Abstract
Objectives
To study total, processed, and unprocessed red meat in relation to risk of coronary heart disease (CHD) and to estimate the effects of substituting other protein sources for red meat with CHD risk.
Design
Prospective cohort study with repeated measures of diet and lifestyle factors.
Setting
Health Professionals Follow-Up Study cohort, United States, 1986-2016.
Participants
43 272 men without cardiovascular disease or cancer at baseline.
Main outcome measures
The primary outcome was total CHD, comprised of acute non-fatal myocardial infarction or fatal CHD. Cox models were used to estimate hazard ratios and 95% confidence intervals across categories of red meat consumption. Substitution analyses were conducted by comparing coefficients for red meat and the alternative food in models, including red meat and alternative foods as continuous variables.
Results
During 1 023 872 person years of follow-up, 4456 incident CHD events were documented of which 1860 were fatal. After multivariate adjustment for dietary and non-dietary risk factors, total, unprocessed, and processed red meat intake were each associated with a modestly higher risk of CHD (hazard ratio for one serving per day increment: 1.12 (95% confidence interval 1.06 to 1.18) for total red meat, 1.11 (1.02 to 1.21) for unprocessed red meat, and 1.15 (1.06 to 1.25) for processed red meat). Compared with red meat, the intake of one serving per day of combined plant protein sources (nuts, legumes, and soy) was associated with a lower risk of CHD (0.86 (0.80 to 0.93) compared with total red meat, 0.87 (0.79 to 0.95) compared with unprocessed red meat, and 0.83 (0.76 to 0.91) compared with processed red meat). Substitutions of whole grains and dairy products for total red meat and eggs for processed red meat were also associated with lower CHD risk.
Conclusions
Substituting high quality plant foods such as legumes, nuts, or soy for red meat might reduce the risk of CHD. Substituting whole grains and dairy products for total red meat, and eggs for processed red meat, might also reduce this risk.
Introduction
Substantial evidence from randomized trials and observational studies suggests that high consumption of red meat, especially processed red meat, is associated with an increased risk of mortality1 2 3 and major chronic diseases,4 5 6 7 8 9 including coronary heart disease (CHD).10 11 12 Consequently, the 2015-20 US Dietary Guidelines for Americans13 encourage dietary patterns that are low in red and processed meat intake. Increases in risk were not, however, seen in Asian populations with low consumption of red meat, or in populations in which consumption of red meat has recently increased.14 15 16 These inconsistencies could be due to the variable amounts and duration of red meat consumed in different populations,15 16 inadequate differentiation between processed and unprocessed red meat,15 17 18 19 differences in the levels of controlling for confounding,15 18 and, importantly, differences in the comparison sources of energy.15 20 21 22 In particular, in most populations, most energy intake come from refined starches, sugar, potatoes, and fats that are highly saturated or partially hydrogenated. Thus, analyses that fail to specify comparison foods are by default mainly comparing red meat with these suboptimal sources of energy intake. Therefore, lack of an association of red meat with disease outcomes simply implies that red meat is as unhealthy as these alternative foods.
To address these problems in study design and analysis, we examined the relation between total, processed, and unprocessed red meat and risk of CHD in the large prospective Health Professionals Follow-up Study cohort with repeated measures of diet during 30 years of follow-up. We estimated the effects of substituting other protein sources for red meat with CHD risk and evaluated the temporal relation of red meat consumption to risk of developing CHD.
Methods
Study population
The Health Professionals Follow-up Study started in 1986 when 51 529 US male health professionals (29 683 dentists, 10 098 veterinary surgeons, 4185 pharmacists, 3745 optometrists, 2218 osteopathic physicians, and 1600 podiatrists) aged 40 to 75 years provided detailed information on their medical history, lifestyle, and typical diet. Questionnaires have been completed biennially to update information on potential risk factors and occurrence of new diseases. A detailed description of the cohort has been published elsewhere.23
Dietary data were not included if participants left more than 70 items blank in the food frequency questionnaire or had implausible total energy intake (<800 kcal/day (1 kcal=4.18 kJ=0.00418 MJ) or >4200 kcal/day)24 in any of the food frequency questionnaires. Participants were excluded if at baseline they had a history of cancer (n=1645), myocardial infarction, angina, or coronary artery bypass graft (CABG, n=3696), or stroke (n=221). A total of 43 272 participants were included and subsequently followed up.
Dietary assessment
Participants completed a semiquantitative food frequency questionnaire in 1986 and every four years thereafter. Participants were asked how often, on average, they had consumed a standard portion of food in the past year. Nine responses were possible and ranged from “never” to “more than six times per day.” The items on processed red meat included beef or pork hotdogs, bacon, salami, bologna, or other processed meat sandwiches, in addition to other processed meats such as sausages and kielbasa. Items on unprocessed red meat included hamburger (lean or extra lean), regular hamburgers, beef, pork, or lamb as a main or mixed dish or sandwich. Total red meat included processed and unprocessed red meat. Other protein sources, apart from red meat, included poultry, fish, eggs, high fat dairy products, low fat dairy products, nuts, legumes, soy, and whole grains (supplemental table 1). Plant based protein foods included nuts, legumes, and soy foods.
The reproducibility and validity of the food frequency questionnaire in measuring food intake have been described in detail previously.25 The correlation coefficients between the questionnaire and multiple dietary records were 0.59 for unprocessed red meat; 0.52 for processed red meat; 0.48 for poultry; 0.74 for fish; 0.56 for eggs; 0.62 for each of high fat and low fat dairy products; 0.46 for legumes, including soybeans and tofu; 0.45 for nuts; and 0.27 for whole grains.24 In this study, we also calculated a modified diet score of the Alternative Healthy Eating Index to assess overall diet quality after removing the red meat components.26
Ascertainment of outcome
The primary outcome for this study was total CHD, comprised of acute non-fatal myocardial infarction or fatal CHD, occurring after the return of the 1986 food frequency questionnaire but before 31 January 2016. Myocardial infarction was initially self-reported and confirmed by medical records documenting symptoms and either diagnostic electrocardiographic changes or raised levels of cardiac specific enzymes. Physicians blinded to the participants’ exposure status reviewed the medical records. For those with unavailable medical records, the diagnosis was considered probable (10.3% of total participants) if supported by telephone interview or other supplemental information. Deaths were identified from searches of vital records, the National Death Index, and reports by the participant’s next of kin or the postal system.12 27 Using these methods, at least 98% of deaths were ascertained.27 Fatal CHD included fatal myocardial infarction, or if CHD was listed as cause of death on the death certificate and there was evidence of previous coronary disease. Sudden death within one hour of the onset of symptoms in men with no other plausible cause of death (other than coronary disease) was considered as fatal CHD.
