Abstract
We provide the first analysis and synthesis of the evolutionary and mechanistic bases for risk of endometriosis in humans, structured around Niko Tinbergen's four questions about phenotypes: phylogenetic history, development, mechanism and adaptive significance. Endometriosis, which is characterized by the proliferation of endometrial tissue outside of the uterus, has its phylogenetic roots in the evolution of three causally linked traits: (1) highly invasive placentation, (2) spontaneous rather than implantation-driven endometrial decidualization and (3) frequent extensive estrogen-driven endometrial proliferation and inflammation, followed by heavy menstrual bleeding. Endometriosis is potentiated by these traits and appears to be driven, proximately, by relatively low levels of prenatal and postnatal testosterone. Testosterone affects the developing hypothalamic–pituitary–ovarian (HPO) axis, and at low levels, it can result in an altered trajectory of reproductive and physiological phenotypes that in extreme cases can mediate the symptoms of endometriosis. Polycystic ovary syndrome, by contrast, is known from previous work to be caused primarily by high prenatal and postnatal testosterone, and it demonstrates a set of phenotypes opposite to those found in endometriosis. The hypothesis that endometriosis risk is driven by low prenatal testosterone, and involves extreme expression of some reproductive phenotypes, is supported by a suite of evidence from genetics, development, endocrinology, morphology and life history. The hypothesis also provides insights into why these two diametric, fitness-reducing disorders are maintained at such high frequencies in human populations. Finally, the hypotheses described and evaluated here lead to numerous testable predictions and have direct implications for the treatment and study of endometriosis.
Lay summary: Endometriosis is caused by endometrial tissue outside of the uterus. We explain why and how humans are vulnerable to this disease, and new perspectives on understanding and treating it. Endometriosis shows evidence of being caused in part by relatively low testosterone during fetal development, that ‘programs’ female reproductive development. By contrast, polycystic ovary syndrome is associated with relatively high testosterone in prenatal development. These two disorders can thus be seen as ‘opposite’ to one another in their major causes and correlates. Important new insights regarding diagnosis, study and treatment of endometriosis follow from these considerations.
Keywords: endometriosis, polycystic ovary syndrome, prenatal testosterone, diametric disorders, sexual dimorphis
INTRODUCTION
Human diseases and disorders have ultimate, evolutionary causes as well as proximate, mechanistic ones. Evolutionary causes of disease include evolutionary legacies (which can constrain adaptation and potentiate maladaptation), tradeoffs (which can prevent optimization of structure or function), mismatches to modernity (which can cause maladaptation via divergence between phenotypes and environments), genetic conflicts (which can cause or generate new scope for maladaptation) and benefits to reproduction that are coupled with health-related costs to the self [1]. Integrated analyses of the proximate and ultimate causes of disease can deepen insights into both disease etiology and evolutionary processes. Such studies are especially important when diseases are human-specific or human-elaborated, and derive, in part, from effects of recent selection along the lineage leading to modern humans.
In this article, we synthesize ultimate with proximate causes in the study of endometriosis. We first describe the symptoms and diagnostic features of endometriosis, and review previous hypotheses on its physiological determinants. Second, we analyze the bases for endometriosis by applying Niko Tinbergen's [2] four questions for analyzing phenotypes: (1) phylogeny and evolutionary history, (2) development, (3) mechanism and (4) adaptive significance. Addressing these four questions allows for an integrative, interdisciplinary analysis of the causes and correlates of endometriosis and the traits that characterize it, in the context of understanding why and how this disorder persists in humans given its high heritability. In doing so, we also describe, evaluate and provide tests of a recently developed hypothesis for a primary cause of endometriosis: that its development is driven by relatively low levels of testosterone in prenatal development [3].
ENDOMETRIOSIS
Endometriosis is found almost exclusively in women. For the purposes of this article, we use the terms ‘women’ and ‘men’, and ‘females’ and ‘males’ (in the context of humans), to refer to the sex of individuals of Homo sapiens who bear XX and XY sex chromosomes respectively, with a functional SRY gene in males. Other chromosome complements exist in humans (such as XXY, and XX/XY mosaicism) but are not considered here. These definitions hold regardless of an individual's gender, which involves aspects of culture.
Endometriosis, which is found in 5–10% of reproductive-aged women, is characterized by endometrial tissue becoming established and growing outside of the uterus, most commonly in the ovaries, pelvic cavity, fallopian tubes, or rectovaginal area [4] (Boxes 1 and 2). Such ‘ectopic’ tissue undergoes menstrual cycle changes like normally situated, ‘eutopic’ endometrium, proliferating under the influence of estradiol, and breaking down during menstrual periods, but with no means of exiting the body. Endometriosis can cause severe pelvic pain, as well as contributing to reduced fertility in a substantial proportion of patients. There is no cure, and management typically involves damping or shutting down the estrogenic stimuli that promote ectopic endometrial tissue proliferation (as well as normal cycling and fertility), surgery to remove the lesions or hysterectomy.
Two main hypotheses have been proposed to help explain the proximate basis of human vulnerability to endometriosis. First, as described by Sampson [10] who originally described and named the disease, endometriosis may be caused by ‘retrograde flow’: endometrial cells that become misplaced during menstruation and establish ectopically. This hypothesis can provide only a partial explanation for endometriosis because at least 90% of women undergo some degree of retrograde menstruation [11], but a much smaller percentage develop the disease [12]; moreover, some endometriotic sites are not accessible to cells via retrograde menstruation. Second, ectopic endometrial tissue may derive from female reproductive system (Müllerian duct) cells that undergo altered differentiation, migration or both, during early in utero development [13]. This hypothesis can explain ectopic lesions at diverse bodily sites other than the peritoneal cavity, as well as (extremely rarely) in males (given that males have Müllerian ducts in early development), and it is consistent with results from some histological studies [13]. However, its general validity remains unknown. The proximate causes of endometriosis thus remain enigmatic, and its ultimate, evolutionary causes have not been addressed previously in any detail.
We propose here that Tinbergen's four questions, taken together in the context of core evolutionary-medical principles, can provide novel and useful insights into the causes and treatments of endometriosis. We consider each question in turn.
PHYLOGENY AND EVOLUTIONARY HISTORY
Tinbergen's first question focuses on how a trait evolved. Evolutionary histories are important to understanding disease because they generate the potential and scope for maladaptations that can manifest as disease and its symptoms. In this context, humans are unusual among mammals in that they exhibit a prominent menstruation process including the monthly discharge of blood and other components of degraded endometrial tissue. The presence of menstruation is rare in mammals, being restricted to some primates, a few bats, elephant shrews and one rodent (the spiny mouse) [14]. Menstruation has been reported only among mammals that exhibit highly invasive (hemochorial) placentation [15], and the comparative association of menstruation with invasive placentation has been interpreted in the context of maternal–fetal conflicts [16]. By this hypothesis, the placenta (which is derived from the fetus) has in some lineages, and especially in humans, evolved relatively deep invasion of endometrial tissue, and spiral artery modification, to enhance resource acquisition by the fetus [17]. Mothers have concomitantly, in response, evolved a more highly developed, thicker endometrium, that decidualizes (differentiates, under the influence of progesterone) spontaneously in preparation for implantation, instead of being induced by implantation, as in most mammals [15, 16, 18]. If implantation does not occur in any given menstrual cycle, the endometrial tissue breaks down.
The unusual uterine endometrial buildup cycle and menstrual bleeding pattern humans may generate risk for endometriosis in several ways:
shed endometrial cells can undergo retrograde flow, as described above, involving relatively high volumes in humans, thus increasing the risk for establishment of ectopic endometrial tissue [11, 12];
endometrial tissue undergoes especially rapid and prolonged proliferation every month during the follicular phase of cycling in humans, such that specialized physiological pathways support its estrogen-fueled growth [19], in both eutopic and ectopic tissues;
breakdown of the endometrial tissue with menstruation is a highly inflammatory process [20], generating the potential for widespread inflammation of endometrial cells at ectopic sites (as seen in endometriosis) that can be facilitated by high estradiol and low testosterone (e.g., [21]) and be unopposed by anti-inflammatory signals [22]; and
embryo implantation into endometrial tissue is also inflammatory, because the fetal-placental unit penetrates deeply into the decidualized endometrium for successful establishment of pregnancy. These observations raise the possibility that in endometriosis, endometrial cells may implant ectopically in ways that facilitate inflammation [23], which is a normal part of their interaction with other tissues including the fetal-placental unit. For example, implantation of embryos in eutopic endometrial tissue can be facilitated by ‘scratching’ (local surgical injury), which elicits inflammation [24]; in parallel, ectopic endometrial tissue (endometriosis) preferentially implants and develops at similarly impacted sites, such as scars from recent surgery [23].
Among nonhuman animals, endometriosis has been reported only among primates that menstruate [25]. Other great apes exhibit invasive placentation as well as menstruation, and endometriosis, or the closely related disease adenomyosis, has been reported in orangutans, chimpanzees and gorillas [26]. However, the prevalence of these disorders in these and other nonhuman primates remains unknown, mainly because diagnosis requires the invasive procedure of laparoscopy (or autopsy).
Several other human-evolved traits may also increase endometriosis risk. First, in humans, the endometrial proliferative phase of the menstrual cycle is especially long, placing the later stages of follicle selection (from many small follicles to a single dominant one) entirely within the follicular stage. This heterochronic change in menstrual cycle timing, along the human lineage, generates the potential for relatively high levels of luteinizing hormone (LH), in some individuals, to generate excessive levels of ovarian androgens that can stall follicle development in the polyfollicular stage and prevent ovulation [15]. Relatively low LH and low ovarian androgen levels may also impact follicular development, though in different ways (e.g., [27]), as described in more detail below. Second, selection along the human lineage for more-intense uterine contractions during childbirth, due to large fetal head circumference, may have pleiotropically led to risk of stronger contractions during menstruation, which is represented as dysmenorrhea in endometriosis [28].
In sum, the human menstrual cycling is characterized by an unusual set of traits. Understanding the molecular mechanisms of rapid endometrial growth, inflammatory signaling in menstruation and implantation, anti-inflammatory signaling after successful implantation, spontaneous, compared to induced, decidualization and uterine contractility during menstruation and childbirth should provide important information concerning risk and causes of endometriosis. These considerations also suggest that current experimental systems for the study of endometriosis (e.g., laboratory mice with endometrial cells inserted ectopically) make limited or misleading models due to their divergence from humans in key aspects of reproductive physiology. The only menstruating rodent, spiny mice, provides an alternative that may be more similar reproductively to humans and may thus provide a better animal model of endometriosis [14].
