Abstract
Hypertension and hyperhomocysteinemia are two independent risk factors of chronic kidney disease (CKD). Our study aimed to evaluate whether hypertension and hyperhomocysteinemia act synergistically toward renal injury. Our analysis included 13 693 subjects from the National Health and Nutritional Survey (NHANES) 1999‐2006. Association was assessed by multivariate logistic regressions. The interaction was investigated on both additive and multiplicative scales. CKD had a prevalence of 17.62% in the NHANES population. After adjusting for age, sex, race, education, physical activity, drinking frequency, current smoking status, poverty‐to‐income ratio, Total cholesterol, high‐density lipoprotein cholesterol, serum folate, vitamin B12, body mass index, waist circumference, and diabetes mellitus, patients with both hypertension and hyperhomocysteinemia had a 5.072 (3.967‐6.486) times risk of CKD than their healthy compartments, higher than that in patients with only hypertension or hyperhomocysteinemia. Moreover, additive interaction of hypertension and hyperhomocysteinemia was significant (relative excess risk due to interaction: 2.107, 95% CI: 1.071‐3.143; the attributable proportion due to interaction: 0.415, 95% CI: 0.270‐0.561; synergy index: 2.072, 95% CI: 1.449‐2.962). Finally, subgroup analyses elucidated the interaction was robust in those with only reduced estimated glomerular filtration rate or albuminuria, and stratification analyses based on gender showed consistency with the main results. Hypertension and hyperhomocysteinemia may act synergistically toward a greater renal injury than the sum of their independent effects. Our findings suggest the coexistence itself also correlates with a deteriorative impact on renal function in addition to the effects of hypertension and diabetes themselves. The results may support the rationality and value of simultaneous tight control of hypertension and hyperhomocysteinemia to prevent CKD.
Keywords: chronic kidney disease, hyperhomocysteinemia, hypertension, synergistic interaction
1. INTRODUCTION
In recent decades, chronic kidney disease (CKD) has emerged as a major threat to global health. CKD reached a worldwide prevalence of 11.0% and contributed to 15% of the mortality in 2012.1 Although we have simple and rapid methods to diagnose CKD, it is still challenging for the prevention of CKD. Hence, finding the risk factors of CKD and understanding their associations and interactions is critical to alleviating the burden of the global healthcare system.
Hypertension (HTN) is one of the most important CKD risk factors. Early studies have revealed that the prevalence of hypertension inversely correlated with estimated glomerular filtration rate (eGFR).2, 3 Moreover, studies have demonstrated the promoting role of hypertension in the development and progression of end‐stage renal disease.4, 5 Epidemiological evidence has also illustrated that lowering of blood pressure could slow down the CKD progression.6, 7 Additionally, a mechanistic study has identified that hypertension could impair the auto‐regulation capacity of renal arterioles, resulting in glomerular pressure overload, leading to glomerulosclerosis and finally developing into CKD.8
Hyperhomocysteinemia (HHcy) is another independent risk factor of CKD. Prior studies have demonstrated that the prevalence of HHcy in CKD patients was significantly higher than that in healthy people, and the HHcy prevalence increased with the elevation of the CKD stages.9, 10 Furthermore, epidemiological studies have also revealed that the plasma homocysteine level was negatively associated with eGFR and positively correlated with the risk of CKD.11, 12, 13 Experimental studies have identified that excessive accumulation of homocysteine could aggravate oxidation reactions and inhibit antioxidant enzymes in the kidney, thereby leading to redox imbalance and subsequent renal injury.14
Recently, a prospective study showed that HHcy could increase the CKD risk among hypertensive patients, implicating the additive effect of HTN and HHcy on renal damage.15 However, no study to date has investigated whether HTN and HHcy have a synergistic interaction toward the exacerbation of renal damage. Thus, our study aims to evaluate whether the coexistence of HTN and HHcy correlates with a greater renal damage than the sum of their independent effects, exploring whether the coexistence itself associates with a deteriorative impact on renal function in addition to the effects of hypertension and diabetes themselves.
2. METHODS
2.1. Study population
The data of the present study were derived from the National Health and Nutritional Examination Survey (NHANES) 1999‐2006, which is a nationwide, representative survey based on the civilian noninstitutionalized American population. Briefly, the NHANES survey is conducted in America every 2 years, and the survey adopts a cross‐sectional, multistage, stratified, and clustered probability sampled study design. More detailed information about NHANES can be acquired at http://www.cdc.gov/nchs/nhanes.htm. In our study, subjects aged more than 20 years old with complete data of blood pressure, serum homocysteine, serum creatinine, urine creatinine, urine albumin, and other associated covariates were finally included into the statistical analysis (n = 13 693). National Center for Health Statistics Ethics Review Board approved the study protocol. All participants provided written informed consent.
2.2. Data collection and measurements
2.2.1. Blood pressure and HTN
Three (sometimes 4) continuous blood pressure measurements (both systolic and diastolic) were performed in the mobile examination center (MEC). For those who were disabled, the blood pressure measurements were conducted during home examinations. Two physicians and two health technologists were trained to collect NHANES blood pressure data at MEC setting and home examination setting, respectively. The measurements were performed according to a standardized protocol. After sitting and resting quietly for 5 minutes, the blood pressure measurements were conducted at the right arm. If the measurements could not be taken in the right arm, they were taken in the left arm. The device used for blood pressure measurement was the Baumanometer Calibrated Manometer. Upon receipt from the factory, these manometers were precisely calibrated to true gravity and were guaranteed by the manufacturer to remain scientifically accurate. All of the blood pressure measurements (including those conducted in participants’ homes) followed the same protocol. A detailed description of the blood pressure measurement procedure was documented in the “Physician Examination Procedures Manual” on the official websites of NHANES (https://wwwn.cdc.gov/nchs/data/nhanes/2005-2006/manuals/PE.pdf). HTN was recognized as mean systolic blood pressure (SBP) ≥140 mm Hg and/or a mean diastolic blood pressure (DBP) ≥90 mm Hg, and participants with self‐reported use of anti‐hypertensive drugs were also regarded as hypertensive patients.16
2.2.2. Serum homocysteine and HHcy
Subjects were examined in the morning after fasting at least 8 hours or more but less than 24 hours. More specific information about specimen collection and processing instructions can be found at the NHANES Laboratory/Medical Technologists Procedures Manual. Serums were frozen (−20°C), stored, and transported to the Division of Environmental Health Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention. For the specimens from 1999 to 2001, homocysteine was quantified by using an Abbott Homocysteine IMX analyzer (Abbott Laboratories). However, for the specimens from 2002 to 2006, the analyzer was changed to an Abbott AxSym analyzer (Abbott Laboratories). The homocysteine data from 2001 were calibrated by the NHANES group before releasing, and the data from 1999 to 2000 were manually calibrated by using the following formula: AxSym = 10**(0.983*log10(IMX) + 0.0418). Finally, HHcy was recognized as serum homocysteine concentration >12 μmol/L.17
2.2.3. Reduced eGFR, albuminuria, and CKD
Assessment of serum creatinine was conducted with a Hitachi Model 917 multichannel analyzer (Roche Diagnostics) at Coulston Foundation, Alamogordo, New Mexico between 1999 and 2001. After 2001, the serum creatinine was quantified by using a Beckman Synchron LX20 (Beckman Instruments, Inc) at Collaborative Laboratory Services in Ottumwa, Iowa. According to the results of Selvin et al,18 the serum creatinine data from 1999 to 2000 and 2005 to 2006 were calibrated. The correction equations were as follows: Standard creatinine = 0.147 + 1.013 × uncalibrated serum creatinine (for data from 1999 to 2000) and Standard creatinine = −0.016 + 0.978 × uncalibrated serum creatinine (for data from 2005 to 2006). Casual urine specimens collected from the subjects were frozen (−20°C), stored, and shipped to the University of Minnesota. Urinary creatinine was tested by a Beckman Synchron CX3 clinical analyzer (Beckman Instruments, Inc). The urinary albumin was quantified by using a fluorometer, Sequoia‐Turner model 450 (Sequoia‐Turner Corp.). eGFR was calculated by following the Chronic Kidney Disease Epidemiology Collaboration creatinine equation.19 Reduced eGFR was determined as eGFR <60 mL/min per 1.73 m*2; albuminuria was defined as urinary albumin‐to‐creatinine ratio ≥3 mg/mmol; CKD referred to the presence of reduced eGFR and/or albuminuria.20
2.2.4. Covariates
Before the health examination, demographic data were collected in the home with a computer‐assisted personal interviewing methodology. If the subjects could not answer the questions by themselves, a proxy would provide related information. Ever smoking was defined as smoked at least 100 cigarettes in life, and current cigarette use (every day or some days) was recognized as current smoking. Ever drinking referred to at least 12 alcohol drinks in 1 year. Drinking frequency was determined as the average drinking times per week in the past 1 year. Poverty‐to‐income ratio (PIR), calculated by family income ratio to the federal poverty threshold, was used to assess the socioeconomic status of subjects.
