Abstract
Klotho was involved in sodium reabsorption and the regulation of blood pressure. Animal studies indicated Klotho deficiency could mediate the development of salt‐sensitive hypertension, indicating its correlation with salt sensitivity. We aimed to explore the responses of Klotho to salt intake through dietary intervention in Chinese adults. Forty‐four participants were enrolled from Lantian county of Shaanxi, China. All participants sequentially underwent a 3‐day normal diet, a 7‐day low‐Na+ diet, and a 7‐day high‐Na+ diet. The concentrations of serum Klotho were assessed by using ELISA kits. Serum level of Klotho was 360.44 ± 93.89 pg/mL at baseline and increased while changed to low‐salt diet (478.65 ± 183.25 vs 360.44 ± 93.89 pg/mL, P < .001). During high‐salt diet, serum Klotho decreased to 354.37 ± 98.16 pg/mL (P < .001, compared to low‐salt diet). The overall responses of Klotho were more prominent in salt‐resistant participants. Serum Klotho of salt‐resistant group changed from 353.92 ± 97.65 pg/mL to 496.76 ± 196.21 pg/mL while changed from normal diet to low‐salt diet (P < .001) and decreased to 350.37 ± 99.50 pg/mL during high‐salt intake (P < .001). Furthermore, the response of serum Klotho to low‐salt intervention was much greater in salt‐resistant individuals than in salt‐sensitive ones. The responses of serum Klotho to dietary salt intervention were influenced by salt sensitivity, which was more prominent in salt‐resistant participants.
Keywords: blood pressure, Klotho, salt intake, salt sensitivity, sodium
1. INTRODUCTION
High‐salt intake is a common risk factor of hypertension. However, individual blood pressure responses to dietary sodium intake are heterogeneous which termed as salt sensitivity. 1 Salt sensitivity is an intermediate phenotype of hypertension, which is the results of the interactions of genetic and environmental factors. 2 Compelling evidence identified that salt‐sensitive individuals had higher risks of hypertension and other cardiovascular disease and were prone to have poorer clinical outcomes. 3 Therefore, a better understanding of salt sensitivity and salt‐sensitive hypertension will contribute to more specific dietary intervention on the prevention and treatment of hypertension.
Klotho, an important anti‐aging protein, was mainly expressed in distal convoluted tubules. Compelling evidence suggested the interaction between Klotho and blood pressure (BP). The expression of Klotho was down‐regulated in spontaneous hypertensive rats (SHRs) and in patients with hypertension. 4 Klotho haplo‐deficiency resulted in the elevation of BP, arterial stiffness, and renal dysfunction, 4 , 5 , 6 whereas exogenous Klotho delivery could mitigate the elevation of BP in SHRs, Otsuka Long–Evans Tokushima Fatty (OLETF) rats and in db/db mice. 7 , 8 Clinical study also indicated the single‐nucleotide polymorphisms of Klotho gene could predict the development of hypertension. 9 These results suggested Klotho gene was intimately involved in the regulation of BP.
In addition, Zhou et al also found Klotho deficiency could mediate the development of salt‐sensitive hypertension. 5 Compared to wild mice, the BP of KL± mice increased significantly after 2% saline intake. Meanwhile, the protein expressions of thiazide‐sensitive NaCl cotransporter (NCC) and other related molecules were upregulated and further increased by high‐salt intake in kidney of Klotho haplo‐deficient mice. This study implied the correlation of Klotho and salt sensitivity.
Recently, Andrukhova et al proposed Klotho could act as the co‐receptor of fibroblast growth factor 23(FGF23) in distal convoluted tubules involving in the reabsorption of sodium through the regulation of NCC. 10 This function might partially mediate the influence of Klotho on BP and salt sensitivity. Previously, we and other researchers have found salt intake could influence serum level of FGF23, and the response of FGF23 was more significant in salt‐resistant participants. 11 Therefore, it is intriguing to figure out the responses of Klotho to dietary salt intake and the influence of salt sensitivity on the responses of Klotho. After that, we would get a better understanding on the regulation of sodium balance and the differences of sodium regulation between salt‐sensitive and salt‐resistant participants. However, no one has focused on this topic.
Thus, we conducted a dietary intervention study to explore the responses of Klotho to dietary salt changes in human for the first time. Besides, we also aimed at elucidate the potent interrelationship of Klotho with salt sensitivity and BP.
