Abstract
Primary aldosteronism (PA) is associated with resistant hypertension and cardiovascular events. There are some limitations of current medical and surgical therapies for PA. To determine the efficacy and safety of catheter‐based adrenal artery ablation for treatment of PA patients who refused both surgery and medical therapy, we performed this prospective cohort study. Thirty‐six PA patients without apparent aldosteronoma were treated by adrenal artery ablation. Primary outcome was postoperative blood pressure and defined daily dose (DDD) of antihypertensive medications after adrenal ablation. Secondary outcome was biochemical success. We assessed outcomes based on Primary Aldosteronism Surgical Outcome (PASO) criteria. Adrenal CT scan, biochemical evaluation, adrenal artery ablation and adrenal venous sampling (AVS) were underwent. After adrenal ablation, complete clinical success (normotension without antihypertensive medication) was achieved in 9/36 (25.0%) patients and partial clinical success (reduction in blood pressure or less antihypertensive medication) in 13/36 (36.1%) patients. Complete biochemical success (correction of hypokalemia and normalization of aldosterone‐to‐renin ratio) was achieved in 16/36 (44.4%) patients. Office‐based and ambulatory blood pressures were reduced by 17/7 and 11/2 mmHg at 6 months after ablation, respectively. The plasma cortisol level in the ablation group decreased slightly, but no patient developed hypoadrenocorticism. Catheter‐based adrenal ablation appears to produce substantial and sustained blood pressure reduction and biochemical improvement, with only minor adverse events in PA patients without apparent aldosteronoma. This therapy could be an important supplement for current PA treatments.
Keywords: adrenal artery ablation, antihypertensive therapy, efficacy and safety, primary aldosteronism
1. INTRODUCTION
Primary aldosteronism (PA) is the main cause of secondary hypertension, affecting 5%‐15% of the general hypertensive population. 1 , 2 The prevalence of PA is 15%‐20% in patients with resistant hypertension. 3 Early diagnosis and treatment are of crucial importance because patients with PA are more susceptible to cardiovascular and cerebrovascular morbidity and mortality than blood pressure‐matched hypertensive patients. 4 , 5 , 6 Currently, mineralocorticoid receptor (MR) antagonists and laparoscopic adrenalectomy are the principal treatments for PA. However, recent studies showed that long‐term MR antagonist therapy did not reduce the risk of cardiometabolic events and death in PA patients with persistent renin suppression, 7 , 8 , 9 and high dose of MR antagonist likely causes side effects. 10 , 11 , 12 , 13 Laparoscopic adrenalectomy is recommended for patients with aldosterone‐producing adenoma (APA) or unilateral adrenal hyperplasia. 1 Incidence of atrial fibrillation, heart failure, chronic kidney disease, and all‐cause mortality is lower with adrenalectomy than with MR antagonist therapy. 7 , 8 , 9 , 14 , 15 During the past two decades, catheter‐based arterial embolization or computed tomography (CT)‐guided radiofrequency thermogenesis has been used for aldosteronomas treatment. 16 , 17 Although these procedures are claimed to be effective for treatment of aldosteronomas, the evidence comes mostly from case reports or small series. 18 , 19 Furthermore, it is unclear whether these procedures can be applied in PA patients without apparent aldosteronoma. In addition, some PA patients refuse surgery and are intolerant to the adverse effects of MR antagonists; others have persistence of PA after adrenelectomy, but respond poorly to MR antagonists. An alternative therapy is needed in such cases. We hypothesized that catheter‐based adrenal artery ablation could be a suitable alternative approach. To our knowledge, adrenal artery ablation has so far not been applied to treat patients without apparent aldosteronoma. This study aimed to evaluate the efficacy and safety of catheter‐based adrenal artery ablation in the treatment of PA patients who are reluctant to surgery and medication.
2. METHODS
2.1. Patients, intervention, and follow‐up
One thousand and twenty hypertensive patients above 30 years of age attending the hypertension center at Chongqing, China between January 2018 and December 2018 were approached for enrollment in this prospective study. Hypertensive patients were willing to participate and then hospitalized for further evaluation. Antihypertensive medications that affect the renin‐angiotensin‐aldosterone system were stopped for two weeks, diuretics and MR antagonists were withdrawn for at least 4 weeks before ARR testing. Hypertensive patients with mild hypertension were prescribed with verapamil and/or terazosin based on Endocrine Society Clinical Practice Guideline. 1 However, some patients with high‐grade hypertension continually administrated antihypertensive medications because of safety concerns. Patients with positive aldosterone‐to‐renin ratio (ARR > 20 ng/dL·ng/mL/h) underwent one of the following confirmatory tests: saline infusion test (2 L of isotonic saline administered within 4 hours) or captopril inhibition test (50 mg of captopril orally). Adrenal CT scan and adrenal venous sampling (AVS) were performed for subtype classification of the PA. The PA patients were counseled on the various treatment options, including surgery, medications, and adrenal artery ablation. Patients were eligible for adrenal ablation only if (a) they refused medication treatment due to intolerance of side effects; (b) they had no apparent aldosteronoma but had lateralization by AVS, and refused the adrenalectomy because of surgical risk; (c) patients had persistent PA after adrenalectomy, but did not appear adrenocortical insufficiency and failed to medication treatment. Exclusion criteria were (a) history of serious contrast agent allergy; (b) complication with severe liver diseases; (c) history of myocardial infarction and stent implantation within the past 3 months; (d) renal insufficiency, with serum creatinine >176 mmo/L; (e) pregnancy or lactation; (f) history of participation in another clinical trial in the past 3 months; or (g) any serious comorbidity.
