Abstract
Background
Globally, decreased susceptibility to ceftriaxone in Neisseria gonorrhoeae is rising. We aimed to compile a global collection of N. gonorrhoeae strains and assess the genetic characteristics associated with decreased susceptibility to ceftriaxone.
Methods
We performed a literature review of all published reports of N. gonorrhoeae strains with decreased susceptibility to ceftriaxone (>0.064 mg/L minimum inhibitory concentration) through October 2019. Genetic mutations in N. gonorrhoeae genes (penA, penB, mtrR, and ponA), including determination of penA mosaicism, were compiled and evaluated for predicting decreased susceptibility to ceftriaxone.
Results
There were 3821 N. gonorrhoeae strains identified from 23 countries and 684 (18%) had decreased susceptibility to ceftriaxone. High sensitivities or specificities (>95%) were found for specific genetic mutations in penA, penB, mtrR, and ponA, both with and without determination of penA mosaicism. Four algorithms to predict ceftriaxone susceptibility were proposed based on penA mosaicism determination and penA or non-penA genetic mutations, with sensitivity and specificity combinations up to 95% and 62%, respectively.
Conclusion
Molecular algorithms based on genetic mutations were proposed to predict decreased susceptibility to ceftriaxone in N. gonorrhoeae. Those algorithms can serve as a foundation for the development of future assays predicting ceftriaxone decreased susceptibility within N. gonorrhoeae globally.
Keywords: Neisseria gonorrhoeae, gonorrhea, ceftriaxone, resistance, decreased susceptibility, algorithms
A global collection of 3821 Neisseria gonorrhoeae strains was compiled and analyzed for penA and non-penA genetic alterations associated with decreased susceptibility to ceftriaxone. Four molecular algorithms were developed to predict decreased susceptibility to ceftriaxone with high sensitivity or specificity.
Neisseria gonorrhoeae is the second most common bacterial sexually transmitted infection in the world [1]. Antimicrobial resistance in N. gonorrhoeae is a major concern, as the organism has developed resistance to every class of antibiotics used for treatment [2]. Currently, dual therapy with ceftriaxone and azithromycin is widely recommended for gonorrhea treatment, although ceftriaxone monotherapy is recommended in some countries [3, 4].
In the past 2 decades, N. gonorrhoeae strains with either decreased susceptibility or resistance to the extended-spectrum cephalosporins (ESCs) have emerged [2]. Although N. gonorrhoeae uses a number of mechanisms to develop resistance to antibiotics, there are primarily 4 genes that contribute to decreased susceptibility to ESCs—penA, mtrR, penB, and ponA [5, 6]. penA and ponA are involved in catalyzing the cross-linking of bacterial cell walls [7], penB in the permeability of porins to antimicrobials [8], and mtrR in transcription of the efflux pump that transports antimicrobials [9]. There is considerable variability in the amino acid alterations within those genes [6], leading to difficulty in developing molecular assays to predict susceptibility to ESCs.
Prior research describing the variability within the genetic loci important for decreased susceptibility to ESCs in N. gonorrhoeae have focused on isolates localized to specific countries or regions [5, 10–16]. There are very few reports that aggregate isolates from multiple countries or regions and none that provide a global aggregate of isolates with resistance or decreased susceptibility to ceftriaxone [17–19]. Many reports have found associations between various genetic loci and ceftriaxone resistance, but those data are limited by small sample sizes, narrow geographic regions, and focus on different gene loci.
Here, we performed a global analysis of all available genetically characterized strains of N. gonorrhoeae with decreased susceptibility to ceftriaxone. Based on that analysis, we identified specific genetic alterations in penA, mtrR, penB, and ponA with high sensitivity or specificity to predict decreased susceptibility to ceftriaxone, and proposed parsimonious molecular algorithms for prediction of N. gonorrhoeae strains with decreased susceptibility to ceftriaxone (CRO-DS strains).
MATERIALS AND METHODS
Definition of Resistance and Decreased Susceptibility
There are 2 different minimum inhibitory concentration (MIC) break points used to define resistance to ceftriaxone in N. gonorrhoeae. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) defines an MIC of ≤0.125 mg/L as susceptible and an MIC of >0.125 mg/L as resistant [20], while the Clinical and Laboratory Standards Institute (CLSI) defines an MIC ≤0.25 mg/L as susceptible and >0.25 mg/L as nonsusceptible [21]. Over time, decreased susceptibility was established to monitor the emergence of resistance as a middle point between susceptibility and resistance or nonsusceptibility. Although not specified by EUCAST or CLSI, investigators have classified decreased susceptibility as >0.064 mg/L [22]. In our report, we also defined decreased susceptibility as MIC values >0.064 mg/L.
