Abstract
The purpose of the present study was to evaluate the performance of the Omron HEM‐9600T, an automatic wrist‐type device for self BP measurement, in the sitting position with the wrist at heart level and supine position according to the ANSI/AAMI/ISO81060‐2:2013 guidelines. In the supine position, we evaluated the device under 3 different conditions: using the supine with sideways palm position, the supine with upwards palm position, and the supine with downwards palm position. After 106 subjects were screened and 21 subjects were excluded, the same 85 subjects (38 men [44.7%] and 47 women [55.3%]) were included in the analyses for each position. The average age of the subjects was 54.5 ± 12.2 years (mean ± SD). The mean wrist circumference was 17.0 ± 2.4 cm. The wrist size distribution fulfilled the requirements of the guidelines. The mean differences between reference BPs and HEM‐9600T readings were 1.0 ± 6.7/1.4 ± 5.7 mm Hg, 6.6 ± 7.2/5.5 ± 6.0 mm Hg, 4.8 ± 7.2/4.9 ± 5.8 mm Hg, and 2.1 ± 7.2/2.8 ± 6.8 mm Hg for SBP/DBP in the sitting position, supine with sideways palm position, supine with upwards palm position, and supine with downwards palm position, respectively. In conclusion, the Omron HEM‐9600T in the sitting position fulfilled the validation criteria of the ANSI/AAMI/ISO81060‐2:2013 guidelines. On the other hand, the accuracies of HEM‐9600T in the supine position differed depending on the positioning of the palm, with only the downwards palm‐position measurement fulfilling both validation criteria of the ANSI/AAMI/ISO81060‐2:2013 guidelines.
Keywords: ANSI/AAMI/ISO81060‐2:2013, blood pressure, home nocturnal blood pressure, self‐measurement, validation, wrist‐type blood pressure monitor
1. INTRODUCTION
Several studies have reported that nighttime ambulatory blood pressure (ABP), which is the nocturnal blood pressure (BP) obtained by a conventional arm‐cuff BP monitoring system at fixed intervals (eg, every 30 minutes) is a stronger predictor of cardiovascular events and prognosis of hypertension than either daytime ABP or office BP.1, 2, 3, 4 Thus, ABP monitoring (ABPM) has historically been the gold standard for measuring nighttime BP. Recently, however, a home BP monitoring device was introduced to measure nighttime BP during sleep, and clinical evidence obtained by home nocturnal BP monitoring has begun to be accumulated.5, 6, 7, 8, 9, 10, 11, 12 The BP level of home nighttime BP measured by a home BP monitor is comparable to that of nighttime BP measured by ABPM, and the home BP monitor and ABPM measurements show similar associations with target organ damage.13
Today, home BP measurement is widely used and recommended for the management of hypertension.1, 2, 14, 15 In addition, because of its relatively low cost and good acceptance by users, it may be more feasible and widely available for routine clinical assessment of nocturnal BP compared with ABPM. However, one of the important remaining problems of arm‐cuff‐based nighttime BP monitoring is its potential interference with the sleep quality of patients, especially in the case of ABPM, which requires frequent BP measurements during sleep.16 Wrist‐type nocturnal BP monitoring might provide a promising solution, since wrist‐cuff devices are expected to cause less discomfort and muscle compression.17 We recently developed a wrist‐type home nocturnal BP monitor (Omron HEM‐9600T) with an automatic BP measurement function during sleep. However, there is no guarantee that this wrist‐cuff oscillometric device will measure the nocturnal BP accurately during sleep. Thus, the purpose of the present study was to validate the performance of HEM‐9600T in the sitting position and the supine position according to the ANSI/AAMI/ISO81060‐2:2013 guidelines.18
2. METHODS
2.1. Features of the device
The Omron HEM‐9600T (Omron Healthcare, Kyoto, Japan) is an automatic oscillometric device for measuring BP at the wrist, with a systolic BP (SBP) range of 60‐260 mm Hg, diastolic BP (DBP) range of 40‐215 mm Hg, and pulse rate (PR) range of 40‐180 beats per minutes. The device measures SBP, DBP, and PR during the inflation period of the cuff. It analyzes the pulse wave detected during inflation using an algorithm for determining SBP and DBP. The cuff is inflated automatically by an electric pump and then deflated by a mechanical valve. The cuff can be used for wrist circumferences in the range of 13.5‐21.5 cm.