2. Methods
2.1. Participants
There were 72 volunteers with similar dietary habits from Lantian county of Shaanxi province going through baseline investigation, and the selection process was depicted in Figure 1. Trained staff collected basic demographic information through a standard questionnaire and conducted anthropometric and blood pressure measurements. The exclusion criteria including hypertension ≥ stage 2, secondary hypertension, taking antihypertensive medicine, history of severe cardiovascular disease, diabetes mellitus, liver or renal dysfunction, infectious disease, and alcohol abuse. At last, 44 participants aged 18‐65 met the criteria and well finished dietary intervention. This study was approved by the ethics committee of the First Affiliated Hospital of Medical School, Xi'an Jiaotong University (Code: 2015‐128) and strictly complied with the Declaration of Helsinki. All participants provided written informed consents before the dietary intervention started.
Figure 1.
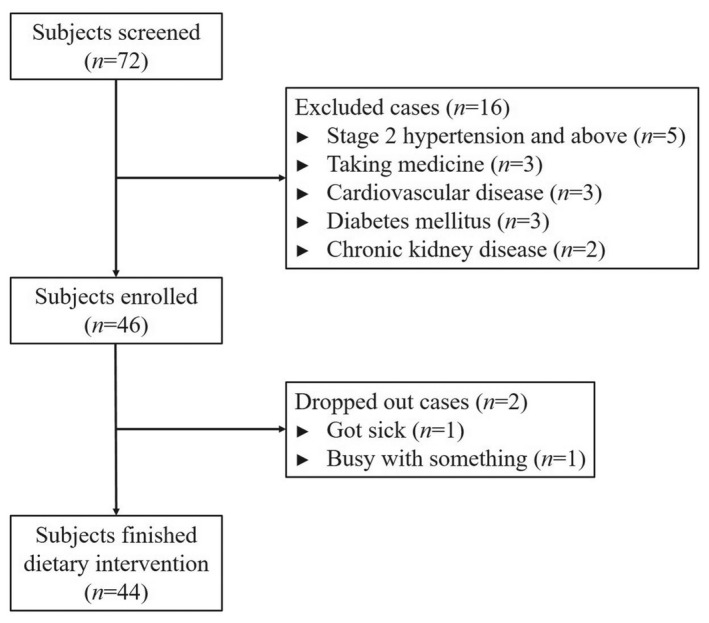
Flow diagram
2.2. Dietary intervention
As described before, this intervention study included three periods, namely, a 3‐day normal diet, a 7‐day low‐Na+ diet (51.3 mmol/d sodium or 3.0 g/d NaCl), and a 7‐day high‐Na+ diet (307.7 mmol/d sodium or 18 g/d NaCl)(Figure 2). 11 Anthropometric data (including height, weight, waist circumference) were collected during baseline period. To ensure the accuracy of dietary intervention, all participants were instructed to avoid extra food and beverages consumption at home and gathered together to eat in the research kitchen during the entire study period. The dieticians of the study prepared food and beverages free from salt. Trained staff prepackaged salt for each participant and added salt to the participant's meal. Participants finished each meal under the supervision of staff.
Figure 2.
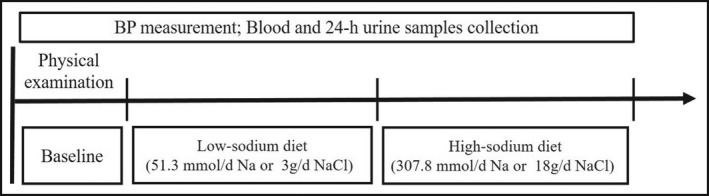
The protocol of dietary intervention. This intervention study had three periods, including baseline for 3 days, low‐sodium diet (51.3 mmol/d sodium or 3.0 g/d NaCl) for 7 days, and high‐sodium diet (307.7 mmol/d sodium or 18 g/d NaCl) for 7 days. Anthropometric data were collected during baseline. BP measured at each day of baseline and the 6th and 7th day of low‐sodium and high‐sodium period. Blood and 24‐hour urine samples were obtained at the last day of each period
2.3. BP measurement
BP of each participant was obtained by 3 certified physicians using standard mercury sphygmomanometer at each day of baseline and the 6th and 7th day of low‐salt and high‐salt period. BP of each day was measured for 3 times with 1‐min interval. The BP of each individual was calculated as the mean of all measurements during each period. Before BP measurement, participants were asked to have a rest in sitting position more than 5 min and avoid exercise, smoking, alcohol, coffee, or tea. Salt‐sensitive participants were defined as an increment of mean arterial pressure (MAP)≥10mmHg while changed from low‐salt diet to high‐salt diet. The others were salt‐resistant individuals. 12 MAP = systolic BP/3 + diastolic BP*2/3.
