Abstract
The Hypertension Cardiovascular Outcome Prevention and Evidence in Asia (HOPE Asia) Network was set up to improve the management of hypertension in Asia with the ultimate goal of achieving “zero” cardiovascular events. Asia is a diverse continent, and the prevalence of hypertension has increased over the last 30 years. There are a number of Asia‐specific features of hypertension and hypertension‐related cardiovascular complications, which means that a region‐specific approach is needed. White‐coat hypertension will become more of an issue over time as Asian populations age, and masked hypertension is more prevalent in Asian than in Western countries. Identifying and treating masked hypertension is important to reduce cardiovascular risk. Abnormal patterns of blood pressure (BP) variability common in Asia include exaggerated early morning BP surge and nocturnal hypertension. These are also important cardiovascular risk factors that need to be managed. Home blood pressure monitoring (HBPM) is an important tool for detecting white‐coat and masked hypertension, and monitoring BP variability, and practices in Asia are variable. Use of HBPM is important given the Asia‐specific features of hypertension, and strategies are needed to improve and standardize HBPM usage. Development of HBPM devices capable of measuring nocturnal BP along with other information and communication technology‐based strategies are key developments in the widespread implementation of anticipation medicine strategies to detect and prevent cardiovascular events in patients with hypertension. Region‐wide differences in hypertension prevalence, control, and management practices in Asia highlight the importance of information sharing to facilitate best practices.
Keywords: Asia, diversity, Hope Asia, hypertension, initiative
1. INTRODUCTION
Hypertension is an important global health issue. Using population data from 154 countries, the estimated number of adults with systolic blood pressure (SBP) of ≥140 mm Hg in 2015 was 874 million, and the number of those with SBP of at least 110‐115 mm Hg was 3.5 billion.1 It is well known that elevated blood pressure (BP) is associated with cardiovascular morbidity and mortality, and that good blood pressure (BP) control is an effective approach to reducing the risk of hypertension‐related target organ damage and cardiovascular events.2, 3 Nevertheless, the cardiovascular complications of hypertension continue to cause significant morbidity and mortality worldwide, largely due to inadequate strategies for the prevention, diagnosis, and control of hypertension in an aging worldwide population, many of whom are experiencing changing social and economic conditions.3
The mission of the Hypertension Cardiovascular Outcome Prevention and Evidence in Asia (HOPE Asia) Network is to improve the management of hypertension and organ protection with the ultimate goal of achieving “zero” cardiovascular events in Asia. There are three main strategies to allow this goal to be achieved: (a) examination and analysis of existing hypertension‐related evidence; (b) development of consensus on clinically relevant themes in hypertension; and (c) performing Asia‐wide clinical studies in the field.4 The HOPE Asia Network is a member of the World Hypertension League (WHL) and will contribute to the WHL's mission of confronting the global hypertension epidemic and the associated high burden of disability and premature death.
Asia is a diverse continent. The majority of countries are lower to upper middle income, but the region includes some high (eg, Japan, Korea) and some lower (eg, Cambodia) income countries. Hypertension prevalence rates vary between countries.5, 6 Perhaps, a more significant issue than high prevalence rates is the low level of awareness and treatment in some countries.7
Diversity in the region in terms of the number of individuals with raised BP was highlighted in a 2015 global analysis of BP trend prevalence.8 South Korea has one of the lowest prevalence rates for raised BP, whereas rates in South Asia and China were much higher.8 The high population density in parts of Asia means that the absolute number of individuals affected by hypertension is substantial. Based on 2015 figures, 258 million (23%) of the 1.13 billion adults with raised BP lived in South Asia (of whom 199 million resided in India) and another 235 million (21%) lived in East Asia (226 million in China).8 Thus, the negative consequences of high BP and poor BP control are significant, highlighting hypertension as an important public health concern for the region. Obesity is another growing issue in Asia. A worldwide population survey showed consistent increases in body mass index over time in Asia and Oceania, although to a lesser extent in the high‐income Asia‐Pacific region.9
2. ASIA‐SPECIFIC FACTORS
There are several differences in the profile of hypertension and hypertension risk in Asians compared with Western populations. In contrast to Western countries, stroke is a more common complication of hypertension than is coronary heart disease (CHD) in some parts of Asia,10 and there is a higher burden of stroke‐related vs CHD‐related mortality in some parts of Asia (Figure 1).10, 11 In addition, the slope of the association between increasing SBP and the rate of cardiovascular events has been shown to be steeper in Asians than in Western/Caucasian populations (Figure 1).12 Another important factor is obesity, the prevalence of which is increasing rapidly in Asia,13 and the impact of obesity on BP may differ between Asians and Caucasians. For example, Asians are likely to develop pre‐hypertension and high BP at a lower BMI and with smaller BMI increments than Europeans.14, 15 Furthermore, obesity and the metabolic syndrome are known to increase salt sensitivity, and Asians are more likely to have a genetic predisposition to salt sensitivity.15 Asians also have a high salt intake (Figure 2), which combines with greater salt sensitivity to increase mean BP to a greater extent in Asians than in Caucasians. Available data also suggest that BP variability (BPV), especially an exaggerated morning BP surge and nocturnal hypertension, is greater in Asians than in Westerners.16, 17, 18, 19, 20 This is relevant because abnormal BPV is known to increase the risk of cardiovascular events.21, 22
Figure 1.
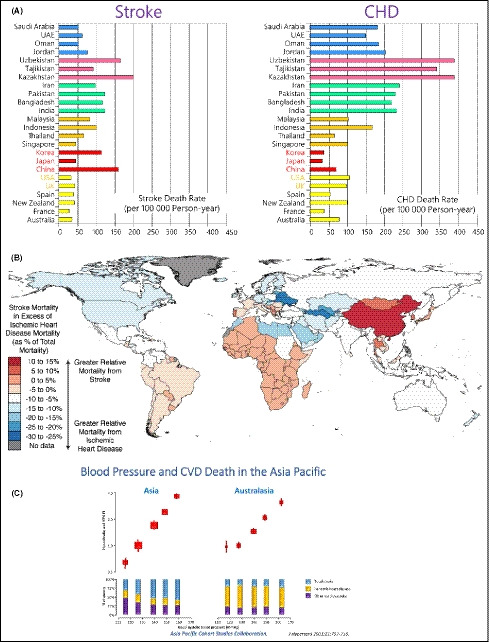
Age‐standardized cardiovascular death rates for stroke and coronary heart disease (CHD; reproduced from Ueshima et al,10 with permission) (A); and excess of stroke mortality compared with CHD mortality (reproduced from Kim,11 with permission) (B); and association between blood pressure and cardiovascular death in Asia vs Australasia in the Asia Pacific Cohort Studies Collaboration (C)12
Figure 2.