ONTOGENY
Tinbergen's second question refers to phenotypic development. Here, the phenotype is, in a broad sense, represented by women's reproductive morphology and physiology, which begins to develop in utero during the first 12 weeks of gestation. This period is characterized first by the divergence between sexes, and next, for females, by development of the HPO axis, which is orchestrated by neurological, hormonal, homeobox-based systems and other transcription factor signaling programs, that determine how it will operate during later life [29]. Early sexual developmental effects, especially early effects of prenatal testosterone, are important because they determine how and why the female HPO axis may become subject to early dysregulation that increases risks for endometriosis and other reproductive disorders.
Sex differentiation is mediated by expression of the transcription factor SRY, that upregulates Sox9, and leads to testosterone production by Leydig cells of the testis, regression of the Müllerian ducts, and development of the testis and other external male genitalia. By contrast, in females, the Wolffian ducts regress and the Müllerian ducts, plus the urogenital sinus, develop into the external genitalia, in part due to antagonism of Sox9 by Foxl2, Wnt4 and Dax1. Differentiation and differences in phenotypes between females and males, with regard to gonads, other bodily systems, the brain and genetic complements, are not dichotomous and deterministic, but show variation along continua that is mediated by a wide range of biological and cultural factors; in this context, each sex also varies in these traits to a considerable degree.
Testosterone is present and active prenatally among females (at lower levels than among males), being produced from fetal and maternal adrenal glands, and maternal ovaries and maternal fat tissue, with maternal sources reaching the fetus via the placenta [30]. Testosterone levels also vary prenatally among females, as evidenced, for example, by effects on sexually dimorphic facial morphology in humans [31], and by extensive experimental studies of other mammals including cows, sheep, mice, monkeys, rats and rabbits (e.g., [32, 33]).
Among both females and males, prenatal testosterone levels have been linked with two main anatomical metrics. Anogenital distance (AGD), usually measured from the scrotal base to the anus in males, or from the distal end of the vagina to the anus in females, is much longer on average in males than females. Longer AGD is strongly associated with higher prenatal testosterone in both sexes [34], with considerable variation within each sex. A second metric of prenatal testosterone, the ratio of the 2nd to the 4th finger length (2D4D digit ratio) is lower among males than females, and lower digit ratio indexes higher prenatal testosterone within each sex as well, though with considerable variability [35]. Some females thus develop under conditions of lower, or higher, prenatal testosterone than others, and lower prenatal testosterone involves a shorter AGD, longer 2D4D and notable effects on development of the HPO axis.
The relevance of within-sex prenatal testosterone variation effects to development of the female HPO axis is clearly indicated by well replicated associations of relatively high prenatal testosterone with the development of polycystic ovary syndrome (PCOS) [36, 37]. PCOS is characterized by anovulation, polyfollicular (polycystic) ovaries, and high serum and ovarian testosterone (Box 2). These traits are associated with the development of phenotypes including hirsutism (increased hair growth on the face, chest and back) and a predominance of abdominal rather than gluteofemoral distribution of fat, which leads to high body mass index (BMI) and waist to hip ratio (WHR) [38]. The HPO axis is altered in PCOS in that GnRH (gonadotropin-releasing hormone) pulsatile secretion rates are increased, leading to higher LH relative to FSH (follicle stimulating hormone), higher AMH (antimüllerian hormone) and lower SHBG (serum hormone binding globulin) in conjunction with higher postnatal serum testosterone and lower oxytocin [3].
PCOS is thus a developmental disorder of the HPO axis, that is mediated by relatively high prenatal testosterone and other factors [36, 37]. AGD is higher in adult women with PCOS compared to controls [3, 39] and in the fetuses of women with PCOS (as measured with ultrasound), and 2D4D digits ratios are lower in women with PCOS [3], all of which demonstrate the importance of higher prenatal testosterone in the etiology of this condition.
Endometriosis, in contrast to PCOS, exhibits a diverse set of hormonal indicators, correlates and risk factors suggesting that it is ‘opposite’ to PCOS in its causes and symptoms [3]. Most notably, women with endometriosis exhibit, relative to controls: (1) shorter AGDs, indicative of lower prenatal testosterone [40, 41], (2) lower LH relative to FSH, (3) higher SHBG, (4) higher serum oxytocin, (5) lower ovarian and serum testosterone, (6) lower AMH, (7) lower WHR and BMI, (8) lower β-endorphin and (9) a suite of other differences, all of which are opposite to the differences found between PCOS versus controls [3]. The patterns of differences between women with PCOS and endometriosis are highly concordant with one another: for example, in women without PCOS or endometriosis, higher postnatal serum testosterone has been associated with lower SHBG, longer AGD, higher LH, lower FSH, higher β-endorphin and higher WHR (e.g., [3, 42, 43]).
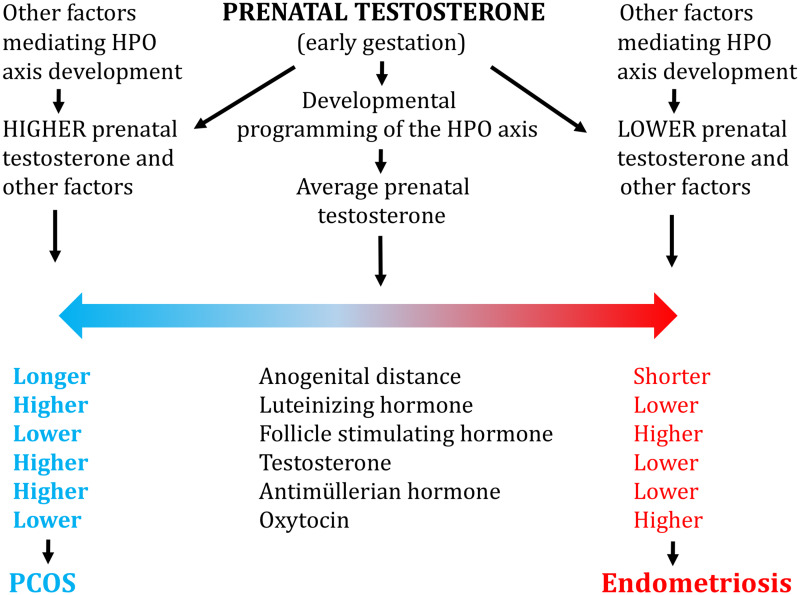
Endometriosis and PCOS thus appear to represent an excellent example of diametric (opposite) disorders such as osteoporosis versus osteoarthritis, pre-eclampsia versus post-partum hemorrhage, and cancer versus neurodegeneration, that are mediated by the tendency for biological systems to vary in two directions in expression or activation, with disorders presenting at the two extremes (e.g., [3, 44, 45]). Like PCOS, the diagnostic features of endometriosis are aligned along a graded continuum, from high severity, to mild forms, to the presence of some endometriosis-associated traits, and to an absence of the disease. By the diametric model, then, endometriosis and PCOS represent the two polar extremes and endpoints of a continuum of reproductive physiological traits that are related, in part, to prenatal testosterone (Fig. 1).
Figure 1.
Model for how prenatal testosterone mediates risks of endometriosis and PCOS, through effects on major hormones that regulate the female HPO axis.
Endometriosis and PCOS thus represent extremes of a continuum of HPO programming effects driven by low versus high prenatal testosterone, respectively. See text for details.
Taken together, the findings described above for endometriosis and PCOS provide evidence that the ontogeny of endometriosis is linked with the quantitative expression of prenatal factors, especially testosterone (and possibly testosterone relative to estrogen), that influence aspects of human sexual dimorphism [3]. As such, the causes, effects and correlates of endometriosis may also be characterized by alterations, and extreme values, in quantitative traits that differ between males and females, and among females, as shown for example by the menstrual, immune, endocrine and physical phenotypes in Table 1.
Table 1.
Evidence salient to the hypothesis that endometriosis involves relative extremes of phenotypes that show evidence of sex differences in humans, due to genetic and environmental effects during development
| Trait | Female–male sex difference, or variation among females | Pattern in endometriosis or its symptoms | References |
|---|---|---|---|
| Anogenital distance | Shorter in females than in males | Shorter in women with endometriosis compared to control women | [40, 41] |
| 2D4D digit ratio | Longer in females than in males | Longer in women with heavy menstrual bleeding and dysmenorrhea (menstrual pain during periods caused by contractions) | [46] |
| Waist-to-hip ratio | Lower in women than in men | Lower in women with endometriosis than in control women | [47] |
| Inflammation levels | Higher in women than in men, during reproductive period of female life history | Higher in women with endometriosis compared to control women and men | [21, 48] |
| Pain (chronic) | Higher sensitivity in women than in men, and lower β-endorphin levels in women | Higher sensitivity to pain, and lower β-endorphin levels, in women with endometriosis compared to controls | See text for details |
| Serum testosterone | Lower in women than men | Lower in women with endometriosis than in controls | [49] |
| Serum oxytocin | Higher in women than in men | Higher in women with endometriosis than in control women | [50, 51] |
| Timing of menarche and menopause | Varies among females | Earlier menarche and menopause in women with endometriosis | [52, 53] |
| Length of menstrual cycle | Varies among females | Shorter menstrual cycle length in women with endometriosis | [54] |
| Dysmenorrhea | Varies among females | Higher levels in women with endometriosis | [55] |
| Bleeding during menstruation | Varies among females | Higher levels in women with endometriosis | [55] |
Pain, which represents one of the primary symptoms of endometriosis, represents a noteworthy example of the interfaces between human sexual dimorphism, hormones and endometriosis. Women report higher sensitivity to pain on average than do men [56, 57], as well as showing lower serum levels of β-endorphin [58], and pain sensitivity is higher under lower levels of serum testosterone in both sexes [59]. In women, testosterone decreases pain perception, and estradiol increases it [60], and in rats, testosterone increases pain thresholds [61] and the androgen receptor antagonist flutamide decreases them [62]. Female rats treated prenatally with testosterone also show pain responses similar to those of untreated males, indicating that pain sensitivity is programmed, in part, during early development [63].
Women with PCOS exhibit higher serum testosterone, as well as higher β-endorphin levels and higher pain thresholds, compared to control women [64]. By contrast, women with endometriosis exhibit lower β-endorphin levels [65], and higher reported sensitivity to pain [66]. Moreover, treatment of endometriosis with the synthetic androgen danazol leads to substantial reductions in chronic pain symptoms [67]. These findings indicate that studying sex differences in pain, and their hormonal bases, should provide novel insights into its etiology and treatment in endometriosis.
