S, Koistinaho M (2008) Transplanted astrocytes internalize deposited beta‐amyloid peptides in a transgenic mouse model of Alzheimer's disease. Glia 56:154–163. [DOI] [PubMed] [Google Scholar]
- 358. Pirttimaki TM, Codadu NK, Awni A, Pratik P, Nagel DA, Hill EJ et al (2013) α7 Nicotinic receptor‐mediated astrocytic gliotransmitter release: Aβ effects in a preclinical Alzheimer's mouse model. PLoS One 8:e81828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Popescu BO, Toescu EC, Popescu LM, Bajenaru O, Muresanu DF, Schultzberg M, Bogdanovic N (2009) Blood‐brain barrier alterations in ageing and dementia. J Neurol Sci 283:99–106. [DOI] [PubMed] [Google Scholar]
- 360. Porchet R, Probst A, Bouras C, Dráberová E, Dráber P, Riederer BM (2003) Analysis of glial acidic fibrillary protein in the human entorhinal cortex during aging and in Alzheimer's disease. Proteomics 3:1476–1485. [DOI] [PubMed] [Google Scholar]
- 361. Prust M, Wang J, Morizono H, Messing A, Brenner M, Gordon E et al (2011) GFAP mutations, age at onset, and clinical subtypes in Alexander disease. Neurology 77:1287–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Rao KV, Panickar KS, Jayakumar AR, Norenberg MD (2005) Astrocytes protect neurons from ammonia toxicity. Neurochem Res 30:1311–1318. [DOI] [PubMed] [Google Scholar]
- 363. Rabchevsky AG, Weinitz JM, Coulpier M, Fages C, Tinel M, Junier MP (1998) A role for transforming growth factor alpha as an inducer of astrogliosis. J Neurosci 18:10541–10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Raoul C, Buhler E, Sadeghi C, Jacquier A, Aebischer P, Pettmann B et al (2006) Chronic activation in presymptomatic amyotrophic lateral sclerosis (ALS) mice of a feedback loop involving Fas, Daxx, and FasL. Proc Natl Acad Sci USA 103:6007–6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Ramon y Cajal S (1897) Histology of the Nervous System of Man and Vertebrates. Oxford University Press: Oxford. [Google Scholar]
- 366. Regan MR, Huang YH, Kim YS, Dykes‐Hoberg MI, Jin L, Watkins AM et al (2007) Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci 27:6607–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Renkawek K, Bosman GJ, de Jong WW (1994) Expression of small heat‐shock protein hsp 27 in reactive gliosis in Alzheimer disease and other types of dementia. Acta Neuropathol 87:511–519. [DOI] [PubMed] [Google Scholar]
- 368. Robel S, Berninger B, Götz M (2011) The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci 12:88–104. [DOI] [PubMed] [Google Scholar]
- 369. Rodríguez A, Pérez‐Gracia E, Espinosa JC, Pumarola M, Torres JM, Ferrer I (2006) Increased expression of water channel aquaporin 1 and aquaporin 4 in Creutzfeldt‐Jakob disease and in bovine spongiform encephalopathy‐infected bovine‐PrP transgenic mice. Acta Neuropathol 112:573–585. [DOI] [PubMed] [Google Scholar]
- 370. Rodríguez JJ, Olabarria M, Chvatal A, Verkhratsky A (2009) Astroglia in dementia and Alzheimer's disease. Cell Death Differ 16:378–385. [DOI] [PubMed] [Google Scholar]
- 371. Rodríguez JJ, Yeh CY, Terzieva S, Olabarria M, Kulijewicz‐Nawrot M, Verkhratsky A (2014) Complex and region‐specific changes in astroglial markers in the aging brain. Neurobiol Aging 35:15–23. [DOI] [PubMed] [Google Scholar]
- 372. Rodríguez‐Arellano JJ, Parpura V, Zorec R, Verkhratsky A (2016) Astrocytes in physiological aging and Alzheimer's disease. Neuroscience 323:170–182. [DOI] [PubMed] [Google Scholar]
- 373. Rojas F, Cortes N, Abarzua S, Dyrda A, van Zundert B (2014) Astrocytes expressing mutant SOD1 and TDP43 trigger motoneuron death that is mediated via sodium channels and nitroxidative stress. Front Cell Neurosci 8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Rolls A, Shechter R, Schwartz M (2009) The bright side of the glial scar in CNS repair. Nat Rev Neurosci 10:235–241. [DOI] [PubMed] [Google Scholar]