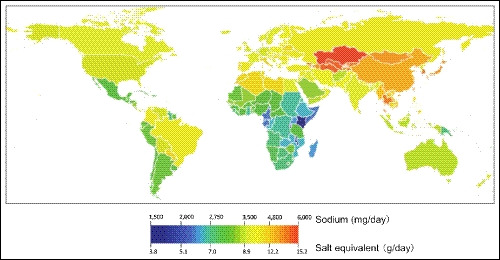
Salt intake in 2010 for adults aged >20 y (reproduced from Powles et al,105 with permission)
In terms of manifestations of cardiovascular disease (CVD), Asians have a lower prevalence of atrial fibrillation (AF) than Caucasians (≈1% vs ≈1%‐2%) but the overall disease burden of AF in Asia is high because of the larger proportion of older individuals in the population.23 Based on data from a large study conducted in Taiwan, approximately one in seven individuals aged >20 years in Asia will develop AF in their lifetime24; these rates are lower than those reported for Caucasians in the Framingham Heart Study25 and the Rotterdam study.26 Rates of heart failure also appear to be slightly lower in Asian vs Western populations but, as for AF, the number of patients in Asia who develop heart failure each year is high, indicating that the worldwide heart failure pandemic includes Asia.27 Given that an increase in CHD prevalence often occurs in parallel with better socioeconomic prosperity, the improving socioeconomic status across much of Asia means that the region has high rates of CHD and CHD mortality.28 It has been estimated that over 60% of the global CHD burden occurs in developing countries, especially in Asia.29
The risk of cardiovascular events shows seasonal variation, usually peaking in winter. However, available data suggest that this interaction is more complex than a direct influence of seasonal factors (eg, daylight exposure, ambient temperature) and actually results from a more complex interaction between an individual and his/her environment.30 Data from Asia show higher absolute BP values in winter,31 winter increases in the incidence of both cardiac and cerebrovascular disease in Japan,32 winter peaks of acute coronary syndrome in China and ischemic heart disease‐ and heart failure‐related events in Hong Kong,33, 34, 35 and associations between mean temperature and the occurrence of intracerebral hemorrhage in South Korea.36
Fine particulate matter <2.5 μg (PM2.5) has been identified as a significant and important contributor to the occurrence of hypertension.37, 38, 39, 40 This is particularly relevant in Asia because PM2.5 levels in China, Pakistan, India, and Bangladesh are among the highest in the world (Figure 3). Evidence for the association between air pollution and cardiovascular disease has recently been comprehensively reviewed.41 Looking at Asian studies, data from Japan showed that the combination of low temperature and high PM2.5 concentration substantially and significantly increased the likelihood of morning hypertension compared with times when temperature was high and PM2.5 concentration was low.42 In Thailand, National Health Examination Survey data show that the prevalence of hypertension is the highest in northern region, which also has a high proportion of days with extremely high PM2.5 levels.
Figure 3.
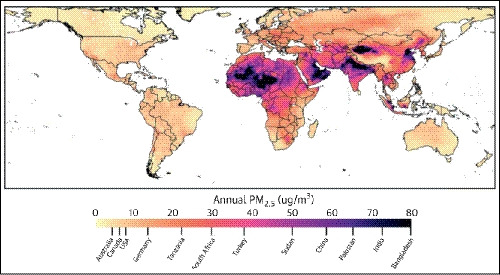
“Heat map” showing annual global levels of fine particulate matter <2.5 μg (PM2.5; reproduced from Rajagopalan S,41 with permission)
The varied factors contributing to hypertension and CVD in Asia are summarized in Figure 4.
Figure 4.
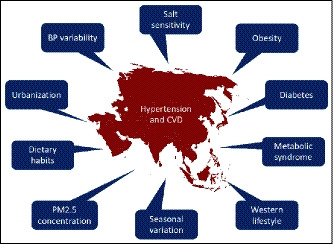
Factors contributing to hypertension and cardiovascular disease (CVD) in Asia. BP, blood pressure; PM2.5, fine particulate matter <2.5 μg
3. WHITE‐COAT HYPERTENSION IN ASIA
The worldwide prevalence of white‐coat hypertension based on data from the Ambulatory blood pressure Registry TEleMonitoring of hypertension and cardiovascular rISk (ARTEMIS) project in patients referred for hypertension was 23%.20 There are limited Asia‐specific data, but the prevalence of white‐coat hypertension in volunteers with no prior cardiovascular disease or antihypertensive treatment in a community‐based survey from Taiwan was somewhat lower at 12%.43 Importantly for the rapidly aging population demographic seen in Asia, the prevalence of white‐coat hypertension is higher in the elderly.20
Currently available evidence suggests that the cardiovascular risk associated with white‐coat hypertension in the absence of other coexisting risk factors is negligible.44, 45, 46, 47 In the Japan Morning Surge‐Home Blood Pressure (J‐HOP) study, white‐coat hypertension detected using home blood pressure monitoring (HBPM) was not significantly associated with stroke risk over a median follow‐up duration of 3.9 years.48 Conversely, data from a population‐based study (also conducted in Japan) documented a significant independent association between white‐coat hypertension diagnosed based on elevated office BP and normal home or ambulatory BP and increased stroke risk.49 Nevertheless, the majority of data suggest no increased risk of CVD events in low‐risk patients who have white‐coat hypertension, as shown by data from a general Japanese population (n = 1332) who were followed for 10 years; there were no significant differences in the risk of cardiovascular mortality and stroke morbidity in individuals with white‐coat hypertension compared with normotensives (RR 1.28, 95% CI 0.76‐2.14).50
Although CVD risk is not usually an issue in patients with white‐coat hypertension in the absence of other risk factors, effective detection of white‐coat hypertension remains important so that over‐treatment can be avoided. Not only does over‐use of antihypertensives have a negative impact on healthcare expenditure, it also unnecessarily exposes individuals to the potential risks associated with antihypertensive drug therapy (eg, hypotension, especially in the elderly).51 Nevertheless, regular (eg, annual) monitoring of patients with white‐coat hypertension might be reasonable to allow detection of progression to sustained hypertension,52, 53, 54 which would be an indication for therapeutic intervention.