Assessment of covariates
In the biennial follow-up questionnaires, we inquired about and updated information on known or potential risk factors for CHD, including body mass index (BMI; <21, 21-22.9, 23-24.9, 25-26.9, 27-29.9, 30-32.9, 33-34.9, 35-39.9, ≥40), cigarette smoking (never smoker, former smoker, current 1-14 cigarettes/day, current 15-24 cigarettes/day, current ≥25 cigarettes/day), alcohol consumption (0, 0.1-4.9, 5.0-9.9, 10-14.9, or ≥15.0 g/day), total energy intake (in fifths), family history of myocardial infarction or stroke (defined as event before age 65 years for a participant’s mother or before age 55 years for a participant’s father), multivitamin use (yes, no), aspirin use (yes, no), race or ethnicity (white, black, Asian, other), work status (full time, part time, retired), profession (dentist, pharmacist, optometrist, podiatrist, veterinary surgeon), living arrangement (lives with family, lives alone, other), and marital status (married, divorced, widowed, never married). Data on physical activity (<3, 3-8.9, 9-17.9, 18-26.9, and ≥27 in metabolic equivalents of task per week) were also collected using the validated physical activity questionnaire.28 In case of missing data, the last value was carried forward for one two year cycle. If the last value was missing, then a missing indicator was created.
Statistical analysis
Age adjusted and multivariate adjusted Cox proportional hazard models were used to estimate hazard ratios and 95% confidence intervals across the fifths of total, processed, and unprocessed red meat consumption in relation to CHD risk. Person years of follow-up were calculated from the return of the 1986 food frequency questionnaire to the date of the first CHD event, death, or end of follow-up, whichever came first. The main models were adjusted for age (in months), calendar time (two year follow-up periods), and energy intake, in addition to BMI, physical activity, smoking status, alcohol intake, family history of myocardial infarction or stroke, multivitamin use, aspirin use, race or ethnicity, work status, profession, living arrangement, and marital status. We further adjusted for other dietary variables, including poultry (unprocessed), fish, egg, high fat dairy, low fat dairy, nuts, legumes, soy, whole grains, fruit, vegetables, coffee, and glycemic index (all in fifths).
To better represent long term diet and minimize within person variation, we calculated the cumulative average of food intake from baseline up to the beginning of each two year follow-up interval. We then investigated the cumulative average intake in relation to risk of CHD from the beginning of each follow-up interval until the next follow-up interval. To minimize the possibility of reverse causation bias, we stopped updating diet after the participant’s diagnosis of cancer or stroke, or after reporting diabetes, angina, or CABG.
We investigated the associations of substituting a single serving of alternative foods for red meat (total, processed, or unprocessed) with CHD risk by including the alternative foods as continuous variables in the same multivariable model and accounting for other dietary and non-dietary variables as well as total energy intake. The difference in the β coefficients of the two foods being compared, and their variances and covariances, were used to estimate the hazard ratio and 95% confidence interval for the substitution.12
Stratified analysis by age (<65, ≥65 years), BMI (<25, ≥25), calendar time (<2000, 2000 or later), and total fiber intake (<28, ≥28 g/day) were also performed. Effect modification was tested after including the multiplicative interaction term between the continuous dietary variables included in the substitution model and each of age, BMI, calendar time, and total fiber intake.
Time lagged analysis with varying non-overlapping lag time periods (0-4 years, 4-8 years, 8-12 years, 12-16 years, 16-20 years, or 20-24 years) was conducted to predict the risk of CHD. For example, for latency of 4-8 years, we used dietary intake of 1986 to predict CHD risk during 1990 to 1994, the dietary intake of 1994 for CHD events occurring from 1998 to 2002, dietary intake in 1998 for CHD events occurring from 2002 to 2006, and so forth. The lagged analyses allow an evaluation of the latency between consumption of a dietary factor and occurrence of the outcome but do not account for correlation of intakes over time.
We also conducted several sensitivity analyses to test the robustness of our results. To check for possible confounding by other aspects of diet, we performed a sensitivity analysis adjusting for a modified diet score of the Alternative Healthy Eating Index that excluded the red meat components. In another sensitivity analysis, we included as covariates baseline history of diabetes, hypertension, and hypercholesterolemia, which might act as intermediates on the pathway linking red meat consumption and risk of CHD. Sensitivity analyses after excluding probable events were similar, so only total numbers of acute myocardial infarction events were presented. In addition to using the cumulative average intake updated until the development of major diseases (ie, incidence of cancer, stroke, diabetes, or angina, or CABG), we used baseline diet; most recent diet; cumulative average, which was continually updated even after the diagnosis of a major disease; and cumulative updated average adjusted for the incidence of major diseases (cancer, stroke, diabetes, angina, or CABG) in the multivariate model.
The proportional hazards assumption was tested by including an interaction term between red meat intake and months to events. To test for linear trend, the median intakes for each fifth were modeled as a single continuous variable. Data were analyzed in SAS software (version 9.4, SAS Institute) at a two tailed α level of 0.05.
Patient and public involvement
No participants were involved in setting the research question or the outcome measures, nor were they involved in the design and implementation of the study. Results from the Health Professionals Follow-Up Study cohort are routinely disseminated to study participants through the study website and social media outlets. We plan to disseminate these findings to participants in our annual newsletter and to the general public in a press release.
Results
During 1 023 872 person years of follow-up of 43 272 participants, 4456 incident CHD events were documented of which 1860 were fatal. At baseline, participants were on average aged 53 (SD 9.5) years and had a mean BMI of 25.5 (SD 3.3). Around 54% were never smokers, 20% had a history of hypertension, 2% had diabetes, and 10% had high cholesterol levels. Those with higher total red meat consumption were more likely to smoke, consume alcohol, have diabetes, and use aspirin. They had higher intakes of total energy and trans fatty acids but were less physically active and less likely to have hypercholesterolemia or a family history of cardiovascular diseases. They had lower intakes of multivitamins, fruit, vegetables, and cereal fiber compared with those in the lower fifths of total red meat intake. Similar distributions were observed with processed and unprocessed red meat consumption (table 1).
Table 1.