The evolutionary developmental framework proposed here for explaining the phenotypes that characterize endometriosis is concordant with the two traditional hypotheses for its risk. Thus, increased levels of retrograde menstruation are expected from the more extreme expression of menstrual characteristics in endometriosis (especially increased menstrual flow), and displacement and altered differentiation of Müllerian stem cells may occur more easily under conditions of lower prenatal testosterone and a more pronounced expression of sexual differentiation during early prenatal development. One possible cause of such effects is the homeobox gene HOXA10, which orchestrates early development of the uterus, as well as endometrial functions, and whose expression is strongly affected by testosterone (e.g., [68]).
MECHANISMS
Tinbergen's third question refers to the causation of a phenotype, which can be considered to encompass its genetic, environmental and physiological causes. We discuss each in turn.
Genetic causes
Endometriosis risk is polygenic, with risk affected by many alleles each of small effect, and a heritability of around 0.5 [69]. Genome wide associations studies have identified a set of alleles (SNPs) that account for a small proportion of this heritability, and that includes genes associated with early sexual development (WNT4, HOXC6), steroid hormone signaling (ESR1, GREB1, KDR), overall growth (IGF1), the HPO axis (FSHB) and other traits [70].
Of the genome-wide significant endometriosis risk genes in the most recent GWAS [70], FSHB emerges as one of special interest because this gene regulates central aspects of female reproduction. The FSHB genetic risk factor for endometriosis involves a large (∼130kb) haplotype with two common alleles at frequencies of about 85 and 15% in European populations [71]. Compared to the minor (lower frequency) haplotype, the major FSHB haplotype has been associated across a large set of studies with higher FSH and lower LH (accounting for 3.5% and 7.1% of their variation respectively, in a typical population), higher risk of endometriosis, a large suite of endometriosis-associated and other traits, and lower risk of PCOS [72–79] (Table 2). This FSHB haplotype thus represents a remarkable example of a specific genetic factor with opposite effects on endometriosis and PCOS risks and their major features. At the whole genome level, endometriosis risk is also genetically correlated (associated via pleiotropy or linkage disequilibrium) with many of its major phenotypic correlates, including earlier menarche, earlier age at first birth, extreme menstrual bleeding, and earlier menopause [70, 81].
Table 2.
Differences in trait expression between women with major versus minor haplotypes of FSHB promoter polymorphism marked by the SNP rs10835638 and tightly linked SNPs
|
Type of trait |
Pattern found in major haplotype compared to minor haplotype |
Comments |
|---|---|---|
|
Endocrine |
Lower luteinizing hormone Higher follicle-stimulating hormone Lower serum testosterone |
All of these phenotypes are associated with endometriosis |
|
Menstrual |
Earlier menarche Shorter menstrual cycles More-excessive menstruation Earlier menopause |
All of these phenotypes are associated with endometriosis |
|
Reproductive |
Earlier age at first parturition/birth Higher lifetime parity (births) Lower risk of nulliparity (no births) Higher rate of dizygotic twinning |
Higher rate of dizygotic twinning may be due to higher rate of two luteinizing hormone surges (which typically indicate ovulation), in women with endometriosis [81] |
|
Disease-related |
Higher risk of endometriosis Lower risk of PCOS |
Rates of endometriosis are also reduced among women diagnosed with PCOS, and involve minimal-to-mild expression (see [3]) |
See text for citations. In PheWAS analysis [80], rs10835638 is also associated with age at first live birth (P = 0.00031), WHR (P = 0.0016), number of older siblings (P = 0.0086), age at last live birth (P = 0.012), having had a bilateral oophorectomy (both ovaries removed) (P = 1.09 x 1 0 −10) and having had a hysterectomy (removal of the uterus) (P = 5.6 x 1 0 −8).
If low versus high prenatal testosterone contributes to the development of endometriosis versus PCOS [3], then genetic markers for correlates of prenatal testosterone (including AGD and 2D4D) should be associated with correlates of these two conditions. This hypothesis can be evaluated using results from the latest GWAS of a correlate of prenatal testosterone, 2D4D digit ratios, in humans, that reported twelve genome-wide significant SNP risk factors ([82]).
Four of the genes harboring digit ratio SNPs (EFNA1, HOXD12/HOXD11, GLI3 and SALL1) show evidence of altered expression in endometriosis; one digit ratio SNP is in the gene TOX3, which is genome-wide significant for risk of PCOS [83], two SNPs are in genes (SMOC1 and HOXA12) that regulate gonad development, and an interaction of HOXA12 with GLI3 mediates development of the vagina and uterus. PheWAS analysis [84] of the twelve SNPs associated with digit ratios, which shows evidence of pleiotropy in their effects, indicates that four of the 12 are associated at P < 0.05 with age at menarche, four with WHR, and nine with BMI (https://atlas.ctglab.nl/PheWAS), suggesting genetically based links of prenatal testosterone with each of these phenotypes, which fits well with the associations of these traits with endometriosis and PCOS at the level of phenotypes.
Concordance among the genetic and phenotypic correlates of endometriosis, in relation to PCOS, can also be analyzed by determining the degree to which disorder risk genes and SNPs are associated with disorder-related phenotypes. Four of 25 total, genome-wide associated endometriosis risk genes (Table 2 in [70]), and three (of 14) PCOS risk genes, are also associated with age of menarche or menopause; moreover, seven endometriosis risk genes, and five PCOS risk genes ([83]), were also associated with WHR or BMI (using Open Targets Genetics Total Association Scores, from https://www.targetvalidation.org). PheWAS analysis ([85], from https://genetics.opentargets.org/) of risk SNPs demonstrated comparable patterns: eight of 27 genome-wide significant endometriosis risk SNPs, and six genome-wide significant PCOS risk SNPs, were pleiotropically associated with age of menarche or menopause (or both, for FSHB and THADA SNPs), and five endometriosis risk SNPs, and two PCOS risk SNPs, were pleiotropically associated with WHR or BMI. Pleiotropic associations have also been reported for endometriosis with WHR [86] and with age at menarche [87]. Remarkably, PheWAS analysis also shows that for endometriosis, 11 (41%) of 27 genome-wide risk SNPs were associated with oophorectomy or hysterectomy. These results provide evidence that, as for effects of the FSHB haplotype described above, genetic risk factors for endometriosis and PCOS are closely associated with the life history and physical-feature correlates of these disorders.
Environmental causes
PCOS is well known to be mediated by increased prenatal testosterone from environmental causes, such as experimental androgen administration in animals or gestational effects of having a mother with PCOS and high serum testosterone [36, 37]. By the hypothesis evaluated here, risk of endometriosis should be associated with lower testosterone, and with higher relative effects from estrogens, during prenatal development. This hypothesis is supported by links of endometriosis with a shorter AGD as described above, and it can be evaluated further using data from studies of prenatal exposures to antiandrogenic and estrogenic endocrine-disrupting chemicals in animals and humans, which are expected to increase risk of endometriosis and expression of its phenotypic correlates including shorter AGD and earlier menarche or estrus, and decreased risk of PCOS. Postnatal exposures may also affect endometriosis or PCOS risk, but are not necessarily relevant to the hypotheses addressed here because they do not involve prenatal programming of the female HPO.
Three endocrine-disrupting agents have been linked with prenatal effects on risk of endometriosis. First, prenatal exposure to the potent synthetic estrogen diethylstilbestrol (DES) is significantly associated with higher endometriosis risk in offspring [88].
Second, prenatal exposure to bisphenol A (BPA), an estrogenic chemical acquired in the diet, leads to shorter AGD in female rats [89], and is associated with earlier first estrus and the generation of endometriosis-like lesions in mice [90–92]. In humans, prenatal BPA leads to shorter AGD in female offspring, when exposure occurs during the first trimester [93].
Third, exposure prenatally to relatively high levels of phthalates, a family of mostly antiandrogenic agents, has been associated with shorter AGD in mice and humans [94–96]. Phthalate levels of women prior to 20 weeks of pregnancy have been linked with increased estradiol and decreased testosterone in infants [97]. Prenatal phthalate exposure has also been associated with reduced risk of PCOS in adolescent female offspring [98], which is consistent with their antiandrogenic effects. Endometriosis has been linked with higher levels of some phthalates in a meta-analysis [99], but none of the relevant studies involved prenatal exposures. Taken together, these studies of DES, BPA and phthalates strongly support the hypothesis that prenatal exposures to evolutionarily novel antiandrogenic and estrogenic chemicals increase risk of endometriosis.
Physiological causes
The findings described above link endometriosis, and its correlates, with effects from reduced prenatal testosterone, due to both genetic and environmental factors. How do these effects translate, mechanistically, into the causes and correlates of endometriosis, in the context of the effects from higher prenatal testosterone that are established for PCOS?
In PCOS, hypothalamic sensitivity to steroid-induced negative feedback is reduced, resulting in an increased frequency and amplitude of GnRH and LH pulses with corresponding increases in levels of LH [100, 101]. Increased LH relative to FSH results in elevated androgen production and arrest of follicular maturation [102]. Immature follicles release high levels of AMH, which increase GnRH pulsatility and inhibit FSH [103]. The capacity to mount an LH surge is thereby impaired, so ovulation is interrupted (leading to anovulation or oligo-ovulation) and menstrual cycles lengthen or disappear [101].
In endometriosis, by contrast, lower LH relative to FSH is associated with lower AMH, signaling lower ovarian reserve and lower ovarian testosterone [3]. In the ovaries, a higher proportion of primordial follicles is recruited into the growing pool and a greater number of maturing follicles degenerate, resulting in quicker depletion of ovarian reserve [104]. Women with endometriosis have fewer pre-ovulatory follicles as well as smaller follicles, relative to unaffected women [105]. Low testosterone predicts elevated pro-apoptotic factors in the follicular fluid of women with endometriosis, which contributes to increased follicular atresia in affected women [49]. Some women with endometriosis also demonstrate premature follicle rupture, which contrasts to the stalled follicles that become cysts in PCOS [106].