2.2. Subject selection
The subjects in the present study were recruited as volunteers. These studies were approved by the Institutional Review Board, and all subjects provided their written informed consent to participate. The inclusion criterion was an age of 20 years or more. Exclusion criteria included arrhythmias based on the interview sheet, a DBP that was unclear of the Korotkoff sound, a wrist circumference smaller than 13.5 cm, and a wrist circumference larger than 21.5 cm.
2.3. Procedure
In the supine position, we evaluated the device under 3 different conditions—that is, with the sideways palm position, upwards palm position, and downwards palm position. The validation studies in the sitting position with the palm upwards and the wrist at heart level, and in the supine with the sideways palm position were performed on Day 1 and Day 2, respectively. The validation studies in the supine with the upwards palm position and the supine with the downwards palm position were performed on Day 3. In the study of the sitting position, subjects were seated in a quiet room at a comfortable room temperature, with their back supported, their legs uncrossed, and their measurement arm supported at the heart level. In all the supine‐position studies, subjects were lying on the bed in a relaxed position with their arms lying comfortable at their sides. In each study, the BP measurements were started after a 5‐minute rest.
2.4. Blood pressure measurements
The devices were validated according to the same‐arm, sequential method of the ANSI/AAMI/ISO 81060‐2:2013 guidelines. For each study, the manufacturer provided standard production device models. The validation team consisted of two nurses and one supervisor who were hired by the manufacturer. Nurses were experienced in performing BP measurements and were trained by the British and Irish Hypertension Society online program (http://www.bihsoc.org). The wrist circumference was measured. All measurements in the sitting position were made on the left wrist at the heart level. All measurements in the supine position were made on the arm on the bed. For the BP measurement by mercury sphygmomanometer, two observers simultaneously measured BP using a Y tube and a calibrated mercury sphygmomanometer. SBP was determined based on phase Ⅰ of the Korotkoff sound. DBP was determined based on phase Ⅴ of the Korotkoff sound, except when the Korotkoff sound was still audible with the cuff deflated, in which case the phase Ⅳ was used. BP measurements were alternated between the mercury sphygmomanometer and the automatic device. The time interval between each set of BP measurements was at least 60 sec. The two observers were blinded to each other's readings, and the third observer served as a supervisor who checked the BP readings by the two observers. BPs measured by the mercury sphygmomanometer were determined as the average value of BPs measured by the two observers.
2.5. Analysis
In each study, data were analyzed according to the ANSI/AAMI/ISO81060‐2:2013 guidelines. First, measurements with the mercury sphygmomanometer and the automatic device were not used in the analysis. In each study, analysis was performed according to criteria 1 and 2.
For criterion 1, the differences defined as the SBP or DBP value of the automatic test device minus the mean value of SBP or DBP measured by mercury sphygmomanometer before and after BP measurement by the test device were calculated. Three difference values were calculated for each subject. The mean value and standard deviation (SD) of these difference values were calculated in each study.
For criterion 2, the reference SBP or DBP value was defined as mean value of SBP or DBP measured by mercury sphygmomanometer in the current and previous sequence. The mean value of three reference SBPs or DBPs and three SBPs or DBPs measured by the test device were calculated per subject, respectively. The differences defined as the mean value of three SBPs or DBPs measured by the test device minus the mean value of three reference SBPs or DBPs were calculated per subject. The mean value and SD of these difference values were calculated in each study.