4. MASKED HYPERTENSION IN ASIA
Masked hypertension is a significant clinical entity given that it has been linked with increased risk of target organ damage and CVD.55 Global prevalence rates for masked hypertension range from 7% to 20%,56, 57, 58, 59, 60, 61 and Asia‐specific rates are at the top end of that range (20% in untreated Chinese outpatients).61 In the ARTEMIS registry, the prevalence of masked hypertension detected using ambulatory BP monitoring (ABPM) showed significant variation between geographic region, being much higher in Asia (16%) than in Europe (9%).20
In contrast to white‐coat hypertension, there is a good body of evidence for the detrimental cardiovascular effects of masked hypertension. Data from Asia are consistent with the overall literature in this area. In terms of target organ damage, impaired flow‐mediated vasodilatation in the brachial artery was documented in a Japanese study of patients with masked hypertension and at least one CVD risk factor.62 In addition, data from a general population (aged ≥40 years) in Japan showed that masked hypertension was significantly associated with the presence of albuminuria (a marker of renal function impairment) after adjustment for other risk factors.63 In another study from Japan conducted in patients with type 2 diabetes, the risk of progression to microalbuminuria was higher in the presence of masked hypertension vs sustained hypertension.64 Retrospective analysis of data from two large Japanese studies suggests that treatment of masked hypertension with intensive antihypertensive therapy targeting morning home BP can improve parameters indicative of target organ damage.65
In the Ohasama study, increases in stroke morbidity and cardiovascular mortality were similar in the presence of masked hypertension and sustained hypertension.50 The increased stroke risk associated with masked hypertension was seen when the phenomenon was detected using ABPM and HBPM, ABPM only or HBPM only.49 Increased stroke risk in patients with masked hypertension was also documented in the J‐HOP study.48
5. MORNING AND NOCTURNAL HYPERTENSION IN ASIA
Due to the influence of a number of factors (eg, neuro‐hormonal effects, environment, behavior), there are diurnal and circadian variations in BP. BP tends to be higher in the morning and lower at night. Variations to this physiological pattern (eg, excessive morning BP surge and/or lack of nocturnal fall [non‐dipper pattern] or an overnight increase [riser pattern] in BP) are pathological forms of BPV that are particularly common in Asian patients. Data from the ARTEMIS study showed that patients with resistant hypertension from Japan have significantly higher morning and moving peak morning systolic BP, and morning and nighttime dynamic BP surge, compared with black and white Americans.16
5.1. Excessive morning BP surge
The association between excessive early morning BP surge and increased stroke risk was first demonstrated by Kario et al66 in elderly hypertensive patients from Japan. In addition, morning BP surge was associated with cerebral hemorrhage in another Japanese population study.67 In studies conducted in Asia (predominantly Japan), exaggerated early morning BP surge has also been associated with target organ damage, including increased left ventricular mass index and left ventricular hypertrophy, impaired diastolic function, increased carotid intima‐media thickness, inflammation, and asymptomatic intracranial stenosis.68, 69, 70, 71, 72
5.2. Nocturnal hypertension
Evaluated using ABPM, nocturnal hypertension and a non‐dipper/riser pattern have been reported to increase the risk of target organ damage and cardiovascular events.73, 74, 75, 76 For example, the proportion of patients with silent cerebral infarcts detected on brain magnetic resonance imaging was significantly higher in elderly Japanese hypertensive patients with a riser vs dipping pattern of nighttime BP (P = .03); riser patients also had a worse prognosis in terms of stroke and cardiac events.74, 77 Furthermore, riser pattern has been shown to closely associate with the development of heart failure with preserved ejection fraction and increase the risk of cardiovascular events in these patients.78, 79 In another Japanese study of elderly patients with hypertension, those with a non‐dipper pattern of both nighttime BP and nighttime pulse rate had the worst cardiovascular prognosis compared with other dipping patterns.80
6. HBPM IN ASIA
HBPM is an effective method to improve awareness and control of hypertension. Management of BP based on HBPM has been shown to result in better BP control (assessed using ABPM) and is therefore recommended for titration of antihypertensive medication.81 HBPM can also help identify white‐coat (uncontrolled) hypertension and masked (uncontrolled) hypertension, and evaluate long‐term BPV.82, 83 Because of the tighter relationship of BP with cardiovascular events, higher prevalence of masked uncontrolled hypertension, higher morning BP and greater BPV in Asian patients, HBPM is likely to be of greater importance in Asian than in Western populations.84 The current status of HBPM in Asia has been reviewed previously,7 and details of current usage of HBPM and local guideline recommendations for each country in the HOPE Asia Network are summarized in specific country reports in this issue.
7. ANTIHYPERTENSIVE MANAGEMENT IN ASIA
Effectively lowering BP, rather than the choice of agent, is the most important goal when trying to reduce cardiovascular risk.85 Lower targets for goal BP are increasingly being recognized and recommended in major hypertension guidelines.54, 81 Preferred antihypertensive agents in Asia are chosen based on their ability to deliver good 24‐hour control of BP in the setting of Asia‐specific hypertension characteristics (eg, salt sensitivity and relative risk of stroke vs CHD). Evaluation of data from the Eastern Asian region comparing a calcium channel blocker (CCB) with an agent from another antihypertensive class showed that reductions in 24‐hour BP were greater with CCBs (by 5 mm Hg for SBP and 3 mm Hg for DBP).86 In addition, data from Thailand showed that response rates to antihypertensive monotherapy were greatest for CCBs followed by diuretics.87 It is perhaps therefore not surprising that CCBs are widely used in Asia, as are angiotensin receptor blockers in most countries (Figure 5). Use of monotherapy varies by region, but is quite high in some countries (Figure 5), despite the fact that combination therapy is often required to achieve BP control. Additional details of antihypertensive therapy by country/region are provided in specific reports in this issue.
Figure 5.