2.4. Laboratory measurements
Blood samples were obtained by peripheral venous puncture after overnight fasting at the last day of each period, which were centrifuged at 3000 × g, 4°C for 10 min. Blood samples were packaged in aliquots and stored at − 80°C for further analysis. Lipid profiles, serum creatinine, uric acid, blood glucose, and C‐reactive protein were analyzed using automatic biochemical analyzer (Hitachi, Ltd.). Human serum Klotho were measured by using commercial enzyme‐linked immunosorbent assay kits (USCN Life Science Inc). Intra‐assay and inter‐assay coefficients of human Klotho ELISA kit were reported to be < 10% and < 12%, respectively. The minimum detectable dose of Klotho was ≤ 6.2 pg/mL.
2.5. Analysis of 24 h urine
The 24‐hour urine samples were collected under the guidance of trained staff during the last two days of each period. After measured the volume, 10 ml urine samples were packaged in aliquots and stored at −40℃. Urine sodium and potassium concentrations were analyzed using ion‐selective electrodes (Hitachi, Ltd). 24 h excretion of sodium/potassium = 24 h total urine volume × sodium/potassium concentration.
2.6. Statistical analyses
All statistical analyses were performed by using SPSS Statistics 22.0 (IBM Corp). Continuous variables were expressed as mean ± standard deviations, and categorical variables were expressed as frequencies with percentages [n (%)]. Differences between SS and SR groups were examined by student's t test or chi‐square test. Alterations among different periods were assessed using one‐way ANOVA with repeated measures. Pearson or Spearman correlation analyses were conducted as appropriate. A two‐sided P < .05 was considered statistical significant.
3. Results
3.1. Profiles of all participants
As previously reported, 44 participants well completed the dietary intervention. Among them, there were 9 salt‐sensitive and 35 salt‐resistant participants. We depicted the characteristics of participants by their salt sensitivity in Table 1. The age, BMI, rate of hypertension, SBP, DBP, and baPWV were higher in salt‐sensitive participants. Biochemical parameters showed no obvious difference between two groups, except for fast blood glucose.
Table 1.
Baseline characteristics of participants by salt sensitivity
| Parameters | Salt sensitive (n = 9) | Salt resistant (n = 35) | P value |
|---|---|---|---|
| Age (y) | 56.9 ± 7.1 | 49.5 ± 13.2 | .117 |
| BMI (kg/m2) | 26.82 ± 3.11 | 24.35 ± 3.05 | .036 |
| Hypertension (%) | 55.6% | 11.4% | .014 |
| SBP (mmHg) | 138.6 ± 14.7 | 120.9 ± 15.0 | .003 |
| DBP (mmHg) | 85.2 ± 9.6 | 77.4 ± 8.0 | .027 |
| BaPWV (cm/s) | 1787.67 ± 385.75 | 1397.17 ± 196.67 | <.001 |
| TC (mmol/L) | 4.52 ± 0.61 | 4.63 ± 0.95 | .734 |
| TG (mmol/L) | 1.80 ± 1.21 | 1.32 ± 0.78 | .153 |
| HDL‐C (mmol/L) | 1.39 ± 0.47 | 1.49 ± 0.41 | .537 |
| LDL‐C (mmol/L) | 2.51 ± 0.41 | 2.61 ± 0.71 | .665 |
| Cre (mmol/L) | 55.78 ± 10.90 | 54.86 ± 9.06 | .795 |
| UA (μmol/L) | 278.79 ± 85.43 | 261.46 ± 84.10 | .586 |
| FBG (mmol/L) | 5.88 ± 0.71 | 5.22 ± 0.71 | .017 |
| CRP (mmol/L) | 0.780 ± 0.776 | 0.879 ± 1.28 | .826 |
| KL (pg/mL) | 389.69 ± 75.11 | 352.92 ± 97.65 | .300 |
| FGF23 (pg/mL) | 83.84 ± 50.19 | 62.07 ± 41.76 | .146 |
Abbreviations: BaPWV, brachial‐ankle pulse wave velocity; BMI, body mass index; CRE, creatinine; CRP, C‐reactive protein; DBP, diastolic blood pressure; FBG, fasting blood glucose; FGF23, fibroblast growth factor 23; HDL‐C, high‐density lipoprotein cholesterol; KL, Klotho; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid.
