Abstract
Nearsightedness, or myopia, is becoming more prevalent worldwide. The eye experiences dynamic growth throughout adolescence, but the etiopathogenesis of myopia progression is not fully understood. Myopia is associated with several pathologic eye conditions, leading to irreversible vision loss. Treatment for preventing myopia progression is reliant on effective screening and initiating treatment early in life. This article will review risk factors for myopia progression and discuss treatment strategies that are most effective in halting its spread.
Background
One of the more staggering trends in ophthalmic care has been the rapid global rise of myopia, also called nearsightedness.1 The prevalence of myopia exceeds 28% globally, and there are projections that approximately half of the world’s population, or five billion people, will have some degree of myopia by 2050.2 With the increase of myopia worldwide, it is concerning that an estimated 10% of the world’s population, or more than 700 million people, do not receive adequate therapy.3 Treatment options for myopia include spectacle correction, contact lenses, or refractive surgery to correct a patient’s refractive error and improve distance vision.
Individuals who are myopic, especially those who are highly myopic with a spherical equivalent of −6.00D or more, have more than just the need for spectacle correction as they may also have chronic pathologic visual changes that include retinal detachment, myopic macular degeneration, glaucoma, amblyopia, and early-onset cataract. Highly myopic individuals are 20 times more likely to have a retinal detachment in their lifetime than an emmetropic individual.6 Pathologic myopia, defined as having high myopia with irreversible retinal atrophy, affects approximately 1% of Caucasians and 1–3% of Asians.4 An increased emphasis on slowing myopic progression with effective childhood screening has been adopted by clinicians in primary care, pediatrics, and ophthalmology.5 Understanding the risk factors for myopia progression and the mechanisms controlling the eye’s axial growth are key to developing therapies for myopia.
The Goal of Emmetropization?
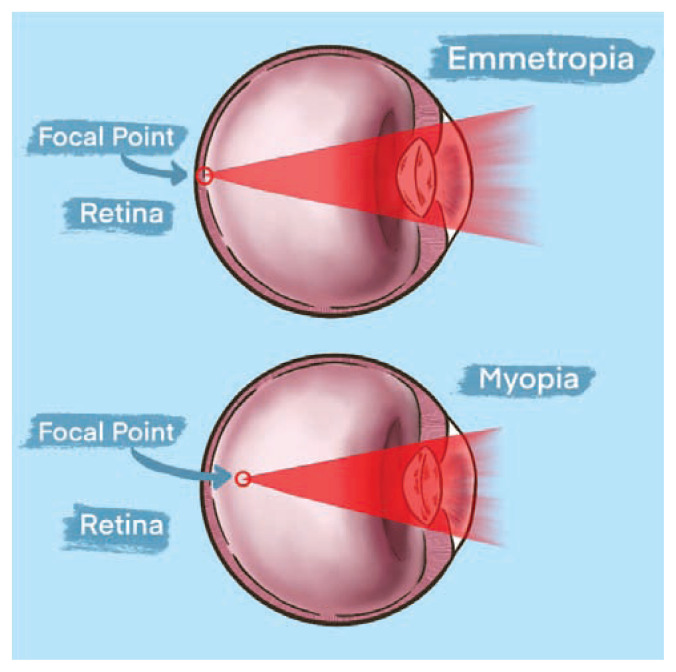
An eye that is focused clearly in the distance (20 feet or >) without correction is said to be emmetropic, and the regulatory process that achieves this goal is called emmetropization. In early development, the globe commonly grows spherically in all directions prior to beginning axial elongation with respect to the corneal and retinal axis.6 The eye experiences dynamic growth throughout childhood and early adulthood. Myopia results in a visual focus landing in front of the retina, caused by either refractive or axial changes to the eye. A schematic showing the differences in focal points between emmetropia and myopia is shown in Figure 1.
Figure 1.
Emmetropia versus myopia. When viewing an object at distance, an emmetropic eye is able to focus a clear image on the retinal surface. In myopic eyes, the image is focused in front of the retinal surface.
This overshoot in eye power or growth, influenced by both environmental stimuli and genetic factors, is referred to as “myopization.”7 Refractive myopia is defined as having abnormally strong dioptric (D) power leading to a misplaced focused image in front of the retina. Axial myopia, the more common cause of myopia, also leads to a focused image in front of the retina due to excessive length of the eye. Therefore, the majority of preventative myopia therapy targets slowing the eye’s axial growth during development.