Age standardized baseline characteristics of participants (n=43 272) by fifths of total, unprocessed, and processed red meat intake. Values are numbers (percentages) unless stated otherwise
| Characteristics | Total red meat intake | Unprocessed red meat intake | Processed red meat intake | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First fifth (n=8599) | Third fifth (n=8331) | Fifth fifth (n=8640) | First fifth (n=9063) | Third fifth (n=9955) | Fifth fifth (n=9840) | First fifth (n=11 069) | Third fifth (n=9503) | Fifth fifth (n=8754) | |||
| Median intake (servings/day) | 0.21 | 0.85 | 1.93 | 0.14 | 0.50 | 1.29 | 0.00 | 0.21 | 0.93 | ||
| Mean (SD) age (years) | 54 (10) | 53 (10) | 52 (9) | 55 (10) | 53 (10) | 52 (9) | 54 (10) | 52 (10) | 53 (9) | ||
| White ethnicity | 8023 (93) | 7393 (95) | 8286 (96) | 8410 (93) | 9487 (95) | 9456 (96) | 10 427 (94) | 9028 (95) | 8386 (96) | ||
| Current smoker | 404 (5) | 783 (9) | 1166 (14) | 489 (5) | 956 (10) | 1132 (12) | 531 (5) | 874 (9) | 1226 (14) | ||
| Mean (SD) physical activity (MET h/wk) | 26 (30) | 19 (23) | 17 (22) | 26 (30) | 19 (24) | 17 (22) | 24 (29) | 20 (24) | 17 (22) | ||
| Body mass index | 25 (3.1) | 26 (3.2) | 26 (3.4) | 25 (3.2) | 26 (3.2) | 26 (3.4) | 25 (3.1) | 26 (3.2) | 26 (3.6) | ||
| Family history of CVD | 1187 (14) | 958 (12) | 968 (11) | 1214 (13) | 1175 (12) | 1141 (12) | 1494 (14) | 1102 (12) | 963 (11) | ||
| History of diabetes | 172 (2) | 175 (2) | 302 (4) | 208 (2) | 239 (2) | 305 (3) | 232 (2) | 209 (2) | 289 (3) | ||
| History of hypertension | 1694 (20) | 1683 (20) | 1737 (20) | 1794 (20) | 1981 (20) | 1988 (20) | 2181 (20) | 1929 (20) | 1716 (20) | ||
| History of hypercholesterolemia | 1273 (15) | 808 (10) | 700 (8) | 1242 (14) | 966 (10) | 856 (9) | 1516 (14) | 950 (10) | 709 (8) | ||
| Multivitamin use | 4282 (50) | 3466 (42) | 3266 (38) | 4504 (50) | 4121 (41) | 3769 (38) | 5346 (48) | 3896 (41) | 3353 (38) | ||
| Aspirin use | 2141 (25) | 2141 (26) | 2436 (28) | 2284 (25) | 2668 (27) | 2745 (28) | 2734 (25) | 2585 (27) | 2495 (29) | ||
| Mean (SD) alcohol intake (g/day) | 9 (13) | 12 (15) | 14 (18) | 9 (13) | 12 (16) | 13 (17) | 9 (13) | 12 (15) | 14 (18) | ||
| Mean (SD) total energy intake (kcal/day) | 1684 (544) | 1906 (521) | 2502 (605) | 1680 (543) | 1899 (530) | 2441 (613) | 1772 (560) | 1943 (569) | 2365 (633) | ||
| Mean (SD) fruit intake (servings/day)* | 2.2 (1.6) | 1.5 (1.1) | 1.2 (1.1) | 2.1 (1.6) | 1.5 (1.1) | 1.2 (1.1) | 2.1 (1.5) | 1.5 (1.1) | 1.2 (1.0) | ||
| Mean (SD) vegetable intake (servings/day)* | 3.8 (2.2) | 3.1 (1.7) | 2.7 (1.7) | 3.7 (2.2) | 3.1 (1.7) | 2.8 (1.8) | 3.7 (2.2) | 3.0 (1.6) | 2.7 (1.7) | ||
| Mean (SD) trans fatty acid intake (g/day)* | 2.1 (1.1) | 3.0 (1.1) | 3.3 (1.0) | 2.2 (1.2) | 2.9 (1.1) | 3.2 (1.0) | 2.3 (1.2) | 2.9 (1.0) | 3.3 (1.0) | ||
| Mean (SD) cereal fiber intake (g/day)* | 7.4 (5.4) | 5.6 (3.4) | 4.8 (2.6) | 7.1 (5.1) | 5.6 (3.5) | 5.0 (2.8) | 7.0 (5.1) | 5.6 (3.5) | 5.0 (2.7) | ||
| Mean (SD) glycemic index* | 53 (4.0) | 53 (3.5) | 54 (3.4) | 53 (4.0) | 53 (3.6) | 54 (3.4) | 53 (4.0) | 53 (3.4) | 53 (3.4) | ||
MET=metabolic equivalents of task; CVD=cardiovascular disease.
Energy adjusted.
In age adjusted analyses, higher intakes of total red meat, unprocessed red meat, and processed red meat were each positively associated with higher risk of CHD (table 2). After further adjustment for non-dietary cardiovascular disease risk factors and energy intake, the associations of total, processed, and unprocessed red meat consumption with CHD risk each remained statistically significant but attenuated. Adjusting for other major dietary variables such as poultry, fish, egg, high fat dairy products, low fat dairy products, nuts, legumes, soy, and whole grains in addition to fruit, vegetables, coffee, and glycemic index further attenuated the associations, but total, unprocessed, and processed red meat remained significantly associated with risk of CHD (comparing the fifth fifth (high intake) with the first fifth, hazard ratio 1.28 (95% confidence interval 1.14 to 1.45, P<0.001 for trend) for total red meat, 1.18 (1.05 to 1.32, P=0.01 for trend) for unprocessed red meat, and 1.19 (1.07 to 1.33, P=0.001 for trend) for processed red meat consumption, see table 2). For an increment of one serving per day, total red meat was associated with a 12% (95% confidence interval 6% to 18%) higher risk of CHD. Similar associations were observed for unprocessed and processed red meat (table 2).
Table 2.
Hazard ratios (95% confidence intervals) for total coronary heart disease associated with fifths of total, unprocessed, and processed red meat intake (n=43 272)
| Fifths of red meat intake | Hazard ratio (95% CI) per 1 serving/day | P for trend* | |||||
|---|---|---|---|---|---|---|---|
| First | Second | Third | Fourth | Fifth | |||
| Total red meat | |||||||
| Median servings/day | 0.21 | 0.52 | 0.78 | 1.14 | 1.72 | ||
| No of events/person years | 811/203 879 | 833/206 108 | 859/203 718 | 865/206 087 | 1087/204 079 | ||
| Age adjusted model† | 1 | 1.08 (0.98 to 1.19) | 1.15 (1.04 to 1.27) | 1.15 (1.04 to 1.27) | 1.47 (1.34 to 1.61) | 1.20 (1.16 to 1.26) | <0.001 |
| Multivariable adjusted model 1‡ | 1 | 1.06 (0.96 to 1.17) | 1.11 (1.00 to 1.23) | 1.09 (0.97 to 1.21) | 1.34 (1.21 to 1.49) | 1.15 (1.09 to 1.21) | <0.001 |
| Multivariable adjusted model 2§ | 1 | 1.06 (0.96 to 1.18) | 1.11 (0.99 to 1.23) | 1.08 (0.97 to 1.21) | 1.28 (1.14 to 1.45) | 1.12 (1.06 to 1.18) | <0.001 |
| Unprocessed red meat | |||||||
| Median servings/day | 0.14 | 0.35 | 0.5 | 0.71 | 1.09 | ||
| No of events/person years | 847/205 918 | 876/199 361 | 840/207 111 | 877/201 942 | 1016/209 540 | ||
| Age adjusted model† | 1 | 1.13 (1.02 to 1.24) | 1.08 (0.98 to 1.19) | 1.17 (1.06 to 1.28) | 1.36 (1.24 to 1.49) | 1.27 (1.18 to 1.35) | <0.001 |
| Multivariable adjusted model 1‡ | 1 | 1.12 (1.01 to 1.23) | 1.05 (0.95 to 1.16) | 1.12 (1.01 to 1.23) | 1.24 (1.12 to 1.37) | 1.17 (1.08 to 1.26) | <0.001 |
| Multivariable adjusted model 2§ | 1 | 1.11 (1.01 to 1.22) | 1.04 (0.94 to 1.16) | 1.09 (0.98 to 1.22) | 1.18 (1.05 to 1.32) | 1.11 (1.02 to 1.21) | 0.01 |
| Processed red meat | |||||||
| Median servings/day | 0.02 | 0.14 | 0.21 | 0.38 | 0.71 | ||
| No of events/person years | 889/224 469 | 734/181 661 | 883/211 353 | 843/201 440 | 1107/204 950 | ||
| Age adjusted model† | 1 | 1.05 (0.95 to 1.15) | 1.14 (1.04, 1.25) | 1.12 (1.02 to 1.24) | 1.39 (1.27 to 1.52) | 1.32 (1.24 to 1.41) | <0.001 |
| Multivariable adjusted model 1‡ | 1 | 1.02 (0.93 to 1.13) | 1.09 (0.99, 1.20) | 1.06 (0.96 to 1.16) | 1.24 (1.12 to 1.36) | 1.20 (1.12 to 1.30) | <0.001 |
| Multivariable adjusted model 2§ | 1 | 1.02 (0.92 to 1.13) | 1.09 (0.98, 1.20) | 1.05 (0.95 to 1.17) | 1.19 (1.07 to 1.33) | 1.15 (1.06 to 1.25) | 0.001 |
P value when each fifth was assigned the median value and treated as a continuous variable.