3.2. Responses of BP,24‐hour urinary sodium and potassium to dietary intervention
SBP of all participants was 124.5 mmHg at baseline, 115.7 mmHg during low‐salt diet, and 127.6 mmHg while high‐salt intake. DBP was 79.0 mmHg, 77.3 mmHg, and 80.0 mmHg, respectively. SBP changed significantly during salt intervention in both salt‐sensitive (P = .004) and salt‐resistant (P = .016) participants, while DBP showed on obvious changes in both groups.
The 24‐hour urinary sodium and potassium excretions were good indicators of dietary salt intake. In the present study, 24‐hour urinary sodium and potassium decreased after low‐salt diet and increased after high‐salt diet well reflecting the compliance of participants to dietary intervention.
Previous studies demonstrated the dysregulation of sodium excretion could partially explain the differences between salt‐sensitive and salt‐resistant participants. In the present study, 24‐hour urinary sodium and potassium excretion were significantly higher in salt‐resistant participants during high‐salt intake (P = .011) (Figure 3A). Furthermore, the responses of 24‐hour urinary sodium excretion were both significant during intervention but more pronounced in salt‐resistant participants, regardless of the response to low‐salt or to high‐salt diet (Table 2, Figure 3B).
Figure 3.
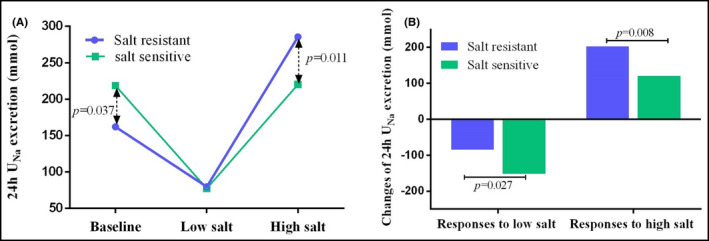
The 24‐hour urinary sodium excretion in salt‐sensitive and salt‐resistant individuals during salt intervention. The 24‐hour UNa decreased after low‐salt diet and increased while high‐salt diet. The changes of 24‐hour UNa excretion were more prominent in salt‐resistant participants, regardless of the response to low‐salt or to high‐salt diet (Figure 3A,B). UNa, urinary sodium
Table 2.
The responses of 24‐hour urinary sodium and potassium to dietary intervention
| Indexes | Salt sensitive | Salt resistant | P value |
|---|---|---|---|
| Baseline | |||
| 24 hours UNa | 218.47 ± 91.51 | 161.97 ± 64.18 | .037 |
| 24 hours UK | 26.63 ± 11.92 | 27.23 ± 7.82 | .855 |
| Low‐salt period | |||
| 24 hours UNa | 77.12 ± 20.19 | 79.61 ± 35.74 | .843 |
| 24 hours UK | 20.99 ± 6.50 | 26.61 ± 9.01 | .088 |
| High‐salt period | |||
| 24 hours UNa | 220.17 ± 75.57 | 285.51 ± 62.33 | .011 |
| 24 hours UK | 25.82 ± 6.70 | 36.56 ± 10.66 | .006 |
| Response to low salt | |||
| 24 hours UNa | −152.46 ± 92.35 | −84.63 ± 75.61 | .027 |
| 24 hours UK | −9.87 ± 8.77 | −1.10 ± 10.03 | .021 |
| Response to high salt | |||
| 24 hours UNa | 120.83 ± 69.56 | 202.88 ± 81.42 | .008 |
| 24 hours UK | 3.05 ± 8.00 | 10.71 ± 12.38 | .086 |
Abbreviations: UK, urinary potassium; UNa, urinary sodium.
3.3. The responses of Klotho to dietary intervention
Serum level of Klotho was 360.44 ± 93.89 pg/mL at baseline and increased while changed to low‐salt diet (478.65 ± 183.25 vs 360.44 ± 93.89 pg/mL, P < .001). During high‐salt diet, serum Klotho decreased to 354.37 ± 98.16 pg/mL(P < .001, compared to low‐salt diet).