Literature and Genetic Analysis
One author (E. Y. L.) performed a literature search of all articles published in PubMed up to 15 October 2019 under the search terms “Neisseria gonorrhoeae” and “ceftriaxone.” All reports (N = 939) were reviewed for information about ceftriaxone MIC and genetic characterization of N. gonorrhoeae. Of the 939 reports, there were 169 unique reports that included genetic information and MIC data, of which 111 contained information about specific genetic alterations in N. gonorrhoeae. All genetically characterized N. gonorrhoeae strains with respect to penA, both susceptible and with decreased susceptibility to ceftriaxone, were recorded. Using information in those reports, or by directly contacting the authors, we also recorded genetic alterations with respect to penB, ponA, and mtrR.
Examining penA Amino Acid Alterations
penA is a gene that codes for the penicillin-binding protein (PBP) 2 and is important for the binding of ESCs [23]. Mutant penA in N. gonorrhoeae has been associated with resistance to ceftriaxone [5]. There are currently 83 specific amino acid positions in penA associated with decreased susceptibility to ceftriaxone [2]; different combinations of alterations at those sites have been classified as Ohnishi sequences, with each assigned a specific roman numeral. In this report, penA amino acid alterations of each genetically characterized strain were obtained from either the Ohnishi sequences or the whole-genome sequences. Ohnishi sequences are defined on the NG-STAR Canadian Web site (https://ng.star.canada) or within the report detailing the site’s development [24]. Ohnishi sequences that were unpublished were obtained by contacting authors.
Whole-genome sequences were accessed through the National Center for Biotechnology Information (https://ncbi.nlm.nih.gov), using the studies’ project codes. The amino acid sites of interest were located, and alterations were recorded.
Previous reports have documented specific amino acid alterations associated with resistance for both mosaic and nonmosaic penA alleles in N. gonorrhoeae, where “mosaic” penA alleles contain up to 62 mutations with respect to wild type, and “nonmosaic” alleles contain only point mutations [24]. Alterations in mosaic strains include A311V (alanine to valine at position 311), I312M, V316T/P, T483P/S, A501V/P/T, N512Y, G545S, and P551L/S [2, 6], while alterations in nonmosaic strains include insD345 (aspartate insertion following position 345), A501V/T, G542S, and P551L/S [5, 6, 22]. In this report, all strains compiled were searched for penA amino acid alterations associated with resistance and separated by mosaic status. Mosaicism was determined if alterations in amino acids 375–377 were found, as detailed by Deng et al [25].
Examining non-penA Amino Acid Alterations
penB (porB1b) is an allele of porB, an outer membrane porin associated with ceftriaxone resistance in N. gonorrhoeae through alterations at G120 and A121 [17], amino acids located within the constriction loop of porin. Alterations at those sites lead to decreased permeability of this porin for antimicrobials [8]. Therefore, any alterations in G120 and A121 of penB were recorded. The ponA gene encodes for PBP1 and, like penA, has a role in β-lactam resistance [7]. However, the effect is considered minor; PBP1 has a 10-fold lower affinity for penicillin than PBP2 [26]. The L421P alteration in ponA has been associated with contributing to ceftriaxone resistance [17], sometimes with respect to mosaic strains specifically [17]. All instances of L421P were recorded. mtrR encodes a major transcriptional repressor of the mtr gene locus. The most common alteration on the mtrR gene associated with increased ceftriaxone resistance is the deletion of an adenine residue from the 13–base pair inverted repeat contained within the promoter region (mtrR delA) [10], resulting in a net increased production of the MtrC-MtrD-MtrE efflux pump, which increases antimicrobial efflux [9]. Because the majority of reports excluded alterations in the mtrR coding region, we only included information on mtrR delA.
Estimation of Algorithm Sensitivity and Specificity
We developed algorithms targeting penA alterations, including mosaicism and nonmosaicism, and non-penA (penB, mtrR, and ponA) gene alterations to predict decreased susceptibility to ceftriaxone in N. gonorrhoeae strains identified in our literature review. Algorithms were developed by using different combinations of the individual genetic alterations with high sensitivities or specificities; we reported the combinations resulting in the highest sensitivities or specificities. Sensitivity was calculated by dividing the number of CRO-DS isolates with the targeted genetic modification by the total number of CRO-DS isolates. Specificity was calculated by dividing the number of ceftriaxone-susceptible (CRO-S) isolates without the targeted genetic modification by the total number of CRO-S isolates.