- 375. Ronco V, Grolla AA, Glasnov TN, Canonico PL, Verkhratsky A, Genazzani AA, Lim D (2014) Differential deregulation of astrocytic calcium signalling by amyloid‐β, TNFα, IL‐1β and LPS. Cell Calcium 55:219–229. [DOI] [PubMed] [Google Scholar]
- 376. Rossi D, Brambilla L, Valori CF, Roncoroni C, Crugnola A, Yokota T et al (2008) Focal degeneration of astrocytes in amyotrophic lateral sclerosis. Cell Death Differ 15:1691–1700. [DOI] [PubMed] [Google Scholar]
- 377. Rossner S, Apelt J, Schliebs R, Perez‐Polo JR, Bigl V (2001) Neuronal and glial beta‐secretase (BACE) protein expression in transgenic Tg2576 mice with amyloid plaque pathology. J Neurosci Res 64:437–446. [DOI] [PubMed] [Google Scholar]
- 378. Rossner S, Lange‐Dohna C, Zeitschel U, Perez‐Polo JR (2005) Alzheimer's disease beta‐secretase BACE1 is not a neuron‐specific enzyme. J Neurochem 92:226–234. [DOI] [PubMed] [Google Scholar]
- 379. Rothstein JD, Dykes‐Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW et al (1996) Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16:675–686. [DOI] [PubMed] [Google Scholar]
- 380. Rothstein JD, Martin LJ, Kuncl RW (1992) Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med 326:1464–1468. [DOI] [PubMed] [Google Scholar]
- 381. Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW (1995) Selective loss of glial glutamate transporter GLT‐1 in amyotrophic lateral sclerosis. Ann Neurol 38:73–78. [DOI] [PubMed] [Google Scholar]
- 382. Rudy CC, Hunsberger HC, Weitzner DS, Reed MN (2015) The role of the tripartite glutamatergic synapse in the pathophysiology of Alzheimer's disease. Aging Dis 6:131–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Rusnakova V, Honsa P, Dzamba D, Stählberg A, Kubista M, Anderova M (2013) Heterogeneity of astrocytes: from development to injury – single cell gene expression. PLOS One 8:e69734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG et al (2009) A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation‐induced death. Cell 137:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H (2011) Astrocytes in the aging brain express characteristics of senescence‐associated secretory phenotype. Eur J Neurosci 34:3–11. [DOI] [PubMed] [Google Scholar]
- 386. Sanders DW, Kaufman SK, DeVos SL, Sharma AM, Mirbaha H, Li A et al (2014) Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 82:1271–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Schipper HM, Liberman A, Stopa EG (1998) Neural heme oxygenase‐1 expression in idiopathic Parkinson's disease. Exp Neurol 150:60–68. [DOI] [PubMed] [Google Scholar]
- 388. Schipper HM, Cissé S, Stopa EG (1995) Expression of heme oxygenase‐1 in the senescent and Alzheimer‐diseased brain. Ann Neurol 37:758–768. [DOI] [PubMed] [Google Scholar]
- 389. Schmidt S, Linnartz B, Mendritzki S, Sczepan T, Lübbert M, Stichel CC, Lübbert H (2011) Genetic mouse models for Parkinson's disease display severe pathology in glial cell mitochondria. Hum Mol Genet 20:1197–1211. [DOI] [PubMed] [Google Scholar]
- 390. Schmitt A, Gofferje V, Weber M, Meyer J, Mössner R, Lesch KP (2003) The brain‐specific protein MLC1 implicated in megalencephalic leukoencephalopathy with subcortical cysts is expressed in glial cells in the murine brain. Glia 44:283–295. [DOI] [PubMed] [Google Scholar]
- 391. Schon EA, Przedborski S (2011) Mitochondria: the next (neurode)generation. Neuron 70:1033–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Schultz C, Ghebremedhin E, Del Tredici K, Rüb U, Braak H (2004) High prevalence of thorn‐shaped astrocytes in the aged human medial temporal lobe. Neurobiol Aging 25:397–405. [DOI] [PubMed] [Google Scholar]