To elucidate the correlations between salt sensitivity and responses of Klotho to dietary intervention, we then conducted subgroup analysis stratified by salt sensitivity. We then found the overall responses of Klotho were more prominent in salt‐resistant participants (Figure 4A). Serum Klotho of salt‐resistant group changed from 353.92 ± 97.65 pg/mL to 496.76 ± 196.21 pg/mL after low‐salt diet (P < .001) and decreased to 350.37 ± 99.50 pg/mL while high‐salt intake (P < .001). The serum Klotho concentrations of salt‐sensitive individuals were 389.69 ± 75.11, 408.21 ± 99.37, and 369.91 ± 96.83 pg/mL from baseline to high‐salt period. Nevertheless, the alteration showed no obvious difference. Furthermore, the response of serum Klotho to low‐salt intervention was much greater in salt‐resistant individuals than in salt‐sensitive ones. However, the difference of response to high‐salt intervention only reached marginal statistical significance (Table 3, Figure 4B).
Figure 4.
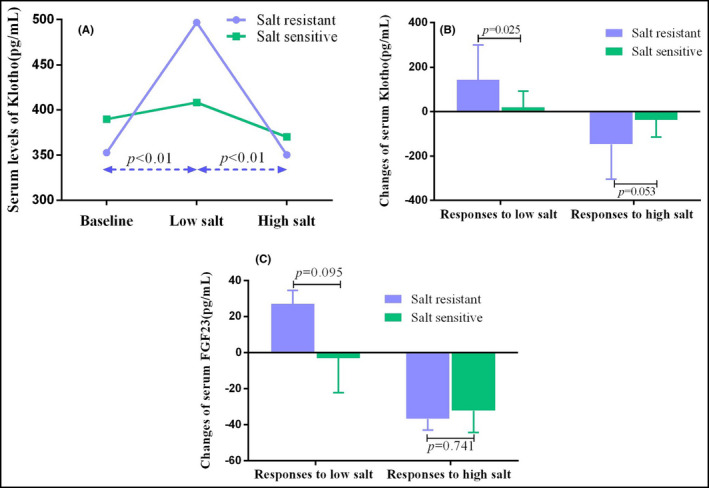
The responses of serum Klotho to dietary intervention in salt‐sensitive and salt‐resistant participants. Serum level of Klotho increased while changed to low‐salt diet and decreased during high‐salt period. The overall responses of Klotho were more prominent in salt‐resistant participants (A). The response of serum Klotho to low‐salt intervention was much greater in salt‐resistant individuals than in salt‐sensitive ones. However, the difference of response to high‐salt intervention only reach marginal statistical significance (B). The separate responses of FGF23 to low‐salt or to high‐salt diet showed no obvious difference between salt‐resistant and salt‐sensitive participants (C). FGF23, fibroblast growth factor 23
Table 3.
The separate response to low‐salt or high‐salt intervention in salt‐sensitive and salt‐resistant groups
| Indexes | Salt sensitive | Salt resistant | P value |
|---|---|---|---|
| Response to low‐salt diet | |||
| Klotho (pg/mL) | 18.52 ± 72.88 | 143.85 ± 156.73 | .025 |
| FGF23 (pg/mL) | −3.12 ± 19.19 | 26.94 ± 7.49 | .095 |
| Response to high‐salt diet | |||
| Klotho (pg/mL) | −38.30 ± 76.39 | −146.39 ± 156.94 | .053 |
| FGF23 (pg/mL) | −32.35 ± 12.00 | −36.92 ± 6.23 | .741 |
Abbreviations: FGF23, fibroblast growth factor 23.
Furthermore, we explored the relationship between Klotho and BP influenced by salt sensitivity. To exclude the influence of dietary factors, we only used the data from low‐salt and high‐salt diet. At last, we found a weak negative correlation between Klotho and SBP after adjusting age, gender, BMI, and hypertension status (r = −0.232, P = .009) (Figure 5A). This correlation was much stronger in SS participants (r = −0.562, P = .015) (Figure 5B) but showed no obvious statistical significance in SR ones (Figure 5C).
Figure 5.