RESULTS
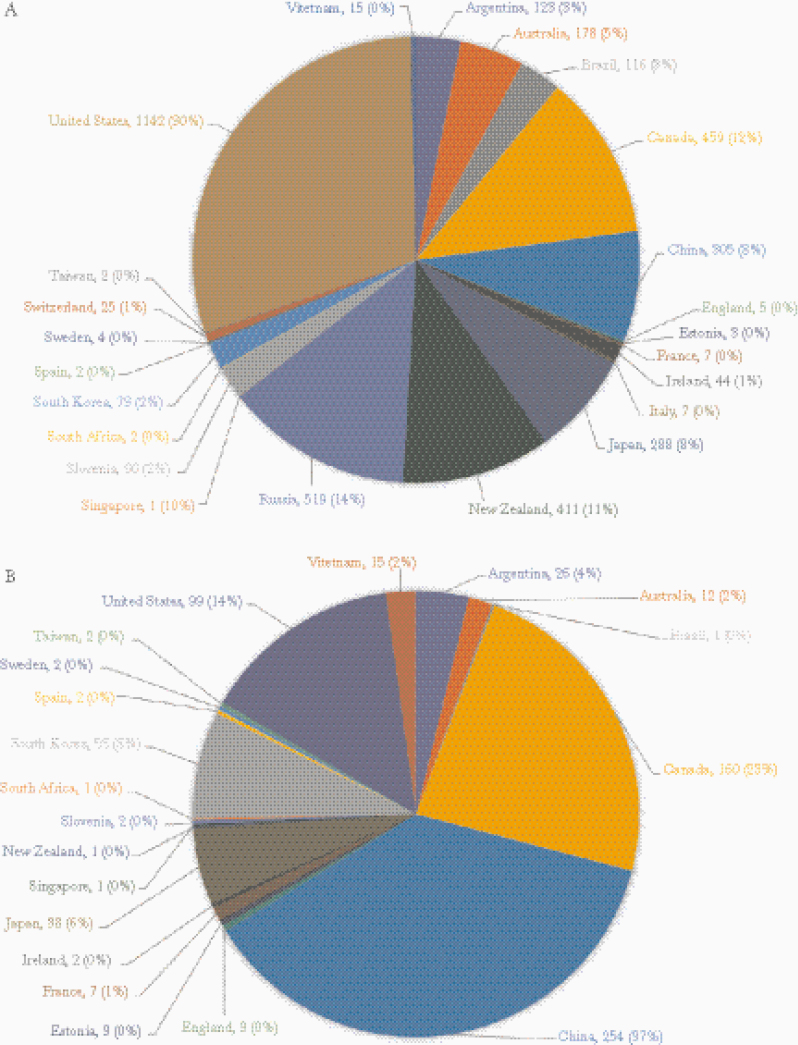
In total, we compiled 3821 sequenced N. gonorrhoeae strains from 23 countries. Five countries accounted for 75% of all characterized strains: the United States (30%), Russia (14%), Canada (12%), New Zealand (11%), and China (8%). (Figure 1) Among all identified strains, 684 (18%) were CRO-DS, originating from 20 countries, of which China (37%), Canada (23%), United States (14%), South Korea (8%), and Japan (6%) were the most frequent. For each strain, we report the specific genetic mutations associated with decreased susceptibility to ceftriaxone in penA, penB, ponA, and mtrR (see Supplementary Data).
Figure 1.
Geographic distribution of Neisseria gonorrhoeae strains with minimum inhibitory concentration data, by country (N = 3821). A, All strains. B, Strains with decreased susceptibility.
Susceptibility Analysis in penA Genes
No penA amino acid mutation or combination of mutations yielded both high sensitivity and specificity for decreased susceptibility to ceftriaxone. The penA amino acid mutations that yielded the highest sensitivities or specificities are listed in Table 1. Among all mosaic CRO-DS strains, the L447V mutation was found in every strain (100% sensitivity, 0.8% specificity). Each of the mutations I312M, V316T/P, H541N, F504L, and A510V yielded similar sensitivities (>95%) and specificities (<5%). In contrast, A311V and T483P/S were not found in any CRO-S mosaic strains, corresponding to 100% specificity, but had sensitivities <10%.
Table 1.