- 393. Schwarz L, Vollmer G, Richter‐Landsberg C (2010) The small heat shock protein HSP25/27 (HspB1) is abundant in cultured astrocytes and associated with astrocytic pathology in progressive supranuclear palsy and corticobasal degeneration. Int J Cell Biol 2010:717520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Scott HA, Gebhardt FM, Mitrovic AD, Vandenberg RJ, Dodd PR (2011) Glutamate transporter variants reduce glutamate uptake in Alzheimer's disease. Neurobiol Aging 32:553.e1–511. [DOI] [PubMed] [Google Scholar]
- 395. Seifert G, Schilling K, Steinhauser C (2006) Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci 7:194–206. [DOI] [PubMed] [Google Scholar]
- 396. Seifert G, Henneberger C, Steinhäuser C (2016) Diversity of astrocyte potassium channels: An update. Brain Res Bull pii:S0361–9230(16)30458–0. [DOI] [PubMed] [Google Scholar]
- 397. Serio A, Bilican B, Barmada SJ, Ando DM, Zhao C, Siller R et al (2013) Astrocyte pathology and the absence of non‐cell autonomy in an induced pluripotent stem cell model of TDP‐43 proteinopathy. Proc Natl Acad Sci USA 110:4697–4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Serrano‐Pozo A, Mielke ML, Gómez‐Isla T, Betensky RA, Growdon JH, Frosch MP, Hyman BT (2011) Reactive glia not only associates with plaques but also parallels tangles in Alzheimer's disease. Am J Pathol 179:1373–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Shankaran M, Di Paolo E, Leoni V, Caccia C, Ferrari Bardile C, Mohammed H et al (2017) Early and brain region‐specific decrease of de novo cholesterol biosynthesis in Huntington's disease: a cross‐validation study in Q175 knock‐in mice. Neurobiol Dis 98:66–76. [DOI] [PubMed] [Google Scholar]
- 400. Shibata N, Kakita A, Takahashi H, Ihara Y, Nobukuni K, Fujimura H et al (2009) Activation of signal transducer and activator of transcription‐3 in the spinal cord of sporadic amyotrophic lateral sclerosis patients. Neurodegener Dis 6:118–126. [DOI] [PubMed] [Google Scholar]
- 401. Shibata N, Yamamoto T, Hiroi A, Omi Y, Kato Y, Kobayashi M (2010) Activation of STAT3 and inhibitory effects of pioglitazone on STAT3 activity in a mouse model of SOD1‐mutated amyotrophic lateral sclerosis. Neuropathology 30:353–360. [DOI] [PubMed] [Google Scholar]
- 402. Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS (2008) Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor‐mediated slow inward currents in pyramidal neurons. J Neurosci 28:6659–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Shigetomi E, Patel S, Khakh BS (2016) Probing the complexities of astrocyte calcium signaling. Trends Cell Biol 26:300–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Shin JY, Fang ZH, Yu ZX, Wang CE, Li SH, Li XJ (2005) Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J Cell Biol 171:1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5:146–156. [DOI] [PubMed] [Google Scholar]
- 406. Silverberg GD, Messier AA, Miller MC, Machan JT, Majmudar SS, Stopa EG et al (2010) Amyloid efflux transporter expression at the blood‐brain barrier declines in normal aging. J Neuropathol Exp Neurol 69:1034–1043. [DOI] [PubMed] [Google Scholar]
- 407. Silverberg GD, Miller MC, Messier AA, Majmudar S, Machan JT, Donahue JE et al (2010) Amyloid deposition and influx transporter expression at the blood‐brain barrier increase in normal aging. J Neuropathol Exp Neurol 69:98–108. [DOI] [PubMed] [Google Scholar]