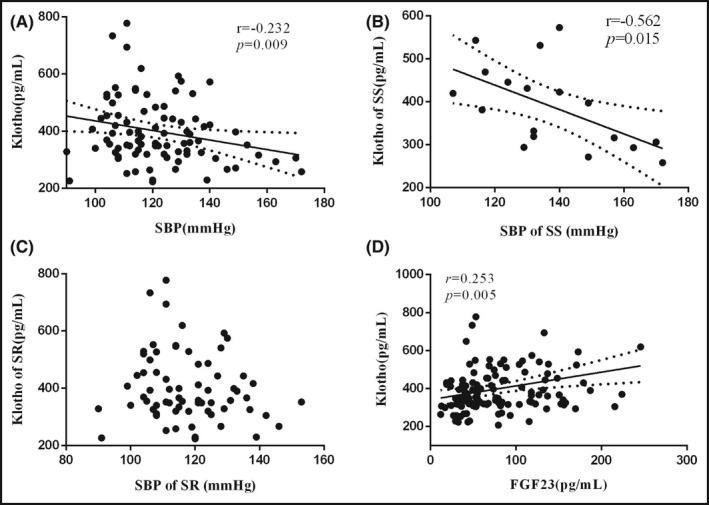
The correlations of Klotho with SBP and FGF23. There was a negative correlation between Klotho and SBP after adjusting age, gender, BMI, and hypertension status (A). This correlation was much stronger in SS participants (B) but showed no obvious statistical significance in SR ones (C). Serum Klotho was positively correlated with serum FGF23 during dietary intervention after adjusting for confounders (D). SBP, systolic blood pressure; FGF23, fibroblast growth factor 23
3.4. Alterations of FGF23 during intervention
Klotho acts as the key co‐receptor of FGF23 while regulating sodium excretion. Therefore, it is also necessary to explore the responses of FGF23 to dietary intervention. Our previous study has found the alterations of FGF23 were also prominent in salt‐resistant participants. In the present study, we found serum Klotho was positively correlated with serum FGF23 during dietary intervention (r = 0.235, P = .005) after adjusting age, gender, BMI, and hypertension status (Figure 5D). We then explored the differences of FGF23 response to low‐salt or to high‐salt intervention between salt‐sensitive and salt‐resistant individuals. Nevertheless, the separate responses to low‐salt diet or to high‐salt diet showed no obvious difference between salt‐resistant and salt‐sensitive participants (Table 3, Figure 4C).
4. Discussion
To the best of our knowledge, this study is the first to explore the responses of Klotho to salt intake and the interactions between Klotho and salt sensitivity in humans. We found serum Klotho increased while low‐salt diet and decreased after high‐salt intake. The responses of Klotho to dietary salt intake especially to low‐salt diet were more pronounced in salt‐resistant participants. Given that serum Klotho went parallel with serum FGF23 during dietary intervention and the responses of FGF23 were also more prominent in salt‐resistant individuals, there were some intrinsic connections between the dysregulation of FGF23/Klotho axis and salt sensitivity.
Longitudinal studies have found salt‐sensitive participants were accompanied by more rapid age‐dependent increase of BP, severe target organ damages and higher risk of mortality. 13 , 14 In this study, the age and BMI of salt‐sensitive participants were higher. Besides, salt‐sensitive individuals had higher BP, rate of hypertension, baPWV, and blood glucose. These results indicated salt‐sensitive participants were often accompanied by more cardiovascular risk factors. Salt sensitivity and these risk factors synergetically contributed to poor clinic outcomes. The dysregulation of renal sodium excretion was one of the main mechanisms of salt‐sensitive hypertension. 15 As with previous studies, we also found the responses of 24‐hour urinary sodium excretion was more pronounced in salt‐resistant participants. During high‐salt diet, 24‐hour urinary sodium and potassium excretion were significantly higher in salt‐resistant participants. That is to say, the abilities to regulate sodium excretion and maintain the balance of sodium were much stronger in salt‐resistant participants.
Klotho was a critical anti‐aging protein, deficiency of which resulted in phenotypes resembling human premature aging syndromes. 16 Klotho was mainly expressed in kidneys and played its physiological roles as single‐transmembrane protein and soluble protein originating from proteolysis or alternative splicing. 17 , 18 Studies have indicated Klotho was involved in the regulation of BP. Deficiency of Klotho led to the elevation of BP, and exogenous Klotho delivery could mitigate or even normalize the elevation of BP in various animal models. 5 , 8 , 19 Furthermore, Zhou et al found haplo‐deficiency of Klotho not only resulted in the development of spontaneous hypertension but also enhanced BP sensitivity to salt intake. 5 High‐salt intake significantly elevated the BP of KL ± mice. These results might attribute to the upregulation of NCC and the attenuated inhibition of renin‐angiotensin system (RAS). 5 , 19 In this study, we found a weak negative correlation between Klotho and SBP after adjusting for age, gender, BMI, and hypertension status. This correlation was much stronger in SS participants but showed no obvious statistical significance in SR ones. This difference might attribute to the reason that salt‐resistant participants exhibited more active responses to dietary salt changes to keep BP to a stable state.