Amino Acid Alterations and Combinations in the penA Gene of Neisseria gonorrhoeae Strains Associated With the Highest Sensitivity and Specificity for Decreased Susceptibility to Ceftriaxone
| Mosaic penA | Nonmosaic penA | All Strains | ||||
|---|---|---|---|---|---|---|
| penA Alterations | Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity |
| L447V | 1 | 0.008 | 0.003 | 0.963 | 0.447 | 0.742 |
| A311V | 0.092 | 1 | 0 | 0.999 | 0.039 | 0.999 |
| I312M | 0.98 | 0.032 | 0 | 0.997 | 0.429 | 0.781 |
| T483P/S | 0.082 | 1 | 0 | 0.997 | 0.036 | 0.998 |
| V316T/P | 0.962 | 0.043 | 0 | 0.997 | 0.416 | 0.793 |
| H541N | 0.957 | 0.017 | 0.068 | 0.907 | 0.464 | 0.699 |
| F504L | 0.957 | 0.012 | 1 | 0.027 | 0.981 | 0.023 |
| A510V | 0.957 | 0·012 | 1 | 0.027 | 0.981 | 0.023 |
| ≥1 of G542S, P551L/S, A501V/T | 0.032 | 0.972 | 0.947 | 0.450 | 0.583 | 0.623 |
| A501V/T | 0.007 | 0.997 | 0.661 | 0.919 | 0.369 | 0.936 |
| ≥1 of G542S, P551L/S, I566V | 0.359 | 0.810 | 0.953 | 0.328 | 0.716 | 0.487 |
| ≥1 of G542S, P551L/S, A574N | 0.359 | 0.582 | 0.950 | 0.207 | 0.723 | 0.276 |
| ≥1 of P551L/S and I566V | 0.360 | 0.582 | 0.908 | 0.386 | 0.690 | 0.422 |
While individual genetic mutations resulted in specificities or sensitivities of ≥95%, they were associated with sensitivity or specificity values of <10%. As a result, we screened various combinations of genetic alterations, and substantial improvements were found for the nonmosaic penA strains. Among those strains, identification of ≥1 of certain amino acid alterations—G542S, P551L/S, or A501V/T—resulted in 94.7% sensitivity and 45% specificity for predicting CRO-DS strains; isolated detection of A501V/T resulted in sensitivity of 66.1% and specificity of 91.9%. Finally, detecting only A510V in nonmosaic strains resulted in similar sensitivity (100%) and specificity (2.7%) to that seen in mosaic strains.
Among all N. gonorrhoeae strains, alterations at F504L or A510V each produced high sensitivities (98.1%). In contrast, detection of A501V/T resulted in a sensitivity of 36.9% and specificity of 93.6%, while detection of A311V or T483P/S resulted in <4% sensitivities but >99.0% specificities (Table 1).
Susceptibility Analysis in Non-penA Genes
Similarly, no non-penA amino acid mutation or combination of mutations yielded both high sensitivities and specificities. As a result, as done with penA, different combinations of non-penA alterations were screened. The non-penA amino acid mutations that yielded the highest sensitivities or specificities are listed in Table 2. Among mosaic strains, detecting ponA L421P, either penB G120 or A121, or both penB alterations all yielded sensitivities >97% and specificities of approximately 10%. In contrast, nonmosaic strains yielded much higher specificity/sensitivity combinations. Detecting mtrR delA resulted in 95% sensitivity and 61% specificity, while additional detection of L421P in ponA slightly decreased sensitivity to 89% but notably increased specificity to 72%. Alternatively, detecting only L421P resulted in a higher rate capture rate of CRO-DS strains (99.6% sensitivity, 45.4% specificity). Among all penA strains, detection of the L421P mutation resulted in 99.6% sensitivity and 45.4% specificity; additional detection of ≥1 of the G120/A121 alterations in penB increased specificity to 60.7% (92.2% sensitivity). (Table 2)
Table 2.
Amino Acid Alterations and Combinations in Non-penA Genes of Neisseria gonorrhoeae Strains Associated With the Highest Sensitivity and Specificity for Decreased Susceptibility to Ceftriaxone
| Mosaic penA | Nonmosaic penA | All Strains | ||||
|---|---|---|---|---|---|---|
| Non-penA Alterations | Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity |
| ponA L421P | 1 | 0.108 | 0.991 | 0.521 | 0.996 | 0.454 |
| mtrR delA | 0.954 | 0.172 | 0.952 | 0.609 | 0.953 | 0.528 |
| mtrR delA + ponA L421P | 0.919 | 0.239 | 0.893 | 0.717 | 0.906 | 0.64 |
| ≥1 penB G120, A121 alteration(s) | 0.988 | 0.111 | 0.945 | 0.486 | 0.966 | 0.421 |
| mtrR delA + penB G120 +A121 alteration(s) | 0.971 | 0.38 | 0.852 | 0.721 | 0.918 | 0.649 |
| penB G120 + A121 alterations | 0.984 | 0.126 | 0.902 | 0.607 | 0.943 | 0.523 |
| ponA L421P + and ≥1 of penB G120, A121 alteration(s) | 0.977 | 0.127 | 0.862 | 0.684 | 0.922 | 0.607 |
Algorithms to Predict Decreased Susceptibility to Ceftriaxone
The sensitivity and specificity results above were used to create several testing algorithms to predict CRO-DS N. gonorrhoeae strains. We present 4 primary algorithms: (1) penA detection, (2) penA detection with mosaicism determination, (3) non-penA detection, and (4) non-penA detection with mosaicism determination. (Figures 2 and 3) Testing for all genetic loci in these algorithms are intended to be done simultaneously.
Figure 2.
Two sets of molecular algorithms using penA. A, Algorithm that excludes mosaicism determination. B, Algorithm that includes mosaicism determination, which can be done by screening penA amino acid positions 375–377. Sensitivity and specificity values are for decreased susceptibility to ceftriaxone. Testing for all genetic loci in these algorithms are intended to be done simultaneously, and not necessarily in a stepwise fashion. Asterisks denote combinations of amino acid alterations for which no strains with decreased susceptibility have been reported.