AVS was performed in PA patients who desire to accept adrenal artery ablation. Traditionally, AVS has been performed via the femoral vein, with which the success rate of bilateral blood collection is reported to be about 31%‐82%. However, a study reported that the success rate of bilateral blood collection by AVS via the anterior cubital vein is 87.6%. 20 We therefore opted for the upper extremity route (median cubital vein or basilic vein). Successful sampling was determined by high selectivity index (cortisol in the adrenal vein/cortisol ≥ 2 subclavian vein without adrenocorticotropic hormone simulation). To correct for the dilution effect of the inferiorphrenic vein flow into the common phrenic trunk, the right and left adrenal vein aldosterone concentrations were divided by the respective cortisol concentrations. These cortisol‐corrected ratios were used to determine the aldosterone lateralization index. A lateralization index ≥ 2 was indicated the excessive unilateral aldosterone production based on an expert consensus on use of AVS for the subtyping of PA. 21 The effect of adrenal ablation was evaluated by repeat AVS in some patients who consented to the examination. Adrenal artery ablation was performed as described previously, 16 , 18 with some modifications. After local anesthesia at the puncture point, a 6F introducer sheath (10 cm; Cordis, USA) was inserted through the radial artery using a modified Seldinger catheterization technique. The sheath was heparinized (50 U/kg). Then, a 5F MPA1 diagnostic catheter (125 cm; Cook Medical lnc., Bloomington, IN, USA) was injected with a 0.035” HiWire angled hydrophilic guidewire(260 cm, Cook Medical, Bloomington, IN, USA), and iodinated contrast agent was injected at the level of T12–L1vertebrae. After confirming the adrenal artery on digital subtraction angiography, the 0.014” peripheral guidewire (300 cm, ASAHI INTECC, Japan) was used to cooperate with 1.7F‐2.1F microcatheter (eV3 Neurovascular, Inc.) to enter the adrenal artery. Under morphine or intravenous anesthesia, 1‐2 mlanhydrous ethanol was injected slowly through the microcatheter, taking care to avoid reflux of anhydrous ethanol into collateral vessels. Successful embolization of the supplying artery was indicated by retention of the contrast agent within the lumen of the supplying artery, with no forward flow. If some forward flow of contrast was detected, anhydrous ethanol was injected again (Figure S1). Blood pressure, electrocardiogram, and patient complaints were closely monitored throughout the procedure and treated as and when necessary. Patients were followed up at 1, 3, and 6 months after the procedure. At each follow‐up visit, patients underwent physical examination, blood pressure measurement, and blood biochemical analysis. Any adverse events were recorded. An independent data and safety monitoring group reviewed the results and serious adverse events. The study protocol was approved by the Institute Research Ethics Committee of Hospital. Informed consent was obtained from all patients before each procedure. This trial was registered with ClinicalTrials.gov, number NCT 03398785.
2.2. Outcomes and definitions
Primary outcome was clinical success of blood pressure reduction. Secondary outcome was biochemical success. Clinical and biochemical outcomes were assessed based on Primary Aldosteronism Surgical Outcome (PASO) criteria and were classified as complete success, partial success, or absent success. 22 Complete clinical success is defined as normotension without the aid of antihypertensive medication. Partial clinical success is defined as the same blood pressure as before treatment with less antihypertensive medication or a reduction in blood pressure with either the same amount or less antihypertensive medication. Absent clinical success is defined as unchanged or increased blood pressure with either the same amount or an increase in antihypertensive medication. Complete biochemical success is defined as correction of hypokalaemia (if present pre‐treatment) and normalization of the ARR; in patients with a raised ARR after treatment, aldosterone secretion should be suppressed in a confirmatory test. Partial biochemical success is defined as correction of hypokalaemia (if present pre‐treatment) and a raised ARR with one or both of the following (compared with pre‐treatment): ≥50% decrease in baseline plasma aldosterone concentration; or abnormal but improved post‐treatment confirmatory test result. Absent biochemical success is defined as persistent hypokalaemia (if present pre‐treatment) or persistent raised ARR, or both, with failure to suppress aldosterone secretion with a post‐treatment confirmatory test.