Standardized measurements were utilized to evaluate weight, height, and waist circumference (WC). Body mass index (BMI) was calculated as weight (kg) ratio to height (m) squared. Total cholesterol (TC) and high‐density lipoprotein cholesterol (HDL‐C) were analyzed at Lipoprotein Analytical Laboratory of Johns Hopkins University School of Medicine. Serum folate and vitamin B12 were measured by using Bio‐Rad Laboratories “Quantaphase II Folate/vitamin B12” radio‐assay kit at the Division of Environmental Health Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention.
2.3. Statistical analysis
In the present work, we used 4 rounds of the NHANES examinations, which were 1999‐2000, 2001‐2002, 2003‐2004, and 2005‐2006. We pooled the data set by combining the data from different rounds of NHANES survey and calculating the weights for 8 years through the methods given by the NHANES official websites (https://www.cdc.gov/nchs/tutorials/nhanes/SurveyDesign/Weighting/Task2.htm). According to the NHANES analytic guidelines, the calculated 8‐year sampling weight was used because of the complex survey design, and the analyses were performed by using the survey data analysis function. Continuous variates were summarized as the mean value with 95% confidence intervals (CI). Categorical variates were shown as the frequency with 95% CI. Multiple comparisons between groups in Figure 1 were conducted by using Kruskal‐Wallis pairwise comparison for homocysteine (skewed distribution) and Bonferroni multiple comparisons in the post hoc analysis of ANOVA for SBP (normal distribution). Multivariate logistic regression models were performed to investigate the associations between HTN, HHcy, and CKD. Covariates that had a P value <.20 for the association with CKD in the univariate models (Table S1) were included in the multivariate models. Furthermore, variates that had shown associations with HTN, HHcy, and CKD in published articles were also included in the multivariate models.
Figure 1.
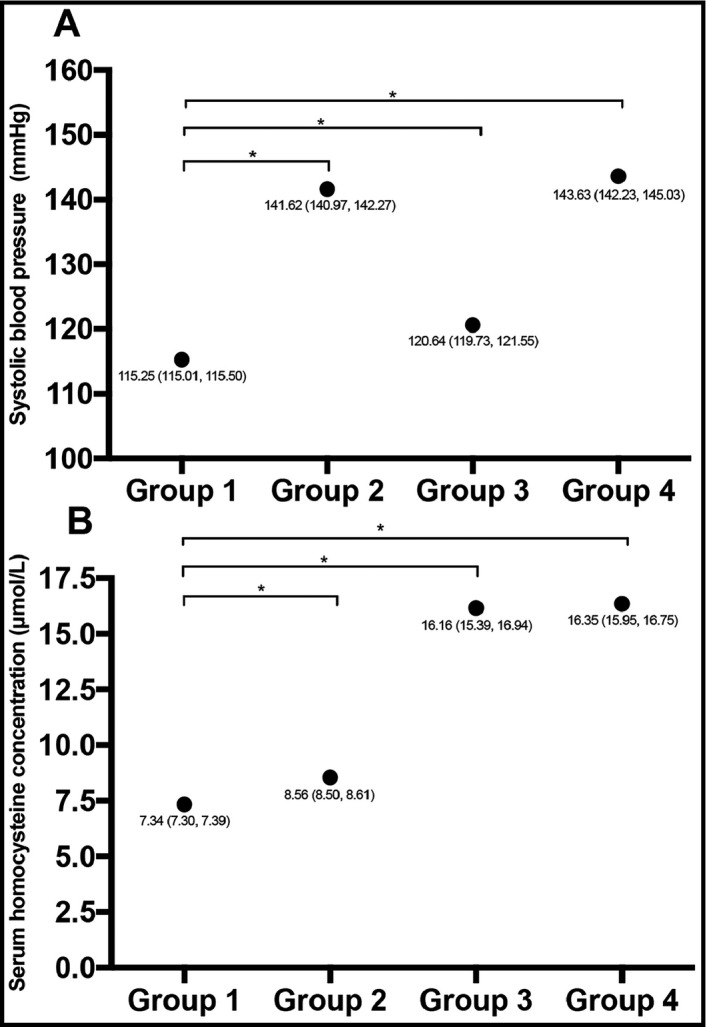
Blood pressure level and serum homocysteine concentration according to the presence or absence of reduced eGFR and albuminuria. Subjects were divided into four groups: Group 1: normal eGFR and non‐albuminuria; Group 2: reduced eGFR and non‐albuminuria; Group 3: normal eGFR and albuminuria; Group 4: reduced eGFR and albuminuria. Each point indicates the mean value of the corresponding group, and the legend beside the point shows the mean value and its 95% confidence interval. The figure suggests that the presence of reduced eGFR and albuminuria is associated with increased SBP level and serum homocysteine concentration. In Group 3, patients still have significantly increased SBP level and homocysteine concentration when eGFR is normal. The line indicated the comparison between the connected two groups. *P for comparison <.001. eGFR, estimated glomerular filtration ratio; SBP, systolic blood pressure
Since the synergistic interaction can be additive or multiplicative,21, 22 the interaction of HTN and HHcy on the risk of CKD and its components were investigated on both additive and multiplicative scales. Additive interaction means the coexistence of 2 risk factors has a significantly greater risk than the sum of their independent impacts. Additive interaction was evaluated by relative excess risk due to interaction (RERI), attributable proportion due to interaction (AP) and synergy index (S index).23, 24 RERI indicated the excessive OR caused by the interaction effect. It was determined as (OR11 − OR10 − OR01) + 1. OR11 represented the risk of CKD when subjects had both HTN and HHcy; OR10 referred to the CKD risk when subjects only had HTN; OR01 indicated the CKD risk of HHcy alone. AP was calculated as RERI/OR11, and it represented the proportion of the total CKD risk that was due to the interaction. S index was calculated as [OR11 − 1]/[(OR10 − 1) + (OR01 − 1)]. For RERI and AP, a 95% CI not across 0 represented significance; for S index, a 95% CI not across 1 indicated significance. For the calculation of multiplicative interaction, HTN × HHcy was included as an interaction term into the multivariate logistic regression models, and the OR of HTN × HHcy was used to evaluate the significance and magnitude of multiplicative interaction. All the analyses were performed by Stata Statistical Software (version 15.1; Stata Corp), SPSS 25.0 software (IBM Corp) and Prism 7.0 software (GraphPad Software, Inc).