Figure 3.
Two sets of molecular algorithms using non-penA genes. A, Algorithm that excludes mosaicism determination. B, Algorithm that includes mosaicism determination, which can be done by screening of penA amino acid positions 375–377. Sensitivity and specificity values are for decreased susceptibility to ceftriaxone. Testing for all genetic loci in these algorithms are intended to be done simultaneously, and not necessarily in a stepwise fashion. Asterisks denote combinations of amino acid alterations for which no strains with decreased susceptibility have been reported.
In strategy 1, detection of A510V and A311V in penA yielded 4% sensitivity and 100% specificity for predicting CRO-DS strains, while detection of A510V without A311V yielded 94% sensitivity and 25% specificity (Figure 2A). In strategy 2, the penA algorithm is first stratified by mosaic strain status, with the results separated according to the presence or absence of mosaic alterations. Mosaic strains with the presence of L447V and ≥1 of G542S, P551L/S, or A501V/T was associated with 97% sensitivity and 7% specificity for predicting CRO-DS strains, while L447V without any of the 3 alterations was associated with 3% sensitivity and 94% specificity. In nonmosaic strains, detection of L447V without G542S, P551L/S, and A501V/T resulted in <1% sensitivity and 98% specificity, and detection of ≥1 of the 3 alterations without L447V resulted in 95% sensitivity and 62% specificity (Figure 2B).
In strategy 3, detection of ponA L421P and ≥1 of G120 or A121 in penB resulted in 92% sensitivity and 61% specificity for CRO-DS. However, without either alteration in penB, the sensitivity and specificity dropped to 2% and 90%, respectively (Figure 3A). In strategy 4, similar to strategy 2, the algorithm is first stratified by mosaic and nonmosaic strain status. Detection of both ponA L421P and mtrR delA in mosaic strains resulted in 95% sensitivity and 31% specificity for CRO-DS strains, while detection of only ponA L421P resulted in 2% sensitivity and 96% specificity. In nonmosaic strains, sensitivity and specificity were 89% and 72% respectively when both alterations were present. However, absence of mtrR delA resulted in substantial reduction in sensitivity (4%) but increased specificity (85%) (Figure 3B).
DISCUSSION
We present findings on 3821 sequenced N. gonorrhoeae strains from around the world with reported MICs to ceftriaxone. For each strain, we reported the genetic mutations associated with decreased susceptibility to ceftriaxone in the penA, penB, ponA, and mtrR genes. We compiled and analyzed the genetic mutations associated with decreased susceptibility to ceftriaxone from a global collection of isolates. Through our analysis, we report the sensitivities and specificities associated with the detection of those genetic determinants. We proposed 4 algorithms for detection of decreased susceptibility to ceftriaxone in N. gonorrhoeae that can guide development of future diagnostic assays.
Our analysis of all CRO-DS strains found 380 mosaic and 305 nonmosaic strains. Previous reports have suggested that a primary mechanism by which N. gonorrhoeae achieves resistance is through acquisition of a mosaic penA allele [23]. However, we found that using mosaic penA to predict CRO-DS strains would result in missing a substantial number of strains with decreased susceptibility. Our findings are consistent with other reports that have found mosaicism insufficient for predicting ceftriaxone resistance and highlight the importance of reviewing isolates from various parts of the world [11].
Our analysis identified high specificities (>86%) associated with detection of A311V, A501P/T/V, and G542S when analyzed among all N. gonorrhoeae strains, suggesting they are viable predictors of decreased susceptibility and higher MIC values. Similarly, Demczuk et al [27] were able to predict (R2 = 0.721) MICs to ceftriaxone in N. gonorrhoeae strains using 5 genetic loci in penA: A311V, A501P/T/V, N512Y, A516G, and G542S. However, our data suggest that N512Y and A516G were associated with lower specificities and might be less important within a global collection of strains. (Supplementary Table 4) The differences in our findings likely reflect the global variability of these alterations, because their analysis was limited to isolates from 4 countries. In addition, we used a categorical classification of decreased susceptibility, whereas their report used MIC values [27].
For mosaic penA strains, we found high sensitivities but very low specificities for CRO-DS strains among alterations I312M, V316T/P, N512Y, and G545S, signifying that while the majority of CRO-DS strains have these alterations, these loci are not key drivers of decreased susceptibility. However, this does not preclude them from influencing ceftriaxone susceptibility. Tomberg et al [28] showed that V316T, G545S, and I312M display epistasis, such that reverting these alterations in mosaic penA completely abolished resistance, while introducing them into wild-type strains had minor effects on MIC. That report also demonstrated decreased MICs after reverting N512Y to wild type in mosaic strains [28]. The mosaic amino acid alterations A311V, T483P/S, A501V/P/T, and P551S all have low sensitivities and high specificities. They are in close proximity to the active site of PBP2 acylation and have been associated with higher ceftriaxone MICs [6, 12, 28, 29]. While rare, they are highly associated with resistance, and are strong candidates to be used in molecular assays to predict CRO-DS strains.