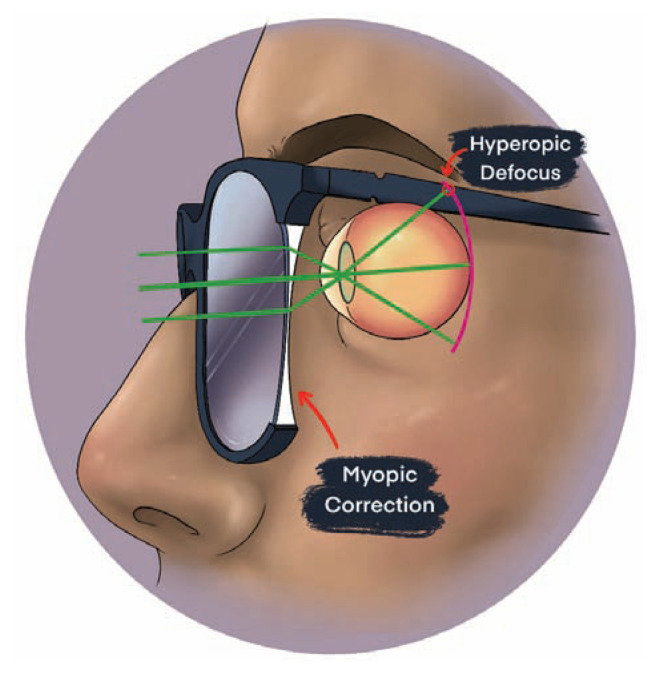
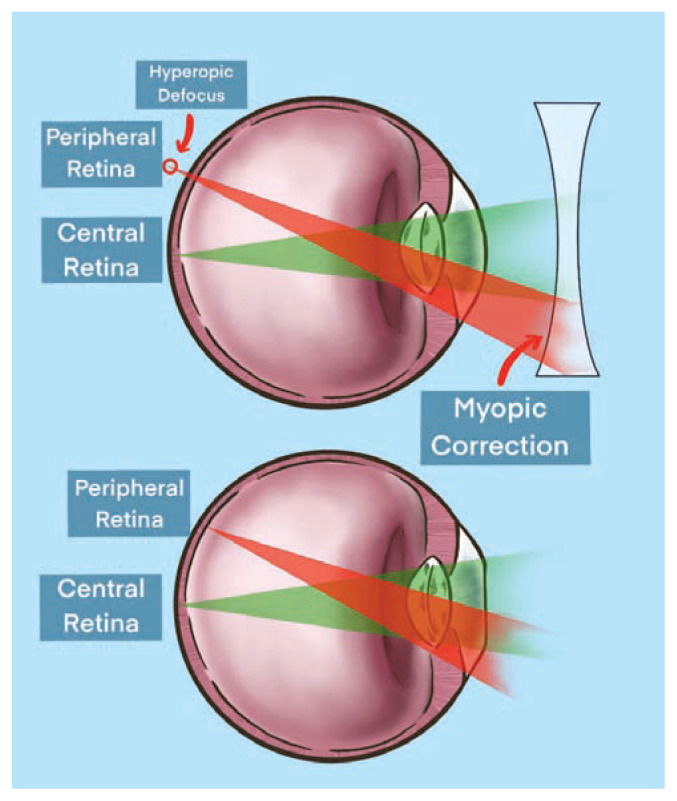
There is evidence to support that myopia represents a dysregulated state influenced by external visual cues. Traditional negative-lens spectacle correction for myopia, has been shown to significantly alter ocular growth and refractive power in children.8 When myopia correction is given to a patient, the eye’s focal point at distance is adjusted more posteriorly in order to land onto the retinal surface and provide clarity for distance viewing. This myopic correction of the central retina incidentally creates hyperopic defocus in mid-peripheral retina with a focal point lying behind the retina as shown in Figure 2.
Figure 2.
Myopic correction creating hyperopic defocus in the mid-peripheral retina. Hyperopic defocus is theorized as a primary driver for myopic progression.
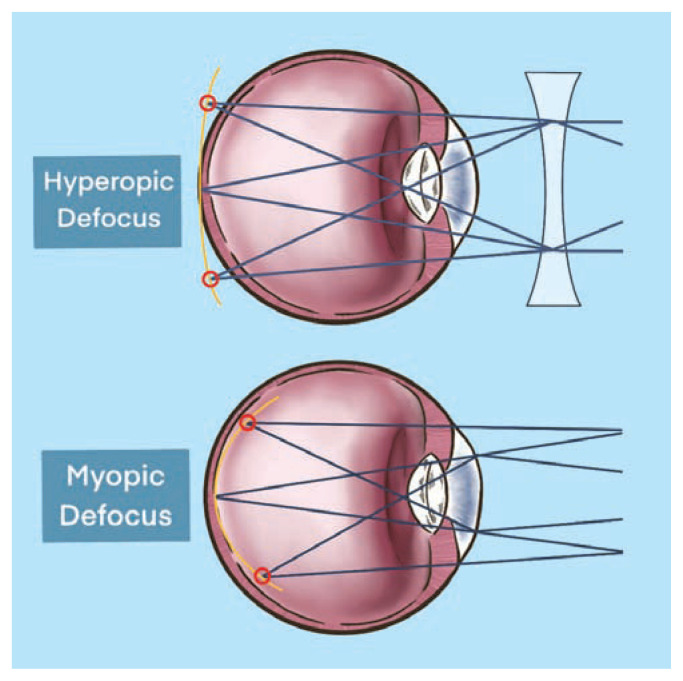
Hyperopic defocus in the mid-peripheral retina driven by negative lens-induced myopia in turn leads to posterior scleral remodeling, choroidal thinning, and eye lengthening. The mid-peripheral retina has been studied as one of the important locations for the visually-guided growth of the eye based on peripheral defocus leading to axial elongation in animal models.9 The mechanisms by which the mid-peripheral retina modulates axial growth is incompletely understood. Treatment strategies aimed at creating myopic defocus at the mid-peripheral retina, meaning to have the eye’s focal point rest in front of the retina, have proven successful in reducing myopic progression.5,9,23,37–42,45–46 A comparison of hyperopic defocus versus myopic defocus is shown in Figure 3.