3. RESULTS
The present study finally included 13 693 subjects into the analysis. Table 1 presented the characteristics of the subjects. Among these participants, the prevalence of CKD was 18.44%. More specifically, 8.63% of the participants suffered from reduced eGFR (eGFR < 60 mL/min per 1.73 m*2) and 12.79% of the subjects had albuminuria. With regard to HTN and HHcy, 30.55% of the total population suffered from HTN, and the CKD group had a higher prevalence of HTN than the non‐CKD group. The prevalence of HHcy was 10.26% among all included subjects and higher in the CKD group than in the non‐CKD group.
Table 1.
Characteristics of subjects divided by CKD
| Variables | Total (n = 13 693) | CKD group (n = 2525) | Non‐CKD group (n = 11 168) |
|---|---|---|---|
| Age (y) | 45.86 (45.27‐46.46) | 59.59 (58.38‐60.81) | 43.65 (43.13‐44.17) |
| Males (%) | 49.47 (48.47‐50.28) | 43.38 (40.93‐45.86) | 50.46 (49.62‐51.29) |
| Race (%) | |||
| Non‐Hispanic white | 74.20 (71.4‐76.9) | 74.15 (70.72‐77.31) | 74.24 (71.38‐76.90) |
| Non‐Hispanic black | 9.70 (8.18‐11.46) | 10.60 (8.63‐12.95) | 9.55 (8.08‐11.26) |
| Mexican American | 7.04 (5.76‐8.59) | 5.95 (4.48‐7.85) | 7.22 (5.94‐8.75) |
| Other Hispanic | 4.76 (3.38‐6.71) | 4.95 (3.38‐7.20) | 4.75 (3.35‐6.69) |
| Others | 4.26 (3.66‐4.96) | 4.36 (3.17‐5.97) | 4.24 (3.62‐4.97) |
| Educational level (%) | |||
| <high school | 17.41 (16.14‐18.76) | 27.42 (24.96‐30.02) | 15.80 (14.58‐17.10) |
| =high school | 25.46 (24.23‐26.74) | 25.39 (23.01‐27.93) | 25.48 (24.09‐26.91) |
| >high school | 57.12 (55.11‐59.11) | 47.19 (44.00‐50.40) | 58.73 (56.69‐60.73) |
| PIR | 3.10 (3.01‐3.19) | 2.74 (2.61‐2.87) | 3.16 (3.08‐3.24) |
| Ever drinking (%) | 75.53 (73.03‐77.87) | 65.50 (62.02‐68.8) | 77.15 (74.62‐79.50) |
| Drinking frequency (per week) | 4.17 (3.58‐4.76) | 3.54 (2.01‐5.08) | 4.27 (3.72‐4.82) |
| Ever smoking (%) | 50.83 (49.16‐52.51) | 53.28 (50.89‐55.66) | 50.44 (48.64‐52.23) |
| Current smoking (%) | 24.98 (23.61‐26.40) | 19.90 (17.80‐22.18) | 25.80 (24.27‐27.38) |
| Physical activity (%) | |||
| Low | 23.44 (22.33‐24.58) | 28.63 (26.11‐31.29) | 22.60 (21.45‐23.80) |
| Moderate | 50.46 (49.35‐51.56) | 53.19 (50.45‐55.91) | 50.02 (48.82‐51.21) |
| High | 26.11 (24.80‐27.46) | 18.18 (16.47‐20.03) | 27.39 (26.01‐28.81) |
| Height (cm) | 169.39 (169.17‐169.62) | 166.78 (166.28‐167.28) | 169.82 (169.59‐170.04) |
| Weight (kg) | 80.87 (80.25‐81.49) | 80.94 (79.58‐82.30) | 80.86 (80.24‐81.48) |
| WC (cm) | 96.67 (96.11‐97.23) | 100.47 (99.36‐101.58) | 96.06 (95.50‐96.62) |
| BMI (kg/m*2) | 28.10 (27.89‐28.31) | 28.93 (28.51‐29.35) | 27.97 (27.76‐28.18) |
| FPG (mmol/L) | 5.28 (5.24‐5.32) | 6.09 (5.96‐6.21) | 5.15 (5.11‐5.19) |
| TC (mmol/L) | 5.22 (5.18‐5.24) | 5.33 (5.26‐5.40) | 5.21 (5.18‐5.24) |
| HDL‐C (mmol/L) | 1.37 (1.36‐1.38) | 1.36 (1.33‐1.38) | 1.37 (1.36‐1.38) |
| Serum folate (nmol/L) | 32.60 (31.80‐33.40) | 38.98 (36.62‐41.34) | 31.57 (30.76‐32.38) |
| Vitamin B12 (pmol/L) | 392.40 (374.43‐410.36) | 415.62 (391.64‐439.60) | 388.66 (368.04‐409.30) |
| Scr (mg/dL) | 0.89 (0.89‐0.90) | 1.09 (1.06‐1.11) | 0.86 (0.86‐0.87) |
| eGFR (mL/min per 1.73 m*2) | 94.06 (93.21‐94.90) | 74.26 (72.61‐75.91) | 97.25 (96.53‐97.96) |
| Urine Scr (mmol/L) | 11.33 (11.09‐11.56) | 10.38 (10.03‐10.73) | 11.48 (11.23‐11.73) |
| Urine albumin (mg/L) | 31.40 (27.04‐35.75) | 171.50 (141.62‐201.38) | 8.81 (8.55‐9.08) |
| UACR (mg/mmol) | 3.15 (2.66‐3.64) | 17.81 (14.49‐21.14) | 0.79 (0.77‐0.80) |
| Glucose‐lowering medication (%) | 4.46 (3.97‐5.00) | 13.13 (11.26‐15.27) | 3.06 (2.64‐3.55) |
| Insulin taking (%) | 1.55 (1.31‐1.83) | 5.91 (4.84‐7.19) | 0.85 (0.67‐1.08) |
| DM (%) | 7.65 (7.04‐8.30) | 22.87 (20.68‐25.22) | 5.19 (4.63‐5.83) |
| SBP (mm Hg) | 122.72 (122.13‐123.30) | 134.35 (133.33‐135.38) | 120.84 (120.26‐121.42) |
| DBP (mm Hg) | 71.53 (71.11‐71.94) | 70.29 (69.19‐71.39) | 71.73 (71.33‐72.13) |
| Anti‐hypertension therapy (%) | 21.54 (20.33‐22.81) | 46.66 (43.65‐49.70) | 17.49 (16.35‐18.70) |
| HTN (%) | 30.55 (29.11‐32.02) | 60.91 (58.15‐63.60) | 25.66 (24.26‐27.11) |
| Hcy (μmol/L) | 8.58 (8.49‐8.67) | 10.81 (10.44‐11.19) | 8.34 (8.24‐8.44) |
| HHcy (%) | 10.26 (9.48‐11.09) | 28.01 (25.08‐31.14) | 7.40 (6.70‐8.17) |
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; HHcy, hyperhomocysteinemia; HTN, hypertension; PIR, poverty‐to‐income ratio; SBP, systolic blood pressure; Scr, serum creatinine; TC, total cholesterol; UACR, urinary albumin‐to‐creatinine ratio; WC, waist circumference.