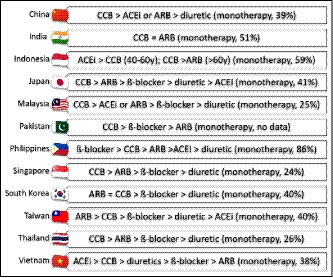
Usage of antihypertensives and proportion of patients receiving antihypertensive monotherapy by country. ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker
8. DEVELOPMENT OF NOCTURNAL HBPM DEVICES AND EVIDENCE
To date, ABPM has been the gold standard for monitoring nighttime BP. HBPM is also recommended for out‐of‐office BP measurement, but has traditionally been used primarily during waking hours. A collaboration between Jichi Medical University and Omron Healthcare Co., Ltd. has seen the development of a semiautomatic nocturnal HBPM device (Medinote; Omron Healthcare Inc). This device automatically measures BP at fixed intervals during sleep using a cuff placed on the patient's arm just before going to bed; BP data are stored in the device memory. It was used to monitor nighttime BP in the J‐HOP study, which showed that self‐measurement of nighttime home BP was feasible and recorded BP values similar to those obtained with ABPM.88, 89, 90, 91 Data from J‐HOP also showed that nighttime home BP was a reliable indicator of target organ damage,89, 90 even more so than nighttime ABPM determined using ABPM.90 The Medinote device was also used in the Japan Target Organ Protection (J‐TOP) trial, and the first time nighttime home BP was assessed in a clinical intervention trial.92, 93 The results showed that nighttime home BP was more closely associated with regression of left ventricular hypertrophy during therapy than clinic BP.93
An updated version of the nocturnal HBPM device (HEM‐7252G‐HP; Omron), which includes a temperature sensor, is now available and was successfully used in studies evaluating the effects of different antihypertensive therapy combinations on uncontrolled nocturnal hypertension or the morning BP surge.94, 95
9. ICT AND FUTURE DIRECTIONS FOR THE HOPE ASIA NETWORK
Recent technology developments suggest that use of information and communication technology (ICT)‐based HBPM devices, which perform automatic, fixed‐interval BP measurement during sleep and store or transmit the data (such as those described above), could facilitate a novel approach to patient management.96 In addition, there are a number of other ICT‐based devices that provide important data for optimization of patient management. These include the addition of a nighttime trigger function (where hypoxia and heart rate are determined using pulse oximetry and BP measurements are taken repeatedly in higher risk situations, as used in a clinical trial of hypertensive patients with sleep apnea97), beat‐by‐beat continuous surge BP monitoring via a wearable device, and determination of environmental factors (eg, temperature, stress, and exercise, Figure 6). Health information technology (HIT) solutions like this are increasingly being recognized as important advances in health care and the important and emerging role of HIT was highlighted in the latest version of the American College of Cardiology/American Heart Association hypertension guidelines.54 BP management using a home BP device with a graphic display of weekly averaged BP values has been shown to facilitate faster BP control than a standard device,98 suggesting the integrated ICT‐based HBPM technologies are an important approach to anticipation medicine. Anticipation medicine is defined as medicine that predicts the time and place of the onset of cardiovascular events based on a time‐series of data to provide a patient and/or physician with advanced warning of potential risk factors, resulting in proactive, real‐time risk reduction (Figure 7).
Figure 6.
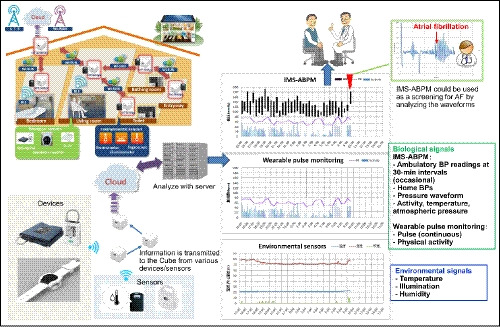
Schematic of a potential information communication technology (ICT)‐based, integrated approach to the individualized management of hypertension (reproduced from Kario et al,102 with permission). ABPM, ambulatory blood pressure monitoring; BP, blood pressure; IMS, ICT‐based multisensor
Figure 7.
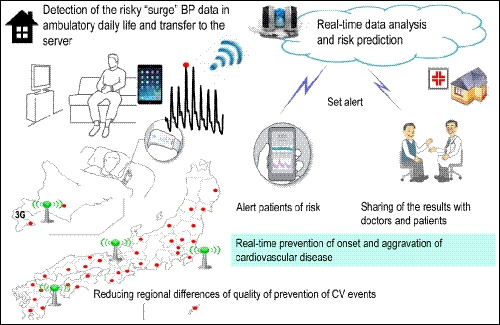
Example of how information communication technology (ICT)‐based technology could be used to facilitate the management of hypertension to reduce the risk of cardiovascular events. BP, blood pressure; CV, cardiovascular
Wearable technologies for evaluation of home BP have recently been validated,99, 100 opening the way for their use in clinical practice. Using these devices and an ICT platform, physicians will be able to obtain data for the evaluation and anticipation of 24‐hour BP control at the individual level, allowing them to work toward the goal of achieving “perfect 24‐hour BP control,” resulting in a “zero” rate of cardiovascular events.101, 102, 103
10. PERSPECTIVES AND CONCLUSIONS
All countries and regions in Asia face the growing problem of non‐communicable diseases (NCDs), of which hypertension and CVD are a significant part. The World Health Organization has proposed nine voluntary global targets to control NCDs by 2025, one of which is to reduce the prevalence of hypertension by 25%.104 Given the unique features of hypertension in Asia, specific evidence is essential to ensure that local guidelines and practice recommend strategies that will be effective in the target populations. Increasing using of HBPM and related ICT‐based advances will provide HIT solutions that maximize the potential to improve outcomes. Using such an approach will contribute to anticipation medicine strategies designed to detect and prevent cardiovascular events in patients with hypertension. Variations in practices and BP control rates across Asia highlight the importance of information sharing in the region to facilitate best practice for the effective management of hypertension and prevention of cardiovascular disease.