Figure 3.
Hyperopic defocus versus myopia defocus. Hyperopic defocus with focal point resting behind mid-peripheral retina versus myopic defocus with focal point resting in front of mid-peripheral retina.
In addition to hyperopic peripheral defocus, other risk factors contributing to increased axial elongation include genetic factors, less outdoor time, weakened accommodation, and increased amount of near work.10
Risk factors
Age
Younger age at onset of myopia is the highest independent risk factor for myopia progression. Patients who have at least −1.25D of myopia, particularly if they are aged 6–7 years old, are at higher risk for faster progression when compared to older children.11 The COMET study involved 426 ethnically diverse children with a baseline myopic spherical equivalent of −1.25D to −4.50D.12 In this study, the mean age of myopia stabilization was 15.61 years. At the time of stabilization, the average amount of myopia was −4.87D. Children who showed myopic progression at age six to seven, had on average 1.70D more myopia at stabilization than children who showed similar progression starting at age eight and older. The vast majority of study participants (96%) received myopia stabilization by age 24.12 The age to initiate treatment for myopia progression is still unknown, although the results of the COMET study helped show that starting preventative myopic therapy at a younger age may be advantageous. There are currently ongoing studies exploring the treatment of pre-myopic children with known risk factors for myopic progression in early childhood.13
Ethnicity
The prevalence of myopia worldwide varies greatly based on regional and ethnic differences. The prevalence of myopia exceeds 80% in some regions in East Asia, and there is a two-times higher prevalence of myopia in East Asians than in Caucasians of similar age.14 In comparison, the estimated prevalence of myopia in studied African populations in 2020 <10%.2 In the COMET study, the African American participant cohort had an earlier average age of myopia stabilization (13.82 years) and consequently the least amount of myopia at stabilization (−4.36D) when compared to other ethnic groups.14
Environmental Factors
Increased amount of time spent outdoors has been shown to be protective against the development of incident myopia.15 Animal studies suggest ultraviolet light may inhibit axial elongation by stabilizing the sclera.16 In a recent meta-analysis review of 25 papers investigating the relationship between outdoor time and myopia, it was shown that increased outdoor time was protective in the incidence of myopia but not effective in slowing the progression of myopia in eyes who were already myopic. Based on the study’s dose-response analysis, spending 76 minutes a day outdoors resulted in a 50% incidence reduction of myopia when compared to control eyes.17
The association between near work and incidence of juvenile-onset myopia has been under investigation for several years. The CLEERE longitudinal study compared visual activities between emmetropic and incident myopic subjects.18 This study concluded that there were little differences in the amount of near work between both groups before myopia onset. Following myopia onset, it was hypothesized that myopic patients adopted more near-work activities based off of their refractive error.18 A meta-analysis of 27 studies found that more time spent doing near work increased an individual’s odds of myopia. Specifically, the odds of myopia increased 2% for every one diopter-hour more near work performed each week.19
Parental Myopia
Several studies have investigated the association between parental myopia and their child’s risk for developing myopia.20–21, 22 A recent meta-analysis of 16 studies reviewing the link between parental myopia and juvenile myopia reported a statistically significant increased risk of myopia in children having one myopic parent (OR = 1.87) and two myopic parents (OR = 2.40).23 Environmental influences, such as children adopting behavioral traits similar to their myopic parents, have been evaluated as additional risk factors for myopic development. Several genetic loci have been linked to ocular axial elongation.24 The complex interaction between an individual’s genes and behavioral habits with the patient’s risk factor for myopic progression requires further investigation.
Preventative Therapies
Preventative therapy for myopia progression currently has three aims: to screen patients with risk factors for myopia progression, to stabilize myopic progression, and to provide optical correction for healthy visual development. There are both medical and optical therapies available which meet that preventative goal, while surgical options for myopia control remain under investigation. In recent literature, scleral crosslinking using topical riboflavin and UV-A light have shown promising results in reducing axial elongation in animal models.25 Posterior scleral contraction surgery with scleral grafts have successfully reduced myopic and axial length progression in limited human studies.26 The COMET study proposed that using progressive addition bifocal lenses (PALs) in children could help reduce myopic progression by treating high accommodative lag seen in myopic patients with near-vision tasks.12 Unfortunately, myopic progression in the PAL-treated group was only reduced by a non-significant 0.20D when compared to single-vision corrective lens (control group) over a 3-year span.27 Despite the insignificant reduction in myopic progression, the COMET study proved that optical therapies can aid in controlling axial growth. The following contemporary medical and optical treatments have shown superiority in controlling myopic progression.