Adjusted for age and year of questionnaire return.
Adjusted for variables in age-adjusted model+race or ethnicity (white, black, Asian, other), marital status (married, divorced, widowed, never married), living arrangement (lives with family, lives alone, other), profession (dentist, pharmacist, optometrist, podiatrist, veterinarian), work status (full time, part time, retired), smoking status (never smoker, former smoker, current 1-14 cigarettes/d, current 15-24 cigarettes/d, current ≥25 cigarettes/d), physical activity(<3, 3-8.9, 9-17.9, 18-26.9, and ≥27 in metabolic equivalents of task/wk), body mass index;(<21, 21-22.9, 23-24.9, 25-26.9, 27-29.9, 30-32.9, 33-34.9, 35-39.9, ≥40), alcohol intake (0, 0.1-4.9, 5.0-9.9, 10-14.9, or ≥15.0 g/d), multivitamin use (yes, no), aspirin use (yes, no), family history of early coronary heart disease or stroke (diagnosis <60 years; yes, no), and total energy intake (fifths).
Adjusted for variables in model 1+intakes of poultry, fish, egg, high fat dairy, low fat dairy, nuts, legumes, soy, whole grains, fruit, vegetables, and coffee, and glycemic index.
Associations of each of total, unprocessed, and processed red meat with fatal CHD were slightly stronger (1.38 (1.15 to 1.66) for total red meat, 1.29 (1.08 to 1.53) for unprocessed red meat, and 1.21 (1.02 to 1.43) for processed red meat) (see supplemental table 2).
In sensitivity analyses, the associations between red meat intake and CHD risk became slightly weaker after including baseline history of diabetes, hypertension, and hypercholesterolemia in the model, or after adjusting for the modified AHEI score (see supplemental table 3).
Compared with intakes of total, unprocessed, or processed red meat, intakes of nuts, legumes, soy, and combined plant protein sources (nuts, legumes, and soy) were each associated with a significantly lower risk of CHD (fig 1, supplemental table 4). Specifically, the hazard ratios for total, unprocessed, and processed red meat intake were 0.86 (0.80 to 0.93), 0.87 (0.79 to 0.95), and 0.83 (0.76 to 0.91) when compared with one serving per day of combined plant protein sources (fig 1). Intake of high fat dairy products, low fat dairy products, and whole grains were also associated with a lower CHD risk compared with intake of total, unprocessed, and processed red meat (fig 1, supplemental table 4). Egg intake was additionally associated with a lower CHD risk compared with intake of processed red meat (0.87 (0.76 to 0.99), fig 1).
Fig 1.
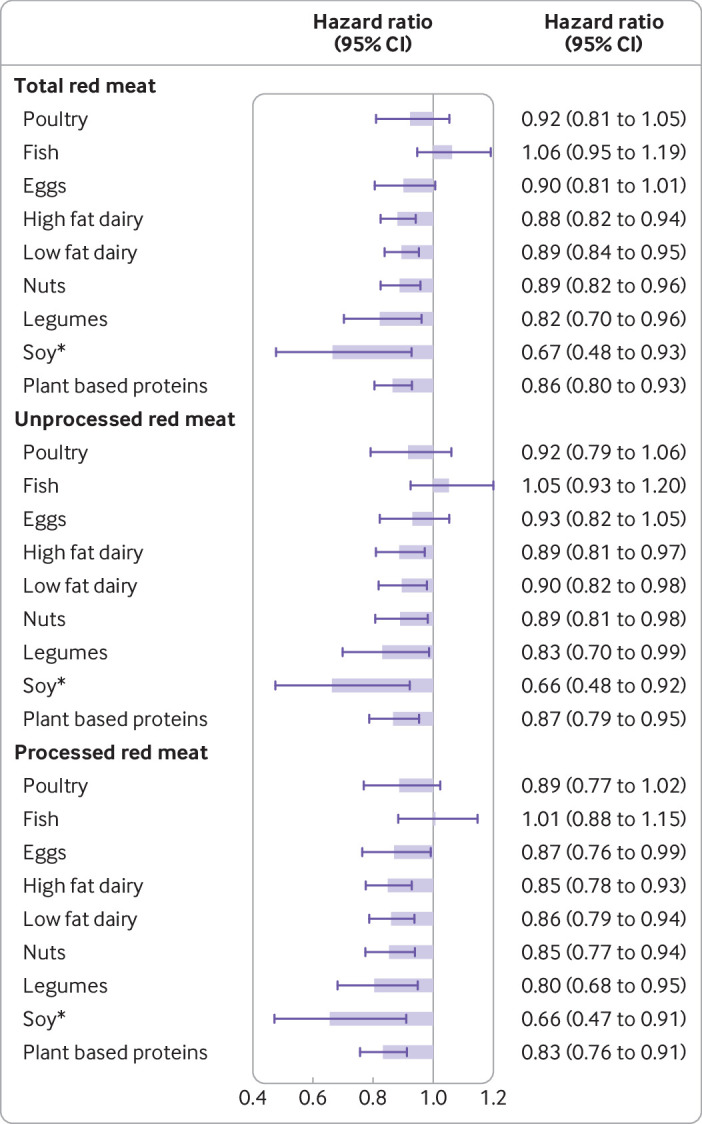
Hazard ratios (95% confidence intervals) for total coronary heart disease associated with replacement of one serving per day of total, unprocessed, and processed red meat with one serving per day of other protein sources. *Replacing ≥2 servings/week of red meat with ≥2 servings/week of soy
Milk (both skimmed and whole), yogurt, and cheese were each associated with a 10% to 22% lower risk of CHD compared with red meat (table 3). These associations were more pronounced when one serving of processed red meat was replaced with one serving of each of these dairy products (table 3).