Data are expressed as mean or frequency with 95% confidence intervals as appropriate.
We divided subjects into four groups according to the presence or absence of reduced eGFR or albuminuria (Group 1: normal eGFR and non‐albuminuria; Group 2: reduced eGFR and non‐albuminuria; Group 3: normal eGFR and albuminuria; Group 4: reduced eGFR and albuminuria). Figure 1 displayed the SBP level and the serum homocysteine concentration in the 4 groups. As for the SBP level, the presence of reduced eGFR or albuminuria was associated with higher levels of SBP, and for patients with albuminuria but without reduced eGFR, the SBP level was still significantly elevated. With regard to the serum homocysteine concentration, the presence of reduced eGFR was associated with increased homocysteine, and the presence of albuminuria alone also related to the increase in homocysteine concentration.
We performed multivariate logistic regression models to reveal the association between HTN, HHcy, and CKD (Table 2). In the crude model, HTN and HHcy caused a 4.515 and a 4.869 times risk of CKD, respectively. After adjusting for age, sex, race, education level, PIR, physical activity, drinking frequency, current smoking, TC, HDL‐C, serum folate, vitamin B12, BMI, WC, and DM, HTN still casts a 1.787 (1.562‐2.044) times risk of CKD, and HHcy also caused a 2.985 (2.472‐3.606) times risk of CKD. As for the diagnostic components of CKD, hypertensive patients had an 81.8% increase in the risk of reduced eGFR and a 95.9% elevation in the risk of albuminuria when compared with normotensive subjects. HHcy patients suffered from a 6.573 (5.075‐8.512) times risk of reduced eGFR and a 1.880 (1.527‐2.314) times risk of albuminuria than patients with normal serum homocysteine level. When adding the multiplicative interaction term into the models, all of the ORs attenuated but remained in significant. Furthermore, we did a stratification analysis according to gender, and the results were consistent with the findings from the whole population (Table S2).
Table 2.
Multivariate logistic regression of HTN or HHcy on CKD and its components
| Risk factors | ORs | 95% CI | P value |
|---|---|---|---|
| Crude model | |||
| CKD | |||
| HTN | 4.515 | 4.004, 5.091 | <.001 |
| HHcy | 4.869 | 4.080, 5.810 | <.001 |
| Reduced eGFR | |||
| HTN | 8.554 | 7.449, 9.822 | <.001 |
| HHcy | 11.269 | 8.916, 14.244 | <.001 |
| Albuminuria | |||
| HTN | 3.366 | 2.930, 3.865 | <.001 |
| HHcy | 2.824 | 2.356, 3.384 | <.001 |
| Multivariate adjusted model without multiplicative interaction term | |||
| CKD | |||
| HTN | 1.787 | 1.562‐2.044 | <.001 |
| HHcy | 2.985 | 2.472‐3.606 | <.001 |
| Reduced eGFR | |||
| HTN | 1.818 | 1.499‐2.203 | <.001 |
| HHcy | 6.573 | 5.075‐8.512 | <.001 |
| Albuminuria | |||
| HTN | 1.959 | 1.659‐2.313 | <.001 |
| HHcy | 1.880 | 1.527‐2.314 | <.001 |
| Multivariate adjusted model with multiplicative interaction term | |||
| CKD | |||
| HTN | 1.594 | 1.363, 1.865 | <.001 |
| HHcy | 2.371 | 1.772, 3.174 | <.001 |
| Reduced eGFR | |||
| HTN | 1.512 | 1.166, 1.962 | <.001 |
| HHcy | 5.180 | 3.391, 7.911 | <.001 |
| Albuminuria | |||
| HTN | 1.844 | 1.532, 2.219 | <.001 |
| HHcy | 1.590 | 1.137, 2.224 | <.001 |
Abbreviations: BMI, body mass index; CI, confidence intervals; CKD, chronic kidney disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration ratio; HDL‐C, high‐density lipoprotein cholesterol; HHcy, hyperhomocysteinemia; HTN, hypertension; PIR, poverty‐to‐income ratio; TC, total cholesterol; WC, waist circumference.
Crude model: no adjustment; multivariate adjusted model without multiplicative interaction term: adjusted by age, sex, race, education level, PIR, physical activity, drinking frequency, current smoking status, TC, HDL‐C, serum folate, vitamin B12, BMI, WC, and DM; multivariate adjusted model with multiplicative interaction term: adjusted by age, sex, race, education level, PIR, physical activity, drinking frequency, current smoking status, TC, HDL‐C, serum folate, vitamin B12, BMI, WC, DM, and interaction term of HTN and HHcy.
To investigate the effect of coexistent HTN and HHcy on CKD and its components, we divided subjects into four categories (non‐HTN and non‐HHcy, HTN and non‐HHcy, non‐HTN and HHcy, and HTN and HHcy) (Table 3). In the crude model, HTN and HHcy group showed a 14.328 (11.588, 17.715) times risk than healthy group for CKD, and 53.387 (39.841, 71.539) and 6.520 (5.267, 8.070) times risk for reduced eGFR and albuminuria, respectively. After adjusting for all covariates, all of the effect values attenuated but remained in significance. With regard to CKD, HTN and HHcy group showed a 5.072 (3.967‐6.486) times risk than the healthy group, much higher than that of HTN and non‐HHcy group (1.594, 95% CI: 1.363‐1.865) and non‐HTN and HHcy group (2.492, 95% CI: 1.879‐3.305). Furthermore, HTN caused significantly elevated OR of CKD in both HHcy and normal Hcy subjects, and the OR of CKD in HHcy subjects was also significantly increased in both HTN and non‐HTN strata. The results of reduced eGFR and albuminuria were consistent with the results of CKD. The coexistence of HTN and HHcy casts an extremely high association with reduced eGFR, with an OR of 10.329 (7.363‐14.491). Similarly, the coexistence of HTN and HHcy caused an OR of 3.452 (2.589‐4.601) for albuminuria, higher than that of HTN and non‐HHcy group (1.844, 95% CI: 1.532‐2.219) and non‐HTN and HHcy group (1.590, 95% CI: 1.137‐2.224). Moreover, we also conducted a stratification analysis based on gender, and the findings were close to the major results derived from the whole population (Table S3).
Table 3.