2.3. Statistical analysis
We assumed at least a different of 20 mmHg and a standard deviation (SD) of 18 mmHg of the change in systolic blood pressure from baseline to 6 months. A sample size of 28 patients is determined to detect the benefit of adrenal artery ablation. Assuming a dropout rate of 20%, a total sample of 34 patients were targeted. The final sample size was 36 patients. The Shapiro‐Wilk test was used to determine the normality of distribution of continuous variables. Normally distributed variables were summarized as means (±SD), and non‐normally distributed variables as medians (interquartile range). Categorical variables were summarized as percentages. The comparisons of clinical success, biochemical improvement, and anthropometric measurement were using 1‐way ANOVA with Games‐Howell multiple comparisons test or Kruskal‐Wallis 1‐way ANOVA (independent k‐samples) to analyze data with an abnormal distribution. The cortisol comparison between the baseline and at 6‐month follow‐up was done by using 2‐tailed unpaired Student t test. Statistical analysis was conducted using SPSS 13.0 (SPSS Inc., Chicago, IL, USA) or GraphPad Prism software, version 5.0 (Graph Pad software Inc., San Diego, CA, USA).Two‐sided P < .05 indicated statistical significance.
3. RESULTS
3.1. Baseline characteristics of the patients
One hundred and one PA patients were screened out from 1020 hypertension patients. Among them, 5 patients refused to participate in this study, 25 patients with APA were recommended for laparoscopic adrenalectomy, 34 patients desired to receive medication, and the remaining 36 patients opted to adrenal artery ablation (Figure 1). Finally, 32 patients who refused medication and surgery, and 4 patients with persistent PA after adrenalectomy and medication were treated with catheter‐based adrenal artery ablation. The baseline characteristics are summarized in Table 1.
FIGURE 1.
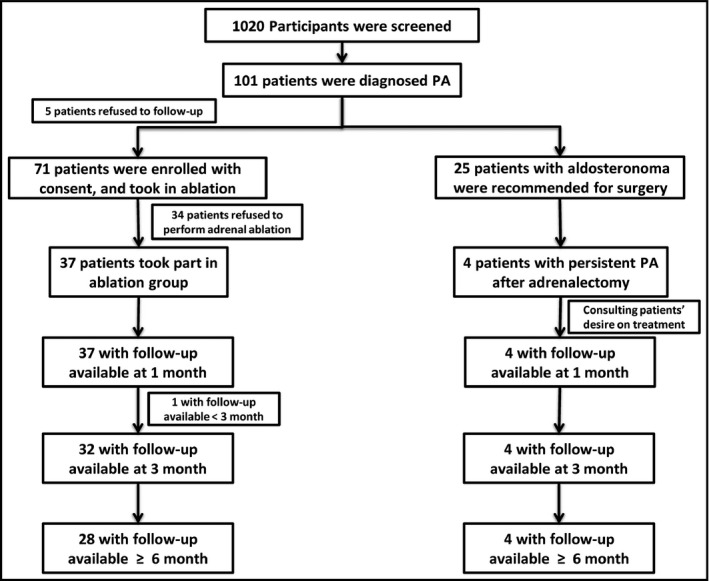
Flowchart of the study. Numbers at each follow‐up are those of patients who had attended each predefined visit at the time. Two patients lost to follow‐up at the visit time. Data from patients who had no less than 3 mo follow‐up were enrolled in the final analysis (n = 36)
TABLE 1.
Baseline characteristics of PA patients without apparent aldosteronoma
| Characteristic | (n = 36) |
|---|---|
| Age—y | 48.4 ± 12.5 |
| Male sex—no. (%) | 21 (58.3) |
| Body‐mass index—kg/m2 | 24.9 ± 3.26 |
| Fasting blood glucose— mmol/L | 5.44 ± 0.72 |
| Waist circumstance—cm | 87 ± 9 |
| Blood pressure—mmHg | |
| Systolic | 149 ± 22 |
| Diastolic | 91 ± 16 |
| Heart rate—beats/min | 80 ± 10 |
| Serum potassium—mmol/L | 3.63 ± 0.61 |
| Plasma aldosterone in supine position— ng/dL | 18.91 ± 3.85 |
| Plasma renin activity in supine position— ng/mL/h | 0.57 (0.17, 0.71) |
| Plasma aldosterone‐to‐renin ratio | 35.00 (26.00, 93.00) |
| Defined daily dose (DDD) | 1.27 (1.00, 1.93) |
| CT imaging features | |
| No change | 11(34.4) |
| Unilateral adrenal hyperplasia | 13 (40.6) |
| Bilateral adrenal hyperplasia | 8 (25.0) |
| Patients receiving (drug class) | |
| α‐1 blockers | 6 (16.7) |
| ACE inhibitors/ARBs | 13 (36.1) |
| β blocker | 5 (13.9) |
| Calcium‐channel blocker | 21 (58.3) |
| Diuretics | 4 (11.1) |
| TC—mmol/L | 4.12 ± 1.18 |
| TG—mmol/L | 2.07 ± 1.22 |
| HDL‐c—mmol/L | 1.08 ± 0.21 |
| LDL‐c—mmol/L | 2.79 ± 0.66 |
Data are mean ± SD, median with interquartile range, or n (%).The body‐mass index is the weight in kilograms divided by the square of the height in meters. The results of CT imaging did not include 4 patients who had PA recurrence after surgery.