Table 3.
Hazard ratios (95% confidence intervals) for total coronary heart disease associated with replacement of one serving per day of total, unprocessed, and processed red meat with one serving per day of each type of dairy product
| Hazard ratio (95% CI) | P value | |
|---|---|---|
| Total red meat | ||
| Dairy products: | ||
| Total milk* | 0.90 (0.85 to 0.96) | 0.002 |
| Skimmed milk | 0.90 (0.85 to 0.96) | 0.002 |
| Whole milk | 0.90 (0.82 to 0.99) | 0.03 |
| Yoghurt† | 0.78 (0.64 to 0.94) | 0.01 |
| Cheese‡ | 0.89 (0.82 to 0.98) | 0.01 |
| Unprocessed red meat | ||
| Dairy products: | ||
| Total milk* | 0.91 (0.83 to 0.99) | 0.03 |
| Skimmed milk | 0.91 (0.83 to 0.99) | 0.04 |
| Whole milk | 0.90 (0.81 to 1.01) | 0.08 |
| Yoghurt† | 0.77 (0.63 to 0.94) | 0.01 |
| Cheese‡ | 0.91 (0.81 to 1.01) | 0.07 |
| Processed red meat | ||
| Dairy products: | ||
| Total milk* | 0.87 (0.80 to 0.95) | 0.001 |
| Skimmed milk | 0.87 (0.80 to 0.95) | 0.002 |
| Whole milk | 0.86 (0.77 to 0.97) | 0.010 |
| Yoghurt† | 0.74 (0.60 to 0.90) | 0.003 |
| Cheese‡ | 0.86 (0.77 to 0.96) | 0.007 |
Models were adjusted for age, year of questionnaire return, race or ethnicity (white, black, Asian, other), marital status (married, divorced, widowed, never married), living arrangement (lives with family, lives alone, other), profession (dentist, pharmacist, optometrist, podiatrist, veterinarian), work status (full time, part time, retired), smoking status (never smoker, former smoker, current 1-14 cigarettes/d, current 15-24 cigarettes/d, current ≥25 cigarettes/d), physical activity(<3, 3-8.9, 9-17.9, 18-26.9, and ≥27 in metabolic equivalents of task /wk), body mass index;(<21, 21-22.9, 23-24.9, 25-26.9, 27-29.9, 30-32.9, 33-34.9, 35-39.9, ≥40), alcohol intake (0, 0.1-4.9, 5.0-9.9, 10-14.9, or ≥15.0 g/d), multivitamin use (yes, no), aspirin use (yes, no), family history of early coronary heart disease or stroke (diagnosis <60 years; yes, no), and total energy intake (fifths), and intakes of poultry, fish, egg, combined plant protein sources of nuts, legumes, and soy, whole grains, fruit, vegetables, and coffee, in addition to total milk, yoghurt, cheese, and other dairy products, and glycemic index. For the analyses of skimmed and whole milk, the models were modified to include these two variables instead of total milk.
Skimmed, low fat, and whole milk.
Flavored and plain yoghurt.
Cottage or ricotta cheese, cream cheese, and other cheese.
Other dairy products included ice cream, sherbert, and cream.
Replacement of red meat with total fish was not associated with CHD risk. In a more detailed analysis according to types of fish (dark meat fish, canned tuna, and other fish), on stratifying by calendar year of follow-up (<2000, ≥2000), intake of dark meat fish was observed to be associated with a lower CHD risk compared with intake of total red meat (0.56 (0.33 to 0.95), unprocessed red meat (0.54 (0.31 to 0.92), and processed red meat (0.52 (0.30 to 0.90; table 4), in 2000 or later but not earlier. Other fish intake was, however, associated with higher CHD risk compared with intake of total, unprocessed, and processed red meat.
Table 4.
Hazard ratios (95% confidence intervals) for total coronary heart disease associated with replacement of one serving per day of total, unprocessed, and processed red meat with one serving per day of each type of fish, stratified by follow-up period (<2000, ≥2000)
| Overall | <2000 | ≥2000 | ||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |||
| Total red meat | ||||||||
| Dark meat fish | 0.89 (0.67 to 1.20) | 0.45 | 1.10 (0.78 to 1.56) | 0.57 | 0.56 (0.33 to 0.95) | 0.03 | ||
| Tuna | 1.03 (0.84 to 1.25) | 0.79 | 1.08 (0.84 to 1.39) | 0.55 | 0.97 (0.70 to 1.35) | 0.85 | ||
| Other fish* | 1.20 (0.95 to 1.51) | 0.12 | 1.03 (0.77 to 1.38) | 0.85 | 1.59 (1.08 to 2.35) | 0.02 | ||
| Unprocessed red meat | ||||||||
| Dark meat fish | 0.88 (0.65 to 1.18) | 0.39 | 1.10 (0.77 to 1.58) | 0.59 | 0.54 (0.31 to 0.92) | 0.02 | ||
| Tuna | 1.01 (0.82 to 1.24) | 0.95 | 1.08 (0.83 to 1.40) | 0.59 | 0.93 (0.66 to 1.31) | 0.67 | ||
| Other fish* | 1.18 (0.93 to 1.49) | 0.17 | 1.02 (0.75 to 1.39) | 0.88 | 1.54 (1.04 to 2.29) | 0.03 | ||
| Processed red meat | ||||||||
| Dark meat fish | 0.84 (0.62 to 1.14) | 0.27 | 1.05 (0.73 to 1.49) | 0.81 | 0.52 (0.30 to 0.90) | 0.02 | ||
| Tuna | 0.98 (0.79 to 1.20) | 0.81 | 1.02 (0.79 to 1.33) | 0.87 | 0.92 (0.65 to 1.29) | 0.62 | ||
| Other fish* | 1.14 (0.90 to 1.45) | 0.28 | 0.97 (0.71 to 1.32) | 0.85 | 1.53 (1.03 to 2.29) | 0.04 | ||
Models were adjusted for age, year of questionnaire return, race or ethnicity (white, black, Asian, other), marital status (married, divorced, widowed, never married), living arrangement (lives with family, lives alone, other), profession (dentist, pharmacist, optometrist, podiatrist, veterinarian), work status (full time, part time, retired), smoking status (never smoker, former smoker, current 1-14 cigarettes/d, current 15-24 cigarettes/d, current ≥25 cigarettes/d), physical activity(<3, 3-8.9, 9-17.9, 18-26.9, and ≥27 in metabolic equivalents of task/wk), body mass index;(<21, 21-22.9, 23-24.9, 25-26.9, 27-29.9, 30-32.9, 33-34.9, 35-39.9, ≥40), alcohol intake (0, 0.1-4.9, 5.0-9.9, 10-14.9, or ≥15.0 g/d), multivitamin use (yes, no), aspirin use (yes, no), family history of early coronary heart disease or stroke (diagnosis <60 years; yes, no), and total energy intake (fifths), and intakes of poultry, dark meat fish, tuna, other fish, egg, high fat dairy, low fat dairy, combined plant protein sources of nuts, legumes, and soy, whole grains, fruit, vegetables, and coffee, and glycemic index.