Multivariate logistic regression models of the joint effect of HTN and HHcy on CKD and its components
| Non‐HTN | HTN | ORs (95% CI) for HTN within strata of HHcy | |||
|---|---|---|---|---|---|
| N cases/non‐cases | OR (95% CI) | N cases/non‐cases | OR (95% CI) | ||
| Crude model | |||||
| CKD | |||||
| Normal Hcy | 652/7515 | 1 | 1034/2781 | 3.808 (3.271, 4.434), P < .001 | 3.808 (3.271, 4.434), P < .001 |
| Hhcy | 177/423 | 3.429 (2.571, 4.575), P < .001 | 662/449 | 14.328 (11.588, 17.715), P < .001 | 4.178 (3.191, 5.470), P < .001 |
| ORs (95% CI) for HHcy within strata of HTN | 3.429 (2.571, 4.575), P < .001 | 3.762 (3.039, 4.658), P < .001 | |||
| Reduced eGFR | |||||
| Normal Hcy | 130/8037 | 1 | 412/3403 | 7.639 (6.217, 9.386), P < .001 | 7.639 (6.217, 9.386), P < .001 |
| Hhcy | 124/476 | 11.235 (7.650, 16.500), P < .001 | 516/595 | 53.387 (39.841, 71.539), P < .001 | 4.752 (3.722, 6.066), P < .001 |
| ORs (95% CI) for HHcy within strata of HTN | 11.235 (7.650, 16.500), P < .001 | 6.989 (5.475, 8.921), P < .001 | |||
| Albuminuria | |||||
| Normal Hcy | 537/7630 | 1 | 738/3077 | 2.983 (2.507, 3.548), P < .001 | 2.983 (2.507, 3.548), P < .001 |
| Hhcy | 90/510 | 1.944 (1.378, 2.741), P < .001 | 387/724 | 6.520 (5.267, 8.070), P < .001 | 3.354 (2.361, 4.764), P < .001 |
| ORs (95% CI) for HHcy within strata of HTN | 1.944 (1.378, 2.741), P < .001 | 2.186 (1.734, 2.756), P < .001 | |||
| Multivariate adjusted model | |||||
| CKD | |||||
| Normal Hcy | 652/7515 | 1 | 1034/2781 | 1.594 (1.363‐1.865), P < .001 | 1.767 (1.507‐2.072), P < .001 |
| Hhcy | 177/423 | 2.731 (1.772‐3.174), P < .001 | 662/449 | 5.072 (3.967‐6.486), P < .001 | 1.652 (1.182‐2.308), P = .004 |
| ORs (95% CI) for HHcy within strata of HTN | 2.492 (1.879‐3.305), P < .001 | 3.108 (2.437‐3.964), P < .001 | |||
| Reduced eGFR | |||||
| Normal Hcy | 130/8037 | 1 | 412/3403 | 1.512 (1.166‐1.962), P = .002 | 1.465 (1.110‐1.934), P = .008 |
| Hhcy | 124/476 | 5.180 (3.391‐7.911), P < .001 | 516/595 | 10.329 (7.363‐14.491), P < .001 | 1.993 (1.423‐2.790), P < .001 |
| ORs (95% CI) for HHcy within strata of HTN | 5.187 (3.244‐8.292), P < .001 | 6.571 (4.905‐8.803), P < .001 | |||
| Albuminuria | |||||
| Normal Hcy | 537/7630 | 1 | 738/3077 | 1.844 (1.532‐2.219), P < .001 | 1.916 (1.590‐2.309), P < .001 |
| Hhcy | 90/510 | 1.590 (1.137‐2.224), P = .008 | 387/724 | 3.452 (2.589‐4.601), P < .001 | 2.052 (1.415‐2.976), P < .001 |
| ORs (95% CI) for HHcy within strata of HTN | 1.654 (1.176‐2.325), P = .005 | 1.773 (1.351‐2.326), P < .001 | |||
Abbreviations: BMI, body mass index; CI, confidence intervals; CKD, chronic kidney disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration ratio; HDL‐C, high‐density lipoprotein cholesterol; HHcy, hyperhomocysteinemia; HTN, hypertension. PIR, poverty‐to‐income ratio; TC, total cholesterol; WC, waist circumference.
Crude model: no adjustment; multivariate adjusted model: adjusted by age, sex, race, education level, PIR, physical activity, drinking frequency, current smoking status, TC, HDL‐C, serum folate, vitamin B12, BMI, WC, and DM.
To demonstrate whether HTN and HHcy have a synergistic interaction toward CKD, we calculated the interaction effect in both additive and multiplicative scale (Table 4). For the additive scale, the RERI 8.090 (95% CI: 5.473‐10.707) for CKD was significant and positive in the crude model. After adjusting for all covariates, the RERI shrank to 2.107 (95% CI: 1.071‐3.143), which meant 2.107 times risk out of the total 5.072 times risk was caused by the interaction between HTN and HHcy. Although the effect value decreased, it was still significant, implicating the synergistic interaction between HTN and HHcy on CKD. Moreover, the AP revealed that the interaction caused 41.5% of the total impact in the HTN and HHcy group. Additionally, the S index for CKD (2.072, 95% CI: 1.449‐2.962) also confirmed the synergistic interaction. The results of reduced eGFR and albuminuria also identified the significant synergistic interaction between HTN and HHcy. The RERI and AP for reduced eGFR were larger than that for CKD, which was consistent with the findings in Table 3. Also corresponding to the findings in Table 3, our results elucidated that the additive interaction toward albuminuria was relatively weak than that toward CKD or reduced eGFR. A clear illustration of the results of addictive synergistic interaction analyses was presented in Figure 2. With regard to the interaction in multiplicative scale, although the interaction term showed a significant and protective effect for reduced eGFR in the crude model, we observed no significant interactions toward CKD, reduced eGFR, and albuminuria in the full model. We then divided our population into males and females and re‐evaluated the interaction effect in both scales (Table S4). In males, the addictive interaction was insignificant toward CKD, reduced eGFR, and albuminuria, but it still revealed a trend to become significant. In females, the addictive interaction was still significant toward CKD and reduced eGFR, accounting for 51.4% and 58.6% of the total impact for CKD and reduced eGFR, respectively. However, the addictive interaction was insignificance toward albuminuria. As for the multiplicative interaction, the results were similar to that from the whole population, and our study detected insignificant multiplicative interaction toward CKD, reduced eGFR, and albuminuria in both genders.
Table 4.
Interaction analysis of HTN and HHcy on the risk of CKD
| CKD | Reduced eGFR | Albuminuria | |
|---|---|---|---|
| Crude model | |||
| Addictive scale | |||
| RERI | 8.090 (5.473, 10.707) | 35.513 (23.875, 47.150) | 2.593 (1.203, 3.984) |
| AP | 0.565 (0.471, 0.658) | 0.665 (0.601, 0.729) | 0.398 (0.236, 0.559) |
| S index | 2.545 (1.991, 3.253) | 3.105 (2.541, 3.793) | 1.886 (1.335, 2.665) |
| Multiplicative scale | |||
| ORs | 1.097 (0.782, 1.539) | 0.622 (0.436, 0.888) | 1.125 (0.732, 1.728) |
| Multivariate adjusted model | |||
| Addictive scale | |||
| RERI | 2.107 (1.071‐3.143) | 4.637 (1.795‐7.479) | 1.018 (0.134‐1.902) |
| AP | 0.415 (0.270‐0.561) | 0.449 (0.254‐0.644) | 0.295 (0.088‐0.502) |
| S index | 2.072 (1.449‐2.962) | 1.988 (1.303‐3.033) | 1.710 (1.071‐2.729) |
| Multiplicative scale | |||
| ORs | 1.342 (0.970‐1.857) | 1.319 (0.817‐2.128) | 1.177 (0.790‐1.754) |
Abbreviations: AP, the attributable proportion due to interaction; BMI, body mass index; CKD, chronic kidney disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration ratio; HDL‐C, high‐density lipoprotein cholesterol; HHcy, hyperhomocysteinemia; HTN, hypertension; PIR, poverty‐to‐income ratio; RERI, the relative excess risk due to interaction; S index, synergy index; TC, total cholesterol; WC, waist circumference.