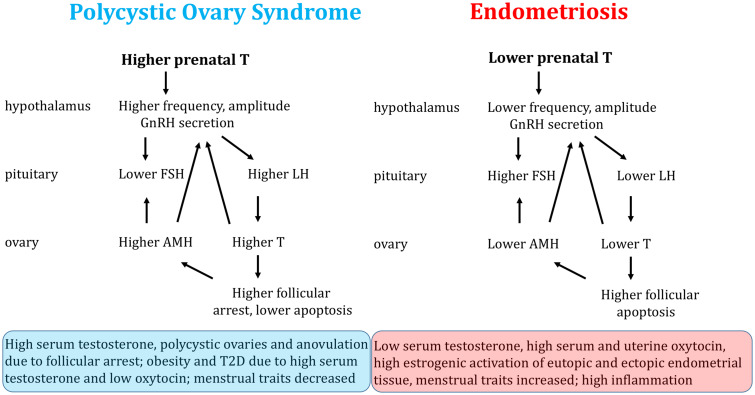
Figure 2 provides a simple depiction of how HPO functions appear to differ between women with endometriosis compared to those with PCOS. Most of the patterns and causal connections shown have been demonstrated from previous work, with notable exceptions. These data gaps include: (1) a paucity of evidence regarding LH pulse frequency and amplitude in women with endometriosis, (2) incomplete knowledge regarding the mechanisms by which ovarian alterations in endometriosis, especially low testosterone and low AMH, influence endometrial function, particularly the establishment, growth and inflammation of endometrial tissue at ectopic sites, and (3) lack of clarity on how variation in ovarian or serum testosterone levels influences eutopic and ectopic endometrial tissues. For example, androgens have recently been shown to regulate the repair of endometrial tissue [107]; Could low levels in women with endometriosis contribute to both high inflammation and reduced repair of ectopic implants, that resemble ‘wounds that never heal’?
Figure 2.
Models showing how risks for PCOS and endometriosis are mediated by effects of higher versus lower prenatal testosterone on development of the female HPO axis, and how these effects may generate the symptoms of each condition.
Details of the mechanistic links of testosterone and other factors with ectopic endometrial implantation, proliferation and inflammation remain to be discerned.
ADAPTIVE SIGNIFICANCE
Tinbergen's fourth question refers to the adaptive significance of a phenotype, in terms of its effects on reproductive success, or fitness. Reproductive disorders like endometriosis or PCOS reduce fertility and fecundity as well as health, so application of this Tinbergen’s criterion also requires considering the fitness-related effects of variation in the nonclinical phenotypes or genotypes that are associated with these two conditions, in populations of women without either disorder. For endometriosis, these effects are known to involve a diverse set of findings:
The major FSHB haplotype associated with higher endometriosis risk is also connected to earlier age at first birth, higher lifetime parity, lower risk of nulliparity (no pregnancies) and a higher rate of dizygotic twinning, as noted above and in Table 2;
A Neanderthal-derived PROGINS progesterone receptor gene haplotype is associated with higher risk of endometriosis, and also with a lower rate of early miscarriage, and having more sisters [108, 109];
A higher 2D4D digit ratio, indicative of lower prenatal testosterone, has been associated with higher reproductive success of women in some populations [110, 111]; and
A lower WHR, as found in endometriosis, positively predicts conception rates [112] and has been associated with measures of higher fecundity and reproductive value in some studies [113–115];
Some animal studies are also relevant to the fitness-related effects of endometriosis-associated traits, even though the subject species do not develop endometriosis in the wild. Thus, in mice and rabbits, females that develop naturally under lower exposure to prenatal testosterone have shorter AGDs and higher fecundity, as well as being preferred by males for mating, compared to females that develop under higher testosterone levels [116–122]. These findings represent comparative evidence that low prenatal testosterone and shorter AGD, as also found in women with endometriosis, are associated with higher fecundity.
Considered together, these lines of evidence suggest that some endometriosis-associated genotypes and phenotypes are associated with increased fertility and fecundity. However, having too many such traits, or too high a level of their expression, apparently leads to maladaptive extremes, and to the lower reproduction observed in endometriosis itself.
How are PCOS-related genotypes and phenotypes associated with fertility and fecundity? The available evidence suggests that, unlike for the correlates of endometriosis, correlates of PCOS are associated with reduced fertility and fecundity; thus:
The minor FSHB haplotype is associated with PCOS risk and higher risk of nulliparity, as noted above;
Higher serum testosterone is associated with lower fertility, in a nonclinical population of women [123];
Women with male co-twins, and thus higher prenatal testosterone, exhibit evidence of lower fecundity than women with female co-twins [124, 125]; and
Higher WHR and BMI, as found in PCOS, are associated in some studies with lower indicators of fecundity and reproductive value (e.g., [115, 126–128]);
Animal studies also demonstrate that mouse and rabbit females developing naturally under exposure to higher prenatal testosterone have longer AGDs and lower fecundity, the opposite of the pattern described above for endometriosis. In addition, females with longer AGDs exhibit lower fertility or fecundity in pigs [129] and cows [130]. These studies of humans and nonhuman animals suggest that PCOS-related genotypes and phenotype are associated with lower fertility and fecundity, in contrast to the findings for endometriosis.
These considerations regarding adaptive significance bear directly on hypotheses for explaining the apparently paradoxical maintenance of endometriosis and PCOS in human populations, given that both of them are highly heritable, persist at frequencies of 5–10%, and substantially reduce reproduction. The maintenance of PCOS has been postulated to be the result of three possible factors. First, PCOS-related physiological traits, which can involve higher WHR and higher levels of visceral, ‘survival fat’, may have provided survivorship benefits in past environments with high fluctuations in food availability and periodic famines, but are deleterious in current environments with plentiful food [131–133]. Second, formerly neutral alleles may have become deleterious in the current, recent human obesogenic environment, generating PCOS phenotypes via maladaptive evolutionary mismatch [9, 134]. And third, PCOS may be maintained by sexually antagonistic selection, whereby selection favors higher fetal testosterone among developing males but disfavors it among developing females, with strong genetic or environmental correlations between the sexes preventing sex-specific optimization [135, 136].
The maintenance of endometriosis may be mediated in part by the fertility and fecundity effects described above, as well as by evolutionary mismatches. Two mismatch models have been proposed. First, risk may be increased by earlier menarche and later childbirth contributing to a higher number of menstrual cycles, that increase risk through more retrograde flow and more-frequent estrogenic stimulation of endometrial tissue [137, 138]. Second, prenatal exposure to anthropogenic estrogenic and antiandrogenic endocrine disrupting chemicals may substantially increase risk, as described above.
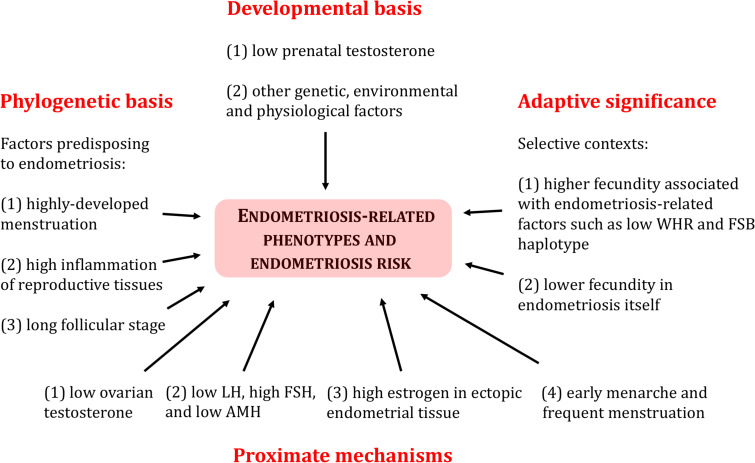
Figure 3 summarizes current understanding of Tinbergen's four questions, as regards endometriosis and the specific phenotypes that characterize it. Insights gained from this interdisciplinary approach provide a robust framework for study of the evolutionary medicine of endometriosis, using disciplines that range from genetics to behavior.
Figure 3.
A summary of Tinbergen's four questions as regards understanding evolved risk, development, adaptive significance and mechanisms of endometriosis and its associated phenotypes
IMPLICATIONS FOR TREATMENT AND RESEARCH
The hypotheses that risk of endometriosis is mediated by low prenatal testosterone, and that it represents a disorder opposite to PCOS [3], have direct implications for the diagnosis, epidemiology, prevention and treatment of both disorders. The first and most important implication of the diametric hypothesis for endometriosis and PCOS is that risk factors for one disorder provide insights into potential treatments for the other disorder. This pattern can be observed in some of the current treatments for each disorder; for example, PCOS is caused in large part by excessive levels of androgens, and the symptoms of endometriosis can be alleviated by treatment with androgenic compounds, including, for example, danazol and letrozole (e.g., [67, 139]). In turn, PCOS symptoms can be reduced by treatment with flutamide, a ‘pure antiandrogen’ that antagonizes the androgen receptor [140], and anti-androgenic, estrogenic chemicals can increase endometriosis risk, as described above. Risk for PCOS is also high in women with congenital adrenal hyperplasia, who exhibit high levels of prenatal testosterone [141]; by contrast, adrenal insufficiency, which involves low testosterone, has been associated with reduced functional ovarian reserve [142], a condition that overlaps with endometriosis in its causes and correlates [143].
Table 3 provides a synopsis of current and proposed treatments for endometriosis and PCOS, that shows the diverse range of evidence for diametric treatments versus causes of the two disorders. More generally, the opposite nature of endometriosis and PCOS provides for reciprocal illumination of their causes and symptoms, such that any new finding for one condition immediately provides potential insights into the other. These insights extend to assisted reproduction; for example, women with low ovarian reserve, endometriosis, or both, may benefit from treatment with dehydroepiandrosterone (DHEA) or other androgens, which show evidence of preventing follicular atresia and facilitating fertility-related outcomes [158, 159].
Table 3.
Evidence that causes of endometriosis represent actual or potential treatments for PCOS, and vice versa
|
Treatment or cause of endometriosis or PCOS |
Effect in endometriosis |
Effect in polycystic ovary syndrome |
Comments |
References (see text for additional details) |
|---|---|---|---|---|
|
Danazol, a synthetic androgen with high affinity for the androgen receptor |
Used to alleviate symptoms of endometriosis; administered orally or vaginally; has androgenic side effects (e.g., hirsutism) |
High androgen levels are a primary cause of PCOS; flutamide, an antagonist of the androgen receptor, is used to treat PCOS |
Ovarian and serum testosterone are lower in women with endometriosis than in controls, and higher in women with PCOS than in controls |
|
|
Valproate, a histone deacetylase inhibitor that suppresses aromatase, used mainly to treat epilepsy and bipolar disorder |
Treatment reduces endometrial lesion size in rat model and reduces proliferation of endometrial stromal cells in vitro. Also reduces menstrual pain in women with adenomyosis, a condition similar to endometriosis |
Treatment induces major symptoms of PCOS, including increased serum testosterone |
A histone deacetylase inhibitor similar to valproate, trichostatin A, induces apoptosis of endometrial cells derived from women with endometriomas |
[145–147] |
|
Oxytocin or oxytocin antagonist (i.e., Atosiban) |
Atosiban treatment reduces size of endometriotic implants in rat model of endometriosis; also increases pregnancy rates in women with endometriosis undergoing embryo transfer |
Oxytocin treatment alleviates obesity-related metabolic traits in rat model of PCOS, and may reduce obesity in women |
Serum oxytocin levels are lower in women with PCOS and higher in women with endometriosis; oxytocin mediates food intake, metabolism and uterine smooth muscle peristalsis |
|
|
Letrozole, an aromatase inhibitor |
Used to treat endometriosis; reduces size of endometriosis implants and pain |
Used to generate PCOS in rodent models |
Aromatase expression is typically elevated in endometriotic tissue, and reduced in ovarian tissue in PCOS |
[150–152] |
|
Mifepristone, a progesterone receptor antagonist |
Used to treat endometriosis; reduces size of endometriosis implants and levels of pain |
Used to generate PCOS in rodent models |
Mifepristone increases the LH/FSH ratio and testosterone/estrogen ratio in rat model and upregulates androgen receptor in human endometrial biopsy tissue |
[153] |
|
Opiates and opiate receptor antagonists (naloxone and naltrexone) |
Opioids used for pain in endometriosis also modulate HPO axis and may affect endometrial proliferation |
Naloxone and naltrexone alleviate PCOS symptoms in humans and in animal models; naltrexone reduces testosterone and LH |
Levels of β-endorphins are higher in PCOS, lower in endometriosis, compared to controls; opioid receptors are expressed in endometrial tissue |
[154–157] |
The table does not include medications for endometriosis (e.g., GnRH agonists, and oral contraceptives) that involve dampening or deactivation of the HPO axis and cessation of menstrual activity, because these do not treat the disorder itself.