Atropine Ophthalmic Drops
Atropine reduces accommodation and increases pupillary diameter. In early studies theorizing the link between accommodation and myopia, low concentration atropine was used to determine if blocking accommodation could help control myopic progression.28 Several experimental trials have since shown that there is a non-accommodative mechanism in the control of myopia and the mechanism of action of atropine is poorly understood.29–30 Despite this lack of clarity about how atropine therapy works, comparative studies exploring treatment options in controlling myopic progression have proven the efficacy and superiority of atropine versus other alternative treatments.31 In the ATOM trials, various low-dose concentrations of topical atropine proved to be beneficial in slowing axial elongation and myopia progression in Asian children with early-onset myopia.32–33 The ATOM1 studied the effect of 1% atropine, while the ATOM2 study stratified treated subjects into receiving 0.01%, 0.1%, and 0.5%. In the initial two-year treatment period in the ATOM2 study, higher concentrations of atropine were more effective in controlling myopia progression, with an average progression of −0.49D, −0.38D, and −0.30D in the 0.01%, 0.1%, and 0.5% concentration groups, respectively. In the following one-year washout period there was less rebound myopic progression in the 0.01% atropine cohort.34 Further studies have shown using 0.01% atropine proved more effective in slowing myopic progression, prevented less rebound myopia after cessation of treatment, and reduced visual side effects.35
The more recent LAMP study compared lower atropine concentrations of 0.01%, 0.025%, and 0.05% on myopia progression.36 Using a similar patient demographic as the ATOM study, the LAMP study found that the 0.05% atropine was doubly more efficacious as 0.01% atropine, showing −1.12D, −0.85D, and −0.55D of myopic progression in a two-year treatment period in the 0.01%, 0.025%, and 0.05% concentration groups, respectively.
Side-effects for atropine therapy include, but are not limited to, photophobia, poor near visual acuity, increased pupillary diameter, and headache.37 Adverse effects to atropine therapy are concentration-dependent. For example, the incidence of photophobia is reported as 6.3% in 0.01% atropine therapy as compared to 43.1% in doses greater than 0.5%.38 There may be additional side-effects of long-term administration of antimuscarinic agents on ocular tissue that have not been reported.39 The side-effect profile of atropine should be considered when monitoring compliance to atropine therapy in treated patients.
Orthokeratology
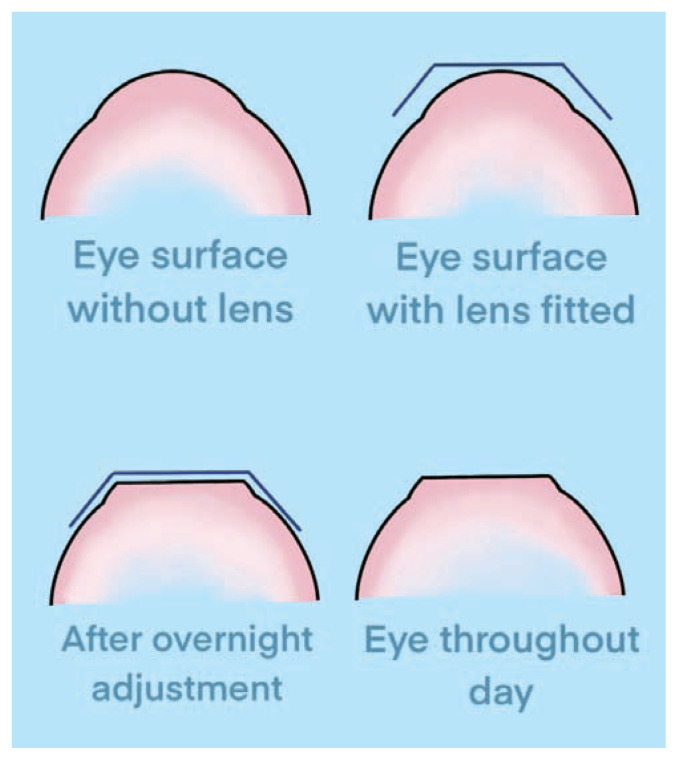
Orthokeratology is the use of an overnight rigid gas permeable (RGP) contact lens for the treatment of low- to moderate-myopia. During overnight wear remodeling of the corneal epithelial surface into a flatter, more oblate and less powerful refractive surface occurs and creates a transient reduction in myopia during the day that lasts until the cornea returns to its natural shape. Following central corneal flattening the focal point of the eye is shifted onto the retinal surface. Orthokeratology corneal remodeling is shown in Figure 4.
Figure 4.
Orthokeratology remodeling. Orthokeratology RGP lens temporary remodeling of the corneal epithelium. The base curve of the central cornea is flattened, leading to a reduction in corneal power. This change allows for a transient reduction in myopia for the user.
Case studies investigating children with myopia who wore an orthokeratology lens in one eye with no correction in the fellow eye showed axial length reduction in the treated eyes when compared to control eyes.40–41 The ROMIO study enrolled 102 myopic patients aged 6 to 10 years to be in orthokeratology lenses or single-vision glasses for a two-year study period.42 On average, the orthokeratology-treated group had slower increase in axial elongation by approximately 43% compared to the single vision-glasses group. A recent meta-analysis reviewing 13 prospective randomized clinical trials found that orthokeratology was effective in reducing axial elongation, on average, by 50% over a two-year period.43 In addition to temporarily flattening the central cornea, orthokeratology also changes the peripheral refractive status of the cornea resulting in less hyperopic defocus of the peripheral retina. This is hypothesized to be a tool in helping control myopic progression as shown in Figure 5.