Crude model: no adjustment; multivariate adjusted model: adjusted by age, sex, race, education level, PIR, physical activity, drinking frequency, current smoking status, TC, HDL‐C, serum folate, vitamin B12, BMI, WC, and DM.
Figure 2.
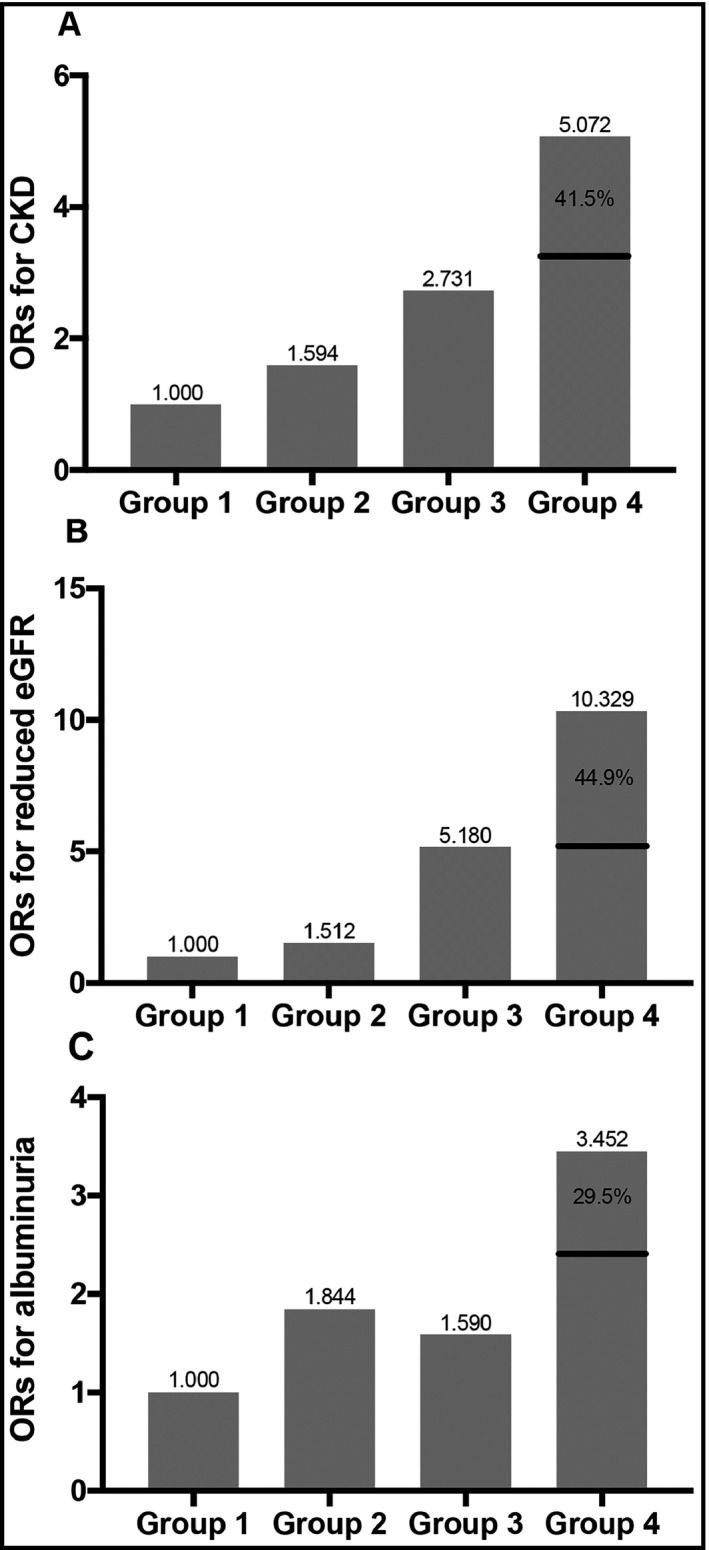
Illustration of the addictive synergistic interaction between HTN and HHcy toward CKD and its components. Group 1: non‐HTN and non‐HHcy; Group 2: HTN and non‐HHcy; Group 3: non‐HTN and HHcy; Group 4: HTN and HHcy. In each panel, Group 4 has a significant higher OR for the outcome (CKD, reduced eGFR, or albuminuria), and a considerable portion of the OR in group 4 is caused by the effect of the synergistic interaction. For CKD, the portion is 41.5%; for reduced eGFR, the portion is 44.9%; for albuminuria, the portion is 29.5%. CKD, chronic kidney disease; eGFR, estimated glomerular filtration ratio; ORs, odds ratios
4. DISCUSSION
Our study verified the positive association between HTN, HHcy, and CKD based on a nationwide and general population. More importantly, our work was the first evidence to suggest the synergistic interaction between HTN and HHcy toward renal injury. Our findings implicate the coexistence of HTN and HHcy may cast a greater impact on renal function than the sum of their independent impact. In other words, the findings suggest the combination of HTN and HHcy may not only add up the impact, but also generate more deteriorative effect. Therefore, the results highlight that the coexistence itself may also correlate with a deteriorative impact on renal function in addition to the effects of HTN and HHcy themselves. Accordingly, our study may support the rationality and value of simultaneous tight control of HTN and HHcy in preventing CKD. In addition, the present work also provides epidemiological evidence for related experimental studies.
The value of interaction analysis is to determine whether the combination of two risk factors causes a significantly higher or lower risk than the sum or product of their independent impacts. Therefore, the interaction effect has two possible scales: additive and multiplicative. Interaction in the additive scale evaluates the difference between the risk of the outcome when both two risk factors exist and the sum of the outcome risks when only one risk factor exists. Interaction in the multiplicative scale indicates whether the risk of the outcome for subjects with both risk factors is significantly distinct from the product of the outcome risks in subjects with only one risk factor.23, 25 Thus, it is possible that only one scale of interaction is significant. Although there is no agreement on which scale is better, additive interaction may have a greater clinical significance, because it directly evaluates the proportion of the risk caused by the synergistic action of the two risk factors.23, 26 Nevertheless, the new STROBE statement advocates authors to provide both additive and multiplicative scale of interaction when investigating the joint effect of two risk factors.27 Accordingly, we reported the results of interaction in both scales. In addition, we facilitated the comparison between our results and other related works by presenting two interaction scales.
The interaction in the additive scale demonstrated a synergistic and significant joint effect of HTN and HHcy on CKD. The results suggest that the combination of HTN and HHcy has a greater effect on CKD than the sum of the impact when only one risk factor exists. Thus, our study implicates that HTN and HHcy may have a synergistic interaction toward greater renal damage. In other words, the combination of HTN and HHcy may not only add up the impact but also produce a more deteriorative effect. Accordingly, except for HTN and HHcy, the coexistence of these two factors may also be an additional factor that correlates with CKD. With this regard, simultaneous tight control of HTN and HHcy may be rational and important to prevent the development and progression of CKD.