Included other types of fish such as cod, haddock, and halibut in addition to store bought breaded fish, fish cakes, fish pieces, and fish sticks.
The associations comparing specific protein sources in relation to risk of CHD did not differ by BMI (<25, ≥25) or period (<2000, ≥2000), (P>0.05 for interaction). However, stronger associations were observed in the comparisons of nuts and plant based proteins with red meat among older men (0.84 (0.76 to 0.92) for nuts and 0.82 (0.75 to 0.90) for plant based proteins) and was attenuated but remained significant among those with low fiber intake (0.93 (0.85 to 1.00) for nuts and 0.92 (0.85 to 1.00) for plant based proteins) (supplemental figures 1 and 2). The associations between red meat and egg intake were stronger among younger men in whom the replacement of red meat with egg was associated with a 20% (95% confidence interval 2% to 35%) lower risk of CHD.
Results were comparable with the primary analysis (in which the updating of diet was stopped after the incidence of intermediate outcomes), when cumulatively updated average diet continued to be used throughout follow-up, with and without adjusting for the incidence of major diseases (supplemental figure 3). Weaker associations were observed when the most recent diet alone (except for poultry) was compared with cumulative updated diet (supplemental figure 3). Total red meat consumption was on average 0.99 (SD 0.73) servings/day at baseline, 0.87 (0.72) servings/day using the most recent diet, and 0.91 (0.64) servings/day using the cumulative average.
Latency analyses were also performed to further evaluate the temporal relation between assessment of diet and diagnosis of CHD. Overall, the associations observed in the substitution analyses did not seem to diminish with up to 20 years of latency; with greater than 20 years, the associations tended to be weaker, but the number of events was relatively small (supplemental figure 4).
Discussion
In this prospective cohort study of men with at least 30 years of follow-up, greater intakes of total, unprocessed, and processed red meat were associated with a higher risk of CHD, independent of other dietary and non-dietary cardiovascular disease risk factors. Compared with intake of total, unprocessed, or processed red meat, intake of high quality plant based protein foods such as nuts, legumes, and soy in addition to whole grains and dairy products were each associated with a lower risk of CHD. Substituting nuts and plant based proteins for total red meat was each associated with a lower CHD risk among those older but not younger than 65 years and remained statistically significant but attenuated among those with low fiber intake. The latency analyses suggested that the inverse associations of substituting red meat with major protein sources did not diminish with lags up to 20 years before the diagnosis of CHD. Also, associations were stronger using cumulative average intakes than with single dietary assessments, likely reflecting the less precise measurement of long term diet when using a single questionnaire compared with using the cumulative average of repeated assessments. This was consistent with the larger standard deviation of total red meat intake observed when single measurements were used (baseline and most recent diet) compared with using the cumulative average of multiple measurements.
The weaker associations observed when using the most recent dietary data might also be influenced by reverse causation bias, as participants could have changed their diet after developing symptoms or a cardiovascular disease related diagnosis.
Comparison with other studies
Our finding of red meat consumption being associated with an increased risk of CHD is in line with several previous studies. In our study, we additionally included substitution analysis that explicitly compared red meat with specific sources of proteins while accounting for total energy intake. Analyses that do not specify a comparison would be implicitly comparing the food under study with a mixture of all other energy contributing foods in the diet, thus making conclusions and dietary recommendations more difficult. In 25 153 California Seventh Day Adventists, daily meat consumption was associated with a 70% (among men) and 37% (among women) higher risk of fatal ischemic heart disease.17 In that study, however, the type of meat was not specified, and the simultaneous adjustment for dietary factors was limited to eggs, cheese, milk, and coffee intake. In a recent analysis of individual level data of six prospective US cohort studies, an additional two servings per week of unprocessed red meat was associated with a 3% greater risk of cardiovascular diseases. Participants who consumed two servings per week of processed meat were also at a 7% higher risk of CVD compared with non-consumers.21 Similar to other studies, substitution analysis was not performed and only baseline data were analyzed. In a meta-analysis study of 17 prospective cohorts, one serving per day of total red meat was associated with a 19% higher risk of cardiovascular disease mortality, and this risk was mostly associated with processed red meat. Unprocessed red meat was associated with higher cardiovascular disease mortality among the US populations only,16 thus highlighting the importance of considering the consumption levels of the populations under study. In an older meta-analysis of observational studies, processed meat intake was associated with an increased risk of CHD,11 and no statistically significant association was observed with unprocessed red meat. However, the included studies were limited by either a small number of events (769 events across all studies), short follow-up, no adjustment for total energy intake, not specifying the comparison food, or not using prospectively collected data. In a recent meta-analysis of prospective cohorts, however, a reduction of three servings per week of unprocessed and processed red meat was each associated with a lower risk of all cause and cardiovascular disease mortality and a lower risk of myocardial infarction.20 In this meta-analysis, the comparison food was not specified, and the results could have been underestimated.
Nonetheless, we previously found that higher intake of total red meat was statistically significantly associated with an increased risk of CHD among 84 136 women of the Nurses’ Health Study cohort, especially when compared with alternative protein sources.12 A prospective study of 409 885 men and women in nine European countries showed that the risk of ischemic heart disease was 19% greater for every 100 g/day increment in the intake of total and processed red meat.29 Substituting 100 kcal/d of fatty fish, yogurt, cheese, or eggs for 100 kcal/d of red and processed meat was associated with a 15-24% lower risk of ischemic heart disease.29 Although the authors did not examine plant sources of proteins, their overall conclusion was consistent with the findings of our study showing that red and processed meat were associated with a higher risk of ischemic heart disease. In another prospective US cohort study, the dietary intake of processed and unprocessed red meat was each associated with higher risk of mortality from heart disease.30 Yet, both prospective cohorts used one dietary measurement at baseline.29 30
Possible explanations and implications
Several mechanisms might contribute to an adverse effect of red meat intake on risk of CHD. A meta-analysis of randomized clinical trials showed that consumption of red meat was associated with increased blood levels of low density lipoprotein cholesterol compared with consumption of plant based protein sources, consistent with the high saturated fat and cholesterol content of red meat.31 In a network meta-analysis of randomized trials, nuts, legumes, and whole grains were each shown to be more effective in reducing low density lipoprotein cholesterol compared with red meat.32 In addition, red meat is low in polyunsaturated fat, and reduction of risk of CHD by replacement of saturated fat with polyunsaturated fat has been supported by both observational cohort studies and randomized trials.33 Dietary heme iron found in red meat has been associated with myocardial infarction and fatal CHD in many epidemiologic studies.34 35 36 Excessive iron intake might catalyze several cellular reactions involved in the production of reactive oxygen species, thus increasing the levels of oxidative stress.37 L-carnitine, which is relatively high in red meat, might be metabolized by intestinal microbiota into proatherogenic compounds, trimethylamine-N-oxide, promoting atherosclerosis.38 Also, the sialic acid N-glycolylneuraminic acid in red meat has been hypothesized to generate a proinflammatory, atherogenic state in humans.39 The high sodium content of processed meats is likely to increase the risk of CHD by increasing blood pressure40 41 and vascular resistance. Preservatives in processed red meat, such as nitrates and nitrate byproducts, have been associated with endothelial dysfunction, atherosclerosis, and insulin resistance in some animal models.42 43
In our analysis, intake of high quality plant based protein foods such as nuts, legumes, and soy was associated with a lower risk of CHD compared with intake of red meat. Such replacement would not only decrease the amounts of saturated fats, cholesterol, and heme iron, but also increase the intake of unsaturated fat, fiber, antioxidants, polyphenols, and many constituents that could reduce the risk of CHD. A reduction in CHD risk with such substitution therefore could be related to multiple changes in intakes of nutrients and phytochemicals. Since hypercholesterolemia, oxidative stress, and endothelial dysfunction increase with age, people older than 65 years might be at a higher risk of developing cardiovascular morbidities. Substitution of plant based proteins for red meat could possibly improve the cardiometabolic profile of this high risk group and consequently lower the risk of CHD, thus explaining the more favorable substitution effect of plant based proteins seen among older men.