Abbreviations: ACE, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; DDD, Defined daily dose; HDL‐c: high‐density lipid‐cholesterol; LDL‐c: low‐density lipid‐cholesterol; SD, standard deviation; TC, total cholesterol; TG, triglycerides.
3.2. Concordance between CT imaging and AVS
CT scan confirmed that these PA patients without apparent aldosteronoma had adrenal hyperplasia and/or nodules. AVS showed the lateralization of aldosterone production was as follows: (a) in 8 cases with bilateral hyperplasia, 6 cases of ipsilateral aldosterone hypersecretion and 2 cases of contralateral aldosterone hypersecretion; (b) in 13 cases of unilateral hyperplasia, 5 cases of ipsilateral aldosterone hypersecretion and 8 cases of contralateral aldosterone hypersecretion; and (c) in 11 cases with normal bilateral adrenals, 5 cases of bilateral aldosterone hypersecretion, and 6 cases of unilateral aldosterone hypersecretion (Figure 2). The rate of inconsistency between CT and AVS was 50% in the 32 cases, excluding 4 cases of persistent PA after adrenalectomy
FIGURE 2.
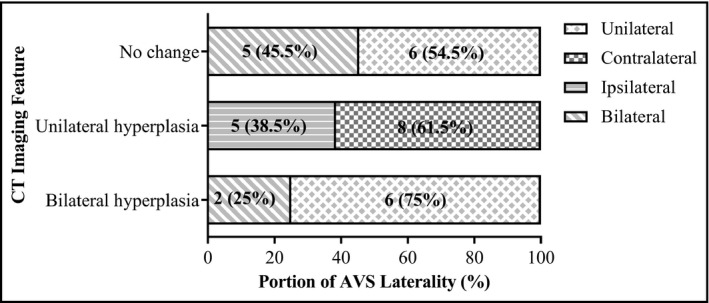
Concordance between CT imaging and AVS laterality. There were 11 patients with normal bilateral adrenals, 13 with unilateral hyperplasia, and 8 with bilateral hyperplasia. In the Figure, data are expressed as n(%). The four APA patients with recurrence PA after surgery were excluded from this analysis. For patients with either no change or bilateral hyperplasia, only AVS can distinguish the ipsilateral or contralateral laterality. APA, aldosterone‐producing adenoma; AVS, adrenal vein sampling; CT, computed tomography
3.3. Effect of adrenal artery ablation on PA
We decided to ablate the lateralized adrenal gland based on AVS. Clinical success in PA patients with ablation was shown in Table 2. After adrenal artery ablation, the reduction in systolic and diastolic blood pressures was 20 ± 17 and 16 ± 10 mmHg in PA patients with complete clinic success, respectively, and the reduction in systolic and diastolic blood pressures was 30 ± 20 and 14 ± 8 mmHg in PA patients with partial clinical success, respectively. The median reduction in office‐based and ambulatory blood pressures was 17/7 and 11/2 mmHg, respectively. The complete clinical success (discontinuation of antihypertensive medication) was achieved in 9/36 (25.0%) patients, and partial clinical success (improvement in blood pressure control) was achieved in 13/36 (36.1%) patients. The overall clinical success rate was 61.1% after adrenal artery ablation in patients. Biochemical improvement in PA patients with ablation was shown in Table 3. Increment of serum potassium was also seen in patients with partial clinical success (pre‐ and post‐ablation change, −0.66 ± 1.05). Reduction of ARR was seen in patients with partial clinical success (30.50 [12.45, 60.40]) in patients. Complete biochemical success was observed in 16/36 (44.4%) patients and partial biochemical success in 1/36 (2.8%) patients. Reduction of excessive aldosterone secretion on the ablation side was confirmed by AVS in the 6 patients who agreed to a repeat examination at 6 months (105.67 ± 64.44 vs 54.40 ± 36.23 ng/mL). Meanwhile, the DDD reduced in both complete and partial clinical success in patients (from 1.00 [0.00, 1.70] to 0 in complete clinical success, and from 1.40 [1.13, 2.54] to 1.00 [0.00, 2.08]). Adrenal artery ablation did not affect the anthropometric parameters (Table S1).
TABLE 2.