As a single‐transmembrane protein, Klotho acted as the co‐receptor of FGF23 to enhance the affinity of FGF23 to its receptors. 20 Andrukhova et al found FGF23/Klotho could regulate the reabsorption of sodium in distal tubules. 10 The influences of Klotho on BP and salt sensitivity might attribute to the regulation of sodium reabsorption. Previously, we, as well as other researchers, found salt intake could influence serum level of FGF23. 11 , 21 , 22 As one of the key factors of FGF23/Klotho axis, we further explored the influences of salt intake on serum Klotho. In line with the changes of FGF23, Klotho elevated during low‐salt diet and decreased after high‐salt diet. During intervention, serum Klotho was positively correlated with serum FGF23. These results reinforce the existence of negative feedback between FGF23/Klotho axis and salt intake. High salt suppressed sodium reabsorption through inhibited the function of FGF23/Klotho axis.
As mentioned above, the dysregulation of sodium excretion played pivotal role in the development of salt sensitivity. We found the regulation of serum FGF23 by salt intake was more prominent in salt‐resistant participants in previous study. Salt‐sensitive individuals showed blunt FGF23 responses to salt intake. Similarly, the response of Klotho to salt intake was also more pronounced in salt‐resistant participants especially the separate effect to low salt. However, the separate effect of FGF23 to low salt or high salt showed no obvious difference. These results suggested an aberrant regulation of FGF23/Klotho axis in salt‐sensitive participants. The different responses of FGF23/Klotho axis to salt intake could influence sodium balance, which might contribute to the progression of salt sensitivity. However, the exact mechanisms of the different responses between salt‐sensitive and salt‐resistant participants were still far from clear. To our knowledge, the interactions between FGF23/Klotho axis and RAS could partially explain the different responses of two groups. Consistent with the changes of FGF23/Klotho axis, studies have found aldosterone decreased in high‐salt period compared with low‐salt period in salt‐resistant participants, which did not change significantly in SS participants. Aldosterone could upregulate the expression of FGF23. Furthermore, the Sgk1‐NCC pathway has been established as an important downstream target of aldosterone/MR, which also was the target of FGF23/Klotho axis. RAS might regulate the function of FGF23/Klotho axis through this common pathway. Additional studies were warranted to elucidate the different responses of FGF23/Klotho axis in salt‐sensitive and salt‐resistant groups.
Though this was the first study that explored the influence of salt intake on serum Klotho in human, several limitations of this study should be stressed. This intervention study was restricted in northern China. The number of participants was quite small. With these limitations, we could not generalize the results of this study to other races. Thus, multiethnic clinical trials were required to determine whether our results could be generalized to populations with multiple backgrounds. Moreover, we observed the changes during intervention but paid little attention to the mechanisms of these changes. Therefore, further researches were still warranted.
Overall, we firstly observed the influence of salt intake on serum Klotho in human, which increased while low‐salt diet and decreased after high‐salt intake. Besides, the responses of Klotho to dietary salt intake especially to low‐salt diet were more pronounced in salt‐resistant participants. Based on our previous study, we thus predicted that salt intake could influence the function of FGF23/Klotho axis. Aberrant responses of FGF23/Klotho axis to salt intake might contribute to the development of salt‐sensitive hypertension.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTIONS
Jia‐Wen Hu and Chao Chu conceived the original idea and completed the manuscript. Jia‐Wen Hu, Chao Chu, Tao Shi, Yang Yan, and Jian‐Jun Mu completed the dietary intervention. Jian‐Jun Mu also designed the experiment and supervised the intervention procedure.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China No. 81570381 (J.‐JM), No. 81600327 (YW), No. 81600574 (HL), and No. 81700368 (CC), the Clinical Research Award of the First Affiliated Hospital of Xi'an Jiaotong University of China No. XJTU1AF‐CRF‐2015‐006 (J.‐JM) and No. XJTU1AF‐CRF‐2017‐021 (YW), Grants 2017YFC1307604 and 2016YFC1300104 from the Major Chronic Non‐communicable Disease Prevention and Control Research Key Project of the Ministry of Science and Technology of the People's Republic of China, and Grant 2017ZDXM‐SF‐107 from the Key Research Project of Shaanxi Province. The sponsor or funding organization had no role in the design or conduct of this research.