Second, the hypothesis of endometriosis and PCOS as diametric disorders provides a basis for prediction and management of both conditions. Endometriosis in particular has been challenging to diagnose; the framework described here provides a large set of established and proposed clinical correlates, including, for example, low AGD, high 2D4D digit ratio, low AMH, high oxytocin, low serum testosterone, a high ratio of FSH to LH, high SHBG, low β-endorphin, low serum testosterone in the mother during early gestation, and other differences [3]. Collectively, these factors can serve as predictors of endometriosis without surgery, and, possibly, as indicators of onset, severity, form or prognosis. Early diagnosis of endometriosis is especially important because it provides the opportunity to halt progression of the disease, using GnRH agonist or combined oral contraceptive (COC) treatments that suppress natural menstrual cycling, until a reproductive opportunity, if and when desired, can be pursued. Diagnosis can also be facilitated by recognizing the expectation of transgenerational effects on endometriosis risk due to low prenatal testosterone, which would mirror the transgenerational effects, due to high testosterone, recently documented in PCOS [160].
The diametric model for endometriosis and PCOS, which follows from their evolutionary underpinnings, is in its infancy, and none of the relevant data have been collected with its predictions in mind. Box 3 provides a list of testable predictions that follow from the hypothesis. We hope that these ideas will spur further research into its scope and application, with benefits to female health, fertility and well-being, as well as leading to enhanced understanding of the diverse evolutionary bases for risks and forms of human disease.
Conflict of interest: None declared.
Box 1. Glossary of terms
Antimüllerian hormone—a glycoprotein that inhibits female reproductive structures during early development (Müllerian ducts) and regulates recruitment of follicles from the resting pool during menstrual cycling.
Anogenital distance—distance from anus to distal end of genitalia; longer in males than in females, and longer within each sex in association with higher prenatal testosterone.
Decidualization—differentiation of endometrial tissues under the influence of progesterone, in preparation for embryo implantation and trophoblast (placental) invasion, or shedding during menstruation if implantation does not occur.
Digit ratio—ratio of the 2nd to 4th finger lengths; shorter in males than females, and shorter within both sexes in association with higher prenatal testosterone.
Endometrium—highly vascularized inner lining of the uterus, that proliferates under the influence of estradiol and progesterone during the menstrual cycle.
β-endorphin—endogenous opioid peptide hormone produced in the central nervous system, that mediate pain perception, as well as GnRH secretion and other aspects of female reproduction.
Follicle stimulating hormone (FSH)—glycoprotein produced by the pituitary under the influence of GnRH that regulates development and function of the reproductive system; during the menstrual cycle, it controls growth and recruitment of ovarian follicles.
Gonadotropin releasing hormone (GnRH)—a peptide hormone released by the hypothalamus that controls production of FSH and LH in the pituitary.
Hypothalamic–pituitary–ovarian (HPO) axis—neural and hormonal system coordinating reproduction and other functions, especially via GnRH release, which affects FSH and LH, impacting ovarian cycles and menstruation.
Luteinizing hormone (LH)—glycoprotein produced by the pituitary under the influence of GnRH that triggers ovulation and the development of the corpus luteum (a temporary endocrine structure that develops from the follicle).
Menarche—a woman's first ovarian cycle resulting in menstrual bleeding or menstruation.
Menstruation—release of degraded endometrial tissue (menses) following menstrual cycle in some mammals.
Ovarian follicle—mass of cells in the ovary that contains the oocyte, as well as granulosa cells and thecal cells.
Retrograde flow—the flow of menstrual blood and endometrial cells back through the fallopian tubes, and into the peritoneal area and pelvic cavity, instead of out of the body.
Box 2. The primary symptoms and diagnostic features of endometriosis and PCOS
Endometriosis
Endometriosis is defined by the presence of endometrial tissue (glands and stroma) outside of the uterine cavity, at so-called ectopic (nonendometrial, noneutopic) sites. Proliferation, inflammation, and degradation of eutopic and ectopic endometrial tissue can cause dysmenorrhea (painful menstrual periods caused by strong uterine contractions, typically referred to as cramps), menorrhagia (heavy menstrual bleeding), dyspareunia (pain during intercourse), chronic pelvic pain, and reduced fertility. Symptoms can vary from minor to highly severe and debilitating, and definitive diagnosis usually requires laparoscopic examination. See Bulun et al. [5] for a useful recent review.
Polycystic ovary syndrome (PCOS)
PCOS is characterized by three primary phenotypes, (1) hyperandrogenism (high levels of testosterone in females, leading to acne, increased body or facial hair, and a lower-pitched voice); (2) polycystic ovaries, which represent ovaries that contain multiple small follicles that have stopped developing at an early stage and resemble cysts; (3) anovulation or oligo-ovulation (absence or rarity of ovulation during menstrual cycling). PCOS is highly variable among women in the presence and degree of expression of these phenotypes, and there is no consensus among medical practitioners concerning its formal definition and diagnostic criteria. This disorder is commonly, but not always, associated with obesity and insulin resistance, and many women exhibit one or more of the primary phenotypes of PCOS but not all of them. PCOS is not a discrete condition biologically, but represents a diagnostic threshold of a continuously varying set of phenotypes, that extend from ‘normality’, to variable development of components of the diagnostic criteria [6, 7]. For example, women with relatively high serum testosterone, but without PCOS, show high LH relative to FSH and low SHBG [6]. See Bellver et al. [8] and Charifson and Trumble [9] for further discussion.
Box 3. Testable predictions that follow from the hypotheses that: (1) low prenatal testosterone mediates the development of endometriosis and (2) endometriosis and PCOS represent diametric (opposite) disorders of the female HPO axis. The listed predictions were chosen to be amenable to robust testing, and to provide insights into both the medical and evolutionary aspects of these two conditions.
Women with endometriosis should exhibit higher 2D4D ratios, which are indicative of lower prenatal testosterone, compared to control women. Higher 2D4D ratio should be positively correlated with lower anogenital distance, and with lower serum and ovarian testosterone. These effects should especially apply to more-severe forms of endometriosis.
Females of nonhuman species, especially those that show natural menstruation (e.g., some primates, and spiny mice), should, when exposed to anti-androgenic or pro-estrogenic chemicals early in gestation, exhibit a higher incidence of endometriosis and its associated traits and correlates, as well as showing shorter anogenital distances. Similar considerations should apply to nonmenstruating animal models (e.g., mice and rats), with regard to correlates of endometriosis and proliferation of experimental ectopic implants.
Genetic risk of endometriosis, as calculated from GWAS summary statistics, should be positively associated with correlates and indicators of lower prenatal testosterone, including for example higher 2D4D digit ratios, lower waist to hip ratio, and lower anogenital distances. Endometriosis risk should show a negative genetic correlation with risk of PCOS.
Endometriosis and PCOS should be associated with sexually dimorphic facial features, as quantified morphometrically. Similar considerations should apply to additional sexually dimorphic traits, such a voice pitch and frequency, whose development is mediated in part by prenatal and postnatal testosterone.
Endometriosis, or high genetic or familial risk of endometriosis, should be characterized by high insulin sensitivity and other phenotypes indicative of low rates of metabolic syndrome and cardiovascular disease, compared to matched controls. This hypothesis is based on contrasts with PCOS, which typically involves high rates of obesity, type 2 diabetes, and related metabolic phenotypes [161], in association with high BMI and WHR.
Rates of endometriosis and PCOS appear to differ substantially among human groups from different geographic areas [162]. Human geographically based, among-group variation in 2D4D digit ratios, and anogenital distances, should parallel among-group variation in the prevalence of endometriosis and PCOS. In particular, across human groups, indicators of lower prenatal testosterone (high 2D4D, and low AGD) should be associated with higher prevalence of endometriosis and lower prevalence of PCOS, and vice versa. This variation should also be reflected in measures of serum or amniotic-fluid testosterone, during gestation.