3. RESULTS
Table 1 shows recruitment details for each study. On day 1 and day 2, 106 subjects were screened for the validation studies; all subjects were screened for both the sitting position and the supine with sideways palm‐position measurements. A total of 21 subjects were excluded for the reasons shown in Table 1, resulting in a final subject group of 38 men (44.7%) and 47 women (55.3%) who participated in both measurement studies. After that, these 85 subjects were included for the validation studies in the supine with upwards palm position and the supine with downwards palm position on day 3, and no one was excluded. In Table 1, “Other reasons” refers to subjects excluded in other studies (not described in the present article) that were conducted to evaluate the performance of the Omron HEM‐9700T, an automatic upper‐arm device for self BP measurement in the sitting and supine position on day 1 and day 2, respectively. In order to make the subjects in the studies using HEM‐9600T and HEM‐9700T comparable, subjects excluded in the study using HEM‐9700T were also excluded in the present study for “Other reasons”. The characteristics of these 85 study subjects are shown in Table 2. Their average age was 54.5 ± 12.2 years (mean ± SD) (range: 21‐78 years). The mean wrist circumference was 17.0 ± 2.4 cm (range: 13.5‐21.2 cm). The percentages of subjects with wrist circumferences of 13.5‐15.4 cm, 13.5‐17.4 cm, 17.5‐21.5 cm, and 19.5‐21.5 cm were 28.2%, 56.5%, 43.5% and 20.0%, respectively, which fulfilled the criteria of ISO81060‐2:2013 (≥20%, ≥40%, ≥40%, and ≥20%, respectively). Table 3 shows reference SBP and DBP values for the subjects in each validation study (n = 255). Figure 1 shows Bland‐Altman plots for the differences between the Omron HEM‐9600T readings and the observer measurements for SBP in the sitting position (A), the supine position with sideways palm (B), the sitting position with upwards palm (C), and the sitting position with downwards palm (D) (N = 255). Figure 2 shows those for DBP in the sitting position (A), the supine position with sideways palm (B), the sitting position with upwards palm (C), and the sitting position with downwards palm (D) (N = 255).
Table 1.
Recruitment details for each validation study
| Screening and recruitment | Validation for HEM‐9600T | |||
|---|---|---|---|---|
| Sitting | Supine with sideways palm | Supine with upward palm | Supine with downward palm | |
| Total screened, n | 106 | 106 | 85 | 85 |
| Total excluded, n | 21 | 21 | 0 | 0 |
| Irregular pulse rate, n | 0 | 1 | 0 | 0 |
| Body movements, n | 1 | 0 | 0 | 0 |
| Reference blood pressure variation, n | 10 | 2 | 0 | 0 |
| Range adjustment | 5 | 5 | 0 | 0 |
| Excluded in the study of sitting position | 0 | 11 | 0 | 0 |
| Excluded in the study of supine with sideways palm | 3 | 0 | 0 | 0 |
| Other reason | 2 | 2 | 0 | 0 |
| Total recruited, n | 85 | 85 | 85 | 85 |
Table 2.
Characteristics of the study subjects (n = 85)
| Age, y (range) | 54.5 ± 12.2 (21‐78) |
| Men: women, n (%) | 38 :47 (44.7, 55.3) |
| Wrist circumference*, cm (range) | 17.0 ± 2.4 (13.5‐21.2) |
| Percentage of the subject with 13.5‐15.4 cm, % | 28.2 |
| Percentage of the subject with 13.5‐17.4 cm, % | 56.5 |
| Percentage of the subject with 17.5‐21.5 cm, % | 43.5 |
| Percentage of the subject with 19.5‐21.5 cm, % | 20.0 |
Data are expressed as the means ± SD or percentages or number.
[Correction updated on January 19, 2019, after initial online publication: The term “Wrist circumstance” was corrected to read as “Wrist circumference”.]
Table 3.
Reference SBP and DBP of the subjects in each validation study (n = 255)
| Validation for HEM‐9600 T | ||||
|---|---|---|---|---|
| Sitting | Supine with sideways palm | Supine with upward palm | Supine with downward palm | |
| Reference SBP, mm Hg (range) | 122.4 ± 23.5 (84.5‐191.3) | 121.7 ± 24.1 (86.3‐207.8) | 123.7 ± 26.0 (83.8‐214.5) | 123.1 ± 25.9 (86.8‐221.0) |
| Percentage for high SBP (≥160 mm Hg), % | 7.1 | 5.9 | 6.7 | 5.9 |
| Percentage for medium SBP (≥140 mm Hg), % | 22.0 | 20.4 | 26.7 | 21.2 |
| Percentage for low SBP (≤100 mm Hg), % | 21.6 | 23.9 | 24.7 | 26.3 |
| Reference DBP, mm Hg (range) | 77.1 ± 12.7 (54.3‐109.8) | 73.1 ± 12.6 (48.5‐109.0) | 72.8 ± 13.6 (47.5‐107.8) | 72.1 ± 14.4 (45.5‐110.8) |
| Percentage for high DBP (≥100 mm Hg), % | 5.5 | 4.3 | 2.4 | 3.5 |
| Percentage for medium DBP (≥85 mm Hg), % | 27.1 | 16.1 | 22.4 | 20.4 |
|
Percentage for low DBP (≤60 mm Hg), % |
7.1 | 15.7 | 18.0 | 23.1 |
Data are expressed as the means ± SD or percentages.