In this study, the replacement of red meat with total fish was not associated with CHD risk. However, when different types of fish were analyzed, intake of dark meat fish was inversely associated with CHD risk compared with intake of red meat in 2000 and later. This could be due to the variation in the method of food preparation over time, as fish were mostly consumed after being deep fried in the earlier years. Other fish intake was positively associated with CHD risk, possibly because this food group also included processed breaded fish, fish cakes, fish pieces, and fish sticks.
Strengths and limitations of this study
Our study has multiple strengths and limitations. The 30 years of follow-up, the large number of CHD events, and the availability of updated dietary data and other risk factors, provided an opportunity to evaluate processed and unprocessed red meat and potential replacements with alternative foods in relation to CHD. The cumulative averages of repeated assessments of intake were used to minimize random measurement error resulting from within person variation and to account for real changes in diet over time. The use of isocaloric models enabled us to interpret food substitution analyses by specification of the comparison foods. Although we are not able to assume causality of the observed relations because of the observational nature of the study, the consistency with findings of randomized studies documenting the benefits on blood lipids when red meat is replaced by plant protein sources supports causality. Inevitable measurement error in dietary assessment leading to inaccurate assessment or misclassification bias, even though reduced by using the average of repeated assessments, would have tended to underestimate the true associations with red meat. Because our study design was prospective, any measurement error would likely be independent of the outcome and therefore would attenuate the observed associations toward the null. Residual and unmeasured confounding cannot be excluded despite the adjustment for important personal and lifestyle factors. Finally, our patients were mostly non-Hispanic white men, drawn from a cohort of health professionals of higher socioeconomic status than the overall population, thus affecting the generalizability of the results to other populations. This homogeneity can, however, help reduce unmeasured confounding related to socioeconomic status.3 Our group has published similar associations with CHD among women of the Nurses’ Health Study cohort,12 and the associations of red meat consumption with all cause, cardiovascular disease, and cancer mortality were also similar among participants of the Nurses’ Health Study and Health Professionals Follow-Up Study.3
Conclusion
We found that greater intakes of total, unprocessed, and processed red meat were each associated with a higher risk of CHD. Compared with total, unprocessed, or processed red meat, other dietary components such as soy, nuts, and legumes were associated with a lower risk of CHD. These associations were stronger among older men. These findings are consistent with the effects of these foods on low density lipoprotein cholesterol levels and support a health benefit of limiting red meat consumption and replacement with plant protein sources; this would also have important environmental benefits.44 We also found that substituting whole grains or dairy products for total red meat and substituting eggs for processed red meat were also associated with a lower CHD risk. Further research on the substitution of dairy products and egg intake for red meat are needed in other cohorts to confirm the generalizability of these findings.
What is already known on this topic
The relation between red meat intake and risk of coronary heart disease (CHD) has long been debated
Discrepant results could be partly due to non-specific characterization of the alternatives to meat sources of protein and energy
What this study adds
Compared with intake of total, unprocessed, or processed red meat, intake of other dietary components such as soy, nuts, and legumes was associated with a lower risk of CHD
Substitutions of whole grains and dairy products for total red meat, and eggs for processed red meat, were also associated with a lower risk of CHD
Acknowledgments
We thank the participants and staff of the Health Professionals Study for their invaluable contributions.
Web extra.
Extra material supplied by authors
Supplementary information: additional four tables and four figures
Contributors: LA, AS, DW, and WCW conceived the study. LA and AS analyzed the data. LA, AS, DW, and WCW provided statistical expertise. LA wrote the first draft of the paper. WCW, EBR, and MJS obtained funding. All authors contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The authors assume full responsibility for analyses and interpretation of these data. LA is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The cohort was supported by the National Institutes of Health (grants U01 CA167552 and R01 HL35464). LA received research support from the National Institutes of Health (training grant T32 HL 098048). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the National Institutes of Health for the submitted work. no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The study protocol was approved by the institutional review board of the Harvard TH Chan School of Public Health. The return of the completed self-administered questionnaire was considered to imply informed consent.
Data sharing: No additional data available.
The lead author (LA) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Findings will be disseminated through the media departments of the authors’ institute. Results from the Health Professionals Follow-Up Study cohort are routinely disseminated to study participants through the study website, Twitter feed, and annual newsletter.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Zheng Y, Li Y, Satija A, et al. Association of changes in red meat consumption with total and cause specific mortality among US women and men: two prospective cohort studies. BMJ 2019;365:l2110. 10.1136/bmj.l2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alshahrani SM, Fraser GE, Sabaté J, et al. Red and Processed Meat and Mortality in a Low Meat Intake Population. Nutrients 2019;11:622. 10.3390/nu11030622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pan A, Sun Q, Bernstein AM, et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med 2012;172:555-63. 10.1001/archinternmed.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pan A, Sun Q, Bernstein AM, et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr 2011;94:1088-96. 10.3945/ajcn.111.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pan A, Sun Q, Bernstein AM, Manson JE, Willett WC, Hu FB. Changes in red meat consumption and subsequent risk of type 2 diabetes mellitus: three cohorts of US men and women. JAMA Intern Med 2013;173:1328-35. 10.1001/jamainternmed.2013.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lo JJ, Park YM, Sinha R, et al. Association between meat consumption and risk of breast cancer: Findings from the Sister Study. Int J Cancer 2020;146;2156-65. 10.1002/ijc.32547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Demeyer D, Mertens B, De Smet S, Ulens M. Mechanisms Linking Colorectal Cancer to the Consumption of (Processed) Red Meat: A Review. Crit Rev Food Sci Nutr 2016;56:2747-66. 10.1080/10408398.2013.873886. [DOI] [PubMed] [Google Scholar]
- 8. Chen GC, Lv DB, Pang Z, Liu QF. Red and processed meat consumption and risk of stroke: a meta-analysis of prospective cohort studies. Eur J Clin Nutr 2013;67:91-5. 10.1038/ejcn.2012.180. [DOI] [PubMed] [Google Scholar]
- 9. Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC. Dietary protein sources in early adulthood and breast cancer incidence: prospective cohort study. BMJ 2014;348:g3437. 10.1136/bmj.g3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes--an updated review of the evidence. Curr Atheroscler Rep 2012;14:515-24. 10.1007/s11883-012-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010;121:2271-83. 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010;122:876-83. 10.1161/CIRCULATIONAHA.109.915165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans. 8th Edition. December 2015. https://health.gov/dietaryguidelines/2015/guidelines/.