Figure 5.
Traditional myopic correction versus orthokeratology correction. Traditional negative-lens myopia correction stimulating hyperopic defocus (top eye) versus Orthokeratology-treated eye reducing hyperopic peripheral defocus (bottom eye).
The rate of rebound myopia and axial elongation after discontinuation of orthokeratology treatment is underreported in orthokeratology literature.44
The safety profile of orthokeratology is reassuring; however, the risk of microbial keratitis with overnight wear or improper handling leading to contamination of a contact lens in children can be visually devastating.45 There is a higher risk of corneal infection with orthokeratology-wear with improper lens or hand hygiene, using expired contact solution, and exposure to a nonsterile solution.46 Other possible adverse effects of orthokeratology include contact lens irritation, dry eye, and corneal epithelial iron deposition.
Contact Lenses (MiSight)
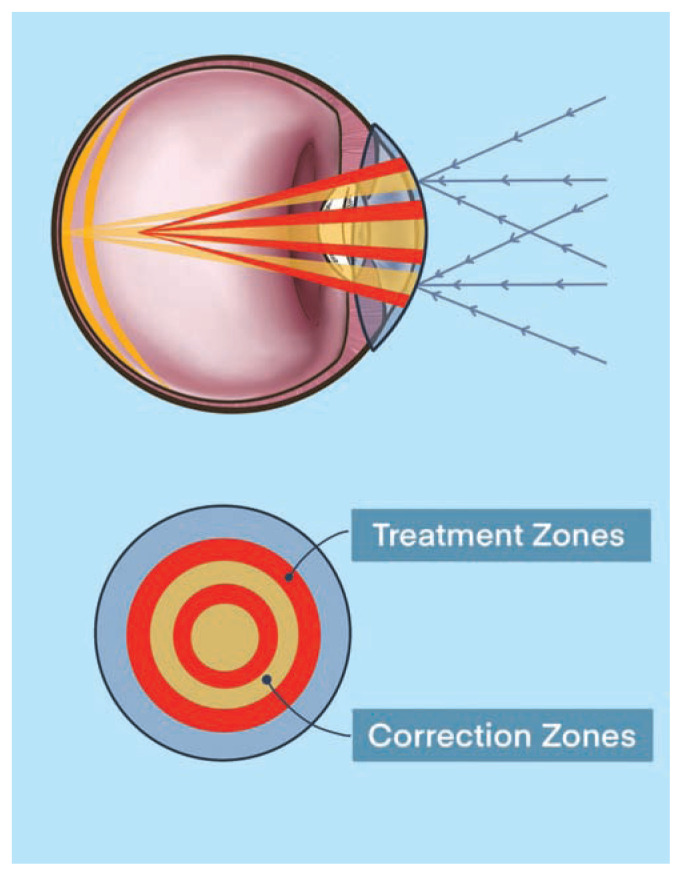
Over the past decade, multifocal, aspheric optics, and bifocal contact lenses have all shown modest potential in the treatment for myopia.47 In 2019, the FDA approved MiSight as the first contact lens available in the treatment for myopia progression.48 The MiSight lens design has “dual-focus optics” in which there is are two zones of myopia correction for all gaze positions and two neighboring concentric treatment zones to eliminate peripheral hyperopic defocus.49 This novel lens design ensures for proper distance visual acuity and normal accommodation for near work in the central corrective zone while simultaneously eliminating hyperopic defocus with plus-powered peripheral rings in the peripheral corrective zone. The MiSight design and optical schematic is shown in Figure 6.
Figure 6.
MiSight dual-focus optical design. The correction zones (yellow) allow for clear distance viewing for myopia. The treatment zones (red) have higher add-powers to stimulate myopic defocus.
In comparison to other soft contact lenses, including bifocal contact lenses, used in the treatment for myopia control, MiSight has shown superiority in slowing myopic progression and slowing axial elongation. In a three-year randomized double-masked clinical trial comparing the MiSight lens to control lens, MiSight showed less myopic progression by 0.40D, 0.54D, and 0.73D at 12 months, 24 months, and 36 months, respectively.50 Additionally, there was an average of 0.32mm less axial elongation in the MiSight treated group.