DBP, diastolic blood pressure; SBP, systolic blood pressure.
Figure 1.
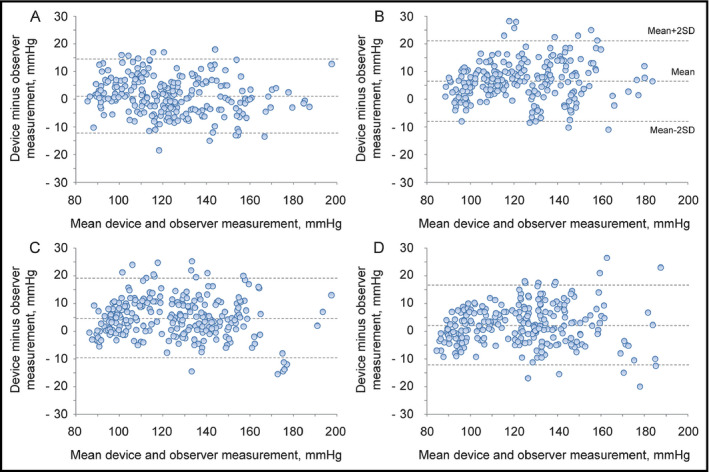
Bland‐Altman plots for the differences between the Omron HEM‐9600T readings and the observer measurements for systolic blood pressure in the sitting position (A), the supine position with sideways palm (B), the supine position with upwards palm (C), and the supine position with downwards palm (D) (N = 255)
Figure 2.
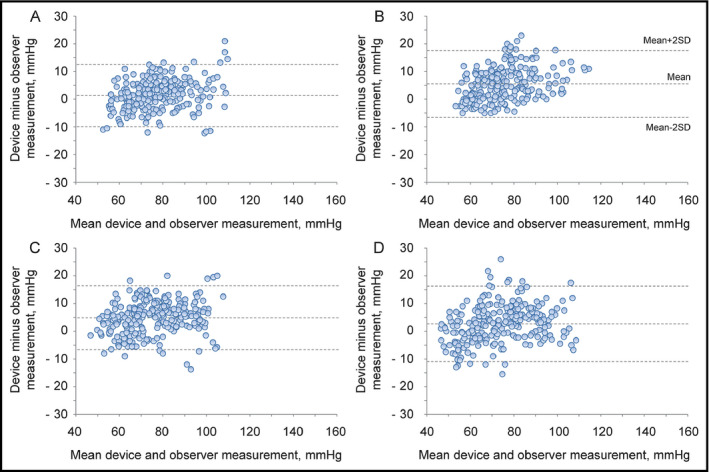
Bland‐Altman plots for the differences between the Omron HEM‐9600T readings and the observer measurements for diastolic blood pressure in the sitting position (A), the supine position with sideways palm (B), the supine position with upwards palm (C), and the supine position with downwards palm (D) (N = 255)
3.1. Validation results of the Omron HEM‐9600T in the sitting position
The differences between the two observers were 0.3 ± 1.5 mm Hg and 0.3 ± 1.5 mm Hg for SBP and DBP, respectively. The mean differences between the reference BPs and HEM‐9600T readings were 1.0 ± 6.7 mm Hg and 1.4 ± 5.7 mm Hg for SBP and DBP according to criterion 1. The mean differences between the reference BPs and HEM‐9600T readings were 1.0 ± 5.6 mm Hg and 1.4 ± 5.2 mm Hg for SBP and DBP according to criterion 2 (Table 4). These results fulfilled the validation criteria of the ISO81060‐2:2013 of ≤5 ± ≤8.0 mm Hg for criterion 1, and smaller SD than 6.8 mm Hg for SBP and DBP for criterion 2.
Table 4.
Results of each validation study
| Validation for HEM‐9600T | ||||
|---|---|---|---|---|
| Sitting | Supine with sideways palm | Supine with upward palm | Supine with downward palm | |
| Difference of SBP for criterion 1, mm Hg | 1.0 ± 6.7 (passed) | 6.6 ± 7.2 (failed) | 4.8 ± 7.2 (passed) | 2.1 ± 7.2 (passed) |
| Difference of SBP for criterion 2, mm Hg | 1.0 ± 5.6 (passed) | 6.6 ± 6.3 (failed) | 4.8 ± 5.9 (failed) | 2.1 ± 6.1 (passed) |
| Difference of DBP for criterion 1, mm Hg | 1.4 ± 5.7 (passed) | 5.5 ± 6.0 (failed) | 4.9 ± 5.8 (passed) | 2.8 ± 6.8 (passed) |
| Difference of DBP for criterion 2, mm Hg | 1.4 ± 5.2 (passed) | 5.5 ± 5.4 (failed) | 4.9 ± 5.2 (failed) | 2.7 ± 6.2 (passed) |
Data are expressed as the means ± SD.