REFERENCES
- 1. Williams GC, Nesse RM.. The dawn of Darwinian medicine. Q Rev Biol 1991;66:1–22. [DOI] [PubMed] [Google Scholar]
- 2. Tinbergen N. On aims and methods of ethology. Z Tierpsychol 2010;20:410–33. [Google Scholar]
- 3. Dinsdale N, Crespi B.. Endometriosis and PCOS are diametrical disorders of the female HPG axis. Evol Appl 2021. (accepted pending minor revision).). [Google Scholar]
- 4. Vercellini P, Viganò P, Somigliana E. et al. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014;10:261–75. [DOI] [PubMed] [Google Scholar]
- 5. Bulun SE, Yilmaz BD, Sison C. et al. Endometriosis. Endocr Rev 2019;40:1048–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun B, Wang F, Sun J. et al. Basal serum testosterone levels correlate with ovarian response but do not predict pregnancy outcome in non-PCOS women undergoing IVF. J Assist Reprod Genet 2014;31:829–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosenfield RL. The polycystic ovary morphology-polycystic ovary syndrome spectrum. J Pediatr Adolesc Gynecol 2015;28:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bellver J, Rodríguez-Tabernero L, Robles A, Group of interest in Reproductive Endocrinology (GIER) of the Spanish Fertility Society (SEF) et al. Polycystic ovary syndrome throughout a woman’s life. J Assist Reprod Genet 2018;35:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charifson MA, Trumble BC.. Evolutionary origins of polycystic ovary syndrome: an environmental mismatch disorder. Evol Med Public Health 2019;2019:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sampson JA. Heterotopic or misplaced endometrial tissue. Am J Obstet Gynecol 1925;10:649–64. [Google Scholar]
- 11. Halme J, Hammond MG, Hulka JF. et al. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol 1984;64:151–4. [PubMed] [Google Scholar]
- 12. Sasson IE, Taylor HS.. Stem cells and the pathogenesis of endometriosis. Annu NY Acad Sci 2008;1127:106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Batt RE, Yeh J.. Müllerianosis: four developmental (embryonic) müllerian diseases. Reprod Sci 2013;20:1030–7. [DOI] [PubMed] [Google Scholar]
- 14. Bellofiore N, Cousins F, Temple-Smith P. et al. A missing piece: the spiny mouse and the puzzle of menstruating species. J Mol Endocrinol 2018;61:R25–41. [DOI] [PubMed] [Google Scholar]
- 15. Barnett DK, Abbott DH.. Reproductive adaptations to a large‐brained fetus open a vulnerability to anovulation similar to polycystic ovary syndrome. Am J Hum Biol 2003;15:296–319. [DOI] [PubMed] [Google Scholar]
- 16. Emera D, Romero R, Wagner G.. The evolution of menstruation: a new model for genetic assimilation: explaining molecular origins of maternal responses to fetal invasiveness. Bioessays 2012;34:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haig D. Maternal-fetal conflict in human pregnancy. Q Rev Biol 1993;68:495–532. [DOI] [PubMed] [Google Scholar]
- 18. Brosens JJ, Parker MG, McIndoe A. et al. A role for menstruation in preconditioning the uterus for successful pregnancy. Am J Obstet Gynecol 2009;200:615–e1. [DOI] [PubMed] [Google Scholar]
- 19. Groothuis PG, Dassen HH, Romano A. et al. Estrogen and the endometrium: lessons learned from gene expression profiling in rodents and human. Hum Reprod Update 2007;13:405–17. [DOI] [PubMed] [Google Scholar]
- 20. Maybin JA, Critchley HO.. Menstrual physiology: implications for endometrial pathology and beyond. Hum Reprod Update 2015;21:748–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. García-Gómez E, Vázquez-Martínez ER, Reyes-Mayoral C. et al. Regulation of inflammation pathways and inflammasome by sex steroid hormones in endometriosis. Front Endocrinol 2019;10:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mor G, Cardenas I, Abrahams V. et al. Inflammation and pregnancy: the role of the immune system at the implantation site. Annu NY Acad Sci 2011;1221:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wardle PG, Hull MG.. Is endometriosis a disease? Baillieres Clin Obstet Gynaecol 1993;7:673–85. [DOI] [PubMed] [Google Scholar]
- 24. Dekel N, Gnainsky Y, Granot I. et al. The role of inflammation for a successful implantation. Am J Reprod Immunol 2014;72:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Story S, Kennedy S.. Animal studies in endometriosis: a review. ILAR J 2004;45:132–8. [DOI] [PubMed] [Google Scholar]
- 26. Lowenstine LJ, McManamon R, Terio KA.. Comparative pathology of aging Great Apes: bonobos, Chimpanzees, Gorillas and Orangutans. Vet Pathol 2016;53:250–76. [DOI] [PubMed] [Google Scholar]
- 27. Prizant H, Gleicher N, Sen A.. Androgen actions in the ovary: balance is a key. J Endocrinol 2014;222:R141–51. [DOI] [PubMed] [Google Scholar]
- 28. Leyendecker G, Wildt L.. Evolutionary aspects in the pathogenesis and pathophysiology of adenomyosis and endometriosis: the importance of primary dysmenorrhea. J Gynecol Endocrinol 2019;29:110–21. [translated from the German]. [Google Scholar]
- 29. Hughes IA. Minireview: sex differentiation. Endocrinology 2001;142:3281–7. [DOI] [PubMed] [Google Scholar]
- 30. Hakim C, Padmanabhan V, Vyas AK.. Gestational hyperandrogenism in developmental programming. Endocrinology 2017;158:199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Whitehouse AJ, Gilani SZ, Shafait F. et al. Prenatal testosterone exposure is related to sexually dimorphic facial morphology in adulthood. Proc R Soc Lond B Biol Sci 2015;282:20151351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. vom Saal FS. Sexual differentiation in litter-bearing mammals: influence of sex of adjacent fetuses in utero. J Anim Sci 1989;67:1824–40. [DOI] [PubMed] [Google Scholar]
- 33. Ryan BC, Vandenbergh JG.. Intrauterine position effects. Neurosci Biobehav Rev 2002;26:665–78. [DOI] [PubMed] [Google Scholar]
- 34. Thankamony A, Pasterski V, Ong KK. et al. Anogenital distance as a marker of androgen exposure in humans. Andrology 2016;4:616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Swift-Gallant A, Johnson BA, Di Rita V. et al. Through a glass, darkly: human digit ratios reflect prenatal androgens, imperfectly. Horm Behav 2020;120:104686. [DOI] [PubMed] [Google Scholar]
- 36. Abbott DH, Dumesic DA, Levine JE.. Hyperandrogenic origins of polycystic ovary syndrome–implications for pathophysiology and therapy. Expert Rev Endocrinol Metab 2019;14:131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abbott DH, Kraynak M, Dumesic DA. et al. In utero androgen excess: a developmental commonality preceding polycystic ovary syndrome? In: Pasquali R, Pignatelli D (eds.). Hyperandrogenism in Women, Vol. 53. Basel, Karger Publishers, 2019, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cosar E, Üçok K, Akgün L. et al. Body fat composition and distribution in women with polycystic ovary syndrome. Gynecol Endocrinol 2008;24:428–32. [DOI] [PubMed] [Google Scholar]
- 39. Wu Y, Zhong G, Chen S. et al. Polycystic ovary syndrome is associated with anogenital distance, a marker of prenatal androgen exposure. Hum Reprod 2017;32:1–43. [DOI] [PubMed] [Google Scholar]
- 40. Sánchez-Ferrer ML, Mendiola J, Jiménez-Velázquez R. et al. Investigation of anogenital distance as a diagnostic tool in endometriosis. Reprod Biomed Online 2017;34:375–82. [DOI] [PubMed] [Google Scholar]
- 41. Crestani A, Arfi A, Ploteau S. et al. Anogenital distance in adult women is a strong marker of endometriosis: results of a prospective study with laparoscopic and histological findings. Hum Reprod Open 2020;2020:hoaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mira‐Escolano MP, Mendiola J, Mínguez‐Alarcón L. et al. Longer anogenital distance is associated with higher testosterone levels in women: a cross‐sectional study. BJOG 2014;121:1359–64. [DOI] [PubMed] [Google Scholar]
- 43. Barbotin AL, Peigné M, Malone SA. et al. Emerging roles of anti-müllerian hormone in hypothalamic-pituitary function. Neuroendocrinology 2019;109:218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crespi BJ, Go MC.. Diametrical diseases reflect evolutionary-genetic tradeoffs: evidence from psychiatry, neurology, rheumatology, oncology and immunology. Evol Med Public Health 2015;2015:216–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abrams ET, Rutherford JN.. Framing postpartum hemorrhage as a consequence of human placental biology: an evolutionary and comparative perspective. Amer Anthropol 2011;113:417–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tabachnik M, Sheiner E, Wainstock T.. The association between second to fourth digit ratio, reproductive and general health among women: findings from an Israeli pregnancy cohort. Sci Rep 2020;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Backonja U, Louis GM, Lauver DR.. Overall adiposity, adipose tissue distribution, and endometriosis: a systematic review. Nurs Res 2016;65:151–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Klein SL, Flanagan KL.. Sex differences in immune responses. Nat Rev Immunol 2016;16:626–38. [DOI] [PubMed] [Google Scholar]
- 49. Ono YJ, Tanabe A, Nakamura Y. et al. A low-testosterone state associated with endometrioma leads to the apoptosis of granulosa cells. PloS One 2014;9:e115618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Leyendecker G, Kunz G, Herbertz M. et al. Uterine peristaltic activity and the development of endometriosis. Ann NY Acad Sci 2004;1034:338–55. [DOI] [PubMed] [Google Scholar]
- 51. Marazziti D, Baroni S, Mucci F. et al. Sex-related differences in plasma oxytocin levels in humans. Clin Pract Epidemiol Ment Health 2019;15:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nnoaham KE, Webster P, Kumbang J. et al. Is early age at menarche a risk factor for endometriosis? A systematic review and meta-analysis of case-control studies. Fertil Steril 2012;98:702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yasui T, Hayashi K, Nagai K. et al. Risk profiles for endometriosis in Japanese women: results from a repeated survey of self-reports. J Epidemiol 2015;25:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wei M, Cheng Y, Bu H. et al. Length of menstrual cycle and risk of endometriosis: a meta-analysis of 11 case-control studies. Medicine 2016;95:e2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bulletti C, De Ziegler D, Polli V. et al. Characteristics of uterine contractility during menses in women with mild to moderate endometriosis. Fertil Steril 2002;77:1156–61. [DOI] [PubMed] [Google Scholar]
- 56. Bartley EJ, Fillingim RB.. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013;111:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hashmi JA, Davis KD.. Deconstructing sex differences in pain sensitivity. Pain 2014;155:10–3. [DOI] [PubMed] [Google Scholar]