Effect of adrenal artery ablation on clinical success in PA patients
| Variable | Clinical success | P value | |||||
|---|---|---|---|---|---|---|---|
| Complete (n = 9, [25.0%]) | Partial (n = 13, [36.1%]) | Absent (n = 14, [38.9%]) | Overall | Completevs Partial | Completevs Absent | Partial vs Absent | |
| Pre‐ablation | |||||||
| Age—y | 48.78 ± 6.48 | 48.15 ± 15.38 | 48.43 ± 13.36 | .994 | .991 | .996 | .999 |
| Blood pressure—mmHg | |||||||
| Systolic | 142 ± 19 | 159 ± 25 | 144 ± 19 | .109 | .184 | .974 | .196 |
| Diastolic | 93 ± 13 | 97 ± 14 | 84 ± 18 | .116 | .815 | .360 | .134 |
| Heart rate—beats/min | 83 ± 12 | 78 ± 8 | 79 ± 11 | .589 | .597 | .746 | .972 |
| DDD | 1.00 [0.00, 1.70] | 1.40 [1.13, 2.54] | 1.00 [0.70, 1.50] | .047 | .029 | .705 | .041 |
| Post‐ablation | |||||||
| Blood pressure—mmHg | |||||||
| Systolic | 122 ± 10 | 129 ± 14 | 140 ± 15 | .012 | .405 | .008 | .136 |
| Diastolic | 77 ± 9 | 83 ± 12 | 89 ± 13 | .062 | .454 | .040 | .356 |
| Heart rate—beats/min | 77 ± 10 | 73 ± 9 | 71 ± 11 | .419 | .671 | .428 | .849 |
| DDD | NA | 1.00 [0.00, 2.08] | 1.00 [0.19, 1.13] | NA | NA | NA | .6539 |
| Pre‐ and post‐ablation change | |||||||
| Blood pressure—mmHg | |||||||
| Systolic | 20 ± 17 | 30 ± 20 | 4 ± 19 | .003 | .396 | .114 | .004 |
| Diastolic | 16 ± 10 | 14 ± 8 | ‐5 ± 11 | <.001 | .891 | <.001 | <.001 |
Data are mean ± SD, or median with interquartile range.
Abbreviations: DDD, Defined daily dose; SD, standard deviation.
TABLE 3.
Effect of adrenal artery ablation on biochemical improvement in PA patients
| Variable | Biochemical improvement | P value | |||||
|---|---|---|---|---|---|---|---|
| Complete (n = 9, [25.0%]) | Partial (n = 13, [36.1%]) | Absent (n = 14, [38.9%]) | Overall | Complete vs Partial | Complete vs Absent | Partial vs Absent | |
| Pre‐ablation | |||||||
| Serum potassium—mmol/L | 4.11 ± 0.33 | 3.29 ± 0.68 | 3.54 ± 0.52 | .006 | .004 | .013 | .548 |
| Plasma aldosterone in supine position—ng/dL | 20.75 ± 4.03 | 17.36 ± 2.11 | 19.07 ± 4.50 | .165 | .126 | .647 | .436 |
| Plasma renin activity in supine position—ng/mL/h | 0.69 [0.64, 0.73] | 0.46 [0.10, 0.57] | 0.54 [0.10, 0.82] | .085 | NA | NA | NA |
| Plasma aldosterone‐to‐renin ratio | 34.00 [25.25, 35.75] | 38.00 [31.00, 120.00] | 37.00 [25.25, 125.00] | .622 | NA | NA | NA |
| Post‐ablation | |||||||
| Serum potassium—mmol/L | 4.22 ± 0.67 | 4.08 ± 0.67 | 4.00 ± 0.41 | .681 | .885 | .656 | .927 |
| Plasma aldosterone in supine position—ng/dL | 15.44 ± 5.05 | 17.50 ± 3.92 | 18.15 ± 4.76 | .388 | .580 | .433 | .925 |
| Plasma renin activity in supine position—ng/mL/h | 1.01 [0.11, 2.88] | 1.65 [0.29, 3.86] | 0.10 [0.10, 1.09] | .048 | 1.000 | .469 | .045 |
| Plasma aldosterone‐to‐renin ratio | 13.00 [6.50, 19.50] | 11.50 [5.00, 66.75] | 90.00 [17.00, 210.00] | .035 | 1.000 | .265 | .037 |
| Pre‐and Post‐ablation Change | |||||||
| Serum potassium—mmol/L | −0.34 ± 0.34 | −0.66 ± 1.05 | −0.16 ± 1.28 | .485 | .604 | .879 | .545 |
| Plasma aldosterone in supine position—ng/dL | 4.75 ± 5.75 | 0.40 ± 5.15 | 0.92 ± 6.13 | .239 | .250 | .343 | .973 |
| Plasma renin activity in supine position—ng/mL/h | −0.19 [−2.61, 0.59] | −1.01 [−3.84, −0.05] | 0.00 [−0.54, 0.39] | .072 | NA | NA | NA |
| Plasma aldosterone‐to‐renin ratio | 14.00 [9.25, 17.50] | 30.50 [12.25, 60.40] | 28.00 [18.50, 48.50] | .085 | NA | NA | NA |
Data are mean ± SD, or median with interquartile range.
Abbreviation: SD, standard deviation.
3.4. Effect of adrenal artery ablation on persistent PA after adrenalectomy
In addition, we also performed adrenal artery ablation in 4 patients who had persistent PA and poorly responded to medical treatment after adrenalectomy (Table S2). A remarkable reduction in office‐based blood pressures was observed after ablation (with mean blood pressure changed from 158/92 mmHg to 128/82 mmHg). Meanwhile, the DDD was also reduced from 105.10 [105.00, 108.90] to 30.00 [11.36, 60.50]. Their biochemical parameters were significantly improved after adrenal ablation. No apparent reduction of plasma cortisol level was found in those 4 patients (from 207.50 ± 46.00 ng/mL to 219.80 ± 42.06 ng/mL). Furthermore, adrenocortical insufficiency did not occur in these patients after follow‐up.