3.2. Validation results of the Omron HEM‐9600T in the supine with sideways palm position
The differences between the two observers were −0.2 ± 1.4 mm Hg and −0.2 ± 1.7 mm Hg for SBP and DBP, respectively. The mean differences between the reference BPs and HEM‐9600T readings were 6.6 ± 7.2 mm Hg and 5.5 ± 6.0 mm Hg for SBP and DBP according to criterion 1. The mean differences between the reference BPs and HEM‐9600T readings were 6.6 ± 6.3 mm Hg and 5.5 ± 5.4 mm Hg for SBP and DBP according to criterion 2 (Table 4). These results did not fulfill the validation criteria of the ISO81060‐2:2013 of ≤5 mm Hg for the mean difference of criterion 1.
3.3. Validation results of the Omron HEM‐9600T in the supine with upwards palm position
The differences between the two observers were −0.1 ± 1.4 mm Hg and −0.1 ± 1.8 mm Hg for SBP and DBP, respectively. The mean differences between the reference BPs and HEM‐9600T readings were 4.8 ± 7.2 mm Hg and 4.9 ± 5.8 mm Hg for SBP and DBP according to criterion 1. The mean differences between the reference BPs and HEM‐9600T readings were 4.8 ± 5.9 mm Hg and 4.9 ± 5.2 mm Hg for SBP and DBP according to criterion 2 (Table 4). These results did not fulfill the validation criteria of the ISO81060‐2:2013 smaller SD than 5.0 mm Hg and 4.9 mm Hg for SBP and DBP for criterion 2.
3.4. Validation results of the Omron HEM‐9600T in the supine with downwards palm position
The differences between the two observers were −0.1 ± 1.4 mm Hg and −0.2 ± 1.9 mm Hg for SBP and DBP, respectively. The mean differences between the reference BPs and HEM‐9600T readings were 2.1 ± 7.2 mm Hg and 2.8 ± 6.8 mm Hg for SBP and DBP according to criterion 1. The mean differences between the reference BPs and HEM‐9600T readings were 2.1 ± 6.1 mm Hg and 2.7 ± 6.2 mm Hg for SBP and DBP according to criterion 2 (Table 4). These results fulfilled the validation criteria of the ISO81060‐2:2013 of ≤5 ± ≤8.0 mm Hg for criterion 1, and smaller SD than 6.6 mm Hg and 6.3 mm Hg for SBP and DBP for criterion 2.
4. DISCUSSION
In the present study, we demonstrated that the Omron HEM‐9600T device, a wrist‐type home nocturnal BP monitor, fulfilled the validation criteria of the ANSI/AAMI/ISO81060‐2:2013 guidelines in the sitting position and in the supine with downwards palm position. However, SBP/DBP measured by HEM‐9600T in the supine with sideways palm position and the supine with upwards position were higher by 6.6/5.5 mm Hg and 4.8/4.9 mm Hg, respectively, than those measured by mercury sphygmomanometer at the upper arm in the supine position, and thus HEM‐9600T did not fulfill the validation criteria of the ANSI/AAMI/ISO81060‐2:2013 guidelines in these two supine positions.
In the present study, the difference between the mean difference in the sitting position and mean difference in the supine with sideways palm position was 5.6 mm Hg for SBP. The degree of differences between the mean values for the sitting and supine positions was less in the cases of the supine with upwards palm position (SBP 3.8 mm Hg and DBP 3.5 mm Hg) and supine with downwards palm position (SBP 1.1 mm Hg and DBP 1.4 mm Hg). This fact indicates that the body position or direction of the palm affects the BP readings measured by the wrist‐type monitor, especially in the supine position, which might be attributed to any of several factors. First, there is a height difference between the wrist and upper arm in the supine position. Some previous studies demonstrated that BP differs by 7 mm Hg if the height difference between the heart level and cuff position is 10 cm19, 20 due to hydrostatic pressure. In the present study, the mean height difference between height from the bed at the surface of the upper arm and height from the bed at the surface of the wrist in the supine position was 2.7 cm. Based on this result, it is estimated that 2 mm Hg of the total difference of 5.6 mm Hg could be attributed to the height difference between the arm and wrist in the supine position.