Similar risks apply to MiSight treatment for preventing myopia as orthokeratology lenses, including microbial keratitis and noncompliance with lens maintenance or usage. The preliminary safety profile of MiSight lenses is encouraging and further long-term studies are needed to determine the viability of this treatment for myopia control.44
Multifocal Contact Lenses
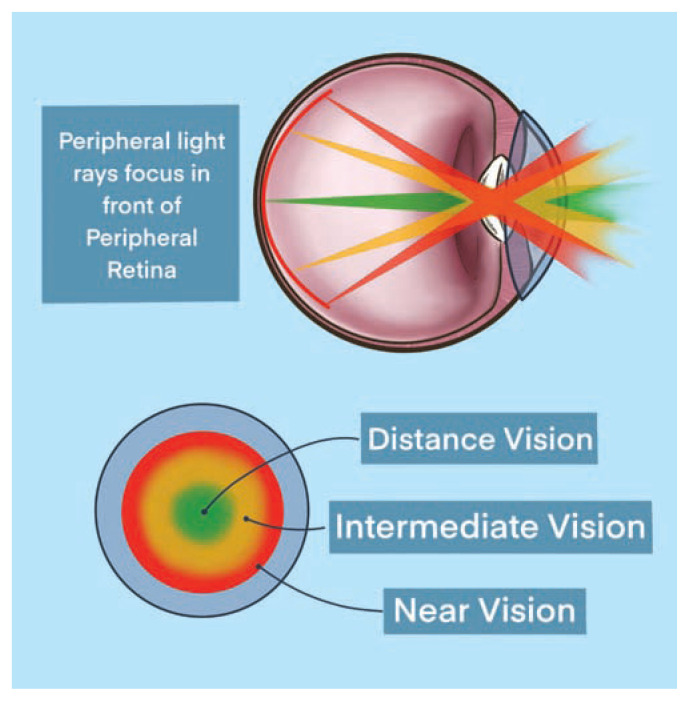
There are several multifocal contact lens designs, each engineered to provide a spectrum of clear vision for both near- and distance-vision tasks. With the multifocal concentric ring design, the center circle of the lens provides distance clarity with neighboring concentric rings offering intermediate and near clarity in a stepwise fashion dependent on the add-power of the respective lens. An example of this contact lens design is shown in Figure 7.
Figure 7.
Multifocal concentric ring contact lens design. Central vision correction followed by concentric rings helping with intermediate- and near-vision.
Recently, the BLINK trial aimed to determine whether soft concentric multifocal contact lenses can slow myopic progression in children and whether the strength of the add-power in the multifocal lens was of importance.51 Similar to MiSight, the central tenet in the BLINK study was using peripheral add-power in the multifocal lens to reduce hyperopic defocus to control axial growth. Over a 3-year investigative period using an ethnically diverse American study population, high add-power (+2.50D) slowed myopic progression by 0.45D and reduced axial length by 0.23mm compared to single-vision contact lenses (control group).
Conclusions
Several pathologic eye conditions are associated with myopia, many of which are related to the eye’s dysregulated axial lengthening. Preventative therapies for myopia progression are aimed at screening at-risk patients at a young age in order to halt axial growth, in return reducing one’s degree of myopia.
Successful treatments for reducing myopia progression have targeted reducing the amount of hyperopic defocus, in which light focused behind the peripheral retina. This article described therapeutic regimens – orthokeratology, atropine ophthalmic drops, multifocal contact lenses, and dual-focus optical contact lenses – which are commonly used and have shown success in treating myopia progression.
Understanding myopia and selecting patients who are at high-risk for myopic progression for treatment is a shared responsibility by primary care providers, opticians, and ophthalmologists. As we continue to move forward with newer treatments and more sophisticated screening, there is hope for a reduction, or reversal, of this optical pandemic.
Acknowledgment
We thank Ashley Akins for her artistic design on this manuscript and Geetha Davis, MD, for acting as a non-author peer reviewer.
Footnotes
James R. Landreneau, MD, (above), Nathan P. Hesemann, MD, and Maggie A. Cardonell, MD, are at the University of Missouri -Columbia School of Medicine and Mason Eye Institute, Columbia, Missouri.
Editor’s Addendum
As the Journal went to press another article was published about myopia during the pandemic. It found major increases in children age 6 to 13 years during COVID-19 “owing to substantially decreased time spent outdoors and increased screen time at home.” Interested readers are referred to: Wang J et al. Progression of Myopia in School-Aged Children After COVID-19 Home Confinement. JAMA Ophthalmol. 2021:139(3):293–300
Disclosure
None reported.