For nonmosaic penA strains, we found A501V/T to be strongly associated with decreased susceptibility to ceftriaxone. The A501V/T residue is located on the β3-β4 loop that has mutations critical for penicillin resistance, with bulkier side chains potentially producing steric clash with the R1 group of ESCs [28]. In addition, introducing A501V/T in vitro increased MIC to ceftriaxone [28]. In contrast, the roles G542S and P551L/S have in contributing to resistance are inconclusive with nondiscriminatory sensitivities and specificities (Supplementary Table 3). However, G542S and P551L/S were previously shown to be associated with nonmosaic CRO-DS strains [22]. Monitoring for those alterations as more genetic and antibiotic susceptibility data emerge will be important. Finally, despite being found in all nonmosaic strains, insD345 does not appear to be linked to decreased ceftriaxone susceptibility [13].
In our report, penB G120/A121, ponA L421P, and mtrR delA were all shown to be important in contributing to N. gonorrhoeae decreased susceptibility to ceftriaxone, with high sensitivities and specificities. Our findings are consistent with the literature identifying the importance of penB, ponA, and mtrR in contributing to decreased susceptibility to ceftriaxone in N. gonorrhoeae [17]. Demczuk et al [27] also found that all 3 of those alterations are important in predicting ceftriaxone MICs in N. gonorrhoeae. The specificities of penB G120/A121, ponA L421P, and mtrR delA all decreased substantially when detected in mosaic strains but increased in nonmosaic penA strains, suggesting interactions between these 3 loci and nonmosaic penA alleles in the development of decreased susceptibility to ceftriaxone.
The molecular algorithms to predict CRO-DS strains are proposed using a global genetic analysis. The primary approach in establishing the molecular algorithms was aimed at maximizing either sensitivity or specificity. High sensitivity ensures that nearly all CRO-DS strains are captured, while high specificity ensures that if detected, the strain will likely have decreased susceptibility. From this approach, we have established targets for a molecular assay that could be powerful and versatile in predicting CRO-DS strains. The proposed algorithms could achieve sensitivities or specificities >95%, with certain sensitivities accompanied by specificities >60%. Those algorithms lay the foundation for the development of molecular assays that could be used in a global population of N. gonorrhoeae strains to predict decreased susceptibility to ceftriaxone. Moreover, by proposing 4 different testing strategies based on 4 different genes (penA, penB, mtrR, and ponA), we present pathways that have the potential to be tailored based on local N. gonorrhoeae epidemiology.
The use of the proposed algorithms in practice will depend on the frequency and distribution of strains with decreased susceptibility in a target population. Based on our reported sensitivities and specificities, positive and negative predictive values could be estimated. For example, using a theoretical 5% and 30% prevalence of CRO-DS strains in a specific population, a testing strategy detecting ponA L421P mutations in nonmosaic strains (99.1% sensitivity and 52.1% specificity), the positive predictive value for decreased susceptibility would be 10% and 47%, respectively. Owing to high sensitivities, the negative predictive value would be >99% in that testing strategy at both 5% and 30% prevalence of decreased susceptibility. The prevalence of mosaic strains is low, comprising 8.6% and 2.8% of the N. gonorrhoeae strains in the United States and China, respectively [14, 15], and as such, false-positives among mosaic strains would have minimal overall impact. Ultimately, the goals of the algorithms are to detect N. gonorrhoeae strains with decreased susceptibility to ceftriaxone, and this depends on the population that is being tested; as prevalence increases, so too does the positive predictive value and the need for a highly sensitive test. While the proposed molecular algorithms are preliminary and will require validation, they can be used as a framework to guide future research aimed at assessing the clinical performance in different populations.
Other molecular algorithms to predict N. gonorrhoeae CRO-DS strains have been reported. Peterson et al [30] reported a molecular assay to predict decreased susceptibility to cephalosporins, including ceftriaxone. Our algorithms achieved similar sensitivities with regard to ponA, mtrR, and penB (>95%), although with lower specificities. However, Peterson et al used only samples from Canada, which might limit generalizability. Doná et al [31] developed a molecular algorithm to predict resistance to ESCs based on screening for the Asp345 deletion (delD345), which is equivalent to a lack of insD345, and G545S in mosaic penA alleles X and XXXIV. Our results agree that detection G545S and delD345 are highly sensitive among mosaic strains (93.8% and 97.6%, respectively) but differ in the extremely low specificity (Supplementary Table 2). However, the algorithm was evaluated only in isolates from Switzerland and focused only on penA mosaic X and XXXIV alleles, which comprise approximately 18.5% and 31.9% of all globally reported mosaic alleles. Thus, by performing our analysis on a global database of isolates, our report expands generalizability and can guide the development of future molecular assays predicting decreased susceptibility to ceftriaxone.