Second, anatomic features of the wrist itself could have played a role. The positional relationship between the radial artery or ulnar artery and radius or tendon in the supine position might differ from those in the sitting position with palm upwards. In some cases, such as when the bend angle of the wrist is different from that in the sitting position, it would be difficult to occlude the radial and ulnar artery compared with those in the sitting position. In such cases, the cuff pressure pulse wave will be shifted to a higher BP level compared with that in the sitting position, resulting in higher SBP and DBP readings. Based on the results of the present study, it can be assumed that this anatomic issue of the wrist would become more conspicuous in the supine with sideways palm position. In the present study, standard deviations of differences between reference BPs and HEM‐9600T readings in the supine position were greater than those in the sitting position. This might also be partially attributed to anatomic issues of the wrist as described above.
The wrist‐type BP monitor has the limitation that patients must set their wrist at their heart level in the sitting position. Moreover, the wrist‐type BP monitor for use in the supine position has some remaining issues as described above. However, it could be a promising device for monitoring the nighttime BP of patients due to its reduction in discomfort and muscle compression compared to ABPM. Further studies will be needed to examine whether our hypothetical factors are actually responsible for the difference in measurement between the sitting position and the supine positions, followed by the development of solutions to minimize any confirmed effects. Moreover, a future study evaluating the difference between nighttime BP measured by a wrist‐type BP monitor and that measured by an upper‐arm BP monitor under real‐world sleeping conditions will be needed in order to assess how the wrist‐type BP monitor is adopted and experienced by users for monitoring nighttime BP.
4.1. Limitation
Although the distribution of reference SBP fulfilled the criteria of ISO81060‐2:2013 in the sitting position and three supine positions, only the distribution of reference DBP in the sitting position fulfilled the criteria of ISO81060‐2:2013.
5. CONCLUSION
In conclusion, the Omron HEM‐9600T fulfilled the validation criteria of the ANSI/AAMI/ISO81060‐2:2013 guidelines when used in the sitting position with the wrist at heart level. On the other hand, the accuracies of HEM‐9600T in the supine position differed depending on the positioning of the palm, with only the downwards palm‐position measurement fulfilling both validation criteria of the ANSI/AAMI/ISO81060‐2:2013 guidelines.
DISCLOSURE
The authors have no disclosures to declare.
ACKNOWLEDGMENTS
We gratefully acknowledge the volunteers who agreed to have their BP measured for the purpose of the present study. In addition, we gratefully acknowledge Mrs. Ayako Okura for her editorial support.
Kuwabara M, Harada K, Hishiki Y, Kario K. Validation of a wrist‐type home nocturnal blood pressure monitor in the sitting and supine position according to the ANSI/AAMI/ISO81060‐2:2013 guidelines: Omron HEM‐9600T. J Clin Hypertens. 2019;21:463–469. 10.1111/jch.13464
Funding information
This research was supported by research funds from Omron Healthcare and was conducted under the auspices of the SURGE (SUper ciRculation monitorinG with high TEchnology) R&D Center developed collaboratively between Jichi Medical University and Omron Healthcare.