CONFLICT OF INTEREST
K Kario received research grants from Omron Healthcare, Fukuda Denshi, A&D, Pfizer Japan, and honoraria from Omron Healthcare. S Park has received honoraria from Pfizer, Daiichi Sankyo, Takeda, Daewon pharmaceutical company, Boryung pharmaceutical company, and Servier. S Siddique has received honoraria from Bayer, Novartis, Pfizer, ICI, and Servier; and travel, accommodation, and conference registration support from Atco Pharmaceutical, Highnoon Laboratories, Horizon Pharma, ICI, Pfizer, and CCL. YC Chia has received honoraria and sponsorship to attend conferences and CME seminars from Abbott, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Menarini, Merck Sharp & Dohme, Novartis, Orient Europharma, Pfizer, and Sanofi; and a research grant from Pfizer. J Nailes has received research grants from Pfizer. J Shin has received honoraria and sponsorship to attend seminars from Daiichi Sankyo, Takeda, Menarini, MSD, Bristol‐Myers Squibb, and Sanofi. CH Chen has served as an advisor or consultant for Novartis Pharmaceuticals Corporation; has served as a speaker or a member of a speakers bureau for AstraZeneca; Pfizer Inc; Bayer AG; Bristol‐Myers Squibb Company; Boehringer Ingelheim Pharmaceuticals, Inc; Daiichi Sankyo, Inc; Novartis Pharmaceuticals Corporation; SERVIER; Merck & Co., Inc; Sanofi; TAKEDA Pharmaceuticals International; and has received grants for clinical research from Microlife Co., Ltd. J Sison has received honoraria from Pfizer, AstraZeneca, AmGen, Boehringer Ingelheim, and Novartis. GP Sogunuru has received a research grant related to hypertension monitoring and treatment from Pfizer. JG Wang has received research grants from Bayer, Merck Sharp & Dohme, Pfizer, and Phillips; and lecture and consulting fees from Bayer, Daiichi Sankyo, Merck Sharp & Dohme, Pfizer, Servier, and Takeda. TD Wang has received honoraria from Abbott, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Medtronic, Menarini, Novartis, Omron, Pfizer, Sanofi, and Servier. Y Zhang has received research grants from Bayer, Novartis, and Shuanghe; and lecture fees from Bayer, Daiichi Sankyo, Novartis, Pfizer, Sanofi, Servier, and Takeda. All other authors report no potential conflicts of interest in relation to this article.
AUTHOR CONTRIBUTIONS
K.K. and J‐G.W. were responsible for the conception and design of the consensus, drafting the manuscript, providing critical review and revision of the manuscript, and the decision to submit the manuscript. Y‐C.C., A.S., Y.T., J.S., C‐H.C., P.B., J.N., S.H., S.S., J.S., A.A.S., G.P.S., J.C.T., B.W.T., Y‐Q.Z., S.P., H.V.M., N.T., T.K., N.V., and T‐D.W. were responsible for drafting the manuscript, providing critical review and revision of the manuscript, and the decision to submit the manuscript.
ACKNOWLEDGMENTS
Medical writing assistance was provided by Nicola Ryan, independent medical writer. Editorial assistance was provided by Ayako Okura, Jichi Medical University.
Kario K, Chia Y‐C, Sukonthasarn A, et al. Diversity of and initiatives for hypertension management in Asia—Why we need the HOPE Asia Network. J Clin Hypertens. 2020;22:331–343. 10.1111/jch.13733
REFERENCES
- 1. Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317:165‐182. [DOI] [PubMed] [Google Scholar]
- 2. Weber MA, Lackland DT. Hypertension: cardiovascular benefits of lowering blood pressure. Nat Rev Nephrol. 2016;12:202‐204. [DOI] [PubMed] [Google Scholar]
- 3. Olsen MH, Angell SY, Asma S, et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet. 2016;388:2665‐2712. [DOI] [PubMed] [Google Scholar]
- 4. Kario K, HOPE Asia (Hypertension Cardiovascular Outcome Prevention and Evidence in Asia) Network . The HOPE Asia Network for "zero" cardiovascular events in Asia. J Clin Hypertens (Greenwich). 2018;20:212‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Angeli F, Reboldi G, Verdecchia P. The 2014 hypertension guidelines: implications for patients and practitioners in Asia. Heart Asia. 2015;7:21‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neupane D, McLachlan CS, Sharma R, et al. Prevalence of hypertension in member countries of South Asian Association for Regional Cooperation (SAARC): systematic review and meta‐analysis. Medicine (Baltimore). 2014;93:e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chia YC, Buranakitjaroen P, Chen CH, et al. Current status of home blood pressure monitoring in Asia: statement from the HOPE Asia Network. J Clin Hypertens (Greenwich). 2017;19:1192‐1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. NCD Risk Factor Collaboration (NCD‐RisC) . Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population‐based measurement studies with 19.1 million participants. Lancet. 2017;389:37‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. NCD Risk Factor Collaboration (NCD‐RisC) . Trends in adult body‐mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population‐based measurement studies with 19.2 million participants. Lancet. 2016;387:1377‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ueshima H, Sekikawa A, Miura K, et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008;118:2702‐2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim AS, Johnston SC. Global variation in the relative burden of stroke and ischemic heart disease. Circulation. 2011;124:314‐323. [DOI] [PubMed] [Google Scholar]
- 12. Lawes CM, Rodgers A, Bennett DA, et al. Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens. 2003;21:707‐716. [DOI] [PubMed] [Google Scholar]
- 13. Ramachandran A, Snehalatha C. Rising burden of obesity in Asia. J Obes. 2010;2010:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishikawa Y, Ishikawa J, Ishikawa S, et al. Prevalence and determinants of prehypertension in a Japanese general population: the Jichi Medical School Cohort Study. Hypertens Res. 2008;31:1323‐1330. [DOI] [PubMed] [Google Scholar]
- 15. Katsuya T, Ishikawa K, Sugimoto K, Rakugi H, Ogihara T. Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens Res. 2003;26:521‐525. [DOI] [PubMed] [Google Scholar]
- 16. Hoshide S, Kario K, de la Sierra A, et al. Ethnic differences in the degree of morning blood pressure surge and in its determinants between Japanese and European hypertensive subjects: data from the ARTEMIS study. Hypertension. 2015;66:750‐756. [DOI] [PubMed] [Google Scholar]
- 17. Kario K, Bhatt DL, Brar S, Bakris GL. Differences in dynamic diurnal blood pressure variability between Japanese and American treatment‐resistant hypertensive populations. Circ J. 2017;81:1337‐1345. [DOI] [PubMed] [Google Scholar]
- 18. Li Y, Staessen JA, Lu L, Li LH, Wang GL, Wang JG. Is isolated nocturnal hypertension a novel clinical entity? Findings from a Chinese population study. Hypertension. 2007;50:333‐339. [DOI] [PubMed] [Google Scholar]
- 19. Li Y, Wang JG. Isolated nocturnal hypertension: a disease masked in the dark. Hypertension. 2013;61:278‐283. [DOI] [PubMed] [Google Scholar]
- 20. Omboni S, Aristizabal D, De la Sierra A, et al. Hypertension types defined by clinic and ambulatory blood pressure in 14 143 patients referred to hypertension clinics worldwide. Data from the ARTEMIS study. J Hypertens. 2016;34:2187‐2198. [DOI] [PubMed] [Google Scholar]
- 21. Parati G, Stergiou GS, Dolan E, Bilo G. Blood pressure variability: clinical relevance and application. J Clin Hypertens (Greenwich). 2018;20:1133‐1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sogunuru GP, Kario K, Shin J, et al. Morning surge in blood pressure and blood pressure variability in Asia: evidence and statement from the HOPE Asia Network. J Clin Hypertens (Greenwich). 2018;21:324‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tse HF, Wang YJ, Ahmed Ai‐Abdullah M, et al. Stroke prevention in atrial fibrillation–an Asian stroke perspective. Heart Rhythm. 2013;10:1082‐1088. [DOI] [PubMed] [Google Scholar]
- 24. Chao TF, Liu CJ, Tuan TC, et al. Lifetime risks, projected numbers, and adverse outcomes in asian patients with atrial fibrillation: a report from the Taiwan Nationwide AF Cohort Study. Chest. 2018;153:453‐466. [DOI] [PubMed] [Google Scholar]
- 25. Lloyd‐Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042‐1046. [DOI] [PubMed] [Google Scholar]
- 26. Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949‐953. [DOI] [PubMed] [Google Scholar]
- 27. Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail. 2015;17:884‐892. [DOI] [PubMed] [Google Scholar]
- 28. Meng Khoo C, Tai ES. Trends in the incidence and mortality of coronary heart disease in Asian Pacific region: the Singapore experience. J Atheroscler Thromb. 2014;21(Suppl 1):S2‐8. [DOI] [PubMed] [Google Scholar]
- 29. Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746‐2753. [DOI] [PubMed] [Google Scholar]
- 30. Stewart S, Keates AK, Redfern A, McMurray JJV. Seasonal variations in cardiovascular disease. Nat Rev Cardiol. 2017;14:654‐664. [DOI] [PubMed] [Google Scholar]
- 31. Iwahori T, Miura K, Obayashi K, et al. Seasonal variation in home blood pressure: findings from nationwide web‐based monitoring in Japan. BMJ Open. 2018;8:e017351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Specified Report of Vital Statistics . Japanese Ministry of Health, Labor and Welfare. 2004.