Our report has several limitations. First, we are limited by the availability of genomic data for both CRO-DS and CRO-S N. gonorrhoeae strains; strains from several countries were reported but not sequenced [10, 16]. Second, our whole-genome sequencing (WGS) analysis focused on the 83 penA amino acid alterations of the Ohnishi sequences only. This method was chosen to encapsulate the results of all studies with genetic characterizations, because most studies have either not performed WGS or have not published their results. We attempted to reach all authors that did not publish their WGS results but did not always receive a reply. Although we used prevalent alterations found in penA, it is possible that other alterations are on the rise and associated with decreased susceptibility to ceftriaxone. Finally, of the 4 proposed diagnostic algorithms, we cannot determine which would be optimal for detection of CRO-S infections, because our data set contains an overrepresentation of CRO-DS strains. Identifying such an algorithm is key, as there are currently limited options available for treatment of CRO-DS infections. Further research on the application of those diagnostic algorithms is therefore needed.
We identified, compiled, and analyzed findings from 3821 global isolates of N. gonorrhoeae with reported MICs to ceftriaxone. We analyzed genetic information for all N. gonorrhoeae isolates with decreased susceptibility to ceftriaxone and generated preliminary molecular algorithms to predict decreased susceptibility to ceftriaxone. The identified genetic targets can be used to guide the development of molecular assays to predict CRO-DS strains, with utility in a variety of global settings.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We would like to acknowledge Yonatan Grad, MD, PhD, for assisting us in accessing the whole-genome sequencing data for hundreds of N. gonorrhoeae strains in 2 of his reports. We also acknowledge Raquel Bonelli, PhD, Ana Paula Ramalho, PhD, Koji Yahara, PhD, Sidharath Dev Thakur, PhD, and Jo-Anne Dillon, PhD, FCAHS, FRSC, for providing us the genetic characterization data from their work.
Financial support. This work was supported by the National Institutes of Health (grants 5P30MH058107 to J. D. K. and T32MH080634 to P. C. A.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Report on global sexually transmitted infection surveillance, 2018. Geneva, Switzerland:World Health Organization, 2018. [Google Scholar]
- 2. Ohnishi M, Golparian D, Shimuta K, et al. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 2011; 55:3538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. WHO guidelines for the treatment of Neisseria gonorrhoeae. Geneva, Switzerland: World Health Organization, 2016. https://apps.who.int/iris/handle/10665/246114. Accessed 1 August 2019. [Google Scholar]
- 4. Fifer H, Saunders J, Soni S, Sadiq ST, FitzGerald M. 2018 UK national guideline for the management of infection with Neisseria gonorrhoeae. Int J STD AIDS 2020; 31:4–15. [DOI] [PubMed] [Google Scholar]
- 5. Jamaludin N, Gedye K, Collins-Emerson J, Benschop J, Nulsen M. Phenotypic and genotypic characterization of Neisseria gonorrhoeae isolates from new zealand with reduced susceptibility to ceftriaxone. Microb Drug Resist 2019; 25:1003–11. [DOI] [PubMed] [Google Scholar]
- 6. Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 2014; 27:587–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zapun A, Morlot C, Taha M-K. Resistance to β-lactams in Neisseria ssp due to chromosomally encoded penicillin-binding proteins. Antibiotics (Basel) 2016; 5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gill MJ, Simjee S, Al-Hattawi K, Robertson BD, Easmon CS, Ison CA. Gonococcal resistance to beta-lactams and tetracycline involves mutation in loop 3 of the porin encoded at the penB locus. Antimicrob Agents Chemother 1998; 42:2799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hagman KE, Pan W, Spratt BG, Balthazar JT, Judd RC, Shafer WM. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 1995; 141(pt 3):611–22. [DOI] [PubMed] [Google Scholar]
- 10. Olsen B, Pham TL, Golparian D, Johansson E, Tran HK, Unemo M. Antimicrobial susceptibility and genetic characteristics of Neisseria gonorrhoeae isolates from Vietnam, 2011. BMC Infect Dis 2013; 13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gianecini RA, Golparian D, Zittermann S, et al. ; Gonococcal Antimicrobial Susceptibility Surveillance Programme-Argentina (GASSP-AR) Working Group . Genome-based epidemiology and antimicrobial resistance determinants of Neisseria gonorrhoeae isolates with decreased susceptibility and resistance to extended-spectrum cephalosporins in Argentina in 2011-16. J Antimicrob Chemother 2019; 74:1551–9. [DOI] [PubMed] [Google Scholar]
- 12. Seike K, Yasuda M, Hatazaki K, et al. Novel penA mutations identified in Neisseria gonorrhoeae with decreased susceptibility to ceftriaxone isolated between 2000 and 2014 in Japan. J Antimicrob Chemother 2016; 71:2466–70. [DOI] [PubMed] [Google Scholar]