3.5. Safety of procedure
We also evaluated intraoperative complications and safety of adrenal artery ablation. Plasma cortisol level decreased slightly from 256.1 ± 87.31 ng/mL before ablation to 214.7 ± 44.18 ng/mL after ablation (P = .01), but no clinical manifestations of adrenal cortical dysfunction (eg, hyponatremia and hypotension) were observed after follow‐up. Adrenal artery ablation by ethanol might cause a transient rise in blood pressure. To avoid the hypertensive crisis, intravenous sodium nitroprusside was injected timely. Very few patients suffered from a decrease in blood pressure and heart rate due to pain‐induced vagal reflex, which can be remitted by administration of atropine or dopamine intravenously. Post‐procedure adverse events mostly occurred within 3 days of adrenal artery ablation, which included back pain (5/36, 13.9%), abdominal distension (6/36, 16.7%), nausea and vomiting (3/36, 8.3%), pruritus (1/36, 2.8%), and numbness of puncture side limbs (1/36, 2.8%). These symptoms generally subsided without treatment within 3 days; however, 2 patients required analgesics after ablation. There were no serious procedure‐related complications.
4. DISCUSSION
Catheter‐based adrenal ablation therapy showed a promising efficacy and did not cause hypocorticism and serious adverse events in PA patients. This procedure could be a useful treatment for PA patients who refuse surgery and show poor compliance with MR antagonist treatment.
MR antagonists have been the main treatment options for PA patients who are not suitable for surgery. 1 , 23 However, recent studies showed that long‐term MR antagonist treatment led to cardiovascular events and target organ damage in PA patients. 5 , 7 , 8 , 9 Currently, there are no perspective randomized control trials on long‐term effect of MR antagonists. Several studies have shown that high‐dose MR antagonist therapy causes gynecomastia, mastodynia, and hyperkalemia commonly occurred in patients treated with spironolactone. 11 , 13 Other nonendocrine and adverse effects include muscle cramps and nonspecific neuropsychiatric symptoms such as fatigue and general weakness. 12 In our study, partial patients opted for adrenal ablation because they were unable to tolerate the side effects of MR antagonists.
For patients with APA or unilateral adrenal hyperplasia, the Endocrine Society guidelines recommend laparoscopic adrenalectomy. 1 It should be noted that the curative effect of adrenalectomy is dependent on successful AVS. Besides functional aldosteronoma, lateralization of the source of the excessive aldosterone secretion could frequently exist in PA patients with adrenal normal‐appearing, micronodularity, bilateral masses, or atypical unilateral mass. 24 However, most centers rely on adrenal CT for determination of fitness for adrenalectomy 21 . But, radiographically identified adrenal nodules are not always the source of the excessive aldosterone. 24 , 25 One systematic review showed an overall discordance between CT and AVS results of ~40% in PA patients. 26 Our results showed that discordance between CT and AVS was 50 % in PA patients without apparent aldosteronoma. Thus, relying only on CT image may lead to inappropriate treatment of substantial patients with PA.
In addition, some patients with PA are reluctant to undergo surgery or long‐term MR antagonist treatment, and partial patients refuse further medication after failure of adrenalectomy. Chemical (ethyl alcohol) or physical (radiofrequency) adrenal ablation might be an alternative treatment in these situations. 16 , 17 Current study was different in that we included PA patients without apparent aldosteronoma. No serious adverse events occurred in our patients during or following ablation. The main complications were postoperative back pain and abdominal pain. Importantly, no patient developed adrenal insufficiency over the follow‐up period. Previous studies have reported that adrenal ablation by ethanol injection might lead to hypertensive crisis because of sympathetic nervous system activation. Therefore, intensive blood pressure monitoring is essential during the procedure.
The complete clinical success rates through adrenalectomy in PA patients varied widely (27%‐52%) in previous reports. 27 , 28 Recently, the PASO study established a set of standardized criteria for reporting clinical and biochemical outcomes of adrenalectomy for unilateral PA. 22 These criteria allow comparison of outcomes of different therapies. 29 , 30 We used the PASO criteria to evaluate clinical and biochemical success in this study. Patients treated with adrenal ablation had more marked decrease in blood pressure and were able to reduce DDD of their antihypertensive drugs. The complete clinical success rate (25%) after adrenal ablation in this study was equivalent to that achieved with unilateral adrenalectomy in a recent study (27%). 28 However, the biochemical success rate was lower with adrenal ablation than with adrenalectomy. It is not surprising because, unlike adrenalectomy, ablation only partially destroys adrenal function.
Catheter‐based adrenal artery ablation has several advantages. First, it is a suitable treatment for patients who refuse medical and surgical treatment and for patients in whom a previous adrenalectomy has failed. Second, it is a minimally invasive and less time‐consuming procedure. Third, adrenalectomy can be undertaken again if adrenal ablation fails. It deserves to be mentioned that full embolization of adrenal arteries can be technical demanding because these vessels are slender and occasionally anatomic variations. Successful adrenal artery ablation requires a multidisciplinary team, comprising endocrinologist, hypertension specialist, urologist, and cardiologist besides the radiologist.