Reference
- 1.Foster PJ, Jiang Y. Epidemiology of myopia. Eye (Lond) 2014;28(2):202–208. doi: 10.1038/eye.2013.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holden BA, Fricke TR, Wilson DA, et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Fricke TR, Holden BA, Wilson DA, et al. Global cost of correcting vision impairment from uncorrected refractive error. Bull World Health Organ. 2012;90(10):728–738. doi: 10.2471/BLT.12.104034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhakal R, Goud A, Narayanan R, Verkicharla PK. Patterns of posterior ocular complications in myopic eyes of Indian population. Sci Rep. 2018;8(1):13700. doi: 10.1038/s41598-018-29536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonas DE, Amick HR, Wallace IF, et al. Vision Screening in Children Aged 6 Months to 5 Years: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2017;318(9):845–858. doi: 10.1001/jama.2017.9900. [DOI] [PubMed] [Google Scholar]
- 6.Wu PC, Chuang MN, Choi J, et al. Update in myopia and treatment strategy of atropine use in myopia control. Eye (Lond) 2019;33(1):3–13. doi: 10.1038/s41433-018-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia [published correction appears in Neuron. 2012 Apr 12; 74(1)207] Neuron. 2004;43(4):447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Smith EL., 3rd Optical treatment strategies to slow myopia progression: effects of the visual extent of the optical treatment zone. Exp Eye Res. 2013;114:77–88. doi: 10.1016/j.exer.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore KE, Benoit JS, Berntsen DA. Spherical Soft Contact Lens Designs and Peripheral Defocus in Myopic. Eyes Optom Vis Sci. 2017;94(3):370–379. doi: 10.1097/OPX.0000000000001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou W, Norton TT, Hyman L, Gwiazda J COMET Group. Axial Elongation in Myopic Children and its Association With Myopia Progression in the Correction of Myopia Evaluation Trial. Eye Contact Lens. 2018;44(4):248–259. doi: 10.1097/ICL.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyman L, Gwiazda J, Hussein M, et al. Relationship of age, sex, and ethnicity with myopia progression and axial elongation in the correction of myopia evaluation trial. Arch Ophthalmol. 2005;123(7):977–987. doi: 10.1001/archopht.123.7.977. [DOI] [PubMed] [Google Scholar]
- 12.Group COMET. Myopia stabilization and associated factors among participants in the Correction of Myopia Evaluation Trial (COMET) Invest Ophthalmol Vis Sci. 2013;54(13):7871–7884. doi: 10.1167/iovs.13-12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chia A, et al. The Use of Atropine 0, 01% in the Prevention and Control of Myopia (ATOM3) https://clinicaltrials.gov/ct2/show/NCT03140358.
- 14.Rudnicka AR, Kapetanakis VV, Wathern AK, et al. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention. Br J Ophthalmol. 2016;100(7):882–890. doi: 10.1136/bjophthalmol-2015-307724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherwin JC, Reacher MH, Keogh RH, Khawaja AP, Mackey DA, Foster PJ. The association between time spent outdoors and myopia in children and adolescents: a systematic review and meta–analysis. Ophthalmology. 2012;119(10):2141–2151. doi: 10.1016/j.ophtha.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Wu M, Zhang L, Liu H, Zhang L, He J. Riboflavin and ultraviolet A irradiation for the prevention of progressive myopia in a guinea pig model Exp Eye Res 20171651–6. [DOI] [PubMed] [Google Scholar]
- 17.Xiong S, Sankaridurg P, Naduvilath T, et al. Time spent in outdoor activities in relation to myopia prevention and control: a meta-analysis and systematic review. Acta Ophthalmol. 2017;95(6):551–566. doi: 10.1111/aos.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones-Jordan LA, Mitchell GL, Cotter SA, et al. Visual activity before and after the onset of juvenile myopia. Invest Ophthalmol Vis Sci. 2011;52(3):1841–1850. doi: 10.1167/iovs.09-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang HM, Chang DS, Wu PC. The Association between Near Work Activities and Myopia in Children-A Systematic Review and Meta-Analysis. PLoS One. 2015;10(10):e0140419. doi: 10.1371/journal.pone.0140419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones-Jordan LA, Sinnott LT, Manny RE, et al. Early childhood refractive error and parental history of myopia as predictors of myopia. Invest Ophthalmol Vis Sci. 2010;51(1):115–121. doi: 10.1167/iovs.08-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pacella R, McLellan J, Grice K, Del Bono EA, Wiggs JL, Gwiazda JE. Role of genetic factors in the etiology of juvenile-onset myopia based on a longitudinal study of refractive error. Optom Vis Sci. 1999;76(6):381–386. doi: 10.1097/00006324-199906000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Kurtz D, Hyman L, Gwiazda JE, et al. Role of parental myopia in the progression of myopia and its interaction with treatment in COMET children. Invest Ophthalmol Vis Sci. 2007;48(2):562–570. doi: 10.1167/iovs.06-0408. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Qu X, Zhou X. Association between parental myopia and the risk of myopia in a child. Exp Ther Med. 2015;9(6):2420–2428. doi: 10.3892/etm.2015.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wojciechowski R. Nature and nurture: the complex genetics of myopia and refractive error. Clin Genet. 2011;79(4):301–320. doi: 10.1111/j.1399-0004.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dotan A, Kremer I, Livnat T, Zigler A, Weinberger D, Bourla D. Scleral cross-linking using riboflavin and ultraviolet-a radiation for prevention of progressive myopia in a rabbit model. Exp Eye Res. 2014;127:190–195. doi: 10.1016/j.exer.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Su Y, Pan A, Wu Y, Zhu S, Zheng L, Xue A. The efficacy of posterior scleral contraction in controlling high myopia in young people Am J Transl Res 201810113628–3634. [PMC free article] [PubMed] [Google Scholar]