- 408. Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M (2003) Signaling at the gliovascular interface. J Neurosci 23:9254–9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Simpson JE, Ince PG, Haynes LJ, Theaker R, Gelsthorpe C, Baxter L, MRC Cognitive Function and Ageing Neuropathology Study Group et al (2010) Population variation in oxidative stress and astrocyte DNA damage in relation to Alzheimer‐type pathology in the ageing brain. Neuropathol Appl Neurobiol 36:25–40. [DOI] [PubMed] [Google Scholar]
- 410. Simpson JE, Ince PG, Lace G, Forster G, Shaw PJ, Matthews F, MRC Cognitive Function and Ageing Neuropathology Study Group et al (2010) Astrocyte phenotype in relation to Alzheimer‐type pathology in the ageing brain. Neurobiol Aging 31:578–590. [DOI] [PubMed] [Google Scholar]
- 411. Simpson JE, Ince PG, Shaw PJ, Heath PR, Raman R, Garwood CJ, MRC Cognitive Function and Ageing Neuropathology Study Group et al (2011) Microarray analysis of the astrocyte transcriptome in the aging brain: relationship to Alzheimer's pathology and APOE genotype. Neurobiol Aging 32:1795–1807. [DOI] [PubMed] [Google Scholar]
- 412. Sims NR, Nilsson M, Muyderman H (2004) Mitochondrial glutathione: a modulator of brain cell death. J Bioenerg Biomembr 36:329–333. [DOI] [PubMed] [Google Scholar]
- 413. Sirko S, Behrendt G, Johansson PA, Tripathi P, Costa M, Bek S et al (2013) Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. Cell Stem Cell 12:426–439. [DOI] [PubMed] [Google Scholar]
- 414. Sofroniew MV (2005) Reactive astrocytes in neural repair and protection. Neuroscientist 11:400–407. [DOI] [PubMed] [Google Scholar]
- 415. Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Sofroniew MV (2014) Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist 20:160–172. [DOI] [PubMed] [Google Scholar]
- 417. Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Song H, Stevens CF, Gage FH (2002) Astroglia induce neurogenesis from adult neural stem cells. Nature 417:39–44. [DOI] [PubMed] [Google Scholar]
- 419. Song YJ, Halliday GM, Holton JL, Lashley T, O'Sullivan SS, McCann H et al (2009) Degeneration in different parkinsonian syndromes relates to astrocyte type and astrocyte protein expression. J Neuropathol Exp Neurol 68:1073–1083. [DOI] [PubMed] [Google Scholar]
- 420. Srinivasan K, Friedman BA, Larson JL, Lauffer BE, Goldstein LD, Appling LL et al (2016) Untangling the brain's neuroinflammatory and neurodegenerative transcriptional responses. Nat Commun 7:11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Sriram K, Benkovic SA, Hebert MA, Miller DB, O'Callaghan JP (2004) Induction of gp130‐related cytokines and activation of JAK2/STAT3 pathway in astrocytes precedes up‐regulation of glial fibrillary acidic protein in the 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine model of neurodegeneration: key signaling pathway for astrogliosis in vivo?. J Biol Chem 279:19936–19947. [DOI] [PubMed] [Google Scholar]
- 422. Staats KA, Van Den Bosch L (2009) Astrocytes in amyotrophic lateral sclerosis: direct effects on motor neuron survival. J Biol Phys 35:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N et al (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131:1164–1178. [DOI] [PubMed] [Google Scholar]
- 424. Strauss U, Zhou FW, Henning J, Battefeld A, Wree A, Köhling R et al (2008) Increasing extracellular potassium results in subthalamic neuron activity resembling that seen in a 6‐hydroxydopamine lesion. J Neurophysiol 99:2902–2915. [DOI] [PubMed] [Google Scholar]
- 425. Strooper BD, Karran E (2016) The cellular phase of Alzheimer's disease. Cell 164:603–615. [DOI] [PubMed] [Google Scholar]
- 426. Sturrock RR (1987) A morphological study of the neostriatum of aged mice with particular reference to neuroglia. J Hirnforsch 28:505–515. [PubMed] [Google Scholar]