REFERENCE
- 1. Staessen JA, Thijs L, Fagard R, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA. 1999;282:539‐546. [DOI] [PubMed] [Google Scholar]
- 2. Kikuya M, Ohkubo T, Asayama K, et al. Ambulatory blood pressure and 10‐year risk of cardiovascular and noncardiovascular mortality: the Ohasama study. Hypertension. 2005;45:240‐245. [DOI] [PubMed] [Google Scholar]
- 3. Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219‐1229. [DOI] [PubMed] [Google Scholar]
- 4. Investigators T‐H, Roush GC, Fagard RH, et al. Prognostic impact from clinic, daytime, and night‐time systolic blood pressure in nine cohorts of 13,844 patients with hypertension. J Hypertens. 2014;32:2332‐2340. [DOI] [PubMed] [Google Scholar]
- 5. Chonan K, Kikuya M, Araki T, et al. Device for the self‐measurement of blood pressure that can monitor blood pressure during sleep. BloodPress Monit. 2001;6:203‐205. [DOI] [PubMed] [Google Scholar]
- 6. Kario K, Hoshide S, Shimizu M, et al. Effect of dosing time of angiotensin II receptor blockade titrated by self‐measured blood pressure recordings on cardiorenal protection in hypertensives: the Japan Morning Surge‐Target Organ Protection (J‐TOP) study. J Hypertens. 2010;28:1574‐1583. [DOI] [PubMed] [Google Scholar]
- 7. Ishikawa J, Hoshide S, Eguchi K, Ishikawa S, Shimada K, Kario K. Nighttime home blood pressure and the risk of hypertensive target organ damage. Hypertension. 2012;60:921‐928. [DOI] [PubMed] [Google Scholar]
- 8. Stergiou GS, Nasothimiou EG, Destounis A, Poulidakis E, Evagelou I, Tzamouranis D. Assessment of the diurnal blood pressure profile and detection of non‐dippers based on home or ambulatory monitoring. Am J Hypertens. 2012;25:974‐978. [DOI] [PubMed] [Google Scholar]
- 9. Kario K, Hoshide S, Haimoto H, et al. J‐HOP study group. Sleep blood pressure self‐measured at home as a novel determinant of organ damage: Japan Morning Surge Home Blood Pressure (J‐HOP) Study. J Clin Hypertens (Greenwich). 2015;17:340‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lindroos AS, Johansson JK, Puukka PJ, et al. The association between home vs. ambulatory night‐time blood pressure and end‐organ damage in the general population. J Hypertens. 2016;34:1730‐1737. [DOI] [PubMed] [Google Scholar]
- 11. Kario K, Tomitani N, Kanegae H, et al. Comparative effects of an angiotensin ii receptor blocker (ARB)/diuretic vs. ARB/calcium‐channel blocker combination on uncontrolled nocturnal hypertension evaluated by information and communication technology‐based nocturnal home blood pressure monitoring—The NOCTURNE Study. Circ J. 2017;81:948‐957. [DOI] [PubMed] [Google Scholar]
- 12. Fujiwara T, Tomitani N, Kanegae H, Kario K. Comparative effects of valsartan plus either cilnidipine or hydrochlorothiazide on home morning blood pressure surge evaluated by information and communication technology‐based nocturnal home blood pressure monitoring. J Clin Hypertens (Greenwich). 2018;20:159‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kollias A, Ntineri A, Stergiou GS. Association of night‐time home blood pressure with night‐time ambulatory blood pressure and target‐organ damage: a systematic review and meta‐analysis. J Hypertens. 2017;35(3):442‐452. [DOI] [PubMed] [Google Scholar]
- 14. Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow‐up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111(14):1777‐1783. [DOI] [PubMed] [Google Scholar]
- 15. Ohkubo T, Imai Y, Tsuji I, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population‐based observation in Ohasama. Japan. J Hypertens. 1998;16:971‐975. [DOI] [PubMed] [Google Scholar]
- 16. Alessi A, Alessi CR, Piana ER, Assis M, Oliveira LR, Cunha CL. Influence of quality of sleep on the nocturnal decline in blood pressure during ambulatory blood pressure monitoring. Arq Bras Cardiol. 2002;78:212‐223. [DOI] [PubMed] [Google Scholar]
- 17. Veerman DP, van Montfrans GA, Wieling W. Effects of cuff inflation on self‐recorded blood pressure. Lancet. 1990;335:451‐453. [DOI] [PubMed] [Google Scholar]
- 18. Association for the Advancement of Medical Instrumentation . American National Standard: Non-invasive sphygmomanometers – part 2: Clinical validation of automated measurement type; ANSI/AAMI/ISO. 81060–2. 2013. http://my.aami.org/store/detail.aspx?xml:id=8106002. Accessed December 11, 2018.
- 19. Kikuya M, Chonan K, Imai Y, Goto E, Ishii M. Research group to assess the validity of automated blood pressure measurement devices in Japan. Accuracy and reliability of wrist‐cuff devices for self‐measurement of blood pressure. J Hypertens. 2002;20:629‐638. [DOI] [PubMed] [Google Scholar]
- 20. Yarows SA. Comparison of the Omron HEM‐637 wrist monitor to the auscultation method with the wrist position sensor on or disabled. Am J Hypertens. 2004;17:54‐58. [DOI] [PubMed] [Google Scholar]