4.1. Limitations
This study was not randomized allocation of patients to the different treatment groups due to ethical reasons. Although we evaluated the subtype of PA by CT scan following the guidelines on PA, the microadenomas were unable to excluded through imaging in this study. It would be preferred to assess adrenal insufficiency by ACTH stimulation test after adrenal ablation. The results may be various which is dependent on a team with experience in AVS and the vascular interventional therapy. A randomized clinical trial comparing catheter‐based adrenal artery ablation versus adrenalectomy and MR antagonist treatment is necessary to confirm the findings of this trial.
5. CONCLUSIONS
Adrenal artery ablation appears to be an effective and safe treatment for PA. The procedure can be applied in PA patients who refuse surgery and cannot tolerate MR antagonist treatment. This therapy might be an important supplement to traditional treatments for PA.
6. PERSPECTIVES
Evidence‐based clinical guidelines for PA treatment focus on medications and surgery. However, long‐term MR antagonist treatment increases the risk for incident cardiovascular events and death. Although adrenalectomy can achieve remission of patients with aldosteronoma, surgery is not indicated in most PA patients, and some patients are reluctant to start long‐term medication. In addition, a proportion of patients with aldosteronoma fail surgical treatment. Catheter‐based adrenal ablation is an alternative to conventional therapies. The results of the present study support the efficacy and safety of this technique. This approach can be useful for patients without aldosteronoma and would help reduce their future cardiovascular risk. Further exploration of the potential of catheter‐based adrenal ablation procedure in the treatment of PA is warranted.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Z.M.Z. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Z.M.Z, H.X.Z., Q.L., and Z.G.Z involved in study concept and design. H.X.Z., Q.L., X.L.L., Z.G.Z., H.B.H., F.S., Y.N.H., X.M.Z., Y.S.L., X.N.B., Z.C.Y., and R.F.S involved in acquisition of data. H.X.Z., Q.L., Z.G.Z., H.B.H., H.T.Z., G.Y.Y., and Z.M.Z involved in analysis and interpretation of data. Z.M.Z., H.X.Z., and Q.L involved in drafting of the manuscript. Z.M.Z., H.T.Z., and G.Y.Y involved in critical revision of the manuscript for important intellectual content. Z.M.Z, H.X.Z., and Q.L involved in statistical analysis. Z.M.Z involved in administrative, technical, and material support.
Additional contributions: We acknowledge the Chongqing EndocrineHypertension Collaborative Team for their contributions to this trial. Dr. Zhiyong Li (Yongchuan Hospital of Chongqing Medical University,Chongqing, China), Dr. Ping Wei (Southwest Hospital, Chongqing, China), Dr. Xiaoli Chen (Yubei District People's Hospital of Chongqing, Chongqing, China), Dr. Peijin Xia (Fuling Central Hospital of Chongqing,, Chongqing, China), Dr. Xiaoyun Fan (The Sixth People's Hospital of Chongqing, Chongqing, China), Dr. Wuquan Deng (Chongqing Emergency Medical Center, Chongqing, China), Dr. Yangjie He (Dazu District People's Hospital of Chongqing, Chongqing, China), Dr. Qingbin Liao (Dadukou District People's Hospital of Chongqing, Chongqing, China), Dr. Xingrong Tan (The Ninth People's Hospital of Chongqing, Chongqing, China), Dr. Liping Zhang (Fengdu County People's Hospital of Chongqing, Chongqing, China), Dr. Yong Luo (Chongqing Three Gorges Central Hospital, Chongqing, China), Dr. Yong Liao (Chongqing Corps Hospital of Armed Police Force, Chongqing, China).
Supporting information
Appendix S1
ACKNOWLEDGMENTS
We also thank Dr. Xiongjing Jiang (Fuwai Cardiovascular Diseases Hospital, Beijing, China) for his assistance in adrenal venous sampling and adrenal artery ablation. Zhu Zhiming designed and directed this study and had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drafting of the manuscript: Zhu Zhiming. Acquisition, analysis, or interpretation of data: others.
Zhang H, Li Q, Liu X, et al; Chongqing Endocrine Hypertension Collaborative Team . Adrenal artery ablation for primary aldosteronism without apparent aldosteronoma: An efficacy and safety, proof‐of‐principle trial. J Clin Hypertens. 2020;22:1618–1626. 10.1111/jch.13960
Zhang and Li contributed equally to this article.