- 33. Chau PH, Wong M, Woo J. Ischemic heart disease hospitalization among older people in a subtropical city–Hong Kong: does winter have a greater impact than summer? Int J Environ Res Public Health. 2014;11:3845‐3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Y, Du T, Lewin MR, et al. The seasonality of acute coronary syndrome and its relations with climatic parameters. Am J Emerg Med. 2011;29:768‐774. [DOI] [PubMed] [Google Scholar]
- 35. Qiu H, Yu IT, Tse LA, Tian L, Wang X, Wong TW. Is greater temperature change within a day associated with increased emergency hospital admissions for heart failure? Circ Heart Fail. 2013;6:930‐935. [DOI] [PubMed] [Google Scholar]
- 36. Han MH, Yi HJ, Ko Y, Kim YS, Lee YJ. Association between hemorrhagic stroke occurrence and meteorological factors and pollutants. BMC Neurol. 2016;16:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cai Y, Zhang B, Ke W, et al. Associations of short‐term and long‐term exposure to ambient air pollutants with hypertension: a systematic review and meta‐analysis. Hypertension. 2016;68:62‐70. [DOI] [PubMed] [Google Scholar]
- 38. Giorgini P, Di Giosia P, Grassi D, Rubenfire M, Brook RD, Ferri C. Air pollution exposure and blood pressure: an updated review of the literature. Curr Pharm Des. 2016;22:28‐51. [DOI] [PubMed] [Google Scholar]
- 39. Liang R, Zhang B, Zhao X, Ruan Y, Lian H, Fan Z. Effect of exposure to PM2.5 on blood pressure. J Hypertens. 2014;32(11):2130‐2140; discussion 2141. [DOI] [PubMed] [Google Scholar]
- 40. Yang BY, Qian Z, Howard SW, et al. Global association between ambient air pollution and blood pressure: a systematic review and meta‐analysis. Environ Pollut. 2018;235:576‐588. [DOI] [PubMed] [Google Scholar]
- 41. Rajagopalan S, Al‐Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2018;72:2054‐2070. [DOI] [PubMed] [Google Scholar]
- 42. Imaizumi Y, Eguchi K, Kario K. Coexistence of PM2.5 and low temperature is associated with morning hypertension in hypertensives. Clin Exp Hypertens. 2015;37:468‐472. [DOI] [PubMed] [Google Scholar]
- 43. Sung SH, Cheng HM, Wang KL, et al. White coat hypertension is more risky than prehypertension: important role of arterial wave reflections. Hypertension. 2013;61:1346‐1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pierdomenico SD, Cuccurullo F. Prognostic value of white‐coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta analysis. Am J Hypertens. 2011;24:52‐58. [DOI] [PubMed] [Google Scholar]
- 45. Asayama K, Li Y, Franklin SS, Thijs L, O'Brien E, Staessen JA. Cardiovascular risk associated with white‐coat hypertension: con side of the argument. Hypertension. 2017;70:676‐682. [DOI] [PubMed] [Google Scholar]
- 46. Franklin SS, Thijs L, Asayama K, et al. The cardiovascular risk of white‐coat hypertension. J Am Coll Cardiol. 2016;68:2033‐2043. [DOI] [PubMed] [Google Scholar]
- 47. Kario K, Thijs L, Staessen JA. Blood pressure measurement and treatment decisions: masked and white coat hypertension. Circ Res. 2019;124:990‐1008. [DOI] [PubMed] [Google Scholar]
- 48. Fujiwara T, Yano Y, Hoshide S, Kanegae H, Kario K. Association of cardiovascular outcomes with masked hypertension defined by home blood pressure monitoring in a Japanese general practice population. JAMA Cardiol. 2018;3:583‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Satoh M, Asayama K, Kikuya M, et al. Long‐term stroke risk due to partial white‐coat or masked hypertension based on home and ambulatory blood pressure measurements: the Ohasama study. Hypertension. 2016;67:48‐55. [DOI] [PubMed] [Google Scholar]
- 50. Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of "masked" hypertension and "white‐coat" hypertension detected by 24‐h ambulatory blood pressure monitoring 10‐year follow‐up from the Ohasama study. J Am Coll Cardiol. 2005;46:508‐515. [DOI] [PubMed] [Google Scholar]
- 51. Cuspidi C, Sala C, Grassi G, Mancia G. White coat hypertension: to treat or not to treat? Curr Hypertens Rep. 2016;18:80. [DOI] [PubMed] [Google Scholar]
- 52. Chiang CE, Wang TD, Lin TH, et al. The 2017 focused update of the guidelines of the Taiwan Society of Cardiology (TSOC) and the Taiwan Hypertension Society (THS) for the management of hypertension. Acta Cardiol Sin. 2017;33:213‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chiang CE, Wang TD, Ueng KC, et al. 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. J Chin Med Assoc. 2015;78:1‐47. [DOI] [PubMed] [Google Scholar]
- 54. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:2199‐2269.29146533 [Google Scholar]
- 55. Cuspidi C, Sala C, Tadic M, et al. Untreated masked hypertension and carotid atherosclerosis: a meta‐analysis. Blood Press. 2015;24:65‐71. [DOI] [PubMed] [Google Scholar]
- 56. Asayama K, Thijs L, Li Y, et al. Setting thresholds to varying blood pressure monitoring intervals differentially affects risk estimates associated with white‐coat and masked hypertension in the population. Hypertension. 2014;64:935‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sega R, Trocino G, Lanzarotti A, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: Data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study). Circulation. 2001;104:1385‐1392. [DOI] [PubMed] [Google Scholar]
- 58. Stergiou GS, Asayama K, Thijs L, et al. International Database on HOme blood pressure in relation to Cardiovascular Outcome Investigators. Prognosis of white‐coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675‐682. [DOI] [PubMed] [Google Scholar]