- 427. Suh SW, Bergher JP, Anderson CM, Treadway JL, Fosgerau K, Swanson RA (2007) Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP‐316,819 ([R‐R*,S*]‐5‐chloro‐N‐[2‐hydroxy‐3‐(methoxymethylamino)‐3‐oxo‐1‐(phenylmethyl)propyl]‐1H‐indole‐2‐carboxamide). J Pharmacol Exp Ther 321:45–50. [DOI] [PubMed] [Google Scholar]
- 428. Sultana R, Butterfield DA (2013) Oxidative modification of brain proteins in Alzheimer's disease: perspective on future studies based on results of redox proteomics studies. J Alzheimers Dis 33: S243–S251. [DOI] [PubMed] [Google Scholar]
- 429. Sultana R, Perluigi M, Butterfield DA (2009) Oxidatively modified proteins in Alzheimer's disease (AD), mild cognitive impairment and animal models of AD: role of Abeta in pathogenesis. Acta Neuropathol 118:131–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Sun W, Cornwell A, Li J, Peng S, Osorio MJ, Aalling N et al (2017) SOX9 is an astrocyte‐specific nuclear marker in the adult brain outside the neurogenic regions. J Neurosci 37:4493–4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Swanson RA, Ying W, Kauppinen TM (2004) Astrocyte influences on ischemic neuronal death. Curr Mol Med 4:193–205. [DOI] [PubMed] [Google Scholar]
- 432. Takano T, Kang J, Jaiswal JK, Simon SM, Lin JH, Yu Y et al (2005) Receptor‐mediated glutamate release from volume sensitive channels in astrocytes. Proc Natl Acad Sci USA 102:16466–16471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433. Talantova M, Sanz‐Blasco S, Zhang X, Xia P, Akhtar MW, Okamoto S et al (2013) Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci USA 110:E2518–E2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434. Terada S, Ishizu H, Yokota O, Tsuchiya K, Nakashima H, Ishihara T et al (2003) Glial involvement in diffuse Lewy body disease. Acta Neuropathol 105:163–169. [DOI] [PubMed] [Google Scholar]
- 435. Thal DR, Schultz C, Dehghani F, Yamaguchi H, Braak H, Braak E (2000) Amyloid beta‐protein (Abeta)‐containing astrocytes are located preferentially near N‐terminal‐truncated Abeta deposits in the human entorhinal cortex. Acta Neuropathol 100:608–617. [DOI] [PubMed] [Google Scholar]
- 436. Thellung S, Florio T, Corsaro A, Arena S, Merlino M, Salmona M et al (2000) Intracellular mechanisms mediating the neuronal death and astrogliosis induced by the prion protein fragment 106–126. Int J Dev Neurosci 18:481–492. [DOI] [PubMed] [Google Scholar]
- 437. Togo T, Dickson DW (2002) Tau accumulation in astrocytes in progressive supranuclear palsy is a degenerative rather than a reactive process. Acta Neuropathol 104:398–402. [DOI] [PubMed] [Google Scholar]
- 438. Tolnay M, Braak H (2011) Argyrophilic grain disease. In: Neurodegeneration, the Molecular Pathology of Dementia and Molecular Disorders. Dickson DW, Weller RO (eds), pp. 165–170. Wiley‐Blackwell, Oxford. [Google Scholar]
- 439. Tong X, Ao Y, Faas GC, Nwaobi SE, Xu J, Haustein MD et al (2014) Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington's disease model mice. Nat Neurosci 17:694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440. Tortarolo M, Veglianese P, Calvaresi N, Botturi A, Rossi C, Giorgini A et al (2003) Persistent activation of p38 mitogen‐activated protein kinase in a mouse model of familial amyotrophic lateral sclerosis correlates with disease progression. Mol Cell Neurosci 23:180–192. [DOI] [PubMed] [Google Scholar]
- 441. Unger JW (1998) Glial reaction in aging and Alzheimer's disease. Microsc Res Tech 43:24–28. [DOI] [PubMed] [Google Scholar]
- 442. Urushitani M, Sik A, Sakurai T, Nukina N, Takahashi R, Julien JP (2006) Chromogranin‐mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci 9:108–118. [DOI] [PubMed] [Google Scholar]
- 443. Valenza M, Cattaneo E (2011) Emerging roles for cholesterol in Huntington's disease. Trends Neurosci 34:474–486. [DOI] [PubMed] [Google Scholar]