REFERENCES
- 1. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(5):1889‐1916. [DOI] [PubMed] [Google Scholar]
- 2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285(2):126‐148. [DOI] [PubMed] [Google Scholar]
- 3. Rossi GP. Primary aldosteronism: JACC State‐of‐the‐Art Review. J Am Coll Cardiol. 2019;74(22):2799‐2811. [DOI] [PubMed] [Google Scholar]
- 4. Mulatero P, Monticone S, Bertello C, et al. Long‐term cardio‐ and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98(12):4826‐4833. [DOI] [PubMed] [Google Scholar]
- 5. Monticone S, D'Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2018;6(1):41‐50. [DOI] [PubMed] [Google Scholar]
- 6. Ohno Y, Sone M, Inagaki N, et al. Prevalence of cardiovascular disease and its risk factors in primary aldosteronism a multicenter study in Japan. Hypertension. 2018;71(3):530‐570. [DOI] [PubMed] [Google Scholar]
- 7. Hundemer GL, Curhan GC, Yozamp N, Wang ML, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(1):51‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. 2018;72(3):658‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rossi GP, Cesari M, Cuspidi C, et al. Long‐term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013;62(1):62‐69. [DOI] [PubMed] [Google Scholar]
- 10. Deinum J, Riksen NP, Lenders JW. Pharmacological treatment of aldosterone excess. Pharmacol Ther. 2015;154:120‐133. [DOI] [PubMed] [Google Scholar]
- 11. Stavropoulos K, Papadopoulos C, Koutsampasopoulos K, Lales G, Mitas C, Doumas M. Mineralocorticoid receptor antagonists in primary aldosteronism. Curr Pharm Des. 2018;24(46):5508‐5516. [DOI] [PubMed] [Google Scholar]
- 12. Parthasarathy HK, Menard J, White WB, et al. A double‐blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J Hypertens. 2011;29(5):980‐990. [DOI] [PubMed] [Google Scholar]
- 13. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709‐717. [DOI] [PubMed] [Google Scholar]
- 14. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of Atrial Fibrillation and Mineralocorticoid Receptor Activity in Patients With Medically and Surgically Treated Primary Aldosteronism. JAMA Cardiol. 2018;3(8):768‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rossi GP, Maiolino G, Flego A, et al. Adrenalectomy lowers incident atrial fibrillation in primary aldosteronism patients at long term. Hypertension. 2018;71(4):585‐591. [DOI] [PubMed] [Google Scholar]
- 16. Hokotate H, Inoue H, Baba Y, Tsuchimochi S, Nakajo M. Aldosteronomas: experience with superselective adrenal arterial embolization in 33 cases. Radiology. 2003;227(2):401‐406. [DOI] [PubMed] [Google Scholar]
- 17. Liu SY, Chu CC, Tsui TK, et al. Aldosterone‐producing Adenoma in Primary Aldosteronism: CT‐guided Radiofrequency Ablation‐Long‐term Results and Recurrence Rate. Radiology. 2016;281(2):625‐634. [DOI] [PubMed] [Google Scholar]
- 18. Fowler AM, Burda JF, Kim SK. Adrenal artery embolization: anatomy, indications, and technical considerations. Am J Roentgenol. 2013;201(1):190‐201. [DOI] [PubMed] [Google Scholar]
- 19. Sacks BA, Sacks AC, Faintuch S. Radiofrequency ablation treatment for aldosterone‐producing adenomas. Curr Opin Endocrinol Diabetes Obes. 2017;24(3):169‐173. [DOI] [PubMed] [Google Scholar]
- 20. Jiang X, Dong H, Peng M, et al. A Novel Method of Adrenal Venous Sampling via an Antecubital Approach. Cardiovasc Intervent Radiol. 2017;40(3):388‐393. [DOI] [PubMed] [Google Scholar]
- 21. Rossi GP, Auchus RJ, Brown M, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63(1):151‐160. [DOI] [PubMed] [Google Scholar]
- 22. Williams TA, Lenders JWM, Mulatero P, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5(9):689‐699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fischer E, Hanslik G, Pallauf A, et al. Prolonged zona glomerulosa insufficiency causing hyperkalemia in primary aldosteronism after adrenalectomy. J Clin Endocrinol Metab. 2012;97(11):3965‐3973. [DOI] [PubMed] [Google Scholar]
- 24. Lim V, Guo Q, Grant CS, et al. Accuracy of adrenal imaging and adrenal venous sampling in predicting surgical cure of primary aldosteronism. J Clin Endocrinol Metab. 2014;99(8):2712‐2719. [DOI] [PubMed] [Google Scholar]
- 25. Nanba AT, Nanba K, Byrd JB, et al. Discordance between imaging and immunohistochemistry in unilateral primary aldosteronism. Clin Endocrinol (Oxf). 2017;87(6):665‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009;151(5):329‐337. [DOI] [PubMed] [Google Scholar]
- 27. Catena C, Colussi G, Lapenna R, et al. Long‐term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension. 2007;50(5):911‐918. [DOI] [PubMed] [Google Scholar]
- 28. Vorselaars WMCM, Nell S, Postma EL, et al. Clinical outcomes after unilateral adrenalectomy for primary aldosteronism. JAMA Surg. 2019;154(4):e185842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burrello J, Burrello A, Stowasser M, et al. The Primary Aldosteronism Surgical Outcome Score for the Prediction of Clinical Outcomes After Adrenalectomy for Unilateral Primary Aldosteronism. Ann Surg. 2019. 10.1097/sla.0000000000003200 [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 30. Benham JL, Eldoma M, Khokhar B, et al. Proportion of patients withhypertension resolution following adrenalectomyfor primary aldosteronism: a systematic review andmeta‐analysis. J Clin Hypertens. 2016;18(12):1205‐1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