- 58. Ritter MM, Sönnichsen AC, Möhrle W. et al. Beta-endorphin plasma levels and their dependence on gender during an enteral glucose load in lean subjects as well as in obese patients before and after weight reduction. Int J Obes 1991;15:421–7. [PubMed] [Google Scholar]
- 59. Cairns BE, Gazerani P.. Sex-related differences in pain. Maturitas 2009;63:292–6. [DOI] [PubMed] [Google Scholar]
- 60. Bartley EJ, Palit S, Kuhn BL. et al. Natural variation in testosterone is associated with hypoalgesia in healthy women. Clin J Pain 2015;31:730–9. [DOI] [PubMed] [Google Scholar]
- 61. Aloisi AM, Ceccarelli I, Fiorenzani P. et al. Testosterone affects formalin-induced responses differently in male and female rats. Neurosci Lett 2004;361:262–4. [DOI] [PubMed] [Google Scholar]
- 62. Neto JO, Garcia JB, de Souza Cartágenes MD. et al. Influence of androgenic blockade with flutamide on pain behaviour and expression of the genes that encode the NaV1.7 and NaV1.8 voltage-dependent sodium channels in a rat model of postoperative pain. J Transl Med 2019;17:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cicero TJ, Nock B, O'Connor L. et al. Role of steroids in sex differences in morphine-induced analgesia: activational and organizational effects. J Pharmacol Exp Ther 2002;300:695–701. [DOI] [PubMed] [Google Scholar]
- 64. Kialka M, Milewicz T, Sztefko K. et al. Metformin increases pressure pain threshold in lean women with polycystic ovary syndrome. Drug Des Devel Ther 2016;10:2483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vercellini P, Sacerdote P, Panerai AE. et al. Mononuclear cell beta-endorphin concentration in women with and without endometriosis. Obstet Gynecol 1992;79:743–6. [PubMed] [Google Scholar]
- 66. van Aken M, Oosterman J, van Rijn T. et al. Experimental pain tolerance is decreased and independent of clinical pain intensity in patients with endometriosis. Fertil Steril 2018;110:1118–28. [DOI] [PubMed] [Google Scholar]
- 67. Barbieri RL, Evans S, Kistner RW.. Danazol in the treatment of endometriosis: analysis of 100 cases with a 4-year follow-up. Fertil Steril 1982;37:737–46. [DOI] [PubMed] [Google Scholar]
- 68. Cermik D, Selam B, Taylor HS.. Regulation of HOXA-10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab 2003;88:238–43. [DOI] [PubMed] [Google Scholar]
- 69. Saha R, Pettersson HJ, Svedberg P. et al. Heritability of endometriosis. Fertil Steril 2015;104:947–52. [DOI] [PubMed] [Google Scholar]
- 70. Rahmioglu N, Banasik K, Paraskevi C. et al. Large-scale genome-wide association meta-analysis of endometriosis reveals 13 novel loci and genetically-associated comorbidity with other pain conditions. BioRxiv 2020:406967. [Google Scholar]
- 71. Grigorova M, Rull K, Laan M.. Haplotype structure of FSHB, the beta‐subunit gene for fertility‐associated follicle‐stimulating hormone: possible influence of balancing selection. Ann Hum Genet 2007;71:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rull K, Grigorova M, Ehrenberg A. et al. FSHB−211 G>T is a major genetic modulator of reproductive physiology and health in childbearing age women. Hum Reprod 2018;33:954–66. [DOI] [PubMed] [Google Scholar]
- 73. Hayes MG, Urbanek M, Ehrmann DA. et al. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Comm 2015;6:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ruth KS, Beaumont RN, Tyrrell J. et al. Genetic evidence that lower circulating FSH levels lengthen menstrual cycle, increase age at menopause, and impact reproductive health: a UK Biobank study. BioRxiv 2015;028001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ruth KS, Campbell PJ, Chew S. et al. Genome-wide association study with 1000 genomes imputation identifies signals for nine sex hormone-related phenotypes. Eur J Hum Genet 2016;24:284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sapkota Y, Steinthorsdottir V, Morris AP. et al. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Comm 2017;8:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gajbhiye R, Fung JN, Montgomery GW.. Complex genetics of female fertility. NPJ Genom Med 2018;3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Laisk T, Kukuškina V, Palmer D. et al. Large-scale meta-analysis highlights the hypothalamic–pituitary–gonadal axis in the genetic regulation of menstrual cycle length. Hum Mol Genet 2018;27:4323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hong SH, Hong YS, Jeong K. et al. Relationship between the characteristic traits of polycystic ovary syndrome and susceptibility genes. Sci Rep 2020;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Watanabe K, Stringer S, Frei O. et al. A global overview of pleiotropy and genetic architecture in complex traits. Nat Genet 2019;51:1339–48. [DOI] [PubMed] [Google Scholar]
- 81. Garitazelaia A, Rueda-Martínez A, Arauzo R. et al. A systematic two-sample Mendelian Randomization Analysis identifies shared genetic origin of endometriosis and associated phenotypes. Life 2021;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Warrington NM, Shevroja E, Hemani G, 23andMe Research Team et al. Genome-wide association study identifies nine novel loci for 2D:4D finger ratio, a putative retrospective biomarker of testosterone exposure in utero. Hum Mol Genet 2018;27:2025–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Day F, Karaderi T, Jones MR, 23andMe Research Team et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet 2018;14:e1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tian D, Wang P, Tang B. et al. GWAS Atlas: a curated resource of genome-wide variant-trait associations in plants and animals. Nucleic Acids Res 2020;48:D927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Carvalho-Silva D, Pierleoni A, Pignatelli M. et al. Open Targets Platform: new developments and updates two years on. Nucleic Acids Res 2019;47:D1056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rahmioglu N, Macgregor S, Drong AW, International Endogene Consortium (IEC), The GIANT Consortium et al. Genome-wide enrichment analysis between endometriosis and obesity-related traits reveals novel susceptibility loci. Hum Mol Genet 2015;24:1185–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ponomarenko I, Reshetnikov E, Polonikov A. et al. Candidate genes for age at menarche are associated with endometrial hyperplasia. Gene 2020;757:144933. [DOI] [PubMed] [Google Scholar]
- 88. Ottolina J, Schimberni M, Makieva S. et al. Early-life factors, in-utero exposures and endometriosis risk: a meta-analysis. Reprod Biomed Online 2020;41:279–89. [DOI] [PubMed] [Google Scholar]
- 89. Christiansen S, Axelstad M, Boberg J. et al. Low-dose effects of bisphenol A on early sexual development in male and female rats. Reproduction 2014;147:477–87. [DOI] [PubMed] [Google Scholar]
- 90. Honma S, Suzuki A, Buchanan DL. et al. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod Toxicol 2002;16:117–22. [DOI] [PubMed] [Google Scholar]
- 91. Signorile PG, Spugnini EP, Mita L. et al. Pre-natal exposure of mice to bisphenol A elicits an endometriosis-like phenotype in female offspring. Gen Comp Endocrinol 2010;168:318–25. [DOI] [PubMed] [Google Scholar]
- 92. Pivonello C, Muscogiuri G, Nardone A. et al. Bisphenol A: an emerging threat to female fertility. Reprod Biol Endocrinol 2020;18:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Barrett ES, Sathyanarayana S, Mbowe O. et al. First-trimester urinary bisphenol A concentration in relation to anogenital distance, an androgen-sensitive measure of reproductive development, in infant girls. Environ Health Perspect 2017;125:077008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Swan SH, Kristensen DM.. Anogenital distance: a marker of steroidal endocrine disruption. Andrology 2018;30:963b72. [Google Scholar]
- 95. Brehm E, Rattan S, Gao L. et al. Prenatal exposure to di(2-ethylhexyl)phthalate causes long-term transgenerational effects on female reproduction in mice. Endocrinology 2018;159:795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Repouskou A, Panagiotidou E, Panagopoulou L. et al. Gestational exposure to an epidemiologically defined mixture of phthalates leads to gonadal dysfunction in mouse offspring of both sexes. Sci Rep 2019;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sathyanarayana S, Butts S, Wang C, TIDES Team et al. Early prenatal phthalate exposure, sex steroid hormones, and birth outcomes. J Clin Endocrinol Metab 2017;102:1870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hart R, Doherty DA, Frederiksen H. et al. The influence of antenatal exposure to phthalates on subsequent female reproductive development in adolescence: a pilot study. Reproduction 2014;147:379–90. [DOI] [PubMed] [Google Scholar]
- 99. Cai W, Yang J, Liu Y. et al. Association between phthalate metabolites and risk of endometriosis: a meta-analysis. Int J Environ Res Public Health 2019;16:3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pastor CL, Griffin-Kork ML, Aloi JA. et al. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 1998;83:582–90. [DOI] [PubMed] [Google Scholar]
- 101. Robinson J. Prenatal programming of the female reproductive neuroendocrine system by androgens. Reproduction 2006;132:539–47. [DOI] [PubMed] [Google Scholar]
- 102. Gilling-Smith CA, Willis DS, Beard RW. et al. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J Clin Endocrinol Metab 1994;79:1158–65. [DOI] [PubMed] [Google Scholar]
- 103. Franks S, Hardy K.. What causes anovulation in PCOS? Curr Opin Endocr Metab Res 2020;12:59–65. [Google Scholar]
- 104. Takeuchi A, Koga K, Satake E. et al. Endometriosis triggers excessive activation of primordial follicles via PI3K-PTEN-Akt-Foxo3 pathway. J Clin Endocrinol Metab 2019;104:5547–54. [DOI] [PubMed] [Google Scholar]
- 105. Stilley JA, Birt JA, Sharpe-Timms KL.. Cellular and molecular basis for endometriosis-associated infertility. Cell Tissue Res 2012;349:849–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Thomas EJ, Lenton EA, Cooke ID.. Follicle growth patterns and endocrinological abnormalities in infertile women with minor degrees of endometriosis. Br J Obstet Gynaecol 1986;93:852–8. [DOI] [PubMed] [Google Scholar]
- 107. Cousins FL, Kirkwood PM, Saunders PT. et al. Evidence for a dynamic role for mononuclear phagocytes during endometrial repair and remodelling. Sci Rep 2016;6:36748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pabalan N, Salvador A, Jarjanazi H. et al. Association of the progesterone receptor gene polymorphism (PROGINS) with endometriosis: a meta-analysis. Arch Gynecol Obstet 2014;290:1015–22. [DOI] [PubMed] [Google Scholar]
- 109. Zeberg H, Kelso J, Pääbo S.. The Neandertal progesterone receptor. Mol Biol Evol 2020;37:2655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Manning JT, Barley L, Walton J. et al. The 2nd:4th digit ratio, sexual dimorphism, population differences, and reproductive success: evidence for sexually antagonistic genes? Evol Hum Behav 2000;21:163–83. [DOI] [PubMed] [Google Scholar]