- 444. Valenza M, Leoni V, Karasinska JM, Petricca L, Fan J, Carroll J et al (2010) Cholesterol defect is marked across multiple rodent models of Huntington's disease and is manifest in astrocytes. J Neurosci 30:10844–10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445. Valenza M, Marullo M, Di Paolo E, Cesana E, Zuccato C, Biella G, Cattaneo E (2015) Disruption of astrocyte‐neuron cholesterol cross talk affects neuronal function in Huntington's disease. Cell Death Differ 22:690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446. Valori CF, Brambilla L, Martorana F, Rossi D (2014) The multifaceted role of glial cells in amyotrophic lateral sclerosis. Cell Mol Life Sci 71:287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447. Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA (2008) Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci 28:13574–13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448. Verardo MR, Lewis GP, Takeda M, Linberg KA, Byun J, Luna G et al (2008) Abnormal reactivity of muller cells after retinal detachment in mice deficient in GFAP and vimentin. Invest Ophthalmol Vis Sci 49:3659–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449. Verkhratsky A, Marutle A, Rodríguez‐Arellano JJ, Nordberg A (2015) Glial asthenia and functional paralysis: a new perspective on neurodegeneration and Alzheimer's disease. Neuroscientist 21:552–568. [DOI] [PubMed] [Google Scholar]
- 450. Verkhratsky A, Olabarria M, Noristani HN, Yeh CY, Rodriguez JJ (2010) Astrocytes in Alzheimer's disease. Neurotherapeutics 7:399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451. Verkhratsky A, Rodríguez JJ, Parpura V (2013) Astroglia in neurological diseases. Future Neurol 8:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452. Verkhratsky A, Rodríguez JJ, Parpura V (2014) Neuroglia in ageing and disease. Cell Tissue Res 357:493–503. [DOI] [PubMed] [Google Scholar]
- 453. Verkhratsky A, Rodríguez‐Arellano JJ, Parpura V, Zorec R (2017) Astroglial calcium signalling in Alzheimer's disease. Biochem Biophys Res Commun 483:1005–1012. [DOI] [PubMed] [Google Scholar]
- 454. Verkhratsky A, Zorec R, Rodríguez JJ, Parpura V (2016a) Astroglia dynamics in ageing and Alzheimer's disease. Curr Opin Pharmacol 26:74–79. [DOI] [PubMed] [Google Scholar]
- 455. Verkhratsky A, Zorec R, Rodríguez JJ, Parpura V (2016b) Pathobiology of neurodegeneration: the role of astroglia. Opera Med Physiol 1:13–22. [PMC free article] [PubMed] [Google Scholar]
- 456. Victoria GS, Arkhipenko A, Zhu S, Syan S, Zurzolo C (2016) Astrocyte‐to‐neuron intercellular prion transfer is mediated by cell‐cell contact. Sci Rep 6:20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457. Vijayan VK, Geddes JW, Anderson KJ, Chang‐Chui H, Ellis WG, Cotman CW (1991) Astrocyte hypertrophy in the Alzheimer's disease hippocampal formation. Exp Neurol 112:72–78. [DOI] [PubMed] [Google Scholar]
- 458. Vincent AJ, Gasperini R, Foa L, Small DH (2010) Astrocytes in Alzheimer's disease: emerging roles in calcium dysregulation and synaptic plasticity. J Alzheimers Dis 22:699–714. [DOI] [PubMed] [Google Scholar]
- 459. Vis JC, Nicholson LF, Faull RL, Evans WH, Severs NJ, Green CR (1998) Connexin expression in Huntington's diseased human brain. Cell Biol Int 22:837–847. [DOI] [PubMed] [Google Scholar]
- 460. Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP Jr (1985) Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol 44:559–577. [DOI] [PubMed] [Google Scholar]