- 14. Lee JE, McLerran DF, Rolland B, et al. Meat intake and cause-specific mortality: a pooled analysis of Asian prospective cohort studies. Am J Clin Nutr 2013;98:1032-41. 10.3945/ajcn.113.062638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abete I, Romaguera D, Vieira AR, Lopez de Munain A, Norat T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: a meta-analysis of cohort studies. Br J Nutr 2014;112:762-75. 10.1017/S000711451400124X. [DOI] [PubMed] [Google Scholar]
- 16. Wang X, Lin X, Ouyang YY, et al. Red and processed meat consumption and mortality: dose-response meta-analysis of prospective cohort studies. Public Health Nutr 2016;19:893-905. 10.1017/S1368980015002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Snowdon DA, Phillips RL, Fraser GE. Meat consumption and fatal ischemic heart disease. Prev Med 1984;13:490-500. 10.1016/0091-7435(84)90017-3 [DOI] [PubMed] [Google Scholar]
- 18. Mann JI, Appleby PN, Key TJ, Thorogood M. Dietary determinants of ischaemic heart disease in health conscious individuals. Heart 1997;78:450-5. 10.1136/hrt.78.5.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fraser GE. Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-day Adventists. Am J Clin Nutr 1999;70(Suppl):532S-8S. 10.1093/ajcn/70.3.532s. [DOI] [PubMed] [Google Scholar]
- 20. Zeraatkar D, Han MA, Guyatt GH, et al. Red and Processed Meat Consumption and Risk for All-Cause Mortality and Cardiometabolic Outcomes: A Systematic Review and Meta-analysis of Cohort Studies. Ann Intern Med 2019;171:703-10. 10.7326/M19-0655. [DOI] [PubMed] [Google Scholar]
- 21. Zhong VW, Van Horn L, Greenland P, et al. Associations of Processed Meat, Unprocessed Red Meat, Poultry, or Fish Intake With Incident Cardiovascular Disease and All-Cause Mortality. JAMA Intern Med 2020;180:503-12. 10.1001/jamainternmed.2019.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whiteman D, Muir J, Jones L, Murphy M, Key T. Dietary questions as determinants of mortality: the OXCHECK experience. Public Health Nutr 1999;2:477-87. 10.1017/S136898009900066X. [DOI] [PubMed] [Google Scholar]
- 23. Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991;338:464-8. 10.1016/0140-6736(91)90542-W. [DOI] [PubMed] [Google Scholar]
- 24. Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 1999;69:243-9. 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 25. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114-26, discussion 1127-36. 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 26. Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009-18. 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol 1994;140:1016-9. 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 28. Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology 1996;7:81-6. 10.1097/00001648-199601000-00014 [DOI] [PubMed] [Google Scholar]
- 29. Key TJ, Appleby PN, Bradbury KE, et al. Consumption of Meat, Fish, Dairy Products, and Eggs and Risk of Ischemic Heart Disease. Circulation 2019;139:2835-45. 10.1161/CIRCULATIONAHA.118.038813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Etemadi A, Sinha R, Ward MH, et al. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: population based cohort study. BMJ 2017;357:j1957. 10.1136/bmj.j1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guasch-Ferré M, Satija A, Blondin SA, et al. Meta-Analysis of Randomized Controlled Trials of Red Meat Consumption in Comparison With Various Comparison Diets on Cardiovascular Risk Factors. Circulation 2019;139:1828-45. 10.1161/CIRCULATIONAHA.118.035225. [DOI] [PubMed] [Google Scholar]
- 32. Schwingshackl L, Hoffmann G, Iqbal K, Schwedhelm C, Boeing H. Food groups and intermediate disease markers: a systematic review and network meta-analysis of randomized trials. Am J Clin Nutr 2018;108:576-86. 10.1093/ajcn/nqy151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sacks FM, Lichtenstein AH, Wu JHY, et al. American Heart Association . Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation 2017;136:e1-23. 10.1161/CIR.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 34. Ascherio A, Willett WC, Rimm EB, Giovannucci EL, Stampfer MJ. Dietary iron intake and risk of coronary disease among men. Circulation 1994;89:969-74. 10.1161/01.CIR.89.3.969. [DOI] [PubMed] [Google Scholar]
- 35. Tzonou A, Lagiou P, Trichopoulou A, Tsoutsos V, Trichopoulos D. Dietary iron and coronary heart disease risk: a study from Greece. Am J Epidemiol 1998;147:161-6. 10.1093/oxfordjournals.aje.a009429. [DOI] [PubMed] [Google Scholar]
- 36. van der A DL, Peeters PHM, Grobbee DE, Marx JJ, van der Schouw YT. Dietary haem iron and coronary heart disease in women. Eur Heart J 2005;26:257-62. 10.1093/eurheartj/ehi027. [DOI] [PubMed] [Google Scholar]
- 37. Rajpathak SN, Crandall JP, Wylie-Rosett J, Kabat GC, Rohan TE, Hu FB. The role of iron in type 2 diabetes in humans. Biochim Biophys Acta 2009;1790:671-81. 10.1016/j.bbagen.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 38. Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576-85. 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kawanishi K, Dhar C, Do R, Varki N, Gordts PLSM, Varki A. Human species-specific loss of CMP-N-acetylneuraminic acid hydroxylase enhances atherosclerosis via intrinsic and extrinsic mechanisms. Proc Natl Acad Sci U S A 2019;116:16036-45. 10.1073/pnas.1902902116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sacks FM, Campos H. Dietary therapy in hypertension. N Engl J Med 2010;362:2102-12. 10.1056/NEJMct0911013. [DOI] [PubMed] [Google Scholar]
- 41. He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013;346:f1325. 10.1136/bmj.f1325. [DOI] [PubMed] [Google Scholar]
- 42. Förstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med 2008;5:338-49. 10.1038/ncpcardio1211. [DOI] [PubMed] [Google Scholar]
- 43. McGrowder D, Ragoobirsingh D, Dasgupta T. Effects of S-nitroso-N-acetyl-penicillamine administration on glucose tolerance and plasma levels of insulin and glucagon in the dog. Nitric Oxide 2001;5:402-12. 10.1006/niox.2001.0360. [DOI] [PubMed] [Google Scholar]
- 44. Willett W, Rockström J, Loken B, et al. Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019;393:447-92. 10.1016/S0140-6736(18)31788-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: additional four tables and four figures