- 27.Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44(4):1492–1500. doi: 10.1167/iovs.02-0816. [DOI] [PubMed] [Google Scholar]
- 28.Donders F. On the Anomalies of accommodation and refraction of the eye. London: The New Sydenham Society; 1864. [Google Scholar]
- 29.Raviola E, Wiesel TN. Neural control of eye growth and experimental myopia in primates. Ciba Found Symp. 1990;155:22–44. doi: 10.1002/9780470514023.ch3. [DOI] [PubMed] [Google Scholar]
- 30.McBrien NA, Moghaddam HO, New R, Williams LR. Experimental myopia in a diurnal mammal (Sciurus carolinensis) with no accommodative ability. J Physiol. 1993;469:427–441. doi: 10.1113/jphysiol.1993.sp019821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J, Wen D, Wang Q, et al. Efficacy Comparison of 16 Interventions for Myopia Control in Children: A Network Meta-analysis. Ophthalmology. 2016;123(4):697–708. doi: 10.1016/j.ophtha.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285–2291. doi: 10.1016/j.ophtha.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 33.Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2) Ophthalmology. 2012;119(2):347–354. doi: 10.1016/j.ophtha.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 34.Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5% Am J Ophthalmol. 2014;157(2):451–457e1. doi: 10.1016/j.ajo.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 35.Chia A, Lu QS, Tan D. Five-Year Clinical Trial on Atropine for the Treatment of Myopia 2: Myopia Control with Atropine 0.01% Eyedrops. Ophthalmology. 2016;123(2):391–399. doi: 10.1016/j.ophtha.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Yam JC, Li FF, Zhang X, et al. Two-Year Clinical Trial of the Low-Concentration Atropine for Myopia Progression (LAMP) Study: Phase 2 Report. Ophthalmology. 2020;127(7):910–919. doi: 10.1016/j.ophtha.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Tan D, Tay SA, Loh KL, Chia A. Topical Atropine in the Control of Myopia. Asia Pac J Ophthalmol (Phila) 2016;5(6):424–428. doi: 10.1097/APO.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 38.Gong Q, Janowski M, Luo M, et al. Efficacy and Adverse Effects of Atropine in Childhood Myopia: A Meta-analysis. JAMA Ophthalmol. 2017;135(6):624–630. doi: 10.1001/jamaophthalmol.2017.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anstice NS, Phillips JR. Effect of dual-focus soft contact lens wear on axial myopia progression in children. Ophthalmology. 2011;118(6):1152–1161. doi: 10.1016/j.ophtha.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 40.Cheung SW, Cho P, Fan D. Asymmetrical increase in axial length in the two eyes of a monocular orthokeratology patient. Optom Vis Sci. 2004;81(9):653–656. doi: 10.1097/01.opx.0000144742.57847.b1. [DOI] [PubMed] [Google Scholar]
- 41.Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res. 2005;30(1):71–80. doi: 10.1080/02713680590907256. [DOI] [PubMed] [Google Scholar]
- 42.Cho P, Cheung SW. Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci. 2012;53(11):7077–7085. doi: 10.1167/iovs.12-10565. [DOI] [PubMed] [Google Scholar]
- 43.VanderVeen DK, Kraker RT, Pineles SL, et al. Use of Orthokeratology for the Prevention of Myopic Progression in Children: A Report by the American Academy of Ophthalmology. Ophthalmology. 2019;126(4):623–636. doi: 10.1016/j.ophtha.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 44.Swarbrick HA, Alharbi A, Watt K, Lum E, Kang P. Myopia control during orthokeratology lens wear in children using a novel study design. Ophthalmology. 2015;122(3):620–630. doi: 10.1016/j.ophtha.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 45.Liu YM, Xie P. The Safety of Orthokeratology--A Systematic Review. Eye Contact Lens. 2016;42(1):35–42. doi: 10.1097/ICL.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jun J, Zhiwen B, Feifu W, Lili L, Fan L. Level of Compliance in Orthokeratology. Eye Contact Lens. 2018;44(5):330–334. doi: 10.1097/ICL.0000000000000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walline JJ, Gaume Giannoni A, Sinnott LT, et al. A Randomized Trial of Soft Multifocal Contact Lenses for Myopia Control: Baseline Data and Methods. Optom Vis Sci. 2017;94(9):856–866. doi: 10.1097/OPX.0000000000001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.FDA approves first contact lens indicated to slow the progression of nearsightedness in children. 2019. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-approves-first-contact-lens-indicated-slow-progression-nearsightedness-children.
- 49.Ruiz-Pomeda A, Pérez-Sánchez B, Valls I, Prieto-Garrido FL, Gutiérrez-Ortega R, Villa-Collar C. MiSight Assessment Study Spain (MASS) A 2-year randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. doi: 10.1007/s00417-018-3906-z. [DOI] [PubMed] [Google Scholar]
- 50.Chamberlain P, Peixoto-de-Matos SC, Logan NS, Ngo C, Jones D, Young G. A 3-year Randomized Clinical Trial of MiSight Lenses for Myopia Control. Optom Vis Sci. 2019;96(8):556–567. doi: 10.1097/OPX.0000000000001410. [DOI] [PubMed] [Google Scholar]
- 51.Walline JJ, Walker MK, Mutti DO, et al. Effect of High Add Power, Medium Add Power, or Single-Vision Contact Lenses on Myopia Progression in Children: The BLINK Randomized Clinical Trial. JAMA. 2020;324(6):571–580. doi: 10.1001/jama.2020.10834. [DOI] [PMC free article] [PubMed] [Google Scholar]