- 59. Tocci G, Presta V, Figliuzzi I, et al. Prevalence and clinical outcomes of white‐coat and masked hypertension: analysis of a large ambulatory blood pressure database. J Clin Hypertens (Greenwich). 2018;20:297‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang YC, Shimbo D, Muntner P, Moran AE, Krakoff LR, Schwartz JE. Prevalence of masked hypertension among US adults with nonelevated clinic blood pressure. Am J Epidemiol. 2017;185:194‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang L, Li Y, Wei FF, et al. Strategies for classifying patients based on office, home, and ambulatory blood pressure measurement. Hypertension. 2015;65:1258‐1265. [DOI] [PubMed] [Google Scholar]
- 62. Kabutoya T, Hoshide S, Ogata Y, Eguchi K, Kario K. Masked hypertension defined by home blood pressure monitoring is associated with impaired flow‐mediated vasodilatation in patients with cardiovascular risk factors. J Clin Hypertens (Greenwich). 2013;15:630‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hata J, Fukuhara M, Sakata S, et al. White‐coat and masked hypertension are associated with albuminuria in a general population: the Hisayama Study. Hypertens Res. 2017;40:937‐943. [DOI] [PubMed] [Google Scholar]
- 64. Ushigome E, Oyabu C, Tanaka T, et al. Impact of masked hypertension on diabetic nephropathy in patients with type II diabetes: a KAMOGAWA‐HBP study. J Am Soc Hypertens. 2018;12:364‐371.e361. [DOI] [PubMed] [Google Scholar]
- 65. Hoshide S, Yano Y, Kanegae H, Kario K. Effect of lowering home blood pressure on subclinical cardiovascular disease in masked uncontrolled hypertension. J Am Coll Cardiol. 2018;71:2858‐2859. [DOI] [PubMed] [Google Scholar]
- 66. Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401‐1406. [DOI] [PubMed] [Google Scholar]
- 67. Metoki H, Ohkubo T, Kikuya M, et al. Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline: the Ohasama study. Hypertension. 2006;47:149‐154. [DOI] [PubMed] [Google Scholar]
- 68. Kuwajima I, Mitani K, Miyao M, Suzuki Y, Kuramoto K, Ozawa T. Cardiac implications of the morning surge in blood pressure in elderly hypertensive patients: relation to arising time. Am J Hypertens. 1995;8:29‐33. [DOI] [PubMed] [Google Scholar]
- 69. Kaneda R, Kario K, Hoshide S, Umeda Y, Hoshide Y, Shimada K. Morning blood pressure hyper‐reactivity is an independent predictor for hypertensive cardiac hypertrophy in a community‐dwelling population. Am J Hypertens. 2005;18:1528‐1533. [DOI] [PubMed] [Google Scholar]
- 70. Yano Y, Hoshide S, Inokuchi T, Kanemaru Y, Shimada K, Kario K. Association between morning blood pressure surge and cardiovascular remodeling in treated elderly hypertensive subjects. Am J Hypertens. 2009;22:1177‐1182. [DOI] [PubMed] [Google Scholar]
- 71. Shimizu M, Ishikawa J, Yano Y, Hoshide S, Shimada K, Kario K. The relationship between the morning blood pressure surge and low‐grade inflammation on silent cerebral infarct and clinical stroke events. Atherosclerosis. 2011;219:316‐321. [DOI] [PubMed] [Google Scholar]
- 72. Chen CT, Li Y, Zhang J, et al. Association between ambulatory systolic blood pressure during the day and asymptomatic intracranial arterial stenosis. Hypertension. 2014;63:61‐67. [DOI] [PubMed] [Google Scholar]
- 73. Kario K, Matsuo T, Kobayashi H, Imiya M, Matsuo M, Shimada K. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension. 1996;27:130‐135. [DOI] [PubMed] [Google Scholar]
- 74. Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852‐857. [DOI] [PubMed] [Google Scholar]
- 75. Hoshide S, Kario K, Hoshide Y, et al. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community‐dwelling normotensives. Am J Hypertens. 2003;16:434‐438. [DOI] [PubMed] [Google Scholar]
- 76. Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24‐h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183‐2189. [DOI] [PubMed] [Google Scholar]
- 77. Kario K, Shimada K. Risers and extreme‐dippers of nocturnal blood pressure in hypertension: antihypertensive strategy for nocturnal blood pressure. Clin Exp Hypertens. 2004;26:177‐189. [DOI] [PubMed] [Google Scholar]
- 78. Komori T, Eguchi K, Saito T, Hoshide S, Kario K. Riser pattern: another determinant of heart failure with preserved ejection fraction. J Clin Hypertens (Greenwich). 2016;18:994‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Komori T, Eguchi K, Saito T, Hoshide S, Kario K. Riser pattern is a novel predictor of adverse events in heart failure patients with preserved ejection fraction. Circ J. 2017;81:220‐226. [DOI] [PubMed] [Google Scholar]
- 80. Kabutoya T, Hoshide S, Ishikawa J, Eguchi K, Shimada K, Kario K. The effect of pulse rate and blood pressure dipping status on the risk of stroke and cardiovascular disease in Japanese hypertensive patients. Am J Hypertens. 2010;23:749‐755. [DOI] [PubMed] [Google Scholar]
- 81. Hirawa N, Umemura S, Ito S. Viewpoint on guidelines for treatment of hypertension in Japan. Circ Res. 2019;124:981‐983. [DOI] [PubMed] [Google Scholar]
- 82. Chinese Hypertension Committee of the Chinese Medical Doctors Association , Chinese Hypertension League , Chinese Society of Cardiology of the Chinese Medical Association . Home blood pressure monitoring: a consensus document of China. Chin J Hypertens. 2012;2012:525‐529. [Google Scholar]
- 83. Kario K, Park S, Buranakitjaroen P, et al. Guidance on home blood pressure monitoring: a statement of the HOPE Asia Network. J Clin Hypertens (Greenwich). 2018;20:456‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Park S, Buranakitjaroen P, Chen CH, et al. Expert panel consensus recommendations for home blood pressure monitoring in Asia: the Hope Asia Network. J Hum Hypertens. 2018;32:249‐258. [DOI] [PubMed] [Google Scholar]