- 461. Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari‐Woodruff S, Sofroniew MV (2009) Reactive astrocytes form scar‐like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci 29:11511–11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462. Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, Takahashi H (2000) NACP/alpha‐synuclein‐positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson's disease brains. Acta Neuropathol 99:14–20. [DOI] [PubMed] [Google Scholar]
- 463. Wang CY, Yang SH, Tzeng SF (2015) MicroRNA‐145 as one negative regulator of astrogliosis. Glia 63:194–205. [DOI] [PubMed] [Google Scholar]
- 464. Wang K, Zhang J, Xu Y, Ren K, Xie WL, Yan YE et al (2013) Abnormally upregulated αB‐crystallin was highly coincidental with the astrogliosis in the brains of scrapie‐infected hamsters and human patients with prion diseases. J Mol Neurosci 51:734–748. [DOI] [PubMed] [Google Scholar]
- 465. Wang L, Lin F, Wang J, Wu J, Han R, Zhu L et al (2012) Truncated N‐terminal huntingtin fragment with expanded‐polyglutamine (htt552–100Q) suppresses brain‐derived neurotrophic factor transcription in astrocytes. Acta Biochim Biophys Sin (Shanghai) 44:249–258. [DOI] [PubMed] [Google Scholar]
- 466. Wang Y, Yang PL, Tang JF, Lin JF, Cai XH, Wang XT, Zheng GQ (2008) Potassium channels: possible new therapeutic targets in Parkinson's disease. Med Hypotheses 71:546–550. [DOI] [PubMed] [Google Scholar]
- 467. Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray‐Thompson Z et al (2013) Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3‐dependent mechanisms after spinal cord injury. J Neurosci 33:12870–12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468. Vaughan DW, Peters A (1974) Neuroglial cells in the cerebral cortex of rats from young adulthood to old age: an electron microscope study. J Neurocytol 3:405–429. [DOI] [PubMed] [Google Scholar]
- 469. Wilhelmsson U, Li L, Pekna M, Berthold CH, Blom S, Eliasson C et al (2004) Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post‐traumatic regeneration. J Neurosci 24:5016–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470. Wilhelmus MM, Otte‐Höller I, Wesseling P, de Waal RM, Boelens WC, Verbeek MM (2006) Specific association of small heat shock proteins with the pathological hallmarks of Alzheimer's disease brains. Neuropathol Appl Neurobiol 32:119–130. [DOI] [PubMed] [Google Scholar]
- 471. Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D et al (2015) GLUT1 reductions exacerbate Alzheimer's disease vasculo‐neuronal dysfunction and degeneration. Nat Neurosci 18:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472. Winter CG, Saotome Y, Levison SW, Hirsh D (1995) A role for ciliary neurotrophic factor as an inducer of reactive gliosis, the glial response to central nervous system injury. Proc Natl Acad Sci USA 92:5865–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473. Wójtowicz AM, Dvorzhak A, Semtner M, Grantyn R (2013) Reduced tonic inhibition in striatal output neurons from Huntington mice due to loss of astrocytic GABA release through GAT‐3. Front Neural Circuits 7:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474. Woltjer RL, Duerson K, Fullmer JM, Mookherjee P, Ryan AM, Montine TJ et al (2010) Aberrant detergent‐insoluble excitatory amino acid transporter 2 accumulates in Alzheimer disease. J Neuropathol Exp Neurol 69:667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475. Wu Z, Guo Z, Gearing M, Chen G (2014) Tonic inhibition in dentate gyrus impairs long‐term potentiation and memory in an Alzheimer's [corrected] disease model. Nat Commun 5:4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476. Wyss‐Coray T, Loike D, Brionne TC, Lu E, Anankov R, Yan F et al (2003) Adult mouse astrocytes degrade amyloid‐beta in vitro and in situ. Nat Med 9:453–457. [DOI] [PubMed] [Google Scholar]