Our study is consistent with established studies. Early studies have elucidated the role of HTN in promoting the development and progression of CKD, and prior research has addressed the importance of HTN control in the management of CKD.4, 5, 28 Previous studies have also identified the association between HHcy and CKD.12 Shankar et al demonstrated the impact of HHcy on CKD was independent of common CKD risk factors such as DM and HTN.29 Furthermore, a study revealed the role of HHcy in predicting renal damage among healthy people.11 More importantly, Xie et al elucidated that HHcy could increase the risk of renal function decline among hypertensive patients, suggesting the additive effect of HTN and HHcy on CKD. However, no study to date has investigated whether the combination of HTN and HHcy correlates with an additional risk of CKD than the sum of their independent impact. In the present study, we expanded the results of the above published articles. Our study confirmed that both HTN and HHcy were independently associated with CKD and its component. Furthermore, our results suggest that HHcy correlates with CKD among HTN patients and vice versa. Most importantly, our study implicates that the coexistence of HTN and HHcy may cast an extremely large impact on CKD, and this impact can be significantly greater than the sum of their independent effects. Our work for the first time reveals the synergistic interaction between HTN and HHcy on CKD, suggesting the rationality and importance of simultaneous tight control of HTN and HHcy to prevent CKD.
It is important to recognize that eGFR may influence the serum homocysteine concentration because homocysteine is excreted by the kidney. Therefore, the association between CKD and HHcy may attribute to the reduced renal excretion of homocysteine. However, the present study demonstrated that the homocysteine level was still statistically increased in patients with albuminuria but without reduced eGFR (Figure 1b), and HHcy was independently associated with the occurrence of albuminuria (Table 2). Thus, it is reasonable to conclude that HHcy and CKD may initiate a vicious circle toward renal damage.
It is also necessary to discuss the unusual findings in Table 1 when interpreting our results. The results showed that the serum creatinine level of the CKD group was within the normal range. However, because the serum creatinine level is influenced by the gender, age, and race, we believe it is more reasonable to regard eGFR rather than serum creatinine concentration as the reflection of renal function. The results revealed the mean eGFR level in the CKD group was lower than that in the non‐CKD group but still higher than 60 mL/min per 1.73 m*2. However, our diagnosis of CKD was based on both reduced eGFR and albuminuria. According to the results, the prevalence of albuminuria (12.79%) was much larger than that of reduced eGFR (8.63%). Therefore, a considerable number of subjects in the CKD group might have an eGFR ≥ 60 mL/min per 1.73 m*2, but they still suffered from albuminuria. We believe this is the major reason why the CKD group still had a serum creatinine within the normal range and a mean eGFR level higher than 60 mL/min per 1.73 m*2.
Our study adopted a stratifying strategy based on gender difference to consolidate the results. The results of stratification analyses were displayed in Tables S2‐S4. In Tables S2 and S3, the results were consistent with the major findings from the association analyses (Table 2) and the interaction analyses (Table 3), suggesting the robustness of these results. However, in the stratification analyses of RERI, AP, and S index (Table S4), we observed some difference from Table 4. In males, the addictive interaction indexes were insignificant. However, they still displayed a trend toward significance. Considering the relatively small simple size of male stratum, we believe the major reason of this insignificance is inadequate power. In females, the interaction indexes were significant toward CKD and reduced eGFR, but insignificant in albuminuria. This intriguing result may suggest the synergistic interaction between HTN and HHcy toward albuminuria does not exist in females. Nevertheless, the sample size of females is half of the total population. Therefore, our findings in female stratum also need larger studies to verify.
There is also some experimental evidence that indirectly supports the synergistic interaction between HTN and HHcy on CKD. Firstly, researchers have found that HHcy may inhibit the vasodilation of renal arteriole, thus enhances the damage of HTN on the kidney. HHcy generates superoxide radicals and inhibits the activity of cellular antioxidant enzymes such as superoxide dismutase. Therefore, HHcy inhibits the endothelial‐dependent nitric oxide‐mediated relaxation of blood vessels.14, 30 Considering that nitric oxide‐mediated efferent arteriole dilation is an important protective mechanism against glomerular hypertension,8 combination of HTN and HHcy may result in a rapid damage of renal function. Secondly, both HTN and HHcy promote the development and progression of atherosclerosis, which is a fundamental pathophysiological process of glomerulosclerosis. On the one hand, HTN promotes endothelial dysfunction, which initiates atherosclerosis.31, 32 On the other hand, HHcy reduces the nitric oxide availability, leading to lipid peroxidation in vessels, and finally accelerating the process of atherosclerosis.33, 34 Thus, the coexistence of HTN and HHcy may provide a suitable environment for the rapid progression of atherosclerosis in renal arterioles, therefore accelerating the development of CKD. Although the above explanations may support our findings, we still lack direct experimental evidence. Thus, more related mechanistic studies are needed to confirm our conclusion.
Our study still has some limitations that we have to mention when interpreting our results. Firstly, due to the cross‐sectional design, our results can only suggest the synergistic interaction between HTN and HHcy on CKD, but the underlying causality still needs prospective studies to confirm. Secondly, the data for our study were collected at least 13 years ago. The prevalence of HTN, HHcy, and CKD might have changed. However, because the NHANES did not collect data of homocysteine concentration after 2008, and it changed its questionnaire at the round of 2007‐2008, we have to use the data from 1999 to 2006. In addition, we believe that the underlying mechanisms of the synergistic interaction between HTN and HHcy on CKD remain unchanged after years. Thirdly, the prevalence of CKD in the present work was slightly higher than 15%. Therefore, the ORs might not accurately reflect the relative risk, and the accuracy of our results could be influenced. However, because of the survey‐weighted design, we could not conduct log‐binomial tests or Poisson regressions with robust variance estimate to overcome this drawback. Instead, we performed a subgroup analysis by separating CKD into reduced eGFR and albuminuria. Both of these 2 CKD components had a prevalence less than 15%, and their results were consistent with the results of CKD. Hence, we think our conclusions are still reliable. Fourthly, the data of eGFR and albuminuria only contained onetime assessment. Thus, our diagnosis of CKD could be inaccurate. However, considering that the data were collected through a standardized procedure, we believe the data are still acceptable. Fifthly, some examinations were conducted repeatedly at different rounds of NHANES surveys, so these examinations may be influenced by the time effect, but since the major determinants (laboratories and equipment) of the test results were kept in same, we believe the time effect will not cast a large fluctuation into our results. Sixthly, although the homocysteine data from 1999 to 2001 were corrected, their inaccuracy may still influence the results. However, because the number of influenced cases is relatively small, a stratification analysis is unfeasible in the present work. Since the influenced cases only constituted a small part of the total population, and the data were corrected before analyses, we believe the results are still reliable. Seventhly, we did not do the power calculations in our study. However, we have used all of the available subjects from NHANES since the protocol was changed from 2007, and our study enrolled a total of 13 693 participants, we believe this number also enables our results to have a considerable power. Lastly, as the same with other observational studies, unmeasured risk factors can bring bias into our work. For example, the NHANES 1999‐2006 did not collect useful data regarding the salt intake; then, our results might be influenced by the difference of salt intake between groups. Therefore, larger studies containing more potential moderators are needed to verify our results.