- 85. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;387:957‐967. [DOI] [PubMed] [Google Scholar]
- 86. Wang JG, Kario K, Lau T, et al. Use of dihydropyridine calcium channel blockers in the management of hypertension in Eastern Asians: a scientific statement from the Asian Pacific Heart Association. Hypertens Res. 2011;34:423‐430. [DOI] [PubMed] [Google Scholar]
- 87. Sukonthasarn A. A comparison of four primary classes of antihypertensive monotherapy in Thai patients. J Med Assoc Thai. 2000;83:1202‐1210. [PubMed] [Google Scholar]
- 88. Hoshide S, Kario K, Yano Y, et al. Association of morning and evening blood pressure at home with asymptomatic organ damage in the J‐HOP Study. Am J Hypertens. 2014;27:939‐947. [DOI] [PubMed] [Google Scholar]
- 89. Kario K, Hoshide S, Haimoto H, et al. Sleep blood pressure self‐measured at home as a novel determinant of organ damage: Japan Morning Surge Home Blood Pressure (J‐HOP) study. J Clin Hypertens (Greenwich). 2015;17:340‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ishikawa J, Hoshide S, Eguchi K, Ishikawa S, Shimada K, Kario K. Nighttime home blood pressure and the risk of hypertensive target organ damage. Hypertension. 2012;60:921‐928. [DOI] [PubMed] [Google Scholar]
- 91. Kario K, Kanegae H, Tomitani N, et al. Nighttime blood pressure measured by home blood pressure monitoring as an independent predictor of cardiovascular events in general practice. Hypertension. 2019;73:1240‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kario K, Hoshide S, Shimizu M, et al. Effect of dosing time of angiotensin II receptor blockade titrated by self‐measured blood pressure recordings on cardiorenal protection in hypertensives: the Japan Morning Surge‐Target Organ Protection (J‐TOP) study. J Hypertens. 2010;28:1574‐1583. [DOI] [PubMed] [Google Scholar]
- 93. Ishikawa J, Shimizu M, Sugiyama Edison E, et al. Assessment of the reductions in night‐time blood pressure and dipping induced by antihypertensive medication using a home blood pressure monitor. J Hypertens. 2014;32:82‐89. [DOI] [PubMed] [Google Scholar]
- 94. Kario K, Tomitani N, Kanegae H, et al. Comparative effects of an angiotensin II receptor blocker (ARB)/diuretic vs. ARB/calcium‐channel blocker combination on uncontrolled nocturnal hypertension evaluated by information and communication technology‐based nocturnal home blood pressure monitoring‐ the NOCTURNE Study. Circ J. 2017;81:948‐957. [DOI] [PubMed] [Google Scholar]
- 95. Fujiwara T, Tomitani N, Kanegae H, Kario K. Comparative effects of valsartan plus either cilnidipine or hydrochlorothiazide on home morning blood pressure surge evaluated by information and communication technology‐based nocturnal home blood pressure monitoring. J Clin Hypertens (Greenwich). 2018;20:159‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kario K. Nocturnal hypertension: new technology and evidence. Hypertension. 2018;71:997‐1009. [DOI] [PubMed] [Google Scholar]
- 97. Kario K, Kuwabara M, Hoshide S, Nagai M, Shimpo M. Effects of nighttime single‐dose administration of vasodilating vs sympatholytic antihypertensive agents on sleep blood pressure in hypertensive patients with sleep apnea syndrome. J Clin Hypertens (Greenwich). 2014;16:459‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kabutoya T, Ishikawa J, Hoshide S, Eguchi K, Shimada K, Kario K. A home blood pressure monitor equipped with a graphic function facilitates faster blood pressure control than the conventional home blood pressure monitor. J Clin Hypertens (Greenwich). 2009;11:422‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kuwabara M, Harada K, Hishiki Y, Kario K. Validation of a wrist‐type home nocturnal blood pressure monitor in the sitting and supine position according to the ANSI/AAMI/ISO81060‐2:2013 guidelines: Omron HEM‐9600T. J Clin Hypertens (Greenwich). 2019;21:463‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kuwabara M, Harada K, Hishiki Y, Kario K. Validation of two watch‐type wearable blood pressure monitors according to the ANSI/AAMI/ISO81060‐2:2013 guidelines: Omron HEM‐6410T‐ZM and HEM‐6410T‐ZL. J Clin Hypertens (Greenwich). 2019;21:853‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kario K. Evidence and perspectives on the 24‐hour management of hypertension: hemodynamic biomarker‐Initiated ‘anticipation medicine’ for zero cardiovascular event. Prog Cardiovasc Dis. 2016;59:262‐281. [DOI] [PubMed] [Google Scholar]
- 102. Kario K, Tomitani N, Kanegae H, Yasui N, Nagai R, Harada H. The further development of out‐of‐office BP monitoring: Japan's ImPACT Program Project's achievements, impact, and direction. J Clin Hypertens (Greenwich). 2019;21:344‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kario K, Tomitani N, Kanegae H, et al. Development of a new ICT‐based multisensor blood pressure monitoring system for use in hemodynamic biomarker‐initiated anticipation medicine for cardiovascular disease: the National IMPACT Program project. Prog Cardiovasc Dis. 2017;60:435‐449. [DOI] [PubMed] [Google Scholar]
- 104. World Health Organization . Noncommunicable diseases and mental health. About 9 voluntary global targets. Global monitoring framework for NCDs. https://www.who.int/nmh/ncd-tools/definition-targets/en/. Accessed June 25, 2019.
- 105. Powles J, Fahimi S, Micha R, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013;3:e003733. [DOI] [PMC free article] [PubMed] [Google Scholar]