- 477. Xiao Q, Yan P, Ma X, Liu H, Perez R, Zhu A et al (2014) Enhancing astrocytic lysosome biogenesis facilitates Aβ clearance and attenuates amyloid plaque pathogenesis. J Neurosci 34:9607–9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478. Xie Z, Morgan TE, Rozovsky I, Finch CE (2003) Aging and glial responses to lipopolysaccharide in vitro: greater induction of IL‐1 and IL‐6, but smaller induction of neurotoxicity. Exp Neurol 182:135–141. [DOI] [PubMed] [Google Scholar]
- 479. Yamada T, McGeer PL, McGeer EG (1992) Appearance of paired nucleated, Tau‐positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci Lett 135:99–102. [DOI] [PubMed] [Google Scholar]
- 480. Yan P, Hu X, Song H, Yin K, Bateman RJ, Cirrito JR et al (2006) Matrix metalloproteinase‐9 degrades amyloid‐beta fibrils in vitro and compact plaques in situ. J Biol Chem 281:24566–24574. [DOI] [PubMed] [Google Scholar]
- 481. Yang Z, Wang K (2015) Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci 38:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482. Yankner BA, Lu T, Loerch P (2008) The aging brain. Annu Rev Pathol 3:41–66. [DOI] [PubMed] [Google Scholar]
- 483. Yata K, Oikawa S, Sasaki R, Shindo A, Yang R, Murata M et al (2011) Astrocytic neuroprotection through induction of cytoprotective molecules; a proteomic analysis of mutant P301S tau‐transgenic mouse. Brain Res 1410:12–23. [DOI] [PubMed] [Google Scholar]
- 484. Yeh CY, Vadhwana B, Verkhratsky A, Rodríguez JJ (2011) Early astrocytic atrophy in the entorhinal cortex of a triple transgenic animal model of Alzheimer's disease. ASN Neuro 3:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485. Yin KJ, Cirrito JR, Yan P, Hu X, Xiao Q, Pan X et al (2006) Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid‐beta peptide catabolism. J Neurosci 26:10939–10948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486. Yoshiyama Y, Zhang B, Bruce J, Trojanowski JQ, Lee VM (2003) Reduction of detyrosinated microtubules and Golgi fragmentation are linked to tau‐induced degeneration in astrocytes. J Neurosci 23:10662–10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487. Zador Z, Stiver S, Wang V, Manley GT (2009) Role of aquaporin‐4 in cerebral edema and stroke. Handb Exp Pharmacol 190:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488. Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32:6391–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489. Zarranz JJ, Ferrer I, Lezcano E, Forcadas MI, Eizaguirre B, Atarés B et al (2005) A novel mutation (K317M) in the MAPT gene causes FTDP and motor neuron disease. Neurology 64:1578–1585. [DOI] [PubMed] [Google Scholar]
- 490. Zeisel A, Muñoz‐Manchado AB, Codeluppi S, Lönnerberg P, La Manno G, Juréus A et al (2015) Brain structure. Cell types in the mouse cortex and hippocampus revealed by single‐cell RNA‐seq. Science 347:1138–1142. [DOI] [PubMed] [Google Scholar]
- 491. Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD et al (2016) Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences in mouse. Neuron 89:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492. Zhao J, O'Connor T, Vassar R (2011) The contribution of activated astrocytes to Aβ production: implications for Alzheimer's disease pathogenesis. J Neuroinflammation 8:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493. Zlokovic BV (2008) The blood‐brain barrier in health and chronic neurodegenerative disorders. Neuron 57:178–201. [DOI] [PubMed] [Google Scholar]
- 494. Zou J, Wang YX, Dou FF, Lü HZ, Ma ZW, Lu PH, Xu XM (2010) Glutamine synthetase down‐regulation reduces astrocyte protection against glutamate excitotoxicity to neurons. Neurochem Int 56:577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]