CONFLICT OF INTEREST
The authors report no relationships that could be construed as a conflict of interest.
AUTHOR CONTRIBUTIONS
In this study, WS and YZ did the study design, statistical analyses, results interpretation, and reviewer comments response. HW and YS participated as analyzing and resolving difficulties of analytic strategies and results discussion. Finally, YC functioned as final reviewer and corresponding author. All authors read and approved the final manuscript.
Supporting information
ACKNOWLEDGMENTS
The authors thank the NHANES participants, staff, and investigators.
Shi W, Zhou Y, Wang H, Sun Y, Chen Y. Synergistic interaction of hypertension and hyperhomocysteinemia on chronic kidney disease: Findings from the National Health and Nutrition Examination Survey 1999‐2006. J Clin Hypertens. 2019;21:1567–1577. 10.1111/jch.13673
Wen‐Rui Shi and Yaping Zhou contributed equally to this work.
[Corrections added on October 12, 2019, after first online publication: “Haoyu Wang” was added as co‐corresponding author and his affiliation was changed from 1 to 3.]
Contributor Information
Haoyu Wang, Email: dallashaoyuwang@163.com.
Yihan Chen, Email: yihanchentongji@gmail.com.
REFERENCES
- 1. Webster A, Nagler E, Morton R, Masson P. Chronic kidney disease. Lancet (London, England). 2017;389(10075):1238‐1252. [DOI] [PubMed] [Google Scholar]
- 2. Muntner P, Anderson A, Charleston J, et al. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2010;55(3):441‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rao MV, Qiu Y, Wang C, Bakris G. Hypertension and CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES), 1999–2004. Am J Kidney Dis. 2008;51(4 suppl 2):S30‐37. [DOI] [PubMed] [Google Scholar]
- 4. Perry HM, Miller JP, Fornoff JR, et al. Early predictors of 15‐year end‐stage renal disease in hypertensive patients. Hypertension. 1995;25(4 Pt 1):587‐594. [DOI] [PubMed] [Google Scholar]
- 5. Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end‐stage renal disease in men. N Engl J Med. 1996;334(1):13‐18. [DOI] [PubMed] [Google Scholar]
- 6. Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol. 2005;16(10):3027‐3037. [DOI] [PubMed] [Google Scholar]
- 7. Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin‐converting enzyme inhibition: a patient‐level meta‐analysis. Ann Intern Med. 2003;139(4):244‐252. [DOI] [PubMed] [Google Scholar]
- 8. Bidani AK, Polichnowski AJ, Loutzenhiser R, Griffin KA. Renal microvascular dysfunction, hypertension and CKD progression. Curr Opin Nephrol Hypertens. 2013;22(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suliman ME, Lindholm B, Barany P, Bergstrom J. Hyperhomocysteinemia in chronic renal failure patients: relation to nutritional status and cardiovascular disease. Clin Chem Lab Med. 2001;39(8):734‐738. [DOI] [PubMed] [Google Scholar]
- 10. Ye Z, Zhang Q, Li Y et al. High prevalence of hyperhomocysteinemia and its association with target organ damage in chinese patients with chronic kidney disease. Nutrients. 2016;8(10):645‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levi A, Cohen E, Levi M, Goldberg E, Garty M, Krause I. Elevated serum homocysteine is a predictor of accelerated decline in renal function and chronic kidney disease: a historical prospective study. Eur J Intern Med. 2014;25(10):951‐955. [DOI] [PubMed] [Google Scholar]
- 12. Ninomiya T, Kiyohara Y, Kubo M, et al. Hyperhomocysteinemia and the development of chronic kidney disease in a general population: the Hisayama study. Am J Kidney Dis. 2004;44(3):437‐445. [PubMed] [Google Scholar]
- 13. Chao M‐C, Hu S‐L, Hsu H‐S, et al. Serum homocysteine level is positively associated with chronic kidney disease in a Taiwan Chinese population. J Nephrol. 2014;27(3):299‐305. [DOI] [PubMed] [Google Scholar]
- 14. Ostrakhovitch EA, Tabibzadeh S. Homocysteine in chronic kidney disease. Adv Clin Chem. 2015;72:77‐106. [DOI] [PubMed] [Google Scholar]
- 15. Xie DI, Yuan Y, Guo J, et al. Hyperhomocysteinemia predicts renal function decline: a prospective study in hypertensive adults. Sci Rep. 2015;5:16268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206‐1252. [DOI] [PubMed] [Google Scholar]
- 17. Morris MS, Bostom AG, Jacques PF, Selhub J, Rosenberg IH. Hyperhomocysteinemia and hypercholesterolemia associated with hypothyroidism in the third US National Health and Nutrition Examination Survey. Atherosclerosis. 2001;155(1):195‐200. [DOI] [PubMed] [Google Scholar]
- 18. Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50(6):918‐926. [DOI] [PubMed] [Google Scholar]
- 19. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825‐830. [DOI] [PubMed] [Google Scholar]
- 21. Kendler KS, Gardner CO. Interpretation of interactions: guide for the perplexed. Br J Psychiatry. 2010;197(3):170‐171. [DOI] [PubMed] [Google Scholar]
- 22. Knol MJ, van der Tweel I, Grobbee DE, Numans ME, Geerlings MI. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int J Epidemiol. 2007;36(5):1111‐1118. [DOI] [PubMed] [Google Scholar]
- 23. Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20(7):575‐579. [DOI] [PubMed] [Google Scholar]
- 24. Assmann SF, Hosmer DW, Lemeshow S, Mundt KA. Confidence intervals for measures of interaction. Epidemiology (Cambridge, Mass). 1996;7(3):286‐290. [DOI] [PubMed] [Google Scholar]
- 25. Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007;17(3):227‐236. [DOI] [PubMed] [Google Scholar]
- 26. Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol. 1980;112(4):467‐470. [DOI] [PubMed] [Google Scholar]
- 27. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Medicine. 2007;4(10):e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28(9):2812‐2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shankar A, Wang JJ, Chua B, Rochtchina E, Flood V, Mitchell P. Positive association between plasma homocysteine level and chronic kidney disease. Kidney Blood Press Res. 2008;31(1):55‐62. [DOI] [PubMed] [Google Scholar]
- 30. Emsley AM, Jeremy JY, Gomes GN, Angelini GD, Plane F. Investigation of the inhibitory effects of homocysteine and copper on nitric oxide‐mediated relaxation of rat isolated aorta. Br J Pharmacol. 1999;126(4):1034‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mordi I, Mordi N, Delles C, Tzemos N. Endothelial dysfunction in human essential hypertension. J Hypertens. 2016;34(8):1464‐1472. [DOI] [PubMed] [Google Scholar]
- 32. Sitia S, Tomasoni L, Atzeni F, et al. From endothelial dysfunction to atherosclerosis. Autoimmun Rev. 2010;9(12):830‐834. [DOI] [PubMed] [Google Scholar]
- 33. Upchurch GR, Welch GN, Fabian AJ, et al. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem. 1997;272(27):17012‐17017. [DOI] [PubMed] [Google Scholar]
- 34. Chen J‐Y, Ye Z‐X, Wang X‐F, et al. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed Pharmacother. 2018;97:423‐428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