- 111. Klimek M, Galbarczyk A, Nenko I. et al. Women with more feminine digit ratio (2D:4D) have higher reproductive success. Am J Phys Anthropol 2016;160:549–53. [DOI] [PubMed] [Google Scholar]
- 112. Zaadstra BM, Seidell JC, Van Noord P. et al. Fat and female fecundity: prospective study of effect of body fat distribution on conception rates. Br Med J 1993;306:484–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Singh D. Female mate value at a glance: relationship of waist-to-hip ratio to health, fecundity and attractiveness. Neuroendocrinol Lett 2002;23:81–91. [PubMed] [Google Scholar]
- 114. Cashdan E. Waist-to-hip ratio across cultures: trade-offs between androgen- and estrogen-dependent traits. Curr Anthropol 2008;49:1099–107. [Google Scholar]
- 115. Andrews TM, Lukaszewski AW, Simmons ZL. et al. Cue-based estimates of reproductive value explain women's body attractiveness. Evol Hum Behav 2017;38:461–7. [Google Scholar]
- 116. vom Saal FS, Bronson FH.. In utero proximity of female mouse fetuses to males: effect on reproductive performance during later life. Biol Reprod 1978;19:842–53. [DOI] [PubMed] [Google Scholar]
- 117. vom Saal FS, Bronson FH.. Sexual characteristics of adult female mice are correlated with their blood testosterone levels during prenatal development. Science 1980;208:597–9. [DOI] [PubMed] [Google Scholar]
- 118. Bánszegi O, Altbäcker V, Bilkó Á.. Intrauterine position influences anatomy and behavior in domestic rabbits. Physiol Behav 2009;98:258–62. [DOI] [PubMed] [Google Scholar]
- 119. Bánszegi O, Altbäcker V, Dúcs A. et al. Testosterone treatment of pregnant rabbits affects sexual development of their daughters. Physiol Behav 2010;101:422–7. [DOI] [PubMed] [Google Scholar]
- 120. Bánszegi O, Szenczi P, Dombay K. et al. Anogenital distance as a predictor of attractiveness, litter size and sex ratio of rabbit does. Physiol Behav 2012;105:1226–30. [DOI] [PubMed] [Google Scholar]
- 121. Drickamer LC. Intra-uterine position and anogenital distance in house mice: consequences under field conditions. Anim Behav 1996;51:925–34. [Google Scholar]
- 122. Drickamer LC, Sessions Robinson A, Mossman CA.. Differential responses to same and opposite sex odors by adult house mice are associated with anogenital distance. Ethology 2001;107:509–19. [Google Scholar]
- 123. Apter DA, Vihko R.. Endocrine determinants of fertility: serum androgen concentrations during follow-up of adolescents into the third decade of life. J Clin Endocrinol Metab 1990;71:970–4. [DOI] [PubMed] [Google Scholar]
- 124. Lummaa V, Pettay JE, Russell AF.. Male twins reduce fitness of female co-twins in humans. Proc Natl Acad Sci USA 2007;104:10915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Bütikofer A, Figlio DN, Karbownik K. et al. Evidence that prenatal testosterone transfer from male twins reduces the fertility and socioeconomic success of their female co-twins. Proc Natl Acad Sci USA 2019;116:6749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Weeden J, Sabini J.. Physical attractiveness and health in Western societies: a review. Psychol Bull 2005;131:635–53. [DOI] [PubMed] [Google Scholar]
- 127. Cloud JM, Perilloux C.. Bodily attractiveness as a window to women’s fertility and reproductive value. In: Weekes-Shackelford VA, Shackelford TK (eds.). Evolutionary Perspectives on Human Sexual Psychology and Behavior. New York, NY, Springer, 2014. [Google Scholar]
- 128. Grillot RL, Simmons ZL, Lukaszewski AW. et al. Hormonal and morphological predictors of women’s body attractiveness. Evol Hum Behav 2014;35:176–83. [Google Scholar]
- 129. Drickamer LC, Arthur RD, Rosenthal TL.. Conception failure in swine: importance of the sex ratio of a female's birth litter and tests of other factors. J Anim Sci 1997;75:2192–6. [DOI] [PubMed] [Google Scholar]
- 130. Gobikrushanth M, Bruinjé TC, Colazo MG. et al. Characterization of anogenital distance and its relationship to fertility in lactating Holstein cows. J Dairy Sci 2017;100:9815–23. [DOI] [PubMed] [Google Scholar]
- 131. Shaw LM, Elton S.. Commentary: polycystic ovary syndrome: a transgenerational evolutionary adaptation. BJOG 2007;115:144–8. [DOI] [PubMed] [Google Scholar]
- 132. Corbett SJ, McMichael AJ, Prentice AM.. Type 2 diabetes, cardiovascular disease, and the evolutionary paradox of the polycystic ovary syndrome: a fertility first hypothesis. Am J Hum Biol 2009;21:587–98. [DOI] [PubMed] [Google Scholar]
- 133. Corbett SJ, Morin-Papunen L.. The polycystic ovary syndrome and recent human evolution. Molec Cel Endocrinol 2013;373:39–50. [DOI] [PubMed] [Google Scholar]
- 134. Fessler DM, Natterson-Horowitz B, Azziz R.. Evolutionary determinants of polycystic ovary syndrome: part 2. Fertil Steril 2016;106:42–7. [DOI] [PubMed] [Google Scholar]
- 135. Casarini L, Simoni M, Brigante G.. Is polycystic ovary syndrome a sexual conflict? A review. Reprod Biomed Online 2016;32:350–61. [DOI] [PubMed] [Google Scholar]
- 136. Mokkonen M, Crespi BJ.. Genomic conflicts and sexual antagonism in human health: insights from oxytocin and testosterone. Evol Appl 2015;8:307–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Jarrell J, Arendt-Nielsen L.. Evolutionary considerations in the development of chronic pelvic pain. Am J Obstet Gynecol 2016;215:201–e1. [DOI] [PubMed] [Google Scholar]
- 138. Scioscia M, Roman H, Somigliana E. et al. Increasing number of menstruations in recent generations may contribute to the development of endometriosis: an evolutionary view from a critical analysis of National Health data. Hum Reprod 2019;34:2549–50. [DOI] [PubMed] [Google Scholar]
- 139. Rasul SG, Yaqub U, Manzoor M. et al. Comparison of letrozole versus anazol for the pain management of females presented with endometriosis. Ann King Edward Med Univ 2018;23:514–8. [Google Scholar]
- 140. Al Khalifah RA, Flórez ID, Dennis B. et al. The effectiveness and safety of treatments used for polycystic ovarian syndrome management in adolescents: a systematic review and network meta-analysis protocol. Syst Rev 2015;4:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Papadakis G, Kandaraki EA, Tseniklidi E. et al. Polycystic ovary syndrome and NC-CAH: distinct characteristics and common findings: a systematic review. Front Endocrinol 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Gleicher N, Kushnir VA, Weghofer A. et al. The importance of adrenal hypoandrogenism in infertile women with low functional ovarian reserve: a case study of associated adrenal insufficiency. Reprod Biol Endocrinol 2016;14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Shah DK. Diminished ovarian reserve and endometriosis: insult upon injury. Semin Reprod Med 2013;31:144–9. [DOI] [PubMed] [Google Scholar]
- 144. Selak V, Farquhar CM, Prentice A. et al. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev 2001;4:CD000068. [DOI] [PubMed] [Google Scholar]
- 145. Isojärvi JI, Taubøll E, Herzog AG.. Effect of antiepileptic drugs on reproductive endocrine function in individuals with epilepsy. CNS Drugs 2005;19:207–23. [DOI] [PubMed] [Google Scholar]
- 146. Wu Y, Guo SW.. Histone deacetylase inhibitors trichostatin A and valproic acid induce cell cycle arrest and p21 expression in immortalized human endometrial stromal cells. Eur J Obstet Gynecol Reprod Biol 2008;137:198–203. [DOI] [PubMed] [Google Scholar]
- 147. Sidhu HS, Srinivasa R, Sadhotra A.. Evaluate the effects of antiepileptic drugs on reproductive endocrine system in newly diagnosed female epileptic patients receiving either Valproate or Lamotrigine monotherapy: a prospective study. Epilepsy Res 2018;139:20–7. [DOI] [PubMed] [Google Scholar]
- 148. Simsek Y, Celik O, Karaer A. et al. Therapeutic efficiency of Atosiban, an oxytocin receptor blocking agent in the treatment of experimental endometriosis. Arch Gynecol Obstet 2012;286:777–83. [DOI] [PubMed] [Google Scholar]
- 149. Iwasa T, Matsuzaki T, Mayila Y et al. The effects of chronic oxytocin administration on body weight and food intake in DHT-induced PCOS model rats. Gynecological Endocrinology : The Official Journal of the International Society of Gynecological Endocrinology 2020; 36:55–60. 10.1080/09513590.2019.1631276 31220962 [DOI] [PubMed] [Google Scholar]
- 150. Kafali H, Iriadam M, Ozardalı I. et al. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res 2004;35:103–8. [DOI] [PubMed] [Google Scholar]
- 151. Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod Biol Endocrinol 2011;9:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Xu J, Dun J, Yang J. et al. Letrozole rat model mimics human polycystic ovarian syndrome and changes in insulin signal pathways. Med Sci Monit 2020;26:e923073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kettel LM, Murphy AA, Morales AJ. et al. Treatment of endometriosis with the antiprogesterone mifepristone (RU486). Fertil Steril 1996;65:23–8. [DOI] [PubMed] [Google Scholar]
- 154. Guido M, Romualdi D, Lanzone A.. Role of opioid antagonists in the treatment of women with glucoregulation abnormalities. Curr Pharm Design 2006;12:1001–12. [DOI] [PubMed] [Google Scholar]
- 155. Matsuzaki S, Canis M, Pouly JL. et al. Both GnRH agonist and continuous oral progestin treatments reduce the expression of the tyrosine kinase receptor B and mu-opioid receptor in deep infiltrating endometriosis. Hum Reprod 2007;22:124–8. [DOI] [PubMed] [Google Scholar]
- 156. Ahmed MI, Duleba AJ, El Shahat O. et al. Naltrexone treatment in clomiphene resistant women with polycystic ovary syndrome. Hum Reprod 2008;23:2564–9. [DOI] [PubMed] [Google Scholar]
- 157. Duan L, Wang J, Sun X. et al. The role and significance of endomorphin-1 and μ-opioid receptor in rats with endometriosis. Gynecol Endocrinol 2016;32:912–5. [DOI] [PubMed] [Google Scholar]
- 158. Sunkara SK, Coomarasamy A, Arlt W. et al. Should androgen supplementation be used for poor ovarian response in IVF? Hum Reprod 2012;27:637–40. [DOI] [PubMed] [Google Scholar]
- 159. Gleicher N, Kim A, Weghofer A. et al. Starting and resulting testosterone levels after androgen supplementation determine at all ages in vitro fertilization (IVF) pregnancy rates in women with diminished ovarian reserve (DOR). J Assist Reprod Genet 2013;30:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Risal S, Pei Y, Lu H. et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med 2019;25:1894–904. [DOI] [PubMed] [Google Scholar]
- 161. Kakoly NS, Khomami MB, Joham AE. et al. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update 2018;24:455–67. [DOI] [PubMed] [Google Scholar]
- 162. Bougie O, Yap MI, Sikora L. et al. Influence of race/ethnicity on prevalence and presentation of endometriosis: a systematic review and meta‐analysis. BJOG 2019;126:1104–15. [DOI] [PubMed] [Google Scholar]