REFERENCES
- 1. Hirohama D, Fujita T. Evaluation of the pathophysiological mechanisms of salt‐sensitive hypertension. Hypertens Res. 2019;42(12):1848‐1857. [DOI] [PubMed] [Google Scholar]
- 2. Gu D, Rice T, Wang S, et al. Heritability of blood pressure responses to dietary sodium and potassium intake in a Chinese population. Hypertension. 2007;50(1):116‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bihorac A, Tezcan H, Ozener C, et al. Association between salt sensitivity and target organ damage in essential hypertension. Am J Hypertens. 2000;13(8):864‐872. [DOI] [PubMed] [Google Scholar]
- 4. Gao D, Zuo Z, Tian J, et al. Activation of SIRT1 attenuates klotho deficiency‐induced arterial stiffness and hypertension by enhancing AMP‐activated protein kinase activity. Hypertension. 2016;68(5):1191‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou X, Chen K, Lei H, et al. Klotho gene deficiency causes salt‐sensitive hypertension via monocyte chemotactic protein‐1/CC chemokine receptor 2‐mediated inflammation. J Am Soc Nephrol. 2015;26(1):121‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen K, Sun Z. Autophagy plays a critical role in Klotho gene deficiency‐induced arterial stiffening and hypertension. J Mol Med (Berl). 2019;97(11):1615‐1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saito Y, Nakamura T, Ohyama Y, et al. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276(2):767‐772. [DOI] [PubMed] [Google Scholar]
- 8. Takenaka T, Kobori H, Miyazaki T, et al. Klotho protein supplementation reduces blood pressure and renal hypertrophy in db/db mice, a model of type 2 diabetes. Acta Physiol (Oxf). 2019;225(2):e13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang HL, Xu Q, Wang Z, et al. A potential regulatory single nucleotide polymorphism in the promoter of the Klotho gene may be associated with essential hypertension in the Chinese Han population. Clin Chim Acta. 2010;411(5–6):386‐390. [DOI] [PubMed] [Google Scholar]
- 10. Andrukhova O, Slavic S, Smorodchenko A, et al. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med. 2014;6(6):744‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu JW, Wang Y, Chu C, et al. Effect of salt intervention on serum levels of fibroblast growth factor 23 (FGF23) in Chinese adults: an intervention study. Med Sci Monit. 2018;24:1948‐1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu JW, Wang Y, Chu C, et al. The responses of the inflammatory marker, pentraxin 3, to dietary sodium and potassium interventions. J Clin Hypertens (Greenwich). 2018;20(5):925‐931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weinberger MH, Fineberg NS. Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension. 1991;18(1):67‐71. [DOI] [PubMed] [Google Scholar]
- 14. Weinberger MH, Fineberg NS, Fineberg SE, et al. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37(2 Pt 2):429‐432. [DOI] [PubMed] [Google Scholar]
- 15. Fujita T. Mechanism of salt‐sensitive hypertension: focus on adrenal and sympathetic nervous systems. J Am Soc Nephrol. 2014;25(6):1148‐1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuro‐o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45‐51. [DOI] [PubMed] [Google Scholar]
- 17. Hu MC, Kuro‐o M, Moe OW. Renal and extrarenal actions of Klotho. Semin Nephrol. 2013;33(2):118‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Loon EP, Pulskens WP, van der Hagen EA, et al. Shedding of klotho by ADAMs in the kidney. Am J Physiol Renal Physiol. 2015;309(4):F359‐F368. [DOI] [PubMed] [Google Scholar]
- 19. Zhou L, Mo H, Miao J, et al. Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin‐angiotensin system. Am J Pathol. 2015;185(12):3211‐3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kinoshita S, Kawai M. The FGF23/KLOTHO regulatory network and its roles in human disorders. Vitam Horm. 2016;101:151‐174. [DOI] [PubMed] [Google Scholar]
- 21. Humalda JK, Lambers HH, Kwakernaak AJ, et al. Fibroblast growth factor 23 and the antiproteinuric response to dietary sodium restriction during renin‐angiotensin‐aldosterone system blockade. Am J Kidney Dis. 2015;65(2):259‐266. [DOI] [PubMed] [Google Scholar]
- 22. Zhang B, Umbach AT, Chen H, et al. Up‐regulation of FGF23 release by aldosterone. Biochem Biophys Res Commun. 2016;470(2):384‐390. [DOI] [PubMed] [Google Scholar]