- 13. Shaskolskiy B, Dementieva E, Kandinov I, et al. Resistance of Neisseria gonorrhoeae isolates to beta-lactam antibiotics (benzylpenicillin and ceftriaxone) in Russia, 2015–2017. PLoS One 2019; 14:e0220339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peng JP, Yin YP, Chen SC, et al. A whole-genome sequencing analysis of Neisseria gonorrhoeae isolates in China: an observational study. EClinicalMedicine 2019; 7:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gose S, Nguyen D, Lowenberg D, Samuel M, Bauer H, Pandori M. Neisseria gonorrhoeae and extended-spectrum cephalosporins in California: surveillance and molecular detection of mosaic penA. BMC Infect Dis 2013; 13:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Laat MM, Wind CM, Bruisten SM, et al. Ceftriaxone reduced susceptible Neisseria gonorrhoeae in the Netherlands, 2009–2017: from penA mosaicism to A501T/V non-mosaicism. Sex Transm Dis 2019; 46:594–601. [DOI] [PubMed] [Google Scholar]
- 17. Lindberg R, Fredlund H, Nicholas R, Unemo M. Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: association with genetic polymorphisms in penA, mtrR, porB1b, and ponA. Antimicrob Agents Chemother 2007; 51:2117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whiley DM, Jennison A, Pearson J, Lahra MM. Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin. Lancet Infect Dis 2018; 18:717–8. [DOI] [PubMed] [Google Scholar]
- 19. Whiley DM, Goire N, Lambert SB, Nissen MD, Sloots TP, Tapsall JW. Reduced susceptibility to ceftriaxone in Neisseria gonorrhoeae is spread internationally by genetically distinct gonococcal populations. J Antimicrob Chemother 2011; 66:1186–7. [DOI] [PubMed] [Google Scholar]
- 20. Kenyon C, Buyze J, Spiteri G, Cole MJ, Unemo M. Population-level antimicrobial consumption is associated with decreased antimicrobial susceptibility in Neisseria gonorrhoeae in 24 European countries: an ecological analysis. J Infect Dis 2019; 221:1107–16. [DOI] [PubMed] [Google Scholar]
- 21. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100. 29th ed. Wayne, PA:Clinical and Laboratory Standards Institute,2019. [Google Scholar]
- 22. Whiley DM, Goire N, Lambert SB, et al. Reduced susceptibility to ceftriaxone in Neisseria gonorrhoeae is associated with mutations G542S, P551S and P551L in the gonococcal penicillin-binding protein 2. J Antimicrob Chemother 2010; 65:1615–8. [DOI] [PubMed] [Google Scholar]
- 23. Singh A, Tomberg J, Nicholas RA, Davies C. Recognition of the β-lactam carboxylate triggers acylation of N. gonorrhoeae penicillin-binding protein 2. J Biol Chem 2019; 294:14020–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Demczuk W, Sidhu S, Unemo M, et al. Neisseria gonorrhoeae sequence typing for antimicrobial resistance, a novel antimicrobial resistance multilocus typing scheme for tracking global dissemination of N. gonorrhoeae strains. J Clin Microbiol 2017; 55:1454–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deng X, Allan-Blitz L-T, Klausner JD. Using the genetic characteristics of Neisseria gonorrhoeae strains with decreased susceptibility to cefixime to develop a molecular assay to predict cefixime susceptibility. Sex Health 2019; 16:488–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dougherty TJ. Involvement of a change in penicillin target and peptidoglycan structure in low-level resistance to beta-lactam antibiotics in Neisseria gonorrhoeae. Antimicrob Agents Chemother 1985; 28:90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Demczuk W, Martin I, Sawatzky P, et al. Equations to predict antimicrobial MICs in Neisseria gonorrhoeae using molecular antimicrobial resistance determinants. Antimicrob Agents Chemother 2020; 64:e02005-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tomberg J, Unemo M, Davies C, Nicholas RA. Molecular and structural analysis of mosaic variants of penicillin-binding protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae: role of epistatic mutations. Biochemistry 2010; 49:8062–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tomberg J, Unemo M, Ohnishi M, Davies C, Nicholas RA. Identification of amino acids conferring high-level resistance to expanded-spectrum cephalosporins in the penA gene from Neisseria gonorrhoeae strain H041. Antimicrob Agents Chemother 2013; 57:3029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peterson SW, Martin I, Demczuk W, et al. Molecular assay for detection of genetic markers associated with decreased susceptibility to cephalosporins in Neisseria gonorrhoeae. J Clin Microbiol 2015; 53:2042–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Donà V, Smid JH, Kasraian S, et al. Mismatch amplification mutation assay-based real-time PCR for rapid detection of Neisseria gonorrhoeae and antimicrobial resistance determinants in clinical specimens. J Clin Microbiol 2018; 56:e00365-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.